Abstract
Trophoblast cells play multiple critical roles in pregnancy, notably modulating blastocyst attachment to the endometrium as well as invading into and actively remodeling the endometrium to facilitate biotransport needs of the growing embryo. Despite the importance of trophoblast invasion for processes essential at early stages of pregnancy, much remains unknown regarding the balance of signaling molecules that may influence trophoblast invasion into the endometrium. The goal of this study was to use three-dimensional trophoblast spheroid motility assays to examine the effect of cues from the maternal–fetal interface on trophoblast motility. We report use of a methacrylamide-functionalized gelatin hydrogel to support quantitative analysis of trophoblast outgrowth area and cell viability. We show that this multidimensional model of trophoblast motility can resolve quantifiable differences in outgrowth area and viability in the presence of a known invasion promoter, epidermal growth factor, and a known invasion inhibitor, transforming growth factor β1. We then investigate the sensitivity of trophoblast motility to cortisol, a hormone associated with exogenous stressors. Together, this approach provides a toolset to investigate the coordinated action of physiological and pathophysiological processes on early stages of trophoblast invasion.
Impact statement
Trophoblast cells from the invading blastocyst play crucial roles in pregnancy, including remodeling the endometrial structure to support embryonic biotransport needs; however, much remains unknown regarding the balance of signaling molecules that may influence trophoblast invasion. We show that this multidimensional model of motility can resolve quantifiable differences in outgrowth area and viability in the presence of known invasion promoters and inhibitors and then investigate motility sensitivity to cortisol, a hormone associated with exogenous stressors. This approach provides a toolset to investigate the coordinated action of molecules from the maternal–fetal interface on trophoblast motility that may be challenging to study in humans.
Keywords: trophoblast, motility, hydrogel, cortisol, maternal–fetal interface, three dimensional
Introduction
Pregnancy is a complex biological process that involves molecular dialogue between trophoblast cells from the invading blastocyst and cells from the target of implantation, the endometrium. This dialogue coordinates the extent of trophoblast invasion into the endometrium. Although variations in trophoblast invasion are believed to impact the success of a pregnancy,1–4 much remains unknown regarding pathological signaling processes that drive trophoblast invasion into the endometrium.
Implantation occurs when the blastocyst establishes a stable connection with the endometrium.1,2 In order for implantation to occur, the endometrium must undergo preparation via hormonal priming and enter what is known as the implantation window, a short 4-day window during the midsecretory phase of a 28-day menstrual cycle.1,5 Human implantation is thought to occur in three phases: apposition, adhesion, and invasion.2,5 Apposition is defined as initial, unstable attachment of the blastocyst to the endometrial luminal epithelium.1,5 Adhesion initiates physical interactions between trophoblast cells from the blastocyst and endometrial epithelium.1,5 Finally, invasion occurs when trophoblast cells breach the endometrial epithelium and subsequently invade into the underlying stroma.1,5
Perturbations in the implantation processes can result in a variety of pregnancy disorders. Implantation failure accounts for ∼75% of failed pregnancies and represents a significant challenge to fertility.1,2 Implantation failure is not clinically recognized as a pregnancy and defective implantation likely causes adverse effects that compound over the course of the pregnancy. These defects can result in poor pregnancy outcomes, including the hypertensive pregnancy disorder preeclampsia, intrauterine growth restriction, and recurrent pregnancy loss.2 Because implantation involves a highly coordinated molecular dialogue between endometrial cells and trophoblast cells, developing a deeper understanding of the biological mechanisms surrounding implantation may provide critical insights into pregnancy and pregnancy disorders.
Implantation has never been observed in humans due to ethical concerns regarding studying pregnancy in humans as well as a lack of tools to study this process in the body.1,6,7 The blastocyst is fully embedded in the endometrial stroma by ∼10 days postconception, which provides a unique challenge to obtaining direct mechanistic evidence regarding what influences trophoblast invasion into the endometrium.1 Rare histological specimens have allowed us to glean some information on implantation in human specimens; however, only a limited number of samples exist and these specimens cannot provide information on implantation in real time.1,6 In addition, inferring mechanistic processes from animal models may not be accurate due to significant differences between human and animal pregnancy, even among humans and nonhuman primates.7 Although we can use two-dimensional (2D) assays, including wound healing assays and Boyden chamber assays, to probe the biological mechanisms of implantation, these traditional types of invasion assays cannot recapitulate the complexity necessary to capture dynamic processes associated with trophoblast invasion such as matrix remodeling. Tissue-engineered models allow for mechanistic studies using rare populations of cells cultured for extended periods of time in three-dimensional (3D) environments. Such models can replicate relevant biophysical properties inspired by the native tissue, including matrix stiffness, extracellular matrix composition, and 3D architecture.
Advanced tissue-engineered platforms have increasingly been utilized to study trophoblast invasion and migration in 3D biomaterial platforms8–11; however, some limitations still remain with existing models. The use of 3D bioprinting8,9 and microfluidic technology10 allows for tracking migration of trophoblast cells toward soluble factor gradients within biomaterials, but these models quantify migration in dissociated cells and hence do not recapitulate the native spherical structure of an invading blastocyst. Existing models that utilize embryos11 utilize Matrigel, which contains a heterogeneous combination of extracellular matrix proteins and exhibits a significant lot to lot variability.12,13 Furthermore, these models did not quantify trophoblast invasion from the embryo. Adaptation of tissue-engineered platforms of increasing complexity can address these issues by using homogeneous, well-characterized materials and replicating native tissue structure and they can provide additional tools to study core processes associated with trophoblast invasion as they relate to pregnancy and pregnancy disorders.
The overall objective of this study was to use methacrylamide-functionalized gelatin (GelMA) hydrogels and advanced trophoblast spheroid motility assays to quantify trophoblast motility and cell viability in the presence of cues from the maternal–fetal interface. We show that this multidimensional model can quantify trophoblast spheroid outgrowth area and viability using known promoters (epidermal growth factor; EGF) and inhibitors (transforming growth factor β1; TGFβ1) of trophoblast motility to demonstrate the relevance of our platform for such studies. EGF is a decidual factor shown to stimulate trophoblast migration.8,14,15 TGFβ superfamily members are expressed in the endometrium, with TGFβ1 present in endometrial epithelial and stromal cells.16 Production and secretion of TGFβ by epithelial cells during the secretory phase suggest that it may play a role in implantation.16 Next, we investigate the effects of cortisol, a steroid hormone produced in response to stressors, on trophoblast motility and quantify trophoblast spheroid outgrowth area and viability. Cortisol is a steroid hormone that increases as part of the physiological stress response. The overlap between the hypothalamic/pituitary/adrenal and hypothalamic/pituitary/ovarian axes makes cortisol highly relevant to reproductive function and thus a variety of processes of pregnancy.17,18 Psychosocial stressors during pregnancy can arise from poverty, intimate partner violence, lack of social support, and structural and interpersonal racism, and have been associated with increased risk for certain pregnancy disorders, such as preterm birth, low birth weight, and preeclampsia.19–22 Nonetheless, poorly understood processes of how psychosocial stressors affect early events in pregnancy such as trophoblast motility motivate our use of a tissue-engineered platform to investigate the role of soluble cues from the maternal–fetal interface on trophoblast invasion.
Materials and Methods
Hydrogel fabrication and characterization
GelMA synthesis and hydrogel fabrication
Methacrylamide-functionalized gelatin with 57% degree of functionalization, determined by 1H nuclear magnetic resonance was synthesized as described previously using the one-pot method developed by Shirahama et al.23,24 Lyophilized GelMA23 was dissolved in phosphate-buffered saline (PBS; 17-516F; Lonza) at 37°C to make 5 wt% polymer solutions. A 0.1% w/v lithium acylphosphinate was used as a photoinitiator.25 Unless otherwise noted, 20 μL prepolymer solution was pipetted onto custom circular Teflon molds (5 mm diameter, 1 mm height). Hydrogels were polymerized under UV light (λ = 365 nm, 7.14 mW/cm2; ULM-3-365; AccuCure Spot System) for 30 s.
Hydrogel characterization
Hydrogel Young's modulus was determined via compression testing using an Instron 5943 mechanical tester with a 5N load cell.26 Hydrogel disks (10 mm diameter, 2 mm height, 100 μL prepolymer solution), fabricated to have approximately the same height as those made using the smaller molds, were submerged in PBS and allowed to swell for 2 h at 37°C. Samples were compressed at a rate of 0.1 mm/min and moduli were quantified from the linear region of the stress/strain curve using a custom MATLAB code that calculates modulus from the linear regime at a load of 0.003 N and offset of 2.5% strain to ensure contact with the hydrogel surface (Supplementary Data S1 and Supplementary Fig. S1). To calculate mass swelling ratio, hydrogels were fabricated using the smaller molds and were hydrated in PBS overnight at 37°C. Swollen hydrogels were weighed, lyophilized, and weighed once more to determine dry mass. Mass swelling ratio was calculated using the ratio of wet polymer mass to dry polymer mass as previously described.23
HTR-8/SVneo spheroid invasion assays
HTR-8/SVneo cell maintenance
HTR-8/SVneo trophoblast cells (ATCC® CRL-3271, used experimentally before passage 6 after purchase) were maintained as per the manufacturer's instructions in phenol red-free RPMI-1640 supplemented with 5% charcoal-stripped fetal bovine serum (F6765; Sigma-Aldrich) and 1% penicillin/streptomycin (15140122; Thermo Fisher). All cultures were grown in 5% CO2 incubators at 37°C. Routine mycoplasma testing was performed every 6 months to ensure cell quality using the MycoAlert™ Mycoplasma Detection Kit (Lonza).
Spheroid motility assays
Spheroid motility assays were performed as previously described by our group.23,27 Four thousand HTR-8/SVneo cells were added to round-bottomed plates (4515; Corning) and placed on a shaker for 48 h to form spheroids. Four thousand cells per spheroid were selected because this generated spheroids with diameters similar to that of an invading blastocyst, which ranges from ∼100–200 μm (Supplementary Data S2; Supplementary Fig. S2).28 Individual spheroids were pipetted onto the Teflon hydrogel molds. Prepolymer solution was added to the mold and spheroids were gently moved to the center of the mold using a pipette tip. Spheroids were moved to ensure they were fully embedded in the hydrogel (e.g., not on top of the hydrogel or attached to the glass slide) because otherwise, they would fall out of the hydrogel. Hydrogels were then polymerized and added to 48-well plates containing 500 μL of cell medium per well. Once all spheroids were encapsulated, spheroids were imaged and medium was replaced with 800 μL of medium with or without (control) biomolecules per well. Medium was not changed at any other time during the experiment unless otherwise noted. Spheroids and encapsulated spheroids were cultured in phenol red-free RPMI-1640 supplemented with 2% charcoal-stripped fetal bovine serum, 1% penicillin/streptomycin, and relevant biomolecules if applicable. No differences in cell growth or morphology were found for cells cultured in medium with 2% fetal bovine serum (Supplementary Data S3 and Supplementary Fig. S3). Recombinant human TGFβ1 (240-B; R&D Systems) and recombinant human EGF (E9644; Sigma-Aldrich) were added to the medium at a concentration of 5 ng/mL. Cortisol (H0888; Sigma-Aldrich) was added to the medium at concentrations of 5, 20, 75, and 150 ng/mL. Control samples were incubated with no added biomolecules. Spheroids were imaged daily using a Leica DMI 4000 B microscope (Leica Microsystems). Total outgrowth area was calculated using the Measure tool in Fiji. Spheroids were manually traced three times and outgrowth area was determined from the average of these three measurements. Image insets were created using the Zoom in Images and Stacks macro for ImageJ.
Viability assay
The CellTiter-Glo® 3D Cell Viability Assay (Promega) was used to quantify spheroid viability on day 3. Samples were equilibrated to room temperature for at least 30 min before running the assay. A stock solution of 1:1 cell medium and CellTiter-Glo® was prepared, medium was removed from each sample well, and 400 μL of the stock solution was added to each sample. Samples were protected from light and incubated for 1 h on a shaker at room temperature. One hundred microliter triplicates were added to an opaque plate and luminescence was read immediately using a plate reader (BioTek Synergy HT Plate Reader and Gen5 software; BioTek Instruments, Inc.). A blank was prepared using stock solution. Relative luminescence for each sample was calculated by subtracting the average luminescence value from the blank wells from the average luminescence values of each sample.
Imaging techniques
Spheroid staining
Spheroids were encapsulated in hydrogels and grown for 1 or 3 days. Samples were fixed in 4% formaldehyde in PBS for 15 min followed by three PBS washes. The following solutions were prepared: Permeabilizing Solution (0.1% Tween 20 [BP337; Fisher Scientific] in PBS) and Working Solution (1 μL Phalloidin-iFluor 488 Reagent [ab176753; Abcam] per 1 mL 1% bovine serum albumin [A4503; Sigma-Aldrich] in PBS). All subsequent steps were performed at room temperature on a shaker. Samples were permeabilized in Permeabilizing Solution for 15 min. After permeabilization, 300 μL of Working Solution was added per well and samples were protected from light and incubated for 90 min. Staining was followed by 4 × 20-min PBS washes. Samples were then stained with Hoechst (1:2000 in PBS; H3570; Thermo Fisher) for 30 min followed by a PBS wash. Samples were stored in PBS at 4°C until imaged. One Z-stack per spheroid was taken using a Zeiss LSM 710 confocal microscope. Maximum intensity projection images were generated using ZEN (blue edition; Zeiss), and median filtering was used to smoothen images.
2D immunofluorescent staining
To determine glucocorticoid receptor expression in HTR-8/SVneo cells, HTR-8/SVneo cells were seeded on six-well plates at a density of 3 × 104 cells/cm2 and cultured in cell growth medium supplemented with 2% charcoal-stripped fetal bovine serum and 1% penicillin/streptomycin until ∼80% confluence. Cells were fixed using 4% formaldehyde for 15 min followed by three PBS washes. Cells were permeabilized in 0.5% Tween 20 in PBS for 15 min followed by 3 × 5-min washes in 0.1% Tween 20 solution in PBS, blocked in blocking solution (2% bovine serum albumin and 0.1% Tween 20 solution in PBS) for 1 h at room temperature, and incubated in primary antibody solution (Abcam ab3578 rabbit polyclonal antiglucocorticoid receptor antibody; 1:20) diluted in blocking solution overnight at 4°C. Following the overnight incubation, 5 × 5-min washes in 0.1% Tween 20 solution in PBS were performed and samples were incubated in a secondary antibody solution [Thermo Fisher A-21428 goat anti-rabbit IgG (H+L) cross-adsorbed secondary antibody Alexa Fluor 555; 1:500] diluted in blocking solution overnight 4°C while protected from light. Washes, 5 × 5 min, in 0.1% Tween 20 solution in PBS were performed followed by a 30-min stain with Phalloidin-iFluor 488 Reagent diluted in Working Solution. PBS washes, 3 × 5 min, were performed followed by a 10-min Hoechst (1:2000) stain and a quick wash with 0.1% Tween 20 solution in PBS. Samples were stored in 0.1% Tween 20 solution in PBS at 4°C until imaged. Two images per well (n = 3 wells each condition) were imaged using identical image settings on a DMi8 Yokogawa W1 spinning disk confocal microscope outfitted with a Hamamatsu EM-CCD digital camera (Leica Microsystems). Images were pseudocolored and overlaid using Fiji.
Statistics
OriginPro 2019 (Origin Lab) and RStudio were used for statistical analysis. Normality was determined using the Shapiro–Wilkes test and homoscedasticity (equality of variance) was determined using Levene's test. For all quantitative data, n = 4–6 spheroids were analyzed per sample group. For each experiment, outgrowth area was compared between groups on the same day. Normal homoscedastic data were analyzed using a one-way analysis of variance (ANOVA) followed by post hoc Tukey test. For data that violated the assumption of normality but maintained homoscedasticity, the Kruskal–Wallis test was used to analyze data followed by Dunn's post hoc test. For normal, heteroscedastic data, Welch's ANOVA was used to analyze data followed by the Games–Howell post hoc test. For non-normal, heteroscedastic data, Welch's heteroscedastic F test with trimmed means and Winsorized variances was used to analyze data followed by the Games–Howell post hoc test. Significance was set as p < 0.05. Outgrowth area results are reported as mean ± standard deviation and cell viability data are reported as box plots.
Results
The development of 3D trophoblast spheroid motility assays allows for quantitative motility analysis
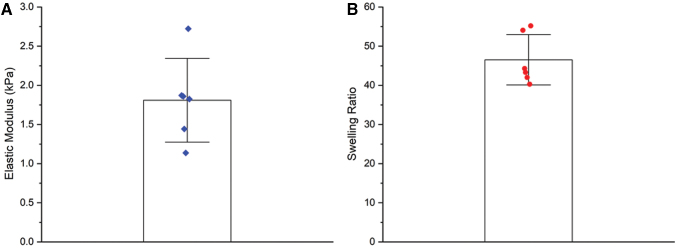
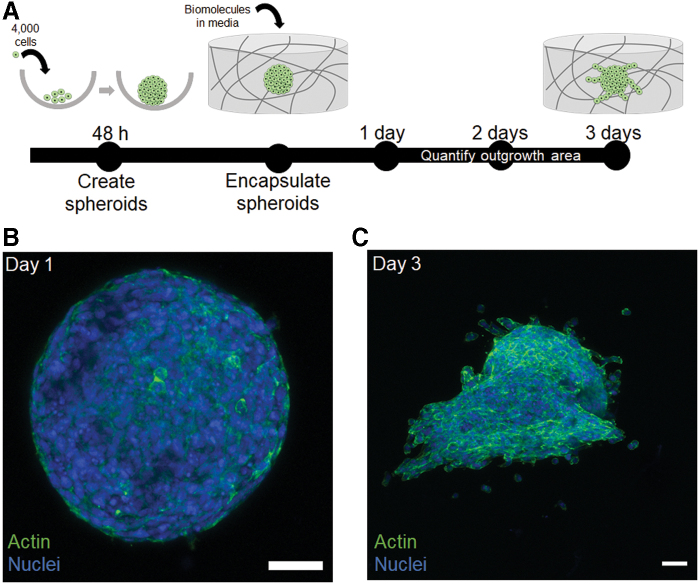
We cultured HTR-8/SVneo trophoblast spheroids in hydrogels with mechanical properties (elastic modulus: 1.8 ± 0.5 kPa; Mass Swelling Ratio: 46.5 ± 6.4) similar to that of native tissue (Fig. 1), which was found to be ∼1.25 kPa for the decidua basalis and 0.171 kPa for the decidua parietalis.29 We cultured spheroids for up to 3 days and observed cell migration into the surrounding hydrogel matrix over time (Fig. 2). These assays provide a platform to quantitatively analyze trophoblast motility in three dimensions. We subsequently examined differences in motility and viability in response to exogenous soluble factors.
FIG. 1.
Mechanical characterization of 5 wt% GelMA hydrogels. (A) Elastic modulus determined by compression testing (n = 6 hydrogels). (B) Mass swelling ratio determined by comparing swollen polymer mass to dry polymer mass (n = 6 hydrogels). Data presented as mean ± standard deviation with individual data points shown. GelMA, methacrylamide-functionalized gelatin. Color images are available online.
FIG. 2.
Three-dimensional trophoblast motility assays in methacrylamide-functionalized gelatin hydrogels. (A) HTR-8/SVneo trophoblast cells were seeded into round-bottomed plates for 48 h to create spheroids. Spheroids were then encapsulated in hydrogels and allowed to migrate into the surrounding hydrogel for up to 3 days in the presence or absence of soluble biomolecules in growth medium. (B) Representative maximum intensity projection image of a spheroid in a hydrogel 1 day postembedding. (C) Representative maximum intensity projection image of a spheroid 3 days postembedding. Green: Phalloidin (actin). Blue: Hoechst (nuclei). Scale: 200 μm. Color images are available online.
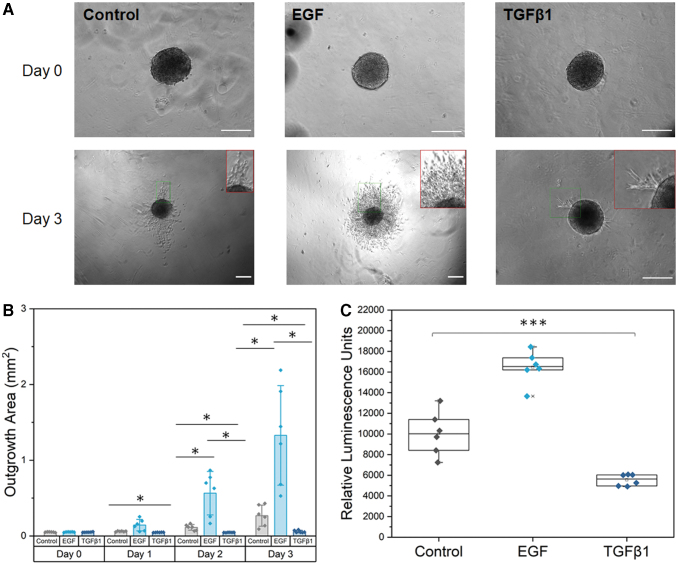
EGF and TGFβ1 modulate trophoblast motility and viability
We quantified shifts in trophoblast motility and viability via the addition of soluble biomolecules in cell media. Control samples had a “thorn-like” migration pattern, with cells migrating away from the spheroid in thorn-like projections. Trophoblast spheroids showed increased motility and increased viability in the presence of known trophoblast motility promoter EGF and showed a “coronal” migration pattern. Trophoblast spheroids showed inhibited motility and decreased viability in the presence of known trophoblast motility inhibitor TGFβ1 by day 2, but still showed a migration pattern of thorn-like projections (Fig. 3). Starting by day 1, the TGFβ1 condition was significantly different compared with control (Welch's ANOVA: p = 0.00064; Games–Howell: p = 0.001). By day 2, all conditions were significantly different (Welch's ANOVA: p = 0.0023; Games–Howell: EGF-control p = 0.027, TGFβ1-control p = 0.018, TGFβ1-EGF p = 0.016) and the conditions were significantly different on day 3 as well (Welch's heteroscedastic F test with trimmed means and Winsorized variances: p = 0.036; Games–Howell: EGF-control p = 0.023, TGFβ1-control p = 0.031, TGFβ1-EGF p = 0.012). Cell viability was significantly different across all three groups (one-way ANOVA: p = 1.78 × 10−8; Tukey test: EGF-control p = 1.05 × 10−5, TGFβ1-control p = 4.6 × 10−4, TGFβ1-EGF p < 1 × 10−8).
FIG. 3.
EGF and TGFβ1 modulate trophoblast outgrowth and viability. (A) Representative phase images of individual HTR-8/SVneo trophoblast spheroids at days 0 and 3. Insets of invading cells are shown for day 3 in red. EGF and TGFβ1 were added to cell medium at day 0. Control samples contained no additional growth factors. (B) Total spheroid outgrowth area at days 0–3 was quantified in Fiji and reported as averages for each condition. Data presented as mean ± standard deviation with individual data points shown. (C) Cell viability data from CellTiter-Glo® 3D Cell Viability Assay measured in encapsulated spheroids on day 3. Data presented as box plots with individual data points shown. *p < 0.05. ***p < 0.001. n = 6 samples per condition. Scale: 200 μm. EGF, epidermal growth factor; TGFβ1, transforming growth factor beta 1. Color images are available online.
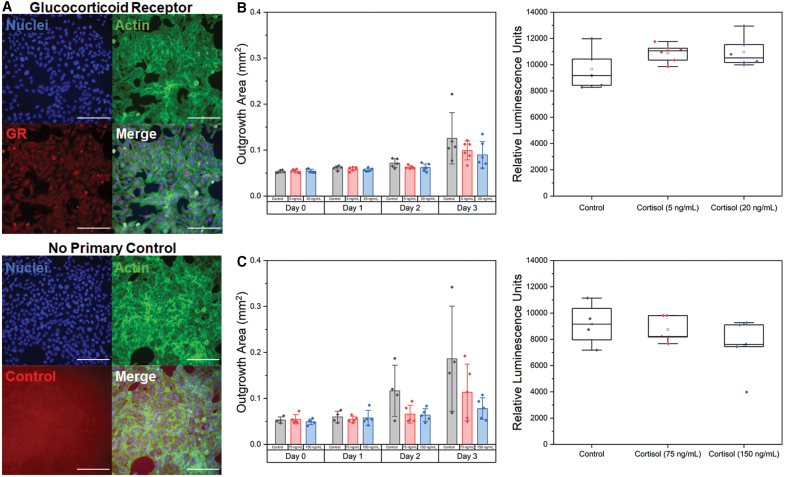
Cortisol has no effect on outgrowth area or viability
We subsequently used immunofluorescence staining of HTR-8/SVneo cells in 2D cell culture well plates to validate their expression of glucocorticoid receptors. Notably, HTR-8/SVneo cells positively express glucocorticoid receptors (Fig. 4A). Furthermore, we observed no evidence of nonspecific antibody binding (samples stained without primary antibody) and observed only minimal background fluorescence. We subsequently quantified trophoblast spheroid outgrowth area and viability in the presence of cortisol. Although sample outgrowth area in the presence of cortisol for lower and physiological concentrations showed a decreasing trend in outgrowth area by day 3 of culture, the effects were not significant. We observed no differences (p > 0.05) in the outgrowth area or viability (p = 0.15) compared with control samples for lower (5 and 20 ng/mL) concentrations at days 0 (p = 0.88), 1 (p = 0.23), 2 (p = 0.08), and 3 (p = 0.28) (Fig. 4B). Furthermore, for physiological (75 and 150 ng/mL) concentrations of cortisol (Fig. 4C), we also observed no differences (p > 0.05) in outgrowth area or viability at days 0 (p = 0.52), 1 (p = 0.83), 2 (p = 0.06), and 3 (p = 0.12) of culture.
FIG. 4.
The effects of cortisol on trophoblast motility. (A) Representative fluorescent images of HTR-8/SVneo trophoblast cells cultured in 6-well plates. HTR-8/SVneo trophoblast cells express glucocorticoid receptors with cytoplasmic staining. The no primary control showed only minimal background fluorescence with no indication of nonspecific binding. Green: Phalloidin (actin). Red: Antiglucocorticoid receptor. Blue: Hoechst (nuclei). (B) Total outgrowth area at days 0–3 and cell viability at day 3 for control, 5 ng/mL cortisol, or 20 ng/mL cortisol. Data presented as mean ± standard deviation with individual data points shown. (C) Total outgrowth area at days 0–3 and cell viability at day 3 for control, 75 ng/mL cortisol, or 150 ng/mL cortisol. Data presented as box plots with individual data points shown. n = 4–6 samples per condition. Scale: 200 μm. Color images are available online.
Discussion
The objective of this study was to adapt a cell spheroid-based trophoblast motility assay to probe how biomolecules from the maternal–fetal interface influence trophoblast motility. Here, we demonstrate that trophoblast motility can be modulated through the use of known promoters and inhibitors of invasion. We then investigate the effects of exogenous cortisol and quantify outgrowth area and cell viability in the presence of this molecule.
For these studies, we use GelMA hydrogels. Gelatin is denatured collagen and hence retains cell binding motifs and degradation sites that allow for cell attachment and matrix remodeling, key processes relevant to invasion.23,27,30–32 The endometrium contains collagens I, III, IV, V, and VI, rendering gelatin relevant in terms of its extracellular matrix composition.33,34 In addition, the biophysical properties of GelMA can be adjusted to fall within the regime of tissue stiffness. Recently, Abbas et al. used atomic force microscopy to demonstrate that the stiffness of the nonpregnant endometrium and placenta has an order of magnitude of 102 Pa, whereas the decidua basalis, the region of the endometrium directly under the placenta that is invaded by trophoblast cells, exhibited an average stiffness on the order of 103 Pa.29 Our hydrogel formulation falls within this range and replicates relevant mechanical properties of the tissue through which trophoblast cells will invade. Nevertheless, the ability to tune the biophysical and biochemical properties of GelMA provides a unique opportunity to modulate hydrogel properties to mimic aspects of disease and disorders that may relate to differences in endometrial and placental tissue variation. We have previously demonstrated that microenvironmental signals within GelMA hydrogels, including biophysical cues, bound and soluble biomolecules, and heterotypic cell–cell interactions, can influence cell behavior and phenotype.35 Microenvironmental tissue variation may relate to the onset and progression of pregnancy disorders, and the ability to probe such questions in an adaptable platform would be crucial for understanding how spatial variation in tissues plays a role in disease. From in vivo studies using shear wave elastography, it was demonstrated that placental stiffness was significantly higher in women with preeclampsia compared with women with normal pregnancies.36 Preeclampsia is a hypertensive pregnancy disorder characterized by high maternal blood pressure and the presence of proteins in urine at or after 20 weeks of gestation.37–40 It is hypothesized that insufficient trophoblast invasion in early pregnancy may be a defect related to the onset of preeclampsia.4,37,41 Using our advanced hydrogel systems, we can create spatially graded hydrogel systems that would allow us to create gradients of stiffness and matrix components to recapitulate biophysical and biochemical variation across endometrial tissue that would occur normally and in diseased states.35,42 Although varying spatial hydrogel properties was beyond the scope of this study, there is potential to assess gradients across hydrogels to determine how the biophysical and biochemical tissue microenvironment plays a role in early pregnancy, implantation, and pregnancy disorders.
Unlike traditional 2D wound healing assays or Boyden chamber assays, spheroid motility assays in biomaterial platforms can replicate the 3D structure of an invading blastocyst and provide a matrix through which cells can invade. We demonstrate reproducible spheroid formation using the HTR-8/SVneo cell line, resulting in spheroid size within the range of an invading blastocyst.28 We use these trophoblast assays to interrogate differences in trophoblast outgrowth area and viability in the presence of biomolecules from the maternal–fetal interface. First, we selected known trophoblast motility promoters and inhibitors: EGF and TGFβ1, and observed quantifiable differences in trophoblast outgrowth area and cell viability. We show that soluble EGF stimulates trophoblast motility and viability at concentrations used previously in literature.8,14,15 In addition, soluble TGFβ1 inhibited trophoblast motility and resulted in decreased viability, similar to what was reported previously in the literature.43,44 Taken together, we show that a multidimensional tissue engineering model can be used to quantify differences in motility and viability using a small number of cells. Furthermore, this platform is able to resolve the effects of other biomolecules from the maternal–fetal interface known to modulate trophoblast invasion.
Subsequently, we included an investigation of the role of cortisol on trophoblast motility. The physiological stress response is an acute-phase response that, when activated repeatedly, can have chronic effects.20,45 Significant psychosocial stressors during pregnancy can be induced by poverty, intimate partner violence, lack of social support, and both structural and interpersonal racism. The downstream effects of this constantly activated physiological stress response have been associated with increased risk for certain pregnancy disorders, including preterm birth, low birth weight, and preeclampsia.19–22 However, much remains unknown regarding how psychosocial stressors affect early events in pregnancy, such as trophoblast invasion. A benefit to our model system is that although stress effects on pregnancy outcomes may not be possible to study in humans or animals due to the complexity of the stress response, models of early implantation could allow us to study how cortisol affects trophoblast invasion. Cortisol is a steroid hormone that elevates as part of the physiological stress response and is functionally significant in multiple processes of reproductive functioning, including during pregnancy.17,18 Previous studies have shown that natural and synthetic glucocorticoids reduce trophoblast motility and proliferation in 2D cultures, wound healing assays, and Matrigel invasion assays.46–48
We first investigated trophoblast motility in the presence of cortisol at concentrations from in vitro studies (5–20 ng/mL).49 Outgrowth area showed a decreasing trend in the presence of cortisol, although not statistically significant, with no changes in viability between groups. We next selected physiological concentrations of cortisol (75–150 ng/mL) comparable with what would occur during early pregnancy.18 We observed a similar trend, with no statistical differences in outgrowth area or viability despite physiologically relevant cortisol concentrations. To our knowledge, no studies to date have quantified cortisol levels in pregnant individuals with high stress levels; therefore, our selected concentrations may be lower than the required threshold to significantly reduce trophoblast motility. Furthermore, 11β-HSD2 activity may have been able to convert these levels of cortisol into cortisone, which could explain why cortisol had a minimal effect on outgrowth area and viability.50 An investigation of 11β-HSD2 activity in the presence and absence of cortisol as well as a further exploration of cortisol dosages may provide additional insight. If no differences are found, it is possible that cortisol does not have an effect on trophoblast motility or proliferation as previously found in the literature. Our system is 3D and captures not only the spherical structure similar to an invading blastocyst but also allows cells to invade and remodel a surrounding gel matrix. Our system may be more applicable to what occurs physiologically and may not have as profound an effect on motility and proliferation as previously demonstrated in less complex models.
We recognize some limitations of our studies. These limitations provide exciting opportunities to improve our platforms to create models of increasing physiological relevance. We first acknowledge that the use of the HTR-8/SVneo cell line may contain a mixed population of cells and may not accurately mimic in vivo trophoblast behavior.51 For future investigations, the physiological relevance of our platform can be increased through the use of primary trophoblast cells. Second, we simplified an increased stress response to external stressors as equivalent to higher levels of cortisol. Although this strategy is easily testable in our platform, literature suggests that cortisol levels exhibit a diurnal pattern and there is some individual variation in cortisol responsiveness based on mental health conditions, for example, post-traumatic stress disorder.52–60 Future work on cortisol may be improved with temporal adjustments of cortisol concentrations to more appropriately mimic what occurs in the body.
Conclusions
Trophoblast invasion is a biological process essential to the establishment and success of a pregnancy. We use GelMA hydrogels to perform advanced quantitative trophoblast motility assays and demonstrate quantifiable differences in trophoblast outgrowth area and viability in the presence of known promoters and inhibitors of trophoblast invasion (EGF and TGFβ1). Furthermore, we begin to probe how cortisol may influence trophoblast motility. The platform proposed herein will provide researchers with a unique tool critical for understanding implantation and will aid researchers in understanding mechanisms that dictate the success or failure of implantation.
Supplementary Material
Acknowledgments
The authors thank Dr. Jee-Wei Emily Chen (U. Illinois) for her incredible mentorship and assistance with method development and Dr. Sara Pedron (U. Illinois) for her assistance with NMR analysis. The authors acknowledge the School of Chemical Sciences Cell Media Facility at the University of Illinois Urbana-Champaign for assistance with cell media and the Core Facilities (Dr. Austin Cyphersmith) at the Institute for Genomic Biology at the University of Illinois Urbana-Champaign for providing assistance with confocal imaging. The authors also gratefully acknowledge additional funding provided by the Department of Chemical & Biomolecular Engineering and the Carl R. Woese Institute for Genomic Biology at the University of Illinois Urbana-Champaign.
Disclaimer
The content herein is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure Statement
No competing financial interests exist.
Funding Information
Research reported herein was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers R01 DK099528 (B.A.C.H.) and by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Numbers R21 EB018481 (B.A.C.H.) and T32 EB019944 (S.G.Z.).
Supplementary Material
References
- 1.Norwitz, E.R., Schust, D.J., and Fisher, S.J.. Implantation and the survival of early pregnancy. N Engl J Med 345,1400, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Cha, J., Sun, X., and Dey, S.K.. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18,1754, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knofler, M.Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol 54,269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman-Wohl, D., and Yagel, S.. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol 187,233, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Su, R.W., and Fazleabas, A.T.. Implantation and establishment of pregnancy in human and nonhuman primates. Adv Anat Embryol Cell Biol 216,189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser, G., and Huppertz, B.. Implantation and extravillous trophoblast invasion: from rare archival specimens to modern biobanking. Placenta 56,19, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright, J.E., and Whitley, G.S.. Strategies for investigating the maternal-fetal interface in the first trimester of pregnancy: what can we learn about pathology? Placenta 60, 145, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo, C.-Y., Eranki, A., Placone, J.K., et al. . Development of a 3D printed, bioengineered placenta model to evaluate the role of trophoblast migration in preeclampsia. ACS Biomater Sci Eng 2,1817, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Kuo, C.Y., Guo, T., Cabrera-Luque, J., et al. . Placental basement membrane proteins are required for effective cytotrophoblast invasion in a three-dimensional bioprinted placenta model. J Biomed Mater Res A 106,1476, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas, Y., Oefner, C.M., Polacheck, W.J., et al. . A microfluidics assay to study invasion of human placental trophoblast cells. J R Soc Interface 14,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, T.A., Bondarenko, G.I., Gerami-Naini, B., et al. . Trophoblast differentiation, invasion and hormone secretion in a three-dimensional in vitro implantation model with rhesus monkey embryos. Reprod Biol Endocrinol 16,24, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vukicevic, S., Kleinman, H.K., Luyten, F.P., Roberts, A.B., Roche, N.S., and Reddi, A.H.. Identification of multiple active growth factors in basement membrane matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res 202,1, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Hughes, C.S., Postovit, L.M., and Lajoie, G.A.. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10,1886, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Cartwright, J.E., Fraser, R., Leslie, K., Wallace, A.E., and James, J.L.. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction 140,803, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Staun-Ram, E., Goldman, S., Gabarin, D., and Shalev, E.. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol 2,59, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, R.L., Stoikos, C., Findlay, J.K., and Salamonsen, L.A.. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction 132,217, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Nepomnaschy, P.A., Welch, K.B., McConnell, D.S., Low, B.S., Strassman, B.I., and England, B.G.. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci U S A 103,3938, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nepomnaschy, P.A., Salvante, K.G., Zeng, L., et al. . Variation in maternal urinary cortisol profiles across the peri-conceptional period: a longitudinal description and evaluation of potential functions. Hum Reprod 30,1460, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Dunkel Schetter, C.Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol 62,531, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Latendresse, G.The interaction between chronic stress and pregnancy: preterm birth from a biobehavioral perspective. J Midwifery Womens Health 54,8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu, Y., Zhang, S., Wang, G., et al. . The combined association of psychosocial stress and chronic hypertension with preeclampsia. Am J Obstet Gynecol 209, 438 e1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aufdenblatten, M., Baumann, M., Raio, L., et al. . Prematurity is related to high placental cortisol in preeclampsia. Pediatric Res 65,198, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Zambuto, S.G., Clancy, K.B.H., and Harley, B.A.C.. A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus 9,2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirahama, H., Lee, B.H., Tan, L.P., and Cho, N.J.. Precise tuning of facile one-pot gelatin methacryloyl (GelMA) synthesis. Sci Rep 6,31036, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahadik, B.P., Pedron Haba, S., Skertich, L.J., and Harley, B.A.. The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials 67,297, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zambuto, S.G., Serrano, J.F., Vilbert, A.C., Lu, Y., Harley, B.A.C., and Pedron, S.. Response of neuroglia to hypoxia-induced oxidative stress using enzymatically crosslinked hydrogels. MRS Commun, 10,83, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen, J.E., Pedron, S., Shyu, P., Hu, Y., Sarkaria, J.N., and Harley, B.A.C.. Influence of hyaluronic acid transitions in tumor microenvironment on glioblastoma malignancy and invasive behavior. Front Mater 5,39, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro, B.S., Daneshmand, S.T., Garner, F.C., Aguirre, M., and Thomas, S.. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil Steril 90,302, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Abbas, Y., Carnicer-Lombarte, A., Gardner, L., et al. . Tissue stiffness at the human maternal-fetal interface. Hum Reprod 34,1999, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen, J.E., Pedron, S., and Harley, B.A.C.. The combined influence of hydrogel stiffness and matrix-bound hyaluronic acid content on glioblastoma invasion. Macromol Biosci 17,1700018, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngo, M.T., and Harley, B.A.. The influence of hyaluronic acid and glioblastoma cell coculture on the formation of endothelial cell networks in gelatin hydrogels. Adv Healthc Mater 6,1700687, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedron, S., and Harley, B.A.. Impact of the biophysical features of a 3D gelatin microenvironment on glioblastoma malignancy. J Biomed Mater Res A 101,3404, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Aplin, J.D., Fazleabas, A.T., Glasser, S.R., and Giudice, L.C.. The Endometrium. 2nd ed. United Kingdom: Informa Healthcare, 2008 [Google Scholar]
- 34.Oefner, C.M., Sharkey, A., Gardner, L., Critchley, H., Oyen, M., and Moffett, A.. Collagen type IV at the fetal-maternal interface. Placenta 36,59, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilchrist, A.E., Lee, S., Hu, Y., and Harley, B.A.C.. Soluble signals and remodeling in a synthetic gelatin-based hematopoietic stem cell niche. Adv Healthc Mater 8,e1900751, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilic, F., Kayadibi, Y., Yuksel, M.A., et al. . Shear wave elastography of placenta: in vivo quantitation of placental elasticity in preeclampsia. Diagn Interv Radiol 21,202, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ananth, C.V., Keyes, K.M., and Wapner, R.J.. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 347,f6564, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lala, P.K., and Nandi, P.. Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: the role of decorin. Cell Adh Migr 10,111, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redman, C.W., and Sargent, I.L.. Latest advances in understanding preeclampsia. Science 308,1592, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Redman, C.W., and Staff, A.C.. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol 213, S9 e1, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann, P., Black, S., and Huppertz, B.. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 69,1, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Pedron, S., Pritchard, A.M., Vincil, G.A., Andrade, B., Zimmerman, S.C., and Harley, B.A.. Patterning three-dimensional hydrogel microenvironments using hyperbranched polyglycerols for independent control of mesh size and stiffness. Biomacromolecules 18,1393, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao, M.R., Qiu, W., Li, Y.X., Zhang, Z.B., Li, D., and Wang, Y.L.. Dual effect of transforming growth factor beta1 on cell adhesion and invasion in human placenta trophoblast cells. Reproduction 132,333, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Lash, G.E., Otun, H.A., Innes, B.A., Bulmer, J.N., Searle, R.F., and Robson, S.C.. Inhibition of trophoblast cell invasion by TGFB1, 2, and 3 is associated with a decrease in active proteases. Biol Reprod 73,374, 2005 [DOI] [PubMed] [Google Scholar]
- 45.McEwen, B.S.Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583,174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gennari-Moser, C., Khankin, E.V., Schuller, S., et al. . Regulation of placental growth by aldosterone and cortisol. Endocrinology 152,263, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Kisanga, E.P., Tang, Z., Guller, S., and Whirledge, S.. Glucocorticoid signaling regulates cell invasion and migration in the human first-trimester trophoblast cell line Sw.71. Am J Reprod Immunol 80,e12974, 2018 [DOI] [PubMed] [Google Scholar]
- 48.Mandl, M., Ghaffari-Tabrizi, N., Haas, J., Nohammer, G., and Desoye, G.. Differential glucocorticoid effects on proliferation and invasion of human trophoblast cell lines. Reproduction 132,159, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Smith, A., Witte, E., McGee, D., Knott, J., Narang, K., and Racicot, K.. Cortisol inhibits CSF2 and CSF3 via DNA methylation and inhibits invasion in first-trimester trophoblast cells. Am J Reprod Immunol 78,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomlinson, J.W., and Stewart, P.M.. Cortisol metabolism and the role of 11beta-hydroxysteroid dehydrogenase. Best Pract Res Clin Endocrinol Metab 15,61, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Abou-Kheir, W., Barrak, J., Hadadeh, O., and Daoud, G.. HTR-8/SVneo cell line contains a mixed population of cells. Placenta 50,1, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Adam, E.K., Hawkley, L.C., Kudielka, B.M., and Cacioppo, J.T.. Day-to-day dynamics of experience—cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A 103,17058, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adam, E.K., Quinn, M.E., Tavernier, R., McQuillan, M.T., Dahlke, K.A., and Gilbert, K.E.. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology 83,25, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bao, A.M., Ji, Y.F., Van Someren, E.J., Hofman, M.A., Liu, R.Y., and Zhou, J.N.. Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Horm Behav 45,93, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Chida, Y., and Hamer, M.. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull 134,829, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Chida, Y., and Steptoe, A.. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol 80,265, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Dahlgren, A., Kecklund, G., Theorell, T., and Akerstedt, T.. Day-to-day variation in saliva cortisol—relation with sleep, stress and self-rated health. Biol Psychol 82,149, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Dmitrieva, N.O., Almeida, D.M., Dmitrieva, J., Loken, E., and Pieper, C.F.. A day-centered approach to modeling cortisol: diurnal cortisol profiles and their associations among U.S. adults. Psychoneuroendocrinology 38,2354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heinrichs, M., Baumgartner, T., Kirschbaum, C., and Ehlert, U.. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 54,1389, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Sin, N.L., Ong, A.D., Stawski, R.S., and Almeida, D.M.. Daily positive events and diurnal cortisol rhythms: examination of between-person differences and within-person variation. Psychoneuroendocrinology 83,91, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.