Abstract
The use of vinyl electrophiles in synthesis has been hampered by the lack of access to a suitable reagent that is practical and of appropriate reactivity. In this work we introduce a vinyl thianthrenium salt as an effective vinylating reagent. The bench-stable, crystalline reagent can be readily prepared from ethylene gas at atmospheric pressure in one step and is broadly useful in the annulation chemistry of (hetero)cycles, N-vinylation of heterocyclic compounds, and palladium-catalyzed cross-coupling reactions. The structural features of the thianthrene core enable a distinct synthesis and reactivity profile, unprecedented for other vinyl sulfonium derivatives.
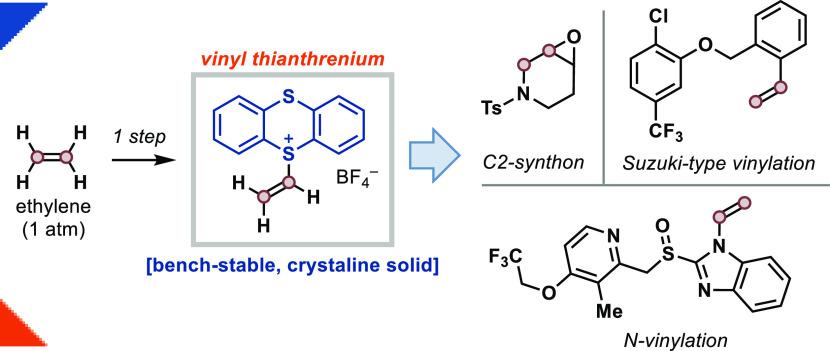
Owing to the rich chemistry of alkenes, the presence of a terminal alkenyl (vinyl, C2H3) substituent enables a myriad of opportunities for diversification and elaboration via dihydroxylation, carbofunctionalization, Heck-type arylation, hydroamination, and metathesis, among others.1−6 However, the introduction of a vinyl group as a C-2 building block is currently difficult, in contrast to the extended use of other substituted alkenyl electrophiles, in view of the lack of suitable reagents with the desired properties and reactivity profile. Here we report the reagent vinyl thianthrenium tetrafluoroborate (vinyl-TT+, 1) that functions as a versatile reagent for different synthetic transformations. Reagent 1 is accessible directly from ethylene (1 atm) in a single step from commercially available material on multigram scale and is a bench-stable, nonhygroscopic solid that can be stored at room temperature in air without signs of decomposition for at least one year. Despite its high stability, 1 displays a rich reactivity profile and has been implemented in several polar and palladium-catalyzed cross-coupling reactions, which differentiates it from all other vinylating reagents reported to date. The unusual direct conversion of ethylene into a versatile building block for organic synthesis sets the approach apart from previous syntheses of alkenylsulfonium salts; in addition, 1 can participate in useful reactions such as a Suzuki cross-coupling that have not been realized with other alkenylthianthrenium salts.
Ethylene is an inexpensive gas (annual production >100 million tons),7 but its use in organic synthesis is rare and typically limited to simple substrates without high levels of complexity.8 One of the main drawbacks of the use of ethylene is the high temperature and pressures that are generally required for its conversion. In fact, reactions engaging ethylene at atmospheric pressure (1 atm) are uncommon and almost exclusive to metal-mediated reactions, owing to the ability of metal centers to activate ethylene via coordination.9−14 Metal-free reactions utilizing ethylene at 1 atm are manily restricted to photochemical cycloadditions with high-energy UV light.15−17 Overall, the general requirement for specialized equipment (high-pressure reactors or UV-photoreactors) has traditionally restricted the use of ethylene as a reagent in organic synthesis involving complex small molecules.
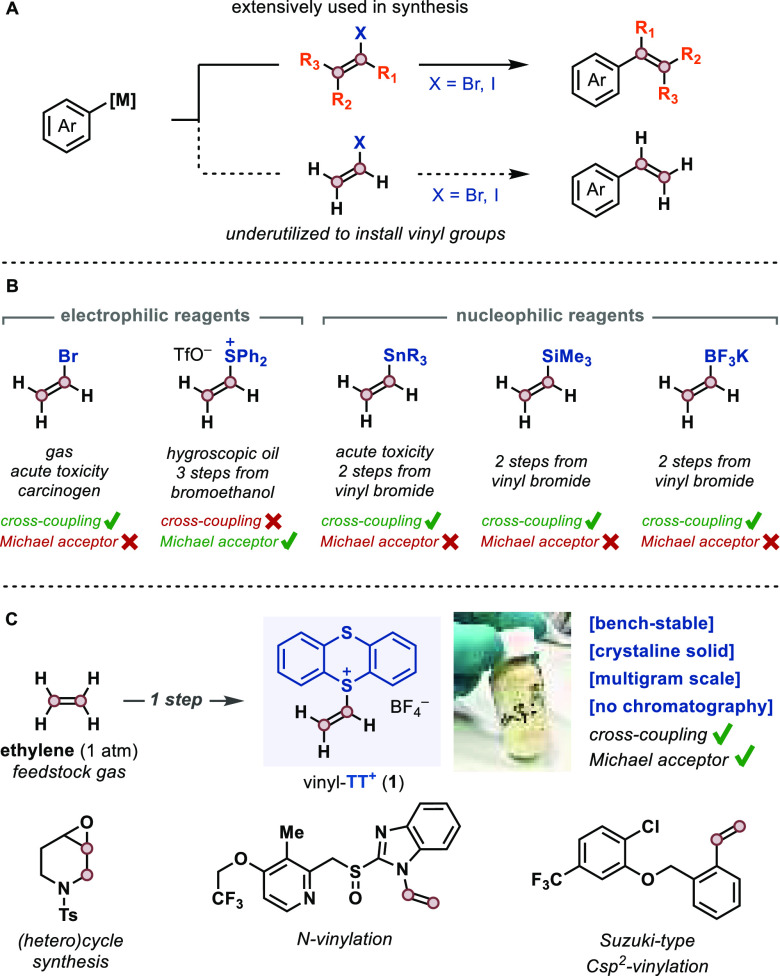
The development of palladium-catalyzed cross-coupling reactions has allowed researchers to reliably construct C–Csp2 bonds (Figure 1A).18,19 However, in contrast to the widespread use of alkenyl (substituted vinyl) derivatives, the use of vinyl halides as electrophiles (e.g., vinyl bromide) is challenging owing to the difficulty of handling the gaseous compounds that are acutely toxic and carcinogenic, which has historically thwarted their utilization in synthesis.20 Alternatively, numerous nucleophilic vinyl-[M] reagents ([M] = SnBu3, SiMe3, B(OR)2, etc.) have been developed over the years,21 but most of them are prepared in several steps from vinyl bromide itself, display high toxicity and low stability, or are poorly reactive (Figure 1B). Moreover, while significant advances have been accomplished with vinyl nucleophiles, the development of electrophilic derivatives that can effectively display the reactivity profile of vinyl halides is significantly less accomplished, and none of them are suitable as Michael acceptors for the direct polar addition of nucleophiles. Jimenez,22 Mukaiyama,23 and Aggarwal24 have developed the use of vinyl diphenylsulfonium salts25 as a 1,2-ethane dication synthon. This hygroscopic oil, prepared in three steps from bromoethanol, displays some practicality issues26 and is often generated in situ from its precursor bromoethyl diphenylsulfonium triflate.27 Over the past two decades, Aggarwal and others have reported a series of elegant transformations applying this reagent to the synthesis of (hetero)cycles.24,28−34 However, neither the reagent nor its precursors have ever been reported as suitable electrophiles in cross-coupling reactions owing to their fundamental reactivity profile (vide infra). In fact, only a few substituted alkenyl sulfonium salts have been successfully engaged in cross-couplings,35−37 but no examples of vinylations have been reported. Our group recently reported the synthesis of alkenyl thianthrenium salts,38 but a general reactivity profile in polar and cross-couling reactions has not been explored yet. Moreover, we were unsuccessful in engaging these salts in efficient couplings with aryl boronic acids via Suzuki-type reactions.
Figure 1.
(A) Use of alkenyl electrophiles in cross-coupling reactions. (B) Commonly used vinylating reagents. (C) Vinyl thianthrenium salt 1 can be accessed directly from ethylene and is a versatile C-2 building block.
We recently aimed to design a strategy to trap ethylene efficiently and convert it into a practical and crystalline reagent (Figure 1C). Ideally, this new species should be stable and easy to handle, while simultaneously exhibiting a rich reactivity profile. We questioned whether vinyl thianthrenium salts (vinyl-TT+) could provide a valuable solution to this task. The perchlorate salt of vinyl-TT+ was published three decades ago while exploring the reactivity of thianthene radical cation (TT•+) with (vinyl)4Sn,39 but only milligram quantities were accessed owing to the involvement of potentially explosive perchlorate salts,40 and its synthetic use has never been reported. While, in comparison with other olefins, ethylene gas typically requires high pressure and an autoclave for cycloaddition reactions,8,41 we sought to capitalize on the high reactivity of the highly electrophilic thianthrenium dication species generated by treatment of thianthrene-S-oxide (2) with activating reagents such as Tf2O (Figure 2A). Following our new protocol, vinyl thianthrenium tetrafluoroborate (1) can now be prepared on multigram scale (50 mmol) with a simple balloon of ethylene (1 atm) in 86% yield. The isolation of 1 as a crystalline solid can be carried out by simple precipitation, to afford an analytically pure compound without the need for further purification. An alternative lab-scale synthetic route from vinyl-SiMe3 (2 equiv) was similarly effective (96% yield, see Supporting Information). The salt 1 is a nonhygroscopic solid that can be stored in the presence of air and moisture without signs of decomposition for at least one year, which makes it practical and easy-to-handle. DSC-TGA reveals that 1 does not decompose at temperatures lower than 280 °C, which underscores a desirable safety profile (Figure S6). In contrast, attempts of implementing this protocol using other sulfoxides such as dibenzothiophene-S-oxide or diphenylsulfoxide were unsuccessful (Figures S7 and S8). The structural features of thianthrene that allow the formation of a [4 + 2] adduct with ethylene seem crucial for a productive reaction, and indeed has enabled the first report on the formation of sulfonium salts directly from ethylene gas. To further confirm the key role of the [4 + 2] cycloadduct under the reaction conditions, we isolated and characterized intermediate 3, the crystal structure of which shows the “snapshot” of ethylene activation by the formal thianthrenium dication (Figure 2B). No other known vinylating reagent can currently be accessed directly from ethylene, and the practical and conceptual advantages of 1 allow a rich and divergent chemistry (see below) while avoiding certain limitations associated with other reagents.
Figure 2.
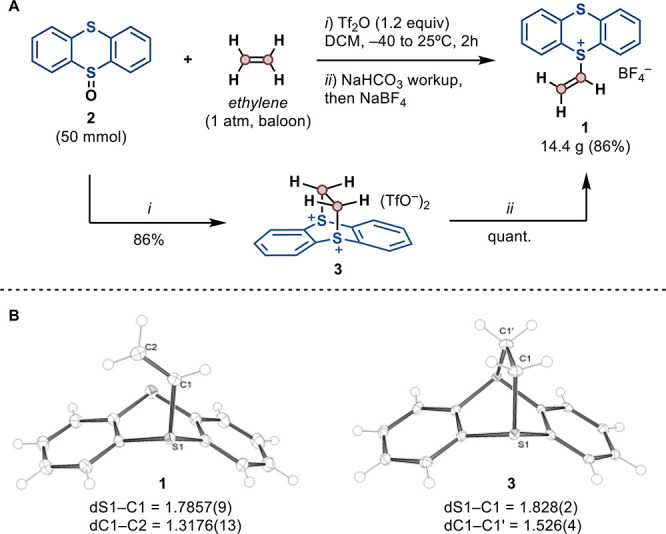
(A) Synthesis of 1 from ethylene, proceeding through a formal [4 + 2] cycloadduct (3) as intermediate. (B) Crystal structures of 1 and 3 obtained by X-ray diffraction (counterions omitted for simplicity).
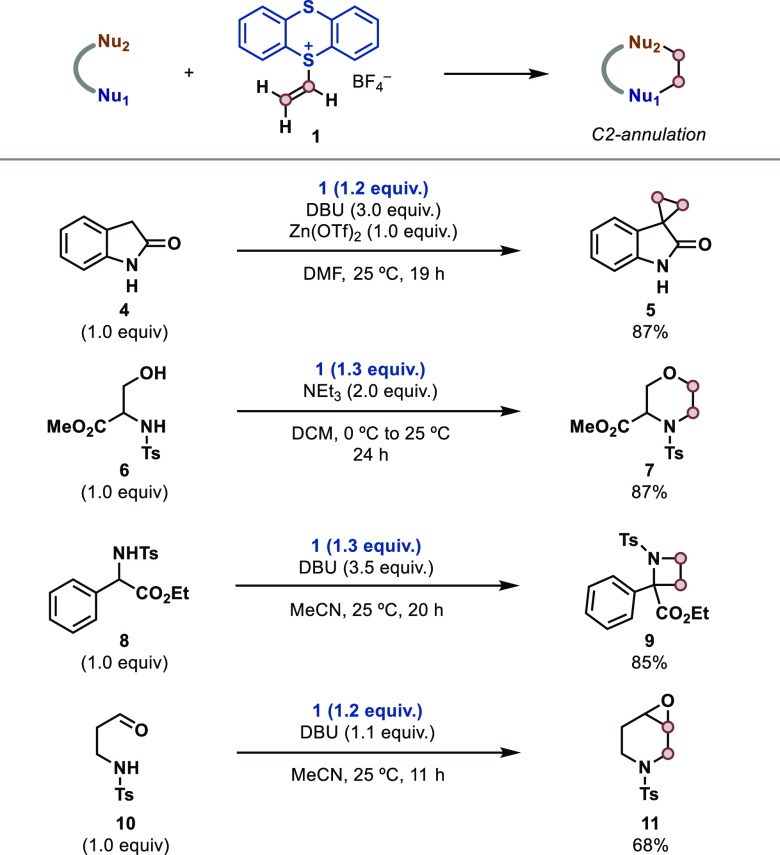
To evaluate the reactivity profile of 1 we started benchmarking the reagent in annulation reactions reported for vinyl-SPh2(OTf) or its precursor, which proceed via sulfonium ylide intermediates.25 As depicted in Figure 3, we can access (hetero)cyclic motifs that are prevalent in bioactive compounds and pharmaceuticals. Selected examples include a cyclopropanation reaction (4 → 5),28 the assembly of morpholine (6 → 7)24 and azetidine (8 → 9)31 scaffolds, and a tandem N-nucleophilic addition/Corey–Chaykovsky epoxidation (10 → 11).32 In all cases, the isolated yields were comparable or superior to those obtained with vinyl-SPh2(OTf) under the same conditions.
Figure 3.
Application of 1 in the annulation of hetero- and carbocycles.
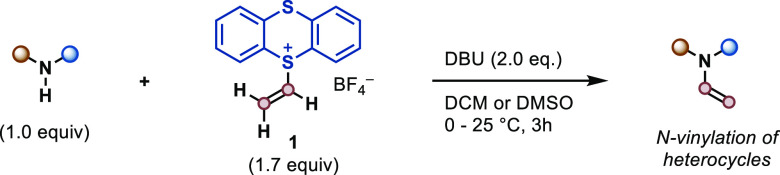
Next, we aimed to implement 1 in new reactions to effectively transfer the vinyl moiety to nucleophilic nitrogen. During his studies on annulation reactions, Aggarwal reported an annulation–vinylation sequence on 1,2-aminoalcohols42 only when Cbz is the N-protecting group, but, beyond these examples, a general platform for N-vinylation of heterocycles using sulfonium salts is not yet established43 and current methods require harsh conditions44−47 or metal-mediated reactions (85–100 °C).48−51 We developed a simple protocol that uses 1 in the presence of a base at room temperature (Table 1). A diverse set of N-vinylated nitrogen heterocycles can now be accessed under mild conditions including azacarbazole (12), indole (13 and 14), imidazole (19), pyrazole (15 and 16), triazole (17), and pyridone (18). A broad tolerance to an array of polar groups was displayed as demonstrated by the compatibility of nitro (13) and aldehyde groups (14), which are not tolerated using calcium carbide,47 or aryl halides (16, 20) that are reactive in SNAr and cross-coupling reactions. Other scaffolds of relevance such as deazapurine (20) or theophylline (21) were also vinylated, as well as the amino acids tryptophan and histidine (22 and 23). Finally, we explored the use of 1 for late-stage N-vinylation. The mild conditions and fast reaction times enabled modification of the drugs metaxalone (24), carvedilol (25), lansoprazole (27), and the laser dye coumarin 7 (26), further showcasing the compatibility with groups such as alcohols, alkylamines, and sulfoxides.
Table 1. Vinylation of N-Heterocycles Using 1a.
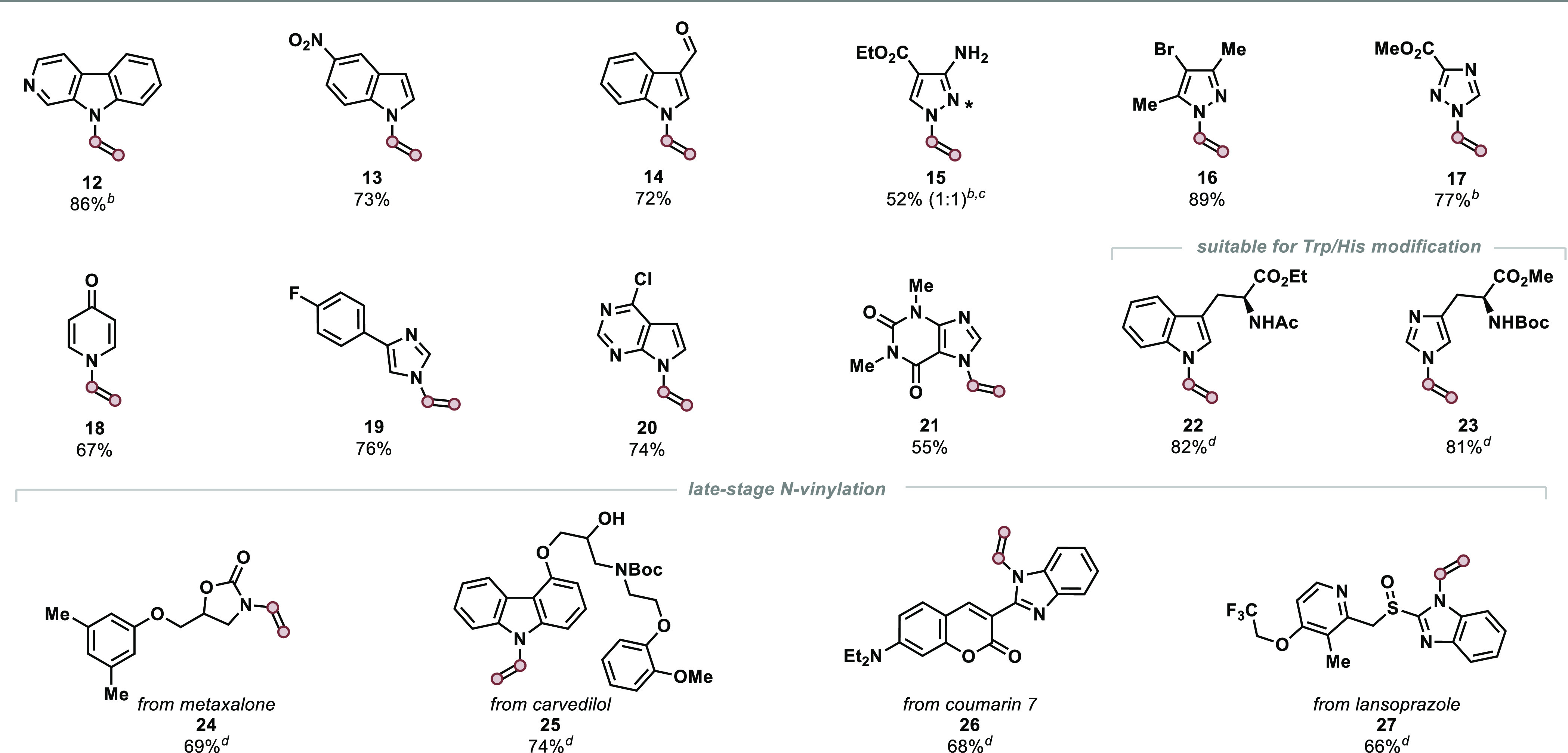
Reaction conditions: 0.300 mmol of N-heterocycle, 1.7 equiv of 1, 2.0 equiv of DBU in CH2Cl2 (3.0 mL, c = 0.10 M), 25 °C, 3 h.
DMSO was used as solvent.
1.2 equiv of 1 were added to a solution of N-heterocycle and DBU.
30 min at 0 °C, then 2.5 h at 25 °C. * denotes the site of vinylation on the constitutional isomer not shown.
Vinylated arenes (styrenes) are activated alkenes with widespread use in transition-metal catalysis,52,53 radical chemistry,54,55 and electrophilic reactions.56,57 In contrast to alkenylation, the assembly of styrenes using vinylating reagents in metal-catalyzed cross-couplings often face several additional challenges,21 such as undesired Heck-type reactivity on the vinyl–[M] reagent or polymerization styrene-type products. Vinyl sulfonium salts are ideally positioned to undergo metal-catalyzed vinylations, but no examples have been reported. One of the main reasons is the unselective cleavage of the different C–S bonds in sulfonium salts,37 which can result in mixtures of products. We conceived 1 as a suitable coupling partner that could overcome the above-mentioned challenges and enable fast oxidative addition in view of its electropositive character (Ered = −1.13 V vs SCE). Moreover, in line with what has been observed in palladium-catalyzed reactions of aryl thianthrenium salts,58−63 cleavage of the Cvinyl–S bond selectively over the two Caryl–S bonds may be explained by irreversible oxidative addition into the vinyl bond but reversible oxidative addition into the aryl bond64,65 of the annulated structure of the thianthrene core (see Supporting Information for a discussion). To demonstrate the performance of 1 in cross-coupling reactions, we developed a Suzuki-type vinylation of aryl boronic acids (Table 2A). The scope of aryl boronic acids encompasses a wide range of (hetero)arenes with different functional groups and substitution patterns (28–36), including electrophilic groups that are not tolerated by Wittig olefination-based synthesis66 (34, 39), with proto-deborylation observed as the main side reaction in those examples with lower yields. The fast rate of oxidative addition of the C–S bond allowed the vinylation of substrates containing C–Br bonds (33) that are otherwise reactive in Suzuki reactions.19 Likewise, a competition experiment between vinyl thianthrenium 1-d3 and vinyl bromide established that the thianthrenium compound reacts substantially faster than vinyl bromide; less than 1% of reaction product based on vinyl bromide could be detected by either NMR spectroscopy or mass spectrometry analysis (Table 2B). Extension of the methodology to other organoboron compounds, such as boronic esters (38) and trifluoroborates (39), is possible. Alkenyl boronic acids were also suitable substrates, yielding valuable dienes (40) that can be employed for further elaboration (e.g., Diels–Alder reactions).
Table 2. Suzuki-Type Vinylation of Organoboron Compoundsa.
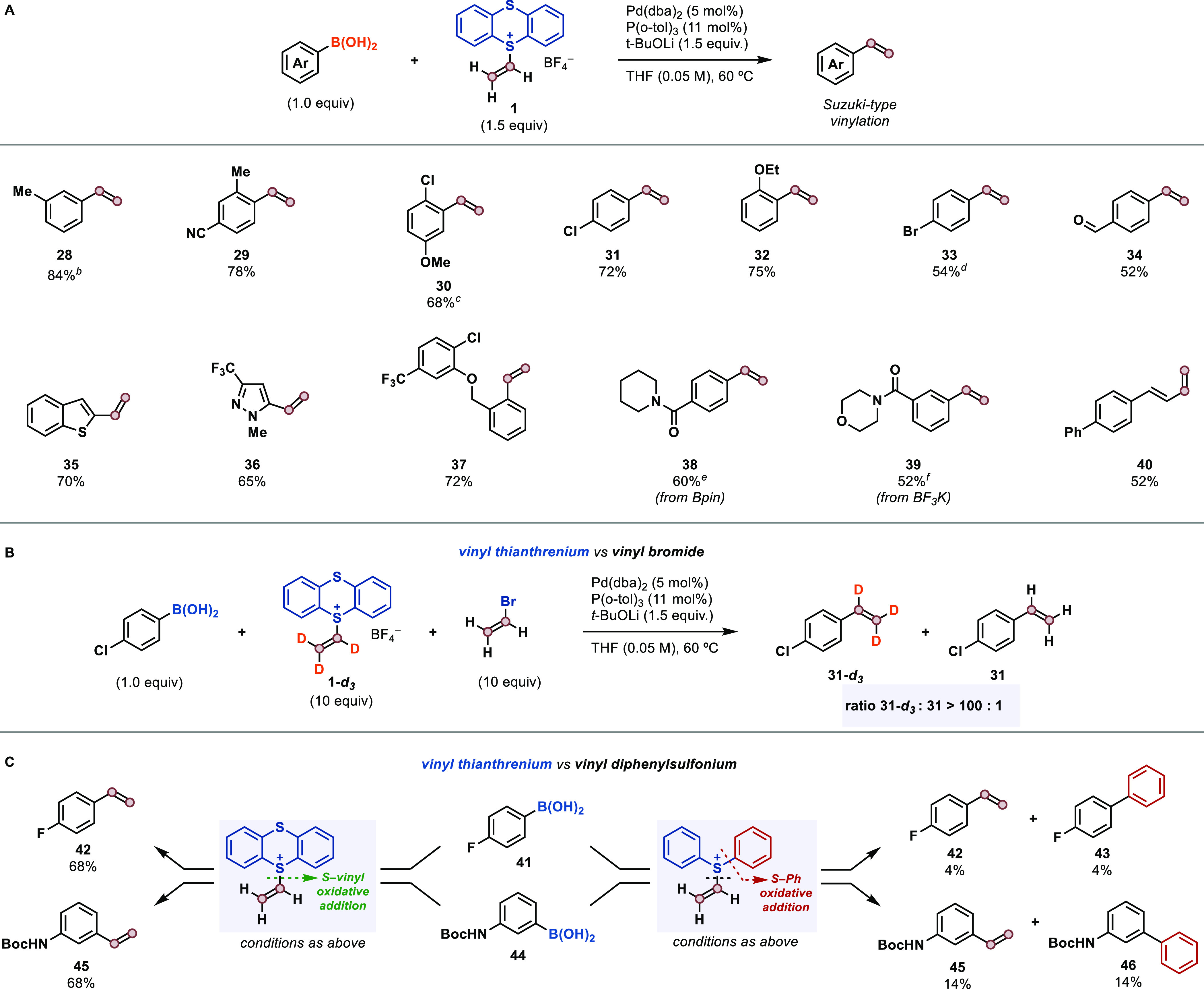
(A) Scope of the transformation. (B) Competition experiment between 1-d3 and vinyl bromide; analysis by NMR spectroscopy and mass spectrometry. (C) Comparison of the reactivity of 1 and vinyl-SPh2(OTf).
Reaction conditions: 0.300 mmol of ArB(OH)2, 1.5 equiv of 1, 0.050 equiv of Pd(dba)2, 0.11 equiv of P(o-tol)3, 1.5 equiv of t-BuOLi in THF (6.0 mL, c = 0.05 M), 60 °C, 16 h.
NMR yield.
K2CO3 was used as base.
1.7 equiv of 1, 50 °C, 24 h.
From ArBpin.
From ArBF3K.
In contrast, the use of vinyl-SPh2(OTf) under the same reaction conditions did not afford the desired products or resulted in <15% yield in all the cases studied (Table 2C). For example, while styrene 42 was isolated in 68% yield when using reagent 1, only a 4% yield could be detected by NMR when using vinyl-SPh2(OTf), which may be the result of a faster reagent decomposition or catalyst poisoning. Moreover, analysis of the reaction mixture revealed the presence of equimolar amounts of side-product 43, arising from aryl–Ph instead of aryl–vinyl bond formation, while no related product resulting from aryl–aryl coupling could be detected in the reaction with 1. A similar outcome was observed with substrate 44. These results underline the key benefits of the structural design of thianthrene electrophiles, effectively channelling the oxidative addition process toward the desired C–S bond and allowing, for the first time, a vinylation reaction based on cross-coupling with vinyl sulfonium salts.
In summary, we have developed a convenient vinyl electrophile reagent that is prepared directly from ethylene and can be stored in the presence of air and moisture. The salt has proven to be an effective vinylating reagent and C-2 synthon for the synthesis of carbo- and heterocycles, N-vinylated products, styrenes, and dienes. The distinct structural features of thianthrenium salts in comparison with other sulfonium salts enable both its unique synthesis from ethylene and its superior performance in cross-coupling reactions. Its one-step synthesis, easy-to-handle features, and robust reactivity make it a valuable and versatile reagent that will find synthetic utility in further organic and transition-metal catalyzed transformations.
Acknowledgments
We thank Dr. Markus Leutzsch and Ms. Petra Philipps for assistance with NMR experiments, Ms. Fei Wang for DSC analysis, Dr. Qiang Cheng and Aboubakr Hamad for helpful discussions, and Dr. Florian Berger for the development of a synthesis of 1 from trimethylvinylsilane. We thank the MPI für Kohlenforschung for their financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c06632.
Experimental procedures and spectral data (PDF)
Accession Codes
CCDC 2075820–2075821 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Open access funded by Max Planck Society.
The authors declare the following competing financial interest(s): T.R. may benefit from royalty payments on sales of thianthrene-related compounds.
Supplementary Material
References
- Patai S.The Chemistry of Alkenes; Wiley: New York, 1964; Vol. 1. DOI: 10.1002/9780470771044. [DOI] [Google Scholar]
- Kolb H. C.; VanNieuwenhze M. S.; Sharpless K. B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94 (8), 2483–2547. 10.1021/cr00032a009. [DOI] [Google Scholar]
- McDonald R. I.; Liu G.; Stahl S. S. Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev. 2011, 111 (4), 2981–3019. 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletskaya I. P.; Cheprakov A. V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100 (8), 3009–3066. 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- Pirnot M. T.; Wang Y.-M.; Buchwald S. L. Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes. Angew. Chem., Int. Ed. 2016, 55 (1), 48–57. 10.1002/anie.201507594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs R. H.; Chang S. Recent advances in olefin metathesis and its application in organic synthesis. Tetrahedron 1998, 54 (18), 4413–4450. 10.1016/S0040-4020(97)10427-6. [DOI] [Google Scholar]
- Zimmermann H.; Walzl R.; Ethylene Ullmann’s Encyclopedia of Industrial Chemistry 2009, 10.1002/14356007.a10_045.pub3. [DOI] [Google Scholar]
- Crimmins M. T.; Kim-Meade A. S. Ethylene. Encyclopedia of Reagents for Organic Synthesis 2001, 1–4. 10.1002/047084289X.re066. [DOI] [Google Scholar]
- Saini V.; Stokes B. J.; Sigman M. S. Transition-Metal-Catalyzed Laboratory-Scale Carbon–Carbon Bond-Forming Reactions of Ethylene. Angew. Chem., Int. Ed. 2013, 52 (43), 11206–11220. 10.1002/anie.201303916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RajanBabu T. V.; Adam Cox G.; Lim H. J.; Nomura N.; Sharma R. K.; Smith C. R.; Zhang A.. 5.32 Hydrovinylation Reactions in Organic Synthesis. In Comprehensive Organic Synthesis II, 2nd ed.; Knochel P., Ed.; Elsevier: Amsterdam, 2014; pp 1582–1620. [Google Scholar]
- Hilt G. Hydrovinylation Reactions – Atom-Economic Transformations with Steadily Increasing Synthetic Potential. Eur. J. Org. Chem. 2012, 2012 (24), 4441–4451. 10.1002/ejoc.201200212. [DOI] [Google Scholar]
- Vaughan B. A.; Webster-Gardiner M. S.; Cundari T. R.; Gunnoe T. B. A rhodium catalyst for single-step styrene production from benzene and ethylene. Science 2015, 348 (6233), 421–424. 10.1126/science.aaa2260. [DOI] [PubMed] [Google Scholar]
- Harper M. J.; Emmett E. J.; Bower J. F.; Russell C. A. Oxidative 1,2-Difunctionalization of Ethylene via Gold-Catalyzed Oxyarylation. J. Am. Chem. Soc. 2017, 139 (36), 12386–12389. 10.1021/jacs.7b06668. [DOI] [PubMed] [Google Scholar]
- Tang S.; Wang D.; Liu Y.; Zeng L.; Lei A. Cobalt-catalyzed electrooxidative C–H/N–H [4 + 2] annulation with ethylene or ethyne. Nat. Commun. 2018, 9 (1), 798. 10.1038/s41467-018-03246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill R. L.; Dalton J. R.; Morton G. H.; Caldwell W. W. Photocyclization of an enone to an alkene. Org. Synth. 2003, 62, 118. 10.1002/0471264180.os062.15. [DOI] [Google Scholar]
- Meyers A. I.; Fleming S. A. Efficient asymmetric (2 + 2) photocycloaddition leading to chiral cyclobutanes. Application to the total synthesis of (−)-grandisol. J. Am. Chem. Soc. 1986, 108 (2), 306–307. 10.1021/ja00262a026. [DOI] [Google Scholar]
- Hoffmann N.; Scharf H.-D.; Runsink J. Chiral induction in photochemical reactions-XII. Synthesis of chiral cyclobutane derivatives from (+)-5-menthyloxy-2-[5H]-furanone and ethylene. Tetrahedron Lett. 1989, 30 (20), 2637–2638. 10.1016/S0040-4039(00)99085-3. [DOI] [Google Scholar]
- Johansson Seechurn C. C. C.; Kitching M. O.; Colacot T. J.; Snieckus V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed. 2012, 51 (21), 5062–5085. 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95 (7), 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]
- World Health Organization International Agency for Research on Cancer . Vinyl bromide. IARC Monogr. Eval. Carcinog. Risks Hum. 1999, 71 Pt 2: 923–8. https://publications.iarc.fr/115. [PMC free article] [PubMed]
- Denmark S. E.; Butler C. R. Palladium- (and nickel-) catalyzed vinylation of aryl halides. Chem. Commun. 2009, 1, 20–33. 10.1039/B809676G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Zhang W.; Colandrea V. J.; Jimenez L. S. Reactivity and rearrangements of dialkyl- and diarylvinylsulfonium salts with indole-2- and pyrrole-2-carboxaldehydes. Tetrahedron 1999, 55 (35), 10659–10672. 10.1016/S0040-4020(99)00605-5. [DOI] [Google Scholar]
- Yamanaka H.; Yamane Y.; Mukaiyama T. A New Method for the Preparation of Nitrogen-containing Heterocycles Using Diphenylsulfonium Triflates. Heterocycles 2004, 63 (12), 2813–2826. 10.3987/COM-04-10232. [DOI] [Google Scholar]
- Yar M.; McGarrigle E. M.; Aggarwal V. K. An Annulation Reaction for the Synthesis of Morpholines, Thiomorpholines, and Piperazines from β-Heteroatom Amino Compounds and Vinyl Sulfonium Salts. Angew. Chem., Int. Ed. 2008, 47 (20), 3784–3786. 10.1002/anie.200800373. [DOI] [PubMed] [Google Scholar]
- Yar M.; McGarrigle E. M.; Aggarwal V. K. Sulfonium, Ethenyldiphenyl-, 1,1,1-Trifluoromethanesulfonate. Encyclopedia of Reagents for Organic Synthesis 2012, 1–6. 10.1002/047084289X.rn01409. [DOI] [Google Scholar]
- “While a large amount of methodology has been developed using diphenylvinylsulfonium triflate, some practical issues remain. Diphenylvinylsulfonium triflate is a slightly hygroscopic brown oil that must be stored under inert atmosphere and kept at low temperature (fridge at 4° C). For this reason, the reagent is difficult to manipulate and hard to synthesize on large scales.” J. V. Matlock, V. K. Aggarwal, E. M. McGarrigle, ref (27).
- Matlock J. V.; Aggarwal V. K.; McGarrigle E. M. (2-Bromoethyl)diphenylsulfonium Trifluoromethanesulfonate. Encyclopedia of Reagents for Organic Synthesis 2016, 1–5. 10.1002/047084289X.rn01921. [DOI] [Google Scholar]
- Zhou M.; En K.; Hu Y.; Xu Y.; Shen H. C.; Qian X. Zinc triflate-mediated cyclopropanation of oxindoles with vinyl diphenyl sulfonium triflate: a mild reaction with broad functional group compatibility. RSC Adv. 2017, 7 (7), 3741–3745. 10.1039/C6RA24985J. [DOI] [Google Scholar]
- Yar M.; McGarrigle E. M.; Aggarwal V. K. Bromoethylsulfonium Salt—A More Effective Annulation Agent for the Synthesis of 6- and 7-Membered 1,4-Heterocyclic Compounds. Org. Lett. 2009, 11 (2), 257–260. 10.1021/ol8023727. [DOI] [PubMed] [Google Scholar]
- Karahan S.; Tanyeli C. Organocatalytic enantioselective construction of isatin-derived N-alkoxycarbonyl 1,3-aminonaphthols via sterically encumbered hydrocarbon-substituted quinine-based squaramide. New J. Chem. 2017, 41 (17), 9192–9202. 10.1039/C7NJ01395G. [DOI] [Google Scholar]
- Fritz S. P.; Moya J. F.; Unthank M. G.; McGarrigle E. M.; Aggarwal V. K. An Efficient Synthesis of Azetidines with (2-Bromoethyl)sulfonium Triflate. Synthesis 2012, 44 (10), 1584–1590. 10.1055/s-0031-1290951. [DOI] [Google Scholar]
- Unthank M. G.; Hussain N.; Aggarwal V. K. The Use of Vinyl Sulfonium Salts in the Stereocontrolled Asymmetric Synthesis of Epoxide- and Aziridine-Fused Heterocycles: Application to the Synthesis of (−)-Balanol. Angew. Chem., Int. Ed. 2006, 45 (42), 7066–7069. 10.1002/anie.200602782. [DOI] [PubMed] [Google Scholar]
- Yar M.; Unthank M. G.; McGarrigle E. M.; Aggarwal V. K. Remote Chiral Induction in Vinyl Sulfonium Salt-Mediated Ring Expansion of Hemiaminals into Epoxide-Fused Azepines. Chem. - Asian J. 2011, 6 (2), 372–375. 10.1002/asia.201000817. [DOI] [PubMed] [Google Scholar]
- Xie C.; Han D.; Hu Y.; Liu J.; Xie T. Synthesis of pyrrolidin-2-ones via tandem reactions of vinyl sulfonium salts under mild conditions. Tetrahedron Lett. 2010, 51 (40), 5238–5241. 10.1016/j.tetlet.2010.07.108. [DOI] [Google Scholar]
- Srogl J.; Allred G. D.; Liebeskind L. S. Sulfonium Salts. Participants par Excellence in Metal-Catalyzed Carbon–Carbon Bond-Forming Reactions. J. Am. Chem. Soc. 1997, 119 (50), 12376–12377. 10.1021/ja9726926. [DOI] [Google Scholar]
- Aukland M. H.; Talbot F. J. T.; Fernández-Salas J. A.; Ball M.; Pulis A. P.; Procter D. J. An Interrupted Pummerer/Nickel-Catalysed Cross-Coupling Sequence. Angew. Chem., Int. Ed. 2018, 57 (31), 9785–9789. 10.1002/anie.201805396. [DOI] [PubMed] [Google Scholar]
- Lin H.; Dong X.; Li Y.; Shen Q.; Lu L. Highly Selective Activation of Vinyl C–S Bonds Over Aryl C–S Bonds in the Pd-Catalyzed Coupling of (E)-(β-Trifluoromethyl)vinyldiphenylsulfonium Salts: Preparation of Trifluoromethylated Alkenes and Dienes. Eur. J. Org. Chem. 2012, 2012 (25), 4675–4679. 10.1002/ejoc.201200758. [DOI] [Google Scholar]
- Chen J.; Li J.; Plutschack M. B.; Berger F.; Ritter T. Regio- and Stereoselective Thianthrenation of Olefins To Access Versatile Alkenyl Electrophiles. Angew. Chem., Int. Ed. 2020, 59 (14), 5616–5620. 10.1002/anie.201914215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochynski S.; Boduszek B.; Shine H. J. Oxidation of organotins (R4Sn, RSnMe3, and R3SnSnR3) by the thianthrene cation radical. J. Org. Chem. 1991, 56 (3), 914–920. 10.1021/jo00003a005. [DOI] [Google Scholar]
- Blauvelt M. L. 5-Thianthrenyl-5-ylium Perchlorate. Encyclopedia of Reagents for Organic Synthesis 2009, 1–6. 10.1002/047084289X.rn01052. [DOI] [Google Scholar]
- Fannes C.; Meerpoel L.; Toppet S.; Hoornaert G. Cycloaddition of Olefinic Compounds to 2H-1,4-Oxazin-2-ones: Synthesis of 2-Oxa-5-azabicyclo[2.2.2]oct-5-en-3-ones. Synthesis 1992, 1992 (07), 705–709. 10.1055/s-1992-26205. [DOI] [Google Scholar]
- Yar M.; Fritz S. P.; Gates P. J.; McGarrigle E. M.; Aggarwal V. K. Synthesis of N-Vinyloxazolidinones and Morpholines from Amino Alcohols and Vinylsulfonium Salts: Analysis of the Outcome’s Dependence on the N-Protecting Group by Nanospray Mass Spectrometry. Eur. J. Org. Chem. 2012, 2012 (1), 160–166. 10.1002/ejoc.201101272. [DOI] [Google Scholar]
- Alkenylation of nitrogen heterocycles has only been reported for styrenyl sulfonium salts:Zhou M.; Tan X.; Hu Y.; Shen H. C.; Qian X. Highly Chemo- and Regioselective Vinylation of N-Heteroarenes with Vinylsulfonium Salts. J. Org. Chem. 2018, 83, 8627–8635. 10.1021/acs.joc.8b00682. [DOI] [PubMed] [Google Scholar]
- Lan T.; Chen X.; Liu Z.; Mao Z. A Novel Facile Synthesis Method of N-Vinylpyrroles. J. Heterocycl. Chem. 2013, 50 (5), 1094–1098. 10.1002/jhet.1102. [DOI] [Google Scholar]
- Martín-Escolano R.; Aguilera-Venegas B.; Marín C.; Martín-Montes Á.; Martín-Escolano J.; Medina-Carmona E.; Arán V. J.; Sánchez-Moreno M. Synthesis and Biological in vitro and in vivo Evaluation of 2-(5-Nitroindazol-1-yl)ethylamines and Related Compounds as Potential Therapeutic Alternatives for Chagas Disease. ChemMedChem 2018, 13 (19), 2104–2118. 10.1002/cmdc.201800512. [DOI] [PubMed] [Google Scholar]
- Shmidt E. Y.; Protsuk N. I.; Vasil’tsov A. M.; Ivanov A. V.; Mikhaleva A. I.; Trofimov B. A. Improved method for the synthesis of 1-vinylindole. Chem. Heterocycl. Compd. 2013, 49 (3), 404–407. 10.1007/s10593-013-1260-y. [DOI] [Google Scholar]
- Rattanangkool E.; Vilaivan T.; Sukwattanasinitt M.; Wacharasindhu S. An Atom-Economic Approach for Vinylation of Indoles and Phenols Using Calcium Carbide as Acetylene Surrogate. Eur. J. Org. Chem. 2016, 2016 (25), 4347–4353. 10.1002/ejoc.201600666. [DOI] [Google Scholar]
- Lebedev A. Y.; Izmer V. V.; Kazyul’kin D. N.; Beletskaya I. P.; Voskoboynikov A. Z. Palladium-Catalyzed Stereocontrolled Vinylation of Azoles and Phenothiazine. Org. Lett. 2002, 4 (4), 623–626. 10.1021/ol0172370. [DOI] [PubMed] [Google Scholar]
- Kimura J.; Nakamichi S.; Ogawa S.; Obora Y. Iridium-Catalyzed Vinylation of Carbazole Derivatives with Vinyl Acetate. Synlett 2017, 28 (06), 719–723. 10.1055/s-0036-1588927. [DOI] [Google Scholar]
- Lam P. Y. S.; Deudon S.; Averill K. M.; Li R.; He M. Y.; DeShong P.; Clark C. G. Copper-Promoted C–N Bond Cross-Coupling with Hypervalent Aryl Siloxanes and Room-Temperature N-Arylation with Aryl Iodide. J. Am. Chem. Soc. 2000, 122 (31), 7600–7601. 10.1021/ja001305g. [DOI] [Google Scholar]
- Arsenyan P.; Petrenko A.; Paegle E.; Belyakov S. Direct N- and C-vinylation with trimethoxyvinylsilane. Mendeleev Commun. 2011, 21 (6), 326–328. 10.1016/j.mencom.2011.11.011. [DOI] [Google Scholar]
- Utsunomiya M.; Kuwano R.; Kawatsura M.; Hartwig J. F. Rhodium-Catalyzed Anti-Markovnikov Hydroamination of Vinylarenes. J. Am. Chem. Soc. 2003, 125 (19), 5608–5609. 10.1021/ja0293608. [DOI] [PubMed] [Google Scholar]
- Rakshit S.; Grohmann C.; Besset T.; Glorius F. Rh(III)-Catalyzed Directed C–H Olefination Using an Oxidizing Directing Group: Mild, Efficient, and Versatile. J. Am. Chem. Soc. 2011, 133 (8), 2350–2353. 10.1021/ja109676d. [DOI] [PubMed] [Google Scholar]
- Tomita R.; Yasu Y.; Koike T.; Akita M. Combining Photoredox-Catalyzed Trifluoromethylation and Oxidation with DMSO: Facile Synthesis of α-Trifluoromethylated Ketones from Aromatic Alkenes. Angew. Chem., Int. Ed. 2014, 53 (28), 7144–7148. 10.1002/anie.201403590. [DOI] [PubMed] [Google Scholar]
- Wang D.; Wu L.; Wang F.; Wan X.; Chen P.; Lin Z.; Liu G. Asymmetric Copper-Catalyzed Intermolecular Aminoarylation of Styrenes: Efficient Access to Optical 2,2-Diarylethylamines. J. Am. Chem. Soc. 2017, 139 (20), 6811–6814. 10.1021/jacs.7b02455. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Loebach J. L.; Wilson S. R.; Jacobsen E. N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J. Am. Chem. Soc. 1990, 112 (7), 2801–2803. 10.1021/ja00163a052. [DOI] [Google Scholar]
- Dewkar G. K.; Narina S. V.; Sudalai A. NaIO4-Mediated Selective Oxidative Halogenation of Alkenes and Aromatics Using Alkali Metal Halides. Org. Lett. 2003, 5 (23), 4501–4504. 10.1021/ol0358206. [DOI] [PubMed] [Google Scholar]
- Ye Y.; Zhu J.; Huang Y. Diverse C–P Cross-Couplings of Arylsulfonium Salts with Diarylphosphines via Selective C–S Bond Cleavage. Org. Lett. 2021, 23 (6), 2386–2391. 10.1021/acs.orglett.1c00748. [DOI] [PubMed] [Google Scholar]
- Berger F.; Plutschack M. B.; Riegger J.; Yu W.; Speicher S.; Ho M.; Frank N.; Ritter T. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 2019, 567 (7747), 223–228. 10.1038/s41586-019-0982-0. [DOI] [PubMed] [Google Scholar]
- Engl P. S.; Häring A. P.; Berger F.; Berger G.; Pérez-Bitrián A.; Ritter T. C–N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts. J. Am. Chem. Soc. 2019, 141 (34), 13346–13351. 10.1021/jacs.9b07323. [DOI] [PubMed] [Google Scholar]
- Alvarez E. M.; Plutschack M. B.; Berger F.; Ritter T. Site-Selective C–H Functionalization–Sulfination Sequence to Access Aryl Sulfonamides. Org. Lett. 2020, 22 (12), 4593–4596. 10.1021/acs.orglett.0c00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmani A.; Gevondian A. G.; Schoenebeck F. Germylation of Arenes via Pd(I) Dimer Enabled Sulfonium Salt Functionalization. Org. Lett. 2020, 22 (12), 4802–4805. 10.1021/acs.orglett.0c01609. [DOI] [PubMed] [Google Scholar]
- Lansbergen B.; Granatino P.; Ritter T. Site-Selective C–H alkylation of Complex Arenes by a Two-Step Aryl Thianthrenation-Reductive Alkylation Sequence. J. Am. Chem. Soc. 2021, 143 (21), 7909–7914. 10.1021/jacs.1c03459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobisu M.; Masuya Y.; Baba K.; Chatani N. Palladium(ii)-catalyzed synthesis of dibenzothiophene derivatives via the cleavage of carbon–sulfur and carbon–hydrogen bonds. Chem. Sci. 2016, 7 (4), 2587–2591. 10.1039/C5SC04890G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J.; Pfeffer M.; DeCian A.; Fischer J. Palladium-Mediated Intramolecular Formation of a C-S Bond: Application to the Selective Syntheses of Six- and Seven-Membered Sulfur-Containing Heterocycles. J. Org. Chem. 1995, 60 (4), 1005–1012. 10.1021/jo00109a036. [DOI] [Google Scholar]
- Broos R.; Anteunis M. A Simplified Wittig Synthesis of Substituted Styrenes. Synth. Commun. 1976, 6 (1), 53–57. 10.1080/00397917608062133. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.