Abstract
The use of serum anti-Helicobacter pylori IgG and pepsinogen (PG) detection as a diagnostic method was evaluated in Sri Lanka. Gastric biopsies were performed (353 patients), and the prevalence of H. pylori infection was 1.7% (culture) and 2.0% (histology). IgG serology testing showed an area under the curve (AUC) of 0.922 (cut-off, 2.95 U/mL; specificity, 91.56%; sensitivity, 88.89%). Histological evaluation showed mild atrophy (34.3%), moderate atrophy (1.7%), metaplasia (1.7%), chronic gastritis (6.2%), and normal tissue (56%). The PGI/PGII ratio was significantly higher in H. pylori-negative patients (p < 0.01). PGII and PGI/PGII levels were lower in patients with metaplasia than in those with normal mucosa (p = 0.049 and p < 0.001, respectively). The PGI/PGII ratio best discriminated metaplasia and moderate atrophy (AUC 0.88 and 0.76, respectively). PGI and PGII alone showed poor discriminative ability, especially in mild atrophy (0.55 and 0.53, respectively) and chronic gastritis (0.55 and 0.53, respectively). The best cut-off to discriminate metaplasia was 3.25 U/mL (95.19% specificity, 83.33% sensitivity). Anti-H. pylori IgG and PG assessment (ABC method) was performed (group B, 2.0%; group A, 92.1%). The new cut-off more accurately identified patients with metaplasia requiring follow-up (group B, 5.4%). Assessment of anti-H. pylori IgG and PG is valuable in countries with a low prevalence of H. pylori infection.
Keywords: Sri Lanka, Helicobacter pylori, prevalence, infectious disease, anti-Hp IgG, gastroduodenal diseases, pepsinogen
1. Introduction
Helicobacter pylori is a rare pathogen that can successfully colonize the human stomach, and infection is an important factor related to the development of various pathological changes in the gastroduodenal tract [1,2]. H. pylori plays a major role in the development of chronic gastritis and peptic ulcers and is also associated with the development of gastric adenocarcinoma [1,3]. This organism is also responsible for gastric mucosal-associated lymphoid tissue lymphoma [4]. H. pylori is believed to infect half of the world’s population, and its prevalence has been reported to be notably higher in developing countries than in developed countries [5].
Previous studies conducted in the South Asian region have shown that the prevalence varies in different geographical locations. In India, the prevalence was reported to range from 58.8% to 85.0% [6]. A high prevalence was also reported in Bhutan and Bangladesh, where the rates were as high as 73.4% and 60.15%, respectively [7,8,9]. Sri Lanka is an island country in South Asia located in the Indian Ocean with a total population of approximately 21.6 million [10]. In previous studies, the prevalence of H. pylori infection in Sri Lanka ranged from 6.5% (12/184) in children by stool diagnosis to 70.1% (40/57) by PCR in 2002 [9,11]. A later study in 2015 reported that the H. pylori prevalence determined by PCR was 19.7% (15/76) [12]. The differences in the diagnostic methods used in each study and the low number of participants might have caused the wide range of prevalence.
Detection of H. pylori infection can be obtained via invasive and non-invasive measures. Detection of H. pylori through upper endoscopy, gastric biopsy, and culture provides evidence of infection [13,14]. It can also reveal the condition of the stomach and allow for the investigation of abnormal development of the gastric mucosa. However, these methods have numerous side effects and are expensive and time consuming. In addition, in the histological examination of a biopsy sample, only a small portion of the stomach is assessed.
The use of antibodies to detect H. pylori infection by serology has shown satisfactory results [15,16]. In a recent study of 2560 cases, among the non-invasive tests, H. pylori IgG detection showed the highest sensitivity (94%) compared to the urea breath test and stool antigen test (64% and 61%, respectively) using the histology results as a reference [17]. It is considered safe and sensitive for screening purposes. Countries with known high H. pylori prevalence, such as Japan, have determined a cut-off value of 10 U/mL [18]. However, the analysis of serology tests from many countries showed a variety of area under the curve (AUC) values [16,19]. Thus, validation and setting up a specific cut-off value for each country is necessary to optimize diagnostic performance [20].
Assessing pepsinogen (PG) levels is also a non-invasive approach for predicting gastric mucosa status [21,22]. PGI is produced by the oxyntic gland in the corpus, while PGII is also produced in the antrum, and the levels change during carcinogenesis [23]. PG levels increase in the presence of infection or inflammation but decrease in gland obliteration [24]. On the basis of this pathogenesis, researchers have proven that the changes in PGI level and PGI/PGII ratio are a good predictor for the development of gastric cancer in many countries such as Japan, Korea, and China [25,26,27,28]. In developing countries where endoscopy facilities are limited, such as in Sri Lanka, the PG test is advantageous for diagnostic and screening purposes. The combination of the PG test with H. pylori infection screening, which is known as the “ABC method”, has also been reported to be accurate and cost-effective [29,30]. Similar to other serology tests, this method also has different sensitivity and specificity ranges in each country [31,32,33] thus, validation is required before application to the general population.
It is important to investigate the burden of H. pylori infection. Eradication of H. pylori is mandatory to prevent gastroduodenal diseases. To our knowledge, this is the first study to validate the serology approach for the diagnosis of H. pylori and mucosal status in Sri Lanka. Hence, this study could help health policy makers in selecting the best strategy to reduce the burden of H. pylori-related diseases.
2. Material and Methods
2.1. Study Participants
The upper endoscopic survey was performed on 400 sequential subjects with dyspeptic symptoms at the University of Peradeniya and Teaching Hospital Peradeniya, Sri Lanka, from November 2017 to January 2018, including 204 males and 184 females with a mean age of 56.4 ± 14.7 and 57.9 ± 15.2 years, respectively. Dyspeptic patients aged >18 years without history of gastrectomy were included in this study. Any intake of proton pump inhibitors, antibiotics, or bismuth-containing compounds was prohibited 4 weeks prior to endoscopy.
Four biopsy specimens were collected during each endoscopy session, including two specimens from the greater curvature of the antrum, one specimen from the greater curvature of the corpus, and one specimen from the angulus. Biopsy from the antrum, corpus, and angulus was used for histopathological examination, while the remaining antrum specimens were used for H. pylori culture. For culture, antrum specimens were immediately placed in transport medium containing brucella broth (Becton Dickinson, Sparks, MD, USA) and glycerol (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) and stored at −80 °C until further use. Fasting serum was collected from each subject on the day of endoscopy and immediately stored at −20 °C until use.
All participants provided written informed consent, and study approval was obtained from the ethics committees of the University of Peradeniya, Sri Lanka, and Oita University Faculty of Medicine, Japan.
2.2. H. pylori Infection Status and Pepsinogen Level
H. pylori infection status was determined using three diagnostic tests: culture, histology, and serology. H. pylori culture was performed as described previously [34]. Briefly, the homogenized antrum specimens were inoculated onto H. pylori-selective media (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) and incubated for up to 10 days at 37 °C under microaerophilic conditions (10% O2, 5% CO2, and 85% N2). H. pylori was then sub-cultured onto Brucella agar (Becton Dickinson, Sparks, MD, USA) supplemented with 7% horse blood (Nippon Bio-test, Tokyo, Japan) without antibiotics. H. pylori was identified on the basis of colony morphology, Gram-negative staining results, bacterial morphology, and positive results for catalase (Sigma Aldrich, Darmstadt, Germany), urease (our laboratory homemade), and oxidase test (Milipore Sigma, Burlington, MA, USA). H. pylori was stored in Brucella broth (Becton Dickinson, Sparks, MD, USA) containing 10% glycerol and 10% horse serum and stored at −80 °C.
For histopathological examination, the biopsy materials were fixed in 10% formalin and embedded them in paraffin. Hematoxylin–eosin (Motu Chemical, Tokyo, Japan) and May–Grünwald–Giemsa (Merck, Darmstadt, Germany) staining were applied to thin slices of paraffin-embedded biopsy specimens. On the basis of the updated Sydney system, an experienced pathologist assessed the bacterial density in each specimen [34]. The neutrophils, monocytes, atrophy, and metaplasia were scored to determine the gastric mucosa status in the antrum, corpus, and angulus. The scoring system for the histology of gastric mucosa was also based on the updated Sydney system [35].
The definitions of each group are as follows: the normal group had a score of zero for all locations; the “chronic gastritis” group had a monocyte score of at least 1 without any atrophy or metaplasia, the “mild atrophic” group had an atrophy score of 1 without any metaplasia, the “moderate atrophic” group had an atrophy score of 2 without any metaplasia, and the “metaplasia” group had a metaplasia score of at least 1 in at least one location.
2.3. Anti-H. pylori IgG Test and PG Test from Serology
The serum antibody to H. pylori was examined using an E-Plate ELISA kit (Eiken Co., Ltd., Tokyo, Japan) following the manufacturer’s instructions. Participants with a serum anti-H. pylori level ≥10 U/mL were classified as H. pylori-positive. PGI and PGII levels were measured using a PG ELISA kit (Eiken, Tokyo, Japan) following the manufacturer’s instructions. The criteria by Miki et al., widely used in Japan, were applied to stratify the cancer risk; a PG I level ≤70 ng/mL and PG I/II ratio ≤3.0 were classified as PG-positive [29]. For further analysis, the group was determined on the basis of the serum anti-H. pylori and serum PG level using the ABC method: group A (H. pylori-negative/PG-negative), group B (H. pylori-positive/PG-negative), group C (H. pylori-positive/PG-positive), and group D (H. pylori-negative/PG-positive) [30].
2.4. Data Analysis
Statistical analyses were performed using R version 3.4.4. The ROC curve and Youden’s index were determined by the “pROC” package. The visualization and statistical comparison were performed using “ggplot”, “pubr”, and “ggsignif”. We analyzed the discrete variables using the chi-squared test, whereas continuous variables were analyzed using the Mann–Whitney U test, and statistical significance was defined as a p-value < 0.05.
3. Results
3.1. H. pylori Infection Status
A total of 400 subjects were recruited in this study. However, due to the incomplete biopsy and serum samples, 47 subjects were excluded. The final dataset of 353 subjects consisted of 164 females with an average age of 57.6 ± 15.0 years and 189 males with an average age of 56.5 ± 14.9 years. The predominant ethnicity of the subjects was Sinhalese (309/353; 87.5%), followed by Muslim (25/353; 7.1%) and Tamil (19/353; 5.4%).
First, a combination of culture and histology tests were used to determine the H. pylori infection status (Table 1). Six subjects had culture-positive results (6/353; 1.7%) and seven subjects had histology-positive results (7/370; 2.0%). With at least one test showing positivity by histology and culture, the percentage of H. pylori infection was 2.5% (9/353), consisting of six males and three females. Among the different ethnicities, Tamil had the highest infection rate (10.5%; 2/25) compared to Muslim (4.0%, 1/19) and Sinhalese (1.9%, 6/309).
Table 1.
H. pylori infection rate by histology and culture.
| Subject Characteristics | Total Subjects | Positive Result (%) | ||
|---|---|---|---|---|
| Culture | Histology | Culture and Histology | ||
| n test positive | 353 | 6 (1.6) | 7 (1.9) | 9 (2.5) |
| Sex | ||||
| Male | 189 | 4 (2.1) | 5 (2.6) | 6 (3.2) |
| Female | 164 | 2 (1.2) | 2 (1.2) | 3 (1.8) |
| p-value | 0.68 | 0.46 | 0.51 | |
| Ethnicity | ||||
| Sinhalese | 309 | 4 (1.3) | 5 (1.6) | 6 (1.9) |
| Muslim | 25 | 1 (4.0) | 1 (4.0) | 1 (4.0) |
| Tamil | 19 | 1 (5.2) | 1 (5.2) | 2 (10.5) |
| p-value | 0.16 | 0.21 | 0.06 | |
3.2. Determination of H. pylori Infection Status by Anti-H. pylori IgG
The isolation of H. pylori bacteria in culture is a definitive approach showing current infection. However, the fastidious nature of H. pylori culture limits its feasibility in daily clinical practice. Histology evaluation showed 99.42% specificity, 57.14% sensitivity, and 98.58% accuracy in detecting H. pylori with the culture method as a reference.
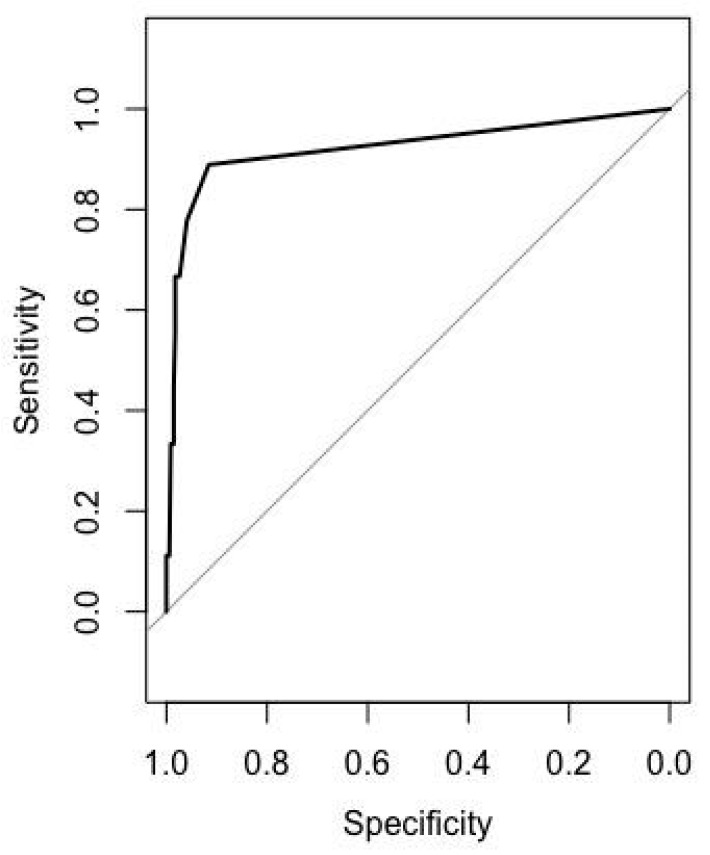
The use of anti-H. pylori IgG in the serum to detect H. pylori infection has not been validated in Sri Lanka. Among all the samples, there was no significant correlation between H. pylori IgG status and age (p = 0.52, r = −0.03). The value of H. pylori IgG was significantly higher in H. pylori-positive patients (median, 6; IQR, 8.5; median, 2.9; IQR, 0.0; p value < 0.001). When the H. pylori IgG levels were plotted into the ROC (receiver operating characteristic) curve with the culture and histology results as the reference, the AUC was 0.922 (Figure 1). In the ABC method, the cut-off value for H. pylori infection was 10.0 U/mL. However, when the cut-off was applied to the Sri Lankan population, the sensitivity was poor (33.33%), even though the specificity was 98.27%. Therefore, in the process of setting a new cut-off for H. pylori infection, we achieved the best sensitivity and specificity (91.6% and 88.9%, respectively) at a value of 2.95 U/mL. The positive likelihood ratio, negative likelihood ratio, positive predictive value, and negative predictive value of the new cut-off were 18.07, 0.17, 23.7, and 99.7%, respectively. The seroprevalence of H. pylori IgG (2.95 U/mL) was 6.2% (22/353).
Figure 1.
ROC curve of the H. pylori IgG to detect H. pylori infection.
3.3. Endoscopy Diagnosis and Histology Examination
Upper endoscopy revealed gastric ulcers and duodenal ulcers in 7.4% (26/353) and 1.1% (4/353) of the subjects, respectively. Gastritis (65.7%; 232/353) and normal-appearing mucosa on endoscopy (25.8%; 91/353) were predominant in this study. However, among the gastric ulcer patients, only 19.2% (5/26) were infected with H. pylori, and in the duodenal ulcer patients, only 25% (1/4) were infected.
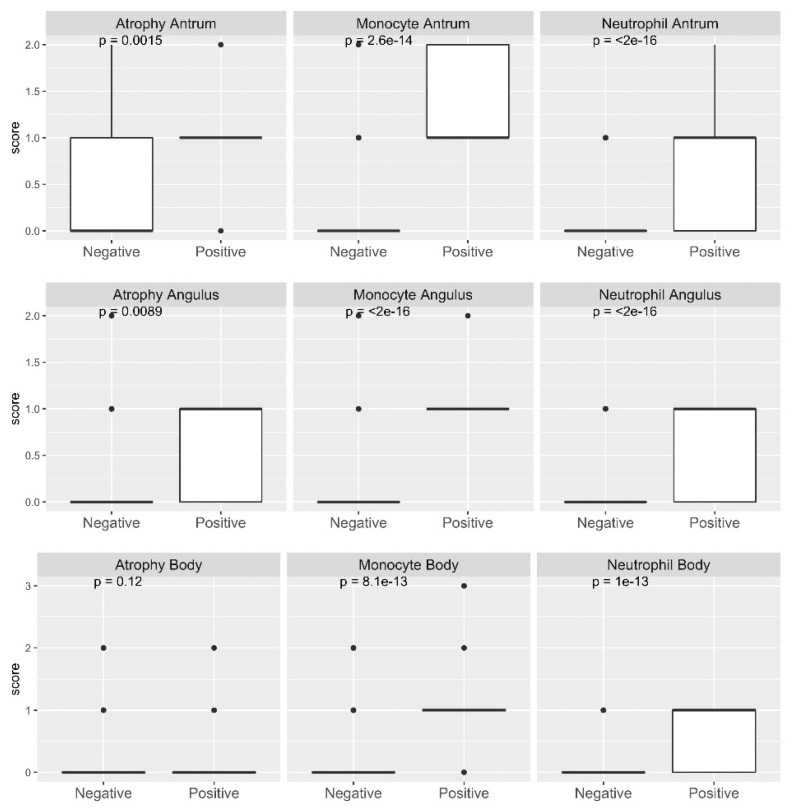
From the histological examination, the score of the severity of inflammation was based on the presence of neutrophils, monocytes, atrophy, and intestinal metaplasia in the antrum, corpus, and angle (Figure 2). The neutrophil and monocyte scores in all locations ranged from 0 to 3, but the scores for atrophy and metaplasia ranged from 0 to 2; there were no patients with severe atrophy and/or metaplasia. The median value and difference among H. pylori infection status based on the serology test (H. pylori-positive patients = 22) are shown in Figure 2. H. pylori infection significantly increased the scores of neutrophils, monocytes, atrophy, and metaplasia. The scores obtained by histology were also used to classify the subjects into five categories according to their severity. Among the 94.8% (331/353) of the H. pylori-negative subjects, the bacteria could not be detected by histology and culture in four subjects, but the severity of gastric mucosal inflammation showed moderate atrophy or metaplasia (Table 2). This phenomenon could have occurred because of past infection.
Figure 2.
Distribution of histology assessment results among H. pylori-positive and -negative samples. A statistically significant difference in neutrophil, monocyte, and atrophy scores between the H. pylori-positive and -negative subjects was observed in the antrum, angulus, and body (p < 0.001) according to at least one positive result from several diagnostic methods. Only one subject was reported to have a metaplasia.
Table 2.
Gastric mucosal status of patients.
| Histology Culture-Negative | Histology Culture-Positive | |||
|---|---|---|---|---|
| H. pylori-IgG Positive | H. pylori-IgG Negative | H. pylori-IgG Positive | H. pylori-IgG Negative | |
| Normal | 2 | 196 | 0 | 0 |
| Chronic gastritis | 1 | 20 | 1 | 0 |
| Mild atrophy | 7 | 108 | 5 | 1 |
| Moderate atrophy | 2 | 3 | 0 | 1 |
| Metaplasia | 2 | 3 | 1 | 0 |
| Total | 14 | 330 | 7 | 2 |
3.4. Analysis of Serum PG and Gastric Cancer Risk Determination by the ABC Method
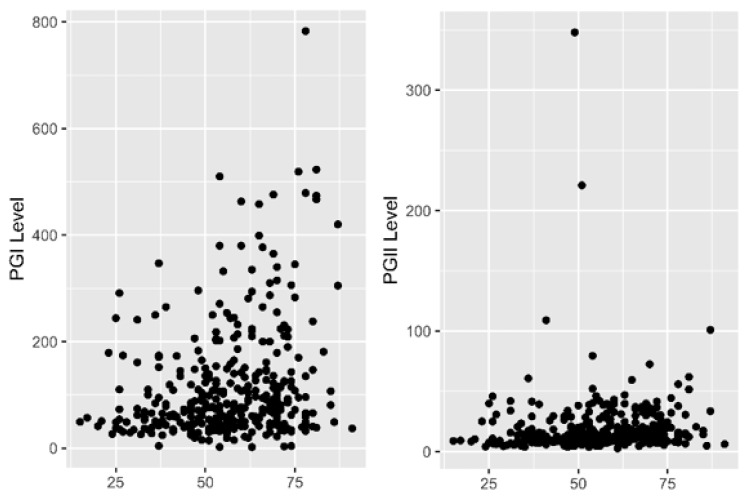
PG levels from the serum could reflect the gastric mucosa status, indicating whether patients have chronic inflammation atrophy or metaplasia. It is also known as a non-invasive approach for identifying the risk of gastric cancer development. Hence, PGI and PGII levels were measured in 353 subjects. The average PGI levels are shown in Table 3. The PGI and PGII levels were significantly higher in male subjects than in female subjects (p = 0.002 and p = 0.009, respectively). For H. pylori infection status, the PGI/PGII ratio was significantly lower in H. pylori-positive patients than in H. pylori-negative patients (p < 0.001) (Table 3). The PGI level and PGI/PGII ratio were positively correlated with age, although the relationship was weak (p < 0.0001 and 0.003; r = 0.29 and 0.17, respectively) (Figure 3).
Table 3.
Pepsinogen level by H. pylori infection status and gender.
| PGI | PGII | PGI/PGII | |
|---|---|---|---|
| All sample summary | |||
| Median | 77.9 | 12.2 | 6.3 |
| Min | 2 | 2.6 | 0.1 |
| Max | 783 | 348 | 25 |
| IQR | 98.5 | 12.4 | 2.8 |
|
H. pylori infection status (median; IQR) |
|||
| Positive (n = 23) * | 69.9; 74.1 | 16.9; 17.5 | 4.1; 1.8 |
| Negative (n = 339) | 78.5; 99.1 | 12; 12.05 | 6.3; 2.9 |
| p-value | 0.31 | 0.19 | <0.001 |
| Gender (median; IQR) | |||
| Male (n = 189) | 92.8; 109.8 | 13.7; 16.7 | 6.3; 2.7 |
| Female (n = 164) | 68.2; 111.3 | 11.2; 18.3 | 6.1; 7.7 |
| p-value | 0.0018 | 0.0089 | 0.28 |
* H. pylori-positive according to at least one positive result (histology, culture, or serology).
Figure 3.
Correlation of pepsinogen (PG) with age. X-axis represents the age and Y-axis represents levels. PGI level and PGI/PGII ratio were weakly correlated with age. PGII was not significantly correlated with age.
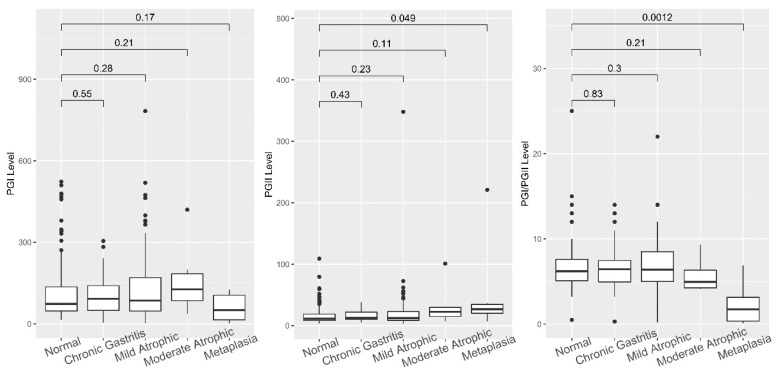
The PG level was analyzed among the different gastric mucosa statuses according to histological evaluation. The subjects were then classified into five groups according to their scores, as shown in Figure 4. PGII increased with the deterioration of mucosa (p = 0.043, r = 0.11) and significantly increased in the presence of metaplasia compared to normal tissue (p = 0.049). Unlike PGII, the PGI and PGI/PGII ratio did not have a significant correlation with the severity of mucosa deterioration (p = 0.3, r = 0.05, and p = 0.84, r = −0.01, respectively). In contrast, the PGI/II ratio in the metaplasia group was significantly lower than that of the normal group (p = 0.0012).
Figure 4.
PGI, PGII, and PGI/PGII ratio among the different histology status groups. The PGI levels among groups were not significantly different. However, a significant difference in PGII and PGI/PGII ratio was observed between the normal and metaplasia groups.
3.5. Diagnostic Value of Pepsinogen to Discriminate Gastric Mucosa Status
The ABC method utilizes a cut-off value for PGI of 10.0 and a PGI/PGII ratio of greater than or equal to 3. This cut-off supposedly discriminates early gastric cancer. To clarify the histological stage of early gastric cancer that can be distinguished by PGI and PGI/PGII ratio according to the criteria by Miki [30], we evaluated the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio with the gastric mucosa histology status as the reference. A new cut-off value was then identified to improve the diagnostic performance of PG to predict histological status.
According to the criteria stated by Miki et al., PGI positivity was defined as surpassing 70 U/mL with a PGI/PGII ratio > 3.0. The sensitivity and specificity analysis of PGI and PGI/PGII ratio to predict the presence of chronic gastritis, mild atrophy, moderate atrophy, and metaplasia are shown in Table 4. Using this cut-off, the specificity of the PGI/PGII ratio to determine chronic gastritis and mild and moderate atrophy was excellent (99.5%, 99.1%, and 97.07%, respectively), but the sensitivity was extremely poor (8.38%, 9.02%, and 33.3%, respectively). Therefore, the accuracy was low. However, the performance of the PGI/PGII ratio was improved when it was applied to discriminate the presence of metaplasia with a sensitivity of 66.67%, specificity of 97.12%, and accuracy of 96.6%.
Table 4.
Diagnostic value of the Miki criteria to predict gastric mucosa status.
| Parameters | Chronic Gastritis | Mild Atrophy | Moderate Atrophy | Metaplasia | ||||
|---|---|---|---|---|---|---|---|---|
| PGI | PGI/PGII Ratio | PGI | PGI/PGII Ratio | PGI | PGI/PGII Ratio | PGI | PGI/PGII Ratio | |
| True positive | 64 | 13 | 56 | 12 | 4 | 4 | 3 | 4 |
| True negative | 104 | 197 | 118 | 218 | 187 | 331 | 192 | 337 |
| False positive | 94 | 1 | 102 | 2 | 154 | 10 | 155 | 10 |
| False negative | 91 | 142 | 77 | 121 | 8 | 8 | 3 | 2 |
| Sensitivity (%) | 41.29 | 8.38 | 42.11 | 9.02 | 33.33 | 33.33 | 50 | 66.67 |
| Specificity (%) | 52.53 | 99.5 | 53.64 | 99.1 | 54.84 | 97.07 | 55.33 | 97.12 |
| Positive likelihood | 0.89 | 16.61 | 0.91 | 9.93 | 0.74 | 11.37 | 1.12 | 23.13 |
| Negative likelihood | 1.18 | 0.93 | 1.08 | 0.92 | 1.21 | 0.69 | 0.9 | 0.3432 |
| PPV | 40.5 | 92.85 | 49.39 | 85.73 | 2.52 | 28.51 | 1.08 | 28.45 |
| NPV | 53.34 | 58.12 | 46.3 | 64.28 | 95.9 | 97.65 | 98.47 | 99.41 |
| Accuracy | 47.59 | 59.49 | 49.29 | 65.16 | 54.11 | 94.9 | 55.24 | 96.6 |
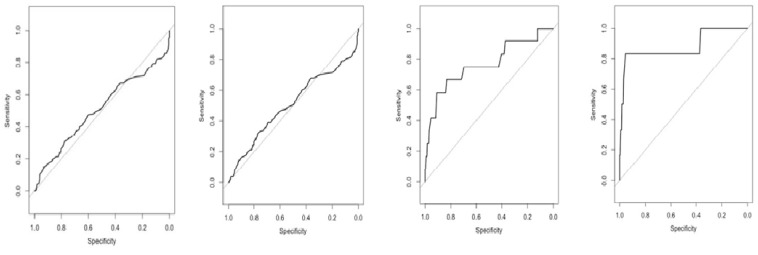
To further define the diagnostic values of PGI, PGII, and the PGI/PGII ratio that are more suitable for application in the Sri Lankan population, we performed ROC curve analysis and determined the new cut-off value by Youden’s index. As depicted in Table 5 and Figure 5, the ROC analysis of PGI, PGII, and the PGI/PGII ratio showed poor discrimination for chronic gastritis and mild atrophy (AUC around 0.5). However, the AUC of PGII and the PGI/PGII ratio to discriminate moderate atrophy increased to 0.70 and 0.76, respectively. The highest AUC was obtained when the PGI, PGII, and PGI/PGII ratio were used to determine metaplasia (0.68, 0.72, and 0.88, respectively). The new cut-off value could improve the sensitivity of PGI, PGII, and the PGI/PGII ratio to determine chronic gastritis, mild and moderate atrophy, and metaplasia. The PGI/PGII ratio is the best candidate for determining moderate atrophy and metaplasia (specificity 83.28% and 95.39%, sensitivity 66.67% and 83.33%, respectively).
Table 5.
Analysis of ROC and cut-off value.
| AUC | New Cut-Off | Specificity (%) | Sensitivity (%) | Control | Case | p-Value | |
|---|---|---|---|---|---|---|---|
| Chronic gastritis | |||||||
| PGI | 0.5335 | 92.45 | 62.12 | 48.38 | 198 | 155 | 0.28 |
| PGII | 0.5552 | 17.45 | 71.22 | 40.64 | 0.07 | ||
| PGI/PGII ratio | 0.5081 | 9.75 | 93.43 | 14.83 | 0.79 | ||
| Mild atrophy | |||||||
| PGI | 0.529 | 92.45 | 60.91 | 48.12 | 220 | 133 | 0.36 |
| PGII | 0.5517 | 17.45 | 71.82 | 42.11 | 0.1 | ||
| PGI/PGII ratio | 0.5055 | 7.65 | 76.36 | 33.08 | 0.86 | ||
| Moderate atrophy | |||||||
| PGI | 0.4785 | 111 | 66.28 | 50 | 341 | 12 | 0.8 |
| PGII | 0.6987 | 14.55 | 60.12 | 83.33 | 0.019 | ||
| PGI/PGII ratio | 0.7652 | 4.75 | 83.28 | 66.67 | 0.0017 | ||
| Metaplasia | |||||||
| PGI | 0.6804 | 15.15 | 98.84 | 50 | 347 | 6 | 0.13 |
| PGII | 0.7207 | 18.15 | 69.16 | 83.33 | 0.064 | ||
| PGI/PGII ratio | 0.8804 | 3.25 | 95.39 | 83.33 | 0.0014 |
Figure 5.
ROC curve of PGI/PGII ratio. From left to right, AUC curve analysis of PGI/PGII ratio for chronic gastritis, mild atrophy, moderate atrophy, and metaplasia. The highest AUC was 0.8804 to discriminate the presence of metaplasia.
3.6. Diagnostic Value of PG to Discriminate Gastric Mucosa Status
We evaluated the use of PG to predict and differentiate gastric mucosa status. Using the criteria reported by Miki that have been widely used in Japan by using the data of PGI, PGI/PGII ratio, and H. pylori infection status by anti-H. pylori IgG, we classified the subjects into groups A to D, as shown in Table 5. The majority of the subjects (94.9%; 335/353) belonged to group A, which had the lowest risk of gastric cancer. Groups B, C, and D comprised 2.0% (7/353), 0.2% (1/353), and 2.8% (10/353) of patients, respectively. From this result, in group A, the normal histology was predominant (58.8%, 197/335), although this group also had a high proportion of mild atrophy (33.1%, 111/335). Among all groups, group D had the highest risk of requiring intervention and surveillance.
As shown in Table 6, the modification of the criteria could capture more moderate atrophy and metaplasia in groups B and C. The moderate atrophy and metaplasia required follow-up to diagnose the development of gastric cancer early; thus, they should not be misclassified into group A.
Table 6.
Gastric mucosa status of each group using the ABC method with the Miki criteria versus the Sri Lanka-modified criteria.
| ABC Method According to Criteria by Miki, n | |||||
| Total | A | B | C | D | |
| Number in each group | 353 | 335 | 7 | 1 | 10 |
| Histology of gastric tissue | |||||
| Metaplasia | 6 | 2 | 1 | 0 | 3 |
| Moderate atrophic | 6 | 4 | 2 | 0 | 0 |
| Mild atrophic | 121 | 111 | 4 | 1 | 5 |
| Chronic gastritis | 22 | 21 | 0 | 0 | 1 |
| Normal | 198 | 197 | 0 | 0 | 1 |
| Sri Lanka-modified criteria, n | |||||
| Total | A | B | C | D | |
| Number in each group | 353 | 323 | 19 | 3 | 8 |
| Histology of gastric tissue | |||||
| Metaplasia | 6 | 1 | 2 | 1 | 2 |
| Moderate atrophic | 6 | 4 | 2 | 0 | 0 |
| Mild atrophic | 121 | 104 | 11 | 2 | 4 |
| Chronic gastritis | 22 | 19 | 2 | 0 | 1 |
| Normal | 198 | 195 | 2 | 0 | 1 |
4. Discussion
This study included the largest number of subjects among the studies performed in Sri Lanka. According to the histology and culture results, the prevalence of H. pylori infection was only 1.69% (7/353). A previous study reported the prevalence in Sri Lanka using the stool antigen test and PCR [9,11,12]. A recent systematic review that compared the accuracy of diagnostic tests ranked the quality according to the superiority index range from 2.17 to 9.94 [13]. For the PCR and stool antigen, the highest superiority index was only 4.99 for the PCR and 3.87 for the stool antigen. The superiority index was lower than that of histology, culture, and serology (7.51, 7.35, and 7.81, respectively). Another study also mentioned that culture was considered to have 100% specificity with a specificity of approximately 85% despite the fastidious nature of H. pylori growth [36]. In the absence of a gold standard, a combination of different methods can be used to detect H. pylori to compensate for the sensitivity and specificity. This study attempted to confirm these results using several diagnostic methods. The first step is to validate the serology test using IgG on the basis of histology and culture.
Our results showed that H. pylori infection was poorly detected using anti-H. pylori IgG with a cut-off of 10 U/mL, as determined by the manufacturer’s instructions [30,37,38]. These criteria have been used in Japan for screening [29]. However, in Sri Lanka, these criteria showed a poor sensitivity (33.3%); thus, a new cut-off of 2.95 U/mL was determined, which improved the sensitivity, specificity, and accuracy. Using the new cut-off, the prevalence of H. pylori according to serology was 6.2% (22/353). Among serology-positive subjects, H. pylori in four subjects could not be detected by histology and culture, but histological examination showed moderate atrophy and metaplasia. This may indicate past infections [39]. A previous study reported that in patients with a high titer of anti-H. pylori IgG, 17.5% of them were previously infected, and the infection had been eradicated [40]. In addition, the current cohort study showed that IgG titers between 3 and 10 U/mL also indicated a higher risk for gastric cancer [40].
Sri Lanka is isolated by the ocean from the mainland of other South Asian countries. As recorded in this study, the subjects predominantly belonged to the native Sinhalese ethnic group. Despite the high prevalence of H. pylori infection in other South Asian countries such as India (81%), Nepal (28.9–38.4%), and Bangladesh (42.1%), Sri Lanka had an extremely low prevalence of H. pylori infection [41,42,43]. This result provides evidence that H. pylori prevalence could vary according to geographical location, indicating the strong roles played by the host and environment in the susceptibility to infection. Only a few countries have reported extremely low prevalence of H. pylori. Malaysia and some regions in Indonesia have also been reported as low-prevalence countries with low gastric cancer risk [34,44,45]. Hence, the low prevalence of H. pylori infection could be attributed to the low age standardized rate of gastric cancer in Sri Lanka.
Histological examination revealed a significantly higher inflammatory and atrophy status in H. pylori-positive cases than in H. pylori-negative cases. Gastric atrophy is the heavy deterioration of gland function in the antrum or corpus, which soon turns into metaplasia [46,47]. Even though the prevalence is considered low, the detection of H. pylori infection must not be neglected in chronic gastritis cases, and it requires immediate eradication [48]. Despite the low prevalence of H. pylori infection, histological evaluation in Sri Lanka showed a high proportion of mild atrophy (34.3%, 121/323) and 1.7% (6/353) moderate atrophy. Among the gastric and duodenal ulcer groups, the proportion of patients infected with H. pylori was also low. These phenomena could be related to other causes, such as consumption of NSAIDs, use of proton pump inhibitors, autoimmune gastritis, and alteration of microbiome composition in the stomach [22,49,50].
For the examination of the gastric mucosa status, assessment of biomarkers such as PG could be an alternative to the histology method [51]. Although the PG value showed a positive correlation with age, the coefficient was low, as mentioned in several previous studies [52,53]. The higher level of PG in H. pylori-positive patients due to inflammation reaction is concordant with previous studies [27,54]. From the analysis of PG considering the inflammation severity of the gastric mucosa, PGI levels significantly decreased in the presence of metaplasia, while PGII levels increased. This phenomenon results in a significantly low PG/PGII ratio [55,56]. From the ROC analysis, the PGI level appeared to have the highest discriminatory ability in determining metaplasia among all gastritis statuses (Table 5). It could be a valuable diagnostic method in the absence of endoscopy facilities, as well as an indicator of high risk and therefore contraindication for endoscopy.
The combination of H. pylori status and PGs (known as the ABC method) has been applied in Japan and other countries as a screening method for gastric cancer [30]. The ABC method has criteria that were supported by a cohort study [40,57]. However, the use of a value of 10 U/mL as the cut-off seems to omit patients with moderate atrophy and severe metaplasia. Severe metaplasia should be followed by endoscopy [30]. Utilizing the best cut-off for the PGI/PGII ratio improved the specificity, sensitivity, and accuracy. The PGI/PGII ratio is recommended for use because it has the highest diagnostic performance compared to PGI and PGII alone. According to the carcinogenesis cascade, atrophy is considered a reversible state, while metaplasia has little chance of returning to normal [46,58]. Early detection by screening is an effective method for preventing cancer [48]. Hence, for gastric cancer screening purposes, the earlier the precursor of the disease is detected, the more beneficial it is for patients [59]. As shown in Table 5 and Figure 3, the highest AUC was achieved in metaplasia followed by moderate atrophy. PGI/PGII can also detect moderate atrophy with 83.3% specificity and 66.7% sensitivity. This result suggests the potential implementation of the PG test for detection of H. pylori infection and early inflammation of the gastric mucosa in Sri Lanka [60].
5. Conclusions
This study revealed the extremely low prevalence of H. pylori infection in Sri Lanka according to three diagnostic methods. However, infection still causes significant damage to the stomach histopathology; thus, effective diagnosis and eradication of H. pylori remains a necessity. In this setting, serology IgG proved its diagnostic value in detecting H. pylori. PG could also be an effective alternative marker to determine gastric mucosa status, especially metaplasia, along with H. pylori IgG.
Author Contributions
Conceptualization, D.D., K.A.F., J.R., M.D.L., T.M. (Takeshi Matsuhisa), and Y.Y.; data curation, V.P.T., J.A. and T.M. (Takashi Matsumoto); formal analysis, K.A.F., J.A., T.M. (Takashi Matsumoto), and T.U.; funding acquisition, T.M. (Takashi Matsumoto) and Y.Y.; investigation, J.R., M.D.L., L.A.W., V.P.T., A.D., E.T.K., B.H.P., S.A. and T.U.; methodology, L.A.W., V.P.T., A.D., E.T.K., B.H.P. and T.U.; resources, J.R., M.D.L. and T.M. (Takeshi Matsuhisa); supervision, Y.Y.; validation, J.A.; writing—original draft, D.D., K.A.F., J.R. and Y.Y.; writing—review and editing, K.A.F., T.M. (Takeshi Matsuhisa) and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by grants from the National Institutes of Health (DK62813) (YY) and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (221S0002, 16H06279, 18KK0266, 19H03473) (Y.Y.), 18K16182 (T.M.), and 17K09353 (J.A.). This work was also supported by the Japan Society for the Promotion of Science Institutional Program for Young Researcher Overseas Visits and the Strategic Funds for the Promotion of Science and Technology Agency (JST) for Y.Y., D.D., E.T.K., A.D., K.A.F. and B.H.P. are Ph.D. students supported by the Japanese Government (MEXT) scholarship programs for 2016 and 2017, respectively.
Institutional Review Board Statement
Study approval was obtained from the ethics committees of the University of Peradeniya, Sri Lanka (2017/EC/91, November 11th 2017), and Oita University Faculty of Medicine, Japan (P-12-10, January 18th 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marshall B., Warren J. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;323:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Yamaoka Y., Ojo O., Fujimoto S., Odenbreit S., Haas R., Gutierrez O., El-Zimaity H.M.T., Reddy R., Arnqvist A., Graham D.Y. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006;55:775–781. doi: 10.1136/gut.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa P., Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Bayerdörffer E., Rudolph B., Neubauer A., Thiede C., Lehn N., Eidt S., Stolte M. MALT Lyphoma Study Group Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. Lancet. 1995;345:1591–1594. doi: 10.1016/S0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 5.Zamani M., Ebrahimtabar F., Zamani V., Miller W., Alizadeh-Navaei R., Shokri-Shirvani J., Derakhshan M. System-atic review with meta–analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018;47:868–876. doi: 10.1111/apt.14561. [DOI] [PubMed] [Google Scholar]
- 6.Satpathi P., Satpathi S., Mohanty S., Mishra S.K., Behera P.K., Maity A.B. Helicobacter pylori infection in dyspeptic patients in an industrial belt of India. Trop. Dr. 2016;47:2–6. doi: 10.1177/0049475515626033. [DOI] [PubMed] [Google Scholar]
- 7.Vilaichone R.-K., Mahachai V., Shiota S., Uchida T., Ratanachu-Ek T., Tshering L., Tung N.L., Fujioka T., Moriyama M., Yamaoka Y. Extremely high prevalence of Helicobacter pyloriinfection in Bhutan. World J. Gastroenterol. 2013;19:2806–2810. doi: 10.3748/wjg.v19.i18.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aftab H., Miftahussurur M., Subsomwong P., Ahmed F., Khan A.K.A., Matsumoto T., Suzuki R., Yamaoka Y. Two pop-ulations of less-virulent Helicobacter pylori genotypes in Bangladesh. PLoS ONE. 2017;12:e0182947. doi: 10.1371/journal.pone.0182947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernando N., Holton J., Vaira D., DeSilva M. Prevalence of Helicobacter pylori in Sri Lanka as Determined by PCR. J. Clin. Microbiol. 2002;40:2675–2676. doi: 10.1128/JCM.40.7.2675-2676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . 2019 Health SDG Profile: Sri Lanka. WHO; Geneva, Switzerland: 2019. [Google Scholar]
- 11.Fernando N., Perera N., Vaira D., Holton J. Helicobacter pylori in School Children From the Western Province of Sri Lanka. Helicobacter. 2001;6:169–174. doi: 10.1046/j.1523-5378.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 12.Ubhayawardana N., Weerasekera M., Weerasekera D., Samarasinghe K., Gunasekera C., Fernando N. Detection of clar-ithromycin-resistant Helicobacter pylori strains in a dyspeptic patient population in Sri Lanka by polymerase chain reac-tion-restriction fragment length polymorphism. Indian J. Med. Microbiol. 2015;33:374–377. doi: 10.4103/0255-0857.158557. [DOI] [PubMed] [Google Scholar]
- 13.Vörhendi N., Soós A., Engh M.A., Tinusz B., Szakács Z., Pécsi D., Mikó A., Sarlós P., Hegyi P., Eröss B. Accuracy of theHelicobacter pyloridiagnostic tests in patients with peptic ulcer bleeding: A systematic review and network meta-analysis. Ther. Adv. Gastroenterol. 2020;13 doi: 10.1177/1756284820965324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas-Rengifo D.F., Mendoza B., Jaramillo C., Rodríguez-Urrego P., Vera-Chamorro J.F., Alvarez J., Delgado M.D.P., Jimenez-Soto L.F. Helicobacter pylori culture as a key tool for diagnosis in Colombia. J. Infect. Dev. Ctries. 2019;13:720–726. doi: 10.3855/jidc.10720. [DOI] [PubMed] [Google Scholar]
- 15.Bessède E., Arantes V., Mégraud F., Coelho L.G. Diagnosis of Helicobacter pylori infection. Helicobacter. 2017;22:e12404. doi: 10.1111/hel.12404. [DOI] [PubMed] [Google Scholar]
- 16.Shiota S., Yamaoka Y. Biomarkers forHelicobacter pyloriinfection and gastroduodenal diseases. Biomark. Med. 2014;8:1127–1137. doi: 10.2217/bmm.14.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch D., Krumm N., Wener M.H., Yeh M.M., Truong C.D., Reddi D.M., Liu Y., Swanson P., Schmidt R.A., Bryan A. Serology Is More Sensitive Than Urea Breath Test or Stool Antigen for the Initial Diagnosis of Helicobacter pylori Gastritis When Compared with Histopathology. Am. J. Clin. Pathol. 2020;154:255–265. doi: 10.1093/ajcp/aqaa043. [DOI] [PubMed] [Google Scholar]
- 18.Hamashima C., for the JPHC Study Group. Sasazuki S., Inoue M., Tsugane S. Receiver operating characteristic analysis of prediction for gastric cancer development using serum pepsinogen and Helicobacter pylori antibody tests. BMC Cancer. 2017;17:1–9. doi: 10.1186/s12885-017-3173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skrebinska S., Daugule I., Santare D., Isajevs S., Liepniece-Karele I., Rudzite D., Kikuste I., Vanags A., Tolmanis I., Atstupens J., et al. Accuracy of two plasma antibody tests and faecal antigen test for non-invasive detection of H. pylori in middle-aged Caucasian general population sample. Scand. J. Gastroenterol. 2018;53:777–783. doi: 10.1080/00365521.2018.1476909. [DOI] [PubMed] [Google Scholar]
- 20.Miftahussurur M., Yamaoka Y. Diagnostic Methods ofHelicobacter pyloriInfection for Epidemiological Studies: Critical Im-portance of Indirect Test Validation. BioMed Res. Int. 2016;2016:1–14. doi: 10.1155/2016/4819423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim N., Jung H.C. The Role of Serum Pepsinogen in the Detection of Gastric Cancer. Gut Liver. 2010;4:307–319. doi: 10.5009/gnl.2010.4.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiota S., Thrift A.P., Green L., Shah R., Verstovsek G., Rugge M., Graham D.Y., El-Serag H.B. Clinical manifesta-tions of Helicobacter pylori–negative gastritis. Clin. Gastroenterol. Hepatol. 2017;15:1037–1046. doi: 10.1016/j.cgh.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Ichinose M., Miki K., Wong R.N.S., Tatematsu M., Furihata C., Konishi T., Matsushima M., Tanji M., Sano J., Kurokawa K., et al. Methylation and Expression of Human Pepsinogen Genes in Normal Tissues and Their Alteration in Stomach Cancer. Jpn. J. Cancer Res. 1991;82:686–692. doi: 10.1111/j.1349-7006.1991.tb01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong Y., Wu Y., Song Z., Yu Y., Yu X. The potential value of serum pepsinogen for the diagnosis of atrophic gastritis among the health check-up populations in China: A diagnostic clinical research. BMC Gastroenterol. 2017;17:88. doi: 10.1186/s12876-017-0641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watabe H., Mitsushima T., Yamaji Y., Okamoto M., Wada R., Kokubo T., Doi H., Yoshida H., Kawabe T., Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen sta-tus: A prospective endoscopic cohort study. Gut. 2005;54:764–768. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu H.K., Park J.W., Lee K.H., Jeon C.H., Lee H.J., Chae H.D. The Role of Serum Pepsinogen in Detection of Gastric Cancer. J. Korean Gastric Cancer Assoc. 2009;9:167. doi: 10.5230/jkgca.2009.9.4.167. [DOI] [Google Scholar]
- 27.Kikuchi S., Kato M., Mabe K., Kawai T., Furuta T., Inoue K., Ito M., Yoshihara M., Kodama M., Murakami K. Optimal Criteria and Diagnostic Ability of Serum Pepsinogen Values for Helicobacter pylori Infection. J. Epidemiol. 2019;29:147–154. doi: 10.2188/jea.JE20170094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X.-Z., Huang C.-Z., Hu W.-X., Liu Y., Yao X.-Q. Gastric Cancer Screening by Combined Determination of Serum Helicobacter pylori Antibody and Pepsinogen Concentrations. Chin. Med. J. 2018;131:1232–1239. doi: 10.4103/0366-6999.231512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi Y., Nagata Y., Hiratsuka R., Kawase Y., Tominaga T., Takeuchi S., Sakagami S., Ishida S. Gastric Cancer Screening by Combined Assay for Serum Anti-Helicobacter pylori IgG Antibody and Serum Pepsinogen Levels-The ABC Method. Digestion. 2016;93:13–18. doi: 10.1159/000441742. [DOI] [PubMed] [Google Scholar]
- 30.Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels-“ABC method”. Proc. Jpn. Acad. Ser. B. 2011;87:405–414. doi: 10.2183/pjab.87.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broutet N., the Eurohepygast Study Group. Plebani M., Sakarovitch C., Sipponen P., Megraud F. Pepsinogen A, pep-sinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br. J. Cancer. 2003;88:1239–1247. doi: 10.1038/sj.bjc.6600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahey M., Hamada G.S., Nishimoto I.N., Kowalski L.P., Iriya K., Gama-Rodrigues J.J., Tsugane S. Ethnic differences in serum pepsinogen levels among Japanese and non-Japanese Brazilian gastric cancer patients and controls. Cancer Detect. Prev. 2000;24:564–571. [PubMed] [Google Scholar]
- 33.Ang T.L., Fock K.M., Dhamodaran S., Teo E.K., Tan J. Racial differences in Helicobacter pylori, serum pepsinogen and gastric cancer incidence in an urban Asian population. J. Gastroenterol. Hepatol. 2005;20:1603–1609. doi: 10.1111/j.1440-1746.2005.03898.x. [DOI] [PubMed] [Google Scholar]
- 34.Syam A.F., Miftahussurur M., Makmun D., Nusi I.A., Zain L.H., Zulkhairi, Akil F., Uswan W.B., Simanjuntak D., Uchida T., et al. Risk Factors and Prevalence of Helicobacter pylori in Five Largest Islands of Indonesia: A Preliminary Study. PLoS ONE. 2015;10:e0140186. doi: 10.1371/journal.pone.0140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stolte M. and A. Meining, The updated Sydney system: Classification and grading of gastritis as the basis of diagnosis and treatment. Can. J. Gastroenterol. 2001;15:591–598. doi: 10.1155/2001/367832. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y.K., Kuo F.C., Liu C.J., Wu M.C., Shih H.Y., Wang S.S., Wu J.Y., Kuo C.H., Huang Y.K., Wu D.C. Diagnosis of Helicobacter pylori infection: Current options and developments. World J. Gastroenterol. 2015;28:11221–11235. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miki K., Ichinose M., Ishikawa K.B., Yahagi N., Matsushima M., Kakei N., Tsukada S., Kido M., Ishihama S., Shimizu Y., et al. Clinical application of serum pepsinogen I and II levels for mass screening to detect gastric cancer. Jpn. J. Cancer Res. 1993;84:1086–1090. doi: 10.1111/j.1349-7006.1993.tb02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miki K., Morita M., Sasajima M., Hoshina R., Kanda E., Urita Y. Usefulness of gastric cancer screening using the serum pepsinogen test method. Am. J. Gastroenterol. 2003;98:735–739. doi: 10.1111/j.1572-0241.2003.07410.x. [DOI] [PubMed] [Google Scholar]
- 39.Inui M., Ohwada S., Ishida F., KUDO S. Serum Helicobacter Pylori IgG Titers are Predictive of H. pylori Infection Status. Showa Univ. J. Med. Sci. 2016;28:233–240. doi: 10.15369/sujms.28.233. [DOI] [Google Scholar]
- 40.Inoue M., Sawada N., Goto A., Shimazu T., Yamaji T., Iwasaki M., Tsugane S., JPHC Study Group High-Negative Anti-Helicobacter pylori IgG Antibody Titers and Long-Term Risk of Gastric Cancer: Results from a Large-Scale Population-Based Cohort Study in Japan. Cancer Epidemiol. Biomark. Prev. 2020;29:420–426. doi: 10.1158/1055-9965.EPI-19-0993. [DOI] [PubMed] [Google Scholar]
- 41.Miftahussurur M., Sharma R.P., Shrestha P.K., Suzuki R., Uchida T., Yamaoka Y. Molecular Epidemiology of Helicobacter pylori Infection in Nepal: Specific Ancestor Root. PLoS ONE. 2015;10:e0134216. doi: 10.1371/journal.pone.0134216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aftab H., Yamaoka Y., Ahmed F., Khan A.A., Subsomwong P., Miftahussurur M., Uchida T., Malaty H.M. Validation of diagnostic tests and epidemiology of Helicobacter pylori infection in Bangladesh. J. Infect. Dev. Ctries. 2018;12:305–312. doi: 10.3855/jidc.9841. [DOI] [PubMed] [Google Scholar]
- 43.Mandal A.K., Kafle P., Puri P., Chaulagai B., Sidhu J.S., Hassan M., Paudel M.S., Kanth R., Gayam V. An association of Helicobacter pylori infection with endoscopic and histological findings in the Nepalese population. J. Fam. Med. Prim. Care. 2019;8:1227–1231. doi: 10.4103/jfmpc.jfmpc_82_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miftahussurur M., Waskito L.A., Syam A.F., Nusi I.A., Wibawa I., Rezkitha Y., Siregar G., Yulizal O.K., Akil F., Uwan W.B., et al. Analysis of risks of gastric cancer by gastric mucosa among Indonesian ethnic groups. PLoS ONE. 2019;14:e0216670. doi: 10.1371/journal.pone.0216670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanafiah A., Binmaeil H., Raja Ali R.A., Mohamed Rose I., Lopes B.S. Molecular characterization and prevalence of antibiotic resistance in Helicobacter pylori isolates in Kuala Lumpur, Malaysia. Infect. Drug Resist. 2019;12:3051–3061. doi: 10.2147/IDR.S219069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correa P., Piazuelo M.B. The gastric precancerous cascade. J. Dig. Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y., Xia R., Zhang B., Li C. Chronic Atrophic Gastritis: A Review. J. Environ. Pathol Toxicol Oncol. 2018;37:241–259. doi: 10.1615/JEnvironPatholToxicolOncol.2018026839. [DOI] [PubMed] [Google Scholar]
- 48.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 49.Gantuya B., El-Serag H.B., Matsumoto T., Ajami N.J., Oyuntsetseg K., Azzaya D., Uchida T., Yamaoka Y. Gastric Microbiota in Helicobacter pylori-Negative and -Positive Gastritis Among High Incidence of Gastric Cancer Area. Cancers. 2019;11:504. doi: 10.3390/cancers11040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miftahussurur M., Waskito L.A. Gastric microbiota and Helicobacter pylori in Indonesian population. Helicobacter. 2020;25:e12695. doi: 10.1111/hel.12695. [DOI] [PubMed] [Google Scholar]
- 51.Leja M., Park J.Y., Murillo R., Liepniece-Karele I., Isajevs S., Kikuste I., Rudzite D., Krike P., Parshutin S., Polaka I., et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: The GISTAR study. BMJ Open. 2017;7:e016999. doi: 10.1136/bmjopen-2017-016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun L.P., Gong Y.H., Wang L., Yuan Y. Serum pepsinogen levels and their influencing factors: A population-based study in 6990 Chinese from North China. World J. Gastroenterol. 2017;13:6562–6567. doi: 10.3748/wjg.v13.i48.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shan J.H., Bai X.J., Han L.L., Yuan Y., Sun X.F. Changes with aging in gastric biomarkers levels and in biochemical factors associated with Helicobacter pylori infection in asymptomatic Chinese population. World J. Gastroenterol. 2017;23:5945–5953. doi: 10.3748/wjg.v23.i32.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang H.X., Pu H., Huh N.H., Yokota K., Oguma K., Namba M. Helicobacter pylori induces pepsinogen secretion by rat gastric cells in culture via a cAMP signal pathway. Int. J. Mol. Med. 2001;7:625–629. doi: 10.3892/ijmm.7.6.625. [DOI] [PubMed] [Google Scholar]
- 55.You W.C., Blot W.J., Zhang L., Kneller R.W., Li J.Y., Jin M.L., Chang Y.S., Zeng X.R., Zhao L., Fraumeni J.F., Jr., et al. Serum pepsinogens in relation to precancerous gastric lesions in a population at high risk for gastric cancer. Cancer Epidemiol. Biomark. Prev. 1993;2:113–117. [PubMed] [Google Scholar]
- 56.Jiang J., Shen S., Dong N., Liu J., Xu Q., Sun L., Yuan Y. Correlation between negative expression of pepsinogen C and a series of phenotypic markers of gastric cancer in different gastric diseases. Cancer Med. 2018;7:4068–4076. doi: 10.1002/cam4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiang T.H., Chiu S.Y., Chen S.L., Yen A.M., Fann J.C., Liu C.Y., Chou C.K., Chiu H.M., Shun C.T., Wu M.S., et al. Serum Pepsinogen as a Predictor for Gastric Cancer Death: A 16-Year Community-based Cohort Study. J. Clin. Gastroenterol. 2019;53:e186–e193. doi: 10.1097/MCG.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 58.Ferreira A.C., Isomoto H., Moriyama M., Fujioka T., Machado J.C., Yamaoka Y. Helicobacter and gastric malignancies. Helicobacter. 2008;13:28–34. doi: 10.1111/j.1523-5378.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taguchi H., Kanmura S., Maeda T., Iwaya H., Arima S., Sasaki F., Nasu Y., Tanoue S., Hashimoto S., Ido A. Helicobacter pylori eradication improves the quality of life regardless of the treatment outcome: A multicenter prospective cohort study. Medicine. 2017;96:e9507. doi: 10.1097/MD.0000000000009507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiso M., Yoshihara M., Ito M., Inoue K., Kato K., Nakajima S., Mabe K., Kobayashi M., Uemura N., Yada T., et al. Characteristics of gastric cancer in negative test of serum anti-Helicobacter pylori antibody and pepsinogen test: A multicenter study. Gastric Cancer. 2017;20:764–771. doi: 10.1007/s10120-016-0682-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.