Abstract
Background: Accumulating evidence indicates that the gut microbiota can synthesize neurotransmitters as well as impact host-derived neurotransmitter levels. In the past, it has been challenging to decipher which microbes influence neurotransmitters due to the complexity of the gut microbiota. Methods: To address whether a single microbe, Bifidobacterium dentium, could regulate important neurotransmitters, we examined Bifidobacteria genomes and explored neurotransmitter pathways in secreted cell-free supernatant using LC-MS/MS. To determine if B. dentium could impact neurotransmitters in vivo, we mono-associated germ-free mice with B. dentium ATCC 27678 and examined fecal and brain neurotransmitter concentrations. Results: We found that B. dentium possessed the enzymatic machinery to generate γ-aminobutyric acid (GABA) from glutamate, glutamine, and succinate. Consistent with the genome analysis, we found that B. dentium secreted GABA in a fully defined microbial media and elevated fecal GABA in B. dentium mono-associated mice compared to germ-free controls. We also examined the tyrosine/dopamine pathway and found that B. dentium could synthesize tyrosine, but could not generate L-dopa, dopamine, norepinephrine, or epinephrine. In vivo, we found that B. dentium mono-associated mice had elevated levels of tyrosine in the feces and brain. Conclusions: These data indicate that B. dentium can contribute to in vivo neurotransmitter regulation.
Keywords: gut–brain axis, neurotransmitters, LC-MS/MS, gut microbiome, Bifidobacteria, GABA
1. Introduction
The communication between the intestine and the brain, known as the gut–brain axis, has recently attracted attention for its crucial role in human health. Intestinal microbes are known to modulate both the enteric nervous system (ENS) and central nervous system (CNS) and have been linked to intestinal motility [1,2,3,4,5,6,7,8,9,10], visceral sensitivity [11,12,13,14,15,16,17,18], anxiety [19,20,21,22,23,24], depression [25,26], neurodegenerative disease [27,28], multiple sclerosis [29,30], and autism spectrum disorder [31]. One way gut microbes can interact with the ENS, and CNS is through the modulation of host neurotransmitters and/or related pathways. Consistent with this notion, select microbes have been found to produce a range of major neuroactive compounds including tyrosine, tryptophan, dopamine, norepinephrine, and GABA. Some neuro-active compounds such as GABA cannot cross the blood–brain barrier and have localized effects within the gut. In contrast, other neuro-active molecules such as tyrosine can cross the blood–brain barrier and can affect both the gut and brain. Most of the work focusing on microbial-derived neurotransmitters has been performed in vitro, typically using rich microbial media which may not reflect the conditions of the gastrointestinal tract. Likewise, in vivo studies using gnotobiotic models have largely focused on complex microbial communities that obscure the ability to identify specific microbes driving neurotransmitter changes. As a result, new mono-associated gnotobiotic animal models are needed to dissect how individual microbes influence intestinal and neuronal neurotransmitter levels.
The communication between the gut and brain is speculated to be particularly important during early life. During this early developmental period, the gut microbiota is dominated by the genus Bifidobacterium (phylum Actinobacteria) [32,33,34,35,36,37,38]. Bifidobacteria species dominant in human infants include B. longum, B. longum subspecies infantis, B. breve, B. bifidum, B. catenulatum, B. adolescentis, B. angulatum, B. animalis, and B. dentium [39,40,41,42,43]. These early-life Bifidobacteria have been shown by several groups to beneficially modulate the gut–brain axis. For example, B. longum strains NCC3001, 1714, and R0175 can modulate anxiety in animal models [44,45,46], and B. longum NCC3001 was found to improve depression scores in patients with IBS [47]. Likewise, B. infantis improved depressive-like behaviors in a rat model of maternal separation [48], while B. longum 1714 was shown to enhance object recognition and cognition in a mouse model [49]. Our previous work has also demonstrated that neonatal colonization with a consortium of human-derived Bifidobacterium species (B. longum subspecies infantis, B. bifidum, B. breve, and B. dentium) affects the CNS synaptic plasticity, microglia activation, and rodent behavior, including anxiety and hyperactivity [50,51]. We have also shown that B. dentium administration to adult mice modulates the serotonergic system and adult behavior [52]. Although these phenotypic effects have been observed, much of the mechanisms by which Bifidobacteria modulate the gut–brain axis remains to be discovered.
To address this knowledge gap, we sought to characterize the neuroactive compounds produced by a single human Bifidobacteria species in vitro and in vivo. We selected B. dentium because it is found in infants and adults [53], has the metabolic capacity to colonize the intestine [54], possesses anti-inflammatory, epithelial-barrier enhancing properties [55,56], and stimulates serotonin release from enterochromaffin cells [52]. The importance of these functions in human health motivated us to characterize the neurotransmitter profile of B. dentium. To effectively address the contribution of B. dentium on neurotransmitter concentrations in vivo, we used a gnotobiotic model (defined microbiota applied to germ-free mice); a well-established model for studying the effects of specific microorganisms on the gut–brain axis [20,21,57]. Our data indicates that B. dentium colonization is associated with a unique gut and neuronal neurotransmitter profile.
2. Materials and Methods
2.1. Bacterial Culture
Bifidobacterium dentium ATCC 27678 (adult human fecal isolate) was cultured in Man, Rogosa, and Sharpe (MRS; Difco) media in an anaerobic workstation (Anaerobe Systems AS-580) at 37 °C with a gas mixture of 5% CO2, 5% H2, and 90% N2. After culturing in MRS media, B. dentium was sub-cultured into a chemically defined media, termed ZMB1 [58], at an optical density (OD600nm) of 0.1 and cultured anaerobically at 37 °C for 18 h. Bacterial cultures were centrifuged at 5000× g for 5 min and the cell-free supernatant was sterile-filtered (0.2 µm-pore PVDF-membrane, Millipore) for LC-MS/MS analysis.
2.2. Mouse Models
Gnotobiotic experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Baylor College of Medicine, Houston, TX, USA. The germ-free mice had ad libitum access to irradiated food (Rodent 50 IF/6F auto 5V0F chow cat# 3002875-703, Lab Diet.com) and water. Adult male germ-free Swiss Webster mice (6–9 months of age) were gavaged with sterile MRS (germ-free controls, n = 5) or 3.2 × 108 CFU (in 0.2 mL) B. dentium ATCC 27678 in MRS (B. dentium mono-associated, n = 5) and maintained in isolators for 17 days as previously described [52]. For the neurotransmitter and neuroactive compounds studies, only male mice were used to limit variation. Colonization of the mice was confirmed by culturing fecal samples by plating. Feces were collected from mice prior to sacrifice. Mice were euthanized via isofluorane asphyxiation and the whole brain and colon were excised. Tissue was fixed in Carnoy’s fixative and paraffin-embedded.
To confirm bacterial colonization, Gram staining was performed on colon tissue sections. Briefly, tissue sections were dehydrated (xylene, 100%, 95%, 75%, 50% ETOH), incubated with crystal violet, gram iodine, decolorized, and counter-stained with gram safranin. Sections were then incubated with picric acid-acetone. After dehydration through a series of alcohols (75%, 95%, 100% ETOH) and xylene, sections were cover-slipped and imaged on an upright Nikon Eclipse 90i microscope at 20× using a Plan Apo (NA 0.75) differential interference contrast (DIC) objective.
2.3. Bifidobacteria Genome Mining
The ability of Bifidobacteria members to generate neuro-active metabolites was estimated using the Integrated Microbial Genomes (IMG) database (http://img.jgi.doe.gov (accessed on 17 January 2021)). The IMG annotates publicly available sequence data and the total number of Bifidobacteria genomes queried are shown in Supplemental Table S1.
2.4. Metabolite Analyses of B. dentium Cultures and Gnotobiotic Mouse Feces and Brain Tissues
2.4.1. LC-MS/MS Equipment
The liquid chromatography-tandem mass spectrometry (LC-MS/MS) system was comprised of a Shimadzu Nexera X2 MP Ultrahigh-Performance Liquid Chromatography (UHPLC) system (Kyoto, Japan) coupled to a Sciex 6500 QTrap hybrid triple-quadrupole/linear ion trap MS system from Danaher (Washington, DC, USA). Operational control of the LC-MS/MS was performed with Analyst® (Ver. 1.6.2), and quantitative analysis was performed using MultiQuant™ (Ver. 3.0.1).
2.4.2. LC-MS/MS Methods
Fecal and tissue homogenization and extraction procedures, and targeted LC-MS/MS-based metabolomics methods used for the quantitative analysis of the glutamate cycle and tyrosine pathway metabolites are described in the Supplemental Section. See Tables S2 and S3 in Supplemental Materials for the molecule-specific selected reaction monitoring (SRM) parameters used to acquire the chromatographic data for the targeted metabolites.
2.4.3. Calibration Standard Preparations
Calibration standards (Calibrators) for the glutamate cycle and tyrosine pathway methods were prepared at concentrations of 0.98, 3.9, 15.6, 62.5, and 1000 ng/mL for all metabolites measured in each method. In each instance, a consistent concentration of method-specific deuterated IS compounds were added to each calibrator and unknown sample—the assay-specific concentration levels for each deuterated IS compound is specified in the Supplemental Materials section.
2.5. Statistics
GraphPad Software (GraphPad Software, Inc. La Jolla, CA, USA) was used to generate graphs and analyze data for statistical significance. Comparisons between the groups were made with one-way or two-way Analysis of Variance (ANOVA), using the Holm-Sidak post-hoc test comparisons, * p < 0.05.
3. Results
3.1. Bifidobacteria Harbor the Enzymatic Machinery to Generate the Neurotransmitter GABA
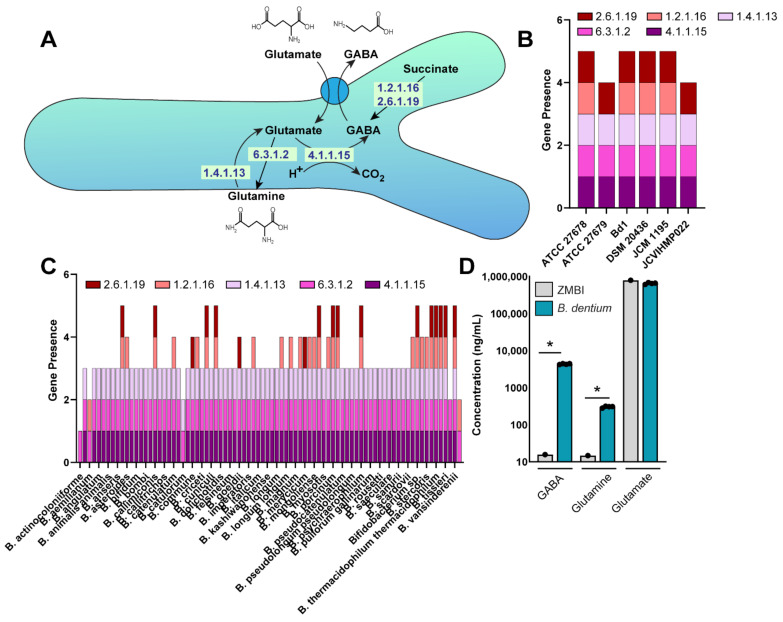
Bifidobacteria are known to colonize the mammalian gastrointestinal tract, but the characterization of Bifidobacteria-specific neuro-active compounds remains incomplete. We first focused on the neurotransmitter GABA, which serves as the major inhibitory neurotransmitter of the ENS and CNS [59]. Select microbes have been demonstrated to generate GABA from glutamate or succinate as a mechanism to decrease intracellular pH [60]. In this process, glutamate is taken into the bacteria via the transporter GadC and can be converted to GABA by the enzyme glutamate decarboxylase (GAD; Enzyme Commission number (EC) 4.1.1.15). Glutamate can also be converted to glutamine (EC 6.3.1.2) or glutamine can be converted to glutamate (EC 1.4.1.13). Alternatively, in some bacteria succinate can be converted into GABA via succinate-semialdehyde dehydrogenase (EC: 1.2.1.16) and 4-aminobutyrate-2-oxoglutarate transaminase (EC 2.6.1.19). To address whether B. dentium harbored the genes for the ECs associated with GABA production, we queried the B. dentium Bd1 genome in the KEGG database (https://www.genome.jp/kegg (accessed on 17 January 2021)) and found that this genome contained the complete enzymatic pathway to generate GABA from glutamate/glutamine and succinate (Figure 1A). Four of the six B. dentium genomes in the Integrated Microbial Genomes (IMG) database (http://img.jgi.doe.gov (accessed on 17 January 2021)), contained all five genes for GABA production from glutamine/glutamate and succinate (Figure 1B). B. dentium ATCC 27679 and JCVIHMP022 did not contain the gene to express succinate-semialdehyde dehydrogenase (EC 1.2.1.16) needed to convert succinate to GABA, although these species could still produce GABA from glutamate.
Figure 1.
(A). Gene pathways in Bifidobacterium dentium as identified via KEGG (https://www.genome.jp/kegg/kegg2.html) related to GABA, glutamate, and glutamine production. (B). Genome analysis of the JGI Integrated Microbial Genomes (IMG) database (http://img.jgi.doe.gov (accessed on 17 January 2021)) of six B. dentium genomes for the enzymes (Enzyme Commission, ECs) involved in glutamate, glutamine, and GABA production. Filled bars represent the presence of at least 1 gene copy of each enzyme. (C). Genome analysis of 83 Bifidobacteria genomes using the JGI Integrated Microbial Genomes (IMG) database. (D). Absolute concentrations of GABA, glutamate, and glutamine in uninoculated ZMB1 and cell-free bacterial conditioned ZMB1 from B. dentium after 18 h of growth. n= 4 biological replicates, two-way ANOVA, * p < 0.05.
To see how common this pathway was among other Bifidobacteria members, we examined the genomes of 83 Bifidobacteria species (total 498 genomes) (Figure 1C, Supplemental Table S1). Thirty out of 83 Bifidobacteria species contained succinate-semialdehyde dehydrogenase (EC 1.2.1.16) for converting succinate to GABA. Eighteen different Bifidobacteria species contained the gene for 4-aminobutyrate-2-oxoglutarate transaminase (EC 2.6.1.19), but not for succinate-semialdehyde dehydrogenase. Seventy-nine Bifidobacteria species (94.1%) possessed the genes to generate GABA from glutamate or glutamine (EC 4.1.1.15, 6.3.1.2, 1.4.1.13), suggesting that the majority of Bifidobacteria species can only generate GABA from glutamate and not succinate (Figure 1C). Four Bifidobacteria species, B. actinocoloniiforme (2 genomes), B. aemilianum (1 genome), B. catulorum (1 genome), and B. xylocopae (1 genome) did not have the ability to generate GABA from glutamate (EC 4.1.1.15) or succinate (EC 2.6.1.19), suggesting that GABA production may not be essential for these strains. In this genome comparison, we found that B. dentium (6 genomes) and 10 other Bifidobacteria species contained all genes required to convert glutamine, glutamate, and succinate into GABA. These findings indicate that B. dentium is well equipped to produce GABA.
To address whether B. dentium could generate GABA in vitro, we grew B. dentium ATCC 27678 in a fully defined bacterial media, termed ZMB1, for 18 h and examined GABA, glutamine, and glutamate levels by LC-MS/MS (Figure 1D). ZMB1 has high concentrations of glutamate (787 µg/mL) and we observed a slight decrease in glutamate concentrations in ZMB1 after the growth of B. dentium. While glutamate concentrations were relatively unchanged, we did observe increased concentrations of GABA and glutamine in B. dentium-conditioned ZMB1, indicating that B. dentium can generate GABA in ZMB1 in vitro.
3.2. Bifidobacterium Dentium ATCC 27678 Influences Fecal GABA Levels in Gnotobiotic Animals
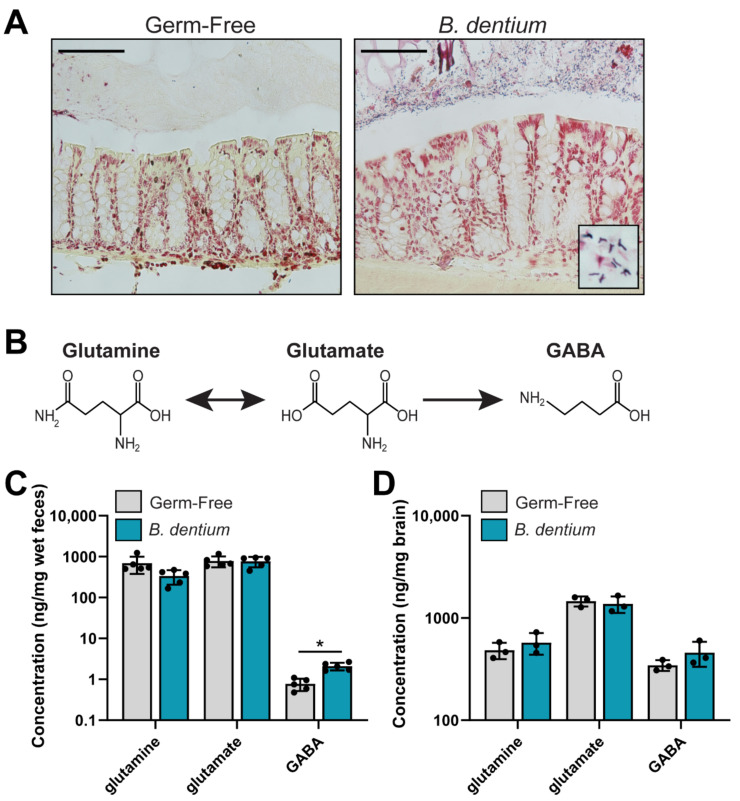
After confirming in vitro production of GABA by B. dentium ATCC 27678, we mono-associated adult mice to determine if B. dentium could modulate GABA in vivo. We examined stool and whole brain homogenates in both B. dentium mono-associated mice and germ-free mice. Colonization was confirmed by Gram staining (Figure 2A). As expected, bifid-shaped microbes were found in the mucus layer above the colonic epithelium in the B. dentium mono-associated mice, while no microbes were present in the germ-free controls (Figure 2A). Next, we assessed the relative concentrations of fecal GABA, glutamate, and glutamine cycle compounds by LC-MS/MS (Figure 2B,C). We found that B. dentium mono-association had no effect on glutamine or glutamate concentrations, both of which were high in the rodent diet (Rodent 50 IF/6F auto 5V0F chow cat# 3002875-703). However, we did observe a significant increase in colonic GABA. We also examined GABA, glutamate, and glutamine in the whole brain homogenate (Figure 2D). Interestingly, no changes were observed in glutamine, glutamate, or GABA concentrations in B. dentium mono-associated brains compared to germ-free controls. This is consistent with the notion that GABA is unable to cross the blood–brain barrier. These data indicate that B. dentium can impact the concentration of GABA in the colon.
Figure 2.
(A). Representative Gram stains of the germ-free and B. dentium mono-associated colon. Scale bar = 100 µm. (B). Depiction of the glutamine, glutamate, GABA pathway. (C). Analysis of glutamine, glutamate, and GABA measured from a known mass of stool (ng/mg wet feces weight) by LC-MS/MS reflecting in vivo levels of these neurotransmitters from the germ-free and B. dentium mono-associated mice. (D). Concentrations of glutamine, glutamate, and GABA in a known mass of whole brain homogenate (ng/mg brain). n = 3–5 mice/group; two-way ANOVA, * p < 0.05.
3.3. Bifidobacteria Harbor the Enzymatic Machinery to Generate the Neuroactive Compoung Tyrosine
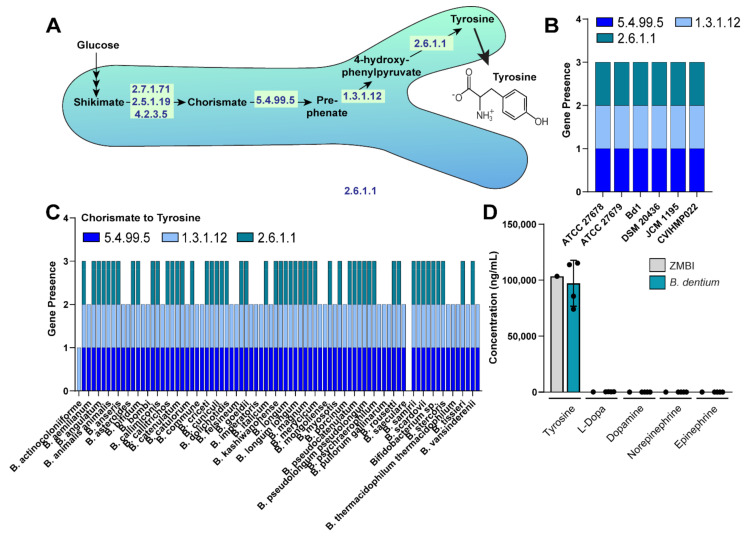
Next, we examined neurotransmitters in the dopamine pathway, including tyrosine, L-dopa, dopamine, norepinephrine, and epinephrine. These neurotransmitters have been shown to act on the enterochromaffin cells and modulate the ENS. We queried the B. dentium Bd1 genome using the KEGG database for the ECs associated with the production of tyrosine, L-dopa, dopamine, norepinephrine, and epinephrine. Analysis of the KEGG pathway revealed that B. dentium could convert chorismate into tyrosine through a three-step process (Figure 3A). However, no genes were identified which could generate L-dopa, dopamine, norepinephrine, or epinephrine. We next searched within the IMG database for the presence of these genes within several B. dentium genomes and found that all six B. dentium genomes, including the B. dentium ATCC 27678 strain used in our gnotobiotic studies, harbored the genes to convert chorismate into tyrosine (Figure 3B).
Figure 3.
(A). Gene pathways in B. dentium as identified via KEGG (https://www.genome.jp/kegg/kegg2.html) related to tyrosine production. (B). Genome analysis of the JGI Integrated Microbial Genomes (IMG) database (http://img.jgi.doe.gov (accessed on 17 January 2021)) of six B. dentium genomes for the enzymes (Enzyme Commission, ECs) involved in tyrosine production. Data represent the number of copies for each EC gene. (C). Genome analysis of 83 Bifidobacteria genomes using the JGI Integrated Microbial Genomes (IMG) database. (D). Absolute concentrations of tyrosine, L-dopa, dopamine, norepinephrine, and epinephrine in uninoculated ZMB1 and cell-free bacterial conditioned ZMB1 from B. dentium after 18 h growth. n= 4 biological replicates, two-way ANOVA.
To determine if tyrosine pathway genes were conserved in the Bifidobacteria genomes, we examined the genomes of the 83 Bifidobacteria species available in the IMG database. Interestingly, 31 (49%) Bifidobacteria species did not have tyrosine aminotransferase (EC 2.6.1.1), which converts 4-hydroxy-phenylpyruvate into tyrosine, indicating that tyrosine production is species dependent. We did not observe any genes related to L-dopa, dopamine, norepinephrine, or epinephrine production in our genome analysis. Next, we examined tyrosine concentrations in our fully defined ZMB1 media (Figure 3C). ZMB1 at baseline contains high concentrations of tyrosine (103 µg/mL). Although we observed that B. dentium had the molecular machinery to produce tyrosine, we found that B. dentium did not generate tyrosine in ZMB1 using LC-MS/MS analysis. As expected, we found no production of L-dopa, dopamine, norepinephrine, and epinephrine, consistent with the absence of the required genes in the B. dentium genome.
3.4. Bifidobacterium Dentium ATCC 27678 Influences Fecal and Neuronal Tyrosine Levels in Gnotobiotic Animals
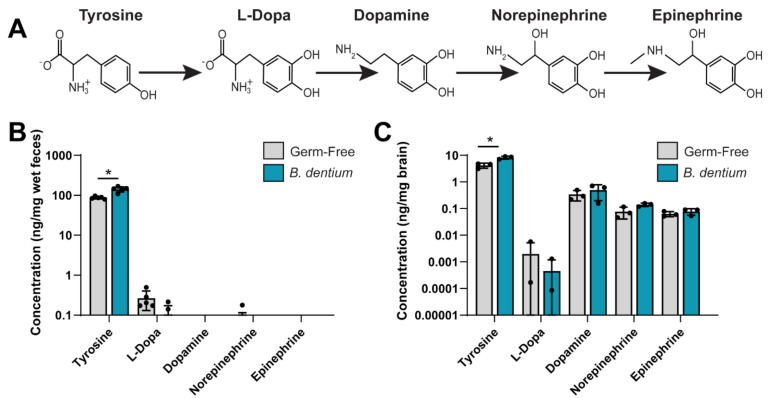
To address whether B. dentium could modulate tyrosine concentrations in vivo, we examined the tyrosine pathway in the stool of B. dentium mono-associated mice by LC-MS/MS and compared them with germ-free controls (Figure 4). We observed elevated levels of tyrosine in the feces of B. dentium-treated mice compared to the germ-free controls (Figure 4B). Tyrosine can be transported through epithelial cells and enter the circulation. Alternatively, tyrosine can enter cells such as enteric neurons and be converted to L-dopa and dopamine, and subsequently to norepinephrine and epinephrine (Figure 4A). Interestingly, no changes were observed in the other neurotransmitters in the stool samples. Since tyrosine can be transported through the blood–brain barrier, we examined the whole brain homogenate by LC-MS/MS (Figure 4C). Similar to our stool, we found that tyrosine was elevated in the brain of B. dentium mono-associated mice compared to the germ-free controls; without changes to the other neurotransmitters. Collectively, these data indicate that B. dentium colonization influences the levels of select gut neurotransmitters (Figure 5).
Figure 4.
(A). Depiction of the tyrosine pathway. (B). Analysis of tyrosine, L-dopa, dopamine, norepinephrine, and epinephrine measured from a known mass of stool (ng/mg wet feces weight) by LC-MS/MS reflecting in vivo levels of these neurotransmitters from germ-free and B. dentium mono-associated mice. (C). Concentrations of tyrosine, L-dopa, dopamine, norepinephrine, and epinephrine in a known mass of whole brain homogenate (ng/mg brain). For all experiments, n = 5 mice/group; two-way ANOVA, * p < 0.05.
Figure 5.
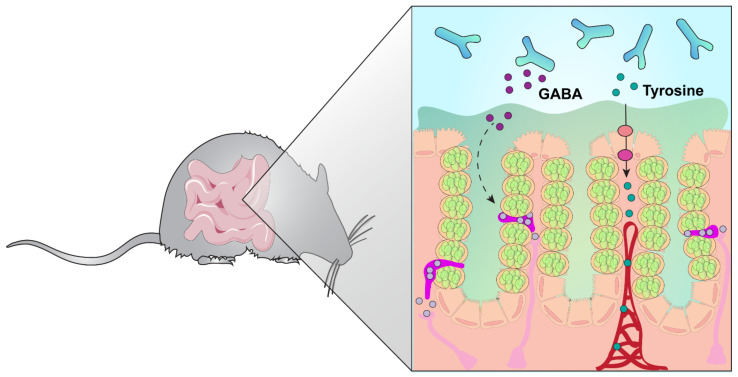
Model diagram of B. dentium colonization. Our data indicate that B. dentium can generate glutamine, GABA, and tyrosine. We speculate that GABA has a local effect on the enteroendocrine cells and enteric neurons in the gut, while tyrosine can be transported through the epithelium, enter the circulation, and be transported into the brain.
4. Discussion
Accumulating evidence indicates that the gut microbiota has a significant impact on the host physiology. One route by which microbes can influence the host is through the production or regulation of neurotransmitters. Herein, we characterize the production of important host-related neurotransmitters by the gut commensal B. dentium. In contrast to other microbes [59], we found that B. dentium was unable to synthesize or modulate dopamine, epinephrine, and norepinephrine. However, B. dentium was able to modulate intestinal concentrations of GABA and tyrosine in vivo. Additionally, we found that B. dentium colonization was associated with an increase in tyrosine in the whole brain homogenate. These findings demonstrate new pathways by which B. dentium can modify the host.
Gnotobiotic models serve as a simple exploratory technique to determine how individual microbes influence the host. Using this approach, we previously found that B. dentium metabolites stimulated the release of serotonin from intestinal enterochromaffin cells [52]. However, the complete profile of intestinal and neuronal neurotransmitters associated with B. dentium colonization was not examined. Herein, we attempted to address this knowledge gap by systemically characterizing stool and whole brain homogenates from germ-free and B. dentium mono-associated mice. We selected the stool as a simplified approach to examine microbial-produced or apically-released neurotransmitters. Because B. dentium was able to produce GABA in vitro in ZMB1 media, we believe that the increased levels of GABA in B. dentium mono-associated stool samples reflect microbially-produced GABA. According to our genome analysis, only 15% of Bifidobacteria harbor all the enzymatic machinery to generate GABA from all sources. We interpret this data to indicate that B. dentium is a more interesting Bifidobacteria candidate for the manipulation of GABA. In vitro, we also observed that B. dentium was able to secrete glutamine, which can enter intestinal epithelial cells via the ASCT2 transporters. Glutamine can be converted to glutamate and subsequently to GABA. Therefore, it is possible that host-derived GABA could contribute to the elevated fecal GABA levels. In the future, it would be interesting to examine how quickly B. dentium produces GABA in vitro and in vivo. This information may inform therapeutic approaches. Our KEGG and IMG pathways analysis revealed that B. dentium could generate glutamate and glutamine, but we did not see any changes in these compounds in our stool samples. Glutamine and glutamate are abundant in the diet, particularly in the diet of germ-free mouse chow, and we speculate that the high availability of these compounds in the intestinal lumen limited the production of these compounds by B. dentium. Additionally, it is possible that B. dentium may be consuming dietary glutamate and glutamine at the same rate as secretion; resulting in no net change in these amino acids. Further studies using stable-isotope labeled substrates would help shed light on the direct contribution of B. dentium to glutamine and glutamate production in vivo. Interestingly, although GABA, glutamate, and glutamine were found in high concentrations in the brain (~1000 ng/mg brain tissue), these levels were unchanged in response to B. dentium colonization. These data suggest that B. dentium colonization in the gut does not influence neuronal GABA concentrations.
Another interesting finding was that B. dentium elevated tyrosine concentrations in the gut and brain. Tyrosine is a non-essential amino acid that can be transported from the intestine into the circulation, cross the blood–brain barrier, and enter neurons, where it is metabolized into catecholamine neurotransmitters. We found that B. dentium harbored the genes to generate tyrosine. We did not observe elevated levels of tyrosine in our ZMB1, which had incredibly high tyrosine levels at baseline. We speculate that the high levels of tyrosine in the ZMB1 limited the production of tyrosine by B. dentium in vitro. Future work optimizing the concentration of tyrosine for growth and secretion would provide an interesting baseline for confirming bacterial tyrosine production. Although we did not observe tyrosine production in vitro, we observed elevated concentrations of tyrosine in the feces of B. dentium-associated mice when compared to the germ-free controls. Since epithelial cells do not appear to have a mechanism to apically secrete tyrosine, we believe that the elevated tyrosine levels in B. dentium-associated mouse stool samples reflect B. dentium synthesis of this amino acid. Although select facultative anaerobes such as Bacillus, Escherichia, and Proteus have been found to produce dopamine and norepinephrine [59], we did not observe detectable levels of dopamine, epinephrine, or norepinephrine in B. dentium-conditioned ZMB1 or in our B. dentium mono-associated mouse feces. These findings are consistent with our KEGG pathway analysis and indicate that B. dentium is unable to synthesize these neurotransmitters.
In vivo, tyrosine is an important regulator of the neuronal catecholamine neurotransmitters, including dopamine, epinephrine, and norepinephrine. Although we saw elevated tyrosine, we did not observe increased concentrations of these catecholamine neurotransmitters in the stool. We speculate that this is because dopamine-producing cells are not commonly found in the intestinal epithelium. Consistent with this notion, we have previously examined a panel of neurotransmitters in human jejunum organoids, also known as enteroids, transduced to increase enteroendocrine cells and we did not detect dopamine, epinephrine, or norepinephrine neurotransmitters [61]. Previous studies have shown that dopamine is largely synthesized in the brain [62]. We found moderate dopamine levels (~1 ng/mg brain tissue) in our germ-free and gnotobiotic mouse brain samples. Epinephrine and norepinephrine are largely produced in sympathetic nerve fibers or adrenal medulla [62]. We also observed moderate concentrations of epinephrine and norepinephrine in the whole brain homogenate. Although we observed increased tyrosine concentration in the B. dentium mono-associated mouse brains, we surprisingly did not see any changes in dopamine, epinephrine, or norepinephrine. This observation could be due to the fact that we examined whole brain neurotransmitter levels. Another possibility is that dopamine concentrations could be elevated in specific brain compartments, but these distinct levels are averaged in a whole brain homogenate. It is also possible that the neuronal cells which generate dopamine require additional metabolite signals generated by other gut microbes to convert tyrosine to dopamine. Finally, since our mice were colonized as adults, it may be that early-life colonization is necessary for the proper wiring of these circuits. Additional studies are necessary to fully tease out this information.
In addition to neurotransmitter production, there are several methods by which the gut microbiota can influence the gut–brain axis, including vagal nerve stimulation, activation of neuropods/direct stimulation of afferent nerves, altering the activity of the stress-associated hypothalamic–pituitary–adrenal (HPA) axis, regulation of the immune cells, and modulating the permeability of the blood–brain barrier [8,24,63,64,65,66,67,68,69,70,71,72,73,74]. Although we have not examined these pathways in our gnotobiotic mouse model, we believe that these routes may be important contributors to the Bifidobacteria–host modulation and should be explored in the future.
In conclusion, this work has provided a unique glimpse into neurotransmitter profiles using germ-free and mono-associated gnotobiotic animals. Our data is among the first to demonstrate that a single microbe is sufficient to modulate select gut and neuronal neurotransmitters in vivo. Previous work with gut microbes has revealed that many microbial effects are strain-specific. As a result, it is important to delineate which microbes are associated with certain effects. We speculate that mono-associated animals and LC-MS/MS analyses may allow researchers to dissect the essential pathways by which commensal microbes influence the gut–brain axis. A better understanding of these pathways may provide novel adjuvant strategies for both gastroenterological and neurologic disorders.
Acknowledgments
The Texas Children’s Hospital Department of Pathology and Immunology provides salary support to Texas Children’s Microbiome Center-Metabolomics Lab staff, and purchased all of the reagents, the consumables and durable supplies, and the LC-MS/MS equipment described.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biom11081091/s1, Table S1: Bifidobacteria species identified in the JGI Integrated Microbial Genomes (IMG) database (http://img.jgi.doe.gov) (accessed on 17 January 2021). Table S2: SRM transition parameters for the glutamate cycle metabolites on the Sciex 6500 QTrap MS. Table S3: SRM transition parameters for the Tyrosine Pathway metabolites on the Sciex 6500 QTrap MS.
Author Contributions
Concept and design, B.L., T.D.H., A.M.H., J.V. and M.A.E.; data acquisition, B.L., T.D.H., K.A.E., W.R., S.J.H., K.M.H. and M.A.E.; data analysis, statistics, and interpretation, B.L., T.D.H., K.A.E., W.R., S.J.H., K.M.H., N.O., J.K.S., A.M.H. and M.A.E.; editing of manuscript, B.L., T.D.H., K.A.E., W.R., S.J.H., K.M.H., N.O., J.K.S., A.M.H., J.V. and M.A.E.; funding, J.V. and M.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the NIH K01K12319501 (M.A.E.). This work was also supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (Grant P30-DK-56338 to the Texas Medical Center Digestive Disease Center), and unrestricted research support from BioGaia AB (Stockholm, Sweden) (J.K.S., J.V.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Baylor College of Medicine, Houston, TX, USA.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
J.V. serves on the scientific advisory board of Seed, a USA-based probiotics/prebiotics company, Biomica, an Israeli informatics enterprise and Plexus Worldwide, a USA-based nutrition company. J.K.S. and J.V. receive unrestricted research support from BioGaia AB, a Swedish probiotics company. The remaining authors have no commercial or financial relationships to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lomasney K.W., Houston A., Shanahan F., Dinan T.G., Cryan J.F., Hyland N.P. Selective influence of host microbiota on cAMP-mediated ion transport in mouse colon. Neurogastroenterol. Motil. 2014;26:887–890. doi: 10.1111/nmo.12328. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A.D., Wang X.Y., Parsons S.P., Khan W.I., Huizinga J.D. Abnormal absorptive colonic motor activity in germ-free mice is rectified by butyrate, an effect possibly mediated by mucosal serotonin. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G896–G907. doi: 10.1152/ajpgi.00237.2017. [DOI] [PubMed] [Google Scholar]
- 3.Collins J., Borojevic R., Verdu E.F., Huizinga J.D., Ratcliffe E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 4.Husebye E., Hellstrom P.M., Sundler F., Chen J., Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 5.Wichmann A., Allahyar A., Greiner T.U., Plovier H., Lunden G.O., Larsson T., Drucker D.J., Delzenne N.M., Cani P.D., Backhed F. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Yajima M., Karaki S.I., Tsuruta T., Kimura S., Nio-Kobayashi J., Kuwahara A., Yajima T. Diversity of the intestinal microbiota differently affects non-neuronal and atropine-sensitive ileal contractile responses to short-chain fatty acids in mice. Biomed. Res. 2016;37:319–328. doi: 10.2220/biomedres.37.319. [DOI] [PubMed] [Google Scholar]
- 7.Dey N., Wagner V.E., Blanton L.V., Cheng J., Fontana L., Haque R., Ahmed T., Gordon J.I. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163:95–107. doi: 10.1016/j.cell.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge X., Ding C., Zhao W., Xu L., Tian H., Gong J., Zhu M., Li J., Li N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017;15:13. doi: 10.1186/s12967-016-1105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge X., Zhao W., Ding C., Tian H., Xu L., Wang H., Ni L., Jiang J., Gong J., Zhu W., et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci. Rep. 2017;7:441. doi: 10.1038/s41598-017-00612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pokusaeva K., Johnson C., Luk B., Uribe G., Fu Y., Oezguen N., Matsunami R.K., Lugo M., Major A., Mori-Akiyama Y., et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017;29 doi: 10.1111/nmo.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luczynski P., Tramullas M., Viola M., Shanahan F., Clarke G., O’Mahony S., Dinan T.G., Cryan J.F. Microbiota regulates visceral pain in the mouse. Elife. 2017;6 doi: 10.7554/eLife.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miquel S., Martin R., Lashermes A., Gillet M., Meleine M., Gelot A., Eschalier A., Ardid D., Bermudez-Humaran L.G., Sokol H., et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci. Rep. 2016;6:19399. doi: 10.1038/srep19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annahazi A., Gecse K., Dabek M., Ait-Belgnaoui A., Rosztoczy A., Roka R., Molnar T., Theodorou V., Wittmann T., Bueno L., et al. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain. 2009;144:209–217. doi: 10.1016/j.pain.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Sessenwein J.L., Baker C.C., Pradhananga S., Maitland M.E., Petrof E.O., Allen-Vercoe E., Noordhof C., Reed D.E., Vanner S.J., Lomax A.E. Protease-Mediated Suppression of DRG Neuron Excitability by Commensal Bacteria. J. Neurosci. 2017;37:11758–11768. doi: 10.1523/JNEUROSCI.1672-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Mahony S.M., Felice V.D., Nally K., Savignac H.M., Claesson M.J., Scully P., Woznicki J., Hyland N.P., Shanahan F., Quigley E.M., et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience. 2014;277:885–901. doi: 10.1016/j.neuroscience.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 17.McKernan D.P., Fitzgerald P., Dinan T.G., Cryan J.F. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol. Motil. 2010;22:1029–1035.e1268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 18.Ait-Belgnaoui A., Eutamene H., Houdeau E., Bueno L., Fioramonti J., Theodorou V. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterol. Motil. 2009;21:567–573. doi: 10.1111/j.1365-2982.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- 19.Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 20.Diaz Heijtz R., Wang S., Anuar F., Qian Y., Bjorkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23:255–264.e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishino R., Mikami K., Takahashi H., Tomonaga S., Furuse M., Hiramoto T., Aiba Y., Koga Y., Sudo N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013;25:521–528. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- 23.Neufeld K.A., Kang N., Bienenstock J., Foster J.A. Effects of intestinal microbiota on anxiety-like behavior. Commun. Integr. Biol. 2011;4:492–494. doi: 10.4161/cib.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly J.R., Borre Y., O’Brien C., Patterson E., El Aidy S., Deane J., Kennedy P.J., Beers S., Scott K., Moloney G., et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., Zeng L., Chen J., Fan S., Du X., et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 27.Harach T., Marungruang N., Duthilleul N., Cheatham V., Mc Coy K.D., Frisoni G., Neher J.J., Fak F., Jucker M., Lasser T., et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minter M.R., Zhang C., Leone V., Ringus D.L., Zhang X., Oyler-Castrillo P., Musch M.W., Liao F., Ward J.F., Holtzman D.M., et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cekanaviciute E., Yoo B.B., Runia T.F., Debelius J.W., Singh S., Nelson C.A., Kanner R., Bencosme Y., Lee Y.K., Hauser S.L., et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA. 2017;114:10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berer K., Mues M., Koutrolos M., Rasbi Z.A., Boziki M., Johner C., Wekerle H., Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Backhed F., Roswall J., Peng Y., Feng Q., Jia H., Kovatcheva-Datchary P., Li Y., Xia Y., Xie H., Zhong H., et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Di Gioia D., Aloisio I., Mazzola G., Biavati B. Bifidobacteria: Their impact on gut microbiota composition and their applications as probiotics in infants. Appl. Microbiol. Biotechnol. 2014;98:563–577. doi: 10.1007/s00253-013-5405-9. [DOI] [PubMed] [Google Scholar]
- 34.Lim E.S., Zhou Y., Zhao G., Bauer I.K., Droit L., Ndao I.M., Warner B.B., Tarr P.I., Wang D., Holtz L.R. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 2015;21:1228–1234. doi: 10.1038/nm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makino H., Kushiro A., Ishikawa E., Kubota H., Gawad A., Sakai T., Oishi K., Martin R., Ben-Amor K., Knol J., et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS ONE. 2013;8:e78331. doi: 10.1371/journal.pone.0078331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuki T. Development of quantitative PCR detection method with 16S rRNA gene-targeted genus- and species-specific primers for the analysis of human intestinal microflora and its application. Nihon Saikingaku Zasshi. Jpn. J. Bacteriol. 2007;62:255–261. doi: 10.3412/jsb.62.255. [DOI] [PubMed] [Google Scholar]
- 37.Turroni F., Foroni E., Pizzetti P., Giubellini V., Ribbera A., Merusi P., Cagnasso P., Bizzarri B., de’Angelis G.L., Shanahan F., et al. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl. Environ. Microbiol. 2009;75:1534–1545. doi: 10.1128/AEM.02216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Favier C.F., Vaughan E.E., De Vos W.M., Akkermans A.D. Molecular monitoring of succession of bacterial communities in human neonates. Appl. Environ. Microbiol. 2002;68:219–226. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagpal R., Kurakawa T., Tsuji H., Takahashi T., Kawashima K., Nagata S., Nomoto K., Yamashiro Y. Evolution of gut Bifidobacterium population in healthy Japanese infants over the first three years of life: A quantitative assessment. Sci. Rep. 2017;7:10097. doi: 10.1038/s41598-017-10711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saturio S., Nogacka A.M., Suarez M., Fernandez N., Mantecon L., Mancabelli L., Milani C., Ventura M., de Los Reyes-Gavilan C.G., Solis G., et al. Early-Life Development of the Bifidobacterial Community in the Infant Gut. Int. J. Mol. Sci. 2021;22:3382. doi: 10.3390/ijms22073382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He M., Li M., Wang S.Y., Zhang L.L., Miao J.J., Shi L., Yu Q., Yao J.R., Huang C.Y., He F. Analyzing Colonization of Bifidobacteria in Infants with Real-time Fluorescent Quantitative PCR. Sichuan Da Xue Xue Bao Yi Xue Ban. 2016;47:527–532. [PubMed] [Google Scholar]
- 42.Turroni F., Peano C., Pass D.A., Foroni E., Severgnini M., Claesson M.J., Kerr C., Hourihane J., Murray D., Fuligni F., et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE. 2012;7:e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinne M.M., Gueimonde M., Kalliomaki M., Hoppu U., Salminen S.J., Isolauri E. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol. Med. Microbiol. 2005;43:59–65. doi: 10.1016/j.femsim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson J.F., Rougeot C., Pichelin M., Cazaubiel M., et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 46.Savignac H.M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 47.Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T., Martin F.P., Cominetti O., Welsh C., Rieder A., et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology. 2017;153:448–459.e448. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Desbonnet L., Garrett L., Clarke G., Kiely B., Cryan J.F., Dinan T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Savignac H.M., Tramullas M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 50.Luk B., Veeraragavan S., Engevik M., Balderas M., Major A., Runge J., Luna R.A., Versalovic J. Postnatal colonization with human “infant-type” Bifidobacterium species alters behavior of adult gnotobiotic mice. PLoS ONE. 2018;13:e0196510. doi: 10.1371/journal.pone.0196510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luck B., Engevik M.A., Ganesh B.P., Lackey E.P., Lin T., Balderas M., Major A., Runge J., Luna R.A., Sillitoe R.V., et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci. Rep. 2020;10:7737. doi: 10.1038/s41598-020-64173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engevik M.A., Luck B., Visuthranukul C., Ihekweazu F.D., Engevik A.C., Shi Z., Danhof H.A., Chang-Graham A.L., Hall A., Endres B.T., et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell. Mol. Gastroenterol. Hepatol. 2021;11:221–248. doi: 10.1016/j.jcmgh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arboleya S., Watkins C., Stanton C., Ross R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engevik M.A., Danhof H.A., Hall A., Engevik K.A., Horvath T.D., Haidacher S.J., Hoch K.M., Endres B.T., Bajaj M., Garey K.W., et al. The metabolic profile of Bifidobacterium dentium reflects its status as a human gut commensal. BMC Microbiol. 2021;21:154. doi: 10.1186/s12866-021-02166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engevik M.A., Luk B., Chang-Graham A.L., Hall A., Herrmann B., Ruan W., Endres B.T., Shi Z., Garey K.W., Hyser J.M., et al. Bifidobacterium dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. mBio. 2019;10:e01087-19. doi: 10.1128/mBio.01087-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engevik M.A., Herrmann B., Ruan W., Engevik A.C., Engevik K.A., Ihekweazu F., Shi Z., Luck B., Chang-Graham A.L., Esparza M., et al. Bifidobacterium dentium-derived y-glutamylcysteine suppresses ER-mediated goblet cell stress and reduces TNBS-driven colonic inflammation. Gut Microbes. 2021;13:1–21. doi: 10.1080/19490976.2021.1902717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Palma G., Blennerhassett P., Lu J., Deng Y., Park A.J., Green W., Denou E., Silva M.A., Santacruz A., Sanz Y., et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 58.Zhang G., Mills D.A., Block D.E. Development of chemically defined media supporting high-cell-density growth of lactococci, enterococci, and streptococci. Appl. Environ. Microbiol. 2009;75:1080–1087. doi: 10.1128/AEM.01416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feehily C., Karatzas K.A. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J. Appl. Microbiol. 2013;114:11–24. doi: 10.1111/j.1365-2672.2012.05434.x. [DOI] [PubMed] [Google Scholar]
- 61.Chang-Graham A.L., Danhof H.A., Engevik M.A., Tomaro-Duchesneau C., Karandikar U.C., Estes M.K., Versalovic J., Britton R.A., Hyser J.M. Human Intestinal Enteroids With Inducible Neurogenin-3 Expression as a Novel Model of Gut Hormone Secretion. Cell. Mol. Gastroenterol. Hepatol. 2019;8:209–229. doi: 10.1016/j.jcmgh.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mittal R., Debs L.H., Patel A.P., Nguyen D., Patel K., O’Connor G., Grati M., Mittal J., Yan D., Eshraghi A.A., et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017;232:2359–2372. doi: 10.1002/jcp.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 66.Wang X., Wang B.R., Zhang X.J., Xu Z., Ding Y.Q., Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J. Gastroenterol. 2002;8:540–545. doi: 10.3748/wjg.v8.i3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verdu E.F., Bercik P., Verma-Gandhu M., Huang X.X., Blennerhassett P., Jackson W., Mao Y., Wang L., Rochat F., Collins S.M. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 69.Akiba Y., Inoue T., Kaji I., Higashiyama M., Narimatsu K., Iwamoto K., Watanabe M., Guth P.H., Engel E., Kuwahara A., et al. Short-chain fatty acid sensing in rat duodenum. J. Physiol. 2015;593:585–599. doi: 10.1113/jphysiol.2014.280792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bellono N.W., Bayrer J.R., Leitch D.B., Castro J., Zhang C., O’Donnell T.A., Brierley S.M., Ingraham H.A., Julius D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170:185–198.e116. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reigstad C.S., Salmonson C.E., Rainey J.F., III, Szurszewski J.H., Linden D.R., Sonnenburg J.L., Farrugia G., Kashyap P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsuruta T., Saito S., Osaki Y., Hamada A., Aoki-Yoshida A., Sonoyama K. Organoids as an ex vivo model for studying the serotonin system in the murine small intestine and colon epithelium. Biochem. Biophys. Res. Commun. 2016;474:161–167. doi: 10.1016/j.bbrc.2016.03.165. [DOI] [PubMed] [Google Scholar]
- 73.Fukumoto S., Tatewaki M., Yamada T., Fujimiya M., Mantyh C., Voss M., Eubanks S., Harris M., Pappas T.N., Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 74.Arentsen T., Qian Y., Gkotzis S., Femenia T., Wang T., Udekwu K., Forssberg H., Diaz Heijtz R. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry. 2017;22:257–266. doi: 10.1038/mp.2016.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request.