Key Points
Question
What is the rate of axial elongation and its risk factors in adults with high myopia?
Findings
In this cohort study of 9161 patient visits by 1877 adults, the mean (SD) annual growth of axial length was 0.05 (0.24) mm, which accounts for 0.17% of the mean value of the entire axial length of eyes with high myopia. A severe axial elongation was associated with sex, baseline axial length, best-corrected visual acuity, presence of myopic maculopathy, and prior choroidal neovascularization.
Meaning
These findings suggest that axial length continues to increase in adults with high myopia, and prevention of myopia seems warranted because risk factors for axial elongation do not appear to be modifiable.
Abstract
Importance
Pathologic myopia due to an excessive increase of axial length is associated with severe visual impairments. Systematic analyses to determine the rate of and the risk factors associated with the axial elongation in adults with high myopia based on long-term follow-up of a large population are needed.
Objective
To determine the risk factors associated with axial elongation in adults with high myopia.
Design, Setting, and Participants
This cohort study used the medical records of 43 201 patient visits in a single-hospital database that were collected from January 3, 2011, to December 28, 2018. A total of 15 745 medical records with the patients’ sex, best-corrected visual acuity (BCVA), axial length, type of myopic maculopathy, and the presence or absence of choroidal neovascularization (CNV) were reviewed. Data were analyzed from April 3, 2019, to August 5, 2020.
Main Outcomes and Measures
Changes in the axial length at each examination were calculated. The significance of the associations between the annual increase of the axial length and age, sex, baseline axial length, types of myopic maculopathy, and a history of CNV was determined. Generalized linear mixed models were used to evaluate the strength of the risk factors associated with an increase of the axial length in high myopia.
Results
Among 1877 patients with 9161 visits included in the analysis, the mean (SD) age was 62.10 (12.92) years, and 1357 (72.30%) were women. The mean (SD) axial length was 29.66 (2.20) mm with a mean (SD) growth rate of 0.05 (0.24) mm/y. Among the 9161 visits, 7096 eyes (77.46%) had myopic maculopathy and 2477 eyes (27.04%) had CNV. The odds ratio for inducing a severe elongation of the axial length was 1.46 (95% CI, 1.38-1.55) for female sex, 0.44 (95% CI, 0.35-0.56) to 0.63 (95% CI, 13 0.50-0.78) for older than 40 years, 1.33 (95% CI, 1.15-1.54) for BCVA of less than 20/400, 1.67 (95% CI, 1.54-1.81) to 2.67 (95% CI, 2.46-2.88) for baseline axial length of 28.15 mm or greater, 1.06 (95% CI, 0.96-1.17) to 1.39 (95% CI, 1.24-1.55) for the presence of maculopathy, and 1.37 (95% CI, 1.29-1.47) for prior CNV.
Conclusions and Relevance
This cohort study found continuing axial elongation in adults with high myopia. The risk factors for elongation do not appear to be modifiable, so prevention of myopia may be the best approach to reduce the incidence of pathologic myopia and its complications in the future.
This cohort study assesses the rate of axial elongation among patients with myopia and the risk factors associated with a severe increase in axial length in adults with high myopia.
Introduction
Myopia has become a great concern worldwide. An increase in the prevalence of myopia and high myopia can result in an increase in the number of eyes with pathologic myopia. This is important because pathologic myopia can lead to myopic maculopathy and progress to severe visual impairment.
Earlier studies have shown that the ocular axial length continues to grow in adults with high myopia or pathologic myopia. For example, an earlier study of 60 adult eyes with high myopia (axial length ≥26.00 mm) found axial elongation by 0.03 to 0.06 mm/y during a 6-year period.1 This is important because a continuous increase in axial length is considered to be a risk factor associated with developing pathologic myopia.
To our knowledge, the risk factors for axial elongation in adults with high myopia have not been determined. Thus, the purpose of this study was to determine the rate of change in axial length among patients with myopia. We also determined the factors that were associated with a severe increase in axial length in adults with high myopia.
Methods
Ethical Approval
The procedures used in this cohort study conformed to the tenets of the Declaration of Helsinki2 and were approved by the ethics committee of Tokyo Medical and Dental University, Tokyo, Japan. The institutional review board and ethics committee approved the methods of medical data collection. All patients provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Acquisition
This was a hospital-based cohort study using data collected in the Advanced Clinical Center for Myopia, Tokyo Medical and Dental University. A schematic diagram of the workflow is provided in Figure 1. All registered patients had at least 1 eye with an axial length greater than 26.5 mm and/or a refractive error (spherical equivalent) of less than −6.0 diopters (D). None of the eyes had any retinochoroidal diseases such as age-related maculopathy or choroiditis. All medical data were collected from January 3, 2011, to December 28, 2018. The number of patient visits was counted daily. Thus, no matter how many times a patients was examined on the same day, each examination was counted as 1 visit. Visits requiring all examinations or treatments were counted, and visits for other purposes such as making surgery appointments or referrals were not counted. Finally, 15 475 of 43 201 medical records of patient visits with axial length data were collected and further paired with information per visit such as age, sex, best-corrected visual acuity (BCVA), myopic maculopathy category, and prior choroidal neovascularization (CNV). All patients were Asian individuals, and patient sex was recorded based on categorizations given on health insurance cards. The increase in the axial length at each examination was calculated by comparing the axial length with that of the nearest past record. The first record of the axial length in each patient was used as the baseline axial length, and the following axial length values were used to calculate the axial length changes at that time and recorded in our database.
Figure 1. Schematic of Workflow.
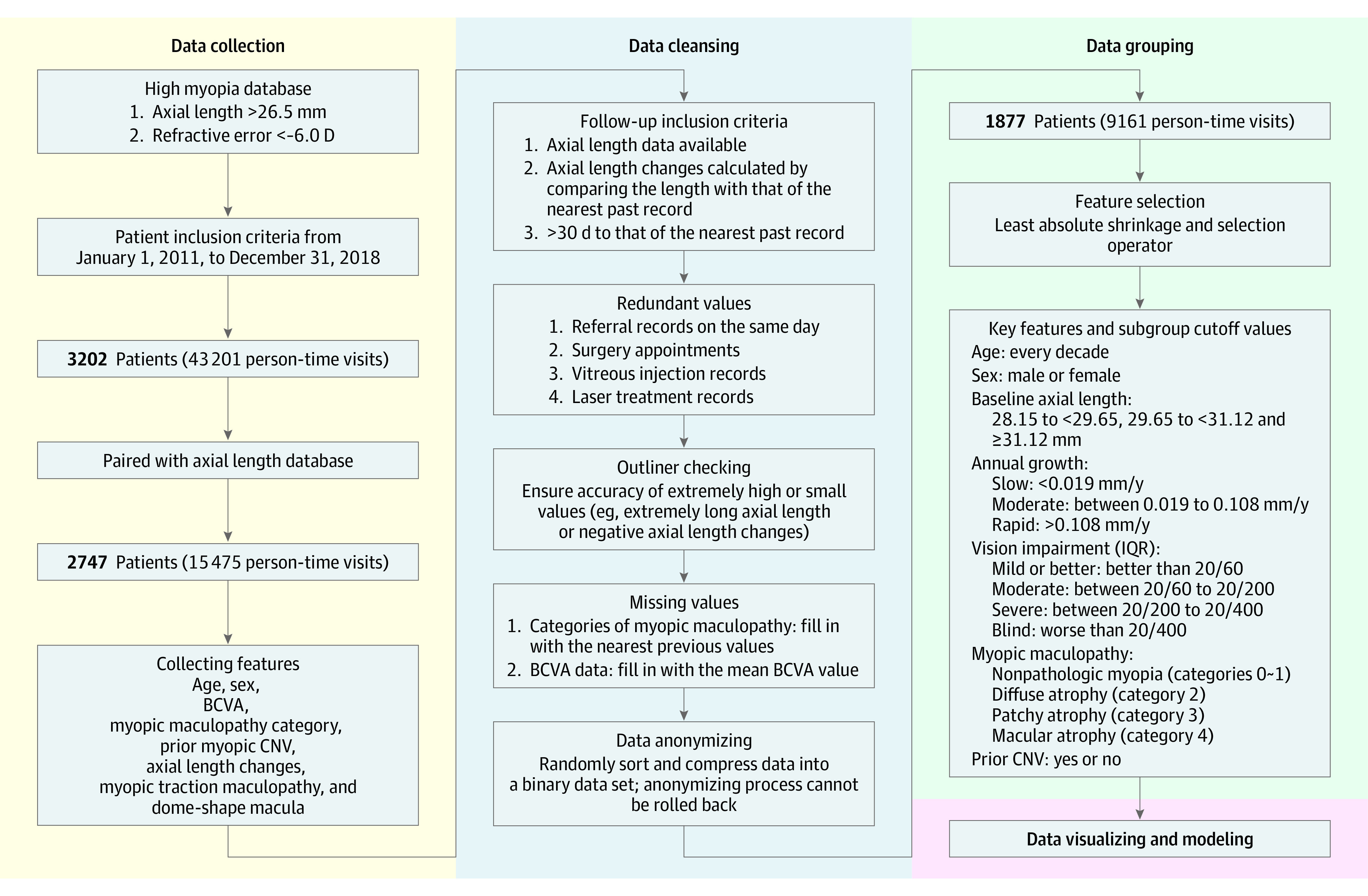
Our project consisted of 4 steps: data collection, data cleansing, data grouping, and data display. First, we collected medical records that included basic information about patient sex, age, and other relevant data. Then, data cleansing was performed, with a particular focus on filtering out redundant values and inserting missing values. The data were then grouped by different criteria, analyzed, and displayed. BCVA indicates best-corrected visual acuity; CNV, choroidal neovascularization; IQR, interquartile range.
For these data, patient age was recorded by comparing the date of birth and the date of the examination. The BCVA was measured with a Snellen chart at 50 cm. The category of myopic maculopathy was recorded after examination of images obtained with a fundus camera (TRC 50DX [Topcon Medical Systems Co] or VX-10i [Kowa Co]) at each examination. Pathologic myopia was classified into 5 categories according to the classification system established by the Meta-analysis for Pathologic Myopia (META-PM) Study Group3: no myopic retinal lesions (category 0), tessellated fundus only (category 1), diffuse atrophy (category 2), patchy atrophy (category 3), and macular atrophy (category 4). An eye was classified as having pathologic myopia when the myopic maculopathy was equal to or more serious than diffuse atrophy (category 2) according to the META-PM study.4 A prior CNV was determined from an examination of the medical records (eg, when an eye was first diagnosed with CNV). The data before this time point were marked as CNV-negative; those after this time point were recorded as CNV-positive. The value of the axial length in each medical record was documented by pairing the results from the biometer (IOLMaster 500; Carl Zeiss Meditec).
Data Cleansing
To decrease the random errors caused by the frequent measurements of axial length, the annual growth rates that were calculated from the values within the nearest examination time (measured within 1 month) were excluded as redundant values. Patients younger than 20 years were also excluded because our research was focused on adults. In addition, eyes with an axial length of less than 21.00 mm were excluded to avoid the influence of microphthalmos.
Missing values were filled in before analyzing. Although we had a complete data set of age, sex, prior CNV, and axial length, we had an incomplete data set for 35.06% of the categories of myopic maculopathy. Considering the regular follow-up examinations and relatively slow changes of the fundus, the missing categories of myopic maculopathy values were filled in with the nearest previous values. In addition, 76 of 15 475 missing values were found for the BCVA data. These missing values were filled in by the mean BCVA value.
After these steps, 9161 records of 1877 patients met the inclusion criteria and were used for the final analyses. Of the 1877 included patients, 1769 had an axial length elongation in both eyes and 108 eyes had an axial length elongation in 1 eye.
Data Grouping and Examinations
We analyzed the mean axial length and annual growth rate at different ages for both sexes. We divided the data by patient ages with decade intervals and observed the distribution of the increase of the axial length at the different age groups. The pathological changes in the myopic fundus and the increase of the axial length may have reciprocal effects, and we compared the axial length and its growth rate for the different categories of myopic maculopathy. In addition, we grouped patients into 4 groups by the 3 BCVA cutoff values of 20/60, 20/200, and 20/400 based on the International Classification of Diseases, 11th Revision. Because no previous study had been performed, we used the 25% quantile values, median values, and 75% quantile values of our database as axial length cutoff values to explore the differences among different axial lengths.
Statistical Analysis
Data were analyzed from April 3, 2019, to August 5, 2020. Statistical processing was performed with R, version 3.6.3 (R Program for Statistical Computing). Among the eyes with different annual rates of axial length growth, descriptive analyses were conducted in determining the risks of age, sex, BCVA, myopic maculopathy categories of axial length growth rates, and prior CNV after guarding the multicollinearity relations by a variance inflation factor of less than 5. We used analysis of covariance to decrease the influences of potential confounding variables, included a test for interactions, and compared the features within each subgroup (2 tailed). Data were made visible through raincloud plots.5 To evaluate the weight of each risk factor in leading to severe axial elongation, factors were binned according to each grouping criteria and regressed using a generalized liner mixed model to test the differences between the continual variables that were controlled for multiple confounders. Unless otherwise indicated, data are expressed as mean (SD). Two-sided P < .05 indicated statistical significance.
Results
Among the 43 201 patient visits, we analyzed 9161 medical records (axial length elongation countable) of 3646 eyes from 1877 patients (1357 women [72.30%] and 520 men [27.70%]). The mean number of countable visits per person was 4.88 (2.81) times with a mean examination interval of 499.77 (289.88) days, and the mean (SD) follow-up time was 3.44 (1.79) years. The mean refractive error (spherical equivalent) among the phakic patients was −2.82 (2.50) D. Of the 9161 records, 2673 (29.18%) were from men and 6488 (70.82%) were from women. The mean age was 62.10 (12.92) years. Among the 9161 visits, 7096 eyes (78.91%) had myopic maculopathy and 2477 eyes (27.04%) had CNV. The mean baseline axial length was 29.66 (2.20) mm, and the mean annual growth rate was 0.05 (0.24) mm/y during a period of 8 years in eyes with high myopia. This accounted for 0.17% of the mean value of the axial length in all eyes. The median BCVA at the baseline was 20/25 (interquartile range, 20/50 to 20/20).
Associations of Age and Sex With Rate of Axial Elongation
The number of women in this study was greater than the number of men in each group (Table 1). Although the number of patients with severe annual growth and the annual growth rate both decreased with increasing age (Figure 2A), the significant interaction (P = .04) between age and BCVA suggests that the axial elongation in eyes with worse BCVA was greater than in eyes with better initial BCVA, but this difference gradually decreased with increased age and was slightly greater in eyes with better BCVAs (mean estimated coefficient values ranged from −0.03 to 0.005). The mean rate of axial elongation in women was 0.06 (0.12) mm/y in those aged 40 to 49 years, 0.07 (0.15) mm/y in those aged 50 to 59 years, and 0.06 (0.29) mm/y in those aged 60 to 69 years, which is faster than in men at 0.04 (0.14) mm/y in those aged 40 to 49 years, 0.04 (0.12) mm/y in those aged 50 to 59 years, and 0.03 (0.18) mm/y in those aged 60 to 69 years (P < .001).
Table 1. Distribution of Patients in Different Age Groups and Different Categories of Myopic Maculopathy.
| Characteristic | No. of eyes | Follow-up times, No./total No. (%)a | Annual growth of axial length, mm, mean (SD) | Axial length, mm, mean (SD) | |||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||
| Age, y | |||||||
| 20-29 | 71 | 51/2673 (1.91) | 81/6488 (1.25) | 0.05 (0.13) | 0.07 (0.13) | 28.40 (1.50) | 28.23 (1.36) |
| 30-39 | 160 | 149/2673 (5.57) | 171/6488 (2.64) | 0.06 (0.13) | 0.07 (0.13) | 29.93 (2.83) | 28.98 (1.78) |
| 40-49 | 550 | 379/2673 (14.18) | 787/6488 (12.13) | 0.04 (0.14) | 0.06 (0.12)b | 29.99 (1.97) | 29.79 (1.80) |
| 50-59 | 956 | 626/2673 (23.42) | 1311/6488 (20.21) | 0.04 (0.12) | 0.07 (0.15)b | 30.41 (1.95) | 30.01 (2.21) |
| 60-69 | 1231 | 881/2673 (32.96) | 1843/6488 (28.41) | 0.03 (0.18) | 0.06 (0.29)b | 30.21 (2.05) | 29.74 (2.08) |
| 70-79 | 1042 | 481/2673 (17.99) | 1736/6488 (26.76) | 0.03 (0.32) | 0.05 (0.33) | 29.95 (2.21) | 29.04 (2.32) |
| ≥80 | 372 | 106/2673 (3.97) | 559/6488 (8.62) | 0.02 (0.24) | 0.01 (0.22) | 29.93 (2.06) | 28.50 (2.40) |
| Category of myopic maculopathyc | |||||||
| 0 or 1 | 938 | 652 (24.39) | 1413/6488 (21.78) | 0.02 (0.13)d | 0.04 (0.12)e | 28.52 (1.72) | 28.05 (1.88) |
| 2 | 1641 | 1186 (44.37) | 2574/6488 (39.67) | 0.05 (0.13) | 0.06 (0.24)f | 30.22 (1.85) | 29.39 (2.07) |
| 3 | 799 | 501 (18.74) | 1338/6488 (20.62) | 0.04 (0.30) | 0.07 (0.33)g | 31.37 (1.96) | 30.73 (2.06) |
| 4 | 634 | 334 (12.50) | 1163/6488 (17.93) | 0.03 (0.30) | 0.05 (0.31) | 30.59 (2.05) | 29.91 (2.06) |
Indicates after baseline examination of axial length values.
P ≤ .001 vs corresponding annual growth among men.
Described in Figure 2.
P = .03 vs annual growth among men in category 3 and P = .02 vs annual growth among men in category 4.
P = .03 vs annual growth among women in category 4.
P = .02 vs annual growth among men in category 2.
P = .02 vs axial length among men in category 3.
Figure 2. Plots of Risk Factors Associated With Axial Elongation.
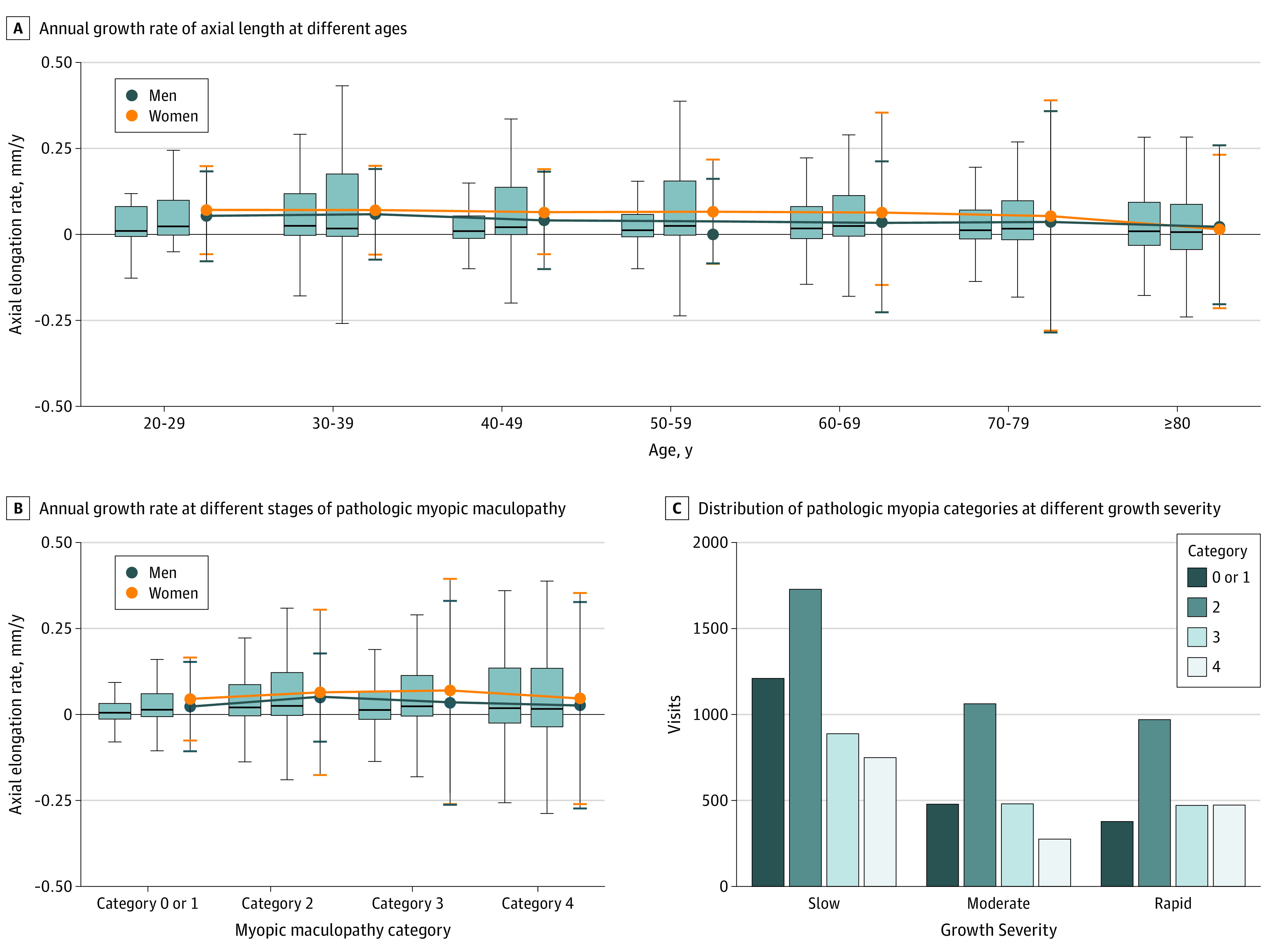
A, The rate of axial elongation is slightly slower after 30 years of age. The mean annual growth of axial length among women is higher than that among men. Box plots represent means and SDs (error bars indicate 95% CIs); dot plots represent medians and interquartile ranges. For each subgroup, the left box plot represents men and the right box plot represents women. B, The annual growth rate was greater in eyes with pathologic myopia, including those with diffuse atrophy (category 2), patchy atrophy (category 3), and macular atrophy (category 4) (maculopathy) than eyes with nonpathologic myopia (category 0) or tessellated fundus (category 1) among men. Among women, the annual growth rate was greater in eyes with categories 2 and 3 maculopathy than eyes with nonpathologic myopia, and no significant difference was seen in the growth rate between women with category 4 or with categories 0 or 1. The annual growth rate among women was greater in eyes with categories 2 (P = .008) and 3 (P < .001) maculopathy compared with that among men. Box plots represent means and SDs (error bars indicate 95% CIs); dot plots represent medians and interquartile ranges. For each subgroup, the left box plot represents men and the right box plot represents women. C, Proportions of eyes with categories 3 and 4 myopic maculopathy in the group with a moderate increase in axial length (0.019 to <0.108 mm) and in the rapid increase group (≥0.108 mm) are compared with the slow increase group (<0.019 mm).
Association Between Axial Elongation Rate and Myopic Maculopathy
The annual growth rate was greater in eyes with pathologic myopia, including those classified as having categories 3 (0.04 [0.30] mm/y) and 4 (0.03 [0.30] mm/y) maculopathy than eyes with nonpathologic myopia (0.02 [0.13] mm/y) in men (Figure 2B and Table 1). However, the annual growth rate among women was greater in eyes with category 4 maculopathy (0.05 [0.31] mm/y) than in eyes with nonpathologic myopia (0.04 [0.12] mm/y). The annual growth rate among women was greater in eyes with category 2 (0.06 [0.24] mm/y; P < .001) and category 3 (0.07 [0.33] mm/y; P = .02) than in those among men (0.05 [0.13] and 0.04 [0.30] mm/y, respectively).
Associations Between BCVA and Axial Length
An examination of the distribution of axial length among the different BCVA groups showed that axial length was longer in the moderate (0.04 [0.27] mm) and blind (0.05 [0.21] mm; P < .001) BCVA groups than that of the mild (0.04 [0.42] mm) or better (0.05 [0.21] mm; P = .03) BCVA groups. In addition, the significant interaction between baseline axial length and BCVA showed that the elongation pace was greater in eyes with longer baseline axial length than in those with shorter baseline axial length (P < .001), and the difference gradually increased with the decrease of BCVA.
Characteristics of Groups Classified According to Annual Growth Rates of Axial Length
We divided the patients into 3 groups according to their annual growth rate of axial length: less than 0.019 mm/y indicated a slow growth rate; 0.019 to less than 0.108 mm/y, a moderate growth rate; and 0.108 mm/y or greater, a rapid growth rate. The axial length was longer in the rapid growth rate group (0.27 [0.34] mm) than in the moderate (0.05 [0.03] mm; P < .001) and slow (−0.06 [0.14] mm; P < .001) growth rate groups. These findings indicated that eyes with longer axial lengths had a faster rate of axial elongation. The proportion of eyes with categories 3 and 4 maculopathy was higher in the moderate and rapid growth rate groups compared with the slow growth rate group (mean estimated coefficient values ranged from 0.01 to 0.03) (Figure 2C). In addition, the rapid growth rate group had a higher percentage of eyes with prior CNV than the slow or moderate growth rate groups (Table 2).
Table 2. Distribution of Patients Among Groups With Different Axial Length Annual Growth Severity and Visual Impairment .
| Characteristic | Annual growth severitya | Visual impairmentb | |||||
|---|---|---|---|---|---|---|---|
| Slow (n = 4575) | Moderate (n = 2295) | Rapid (n = 2291) | Mild or better (n = 6981) | Moderate (n = 1366) | Severe (n = 541) | Blindness (n = 273) | |
| Axial length, mean (SD), mm | 29.34 (2.31) | 29.78 (2.16)c | 30.15 (1.91) | 29.50 (2.21)d | 30.21 (2.19) | 30.07 (1.94) | 29.92 (2.08) |
| Age, mean (SD), y | 62.57 (13.03) | 61.59 (12.71) | 61.67 (12.88) | 60.21 (12.87) | 66.66 (11.43) | 69.74 (9.83) | 72.36 (10.54) |
| BCVA, median (IQR) | 20/25 (20/60 to 20/20) | 20/22 (20/40 to 20/20) | 20/25 (20/60 to 20/20) | 20/20 (20/25 to 20/16) | 20/125 (20/200 to 20/100) | 20/250 (20/333 to 20/250) | 20/1000 (20/1000 to 20/500) |
| Annual growth, mean (SD), mm/y | –0.06 (0.14)e | 0.05 (0.03) | 0.27 (0.34) | 0.05 (0.210) | 0.04 (0.27) | 0.06 (0.38) | 0.04 (0.42)d |
| Prior CNV, No. (%) | 1147 (25.07) | 554 (24.14) | 776 (33.87) | 1192 (17.07) | 715 (52.34) | 378 (69.87) | 192 (70.33) |
| Category of PM, No. (%)f | |||||||
| Non-PM | 1210 (26.45) | 478 (22.83) | 377 (16.46) | 1952 (27.96) | 83 (6.08) | 16 (2.96) | 14 (5.13) |
| Category 2 | 1728 (37.77) | 1062 (46.27) | 970 (42.34) | 3352 (48.02) | 320 (23.43) | 64 (11.83) | 24 (8.79) |
| Category 3 | 888 (19.41) | 480 (20.92) | 471 (20.56) | 1365 (19.55) | 353 (25.84) | 99 (18.30) | 22 (8.06) |
| Category 4 | 749 (16.37) | 275 (11.98) | 473 (20.65) | 312 (4.47) | 610 (44.66) | 362 (66.91) | 213 (78.02) |
Abbreviations: BCVA, best-corrected visual acuity; CNV, choroidal neovascularization; IQR, interquartile range; PM, pathologic myopia.
Slow indicates less than 0.019 mm; moderate, 0.019 to less than 0.108 mm; and rapid, 0.108 mm or greater.
Mild or better indicates BCVA better than 20/60; moderate, BCVA worse than 20/60 to 20/200; severe, BCVA worse than 20/200 to 20/400; and blindness, BCVA worse than 20/400.
P < .001 vs axial length in slow and rapid groups.
P < .001 vs the mild or better group and P = .03 vs the blindness group.
The negative value is attributable to fluctuating data. A stable axial length with a small measure error between 2 follow-ups would magnify to a small negative value.
Described in Figure 2.
Factors Associated With Rapid Annual Rate of Axial Elongation
To determine the risks associated with severe axial elongation, we examined the sex, age, BCVA, axial length, categories of myopic maculopathy, and CNV history between patients with annual growth of 0.108 mm or greater compared with those with an annual rate of less than 0.108 mm by adjusting the confounding factors through logistic regression (Figure 3). Female sex (odds ratio [OR], 1.46; 95% CI, 1.38-1.55), older than 40 years (OR range, 0.44 [95% CI, 0.35-0.56] for 80 years or older to 0.63 [95% CI, 13 0.50-0.78] for 50 to <60 years of age), BCVA of worse than 20/400 (OR, 1.33; 95% CI, 1.15-1.54), baseline axial length of greater than 28.15 mm (OR for 28.15 to <29.65 mm, 1.67 [95% CI, 1.54-1.81]; OR for 29.65 to <31.12 mm, 2.67 [95% CI, 2.46-2.88]; OR for ≥31.12 mm, 2.39 [95% CI, 2.20-2.60]), presence of myopic maculopathy equal to or more serious than category 2 (OR for category 2, 1.25 [95% CI, 1.16-1.35]; OR for category 3, 1.06 [95% CI, 0.96-1.17]; OR for category 4, 1.39 [95% CI, 1.24-1.55]), and prior CNV (OR, 1.37; 95% CI, 1.29-1.47) were factors associated with a rapid rate of axial length growth.
Figure 3. Odds Ratios (ORs) of Risk Factors Between Groups With and Without Severe Axial Elongation.
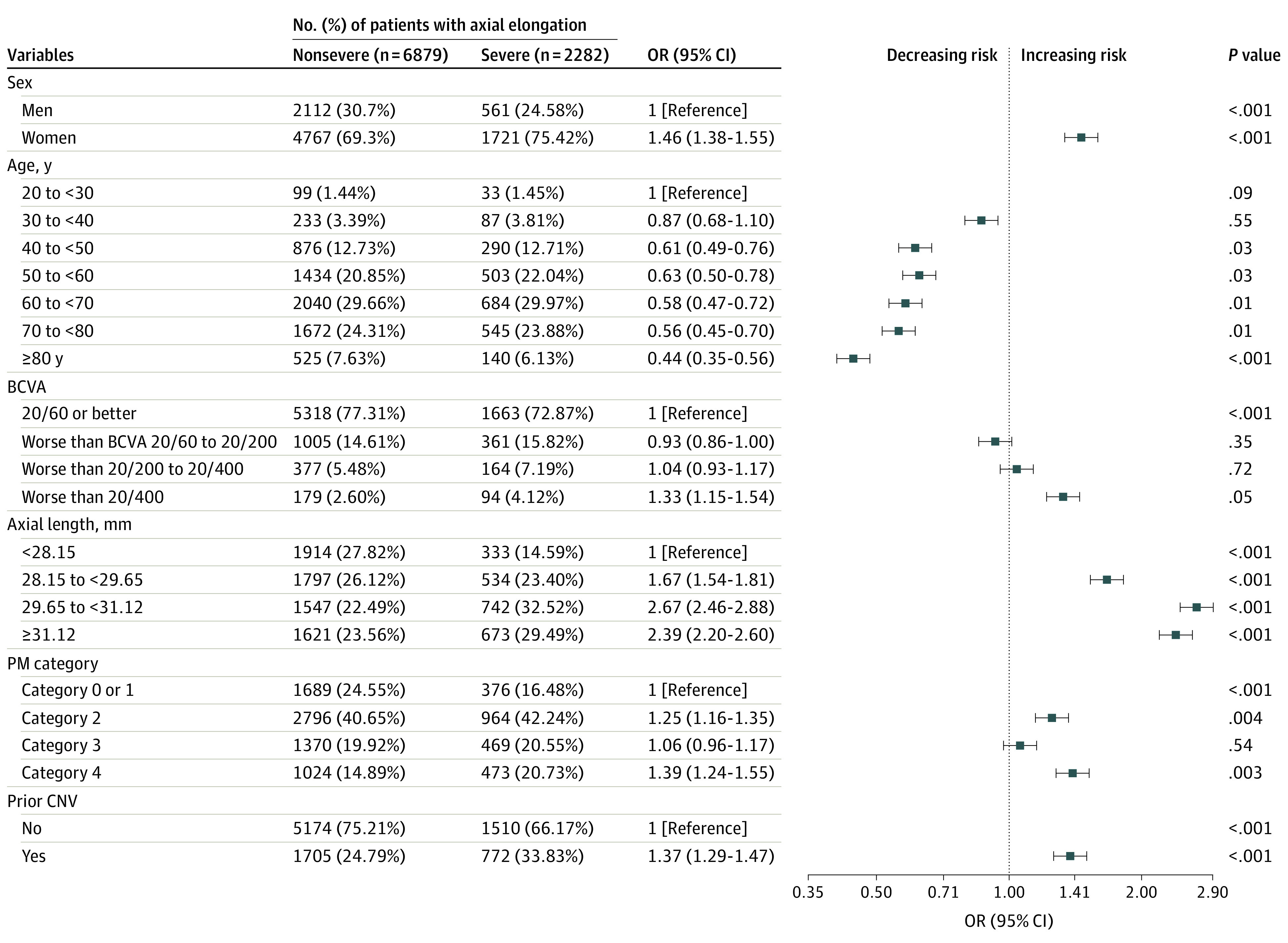
Severe axial elongation was 0.108 mm/y or greater; nonsevere, less than 0.108 mm/y. Factors were categorized according to each grouping criteria and regressed by using logistic regression to test the differences between the continuous variables that are controlled for multiple confounders. BCVA indicates best-corrected visual acuity; CNV, choroidal neovascularization; PM, pathologic myopia.
Discussion
We evaluated axial length and its annual growth rate in 3646 eyes from 1877 adults in an 8-year follow-up study, and the results show that axial length continued to increase throughout this period in patients with high myopia. Specifically, sex, poor BCVA, increased axial length, presence of myopic maculopathy, and prior CNV may be risk factors for severe changes in axial length among adults with high myopia.
Association of Sex and Age With Axial Length
Women were predominant in the overall database and in each age group, consistent with epidemiological studies of the general population that have shown female sex to be a risk factor for high myopia.6,7,8,9,10 Ohsugi et al11 reported an annual increase in axial length in 316 eyes of 316 Japanese patients with a mean age of 63.8 (9.0) years with and without high myopia. In the present study, multiple regression analyses showed that eyes with prior CNV and eyes in female patients had a greater annual rate of axial elongation. In addition, women had a greater rate of axial elongation than men among patients with myopia aged 40 to 70 years. Our results suggest an association between female sex and severe axial growth rate and that women with high myopia are 1.51 times more likely to develop severe axial elongation than men. This disparity should be taken into account when monitoring changes in axial length.
Our results also show an association between age and annual changes in axial length. Patients aged 20 to 30 years were more likely to be observed with axial length growth greater than 0.108 mm/y than patients older than 40 years. In addition, there was an inverse association between age and eyes with rapid axial growth rates. Another possible reason for the changes in axial length in the group aged 20 to 30 years is that they are from the generation with more high myopia and prolonged use of visual display terminals of computers and mobile phones. Although our results and those of Morgan et al12 suggest that the myopia epidemic is confined to the developed countries of East and Southeast Asia, sex and age should be taken into account when examining axial elongation in adults with myopia throughout the world.
Association of Myopic Maculopathy With Axial Length
Studies have shown that there is an association between axial length and choroidal thickness in healthy eyes as well as eyes with pathologic myopia.13,14,15 In addition, axial length elongation has been associated with defects of the Bruch membrane in the retroequatorial region.16,17 These defects could lead to thinning of the retina and a reduction in the density of retinal pigment epithelial cells.16,17 Thus, it is understandable that axial length changes are more common in eyes with diffuse and patchy atrophy, because diffuse atrophy is characterized by an extreme thinning of the choroid, whereas patchy atrophy is more likely to be found with Bruch membrane and retinal pigment epithelial defects.18,19 Therefore, there may be an association between increased axial length and progression of myopic maculopathy. In addition, the progression of myopic maculopathy and further thinning of the choroid may facilitate an increase in axial length. Because the presence of diffuse atrophy is an important gateway to pathologic myopia,4,18,20 our results suggest that when myopia progresses to pathologic myopia, the rate of axial elongation may increase.
Association of CNV With Axial Length
Eyes with severe axial length growth were more likely to have had a longer baseline axial length, and some of them had myopic maculopathy and prior CNV. However, an association between prior CNV and changes in axial length was not determined. We did confirm that prior CNV was a risk factor for axial elongation during a long follow-up period, even though these results still need to be confirmed by basic research at the micro level. In 2017, Jonas et al17 reported that ocular tissues actively regulated axial elongation and that myopization was due to defects of the Bruch membrane. Thus, once Bruch membrane defects develop and enlarge in the foveal area, they may facilitate axial elongation.
Association of BCVA With Axial Length
Our results showed that a BCVA of 20/400 was associated with the highest risk for axial elongation when compared with BCVA of better than 20/60 (Figure 3). Because the refractive error and axial length were not matched, we believe that the emmetropic process may lead to axial elongation. This may also explain the increased axial length among eyes with BCVA of worse than 20/60 (Table 2). Because BCVA is a routine examination procedure, changes in axial length should be monitored in eyes with poor BCVA in the management of myopia. Although the pathogenesis of CNV-related macular atrophy is not fully determined, mechanical stress at and around the scarred CNV may be associated with the enlargement of the hole in Bruch membrane that is commonly found in CNV-related macular atrophy.21 Thus, a prior CNV and subsequent CNV-related macular atrophy may result in a greater increase of the axial length in this group with poor BCVA.
Limitations
This study has some limitations. First, we collected all registered patients regardless of prior measurement biases caused by an epiretinal membrane, artificial lens, or prior surgical procedures such as vitreoretinal operations (postsurgical eyes, <1%), and we believed that these occasional outliers did not affect the results of our study. Second, only 30% of our eyes had a definite staphyloma, which is insufficient for analyzing their association with axial lengths and their changes. Previous studies have reported no significant difference in the axial length between eyes with and without a staphyloma based on 3-dimensional magnetic resonance imaging and Optos imaging with a scanning laser ophthalmoscope (200Tx; Optos PLC).22,23 Further studies are needed to confirm the lack of association of staphylomas with the axial length changes after routine widefield fundus imaging and ultra-widefield ocular coherence tomography. Third, the presence of concomitant abnormalities such as amblyopia or glaucoma were not analyzed because the diagnosis of these conditions is difficult in patients with pathologic myopia. Last, because our research was conducted in the Advanced Clinical Center for Myopia of our university, these results might not reflect the tendency in the general population of individuals with myopia.
Conclusions
In this cohort study, we evaluated axial length and its annual growth rate in 9161 medical records of 3646 eyes from 1877 patients. Although there was an association between decreased annual growth of axial length and age, high myopia was associated with axial length elongation. The risk factors for elongation do not appear to be modifiable, so prevention of myopia may be the best approach to reduce the prevalence of pathologic myopia and its complications in the future.
References
- 1.Lee MW, Lee SE, Lim HB, Kim JY. Longitudinal changes in axial length in high myopia: a 4-year prospective study. Br J Ophthalmol. 2020;104(5):600-603. doi: 10.1136/bjophthalmol-2019-314619 [DOI] [PubMed] [Google Scholar]
- 2.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 3.Ohno-Matsui K, Kawasaki R, Jonas JB, et al. ; META-analysis for Pathologic Myopia (META-PM) Study Group . International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(5):877-83.e7. doi: 10.1016/j.ajo.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 4.Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156-187. doi: 10.1016/j.preteyeres.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Allen M, Poggiali D, Whitaker K, Marshall TR, Kievit RA. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Res. 2019;4(63):63. doi: 10.12688/wellcomeopenres.15191.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong YL, Saw SM. Epidemiology of pathologic myopia in Asia and worldwide. Asia Pac J Ophthalmol (Phila). 2016;5(6):394-402. doi: 10.1097/APO.0000000000000234 [DOI] [PubMed] [Google Scholar]
- 7.Ueda E, Yasuda M, Fujiwara K, et al. Trends in the prevalence of myopia and myopic maculopathy in a Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci. 2019;60(8):2781-2786. doi: 10.1167/iovs.19-26580 [DOI] [PubMed] [Google Scholar]
- 8.Wong YL, Sabanayagam C, Ding Y, et al. Prevalence, risk factors, and impact of myopic macular degeneration on visual impairment and functioning among adults in Singapore. Invest Ophthalmol Vis Sci. 2018;59(11):4603-4613. doi: 10.1167/iovs.18-24032 [DOI] [PubMed] [Google Scholar]
- 9.Han X, Guo X, Lee PY, Morgan IG, He M. Six-year changes in refraction and related ocular biometric factors in an adult Chinese population. PLoS One. 2017;12(8):e0183364. doi: 10.1371/journal.pone.0183364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mo Y, Wang MF, Zhou LL. Risk factor analysis of 167 patients with high myopia. Int J Ophthalmol. 2010;3(1):80-82. doi: 10.3980/j.issn.2222-3959.2010.01.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohsugi H, Ikuno Y, Shoujou T, Oshima K, Ohsugi E, Tabuchi H. Axial length changes in highly myopic eyes and influence of myopic macular complications in Japanese adults. PLoS One. 2017;12(7):e0180851. doi: 10.1371/journal.pone.0180851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan IG, French AN, Ashby RS, et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134-149. doi: 10.1016/j.preteyeres.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Tham YC, Chong C, et al. Patterns and determinants of choroidal thickness in a multiethnic Asian population: the Singapore Epidemiology of Eye Diseases study. Ophthalmol Retina. 2021;5(5):458-467. doi: 10.1016/j.oret.2020.08.012 [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Cano A, Orduna E, Segura F, et al. Choroidal thickness and volume in healthy young white adults and the relationships between them and axial length, ammetropy and sex. Am J Ophthalmol. 2014;158(3):574-83.e1. doi: 10.1016/j.ajo.2014.05.035 [DOI] [PubMed] [Google Scholar]
- 15.Tan CS, Cheong KX. Choroidal thickness and volume in healthy young white adults and the relationships between them and axial length, ammetropy and sex. Am J Ophthalmol. 2015;159(4):817-818. doi: 10.1016/j.ajo.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 16.Chalam KV, Sambhav K. Choroidal thickness measured with swept source optical coherence tomography in posterior staphyloma strongly correlates with axial length and visual acuity. Int J Retina Vitreous. 2019;5:14. doi: 10.1186/s40942-019-0166-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas JB, Ohno-Matsui K, Jiang WJ, Panda-Jonas S. Bruch membrane and the mechanism of myopization: a new theory. Retina. 2017;37(8):1428-1440. doi: 10.1097/IAE.0000000000001464 [DOI] [PubMed] [Google Scholar]
- 18.Fang Y, Du R, Nagaoka N, et al. OCT-based diagnostic criteria for different stages of myopic maculopathy. Ophthalmology. 2019;126(7):1018-1032. doi: 10.1016/j.ophtha.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 19.Du R, Fang Y, Jonas JB, et al. Clinical features of patchy chorioretinal atrophy in pathologic myopia. Retina. 2020;40(5):951-959. doi: 10.1097/IAE.0000000000002575 [DOI] [PubMed] [Google Scholar]
- 20.Fang Y, Yokoi T, Nagaoka N, et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology. 2018;125(6):863-877. doi: 10.1016/j.ophtha.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 21.Ohno-Matsui K, Jonas JB, Spaide RF. Macular Bruch membrane holes in choroidal neovascularization-related myopic macular atrophy by swept-source optical coherence tomography. Am J Ophthalmol. 2016;162:133-139.e1. doi: 10.1016/j.ajo.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 22.Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology. 2014;121(9):1798-1809. doi: 10.1016/j.ophtha.2014.03.035 [DOI] [PubMed] [Google Scholar]
- 23.Ohno-Matsui K, Alkabes M, Salinas C, et al. Features of posterior staphylomas analyzed in wide-field fundus images in patients with unilateral and bilateral pathologic myopia. Retina. 2017;37(3):477-486. doi: 10.1097/IAE.0000000000001327 [DOI] [PubMed] [Google Scholar]


