Abstract
The first formal description of the microbicidal activity of extracellular traps (ETs) containing DNA occurred in neutrophils in 2004. Since then, ETs have been identified in different populations of cells involved in both innate and adaptive immune responses. Much of the knowledge has been obtained from in vitro or ex vivo studies; however, in vivo evaluations in experimental models and human biological materials have corroborated some of the results obtained. Two types of ETs have been described—suicidal and vital ETs, with or without the death of the producer cell. The studies showed that the same cell type may have more than one ETs formation mechanism and that different cells may have similar ETs formation mechanisms. ETs can act by controlling or promoting the mechanisms involved in the development and evolution of various infectious and non-infectious diseases, such as autoimmune, cardiovascular, thrombotic, and neoplastic diseases, among others. This review discusses the presence of ETs in neutrophils, macrophages, mast cells, eosinophils, basophils, plasmacytoid dendritic cells, and recent evidence of the presence of ETs in B lymphocytes, CD4+ T lymphocytes, and CD8+ T lymphocytes. Moreover, due to recently collected information, the effect of ETs on COVID-19 is also discussed.
Keywords: extracellular traps (ETs), neutrophils ETs, macrophage ETs, mast cell ETs, eosinophil ETs, lymphocyte ETs, basophil ETs, dendritic cell ETs, COVID-19
1. Preamble
With the great impetus given to the understanding of cellular functions in the immune system in the early 1950s, much information has been obtained; nevertheless, some mechanisms have not yet been fully elucidated. This knowledge is crucial to understand the mechanisms of disease and/or protection, since knowing how to recognize in what way diseases occur develop new treatments and effective vaccines with fewer adverse effects. Thus, paraphrasing Stephen Hawking [1], cells are a universe involved in a plasma membrane, and new components and functions are described every day. The formation of extracellular traps (ETs) containing DNA is clear evidence. The mechanisms described are varied and can be identified as inhibitors or facilitators of lesions, and some recent publications describe details of the ETs formation, their mechanisms, and structure [2,3,4,5]. The ETs formation seems to be a fast event, and perhaps, for this reason, it has not been easily observed in vivo until now. In addition, some cells in which their formation has been described are difficult to handle. Thus, much of the knowledge was obtained from in vitro or ex vivo studies with different protocols (reviewed in [2]). However, evaluations in experimental models and human samples have corroborated many of these results [6]. Although some mechanisms and effects of ETs release still need further elucidation, what is already known shows the importance of ETs in the control and/or development of the immune response.
2. General Background
The term ETosis was coined in the late 2000s [7,8] to designate a type of cell death promoted by decondensation of nucleic DNA called ETs. Based on the first description of the microbicidal activity of neutrophils [9], several studies showed that ETs play a pivotal role in infection control through an innate immune response, and their understanding, scope, and particularities have been described over subsequent years. Additionally, what has been previously described as having a beneficial action upon the resolution of inflammation is now known to be also capable of expanding inflammatory processes. It is also involved in the pathogenesis of various infectious and non-infectious diseases, such as autoimmune, cardiovascular, thrombotic, and neoplastic diseases, etc. [2,10,11,12,13]. It is known that DNA must be removed from the system rapidly, since it may stimulate an inflammatory response, as observed in the suggested relationship between the presence of extracellular DNA and several autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), as well as HIV infection, cardiovascular diseases, and neoplasms [14,15,16,17].
Besides neutrophils, ETs formation has already been identified in macrophages, mast cells, eosinophils, basophils, plasmacytoid dendritic cells, and recently in lymphocytes. Hence, the term originally coined (NETs—neutrophil extracellular traps) was adapted according to the cell type involved. The cell death resulting from this process was called ETosis to differentiate it from other types of cell death, such as necrosis, apoptosis, necroptosis, and pyroptosis [7,8,13,18].
It is worth noting that recent studies suggest that the intensity and type of inflammatory response produced by DNA traps depend on the type of producing cell, the origin of the DNA involved, and on the presence of other associated products or not with DNA, such as enzymes, plasma proteins, histones, etc. Lately, it has been found that not all ETs formation produces cell death [19,20,21], and a new classification of DNA types released into the extracellular environment has been organized [2,21]. In summary, the previously detected types of DNA release are divided into: (1) Suicidal ETosis—nuclear DNA release with histones, which occurs 3–8 h after cell activation. The nuclear chromatin is decondensed, and after expanding into the cytoplasm, it associates with cytoplasmic and granular proteins. As expansion continues, the plasma membrane breaks, causing the death of the involved cell and the release of the DNA associated with histones and other proteins into the extracellular environment, thus forming filament traps in continuous expansion. This can be detected after stimulation by phorbol myristate acetate (PMA), concanavalin A (ConA), interferon (IFN), infectious agents, immune complexes, and autoantibodies, among others [2,10]; (2) vital ETosis—release of DNA, maintaining cell viability. The vital ETs formation is an early/rapid process that usually occurs between 5–60 min after cell activation, which continues to perform its functions, such as chemotaxis and phagocytosis after externalization of DNA (nuclear or mitochondrial) simultaneously as degranulation and protein release [2,19,20,21,22]. Two main forms have been described: (1) Extracellular environment release of vesicles containing nuclear DNA, which expands to form extracellular traps. Initially, it maintains cell viability and functions as chemotaxis, adherence, and phagocytosis. It has been well described for neutrophils, even when anuclear cells are identified, since the granules and outer membrane are preserved [19,20,21]. In vitro studies suggest a rapid process when compared to ETs produced by suicidal ETosis. It has already been described that later, these cells also die and are usually phagocytosed by macrophages; (2) produced from mitochondrial DNA (mtDNA) in a mechanism dependent on reactive oxygen species (ROS), which is also related to the maintenance of cell viability. Essentially, two forms of mtDNA exteriorization are proposed: (A) Initial release into cytosol and thereafter into vesicles that merge with the plasma membrane, exteriorizing their content; and (B) mitochondrial and plasma membrane fusion, producing the direct release of DNA content, into the extracellular environment. However, the latter has not yet been fully proven [2,3,4,5,10,13,23,24].
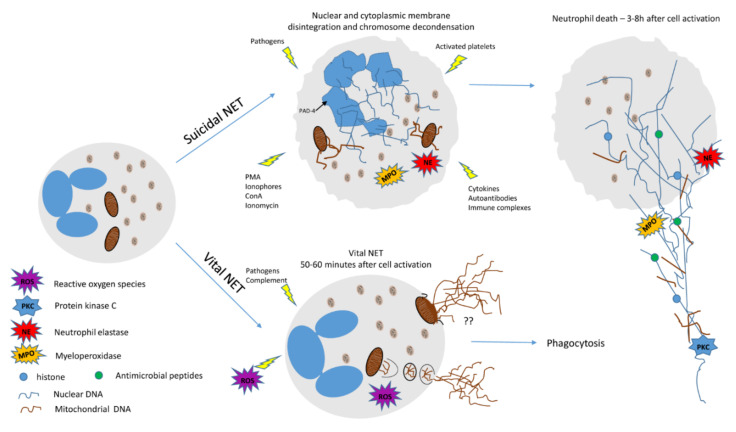
Data collection has shown that the same cell type can present more than one ETs formation mechanism simultaneously or consecutively, and that different cell types can present similar ETs formation mechanisms [2,14,25,26]. Nevertheless, further studies are needed to better understand the relationship between the origin of ETs formation and its role in cell viability and in the immune response. Figure 1 shows a scheme of the different types of ETs produced by neutrophils.
Figure 1.
Scheme showing the formation of suicidal and vital NETs. Suicidal NETs: Occurs 3–8 h after cell activation and ends with the death of neutrophils. It starts with chromosomal decondensation and nuclear membrane disintegration, followed by decondensation and release of mitochondrial DNA to the cytosol. Finally, the cytoplasmic membrane disintegrates, releasing nuclear and mitochondrial DNA, as well as granular content into the extracellular environment. Vital NETs: Occurs 50–60 min after cell activation maintaining neutrophil viability and phagocytosis. Two forms of exteriorization of mitochondrial DNA are proposed: a-Initial release into the cytosol and thereafter into vesicles that merge with the plasma membrane, exteriorizing their content; and b-fusion of the mitochondrial and plasma membrane, producing the direct release of the content of DNA into the extracellular environment. However, the latter has not yet been fully proven. Various stimuli for the formation of suicidal and vital NETs have already been described, the most commonly seen in the figure. Some variations related to stimuli and composition can occur with ETs produced by other types of cells.
Concerning infectious diseases, releasing ETs into the extracellular environment promotes the capture and death of the surrounding microorganisms [27,28,29,30,31]. Moreover, the presence of DNA and other proteins can lead to an increment in local inflammation, including exacerbation of the disease [10,32]. Even so, it has been described that the evasion of microorganisms by DNase production, the inhibition of cell recruitment from the immune response, as well as the evasion of bacteria by modification of the cell wall with alteration of the ionic charge, hampers the coupling of the microorganism to ETs (reviewed by the authors of [33]).
In terms of location, ETs can be virtually seen in any compartment of the human body, such as solid organs and blood, where clusters of ETs are cell-free and may be involved in the formation of thrombus and tissue injury [5,24]. On the other hand, the presence of enzymes can degrade inflammatory mediators, which could lead to a decrease in the inflammatory process, promoting the resolution of lesions. We will discuss the presence of ETs in neutrophils, macrophages, mast cells, eosinophils, basophils, plasmacytoid dendritic cells, and new evidence of the presence of ETs in B lymphocytes, CD4+ T lymphocytes, and CD8+ T lymphocytes. Moreover, due to recent data, the effect of ETs on COVID-19 will also be discussed.
3. Neutrophils
Neutrophils are the first cells attracted to the site of tissue injury. They are polymorphonuclear leukocytes originated from a myeloid progenitor in the bone marrow, and present nucleic acid organized in a multilobulated form containing three to five lobes, connected by chromatin. Indeed, about 60% of the white blood cells produced by bone marrow are neutrophils, although this number may change depending on the stimulus [34,35]. Though there is a plethora of neutrophils, they have a fairly short lifespan, and in the absence of signs of infection or inflammation, they die 6 to 8 h later via a programmed cell death process [36]. They are removed from the tissues by macrophages, preventing the release of their potentially harmful content into the tissues [37]. The maintenance of neutrophil cell debris in tissues has been associated with developing diseases, such as cystic fibrosis, chronic obstructive pulmonary disease (COPD), and RA [38].
Neutrophils act upon the innate immune response with inflammatory responses against pathogens (protozoa, bacteria, fungi, viruses) via intra- and extracellular mechanisms, such as phagocytosis, secretion of granular enzymes, ROS production, and NETs formation [9,39,40]. After the onset of the early stage of tissue injury, neutrophils rapidly migrate into tissues through the expression of chemotactic factors and adhesion molecules (P-selectin and E-selectin) expressed in endothelial cells [41,42]. Thus, neutrophils express the chemokine receptors CXCR1 and CXCR2 that bind to CXCL1 and CXCL8; the main chemokine that maintains migration of neutrophils into the affected tissue [43,44]. Classically, within the tissues, neutrophils initiate the process of phagocytosis, which can occur through recognizing PAMP (Pathogen-Associated Molecular Patterns), through TLR (Toll-like Receptors), or through opsonization and connection with Fc receptors, complement receptors (CR1 and CR3), and C-type lectins. The pathogen is then destroyed by the enzymes present in its granules and by the formation of ROS [39,40]. These granules are classified into azurophils (or primary), specific (or secondary), and gelatinase (or tertiary). Azurophilic granules consist of myeloperoxidase (MPO), defensins, lysozymes, and antibacterial proteins with serine protease activity (NE-neutrophil elastase, Proteinase 3, and cathepsin G) [45,46]. However, specific granules have lactoferrin and lysozymes, and gelatinase granules consist of very few antimicrobial substances, which function as storage for metalloproteases (gelatinase and leucolysin) [38,47].
During the pathogen internalization/neutrophil activation process, Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase is activated by converting molecular oxygen into superoxide anion, hydrogen peroxide (H2O2), and free radicals (such as ROS) [35,48] in the oxidative burst with active participation in the elimination of pathogens. Furthermore, the formation of hypochlorous acid (HOCl) occurs through the catalysis of hydrogen peroxide [49].
In 2004, Brinkmann et al. described the control of aggressive agents by neutrophils stemming from the decondensation and release of DNA called NETs. The release of nuclear DNA by neutrophils had been previously verified by Takei et al. and described as a new form of cell death called NETosis [9,50]. Subsequently, several studies have analyzed their formation mechanisms and function [5,10,22].
Typically, NETs are 3D structures composed of DNA, histones (H1, H2A, H2B, H3, and H4), proteins of three types of granules, such as NE, MPO, cathepsin G, leukocyte proteinase 3 (PR3), azurocidin, lysozyme C, and antimicrobial peptides, i.e., defensins and cathelicidins, that act as secreted physical barriers to restrain the spread of free pathogens present in the extracellular environment or that evaded phagosomes [2,3,5]. The mechanism of NETs formation can be induced by several stimuli: Microbial (bacteria, fungi, protozoa, viruses) [3,23], immune complexes [6,51], cytokines [6,51], damage-associated molecular patterns (DAMPs) [52], activated platelets [20,21], microcrystals (cholesterol, calcium carbonate) [6,23,53], among others. The phorbol ester (mainly PMA) and ionophores (A23187, nigericin) are important inducers of NETs in vitro [4,5,23] (Figure 1).
Although ETs are widely studied, NETs are a complex phenomenon, and there are still questions to be elucidated about the mechanisms involved in their formation (origin of the DNA, programmed cell death, signaling pathways), their role in host defense, and the pathophysiology of some diseases [6]. Many studies have been carried out in recent years; however, factors, such as the origin of the neutrophils used in the experiments, the isolation methods, the culture media, and/or the cell reactivation may influence the results, making it difficult to compare the results and the outline of unified knowledge about NETs [54,55]. Despite variations in nomenclature and classification, the mechanisms can share pathways, resulting in the release of extracellular DNA [2,5,10,20,22,23,55]. In order to standardize, the Nomenclature Committee on Cell Death (NCCD) recommends that the term “NETosis” be replaced by NETs formation, since NETs can be produced without cell death [21,56].
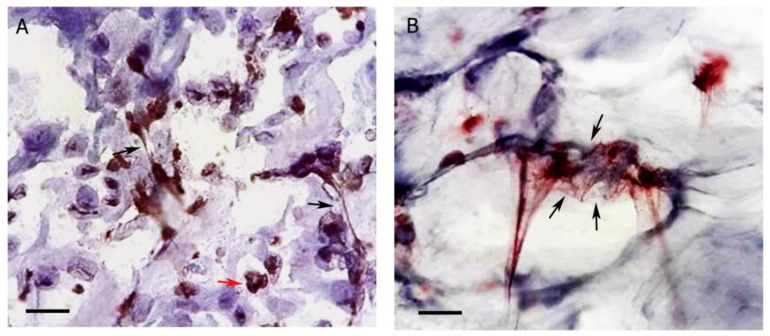
Morphologically, several types of NETs have been described, roughly dense, wider, or more delicate, isolated, or forming aggregates, etc., and can be organized according to the stimulus, pH, neutrophil concentration, and pathogens present at the site of NETs formation [24]. For example, tapering formations called spike NETs can be induced by the complement factor C5a (C5a), among other stimuli. However, the alkaline pH, commonly observed in chronic wounds, favors the formation of cloudy NETs and giant bicarbonate-induced aggregated NETs (aggNETs), which can reduce necrotic areas [22,24,57]. AggNETs are large cloudy or clumpy traps that form in places with high-density neutrophils and contain viable neutrophils, microorganisms, and enzymes. They act upon the elimination of pathogens and the degradation of inflammatory mediators, favoring healing. However, they can also cause vessel and duct obstruction, due to their size and sticky nature [24,58,59]. The formation of NETs with different morphology can be observed in active cutaneous lesions of American Tegumentary Leishmaniasis (ATL) [29] (Figure 2).
Figure 2.
Neutrophils and NETs in skin lesions of patients with Cutaneous Leishmaniasis. Neutrophil elastase was used as a marker of neutrophils and NETs by immunohistochemistry (brown staining—aminoethyl carbazole—AEC). (A) Apparent whole neutrophils (red arrows) and NETs with a spiky aspect (black arrows). (B) Aggregated NETs (black arrows). Magnification bar A = 25 μm and magnification bar B = 10 μm. Counterstaining was carried out using Meyer’s hematoxylin.
The cellular pathways involved in NETs formation are complex, and their protein composition seems to be stimulus-dependent [10,55]. Several enzymes and signaling proteins, such as protein kinase C (PKC), extracellular signal-regulated kinase (ERK), MEK (MAPK/ERK kinase), participate in the process. NE and MPO play a critical role in histone cleavage and inactivation, chromatin decondensation, and nuclear membrane degradation, enabling the combination of nuclear and cytoplasmic contents. Protein-arginine deiminase type 4 (PAD-4) migrates to the nucleus of neutrophils to induce an increase in the permeability of the nuclear membrane and also takes part in the chromatin decondensation process through the citrullination of histones. Despite being essential in the NETs formation, there is still no clear consensus on whether its role is fundamental in suicidal NETs formation [5,10,22,55].
NETs can also be classified as NADPH (NOX)-dependent and NOX-independent according to ROS production, since the presence or absence of ROS produced by NADPH oxidase in the cytoplasm or mitochondria seems to influence their formation [3,4,5,22,23,54]. In the formation of NOX-dependent NETs, various stimuli (e.g., PMA, cholesterol crystals, fungi, bacteria) induce ROS production by NADPH oxidase [10,23,54,55]. It has been found that NOX-independent NETs formation can be induced by calcium ionophores (e.g., A23128, A23187, ionomycin), uric acid crystals, nicotine, and immune complexes [22,25,55,60], but it is still questionable whether these mechanisms are ROS-independent, or whether mitochondrial ROS (mtROS) are produced [54]. Vital NETs produced from mtDNA seem to depend on mtROS [61,62,63]. On the other hand, vital NETs with nuclear DNA seem to be ROS-independent [19,20,21]. Moreover, it has been reported that Candida albicans, gram-positive bacteria Group B Streptococcus, and Leishmania amazonensis may induce the formation of these traps with little or no ROS production by neutrophils, possibly because these pathogens can generate their own ROS [4,64].
In candidiasis, neutrophils are the major cells recruited to destroy yeasts and hyphae of the fungus, although the latter form cannot be phagocyted, due to its size. However, hyphae are destroyed by the NETs formation even independently of opsonization, which may occur in both hyphae and yeast. Thus, the elimination of these fungi results from the activity of the granules, mainly calprotectin. This process depends on recognizing β-glucan by CR3, fibronectin, and ERK, but is ROS-independent and the NETs formed are classified as vital [65]. Though, Aspergillus fumigatus, a fungus that causes aspergillosis, an opportunistic disease that can lead to severe lung involvement, stimulates the NETs formation from β-glucan in a ROS-dependent process. However, it has been demonstrated that these NETs cannot kill the fungus but can prevent its spread and growth [66]. The presence of NETs in active sporotrichosis lesions caused by Sporothrix schenckii in both fixed and sporotrichoid forms has also been demonstrated [30].
Infections by Protozoa of the genus Leishmania, on the other hand, may stimulate NETs dependent on NE activity, but independent of ROS from NADPH oxidase and PAD-4. NETs have been described as having a protective function against this protozoosis, being able to capture and destroy parasites, except Leishmania mexicana, which can escape from this mechanism. Neutrophils have a close relationship with Leishmania spp. from the early stages of infection, as they are rapidly recruited into the skin after the entry of the protozoan. Interestingly, it has already been demonstrated that the saliva of Lutzomyia longipalpis, one of the insects that transmit this parasite, has endonucleases capable of degrading NETs, which could indirectly act on the pathogenesis of the disease [64,67,68,69,70]. In addition, NETs are also observed in ATL lesions presenting different evolution times, suggesting a continuous role of neutrophils in tissue inflammation [29]. In recent work, da Fonseca-Martins et al. demonstrated that protozoa of the genus Leihmania may increase the expression of programmed death ligand-1 (PD-L1) by neutrophils, in favor of their survival, with a consequent suppressor effect associated with progression of the lesion [71].
The protective role of NETs in innate immunity is associated with the resolution of inflammation and healing, along with antimicrobial activities. Nevertheless, if there is no balance between their formation/degradation, NETs can promote tissue damage and inflammation, implicating the pathophysiology of several diseases [3,24,72]. It is the case of infection by the causative agent of Malaria—Plasmodium falciparum—which induces NETs formation independent of ROS, but dependent on the MEK/ERK pathway. The components released by neutrophils during the process can cause tissue damage, mainly in the liver, further aggravating the condition [73]. This imbalance in the formation and extravasation of NETs is also evident in cases of sepsis. Despite having an important role in containing bacteria, the content of neutrophils released into the external environment can increase inflammation, cause thrombosis, and, in the worst case, lead to the failure of several organs [74,75].
NETs may also act as self-antigens and induce an immune response with the production of autoantibodies (e.g., anti-damaged-DNA/RNA ribonucleoprotein antibody immune complexes) and DAMPs capable of activating neutrophils and triggering the formation of new NETs. A vicious cycle is created and may exacerbate inflammation and lead to the development of autoimmune diseases, such as SLE [25,51,76]. In the literature, SLE is a well-reported example of loss of tolerance to self-antigens. In these patients, anti-DNA antibodies may deactivate the DNase enzyme, which cleaves NETs. When NETs are not cleaved, they can become a source of self-antigens, thus stimulating the higher production of anti-DNA antibodies [77].
Activated platelets and neutrophils may contribute to an increased risk of adverse cardiovascular events after acute myocardial infarction [78]. NETs are an important product of the platelet-neutrophil axis and contribute to vascular damage in cardiovascular disease [78]. During transmigration, endothelial cells interact with neutrophils and may stimulate the formation of the NETs within the microcirculation and generate an occlusion, leading to thrombotic diseases. Immune-thrombi formation occurs after contact of activated platelets with polymorphonuclear cells [79]. Many works on sepsis have also demonstrated this phenomenon [24,80,81,82,83]. In addition, the participation of interleukin-1β (IL-1β), a pro-inflammatory cytokine, in the tissue factor (TF) release and NETs formation in atherothrombotic events has also been indicated [84]. IL-1β recruits cells by inducing the expression of adhesion molecules on endothelial cells [85] and has already been suggested as a stimulus for NETs production in abdominal aortic aneurysms [86]. In a murine model, NETs and inflammasomes have been shown to cooperatively contribute to venous thrombosis [87]. The stimulation of neutrophils led to the formation of NETs, which, through their histones, promoted a robust activation of caspase-1 in platelets. Using intravital microscopy, the study showed that NETs were colocalized with caspase-1 and platelets at the site of thrombosis [87].
The interaction of activated platelets and neutrophils as causes of vascular damage is also described in myocardial infarction and in autoimmune processes, such as systemic sclerosis, where the mobility group box 1 (HMGB1) protein induces the formation of NETs mediated by autophagy [79,88].
Neutrophil autophagy is a process already discussed by some authors and seems to be related to the production of NETs in several situations, such as sepsis, gout, and fibrotic processes. Fibrosis occurs because of the activation of fibroblasts by the exteriorized content of neutrophils. The process can be harmful, especially in lung diseases with an inflammatory process, and has the participation of components, such as chromatin, histones, MPO, and IL-17 [89,90].
In tumors, it has been found that NETs-derived granule proteins may contribute to the migration of tumor cells from the primary site to other sites, favoring the formation of metastases [11,12,91]. The type of disease associated with NETs is related to the trap formation site and their degradation mechanisms [24].
NETs and COVID-19
Recent studies reinforce a body of evidence pointing to the participation of neutrophils and especially NETs in COVID-19 [92,93,94,95,96,97,98,99]. As mentioned above, when NETs are excessively induced in vital organs, such as the lung, they are harmful to the body. Patients with severe cases of COVID-19 are predisposed to thrombosis, which is the obstruction of veins and arteries, due to excessive formation of blood clots [100], which is frequently considered one of the main negative consequences of the formation of NETs. Severely infected patients develop an uncontrolled and damaging inflammatory response to host tissues, similar to those seen in cases of sepsis. This excessive damage has been credited to the participation of neutrophils in the acute phase of infection [101].
Severe COVID-19 is also associated with a cytokine storm, characterized by increased plasma concentrations of various inflammatory mediators. Some of them involved in the regulation of neutrophil activity and the expression of chemoattractants that increase the trafficking of neutrophils. Since NETs can induce macrophages to secrete IL1β, and IL1β enhances NET formation in various diseases [84,86], it is possible that a NET–IL1β loop is activated in severe COVID-19, and can participate in the formation of microthrombi and respiratory decompensation.
The significant increase of neutrophils in the bloodstream is one of the markers of COVID-19 severity, being associated with a higher risk of death. The increase of molecules that indicate the presence of NETs has been detected in the sera of critically ill patients [92,94,95,96,97,98], and increased levels of plasma NETs markers correlated with increased COVID-19 severity [96,97]. NETs formation could also be observed in lung autopsies tissues of COVID-19 patients [94,95,96].
The presence and excessive activation of neutrophils by complement, together with platelets and NET formation, have also been associated with severity in SARS-CoV-2 infection, in which TF plays an important role in the thrombogenic activity [102,103]. The results produced by Skendros et al. suggest that the inhibition of C3 may interrupt neutrophil TF release and prevent complement activation [103]. Morrissey et al. identified a population of low-intensity inflammatory neutrophils in COVID-19 patients. These cells expressed intermediate levels of CD16 (CD16Int), an inflammatory profile, and were associated with platelet activation, spontaneous formation of NETs, increased phagocytic capacity, and cytokine production [104]. Furthermore, neutrophils were the main cells found in bronchoalveolar lavage fluid (BALF) and in peripheral blood, associated with high levels of D-dimer, ferritin, and inflammatory cytokines (such as tumor necrosis factor-TNF and IL-6), suggesting the participation of these cells in coagulopathy, systemic inflammation, and severe acute respiratory syndrome associated with COVID-19 [104].
Neutrophil exposure to serum from severe COVID-19 patients was also shown to induce functionally active NETs [93,99]. This can occur either because of the presence of active viral particles in the serum or the presence of inflammatory factors capable of activating the formation of NETs. Other results further indicated that SARS-CoV-2 alone can directly activate NETs in neutrophils incubated with the virus [92,94], indicating in an unprecedented way that the new coronavirus can stimulate neutrophils to release ROS, together with NETs [92]. Another class of weapon used by defense cells to fight infections, these substances act directly to kill invading microorganisms, and simultaneously, stimulate the formation of NETs in the process of activating the blood coagulation cascade, a hallmark of severe cases of COVID-19 [93,100].
These results alert that the activation of neutrophils to release NETs and ROS is possibly one of the important causes of thrombosis in COVID-19 [92,93,94,95,96]. Therefore, it is possible to apply therapeutic strategies on these targets to avoid as much as possible the formation of vascular thrombosis, a harmful response for patients. Affecting organs, such as lungs, kidneys, heart, and brain, the phenomenon is associated with death by cardiorespiratory failure or multiple organ failure [100]. It is important to note that these works point to a biochemical pathway that may target developing new therapies to combat clot formation. NETs can be attacked by existing drugs in different ways. Among the options that can be evaluated, we can consider the medications used to treat cystic fibrosis, which works by disrupting neutrophilic networks and released antioxidants [105]. Colchicin and Anakinra are other existing drugs that could be used as blockers of the inflammatory loop between NETs and IL1β, with several ongoing clinical trials (ClinicalTrials.gov identifiers (accessed on 25 June 2021): NCT02735707, NCT04322565, NCT04322682, NCT04324021, NCT04326790, NCT04328480, NCT04330638) [106].
Examples of NETs and their role in host defense and disease are described in Table 1.
Table 1.
Neutrophil extracellular traps in host defense and disease.
| Cell | Mechanism of ETs Formation | Stimulus/Models | Biological Effect Protective Deleterious | |
|---|---|---|---|---|
| Neutrophil in Cancer | Suicidal (ROS-dependent) [91,107,108,109,110] Early/rapid ROS-independent (but may alternatively be dependent on autophagy) [111] Mitochondrial NETs [61] |
In vivo Murine models of: breast cancer [91], lung carcinoma [107], metastatic colorectal cancer [109,110], lung carcinoma [108] Ex vivo Serum samples of patients with metastatic colorectal [109,110] and human tissue samples of breast cancer [91] In vitro Cancer cells [91], pancreatic cancer cells [111], anaplastic thyroid cancer cells [61] |
Entrapment of tumor cells [107] | Association with an aggressive subtype of breast cancer [91] Tumor progression [61,110] Metastasis [91,107,108,109,110] Reduction in disease-free survival [109] Cancer-associated thrombosis [111] |
| Neutrophil in Central Nervous System Diseases | ROS-dependent [112] Nuclear DNA [113,114,115,116] |
In vivo Murine model of Alzheimer’s disease, meningitis and [112,116] Piglet model of S. suis meningitis [113] In vitro Thrombi from patients with acute ischemic stroke [114,115]; paraffin sections of human cortex from Alzheimer’s disease brains [116] CSF of patients with S. pneumoniae meningitis [112] Modified human BCSFB model [113] |
Entrapment of streptococci [113] | Alzheimer’s disease pathogenesis [116] Impairment of pneumococci clearance in meningitis [112] Poorer clinical outcomes and inflammation aggravation in patients with acute ischemic stroke [115]; Important constituents of cerebral thrombi [114] |
| Neutrophil in Pulmonary Diseases | Suicidal, ROS-dependent [117,118] ROS-dependent [119] Nuclear DNA [120,121,122] |
In vivo Murine and human model of rhinovirus-induced allergic asthma exacerbation [122], murine model of S. pneumoniae induced pneumonia [119], and PTB [121] Ex vivo Human lung samples [121] In vitro Sputum samples of asthma patients/human airway epithelial cells [117] Sputum samples of COPD patients [118,120] |
Asthma severity and exacerbation [117,122] Airway epithelial and endothelial damage [117] Severity of S. pneumoniae induced pneumonia [119] COPD severity and airway flow limitation [118,120] PTB pathogenesis and severity [121] |
|
| Neutrophil in Autoimmune Diseases | ROS-dependent [123] Mitochondrial NETs (mtDNA, mtROS) [25] Not described [15] |
In vitro Immune complexes (Anti-LL-37, anti-HNP, PR3 and MPO, ANCAs) [123] Healthy and lupus neutrophils (PMA and immune complexes) [25] Healthy and rheumatoid arthritis neutrophils (PMA and A23187) [15] |
Autoimmune diseases (systemic lupus erythematosus, psoriasis, vasculitis, rheumatoid arthritis) [15,25,123,124] |
|
| Neutrophil in Thrombosis/Cardiovascular Disorders | Nuclear DNA [125] ROS-dependent [126] |
In vitro Blood neutrophils and platelets [125] In vivo Deep vein thrombosis model (Baboons) [125] In vivo Murine model (cholesterol crystals) [126] |
Thrombosis [125] Atherosclerosis [126] |
|
| Neutrophil and Virus | ROS-dependent [127,128] Suicidal, ROS-dependent [92] PAD-4 dependent [94] Suicidal, presence of Cit-H3 and MPO-DNA complexes [94,95,96,97,98,99] |
In vivo Murine model of influenza A virus H1N1pneumonia [127] and Chikungunya virus infection [128] In vitro Neutrophils + influenza virus–primed epithelial cells [127] Serum samples and/or nasal swab specimens from COVID-19 patients [92,93,94,95,96,97,98,99] Neutrophils + SARS-CoV-2 [92,94] Neutrophils + Chikungunya virus [128] Ex vivo BALF and lung autopsies from COVID-19 patients [94,95,96] |
Virus capture, Neutralization and reduction of viral load in the blood. [128] |
Lung injury [127] Thrombosis formation in COVID-19 [92,96,99] COVID-19 Pneumonia [97] COVID-19 severity and vascular damage [94,95,98,99] |
| Neutrophil and Fungi | Suicidal, ROS-dependent [66,129,130] Vital NETs, ROS-independent [65] Not described [30] |
In vivo Murine model of A. fumigatus [66] Murine model of C. albicans infection [129] In vitro A fumigatus conidia [130] C. albicans (β-glucan) [65] Ex vivo Active sporotrichosis lesion [30] |
Entrapment of conidia, the only fungistatic effect [66,130] Capture and kill C. albicans yeast and hyphal forms [65,129] Antimicrobial effect [30] |
|
| Neutrophil and Protozoa | Early/rapid, ROS-independent, and late ROS-dependent [68] Suicidal, ROS-dependent [64] ROS-dependent [28,73,131] ROS-independent [132] Not described [27,29,67] |
In vivo Murine model of T. cruzi [131] Murine model of Malaria with P. berghei [132] and P. chabaudi [73] Murine model of T. gondii [28] Ex vivo ATL active cutaneous lesions [29] In vitro Leishmania spp.—amastigotes, promastigote/lipophosphoglycan [64,67,68] T. cruzi [131] Blood samples from patients infected with P. falciparum [73,132] |
Containment of promastigotes at the inoculation site and Leishmania killing [64,68] Limits infection by affecting the parasite’s pathogenicity [131] Antimicrobial effect [29,73,132] Interferes with the parasite’s ability to invade cells [28] |
Activation of emergency granulopoiesis via GM-CSF production, and induction of the endothelial cytoadhesion receptor ICAM-1 [73] Stimulus of ANA production, which may lead to autoimmunity [27] |
ETs, extracellular traps; ROS, reactive oxygen species; NETs, neutrophil extracellular traps; CSF, cerebrospinal fluid; BCSFB, blood-cerebrospinal fluid barrier; PTB, pulmonary tuberculosis; COPD, chronic obstructive pulmonary disease; mtDNA, mitochondrial DNA; GM-CSF, granulocyte macrophage colony-stimulating factor; CF5a, complement factor 5a; LPS, lipopolysaccharide; TLR4, toll like receptor 4; anti-LL-37, antimicrobial peptide, anti-HNP, human neutrophil peptide; PR3, proteinase-3; BALF, bronchoalveolar lavage fluid; Cit-H3, citrullinated histone H3; MPO, myeloperoxidase; ANCAs, anti-neutrophil cytoplasmic antibodies; PMA, phorbol-12-myristate-13-acetate; oxLDL, oxidized low-density lipoprotein; ICAM-1, intercellular Adhesion Molecule 1; ANA, antinuclear Antibodies; ATL, American Tegumentary Leishmaniasis.
4. Macrophages
Macrophages are leukocytes produced in the bone marrow from myeloid progenitors. They leave the bone marrow as peripheral blood monocytes, and, when located in tissues, differentiate into macrophages. In some tissues, they remain for variable periods, being called resident macrophages, and may receive specific denominations according to the tissue, such as histiocytes in the skin, or Kupffer cells in the liver. Macrophages were initially identified by their phagocytosis ability, which can be easily visualized under an optical microscope. As studies on the immune system advanced, macrophages were found to participate in several stages of the immune response to infectious agents, from the initial stimuli to naive T lymphocytes for their differentiation in activated T cells, subsequently acting upon the effector phase of the immune response with intense secretory and microbicidal activity. Finally, when pathogens are eliminated, macrophages act upon the removal of cell debris in the healing process and return to homeostasis. They are involved in the remodeling of the extracellular matrix, angiogenesis, and stimulation of fibroblasts. Moreover, they act significantly on non-infectious inflammatory processes, secreting mediators, and phagocyting cellular debris, among other functions. Macrophage subpopulations have been described, being M1 and M2 the best-known expression profiles (reviewed by the authors of [133]). In brief, the M1 profile is composed of macrophages, activated by the classical IFN-γ pathway and TLR microbial ligands that can express inflammatory cytokines, the inducible nitric oxide synthase (iNOS) enzyme, and the production of nitric oxide (NO) [134]. The M2 profile, however, is activated via an alternative route. The known stimuli are cytokines IL-13 and IL-4, and this cell can express arginase-1, TGF-β, and IL-10 [134]. Typically, the M2 profile is related to pathogens eliminated by the Th2 cells-mediated immune response. Another function described for M2 cells is to induce healing, as they produce fibroblast growth factors, stimulate collagen synthesis, and angiogenesis [135].
Recently, an additional effector function has been described for macrophages: the capacity to release their DNA content to form extracellular traps called METs (macrophage extracellular traps). METs are mostly composed of DNA and histones, but also of MPO, lysozymes, and citrullinated histones (H4Cit3, CitH3) [136,137,138,139,140,141,142,143]. Macrophages can release both nuclear DNA and mtDNA to form METs, which can be composed only of mtDNA, or the association of mtDNA and nuclear DNA [136]. The stimuli for METs formation described in in vitro studies can be NETs, NE, citrullinated histones, ROS, MPO, PMA, HOCl, IL-8, TNF, and IFN-γ [136,137,139,140,144,145,146]. Several infectious agents were also able to stimulate METs in vitro, as shown in Table 2.
Table 2.
Macrophage extracellular traps in host defense and disease.
| Cell | Mechanism of ETs Formation | Stimulus/Models | Biological Effect | |
|---|---|---|---|---|
| Protective | Deleterious | |||
| Monocytes/Macrophages and Fungi | Not described [137] ROS and NADPH oxidase-independent manner, mtDNA only or mtDNA and nuclear DNA [136] |
In vitro C. albicans [136,137] |
C albicans load control in vitro [137] Entrapment of C. albicans [136,137] |
|
| Monocytes/Macrophages and Bacteria | mtDNA only or mtDNA and nuclear DNA, ROS, and NADPH oxidase-independent manner [136] Not described [144] Elastase activity and M. tuberculosis ESX-1 [144] |
In vitro E. coli [136,142] M tuberculosis [144] IFN-γ [144] Ex vivo U. urealyticum and C. trachomatis [142] |
E. coli load control in vitro [136] Entrapment of E. coli and M. tuberculosis [136,142,144] |
|
| Monocytes/Macrophages and Protozoa | MPO, ROS, and NADPH oxidase-dependent manner [139,140] | In vitro E. ninakohlyakimovae [140] B. besnoiti/E. bovis [139] |
Entrapment of E. ninakohlyakimova, B besnoiti/E bovis [139,140] | |
| Monocytes/Macrophages in Diabetes and Obesity | PAD2/PAD4 mediated histone hypercitrulination [138] Not described [143] |
In vitro TNF [138] Not described [143] |
Induction of inflammation and insulin resistance [143] Acceleration of inflammation associated with obesity [138] |
|
| Monocytes/Macrophages in Thrombosis | Not described [141,147] | Not described [141,147] | Arteriosclerotic plaques and coronary thrombosis formation [141,147] Thrombus instability [147] |
|
ETs, extracellular traps; ROS, reactive oxygen species; NADPH, nicotinamide adenine dinucleotide phosphate mtDNA, mitochondrial DNA; METs, macrophage extracellular traps; ESX-1, ESAT-6 secretion system 1; IFN-γ, interferon gamma; MPO, myeloperoxidase; PAD2, peptidyl arginine deiminase 2; PAD4, peptidyl arginine deiminase 4; TNF, tumor necrosis factor; PMA, phorbol-12-myristate-13-acetate; —HOCl, hypochlorous acid, IL-8, interleukin.
METs have already been described in some inflammatory/infectious conditions. In acute epididymitis caused by Ureaplasma urealyticum, Chlamydia trachomatis, and E. coli, METs and NETs were observed in the semen of patients [142]. Three patterns of ETs formation were observed by macrophages and neutrophils in the semen: (1) Spread ETs—structures in the form of elongated bands of decondensed chromatin associated with antimicrobial proteins and composed of fine fibers 15–17 μm diameter; (2) Diffuse ETs—decondensed extracellular chromatin traps, associated with globular antimicrobial proteins and measuring 15–20 μm in diameter; (3) aggregated ETs—high-density release of ETs forming aggregates. However, METs were mainly diffuse and composed of DNA, histones, H4Cit3, and MPO [142].
A recently published protocol demonstrated the METs formation in vitro from macrophages derived from human monocytes [146]. Macrophages were polarized to the M1 profile, and then METs release was stimulated by inflammatory compounds (PMA, HOCl, IL-8, and TNF) [146]. In an experimental in vitro infection model, C. albicans stimulated the METs formation in macrophage cell line J774, peritoneal macrophages, and bone marrow-derived from BALB/c mice [136,137]. The METs formation occurred at the beginning of the assay, increased progressively over time, increasing the yeast: Macrophage ratio, and presenting a significant antimicrobial effect [137]. The authors also show the ability of the fungus to degrade METs—when comparing the ETs formation with live and dead yeasts, greater METs formation after stimulation with dead yeasts was observed. Moreover, they showed that there was arbitrary DNA degradation when cocultivation was performed with live yeasts, and there was no change in the amount of arbitrary DNA when performed with dead yeasts. Subsequently, they suggested that C. albicans can degrade METs, describing it as a virulence factor and escape mechanism [137]. However, in the study by Liu et al., METs could not control fungal load, despite restraining C. albicans [136]. Possibly, the restraint of these pathogens by METs reduces the probability of spreading through the organism, which would be an effector action that contributes to the control the infection, despite having no direct effect on the fungal load. Interaction studies between neutrophils and macrophages in this infection could elucidate some questions, such as whether the yeasts in METs and NETs could be delivered to the macrophages not involved in the formation of ETs, but involved in phagocytosis, as described by Loureiro et al. [137].
Eimeria ninakohlyakimovae also induced the METs formation in vitro from goat monocytes [140]. METs were observed after stimulation with different viable evolutionary forms: Sporozoites, sporocysts, and oocysts and confirmed by the colocalization of DNA, MPO, and histones in ETs. The authors confirmed that ROS stimulated the METs formation, since the traps decreased in the presence of the NADPH-oxidase inhibitor: Diphenylene iodondium (DPI). Despite observing the restraint of this protozoan in METs, no direct toxic effect or in vitro control of the parasitic load was verified [140]. The same was observed in the in vitro infection of bovine monocytes by Besnoitia besnoiti and Eimeria bovis [139]. In addition to the effect of ROS, the authors also confirmed the role of MPO in the induction of METs using a specific inhibitor. In this study, the impact of METs on the parasitic load was small, with only a 2% reduction in parasite numbers [139].
Mycobacterium tuberculosis (Mtb) could also stimulate MET formation by human macrophages [144]. This process occurred in highly parasitized macrophages and was induced by INF-γ, regulated by elastase activity, and required the Mtb ESX-1 secretion system. ESX-1 is a virulence factor of Mtb and encodes a protein secretion system that triggers the cell death pathway independent of caspase-1. In the presence of IFN-γ, there was synergism with ESX-1, leading to the macrophage METs formation [144]. Taken together, the data suggest a possible role of METs in the innate immune response to different infectious agents, since METs in vitro have led to the restraint of pathogens, and, in some models, to control the parasitic load.
In the breast and visceral adipose tissue of obese patients, macrophage infiltrates were often observed surrounding dead adipocytes, forming “crown-shaped structures” (CLS), and the presence of these lesions was associated with elevated levels of inflammatory mediators. The authors argue that obesity-induced inflammation of adipose tissue promotes the METs formation within CLS lesions via PAD-4-mediated hypercitrullination of histones [138]. In a db/db mouse model of diabetes, METs were found in adipose tissue and associated with a deleterious effect on inflammation and insulin resistance [146]. In this study, the authors indicate that silencing the hepcidin gene reduced the recruitment of macrophages and inhibited the METs formation, resulting in decreased inflammation (decreased IL-1β and TNF) and insulin resistance [146]. Hepcidin is a peptide primarily produced by hepatocytes and is the key regulator of iron metabolism. It binds to ferroportin on the surface of macrophages and other cells, and consequently, prevents iron leakage, leading to the accumulation of intracellular iron [148]. Iron accumulation in tissues is related to the remodeling in adipocytes and the accumulation of macrophages, which increases the secretion of inflammatory cytokines and oxidative stress [143]. In a study by Zhang et al., the authors discuss a possible role of hepcidin in regulating the METs formation [143]. Although interesting, further studies are needed to prove whether hepcidin has a direct or indirect effect on the induction of METs.
Published data have shown that, depending on the disease model studied, METs can be beneficial or harmful to the body [136,137,138,141,143]. The balance between the ETs formation and their degradation is essential to prevent infections and inflammatory diseases. Thus, an excessive formation or delayed degradation of ETs may cause tissue damage, due to the toxic components associated with this structure. Therefore, macrophages play a key role in removing ETs from different cell types.
Cooperation between Macrophages and Neutrophils in the Extracellular Traps Context
Macrophages, besides participating in the inflammatory response, play an essential role in removing cellular debris and toxic products, potentially harmful to the organism, since they can perpetuate the stimulus to inflammation. Cell-free DNA, the main component of ETs, is recognized as DAMP and induces tissue injury [145]. In a diabetes model in mice, it was observed that NETs that promoted inflammation and progression of atherosclerosis were more abundant and prevented the resolution of inflammation during the wound healing process [149]. The NETs+ areas were enriched by NOS2+ macrophages and by the increased activation of inflammasomes, suggesting that NETs exacerbate the inflammation of macrophages and induce their differentiation to the M1 profile. In this study, the use of DNase 1 decreased the number of NETs, due to the degradation of chromatin fibers, thus reducing inflammation and disease severity [149]. In Behcet’s disease, NETs have been shown to stimulate macrophages to produce high levels of IL-8 and TNF, and induce the differentiation of CD4+ T lymphocytes into IFN-γ producing lymphocytes [150]. However, the data published in the literature remain questionable and depend on the disease model studied. In an acute myocardial infarction model, NETs induced macrophage polarization to the M2 profile, and the deficiency in NETs formation worsened acute inflammation and tissue damage after myocardial infarction, suggesting a protective effect exerted by NETs in this model [151].
Several studies have shown the cooperation between macrophages and neutrophils, and recently, in a thrombosis model in mice, it was observed that even non-polarized macrophages were capable of degrading NETs, but macrophages with a pro-inflammatory profile had a greater degradation capacity [134]. NETs degradation occurred through DNases, and the DNA fragments were internalized via macrophage macropinocytosis. Since NETs work as structures that activate clotting factors, their presence can contribute to thrombus formation. In this regard, the inhibition of macropinocytosis in monocytes led to an increase in NETs load and a reduction in thrombus resolution in vivo [134].
Coronary thrombosis occurs, due to the rupture of the atherosclerotic plaque [147]. NETs and METs are involved in the formation of atherosclerotic plaques and coronary thrombosis in patients who died from acute myocardial infarction, and although other cells have also been related, neutrophil and macrophage ETs were the most frequent [141,147]. NETs predominate in early thrombosis, while METs predominate in chronic thrombosis. METs were more abundant in the intact plaques (lipid core) and in the organized thrombus, since macrophage death contributes to the growth of the lipid core in atherosclerotic plaque [141]. The authors also propose the use of ETs formation as a biomarker for the progression of coronary thrombosis [147]. Activated platelets can also induce ETs formation by macrophages. A study in the murine model of rhabdomyolysis showed that the heme generated by muscle lysis led to platelet activation, which in turn induced the formation of METs, contributing to kidney damage [152]. Another curious fact regarding the cooperation between neutrophils and macrophages is the effect of the proteins present in NETs on the triggering ETs formation in monocytes in vitro [145]. In an experiment designed to show the participation of monocytes in NETs degradation, the exposure to NETs stimulated the formation of extracellular traps by the monocytes themselves. The authors demonstrated that the supernatant of the NETs was capable of stimulating ETs in monocytes, which was caused by citrullinated histones and elastase [145].
Despite all the knowledge already described METs and NETs, it is still unclear whether the interaction between these two effector mechanisms is beneficial or harmful. This is likely to depend on the model of infection or inflammatory disease. This knowledge may be used in new therapeutic or diagnosis/prognosis strategies, as described by Tian et al., who detected CitH3 in the serum of patients with septic shock, and could associate the levels of this compound with the severity and the prognosis of the disease [153].
5. Mast Cells
Mast cells (MCs) are derived from the myeloid progenitor in the bone marrow. They circulate in the blood as precursor cells, and when they reach the target tissues, they mature into effector granular cells. They are cells with a monolobulated nucleus, with specific granules and the absence of cytoplasmic glycogen aggregates. Their granules are composed of histamine, heparin, tryptase, and chymase. There are different subclasses of MCs according to the composition of the proteases contained in their granules, morphology, location, and degranulation potential [154,155].
Initially recognized for their role in allergic reactions, it is now widely discussed that long-lived resident MCs are involved in several initial immune responses to various pathogens. Their presence close to the vascular and lymphatic endothelium spreads their products to other locations, enabling MCs to act both locally and remotely [156]. MCs are located at the host-environment interfaces, being abundant in the skin, intestinal mucosa, and respiratory tract, working as sentinel cells. They have direct pivotal microbicidal activity, but can also interact, activate, and recruit other cells to the site of infection through the release of mediators. MCs participate in tissue repair and the regulation of angiogenesis and may influence the progression of tumors and chronic inflammation observed in some types of cancer [157,158].
Several stimuli, such as drugs, food, fungi, viruses, and bacteria, can trigger MC degranulation or activation without degranulation [159]. When stimulated, they present a biphasic response. In an initial phase, they promptly respond to the stimulus through degranulation and the release of preformed inflammatory mediators. Moreover, in a second moment, they secrete de novo synthesized mediators [156]. MCs can secrete β-hexosaminidase, histamine, TNF, tryptase, and prostaglandin D2 within minutes, besides being the only cells capable of storing preformed TNF, making them the first cells to release TNF. After stimulation, they can also secrete cytokines, chemokines, and several growth factors, actively taking part in the initial profile of inflammatory mediators [160,161]. Immune responses to bacteria, viruses, or parasites started by MCs involve different triggering mechanisms and different mediator releases [157,162].
Along with degranulation and mediator release mechanisms, MCs can produce ETs from stimulation with H2O2, PMA, and various pathogens. ETs in MCs are known as MCETs (mast cell extracellular traps). The presence of MCETs in vitro related to various infections caused by bacteria, protozoa, fungi, and also in other pathological conditions has already been described [163]. MCETs are comprised of nuclear DNA, tryptase, histones, and cathelicidins. Because of the presence of tryptase, both DNase and tryptase-specific proteinases are required for the complete degradation of MCETs [33]. Cathelicidins are antimicrobial peptides (AMPs) that have an antimicrobial effect on bacteria, fungi, enveloped viruses, and protozoa. In humans, cathelicidin LL-37 has already been identified, as well as cathelicidin-related AMP (CRAMP) in mice [164]. Typically, the formation of MCETs is ROS-dependent [163], and MCs undergo nuclear membrane rupture and subsequently cell death [33].
At first, MCETs were observed in a study with Streptococcus pyogenes, a bacterium responsible for different human infections, from impetigo to acute necrotizing fasciitis and septic shock. Von Köckritz-Blickwede et al. investigated the in vitro induction of MCETs in response to the human MC (HMC-1) and bone marrow-derived MCs (BMMCs) lines to S. pyogenes, and observed a proximity-dependent mechanism, which was not phagocytosis, where MCs were able to inhibit the growth of bacteria. ROS-dependent MCETs induction occurred, since the previous treatment of cultures with NADPH oxidase inhibitor destroyed the antimicrobial effect. Moreover, the addition of DNase and MPO to cultures also destroyed the antimicrobial effect. During MCETs formation, MCs died, due to the rupture of the nuclear membrane, as already described for NETs. The induction of MCETs also occurred in the presence of other human pathogens, such as Pseudomonas aeruginosa and Staphylococcus aureus. Besides the direct antimicrobial role of MCETs shown by the presence of dead bacteria in MCETs, the authors suggested that such structures could also be useful to restrain harmful substances released by MCs, thus mitigating possible tissue damage [33].
Several studies have corroborated the aforementioned findings demonstrating the importance of MCETs formation by HMC1 and BMMC cells, decreasing the viability of S. aureus. The direct microbicidal effect of MCETs associated with the secretion of compounds, such as β-hexosaminidase, tryptase, and TNFα on MC degranulation seems to be an important mechanism for the initial control of S. aureus infection. Nevertheless, as an escape mechanism, bacteria are internalized via an active process by MCs and survive in cytosol, which may lead to persistent infection [165]. This escape mechanism was also observed in a study on the cellular mechanisms involved in the pathogenesis of nasal polyps by internalization of S. aureus, which allows the survival of bacteria [166]. In vitro, HMC1 cells were able to trap the bacteria within MCETs and then internalize S. aureus. The infection would be maintained by cycles of cell disruption, bacterium release, trapping within the MCETs, internalization, and further disruption [166]. The role of hypoxia-inducible factor-1-α (HIF-1α) in MCETs formation in the MC-S. aureus interaction was also demonstrated [167]. HIF-1α induces ROS-dependent MCETs formation, for the use of HIF-1α-deficient BMMC antagonists or cells eliminate antimicrobial capacity. The increase in the expression HIF-1α can strengthen the antimicrobial activity, showing its influence on the growth control of S. aureus. The authors, therefore, suggest that understanding such interactions may lead to developing new drugs capable of controlling or inhibiting bacterial growth, restraining infections that can reach variable severity, including severe prognosis [167].
Since most of the studies showed that MCETs formation is ROS-dependent and that pathogens can induce different MC responses, Garcia-Rodrigues et al. analyzed the response pattern of human MCs obtained from blood mononuclear cells and differentiated in vitro (hMC) according to DNA and chemokine release, degranulation, and the presence or absence of ROS vis-à-vis pathogens from different tissues. The authors observed that each pathogen-induced a type of MC response [168]. Whereas L. monocytogenes-induced degranulation and large DNA release in the absence of ROS, S. pneumoniae could not induce degranulation, despite a minimal DNA and ROS release. E. coli induced low levels of degranulation with the secretion of IL-8 and MCP-1, with no DNA and ROS release. S. aureus induced DNA release and PGD2 secretion by hMCs. These results showed that these MC response mechanisms can be activated independently, as well as that the stimulus has a direct influence on the type of response of hMCs. Based on the results, the authors suggested that hMC cells would present both suicidal and vital MCETs in response to L. monocytogenes, being rapid DNA release important for mediator secretion and antimicrobial activity [168]. It had previously been demonstrated that L. monocytogenes can induce MCETs formation in a ROS-dependent process with membrane rupture and death of HMC1-dependent, in part based on the release and activity of β-hexosaminidase, as its blockade restored bacterial growth [169]. Opposite results of whether the process is ROS-dependent or not can be caused by the different sources of MCs used in the studies, since in vivo MCs are known to have specific responses according to their granules and tissue location.
MCETs formation has also been described in the group A Streptococcus (GAS), gram-positive bacteria capable of producing various infections in humans, ranging from skin infections and pharyngitis to endocarditis and septicemia. An in vitro study on the role of GAS M1 protein in MCETs induction compared wild-type bacteria with mutant bacteria in M1 expression or treatment with purified M1 [170]. The results showed that the expression of this protein played an important role in the induction of MCETs by HMC1. Moreover, it was observed that GAS strains associated with invasive forms of infection were resistant to the antimicrobial effect of cathelicidin LL37 and death by MCETs. The loss of M1 expression was able to confer susceptibility to death by MCETs once again. Thus, the M1 protein strain and the origin of the bacterial isolates could influence resistance to LL37, making certain GAS strains capable of escaping the antimicrobial effect of LL37 and death by MCETs, and as a result, with no control of infection [170]. Moreover, another study demonstrated that MCETs play a fundamental role in the control of GAS infection through changes in the integrity of the membrane produced by LL-37, since the inability to promote MC degranulation during the onset of infection does not allow the control of GAS infection [171].
It has been shown that MCs can control Enterococcus faecalis growth via MCETs formation. It was not the only mechanism involved, since, in addition to the evidence of dead bacteria beyond the MCETs, the disruption of these structures partially inhibited growth. Significant degranulation was observed in vitro, suggesting that both; the release of antimicrobial components into the extracellular medium and the induction of MCETs would be important mechanisms to control an E. faecalis infection [172].
In vitro studies with Mtb demonstrated that heat-killed Mtb (HK-Mtb) could induce DNA release and that this release also contained tryptase and histones, being consistent with MCETs. This process was H2O2-dependent, since the inhibition of NADPH oxidase decreased the release of DNA. However, MCETs induced by HK-Mtb and PMA were unable to kill the bacteria. On the other hand, viable Mtb did not produce H2O2 or induce MCETs. The inhibition of MCETs formation through viable Mtb was related to H2O2 decomposition by catalase activity in microorganisms. This inhibition would act as a mechanism to evade Mtb from the microbicidal effects of MCETs [169].
MCETs seem to play a role not only in infections caused by bacteria, but also by fungi and protozoa. MCETs formation in the presence of C. albicans was observed in vitro, but these structures were not able to decrease the viability of the fungi, which suggests that MCETs formation in C. albicans would work as a mechanism of physical restraint of the fungi, so it cannot directly inhibit growth [173]. In Leishmania spp., both L. donovani and L. tropica were able to induce MC death and MCETs formation. The extracellular killing of the parasites in both species was MCETs-dependent, as treatment with DNase increased the viability of promastigotes, both in cultures of peritoneal MCs and in cultures with rat basophilic leukemia (RBL-2H3) mast cell line. The authors suggested that MCETs could be important in the innate immune response formed by MCs towards Leishmania spp., since these cells are present in the skin and MCETs can kill promastigotes and arresting them. The signaling mechanisms, as well as the evasion of parasites towards them, might contribute to different outcomes of Leishmania spp. infections [174].
All the results discussed refer to in vitro studies, and a direct correlation between these findings and the development of infections caused by these pathogens is not possible. There is still scarce information about the role of MCs and MCETs in vivo. There are three murine models for in vivo studies of MCs: C-kit-dependent MC-deficient mice, c-kit independent MC-deficient mice, and mice with restricted MC mediators. Unfortunately, in humans, most studies use in vitro assessments of human cell lines, such as HMC1 [175]. However, two studies have shown evidence of the possible in vivo role of MCETs. In skin biopsies from patients with psoriasis, cellular expression of interleukin 17 (IL-17) has been demonstrated. This cytokine plays an essential role in the pathogenesis of this disease. Most of the IL17+ cells were MCs, and MCETs formation was observed, especially in normal-looking symptomless psoriatic skin and psoriasis plaques. Besides being more compact than MCETs formed in vitro, in vivo MCETs were a release mechanism for IL-17 by MCs, and were induced by the action of IL-23 and IL-1β. The authors suggested that a possible therapeutic mechanism with targeted drugs for IL-23 might work to decrease NETs and MCETs formations, modulating the effect of these structures on psoriasis lesions [176].
In cardiovascular diseases, the role of NETs in coronary atherosclerosis has already been described [141]. Recognizing that other cell types can form ETs, their role in atherothrombosis was evaluated by immunohistochemistry in coronary plaques from autopsy and in thrombus aspiration samples from patients who died of myocardial infarction. A greater number of ETs were observed in atheromatous plaques that presented thrombotic complications compared to intact plaques, with NETs, METs, MCETs, and EETs (eosinophil extracellular traps) being observed in descending order. Additionally, all types of ETs were also observed in coronary thrombus aspirates, but their presence varied according to the type of cell, as well as the age of the thrombus. Although METs and NETs outweighed MCETs and EETs, MCETs appeared in higher numbers in the organized thrombi. Thus, the authors suggest that ETs formation is involved in thrombus progression and maturation and that MCETs might help destabilize the coronary plaque by releasing anti-inflammatory cytokines and mediators by MCs [141].
MCETs formation seems to be an active process induced or inhibited by different stimuli. It is followed by a series of variations in the production of mediators and ROS, as well as degranulation, and induction or not of cell death (respectively suicidal or vital MCETs). Hence, their role in protecting or worsening a given infection seems to depend on the type of stimulus, the type of MC, and probably in vivo on the type of resident cells or on migration into the site of the infection. The development of conditions for the in vivo study of these cells can provide valuable information for the understanding of MCETs formation. Table 3 shows a summary of the stimuli and types of MCETs observed in vitro and in vivo.
Table 3.
Mast cell extracellular traps in host defense and disease.
| Cell | Mechanism of ETs Formation | Stimulus/Models | Biological Effect | |
|---|---|---|---|---|
| Protective | Deleterious | |||
| Mast cell and Bacteria | ROS-dependent [33,167,169,177] Suicidal MCETs [33,166] Not described, probably suicidal because DNA released was linked to dead cell staining or nuclear changes were observed [165,167,177] Not described [33,169,170,171,172] Suicidal and vital MCETs, ROS-independent [168] |
In vitro HMC1 and BMMC lines + S. pyogenes/S. aureus/P. aeruginosa [33] HMC1 + GAS/Purified M1 GAS protein/L. lactis [170] HMC1 and BMMC lines + S.aureus [165,167] HMC-1 and BMMC lines + E. faecalis [172] HMC1 line + L. monocytogenes [177] HMC-1 and BMMC lines + Mtb (viable and HK-Mtb)/S.aureus [169] HMC-1 and BMMC lines + GAS/L.lactis/S.aureus [171] HMC-1 + S.aureus [166] HMC-1 + L.monocytogenes/E. coli/S.aureus/S. pneumoniae [168] |
Antimicrobial effect [33,165,167,168,171,172,177] | M1 GAS protein contributes to GAS survival—invasive forms of infection [170] Mtb inhibit MCET formation—bacteria survival [169] Capture, phagocytosis, maintenance of infection [166] |
| Mast cell and Fungi | Not described, probably suicidal, but dead MC numbers were higher than MCETs observed [173] | In vitro HMC1 + C. albicans [173] |
Physical restraint only [173] | |
| Mast cell and Protozoa | Suicidal MCETs ROS-dependent [174] |
In vitro RBL MC line + L. donovani/L. tropica [174] |
Antimicrobial effect [174] | |
| Mast cell and Psoriasis | Not described, probably suicidal because it was observed that MCs were not intact in lesions [176] | Ex vivo MCs from psoriasis lesions [176] |
IL-17 release, leading to pathogenic effect [176] | |
| Mast cell And Atherothrombosis | Not described [141] | Ex vivo MCs from coronary plaques and thrombus [141] |
Thrombus progression and maturation [141] | |
Mtb, mycobacterium tuberculosis; ROS, reactive oxygen species; MCETs, mast cell extracellular traps; MC, mast cell; PMA, phorbol myristate acetate; HMC1, human MC line; BMMC, bone marrow–derived MC; RBL, rat basophilic leukemia mast cell line; HK-Mtb, heat-killed Mtb; GAS, group A streptococcus; IL-17, interleukin 1.
6. Eosinophils
Eosinophils are granulocytes derived from the myeloid progenitor in the bone marrow, whose production is regulated by the secretion of hematopoietic growth factors, GM-CSF, IL-3, and IL-5. Although GM-CSF and IL-3 also increase the production of other myeloid cells, IL-5 only increases the production of eosinophils. Under normal conditions, they are cells that are found at low frequency in the blood (1–5% of circulating leukocytes) and other tissues, such as lungs, gastrointestinal tract, thymus, adipose tissue, and in secondary lymphoid organs [178]. Moreover, they have a bilobed nucleus, and cytoplasmic granules that contain primary basic proteins (primary granules) and eosinophilic cationic proteins (secondary granules) that are toxic to various parasites and mammalian cells. The primary granules comprise Charcot-Leyden crystal protein, also known as galectin 10, and eosinophil peroxidase (EPO), while the secondary granules contain, in addition to EPO, major basic protein (MBP), eosinophilic cationic protein (ECP), and eosinophil-derived neurotoxin (EDN) [178].
Typically, the increase of eosinophils in the blood or the presence of eosinophilic infiltrate in tissues is observed in allergic reactions, such as asthma and chronic rhinosinusitis, in helminth infections, and also in some bacterial and fungal infections [179,180]. Immunoregulatory actions for eosinophils, such as lymphocyte recruitment and tissue repair, have also been described [181].
Unlike neutrophils, eosinophils are not phagocytic cells, performing their defensive activity by the selective release of granular content into the extracellular environment. During degranulation, EPO, which differs significantly from the peroxidase in other granulocytes, interacts with H2O2, generating cytotoxic oxygen radicals for tumor cells, HIV, and schistosomula of Schistosoma mansoni. EDN has ribonuclease activity that acts against single-stranded RNA viruses, such as HIV and respiratory syncytial viruses, while ECP has antiparasitic and antibacterial activities. MBP-1 is toxic to bacteria, schistosomula of S. mansoni, and can injure host tissues with eosinophilic infiltrate. Moreover, MBP-1 has immunoregulatory activity, such as an increase in the pro-inflammatory cytokine IL-8. Charcot-Leyden crystals are essentially composed of phospholipase B and are found in phlegm, tissues, and feces in diseases that have an intense inflammatory response, indirectly evidencing the release of eosinophil granules (reviewed by the authors of [182]).
In 2008, Yousefi et al. reported that degranulation was not the only way eosinophils acted, demonstrating both ex vivo (using colon biopsy from patients with schistosomiasis, Crohn’s disease, or intestinal spirochetes), as well as in vitrostudies on colocalization, the presence of EETs, and that the DNA present was mtDNA, with eosinophil granule proteins, such as MBP and ECP, incorporated into the multiple extracellular DNA fibers observed. The reaction depended on the activation of NADPH oxidase and release of ROS. Eosinophils remained viable during the process (vital EETs) [183]. Stimulation of human eosinophils with thymic stromal lymphopoietin also induced the release of mitochondrial-originated EETs [184].
Subsequently, a process of release of EETs with nuclear origin occurring with cell death was described, which was initially called EETosis (as the mechanism observed for neutrophils) [185]. In this study, EETs released by human eosinophils were observed after in vitro stimulation with immobilized immunoglobulins (IgG and IgA), platelet-activating factor (PAF), calcium ionophore, or PMA. Eosinophil cytolysis (suicidal EETs) was observed, and EETs were composed of nuclear DNA associated with histones and eosinophil granules. The process was also NADPH oxidase-dependent [185].
The production of vital EETs (mtDNA) or suicidal EETs has been associated with allergic eosinophilic diseases, such as allergic asthma, rhinosinusitis with nasal polyps, eosinophilic esophagitis, chronic obstructive pulmonary disease, allergic bronchopulmonary aspergillosis, and eosinophilic otitis media [186,187,188,189,190]. Recently, suicidal EETs formation by murine and human eosinophils has been observed in the presence of microfilariae and infective L3 larvae of Litomosoides sigmodontis and microfilariae of Dirofilaria immitis, in a Dectin-1-dependent manner [191].
In eosinophilic esophagitis, EETs formation with mtDNA was correlated with the number of eosinophils in the tissue. An inverse correlation of the serine protease inhibitor protein LEKTI with a number of EETs suggested a possible protective role of eosinophils against invading pathogens, regarding disruption of the epithelial barrier, where EETs would work as a secondary barrier [186].
Airway inflammation resulting from eosinophilia is closely related to Severe Eosinophilic Asthma (SEA). The high production of granular proteins, such as ECP, EDN, and MBP, identified in patients with SEA indicates activation and degranulation of eosinophils. Moreover, patients with asthma, chronic lung diseases, and viral respiratory infections produce large amounts of IL-8 at inflammatory sites, a cytokine closely related to EETs production. The high level of eosinophil activation observed in SEA leads to an increase in ROS production and EETs formation, resulting in inflammation and airway obstruction in patients with SEA, in a NADPH oxidase-dependent manner [188]. In a murine model of acute asthma, EETs have been shown to increase mucin secretion in the airways of animals after the OVA challenge [192]. Controlling EETs formation and its activity may provide innovative treatment methods for patients with asthma [192,193].
The correlation between the viscosity of eosinophil-rich exudates and EETs formation has been demonstrated microscopically in secretions obtained from patients with chronic eosinophilic rhinosinusitis (ECRS) and eosinophilic otitis media (EOM) [185,187,190,194]. The authors demonstrated that EETs were composed of thick fibers associated with eosinophil granules and H1 histone, indicating nuclear DNA with cell death (suicidal EETs). Regarding ECRS associated with S. aureus, it has been suggested that eosinophils are likely to be specifically recruited for S. aureus and possibly for other microorganisms, thus forming EETs at epithelial damage sites to protect the host from infection [195].
Allergic bronchopulmonary aspergillosis (ABPA) affects asthmatic patients and individuals with cystic fibrosis in response to several antigens of A. fumigatus, which colonize the bronchial mucus. The assessment of mucus, obtained from the airways of patients with ABPA, showed suicidal EETs formation, with citrullinated histone 3 and intact eosinophil granules [189,196]. Eosinophils stimulated in vitro with A. fumigatus antigens did not induce ROS production, since inhibition of NADPH oxidase activity or mtROS generation did not inhibit EETs formation. However, it is dependent on the pathway of CD11b and Syk tyrosine kinase. Interestingly, these fungus-stimulated EETs did not show fungicidal or fungistatic activity towards A. fumigatus [189]. In a recent study on the characterization of the mechanisms involved in EETs formation in ABPA, the dependence on the signaling pathways p38 MAPK, Akt, Src, calcium, and PI3 was demonstrated, regardless of the viability of the fungus. Remarkably, the release of EETs was independent of histone citrullination by PAD-4 [197]. In concert, the results suggest that EETs may be produced by several pathways in response to antigenic stimuli.
EETs have also been identified in non-allergic inflammatory processes, such as sepsis and colitis [183], atherosclerotic plaque formation, and thrombosis [141,198]. In atherothrombosis, eosinophils form ETs after interacting with platelets, and eosinophils participate in platelet activation. The formed EETs comprise a significant part of the DNA traps found in human and murine thrombi, presenting a large amount of main basic protein (MBP) adhered to DNA filaments [198]. Moreover, the origin of DNA (mitochondrial or nuclear) has not been evaluated.
EETs formation has also been shown in some atopic dermatitis [199], such as bullous delayed pressure urticaria lesions, where EETs formation seems to be related to the apoptosis in keratinocytes and blister formation. However, the mechanisms involved in EETs formation and function have not yet been elucidated [199,200,201].
Despite the need for more information, the study on EETs induction mechanisms in eosinophilic diseases, whether allergic or not, or in autoimmune and cardiovascular diseases, has received a great deal of attention in the last decade because it may bring new alternatives to treat these diseases. Table 4 shows a summary of EETs and their possible roles in eosinophilic, autoimmune, and cardiovascular diseases discussed in this document.
Table 4.
Eosinophil extracellular traps in host defense and disease.
| Cell | Mechanism of ETs Formation | Stimulus/Models | Biological Effect | |
|---|---|---|---|---|
| Protective | Deleterious | |||
| Eosinophil in Intestinal (Colon) Diseases | Vital (mtDNA) ROS-dependent [183] |
Ex vivo Colon Biopsies from Crohn’s disease, schistosomiasis, and intestinal spirochetosis patients |
Entrapment of bacteria [183] | |
| Eosinophil In vitro (Human PBMC) | Vital (mtDNA) ROS-dependent [183] NADPH oxidase-dependent [184,188] Suicidal (Nuclear DNA) dependent of histone citrullination, CD11b, and the Syk tyrosine kinase pathway [185,187,189] Suicidal-independent of PAD4 histone citrullination and depends on the Src family, Akt, Ca, and p38 MAPK signaling pathways [197] |
LPS, C5a, cotaxin/CCL11 [183] Opsonized E. coli [183] A. fumigatus [189,197] Thymic stromal lymphopoietin [184] Immobilized immunoglobulins (IgG, IgA), cytokines with PAF, Ca ionophore, or PMA [185,187] IL-5 and LPS [188] |
Bactericidal activity [183] Entrapment of fungi [197] |
Airway inflammation and obstruction in Asthma [188] |
| Eosinophils in Eosinophilic Diseases | Suicidal (Nuclear DNA) [187,194] Not described [186,190,195,202] |
Ex vivo Secretions and tissue slides ECRS patients [187,190,195] Secretions from EOM patients Tissue slides [187,194] Biopsies from EOE patients [186] Skin biopsy tissues of 25 different eosinophilic skin diseases [202] |
Firewall against the invasion of pathogens [186,195] | Increase in secretion viscosity [187,194] Inflammation [202] |
| Eosinophils in Allergic Bronchopulmonary Diseases | Suicidal (Nuclear DNA) [189,196] Dependent of histone citrullination, CD11b, and the Syk tyrosine kinase pathway [189] Not described [193] |
Ex vivo BALF [196] Bronchial mucus plugs [189] In vivo Murine animal model of Asthma [193] |
Increase in secretion viscosity [189,196] Asthma exacerbation [193] |
|
| Eosinophils in Atherothrombosis | Suicidal (Nuclear DNA) [141,198] | In vivo Murine model [198] Ex vivo Human autopsy [141] |
Thrombus formation [141,198] | |
mtDNA, mitochondrial DNA; BALF, bronchial lavage fluid; C5a, complement component C5a; CCL11, C-C motif chemokine ligand 11; ECRS, eosinophilic chronic rhinosinusitis; EOE, eosinophilic esophagitis; EOM, eosinophilic otitis media; ETs, extracellular traps; LPS, lipopolysaccharides; PAD4, protein arginine deiminase 4; PAF, platelet-activating factor; PBMC, peripheral blood mononuclear cells; PMA, phorbol myristate acetate; ROS, reactive oxygen species.
7. Lymphocytes
T and B lymphocytes result from stimulation of lymphoid progenitors in the bone marrow, and the selection and clonal maturation of which occurs in the bone marrow (B lymphocytes) or in the thymus (T lymphocytes). They are mononuclear cells known as the major orchestrators of the immune response, as they participate in both, stimulation/regulation and in the effector function of the inflammatory process. In short, lymphocytes are involved in the presentation of antigens via the major histocompatibility complex (MHC) class II (B lymphocytes), cytokine production (B and T lymphocytes), a stimulus to the effector phase of other lymphocytes, as well as other cells associated with immune response, such as macrophages and granulocytes. They are also capable of exerting cytotoxicity on target cells by MHC class I recognition followed by direct degranulation, or by receptor-ligand binding, such as Fas-FasL, TRAIL, and others. Besides, they can form and maintain immune memory, and act in both humoral (B lymphocytes) and cellular (T lymphocytes) immune responses. T lymphocytes are now considered as central cells in the organization, targeting, and modulation of inflammation. There has not been enough evidence in the last few years that lymphocytes might produce ETs. Many of the results were obtained in vitro, but some direct or indirect evidence of their in vivo role has been identified. Nevertheless, many questions need to be clarified, although the first results point to the possibility that DNA extravasation plays a role in infectious and non-infectious diseases, especially in autoimmune diseases. According to the authors, the phenomenon has been named differently, but the term lymphocyte-derived extracellular traps (LETs) has now been used, and will, therefore, be referred to in this document [32].
To our knowledge, the first evidence of the participation of lymphocytes in ETs formation emerged in 2017 [202], when the formation of extracellular structures rich in DNA from B lymphocytes stimulated with PMA, ionomycin, anti-IgM, LPS, or serum from patients with SLE, as well as with serum from other types of autoimmune diseases, such as cryoglobulinemic vasculitis and Sjögren’s syndrome, all characterized by the formation of immune complexes, was verified in vitro. In the same study, other autoimmune diseases, such as RA and dermatomyositis, did not have a similar effect on B cells. The authors were able to detect plasma membrane damage in B lymphocytes. In this paper, similar data were identified in T cells. Nevertheless, the authors did not evaluate other protein molecules associated with extravasated DNA, although the data suggest ETs formation and B lymphocyte death by ETosis [202].
In 2018, Ingelssom et al. demonstrated the extracellular release of mtDNA by B lymphocytes. mtDNA is known to be rich in CpG motifs, which are recognized by TLR-9 [14]. Stimulation by oligodeoxynucleotides with or without CpG is observed in cells, such as B lymphocytes, T lymphocytes, NK, neutrophils, and macrophages. The authors described that the formation of filaments was different from that observed in neutrophils, and their presence was not stimulated by lipopolysaccharides (LPS) or PMA. B cells showed the release of long filaments produced independently of BCR, ROS, and without evidence of cell death. Due to the absence of toxic proteins coupled to DNA filaments, the authors suggested that this type of DNA extravasation would work as a DAMP, associated with the triggering of an innate immune response and stimulating the production of IFN-1 [14].
The characterization of LETs formation in activated T lymphocytes was subsequently published for both CD4+ T cells [32,203] and CD8+ T cells [32], and with this description of LETs formation, another function was associated with a plethora of important functions of lymphocytes.
Costanza et al. demonstrated the presence of LETs in CD4+ cells both in vitro from cell stimulation with anti-CD3/anti-CD28, and in the experimental model of autoimmune encephalomyelitis (EAE) [203]. In vitro, DNA expression was verified with an association of histones and identification of damage to the plasma membrane, and the formation of several filaments involving activated CD4+ T lymphocytes connected to the adjacent lymphocytes. The extracellular DNA was destroyed by DNase, and resting CD4+ T lymphocytes were weakly positive in the stains performed, evidencing activation as a key orchestrator for DNA release. The phenomenon also seems to be governed by ROS, since its inhibition reduces DNA release without altering cell activation and proliferation. It also showed an increase in the production of IL-2, GM-CSF, IFN-γ, and TNF-α in CD4+ cell cultures with LETs, suggesting that LETs formation might work as a second signal for effective action upon lymphocytes. Interestingly, tagged mtDNA showed that it was part of the LETs produced by CD4+ cells [203]. The authors identified that the inhibition of mtROS entailed a decrease in lymphocyte activation in vitro, cytokine production, and LETs formation. In vivo, the cells maintained the proliferation capacity, but not the cytokine production. The presence of LETs containing DNA and histones was detected in CD4+ cells present in lymph nodes of mice with EAE. The inhibition of LETs formation caused an improvement in the condition of EAE, which evidences the implication of LETs in the pathogenesis of the disease. Due to the concurrent presence of histones and mtDNA, the authors were unable to determine whether the phenomenon in CD4+ lymphocytes was suicidal or vital [203]. The presence of a combination of mtDNA and DNA containing histone in other cell types, such as neutrophils, has already been described [25]. Moreover, as LETs formation is quite fast, the results on various cell types published so far suggest that the phenomenon might occur simultaneously or be organized as a sequence of temporal events, due to the evolution of cellular structures, since damage to the plasma membrane is evident.
Koh et al. has recently identified that after in vitro stimulation with anti-CD4/anti-CD28, CD4+ cells produce diffuse ETs that surround the cell like a halo and with evidence of cell death, and that this formation differs from ETs in CD8+ T lymphocytes, whose ETs form filaments [32].
In addition to cytokine production, CD8+ cells feature as one of the main effector functions of direct cytotoxicity (CTL) from the T-cell receptor (TCR) recognition of MHC class I molecules containing antigen on the surface of infected/altered cells, called target cells, with the association of costimulatory molecules. Subsequently, the granular content is released into the area of contact between the cells and the action of enzymes in a cascade of events that result in nuclear DNA degradation and the rupture of the plasma membrane, leading to cell destruction. This classic mechanism of CTL was added to the description of ETs production by CD8+ T lymphocytes by Koh et al., who evaluated LETs formation by CD8+ T lymphocytes both in vitro by stimulation with anti-CD3/anti-CD28 and in vivo in ATL lesions [32]. The authors detected the formation of long filaments of extracellular DNA containing enzymes of granular content shown by the colocalization of CD107a, resulting in cell death by LETs. Electron microscopy confirmed ETs of CD8+ T lymphocytes via disruption of the cell membrane and polarization of organelles. This action was also verified ex vivo by the in situ study on ATL lesions of various clinical presentations. The comparative evaluation showed that the presence of LETs was associated with greater severity of the lesions, leading to a correlation between a higher concentration of CD8+ cells forming LETs and the most exuberant inflammatory conditions in ATL. The in vitro study also identified the association of LETs formation with an increase in intracellular Ca++ and the absence of association with NOS2 and ROS, showing particular features of extracellular DNA formation and release structure in CD8+ cells when compared to other cell types [32].
Unlike the CTL mechanism, LETs formation can reach cells at a distance, increasing the action capacity of CD8+ T cells. Furthermore, disruption of both the target cell and the CD8+ cell leads to the release of intracytoplasmic content and can cause additional inflammatory stimulation [32].
Other studies should be performed to elucidate the unclear points concerning LETs formation by T and B lymphocytes and their action on the inflammatory process of different etiologies. The results published so far point out that this possibility is quite robust, and this intensity and regulation role of the immune response should be considered. Clarifying whether they play a role in the protection and/or exacerbation of different diseases may bring subsidies to the design and production of therapeutic targets able to modulate the immune response and consequently tissue damage caused by inflammatory phenomena. Table 5 shows a summary of the data discussed concerning LETs formation and function.
Table 5.
Lymphocyte extracellular traps in host defense and disease.
| Cell | Mechanism of ETs Formation | Stimulus/Models | Biological Effect | |
|---|---|---|---|---|
| Protective | Deleterious | |||
| B lymphocytes | Not described, probably suicidal, since membrane damage is described [202] Vital [14] |
In vitro PMA, ionomycin,, anti-IgM,, LPS, SLE serum [202] CPG motifs [14] |
Probably autoimmune diseases, SLE, cryoglobulemic vasculitis, and Sjögren syndrome [202] Autoimmune diseases [14] |
|
| CD4 T lymphocytes | In vitro antiCD3/antiCD28 [203] antiCD4/antiCD28 [32] In vivo Experimental model of encephalomyelitis [203] |
Autoimmune diseases [203] American Tegumentary Leishmaniasis [32] |
||
| CD8 T lymphocytes | Suicidal [32] | In vitro antiCD3/antiCD28 [32] Ex vivo American Tegumentary Leishmaniasis lesions [32] |
American Tegumentary Leishmaniasis [32] | |
ETs, extracellular traps; PMA, phorbol-12-myristate-13-acetate; anti-IgM, anti-immunoglobulin M; LPS, lipopolysaccharide; SLE, systemic lupus erythematosus; mtDNA, mitochondrial DNA.
8. Other Cells Involved in the Immune Response Whereby the Formation of Extracellular Traps Has Been Identified
Basophils and plasmacytoid dendritic cells have been identified as capable of producing ETs, requiring a greater understanding of the formation, stimuli, and application in disease or protection during the inflammatory process. The information regarding the ETs produced by these cell types is shown in Table 6.
Table 6.
Basophils and plasmacytoid dendritic cell extracellular traps in host defense and disease.
| Cell | Mechanism of ETs Formation | Stimulus/Models | Biological Effect | |
|---|---|---|---|---|
| Protective | Deleterious | |||
| Basophils | Vital (mtDNA), NADPH oxidase independent [204] Not described [205,206] |
In vitro (human blood) Monosodium urate [205] Staphylococcus aureus [206] In vitro (human blood and murine Hoxb8-immortalized myeloid progenitors derived basophils) IL-3 priming and subsequent activation of the C5a receptor or FcεRI [204] |
Bactericidal activity [206] | |
| Plasmacytoid dendritic cells | Suicidal (Nuclear DNA) Citrullinated histone H3 Dectin-2-dependent [207] | In vitro (Human PBMC) Aspergillus fumigatus [207] |
Antifungal activity [207] | |
mtDNA, mitochondrial DNA; C5a, complement component C5a; ETs, extracellular Traps; PBMC, peripheral blood mononuclear cells.
8.1. Basophils
Basophils are scarce blood leukocytes (about 2%), produced in the bone marrow from myeloid progenitors. Their granules are metachromatic, larger than other granulocytes, and contain hydrolytic enzymes, chemotactic factors for neutrophils and eosinophils, heparin, and histamine. They have receptors on the plasma membrane to bind to immunoglobulin E (IgE), which after subsequent exposure to the allergen, release their granules, leading to vascular disorders associated with hypersensitivity and anaphylaxis [208].
Besides, basophils can produce ETs within a few minutes of stimulation by IgE, chemokines, TLRs, cytokines, and lipid mediators. Basophil extracellular traps (BETs) are formed from mitochondria and by a mtROS-dependent and NADPH oxidase-independent mechanism [204,205]. However, there is still little information about the mechanisms and implications of this function in basophils. Considering that they are not capable of intracellular killing of bacteria like neutrophils, basophils can trap and kill microorganisms through BETs, as already demonstrated against E. coli and S. aureus [208]. The development of methodologies to study these cells will enable us to obtain data that may provide a better understanding of the action of basophils upon inflammatory processes, including the participation of BETs.
8.2. Plamacytoid Dendritic Cells
Dendritic cells (DCs) are bone marrow-derived from pluripotent hematopoietic stem cells considered one of the major antigen-presenting cells of the immune system. Although they account for less than 1% of leukocytes in peripheral blood, these cells are located in other tissues where they act as sentinels of the immune system, patrolling the presence of antigens for presentation to T lymphocytes, playing a critical role in linking innate and adaptive immune responses. They are classified into subpopulations according to location, function, etc. Recently, an article identified in vitro ETs formation derived from plasmacytoid dendritic cells after recognizing hyphae of A. fumigatus. The recognition was Dectin-2-dependent and led to the ETs formation comprised of nuclear DNA and citrullinated histone H3 [207]. Further studies should be conducted to elucidate the mechanisms and participation of dendritic cell extracellular traps (DCETs) in the inflammatory process.
9. Concluding Remarks
All these years of studies and data collection on ETs identify a variety of cell types involved, as well as formation mechanisms and potential actions of releasing extracellular traps upon protection and disease. However, information capable of elucidating some important mechanisms is still deficient. While the mechanisms involved have been formerly associated only with the innate immune response, nowadays, their involvement in a specific immune response are discussed—mainly at the expense of recent descriptions of ETs in lymphocytes. Moreover, considering that there are not enough in vivo studies, it is essential to develop experimental models and studies on human biological material that can verify the influence of ETs on the pathophysiology of infectious and non-infectious diseases, such as autoimmune diseases. Much is already known, but much more needs to be known to understand the dynamics of ETs in inflammation. For instance, how the cell is stimulated to develop ETs and not another effector mechanism, the effect of these traps on the infectious/inflammatory process, and the impact of these extracellular traps on the other types of cells involved in the immune response. On the other hand, there is no doubt that all the knowledge generated so far regarding the formation of ETs. Many data that are generated daily on this subject, point to the possibility of developing new drugs, or repositioning already known drugs, capable of preventing the development of ETs or leading to their dissolution, as immunotherapeutic alternatives in a variety of infectious and non-infectious diseases, notably those with immunothrombotic characteristics, such as COVID-19. The immune system sets its traps; however, we still do not fully understand how and what the consequences of this movement are.
Acknowledgments
A.M. is supported by grants from CNPq, Capes, Faperj, and Focem/Mercosur.
Author Contributions
Conceptualization: F.C.-S., C.S.M.R., P.M.D.L., J.L.-S., M.A.S., A.M., and F.N.M.; writing—original draft preparation: F.C.-S., C.S.M.R., P.M.D.L., J.L.-S., M.A.S., A.M., and F.N.M.; writing—review and editing: F.C.-S., C.S.M.R., P.M.D.L., J.L.-S., M.A.S., A.M., and F.N.M.; funding acquisition: F.C.-S., F.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by PAEF-IOC-Fiocruz (IOC-023-FIO-18-2-47) and by Programa Jovem Cientista do Nosso Estado (Faperj-E-26/202.760/2019).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hawking S. The Universe in a Nutshell. 1st ed. Transworld Publishers Ltd.; London, UK: 2001. [Google Scholar]
- 2.Tan C., Aziz M., Wang P. The Vitals of NETs. J. Leukoc. Biol. 2020:1–12. doi: 10.1002/JLB.3RU0620-375R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V. Neutrophil Extracellular Traps in the Second Decade. J. Innate Immun. 2018;10:414–421. doi: 10.1159/000489829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenny E.F., Herzig A., Krüger R., Muth A., Mondal S., Thompson P.R., Brinkmann V., von Bernuth H., Zychlinsky A. Diverse Stimuli Engage Different Neutrophil Extracellular Trap Pathways. eLife. 2017;6:e24437. doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgener S.S., Schroder K. Neutrophil Extracellular Traps in Host Defense. Cold Spring Harb. Perspect. Biol. 2020;12:a037028. doi: 10.1101/cshperspect.a037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousefi S., Simon D., Stojkov D., Karsonova A., Karaulov A., Simon H.-U. In Vivo Evidence for Extracellular DNA Trap Formation. Cell Death Dis. 2020;11:300. doi: 10.1038/s41419-020-2497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schuize I., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel Cell Death Program Leads to Neutrophi Extracellular Traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wartha F., Henriques-Normark B. ETosis: A Novel Cell Death Pathway. Sci. Signal. 2008;1:pe25. doi: 10.1126/stke.121pe25. [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 10.Papayannopoulos V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 11.Rawat K., Syeda S., Shrivastava A. Neutrophil-Derived Granule Cargoes: Paving the Way for Tumor Growth and Progression. Cancer Metastasis Rev. 2021;40:221–244. doi: 10.1007/s10555-020-09951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M., Ma M., Tan Z., Zheng H., Liu X. Neutrophil: A New Player in Metastatic Cancers. Front. Immunol. 2020;11:565165. doi: 10.3389/fimmu.2020.565165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldmann O., Medina E. The Expanding World of Extracellular Traps: Not Only Neutrophils but Much More. Front. Immunol. 2012;3:420. doi: 10.3389/fimmu.2012.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingelsson B., Söderberg D., Strid T., Söderberg A., Bergh A.-C., Loitto V., Lotfi K., Segelmark M., Spyrou G., Rosén A. Lymphocytes Eject Interferogenic Mitochondrial DNA Webs in Response to CpG and Non-CpG Oligodeoxynucleotides of Class C. Proc. Natl. Acad. Sci. USA. 2018;115:E478–E487. doi: 10.1073/pnas.1711950115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bont C.M., Stokman M.E.M., Faas P., Thurlings R.M., Boelens W.C., Wright H.L., Pruijn G.J.M. Autoantibodies to Neutrophil Extracellular Traps Represent a Potential Serological Biomarker in Rheumatoid Arthritis. J. Autoimmun. 2020;113:102484. doi: 10.1016/j.jaut.2020.102484. [DOI] [PubMed] [Google Scholar]
- 16.Hofbauer T.M., Ondracek A.S., Mangold A., Scherz T., Nechvile J., Seidl V., Brostjan C., Lang I.M. Neutrophil Extracellular Traps Induce MCP-1 at the Culprit Site in ST-Segment Elevation Myocardial Infarction. Front. Cell Dev. Biol. 2020;8:564169. doi: 10.3389/fcell.2020.564169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L., Liu Q., Zhang X., Liu X., Zhou B., Chen J., Huang D., Li J., Li H., Chen F., et al. DNA of Neutrophil Extracellular Traps Promotes Cancer Metastasis via CCDC25. Nature. 2020;583:133–138. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Figueroa E., Álvarez-Carrasco P., Ortega E., Maldonado-Bernal C. Neutrophils: Many Ways to Die. Front. Immunol. 2021;12:631821. doi: 10.3389/fimmu.2021.631821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilsczek F.H., Salina D., Poon K.K.H., Fahey C., Yipp B.G., Sibley C.D., Robbins S.M., Green F.H.Y., Surette M.G., Sugai M., et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus Aureus. J. Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 20.Yipp B.G., Petri B., Salina D., Jenne C.N., Scott B.N.V., Zbytnuik L.D., Pittman K., Asaduzzaman M., Wu K., Meijndert H.C., et al. Infection-Induced NETosis Is a Dynamic Process Involving Neutrophil Multitasking in Vivo. Nat. Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yipp B.G., Kubes P. NETosis: How Vital Is It? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 22.Ravindran M., Khan M.A., Palaniyar N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules. 2019;9:365. doi: 10.3390/biom9080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vorobjeva N.V., Chernyak B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry. 2020;85:1178–1190. doi: 10.1134/S0006297920100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel C., Leppkes M., Muñoz L.E., Schley G., Schett G., Herrmann M. Extracellular DNA Traps in Inflammation, Injury and Healing. Nat. Rev. Nephrol. 2019;15:559–575. doi: 10.1038/s41581-019-0163-2. [DOI] [PubMed] [Google Scholar]
- 25.Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K., Malech H.L., Ledbetter J.A., Elkon K.B., Kaplan M.J. Neutrophil Extracellular Traps Enriched in Oxidized Mitochondrial DNA Are Interferogenic and Contribute to Lupus-like Disease. Nat. Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West A.P., Shadel G.S. Mitochondrial DNA in Innate Immune Responses and Inflammatory Pathology. Nat. Rev. Immunol. 2017;17:363–375. doi: 10.1038/nri.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker V.S., Imade G.E., Molta N.B., Tawde P., Pam S.D., Obadofin M.O., Sagay S.A., Egah D.Z., Iya D., Afolabi B.B., et al. Cytokine-Associated Neutrophil Extracellular Traps and Antinuclear Antibodies in Plasmodium Falciparum Infected Children under Six Years of Age. Malar. J. 2008;7:41. doi: 10.1186/1475-2875-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abi Abdallah D.S., Lin C., Ball C.J., King M.R., Duhamel G.E., Denkers E.Y. Toxoplasma Gondii Triggers Release of Human and Mouse Neutrophil Extracellular Traps. Infect. Immun. 2012;80:768–777. doi: 10.1128/IAI.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgado F.N., Nascimento M.T.C., Saraiva E.M., de Oliveira-Ribeiro C., de Fátima Madeira M., da Costa-Santos M., Vasconcellos E.C.F., Pimentel M.I.F., Rosandiski Lyra M., Schubach A.d.O., et al. Are Neutrophil Extracellular Traps Playing a Role in the Parasite Control in Active American Tegumentary Leishmaniasis Lesions? PLoS ONE. 2015;10:e0133063. doi: 10.1371/journal.pone.0133063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgado F.N., Schubach A.O., Barros M.B.L., Conceição-Silva F. The in Situ Inflammatory Profile of Lymphocutaneous and Fixed Forms of Human Sporotrichosis. Med. Mycol. 2011;49:612–620. doi: 10.3109/13693786.2011.552532. [DOI] [PubMed] [Google Scholar]
- 31.Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., et al. Neutrophil Extracellular Traps Mediate a Host Defense Response to Human Immunodeficiency Virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Koh C.C., Wardini A.B., Vieira M., Passos L.S.A., Martinelli P.M., Neves E.G.A., do Vale Antonelli L.R., Barbosa D.F., Velikkakam T., Gutseit E., et al. Human CD8+ T Cells Release Extracellular Traps Co-Localized with Cytotoxic Vesicles That Are Associated with Lesion Progression and Severity in Human Leishmaniasis. Front. Immunol. 2020;11:594581. doi: 10.3389/fimmu.2020.594581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Köckritz-Blickwede M., Goldmann O., Thulin P., Heinemann K., Norrby-Teglund A., Rohde M., Medina E. Phagocytosis-Independent Antimicrobial Activity of Mast Cells by Means of Extracellular Trap Formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 34.Hellebrekers P., Vrisekoop N., Koenderman L. Neutrophil Phenotypes in Health and Disease. Eur. J. Clin. Investig. 2018;48:e12943. doi: 10.1111/eci.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liew P.X., Kubes P. The Neutrophil’s Role during Health and Disease. Physiol. Rev. 2019;99:1223–1248. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 36.Pillay J., den Braber I., Vrisekoop N., Kwast L.M., de Boer R.J., Borghans J.A.M., Tesselaar K., Koenderman L. In Vivo Labeling with 2H2O Reveals a Human Neutrophil Lifespan of 5.4 Days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 37.Brinkmann V., Zychlinsky A. Beneficial Suicide: Why Neutrophils Die to Make NETs. Nat. Rev. Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 38.Amulic B., Cazalet C., Hayes G.L., Metzler K.D., Zychlinsky A. Neutrophil Function: From Mechanisms to Disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 39.Cruvinel W.D.M., Mesquita D., Jr., Araújo J.A.P., Catelan T.T.T., De Souza A.W.S., Da Silva N.P., Andrade L.E.C. Immune System-Part I Fundamentals of Innate Immunity with Emphasis on Molecular and Cellular Mechanisms of Inflammatory Response. Bras. J. Rheumatol. 2010;50:434–461. [PubMed] [Google Scholar]
- 40.Mócsai A. Diverse Novel Functions of Neutrophils in Immunity, Inflammation, and Beyond. J. Exp. Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dejana E. Endothelial Cell–Cell Junctions: Happy Together. Nat. Rev. Mol. Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 42.Mayadas T.N., Cullere X., Lowell C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palomino D.C.T., Marti L.C. Chemokines and Immunity. Einstein (São Paulo) 2015;13:469–473. doi: 10.1590/S1679-45082015RB3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbas A.K., Lichtman A.H., Pillai S. Circulação de Leucócitos e migração para os tecidos. In: Koogan G.G., editor. Imunologia Celular E Molecular. Elsevier; Rio de Janeiro, Brazil: 2019. pp. 39–56. [Google Scholar]
- 45.Faurschou M., Borregaard N. Neutrophil Granules and Secretory Vesicles in Inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Kolaczkowska E., Kubes P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 47.Borregaard N. Neutrophils, from Marrow to Microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Segal A.W. How Neutrophils Kill Microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordenfelt P., Tapper H. Phagosome Dynamics during Phagocytosis by Neutrophils. J. Leukoc. Biol. 2011;90:271–284. doi: 10.1189/jlb.0810457. [DOI] [PubMed] [Google Scholar]
- 50.Takei H., Araki A., Watanabe H., Ichinose A., Sendo F. Rapid Killing of Human Neutrophils by the Potent Activator Phorbol 12-Myristate 13-Acetate (PMA) Accompanied by Changes Different from Typical Apoptosis or Necrosis. J. Leukoc. Biol. 1996;59:229–240. doi: 10.1002/jlb.59.2.229. [DOI] [PubMed] [Google Scholar]
- 51.Cahilog Z., Zhao H., Wu L., Alam A., Eguchi S., Weng H., Ma D. The Role of Neutrophil NETosis in Organ Injury: Novel Inflammatory Cell Death Mechanisms. Inflammation. 2020;43:2021–2032. doi: 10.1007/s10753-020-01294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S.-W., Lee J.-K. Role of HMGB1 in the Interplay between NETosis and Thrombosis in Ischemic Stroke: A Review. Cells. 2020;9:1794. doi: 10.3390/cells9081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rada B. Neutrophil Extracellular Traps and Microcrystals. J. Immunol. Res. 2017;2017:1–7. doi: 10.1155/2017/2896380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosazza T., Warner J., Sollberger G. NET Formation-Mechanisms and How They Relate to Other Cell Death Pathways. FEBS J. 2020;288:3334–3350. doi: 10.1111/febs.15589. [DOI] [PubMed] [Google Scholar]
- 55.Petretto A., Bruschi M., Pratesi F., Croia C., Candiano G., Ghiggeri G., Migliorini P. Neutrophil Extracellular Traps (NET) Induced by Different Stimuli: A Comparative Proteomic Analysis. PLoS ONE. 2019;14:e0218946. doi: 10.1371/journal.pone.0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naffah de Souza C., Breda L.C.D., Khan M.A., de Almeida S.R., Câmara N.O.S., Sweezey N., Palaniyar N. Alkaline PH Promotes NADPH Oxidase-Independent Neutrophil Extracellular Trap Formation: A Matter of Mitochondrial Reactive Oxygen Species Generation and Citrullination and Cleavage of Histone. Front. Immunol. 2018;8:1849. doi: 10.3389/fimmu.2017.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leppkes M., Maueröder C., Hirth S., Nowecki S., Günther C., Billmeier U., Paulus S., Biermann M., Munoz L.E., Hoffmann M., et al. Externalized Decondensed Neutrophil Chromatin Occludes Pancreatic Ducts and Drives Pancreatitis. Nat. Commun. 2016;7:10973. doi: 10.1038/ncomms10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiménez-Alcázar M., Rangaswamy C., Panda R., Bitterling J., Simsek Y.J., Long A.T., Bilyy R., Krenn V., Renné C., Renné T., et al. Host DNases Prevent Vascular Occlusion by Neutrophil Extracellular Traps. Science. 2017;358:1202–1206. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 60.Douda D.N., Khan M.A., Grasemann H., Palaniyar N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. USA. 2015;112:2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cristinziano L., Modestino L., Loffredo S., Varricchi G., Braile M., Ferrara A.L., de Paulis A., Antonelli A., Marone G., Galdiero M.R. Anaplastic Thyroid Cancer Cells Induce the Release of Mitochondrial Extracellular DNA Traps by Viable Neutrophils. J. Immunol. 2020;204:1362–1372. doi: 10.4049/jimmunol.1900543. [DOI] [PubMed] [Google Scholar]
- 62.McIlroy D.J., Jarnicki A.G., Au G.G., Lott N., Smith D.W., Hansbro P.M., Balogh Z.J. Mitochondrial DNA Neutrophil Extracellular Traps Are Formed after Trauma and Subsequent Surgery. J. Crit. Care. 2014;29:1133.e1–1133.e5. doi: 10.1016/j.jcrc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H.U. Viable Neutrophils Release Mitochondrial DNA to Form Neutrophil Extracellular Traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 64.Rochael N.C., Guimarães-Costa A.B., Nascimento M.T.C., DeSouza-Vieira T.S., Oliveira M.P., Garcia e Souza L.F., Oliveira M.F., Saraiva E.M. Classical ROS-Dependent and Early/Rapid ROS-Independent Release of Neutrophil Extracellular Traps Triggered by Leishmania Parasites. Sci. Rep. 2016;5:18302. doi: 10.1038/srep18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byrd A.S., O’Brien X.M., Johnson C.M., Lavigne L.M., Reichner J.S. An Extracellular Matrix–Based Mechanism of Rapid Neutrophil Extracellular Trap Formation in Response to Candida Albicans. J. Immunol. 2013;190:4136–4148. doi: 10.4049/jimmunol.1202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruns S., Kniemeyer O., Hasenberg M., Aimanianda V., Nietzsche S., Thywißen A., Jeron A., Latgé J.-P., Brakhage A.A., Gunzer M. Production of Extracellular Traps against Aspergillus Fumigatus In Vitro and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA. PLoS Pathog. 2010;6:e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guimaraes-Costa A.B., Nascimento M.T.C., Froment G.S., Soares R.P.P., Morgado F.N., Conceicao-Silva F., Saraiva E.M. Leishmania Amazonensis Promastigotes Induce and Are Killed by Neutrophil Extracellular Traps. Proc. Natl. Acad. Sci. USA. 2009;106:6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabriel C., McMaster W.R., Girard D., Descoteaux A. Leishmania Donovani Promastigotes Evade the Antimicrobial Activity of Neutrophil Extracellular Traps. J. Immunol. 2010;185:4319–4327. doi: 10.4049/jimmunol.1000893. [DOI] [PubMed] [Google Scholar]
- 69.Regli I.B., Passelli K., Hurrell B.P., Tacchini-Cottier F. Survival Mechanisms Used by Some Leishmania Species to Escape Neutrophil Killing. Front. Immunol. 2017;8:1558. doi: 10.3389/fimmu.2017.01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Passelli K., Billion O., Tacchini-Cottier F. The Impact of Neutrophil Recruitment to the Skin on the Pathology Induced by Leishmania Infection. Front. Immunol. 2021;12:649348. doi: 10.3389/fimmu.2021.649348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Da Fonseca-Martins A.M., de Souza Lima-Gomes P., Antunes M.M., de Moura R.G., Covre L.P., Calôba C., Rocha V.G., Pereira R.M., Menezes G.B., Gomes D.C.O., et al. Leishmania Parasites Drive PD-L1 Expression in Mice and Human Neutrophils with Suppressor Capacity. Front. Immunol. 2021;12:598943. doi: 10.3389/fimmu.2021.598943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahn J., Schauer C., Czegley C., Kling L., Petru L., Schmid B., Weidner D., Reinwald C., Biermann M.H.C., Blunder S., et al. Aggregated Neutrophil Extracellular Traps Resolve Inflammation by Proteolysis of Cytokines and Chemokines and Protection from Antiproteases. FASEB J. 2019;33:1401–1414. doi: 10.1096/fj.201800752R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knackstedt S.L., Georgiadou A., Apel F., Abu-Abed U., Moxon C.A., Cunnington A.J., Raupach B., Cunningham D., Langhorne J., Krüger R., et al. Neutrophil Extracellular Traps Drive Inflammatory Pathogenesis in Malaria. Sci. Immunol. 2019;4:eaaw0336. doi: 10.1126/sciimmunol.aaw0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma A.C., Kubes P. Platelets, Neutrophils, and Neutrophil Extracellular Traps (NETs) in Sepsis. J. Thromb. Haemost. 2008;6:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 75.Caudrillier A., Kessenbrock K., Gilliss B.M., Nguyen J.X., Marques M.B., Monestier M., Toy P., Werb Z., Looney M.R. Platelets Induce Neutrophil Extracellular Traps in Transfusion-Related Acute Lung Injury. J. Clin. Investig. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith C.K., Kaplan M.J. The Role of Neutrophils in the Pathogenesis of Systemic Lupus Erythematosus. Curr. Opin. Rheumatol. 2015;27:448–453. doi: 10.1097/BOR.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 77.Leffler J., Martin M., Gullstrand B., Tydén H., Lood C., Truedsson L., Bengtsson A.A., Blom A.M. Neutrophil Extracellular Traps That Are Not Degraded in Systemic Lupus Erythematosus Activate Complement Exacerbating the Disease. J. Immunol. 2012;188:3522–3531. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 78.Hally K.E., Parker O.M., Brunton-O’Sullivan M.M., Harding S.A., Larsen P.D. Linking Neutrophil Extracellular Traps and Platelet Activation: A Composite Biomarker Score for Predicting Outcomes after Acute Myocardial Infarction. Thromb. Haemost. 2021 doi: 10.1055/s-0041-1728763. [DOI] [PubMed] [Google Scholar]
- 79.Stakos D., Skendros P., Konstantinides S., Ritis K. Traps N’ Clots: NET-Mediated Thrombosis and Related Diseases. Thromb. Haemost. 2020;120:373–383. doi: 10.1055/s-0039-3402731. [DOI] [PubMed] [Google Scholar]
- 80.Skendros P., Mitroulis I. Host Cell Autophagy in Immune Response to Zoonotic Infections. Clin. Dev. Immunol. 2012;2012:910525. doi: 10.1155/2012/910525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinod K., Wagner D.D. Thrombosis: Tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moschonas I.C., Tselepis A.D. The Pathway of Neutrophil Extracellular Traps towards Atherosclerosis and Thrombosis. Atherosclerosis. 2019;288:9–16. doi: 10.1016/j.atherosclerosis.2019.06.919. [DOI] [PubMed] [Google Scholar]
- 83.Chen Z., Zhang H., Qu M., Nan K., Cao H., Cata J.P., Chen W., Miao C. Review: The Emerging Role of Neutrophil Extracellular Traps in Sepsis and Sepsis-Associated Thrombosis. Front. Cell. Infect. Microbiol. 2021;11:653228. doi: 10.3389/fcimb.2021.653228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liberale L., Holy E.W., Akhmedov A., Bonetti N.R., Nietlispach F., Matter C.M., Mach F., Montecucco F., Beer J.H., Paneni F., et al. Interleukin-1β Mediates Arterial Thrombus Formation via NET-Associated Tissue Factor. J. Clin. Med. 2019;8:2072. doi: 10.3390/jcm8122072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu Z., Murakami T., Tamura H., Reich J., Kuwahara-Arai K., Iba T., Tabe Y., Nagaoka I. Neutrophil Extracellular Traps Induce IL-1β Production by Macrophages in Combination with Lipopolysaccharide. Int. J. Mol. Med. 2017;39:549–558. doi: 10.3892/ijmm.2017.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meher A.K., Spinosa M., Davis J.P., Pope N., Laubach V.E., Su G., Serbulea V., Leitinger N., Ailawadi G., Upchurch G.R. Novel Role of IL (Interleukin)-1β in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. Arter. Thromb. Vasc. Biol. 2018;38:843–853. doi: 10.1161/ATVBAHA.117.309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campos J., Ponomaryov T., De Prendergast A., Whitworth K., Smith C.W., Khan A.O., Kavanagh D., Brill A. Neutrophil Extracellular Traps and Inflammasomes Cooperatively Promote Venous Thrombosis in Mice. Blood Adv. 2021;5:2319–2324. doi: 10.1182/bloodadvances.2020003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maugeri N., Campana L., Gavina M., Covino C., De Metrio M., Panciroli C., Maiuri L., Maseri A., D’Angelo A., Bianchi M.E., et al. Activated Platelets Present High Mobility Group Box 1 to Neutrophils, Inducing Autophagy and Promoting the Extrusion of Neutrophil Extracellular Traps. J. Thromb. Haemost. 2014;12:2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 89.Mitroulis I., Kambas K., Chrysanthopoulou A., Skendros P., Apostolidou E., Kourtzelis I., Drosos G.I., Boumpas D.T., Ritis K. Neutrophil Extracellular Trap Formation Is Associated with IL-1β and Autophagy-Related Signaling in Gout. PLoS ONE. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chrysanthopoulou A., Mitroulis I., Apostolidou E., Arelaki S., Mikroulis D., Konstantinidis T., Sivridis E., Koffa M., Giatromanolaki A., Boumpas D.T., et al. Neutrophil Extracellular Traps Promote Differentiation and Function of Fibroblasts: NETs Induce Fibrosis via Differentiation of Fibroblasts. J. Pathol. 2014;233:294–307. doi: 10.1002/path.4359. [DOI] [PubMed] [Google Scholar]
- 91.Park J., Wysocki R.W., Amoozgar Z., Maiorino L., Fein M.R., Jorns J., Schott A.F., Kinugasa-Katayama Y., Lee Y., Won N.H., et al. Cancer Cells Induce Metastasis-Supporting Neutrophil Extracellular DNA Traps. Sci. Transl. Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., dos Reis M.C., de Castro G.M.M., da Silva Fontes Y., Todeschini A.R., Freire-de-Lima L., Decoté-Ricardo D., et al. The Emerging Role of Neutrophil Extracellular Traps in Severe Acute Respiratory Syndrome Coronavirus 2 (COVID-19) Sci. Rep. 2020;10:19630. doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C.N., Weber A., Barnes B.J., Egeblad M., et al. Neutrophil Extracellular Traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetité D., Tavares L.A., Paiva I.M., et al. SARS-CoV-2–Triggered Neutrophil Extracellular Traps Mediate COVID-19 Pathology. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I., Stürzl M., Staats L., Mahajan A., Schauer C., et al. Vascular Occlusion by Neutrophil Extracellular Traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Godement M., Zhu J., Cerf C., Vieillard-Baron A., Maillon A., Zuber B., Bardet V., Geri G. Neutrophil Extracellular Traps in SARS-CoV2 Related Pneumonia in ICU Patients: The NETCOV2 Study. Front. Med. 2021;8:615984. doi: 10.3389/fmed.2021.615984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guéant J., Guéant-Rodriguez R., Fromonot J., Oussalah A., Louis H., Chery C., Gette M., Gleye S., Callet J., Raso J., et al. Elastase and Exacerbation of Neutrophil Innate Immunity Are Involved in Multi-visceral Manifestations of COVID-19. Allergy. 2021;76:1846–1858. doi: 10.1111/all.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petito E., Falcinelli E., Paliani U., Cesari E., Vaudo G., Sebastiano M., Cerotto V., Guglielmini G., Gori F., Malvestiti M., et al. Association of Neutrophil Activation, More than Platelet Activation, With Thrombotic Complications in Coronavirus Disease 2019. J. Infect. Dis. 2021;223:933–944. doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Connors J.M., Levy J.H. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., Luo M., Chen L., Zhao Y. Neutrophil-to-Lymphocyte Ratio as an Independent Risk Factor for Mortality in Hospitalized Patients with COVID-19. J. Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ackermann M., Anders H.-J., Bilyy R., Bowlin G.L., Daniel C., De Lorenzo R., Egeblad M., Henneck T., Hidalgo A., Hoffmann M., et al. Patients with COVID-19: In the Dark-NETs of Neutrophils. Cell Death Differ. 2021 doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., Ntinopoulou M., Sertaridou E., Tsironidou V., Tsigalou C., et al. Complement and Tissue Factor–Enriched Neutrophil Extracellular Traps Are Key Drivers in COVID-19 Immunothrombosis. J. Clin. Investig. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morrissey S.M., Geller A.E., Hu X., Tieri D., Ding C., Klaes C.K., Cooke E.A., Woeste M.R., Martin Z.C., Chen O., et al. A Specific Low-Density Neutrophil Population Correlates with Hypercoagulation and Disease Severity in Hospitalized COVID-19 Patients. JCI Insight. 2021;6:e148435. doi: 10.1172/jci.insight.148435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang C., Montgomery M. Dornase Alfa for Cystic Fibrosis. Cochrane Database Syst. Rev. 2018;6:CD001127. doi: 10.1002/14651858.CD001127.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.ClinicalTrials.Gov. [(accessed on 25 June 2021)]; Available online: https://clinicaltrials.gov/
- 107.Cools-Lartigue J., Spicer J., McDonald B., Gowing S., Chow S., Giannias B., Bourdeau F., Kubes P., Ferri L. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells and Promote Metastasis. J. Clin. Investig. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Najmeh S., Cools-Lartigue J., Rayes R.F., Gowing S., Vourtzoumis P., Bourdeau F., Giannias B., Berube J., Rousseau S., Ferri L.E., et al. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells via B1-Integrin Mediated Interactions. Int. J. Cancer. 2017;140:2321–2330. doi: 10.1002/ijc.30635. [DOI] [PubMed] [Google Scholar]
- 109.Tohme S., Yazdani H.O., Al-Khafaji A.B., Chidi A.P., Loughran P., Mowen K., Wang Y., Simmons R.L., Huang H., Tsung A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yazdani H.O., Roy E., Comerci A.J., van der Windt D.J., Zhang H., Huang H., Loughran P., Shiva S., Geller D.A., Bartlett D.L., et al. Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Res. 2019;79:5626–5639. doi: 10.1158/0008-5472.CAN-19-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abdol Razak N., Elaskalani O., Metharom P. Pancreatic Cancer-Induced Neutrophil Extracellular Traps: A Potential Contributor to Cancer-Associated Thrombosis. Int. J. Mol. Sci. 2017;18:487. doi: 10.3390/ijms18030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohanty T., Fisher J., Bakochi A., Neumann A., Cardoso J.F.P., Karlsson C.A.Q., Pavan C., Lundgaard I., Nilson B., Reinstrup P., et al. Neutrophil Extracellular Traps in the Central Nervous System Hinder Bacterial Clearance during Pneumococcal Meningitis. Nat. Commun. 2019;10:1667. doi: 10.1038/s41467-019-09040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Buhr N., Reuner F., Neumann A., Stump-Guthier C., Tenenbaum T., Schroten H., Ishikawa H., Müller K., Beineke A., Hennig-Pauka I., et al. Neutrophil Extracellular Trap Formation in the Streptococcus Suis-Infected Cerebrospinal Fluid Compartment: NETs in Strep. Suis-Infected Cerebrospinal Fluid. Cell. Microbiol. 2017;19:e12649. doi: 10.1111/cmi.12649. [DOI] [PubMed] [Google Scholar]
- 114.Laridan E., Denorme F., Desender L., François O., Andersson T., Deckmyn H., Vanhoorelbeke K., De Meyer S.F. Neutrophil Extracellular Traps in Ischemic Stroke Thrombi: NETs in Stroke. Ann. Neurol. 2017;82:223–232. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 115.Novotny J., Oberdieck P., Titova A., Pelisek J., Chandraratne S., Nicol P., Hapfelmeier A., Joner M., Maegdefessel L., Poppert H., et al. Thrombus NET Content Is Associated with Clinical Outcome in Stroke and Myocardial Infarction. Neurology. 2020;94:e2346–e2360. doi: 10.1212/WNL.0000000000009532. [DOI] [PubMed] [Google Scholar]
- 116.Zenaro E., Pietronigro E., Bianca V.D., Piacentino G., Marongiu L., Budui S., Turano E., Rossi B., Angiari S., Dusi S., et al. Neutrophils Promote Alzheimer’s Disease–like Pathology and Cognitive Decline via LFA-1 Integrin. Nat. Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 117.Lachowicz-Scroggins M.E., Dunican E.M., Charbit A.R., Raymond W., Looney M.R., Peters M.C., Gordon E.D., Woodruff P.G., Lefrançais E., Phillips B.R., et al. Extracellular DNA, Neutrophil Extracellular Traps, and Inflammasome Activation in Severe Asthma. Am. J. Respir. Crit. Care Med. 2019;199:1076–1085. doi: 10.1164/rccm.201810-1869OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Obermayer A., Stoiber W., Krautgartner W.-D., Klappacher M., Kofler B., Steinbacher P., Vitkov L., Grabcanovic-Musija F., Studnicka M. New Aspects on the Structure of Neutrophil Extracellular Traps from Chronic Obstructive Pulmonary Disease and In Vitro Generation. PLoS ONE. 2014;9:e97784. doi: 10.1371/journal.pone.0097784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moorthy A.N., Rai P., Jiao H., Wang S., Tan K.B., Qin L., Watanabe H., Zhang Y., Teluguakula N., Chow V.T.K. Capsules of Virulent Pneumococcal Serotypes Enhance Formation of Neutrophil Extracellular Traps during in vivo Pathogenesis of Pneumonia. Oncotarget. 2016;7:19327–19340. doi: 10.18632/oncotarget.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grabcanovic-Musija F., Obermayer A., Stoiber W., Krautgartner W.-D., Steinbacher P., Winterberg N., Bathke A.C., Klappacher M., Studnicka M. Neutrophil Extracellular Trap (NET) Formation Characterises Stable and Exacerbated COPD and Correlates with Airflow Limitation. Respir. Res. 2015;16:59. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moreira-Teixeira L., Stimpson P.J., Stavropoulos E., Hadebe S., Chakravarty P., Ioannou M., Aramburu I.V., Herbert E., Priestnall S.L., Suarez-Bonnet A., et al. Type I IFN Exacerbates Disease in Tuberculosis-Susceptible Mice by Inducing Neutrophil-Mediated Lung Inflammation and NETosis. Nat. Commun. 2020;11:5566. doi: 10.1038/s41467-020-19412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toussaint M., Jackson D.J., Swieboda D., Guedán A., Tsourouktsoglou T.-D., Ching Y.M., Radermecker C., Makrinioti H., Aniscenko J., Bartlett N.W., et al. Host DNA Released by NETosis Promotes Rhinovirus-Induced Type-2 Allergic Asthma Exacerbation. Nat. Med. 2017;23:681–691. doi: 10.1038/nm.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Behnen M., Leschczyk C., Möller S., Batel T., Klinger M., Solbach W., Laskay T. Immobilized Immune Complexes Induce Neutrophil Extracellular Trap Release by Human Neutrophil Granulocytes via FcγRIIIB and Mac-1. J. Immunol. 2014;193:1954–1965. doi: 10.4049/jimmunol.1400478. [DOI] [PubMed] [Google Scholar]
- 124.Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J., Meller S., Chamilos G., Sebasigari R., Riccieri V., et al. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA-Peptide Complexes in Systemic Lupus Erythematosus. Sci. Transl. Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D.J., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA Traps Promote Thrombosis. Proc. Natl. Acad. Sci. USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Inflammation. Neutrophil Extracellular Traps License Macrophages for Cytokine Production in Atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.-A., Phoon M.C., van Rooijen N., Chow V.T. Excessive Neutrophils and Neutrophil Extracellular Traps Contribute to Acute Lung Injury of Influenza Pneumonitis. Am. J. Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hiroki C.H., Toller-Kawahisa J.E., Fumagalli M.J., Colon D.F., Figueiredo L.T.M., Fonseca B.A.L.D., Franca R.F.O., Cunha F.Q. Neutrophil Extracellular Traps Effectively Control Acute Chikungunya Virus Infection. Front. Immunol. 2020;10:3108. doi: 10.3389/fimmu.2019.03108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guiducci E., Lemberg C., Küng N., Schraner E., Theocharides A.P.A., LeibundGut-Landmann S. Candida Albicans-Induced NETosis Is Independent of Peptidylarginine Deiminase 4. Front. Immunol. 2018;9:1573. doi: 10.3389/fimmu.2018.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Silva J.C., Rodrigues N.C., Thompson-Souza G.A., Muniz V.D.S., Neves J.S., Figueiredo R.T. Mac-1 Triggers Neutrophil DNA Extracellular Trap Formation to Aspergillus fumigatus Independently of PAD4 Histone Citrullination. J. Leukoc. Biol. 2020;107:69–83. doi: 10.1002/JLB.4A0119-009RR. [DOI] [PubMed] [Google Scholar]
- 131.Sousa-Rocha D., Thomaz-Tobias M., Diniz L.F.A., Souza P.S.S., Pinge-Filho P., Toledo K.A. Trypanosoma Cruzi and Its Soluble Antigens Induce NET Release by Stimulating Toll-Like Receptors. PLoS ONE. 2015;10:e0139569. doi: 10.1371/journal.pone.0139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodrigues D.A.S., Prestes E.B., Gama A.M.S., de Souza Silva L., Pinheiro A.A.S., Ribeiro J.M.C., Campos R.M.P., Pimentel-Coelho P.M., De Souza H.S., Dicko A., et al. CXCR4 and MIF Are Required for Neutrophil Extracellular Trap Release Triggered by Plasmodium-Infected Erythrocytes. PLoS Pathog. 2020;16:e1008230. doi: 10.1371/journal.ppat.1008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han X., Ding S., Jiang H., Liu G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021;9:625423. doi: 10.3389/fcell.2021.625423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haider P., Kral-Pointner J.B., Mayer J., Richter M., Kaun C., Brostjan C., Eilenberg W., Fischer M.B., Speidl W.S., Hengstenberg C., et al. Neutrophil Extracellular Trap Degradation by Differently Polarized Macrophage Subsets. Arter. Thromb. Vasc. Biol. 2020;40:2265–2278. doi: 10.1161/ATVBAHA.120.314883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang S., Chen L., Zhang G., Zhang B. Umbilical Cord-Matrix Stem Cells Induce the Functional Restoration of Vascular Endothelial Cells and Enhance Skin Wound Healing in Diabetic Mice via the Polarized Macrophages. Stem Cell Res. 2020;11:39. doi: 10.1186/s13287-020-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu P., Wu X., Liao C., Liu X., Du J., Shi H., Wang X., Bai X., Peng P., Yu L., et al. Escherichia Coli and Candida Albicans Induced Macrophage Extracellular Trap-Like Structures with Limited Microbicidal Activity. PLoS ONE. 2014;9:e90042. doi: 10.1371/journal.pone.0090042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loureiro A., Pais C., Sampaio P. Relevance of Macrophage Extracellular Traps in C. Albicans Killing. Front. Immunol. 2019;10:2767. doi: 10.3389/fimmu.2019.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mohanan S., Horibata S., McElwee J.L., Dannenberg A.J., Coonrod S.A. Identification of Macrophage Extracellular Trap-Like Structures in Mammary Gland Adipose Tissue: A Preliminary Study. Front. Immunol. 2013;4:67. doi: 10.3389/fimmu.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muñoz-Caro T., Silva L.M.R., Ritter C., Taubert A., Hermosilla C. Besnoitia Besnoiti Tachyzoites Induce Monocyte Extracellular Trap Formation. Parasitol. Res. 2014;113:4189–4197. doi: 10.1007/s00436-014-4094-3. [DOI] [PubMed] [Google Scholar]
- 140.Pérez D., Muñoz M.C., Molina J.M., Muñoz-Caro T., Silva L.M.R., Taubert A., Hermosilla C., Ruiz A. Eimeria Ninakohlyakimovae Induces NADPH Oxidase-Dependent Monocyte Extracellular Trap Formation and Upregulates IL-12 and TNF-α, IL-6 and CCL2 Gene Transcription. Vet. Parasitol. 2016;227:143–150. doi: 10.1016/j.vetpar.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 141.Pertiwi K.R., de Boer O.J., Mackaaij C., Pabittei D.R., de Winter R.J., Li X., van der Wal A.C. Extracellular Traps Derived from Macrophages, Mast Cells, Eosinophils and Neutrophils Are Generated in a Time-Dependent Manner during Atherothrombosis. J. Pathol. 2019;247:505–512. doi: 10.1002/path.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zambrano F., Schulz M., Pilatz A., Wagenlehner F., Schuppe H.-C., Conejeros I., Uribe P., Taubert A., Sánchez R., Hermosilla C. Increase of Leucocyte-Derived Extracellular Traps (ETs) in Semen Samples from Human Acute Epididymitis Patients—a Pilot Study. J. Assist. Reprod. Genet. 2020;37:2223–2231. doi: 10.1007/s10815-020-01883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang X., Zhang L., Tan Y., Liu Y., Li J., Deng Q., Yan S., Zhang W., Han L., Zhong M. Hepcidin Gene Silencing Ameliorated Inflammation and Insulin Resistance in Adipose Tissue of Db/Db Mice via Inhibiting METs Formation. Mol. Immunol. 2021;133:110–121. doi: 10.1016/j.molimm.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 144.Wong K.-W., Jacobs W.R. Mycobacterium Tuberculosis Exploits Human Interferon γ to Stimulate Macrophage Extracellular Trap Formation and Necrosis. J. Infect. Dis. 2013;208:109–119. doi: 10.1093/infdis/jit097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Haritha V.H., Seena P., Shaji B.V., Nithin T.U., Hazeena V.N., Anie Y. Monocyte Clearance of Apoptotic Neutrophils Is Unhindered in the Presence of NETosis, but Proteins of NET Trigger ETosis in Monocytes. Immunol. Lett. 2019;207:36–45. doi: 10.1016/j.imlet.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y., Rayner B.S., Jensen M., Hawkins C.L. In Vitro Stimulation and Visualization of Extracellular Trap Release in Differentiated Human Monocyte-Derived Macrophages. J. Vis. Exp. 2019;1:60541. doi: 10.3791/60541. [DOI] [PubMed] [Google Scholar]
- 147.Pertiwi K.R., de Boer O.J., Gabriels P.A.M., van der Wal A.C. Etosis, Rather than Apoptosis or Cell Proliferation, Typifies Thrombus Progression—An Immunohistochemical Study of Coronary Aspirates. Int. J. Cardiol. Heart Vasc. 2020;26:100439. doi: 10.1016/j.ijcha.2019.100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Antunes S.A., Canziani M.E.F. Hepcidin: An Important Iron Metabolism Regulator in Chronic Kidney Disease. J. Bras. Nefrol. 2016;38:351–355. doi: 10.5935/0101-2800.20160053. [DOI] [PubMed] [Google Scholar]
- 149.Josefs T., Barrett T.J., Brown E.J., Quezada A., Wu X., Voisin M., Amengual J., Fisher E.A. Neutrophil Extracellular Traps Promote Macrophage Inflammation and Impair Atherosclerosis Resolution in Diabetic Mice. JCI Insight. 2020;5:e134796. doi: 10.1172/jci.insight.134796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li L., Yu X., Liu J., Wang Z., Li C., Shi J., Sun L., Liu Y., Zhang F., Chen H., et al. Neutrophil Extracellular Traps Promote Aberrant Macrophages Activation in Behçet’s Disease. Front. Immunol. 2021;11:590622. doi: 10.3389/fimmu.2020.590622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eghbalzadeh K., Georgi L., Louis T., Zhao H., Keser U., Weber C., Mollenhauer M., Conforti A., Wahlers T., Paunel-Görgülü A. Compromised Anti-Inflammatory Action of Neutrophil Extracellular Traps in PAD4-Deficient Mice Contributes to Aggravated Acute Inflammation After Myocardial Infarction. Front. Immunol. 2019;10:2313. doi: 10.3389/fimmu.2019.02313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oishi S., Tsukiji N., Otake S., Oishi N., Sasaki T., Shirai T., Yoshikawa Y., Takano K., Shinmori H., Inukai T., et al. Heme Activates Platelets and Exacerbates Rhabdomyolysis-Induced Acute Kidney Injury via CLEC-2 and GPVI/FcRγ. Blood Adv. 2021;5:2017–2026. doi: 10.1182/bloodadvances.2020001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tian Y., Russo R.M., Li Y., Karmakar M., Liu B., Puskarich M.A., Jones A.E., Stringer K.A., Standiford T.J., Alam H.B. Serum Citrullinated Histone H3 Concentrations Differentiate Patients with Septic Verses Non-Septic Shock and Correlate with Disease Severity. Infection. 2021;49:83–93. doi: 10.1007/s15010-020-01528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li L., Krilis S. Mast-cell Growth and Differentiation. Allergy. 1999;54:306–313. doi: 10.1034/j.1398-9995.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 155.Féger F., Varadaradjalou S., Gao Z., Abraham S.N., Arock M. The Role of Mast Cells in Host Defense and Their Subversion by Bacterial Pathogens. Trends Immunol. 2002;23:151–158. doi: 10.1016/S1471-4906(01)02156-1. [DOI] [PubMed] [Google Scholar]
- 156.Kunder C.A., St John A.L., Abraham S.N. Mast Cell Modulation of the Vascular and Lymphatic Endothelium. Blood. 2011;118:5383–5393. doi: 10.1182/blood-2011-07-358432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dudeck A., Köberle M., Goldmann O., Meyer N., Dudeck J., Lemmens S., Rohde M., Roldán N.G., Dietze-Schwonberg K., Orinska Z., et al. Mast Cells as Protectors of Health. J. Allergy Clin. Immunol. 2019;144:S4–S18. doi: 10.1016/j.jaci.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 158.Varricchi G., Rossi F.W., Galdiero M.R., Granata F., Criscuolo G., Spadaro G., de Paulis A., Marone G. Physiological Roles of Mast Cells: Collegium Internationale Allergologicum Update 2019. Int. Arch. Allergy Immunol. 2019;179:247–261. doi: 10.1159/000500088. [DOI] [PubMed] [Google Scholar]
- 159.Theoharides T.C., Tsilioni I., Ren H. Recent Advances in Our Understanding of Mast Cell Activation—or Should It Be Mast Cell Mediator Disorders? Expert Rev. Clin. Immunol. 2019;15:639–656. doi: 10.1080/1744666X.2019.1596800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.John A.L.S., Abraham S.N. Innate Immunity and Its Regulation by Mast Cells. J. Immunol. 2013;190:4458–4463. doi: 10.4049/jimmunol.1203420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mukai K., Tsai M., Saito H., Galli S.J. Mast Cells as Sources of Cytokines, Chemokines, and Growth Factors. Immunol. Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dawicki W., Marshall J.S. New and Emerging Roles for Mast Cells in Host Defence. Curr. Opin. Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 163.Nija R.J., Sanju S., Sidharthan N., Mony U. Extracellular Trap by Blood Cells: Clinical Implications. Tissue Eng. Regen. Med. 2020;17:141–153. doi: 10.1007/s13770-020-00241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Di Nardo A., Vitiello A., Gallo R.L. Cutting Edge: Mast Cell Antimicrobial Activity Is Mediated by Expression of Cathelicidin Antimicrobial Peptide. J. Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 165.Abel J., Goldmann O., Ziegler C., Höltje C., Smeltzer M.S., Cheung A.L., Bruhn D., Rohde M., Medina E. Staphylococcus Aureus Evades the Extracellular Antimicrobial Activity of Mast Cells by Promoting Its Own Uptake. J. Innate Immun. 2011;3:495–507. doi: 10.1159/000327714. [DOI] [PubMed] [Google Scholar]
- 166.Hayes S.M., Biggs T.C., Goldie S.P., Harries P.G., Walls A.F., Allan R.N., Pender S.L.F., Salib R.J. Staphylococcus Aureus Internalization in Mast Cells in Nasal Polyps: Characterization of Interactions and Potential Mechanisms. J. Allergy Clin. Immunol. 2020;145:147–159. doi: 10.1016/j.jaci.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 167.Branitzki-Heinemann K., Okumura C.Y., Völlger L., Kawakami Y., Kawakami T., Naim H.Y., Nizet V., Von Köckritz-Blickwede M. A Novel Role for the Transcription Factor HIF-1α in the Formation of Mast Cell Extracellular Traps. Biochem. J. 2012;446:159–163. doi: 10.1042/BJ20120658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garcia-Rodriguez K.M., Bahri R., Sattentau C., Roberts I.S., Goenka A., Bulfone-Paus S. Human Mast Cells Exhibit an Individualized Pattern of Antimicrobial Responses. Immun. Inflamm. Dis. 2020;8:198–210. doi: 10.1002/iid3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Campillo-Navarro M., Leyva-Paredes K., Donis-Maturano L., Rodríguez-López G.M., Soria-Castro R., García-Pérez B.E., Puebla-Osorio N., Ullrich S.E., Luna-Herrera J., Flores-Romo L., et al. Mycobacterium Tuberculosis Catalase Inhibits the Formation of Mast Cell Extracellular Traps. Front. Immunol. 2018;9:1161. doi: 10.3389/fimmu.2018.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lauth X., von Köckritz-Blickwede M., McNamara C.W., Myskowski S., Zinkernagel A.S., Beall B., Ghosh P., Gallo R.L., Nizet V. M1 Protein Allows Group A Streptococcal Survival in Phagocyte Extracellular Traps through Cathelicidin Inhibition. J. Innate Immun. 2009;1:202–214. doi: 10.1159/000203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Clark M., Kim J., Etesami N., Shimamoto J., Whalen R.V., Martin G., Okumura C.Y.M. Group A Streptococcus Prevents Mast Cell Degranulation to Promote Extracellular Trap Formation. Front. Immunol. 2018;9:327. doi: 10.3389/fimmu.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Scheb-Wetzel M., Rohde M., Bravo A., Goldmann O. New Insights into the Antimicrobial Effect of Mast Cells against Enterococcus Faecalis. Infect. Immun. 2014;82:4496–4507. doi: 10.1128/IAI.02114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lopes J.P., Stylianou M., Nilsson G., Urban C.F. Opportunistic Pathogen Candida Albicans Elicits a Temporal Response in Primary Human Mast Cells. Sci. Rep. 2015;5:12287. doi: 10.1038/srep12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Naqvi N., Ahuja K., Selvapandiyan A., Dey R., Nakhasi H., Puri N. Role of Mast Cells in Clearance of Leishmania through Extracellular Trap Formation. Sci. Rep. 2017;7:13240. doi: 10.1038/s41598-017-12753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Piliponsky A.M., Romani L. The Contribution of Mast Cells to Bacterial and Fungal Infection Immunity. Immunol. Rev. 2018;282:188–197. doi: 10.1111/imr.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin A.M., Rubin C.J., Khandpur R., Wang J.Y., Riblett M., Yalavarthi S., Villanueva E.C., Shah P., Kaplan M.J., Bruce A.T. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J. Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Campillo-Navarro M., Leyva-Paredes K., Donis-Maturano L., González-Jiménez M., Paredes-Vivas Y., Cerbulo-Vázquez A., Serafín-López J., García-Pérez B., Ullrich S.E., Flores-Romo L., et al. Listeria Monocytogenes Induces Mast Cell Extracellular Traps. Immunobiology. 2017;222:432–439. doi: 10.1016/j.imbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 178.Weller P.F., Spencer L.A. Functions of Tissue-Resident Eosinophils. Nat. Rev. Immunol. 2017;17:746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nakayama T., Yoshikawa M., Asaka D., Okushi T., Matsuwaki Y., Otori N., Hama T., Moriyama H. Mucosal Eosinophilia and Recurrence of Nasal Polyps—New Classification of Chronic Rhinosinusitis. Rhinology. 2011;49:392–396. doi: 10.4193/Rhino10.261. [DOI] [PubMed] [Google Scholar]
- 180.Figueiredo R.T., Neves J.S. Eosinophils in Fungal Diseases: An Overview. J. Leukoc. Biol. 2018;104:49–60. doi: 10.1002/JLB.4MR1117-473R. [DOI] [PubMed] [Google Scholar]
- 181.Strandmark J., Rausch S., Hartmann S. Eosinophils in Homeostasis and Their Contrasting Roles during Inflammation and Helminth Infections. Crit. Rev. Immunol. 2016;36:193–238. doi: 10.1615/CritRevImmunol.2016018726. [DOI] [PubMed] [Google Scholar]
- 182.Gigon L., Yousefi S., Karaulov A., Simon H.-U. Mechanisms of Toxicity Mediated by Neutrophil and Eosinophil Granule Proteins. Allergol. Int. 2021;70:30–38. doi: 10.1016/j.alit.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 183.Yousefi S., Gold J.A., Andina N., Lee J.J., Kelly A.M., Kozlowski E., Schmid I., Straumann A., Reichenbach J., Gleich G.J., et al. Catapult-like Release of Mitochondrial DNA by Eosinophils Contributes to Antibacterial Defense. Nat. Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 184.Morshed M., Yousefi S., Stöckle C., Simon H.-U., Simon D. Thymic Stromal Lymphopoietin Stimulates the Formation of Eosinophil Extracellular Traps. Allergy. 2012;67:1127–1137. doi: 10.1111/j.1398-9995.2012.02868.x. [DOI] [PubMed] [Google Scholar]
- 185.Ueki S., Melo R.C.N., Ghiran I., Spencer L.A., Dvorak A.M., Weller P.F. Eosinophil Extracellular DNA Trap Cell Death Mediates Lytic Release of Free Secretion-Competent Eosinophil Granules in Humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Simon D., Radonjic-Hösli S., Straumann A., Yousefi S., Simon H.-U. Active Eosinophilic Esophagitis Is Characterized by Epithelial Barrier Defects and Eosinophil Extracellular Trap Formation. Allergy. 2015;70:443–452. doi: 10.1111/all.12570. [DOI] [PubMed] [Google Scholar]
- 187.Ueki S., Konno Y., Takeda M., Moritoki Y., Hirokawa M., Matsuwaki Y., Honda K., Ohta N., Yamamoto S., Takagi Y., et al. Eosinophil Extracellular Trap Cell Death-Derived DNA Traps: Their Presence in Secretions and Functional Attributes. J. Allergy Clin. Immunol. 2016;137:258–267. doi: 10.1016/j.jaci.2015.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Choi Y., Le Pham D., Lee D.-H., Lee S.-H., Kim S.-H., Park H.-S. Biological Function of Eosinophil Extracellular Traps in Patients with Severe Eosinophilic Asthma. Exp. Mol. Med. 2018;50:1–8. doi: 10.1038/s12276-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Muniz V.S., Silva J.C., Braga Y.A.V., Melo R.C.N., Ueki S., Takeda M., Hebisawa A., Asano K., Figueiredo R.T., Neves J.S. Eosinophils Release Extracellular DNA Traps in Response to Aspergillus Fumigatus. J. Allergy Clin. Immunol. 2018;141:571–585.e7. doi: 10.1016/j.jaci.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 190.Hwang C.S., Park S.C., Cho H.-J., Park D.-J., Yoon J.-H., Kim C.-H. Eosinophil Extracellular Trap Formation Is Closely Associated with Disease Severity in Chronic Rhinosinusitis Regardless of Nasal Polyp Status. Sci. Rep. 2019;9:8061. doi: 10.1038/s41598-019-44627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ehrens A., Lenz B., Neumann A.-L., Giarrizzo S., Reichwald J.J., Frohberger S.J., Stamminger W., Buerfent B.C., Fercoq F., Martin C., et al. Microfilariae Trigger Eosinophil Extracellular DNA Traps in a Dectin-1-Dependent Manner. Cell Rep. 2021;34:108621. doi: 10.1016/j.celrep.2020.108621. [DOI] [PubMed] [Google Scholar]
- 192.Da Cunha A.A., Nuñez N.K., de Souza R.G., Moraes Vargas M.H., Silveira J.S., Antunes G.L., da Silva Durante L., Porto B.N., Marczak E.S., Jones M.H., et al. Recombinant Human Deoxyribonuclease Therapy Improves Airway Resistance and Reduces DNA Extracellular Traps in a Murine Acute Asthma Model. Exp. Lung Res. 2016;42:66–74. doi: 10.3109/01902148.2016.1143537. [DOI] [PubMed] [Google Scholar]
- 193.Yousefi S., Sharma S.K., Stojkov D., Germic N., Aeschlimann S., Ge M.Q., Flayer C.H., Larson E.D., Redai I.G., Zhang S., et al. Oxidative Damage of SP-D Abolishes Control of Eosinophil Extracellular DNA Trap Formation. J. Leukoc. Biol. 2018;104:205–214. doi: 10.1002/JLB.3AB1117-455R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ohta N., Ueki S., Konno Y., Hirokawa M., Kubota T., Tomioka-Matsutani S., Suzuki T., Ishida Y., Kawano T., Miyasaka T., et al. ETosis-Derived DNA Trap Production in Middle Ear Effusion Is a Common Feature of Eosinophilic Otitis Media. Allergol. Int. 2018;67:414–416. doi: 10.1016/j.alit.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 195.Gevaert E., Zhang N., Krysko O., Lan F., Holtappels G., De Ruyck N., Nauwynck H., Yousefi S., Simon H.-U., Bachert C. Extracellular Eosinophilic Traps in Association with Staphylococcus Aureus at the Site of Epithelial Barrier Defects in Patients with Severe Airway Inflammation. J. Allergy Clin. Immunol. 2017;139:1849–1860.e6. doi: 10.1016/j.jaci.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 196.Omokawa A., Ueki S., Kikuchi Y., Takeda M., Asano M., Sato K., Sano M., Ito H., Hirokawa M. Mucus Plugging in Allergic Bronchopulmonary Aspergillos is: Implication of the Eosinophil DNA Traps. Allergol. Int. 2018;67:280–282. doi: 10.1016/j.alit.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 197.Barroso M.V., Gropillo I., Detoni M.A.A., Thompson-Souza G.A., Muniz V.S., Vasconcelos C.R.I., Figueiredo R.T., Melo R.C.N., Neves J.S. Structural and Signaling Events Driving Aspergillus Fumigatus-Induced Human Eosinophil Extracellular Trap Release. Front. Microbiol. 2021;12:633696. doi: 10.3389/fmicb.2021.633696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marx C., Novotny J., Salbeck D., Zellner K.R., Nicolai L., Pekayvaz K., Kilani B., Stockhausen S., Bürgener N., Kupka D., et al. Eosinophil-Platelet Interactions Promote Atherosclerosis and Stabilize Thrombosis with Eosinophil Extracellular Traps. Blood. 2019;134:1859–1872. doi: 10.1182/blood.2019000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Simon D., Hoesli S., Roth N., Staedler S., Yousefi S., Simon H.-U. Eosinophil Extracellular DNA Traps in Skin Diseases. J. Allergy Clin. Immunol. 2011;127:194–199. doi: 10.1016/j.jaci.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 200.Jones V.A., Patel P.M., Amber K.T. Eosinophils in Bullous Pemphigoid. Panminerva Med. 2020 doi: 10.23736/S0031-0808.20.03997-X. [DOI] [PubMed] [Google Scholar]
- 201.Kerstan A., Simon H.-U., Yousefi S., Leverkus M. Extensive Accumulation of Eosinophil Extracellular Traps in Bullous Delayed-Pressure Urticaria: A Pathophysiological Link? Br. J. Derm. 2012;166:1151–1152. doi: 10.1111/j.1365-2133.2012.10848.x. [DOI] [PubMed] [Google Scholar]
- 202.Rocha Arrieta Y.C., Rojas M., Vasquez G., Lopez J. The Lymphocytes Stimulation Induced DNA Release, a Phenomenon Similar to NETosis. Scand. J. Immunol. 2017;86:229–238. doi: 10.1111/sji.12592. [DOI] [PubMed] [Google Scholar]
- 203.Costanza M., Poliani P.L., Portararo P., Cappetti B., Musio S., Pagani F., Steinman L., Colombo M.P., Pedotti R., Sangaletti S. DNA Threads Released by Activated CD4(+) T Lymphocytes Provide Autocrine Costimulation. Proc. Natl. Acad. Sci. USA. 2019;116:8985–8994. doi: 10.1073/pnas.1822013116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Morshed M., Hlushchuk R., Simon D., Walls A.F., Obata-Ninomiya K., Karasuyama H., Djonov V., Eggel A., Kaufmann T., Simon H.-U., et al. NADPH Oxidase–Independent Formation of Extracellular DNA Traps by Basophils. J. Immunol. 2014;192:5314–5323. doi: 10.4049/jimmunol.1303418. [DOI] [PubMed] [Google Scholar]
- 205.Schorn C., Janko C., Latzko M., Chaurio R., Schett G., Herrmann M. Monosodium Urate Crystals Induce Extracellular DNA Traps in Neutrophils, Eosinophils, and Basophils but Not in Mononuclear Cells. Front. Immunol. 2012;3:277. doi: 10.3389/fimmu.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yousefi S., Morshed M., Amini P., Stojkov D., Simon D., von Gunten S., Kaufmann T., Simon H.-U. Basophils Exhibit Antibacterial Activity through Extracellular Trap Formation. Allergy. 2015;70:1184–1188. doi: 10.1111/all.12662. [DOI] [PubMed] [Google Scholar]
- 207.Loures F.V., Röhm M., Lee C.K., Santos E., Wang J.P., Specht C.A., Calich V.L.G., Urban C.F., Levitz S.M. Recognition of Aspergillus Fumigatus Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps. PLoS Pathog. 2015;11:e1004643. doi: 10.1371/journal.ppat.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Karasuyama H., Miyake K., Yoshikawa S., Yamanishi Y. Multifaceted Roles of Basophils in Health and Disease. J. Allergy Clin. Immunol. 2018;142:370–380. doi: 10.1016/j.jaci.2017.10.042. [DOI] [PubMed] [Google Scholar]