Abstract
Significance: Wound healing involves the phasic production of growth factors (GFs) and cytokines to progress an acute wound to a resolved scar. Dysregulation of these proteins contributes to both wound chronicity and excessive scarring. Direct supplementation of GFs and cytokines for treatment of healing and scarring complications has, however, been disappointing. Failings likely relate to an inability to deliver recombinant proteins at physiologically relevant levels to an environment conducive to healing.
Recent Advances: Inspired by the extracellular matrix, natural biomaterials have been developed that resemble human skin, and are capable of delivering bioactives. Hybrid biomaterials made using multiple polymers, fabrication methods, and proteins are proving efficacious in animal models of acute and impaired wound healing.
Critical Issues: For clinical translation, these delivery systems must be tailored for specific wound indications and the correct phase of healing. GFs and cytokines must be delivered in a controlled manner that will target specific healing or scarring impairments. Preclinical assessment in clinically relevant animal models of impaired or excessive healing is critical.
Future Directions: Clinical success will likely depend on the GF or cytokine selected, their compatibility with the chosen biomaterial(s), degradation rate of the fabricated system, and the degree of control over release kinetics. Further testing is essential to assess which wound indications are most suited to specific delivery systems and to prove whether they provide superior efficacy over direct protein therapies.
Keywords: growth factor, cytokine, skin, wound, scar, biomaterial, delivery

Lyn Wise, PhD
Scope and Significance
This review introduces growth factors (GFs) and cytokines involved in the skin wound healing response and summarizes attempts to harness them as wound therapeutics from studies that meet 50% or more of the CONSORT randomized control trial reporting standards. Natural biomaterials developed as GF and cytokine delivery systems are then critiqued as to whether they offer therapeutic advantages over direct protein administration. Finally, a preclinical development pathway is proposed to aid translation of these wound and scar therapies.
Translational Relevance
Much is known about the roles of GFs and cytokines in successful, impaired, and excessive healing responses. Yet, attempts to harness them as wound therapeutics have yielded disappointing results. To address limitation associated with their administration, biomaterial-based delivery systems that offer tailored protein release, proteolytic and immune protection, and an extracellular matrix (ECM) replacement have been developed. While these systems are proving efficacious in preclinical models, it is unclear whether this will translate to human wound indications. To ensure success, it is critical that testing occurs in a standardized manner in clinically relevant wound models, in comparison to direct protein administration.
Clinical Relevance
Wound skin complications are highly prevalent and include surgical site infections, traumatic or combat injuries, burns, pressure ulcers in the immobile, foot and limb ulcers in the elderly, obese, and diabetic, as well as fibrotic and scarring disorders. While numerous GFs and cytokines have been tested for these pathologies, only selected treatments are approved for clinical use. Presently, Regranex® is the only GF product that is FDA-approved for treatment of neuropathic diabetic ulcers, with two others available in selected countries. As yet, no such treatments are available for pressure ulcers, or for surgical, traumatic, burn, infected, or scar-prone wounds.
The reasons why GF and cytokine therapies may have failed are numerous. Contributing factors likely include trial design limitations, poor patient compliance, risk of systemic action and immunogenicity, proteolytic degradation, and variability in the responsiveness and support for healing provided by the surrounding tissue. The net result being a failure to deliver therapeutically relevant levels of these proteins to a wound receptive to that intervention. Given the clinical need, it is critical that issues identified with GF and cytokine use be addressed. Natural biomaterials that provide sustained protein retention and delivery, along with a physical environment supportive of healing, thus offer hope in overcoming these limitations and addressing the growing health burden associated with cutaneous wounds.
Background
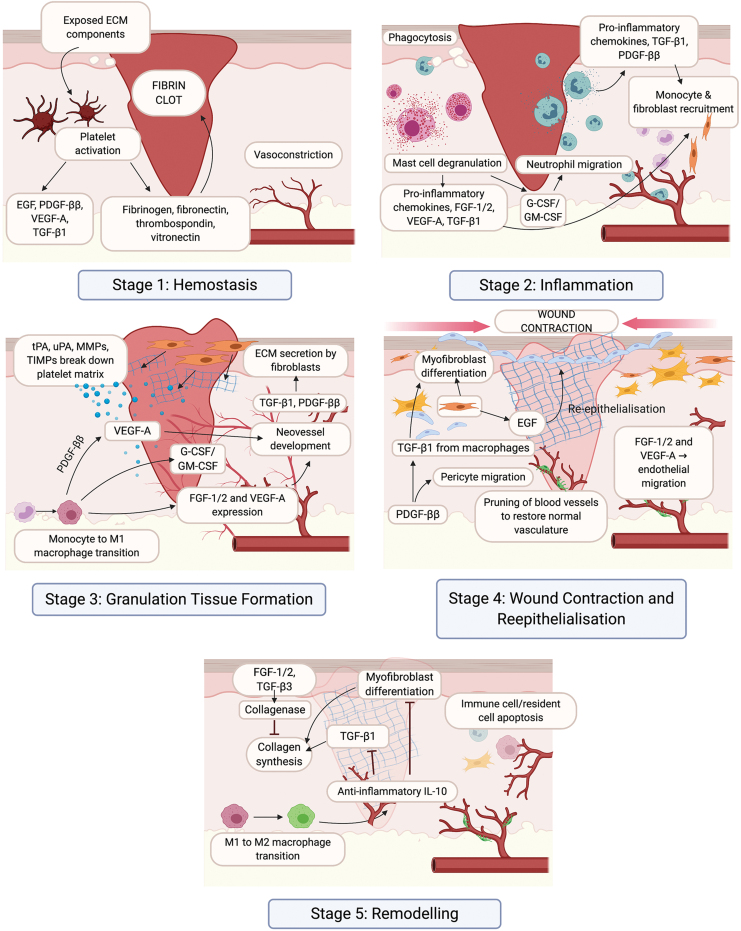
Cutaneous wounding stimulates a complex series of events that regulate the progression from an acute wound to a resolved scar (Fig. 1). This process involves a diverse number of cell types, ECM components, the regulatory GFs, and cytokines that direct their interactions.1
Figure 1.
The wound healing response of the skin. There are five key stages in the wound healing response: hemostasis (1), inflammation (2), granulation tissue formation (3), wound contraction and reepithelialization (4), and remodeling (5). Critical cell types, GFs, and cytokines that regulate each stage of the wound healing response are indicated. ECM, extracellular matrix; EGF, epidermal growth factor; FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HA, hyaluronic acid; IL, interleukin; MMP, matrix metalloproteinases; PDGF, platelet-derived growth factor; TGF, transforming growth factor; tPA, tissue plasminogen activator; uPA, urokinase-type plasminogen activator; VEGF, vascular endothelial growth factor. Color images are available online.
Successful cutaneous wound healing
Following injury, the skin must reach hemostasis before it can begin to heal (Fig. 1).2 Disruption of the endothelium exposes ECM components that activate platelets, initiating the coagulation cascade, with cleavage of fibrinogen by thrombin and release of fibronectin, thrombospondin, and vitronectin. This leads to platelet aggregation and formation of a fibrin clot to prevent blood loss, protects from microbial infiltration, and provides a scaffold for invasion of inflammatory cells in response to inflammatory cytokines and GFs released by platelets.
During the inflammatory phase of wound healing (Fig. 1),3 damaged endothelial cells facilitate entry of neutrophils to phagocytose debris and invading bacteria, and express proinflammatory cytokines that recruit monocytes and fibroblasts to initiate granulation tissue formation. Monocytes then differentiate into proinflammatory (M1) macrophages that produce matrix metalloproteinases (MMPs) to enhance migration through the fibrin clot.4 M1 macrophages also produce GFs and cytokines that recruit endothelial cells into the provisional ECM, with the new blood vessels giving the tissue its highly vascularized and granular appearance.5
Once granulation tissue formation has commenced, wound closure occurs through reepithelialization and contraction (Fig. 1). Keratinocytes migrate across the provisional matrix in response to GFs to restore the epithelial barrier.6 This process is assisted by differentiation of fibroblasts into alpha-smooth muscle actin-expressing myofibroblasts that support wound contraction.7 Transition of M1 macrophages into a M2 phenotype allows for expression of cytokines that dampen inflammation and cell proliferation.4
GFs activate fibroblasts to secrete a new collagen-rich ECM that undergoes substantial remodeling (Fig. 1), a process that can last for years.8 During this time, cellular recruitment ceases, with much of the infiltrate undergoing apoptosis. Recruitment of pericytes to the endothelium allows for maturation of its function.9 Differentiation of the epithelium reestablishes barrier functionality, although regeneration of epithelial appendages, such as hair follicles and sweat glands, is minimal.6 Fibroblasts continue to synthesize ECM, which transitions from type III to type I collagen, increasing tensile strength and structural integrity. GFs and MMPs produced by M2 macrophages regulate the realignment of collagen into highly organized bundles that strengthen the scar.8
Cutaneous wound complications: impaired and excessive healing
Healing complications emerge in patients with vascular insufficiency and diabetic neuropathy, but chronicity can also occur in acute wounds following surgical, traumatic, or burn injury.10–12
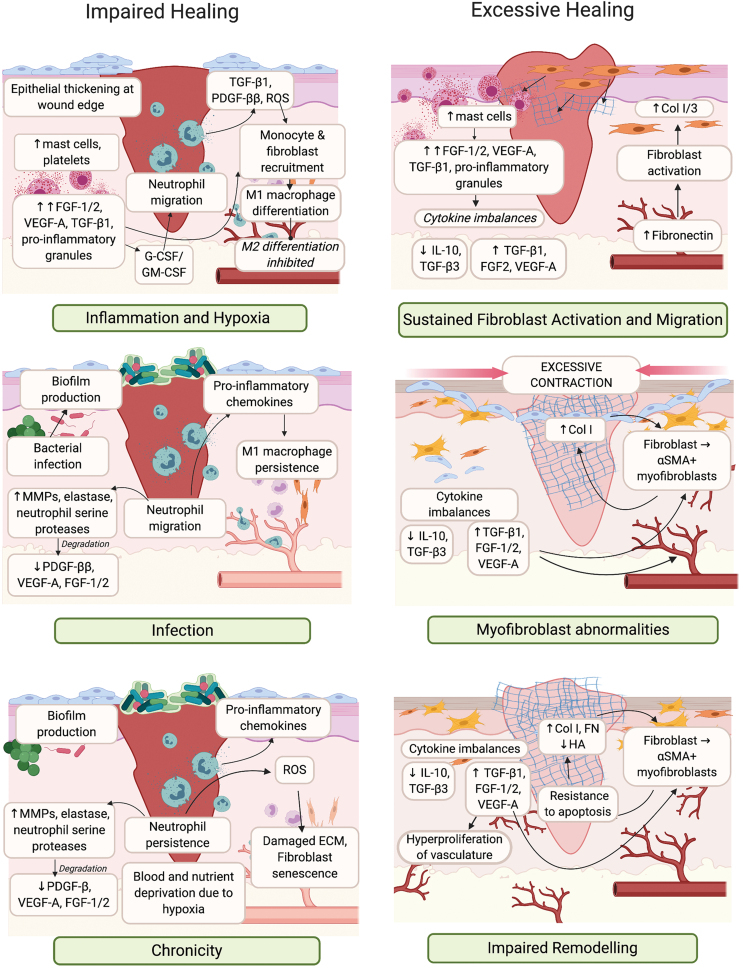
Impaired healing results from disruptions in the transition between stages, leaving the wound in perpetual inflammation and hypoxia (Fig. 2).3,12 Inflammatory and oxidative complications are driven by excess platelet activation, neutrophil and monocyte recruitment, production of proinflammatory cytokines, lipid mediators, reactive oxygen species (ROS), as well as MMP-mediated degradation of GFs, cytokines, and ECM. Damage to the vasculature results in a failure to deliver sufficient oxygen and nutrients to support repair. Persisting neutrophils and ROS production also damages the ECM causing cellular senescence. M1 macrophage persistence is observed, as these cells fail to differentiate into the proresolving M2 phenotype. Transition to a chronic state is also associated with formation of antibiotic-recalcitrant bacterial biofilm communities, which produce proteases that degrade GFs and cytokines.13,14
Figure 2.
Impaired or excessive healing of skin wounds. Impaired healing occurs due to inflammation and infection, resulting in wound chronicity (left panel). Excessive healing occurs due to sustained inflammation and myofibroblast abnormalities, resulting in impaired remodeling (right panel). Critical cell types, GFs and cytokines that are dysregulated each stage of the wound healing response are indicate. αSMA, alpha-smooth muscle actin; Col, collagen; FN, fibronectin; ROS, reactive oxygen species. Color images are available online.
Cumulatively, the failure to produce ECM confounds GF and cytokine dysfunction, leading to defective granulation tissue formation and a hyperproliferative epidermis that prevents reepithelialization.
Where chronicity is defined as a lack of healing, excessive scarring is a case of exaggerated healing.15 Hypertrophic scars are characterized by hyperproliferation of the ECM and surrounding vasculature, and form 1–3 months after tissue insult. However, unlike keloid scars, they do not extend outside of the original wound boundary.16 These scars emerge as a result of excessive inflammation and ECM deposition (Fig. 2). Mast cell infiltration and degranulation contributes to increased histamine, proteoglycans, proteinases, proinflammatory cytokine, and GF levels.17 Excess in these mediators sustains the activation of M2 macrophages, endothelial cells, and fibroblasts.18–20
Normal, hypertrophic, and keloid scars, in turn, are associated with greater extent, persistence, and dysfunction of the vascular and fibrotic responses, which leads to pronounced type I collagen deposition and bundle thickening, resulting in a characteristic lack of flexibility.
GFs and cytokines in the treatment of human wound indications
A multitude of GFs and cytokines are critical to skin repair, with their dysregulation contributing to impaired or excessive healing (Figs. 1 and 2).21–23 Their direct administration has also been trialled as treatments for chronic ulcers, burns, or scar-prone wounds, and those trials that meet 50% or more of the CONSORT reporting standards to clinical trials are listed in Table 1 (with the full CONSORT checklist provided in Supplementary Table S1).24 Of particular importance are the epidermal growth factor (EGF), colony-stimulating factor (CSF), fibroblast growth factor (FGF), interleukin (IL), platelet-derived growth factor (PDGF), transforming growth factor (TGF), and vascular endothelial growth factor (VEGF) families.
Table 1.
Randomized control trials of cutaneous wound and scar treatments utilizing growth factors or cytokines
| Treatment | Clinical Indication | Delivery | Clinical Outcome | CONSORT Standards Total Scorec/2524 |
|---|---|---|---|---|
| PDGF-ββ | Pressure ulcers (Wagner stage III–IV) | Topical spray, daily for 28 days | Trend toward faster healing than vehicle control30 | 14.5 |
| Pressure ulcers (III–IV) | Topical hydrogel (Regranex®), 1–2 × daily for 16 weeksa | Faster and more complete healing than hydrogel control31 | 15.25 | |
| Diabetic foot ulcers (1–100 cm2) | Topical hydrogel, daily for 20 weeksa | Complete healing increased relative to hydrogel control28 | 18 | |
| Diabetic foot ulcers—chronic | Topical hydrogel (Regranex), 2 × daily for 20 weeks | Complete healing increased relative to hydrogel control27 | 13.75 | |
| Diabetic foot ulcers (III–IV)—neuropathic | Topical hydrogel (Regranex), daily for 20 weeksa | Faster and more complete healing than hydrogel control29 | 17 | |
| Ulcers—hypertensive | Topical hydrogel (Regranex), daily for 8 weeks | No change in healing relative to Duoderm hydrogel control32 | 19.25 | |
| Traumatic wounds | Topical hydrogel (Plermin™)b | Faster re-epithelialization relative to saline control33 | 17.25 | |
| EGF | Diabetic foot ulcers (I–II) | Actovegin cream, daily for 12 weeksa | Complete healing increased relative to cream control38 | 15.75 |
| Diabetic foot ulcers (I–II) | Topical hydrogel (Regen-D™), 2 × daily for 15 weeksa | Complete healing increased relative to hydrogel control42 | 15.25 | |
| Diabetic foot ulcers (I–II) | Intralesional injection (Heberprot-P®), 3 × weekly for 8 weeks | Complete healing increased relative to vehicle control40 | 15 | |
| Diabetic foot ulcers (I–II) | Topical spray (Easyef®), 2 × daily for 12 weeksa | Complete healing increased relative to saline control39 | 22.25 | |
| Diabetic foot ulcers (I–II) | Topical hydrogel (Regen-D), daily for 30 days | Trend toward faster healing than hydrogel control41 | 14 | |
| Thyroidectomy incision | Topical, daily for 4 days | Trend toward improved scar pliability and thickness relative to standard care43 | 14.25 | |
| FGF-1 | Burns—partial thickness Donor site wounds |
Topical spray, daily for 3 weeksa | Faster healing than vehicle control49 | 16.25 |
| Diabetic chronic wounds (>2 cm2) | Topical, daily for 6 weeksa | Faster healing than FGF-2 control50 | 14.5 | |
| FGF-2 | Pressure ulcers (III–IV) | Topical spray, tiered dosing and length | Trend toward faster healing than vehicle control46 | 15 |
| Pressure ulcers (III–IV) | Topical spray, daily for 35 days | Faster healing than vehicle control45 | 12.5 | |
| Diabetic foot ulcers (I–III)—neuropathic | Topical spray, daily for 6 weeks, 2 × daily for 12 weeks | No change in healing relative to saline control47 | 15.75 | |
| Traumatic wounds | Collagen spongeb | Complete healing increased relative to standard care79 | 17.5 | |
| Burns—partial thickness | Topical spray (Fiblast®)b | Faster healing than standard care, with improved scar extension and elasticity48 | 13 | |
| VEGF-A | Diabetic foot ulcers (I) | Topical CMC hydrogel (Telbermin), every 2 days for 6 weeks | Trend toward faster healing than hydrogel control52 | 16.5 |
| G-CSF | Infected diabetic foot ulcer | SC injection (Neupogen®), daily for 7 days | Faster resolution of infection than saline56 | 18.75 |
| Infected diabetic foot ulcer | SC injection (Granocyte®), daily for 21 days | No change in infection or rate of healing relative to standard care55 | 15.75 | |
| Infected diabetic foot ulcer | SC injection (Neupogen), daily for 10 days | No change in infection, but trend toward faster healing than saline control57 | 14.75 | |
| GM-CSF | Chronic ulcers—mixed | SC injection (Leucomax®), day 0 | Trend toward faster healing than vehicle control58 | 14.75 |
| Venous ulcers (3–30 cm2) | SC injection (Leucomax), weekly for 4 weeksa | Complete healing increased relative to vehicle control59 | 19.25 | |
| Pressure ulcers (III-IV) | Topical spray, daily for 35 days | No change in healing relative to vehicle control45 | 12.5 | |
| Chronic ulcers—mixed | Alginate dressingb | Faster healing and lower pain score than GM-CSF paste control and standard care80 | 13.5 | |
| Burns—partial thickness | Gelatin hydrogel, dailyb | Complete healing increased relative to hydrogel control83 | 18 | |
| Burns—partial thickness | Gelatin hydrogel, dailya | Faster and more complete healing than hydrogel control82 | 13.75 | |
| Burns—partial thickness | Gelatin hydrogel, 4 weeksb | Faster and more complete healing than vehicle control81 | 14.5 | |
| TGF-β3 | Pressure ulcers (15–120 cm2) | Topical hydrogel, daily for 16 weeksa | Faster healing than hydrogel control63 | 12.5 |
| Incisions | ID injection (Avotermin), before+after incision | Scar score improved relative to vehicle control64 | 17.75 | |
| Incisions | ID injection (Avotermin), before+after incision | Scar score improved relative to vehicle control65 | 17.75 | |
| Bilateral varicose vein surgery | ID injection (Avotermin), day 0 | Scar score improved relative to vehicle control66 | 17 | |
| Scar revision surgery | ID injection (Avotermin), before+after incision | Scar score transiently improved relative to vehicle control67 | 18.5 | |
| Scar treatment | Silicone HA cream, 2 × daily for 12 weeks | Scar score improved relative to silicone control84 | 17.25 | |
| IL-10 | Incisions | ID injection (Ilodecakin), before+after incision | Scar score and redness improved relative to vehicle control69 | 15.25 |
| Incisions | ID injection (Ilodecakin), 2 × after incision | Trend toward reduced scar width and transient improvement in scar score relative to vehicle control70 | 16 |
Until primary endpoint was reached.
Treatment timing or length not evident.
Adherence to CONSORT reporting standards.
CMC, carboxymethylcellulose; EGF, epidermal growth factor; FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HA, hyaluronic acid; IL, interleukin; PDGF, platelet-derived growth factor, TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
The only FDA-approved GF treatment for neuropathic ulcers is recombinant human (rh)PDGF-ββ delivered in a carboxymethylcellulose (CMC) hydrogel (Regranex). A biosimilar (Plermin™) was launched in India in 2013. PDGF-ββ is released during hemostasis by activated platelets to promote migration of neutrophils, macrophages, and fibroblasts, induce granulation tissue formation, and recruit pericytes to new blood vessels (Fig. 1).25 Expression of PDGF-ββ is decreased in chronic wounds (Fig. 2).26
Once or twice daily application of rhPDGF-ββ hydrogel led to more complete healing of nonhealing diabetic foot ulcers (Table 1),27–29 with a trend toward faster healing in pressure ulcers30,31 but not hypertensive ulcers.32 Faster reepithelialization of traumatic wounds was also observed following hydrogel treatment.33 It has noted that in these trials, there was extensive variability in wound closure rates for placebo and standard care controls, and in response to rhPDGF-ββ hydrogel, meaning that large trials were needed to demonstrate a treatment effect.34
An increased risk of death from cancer was also observed,35 which was postulated to result from systemic leakage of PDGF-ββ from the wound stimulated the growth of preexisting cancers expressing its receptor. As a result, Regranex was issued a black-box warning by the FDA in 2008, but this was removed in 2018 in response to postmarketing analysis that showed no increased risk of cancer or cancer mortality.36
Three products containing rhEGF are available for treatment of diabetic foot ulcers. Heberprot-P® is registered in 15 countries and is administered by intralesional injection to recalcitrant ulcers. Easyef® is a topical spray approved for diabetic foot ulcers in four Asian countries. Regen-D™ is a topical hydrogel commercialized in India. EGF is produced by activated platelets, M1 and M2 macrophages, and fibroblasts and drives reepithelialization (Fig. 1).37 Expression of EGF is limited in chronic wounds (Fig. 2).26
An early clinical study with rhEGF administered topically once or twice daily to diabetic foot ulcers showed more complete healing than the cream control (Table 1).38 Complete healing of superficial ulcers in diabetic patients was also accelerated by rhEGF when administered by twice daily topical spray39 or intralesional injection three times per week,40 with variable findings from the topical hydrogel.41,42 Short-term topical administration of rhEGF in thyroidectomy incisions led to improvements in scar parameters relating to pliability and thickness.43
A topical spray containing rhFGF-2 (Fiblast®) is marketed for skin ulcers in Japan, while rhFGF-1 has been evaluated for skin ulcers and burns. FGF-1 and FGF-2, also known as acidic and basic FGF, respectively, are produced by most skin cells. These GFs increase keratinocyte, endothelial, and fibroblast motility to promote reepithelialization and granulation tissue formation (Fig. 1).44 FGF-2 also regulates the production of collagenase/MMP1 to refine the newly synthesised ECM.
Decreased expression of FGF-2 has been observed in chronic wounds (Fig. 2).26 Following daily application of rhFGF-2 using topical sprays (Table 1), severe pressure ulcers showed trends toward faster healing,45,46 while diabetic patients with neuropathic ulcers of mixed severity showed no change in healing.47 Treatment of partial-thickness burns with topical rhFGF-2 spray, also elicited improvements in healing rate and scar flexibility.48 Daily topical administration of rhFGF-1 accelerated healing in partial-thickness burns and skin graft donor sites (Table 1)49 and showed greater healing improvements than rhFGF-2 in chronic wounds of diabetic patients.50
A topical hydrogel administering rhVEGF-A (Telbermin) was discontinued following a Phase II trial. VEGF-A is produced by most skin cells and mediates fibrin deposition, MMP production, monocyte recruitment, reepithelialization, and angiogenesis (Fig. 1).8,51 Chronic wounds show abnormally low levels of VEGF-A protein,26 while excess VEGF-A has been implicated in wound inflammation, edema, and scar formation (Fig. 2).20 Diabetic foot ulcers only showed a trend toward faster healing following treatment every second day with this hydrogel (Table 1).52 This indicates the lack of progression for Telbermin and may relate to the treatment's failure to achieve complete wound closure.
Granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) are cytokines developed as therapeutics for other indications.53,54 Leucomax® (rhGM-CSF) is a FDA-approved immune stimulator administered following chemotherapy and bone marrow transplantation, while Neupogen® and Granocyte® (rhG-CSF) are indicated to reduce neutropenia. Produced by a range of skin cells, these cytokines stimulate neutrophil proliferation, differentiation, and infiltration of the wound bed (Fig. 1).53,54 GM-CSF also promotes reepithelialization and vascularization, and myofibroblast-mediated wound contraction.
Trials in patients with infected diabetic foot ulcers yielded inconsistent results following daily treatment with rhG-CSF (Table 1).55–57 Treatment of chronic and pressure ulcers with rhGM-CSF also yielded variable results,45,58 but complete healing was observed with venous ulcers following weekly administration.59 These inconsistent results may, in part, be due to the production of neutralizing antibodies against the rhGM-CSF, which have been observed following repeated subcutaneous administrations to immune-competent individuals.60
Other cytokines have been explored as antiscarring therapies. Avotermin, a rhTGF-β3 preparation, showed promise in Phase II trials, but failed to improve scar appearance following scar-revision surgery in Phase III trials.61 The TGF-β family are multifunctional cytokines produced by inflammatory and resident skin cells, that recruit inflammatory cells to the wound, and induce blood vessel formation (Fig. 1).62 While TGF-β1 promotes collagen deposition, TGF-β3 inhibits this and promotes ECM remodeling (Fig. 2).
rhTGF-β3 was first trialled in pressure ulcers, with accelerated healing observed following daily administration with a topical hydrogel (Table 1).63 Intradermal administration of rhTGF-β3, before and after skin incisions, varicose vein, or scar revision surgery improved scar aesthetics for 5–12 months (Table 1).64–67 Inconsistencies between the design of the Phase II and Phase III trials, such as in the timing of treatments and the timing and measurement of scarring outcomes, may have contributed to the lack of progression for Avotermin.
Another cytokine explored for antiscarring purposes is IL-10 (Ilodecakin), which following a Phase II trial did not progress further after Avotermin failed. IL-10 is an anti-inflammatory cytokine produced by leukocytes, which suppresses inflammatory and fibrotic gene expression, reducing myofibroblast contractility, and limits collagen deposition through induction of MMP1 (Fig. 1).68 IL-10 is considered necessary for scarless fetal wound healing and has a protective role for skin conditions such as psoriasis. Intradermal administration of rhIL-10, before or after skin incisions, showed a reduced scar width and transient improvements in scar aesthetics at selected doses (Table 1).69,70
In summary, with numerous trials conducted in patients with chronic ulcers, only three GF therapies increased complete healing (Table 1), and are now approved for clinical use, although for specific indications or in limited countries. Importantly, no GF or cytokine therapies enhanced healing of pressure ulcers, traumatic, infected, and burn wounds sufficiently to warrant approval. In addition, GFs and cytokines therapies showed only partial or temporary improvements in scarring following experimental or surgical incisions. Many reasons have been proposed as to why GF and cytokine therapies failed to achieve meaningful outcomes. These include difficulties designing clinical trials for wound therapies, defining appropriate measures of treatment success, the costs associated with development and testing, and issues with efficacy or safety.71
Most trials have attempted to standardize protocols, but as evidence in the trials with rhPDGF-ββ,34 differences in ulcer duration, size, and intrinsic healing capacity greatly influence healing. Interpatient variability, with regard to this wound microenvironment, also results in differences in responsiveness to GF and cytokine interventions. The ability to stratify wounds into those likely to be responsive to a specific GF or cytokine treatment is thus critical to clinical success. Identification of molecular biomarkers that may indicate whether wounds are likely to heal or not heal will greatly help selection of trial participants.72
Another significant issue is that the FDA currently only accepts complete wound healing as an endpoint for chronic ulcer trials.73 As shown with the trials assessing rhVEGF-A,52,73 it can be difficult to demonstrate a significant difference between groups if patients do not heal within the study timeframe. GF and cytokine therapies may progress further if patient follow-up is extended and alternative outcomes such as time to heal, rate of wound size reduction, or health-related quality of life are deemed acceptable. Trials of scar therapies are also limited in that there is at present no standardized outcome measure to indicate treatment success.74 Differences between the trials with rhTGF-β3 indicate how scar assessment protocols can alter trial outcomes.61,64,67
Financial implications can also influence whether and how GF or cytokine wound healing therapies are trialled.71 Randomized controlled trials are very costly and investors are looking for strong intellectual property, and the shortest, most cost-effective route to the largest potential market for a given product. Much of the knowledge regarding GFs and cytokines is already in the public domain presenting limited scope for patents, which is likely to reduce investment. Thus innovative production or delivery systems and novel treatment combinations tailored for specific indications are essential to maximizing patent claims and obtaining trial sponsorship.75 Commercial considerations can also influence clinical trial design.71 Instead of choosing the subset of patients most likely to benefit from the treatment, a study population maybe chosen to support future marketing claims, or adjusted during the course of the trial to meet enrolment targets.
The therapeutic efficacy of recombinant proteins can also be compromised by the proteolytic nature of chronic wounds,14 and by systemic or immunogenic clearance.60,76 The net result being that GF and cytokines may not have been delivered to receptive wounds at therapeutically relevant levels. This issue can be overcome by increasing the frequency or dosage of the protein administered.21 But these changes in the treatment regimen likely reduce patient compliance,10,11,77 and may exasperate local hypersensitivity reactions and the risk of systemic action on healthy or cancerous tissue.35,76
Given the extreme clinical need, and the promise offered by GF and cytokine therapies across a broad range of wound complication, it is critical that the issues identified with their clinical application be addressed. Natural biomaterials can retain GFs and cytokines within the wound, while providing sustained local delivery and a physical environment supportive of healing processes (Fig. 3). Innovative combination therapies may therefore offer greater efficacy, safety, and patentability, thus addressing many of the limitations associated with direct GF or cytokine administration.
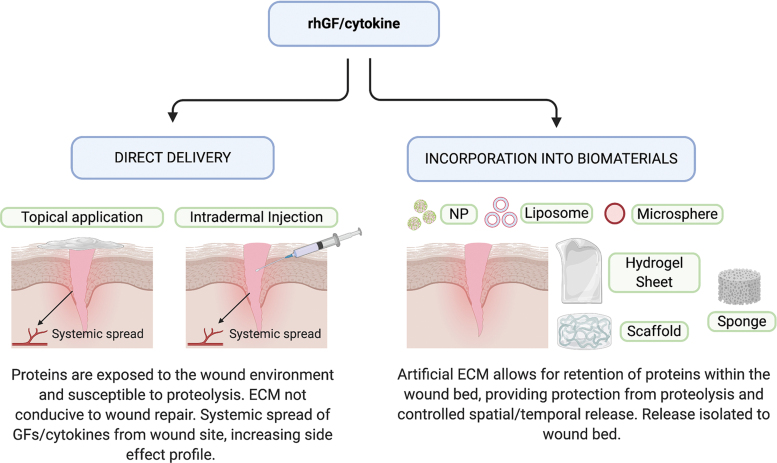
Figure 3.
Methods for delivering GFs and cytokines to skin wounds. Direct delivery of rhGFs or cytokines is achieved via topical application or intradermal injection (left), with only short-term bioactivity, due to proteolysis and an ECM not able to support their action. Biomaterial-based delivery is achieved through the incorporation of rhGFs or cytokines into an ECM-like hydrogel, scaffold, sponge, or particle (right) that offers proteolytic protection and structural support for sustained bioactivity. ECM, extracellular matrix; NP, nanoparticles to list of abbreviations; rh, recombinant human. Color images are available online.
Such GF and cytokine delivery systems are in development with the first in human clinical trials (Table 1). For example, administration of FGF-2 applied in a collagen sponge to chronic ulcers78 and to traumatic wounds79 showed greater closure relative to the sponge alone. GM-CSF delivery with an alginate dressing was trialled in chronic ulcers,80 and a GM-CSF-gelatin hydrogel accelerated closure of partial thickness burns when applied daily.81–83 The appearance of established scars was also improved following 12 weeks of twice daily treatment with rhTGF-β3 in a silicone-hyaluronate cream.84 GF and cytokine delivery systems therefore offer potential as therapies for cutaneous wound indications. But to ensure their success, it is critical to evaluate which biomaterials are most compatible with specific GF and cytokine delivery systems, and to prove their superior efficacy and safety relative to direct protein therapies.
Discussion
Biomaterials as repositories for GF and cytokine delivery
Biomaterial-based scaffolds have become very popular due to their unique biocompatibility, ease of functionalization, and release capability. When fabricated, these biomaterials function as a three-dimensional (3D) biomimetic ECM, simulating the arrangement and role of their native counterpart. The ECM is composed of fibrous proteins, as well as an abundance of proteoglycan.85 The proteins provide important adhesive domains that anchor cells to the ECM, and with proteoglycans, direct cellular function through their interactions with GFs and cytokines. When a wound occurs, both cells and surrounding ECM are lost. Reconstruction of the ECM while simultaneously supplying GFs or cytokines is thus an attractive strategy to promote wound healing.
Natural biomaterials
Two biomaterial groups are commonly used for GF and cytokine release, categorized as either natural or synthetic polymers. This review will concentrate on biomaterials made up partly with natural polymers, as they have been established to stimulate wound healing.86 Natural biomaterials possess inherent advantages such as being nontoxic, susceptible to cell-triggered proteolytic degradation and natural remodeling, and able to present ligands to cells.87 Natural wound biomaterials are composed of either polysaccharides, such as chitosan, alginate, glycosaminoglycans (GAGs) and cellulose; or proteins, such as collagen, gelatin, and fibrin (Table 2).88 The most utilized subsets are collagen, hyaluronate/hyaluronic acid (HA), and chitosan due to their low immunogenicity, biocompatibility, and biodegradability. These macromolecules are typically used to form hydrogels, composed of 3D hydrophilic polymeric network that simulates ECM and absorb large amounts of fluids.89
Table 2.
Details of natural polymers commonly used for growth factor and cytokine delivery
| Polymer | Class | Properties | Forms | Crosslinking Mechanisms |
|---|---|---|---|---|
| Chitosan | Polysaccharide | Polycationic polymer with antimicrobial activity but weak mechanical strength and stability | Hydrogels, films | Covalent: GA, dextran aldehyde, ionic: oxalic acid90 |
| Alginate | Polysaccharide | Negatively charged polymer, adjustable mechanical and biological properties by varying the content of two monomers | Beads, hydrogel, sponge | Physical: Calcium chloride or other divalent metal chlorides94,95 |
| Hyaluronate | Polysaccharide | Negatively charged GAG, main connective tissue ECM component, good biocompatibility and mechanical properties | Hydrogel, film | Covalent: amine-modified HA reacted with oxidized GAG to form imine bonds99 |
| Heparin | Polysaccharide | Negatively charged sulphated GAG | Hydrogel | Covalent: disulphide bond between cysteine residues101 |
| Collagen | Protein | Slightly anionic, most abundant protein in the body, provides cell-matrix and matrix–matrix interactions, good biocompatibility, low immunogenicity, poor mechanical properties | Scaffold, sponges, membranes, hydrogel | Covalent: GA, TG, three polypeptide chains that form pH and temperature dependent hydrogels103 |
| Gelatin | Protein | Anionic, denaturalized form of collagen, similar properties as collagen | Microspheres, sheets, sponge | Covalent: genipin, GA112,114 |
| Fibrin/fibrinogen | Protein | Anionic, component of tissue architecture, cell-matrix and matrix–matrix interactions, good biocompatibility | Matrix, hydrogel | Initiated by thrombin115 |
ECM, extracellular matrix; GA, glutaraldehyde; GAG, glycosaminoglycan; TG, transglutiminase.
Numerous hydrogel delivery systems have been explored for wound healing applications, the most common of which being chitosan. Chitosan, derived from the alkaline deacetylation of chitin, is a linear polysaccharide consisting of (1,4)-linked 2-amino-deoxy-β-d-glucan. Depending on its application, the chemical characteristics of chitosan can be altered by modifying its degree of acetylation and molecular weight.90 Chitosan gelation can occur easily via physical or chemical means by mixing or crosslinking with appropriate reagents. Some examples evaluated for wound healing applications as GF or cytokine delivery systems include the following (Tables 3–5): a chitosan hydrogel containing EGF,91 FGF-2 within a photocrosslinkable chitosan gel,92 and an injectable chitosan hydrogel encompassing both FGF-2 and heparin.93
Table 3.
Natural biomaterial delivery systems evaluated for cutaneous wound and scarring indications
| Biomaterial | Fabrication Method | Treatment(s) | Experimental Parameters Tested and Outcomes |
|---|---|---|---|
| Chitosan | Film | EGF |
In vitro release within 24 h Porcine full-thickness excisional wound model—daily treatment led to faster healing relative to control149 |
| Film | FGF-2 | Diabetic mouse full-thickness excisional wound model—treatment three times per week led to faster healing relative to hydrogel control166 | |
| Hydrogel | EGF |
In vitro release—within 3 h Rat partial-thickness thermal burn—daily treatment increased epithelialization relative to hydrogel and EGF controls91 |
|
| Hydrogel | FGF-2 | Diabetic db/db mouse full-thickness excisional wound model—single treatment led to faster healing relative to hydrogel control92 | |
| Hydrogel | FGF-2+heparin | Rat hind limb ischemia model—single treatment led to increased vascularization relative to hydrogel controls93 | |
| Alginate | Bead | VEGF-A | In vitro release+serum—cumulative over 14 days97 |
| Hydrogel | VEGF-A |
In vitro release+mechanical stimulation—pulsatile over 1 h Mouse hind limb ischemia model—daily stimulation increased vascularization relative to placebo control96 |
|
| HA | Heparin hydrogel | FGF-2 | In vitro release+serum+hyaluronase—over 14 days100 |
| Heparin | Hydrogel | VEGF-A |
In vitro release within 24 h Diabetic db/db mouse full-thickness excisional wound model—single treatment led to increase in vascularization relative to hydrogel control102 |
| Collagen | Hydrogel | FGF-2 |
In vitro culture—attachment of adipocytes Mouse full-thickness excisional wound model—single treatment led to faster healing relative to hydrogel control160 |
| Membrane | CBD-PDGF-ββ | Rabbit ischemic full-thickness excisional wound model—single treatment led to faster repair relative to membrane control106 | |
| Scaffold | VEGF-A | Rat SC implant model—increased vascularization relative to scaffold and unconjugated VEGF-A controls107 | |
| Scaffold | EGF |
In vitro release—within 8 h In vitro culture—fibroblast and keratinocyte proliferation Rat full-thickness excisional wound model—single treatment led to enhanced cellularity relative to scaffold control108 |
|
| Sponge | EGF | In vitro release+collagenase—cumulative over 30 days, induces fibroblast proliferation104 | |
| TFA-sponge | FGF-2 | In vitro release+serum—burst within 4 days, cumulative over 18 days, induces endothelial cell proliferation105 | |
| Gelatin | Microsphere | FGF-2 | Diabetic db/db mouse full-thickness excisional wound model—single injection led to increase in repair processes relative to microsphere control110 |
| Microsphere | VEGF-A | In vitro release+collagenase—over 14 days112 | |
| Sheet | FGF-2 | Diabetic db/db mouse full-thickness excisional wound model—cumulative over 7 days in plasma, single treatment led to faster healing relative to sheet control113 | |
| Fibrin | Hydrogel | VEGF-A+Factor XIIIa substrate |
In vitro release—cumulative over 7 days Mouse ischemic hind limb and skin flap models—single treatment led to increase in vascularization relative to hydrogel control151 |
| Lipid | Multi-lamellar liposome | EGF | Rat full-thickness incisional wound model (nonsutured)—single treatment led to cumulative release in wound over 5 days, increased tensile strength relative to liposome control121 |
CBD, collagen-binding domain; TFA, trifluoroacetic acid.
Table 5.
Combination therapies evaluated for cutaneous wound and scarring indications
| Biomaterial(s) | Fabrication Method(s) | Treatment(s) | Experimental Parameters Tested and Outcomes |
|---|---|---|---|
| Chitosan | |||
| Hydrogel | PDGF-ββ+VEGF-A | Rat full-thickness incisional wound model (sutured)—single treatment led to oxidative changes relative to hydrogel and untreated controls164 | |
| PAAm | Hydrogel | EGF+Piperacillin | In vitro release—antibiotic within 10 h, EGF cumulative over 10 days, increase in fibroblast proliferation157 |
| PEG | Scaffold | FGF-2+VEGF-A+heparin |
In vitro culture—keratinocyte proliferation and migration Rat full-thickness excisional wound model—single treatment led to faster healing relative to scaffold control138 |
| HA+PLGA | Hydrogel+microsphere | VEGF-A+vancomycin |
In vitro release—antibiotic and VEGF cumulative over 7 and 20 days Infected rat full-thickness splinted excisional wound model—single treatment led to faster healing, with increased granulation tissue relative to hydrogel, hydrogel+antibiotic and hydrogel+VEGF microsphere controls158 |
| PEO+PLGA | Nanofiber+NP | PDGF-ββ+VEGF-A |
In vitro release—VEGF-A in 24 h, PDGF-ββ 40% by 7.5 days, In vitro culture—supported fibroblast proliferation Rat full-thickness excisional wound model—single treatment led to faster healing relative to fiber control135 |
| Alginate | |||
| Microparticle | FGF-2+Rifamycin |
In vitro release in serum—induced fibroblast proliferation and migration Rat full-thickness excisional wound model—single treatment led to faster healing than untreated control98 |
|
| Collagen | |||
| Membrane | FGF-2+FGF-7 |
In vitro release—cumulative over 28 days Rat full-thickness splinted excisional wound model—single treatment led to faster healing relative to membrane, membrane+FGF-2 or FGF-7 controls155 |
|
| HA | Hydrogel | VEGF-E+vIL-10 | Horse bandaged full-thickness excisional limb wound model—single treatment led to faster repair processes and resolution of EGT formation relative to hydrogel control170 |
| HA+gelatin | Nanofiber+NP | EGF+FGF-2 PDGF-ββ+VEGF-A |
In vitro release—within 5 days for FGF-2 and cumulative over 25 days for others In vitro culture—supports endothelial tube formation STZ-induced diabetic rat full-thickness excisional wound model—single treatment led to faster healing relative to nanofibers, nanofibers+FGF-2/EGF controls126 |
| Fibrin | |||
| Matrix | EGF+keratinocytes |
In vitro release—burst within 24 h, cumulative over 7 days Mouse full-thickness excisional wound model—single treatment led to faster healing relative to keratinocytes+matrix, EGF+matrix and matrix controls117 |
|
| Sealant | PDGF-ββ+fibroblasts | Rabbit full-thickness excisional wound model—single treatment led to faster healing relative to sealant and sealant+fibroblasts controls116 | |
| Fibrinogen fragments | Matrix | VEGF-A+PDGF-ββ |
In vitro culture—supports tubule formation by endothelial cells or sprout formation by smooth muscle cells Diabetic db/db mouse full-thickness excisional wound model—single treatment led to faster healing relative to matrix, matrix+FN and matrix+GF controls161 |
| HBD+PEG | Matrix | Factor XIIIa substrate+FGF-2+PlGF |
In vitro release+plasmin—burst release within 24 h, retention over 7 days Diabetic db/db mouse full-thickness excisional wound model—single treatment led to faster healing with fibrin+GFs and PEG+HBD+GFs relative to matrix, matrix+FGF-2 or PlGF controls137 |
| Collagen | Sealant+matrix | FGF-2+VEGF-A |
In vitro release—within 24 h, cumulative over 8 days Mouse full-thickness excisional wound model—single treatment led to prolonged vascularization relative to matrix+GFs, matrix+sealant and matrix controls156 |
| PEtU-PDMS+PLGA | Scaffold+NP | FGF-2+VEGF-A+heparin | Diabetic db/db mouse full-thickness excisional wound model—single treatment led to faster healing relative to scaffold, scaffold+GFs and scaffold with NP controls127 |
EGT, exuberant granulation tissue formation; HBD, heparin-binding domain; PAAm, polyacrylamide; PDMS, polydimethylsiloxane; PEG, poly(ethylene glycol); PEO, poly(ethylene oxide); PEtU, polyetherurethane; PLGA, poly(lactic-co-glycolic acid); PlGF, placental growth factor; STZ, streptozotocin.
Another commonly used natural polymer is alginate, an algae or bacteria-derived polysaccharide, composed of a linear copolymer of homopolymeric blocks of 1,4-linked β-d-mannuronate and α-l-guluronate residues. Alginates are gelled with multivalent cations via an “egg-box” model,94 and can be chemically and physically modified to alter their properties.95 Examples explored as wound therapeutics (Tables 3–5) include the following: VEGF-A encapsulated within an alginate hydrogel or beads96,97 and pH responsive alginate microparticles developed for dual release of FGF-2 and an antibiotic.98
Another commonly used polysaccharide is HA. HA is a linear, nonsulfated GAG composed of repeated units of glucoronic acid and N-acetylglucosamine. HA has important biological functions, as it is the main nonprotein component of ECM and contributes to elastoviscosity, hydration, and cellular function.99 HA has been used for selected wound delivery systems (Tables 3 and 4), with conjugation of FGF-2 to an amine-modified HA, coupled with oxidized heparin, used to produce a HA-heparin hydrogel, with increased GF stability and activity.100
Table 4.
Composite biomaterial delivery systems evaluated for cutaneous wound and scarring indications
| Biomaterial(s) | Fabrication Method(s) | Treatment (s) | Experimental Parameters Tested and Outcomes |
|---|---|---|---|
| Chitosan | |||
| CMC-chitosan | Hydrogel+NP | EGF |
In vitro release+proteinases—within 48 h, induced fibroblast proliferation STZ-induced diabetic rat full-thickness excisional wound model—treatment every 2 days led to faster healing relative to hydrogel and hydrogel+EGF controls124 |
| Cellulose+HA | NP+composite | GM-CSF |
In vitro release—within 48 h Rat full-thickness excisional wound model—treatment every 2 days led to faster healing relative to NP+composite control123 |
| Gelatin/PVA+PCL | Hydrogel+microsphere | FGF-2 |
In vitro release—cumulative over 25 days, nontoxic for fibroblasts Rat full-thickness excisional wound model—single treatment led to faster healing relative to hydrogel and dressed controls154 |
| Gelatin+diamond | Hydrogel+NP | VEGF-A | In vitro release—within 3 days, induces endothelial cell attachment and proliferation128 |
| Alginate | |||
| CMC-chitosan | Hydrogel | EGF |
In vitro release—within 12 h, nontoxic to fibroblasts, induces RBC clotting Rat partial-thickness scald burn model—daily treatment led to faster healing relative to hydrogel and EGF controls148 |
| CMC-chitosan+PVA | Microsphere+hydrogel | FGF-2 |
In vitro release—burst release within 48 h, cumulative over 12 days, induces fibroblast proliferation Rat full-thickness thermal burn model—single treatment led to faster healing relative to hydrogel and hydrogel+FGF-2 controls134 |
| HA | |||
| Heparin+PEGDA | Hydrogel | FGF-2 | In vitro release+hyaluronidase—cumulative over 35 days, increases fibroblast proliferation150 |
| Collagen | |||
| PCL+chitosan | Hydrogel+NP | G-CSF |
In vitro release—cumulative over 15 days, nontoxic for mesenchymal stem cells Rat full-thickness excisional wound model—single treatment led to faster healing relative to nanofiber control125 |
| Gelatin | |||
| Gelatin | Microsphere+sponge | EGF | Rat full-thickness excisional wound model—single treatment led to faster healing, with increased tensile strength relative to sponge control111 |
| Collagen | Microsphere+hydrogel+matrix | EGF |
In vitro release—cumulative over 14 days In vitro culture—enhanced proliferation of keratinocytes relative to fibroblasts Rat full-thickness excisional wound model—single treatment led to faster healing relative to matrix, and matrix+microsphere controls152 |
| EUP polysaccharide | Sponge+EUP fibers | PDGF-ββ |
In vitro release+collagenase—within 48 h, induced fibroblast proliferation Mouse full-thickness excisional wound model—EUP fibers in sponge sequestered PDGF-ββ114 |
| Fibrin | |||
| HA+protein | Hydrogel+NP | VEGF-A | Mouse splinted full-thickness excisional wound model—single treatment led to faster healing than hydrogel control122 |
| Fibrinogen+anti-VEGF aptamer | Hydrogel+macromer | VEGF-A |
In vitro release in serum—cumulative over 15 days, induces endothelial cell migration In vitro culture—growth of keratinocytes, fibroblasts and enhanced that of endothelial cells Mouse full-thickness excisional wound model—single treatment led to faster healing relative to hydrogel and hydrogel+VEGF-A controls153 |
| Lipid | |||
| Silk fiboid | Liposome+SF core | FGF-2 |
In vitro release+wound fluid—protection, supported fibroblast survival Mouse partial-thickness thermal burn—treatment every 3 days led to faster healing relative to FGF-2 control120 |
CMC, carboxymethylcellulose; EUP, galacturonic acid-containing polysaccharide; NP, nanoparticle; PCL, polycaprolactone; PEGDA, poly(ethylene glycol) diacrylate; PVA, poly(vinyl alcohol); SF, silk fibroid.
Heparin is another commonly used linear polysaccharide and is composed of a complex heterogeneous mixture of highly sulfated repeating disaccharide units, consisting of an uronic acid (d-glucoronic or l-iduronic) and d-glucosamine or N-acetyl-glucosamine (Table 3).101 These sulfation patterns dictate the interaction of heparin with various signaling molecules, such as VEGF-A. Taking advantage of this feature, a set of selectively desulfated heparins was developed to form biohybrid hydrogels with star poly(ethylene glycol) (PEG) polymers. Specific removal of single sulfate groups resulted in higher VEGF release rates.102
Collagen, the most abundant ECM protein, has garnered much interest for biomedical applications. Collagen fibers can sustain mechanical stress, support different cell types, and function as anchors for various GFs and cytokines.103 Collagen is classified into type I to XXVIII types, among these type I is the most utilized. Much research has been reported on collagen constructs for GF and cytokine release for wound healing applications (Tables 3–5). EGF and FGF-2 have been incorporated into denatured collagen sponges.104,105 Collagen membranes have been loaded with PDGF-ββ,106 VEGF-A in sulfhydryl-modified scaffolds,107 and EGF with a scaffold that comprised collagen and elastin.108
Gelatin-based hydrogels derived from collagen hydrolysis have also been extensively used. GFs or cytokines can be loaded into gelatin-based delivery systems via electrostatic interactions and coupling reactions (Tables 3 and 4).109 Specific examples include fabrication of gelatin microspheres to deliver FGF-2 and EGF,110,111 genipin-crosslinked gelatin micropheres incorporating VEGF-A,112 and gelatin sheets to deliver FGF-2.113 A sponge composed of electrospun fibers of plant-derived polysaccharide crosslinked using gluteraldehyde (GA) to gelatin was also created to specifically bind PDGF-ββ.114
Another commonly used biomaterial is fibrin, a structural protein involved in the clotting process that is formed by polymerization of the plasma protein, fibrinogen. Fibrin is the primary component in clots and provides a natural scaffold for infiltrating blood and skin cells and has been extensively investigated as a sealant (Tables 3–5).115 A fibrin sealant was combined with fibroblasts and PDGF-ββ to support granulation tissue formation,116 while a fibrin matrix delivering keratinocytes and EGF was used to enhance reepithelialization.117
Biomaterial delivery system platforms
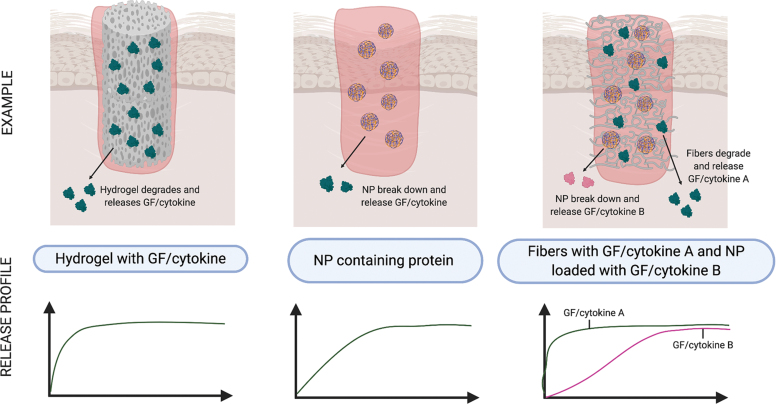
Naturally based hydrogels are mechanically weak; therefore control of their physicomechanical and biological characteristics is a major drawback. In an attempt to improve their biological performance, researchers have turned to fabrication techniques such as nanoparticle (NP) technology, which includes liposomes, microspheres, and bead formation. Interest in NP research for biomedical purposes relates to their nanoscale dimension, and ability to diffuse across membranes, and carry bioactive agents (Fig. 4).118 NP composition can differ depending on the source material (Tables 3–5).
Figure 4.
Tailored release of GFs and cytokines to skin wounds using composite biomaterials. Hydrogels containing GF or cytokines allow for rapid diffusion into the wound bed upon absorption of wound fluids (left panel). NPs encapsulating GFs or cytokines allow for sustained release coinciding with degradation of the NP (center panel). Hybrid biomaterials consisting of fibers containing GFs or cytokines and NPs encapsulating GFs or cytokines allows for biphasic release following degradation of the fiber then the NP (right panel). NP, nanoparticle. Color images are available online.
Liposomes, composed of a natural or synthetic phospholipid shell, can entrap hydrophilic GFs and cytokines in their aqueous core. Liposomes are made by introducing a aqueous buffer into a mixture of phospholipid and organic solvent; with the organic solvent then removed by evaporation under reduced pressure.119 For wound healing applications, lipid NPs have been used to encapsulate EGF.120,121 Other nanocarriers trialled for GFs and cytokines include those made from proteins or polysaccharides,122–126 synthetic polymers,127 and carbon.128
Biodegradable microspheres are colloidal systems made using either natural or synthetic materials, such as poly(lactic-co-glycolic acid) (PLGA), gelatin, or other polymer composites (Tables 4 and 5).129 They are able to load, protect, and control the release of GFs and cytokines (Fig. 4). Passive delivery can occur with diffusion of the protein through the biomaterial as they degrade. As an example, gelatin microspheres can be prepared by creating a gelatin-olive oil emulsion, then adding acetone to induce gelation, followed by crosslinking using GA. The microspheres are then incubated with a protein solution to facilitate absorption. EGF-loaded gelatin microspheres were fabricated using this method.110
To improve their structural performance, natural biomaterials are used to develop scaffolds, such as sponges, sheets/films, and composite hydrogels (Fig. 4).129 The simplest method to encapsulate proteins within these is to mix proteins with the polymers before gelation or solidification (Fig. 4 and Tables 3–5). Once encapsulated, the biomaterial can be prepared using phase separation, particulate leaching and solvent casting, gas foaming, freeze drying, and/or melt molding.130 Collagen sponge fabrication, structural, and protein release rate are influenced by the collagen type, amount, concentration, and the crosslinking agents used.131 As an example, various crosslinking agents, namely GA, genipin, and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, were mixed with a collagen solution, cooled and cast into Teflon molds, with EGF solution added dropwise to the crosslinked sponges.104
Physically crosslinked hydrogels are formed by molecular self-assembly due to ionic or hydrogen bonds; while chemical gels are formed by covalent bonds.132 Ionic bonds are made by crosslinking an anionic polymer, such as alginate, with various divalent cations, for example, calcium. These can occur via a number of routes and include photopolymerization, enzyme-induced crosslinking, “click” chemistry, including Michael type-addition, Diels-Alder “click” reaction, oxime formation, and Schiff base formation.133 GFs and cytokines can be freely incorporated within the network allowing for natural interactions with the polymer, or can be also crosslinked to the polymer (Fig. 4 and Tables 3–5). In an example of Schiff base formation, low molecular weight water-soluble chitosan was functionalized with amino groups using lactose introduced through a condensation reaction. Sodium periodate-treated heparin and FGF-2 solutions were then mixed with the modified chitosan to create a crosslinked hydrogel.93
Hybrid delivery systems
Current efforts in biomaterial development combine mechanically strong synthetic polymers with the biomemetic natural polymers, so to as to retain tissue biocompatibility, while enhancing the longevity of the scaffold. In parallel, phasic delivery of GFs and cytokines is enabled through their incorporation into nanocarriers, such as liposomes, NPs, or nanofibers embedded within each polymer (Fig. 4 and Tables 4 and 5). For example, FGF-2-loaded alginate microspheres encapsulated in a composite CMC-chitosan and poly(vinyl alcohol) (PVA) hydrogel.134 In addition, hybrid delivery systems encompassing multiple bioactive components have been investigated (Figs. 4 and 5 and Table 5).
Figure 5.
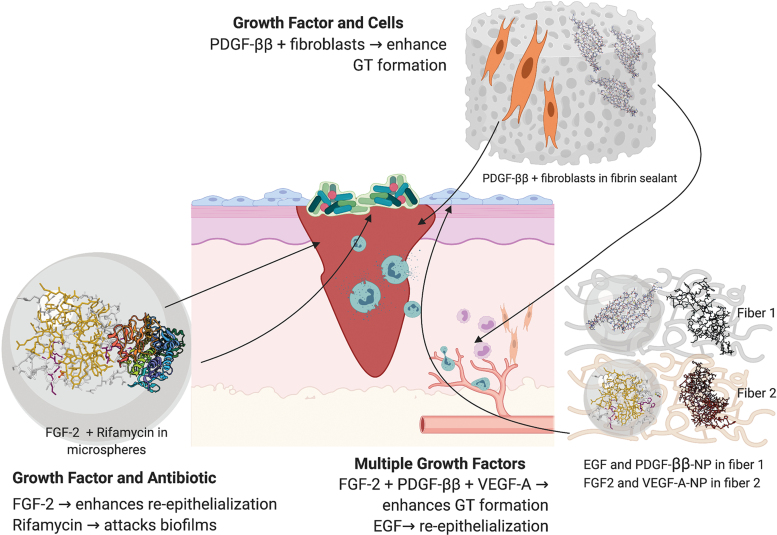
Hybrid biomaterials for delivery of GFs, cytokines, drugs, and/or cells to skin wounds. Examples include delivery of: PDGF-ββ with fibroblasts in a fibrin sealant to enhance GT formation,116 FGF-2 and rifamycin in alginate microparticles for combined targeting of wound reepithelialization and biofilm infection,98 and delivery of multiple GFs to enhance both epidermal and dermal repair using collagen nanofibers containing EGF and PDGF-ββ-NPs, and HA nanofibers containing FGF-2 and VEGF-A-NPs.126 GT, granulation tissue. Color images are available online.
In a simple example, the antibiotic, rifamycin, was suspended in a CaCO3 solution, as was FGF-2, which was then used to solidify alginate droplets prepared using a microfluidics device.98 But to create a stage-wise release pattern of multiple GFs, a NP-in-nanofiber structure was developed where VEGF-A and PDGF-ββ were individually encapsulated into gelatin NPs, which were mixed with a HA solution containing FGF-2, or a collagen EGF solution, before incorporation within nanofibers.126 In a further example of this strategy, a composite chitosan-poly(ethylene oxide) (PEO) nanofibrous scaffold was embedded with VEGF, as well as PDGF-ββ-containing NPs.135
Functionalization of wound biomaterials is becoming a major priority, so as to enhance the integratation of both protein and cellular therapies. Heparin and fibronectin fragments are commonly used to immobilize GFs within various scaffolds (Tables 3–5).127,136–138 GFs have also been added to fibrin scaffolds so as to support keratinocyte or fibroblast viability (Table 5).116,117,139 Integrin-binding peptides, such as arginylglycylaspartic acid, have also been incorporated, along with GFs, to enhance the biocompatability of PEG hydrogels for endothelial progenitor cells.140 Similarly, conjugation of anti-CD29 antibodies into collagen/PLGA-blended nanofibrous scaffolds was used to enrich the attachment of bone marrow-derived stem cells.141
Alternative GF and cytokine sources, and methods of delivery, will continue to be explored. This includes the production of biomaterials containing platelet-rich plasma, which is rich in GFs and cytokines and can be derived directly from and administered to the same patient.142 In another example, biomaterials made from the amniotic membrane from human placenta, including intact or decellularized matrices or hydrogels, naturally produce, or can be enriched with, GFs, cytokines, drugs, or stem cells.143
In addition, natural and synthetic scaffolds are being used to increase the transfection efficiency of plasmid deoxyribonucleic acid (DNA) encoding angiogenic GFs for administration to ischemic or wounded tissue.144,145 Stems cells are also being genetically engineered to produce angiogenic GFs and then applied directly, or within scaffolds, to skin wounds.146,147 As illustrated by these innovations, future therapies will likely be tailored to specific wound environments and encompass multiple functionalized biomaterials, delivering selected biotherapeutic combinations through a range of mechanisms.
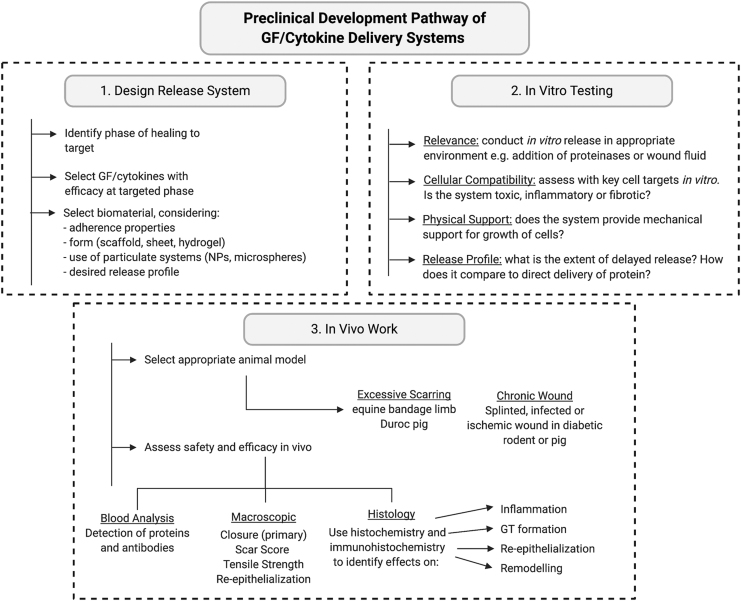
Several challenges, however, remain for the translation of these advanced biomaterial delivery systems. For example, more care is needed in the physicochemical characterization and synthesis of the scaffold with regard to polymer arrangement, orientation, and porosity. This must be performed in concert with the nanosystem developed for controlled or multistage release of the appropriate amount of protein, DNA and/or stem cells. Finally, comprehensive preclinical testing is vital to ensure the safety and efficacy of these complex systems before their entering into clinical trials.
Suitability of GF and cytokine delivery systems for cutaneous wound indications
As described, numerous studies have addressed the limitations associated with GF and cytokine therapies, by incorporating proteins into natural biomaterials fabricated into scaffolds, hydrogels, or various size particles. In the clinic, these combination therapies may provide superior efficacy relative to direct protein treatment. This section will evaluate the preclinical evidence as to the therapeutic advantages of GF and cytokine delivery systems for the treatment of ulcers, burns, and scar-prone wounds.
Tailored delivery of GFs and cytokines
Direct GF and cytokine treatments are not compatible with standard wound care, as they require specialized administration and frequent dressing changes. This can increase pain and discomfort for the patient and may prolong the healing process.10,11,77 Sustained delivery systems could offer improved usability and patient compliance and as such enhance healing responses.
Natural biomaterials have been formulated, which provide for a burst release of proteins that would be compatible with acute wound care (Fig. 4 and Tables 3 and 4). For example, chitosan hydrogels, films, and NPs were shown to release EGF in vitro within 3–48 h.91,123,124,148,149 Mechanical stimulation of an alginate hydrogel offered pulsatile release of VEGF-A.96 Controlled release from biomaterials can be provided by degradative enzymes within the wound environment. Release of VEGF-A from gelatin microspheres was shown to be substantially faster in the presence of collagenase,112 as was FGF-2 from a collagen sponge,104 and PDGF-ββ from galacturonic acid-containing polysaccharide (EUP) fibers within a gelatin sponge.114 Hyaluronidase also facilitated the release of FGF-2 from a HA hydrogel.150 Cleavage domains engineered between protein and biomaterial can also provide for enzyme-triggered release. For example, fusion of an α2-plasmin inhibitor sequence to VEGF-A or FGF-2, crosslinked to fibrin gels or a composite matrix, enabled release by coagulation factor XIIIa.137,151
Importantly, delivery systems combining different biomaterials and fabrication techniques provide sustained release profiles that may have utility for slow-to-heal wounds (Fig. 4 and Tables 3 and 4). For example, EGF encapsulated in gelatin microspheres within a gelatin sponge provided release over 14 days, compared to the burst release offered by the sponge alone.152 While cumulative release of VEGF-A from a fibrin hydrogel was observed over 7 days,151 the incorporation of an anti-VEGF aptamer-fibrinogen macromer into the hydrogel extended release to 15 days.153
A greater improvement was observed with an HA hydrogel, where the addition of heparin and synthetic polymers, extended the release of FGF-2 from 14 to over 35 days.150 Chitosan NPs embedded in collagen nanofibers also offered extended release of G-CSF over 14 days.125 Incorporation of synthetic polymers during fabrication presents another approach to extending delivery kinetics, as demonstrated by the encapsulation of FGF-2 in a polycaprolactone (PCL) microsphere within a composite chitosan/gelatin/PVA hydrogel, which provided protein release beyond 25 days.154 These findings indicate that composite biomaterials offer the greatest delivery time-span, which may provide greatest compatibility with the care of chronic wounds.
Tailored delivery of GFs, cytokines, drugs, and/or cells
Impaired and excessive healing are exasperated by a range of factors, which suggests that future interventions will require a combinatorial approach, involving therapeutic proteins, drugs, and cells. Delivery systems can thus be designed to provide the benefit of an ECM with GFs and cytokines, while addressing other local factors that may limit their efficacy.
A number of studies have evaluated natural and composite biomaterials for the delivery of two or more GFs or cytokines to skin wounds (Fig. 5 and Table 5). A collagen membrane was developed to deliver EGF and FGF-2 over a 28-day period.155 Proangiogenic factors, FGF-2 and VEGF-A, were combined in fibrin sealant, composite fibrin or chitosan scaffolds, and PLGA NPs in a composite fibrin sealant, offering cumulative release of both factors over 8 days.127,138,156 A chitosan/PEO nanofiber system provided immediate release of VEGF-A, but extended release of PDGF-ββ beyond 7.5 days through its incorporation in PLGA NPs.135 A multifactor delivery system encompassed collagen fibers releasing FGF-2 and VEGF-A NPs over 5 and 25 days, respectively, with HA fibers eluting EGF- and PDGF-ββ-NPs also over 25 days.126 These tailored release systems allow for greater GF and cytokine control of wound progression through the phases of healing.
Other studies have evaluated natural and composite biomaterials for the delivery of GFs or cytokines with antibiotics (Fig. 5 and Table 5). A chitosan/polyacrylamide hydrogel was developed to deliver EGF along with piperacillin–tazobactam, showing release of the antibiotic within 10 h, and cumulative release of the EGF over 10 days.157 VEGF-A-loaded PLGA microspheres within a chitosan HA hydrogel containing vancomycin showed cumulative release of the antibiotic and protein over 7 and 20 days, respectively.158 As previously mentioned, biomaterials are also capable of delivering skin cells in parallel with GFs and cytokines (Fig. 5 and Table 5), so as to enhance both cell viability and healing responses. This includes fibroblasts embedded with PDGF-ββ in a fibrin sealant116 and keratinocytes delivered with EGF in a fibrin matrix.117 These findings give support to the concept of multifactorial delivery systems as wound therapeutics.
Retention and proteolytic protection of GFs and cytokines
The wound environment is harsh on directly applied proteins due to excess production of proteases, in particular, plasmin, MMPs, elastases, and bacterial proteases.14 Biomaterials that offer proteolytic protection would therefore allow more physiologically relevant doses to be administered. In providing this protection, biomaterials will also aid the retention of the GFs and cytokines within the wound, thus preventing their systemic distribution and risk of inducing malignancy or immunogenicity.
While the GF and cytokine release profiles from biomaterials are often evaluated in vitro, they are rarely conducted in vivo or in a proteolytic environment (Tables 3–5). Most studies that evaluate release kinetics have done so in vitro using standard buffers. Physiologically relevant additions have included MMP-containing serum97 or culture medium.98,100,105,153 Protection of GFs in biomaterials has also been demonstrated in the presence of a serine protease, savinase,124 and in wound fluid.120
Limited studies have directly evaluated protein release from biomaterials within animal wounds. But FGF-2 released from gelatin sheets within diabetic mouse wounds was shown to be stable for 7 days,113 EUP-fibers within a gelatin sponge sequestered PDGF-ββ in murine excisional wounds,114 while liposome-delivered EGF persisted within rat incisional wounds for 5 days.121
Although wound and plasma analyses in treated animals revealed release of GFs or cytokines from the biomaterial,113,121 none of the studies reviewed examined the systemic distribution of these proteins. In addition, there were no attempts to detect antibody production in response to short- or long-term exposure to the biomaterial(s), or to the GFs and cytokines delivered. Further analyses are thus needed to determine whether biomaterials do indeed provide protection and retention of GF and cytokines within the wound bed.
Structural support for GF and cytokine action
A limitation of GF and cytokine therapies is that their action is dependent on ECM interactions, which in clinical scenarios, can exhibit altered structure and function.8,159 Natural biomaterials that mimic the ECM could synergize with GFs and cytokines providing spatial cues to direct the migration and proliferation of skin cells into the wound bed.
Of the many GFs and cytokines delivery systems developed, only a few have been directly assessed for their biocompatibility, and ability to support skin cell attachment, migration, or proliferation in vitro (Tables 3–5). Toxicity of G-CSF-loaded chitosan NPs in a collagen/PCL hydrogel was evaluated for mesenchymal cells,125 as was a chitosan/gelatin/PVA hydrogel containing FGF-2 for fibroblasts.154 An alginate/chitosan hydrogel containing EGF was incubated with red blood cells, demonstrating clotting and hemostatic potential.124 Cell attachment was demonstrated to a collagen hydrogel containing FGF-2 using adipocytes,160 and to a chitosan/gelatin hydrogel containing diamond NPs encapsulating VEGF-A using endothelial cells.128 Martino et al., went further still to show that a fibrin matrix containing VEGF-A+PDGF-ββ supported both endothelial cell and smooth muscle cell function in vitro.161
More extensive analysis of the differential effects of biomaterials on skin cells has been performed for some delivery systems (Tables 3–5). The addition of EGF-loaded microspheres to a gelatin hydrogel-coated collagen matrix enhanced keratinocyte proliferation, while fibroblast proliferation was greatest on collagen matrix in the absence of gelatin.152 The addition of anti-VEGF aptamer-fibrinogen macromers, so as to sequester VEGF-A, accelerated the growth of endothelial cells and keratinocytes, but not fibroblasts, within a fibrin hydrogel.153
Collagen scaffolds incorporating EGF also enhanced the proliferation of keratinocytes and fibroblasts, with coculture of the cells maintained in vitro for 21 days.108 This study went further to show that these cell types were also detected within the EGF scaffold in rat excisional wounds after 3 days. By contrast, a FGF-2-incorporated chitosan hydrogel was not retained in diabetic mouse wounds, as reepithelialization of the wound occurred beneath the biomaterial.92 As these studies have been limited, it is not yet clear which biomaterials provide the best support for the growth of different skin layers. Furthermore, the impact of different biomaterials on inflammation, which can contribute to chronicity and scarring, has not been compared. In addition, it is not evident which biomaterials integrate into the wound, as opposed to providing topical GF and cytokine delivery.
GF and cytokine delivery systems as wound healing treatments
GF and cytokine therapies have been underwhelming with regard to their efficacy as therapeutics for chronic ulcers, traumatic burns, and scar-prone surgical incisions. One reason proposed for their clinical failings has been an inadequate translation from preclinical models.162,163 These difficulties can be explained by preferential use of rodent models, when there is poor concordance between wound healing in rodents with that in humans, with rodent skin healing mostly by contraction, and human skin by reepithelialization. Larger animals offer greater similarity to human healing processes, but are less commonly used due to logistics.
There is also a discordance between the healing parameters evaluated in animal models, relative to humans. Treatment success in humans is evaluated as the time till complete wound closure. While this measurement is possible in porcine wound models, it is less clear in rodents due to skin contraction. In addition, while wound inflammation, vascularization, reepithelialization, and scarring can be observed macroscopically to a certain extent in humans and pigs, the size of rodent wounds compromises these measures, meaning that wound biopsies must be taken from euthanized animals to facilitate each type and stage of wound analysis. Thus large numbers of animals are required to fully assess phasic wound responses to a given treatment.
The translatability of biomaterial-based GF and cytokine delivery systems to clinical use may therefore still be dependent on the preclinical models, in which they are tested, the healing parameters evaluated in them, and the number of animals that can be cost-effectively sourced for a given study.
The efficacy of GF and cytokine delivery systems has been evaluated in acute surgical wounds in the rat. A chitosan hydrogel encapsulating PDGF-ββ and VEGF-A improved the oxidative status of sutured incisions relative to hydrogel-treated controls.164 Most GF and cytokine delivery systems have, however, been evaluated in acute full-thickness excisional wounds, either in the rat or mouse.
For example, FGF-2 and rifamycin-laden alginate microparticles accelerated healing relative to untreated excisional wounds in rats, with enhanced granulation tissue formation and angiogenesis relative to untreated controls.98 Chitosan NPs delivering G-CSF or GM-CSF, embedded in a range of natural and synthetic fibers, induced faster healing in rats relative to empty biomaterial controls, with increased reepithelialization and granulation tissue formation observed.123,125 Composite chitosan scaffolds delivering FGF-2 microspheres, FGF-2 with VEGF-A, and VEGF-A with PDGF-ββ-NPs induced healing of rat wounds to a greater extent than their scaffold controls,135,138,154 with increases in wound contraction, granulation tissue formation, vascularization, and fibrosis reported.
Collagen-based systems incorporating EGF or FGF-2 also enhanced rat wound healing relative to their delivery controls.108,160 EGF-loaded gelatin microspheres, delivered in either a gelatin sponge or collagen matrix, also accelerated healing of excisional wounds in rats when compared to the vehicles lacking EGF microspheres.111,152
Fibrin hydrogels and matrices have instead been tested in mouse wounds, particularly for their capacity to delivery VEGF-A-encapsulated macromers or EGF with keratinocytes.117,153 Faster healing was observed relative to individual components, with increased vascularization and collagen production, and epidermal regeneration observed for the macromer and EGF cell therapy, respectively. Incorporation of VEGF-A and FGF-2 into a fibrin sealant also sustained vascularization of a collagen matrix in a mouse wound model.156 A rabbit excisional wound model has also been used to assess delivery of PDGF-ββ and fibroblasts using fibrin sealant,116 with faster healing and granulation tissue formation observed after a single administration relative to sealant with or without keratinocytes.
It is important to note that across these studies, there is very little consistency in how wound healing was measured, and at which time-point each parameter was examined. As an example, Dehkordi et al., calculated the percentage wound closure, every second day for 13 days,123 while Dogan et al., compared wound areas at day 7 and 14, postwounding.111 Vijayan et al., evaluated histological parameters every 5 days from day 5 to 20, postwounding,138 while Wilcke et al., examined wound vascularization after 2 and 4 weeks.156 It is important that a consensus be reached regarding the most appropriate types and time frame for these analyses, so as to enable accurate comparisons between different therapeutics.
To make the rodent model more clinically relevant, a splinted full-thickness excisional wound model has been created, in which a silicon or steel ring is sutured to the skin around the wound, so as to limit contraction, thus enabling macroscopic measurements of reepithelialization.165 This model was used in rats to examine the efficacy of a collagen membrane for dual delivery of EGF and FGF-7 over an extended course of 4 weeks.155 This dual GF therapy led to faster wound healing, with improved vascularization and epidermal regeneration relative to the membrane with or without the individual GFs.
A splinted wound model was also used in mice to evaluate VEGF-A NPs in a composite fibrin-HA hydrogel and showed faster healing and a more mature vasculature than compared to wounds treated with the hydrogel alone.122 This model was further adapted by inoculation of splinted rat wounds with Staphylococcus aureus derived from a patient with infected skin, so as to greater mimic an infected human ulcer.158 The infected wound model was then used to test the efficacy of VEGF-A-loaded PLGA microspheres incorporated in a chitosan-HA hydrogel containing vancomycin, with a single treatment leading to faster healing and increased granulation tissue and vasculature formation relative to the hydrogel with and without each active component.158
GF and cytokine delivery systems have also been evaluated in rodent burn models, with most common method of application being scald or thermal rod burns.91,120,134,148 Scald burns offer the greatest translational relevance, while thermal rod burns provide greater control of burn depth. Partial-thickness scald burn in rats showed faster healing after daily treatment with alginate/chitosan hydrogel containing EGF relative to the hydrogel and EGF controls.148 EGF delivered daily using a chitosan gel also increased reepithelialization of partial-thickness thermal burn in rats relative to hydrogel and EGF controls.91 In an equivalent mouse model, liposomes encompassing FGF-2 within a silk fibroid core lead to faster healing and vascularization than FGF-2–treated control burns.120 FGF-2-laden alginate/chitosan microspheres in a PVA hydrogel also accelerated healing in full-thickness thermal burns in rats when compared to hydrogel with or without FGF-2 controls.134
A few studies have utilized chemical ablation of pancreatic beta cells through streptozotocin (STZ) injection to model type 1 diabetes in rats, so as to delay healing of full-thickness excisional wounds.163 Delivery of EGF in chitosan NPs within a chitosan hydrogel to STZ-induced diabetic rat wounds showed faster healing relative to the hydrogel with or without EGF.124 The combination therapy of collagen nanofibers containing EGF and PDGF-ββ-NPs, with HA nanofibers containing FGF-2 and VEGF-A-NPs, was also tested in STZ-induced diabetic rats, showing faster wound healing and vasculature improvements relative to the fibers with or without FGF-2 and EGF.126
Leptin-deficient db/db mice, a monogenic model of diabetes exhibiting extreme obesity, are also commonly used, as full-thickness excisional wounds in these mice exhibit a substantial delay in repair processes.163 Chitosan fabrications delivering FGF-2 led to faster healing and increased granulation tissue formation and vascularization of the diabetic wounds when compared to the chitosan alone.92,166 Composite fibrin matrices delivering FGF-2 and placental growth factor (PlGF), VEGF-A and PDGF-ββ, or FGF-2/VEGF-A-laden NPs, also accelerated healing in the db/db mouse, with increased epidermal, granulation tissue, and vasculature formation than in wounds treated with the matrices alone.127,137,161 Gelatin-based systems incorporating FGF-2 also enhanced healing of diabetic mice, increasing wound reepithelialization and vascularization relative to gelatin controls.110,113
It should, however, be noted that these animals do not accurately represent type 2 diabetes, which is a polygenic metabolic disorder that can occur in the absence of obesity. It might be more relevant to therefore investigate GF and cytokine delivery systems in TALLYHO/JngJ or NONcNZO10/LtJ mice.167 These mice better represent the polygenic nature of type 2 diabetes and exhibit impaired wound reepithelialization and granulation tissue formation, which in the case of NONcNZO10/LtJ mice is accelerated by topical administration of FGF-1.168,169
Other studies utilized rodent models with local tissue hypoxia to assess the efficacy of delivery systems aimed at treating ulcers with vascular deficiency. In the skin flap model, an ischemic gradient was created in a flap of skin by severing the supplying blood vessels, and delivery of VEGF-A with a fibrin hydrogel reduced skin necrosis and increase vascularization in mice relative to the sealant control.151 In the hind limb model, ischemia was created by ligation of the femoral artery, and here too, delivery of VEGF-A with an alginate or fibrin hydrogel, or FGF-2 in a chitosan gel, enhanced vascularization of mouse and rat limbs relative to biomaterial controls, respectively.93,96,151 Delivery of PDGF-ββ engineered with a collagen-binding domain with a collagen membrane to an ischemic version of the rabbit excisional wound model showed treatment at the time of wounding led to faster reepithelialization and vascularization than with the membrane or native PDGF-ββ.106
Only two studies have evaluated GF and cytokine delivery systems in nonrodent models. A pig excisional wound model was used to test a chitosan film containing EGF and showed that daily administration accelerated healing relative to wounds treated with the film only.149 A viral GF and cytokine combination, VEGF-E and vIL-10, was administered in a collagen/HA hydrogel to limb wounds of horses, showing increased reepithelialization than with hydrogel alone.170 The porcine and equine models better represent the rate of healing for human ulcers, with closure achieved on average after 27 and 50 days, respectively.
Most GF and cytokine delivery systems described above are tailored to specific aspects of the healing response, that is, angiogenesis, while very few aim to progress the wound through all phases of healing. Therapeutic success will likely depend on suppression of infection and inflammation, relief of hypoxia, and the promotion of granulation tissue and epidermal repair to facilitate wound closure. This will require the phasic delivery of multiple bioactives using composite biomaterial and nanodelivery systems. It is evident that the most accurate assessment of these therapeutics will be in slow-to-heal, potentially ischemic, wounds in diabetic rodents or large animals that provide the best representation of human ulcers.
GF and cytokine delivery systems as antiscar treatments
The impact of GF and delivery systems on cutaneous scarring has not been extensively investigated. This may, in part, be due to the high use of models that utilize rodents, which heal with minimal scarring due to the lack of tension provided by their loose skin.171 In rodents, the minimal scar size and hair regeneration around the wound limit the use of macroscopic assessments such as scar scales.74 Most preclinical studies have, therefore, evaluated the impact of the delivery system on scarring processes and the quality of skin repair using histological analyses.
Excess inflammation is a hallmark of excessive healing. Neutrophil and macrophage levels were investigated in wounds treated with fibrin hydrogels delivering VEGF-A-encapsulated macromers, showing no increase relative to untreated wounds.153 Scarring is also characterized by a lack of remodeling, with impairments in the maturation of the blood vessels, differentiation of the epidermis and reorganization of collagen fibers.
FGF-2 and FGF-7 within a collagen membrane, and VEGF-A macromers in a fibrin hydrogel, were reported to increase epidermal differentiation in healed wounds in rodents, as evidenced by increased suprabasal and squamous cell production of keratin 10 and involucrin and pronounced hair follicle formation.117,153,155
Blood vessel maturation is measured by evaluating the association of endothelial cells with pericytes. Vessel maturity was increased following treatment of rodent wounds with collagen membrane containing FGF-2 and FGF-7, fibrin-HA hydrogel containing VEGF-A-NPs, and fibrin matrix delivering FGF-2 and PlGF.122,137,155 In addition, more mature blood vessels were noted following treatment of rat burns with FGF-2-laden microspheres in a composite alginate sponge.134
Collagen remodeling, with collagen I to III ratios and thickening fibers organized in a “basket-weave” pattern approaching that of unwounded skin, was also observed following burns in rats treated with liposomes encapsulating FGF-2.120 Similarly EGF-loaded liposomes increased the tensile strength of healed incisions relative to liposome and saline controls.121 These analyses show greater translatability, and similar histological and biomechanical analyses are performed in human scar therapy trials.74
As rodents heal with very little fibrosis or scarring, large animal models may better represent scarring in humans. For example, skin wounds in the Duroc pig produce raised, hyperpigmented scars with collagen organization similar to that in hypertrophic scars.172 By contrast, horses are the only animal, other than humans, to naturally develop fibroproliferative skin lesions known as exuberant granulation tissue (EGT), which are reminiscent of keloids.173 Administration of a viral GF and cytokine to equine limb wounds before bandaging led to faster resolution of EGT formation, with greater blood vessel integrity observed relative to the hydrogel alone.170 To our knowledge, no GF or cytokine delivery systems have been trialled in the porcine scarring model.
These findings indicate that scar prevention is also dependent on timely progression through the phases of the wound healing response. Once again therapies must suppress inflammation, relieve hypoxia, and control granulation tissue and epidermal repair so as to support scar remodeling. This will also likely require the phasic delivery of multiple bioactives using composite biomaterial and nanodelivery systems. Further research is, however, required as to the impact of different biomaterials on scarring processes, as well as optimization of dosage and timing of GF and cytokine delivery. Assessment of these parameters would ideally be conducted in large animals that exhibit excessive scarring more like hypertrophic scars or keloids in human.
Summary
Healing of the skin requires phasic production of GFs and cytokines, and their dysregulation can lead to impaired or excessive healing. Direct administration of GFs and cytokines to skin ulcers, burns, and surgical incisions increased closure rates and improved scar aesthetics, but underwhelming results mean that only three products have progressed to clinical use. To address the limitations associated with direct protein administration, delivery systems have been developed from natural and composite biomaterials. The findings from this review indicate that biomaterials can provide differential GF and cytokine release kinetics, and extend protein half-life in the presence of proteinases or wound fluid. The incorporation of GFs or cytokines into a biomaterial, in most instances, also increased the reparative functionality of that biomaterial, particularly in acute wounds in rodents. There was, however, limited evidence to suggest that the delivery systems would offer greater efficacy as wound or scar therapeutics, as most studies do not compare the product to direct administration of the same proteins. It was also unclear which GF or cytokine delivery system could, or should, be tailored for specific wound indications, as only a few were assessed in models that exhibit impaired or excessive healing, and no comparisons between different natural or synthetic biomaterials were made. In addition, no studies examined the retention of GFs or cytokines within the biomaterial or the wound site nor did they evaluate the immunogenicity for delivery system components. As such, there is insufficient evidence to conclude whether GF and cytokine delivery systems will offer greater clinical success than the direct therapies that were originally trialled.
To increase the likelihood of GF and cytokine delivery systems succeeding in clinical trials, it is important that they are rigorously tested at the preclinical stage (Fig. 6). Interventions must be carefully designed for a specific wound indication, and phase of healing at which the healing response has stalled. Appropriate biomaterials, proteins, drugs, and cells must then be chosen to address that wound complication. The fabrication method(s) selected must allow for both tailored delivery of the GFs, cytokines, and drugs and physicomechanical support for delivered or invading cells. Delivery systems should then be assessed in vitro, for their ability to protect GFs and cytokines within a proteolytic environment, prevent systemic protein leakage and the induction of immunogenicity, and to support the migration, growth, and differentiation of their skin cell targets.
Figure 6.
Proposed preclinical development pathway for biomaterial-based GF and cytokine delivery systems. Pathway includes three stages: design of the release system with regard to selection of wound indication, desired GF and/or cytokine release profile, biomaterial, and fabrication methods (1), in vitro testing in proteolytic environments and with skin cells (2) and in vivo work in clinically relevant chronic wound or excessive scarring models with macroscopic and histological readouts of healing and scarring outcomes along with blood analysis of protein and antibody levels (3). Note that testing should be conducted in comparison to direct protein administration.
These parameters should then be confirmed in vivo within the context of a healing or nonhealing wound. Acute wound models in rodents can allow for mechanistic insight into the effects of the biomaterial and GF/cytokine on key healing processes such as inflammation, reepithelialization, vascularization, and collagen remodeling. It is, however, important that parameters such as complete closure, scar esthetics, and tensile strength, that are translatable to human wounds, also be assessed. Healing should also be evaluated using standardized units of measure and at agreed upon stages of healing. These analyses are most appropriate in cutaneous wounds that exhibit delayed healing, such as in the diabetic rodent, or those with exasperated scarring, such as the Duroc pig.
Should a delivery system prove more efficacious than direct administration of a GF or cytokine when following a preclinical development plan such as that proposed, the findings may then indeed translate to human trials. These products will likely offer greater efficacy, safety, and patentability than direct protein administration. But they will likely be more costly to produce and may face more regulatory hurdles. Whether the clinical benefit generated by such combination therapies will be sufficient to justify the costs associated with their development, and the clinical testing for products that contain more than one bioactive component, will still need to be answered. Future success with GF and cytokine delivery systems as wound healing and antiscarring therapeutics will, however, still depend on advancements in clinical trial design and regulatory requirements, with regard to science-based patient selection, and quality of life-based healing outcomes.
Take Home Messages
GFs and cytokines are critical regulators of skin repair, and their dysregulation can contribute to wound chronicity and excessive scarring.
Direct administration of recombinant GFs and cytokines to skin ulcers, burns, and surgical incisions has led to underwhelming results in human trials.
Delivery systems made from natural biomaterials offer sustained GF and cytokine release, proteolytic protection, and a matrix supportive of wound repair processes.
Delivery systems have proved efficacious in promoting healing of acute, diabetic, and ischemic wounds in rodent models, but have rarely been tested in large animal models or for antiscarring action nor compared to direct protein therapy or other delivery systems.
Further preclinical and clinical testing is required to determine whether GF and cytokine delivery systems will be more efficacious and safer than direct protein administration, and if so, for which skin wound indications.
Supplementary Material
Abbreviations and Acronyms
- 3D
three-dimensional
- CBD
collagen-binding domain
- CMC
carboxymethylcellulose
- CSF
colony-stimulating factor
- DNA
deoxyribonucleic acid
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EUP
galacturonic acid-containing polysaccharide
- FGF
fibroblast growth factor
- GA
gluteraldehyde
- GAG
glycosaminoglycan
- G-CSF
granulocyte colony-stimulating factor
- GF
growth factors
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HA
hyaluronate/hyaluronic acid
- HBD
heparin-binding domain
- IL
interleukin
- M1
proinflammatory macrophages
- M2
anti-inflammatory macrophages
- MMP
matrix metalloproteinases
- NP
nanoparticle
- PAAm
polyacrylamide
- PCL
polycaprolactone
- PDGF
platelet-derived growth factor
- PDMS
polydimethylsiloxane
- PEG
poly(ethylene glycol)
- PEGDA
poly(ethylene glycol) diacrylate
- PEO
poly(ethylene oxide)
- PEtU
polyetherurethane
- PLGA
poly(lactic-co-glycolic acid)
- PlGF
placental growth factor
- PVA
poly(vinyl alcohol)
- rh
recombinant human
- ROS
reactive oxygen species
- SF
silk fibroid
- STZ
streptozotocin
- TFA
trifluoroacetic acid
- TG
transglutiminase
- TGFβ
transforming growth factor beta
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
This work was supported by the Health Research Council of New Zealand (13/774, 17/649) and New Zealand Lottery Health Research (2019-98814).
Author Disclosure and Ghost Writing
The authors do not have any commercial or financial conflicts of interests to declare. The named authors wrote this article and no ghostwriters were used.
About the Authors
Caitlin Berry-Kilgour, BSc, is a graduate student in the Department of Pharmacology and Toxicology of the University of Otago. She is investigating the efficacy of a natural biomaterial for dual of GF and cytokine delivery with the intent of reducing surgical wound complications. Jaydee Cabral, PhD, has a joint appointment in the Departments of Food Science and Chemistry at the University of Otago. She has extensive experience in the synthesis, physicochemical characterization, and in vitro/in vivo biocompatibility of medical hydrogels and biomaterials. Lyn Wise, PhD, is a senior lecturer in the Department of Pharmacology and Toxicology at the University of Otago. She leads a laboratory focused on the development of GF, cytokine, and lipid therapies to enhance skin repair and resolve skin pathologies, with broad ranging expertise in cellular and animal models of healing and scarring processes.
Supplementary Material
References
- 1.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014;6:265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica 2020;105:284–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science 2017;356:1026–1030 [DOI] [PubMed] [Google Scholar]
- 4.Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci 2013;72:206–217 [DOI] [PubMed] [Google Scholar]
- 6.Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care 2014;3:445–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochaton-Piallat ML, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Res 2016;5:F1000.Faculty Rev-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care 2015;4:119–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 2014;7:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg 2006;117:193S–209S; discussion 210S–211S. [DOI] [PubMed] [Google Scholar]
- 11.Greenhalgh DG.Management of burns. New Eng J Med 2019;380:2349–2359 [DOI] [PubMed] [Google Scholar]
- 12.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalan LR, Brennan MB. The role of the microbiome in nonhealing diabetic wounds. Ann NY Acad Sci 2019;1435:79–92 [DOI] [PubMed] [Google Scholar]
- 14.McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care (New Rochelle) 2013;2:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghazawi FM, Zargham R, Gilardino MS, Sasseville D, Jafarian F. Insights into the pathophysiology of hypertrophic scars and keloids: how do they differ? Adv Skin Wound Care 2018;31:582–595 [DOI] [PubMed] [Google Scholar]
- 16.Zhu Z, Ding J, Tredget EE. The molecular basis of hypertrophic scars. Burns Trauma 2016;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilgus TA, Wulff BC. The importance of mast cells in dermal scarring. Adv Wound Care 2014;3:356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes LA, Marshall CD, Leavitt T, et al. Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Adv Wound Care 2018;7:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Y, Sun Z-L, Liu S-Y, et al. Direct and indirect roles of macrophages in hypertrophic scar formation. Front Physiol 2019;10:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilgus TA.Vascular endothelial growth factor and cutaneous scarring. Adv Wound Care 2019;8:671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014;22:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing therapeutic potential and methods of delivery. Adv Skin Wound Care 2012;25:349–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870 [DOI] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008;22:1276–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 27.D'Hemecourt PA, Smiell JM, Karim MR. Sodium carboxymethylcellulose aqueous-based gel vs. becaplermin gel in patients with nonhealing lower extremity diabetic ulcers. Wounds 1998;10:69–75 [Google Scholar]
- 28.Steed DL.Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg 1995;21:71–78; discussion 79–81. [DOI] [PubMed] [Google Scholar]
- 29.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers: a phase III randomized placebo-controlled double-blind study. Diabetes Care 1998;21:822–827 [DOI] [PubMed] [Google Scholar]
- 30.Mustoe TA, Cutler NR, Allman RM, et al. A phase II study to evaluate recombinant platelet-derived growth factor-BB in the treatment of stage 3 and 4 pressure ulcers. Arch Surg 1994;129:213–219 [DOI] [PubMed] [Google Scholar]
- 31.Rees RS, Robson MC, Smiell JM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled study. Wound Reg Regen 1999;7:141–147 [DOI] [PubMed] [Google Scholar]
- 32.Senet P, Vicaut E, Beneton N, Debure C, Lok C, Chosidow O. Topical treatment of hypertensive leg ulcers with platelet-derived growth factor-BB: a randomized controlled trial. Arch Dermatol 2011;147:926–930 [DOI] [PubMed] [Google Scholar]
- 33.Langer V, Rajagopalan S. Evaluation of recombinant human platelet-derived growth factor as an agent for wound bed preparation in traumatic wounds. Indian J Plast Surg 2012;45:203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang RC, Galiano RD. A review of becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Biologics 2008;2:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papanas D, Maltezos E. Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Safety 2010;33:455–461 [DOI] [PubMed] [Google Scholar]
- 36.Ziyadeh N, Fife D, Walker AM, Wilkinson GS, Seeger JD. A matched cohort study of the risk of cancer in users of becaplermin. Adv Skin Wound Care 2011;24:31–39 [DOI] [PubMed] [Google Scholar]
- 37.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol 2008;128:1365–1374 [DOI] [PubMed] [Google Scholar]
- 38.Tsang MW, Wong WK, Hung CS, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 2003;26:1856–1861 [DOI] [PubMed] [Google Scholar]
- 39.Park KH, Han SH, Hong JP, et al. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: a phase III multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res Clin Prac 2018;142:335–344 [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Villa R, Aguilar-Rebolledo F, Lozano-Platonoff A, et al. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double-blinded controlled trial. Wound Repair Regen 2014;22:497–503 [DOI] [PubMed] [Google Scholar]
- 41.Viswanathan V, Juttada U, Babu M. Efficacy of recombinant human epidermal growth factor (Regen-D 150) in healing diabetic foot ulcers: a hospital-based randomized controlled trial. Int J Low Extrem Wounds 2019;19:158–164 [DOI] [PubMed] [Google Scholar]
- 42.Viswanathan V, Pendsey S, Sekar N, Murthy GSR. A phase III study to evaluate the safety and efficacy of recombinant human epidermal growth factor (REGEN-D™ 150) in healing diabetic foot ulcers. Wounds 2006;18:186–196 [Google Scholar]
- 43.Shin JU, Kang SW, Jeong JJ, Nam KH, Chung WY, Lee JH. Effect of recombinant human epidermal growth factor on cutaneous scar quality in thyroidectomy patients. J Dermatol Treat 2015;26:159–164 [DOI] [PubMed] [Google Scholar]
- 44.Nunes QM, Li Y, Sun C, Kinnunen TK, Fernig DG. Fibroblast growth factors as tissue repair and regeneration therapeutics. PeerJ 2016;4:e1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robson MC, Hill DP, Smith PD, et al. Sequential cytokine therapy for pressure ulcers: clinical and mechanistic response. Ann Surg 2000;231:600–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robson MC, Phillips LG, Lawrence WT, et al. The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Ann Surg 1992;216:401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richard JL, Parer-Richard C, Daures JP, et al. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot, randomized, double-blind, placebo-controlled study. Diabetes Care 1995;18:64–69 [DOI] [PubMed] [Google Scholar]
- 48.Akita S, Akino K, Imaizumi T, Hirano A. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen 2008;16:635–641 [DOI] [PubMed] [Google Scholar]
- 49.Bing M, Da-Sheng C, Zhao-Fan X, et al. Randomized, multicenter, double-blind, and placebo-controlled trial using topical recombinant human acidic fibroblast growth factor for deep partial-thickness burns and skin graft donor site. Wound Repair Regen 2007;15:795–799 [DOI] [PubMed] [Google Scholar]
- 50.Tan Y, Xiao J, Huang Z, et al. Comparison of the therapeutic effects recombinant human acidic and basic fibroblast growth factors in wound healing in diabetic patients. J Health Sci 2008;54:432–440 [Google Scholar]
- 51.Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care 2014;3:647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanft JR, Pollak RA, Barbul A, et al. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care 2008;17:30–32, 34–37. [DOI] [PubMed] [Google Scholar]
- 53.Brem H, Howell R, Criscitelli T, et al. Practical application of granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with wounds. Surg Technol Int 2018;32:61–66 [PubMed] [Google Scholar]
- 54.Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev 2013:Cd006810. DOI: 10.1002/14651858.CD006810.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Lalla F, Pellizzer G, Strazzabosco M, et al. Randomized prospective controlled trial of recombinant granulocyte colony-stimulating factor as adjunctive therapy for limb-threatening diabetic foot infection. Antimicrob Agents Chemother 2001;45:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gough A, Clapperton M, Rolando N, Foster AV, Philpott-Howard J, Edmonds ME. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet 1997;350:855–859 [DOI] [PubMed] [Google Scholar]
- 57.Kästenbauer T, Hörnlein B, Sokol G, Irsigler K. Evaluation of granulocyte-colony stimulating factor (Filgrastim) in infected diabetic foot ulcers. Diabetologia 2003;46:27–30 [DOI] [PubMed] [Google Scholar]
- 58.da Costa RM, Jesus FM, Aniceto C, Mendes M. Double-blind randomized placebo-controlled trial of the use of granulocyte-macrophage colony-stimulating factor in chronic leg ulcers. Am J Surg 1997;173:165–168 [DOI] [PubMed] [Google Scholar]
- 59.da Costa RM, Jesus FMR, Aniceto C, Mendes M. Randomized, double-blind, placebo-controlled, dose-ranging study of granulocyte-macrophage colony stimulating factor in patients with chronic venous leg ulcers. Wound Repair Regen 1999;7:17–25 [DOI] [PubMed] [Google Scholar]
- 60.Wadhwa M, Skog A-LH, Bird C, et al. Immunogenicity of granulocyte-macrophage colony-stimulating factor (GM-CSF) products in patients undergoing combination therapy with GM-CSF. Clin Canc Res 1999;5:1353. [PubMed] [Google Scholar]
- 61.Lichtman MK, Otero-Vinas M, Falanga V. Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair Regen 2016;24:215–222 [DOI] [PubMed] [Google Scholar]
- 62.Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care 2013;2:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirshberg J, Coleman J, Marchant B, Rees RS. TGF-beta3 in the treatment of pressure ulcers: a preliminary report. Adv Skin Wound Care 2001;14:91–95 [DOI] [PubMed] [Google Scholar]
- 64.Ferguson MW, Duncan J, Bond J, et al. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet 2009;373:1264–1274 [DOI] [PubMed] [Google Scholar]
- 65.Bush J, Duncan JAL, Bond JS, et al. Scar-Improving efficacy of avotermin administered into the Wound margins of skin incisions as evaluated by a randomized, double-Blind, placebo-Controlled, phase II clinical trial. Plast Reconstruct Surg 2010;126:1604–1615 [DOI] [PubMed] [Google Scholar]
- 66.McCollum PT, Bush JA, James G, et al. Randomized phase II clinical trial of avotermin versus placebo for scar improvement. Br J Surg 2011;98:925–934 [DOI] [PubMed] [Google Scholar]
- 67.So K, McGrouther DA, Bush JA, et al. Avotermin for scar improvement following scar revision surgery: a randomized, double-blind, within-patient, placebo-controlled, phase II clinical trial. Plast Reconstruct Surg 2011;128:163–172 [DOI] [PubMed] [Google Scholar]
- 68.King A, Balaji S, Le LD, Crombleholme TM, Keswani SG. Regenerative wound healing: the role of interleukin-10. Adv Wound Care 2014;3:315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kieran I, Knock A, Bush J, et al. Interleukin-10 reduces scar formation in both animal and human cutaneous wounds: results of two preclinical and phase II randomized control studies. Wound Repair Regen 2013;21:428–436 [DOI] [PubMed] [Google Scholar]
- 70.Kieran I, Taylor C, Bush J, et al. Effects of interleukin-10 on cutaneous wounds and scars in humans of African continental ancestral origin. Wound Repair Regen 2014;22:326–333 [DOI] [PubMed] [Google Scholar]
- 71.Maderal AD, Vivas AC, Eaglstein WH, Kirsner RS. The FDA and designing clinical trials for chronic cutaneous ulcers. Semin Cell Dev Biol 2012;23:993–999 [DOI] [PubMed] [Google Scholar]
- 72.Patel S, Maheshwari A, Chandra A. Biomarkers for wound healing and their evaluation. J Wound Care 2016;25:46–55 [DOI] [PubMed] [Google Scholar]
- 73.Eaglstein WH, Kirsner RS, Robson MC. Food and Drug Administration (FDA) drug approval end points for chronic cutaneous ulcer studies. Wound Repair Regen 2012;20:793–796 [DOI] [PubMed] [Google Scholar]
- 74.Junker JPE, Philip J, Kiwanuka E, Hackl F, Caterson EJ, Eriksson E. Assessing quality of healing in skin: review of available methods and devices. Wound Repair Regen 2014;22:2–10 [DOI] [PubMed] [Google Scholar]
- 75.Gupta H, Kumar S, Roy SK, Gaud RS. Patent protection strategies. J Pharm Bioallied Sci 2010;2:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tovey MG, Lallemand C. Immunogenicity and other problems associated with the use of biopharmaceuticals. Ther Adv Drug Saf 2011;2:113–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns 2014;40:1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akita S, Akino K, Tanaka K, Anraku K, Hirano A. A basic fibroblast growth factor improves lower extremity wound healing with a porcine-derived skin substitute. J Trauma 2008;64:809–815 [DOI] [PubMed] [Google Scholar]
- 79.Yao C, Yao P, Wu H, Zha Z. Acceleration of wound healing in traumatic ulcers by absorbable collagen sponge containing recombinant basic fibroblast growth factor. Biomed Mater 2006;1:33–37 [DOI] [PubMed] [Google Scholar]
- 80.Huang G, Sun T, Zhang L, et al. Combined application of alginate dressing and human granulocyte-macrophage colony stimulating factor promotes healing in refractory chronic skin ulcers. Exp Ther Med 2014;7:1772–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan D, Liu S, Zhao X, et al. Recombinant human granulocyte macrophage colony stimulating factor in deep second-degree burn wound healing. Medicine 2017;96:e6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan H, Chen J, Peng X. Recombinant human granulocyte-macrophage colony-stimulating factor hydrogel promotes healing of deep partial thickness burn wounds. Burns 2012;38:877–881 [DOI] [PubMed] [Google Scholar]
- 83.Zhang L, Chen J, Han C. A multicenter clinical trial of recombinant human GM-CSF hydrogel for the treatment of deep second-degree burns. Wound Repair Regen 2009;17:685–689 [DOI] [PubMed] [Google Scholar]
- 84.Zoumalan CI, Tadayon SC, Roostaeian J, Rossi AM, Gabriel A. Safety and efficacy of a scar cream consisting of highly selective growth factors within a silicone cream matrix: a double-blinded, randomized, multicenter study. Aesth Surg J 2019;39:319–330 [DOI] [PubMed] [Google Scholar]
- 85.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010;123:4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zarei F, Soleimaninejad M. Role of growth factors and biomaterials in wound healing. Artif Cell Nanomed Biotech 2018;46:906–911 [DOI] [PubMed] [Google Scholar]
- 87.Phillips PL, Wolcott RD, Cowan LJ, Schultz GS. 3—Biofilms in wounds and wound dressing. In: Ågren MS, ed. Wound Healing Biomaterials. Cambridge, UK: Woodhead Publishing, 2016:55–78 [Google Scholar]
- 88.Uebersax L, Merkle HP, Meinel L. Biopolymer-based growth factor delivery for tissue repair: from natural concepts to engineered systems. Tissue Eng Part B 2009;15:263–289 [DOI] [PubMed] [Google Scholar]
- 89.Cabral J, Moratti SC. Hydrogels for biomedical applications. Future Med Chem 2011;3:1877–1888 [DOI] [PubMed] [Google Scholar]
- 90.Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W: chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003;4:1457–1465 [DOI] [PubMed] [Google Scholar]
- 91.Alemdaroělu C, Deěim Z, Çelebi N, Zor F, Öztürk S, Erdoěan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 2006;32:319–327 [DOI] [PubMed] [Google Scholar]
- 92.Obara K, Ishihara M, Fujita M, et al. Acceleration of wound healing in healing-impaired db/db mice with a photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2. Wound Repair Regen 2005;13:390–397 [DOI] [PubMed] [Google Scholar]
- 93.Fujita M, Ishihara M, Shimizu M, et al. Therapeutic angiogenesis induced by controlled release of fibroblast growth factor-2 from injectable chitosan/non-anticoagulant heparin hydrogel in a rat hindlimb ischemia model. Wound Repair Regen 2007;15:58–65 [DOI] [PubMed] [Google Scholar]
- 94.Grant GT, Morris ER, Rees DA, Smith PJC, Thom D. Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett 1973;32:195–198 [Google Scholar]
- 95.Wawrzynska E, Kubies D. Alginate matrices for protein delivery—a short review. Physiol Res 2018;67:S319–s334 [DOI] [PubMed] [Google Scholar]
- 96.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature 2000;408:998–1000 [DOI] [PubMed] [Google Scholar]
- 97.Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Control Release 2004;96:463–472 [DOI] [PubMed] [Google Scholar]
- 98.Shi M, Zhang H, Song T, et al. Sustainable dual release of antibiotic and growth factor from pH-responsive uniform alginate composite microparticles to enhance wound healing. ACS App Mat Interfaces 2019;11:22730–22744 [DOI] [PubMed] [Google Scholar]
- 99.Necas J, Bartosikova L, Brauner P, Kolář J. Hyaluronic acid (Hyaluronan): a review. Vet Med Czech 2008;53:397–411 [Google Scholar]
- 100.Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2). J Biomed Mat Res 2002;62:128–135 [DOI] [PubMed] [Google Scholar]
- 101.He C, Ji H, Qian Y, et al. Heparin-based and heparin-inspired hydrogels: size-effect, gelation and biomedical applications. J Mater Chem B 2019;7:1186–1208 [DOI] [PubMed] [Google Scholar]
- 102.Freudenberg U, Zieris A, Chwalek K, et al. Heparin desulfation modulates VEGF release and angiogenesis in diabetic wounds. J Control Release 2015;220:79–88 [DOI] [PubMed] [Google Scholar]
- 103.Bauer AJP, Liu J, Windsor LJ, Song F, Li B. Current development of collagen-based biomaterials for tissue repair and regeneration. Soft Mater 2014;12:359–370 [Google Scholar]
- 104.Yang CH.Evaluation of the release rate of bioactive recombinant human epidermal growth factor from crosslinking collagen sponges. J Mater Sci 2008;19:1433–1440 [DOI] [PubMed] [Google Scholar]
- 105.Côté MF, Laroche G, Gagnon E, Chevallier P, Doillon CJ. Denatured collagen as support for a FGF-2 delivery system: physicochemical characterizations and in vitro release kinetics and bioactivity. Biomaterials 2004;25:3761–3772 [DOI] [PubMed] [Google Scholar]
- 106.Sun W, Lin H, Xie H, et al. Collagen membranes loaded with collagen-binding human PDGF-BB accelerate wound healing in a rabbit dermal ischemic ulcer model. Growth factors 2007;25:309–318 [DOI] [PubMed] [Google Scholar]
- 107.He Q, Zhao Y, Chen B, et al. Improved cellularization and angiogenesis using collagen scaffolds chemically conjugated with vascular endothelial growth factor. Acta Biomater 2011;7:1084–1093 [DOI] [PubMed] [Google Scholar]
- 108.Yoon D, Yoon D, Cha HJ, Lee JS, Chun W. Enhancement of wound healing efficiency mediated by artificial dermis functionalized with EGF or NRG1. Biomed Mater 2018;13:045007. [DOI] [PubMed] [Google Scholar]
- 109.Subbiah R, Guldberg RE. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv Healthc Mater 2019;8:e1801000. [DOI] [PubMed] [Google Scholar]
- 110.Huang C, Orbay H, Tobita M, et al. Proapoptotic effect of control-released basic fibroblast growth factor on skin wound healing in a diabetic mouse model. Wound Repair Regen 2016;24:65–74 [DOI] [PubMed] [Google Scholar]
- 111.Dogan S, Demirer S, Kepenekci I, et al. Epidermal growth factor-containing wound closure enhances wound healing in non-diabetic and diabetic rats. Int Wound J 2009;6:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Turner PA, Thiele JS, Stegemann JP. Growth factor sequestration and enzyme-mediated release from genipin-crosslinked gelatin microspheres. J Biomater Sci 2017;28:1826–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miyoshi M, Kawazoe T, Igawa HH, Tabata Y, Ikada Y, Suzuki S.. Effects of bFGF incorporated into a gelatin sheet on wound healing. J Biomater Sci.2005;16:893–907 [DOI] [PubMed] [Google Scholar]
- 114.Li Q, Niu Y, Diao H, et al. In situ sequestration of endogenous PDGF-BB with an ECM-mimetic sponge for accelerated wound healing. Biomaterials 2017;148:54–68 [DOI] [PubMed] [Google Scholar]
- 115.Sproul E, Nandi S, Brown A. 6—Fibrin biomaterials for tissue regeneration and repair. In: Barbosa MA, Martins MCL, eds. Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair. Cambridge, UK: Woodhead Publishing, 2018:151–173 [Google Scholar]
- 116.Mogford JE, Tawil B, Jia S, Mustoe TA. Fibrin sealant combined with fibroblasts and platelet-derived growth factor enhance wound healing in excisional wounds. Wound Repair Regen 2009;17:405–410 [DOI] [PubMed] [Google Scholar]
- 117.Gwak SJ, Kim SS, Sung K, Han J, Choi CY, Kim BS. Synergistic effect of keratinocyte transplantation and epidermal growth factor delivery on epidermal regeneration. Cell Transplant 2005;14:809–817 [DOI] [PubMed] [Google Scholar]
- 118.De Jong WH, Borm PJA. Drug delivery and nanoparticles:applications and hazards. Int J Nanomed 2008;3:133–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szoka F Jr., Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A 1978;75:4194–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu HL, Chen PP, Wang LF, et al. Skin-permeable liposome improved stability and permeability of bFGF against skin of mice with deep second degree scald to promote hair follicle neogenesis through inhibition of scar formation. Colloids Surf 2018;172:573–585 [DOI] [PubMed] [Google Scholar]
- 121.Brown GL, Curtsinger LJ, White M, et al. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg 1988;208:788–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cam C, Zhu S, Truong NF, Scumpia PO, Segura T. Systematic evaluation of natural scaffolds in cutaneous wound healing. J Mater Chem B 2015;3:7986–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dehkordi KN, Minaiyan M, Talebi A, Akbari V, Taheri A. Nanocrystalline cellulose-hyaluronic acid composite enriched with GM-CSF loaded chitosan nanoparticles for enhanced wound healing. Biomed Mater 2019;14:035003. [DOI] [PubMed] [Google Scholar]
- 124.Hajimiri M, Shahverdi S, Esfandiari MA, et al. Preparation of hydrogel embedded polymer-growth factor conjugated nanoparticles as a diabetic wound dressing. Drug Dev Ind Pharm 2016;42:707–719 [DOI] [PubMed] [Google Scholar]
- 125.Tanha S, Rafiee-Tehrani M, Abdollahi M, et al. G-CSF loaded nanofiber/nanoparticle composite coated with collagen promotes wound healing in vivo. J Biomed Mater Res Part A 2017;105:2830–2842 [DOI] [PubMed] [Google Scholar]
- 126.Lai HJ, Kuan CH, Wu HC, et al. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater 2014;10:4156–4166 [DOI] [PubMed] [Google Scholar]
- 127.Losi P, Briganti E, Errico C, et al. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater 2013;9:7814–7821 [DOI] [PubMed] [Google Scholar]
- 128.Pacelli S, Acosta F, Chakravarti AR, et al. Nanodiamond-based injectable hydrogel for sustained growth factor release: preparation, characterization and in vitro analysis. Acta Biomater 2017;58:479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Z, Wang Z, Lu WW, Zhen W, Yang D, Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater 2017;9:e435 [Google Scholar]
- 130.Koria P.Delivery of growth factors for tissue regeneration and wound healing. BioDrugs 2012;26:163–175 [DOI] [PubMed] [Google Scholar]
- 131.Wang H-M, Chou Y-T, Wen Z-H, Wang Z-R, Chen C-H, Ho M-L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS One 2013;8:e56330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Silva AK, Richard C, Bessodes M, Scherman D, Merten OW. Growth factor delivery approaches in hydrogels. Biomacromolecules 2009;10:9–18 [DOI] [PubMed] [Google Scholar]
- 133.Hu W, Wang Z, Xiao Y, Zhang S, Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater Sci 2019;7:843–855 [DOI] [PubMed] [Google Scholar]
- 134.Liu Q, Huang Y, Lan Y, et al. Acceleration of skin regeneration in full-thickness burns by incorporation of bFGF-loaded alginate microspheres into a CMCS-PVA hydrogel. J Tissue Eng Regen Med 2017;11:1562–1573 [DOI] [PubMed] [Google Scholar]
- 135.Xie Z, Paras CB, Weng H, et al. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater 2013;9:9351–9359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Briganti E, Spiller D, Mirtelli C, et al. A composite fibrin-based scaffold for controlled delivery of bioactive pro-angiogenetic growth factors. J Control Release 2010;142:14–21 [DOI] [PubMed] [Google Scholar]
- 137.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A 2013;110:4563–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vijayan A, Sabareeswaran A, Kumar GSV. PEG grafted chitosan scaffold for dual growth factor delivery for enhanced wound healing. Sci Rep 2019;9:19165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aduba DC, Yang H. Polysaccharide fabrication platforms and biocompatibility assessment as candidate wound dressing materials. Bioengineering 2017;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ouyang L, Dan Y, Shao Z, et al. MMP-sensitive PEG hydrogel modified with RGD promotes bFGF, VEGF and EPC-mediated angiogenesis. Exp Ther Med 2019;18:2933–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ma K, Liao S, He L, Lu J, Ramakrishna S, Chan CK. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng Part A 2011;17:1413–1424 [DOI] [PubMed] [Google Scholar]
- 142.Miller ED, Song F, Smith JD, et al. Plasma-based biomaterials for the treatment of cutaneous radiation injury. Wound Repair Regen 2019;27:139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Farhadihosseinabadi B, Farahani M, Tayebi T, et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif Cells Nanomed Biotechnol 2018;46:431–440 [DOI] [PubMed] [Google Scholar]
- 144.Kasahara H, Tanaka E, Fukuyama N, et al. Biodegradable gelatin hydrogel potentiates the angiogenic effect of fibroblast growth factor 4 plasmid in rabbit hindlimb ischemia. J Am Coll Cardiol 2003;41:1056–1062 [DOI] [PubMed] [Google Scholar]
- 145.Kobsa S, Kristofik NJ, Sawyer AJ, Bothwell ALM, Kyriakides TR, Saltzman WM. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials 2013;34:3891–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han Y, Tao R, Han Y, et al. Microencapsulated VEGF gene-modified umbilical cord mesenchymal stromal cells promote the vascularization of tissue-engineered dermis: an experimental study. Cytotherapy 2014;16:160–169 [DOI] [PubMed] [Google Scholar]
- 147.Li Y, Zheng L, Xu X, et al. Mesenchymal stem cells modified with angiopoietin-1 gene promote wound healing. Stem Cell Res Ther 2013;4:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu Y, Zhang Z, Li Y, et al. Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications. Macromol Rapid Commun 2018;39:e1800069. [DOI] [PubMed] [Google Scholar]
- 149.Hong JP, Kim YW, Lee SK, Kim SH, Min KH. The effect of continuous release of recombinant human epidermal growth factor (rh-EGF) in chitosan film on full thickness excisional porcine wounds. Ann Plast Surg 2008;61:457–462 [DOI] [PubMed] [Google Scholar]
- 150.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005;26:6054–6067 [DOI] [PubMed] [Google Scholar]
- 151.Sacchi V, Mittermayr R, Hartinger J, et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc Natl Acad Sci U S A 2014;111:6952–6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang S, Zhang Y, Tang L, et al. Functional bilayered skin substitute constructed by tissue-engineered extracellular matrix and microsphere-incorporated gelatin hydrogel for wound repair. Tissue Eng Part A 2009;15:2617–2624 [DOI] [PubMed] [Google Scholar]
- 153.Zhao N, Coyne J, Xu M, et al. Assembly of bifunctional aptamer-fibrinogen macromer for VEGF delivery and skin wound healing. Chem Mater 2019;31:1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shamloo A, Sarmadi M, Aghababaie Z, Vossoughi M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int J Pharm 2018;537:278–289 [DOI] [PubMed] [Google Scholar]
- 155.Qu Y, Cao C, Wu Q, et al. The dual delivery of KGF and bFGF by collagen membrane to promote skin wound healing. J Tissue Eng Regen Med 2018;12:1508–1518 [DOI] [PubMed] [Google Scholar]
- 156.Wilcke I, Lohmeyer JA, Liu S, et al. VEGF(165) and bFGF protein-based therapy in a slow release system to improve angiogenesis in a bioartificial dermal substitute in vitro and in vivo. Langenbecks Arch Surg 2007;392:305–314 [DOI] [PubMed] [Google Scholar]
- 157.Pulat M, Kahraman AS, Tan N, Gumusderelioglu M. Sequential antibiotic and growth factor releasing chitosan-PAAm semi-IPN hydrogel as a novel wound dressing. J Biomater Sci 2013;24:807–819 [DOI] [PubMed] [Google Scholar]
- 158.Huang J, Ren J, Chen G, et al. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mater Sci Eng C 2018;89:213–222 [DOI] [PubMed] [Google Scholar]
- 159.Wilgus TA.Growth factor-extracellular matrix interactions regulate wound repair. Adv Wound Care 2012;1:249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guo Y, Xu B, Wang Y, et al. Dramatic promotion of wound healing using a recombinant human-like collagen and bFGF cross-linked hydrogel by transglutaminase. J Biomater Sci 2019;30:1591–1603 [DOI] [PubMed] [Google Scholar]
- 161.Martino MM, Tortelli F, Mochizuki M, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 2011;3:100ra89. [DOI] [PubMed] [Google Scholar]
- 162.Greek R, Menache A. Systematic reviews of animal models: methodology versus epistemology. Int J Med Sci 2013;10:206–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ansell DM, Holden KA, Hardman MJ. Animal models of wound repair: are they cutting it? Exp Dermatol 2012;21:581–585 [DOI] [PubMed] [Google Scholar]
- 164.Dindar B, Kaltalioğlu K, Coşkun Cevher Ş. Effect of dual growth factor administration on oxidative markers during acute stage wound healing in rats. Turk J Zool 2017;41:841–847 [Google Scholar]
- 165.Davidson JM, Yu F, Opalenik SR. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv Wound Care 2013;2:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mizuno K, Yamamura K, Yano K, et al. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res A 2003;64:177–181 [DOI] [PubMed] [Google Scholar]
- 167.Leiter EH, Strobel M, O'Neill A, Schultz D, Schile A, Reifsnyder PC. Comparison of two new mouse models of polygenic type 2 diabetes at the Jackson Laboratory, NONcNZO10Lt/J and TALLYHO/JngJ. J Diabetes Res 2013;2013:165327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Blaber SI, Diaz J, Blaber M. Accelerated healing in NONcNZO10/LtJ type 2 diabetic mice by FGF-1. Wound Repair Regen 2015;23:538–549 [DOI] [PubMed] [Google Scholar]
- 169.Buck DW, 2nd, Jin DP, Geringer M, Hong SJ, Galiano RD, Mustoe TA. The TallyHo polygenic mouse model of diabetes: implications in wound healing. Plast Reconstr Surg 2011;128:427e. [DOI] [PubMed] [Google Scholar]
- 170.Wise LM, Bodaan CJ, Stuart GS, et al. Treatment of limb wounds of horses with orf virus IL-10 and VEGF-E accelerates resolution of exuberant granulation tissue, but does not prevent its development. PLoS One 2018;13:e0197223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Padmanabhan J, Maan ZN, Kwon SH, Kosaraju R, Bonham CA, Gurtner GC. In vivo models for the study of fibrosis. Adv Wound Care 2019;8:645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li J, Wang J, Wang Z, et al. Experimental models for cutaneous hypertrophic scar research. Wound Repair Regen 2020;28:126–144 [DOI] [PubMed] [Google Scholar]
- 173.Theoret CL, Wilmink JM. Aberrant wound healing in the horse: naturally occurring conditions reminiscent of those observed in man. Wound Repair Regenn 2013;21:365–371 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.