Abstract
Edible packaging is a sustainable product and technology that uses one kind of “food” (an edible material) to package another kind of food (a packaged product), and organically integrates food with packaging through ingenious material design. Polysaccharides are a reliable source of edible packaging materials with excellent renewable, biodegradable, and biocompatible properties, as well as antioxidant and antimicrobial activities. Using polysaccharide-based materials effectively reduces the dependence on petroleum resources, decreases the carbon footprint of the “product-packaging” system, and provides a “zero-emission” scheme. To date, they have been commercialized and developed rapidly in the food (e.g., fruits and vegetables, meat, nuts, confectioneries, and delicatessens, etc.) packaging industry. However, compared with petroleum-based polymers and plastics, polysaccharides still have limitations in film-forming, mechanical, barrier, and protective properties. Therefore, they need to be improved by reasonable material modifications (chemical or physical modification). This article comprehensively reviews recent research advances, hot issues, and trends of polysaccharide-based materials in edible packaging. Emphasis is given to fundamental compositions and properties, functional modifications, food-packaging applications, and safety risk assessment of polysaccharides (including cellulose, hemicellulose, starch, chitosan, and polysaccharide gums). Therefore, to provide a reference for the development of modern edible packaging.
Keywords: polysaccharide-based materials, edible packaging, cellulose, hemicellulose, starch, chitosan, polysaccharide gums
1. Introduction
Since the 19th century, petroleum-based polymers and plastics have occupied a major position in food packaging, but most are non-renewable, non-biodegradable, difficult to recycle, and carelessly discarded as garbage after use, thereby contributing to ecological environmental deterioration and possible health hazards [1]. Under various natural and anthropogenic forces, plastic fragments (from waste plastic containers, sheets, and films) break down into small particle sizes, further generating microplastics with a diameter smaller than 5 mm [1,2,3]. According to Lebreton et al. [4], over 79,000 tons of plastic waste float on the Great Pacific Garbage Patch, and the content of marine microplastics has increased rapidly from 0.4 kg/km2 in the 1970s to 1.23 kg/km2 in 2015. Then Barrett et al. [5] estimated that there could be as much as 14.4 million tonnes of microplastics in the top 9 cm of sediment throughout the global ocean, which was 34–57 times more than that at the ocean surface. Moreover, microplastics have been ubiquitously detected in oceans (from the continental shelf to deep-sea waters [6], from the eastern North Pacific Ocean [3] to the Indian Ocean [7], and from coral reef to whales [8]), freshwater systems [9], airborne [10], plants, animals, and even humans [11,12]. Unfortunately, the plastic (including microplastics) pollution is posing a serious threat to the global environment and human health. Therefore, it is of great significance for packaging to develop a series of renewable environment-friendly materials to replace the traditional petrochemical-based materials, among which the edible material is one of the most promising materials.
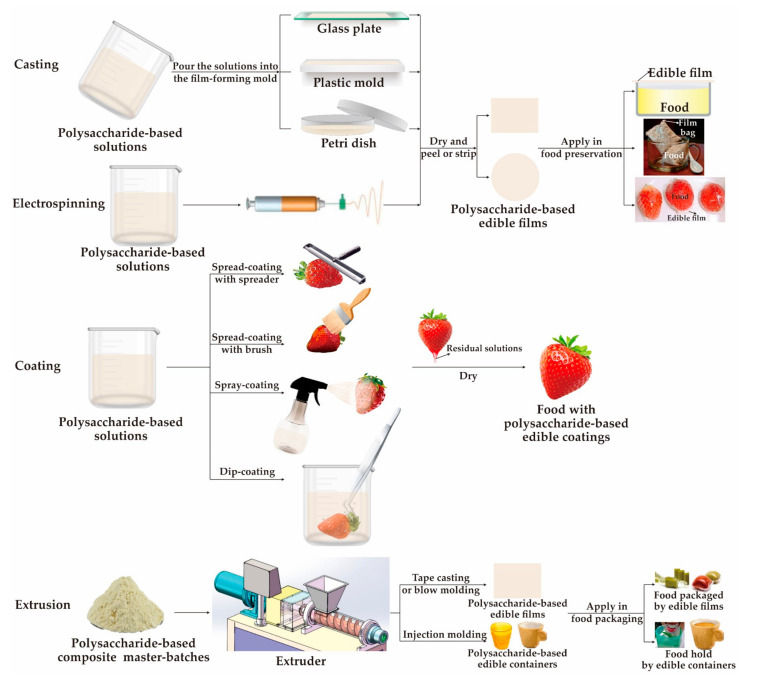
Edible packaging material is a kind of sustainable material that takes natural, edible and digestible “food” as raw material and is processed by modern material forming technology. It has excellent biocompatibility and biodegradability and can be consumed by animals or humans along with the food, while satisfying the basic functions of packaging (e.g., protection and transport), thus avoiding packaging waste pollution [13,14]. The design of edible packaging was originally inspired by the “peel/skin” of fruits and vegetables, and now edible packaging has been widely applied to various forms of food packaging (e.g., films, coatings, sheets, bags, cups, trays, and lids), as shown in Figure 1. In addition, edible packaging materials are non-toxic harmless, can be in direct contact with food, and even can be used as carriers of some antioxidative, antibacterial and/or nutritional factors to improve the sensory quality and nutritional value of foods [14,15].
Figure 1.
The major sources, types, processing methods, product forms, and food preservation applications of polysaccharide-based edible packaging.
To date, edible packaging materials include three natural biopolymers: polysaccharides, proteins, and lipids, among which polysaccharides (the most abundant natural macromolecules in nature, low processing cost and special function) occupy the most important position [13]. Polysaccharides are complex carbohydrates with varying degrees of polymerization and are composed of monosaccharides linked by α-1,4-, β-1,4-, or α-1,6-glycosidic bonds [16]. The polysaccharides commonly applied in edible packaging are cellulose, hemicellulose, starch, chitosan, and polysaccharide gums, which are used as the main matrix of packaging materials, and processed into polysaccharide-based edible films or layers by casting, coating, electrospinning, or extrusion technologies (Figure 1) [15,16,17,18].
The main value of polysaccharide-based edible packaging materials is to protect the quality of food, prolong their shelf life, and improve the functional characteristics, economic benefits, and sustainability of the packaging [15,19]. Compared with traditional packaging materials (such as paper, plastic, metal, and glass), polysaccharide-based materials have two significant advantages: Edibility and environmentally friendly performance. Compared with protein- and lipid-based packaging materials, polysaccharides have better chemical stability and processing adaptability, a greater range of sources, and lower cost. According to relevant studies, polysaccharide-based materials have good gases, aromas, and lipids barrier properties [20,21,22,23,24]; and even some polysaccharides and their derivatives have antioxidant and antimicrobial activities, which can effectively protect foods (e.g., fruits, vegetables, meat, aquatic products, nuts, confectioneries, and delicatessens), and extend their shelf life [15,19]. Furthermore, developing polysaccharide-based materials effectively reduces the dependence on petroleum resources, decreases the carbon footprint of the “product-packaging” system, and meets the strategic requirements of global sustainable packaging.
This article reviews the latest advances in the major polysaccharide-based edible packaging materials (cellulose, hemicellulose, starch, chitosan, and polysaccharide gums) from the viewpoints of fundamental compositions, properties, functional modification, application, and safety, highlights the potential of polysaccharides in food packaging, and provides the trends of these materials in modern packaging technology.
2. Fundamental Compositions and Properties of Various Polysaccharides
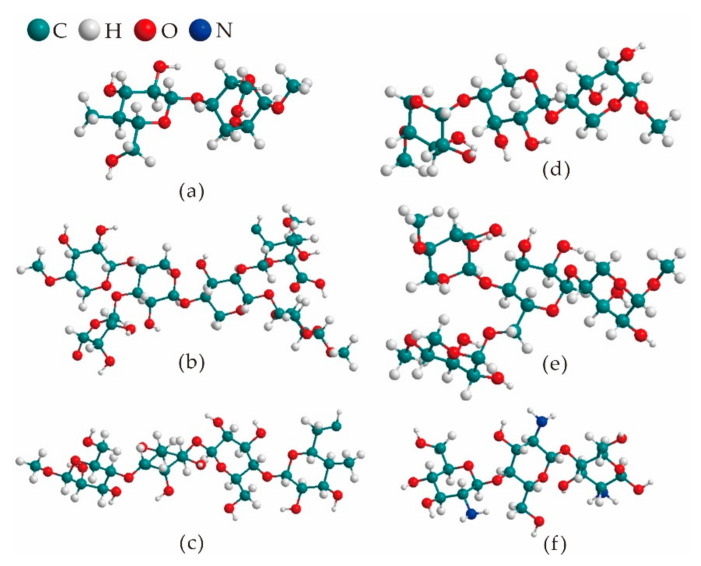
The functional characteristics of food packaging are not only related to the properties and main deterioration modes of packaged foods, but also depend on the compositions and properties of the packaging materials. Therefore, the relevant discussion of various polysaccharides has important guiding significance for analyzing the applicability of different polysaccharides in food packaging, as well as the selection of corresponding modification and application schemes. The major and minor sources, similarities and differences in compositions and structures of five polysaccharides, as well as their outstanding advantages as edible packaging are shown in Table 1. Meanwhile, the molecular structure models of different polysaccharides are shown in Figure 2.
Table 1.
Sources, compositions, structures, and outstanding characteristics of five polysaccharides for edible packaging application.
| Polysaccharides | Sources | Molecular Structure Characteristics | Functional Advantages | |
|---|---|---|---|---|
| Cellulose |
|
|
||
| Hemicellulose | Xylan | |||
| Glucomannan | Softwoods, tubers and seeds of Amorphophallus konjac plants [30,31,33] | |||
| Starch | Amylose | |||
| Amylopectin | ||||
| Chitosan |
|
|
||
| Polysaccharide gums | Pectin |
|
|
|
| Alginate | ||||
| Carrageenan | Cell walls of marine red algae, such as Eucheuma, Chondrus, Gigartina, Gelidium, and Hypnea [15,79] |
|
||
| Agar | Marine red algae, such as ferns, asparagus, laver, Gelidium, and Gracilaria [80] | |||
Figure 2.
Three-dimensional models of the molecular structure of various polysaccharides. (a): Cellulose (b): Xylan (c): Glucomannan (d): Amylose (e): Amylopectin (f): Chitosan.
Although the reported polysaccharides differ in source, composition, structure, and characteristics, they generally have good gelation, film-forming, mechanical, and barrier properties, and are abundant, renewable, edible and biodegradable. In particular, there are many kinds of hemicellulose and polysaccharide gums, but the ones commonly used in packaging are xylan, glucomannan, pectin, alginate, carrageenan, and agar. These polysaccharides can be processed into different forms of packaging (including films, coatings, containers, sponges, and gels) through various material technologies, and have tremendous potential in the development and application of edible packaging in the future.
However, compared with traditional petroleum-based polymers and plastics, polysaccharide-based materials still have many disadvantages, mainly including the following:
-
(1)
The chemical and thermal stability of polysaccharides are poor, which is not conducive to their subsequent molding processing. In particular, the materials formed by only one kind of polysaccharide are often brittle, easy to crack or wrinkle, have high shrinkage after molding, and have poor mechanical properties.
-
(2)
Polysaccharide-based materials contain many hydroxyl, amino or carboxyl groups, which result in high hydrophilicity, easy swelling by moisture, and poor water vapor barrier and moisture resistance. Moreover, they are sensitive to water, and their hydrogen bonding actions, microstructures and internal stress would change after moisture absorption; which resulted in a significant decrease in the mechanical strength of polysaccharide-based materials at high relative humidity [81,82,83].
-
(3)
Cellulose, hemicellulose, starch, agar, and other polysaccharides (except chitosan, pectin, and their derivatives) would provide nutrients and facilitate the growth and reproduction of microorganisms, which is not conducive to food storage.
3. Modifications of Various Polysaccharide-Based Materials for Edible Packaging
Given the above limitations, polysaccharide-based materials should be modified based on the actual application requirements to optimize their functional properties and promote their application in edible packaging. The existence of functional groups such as hydroxyl, amino, acetylamino, and carboxyl groups in polysaccharides creates conditions for their material modification. Currently, the commonly used modification techniques are chemical and physical modifications.
3.1. Chemical Modifications of Polysaccharide-Based Materials
Common methods of polysaccharide chemical modification include functional group modification, graft copolymerization, and cross-linking (Table 2). Functional group modification refers to the modification of some functional groups on the main chain and/or side chain of polysaccharides to obtain modified polysaccharides with improved physical and chemical properties through etherification (e.g., carboxymethylation and hydroxypropylation), esterification (e.g., organic acid and anhydride esterification), quaternization, and acylation [32,84,85,86]. Graft copolymerization refers to the process by which the polysaccharide active groups (e.g., hydroxyl, amino, and carboxyl) react with other monomers to obtain target polysaccharides under the action of an initiator or radiation [32,87]. Cross-linking refers to the process in which polysaccharides are polymerized within themselves or with macromolecules of other materials under the action of cross-linking agents (which can improve the cross-linking degree between substances) to obtain cross-linked polysaccharides with a network structure, thus enhancing the stability and physical properties of polysaccharides [85]. For example, polysaccharides are linked with proteins (whose mechanical properties are often better than polysaccharides) to obtain polysaccharide-protein complexes with optimized properties based on reducing the electrostatic free energy of the system by electrostatic interaction. In particular, during cross-linking, the thermal and mechanical properties of polysaccharide-based materials can be further improved by using carboxylic acid or calcium ions as cross-linking agents [88].
Table 2.
Chemical modification methods and effects of various polysaccharides.
| Polysaccharides | Modification Methods | Edible Packaging Materials | Th | MC/% | WS/% | TS | EB/% | WVP | Functional Characteristics |
|---|---|---|---|---|---|---|---|---|---|
| Cellulose | Methylation (etherification) | Methylcellulose (MC) [89] | 0.041 | 27.3 | 100 | 55 | 36 | 2.78 × 10−10 | Better water solubility and mechanical properties than native cellulose |
| MC [90] | 0.048 | 98.9 | 31.4 | 16.2 | 7.95 × 10−11 | ||||
| MC [91] | 0.062 | 100 | 15.78 | 15.4 | 1.19 × 10−4 | ||||
| Carboxymethylation | Carboxymethyl cellulose (CMC) [92] | 0.142 | 16.55 | 10.48 | 42.37 | 1.198 × 10−3 | Improve transparency, thermal stability, salt tolerance and acid resistant properties | ||
| CMC [93] | 0.097 | 21.19 | 0.23 | 60.21 | 7.41 × 10−7 | ||||
| CMC [94] | 0.05 | 56 | 6.5 | 11.18 × 10−11 | |||||
| CMC [95] | 0.070 | 22.71 | 75.08 | 14.18 | 10.54 | 3.36 × 10−10 | |||
| Hydroxyethylation (etherification) | Hydroxyethyl cellulose (HEC) [96] | 0.07 | 93.26 | WVTR: 18.94 |
|
||||
| Hydroxypropylation (etherification) | Hydroxypropylated cellulose (HPC) [87] | 0.04 | 7.0 | 7.5 | 7.52 × 10−5 | Enhance mechanical and barrier properties | |||
| Hydroxypropyl methyl cellulose (HPMC) [92] | 0.110 | 24.54 | 19.25 | 37.56 | 95.66 × 10−5 | ||||
| HPMC [97] | 100 | 33.0 | 13.4 | 1.34 × 10−10 | |||||
| Average methoxyl content/hydroxypropyl content (M/HP): 3.05 [83] | 0.025 | 30.83 | 6.06 | 2.036 × 10−4 | |||||
| M/HP: 2.26 [83] | 0.044 | 52.13 | 11.89 | 4.136 × 10−4 | |||||
| M/HP: 3.05 [83] | 0.030 | 67.28 | 17.37 | 2.566 × 10−4 | |||||
| HPMC [98] | 0.079 | 53.02 | 10.32 | 6.85 × 10−5 | |||||
| Acetic acid esterification | Acetylated cellulose (DS 0.54) [82] | 0.04–0.12 |
|
||||||
| Cross-linking | Tea catechins-cross-linked MC [90] | 0.060 | 25.5 | 73.7 | 2.8 | 2.84 × 10−11 | Light barrier, antioxidant and antibacterial properties | ||
| Dialdehyde carboxymethyl cellulose (DCMC) crosslinkedfeather keratin (FK) [99] | 0.09–0.15 | 17.9 | 43.8 | 2.1 | 26.8 | 3.3 × 10−10 |
|
||
| Graft copolymerization | MC-g-2-hydroxyethyl methacrylate [100] | 0.025 | 8.6 × 10−5 |
|
|||||
| Hemicellulose | Carboxymethylation | Carboxymethyl xylan (DS 0.30) [101] | 0.052 | 28.0 | 1.6 | 1.6 × 10−5 |
|
||
| Hydroxypropylation | Hydroxypropylated birch xylan [87] | 0.04 | 39.0 | 4.5 | 1.5 × 10−5 |
|
|||
| Esterification | 2-dodecenyl succinic anhydride-modified xylan (DS 0.31) [101] | 0.057 | 29.3 | 6.0 | 0.69 × 10−5 |
|
|||
| Acetylation | Acetylated bleached hemicellulose (DS 1.8) [82] |
0.04–0.12 | 44.1 | 5.7 |
|
||||
| Cross-linking | Add citric acid into wheat straw hemicelluloses matrix containing cellulose nanocrystals [102] | 7.0–7.5 | 47.41 | 9.76 | 3.94 | 4.09 × 10−4 |
|
||
| Starch | Carboxymethylation | Carboxymethyl starch (as functional master batch or raw material) [103] |
|
||||||
| Hydroxypropylation | Hydroxypropylated rice starch (Molar substitution: 0.022–0.033) [104] | 4.46–5.97 | 3.88–5.53 | 79.57–132.58 | 4.19–5.75 × 10−5 |
|
|||
| Acetylation | Acetylated cassava starch [96] | 0.04 | 28.73 | WVTR: 12.84 |
|
||||
| Esterification | Thermoplastic/succinated cassava starch (as functional master batch or raw material) [84] |
|
|||||||
| Starch-laurate esters (DS 0.45–2.92) [105,106] |
|
||||||||
| Cross-linking | Add citric acid (as crosslinker and plasticizer) into carboxymethyl potato starch (DS 0.5) matrix [107] | 0.2–0.3 | 58 | 0.16 | 26 |
|
|||
| Add citric acid into corn starch (DS ≈ 0.98), and then blended with grape juice [108] | 0.17 | 59 | 0.24 | 63.68 | 4.7 × 10−4 |
|
|||
| Add sodium trimetaphosphate into corn starch (DS 0.95), and then the modified starch was blended with grape juice [108] | 0.17 | 55 | 0.38 | 16.47 | 3.84 × 10−4 |
|
|||
| Click chemistry: Cu(I) catalyzed azide-alkyne [3 + 2] cycloaddition (CuAAC) | Amphiprotic starch derivatives linked 1,2,3-triazole (as antibacterial raw material) [109] |
|
|||||||
| Chitosan (CS) | Carboxymethylation | Carboxymethyl chitosan (DS 0.49) [110] | 0.159 | 21.25 | 42 | 6.65 × 10−11 |
|
||
| Graft copolymerization | Ascorbic acid was chemically grafted into CS backbones to form chitosan ascorbate (DS 0.88) [111] | 0.067 | 10.7 | 43.0 | 22 | 12.0 | 6.3 × 10−10 |
|
|
| Chitosan acetate (DS 0.60) [111] | 0.070 | 24.3 | 20.4 | 43 | 31 | 8.6 × 10−10 |
|
||
| Cross-linking | Add fulvic acid (as crosslinker) into konjac glucomannan/chitosan matrix [33] | 57.79 | 21.04 | 5.25 |
|
||||
| Polysaccharide gums | Carboxymethylation | Carboxymethyl agar (CMA) [112] |
|
||||||
| Cross-linking | Add calcium chloride (as crosslinker) into citrus pectin/CMC composite matrix [113] | 468 | 10.6 | 4.45 × 10−11 |
|
Note: DS: Degree of substitution; Th: Thickness, mm; MC: Moisture content; WS: Water solubility; TS: Tensile strength, MPa; EB: Elongation at break; WVP: Water vapor permeability, g·m−1·s−1·Pa−1; WVTR: Water vapor transmission rate, g·h−1·m−2; OP: Oxygen permeability, cm3·m−1·d−1·Pa−1; SWCA: Static water contact angle; EC50: Antioxidant value against the DPPH radical (namely, the mass concentration of antioxidants produced a 50% scavenging effect against active free radicals), mg/mL; E: Young’s modulus; HMTST: 6-hydroxymethyltriazole-6-deoxy starch; BMTST: 6-bromomethyltriazole-6-deoxy starch; CMTST: 6-chloromethyltriazole-6-deoxy starch; CBTST: 6-carboxyltriazole-6-deoxy starch.
For cellulose, the goal of chemical modification is to reduce the hydrogen bond strength and improve the processing adaptability of the materials. Various properties of cellulose-based materials (e.g., permeability, solubility, mechanical properties, barrier properties, and thermoplastic behavior) can be adjusted by changing the degree of substitution, type of chemicals, and polymer chain length [114]. Methylation, carboxymethylation, hydroxypropylation, and acetic acid esterification are often used to replace the hydroxyl groups of cellulose (Table 2). For instance, the mechanical and water vapor barrier properties of edible films, prepared using modified hydroxypropyl methylcellulose (HPMC), obtained by increasing the degree of hydroxyl substitution and relative molecular weight, were significantly improved [83]. The modified methylcellulose (MC) has high solubility and efficient oxygen and lipid barrier properties. A water-soluble edible packaging bag made of MC/HPMC composites has better mechanical and barrier properties, which are suitable for packaging dry food ingredients [81]. Furthermore, compared with other polymers in the previous literature, the tensile strength of MC films (15.78 MPa) was better than that of collagen and whey protein films [91], and even was higher than that of low-density polyethylene films (0.9–14 MPa) and poly(ε-caprolactone) (14 MPa). Moreover, the corresponding elongation at break (15.4%) was superior to polystyrene (2–3%), poly (3-hydroxybutyrate) (5–8%), and poly(L-lactic acid) (9%) [115,116]. Besides, the cross-linking [90] and graft copolymerization [100] could give cellulose-based materials better surface morphology and mechanical properties, even light resistance, antioxidant, and/or antimicrobial activities.
Chemical modification of hemicellulose is often conducted through carboxymethylation, hydroxypropylation, esterification, acetylation, and cross-linking (Table 2) [32,87,101,102]. Ramos et al. [101] prepared two kinds of functional xylans, carboxymethyl xylan (CMX) and 2-dodecenyl succinic anhydride-modified xylan (X-2-DSA), using beech xylan as the raw material, and then prepared different films. The results showed that X-2-DSA film possessed similar tensile strength and oxygen permeability to CMX film. Whereas the elongation at break of X-2-DSA film was almost 3.75 times that of the latter one, and the water vapor permeability of CMX film was about 2.3 times that of the former. These phenomena might be due to the replacement of some hydroxyl groups by non-polar long aliphatic carbon chains of dodecenyl succinic anhydride, which obtains plasticizing effect and makes the xylan less polar. Additionally, Mikkone et al. [87] modified xylan by hydroxypropylation (playing an internal plasticization role) and sorbitol was added as an external plasticizer to prepare a xylan-based barrier film via the casting method. The results indicated that the combination of xylan and sorbitol with a certain degree of hydroxypropyl substitution (from low to medium is 0.3 to 1.1) improved the film formability, flexibility, thermal stability, and barrier properties of the composite films. In particular, the composite film with the lowest hydroxypropyl substitution degree (0.3) had the best comprehensive properties (e.g., the highest tensile strength and the lowest oxygen and water vapor permeabilities), and the best biomass use and biodegradability.
The objective of chemical modification of the original starch is to reduce its moisture absorption and water sensitivity, heighten the compatibility of starch with other hydrophobic materials, and improve its processing adaptability [117]. Therefore, researchers often use highly hydrophobic groups to replace hydrophilic -OH groups through chemical modification methods, such as carboxymethylation, acetylation, esterification [84,106], polymer grafting, cross-linking, and “click chemistry”, which reduce the polarity of starch-based materials and improve their mechanical properties (Table 2). Liu et al. [118] first prepared carboxylated starch (which has higher hydrophilicity and polarity than that of native starch, but lower gelatinization temperature and enthalpy) by bio-α-amylase catalysis, and then introduced CMC into the modified starch matrix to enhance the hydrophobicity, thermal stability and mechanical strength of starch-based materials. In particular, the tensile strength of carboxylated starch composite films reached a maximum value of 44.8 MPa at 15% CMC addition, the hydrophobic property was effectively improved when CMC > 10%, and the static water contact angle was 66.8° at 35% CMC addition. Similarly, other researchers have modified starch by chemical methods first, but then combined it with the unmodified natural starch to produce better composites [17,119,120]. Notably, the FDA has limitations on the reagents and reactions, which are used for the manufacturing of food-grade modified starch [46], so we should follow the applicable regulations and standards when preparing starch-based edible packaging, as well as other polysaccharides edible packaging.
The purpose of the chemical modification of chitosan is to increase its water solubility, thermal stability, mechanical properties, barrier properties, and antibacterial activity, and the main chemical methods include carboxymethylation, acylation, quaternization, graft copolymerization, and cross-linking (Table 2) [58,88,121]. For example, carboxymethyl chitosan was formed by introducing carboxymethyl into N or O atoms of the chitosan skeleton through reactions of halogenated acetic acid or glyoxylic acid, thus enhancing the water solubility and adhesion [58]. This modification could also improve the antibacterial properties, with a wide range of carboxymethylation degrees. O-carboxymethyl and N,O-carboxymethyl chitosans showed better antibacterial activity than ordinary chitosan, and with the increase in carboxymethylation, the antibacterial activity of O-carboxymethyl chitosan increased first, then decreased [122]. Likewise, the water solubility and antimicrobial activity of the original chitosan also improved by grafting glycidyltrimethylammonium chloride [123] or nisin [124] onto the chitosan chain. Furthermore, Li et al. [125] introduced monophenol and ortho-diphenol to chitosan to obtain functionalized chitosan derivatives owned high antioxidant activity, which the EC50 of inhibition of DPPH, hydroxyl (·OH), and superoxide (O2·-) radical-scavenging was 0.041–0.172, 0.010–0.089, and 0.014–0.038 mg/mL, respectively. Tan et al. [126] synthesized amino- and acylhydrazine-functionalized chitosan derivatives via 1,2,3-triazole and 1,2,3-triazolium by Cuprous-catalyzed azide-alkyne cycloaddition and N-methylation, which displayed stronger antioxidant capacity (especially against superoxide anion radical) than pristine and hydroxyl-modified chitosan. Besides, N-methylation of 1,2,3-triazoles further strengthened their antioxidant action. These chitosan derivatives had no cytotoxicity on L929 (at 0.0625 mg/mL) or HaCaT (at 0.1 mg/mL) cells, showing bright prospect in novel antioxidant edible packaging. In addition, the reaction of amino and hydroxyl groups in chitosan with polyaldehydes, polyesters, or polyethers can lead to cross-linking in the composite system, forming a three-dimensional network structure, thus, enhancing the thermal stability, mechanical properties, and barrier properties of chitosan [33,88]. Notably, the introduction of a cross-linking agent can further improve the properties of chitosan-based materials [33,127]. Chen et al. [33] added fulvic acid as a cross-linking agent to a konjac glucomannan/chitosan matrix to improve the thermostability, optical properties, and tensile strength (57.79 MPa, increased by 41.16%) of the composite film, while reduced its WVP (as low as 5.25 g·Pa−1·s−1·m−1, decreased by 39.31%).
In addition, the chemical modifications of polysaccharide gums (e.g., pectin, alginate, carrageenan, and agar) are mainly carboxymethylation, hydroxylation, acylation, esterification, graft copolymerization, and cross-linking (Table 2) [86,88,113,128]. For instance, Cao et al. [112] modified the original agar via carboxymethylation, while decreasing the dissolving temperature, gelling temperature, gel strength, hardness, fragility, adhesiveness, gumminess, and chewiness of carboxymethyl agar (CMA) by increasing carboxymethyl groups, conversely improving the springiness and cohesiveness of CMA, and enhancing the compactness of CMA skeleton structures. Based on polysaccharide gums and carboxymethyl cellulose being rich in active groups (-COOH and -OH) and have polyanion properties, Šešlija et al. [113] modified pectin with carboxymethyl cellulose and added glycerol and calcium chloride (which promote cross-linking through calcium ions), thus improving the thermal stability and mechanical strength of the composite film.
3.2. Physical Modifications of Polysaccharide-Based Materials
The most common and simple method for physical modification of polysaccharides is blending, namely blending one kind of polysaccharide with another or more edible materials (e.g., another polysaccharide, protein, and lipid), while supplementing with edible plasticizers, compatibilizers, antioxidants or antibacterial agents, and other small molecular additives (e.g., glycerin, essential oil, and other plant extracts). Therefore, complementary advantages of different materials are achieved while optimizing their comprehensive functions (Table 3) [81]. For example, proteins and polysaccharides are blended to form edible composites, in which positively charged proteins and anionic polysaccharides are attracted to each other to form highly structured compounds, and the water solubility, interfacial properties, adsorption, mechanical properties, and barrier properties of the composites are better than those of a single material [129,130]. Furthermore, when adding lipids into the polysaccharide/protein matrix, polysaccharides or proteins with high surface activity reduces the surface tension in the lipid emulsion, forms a space layer around the lipid droplets to enhance the emulsifying ability, promotes the stability of the emulsion, and ensure the mechanical strength and structural integrity of the composites. However, hydrophobic lipids reduce water migration and enhance the water resistance and water vapor barrier properties of the composites [131,132]. Overall, the water resistance, barrier properties, mechanical properties, heat sealing properties, and transparency of the polysaccharide-based composites could be further optimized, and even new functional activities could be developed by adjusting the composition and proportion of raw materials during blending. In general, the cohesion of a complex material increases with an increase in the length and polarity of the polymer chain, thus, improving the strength and abrasion resistance of its products, as well as the barrier properties to gas, water vapor, and solute. However, the enhancement of structural cohesion would lead to a decrease in the flexibility, porosity, and transparency of materials. Therefore, the types, proportions, and processing methods of raw materials should be explored according to the application requirements of polysaccharide-based edible packaging.
Table 3.
Physical modification methods and effects of various polysaccharides.
| Polysaccharides | Modification Methods | Th | MC/% | WS/% | TS | EB/% | WVP | Functional Characteristics |
|---|---|---|---|---|---|---|---|---|
| Cellulose | Blend CMC with gelatin and add Dianthus barbatus essential Oil [93] | 0.100 | 9.86 | 0.16 | 68.37 | 2.19 × 10−7 |
|
|
| Add dipalmitoyl lecithin liposomes loaded with quercetin and rutin to CMC matrix [133] | 0.035–0.045 |
|
||||||
| Add α-tocopherol and a mixture of polysorbate 80 and lecithin to CMC matrix [94] | 44 | 18.5 | 12.45 × 10−11 |
|
||||
| Add spent coffee grounds polysaccharides to CMC matrix [95] | 0.070 | 21.63 | 50.52 | 26.04 | 6.84 | 3.36 × 10−10 | Light barrier, antioxidant and antimicrobial properties | |
| Add cypress (Cupressus sempervirens) cone seeds extracts to HPMC matrix [98] | 0.084 | 61.04 | 7.67 | 5.16 × 10−5 | Light barrier and antioxidant properties | |||
| Hemicellulose | Add cellulose nanocrystals into wheat straw hemicelluloses matrix [102] | 7.0–7.5 | 93.75 | 11.25 | 3.13 | 8.376 × 10−4 | Improved tensile strength, modulus, water resistance, and water vapor barrier property | |
| Blend acetylated hemicellulose (DS 1.7) with acetylated nanocellulose (DS 2.34) [134] | 0.250 | 17.67 | 10.59 | 15.49 | Increasing DS and loading of acetylated nanocellulose, increased hydrophobicity (SWCA 68.29°) of composite and reduced its solubility in food simulants | |||
| Blend konjac glucomannan (KGM) with microcrystalline cellulose [135] | 40.53 | 5.12 | WVTR: 3.38 | Improved thermal stability, barrier and mechanical properties compared pure KGM film | ||||
| Add polydopamine functionalized microcrystalline cellulose into KGM matrix [135] | 43.01 | 8.51 | WVTR: 1.67 |
|
||||
| Add CS/gallic acid nanoparticles into KGM matrix [127] | 42.50 | 26.61 | 11.25 × 10−11 |
|
||||
| Blend KGM with zein and add curcumin [136] | 7.34 |
|
||||||
| Blend KGM with pectin [137] | 0.048 | 17.91 | 15.75 | 22 | 1.76 × 10−10 |
|
||
| Add tea polyphenol into KGM/pectin matrix [137] | 0.061 | 16.13 | 21.03 | 16.94 | 1.37 × 10−10 |
|
||
| Blend KGM with shellac [138] | 0.106 | 13.8 | 20.5 | 11.28 × 10−5 |
|
|||
| Starch | Blend acetylated cassava starch with hydroxyethyl cellulose [96] | 0.06 | 61.24 | WVTR: 16.27 |
|
|||
| Blend carboxymethyl potato starch (DS 0.8) with carboxymethyl cellulose (DS 2.6) and add citric acid and glycerol [139] | 0.2–0.3 | 3.4 | 29 |
|
||||
| Blend octenylsuccinated- (DS 0.0425) with native- sweet potato starch and add glycerol [120] | 0.091 | 13.41 | 15.25 | 0.72 | 260 | 5.69 × 10−11 |
|
|
| Blend acetylated- with native- corn starches and add glycerol to form thermoplastic corn starch [17] | 0.129 | 9.26 | 23.99 | 6.14 | 1.20 × 10−10 |
|
||
| Add CS into thermoplastic corn starch [140] | 0.138 | 12.5 | 1.64 | 0.87 × 10−9 |
|
|||
| Add chitin into thermoplastic corn starch [140] | 0.121 | 12.6 | 1.86 | 0.59 × 10−9 | ||||
| Blend rice starch with carboxymethyl chitosan (DS 0.49) [110] | 0.143 | 18.5 | 35 | 4.70 × 10−11 |
|
|||
| Blend hydroxypropyl high-amylose starch with pomegranate peel [141] | 0.11 | 24.32 | 9.39 |
|
||||
| Chitosan | Blend CS ascorbate (DS 0.80) with MC [89] | 0.044 | 21.9 | 61 | 35 | 24.4 | 2.93 × 10−10 |
|
| Blend CS with carboxymethyl chitosan and add nisin [142] | 0.048 | 45.4 | 9.2 | 19.8 | 7.65 × 10−10 |
|
||
| Blend CS with carboxymethyl chitosan [142] | 0.021 | 15.4 | 25.4 | 58.4 | 3.43 × 10−10 | |||
| Add nisin into CS matrix [142] | 0.043 | 37.5 | 11.4 | 15.3 | 6.35 × 10−10 | |||
| Blend CS with gelatin and add thymol [143] | 0.104 | WVTR: 2.18 |
|
|||||
| Blend CS with starch and add thymol [143] | 0.108 | WVTR: 1.32 | ||||||
| Blend CS with propolis extract [144] | 17.5 | 12.1 | 0.578 × 10−8 OP: 0.21 × 10−8 |
|
||||
| Polysaccharide gums | Blend agar with acid hydrolyzed cotton linter cellulose nanocrystals (which neutralized with NaOH) [145] | 0.052 | 33.7 | 30.7 | 1.9 5 × 10−9 |
|
||
| Blend agarose with CS [146] | 0.013 | 42.35 | 16 | 6.95 × 10−11 |
|
|||
| Blend pectin (75–80% degree of esterification) with corn flour [147] | 0.06 | 21.2 | 70.7 | 7.47 | 0.022 × 10−7 |
|
||
| Blend CS (prepared from Callinectes sapidus) with (high methoxyl pectin (prepared from Citruis sinensis Osbeck peel) [148] | 0.082 | 16.9 | 17.5 | 35 | 0.97 × 10−15 |
|
||
| Blend gum tragacanth with locust bean gum [149] | 0.047 | 13.07 | 20.28 | 1.10 | 0.83 × 10−4 |
|
||
| Blend low methoxyl with pectin sodium caseinate at pH 3 [150] and pH 7 [151] | 0.040 | 14.5 | 15.64 | 9.35 |
|
|||
| Add Origanum vulgare subsp. viride essential oil into basil seed gum [152] | 0.060 | 17.92 | 3.69 × 10−11 |
|
||||
| Add fish protein hydrolysate into agar matrix [153] | 0.044 | 48.86 | 19.89 | 42.70 | 10.04 × 10−11 |
|
||
| Add clove essential oil into agar matrix [153] | 0.061 | 20.86 | 10.16 | 3.93 | 9.37 × 10−11 | Better hydrophobicity, antioxidant and antimicrobial activities |
DS: Degree of substitution; Th: Thickness, mm; MC: Moisture content; WS: Water solubility; TS: Tensile strength, MPa; EB: Elongation at break; WVP: Water vapor permeability, g·m−1·s−1·Pa−1; WVTR: Water vapor transmission rate, g·h−1·m−2; OP: Oxygen permeability, cm3·m−1·d−1·Pa−1; SWCA: Static water contact angle; EC50: Antioxidant value against the DPPH radical (namely, the mass concentration of antioxidants produced a 50% scavenging effect against active free radicals), mg/mL; DPPH value: 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity; E: Young’s modulus.
For cellulose, the functional properties of cellulose-based packaging materials can be further optimized through physical blending reinforcers, barrier factors, antioxidants, or antimicrobials into the cellulose matrix (Table 3). Esther et al. [154] significantly improved the antioxidant, antibacterial, and barrier properties of carboxymethyl cellulose-based edible films by adding concentrated bay leaf essential oil. The results showed that when the content of essential oil was 15% (w/w), compared with the unmodified carboxymethyl cellulose film, the antioxidant activity of the composite film was improved (as high as 99%), which slowed down lipid oxidation in food and effectively inhibited the growth of Escherichia coli and Candida glabrata, the water vapor barrier property was increased by 50%, and almost 100% ultraviolet light was blocked. Other studies have found that the antioxidant and antibacterial activities of cellulose-based materials can also be improved by adding dipalmitoyl lecithin liposomes (loaded with quercetin and rutin) [133], α-tocopherol [94], spent coffee grounds’ polysaccharides [95] and bacteriocin (from Bacillus methylotrophicus BM47) [27].
Functional hemicellulose-based edible materials can be obtained by the physical blending of hemicellulose with other polysaccharides, proteins, lipids, or other animal and plant extracts (Table 3) [127,135,136]. Along with konjac glucomannan (KGM), Wang et al. [135] improved the thermal stability, mechanical and water vapor barrier properties of KGM-based edible films by introducing microcrystalline cellulose loaded with polydopamine. Wu et al. [127] integrated chitosan/gallic acid nanoparticles with a KGM matrix to reduce the free volume of this blending system, significantly improving the mechanical and barrier properties of the edible composite film, while endowing the films with good antibacterial activity (for Staphylococcus aureus and E. coli O157:H7). Likewise, electrospun KGM/zein edible nanofiber films loaded with curcumin were prepared by Wang et al. [136] for application in food packaging. The addition of zein caused an increase in the thermal properties and hydrophobicity based on the interactions of hydrogen bonds between KGM and zein, whereas curcumin functioned as an antioxidant [2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity increased by about 15%] and antibacterial (the bacteriostatic zone for E. coli and S. aureus was 12–20 mm).
Various extracts or processing residues of animals and plants, such as cellulose, chitosan, propolis, protein, gallic acid, resveratrol, curcumin, and essential oils are often used in the blending modification of starch (Table 3) [108,141,155,156]. They have a wide range of sources and low cost, which could enhance the stability, mechanical, and barrier properties of starch-based materials, and even give them antioxidant, antibacterial, or ultraviolet light-shielding performances. For instance, Ali et al. [141] increased the mechanical properties (e.g., Young’s modulus, tensile strength, stiffness, and drop impact strength) of hydroxypropyl high-amylose starch-based films by adding pomegranate peel ground powder, and endowed the films with an inhibitory effect on the growth of S. aureus and Salmonella.
Chitosan is often uniformly blended with small molecular additives (e.g., glycerol, essential oils, and other plant extracts), or with other natural polymers (e.g., other polysaccharides, proteins, and lipids) to improve the comprehensive properties of chitosan-based composites (Table 3) [51,144,157,158]. Siripatrawan et al. [144] improved the functional properties of chitosan-based edible films by incorporating propolis containing high polyphenols, specifically enhancing the tensile strength, elongation at break, total phenol content, and antioxidant and antibacterial activities of the composite films, while reducing their oxygen and WVP. Likewise, Rambabu et al. [157] added mango leaf extract (MLE) to chitosan to significantly improve the tensile strength and surface hydrophobicity of the chitosan-based composite film and reduce its WVP, water solubility, and elongation at break. Moreover, the antioxidant activity of the composite film was higher than both the original chitosan and commercial PA/PE films (in which the antioxidant activity of the edible composite film containing 5% extracts was 56% higher than the PA/PE film).
In addition, polysaccharide-gum based composites with improved performance can be obtained by uniformly blending cellulose [113,145,159], starch [147,160,161], chitosan [146,148,162], another polysaccharide gum [149,163], as well as proteins [150,151], lipids [164,165,166,167], essential oils, and probiotics [64,152,153,168,169] with the original polysaccharide-gum matrix (Table 3). By adding nanocellulose (usually ≤ 5% w/w) to the agar matrix, Oun [145] and Shankar [159] et al. significantly improved the tensile strength, water vapor barrier and thermal stability of the agar-based edible films. Likewise, the addition of starch to agar by Phan [160] and Fekete [161] also enhanced the water resistance and water vapor barrier properties of the composite films and reduced the overall cost of the composites. When the amount of cassava starch was 20% (w/w), the WVP at 57–22% relative humidity differential of the composite film was reduced to about 3.33 × 10−11 g·m−1·s−1·Pa−1, which is 53.8% less than pure agar film. When the added amount was 50% (w/w), the WVP was about 2.99 × 10−11 g·m−1·s−1·Pa−1, which was 58.5% less than pure agar film [160]. Furthermore, the blending of chitosan (an alkali-soluble polysaccharide) and acidic polysaccharide gums produces electrostatic interactions, which makes the structure of the composite compact and without phase separation, thus, leading to better mechanical and barrier properties than a single material, and even improves the antibacterial and ultraviolet light-shielding properties of the composite [146,148,162]. Sodium caseinate was introduced into low methoxyl pectin by Eghbal et al. [150,151] to adjust the water content and absorption, as well as the mechanical and optical properties of the composites. The results indicated that the protein content affected the properties of the composites; the highest amount of complex coacervates of blending liquids was formed at a sodium caseinate/low methoxy pectin ratio of 2, at which the ζ-potential value was zero and the turbidity reached the highest value. While the ratio was 0.05, the Young’s modulus (182.97 ± 6.48 MPa) and tensile strength (15.64 ± 1.74 MPa) of the composite films were the highest, which were all higher than those of the pure pectin film. In addition, lipids (e.g., beeswax, shortening, and shellac) are the most effective natural substances to enhance the water and moisture resistances of polysaccharide gums [165,166,167]. Active extracts (e.g., various plant essential oils) could not only strengthen the thermal stability, and mechanical and barrier properties of polysaccharide gum-based materials, but also improve their antioxidative, antibacterial, and other functional characteristics [64,152,153,168,169].
4. Applications of Various Polysaccharide-Based Materials in Edible Packaging
Original polysaccharides can form self-assembled films, coatings, or microcapsules under the action of hydrogen bonding, van der Waals, or electrostatic forces, form hydrogels with a three-dimensional network structure through gelation, and form composites by combining them with other edible materials (e.g., proteins, lipids, probiotics, and other natural active small molecule substances), which can apply to different food packaging (Figure 1). The predominant use of polysaccharide-based edible materials is to serve as an auxiliary means of packaging. They can effectively delay the migration of water, gas, oil, and solute by providing a selective barrier, retain volatile flavor compounds and mechanical integrities of foods, improve treatment properties of foods, or even be used as non-toxic carriers of food additives (e.g., antioxidants, anti-browning, and antimicrobial agents) integrated into the packaging to improve the sensory properties of foods and extend their shelf lives.
In the following section, the potential application of five polysaccharides (cellulose, hemicellulose, starch, chitosan, and polysaccharide gums) in targeting the edible packaging sector are briefly described. Whereas, Table 4 includes the main preparation methods, packaging forms, packaged objects, and packaging effects of the different polysaccharide-based materials; the main preparation methods are shown in Figure 3.
Table 4.
Applications of five kinds of polysaccharide-based edible materials in food packaging.
| Food | Edible Packaging & Preparation Method | St | Mass Loss/% | Dp/% | TSS/% | TA/% | pH | Vc Mass | TSP | Packaging Effects | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit | Strawberry | CMC/bacteriocin from Bacillus methylotrophicus BM47 coating; Dip-coating [27] |
4–16 °C | 10.5; 12 d | 0; 12 d |
8.6; 12 d | 1.09; 12 d | 3.34; 12 d | 24.5; 12 d | 9–10; 12 d |
|
| KGM/pullulan film; Casting [170] | 4–14 °C | 25 | 6 | 0.55 | 0.015 μg/mL |
|
|||||
| CS/gelatin/thymol coating; Dip-coating [143] | 4–7 °C | 1–2 | 1.67 | 7.16 | 6.71 |
|
|||||
| CS/starch/thymol coating; Dip-coating [143] |
4–7 °C | 0.61 | 0 | 6.95 | 7.06 | ||||||
| Grape | CS/Mentha (piperita L. or x villosa Huds) essential oil coating; Dip-coating [171] | 25–12 °C; 12–24 °C | 11.2–12.6° Brix | 42.9–47.3 mmol H+/100 g food |
|
||||||
| Banana | Rice starch/ι-carrageenan/sucrose fatty acid esters coating; Spray-coating [172] |
20–14 °C | 4.5 | 20.5°Brix | 0.25 |
|
|||||
| Guava | Acetylated cassava starch/hydroxyethyl cellulose coating; Dip-coating [96] | 25–13 °C | 13.15 | 8.0 | 0.66 | 20.5 |
|
||||
| Apricot | Basil seed gum/Origanum vulgare subsp. viride essential oil coating; Dip-coating [152] | 4–8 °C | 6.9 | 15 | 230 |
|
|||||
| Vegetable | Cherry tomato | KGM/nisin coating; Spread-coating [173] | 25–16 °C | 9.5 | Decay index: 0.133 | 6.22 |
|
||||
| Cucumber | Konjac glucomannan/saffron petal extract coating; Spread-coating [174] | 4–5 °C | 17.56 | 0.17 |
|
||||||
| Tomato/Chilly/Brinjal | CS nanoparticles coating; Dip-coating [56] | 25–5 °C | 0.21/ 3.3/ 0.53 |
|
|||||||
| Food |
Edible Packaging &
Preparation Method |
St | PV | TBARS | TVB-N | DPPH/% | ABTS/% | pH | TSP | Packaging Effects | |
| Nut | Pistachio | CMC/gelatin/Dianthus barbatus essential oil coating; Dip-coating [93] | 25 °C–6 months | 0.1–3.5 |
|
||||||
| Cashew nut | CS/mango leaf extract film; Casting [157] | 30–28 °C | 2.88 |
|
|||||||
| Meat | Chicken breast | Rye starch/Rosehip extract film; Casting [175] | 4–9 °C | 0.59 | 80.22 | 96.87 |
|
||||
| Corn starch/gelatin/N-α-lauroyl-l-arginine ethyl ester monohydrochloride film; Casting [155] | 4–19 °C | 0.2 | 5.88 |
|
|||||||
| Oxidized corn starch/gelatin/N-α-lauroyl-l-arginine ethyl ester monohydrochloride coating; Spread-coating [155] | 4–19 °C | 1.52; 9 d | 5.72; 9 d |
|
|||||||
| Pork | Cassava starch/Lycium ruthenicum Murr anthocyanins film; Casting [176] | 25 °C–48 h | 10.89–16 h; 17.21–24 h | 6.15 −16 h; 6.49 −24 h |
|
||||||
| Beef loin | CS/cumin essential oil-loaded nanoemulsion film; Casting [177] | 3–21 °C | 1.39 | 12 | 5.4 |
|
|||||
| Ham | Iota-carrageenan/rosemary extract coating; Dip-coating [178] | 5–15 °C |
|
||||||||
| Goat meat sausage | Maltodextrin/calcium alginate/Tinospora cordifolia extracts film; Casting [179] | −18–21 °C | 0.54 | 6.79 |
|
||||||
| Oil | Olive oil | HPMC/cypress seed extract film; Casting [98] | 23–23 °C | <20 (legal limit) |
|
||||||
| Soybean oil | Pomelo peel flours/tea polyphenol film; Casting [64] | 23–15 °C | 31.58 | 74.39 |
|
||||||
| Lime peel pectin/coconut water/lime peel extract film; Casting [180] | 27–30 °C | 3.39 |
|
||||||||
| Aquatic product | Salmon | Cowpea starch/maqui berry extract film; Casting [181] | 4–6 °C | 1 | 0.63 | 42.39 | 88.46 |
|
|||
| Hake | Agar/green tea extract/probiotic bacteria film; Casting [169] | 4–15 °C | 25 | 7.01 |
|
||||||
| Flounder fillets | Agar/fish protein hydrolysate film; Casting [153] | 5–15 °C | 29.80 | 7.05 |
|
||||||
| Agar/clove essential oil film; Casting [153] | 5–15 °C | 25.83 | 6.76 | ||||||||
| Beluga sturgeon fillets | Jujube gum/nettle oil-loaded nanoemulsions coating; Coating [182] | 4–15 °C | 2.64 | 1.22 | 16.42 mg N/100 g | 6.42 |
|
||||
| Shrimp | Sweet potato starch/thyme essential oil coating; Dip-coating [183] | 4–8 °C | 0.3–0.5 | 8 |
|
||||||
Note: St: Storage time, day; Dp: Decay percentage; TSS: Total soluble solids; TA: Titratable acidity; TSP: Total soluble phenolic, mg Gallic acid equivalent (GAE)/100 g food; Vitamin C mass: Vc mass, mg/100 g food; PV: Peroxide value, meq (peroxides or O2)/kg food; TBARS: Thiobarbituric acid reactive substances, mg malondialdehyde (MDA)/kg food. TVB-N: Total volatile basic nitrogen, mg/100 g food; TMA-N: Trimethylamine nitrogen, mg/100 g food; ABTS value: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity; DPPH value: 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity.
Figure 3.
Different manufacture methods of polysaccharide-based edible packaging.
4.1. Applications of Cellulose
Cellulose is commonly applied in food packaging (e.g., fruits, vegetables, and oils) as edible films, coatings, and emulsions to protect the sensory qualities of foods and extend their shelf lives (Table 4). Rhimi et al. [98] added cypress seed extract to an HPMC matrix and prepared edible composite films using the casting method, and then applied them in olive oil packaging (as shown in Figure 3). The results indicated that compared with pure HPMC films, the tensile strength of the composite films was significantly improved (up to 15.13%) and the WVP was reduced (24.66% at most), which slowed down the oxidation of olive oil during 23 days storage. The lowest WVP, greatest opacity, and highest antioxidant capacity of the composite films were obtained with the highest extract concentration. Therefore, the peroxide value of olive oil sealed with composite films (containing 2% w/v extract) after accelerated storage for 11 days was 10 times lower than when sealed with pure HPMC films.
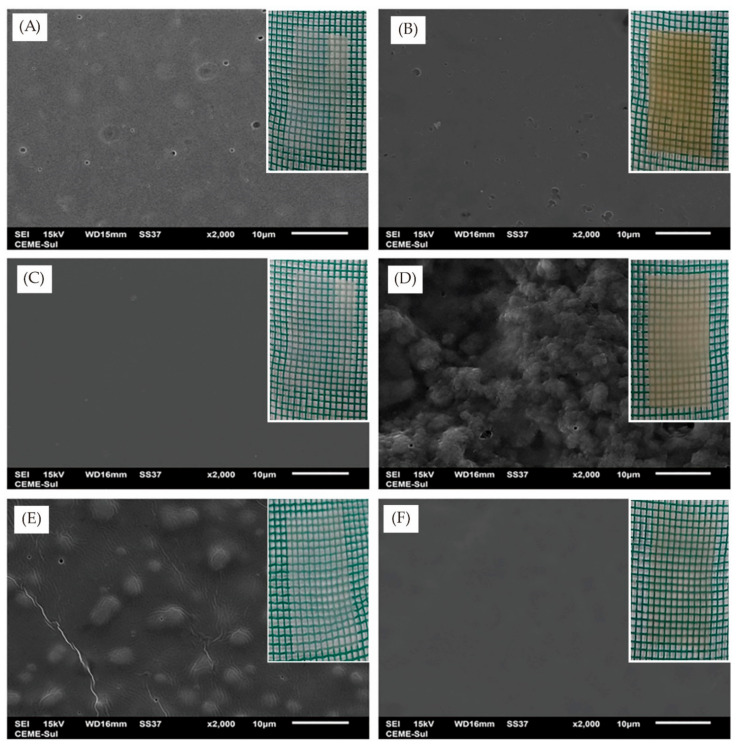
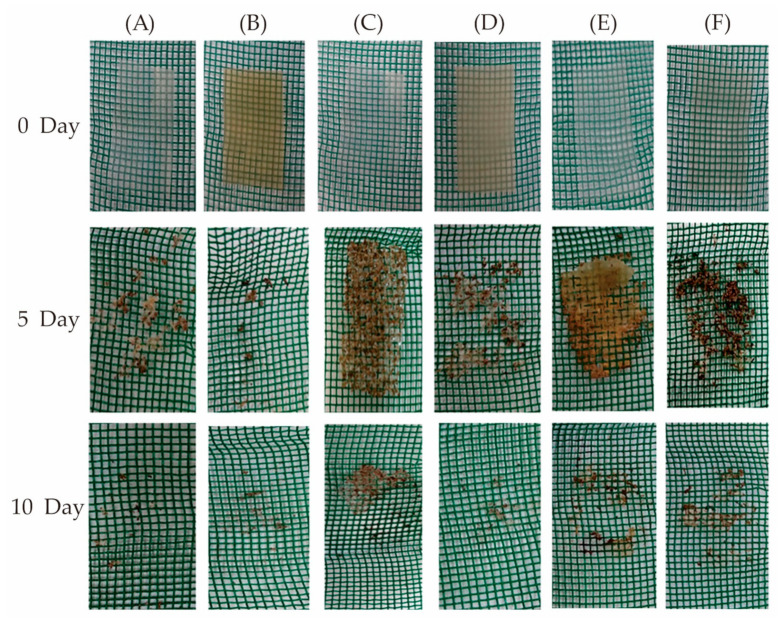
It is also noteworthy that cellulose is usually added to other edible materials as a reinforcing or toughening agent to improve the properties of composites. In the blends with collagen and whey protein, methylcellulose was responsible for the increase in tensile strength, water vapor barrier, and thermal properties. While, the prepared methylcellulose-based edible materials (Figure 4) could maintain their integrity for months, be completely biodegraded in 10 days in soil (Figure 5), and when immersed in hot or cold water showed total solubilization in around 30 s upon manual shaking [91]. The edible packaging has immense potential applications in soluble sachets for powdered foods, as well as oil containers and capsules for instant foods (Figure 6). Furthermore, the addition of cellulose nanocrystals to soybean protein could improve the tensile strength and barrier properties (the static water contact angle increased, and the moisture content, WVP, and reduced oxygen permeability) of the edible composite film, and enable the film to obtain ultraviolet light-shielding performance on the premise of appropriate transparency [184]. In addition, the creaming stability and ability to form an elastic gel-like network of beeswax-in-water (O/W) Pickering emulsions could be improved by blending with cellulose nanofibrils/carboxymethyl chitosan. Meanwhile, the complex edible films cast by modified emulsions had good tensile strength (5.0 MPa at a strain of 2.2%) and low WVP (<2 × 10−7 g∙h−1∙m−1∙Pa−1), and could inhibit the growth of S. aureus and E. coli, a promising application for antiseptic and fresh-keeping packaging for berry fruits [185].
Figure 4.
Scanning electron microscopy images (×2000) and physical photos of different edible films. (A) Collagen film; (B) Whey protein film; (C) Methylcellulose film; (D) Collagen/whey protein blend film; (E) Collagen/methylcellulose blend film; (F) Whey protein/methylcellulose blend film. (Adapted with permission from Filipini [91]; published by John Wiley and Sons, 2020).
Figure 5.
Biodegradability in the soil of different edible films. (A) Collagen film; (B) Whey protein film; (C) Methylcellulose film; (D) Collagen/whey protein blend film; (E) Collagen/methylcellulose blend film; (F) Whey protein/methylcellulose blend film. (Adapted with permission from Filipini [91]; published by John Wiley and Sons, 2020).
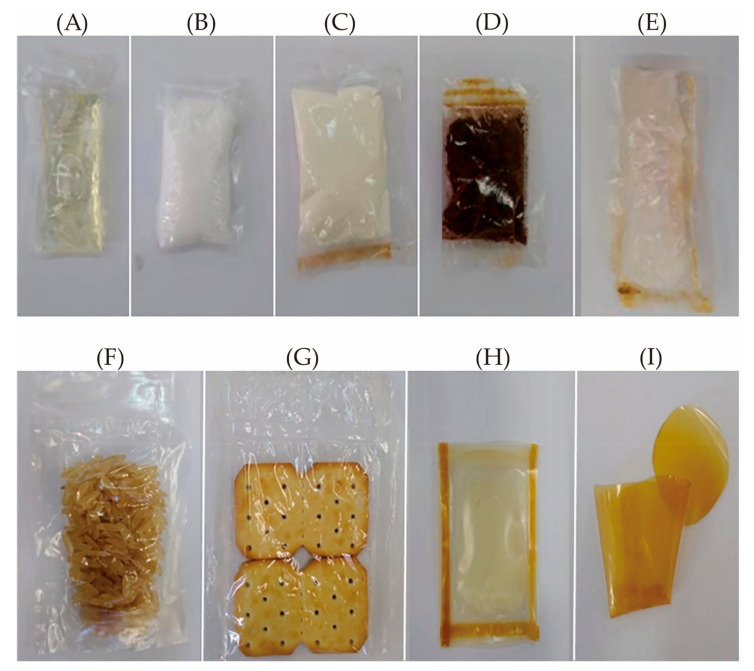
Figure 6.
Prototype photos of different edible packaging. From (A–G) are methylcellulose sachets containing soybean oil, salt, whey protein, powdered coffee, powdered juice, rice, and cookies, respectively; (H) Whey protein/methylcellulose edible sachet containing oil; (I) Whey protein edible film for the coffee capsule. (Reproduced with permission from Filipini [91]; published by John Wiley and Sons, 2020).
4.2. Applications of Hemicellulose
Hemicellulose is usually used in edible packaging as films, coatings, or modifying additives, which is like cellulose (Table 4). Taking KGM as an example, Yan et al. [170] introduced pullulan into the KGM matrix to cast edible composite films for strawberry preservation. They showed that the mechanical and barrier properties of the composite films were markedly enhanced because of the intermolecular interaction between KGM and pullulan; 1% (w/v) KGM/pullulan (with a mass ratio of 2:1) composite film significantly decreased the weight loss and maintained the titratable acidity, soluble solids, ascorbic acid, and skin color on strawberry preservation, thus slowing fruit aging, improving the quality during storage, and extending their shelf life to 14 days. Hashemi et al. [174] blended saffron petal extract with a KGM matrix to cast edible complex films, while coating fresh-cut cucumbers (as shown in Figure 3). The results indicated that saffron petal extracts markedly improved the transparency and moisture content of the complex films, reduced their WVP, and even endowed them with promising antioxidant and antimicrobial properties. Furthermore, this composite coating reduced mesophilic bacterial and fungal populations during cucumber storage (in which 4% extracts were considered as the most effective additives), improved the soluble solids content, antioxidant activity, and soluble phenols of coated sliced cucumbers, thus decreasing their spoilage, maintaining their quality features, and prolonging their shelf lives. Wang et al. [18] introduced zein into KGM matrix by electrospinning to form stable homogeneous nanofibril films, which the hydrophobicity was improved (SWCA of the composite film increased from 7.5° to 57.5°). Furthermore, they added curcumin into the above nanofibers to form a functional nanofilm with advanced antioxidant (scavenging activity increased about 15%) and antibacterial (a large inhibitory zone of 12–20 mm for E. coli and S. aureus) activities, as well as better thermal stability, water resistance and tensile strength.
4.3. Applications of Starch
Starch is often compounded with other edible materials to fabricate edible films or coatings, which are widely used in different food packagings, such as fruits, vegetables, meat, seafood, confectioneries, cakes, and pastries to block the migration of oxygen and grease and help improve the appearance, texture, and processing performance of foods (Table 4). Go et al. [175] added rosehip extracts to rye starch matrix to cast edible composite films and applied them in chicken breast packaging. The flexibility, optical properties, and antioxidant activity of the composite films were improved, and the highest ABTS and DPPH radical scavenging activities were observed in films containing 1.0% extracts (96.87% and 80.22%, respectively). Moreover, chicken breasts packaged with these films had lower peroxide and thiobarbituric acid reactive substance values than those packaged with original rye starch film, as well as the non-packaged control, suggesting that the edible composite films could effectively inhibit lipid oxidation and prolong its shelf life. Likewise, incorporating maqui berry extract [181], carvacrol, and chitosan [186] in starch-based edible composites (e.g., edible films and coatings) retarded lipid oxidation in fish, ham, and other foods, inhibited the growth of foodborne pathogens, and extended the shelf life of foods. Qin et al. [176] added Lycium ruthenicum Murr anthocyanins to cassava starch to manufacture a freshness indicator film with both intelligent pH sensitivity and edibility for pork packaging. The results showed that the barrier ability, tensile strength, and antioxidant activity of the composite film were improved by hydrogen bond interactions between anthocyanins and starch chains. Moreover, this composite film achieved real-time and visual monitoring of pork freshness based on its color change with pork quality during storage.
Furthermore, a significant difference from other polysaccharides is that original starch exposed to shear and high temperature (supplemented with water and processing aids) could be converted into thermoplastic starch-based materials, and then various starch-based edible packaging containers (e.g., film, cup, tray, and plate) can be obtained through extrusion, compression, or injection molding (Figure 3) [17,140,187,188].
4.4. Applications of Chitosan
Currently, chitosan-based edible packaging (as a film and coating) has been widely used in the packaging of fruits (e.g., strawberries, apples, kiwi, and grapes), vegetables (e.g., tomato, pepper, and eggplant), meats, and nuts to retain food quality and prolong their shelf life (Table 4) [56,171,177,189,190,191,192]. These edible packages mainly achieve food preservation by reducing the transpiration rate, delaying browning or lipid oxidation, and inhibiting the growth of spoilage microorganisms.
Divya et al. [56] coated chitosan nanoparticle solutions on the surfaces of tomatoes, chilies, and brinjals using the dip-coating method (Figure 3). The edible coatings had a good inhibitory effect on Rhizoctonia solani, Fusarium oxysporum, Collectotrichum acutatum, and Phytophthora infestans during 5 days of storage, had significant antioxidant activity, reduced the weight loss of these vegetables, and prolonged their shelf lives. Perdones et al. [189] applied chitosan-lemon essential oil dip-coatings to strawberry preservation. The results indicated that these edible coatings could control strawberry fungal decay during storage and affect the metabolic pathways and volatile profile by promoting the formation of esters and dimethyl furfural and incorporating terpenes into the fruit volatiles in a short time. Likewise, Dini et al. [177] packaged beef loins in chitosan-based edible films containing cumin essential oil nanoemulsions supplemented with irradiation treatment. The results showed that the edible composite films could withstand low-dose gamma irradiation at 2.5 kGy, while inhibiting the growth of L. monocytogenes, E. coli O157:H7, and Salmonella typhimurium in beef loins during the 21/days refrigerated storage, and slowed down the increasing level of total volatile basic nitrogen and pH value of beef, thus effectively enhancing the microbiological safety, quality, and storage life.
4.5. Applications of Polysaccharide Gums
Polysaccharide gums (e.g., pectin, alginate, carrageenan, and agar) are commonly used in edible packaging as gels, films, and coatings for food preservation of fruits (e.g., apple, peach, cherry), vegetables (e.g., tomato, papaya, and lettuce), meats, and seafood (Table 4), and even have commoditized packaging of pure water and other beverages. These polysaccharide-gum based edible materials could effectively reduce the dryness degree of food surfaces, prevent food from water loss and atrophy, and are beneficial to slow down lipid oxidation and surface discoloration of foods, as well as inhibit the reproduction of spoilage microorganisms, thereby extending the shelf life of foods [19,70].
López et al. [169] added green tea extract to an agar solution containing glycerin and glucose to prepare the substrates by casting, and then coated the substrates with probiotic strains (Lactobacillus paracasei L26 and Bifidobacterium lactis B94) to acquire the edible composite films and further apply in hake packaging. The results showed that during 15 days of storage, the edible composite films effectively inhibited the growth of spoilage microorganisms, especially the H2S-producing bacteria, causing a decrease in TVB-N, trimethylamine nitrogen (TMA-N), and pH value of the hake, and increased the beneficial lactic acid bacteria, thus leading to its shelf life extension for at least a week. Additionally, maltodextrin/calcium alginate edible casting films containing Tinospora cordifolia extracts were fabricated by Kalem et al. [179] and then used as casings substitutes for goat meat sausages. It was found that edible films with antibacterial and antioxidant properties could significantly reduce the production of thiobarbituric acid reacting substances and free fatty acids in sausages during storage, inhibit the reproduction of microorganisms (total plate, psychrophilic, and yeast and mold), and maintain the sensory quality of goat meat sausages. Similarly, the cooked ham portions were dipped in iota-carrageenan-based coating solutions containing rosemary extract, ascorbic acid, calcium chloride, α-tocopherol, and glycerol by Carocho et al. [178] for food preservation. The results showed the edible coating based on the above solutions inhibited the growth of microorganisms and retained the sensory quality of hams over the 15-days of storage.
5. Safety Risk Assessment of Polysaccharide-Based Edible Packaging
Edible packaging serves to protect food and act as a ready-to-eat “food”, which provides valuable nutrients and energy [193]. In theory, food-grade polysaccharides made from natural edible constituents used in most studies are non-toxic, and edible packaging prepared from these polysaccharides could be consumed by animals or humans without health risk [15]. However, to be edible actually, the materials (including substrates and additives) used in the formulations should be green, non-toxic, safe and meet applicable regulations or standards (e.g., GRAS—Generally Recognized as Safe by the FDA-U.S. Food and Drug Administration).
Uncertainties and knowledge gaps on the possible health effects and long-term safety of polysaccharides and their modifying additives, when used in edible packaging, are still the most important concern. To date, very few studies have been published regarding the effects of polysaccharides-based edible packaging upon ingestion, and the absorption, distribution, metabolism, and excretion after oral exposure, and the potential interactions of polysaccharides with packaged food components [194]. Most edible films and coatings, discussed in this review, focus on the preparation and characterization of materials, with little follow-up food safety risk assessment.
Therefore, polysaccharides-based edible packaging must be exhaustively studied, they are easier to transfer constituents into foods than petroleum-based polymers. The first step in assessing the potential hazard of polysaccharides-based packaging for a comprehensive risk assessment, in terms of consumer safety, is to evaluate their potential migration into food (usually according to Regulation (EU) No. 10/2011 on plastic materials and articles) [195]. In particular, the solubility of polysaccharides that migrate in the food matrix and/or upon gastrointestinal passage is a crucial factor.
In addition, toxicological risk and dietary exposure assessment are important for polysaccharides edible packaging. Barreto et al. [196] prepared two kinds of onion (Allium cepa L.) puree-based edible films by casting, namely unwashed hydrothermally treated pulp (HTP) and washed hydrothermally treated pulp (W-HTP), and then assessed their genotoxicological safety. The cellular viability demonstrated that HTP films showed greater cytotoxicity than W-HTP films; and the mutagenic activity indicated that both HTP and W-HTP films were not able to statistically increase the frequencies of the biomarkers for chromosome damage (micronucleus test) at the tested concentrations. However, the HTP films showed signs of mutagenicity in the Ames test (gene mutations), suggesting caution in their use. Therefore, W-HTP onion-based edible films are harmless and possess safety potential application in food packaging, supporting the first level of evidence. For the additives, Sohrabi et al. [197] evaluated the potential cyto-genotoxicity of ascorbyl palmitate (AP, a widely used food additive) on Human Umbilical Vein Endothelial Cells (HUVECs). The results indicated that the growth of HUVECs was decreased upon treatment with AP in dose-and time-dependent manner, and AP induced apoptosis by up-regulation of caspase-3, 9 and down-regulation of Bcl-2 ratio. Therefore, AP application in the edible packaging industry should be carefully considered.
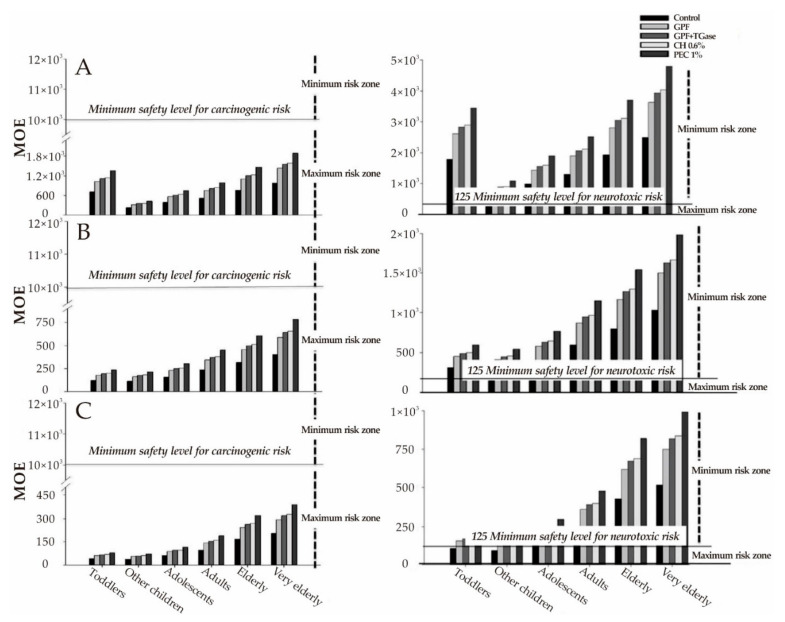
Zheng et al. [198] prepared hydroxypropylated-Phosphated-modified glutinous rice starch and evaluated its safety through acute and 28-day repeated oral toxicity tests. The results showed that the modified starch possessed more than 10,000 mg/kg LD50 value, was belong to non-toxic. Moreover, its acceptable daily intake for a normal person (70 kg) should be less than 38,900 mg, which means that the recommended intake (RNI) is no more than 38,900 mg/d. Asmar et al. [199] dipped the potato sticks into chitosan or pectin hydrocolloid coating solutions before frying to reduce the acrylamide and oil content of French fries. Then, the Daily Intake (DI) (Table 5) and Margin of Exposure (MOE) (Figure 7) were further calculated by considering the six following age groups (as stated from EFSA) to estimate variations in risk assessment by applying coating solutions. The results showed that, compared with the control sample (reached highest acrylamide concentration 2089 µg·kg−1), the edible polysaccharides coating reduced the acrylamide content by 48% for pectin and >38% for chitosan, respectively. Moreover, the increasing MOE value indicated that recurring coatings could provide advantages to consumers, especially for the ones from 1 to 65 years old, and the pectin coating was the most effective.
Table 5.
Dietary intake of acrylamide consumption [ng·(kg·body·weight)−1·day−1] based on the median of the estimated consumption of fried potatoes treated with the coating solutions. (Adapted with permission from Al-Asmar [199]; published by MDPI, 2018).
| Age Groups | Control | Chitosan Coating | Pectin Coating |
|---|---|---|---|
| Toddlers | 1387 | 858 | 720 |
| Other children | 1521 | 941 | 790 |
| Adolescents | 1072 | 663 | 557 |
| Adults | 719 | 445 | 374 |
| Elderly | 536 | 332 | 279 |
| Very elderly | 417 | 258 | 217 |
Figure 7.
MOE values for carcinogenicity (left panel) and neurotoxic (right panel) of acrylamide through the consumption of French fries that were both uncoated and coated with hydrocolloid coating solutions. Samples were coated with different polysaccharides-based coatings made of PEC, pectin; and CH, chitosan. “Uncoated” represents the control sample dipped in distilled water, across different consumer age groups: (A) minimum, (B) median, and (C) maximum of consumption levels estimated from the 2015 EFSA report. (Adapted with permission from Al-Asmar [199]; published by MDPI, 2018).
Overall, a series of safety studies can be conducted on edible materials based on relevant regulations and standards (e.g., FDA for Preparation of Food Contact Notifications for Food Contact Substances-Toxicology Recommendations), such as composition analysis (including nutritional composition and possible natural toxic substances), hygienic tests (heavy metals, pesticide residues), and toxicological tests [including acute oral toxicity test, three genetic toxicity tests (Ames test, mammalian red blood cell micronucleus test and mouse spermatocyte chromosome aberration test), 90 d oral toxicity test and teratogenicity test], and further combined with the population, history of consumption, and the survey results of adverse reactions to assess the safety of polysaccharides-based edible packaging comprehensively.
6. Conclusions and Prospects
Edible packaging is a vital component of sustainable packaging. It significantly expands the source of packaging materials, reduces the dependence on non-renewable petroleum resources, and efficiently uses food processing waste. Polysaccharides are the major study objects of edible packaging materials. Considering the advantages and limitations of polysaccharides, researchers currently use various modifications to optimize the material’s comprehensive properties, such as film-forming, mechanical and barrier properties, and antioxidant and antibacterial activities. They have successfully developed a variety of polysaccharide-based edible packaging materials such as ink, microcapsules, coatings, films, and sheets, which are applied to food packaging. These materials can provide selective barriers to prevent the migration of water, gas, and lipid in the food-packaging system, effectively retain the flavor and nutrition of food, and extend its shelf life (e.g., fruits, vegetables, meat, aquatic products, nuts, confectioneries, and delicatessens, etc.).
In general, polysaccharide-based edible packaging plays a key role in the environmental protection of food packaging and the high value of food processing waste, and it is one of the best alternative non-renewable resources. Although numerous studies on polysaccharide-based edible packaging have been reported in the past 10 years, they are still mainly on the laboratory scale and are less industrialized. Herein, trends of research and application of polysaccharide-based edible packaging will mainly focus on the following four aspects:
-
(1)
Development of more edible polysaccharide-based materials: To date, the primary sources of polysaccharide-based edible packaging are plants and animals. Microorganisms, such as bacteria and fungi are also a great potential source, especially for the development of marine microorganisms.
-
(2)
Multi-functional modification of polysaccharide-based materials: Modification of materials based on practical application requirements is a hot topic in material science. Defects in mechanical properties and water resistance are difficulties in the application of polysaccharide-based materials. Whereas, hydrophilicity is a key factor influencing these performances. Therefore, selecting the most reasonable modification technology (e.g., high stability, low cost, safety, convenience, and easy industrialization) for new polysaccharide-based materials to improve their properties would be an important topic for future research on polysaccharide-based edible packaging. Therefore, the design of the binding mode between polymer chains, the design of monomer molecular group structures, and the realization of more functional effects according to different food requirements (e.g., flavor regulation, acid and alkali resistance, amphiphobicity, and controlled release of functional factors) are the development trend of the future modification of polysaccharide-based materials.
-
(3)
Application expansion and comprehensive evaluation of polysaccharide-based edible packaging: To conduct engineering research on cost reduction and large-scale production, and to evaluate the economic, environmental, and social benefits of polysaccharide-based edible packaging (the life cycle sustainability assessment theoretical model is recommended here), is the only way for the application and promotion of polysaccharides in packaging and food fields in the future.
-
(4)
A deeper knowledge and practice of safety risk assessment for polysaccharides: Understand the potential exposure of polysaccharides through migration into food, the interaction of polysaccharides with food constituent, and their effects upon ingestion, which could verify polysaccharides-based edible packaging safety for commercial purposes and provide a reference for dietary reference intake of residents.
Author Contributions
Conceptualization and writing—original draft preparation, Y.Z.; investigation, B.L., C.L., Y.X. and Y.L.; writing—review and editing, Y.Z., B.L. and D.L.; supervision and funding acquisition, D.L. and C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (Grant number 31960484) and the Natural Science Foundation of Guangxi Province (Grant number 2019JJD120012).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jang M., Shim W.J., Cho Y., Han G.M., Song Y.K., Hong S.H. A close relationship between microplastic contamination and coastal area use pattern. Water Res. 2020;171:115400. doi: 10.1016/j.watres.2019.115400. [DOI] [PubMed] [Google Scholar]
- 2.Eckert E.M., Di Cesare A., Kettner M.T., Arias-Andres M., Fontaneto D., Grossart H.-P., Corno G. Microplastics increase impact of treated wastewater on freshwater microbial community. Environ. Pollut. 2018;234:495–502. doi: 10.1016/j.envpol.2017.11.070. [DOI] [PubMed] [Google Scholar]
- 3.Egger M., Nijhof R., Quiros L., Leone G., Royer S.-J., McWhirter A.C., Kantakov G.A., Radchenko V.I., Pakhomov E.A., Hunt B.P.V., et al. A spatially variable scarcity of floating microplastics in the eastern North Pacific Ocean. Environ. Res. Lett. 2020;15:114056. doi: 10.1088/1748-9326/abbb4f. [DOI] [Google Scholar]
- 4.Lebreton L., Slat B., Ferrari F., Sainte-Rose B., Aitken J., Marthouse R., Hajbane S., Cunsolo S., Schwarz A., Levivier A., et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018;8:4666. doi: 10.1038/s41598-018-22939-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett J., Chase Z., Zhang J., Holl M.M.B., Willis K., Williams A., Hardesty B.D., Wilcox C. Microplastic Pollution in Deep-Sea Sediments From the Great Australian Bight. Front. Mar. Sci. 2020;7:808. doi: 10.3389/fmars.2020.576170. [DOI] [Google Scholar]
- 6.Eo S., Hong S.H., Song Y.K., Han G.M., Seo S., Shim W.J. Prevalence of small high-density microplastics in the continental shelf and deep sea waters of East Asia. Water Res. 2021;200:117238. doi: 10.1016/j.watres.2021.117238. [DOI] [PubMed] [Google Scholar]
- 7.Connan M., Perold V., Dilley B.J., Barbraud C., Cherel Y., Ryan P.G. The Indian Ocean ‘garbage patch’: Empirical evidence from floating macro-litter. Mar. Pollut. Bull. 2021;169:112559. doi: 10.1016/j.marpolbul.2021.112559. [DOI] [PubMed] [Google Scholar]
- 8.Auta H.S., Emenike C.U., Fauziah S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017;102:165–176. doi: 10.1016/j.envint.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Yan Z., Chen Y., Bao X., Zhang X., Ling X., Lu G., Liu J., Nie Y. Microplastic pollution in an urbanized river affected by water diversion: Combining with active biomonitoring. J. Hazard. Mater. 2021;417:126058. doi: 10.1016/j.jhazmat.2021.126058. [DOI] [PubMed] [Google Scholar]
- 10.Chen G., Feng Q., Wang J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total. Environ. 2020;703:135504. doi: 10.1016/j.scitotenv.2019.135504. [DOI] [PubMed] [Google Scholar]
- 11.Amato-Lourenço L.F., Carvalho-Oliveira R., Júnior G.R., dos Santos Galvão L., Ando R.A., Mauad T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021;416:126124. doi: 10.1016/j.jhazmat.2021.126124. [DOI] [PubMed] [Google Scholar]
- 12.Ragusa A., Svelato A., Santacroce C., Catalano P., Notarstefano V., Carnevali O., Papa F., Rongioletti M.C.A., Baiocco F., Draghi S., et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- 13.Hassan B., Chatha S.A.S., Hussain A.I., Zia K.M., Akhtar N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018;109:1095–1107. doi: 10.1016/j.ijbiomac.2017.11.097. [DOI] [PubMed] [Google Scholar]
- 14.Garcia M.P.M., GómezGuillén M.C., LópezCaballero M.E., BarbosaCánovas G.V. Edible Films and Coatings: Fundamentals and Applications. 1st ed. CRC Press; Boca Raton, FL, USA: 2017. [Google Scholar]
- 15.Mohamed S.A.A., El-Sakhawy M., El-Sakhawy M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020;238:116178. doi: 10.1016/j.carbpol.2020.116178. [DOI] [PubMed] [Google Scholar]
- 16.Nešić A., Cabrera-Barjas G., Dimitrijević-Branković S., Davidović S., Radovanović N., Delattre C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules. 2020;25:135. doi: 10.3390/molecules25010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López O.V., Zaritzky N.E., Grossmann M.V.E., García M.A. Acetylated and native corn starch blend films produced by blown extrusion. J. Food Eng. 2013;116:286–297. doi: 10.1016/j.jfoodeng.2012.12.032. [DOI] [Google Scholar]
- 18.Wang P., Li Y., Zhang C., Feng F., Zhang H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020;308:125599. doi: 10.1016/j.foodchem.2019.125599. [DOI] [PubMed] [Google Scholar]
- 19.Dehghani S., ValiHosseini S.M. Regenstein, J. Edible films and coatings in seafood preservation: A review. Food Chem. 2018;240:505–513. doi: 10.1016/j.foodchem.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Benbettaïeb N., Gay J.-P., Karbowiak T., Debeaufort F. Tuning the Functional Properties of Polysaccharide–Protein Bio-Based Edible Films by Chemical, Enzymatic, and Physical Cross-Linking. Compr. Rev. Food Sci. Food Saf. 2016;15:739–752. doi: 10.1111/1541-4337.12210. [DOI] [PubMed] [Google Scholar]
- 21.Embuscado M.E., Huber K.C. Edible Films and Coatings for Food Applications. 1st ed. Springer; New York, NY, USA: 2009. [DOI] [Google Scholar]
- 22.Nechita P., Roman M. Review on Polysaccharides Used in Coatings for Food Packaging Papers. Coatings. 2020;10:566. doi: 10.3390/coatings10060566. [DOI] [Google Scholar]
- 23.Rastogi V.K., Samyn P. Bio-Based Coatings for Paper Applications. Coatings. 2015;5:887–930. doi: 10.3390/coatings5040887. [DOI] [Google Scholar]
- 24.Lavoine N., Desloges I., Dufresne A., Bras J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012;90:735–764. doi: 10.1016/j.carbpol.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Habibi Y., Lucia L.A., Rojas O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010;110:3479–3500. doi: 10.1021/cr900339w. [DOI] [PubMed] [Google Scholar]
- 26.Brown R.M. The Biosynthesis of Cellulose. J. Macromol. Sci. Part A. 1996;33:1345–1373. doi: 10.1080/10601329608014912. [DOI] [Google Scholar]
- 27.Tumbarski Y., Nikolova R., Petkova N., Ivanov I., Lante A. Biopreservation of Fresh Strawberries by Carboxymethyl Cellulose Edible Coatings Enriched with a Bacteriocin from Bacillus methylotrophicus BM47. Food Technol. Biotechnol. 2019;57:230–237. doi: 10.17113/ftb.57.02.19.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H.-Y., Zhang H., Song M.-L., Zhou Y., Yao J., Ni Q.-Q. From Cellulose Nanospheres, Nanorods to Nanofibers: Various Aspect Ratio Induced Nucleation/Reinforcing Effects on Polylactic Acid for Robust-Barrier Food Packaging. ACS Appl. Mater. Interfaces. 2017;9:43920–43938. doi: 10.1021/acsami.7b09102. [DOI] [PubMed] [Google Scholar]
- 29.Ebringerová A. Structural Diversity and Application Potential of Hemicelluloses. Macromol. Symp. 2005;232:1–12. doi: 10.1002/masy.200551401. [DOI] [Google Scholar]
- 30.Scheller H.V., Ulvskov P. Hemicelluloses. Annu. Rev. Plant Biol. 2010;61:263–289. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 31.Ebringerová A., Hromádková Z., Heinze T. Hemicellulose. In: Heinze T., editor. Polysaccharides I: Structure, Characterization and Use. Springer; Berlin/Heidelberg, Germany: 2005. [DOI] [Google Scholar]
- 32.Mikkonen K.S., Tenkanen M. Sustainable food-packaging materials based on future biorefinery products: Xylans and mannans. Trends Food Sci. Technol. 2012;28:90–102. doi: 10.1016/j.tifs.2012.06.012. [DOI] [Google Scholar]
- 33.Chen X., Pang J., Wu C. Fabrication and characterization of antimicrobial food packaging materials composed of konjac glucomannan, chitosan and fulvic acid. Food Sci. 2021;42:232–239. doi: 10.7506/spkx1002-6630-20200417-226. [DOI] [Google Scholar]
- 34.Dai L., Zhang J., Cheng F. Effects of starches from different botanical sources and modification methods on physicochemical properties of starch-based edible films. Int. J. Biol. Macromol. 2019;132:897–905. doi: 10.1016/j.ijbiomac.2019.03.197. [DOI] [PubMed] [Google Scholar]
- 35.Zhu F. Underutilized and unconventional starches: Why should we care? Trends Food Sci. Technol. 2020;100:363–373. doi: 10.1016/j.tifs.2020.04.018. [DOI] [Google Scholar]
- 36.Zhu F., Cui R. Comparison of molecular structure of oca (Oxalis tuberosa), potato, and maize starches. Food Chem. 2019;296:116–122. doi: 10.1016/j.foodchem.2019.05.192. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q., Zheng Y., Zhuang W., Lu X., Luo X., Zheng B. Genome-wide transcriptional changes in type 2 diabetic mice supplemented with lotus seed resistant starch. Food Chem. 2018;264:427–434. doi: 10.1016/j.foodchem.2018.05.056. [DOI] [PubMed] [Google Scholar]
- 38.Nakthong N., Wongsagonsup R., Amornsakchai T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crop. Prod. 2017;105:74–82. doi: 10.1016/j.indcrop.2017.04.048. [DOI] [Google Scholar]
- 39.Zhang Y., Li B., Xu F., He S., Zhang Y., Sun L., Zhu K., Li S., Wu G., Tan L. Jackfruit starch: Composition, structure, functional properties, modifications and applications. Trends Food Sci. Technol. 2021;107:268–283. doi: 10.1016/j.tifs.2020.10.041. [DOI] [Google Scholar]
- 40.Leloup V.M., Colonna P., Ring S.G., Roberts K., Wells B. Microstructure of amylose gels. Carbohydr. Polym. 1992;18:189–197. doi: 10.1016/0144-8617(92)90063-V. [DOI] [Google Scholar]
- 41.Pérez S., Bertoft E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch-Stärke. 2010;62:389–420. doi: 10.1002/star.201000013. [DOI] [Google Scholar]
- 42.Jiang Z., Neetoo H., Chen H. Efficacy of freezing, frozen storage and edible antimicrobial coatings used in combination for control of Listeria monocytogenes on roasted turkey stored at chiller temperatures. Food Microbiol. 2011;28:1394–1401. doi: 10.1016/j.fm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Neetoo H., Ye M., Chen H. Bioactive alginate coatings to control Listeria monocytogenes on cold-smoked salmon slices and fillets. Int. J. Food Microbiol. 2010;136:326–331. doi: 10.1016/j.ijfoodmicro.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Zhu F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Technol. 2018;78:234–242. doi: 10.1016/j.tifs.2018.05.024. [DOI] [Google Scholar]
- 45.Singh S., Singh N., Isono N., Noda T. Relationship of Granule Size Distribution and Amylopectin Structure with Pasting, Thermal, and Retrogradation Properties in Wheat Starch. J. Agric. Food Chem. 2010;58:1180–1188. doi: 10.1021/jf902753f. [DOI] [PubMed] [Google Scholar]
- 46.Kumar N. Polysaccharide-based component and their relevance in edible film/coating: A review. Nutr. Food Sci. 2019;49:793–823. doi: 10.1108/NFS-10-2018-0294. [DOI] [Google Scholar]
- 47.Le Corre D., Bras J., Dufresne A. Starch Nanoparticles: A Review. Biomacromolecules. 2010;11:1139–1153. doi: 10.1021/bm901428y. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Li B., Zhang Y., Xu F., Zhu K., Li S., Tan L., Wu G., Dong W. Effect of degree of polymerization of amylopectin on the gelatinization properties of jackfruit seed starch. Food Chem. 2019;289:152–159. doi: 10.1016/j.foodchem.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 49.Sandford P. In: Chitin and Chitosan: Commercial Uses and Potential Applications in Chitin and Chitosan: Sources, Chemistry, Biochemistry, Physical Properties and Applicaties. Skjak-braek G., Anthonsen T., Sandford P., editors. Elsevier Applied Science; New York, NY, USA: 1989. pp. 51–69. [Google Scholar]
- 50.Yen M.-T., Yang J.-H., Mau J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009;75:15–21. doi: 10.1016/j.carbpol.2008.06.006. [DOI] [Google Scholar]
- 51.Wang H., Qian J., Ding F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018;66:395–413. doi: 10.1021/acs.jafc.7b04528. [DOI] [PubMed] [Google Scholar]
- 52.Szymańska E., Winnicka K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs. 2015;13:1819–1846. doi: 10.3390/md13041819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayakumar R., Menon D., Manzoor K., Nair S.V., Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010;82:227–232. doi: 10.1016/j.carbpol.2010.04.074. [DOI] [Google Scholar]
- 54.Homez-Jara A., Daza L.D., Aguirre D.M., Muñoz J.A., Solanilla J.F., Váquiro H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018;113:1233–1240. doi: 10.1016/j.ijbiomac.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 55.O’Callaghan K.A.M., Kerry J.P. Preparation of low- and medium-molecular weight chitosan nanoparticles and their antimicrobial evaluation against a panel of microorganisms, including cheese-derived cultures. Food Control. 2016;69:256–261. doi: 10.1016/j.foodcont.2016.05.005. [DOI] [Google Scholar]
- 56.Divya K., Smitha V., Jisha M.S. Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int. J. Biol. Macromol. 2018;114:572–577. doi: 10.1016/j.ijbiomac.2018.03.130. [DOI] [PubMed] [Google Scholar]
- 57.Dutta P.K., Tripathi S., Mehrotra G.K., Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114:1173–1182. doi: 10.1016/j.foodchem.2008.11.047. [DOI] [Google Scholar]
- 58.Sahariah P., Másson M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules. 2017;18:3846–3868. doi: 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- 59.Pérez-Jiménez J., Saura-Calixto F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018;111:148–152. doi: 10.1016/j.foodres.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 60.Abboud K.Y., Iacomini M., Simas F.F., Cordeiro L.M.C. High methoxyl pectin from the soluble dietary fiber of passion fruit peel forms weak gel without the requirement of sugar addition. Carbohydr. Polym. 2020;246:116616. doi: 10.1016/j.carbpol.2020.116616. [DOI] [PubMed] [Google Scholar]
- 61.Rodsamran P., Sothornvit R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019;278:364–372. doi: 10.1016/j.foodchem.2018.11.067. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen B.M.N., Pirak T. Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food Agric. 2019;5:1633076. doi: 10.1080/23311932.2019.1633076. [DOI] [Google Scholar]
- 63.Gharibzahedi S.M.T., Smith B., Guo Y. Pectin extraction from common fig skin by different methods: The physicochemical, rheological, functional, and structural evaluations. Int. J. Biol. Macromol. 2019;136:275–283. doi: 10.1016/j.ijbiomac.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 64.Wu H., Lei Y., Zhu R., Zhao M., Lu J., Xiao D., Jiao C., Zhang Z., Shen G., Li S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019;90:41–49. doi: 10.1016/j.foodhyd.2018.12.016. [DOI] [Google Scholar]
- 65.Presentato A., Scurria A., Albanese L., Lino C., Sciortino M., Pagliaro M., Zabini F., Meneguzzo F., Alduina R., Nuzzo D., et al. Superior Antibacterial Activity of Integral Lemon Pectin Extracted via Hydrodynamic Cavitation. ChemistryOpen. 2020;9:628–630. doi: 10.1002/open.202000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X., Qi Y., Zhu C., Wang Q. Effect of ultrasound on the properties and antioxidant activity of hawthorn pectin. Int. J. Biol. Macromol. 2019;131:273–281. doi: 10.1016/j.ijbiomac.2019.03.077. [DOI] [PubMed] [Google Scholar]
- 67.Muñoz-Almagro N., Prodanov M., Wilde P.J., Villamiel M., Montilla A. Obtainment and characterisation of pectin from sunflower heads purified by membrane separation techniques. Food Chem. 2020;318:126476. doi: 10.1016/j.foodchem.2020.126476. [DOI] [PubMed] [Google Scholar]
- 68.Chen J. Ph.D. Thesis. Nanchang University; Nanchang, China: 2013. Extraction and Physicochemical Characterization of Pectins from some Special Resources and the Mechanism of Pectin Degradation Induced by Dynamic High Pressure Microfluidization. [Google Scholar]
- 69.Mostafavi F.S., Zaeim D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020;159:1165–1176. doi: 10.1016/j.ijbiomac.2020.05.123. [DOI] [PubMed] [Google Scholar]
- 70.Tavassoli-Kafrani E., Shekarchizadeh H., Masoudpour-Behabadi M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016;137:360–374. doi: 10.1016/j.carbpol.2015.10.074. [DOI] [PubMed] [Google Scholar]
- 71.Nieto M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. In: Huber K., Embuscado M., editors. Edible Films and Coatings for Food Applications. Springer; New York, NY, USA: 2009. pp. 57–112. [Google Scholar]
- 72.Ciriminna R., Fidalgo A., Meneguzzo F., Presentato A., Scurria A., Nuzzo D., Alduina R., Ilharco L.M., Pagliaro M. Pectin: A Long-Neglected Broad-Spectrum Antibacterial. ChemMedChem. 2020;15:2228–2235. doi: 10.1002/cmdc.202000518. [DOI] [PubMed] [Google Scholar]
- 73.Martinov J., Krstić M., Spasić S., Miletić S., Stefanović-Kojić J., Nikolić-Kokić A., Blagojević D., Spasojević I., Spasić M.B. Apple pectin-derived oligosaccharides produce carbon dioxide radical anion in Fenton reaction and prevent growth of Escherichia coli and Staphylococcus aureus. Food Res. Int. 2017;100:132–136. doi: 10.1016/j.foodres.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 74.Senturk Parreidt T., Müller K., Schmid M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods. 2018;7:170. doi: 10.3390/foods7100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kloareg B., Quatrano R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. Annu. Rev. 1988;26:259–315. [Google Scholar]
- 76.Abraham R.E., Su P., Puri M., Raston C.L., Zhang W. Optimisation of biorefinery production of alginate, fucoidan and laminarin from brown seaweed Durvillaea potatorum. Algal Res. 2019;38:101389. doi: 10.1016/j.algal.2018.101389. [DOI] [Google Scholar]
- 77.Comaposada J., Marcos B., Bou R., Gou P. Influence of surfactants and proteins on the properties of wet edible calcium alginate meat coatings. Food Res. Int. 2018;108:539–550. doi: 10.1016/j.foodres.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Costa M.J., Marques A.M., Pastrana L.M., Teixeira J.A., Sillankorva S.M., Cerqueira M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018;81:442–448. doi: 10.1016/j.foodhyd.2018.03.014. [DOI] [Google Scholar]
- 79.Delattre C., Fenoradosoa T.A., Michaud P. Galactans: An overview of their most important sourcing and applications as natural polysaccharides. Braz. Arch. Biol. Technol. 2011;54:1075–1092. doi: 10.1590/S1516-89132011000600002. [DOI] [Google Scholar]
- 80.Armisén R., Gaiatas F. 4-Agar. In: Phillips G.O., Williams P.A., editors. Handbook of Hydrocolloids. 2nd ed. Woodhead Publishing; Sawston, UK: 2009. [DOI] [Google Scholar]
- 81.Janjarasskul T., Krochta J.M. Edible Packaging Materials. Annu. Rev. Food Sci. Technol. 2010;1:415–448. doi: 10.1146/annurev.food.080708.100836. [DOI] [PubMed] [Google Scholar]
- 82.Gordobil O., Egüés I., Urruzola I., Labidi J. Xylan–cellulose films: Improvement of hydrophobicity, thermal and mechanical properties. Carbohydr. Polym. 2014;112:56–62. doi: 10.1016/j.carbpol.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 83.Otoni C.G., Lorevice M.V., Moura M.R.d., Mattoso L.H.C. On the effects of hydroxyl substitution degree and molecular weight on mechanical and water barrier properties of hydroxypropyl methylcellulose films. Carbohydr. Polym. 2018;185:105–111. doi: 10.1016/j.carbpol.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Fonseca-Florido H.A., Soriano-Corral F., Yañez-Macías R., González-Morones P., Hernández-Rodríguez F., Aguirre-Zurita J., Ávila-Orta C., Rodríguez-Velázquez J. Effects of multiphase transitions and reactive extrusion on in situ thermoplasticization/succination of cassava starch. Carbohydr. Polym. 2019;225:115250. doi: 10.1016/j.carbpol.2019.115250. [DOI] [PubMed] [Google Scholar]
- 85.Li S., Xiong Q., Lai X., Li X., Wan M., Zhang J., Yan Y., Cao M., Lu L., Guan J., et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016;15:237–250. doi: 10.1111/1541-4337.12161. [DOI] [PubMed] [Google Scholar]
- 86.Cumpstey I. Chemical Modification of Polysaccharides. ISRN Org. Chem. 2013;2013:417672. doi: 10.1155/2013/417672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mikkonen K.S., Laine C., Kontro I., Talja R.A., Serimaa R., Tenkanen M. Combination of internal and external plasticization of hydroxypropylated birch xylan tailors the properties of sustainable barrier films. Eur. Polym. J. 2015;66:307–318. doi: 10.1016/j.eurpolymj.2015.02.034. [DOI] [Google Scholar]
- 88.Garavand F., Rouhi M., Razavi S.H., Cacciotti I., Mohammadi R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017;104:687–707. doi: 10.1016/j.ijbiomac.2017.06.093. [DOI] [PubMed] [Google Scholar]
- 89.Tan W., Zhang J., Zhao X., Li Q., Dong F., Guo Z. Preparation and physicochemical properties of antioxidant chitosan ascorbate/methylcellulose composite films. Int. J. Biol. Macromol. 2020;146:53–61. doi: 10.1016/j.ijbiomac.2019.12.044. [DOI] [PubMed] [Google Scholar]
- 90.Yu S.-H., Tsai M.-L., Lin B.-X., Lin C.-W., Mi F.-L. Tea catechins-cross-linked methylcellulose active films for inhibition of light irradiation and lipid peroxidation induced β-carotene degradation. Food Hydrocoll. 2015;44:491–505. doi: 10.1016/j.foodhyd.2014.10.022. [DOI] [Google Scholar]
- 91.da Silva Filipini G., Romani V.P., Guimarães Martins V. Blending collagen, methylcellulose, and whey protein in films as a greener alternative for food packaging: Physicochemical and biodegradable properties. Packag. Technol. Sci. 2021;34:91–103. doi: 10.1002/pts.2541. [DOI] [Google Scholar]
- 92.Zheng H., Zhang G., Zhuang C., Yang T., Zheng Y., Zheng C., Zhong Y. Effects of Different Substrates on the Properties of Edible Films for Oily Foods. Food Res. Dev. 2018;39:18–23. doi: 10.3969/j.issn.1005-6521.2018.13.004. [DOI] [Google Scholar]
- 93.Mohammadi M., Azizi M.H., Zoghi A. Antimicrobial activity of carboxymethyl cellulose-gelatin film containing Dianthus barbatus essential oil against aflatoxin-producing molds. Food Sci. Nutr. 2020;8:1244–1253. doi: 10.1002/fsn3.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martelli S.M., Motta C., Caon T., Alberton J., Bellettini I.C., do Prado A.C.P., Barreto P.L.M., Soldi V. Edible carboxymethyl cellulose films containing natural antioxidant and surfactants: α-tocopherol stability, in vitro release and film properties. LWT. 2017;77:21–29. doi: 10.1016/j.lwt.2016.11.026. [DOI] [Google Scholar]
- 95.Ballesteros L.F., Cerqueira M.A., Teixeira J.A., Mussatto S.I. Production and physicochemical properties of carboxymethyl cellulose films enriched with spent coffee grounds polysaccharides. Int. J. Biol. Macromol. 2018;106:647–655. doi: 10.1016/j.ijbiomac.2017.08.060. [DOI] [PubMed] [Google Scholar]
- 96.Francisco C.B., Pellá M.G., Silva O.A., Raimundo K.F., Caetano J., Linde G.A., Colauto N.B., Dragunski D.C. Shelf-life of guavas coated with biodegradable starch and cellulose-based films. Int. J. Biol. Macromol. 2020;152:272–279. doi: 10.1016/j.ijbiomac.2020.02.249. [DOI] [PubMed] [Google Scholar]
- 97.Zhai X., Qin Y., Lu H., Dai Y., Zhang H., Wang W., Hou H., Chen N. Preparation and Properties of Edible Films of High-Amylose Corn Starch/HPMC (Hydroxypropyl Methylcellulose) J. Chin. Cereals Oils Assoc. 2019;34:33–38. [Google Scholar]
- 98.Rhimi W., Boulila A., Gheribi R., Khwaldia K. Development, characterization and application of hydroxypropylmethylcellulose films enriched with cypress seed extract. Rsc Adv. 2018;8:23615–23622. doi: 10.1039/C8RA04369H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dou Y., Zhang L., Zhang B., He M., Shi W., Yang S., Cui Y., Yin G. Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers. 2020;12:158. doi: 10.3390/polym12010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salmieri S., Khan R.A., Safrany A., Lacroix M. Gamma rays-induced 2-hydroxyethyl methacrylate graft copolymerization on methylcellulose-based films: Structure analysis and physicochemical properties. Ind. Crop. Prod. 2015;70:64–71. doi: 10.1016/j.indcrop.2015.02.056. [DOI] [Google Scholar]
- 101.Ramos A., Sousa S., Evtuguin D.V., Gamelas J.A.F. Functionalized xylans in the production of xylan-coated paper laminates. React. Funct. Polym. 2017;117:89–96. doi: 10.1016/j.reactfunctpolym.2017.06.006. [DOI] [Google Scholar]
- 102.Pereira P.H.F., Waldron K.W., Wilson D.R., Cunha A.P., Brito E.S.d., Rodrigues T.H.S., Rosa M.F., Azeredo H.M.C. Wheat straw hemicelluloses added with cellulose nanocrystals and citric acid. Effect on film physical properties. Carbohydr. Polym. 2017;164:317–324. doi: 10.1016/j.carbpol.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 103.Milotskyi R., Bliard C., Tusseau D., Benoit C. Starch carboxymethylation by reactive extrusion: Reaction kinetics and structure analysis. Carbohydr. Polym. 2018;194:193–199. doi: 10.1016/j.carbpol.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 104.Woggum T., Sirivongpaisal P., Wittaya T. Characteristics and properties of hydroxypropylated rice starch based biodegradable films. Food Hydrocoll. 2015;50:54–64. doi: 10.1016/j.foodhyd.2015.04.010. [DOI] [Google Scholar]
- 105.Ojogbo E., Blanchard R., Mekonnen T. Hydrophobic and Melt Processable Starch-Laurate Esters: Synthesis, Structure–Property Correlations. J. Polym. Sci. Part A: Polym. Chem. 2018;56:2611–2622. doi: 10.1002/pola.29237. [DOI] [Google Scholar]
- 106.Blohm S., Heinze T. Synthesis and properties of thermoplastic starch laurates. Carbohydr. Res. 2019;486:107833. doi: 10.1016/j.carres.2019.107833. [DOI] [PubMed] [Google Scholar]
- 107.Wilpiszewska K., Antosik A.K., Zdanowicz M. The Effect of Citric Acid on Physicochemical Properties of Hydrophilic Carboxymethyl Starch-Based Films. J. Polym. Environ. 2019;27:1379–1387. doi: 10.1007/s10924-019-01436-9. [DOI] [Google Scholar]
- 108.Yıldırım-Yalçın M., Şeker M., Sadıkoğlu H. Development and characterization of edible films based on modified corn starch and grape juice. Food Chem. 2019;292:6–13. doi: 10.1016/j.foodchem.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 109.Tan W., Li Q., Wang H., Liu Y., Zhang J., Dong F., Guo Z. Synthesis, characterization, and antibacterial property of novel starch derivatives with 1,2,3-triazole. Carbohydr. Polym. 2016;142:1–7. doi: 10.1016/j.carbpol.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Suriyatem R., Auras R.A., Rachtanapun P. Improvement of mechanical properties and thermal stability of biodegradable rice starch–based films blended with carboxymethyl chitosan. Ind. Crop. Prod. 2018;122:37–48. doi: 10.1016/j.indcrop.2018.05.047. [DOI] [Google Scholar]
- 111.Tan W., Dong F., Zhang J., Zhao X., Li Q., Guo Z. Physical and Antioxidant Properties of Edible Chitosan Ascorbate Films. J. Agric. Food Chem. 2019;67:2530–2539. doi: 10.1021/acs.jafc.8b04567. [DOI] [PubMed] [Google Scholar]
- 112.Cao M., Liu X., Luan J., Zhang X. Characterization of physicochemical properties of carboxymethyl agar. Carbohydr. Polym. 2014;111:449–455. doi: 10.1016/j.carbpol.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 113.Šešlija S., Nešić A., Škorić M.L., Krušić M.K., Santagata G., Malinconico M. Pectin/Carboxymethylcellulose Films as a Potential Food Packaging Material. Macromol. Symp. 2018;378:1600163. doi: 10.1002/masy.201600163. [DOI] [Google Scholar]
- 114.Ling X., Yang C., Ning J. Research progress in modification of cellulose and application. J. Text. Sci. Eng. 2020;37:60–85. [Google Scholar]
- 115.Dias M.V., de Medeiros H.S., Soares N.d.F.F., Melo N.R.d., Borges S.V., Carneiro J.d.D.S., Pereira J.M.T.d.A.K. Development of low-density polyethylene films with lemon aroma. LWT Food Sci. Technol. 2013;50:167–171. doi: 10.1016/j.lwt.2012.06.005. [DOI] [Google Scholar]
- 116.Bastarrachea L., Dhawan S., Sablani S.S. Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging: A Review. Food Eng. Rev. 2011;3:79–93. doi: 10.1007/s12393-011-9034-8. [DOI] [Google Scholar]
- 117.Shah U., Naqash F., Gani A., Masoodi F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016;15:568–580. doi: 10.1111/1541-4337.12197. [DOI] [PubMed] [Google Scholar]
- 118.Liu C., Qin S., Xie J., Lin X., Zheng Y., Yang J., Kan H., Shi Z. Using Carboxymethyl Cellulose as the Additive With Enzyme-Catalyzed Carboxylated Starch to Prepare the Film With Enhanced Mechanical and Hydrophobic Properties. Front. Bioeng. Biotechnol. 2021;9:638546. doi: 10.3389/fbioe.2021.638546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gurgel de Carvalho L.G., do Nascimento Marques N., da Silva Fernandes R., Villetti M.A., Men de Sá Moreira de Souza F., de Carvalho Balaban R. Effect of Starch Laurate Addition on the Properties of Mango Kernel Starch Films. Materials Research. Mater. Res. 2021;24:e20200331. doi: 10.1590/1980-5373-mr-2020-0331. [DOI] [Google Scholar]
- 120.Li J., Ye F., Liu J., Zhao G. Effects of octenylsuccination on physical, mechanical and moisture-proof properties of stretchable sweet potato starch film. Food Hydrocoll. 2015;46:226–232. doi: 10.1016/j.foodhyd.2014.12.017. [DOI] [Google Scholar]
- 121.Guo L.-Y., Lu H.-Q., Rackemann D., Shi C., Li W., Li K., Doherty W.O.S. Quaternary ammonium-functionalized magnetic chitosan microspheres as an effective green adsorbent to remove high-molecular-weight invert sugar alkaline degradation products (HISADPs) Chem. Eng. J. 2021;416:129084. doi: 10.1016/j.cej.2021.129084. [DOI] [Google Scholar]
- 122.Anitha A., Divya Rani V.V., Krishna R., Sreeja V., Selvamurugan N., Nair S.V., Tamura H., Jayakumar R. Synthesis, characterization, cytotoxicity and antibacterial studies of chitosan, O-carboxymethyl and N,O-carboxymethyl chitosan nanoparticles. Carbohydr. Polym. 2009;78:672–677. doi: 10.1016/j.carbpol.2009.05.028. [DOI] [Google Scholar]
- 123.Tang R., Zhang Y., Zhang Y., Yu Z. Synthesis and characterization of chitosan based dye containing quaternary ammonium group. Carbohydr. Polym. 2016;139:191–196. doi: 10.1016/j.carbpol.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 124.Zhu X., Wu H., Yang J., Tong J., Yi J., Hu Z., Hu J., Wang T., Fan L. Antibacterial activity of chitosan grafting nisin: Preparation and characterization. React. Funct. Polym. 2015;91–92:71–76. doi: 10.1016/j.reactfunctpolym.2015.04.009. [DOI] [Google Scholar]
- 125.Li Q., Mi Y., Tan W., Guo Z. Highly efficient free radical-scavenging property of phenolic-functionalized chitosan derivatives: Chemical modification and activity assessment. Int. J. Biol. Macromol. 2020;164:4279–4288. doi: 10.1016/j.ijbiomac.2020.08.250. [DOI] [PubMed] [Google Scholar]
- 126.Tan W., Zhang J., Zhao X., Dong F., Li Q., Guo Z. Synthesis and antioxidant action of chitosan derivatives with amino-containing groups via azide-alkyne click reaction and N-methylation. Carbohydr. Polym. 2018;199:583–592. doi: 10.1016/j.carbpol.2018.07.056. [DOI] [PubMed] [Google Scholar]
- 127.Wu C., Li Y., Du Y., Wang L., Tong C., Hu Y., Pang J., Yan Z. Preparation and characterization of konjac glucomannan-based bionanocomposite film for active food packaging. Food Hydrocoll. 2019;89:682–690. doi: 10.1016/j.foodhyd.2018.11.001. [DOI] [Google Scholar]
- 128.Chen J., Liu W., Liu C.-M., Li T., Liang R.-H., Luo S.-J. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015;55:1684–1698. doi: 10.1080/10408398.2012.718722. [DOI] [PubMed] [Google Scholar]
- 129.Turgeon S.L., Beaulieu M., Schmitt C., Sanchez C. Protein–polysaccharide interactions: Phase-ordering kinetics, thermodynamic and structural aspects. Curr. Opin. Colloid Interface Sci. 2003;8:401–414. doi: 10.1016/S1359-0294(03)00093-1. [DOI] [Google Scholar]
- 130.Ye A. Complexation between milk proteins and polysaccharides via electrostatic interaction: Principles and applications—A review. Int. J. Food Sci. Technol. 2008;43:406–415. doi: 10.1111/j.1365-2621.2006.01454.x. [DOI] [Google Scholar]
- 131.Wu Y., Weller C.L., Hamouz F., Cuppett S.L., Schnepf M. Advances in Food and Nutrition Research. Volume 44. Academic Press; Cambridge, MA, USA: 2002. Development and application of multicomponent edible coatings and films: A review; pp. 347–394. [DOI] [PubMed] [Google Scholar]
- 132.Alizadeh Sani M., Ehsani A., Hashemi M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017;251:8–14. doi: 10.1016/j.ijfoodmicro.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 133.Silva-Weiss A., Quilaqueo M., Venegas O., Ahumada M., Silva W., Osorio F., Giménez B. Design of dipalmitoyl lecithin liposomes loaded with quercetin and rutin and their release kinetics from carboxymethyl cellulose edible films. J. Food Eng. 2018;224:165–173. doi: 10.1016/j.jfoodeng.2018.01.001. [DOI] [Google Scholar]
- 134.Mugwagwa L.R., Chimphango A.F.A. Enhancing the functional properties of acetylated hemicellulose films for active food packaging using acetylated nanocellulose reinforcement and polycaprolactone coating. Food Packag. Shelf Life. 2020;24:100481. doi: 10.1016/j.fpsl.2020.100481. [DOI] [Google Scholar]
- 135.Wang L., Lin L., Chen X., Tong C., Pang J. Synthesis and characteristics of konjac glucomannan films incorporated with functionalized microcrystalline cellulose. Colloids Surf. A: Physicochem. Eng. Asp. 2019;563:237–245. doi: 10.1016/j.colsurfa.2018.12.015. [DOI] [Google Scholar]
- 136.Wang L., Mu R.-J., Li Y., Lin L., Lin Z., Pang J. Characterization and antibacterial activity evaluation of curcumin loaded konjac glucomannan and zein nanofibril films. LWT. 2019;113:108293. doi: 10.1016/j.lwt.2019.108293. [DOI] [Google Scholar]
- 137.Lei Y., Wu H., Jiao C., Jiang Y., Liu R., Xiao D., Lu J., Zhang Z., Shen G., Li S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019;94:128–135. doi: 10.1016/j.foodhyd.2019.03.011. [DOI] [Google Scholar]
- 138.Du Y., Wang L., Mu R., Wang Y., Li Y., Wu D., Wu C., Pang J. Fabrication of novel Konjac glucomannan/shellac film with advanced functions for food packaging. Int. J. Biol. Macromol. 2019;131:36–42. doi: 10.1016/j.ijbiomac.2019.02.142. [DOI] [PubMed] [Google Scholar]
- 139.Wilpiszewska K., Antosik A.K., Schmidt B., Janik J., Rokicka J. Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymers. 2020;12:2447. doi: 10.3390/polym12112447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lopez O., Garcia M.A., Villar M.A., Gentili A., Rodriguez M.S., Albertengo L. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT Food Sci. Technol. 2014;57:106–115. doi: 10.1016/j.lwt.2014.01.024. [DOI] [Google Scholar]
- 141.Ali A., Chen Y., Liu H., Yu L., Baloch Z., Khalid S., Zhu J., Chen L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019;129:1120–1126. doi: 10.1016/j.ijbiomac.2018.09.068. [DOI] [PubMed] [Google Scholar]
- 142.Zimet P., Mombrú Á.W., Mombrú D., Castro A., Villanueva J.P., Pardo H., Rufo C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/carboxymethyl chitosan films. Carbohydr. Polym. 2019;219:334–343. doi: 10.1016/j.carbpol.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 143.Badawy M.E.I., Rabea E.I., El-Nouby M., Ismail R.I.A., Taktak N.E.M. Strawberry Shelf Life, Composition, and Enzymes Activity in Response to Edible Chitosan Coatings. Int. J. Fruit Sci. 2017;17:117–136. doi: 10.1080/15538362.2016.1219290. [DOI] [Google Scholar]
- 144.Siripatrawan U., Vitchayakitti W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016;61:695–702. doi: 10.1016/j.foodhyd.2016.06.001. [DOI] [Google Scholar]
- 145.Oun A.A., Rhim J.-W. Effect of post-treatments and concentration of cotton linter cellulose nanocrystals on the properties of agar-based nanocomposite films. Carbohydr. Polym. 2015;134:20–29. doi: 10.1016/j.carbpol.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 146.Cao Q., Zhang Y., Chen W., Meng X., Liu B. Hydrophobicity and physicochemical properties of agarose film as affected by chitosan addition. Int. J. Biol. Macromol. 2018;106:1307–1313. doi: 10.1016/j.ijbiomac.2017.08.134. [DOI] [PubMed] [Google Scholar]
- 147.Rai S.K., Chaturvedi K., Yadav S.K. Evaluation of structural integrity and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll. 2019;91:127–135. doi: 10.1016/j.foodhyd.2019.01.022. [DOI] [Google Scholar]
- 148.Baron R.D., Pérez L.L., Salcedo J.M., Córdoba L.P., Sobral P.J.d.A. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. Int. J. Biol. Macromol. 2017;98:676–683. doi: 10.1016/j.ijbiomac.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 149.Mostafavi F.S., Kadkhodaee R., Emadzadeh B., Koocheki A. Preparation and characterization of tragacanth–locust bean gum edible blend films. Carbohydr. Polym. 2016;139:20–27. doi: 10.1016/j.carbpol.2015.11.069. [DOI] [PubMed] [Google Scholar]
- 150.Eghbal N., Yarmand M.S., Mousavi M., Degraeve P., Oulahal N., Gharsallaoui A. Complex coacervation for the development of composite edible films based on LM pectin and sodium caseinate. Carbohydr. Polym. 2016;151:947–956. doi: 10.1016/j.carbpol.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 151.Eghbal N., Degraeve P., Oulahal N., Yarmand M.S., Mousavi M.E., Gharsallaoui A. Low methoxyl pectin/sodium caseinate interactions and composite film formation at neutral pH. Food Hydrocoll. 2017;69:132–140. doi: 10.1016/j.foodhyd.2017.01.033. [DOI] [Google Scholar]
- 152.Hashemi S.M.B., Mousavi Khaneghah A., Ghaderi Ghahfarrokhi M., Eş I. Basil-seed gum containing Origanum vulgare subsp. viride essential oil as edible coating for fresh cut apricots. Postharvest Biol. Technol. 2017;125:26–34. doi: 10.1016/j.postharvbio.2016.11.003. [DOI] [Google Scholar]
- 153.da Rocha M., Alemán A., Romani V.P., López-Caballero M.E., Gómez-Guillén M.C., Montero P., Prentice C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocoll. 2018;81:351–363. doi: 10.1016/j.foodhyd.2018.03.017. [DOI] [Google Scholar]
- 154.Rincón E., Serrano L., Balu A.M., Aguilar J.J., Luque R., García A. Effect of Bay Leaves Essential Oil Concentration on the Properties of Biodegradable Carboxymethyl Cellulose-Based Edible Films. Materials. 2019;12:2356. doi: 10.3390/ma12152356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moreno O., Atarés L., Chiralt A., Cruz-Romero M.C., Kerry J. Starch-gelatin antimicrobial packaging materials to extend the shelf life of chicken breast fillets. LWT. 2018;97:483–490. doi: 10.1016/j.lwt.2018.07.005. [DOI] [Google Scholar]
- 156.Panrong T., Karbowiak T., Harnkarnsujarit N. Thermoplastic starch and green tea blends with LLDPE films for active packaging of meat and oil-based products. Food Packag. Shelf Life. 2019;21:100331. doi: 10.1016/j.fpsl.2019.100331. [DOI] [Google Scholar]
- 157.Rambabu K., Bharath G., Banat F., Show P.L., Cocoletzi H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019;126:1234–1243. doi: 10.1016/j.ijbiomac.2018.12.196. [DOI] [PubMed] [Google Scholar]
- 158.Souza V.G.L., Pires J.R.A., Rodrigues C., Coelhoso I.M., Fernando A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers. 2020;12:417. doi: 10.3390/polym12020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shankar S., Rhim J.-W. Preparation of nanocellulose from micro-crystalline cellulose: The effect on the performance and properties of agar-based composite films. Carbohydr. Polym. 2016;135:18–26. doi: 10.1016/j.carbpol.2015.08.082. [DOI] [PubMed] [Google Scholar]
- 160.Phan The D., Debeaufort F., Voilley A., Luu D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. J. Food Eng. 2009;90:548–558. doi: 10.1016/j.jfoodeng.2008.07.023. [DOI] [Google Scholar]
- 161.Fekete E., Bella É., Csiszár E., Móczó J. Improving physical properties and retrogradation of thermoplastic starch by incorporating agar. Int. J. Biol. Macromol. 2019;136:1026–1033. doi: 10.1016/j.ijbiomac.2019.06.109. [DOI] [PubMed] [Google Scholar]
- 162.Liu K., Lin X., Chen L., Huang L., Cao S. Dual-functional chitosan–methylisothiazolinone/microfibrillated cellulose biocomposites for enhancing antibacterial and mechanical properties of agar films. Cellulose. 2014;21:519–528. doi: 10.1007/s10570-013-0145-7. [DOI] [Google Scholar]
- 163.Sousa A.M.M., Gonçalves M.P. Strategies to improve the mechanical strength and water resistance of agar films for food packaging applications. Carbohydr. Polym. 2015;132:196–204. doi: 10.1016/j.carbpol.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 164.Luangtana-anan M., Soradech S., Saengsod S., Nunthanid J., Limmatvapirat S. Enhancement of Moisture Protective Properties and Stability of Pectin through Formation of a Composite Film: Effects of Shellac and Plasticizer. J. Food Sci. 2017;82:2915–2925. doi: 10.1111/1750-3841.13956. [DOI] [PubMed] [Google Scholar]
- 165.Pérez-Gago M.B., Rhim J.-W. Chapter 13—Edible Coating and Film Materials: Lipid Bilayers and Lipid Emulsions. In: Han J.H., editor. Innovations in Food Packaging, 2nd ed. Academic Press; San Diego, CA, USA: 2014. [DOI] [Google Scholar]
- 166.Galus S., Kadzińska J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015;45:273–283. doi: 10.1016/j.tifs.2015.07.011. [DOI] [Google Scholar]
- 167.Zhang R., Wang W., Zhang H., Dai Y., Dong H., Hou H. Effects of hydrophobic agents on the physicochemical properties of edible agar/maltodextrin films. Food Hydrocoll. 2019;88:283–290. doi: 10.1016/j.foodhyd.2018.10.008. [DOI] [Google Scholar]
- 168.Nisar T., Wang Z.-C., Yang X., Tian Y., Iqbal M., Guo Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018;106:670–680. doi: 10.1016/j.ijbiomac.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 169.López de Lacey A.M., López-Caballero M.E., Montero P. Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT Food Sci. Technol. 2014;55:559–564. doi: 10.1016/j.lwt.2013.09.028. [DOI] [Google Scholar]
- 170.Yan Y., Duan S., Zhang H., Liu Y., Li C., Hu B., Liu A., Wu D., He J., Wu W. Preparation and characterization of Konjac glucomannan and pullulan composite films for strawberry preservation. Carbohydr. Polym. 2020;243:116446. doi: 10.1016/j.carbpol.2020.116446. [DOI] [PubMed] [Google Scholar]
- 171.Guerra I.C.D., de Oliveira P.D.L., Santos M.M.F., Lúcio A.S.S.C., Tavares J.F., Barbosa-Filho J.M., Madruga M.S., de Souza E.L. The effects of composite coatings containing chitosan and Mentha (piperita L. or x villosa Huds) essential oil on postharvest mold occurrence and quality of table grape cv. Isabella. Innov. Food Sci. Emerg. Technol. 2016;34:112–121. doi: 10.1016/j.ifset.2016.01.008. [DOI] [Google Scholar]
- 172.Thakur R., Pristijono P., Bowyer M., Singh S.P., Scarlett C.J., Stathopoulos C.E., Vuong Q.V. A starch edible surface coating delays banana fruit ripening. LWT. 2019;100:341–347. doi: 10.1016/j.lwt.2018.10.055. [DOI] [Google Scholar]
- 173.Pan T., Shi T., Wen D., Yan G. Study on Preservation of Cherry Tomato by Konjac Glucomannan Composite Coating Solution. Food Ind. 2020;41:60–63. [Google Scholar]
- 174.Hashemi S.M.B., Jafarpour D. The efficacy of edible film from Konjac glucomannan and saffron petal extract to improve shelf life of fresh-cut cucumber. Food Sci. Nutr. 2020;8:3128–3137. doi: 10.1002/fsn3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Go E.-J., Song K.B. Antioxidant Properties of Rye Starch Films Containing Rosehip Extract and Their Application in Packaging of Chicken Breast. Starch-Stärke. 2019;71:1900116. doi: 10.1002/star.201900116. [DOI] [Google Scholar]
- 176.Qin Y., Liu Y., Yong H., Liu J., Zhang X., Liu J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019;134:80–90. doi: 10.1016/j.ijbiomac.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 177.Dini H., Fallah A.A., Bonyadian M., Abbasvali M., Soleimani M. Effect of edible composite film based on chitosan and cumin essential oil-loaded nanoemulsion combined with low-dose gamma irradiation on microbiological safety and quality of beef loins during refrigerated storage. Int. J. Biol. Macromol. 2020;164:1501–1509. doi: 10.1016/j.ijbiomac.2020.07.215. [DOI] [PubMed] [Google Scholar]
- 178.Carocho M., Heleno S., Rodrigues P., Barreiro M.F., Barros L., Ferreira I.C.F.R. A novel natural coating for food preservation: Effectiveness on microbial growth and physicochemical parameters. LWT. 2019;104:76–83. doi: 10.1016/j.lwt.2019.01.031. [DOI] [Google Scholar]
- 179.Kalem I.K., Bhat Z.F., Kumar S., Wang L., Mudiyanselage R.J., Bhat H.F. Tinospora cordifolia: A novel bioactive ingredient for edible films for improved lipid oxidative and microbial stability of meat products. J. Food Process. Preserv. 2018;42:e13774. doi: 10.1111/jfpp.13774. [DOI] [Google Scholar]
- 180.Rodsamran P., Sothornvit R. Lime peel pectin integrated with coconut water and lime peel extract as a new bioactive film sachet to retard soybean oil oxidation. Food Hydrocoll. 2019;97:105173. doi: 10.1016/j.foodhyd.2019.105173. [DOI] [Google Scholar]
- 181.Baek S.-K., Kim S., Song K.B. Cowpea starch films containing maqui berry extract and their application in salmon packaging. Food Packag. Shelf Life. 2019;22:100394. doi: 10.1016/j.fpsl.2019.100394. [DOI] [Google Scholar]
- 182.Gharibzahedi S.M.T., Mohammadnabi S. Effect of novel bioactive edible coatings based on jujube gum and nettle oil-loaded nanoemulsions on the shelf-life of Beluga sturgeon fillets. Int. J. Biol. Macromol. 2017;95:769–777. doi: 10.1016/j.ijbiomac.2016.11.119. [DOI] [PubMed] [Google Scholar]
- 183.Alotaibi S., Tahergorabi R. Development of a sweet potato starch-based coating and its effect on quality attributes of shrimp during refrigerated storage. LWT. 2018;88:203–209. doi: 10.1016/j.lwt.2017.10.022. [DOI] [Google Scholar]
- 184.Yu Z., Sun L., Wang W., Zeng W., Mustapha A., Lin M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crop. Prod. 2018;112:412–419. doi: 10.1016/j.indcrop.2017.12.031. [DOI] [Google Scholar]
- 185.Xie B., Zhang X., Luo X., Wang Y., Li Y., Li B., Liu S. Edible coating based on beeswax-in-water Pickering emulsion stabilized by cellulose nanofibrils and carboxymethyl chitosan. Food Chem. 2020;331:127108. doi: 10.1016/j.foodchem.2020.127108. [DOI] [PubMed] [Google Scholar]
- 186.Zhao Y., Teixeira J.S., Saldaña M.D.A., Gänzle M.G. Antimicrobial activity of bioactive starch packaging films against Listeria monocytogenes and reconstituted meat microbiota on ham. Int. J. Food Microbiol. 2019;305:108253. doi: 10.1016/j.ijfoodmicro.2019.108253. [DOI] [PubMed] [Google Scholar]
- 187.Volpe V., De Feo G., De Marco I., Pantani R. Use of sunflower seed fried oil as an ecofriendly plasticizer for starch and application of this thermoplastic starch as a filler for PLA. Ind. Crop. Prod. 2018;122:545–552. doi: 10.1016/j.indcrop.2018.06.014. [DOI] [Google Scholar]
- 188.Meng L., Liu H., Yu L., Duan Q., Chen L., Liu F., Shao Z., Shi K., Lin X. How water acting as both blowing agent and plasticizer affect on starch-based foam. Ind. Crop. Prod. 2019;134:43–49. doi: 10.1016/j.indcrop.2019.03.056. [DOI] [Google Scholar]
- 189.Perdones Á., Escriche I., Chiralt A., Vargas M. Effect of chitosan–lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016;197:979–986. doi: 10.1016/j.foodchem.2015.11.054. [DOI] [PubMed] [Google Scholar]
- 190.Duran M., Aday M.S., Zorba N.N.D., Temizkan R., Büyükcan M.B., Caner C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016;98:354–363. doi: 10.1016/j.fbp.2016.01.007. [DOI] [Google Scholar]
- 191.Sahraei Khosh Gardesh A., Badii F., Hashemi M., Ardakani A.Y., Maftoonazad N., Gorji A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT. 2016;70:33–40. doi: 10.1016/j.lwt.2016.02.002. [DOI] [Google Scholar]
- 192.Kaya M., Česonienė L., Daubaras R., Leskauskaitė D., Zabulionė D. Chitosan coating of red kiwifruit (Actinidia melanandra) for extending of the shelf life. Int. J. Biol. Macromol. 2016;85:355–360. doi: 10.1016/j.ijbiomac.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 193.Ciurzyńska A., Cieśluk P., Barwińska M., Marczak W., Ordyniak A., Lenart A., Janowicz M. Eating Habits and Sustainable Food Production in the Development of Innovative “Healthy” Snacks. Sustainability. 2019;11:2800. doi: 10.3390/su11102800. [DOI] [Google Scholar]
- 194.Pitkänen L., Heinonen M., Mikkonen K.S. Safety considerations of plant polysaccharides for food use: A case study on phenolic-rich softwood galactoglucomannan extract. Food Funct. 2018;9:1931–1943. doi: 10.1039/C7FO01425B. [DOI] [PubMed] [Google Scholar]
- 195.Picó Y. Chapter 9—Safety Assessment and Migration Tests. In: Cerqueira M.Â.P.R., Lagaron J.M., Pastrana Castro L.M., de Oliveira Soares Vicente A.A.M., editors. Nanomaterials for Food Packaging. Elsevier; Amsterdam, The Netherlands: 2018. [DOI] [Google Scholar]
- 196.Barreto M.R., Aleixo N.A., Silvestre R.B., Fregonezi N.F., Barud H.d.S., Dias D.d.S., Ribeiro C.A., Resende F.A. Genotoxicological safety assessment of puree-only edible films from onion bulb (Allium cepa L.) for use in food packaging-related applications. J. Food Sci. 2020;85:201–208. doi: 10.1111/1750-3841.14977. [DOI] [PubMed] [Google Scholar]
- 197.Sohrabi Y., Mohammadzadeh-Aghdash H., Baghbani E., Dehghan P., Ezzati Nazhad Dolatabadi J. Cytotoxicity and Genotoxicity Assessment of Ascorbyl Palmitate (AP) Food Additive Adv. Pharm. Bull. 2018;8:341–346. doi: 10.15171/apb.2018.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zheng X., Zhou Y., Yang L. Safty evaluation of hydroxypropylated-phosphorylated modified glutinous rice starch in KM mice. Food Sci. Technol. 2017;42:229–233. [Google Scholar]
- 199.Al-Asmar A., Naviglio D., Giosafatto C.V.L., Mariniello L. Hydrocolloid-Based Coatings are Effective at Reducing Acrylamide and Oil Content of French Fries. Coatings. 2018;8:147. doi: 10.3390/coatings8040147. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.