This cross-sectional study evaluates the role of the components of the olfactory pathway structures in impaired olfactory function.
Key Points
Question
What are the neuroanatomical correlates of olfactory function in the general population?
Findings
In this cross-sectional study of 541 participants who underwent brain imaging and olfactory assessment, olfactory bulb volume was independently associated with olfactory function and a robust mediator of the association between volumes of central olfactory structures (amygdala, hippocampus, insular cortex, and medial orbitofrontal cortex) and olfactory function.
Meaning
Olfactory dysfunction may primarily originate from the pathology of the olfactory bulb or more distal structures, whereas olfactory bulb volume may serve as a preclinical marker for the identification of individuals who are at an increased risk for developing neurodegenerative diseases later in life.
Abstract
Importance
Olfactory dysfunction is a prodromal manifestation of many neurodegenerative disorders, including Alzheimer and Parkinson disease. However, its neuroanatomical basis is largely unknown.
Objective
To assess the association between olfactory brain structures and olfactory function in adults 30 years or older and to examine the extent to which olfactory bulb volume (OBV) mediates the association between central olfactory structures and olfactory function.
Design, Setting, and Participants
This cross-sectional study analyzed baseline data from the first 639 participants with brain magnetic resonance imaging (MRI) in the Rhineland Study, an ongoing population-based cohort study in Bonn, Germany. Participants were enrolled between March 7, 2016, and October 31, 2017, and underwent brain MRI and olfactory assessment. Data were analyzed from March 1, 2018, to June 30, 2021.
Exposure
Volumetric measures were derived from 3-T MRI T1-weighted brain scans, and OBV was manually segmented on T2-weighted images. The mean volumetric brain measures from the right and left sides were calculated, adjusted by head size, and normalized to all participants.
Main Outcomes and Measures
Performance on the 12-item smell identification test (SIT-12) was used as a proxy for olfactory function.
Results
A total of 541 participants with complete data on MRI-derived measures and SIT-12 scores were included. This population had a mean (SD) age of 53.6 (13.1) years and comprised 306 women (56.6%). Increasing age (difference in SIT-12 score, –0.04; 95% CI, –0.05 to –0.03), male sex (–0.26; 95% CI, –0.54 to 0.02), and nasal congestion (–0.28; 95% CI, –0.66 to 0.09) were associated with worse olfactory function (SIT-12 scores). Conversely, larger OBV was associated with better olfactory function (difference in SIT-12 score, 0.46; 95% CI, 0.29-0.64). Larger volumes of amygdala (difference in OBV, 0.12; 95% CI, 0.01-0.24), hippocampus (0.16; 95% CI, 0.04-0.28), insular cortex (0.12; 95% CI, 0.01-0.24), and medial orbitofrontal cortex (0.10; 95% CI, 0.00-0.20) were associated with larger OBV. Larger volumes of amygdala (volume × age interaction effect, 0.17; 95% CI, 0.03-0.30), parahippocampal cortex (0.17; 95% CI, 0.03-0.31), and hippocampus (0.21; 95% CI, 0.08-0.35) were associated with better olfactory function only in older age groups. The age-modified association between volumes of central olfactory structures and olfactory function was largely mediated through OBV.
Conclusions and Relevance
This cross-sectional study found that olfactory bulb volume was independently associated with odor identification function and was a robust mediator of the age-dependent association between volumes of central olfactory structures and olfactory function. Thus, neurodegeneration-associated olfactory dysfunction may primarily originate from the pathology of peripheral olfactory structures, suggesting that OBV may serve as a preclinical marker for the identification of individuals who are at an increased risk of neurodegenerative diseases.
Introduction
Olfactory dysfunction has a high prevalence in the general population, especially in older adults.1 Impaired olfactory function is among the earliest signs of many neurodegenerative disorders, including Alzheimer disease and Parkinson disease, and often precedes formal diagnosis by many years.2 Moreover, olfactory dysfunction has recently emerged as one of the earliest and most frequent neurological signs of SARS-CoV-2 infection, which may result from viral invasion of the olfactory pathway.3,4 However, little is known about the neuroanatomical basis of olfactory dysfunction in the general population, elucidation of which could not only provide insights into its underlying causes but also facilitate the identification of individuals who are at an increased risk of neurodegenerative conditions.
Olfactory function has been evaluated through various psychophysical tests in both clinical and research settings, yet there are few studies on the structural changes of the olfactory system. With the advent of high-field magnetic resonance imaging (MRI), it is now possible to obtain an objective and reliable assessment of the neuroanatomical correlates of olfactory dysfunction in large groups of individuals. Smaller volumes of central olfactory structures (ie, hippocampus and several other mesial temporal lobe structures) were associated with impaired odor identification.5 The olfactory bulb, as the first relay of the olfactory pathway, plays a role in the integration of peripheral and central processing of odor information. Olfactory bulb volume (OBV) was also associated with olfactory dysfunction in both healthy participants and patients with various neurological conditions in previous small-scale studies,6,7 although this presumed association between structure and function has not been consistently found in clinical cohorts.8 Thus far, only a few small-scale imaging studies have investigated the role of central and peripheral olfactory structures simultaneously in olfactory function.7 Although these studies described the associations between olfactory performance and volumes of gray matter in the right orbitofrontal cortex and olfactory bulb, evidence is still lacking in the general population. Moreover, the association between the structural integrity of peripheral and central components of the olfactory system remains to be elucidated.
Given the appearance of neuropathological markers, including neurofibrillary tangles and α-synuclein aggregates, in the peripheral olfactory region (ie, olfactory epithelium and olfactory bulb) before their emergence in many other brain regions,9,10 it has been hypothesized that environmental insults might trigger olfactory bulb damage that could then spread to connected brain regions in a prion-like manner.9 For example, recently, in the early stages of a SARS-CoV-2 infection, a hyperintense signal was detected in the olfactory bulbs; the signal later resolved but left a trace of olfactory bulb damage with reduced volumes.3,11 Similar to many other viruses, SARS-CoV-2 appears to invade the brain through the olfactory pathway.12 Thus, pathology of the olfactory bulb may be the starting site of the cascade of events that ultimately leads to olfactory dysfunction and further upstream central nervous system pathology.
To gain a better understanding of the neuroanatomical basis of impaired olfaction and disentangle the role of the different components of the olfactory pathway structures in impaired olfaction, we conducted a cross-sectional study. Our aim was to (1) assess the association between olfactory brain structures and olfactory function in adults 30 years or older and (2) to examine the extent to which OBV mediates the association between central olfactory structures and olfactory dysfunction.
Methods
Approval to undertake this study was granted by the ethics committee of the University of Bonn Medical Faculty. The study was conducted in accordance with the recommendations of the International Conference on Harmonization Good Clinical Practice standards. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.13
Study Design and Participants
This cross-sectional study analyzed baseline data from the first 639 participants of the Rhineland Study, an ongoing population-based cohort study that invites the participation of all inhabitants of 2 geographically defined areas in the city of Bonn, Germany, who are 30 years or older.14 The only exclusion criterion was insufficient command of the German language that would prevent participants from providing informed consent. The Rhineland Study aims to investigate the cause and prediction of age-related (neurodegenerative) diseases and to assess the normal and pathological (brain) structure and function over the adult life course. Each participant in the Rhineland Study undergoes a comprehensive 8-hour protocol, including detailed brain imaging, except in case of contraindications for MRI (eTable 1 in the Supplement).
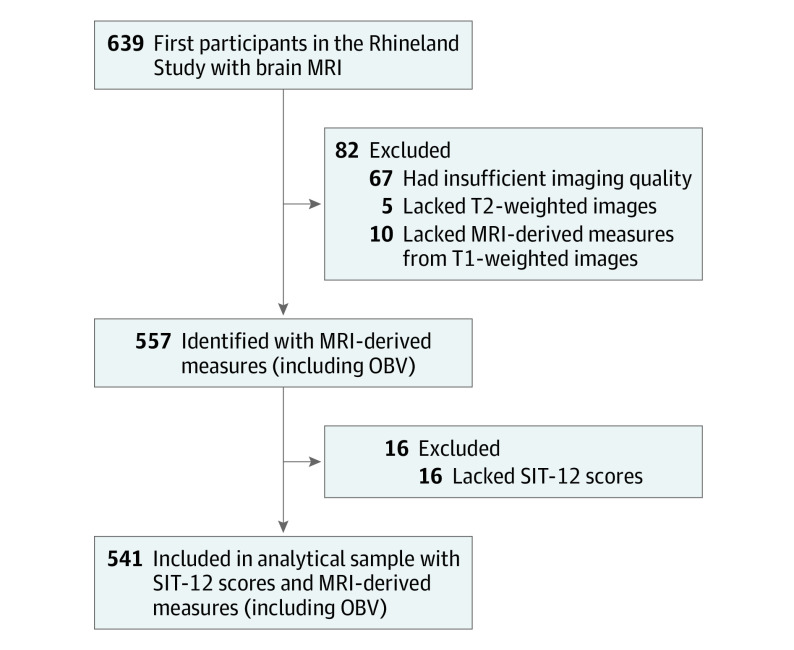
The first 639 participants were enrolled between March 7, 2016, and October 31, 2017, and underwent brain imaging. Blinded to the outcomes of olfactory testing and demographic information, we (including R.L.) estimated OBV on the basis of bilateral manual segmentation of T2-weighted images. We selected 541 participants with complete data on all MRI-derived measures (including OBV) and the 12-item smell identification test (SIT-12) as the focus of subsequent analyses (Figure 1).
Figure 1. Selection of Participants From the Rhineland Study.
MRI indicates magnetic resonance imaging; OBV, olfactory bulb volume; and SIT-12, 12-item smell identification test.
Before undergoing clinical examination, participants answered a detailed questionnaire about relevant health-related issues under the supervision of trained technicians. All smoking and nasal patency information was self-reported. Smoking categories in the questionnaire were current, former, or nonsmoker. Nasal patency was reported as blocked or free.
Image Acquisition and Processing
Magnetic resonance imaging scans were collected on two 3-T MRI scanners with 64-channel head-neck coils (MAGNETOM Prisma; Siemens Healthcare) at 2 examination sites in Bonn, Germany. The standardized protocol included a T1-weighted, multiecho, magnetization-prepared rapid gradient-echo sequence15 with 2-dimensional acceleration16 (acquisition time of 6.5 minutes, 4 echoes, repetition time of 2560 ms, inversion time of 1100 ms, flip angle of 7°, matrix size of 320 × 320 × 224, and voxel size of 0.8 × 0.8 × 0.8 mm3) and a bandwidth-matched, T2-weighted, 3-dimensional turbo-spin-echo sequence using variable flip angles17 (acquisition time of 5 minutes, repetition time of 2800 ms, echo time of 405 ms, turbo factor of 282, matrix size of 320 × 320 × 224, and voxel size of 0.8 × 0.8 × 0.8 mm3). Both sequences used elliptical sampling16 for faster acquisition.
All T1-weighted images were processed with FreeSurfer software, version 6.0 (FreeSurfer) to derive quantitative volumetric measures. Because of their known role in the central processing of olfactory information, the entorhinal cortex, amygdala, parahippocampal cortex, hippocampus, insular cortex, and lateral and medial orbitofrontal cortex were selected as regions of interest for subsequent analyses.18 We used the estimated total intracranial volume (eTIV) generated by FreeSurfer as a proxy for head size.
Left- and right-side OBVs were manually segmented on T2-weighted images using a visualization tool (Freeview; FreeSurfer). The olfactory bulbs are mostly spindle-shaped structures that are symmetrically located at the base of the forebrain (eFigure 1 in the Supplement). The boundaries were demarcated according to surrounding cerebrospinal fluid and the underlying cribriform plate. The abrupt change in diameter of the olfactory bulb was used as the marker of the posterior end.19 Using a voxelwise approach, one of us (R.L.), who was blinded to all other participant information, manually labeled all slices through the olfactory bulb. The labeled voxels were summed to obtain the volume in cubic millimeters (mm3). In cases in which the olfactory bulb could not be detected on the consecutive MRI scans in the olfactory region, OBV was defined as 0 (ie, absent). Fifty scans were randomly chosen for repeated measurements by the same rater (R.L.) to assess intrarater reliability and by another rater to assess interrater reliability. The intraclass correlation coefficients showed good to excellent agreement: 0.976 (95% CI, 0.962-0.984) for intrarater reliability and 0.862 (95% CI, 0.802-0.905) for interrater reliability.
Odor Identification Function
The SIT-12 (Sniffin’ Sticks; Burghart Messtechnik GmbH) is a widely used screening test for odor identification function.20 Each of the 12 felt-tip sticks from the SIT-12 test kit was positioned approximately 2 cm in front of both nostrils for 3 to 4 seconds by trained technicians in a well-ventilated room. Participants were then asked to choose only 1 of the 4 answer options for each odorant. The time interval between 2 consecutive odor presentations was at least 20 seconds. The final score was generated as the total number of correct answers (range 0-12, with higher scores indicating better odor identification function).
Statistical Analysis
Descriptive data were expressed as mean (SD) for continuous variables or number (percentage) for categorical variables. Intergroup differences were compared with an unpaired, 2-tailed t test for continuous variables and with the Pearson χ2 test for categorical variables. We used multivariable linear regression models to examine the association between previously reported factors of odor identification function (age, sex, self-reported nasal patency, and smoking status)21 and SIT-12 score, as well as to assess sex differences in OBV while accounting for age and head size of the participants. The magnitude of the difference between compared groups or the strength of the association between factors and outcomes was described by effect size and its corresponding 95% CI.
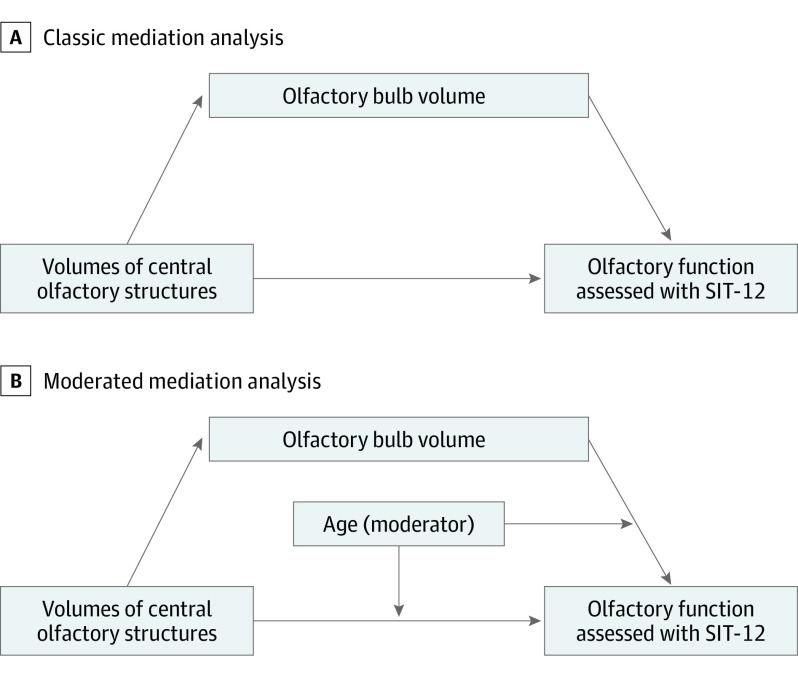
To assess the extent to which the association between central olfactory structures and olfactory function was mediated through the olfactory bulb, we used structural equation modeling (Figure 2).22 First, using multiple regression analysis, we confirmed the associations among the volumes of central olfactory structures, OBV, and olfactory function (as assessed by the SIT-12) while accounting for potential confounders (including age, sex, and nasal patency). We included interaction terms to assess whether age and sex modified the associations between olfactory structures and olfactory function. Second, we performed a classical mediation analysis by constructing models with the volume of each central olfactory structure as the independent variable, OBV as the mediator, and the odor identification function (SIT-12 score) as the outcome variable (Figure 2A) while adjusting for age, sex, and nasal patency. Because we found that age moderated the association between volumes of olfactory structures and olfactory function in the multivariable regression analyses, we performed a moderated mediation analysis23 by adding age as a moderator to the paths directed from volumes of central olfactory structures and OBV to the odor identification function (Figure 2B) while adjusting for sex and nasal patency. Moreover, we assessed an alternative scenario with volumes of central olfactory structures as mediators of the association between OBV and olfactory dysfunction.
Figure 2. Mediation Analysis Models.
SIT-12 indicates 12-item smell identification test.
Moderation by age was described by depicting model-estimated values at 1 SD below and 1 SD above the mean age. The 95% CIs of all of the (moderated) mediation analysis estimates were based on nonparametric bias–corrected accelerated bootstrapping with 1000 resamplings. In the mediation analyses, the mean between the left- and right-side volumes of olfactory bulb and central olfactory brain structures were calculated, adjusted by head size, and normalized to the study population as follows:
| Volumeadjusted = Volume / eTIV × eTIVmean. |
To evaluate the robustness of the findings, we performed a sensitivity analysis by excluding participants with no olfactory bulb. All statistical analyses were performed in R, version 3.4 (R Foundation for Statistical Computing)24 using the lavaan package for structural equation modeling.25 Data were analyzed from March 1, 2018, to June 30, 2021.
Results
A total of 541 participants were included in the study, of whom 306 were women (56.6%) and 235 were men (43.4%), with a mean (SD) age of 53.6 (13.1) years (Table 1). The mean (SD) SIT-12 score of the participants was 9.8 (1.7).
Table 1. Characteristics of the Study Population.
| Characteristica | Mean (SD) | Mean difference (95% CI)a | ||
|---|---|---|---|---|
| Overall | Women | Men | ||
| No. of participants (%) | 541 (100) | 306 (56.6) | 235 (43.4) | NA |
| Age, y | 53.6 (13.1) | 53.2 (13.1) | 54.0 (13.1) | −0.82 (−3.05 to 1.41) |
| SIT-12 score | 9.8 (1.7) | 9.9 (1.6) | 9.6 (1.8) | 0.31 (0.01 to 0.61) |
| Nasal patency as free, No. (%) | 453 (83.7) | 262 (85.6) | 191 (81.3) | NA |
| Smoking status, No. (%)b | ||||
| Current smoker | 228 (50.2) | 131 (50.2) | 97 (50.3) | NA |
| Former smoker | 166 (36.6) | 94 (36.0) | 72 (37.3) | NA |
| Nonsmoker | 60 (13.2) | 36 (13.8) | 24 (12.4) | NA |
| Left-side OBV, mm3 | 26.1 (8.4) | 24.4 (7.7) | 28.3 (8.9) | −3.82 (−5.25 to −2.40) |
| Right-side OBV, mm3 | 27.6 (9.0) | 26.0 (8.3) | 29.7 (9.4) | −3.72 (−5.25 to −2.20) |
| eTIV, L | 1.54 (0.15) | 1.46 (0.11) | 1.65 (0.12) | −0.19 (−0.21 to −0.17) |
Abbreviations: eTIV, estimated total intracranial volume; NA, not applicable; OBV, olfactory bulb volume; SIT-12, 12-item smell identification test.
Differences between women and men were assessed with an unpaired, 2-tailed t test for continuous variables and with Pearson χ2 test for categorical variables. Mean difference (95% CI) was reported by continuous variables.
Overall, 87 participants (45 women and 42 men) had missing data on smoking status.
Compared with the 515 participants who scored 7 or higher on the SIT-12, those 26 participants who scored 6 or lower were significantly older (65.5 [11.4] vs 53.0 [12.9] years; mean difference, 12.5 [95% CI, 7.8-17.3] years) and more likely to be men (16 of 26 [61.5%] vs 219 of 515 [42.5%]). Overall, women had higher SIT-12 scores than men (9.9 [1.6] vs 9.6 [1.8]; mean difference, 0.31 [95% CI, 0.01-0.61]), whereas no meaningful differences were found in age (53.2 [13.1] vs 54.0 [13.1] years; mean difference, −0.82 [95% CI, −3.05 to 1.41] years), self-reported nasal patency (262 [85.6%] vs 191 [81.3%]), or smoking status (eg, current smoker: 131 [50.2%] vs 97 [50.3%]) between the sexes (Table 1). Among the 541 participants, 13 (2.4%) had a self-reported diagnosis of a central nervous system disorder, including dementia (n = 1), Parkinson disease (n = 2), multiple sclerosis (n = 3), ischemic stroke (n = 3), and intracranial hemorrhage (n = 5).
In general, olfactory function decreased with increasing age (difference in SIT-12 score, –0.04; 95% CI, –0.05 to –0.03) and was worse in men than in women (difference in SIT-12 score, –0.26; 95% CI, –0.54 to 0.02) and those with nasal congestion (difference in SIT-12 score, –0.28; 95% CI, –0.66 to 0.09), but it was not associated with smoking status (former vs never smoker: 0.18 [95% CI, –0.16 to 0.51]; current vs never smoker: 0.06 [95% CI, –0.41 to 0.53]) (eTable 2 in the Supplement). Given that participants with nasal congestion tended to perform worse on the SIT-12, we also assessed the association between odor identification function and nasal congestion in the first 2000 participants of the Rhineland Study, of whom 1915 had valid SIT-12 results. An inverse association was observed between nasal patency and olfactory function (difference in SIT-12 score, −0.21; 95% CI, −0.41 to −0.02) (eTable 2 in the Supplement). Therefore, nasal patency, but not smoking, was included as a covariate in subsequent analyses.
The OBVs varied widely among participants (left side: from 0 to 55.30 mm3; right side: from 0 to 55.81 mm3), but left- and right-side OBVs were correlated within each individual (Pearson correlation coefficient [r] = 0.87; 95% CI, 0.84-0.89). Men had larger OBVs than women on both left (age-adjusted difference, 3.97 mm3; 95% CI, 2.62-5.31 mm3) and right (age-adjusted difference, 3.86 mm3; 95% CI, 2.40-5.31 mm3) sides. These differences became smaller after accounting for head size (age- and head size–adjusted difference, left-side OBV: 2.20 mm3 [95% CI, 0.47-3.92 mm3]; right-side OBV: 2.55mm3 [95% CI, 0.68-4.42 mm3]). In general, the right-side OBV was slightly larger (mean difference, 1.53 mm3; 95% CI, 1.15-1.91 mm3). Larger OBV was associated with better olfactory function (difference in SIT-12 score, 0.46; 95% CI, 0.29-0.64).
Using the Youden index, we identified that the optimal cutoff points for detecting hyposmia (defined as a SIT-12 score of 9 or lower) on the basis of mean OBV were 23.81 mm3 or less for women and 27.39 mm3 or less for men. These values corresponded to an area under the curve of the receiver operating characteristic curve of 0.63 (95% CI, 0.57-0.70) for women and 0.69 (95% CI, 0.62-0.76) for men (eFigure 2A and B in the Supplement). Similarly, the optimal cutoff points for detecting anosmia (defined as a SIT-12 score of 6 or lower) on the basis of mean OBV were 20.22 mm3 or less for women and 23.04 mm3 or less for men. These values corresponded to the areas under the curve of the receiver operating characteristic curve of 0.90 (95% CI, 0.85-0.96) for women and 0.81 (95% CI, 0.73-0.90) for men (eFigure 2C and D in the Supplement).
Association Between Volumes of Olfactory Brain Structures and Olfactory Function
After adjusting for age, sex, and nasal patency, we found that OBV was associated with olfactory function (difference in SIT-12 score, 0.51; 95% CI, 0.38-0.65). No associations were observed between volumes of other central olfactory structures and olfactory function (eTable 3 in the Supplement). However, we observed age-dependent associations between odor identification function and OBV (volume × age interaction effect, 0.17; 95% CI, 0.03-0.30), amygdalar volume (volume × age interaction effect, 0.17; 95% CI, 0.03-0.30), parahippocampal cortex volume (volume × age interaction effect, 0.17; 95% CI, 0.03-0.31), and hippocampal volume (volume × age interaction effect, 0.21; 95% CI, 0.08-0.35), with the magnitude of each association being stronger in older participants (Figure 3; eTable 3 in the Supplement). These associations were not modified by sex.
Figure 3. Association Between Olfactory Structure Volumes and Olfactory Function Stratified by Age.
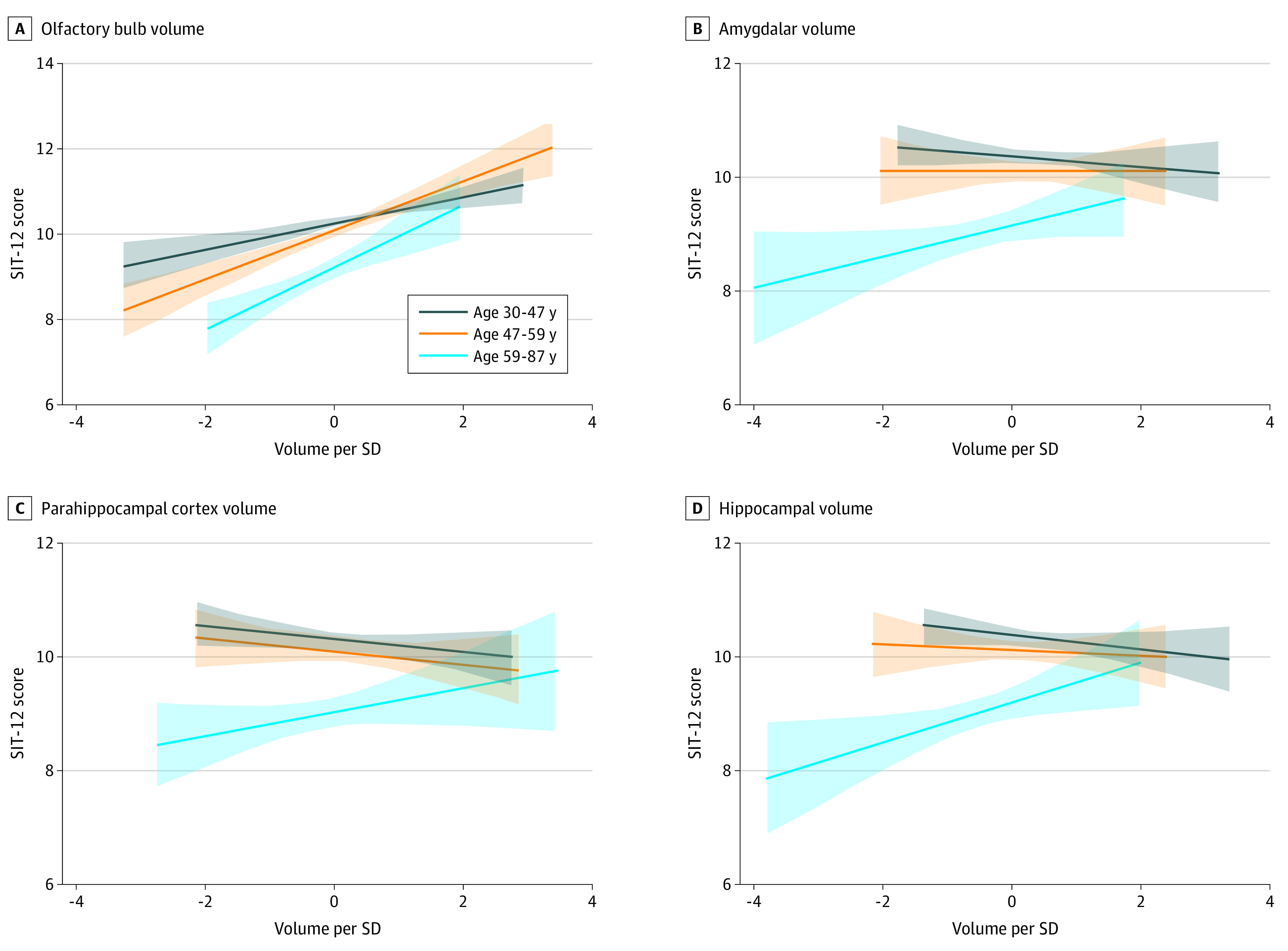
Lines represent the linear association between volumes and function, as assessed with the 12-item smell identification test (SIT-12), for each age tertile, and the shaded areas represent the accompanying 95% CIs. The mean volumetric measures from the right and left sides were calculated, adjusted by head size, and normalized to the 541 participants.
The volumes of amygdala (difference in OBV, 0.12; 95% CI, 0.01-0.24), hippocampus (difference in OBV, 0.16; 95% CI, 0.04-0.28), insular cortex (difference in OBV, 0.12; 95% CI, 0.01-0.24), and medial orbitofrontal cortex (difference in OBV, 0.10; 95% CI, 0.00-0.20) were associated with OBV after accounting for age, sex, and nasal patency (eTable 4 in the Supplement).
Olfactory Bulb Volume as the Mediator
Classical mediation analysis (Figure 2A) showed that the association between volumes of central olfactory structures (ie, amygdala, hippocampus, insular cortex, and medial orbitofrontal cortex) and odor identification function (as assessed with SIT-12 score) was mediated by OBV (indirect effect estimate, amygdala: 0.039 [95% CI, 0.001-0.087]; hippocampus: 0.054 [95% CI, 0.014-0.107]; insular cortex: 0.065 [95% CI, 0.025-0.124]; and medial orbitofrontal cortex: 0.047 [95% CI, 0.002-0.109]) (Table 2). In addition, the moderated mediation analysis (Figure 2B) showed that the magnitude of the mediation through OBV was largely dependent on age: the indirect effect (ie, the mediation effect through OBV) was consistently larger in older individuals (estimate for 1 SD above mean age, amygdala: 0.053 [95% CI, 0.001-0.125]; hippocampus: 0.072 [95% CI, 0.020-0.151]; insular cortex: 0.090 [95% CI, 0.025-0.154]; and medial orbitofrontal cortex: 0.065 [95% CI, 0.005-0.150]). An age-modified direct effect was seen only for the association between hippocampal volume and olfactory function (1 SD below mean age: −0.284 [95% CI, −0.473 to −0.132]; mean age: −0.046 [95% CI, −0.191 to 0.099]; 1 SD above mean age: 0.191 [95% CI, −0.013 to 0.430]), with the magnitude of the association increasing with age. The association between OBV and olfactory function performance was not mediated by volumes of central olfactory structures (eTables 5 and 6 in the Supplement). Similar results were obtained after the exclusion of 5 participants who had no olfactory bulb.
Table 2. Mediation of Olfactory Bulb Volume in the Association Between Central Olfactory Structure Volumes and Olfactory Function.
| Volume of olfactory structurea | Modelb | Indirect effect size estimate (95% CI) | Direct effect size estimate (95% CI) | Total effect size estimate (95% CI) | Moderated mediation indexc |
|---|---|---|---|---|---|
| Amygdala | Model A | 0.039 (0.001 to 0.087) | 0.050 (−0.094 to 0.213) | 0.089 (−0.065 to 0.269) | NA |
| Model B | NA | NA | NA | 0.011 (−0.002 to 0.043) | |
| 1 SD below | 0.031 (0.003 to 0.080) | −0.134 (−0.350 to 0.045) | −0.103 (−0.324 to 0.085) | NA | |
| Mean age | 0.042 (0.001 to 0.090) | 0.017 (−0.121 to 0.182) | 0.059 (−0.095 to 0.237) | NA | |
| 1 SD above | 0.053 (0.001 to 0.125) | 0.168 (−0.030 to 0.391) | 0.221 (0.015 to 0.445) | NA | |
| Hippocampus | Model A | 0.054 (0.014 to 0.107) | −0.016 (−0.165 to 0.138) | 0.038 (−0.109 to 0.201) | NA |
| Model B | NA | NA | NA | 0.013 (−0.003 to 0.045) | |
| 1 SD below | 0.045 (0.011 to 0.099) | −0.284 (−0.473 to −0.132) | −0.238 (−0.411 to −0.067) | NA | |
| Mean age | 0.059 (0.015 to 0.114) | −0.046 (−0.191 to 0.099) | 0.013 (−0.128 to 0.166) | NA | |
| 1 SD above | 0.072 (0.020 to 0.151) | 0.191 (−0.013 to 0.430) | 0.263 (0.045 to 0.514) | NA | |
| Insular cortex | Model A | 0.065 (0.025 to 0.124) | −0.113 (−0.264 to 0.035) | −0.048 (−0.202 to 0.096) | NA |
| Model B | NA | NA | NA | 0.021 (−0.001 to 0.043) | |
| 1 SD below | 0.048 (0.008 to 0.088) | −0.082 (−0.273 to 0.109) | −0.034 (−0.226 to 0.158) | NA | |
| Mean age | 0.069 (0.020 to 0.117) | −0.109 (−0.242 to 0.024) | −0.040 (−0.180 to 0.099) | NA | |
| 1 SD above | 0.090 (0.025 to 0.154) | −0.136 (−0.307 to 0.035) | −0.046 (−0.227 to 0.134) | NA | |
| Medial orbitofrontal cortex | Model A | 0.047 (0.002 to 0.109) | −0.018 (−0.196 to 0.139) | 0.029 (−0.150 to 0.195) | NA |
| Model B | NA | NA | NA | 0.016 (−0.001 to 0.048) | |
| 1 SD below | 0.034 (0.003 to 0.098) | 0.064 (−0.133 to 0.240) | 0.098 (−0.112 to 0.290) | NA | |
| Mean age | 0.050 (0.003 to 0.113) | −0.004 (−0.171 to 0.145) | 0.046 (−0.128 to 0.205) | NA | |
| 1 SD above | 0.065 (0.005 to 0.150) | −0.071 (−0.365 to 0.174) | −0.006 (−0.300 to 0.238) | NA |
Abbreviation: NA, not applicable.
Separate mediation analyses (with the volume of each central olfactory structure as the independent variable, olfactory bulb volume as the mediator, and 12-item smell identification test score as the outcome variable) were performed for each central olfactory structure. The mean volumetric measures, including olfactory bulb volume from the right and left sides, were calculated, adjusted by head size, and normalized to all participants.
Model A was not moderated by age, and model B was moderated by age.
The estimate (95% CI) of moderated mediation analysis is reported for mean age and 1 SD below or above the mean age. The moderated mediation index and interaction effects are presented only for model B.
Discussion
In this large, cross-sectional study of adults 30 years or older, we found an association between larger OBV and better odor identification function that was independent of other established factors of olfactory function, such as sex, nasal patency, and smoking status. We also found an age-dependent association between the volumes of several key central olfactory brain structures (ie, amygdala, parahippocampal cortex, and hippocampus) and olfactory function that was predominantly present in older age groups. Furthermore, we identified OBV as a robust mediator of the association between the volumes of amygdala, hippocampus, insular cortex, and medial orbitofrontal cortex and odor identification function, whereby the mediation effect consistently increased with older age.
To our knowledge, this cross-sectional study was the first to evaluate the interconnections between OBV and central olfactory structure volumes and odor identification function simultaneously. The findings highlight the unique mediatory role of OBV in the odor pathway: it was not only consistently associated with olfactory function and the volumes of the core components of the central olfactory system, but it also largely mediated the association between the volumes of central olfactory structures and olfactory function.
Unlike other subcortical and cortical brain structures that have been associated with olfactory function in previous structural and functional MRI studies,5,26 the olfactory bulb is exclusively dedicated to olfaction. Serving as the first relay in the odor pathway, it receives and processes afferent odor information from peripheral olfactory receptor neurons and then conveys this information directly to several subcortical and cortical regions without intermediary synapsing at the level of the thalamus.27 Damage to the olfactory bulb has been associated with severely impaired olfactory function.28 In this study, OBV showed a stronger and more consistent association with olfactory function than volumes of its central counterparts in the olfactory pathway. Moreover, OBV’s role in mediating the association between central olfactory structure volumes and odor identification function performance underscores the innate close link between the olfactory bulb and other central components of the olfactory pathway, including a top-down neuromodulatory cortical feedback loop.29 This role is in line with the finding from this study that OBV mediates the association between volumes of central olfactory structures and olfactory function, whereas the path from OBV to olfactory function does not go through volumes of central olfactory structures.
After accounting for the apparent indirect associations through OBV, we found that the direct association between the volumes of central olfactory structures and olfactory function was more complex than previously reported. The association between hippocampal volume and olfactory function was modified by age; although only in younger adults, a statistically significant direct association was found between hippocampal volume and odor identification. We observed a similar age dependency of the direct association for the amygdalar volume. These findings suggest that the pathology of central olfactory structures, especially the hippocampus, in old age may also contribute to disturbed olfactory function, perhaps through impaired odor memory.4 This age-dependent association may also explain some discrepancies in previous studies.5,7,30 For example, it was reported that, in older adults without dementia, the volumes of the amygdala, parahippocampal cortex, and hippocampus were associated with odor identification,5 whereas other studies failed to replicate these findings in younger individuals.7,30 However, given the relatively large variability of the direct effect estimates (likely resulting from their stronger age dependency) and the cross-sectional design of this study, future longitudinal examinations involving larger groups of participants across the entire age spectrum are warranted.
Accumulating evidence suggests that early deficits in olfactory function are a frequent hallmark of many neurodegenerative diseases and may serve as a preclinical marker for cognitive impairment and assist in estimating the rate of cognitive decline.5,9 Postmortem studies in patients with Alzheimer and Parkinson disease have demonstrated the presence of neurofibrillary tangles and α-synuclein aggregates, respectively, in both central olfactory regions and the olfactory bulb.31,32 The olfactory bulb is among the first regions in which these neuropathological changes occur.31,32 Degeneration of olfactory bulb neurons and a lower volume of its glomerular component have also been reported in Alzheimer and Parkinson disease,32,33 findings that are further corroborated by clinical evidence from imaging studies that demonstrated smaller OBVs in patients compared with healthy control participants.19 Although it is still unclear which factors trigger olfactory bulb pathology in neurodegenerative diseases, viral infections of the olfactory pathway may be implicated.9,10 Decreased OBV was recently reported in individuals who had developed anosmia after a SARS-CoV-2 infection.3,11 This report, together with findings from the present study, suggest that involvement of the olfactory bulb is likely to be one of the earliest underlying neuroanatomical substrates of olfactory dysfunction. Thus, OBV could be a clinically relevant quantitative marker of early olfactory dysfunction that may prove useful in the identification of individuals who are at an increased risk of several neurodegenerative disorders.
In accordance with findings of previous population-based studies, we found sex differences in odor identification function.34,35 Women outperformed men in odor identification ability despite men having slightly larger OBVs, even after adjustment for head size. This discrepancy may be partly associated with endocrine differences between the sexes given that odor sensitivity has been reported to vary with fluctuations of sex hormone levels in women.36 Social behavior and learning efficiency may also play a role because, compared with men, women tended to have a higher interest in the sense of smell37 and had a larger improvement in sensitivity to odorants with repeated test exposures.38 These sex-based differences could also have a neuroanatomical basis given that women were found to have a higher number of both neurons and glial cells in their olfactory bulb, even after accounting for olfactory bulb mass,39 emphasizing the importance of sex-specific analyses in olfactory research.
Limitations
This study has several limitations. First, the analyses were based on cross-sectional data, limiting the assessment of the temporal evolution of the associations between olfactory structure and function. Nevertheless, as a large-scale study in the general population that explored the mutual interconnections among OBV, volumes of central olfactory structures, and odor identification function, it provides a solid foundation for future longitudinal studies. Second, we used odor identification ability, a subtask of the complete olfactory testing battery, as a proxy for olfactory function. Compared with odor threshold and discrimination methods, testing for odor identification is less time consuming and more practical in both the general population and clinical settings.40 Moreover, SIT-12 has been extensively validated and widely used as a reliable screening test for olfactory dysfunction.41 Third, nasal patency was self-reported by participants rather than assessed objectively through clinical examination or nasal endoscopy. The subjective assessment of nasal patency might have led to less precise estimates of the associations between imaging measures and olfactory function in this study. Therefore, further investigations that apply more objective measures of nasal patency are warranted to confirm and extend these findings. Fourth, the sequences of the brain MRI scans were not specifically designed for optimal imaging of the olfactory bulb. However, the T2-weighted, high-resolution images allowed for reliable and accurate detection of the olfactory bulb with low intrarater and interrater variability.
Conclusions
The findings of this cross-sectional study indicate that OBV is independently associated with better odor identification function in the general population and is a robust mediator of the age-dependent association between volumes of central olfactory structures and olfactory function. Given that olfactory dysfunction is a common and early feature of many neurodegenerative diseases, OBV may serve as a preclinical marker for the identification of individuals who are at an increased risk for developing neurodegenerative conditions later in life.
eTable 1. Exclusion Criteria for Magnetic Resonance Imaging (MRI) in the Rhineland Study
eTable 2. The Association of Determinants With Olfactory Function Using Multivariable Linear Regression (n = 1915)
eTable 3. Relation Between Volumes of Olfactory Brain Structures and Olfactory Function
eTable 4. Relation Between Volumes of Central Olfactory Structures and Olfactory Bulb Volume
eTable 5. Relation Between Olfactory Bulb Volume and Volumes of Central Olfactory Structures
eTable 6. Mediation Effect of Central Olfactory Structure Volumes of the Association Between Olfactory Bulb Volume and Olfactory Function
eFigure 1. Coronal and Sagittal Depictions of the Annotated Olfactory Bulb Volumes (OBVs) on T2-Weighted Images
eFigure 2. The Optimal Cut-off Points for Detecting Hyposmia and Anosmia Based on Mean OBV
References
- 1.Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ. The prevalence of olfactory dysfunction in the general population: a systematic review and meta-analysis. Am J Rhinol Allergy. 2021;35(2):195-205. doi: 10.1177/1945892420946254 [DOI] [PubMed] [Google Scholar]
- 2.Doty RL. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 2017;16(6):478-488. doi: 10.1016/S1474-4422(17)30123-0 [DOI] [PubMed] [Google Scholar]
- 3.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020;77(8):1028-1029. doi: 10.1001/jamaneurol.2020.2125 [DOI] [PubMed] [Google Scholar]
- 4.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018-1027. doi: 10.1001/jamaneurol.2020.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dintica CS, Marseglia A, Rizzuto D, et al. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology. 2019;92(7):e700-e709. doi: 10.1212/WNL.0000000000006919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazal PP, Haehner A, Hummel T. Relation of the volume of the olfactory bulb to psychophysical measures of olfactory function. Eur Arch Otorhinolaryngol. 2016;273(1):1-7. doi: 10.1007/s00405-014-3325-7 [DOI] [PubMed] [Google Scholar]
- 7.Seubert J, Freiherr J, Frasnelli J, Hummel T, Lundström JN. Orbitofrontal cortex and olfactory bulb volume predict distinct aspects of olfactory performance in healthy subjects. Cereb Cortex. 2013;23(10):2448-2456. doi: 10.1093/cercor/bhs230 [DOI] [PubMed] [Google Scholar]
- 8.Servello A, Fioretti A, Gualdi G, et al. Olfactory dysfunction, olfactory bulb volume and Alzheimer’s disease: is there a correlation? A pilot study. J Alzheimers Dis. 2015;48(2):395-402. doi: 10.3233/JAD-150232 [DOI] [PubMed] [Google Scholar]
- 9.Rey NL, Wesson DW, Brundin P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis. 2018;109(pt B):226-248. doi: 10.1016/j.nbd.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rey NL, George S, Steiner JA, et al. Spread of aggregates after olfactory bulb injection of α-synuclein fibrils is associated with early neuronal loss and is reduced long term. Acta Neuropathol. 2018;135(1):65-83. doi: 10.1007/s00401-017-1792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu A, Fischbein N, Wintermark M, Zaharchuk G, Yun PT, Zeineh M. COVID-19-induced anosmia associated with olfactory bulb atrophy. Neuroradiology. 2021;63(1):147-148. doi: 10.1007/s00234-020-02554-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277-287. doi: 10.1002/path.4461 [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Breteler MM, Stöcker T, Pracht E, Brenner D, Stirnberg R. MRI in the Rhineland Study: a novel protocol for population neuroimaging. Alzheimers Dement. 2014;10(4S):P92. doi: 10.1016/j.jalz.2014.05.172 [DOI] [Google Scholar]
- 15.van der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559-569. doi: 10.1016/j.neuroimage.2007.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner D, Stirnberg R, Pracht ED, Stöcker T. Two-dimensional accelerated MP-RAGE imaging with flexible linear reordering. MAGMA. 2014;27(5):455-462. doi: 10.1007/s10334-014-0430-y [DOI] [PubMed] [Google Scholar]
- 17.Mugler JP, Brookeman JR. Efficient spatially-selective single-slab 3D turbo-spin-echo imaging. Proc Intl Soc Magn Reson Med. 2004;11:695. [Google Scholar]
- 18.Wilson DA, Chapuis J, Sullivan RM. Cortical olfactory anatomy and physiology. In: Doty R, ed. Handbook of Olfaction and Gustation. 3rd ed. Wiley-Liss; 2015:209-226. doi: 10.1002/9781118971758.ch10 [DOI] [Google Scholar]
- 19.Wang J, You H, Liu JF, Ni DF, Zhang ZX, Guan J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am J Neuroradiol. 2011;32(4):677-681. doi: 10.3174/ajnr.A2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110(10):976-981. doi: 10.1177/000348940111001015 [DOI] [PubMed] [Google Scholar]
- 21.Doty RL, Laing DG. Psychophysical measurement of human olfactory function. In: RL D, ed. Handbook of Olfaction and Gustation. 3rd ed. Wiley-Liss; 2015:229-261. doi: 10.1002/9781118971758.ch11 [DOI] [Google Scholar]
- 22.Kline R. Principles and Practice of Structural Equation Modeling. 3rd ed. Guilford Press; 2011. [Google Scholar]
- 23.Hayes AF. Methodology in the Social Sciences. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. Guilford Press; 2013. [Google Scholar]
- 24.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017. [Google Scholar]
- 25.Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(2):1-36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- 26.Zhou G, Lane G, Cooper SL, Kahnt T, Zelano C. Characterizing functional pathways of the human olfactory system. Elife. 2019;8:e47177. doi: 10.7554/eLife.47177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286(5440):711-715. doi: 10.1126/science.286.5440.711 [DOI] [PubMed] [Google Scholar]
- 28.Ueha R, Mukherjee S, Ueha S, et al. Viral disruption of olfactory progenitors is exacerbated in allergic mice. Int Immunopharmacol. 2014;22(1):242-247. doi: 10.1016/j.intimp.2014.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76(6):1175-1188. doi: 10.1016/j.neuron.2012.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smitka M, Puschmann S, Buschhueter D, et al. Is there a correlation between hippocampus and amygdala volume and olfactory function in healthy subjects? Neuroimage. 2012;59(2):1052-1057. doi: 10.1016/j.neuroimage.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 31.Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z. Alpha-synuclein pathology in the olfactory pathways of dementia patients. J Anat. 2007;211(1):117-124. doi: 10.1111/j.1469-7580.2007.00748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ubeda-Bañon I, Saiz-Sanchez D, Flores-Cuadrado A, et al. The human olfactory system in two proteinopathies: Alzheimer’s and Parkinson’s diseases. Transl Neurodegener. 2020;9(1):22. doi: 10.1186/s40035-020-00200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zapiec B, Dieriks BV, Tan S, Faull RLM, Mombaerts P, Curtis MA. A ventral glomerular deficit in Parkinson’s disease revealed by whole olfactory bulb reconstruction. Brain. 2017;140(10):2722-2736. doi: 10.1093/brain/awx208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chem Senses. 2012;37(4):325-334. doi: 10.1093/chemse/bjr102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson M, Nilsson LG, Olofsson JK, Nordin S. Demographic and cognitive predictors of cued odor identification: evidence from a population-based study. Chem Senses. 2004;29(6):547-554. doi: 10.1093/chemse/bjh059 [DOI] [PubMed] [Google Scholar]
- 36.Doty RL, Cameron EL. Sex differences and reproductive hormone influences on human odor perception. Physiol Behav. 2009;97(2):213-228. doi: 10.1016/j.physbeh.2009.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo HS, Guarneros M, Hudson R, et al. Attitudes toward olfaction: a cross-regional study. Chem Senses. 2011;36(2):177-187. doi: 10.1093/chemse/bjq112 [DOI] [PubMed] [Google Scholar]
- 38.Dalton P, Doolittle N, Breslin PA. Gender-specific induction of enhanced sensitivity to odors. Nat Neurosci. 2002;5(3):199-200. doi: 10.1038/nn803 [DOI] [PubMed] [Google Scholar]
- 39.Oliveira-Pinto AV, Santos RM, Coutinho RA, et al. Sexual dimorphism in the human olfactory bulb: females have more neurons and glial cells than males. PLoS One. 2014;9(11):e111733. doi: 10.1371/journal.pone.0111733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hummel T, Welge-Lüssen A. Assessment of olfactory function . In: Hummel T, Welge-Lüssen A, eds. Taste and Smell. An Update. Vol 63. Karger; 2006:84-98. doi: 10.1159/000093752 [DOI] [PubMed] [Google Scholar]
- 41.Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Exclusion Criteria for Magnetic Resonance Imaging (MRI) in the Rhineland Study
eTable 2. The Association of Determinants With Olfactory Function Using Multivariable Linear Regression (n = 1915)
eTable 3. Relation Between Volumes of Olfactory Brain Structures and Olfactory Function
eTable 4. Relation Between Volumes of Central Olfactory Structures and Olfactory Bulb Volume
eTable 5. Relation Between Olfactory Bulb Volume and Volumes of Central Olfactory Structures
eTable 6. Mediation Effect of Central Olfactory Structure Volumes of the Association Between Olfactory Bulb Volume and Olfactory Function
eFigure 1. Coronal and Sagittal Depictions of the Annotated Olfactory Bulb Volumes (OBVs) on T2-Weighted Images
eFigure 2. The Optimal Cut-off Points for Detecting Hyposmia and Anosmia Based on Mean OBV