Abstract
Practical relevance:
Open wounds and their treatment present a common challenge in veterinary practice. Approaching 15 years ago negative pressure wound therapy (NPWT) started to be incorporated into clinical veterinary medicine, and its availability is becoming more widespread in Europe and the USA. Use of this therapy has the potential to significantly increase the healing rate of open wounds as well as free skin grafts in small animals, and it has been occasionally described for the management of feline wounds.
Aim:
This review describes the mechanisms of action of, and indications for, NPWT, and offers recommendations for NPWT specific to feline patients.
Evidence base:
The information presented is based on the current evidence and the author’s clinical experience of the technique gained over the past 12 years. Comparative studies of different treatment options are lacking and, since wound healing in cats and dogs differs, cat-specific studies are especially needed. Well-designed wound healing studies comparing different advanced techniques will improve open wound healing in cats in the future, and potentially allow better understanding of the role of NPWT in this setting.
Keywords: Open wound treatment, cat wound healing, skin grafts, negative pressure wound therapy
Introduction
Treatment of wounds is a common activity in clinical veterinary practice. Although the majority of wounds are minor, and can be closed after initial treatment (ie, healing by primary intention), more challenging cases require appropriate open wound therapy for successful management.1–3 Modern veterinary open wound treatment encompasses a broad range of indications, including extensive acute trauma and polytrauma, chronic non-healing wounds, burns and surgical site or wound-associated infections.2,4–10 Unfortunately, there is currently no study available that reports the most common causes, or the incidence, of wounds necessitating open wound treatment in cats. In the author’s experience, cats requiring open wound treatment are commonly polytrauma patients. There may be a history of high velocity trauma (eg, road traffic accident) causing degloving injuries, open fractures and crush/tear injuries. Other cases involve blunt trauma leading to fat tissue necrosis; high-rise syndrome associated with open fractures and impalement injuries; or bite wounds, resulting in crush/shearing injuries. Infection (either secondary wound infection after minor injury/bite or surgical site infection) is another common indication for open wound treatment in the author’s experience. Complications such as multidrug-resistant wound (or systemic) infections with pathogens of the so-called ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) are frequent, especially in patients pretreated with antibiotics.7,9–11
Effective open wound management options no longer involve simply covering a wound until the body has healed of its own accord. Rather, they should actively promote the healing process and improve immune function to prevent – or treat – local or systemic infection.4,12–15
Basics of wound healing and special considerations in cats
Wound healing can be divided into three phases: inflammation, proliferation and maturation.15–17 Although these healing phases apply to all wounds, they are especially relevant to consider in open wound management, as treatment decisions can actively support or suppress them. 3 For example, sufficient proliferation cannot happen if the inflammatory phase is not completed,3,4,13 and prolonged subinflammatory states with poor peaks of inflammation at the beginning of healing can be linked to poor granulation tissue formation and healing.18,19 The phase of proliferation starts with neovascularisation, followed by proliferation of fibroblasts and formation of myofibroblasts, collagen synthesis and subsequently epithelialisation and contracture.15,20,21 Contraction and epithelialisation happen simultaneously and to different extents depending on wound tension and location. Trunk wounds in cats, for example, heal to a large degree by contraction, but this effect is less important than epithelialisation at the distal extremities.9,22,23 Finally, the scar matures over several years.3,4,13
While the fundamental patterns and phases of healing are comparable in most species, there are major differences in the overall healing capacity, speed and the proportion of contraction and epithelialisation seen in individual species. 22 This is of importance, especially when evaluating the clinical benefits of treatments, as different species might need different support of the species-specific weak points during healing. 22 Unfortunately, studies establishing the precise mediators and progression of cellular events in wound healing in cats are not available. Bohling et al demonstrated that primary wound healing in cats differs substantially from that in dogs.24–26 While the inflammatory response in clean wounds in dogs is rapid and robust, inflammation in cats is weak. 22 As a result, cats not only build up less granulation tissue, but when formed it is poorly vascularised and more fibrotic compared with that of dogs.24–26 In addition, healing in cats is relatively impaired in terms of rates of contraction (18% after 7 days in cats vs 41% in dogs) and epithelialisation (13% after day 14 in cats vs 44% in dogs).24–26 Finally, while resection of subcutaneous tissue slows healing in both species, this effect is much more pronounced in cats. 26
These various factors have an important bearing on wound treatment techniques: it is not valid to assume that a technique that has proved superior in rats or pigs will also perform well in a cat. 22 Given that veterinarians treat wounds commonly, this reveals a tremendous gap in the knowledge available to inform optimal decision-making in feline open wound management, as studies performed in the target species are lacking. Interestingly, among the few papers published on open wound healing in cats, negative pressure wound therapy (NPWT) is one of the most frequently mentioned techniques (Table 1).7,27–34
Table 1.
Overview of the current literature reporting on open wound management in cats
| Study | Publication type | Indication(s) for open wound management | Wound healing product(s)/ technique | Outcome |
|---|---|---|---|---|
| Gemignani et al (2017) 27 | Case report | Unknown, contaminated wound | Platelet-rich plasma and wet-to-dry bandage | Wound healed in 20 days |
| Nolff et al (2017) 7 | Retrospective match-controlled clinical study
(n = 20) |
Trauma, infection (SSI), fat tissue necrosis | NPWT vs polymer foam | Significantly faster closure under NPWT (mean 25.8 days [range 11–57]) than under foam (mean 39.5 days [range 28–75]) |
| Tsioli et al (2016) 28 | Experimental controlled
study (n = 10) |
Not applicable | Hydrocolloid vs semiocclusive pad | No differences in planimetry; more oedema with hydrocolloid |
| Nolff and Meyer-Lindenberg (2015) 29 | Case series (n = 6) | Trauma | NPWT followed by polyurethane foam and NPWT-augmented skin grafting | Mean duration of open wound management was 21 days (range 3–43). Mean graft take rate was 97% (range 80–100%; 100% in 7/10 grafts) |
| Nolff and Meyer-Lindenberg (2015) 30 | Case report | Necrotising fasciitis | NPWT | Wound grafted after 29 days; 100% graft take |
| Jordan et al (2012) 31 | Case report | Peristomal urine-induced tissue necrosis | Polymer foam followed by NPWT | Vancomycin-resistant Enterococcus faecalis infection under open therapy with foam for 5 days. Switched to NPWT for 9 days. Wound closed with flank skin fold flap |
| Owen et al (2009) 32 | Case report | Urine-induced skin and muscle necrosis | NPWT | 40.3% wound contraction and granulation after 8 days. Wound closed with flap |
| Guille et al (2007) 33 | Case report | Traumatic wound (RTA) | NPWT | Wound successfully grafted (NPWT assisted) 40 days after trauma |
| Siegfried et al (2004) 34 | Case series (n = 5) | Four traumatic wounds (degloving), one open treatment after sarcoma resection | Wet-to-dry bandages followed by skin grafts | Mean duration of open wound management until stable granulation achieved was 14 days (range 7–21). Mean graft take rate was 94% (range 90–100%) |
NPWT = negative pressure wound therapy; SSI = surgical site infection; RTA = road traffic accident
The presence of dressings and requirement for frequent dressing changes can cause substantial stress for feline patients, and this is an important consideration when choosing the right treatment regimen. Dressing changes should be performed in a quiet environment and with the least restraint possible. In cats with severe wounds, sedation and adequate procedural analgesia are mandatory for potentially painful dressing changes. Treatment options that require less frequent dressing changes owing to improved exudate management should be favoured over standard bandages that require daily or even more frequent changes.
General principles of open wound treatment
The three principal pillars of open wound treatment are debridement, lavage and dressing.4,12,17
Debridement
Debridement is needed to remove all necrotic tissue from the wound, thus helping the body to transition efficiently from the inflammatory phase of wound healing to the proliferative phase.13,15,35,36 If debridement is not carried out, or is performed poorly, necrotic tissue keeps the wound in an inflammatory state and provides a surface for biofilm formation. 17
Lavage
Lavage is the process of decreasing the bioburden and cleaning the wound. Different lavage solutions are available and, once again, the evidence for ‘which to use in which indication’ in the veterinary literature is sparse,37,38 and also somewhat contentious in the light of recommendations in human medicine. 39 In general, and based on current human guidelines, saline or lactated Ringer’s solution can be used in all settings (even when pockets are present or joints, body cavities or nerves might be involved),17,40 as long as the wound is not infected (ie, for contaminated or colonised wounds [Table 2]).
Table 2.
Definition of wound bacterial status and recommendations for treatment
| Bacterial status | Treatment recommendations | |
|---|---|---|
| Contamination | Bacteria sit on the wound without causing harm | Debridement; potentially lavage |
| Colonisation | Bacteria replicate in the wound; no effect on healing | Debridement; potentially lavage |
| Critical contamination | Bacteria replicate in the wound; impaired healing | Debridement, lavage, antiseptics |
| Infection | Bacteria cause a local or systemic infection | Debridement, antiseptics ± antibiotics |
| Biofilm | Default mode of growth, probably present in chronic wounds | Debridement; no other options for effective treatment |
Based on recommendations by Kramer et al 39
Where there is local infection, wound antiseptics may be warranted.39,40 In veterinary medicine, use of chlorhexidine 0.05% has traditionally been advocated, based on two experimental studies dating back to 1988 (Sanchez et al 37 [iodine vs chlorhexidine, n = 6 dogs]) and 1992 (Lozier et al 38 [chlorhexidine vs lactated Ringer’s solution, n = 6 dogs]). Since then, several new wound antiseptics have been released and are now recommended in human medicine due to improved performance and fewer issues with resistance formation compared with chlorhexidine.39,41 However, investigations of these new compounds in veterinary medicine are rare. A recent blinded clinically controlled study of dog bite wounds underlines the powerful effect of saline alone, but also demonstrates the superior performance of polyhexanide compared with saline; 40 polyhexanide is a biguanide (part of the same pharmaceutical family as chlorhexidine), and has shown promising performance as a wound antiseptic.39,42,43 Feline studies and/or comparative studies in dogs or cats comparing chlorhexidine and new wound antiseptics are lacking, and should be a focus of future research in order to evaluate if current recommendations regarding chlorhexidine are still valid. The author’s preference is polyhexanide biguanide based on the evidence in human medicine.
Choice of dressing
The job of the dressing is to impart oncotic and thermal stability and keep the wound moist, while creating an environment that supports the cellular events of healing.12–15,17 This is mainly achieved by using ‘interactive’ wound dressings, which alter the wound environment and interact with the wound surface. A study in dogs was able to demonstrate that minor wounds healed faster under interactive dressings (polymer foam) than when passive dressings (silicone gauze and bandage) were used. 10 There is a plethora of modern wound dressings available, but unfortunately there is insufficient data on the application of these in dogs and cats to make evidence-based recommendations.12,17 This becomes even more problematic given what little is known about the specifics of feline open wound care.
So far, only two studies are available that have investigated the effect of different dressings in cats. In a controlled experimental study in 10 cats, Tsioli et al compared healing of 2 x 2 cm wounds on the dorsum under hydrocolloid or semiocclusive cotton-polyester pads. 28 These investigators were not able to demonstrate differences in healing rate; however, wounds treated with hydrocolloid showed more oedema than control wounds. 28 In a retrospective clinical study comparing NPWT (n = 10 cats) and polyurethane foam dressings (n = 10 cats) for open wound management, the author’s own group was able to document that wounds treated with NPWT had a significantly shorter time to closure than wounds managed with an interactive dressing. 7 Besides these two studies, all other published information on open wound care in cats consists of case reports or small non-controlled case series.27,29–34
Owing to the lack of evidence, the selection of an ‘appropriate’ wound dressing is often based on personal preference and historical choices, rather than on evidence of effectiveness in the species in question. Nevertheless, among the many different treatment options, NPWT (an active wound dressing system) has proven especially interesting in treating complex wounds. Despite this form of therapy having until recently only been available in selected veterinary centres in Europe and the USA, there are now more experimental and clinical studies available regarding its efficacy in dogs than for any other open wound management technique.9,10,44–47 In cats, there is only one clinical study to date, 7 but seven out of the nine available publications on wound care in cats, including case reports and case series, have involved NPWT.7,27–34
The following sections of this review describe the technique, as well as the mode of action of NPWT, and seek to highlight indications for NPWT in open wound management in cats.
NPWT for open wound management
The main indications for NPWT in people, dogs and also cats are acute traumatic, chronic or infected wounds requiring open wound treatment. The system is especially effective with regard to infection control and for wound bed preparation prior to reconstruction of large defects.7,9,10,48–50 This is an important consideration, as surgical site infections or acute infected wounds causing local tissue damage and sepsis represent a challenging situation.
Mode of action
The mode of action of NPWT is not yet completely understood, but there are different proven effects (mostly in mice and pigs or ex vivo) that lead to improved wound healing. The first (simple) effect is that the wound is protected against thermal and oncotic fluctuations (thus preventing fluid evaporation and scab formation) and is kept moist.51,52
A more specific effect of the vacuum is that three-dimensional contraction of the wound is stimulated, thereby bringing the wound edges together, while antagonising the physiological mechanisms leading to skin retraction (macrodeformation).50,53 This contraction effect has been demonstrated in studies in pigs, as well as in dogs, and substantially aids secondary closure after NPWT in dogs.9,53 However, it is strongly dependent on wound geometry. While NPWT greatly increases contraction in deep, three-dimensional wounds, such as those involving the trunk (where the foam and vacuum are centred deep within the wound), this effect is not seen in superficial skin wounds at the extremities or very flat wounds with just skin loss (where the foam and vacuum are placed on top of the wound). 9
In addition, the vacuum decreases interstitial pressure, leading to decreased wound oedema and improved transport of exudate away from the wound.50,52–55 Owing to the interstitial pressure gradient, a mechanical deformation of the fibroblasts and collagen network occurs, inducing ion flux.50,52,53,55,56 The cells at the tissue/foam border undergo microdeformation, leading to mechanical stress (5–20% mechanical stress) that triggers ion flux mechanotransduction and cell stimulation. 57 Recent studies in pigs and dogs have even shown that NPWT modulates mediators of wound healing, increasing the concentration of cytokines (interleukin [IL]-10, IL-6, IL-8, vascular endothelial growth factor [VEGF] and fibroblast growth factor [FGF]-2)47,58,59 and decreasing the matrix-metalloproteinase (MMP) concentration (MMP-9, MMP-1 and MMP-13).60,61 The net effect of these processes is inhibition of apoptosis, increased intra-cellular signal transduction, changes in gene expression and, ultimately, increased cell proliferation and granulation. 57 Depending on the wound, species and study design, increases in granulation rate of between 60% and 200% have been documented.58,62 Finally, NPWT increases tissue perfusion and neovascularisation, and speeds up tissue organisation and maturation within the wound.53,63,64
Technique
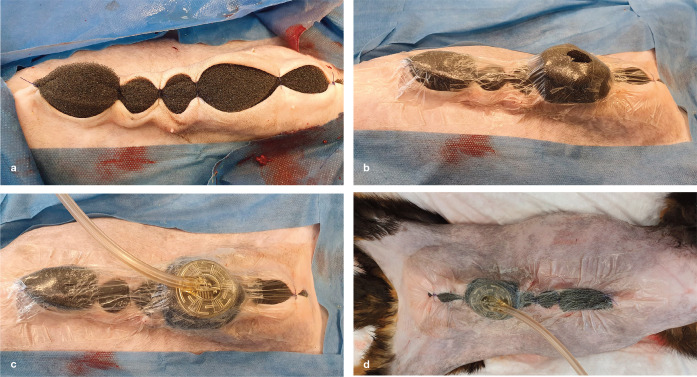
Before application of the NPWT dressing (Figure 1), the wound needs to be debrided and lavaged. The area around the wound is generously clipped (wet shaving is not recommended due to risk of skin trauma), and the skin surrounding the wound is completely dried. Skin protective adhesives can be used to help fix the system in place (eg, Cavilon Skin Protective [3M] or Opsite [Smith and Nephew]). For challenging areas, the use of stoma rings or paste applied around the wound edges on the skin (eg, Brava series [Coloplast]) can help to seal the dressing. A piece of foam is cut to match the size of the defect and placed within the wound (avoiding overlap with the skin). It is important to ensure good contact between the foam and all areas of the wound. Finally, the foam is secured to the wound by applying NPWT foil. This is best achieved by using small overlapping pieces of the sticky foil, rather than trying to use one big piece. Careful application is needed to ensure an airtight seal. A hole is cut into the foil centrally over the foam, and the pressure transducer is applied. Once the pressure transducer has been connected to the vacuum device, therapy is started. Figure 2 shows application of an NPWT dressing in a cat undergoing open wound treatment.
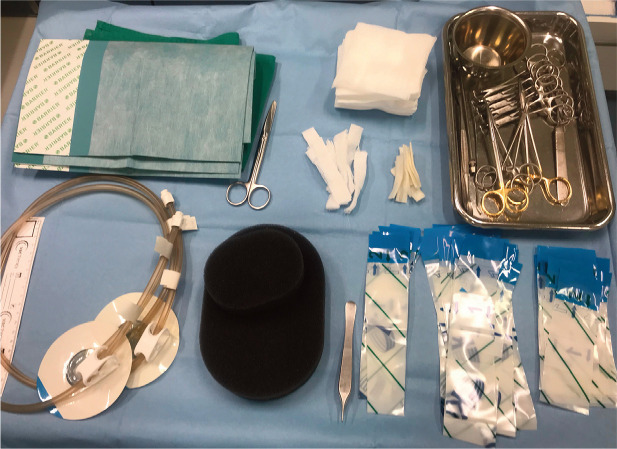
Figure 1.
The negative pressure wound therapy system most frequently used by the author consists of the so-called TRAC pad (for connection of the dressing to the machine, bottom left), a grey polyurethane foam (bottom middle) and adhesive foils (bottom right) to seal the wounds
Figure 2.
(a) Grey foam (VAC Granufoam Dressing; KCI-3M) is secured in place in the wound. When dealing with cavitating lesions, it is important to ensure that the foam has good contact with all areas of the wound cavity. (b) The next step is application of the negative pressure wound therapy foil and incision to allow (c) placement of the TRAC pad (VAC Sensa TRAC; KCI-3M). (d) After connecting the device, a vacuum is established at a setting of −125 mmHg
A pressure setting of −125 mmHg has been demonstrated to be ideal for achieving optimal tissue perfusion and granulation,57,62,64 and the author recommends continuous mode therapy, as this is better tolerated by most patients than intermittent mode therapy. It is essential that the therapy is not stopped for longer than 2 h a day. If the device is not properly working, bacteria within the wound are granted optimal conditions to proliferate, resulting in wound infection and tissue maceration. At the author’s facility, NPWT is performed in-house for the initial one or two dressing changes. If the dressing is well tolerated and no problems occur, the treatment is then offered on an out-patient basis in stable patients. This significantly reduces the cost of treatment, and also reduces the stress associated with a hospital stay. Dressing changes are performed every 3–4 days under sedation or general anaesthesia, depending on the size of the defect.
NPWT for skin graft augmentation
One of the earliest applications of NPWT in human medicine, besides open wound care, was skin graft augmentation; as recorded in a literature search by Azzopardi et al, 65 this dates back 23 years. Indeed, in the first published case of NPWT usage in a cat, in 2007, the technique was applied to a grafted wound. 33 NPWT facilitates graft fixation and encourages graft ‘take’.65–68 This effect becomes especially important for complex three-dimensional wounds that generally do not allow secure graft fixation by any other method. Increased graft take rates under NPWT compared with controls (standard bolster dressing) have been documented for humans65–68 and dogs. 46
Mode of action
The mechanisms responsible for improved graft healing under NPWT can be divided into two broad categories: 65
✜ Active stimulation of cellular proliferation, revascularisation and microcirculation. A demonstration of these effects was provided by Saaiq et al’s 2010 study, which showed that pretreatment of the recipient bed using NPWT increased graft take in human patients. 69
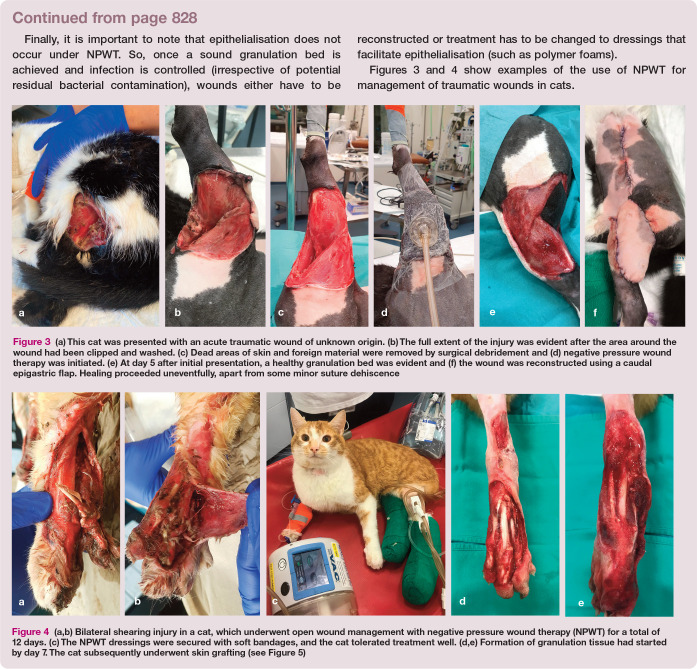
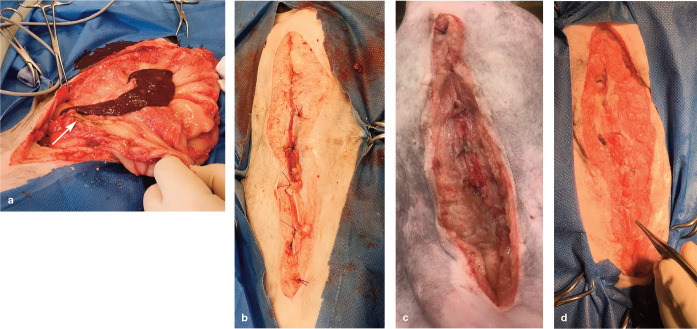
Figure 3.
(a) This cat was presented with an acute traumatic wound of unknown origin. (b) The full extent of the injury was evident after the area around the wound had been clipped and washed. (c) Dead areas of skin and foreign material were removed by surgical debridement and (d) negative pressure wound therapy was initiated. (e) At day 5 after initial presentation, a healthy granulation bed was evident and (f) the wound was reconstructed using a caudal epigastric flap. Healing proceeded uneventfully, apart from some minor suture dehiscence
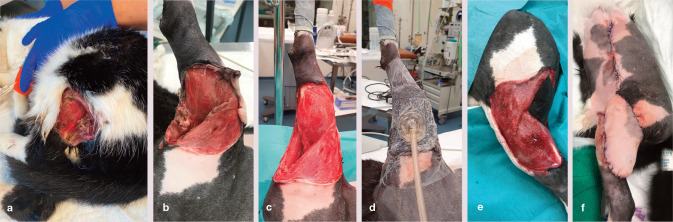
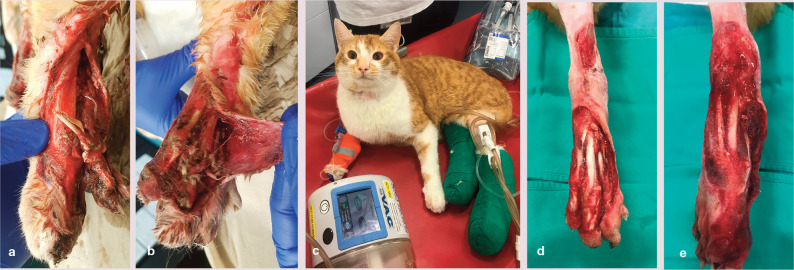
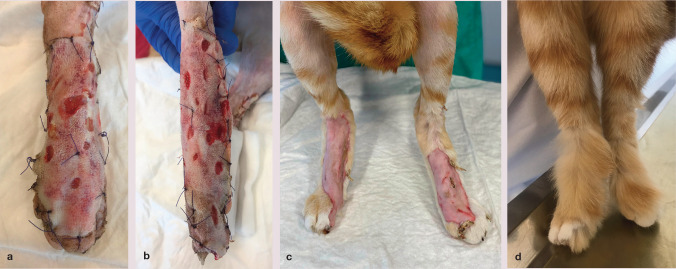
Figure 4.
(a,b) Bilateral shearing injury in a cat, which underwent open wound management with negative pressure wound therapy (NPWT) for a total of 12 days. (c) The NPWT dressings were secured with soft bandages, and the cat tolerated treatment well. (d,e) Formation of granulation tissue had started by day 7. The cat subsequently underwent skin grafting (see Figure 5)
✜ Prevention of complications, such as graft lift-off and fluid accumulation beneath the grafted skin, and neutralisation of shear forces. In contrast to conventional dressings, NPWT allows uniform pressure distribution across the entire graft surface, and thus is effective at neutralising shear forces.65–68
NPWT-augmented skin transplants exhibit improved healing, based on histological evaluation, as early as 3 days after grafting, when compared with controls (standard bolster dressing). 70 Becker et al reported faster revascularisation and improved take under NPWT, although the researchers also found that oxygen tension within NPWT grafts was inferior to controls during the first 3 days. 67
Technique
After skin transplantation is completed, the graft is covered with a protective, non-adhesive silicone dressing. 17 Note that NPWT foam should never be placed in direct contact with the skin, since this can cause skin maceration and risk graft lift-off during dressing changes. The pre-cut foam is placed upon non-adhesive gauze and secured as described earlier for standard NPWT dressings. Both grey (polyurethane) and white (polyvinyl alcohol) foam can be used for graft augmentation, although the author favours grey foam, owing to its greater flexibility, at a pressure setting of −125 mmHg. Some difference of opinion exists regarding the optimal pressure for skin graft augmentation. Stanley et al worked with a pressure of −65 mmHg, which was further reduced to −45 mmHg, in their canine study. 46 This was based on the manufacturer’s advice, but the group no longer works with these settings (Bj Stanley, personal communication). In human medicine, the advocated pressure setting ranges between −100 and −125 mmHg, based on studies investigating the effects of NPWT on graft take,65,66,68,70 and this is also the standard range of settings for skin graft augmentation in the author’s institution.29,30
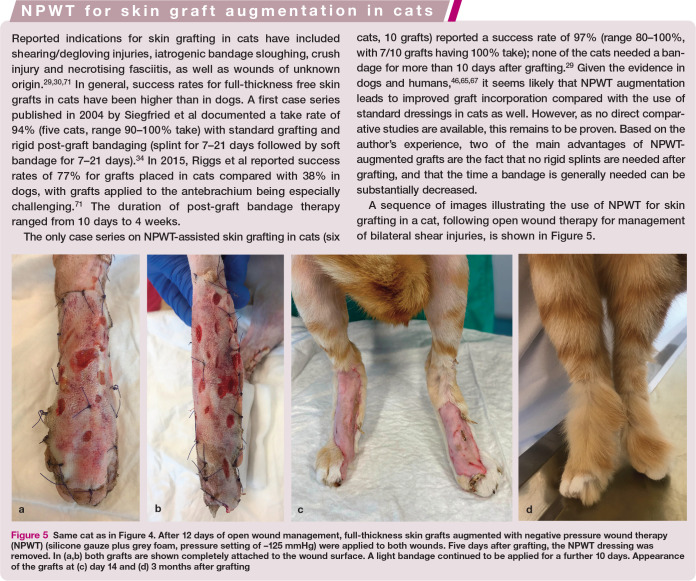
Figure 5.
Same cat as in Figure 4. After 12 days of open wound management, full-thickness skin grafts augmented with negative pressure wound therapy (NPWT) (silicone gauze plus grey foam, pressure setting of −125 mmHg) were applied to both wounds. Five days after grafting, the NPWT dressing was removed. In (a,b) both grafts are shown completely attached to the wound surface. A light bandage continued to be applied for a further 10 days. Appearance of the grafts at (c) day 14 and (d) 3 months after grafting
The dressing is kept in place for 3 days. Dressing changes are performed under mild sedation, and therapy is discontinued once granulation of the graft incisions has reached the skin level (usually after 3 days). If the graft is not securely integrated after 3 days, a further 3 days of NPWT is carried out. The graft is then covered with a silicone gauze or polymer foam dressing for another 7 days, after which no more bandaging is needed. The author does not use splints or rigid bandaging, unless the orthopaedic situation requires it.
NPWT for treatment of septic peritonitis
Another potential application of NPWT is in the treatment of septic peritonitis. In the human medical field, use of negative pressure has greatly reduced morbidity and mortality in patients managed with an open abdomen, 25 and an initial veterinary case series has documented the use of NPWT to manage open abdomens in dogs and cats as well. 72 Although the precise mode of action has yet to be determined, NPWT appears to be safe in these patients, and improves management of exudate, while keeping patients mobile, dry and comfortable. The device can either be placed directly within the abdominal cavity (a special foil-coated abdominal foam is needed in this case), or on top of a partially closed coeliotomy, as reported by Cioffi et al. 72
The author has limited experience of using NPWT in feline patients with septic abdomen (three cases to date). The technique described by Cioffi et al 72 proved effective in evacuating the abdominal cavity and led to a rapid recovery in all three cats (Figure 6). Although this application is very interesting, future studies are needed to evaluate if a real benefit can be expected in cats with septic peritonitis.
Figure 6.
(a) Septic abdomen in a cat owing to a migrating grass awn (arrow). After thorough lavage, the coeliotomy was closed with four interrupted sutures placed approximately 3 cm apart (b). The negative pressure wound therapy dressing was placed as illustrated in Figure 2. In this case, the dressing was changed the next day to allow repeated lavage of the abdominal cavity (c) and a second time at day 4 (d), at which point the wound was closed
Key Points
Although the majority of wounds are minor, and can be closed after initial treatment, more challenging cases require appropriate open wound therapy for successful management. There is a considerable gap, however, in existing knowledge of open wound care in cats.
While there are few papers published on open wound healing in cats, NPWT is one of the most frequently mentioned techniques, and its application for open wound treatment, as well as graft augmentation and indications such as septic peritonitis, seems promising.
Further controlled studies are required to investigate the full potential of NPWT in feline patients.
Footnotes
The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author received no financial support for the research, authorship and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
- 1. Balsa IM, Culp WT. Wound care. Vet Clin North Am Small Anim Pract 2015; 45: 1049–1065. [DOI] [PubMed] [Google Scholar]
- 2. Corr S. Intensive, extensive, expensive. Management of distal limb shearing injuries in cats. J Feline Med Surg 2009; 11: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nolff MC. Wundmanagement. In: Modernes Wundmanagement bei Hund und Katze. Stuttgart: Thieme, 2020, pp 86–113. [Google Scholar]
- 4. Pavletic MM. Atlas of small animal wound management and reconstructive surgery. Hoboken, NJ: Wiley, 2018. [Google Scholar]
- 5. Davidson JR. Current concepts in wound management and wound healing products. Vet Clin North Am Small Anim Pract 2015; 45: 537–564. [DOI] [PubMed] [Google Scholar]
- 6. Lascelles BD, Davison L, Dunning M, et al. Use of omental pedicle grafts in the management of non-healing axillary wounds in 10 cats. J Small Anim Pract 1998; 39: 475–80. [DOI] [PubMed] [Google Scholar]
- 7. Nolff MC, Fehr M, Reese S, et al. Retrospective comparison of negative pressure wound therapy and silver-coated foam dressings in open-wound treatment in cats. J Feline Med Surg 2017; 19: 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pope ER. Head and facial wounds in dogs and cats. Vet Clin North Am Small Anim Pract 2006; 36: 793–817. [DOI] [PubMed] [Google Scholar]
- 9. Nolff MC, Albert R, Reese S, et al. Comparison of negative pressure wound therapy and silver-coated foam dressings in open wound treatment in dogs: a prospective controlled clinical trial. Vet Comp Orthop Traumatol 2018; 31: 229–238. [DOI] [PubMed] [Google Scholar]
- 10. Nolff MC, Fehr M, Bolling A, et al. Negative pressure wound therapy, silver coated foam dressing and conventional bandages in open wound treatment in dogs. A retrospective comparison of 50 paired cases. Vet Comp Orthop Traumatol 2015; 28: 30–38. [DOI] [PubMed] [Google Scholar]
- 11. Nolff MC, Reese S, Fehr M, et al. Assessment of wound bio-burden and prevalence of multi-drug resistant bacteria during open wound management. J Small Anim Pract 2016; 57: 255–259. [DOI] [PubMed] [Google Scholar]
- 12. Fahie MA, Shettko D. Evidence-based wound management: a systematic review of therapeutic agents to enhance granulation and epithelialization. Vet Clin North Am Small Anim Pract 2007; 37: 559–577. [DOI] [PubMed] [Google Scholar]
- 13. Pitzer GB, Patel KG. Proper care of early wounds to optimize healing and prevent complications. Facial Plast Surg Clin North Am 2011; 19: 491–504. [DOI] [PubMed] [Google Scholar]
- 14. Bolton LL, Monte K, Pirone LA. Moisture and healing: beyond the jargon. Ostomy Wound Manage 2000; 46 1A Suppl: 51S-62S; quiz 3S-4S. [PubMed] [Google Scholar]
- 15. Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature 2008; 453: 314–321. [DOI] [PubMed] [Google Scholar]
- 16. Pavletic MM, Trout NJ. Bullet, bite, and burn wounds in dogs and cats. Vet Clin North Am Small Anim Pract 2006; 36: 873–893. [DOI] [PubMed] [Google Scholar]
- 17. Nolff MC. Unterdrucktherapie. In: Modernes Wundmanagement bei Hund und Katze. Stuttgart: Thieme, 2020, pp 131–140. [Google Scholar]
- 18. Theoret CL, Wilmink JM. Aberrant wound healing in the horse: naturally occurring conditions reminiscent of those observed in man. Wound Repair Regen 2013; 21: 365–371. [DOI] [PubMed] [Google Scholar]
- 19. Wilmink JM, van Weeren PR. Second-intention repair in the horse and pony and management of exuberant granulation tissue. Vet Clin North Am Equine Pract 2005; 21: 15–32. [DOI] [PubMed] [Google Scholar]
- 20. Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability 2011; 20: 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong VW, Akaishi S, Longaker MT, et al. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol 2011; 131: 2186–2196. [DOI] [PubMed] [Google Scholar]
- 22. Volk SW, Bohling MW. Comparative wound healing – are the small animal veterinarian’s clinical patients an improved translational model for human wound healing research? Wound Repair Regen 2013; 21: 372–381. [DOI] [PubMed] [Google Scholar]
- 23. Wilmink JM, Nederbragt H, van Weeren PR, et al. Differences in wound contraction between horses and ponies: the in vitro contraction capacity of fibroblasts. Equine Vet J 2001; 33: 499–505. [DOI] [PubMed] [Google Scholar]
- 24. Bohling MW, Henderson RA. Differences in cutaneous wound healing between dogs and cats. Vet Clin North Am Small Anim Pract 2006; 36: 687–692. [DOI] [PubMed] [Google Scholar]
- 25. Bohling MW, Henderson RA, Swaim SF, et al. Cutaneous wound healing in the cat: a macroscopic description and comparison with cutaneous wound healing in the dog. Vet Surg 2004; 33: 579–587. [DOI] [PubMed] [Google Scholar]
- 26. Bohling MW, Henderson RA, Swaim SF, et al. Comparison of the role of the subcutaneous tissues in cutaneous wound healing in the dog and cat. Vet Surg 2006; 35: 3–14. [DOI] [PubMed] [Google Scholar]
- 27. Gemignani F, Perazzi A, Iacopetti I. Use of canine sourced platelet-rich plasma in a feline contaminated cutaneous wound. Canadian Vet J 2017; 58: 141–144. [PMC free article] [PubMed] [Google Scholar]
- 28. Tsioli V, Gouletsou PG, Galatos AD, et al. Effects of two occlusive, hydrocolloid dressings on healing of full-thickness skin wounds in cats. Vet Comp Orthop Traumatol 2016; 29: 298–305. [DOI] [PubMed] [Google Scholar]
- 29. Nolff MC, Meyer-Lindenberg A. Negative pressure wound therapy augmented full-thickness free skin grafting in the cat: outcome in 10 grafts transferred to six cats. J Feline Med Surg 2015; 17: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nolff MC, Meyer-Lindenberg A. Necrotising fasciitis in a domestic shorthair cat – negative pressure wound therapy assisted debridement and reconstruction. J Small Anim Pract 2015; 56: 281–284. [DOI] [PubMed] [Google Scholar]
- 31. Jordan CJ, Kulendra E, Perry KL, et al. Management of peristomal tissue necrosis following prepubic urethrostomy in a cat. Vet Comp Orthop Traumatol 2012; 25: 433–437. [DOI] [PubMed] [Google Scholar]
- 32. Owen L, Hotston-Moore A, Holt P. Vacuum-assisted wound closure following urine-induced skin and thigh muscle necrosis in a cat. Vet Comp Orthop Traumatol 2009; 22: 417–421. [DOI] [PubMed] [Google Scholar]
- 33. Guille AE, Tseng LW, Orsher RJ. Use of vacuum-assisted closure for management of a large skin wound in a cat. J Am Vet Med Assoc 2007; 230: 1669–1673. [DOI] [PubMed] [Google Scholar]
- 34. Siegfried R, Schmokel H, Rytz U, et al. Treatment of large distal extremity skin wounds with autogenous full-thickness mesh skin grafts in 5 cats. Schweiz Archiv Tierheilkd 2004; 146: 277–283. [DOI] [PubMed] [Google Scholar]
- 35. Granick M, Boykin J, Gamelli R, et al. Toward a common language: surgical wound bed preparation and debridement. Wound Repair Regen 2006; 14 Suppl 1: S1–S10. [DOI] [PubMed] [Google Scholar]
- 36. Granick MS, Tran BNN, Alvarez OM. Latest advances in wound debridement techniques. Surg Technol Int 2020; 36: 37–40. [PubMed] [Google Scholar]
- 37. Sanchez IR, Swaim SF, Nusbaum KE, et al. Effects of chlorhexidine diacetate and povidone-iodine on wound healing in dogs. Vet Surg 1988; 17: 291–295. [DOI] [PubMed] [Google Scholar]
- 38. Lozier S, Pope E, Berg J. Effects of four preparations of 0.05% chlorhexidine diacetate on wound healing in dogs. Vet Surg 1992; 21: 107–112. [DOI] [PubMed] [Google Scholar]
- 39. Kramer A, Dissemond J, Kim S, et al. Consensus on wound antisepsis: update 2018. Skin Pharmacol Physiol 2018; 31: 28–58. [DOI] [PubMed] [Google Scholar]
- 40. Nolff MC, Winter S, Reese S, et al. Comparison of poly-hexanide, cold atmospheric plasma and saline in the treatment of canine bite wounds. J Small Anim Pract 2019; 60: 348–355. [DOI] [PubMed] [Google Scholar]
- 41. Bhardwaj P, Ziegler E, Palmer KL. Chlorhexidine induces vana-type vancomycin resistance genes in enterococci. Antimicrob Agents Chemother 2016; 60: 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winter S, Meyer-Lindenberg A, Wolf G, et al. In vitro evaluation of the decontamination effect of cold atmospheric argon plasma on selected bacteria frequently encountered in small animal bite injuries. J Microbiol Methods 2020; 169: 105728. DOI: 10.1016/j.mimet.2019.105728. [DOI] [PubMed] [Google Scholar]
- 43. Chindera K, Mahato M, Sharma AK, et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci Rep 2016; 6: 23121. DOI: 10.1038/srep23121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demaria M, Stanley BJ, Hauptman JG, et al. Effects of negative pressure wound therapy on healing of open wounds in dogs. Vet Surg 2011; 40: 658–669. [DOI] [PubMed] [Google Scholar]
- 45. Coutin JV, Lanz OI, Magnin-Bissel GC, et al. Cefazolin concentration in surgically created wounds treated with negative pressure wound therapy compared to surgically created wounds treated with nonadherent wound dressings. Vet Surg 2015; 44: 9–16. [DOI] [PubMed] [Google Scholar]
- 46. Stanley BJ, Pitt KA, Weder CD, et al. Effects of negative pressure wound therapy on healing of free full-thickness skin grafts in dogs. Vet Surg 2013; 42: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nolff M, Albert R, Wohlsein P, et al. Histomorphometric evaluation of MMP-9 and CD31 expression during healing under negative pressure wound therapy in dogs. Schweiz Arch Tierheilkd 2018; 160: 525–532. [DOI] [PubMed] [Google Scholar]
- 48. Liu D, Zhang L, Li T, et al. Negative-pressure wound therapy enhances local inflammatory responses in acute infected soft-tissue wound. Cell Biochem Biophys 2014; 70: 539–547. [DOI] [PubMed] [Google Scholar]
- 49. Ma Z, Shou K, Li Z, et al. Negative pressure wound therapy promotes vessel destabilization and maturation at various stages of wound healing and thus influences wound prognosis. Exp Ther Med 2016; 11: 1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lalezari S, Lee CJ, Borovikova AA, et al. Deconstructing negative pressure wound therapy. Int Wound J 2017; 14: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scherer SS, Pietramaggiori G, Mathews JC, et al. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg 2008; 122: 786–797. [DOI] [PubMed] [Google Scholar]
- 52. Thompson G. An overview of negative pressure wound therapy (NPWT). Br J Community Nurs 2008; 13: S23–S24, S6, S8–S30. [DOI] [PubMed] [Google Scholar]
- 53. Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997; 38: 553–562. [DOI] [PubMed] [Google Scholar]
- 54. Yang CC, Chang DS, Webb LX. Vacuum-assisted closure for fasciotomy wounds following compartment syndrome of the leg. J Surg Orthop Adv 2006; 15: 19–23. [PubMed] [Google Scholar]
- 55. Lessing MC, James RB, Ingram SC. Comparison of the effects of different negative pressure wound therapy modes – continuous, noncontinuous, and with instillation – on porcine excisional wounds. Eplasty 2013; 13: e51. [PMC free article] [PubMed] [Google Scholar]
- 56. Lu F, Ogawa R, Nguyen DT, et al. Microdeformation of three-dimensional cultured fibroblasts induces gene expression and morphological changes. Ann Plast Surg 2011; 66: 296–300. [DOI] [PubMed] [Google Scholar]
- 57. Saxena V, Hwang CW, Huang S, et al. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004; 114: 1086–1096; discussion 1097–1098. [DOI] [PubMed] [Google Scholar]
- 58. Jacobs S, Simhaee DA, Marsano A, et al. Efficacy and mechanisms of vacuum-assisted closure (VAC) therapy in promoting wound healing: a rodent model. J Plast Reconstr Aesth Surg 2009; 62: 1331–1338. [DOI] [PubMed] [Google Scholar]
- 59. Kilpadi DV, Bower CE, Reade CC, et al. Effect of vacuum assisted closure therapy on early systemic cytokine levels in a swine model. Wound Repair Regen 2006; 14: 210–215. [DOI] [PubMed] [Google Scholar]
- 60. Greene AK, Puder M, Roy R, et al. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006; 56: 418–422. [DOI] [PubMed] [Google Scholar]
- 61. Glass GE, Murphy GF, Esmaeili A, et al. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg 2014; 101: 1627–1636. [DOI] [PubMed] [Google Scholar]
- 62. Morykwas MJ, Faler BJ, Pearce DJ, et al. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plastic Surg 2001; 47: 547–551. [DOI] [PubMed] [Google Scholar]
- 63. Ichioka S, Watanabe H, Sekiya N, et al. A technique to visualize wound bed microcirculation and the acute effect of negative pressure. Wound Repair Regen 2008; 16: 460–465. [DOI] [PubMed] [Google Scholar]
- 64. Wackenfors A, Gustafsson R, Sjogren J, et al. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg 2005; 79: 1724–1730; discussion 1730–1731. [DOI] [PubMed] [Google Scholar]
- 65. Azzopardi EA, Boyce DE, Dickson WA, et al. Application of topical negative pressure (vacuum-assisted closure) to split-thickness skin grafts: a structured evidence-based review. Ann Plast Surg 2013; 70: 23–29. [DOI] [PubMed] [Google Scholar]
- 66. Blume PA, Key JJ, Thakor P, et al. Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: comparing VAC®) therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J 2010; 7: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Becker ST, Rennekampff HO, Alkatout I, et al. Comparison of vacuum and conventional wound dressings for full thickness skin grafts in the minipig model. Int J Oral Max Surg 2010; 39: 699–704. [DOI] [PubMed] [Google Scholar]
- 68. Llanos S, Danilla S, Barraza C, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg 2006; 244: 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saaiq M, Hameed Ud D, Khan MI, et al. Vacuum-assisted closure therapy as a pretreatment for split thickness skin grafts. J Coll Physicians Surg Pak 2010; 20: 675–679. [PubMed] [Google Scholar]
- 70. Kim EK, Hong JP. Efficacy of negative pressure therapy to enhance take of 1-stage allodermis and a split-thickness graft. Ann Plast Surg 2007; 58: 536–540. [DOI] [PubMed] [Google Scholar]
- 71. Riggs J, Jennings JL, Friend EJ, et al. Outcome of full-thickness skin grafts used to close skin defects involving the distal aspects of the limbs in cats and dogs: 52 cases (2005–2012). J Am Vet Med Assoc 2015; 247: 1042–1047. [DOI] [PubMed] [Google Scholar]
- 72. Cioffi KM, Schmiedt CW, Cornell KK, et al. Retrospective evaluation of vacuum-assisted peritoneal drainage for the treatment of septic peritonitis in dogs and cats: 8 cases (2003-2010). J Vet Emerg Crit Care 2012; 22: 601–609. [DOI] [PubMed] [Google Scholar]