Abstract
Background:
Previous studies reported that melatonin exerts its effect on mesenchymal stem cell (MSC) survival and differentiation into osteogenic and adipogenic lineage. In the current study we aimed to explore the effect of melatonin on osteoporosis and relevant mechanisms.
Methods:
Real-time qualitative polymerase chain reaction (RT-qPCR) and Western blot analysis were conducted to determine expression of HGF, PTEN, and osteoblast differentiation-related genes in ovariectomy (OVX)-induced osteoporosis mice and the isolated bone marrow MSCs (BMSCs). Pre-conditioning with melatonin (1 μmol/l, 10 μmol/l and 100 μmol/l) was carried out in OVX mice BMSCs. Bone microstructure was analyzed using micro-computed tomography and the contents of alkaline phosphatase (ALP) and tartrate-resistant acid phosphatase 5b (TRAP5b) were detected by enzyme-linked immunosorbent assay in serum. BMSC proliferation was measured by cell-counting kit (CCK)-8 assay. Alizarin red S (ARS) staining and ALP activity assay were performed to assess BMSC mineralization and calcification. The activity of the Wnt/β-catenin pathway was evaluated by dual-luciferase reporter assay.
Results:
Melatonin prevented bone loss in OVX mice. Melatonin increased ALP expression and reduced TRAP5b expression. HGF and β-catenin were downregulated, while PTEN was upregulated in the femur of OVX mice. Melatonin elevated HGF expression and then stimulated BMSC proliferation and osteogenic differentiation. Additionally, HGF diminished the expression of PTEN, resulting in activated Wnt/β-catenin pathway both in vitro and in vivo. Furthermore, melatonin was shown to ameliorate osteoporosis in OVX mice via the HGF/PTEN/Wnt/β-catenin axis.
Conclusion:
Melatonin could potentially enhance osteogenic differentiation of BMSCs and retard bone loss through the HGF/PTEN/Wnt/β-catenin axis.
Keywords: bone marrow mesenchymal stem cells, HGF, melatonin, osteoporosis, PTEN, Wnt/β-catenin
Introduction
Osteoporosis is a common systemic skeletal disorder characterized by low bone mass and microarchitectural deterioration in bone tissues, resulting in bone fragility and an enhanced fracture risk.1 Postmenopausal women are more likely than men to suffer from osteoporosis.2 Risk factors for the disease include age, glucocorticoid use, and decreased bone mineral density (BMD).3 The impairment of osteoblast differentiation and bone formation is also a cause of osteoporosis.4 Mesenchymal stem cells (MSCs) have shown crucial roles in the maintenance of the dynamic balance of bone metabolism.5 Bone marrow MSCs (BMSCs) are capable of differentiating into mainly osteoblasts and adipocytes in bone and fat formation, whereupon a decline of BMSC differentiation into osteoblasts triggers the impaired bone formation in osteoporosis.6 Therefore, it is useful to rule out the therapies targeting osteogenic differentiation of BMSCs for osteoporosis.
Melatonin is a ubiquitous hormone that is reported to be associated with a variety of functions such as sleep disorders, circadian rhythm regulation, anti-inflammation, anti-oxidation, anti-tumor, anti-depression, and vasodilation through its receptors.7 Melatonin, which regulates dark signals and provides night-time information, is also considered an ‘endogenous synchronizer’ that stabilizes and enhances various circadian rhythms in the body.8 It has been documented that melatonin is capable orchestrating bone mass through melatonin receptor 2 (MT2) receptors.9 Also, melatonin has been identified as a safe and effective bone loss therapy because of its osteoblast-inducing, bone-enhancing effects and improvement in quality of life through MT2 receptors.10 Melatonin demonstrated promoting effects on the osteogenic differentiation of BMSCs.11 Melatonin treatment increases the number of trabecular bones, improves the microstructure of the femur and vertebra, and enhances bone mass density in osteoporosis mice.12 Adipose tissue-derived MSCs treated with 5 μmol melatonin presented with upregulated hepatocyte growth factor (HGF), a key mitogenic factor promoting proliferation of many types of cells.13 Systemic infusion of HGF has been highlighted to have potential therapeutic properties to relieve bone loss in the early phase of ovariectomy (OVX)-induced osteoporosis.14 The increased expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN; known as a dual lipid and protein phosphatase) induced by inhibition of miR-221 or miR-26b can be counteracted following HGF treatment in MSCs.15 Knockdown of PTEN results in promoted bone formation and ameliorates alcohol-induced osteopenia in a mouse model.16 Diminished PTEN expression is accompanied by activation of the Wnt/β-catenin pathway in gastric cancer cells.17 The Wnt/β-catenin pathway has crucial capacity in orchestrating osteogenic differentiation of BMSCs, bone formation, and bone metabolism disorders.18 The Wnt/β-catenin pathway activation could accelerate the osteogenic differentiation in human BMSCs.19 Based on the aforementioned information, we postulated that melatonin may induce the osteogenic differentiation of BMSCs and depress OVX-induced bone loss in the pathogenesis of osteoporosis involving the regulation of HGF/PTEN/Wnt/β-catenin axis. To address this hypothesis, we implemented both in vitro and in vivo experiments to investigate the mechanism by which the melatonin/HGF/PTEN/Wnt/β-catenin axis participates in osteoporosis, shedding light on the development of a novel therapeutic strategy to prevent osteoporosis.
Materials and methods
Ethics statement
The current study was implemented under the ratification from the ethics committee of The Second Hospital of Jilin University (approval number 201908013) following the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Extensive efforts were made to minimize the suffering of the included animals.
Establishment of OVX-induced osteoporosis mouse model
A total of 95 female C57BL/6 mice (aged 8-weeks old) purchased from Animal Center of Suzhou University (Jiangsu, China) were used in the study. These mice were housed under specific pathogen-free conditions (22–24°C, 50–60% humidity and a 12 h light/dark cycle) with ad libitum access to water and food. After 2 weeks of adaptation, the mice were treated with sham operation (n = 20) and OVX (n = 75). During the OVX operation, the mice were anesthetized with pentobarbital sodium (Sigma-Aldrich, St. Louis, USA) for 1 h (0.3 g/kg body weight in the peritoneum, Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China). The dorsal midline of the rat was curetted, and the incision was about 2 cm. After the incision was pulled to the left, a small incision of about 10 mm was cut through the abdominal wall. The ovary was exposed, and a silk knot was attached between the end of the uterine horn and the ovarian artery. After the ovaries were excised, the abdominal wall was sutured with absorbable suture, and the skin was pulled to the other side. The ovaries on the other side were removed in the same way. After the operation, the wound was sutured, and mice were intramuscularly injected with penicillin for 3 consecutive days (80,000 units/mouse). The same procedure was performed on mice (aged 10 weeks and weighing 220–230 g), excluding ligation and ovariectomy, for sham operation. At 3 months after OVX operation, the mice were euthanized, after which the blood samples were collected, and their longitudinal bones were dissected for subsequent experiments. Seventy mice were successfully modeled, and the success rate was 92.31%.
OVX-induced osteoporosis mouse treatment
Seventy OVX mice were further grouped into OVX (n = 20), melatonin (Met)-1 μmol/l, Met-10 μmol/l, Met-100 μmol/l, Met-100 μmol/l + small interfering ribonucleic acid (RNA)-negative control (si-NC), and Met-100 μmol/l + si-HGF groups. Melatonin was dissolved in double-distilled H2O (ddH2O) to prepare melatonin solution at concentration of 1 mmol/l, 10 mmol/l, and 100 mmol/l. At 1 week after the operation, the mice undergoing OVX were injected with melatonin solution via tail vein at a dose of 10 mg/kg twice a week for 3 months. The sham-operated mice without melatonin treatment and the mice in the OVX group were injected with the same amount of normal saline via tail vein. The lentiviral transfection complex was diluted in the light of the protocols of the in vivo transfection reagent (En-transterTM in vivo), and the lentiviral infection was performed by tail vein injection. Each group of mice was protectively injected with a 3 µl phosphate-buffered saline mixture containing 0.5 nmol/l transfectant, strictly following manufacturer instructions, with approximately 100 µl viruses per mouse. The sham-operated mice without melatonin treatment and the mice in the OVX group were injected with the same amount of phosphate-buffered saline. The injection continuously lasted for 3 days. All oligonucleotides and plasmids were synthesized by Genechem (Shanghai, China). At 1 day before euthanasia, mice were placed in metabolic cages, followed by attaining of 24 h urine. Blood was harvested from the right ventricle before euthanasia, and serum was separated for biochemical analysis. Long bones were dissected after euthanasia, placed in 0.01% sodium azide saline, and stored in a refrigerator at 4°C for histological and micro-computed tomography (micro-CT) analysis and three-dimensional (3D) reconstruction.
Biochemical measurements
Serum bone-specific alkaline phosphatase (BALP) activity was determined on the biochromatic analyzer using lectin precipitation procedure that also quantified total alkaline phosphatase (ALP) activity (tALP, Abbott VP Biochromatric Analyser, Abbott Laboratories Diagnostic Division, USA). The calcium (Ca; CA 590 Calcium kit, Randox Laboratory Ltd., UK) and phosphorus (P; PH 1016 Inorganic phosphorus kit, Randox Laboratory Ltd., UK) concentrations were evaluated in serum and urine samples with colorimetric methods. Urinary excretion of deoxypridinoline (Dpd) was assessed by immunoassay method (8007 Pyrilinks-D kit, Metra Biosystems Inc., USA). Duplicate assays were implemented for serum BALP and Ca, and urinary Ca and P, whereas a single assay was carried out for urinary Dpd.
BMSC isolation and culture
Three months after OVX, the mice were euthanized by intraperitoneal injection of pentobarbital sodium (Sigma-Aldrich) under anesthesia. Bone marrow cells were isolated from the femur by washing with α-minimum essential medium (α-MEM; M0894, Sigma-Aldrich), and the red blood cells were removed with red blood cell lysis buffer (R7757, Sigma-Aldrich). After washing, the cells were plated in standard growth medium [α-MEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin] at 37°C. The mouse primary BMSCs were then detached with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA), and then re-plated.
BMSC treatment
As per the directions of the Lipofectamine 2000 reagents (11668-019, Invitrogen, NY, CA, USA), BMSCs were arranged into Met-1 μmol/l (BMSCs cultured in standard growth medium + 1 mmol/l melatonin), Met-10 μmol/l (BMSCs cultured in standard growth medium + 10 mmol/l melatonin), Met-100 μmol/l (BMSCs cultured in standard growth medium + 100 mmol/l melatonin), Met-100 μmol/l + small interfering RNA (si)-HGF (BMSCs transfected with si-HGF plasmid and then cultured in standard growth medium + 100 mmol/l melatonin), Met-100 μmol/l + overexpression (oe)-PTEN (BMSCs transfected with oe-PTEN plasmid and then cultured in standard growth medium + 100 mmol/l melatonin), si-PTEN (BMSCs cultured in standard growth medium after transfection with si-PTEN plasmid), and Met-100 μmol/l + oe-DKK1 (BMSCs transfected with oe-DKK1 plasmid and then cultured in standard growth medium + 100 mmol/l melatonin) groups, as well as their controls, including control (BMSCs cultured in standard growth medium without other components), control + oe-NC [BMSCs transfected with negative control overexpression (oe-NC) plasmid and then cultured in standard growth medium], Met-100 μmol/l + si-NC (BMSCs transfected with si-NC plasmid and then cultured in standard growth medium + 100 mmol/l melatonin), control + si-NC (BMSCs transfected with si-NC plasmid and then cultured in standard growth medium), Met-100 μmol/l + oe-NC (BMSCs transfected with oe-NC plasmid and then cultured in standard growth medium + 100 mmol/l melatonin) and si-NC (BMSCs transfected with si-NC plasmid and then cultured in standard growth medium) groups. The expression plasmid vectors pcDNA3.1, the overexpression plasmid GV141, and the silencing plasmid GV248 (final concentration of 50 nmol/l and transfection amount of 500 ng) were purchased from Genechem. After 48 h, the cells were used for the subsequent experiments.
Osteogenic differentiation induction
BMSCs were cultured in 12-well plates at a density of 1 × 104 cells/cm2. Upon reaching 80% confluence, the cells were cultured in osteogenic medium containing 10% FBS, 10 mmol/l β-glycerophosphate, 100 nmol/l dexamethasone, and 50 μg/ml dulbecco’s modified Eagle’s medium [DMEM] ascorbic acid) for induction, lasting for 14 days.
Cell-counting kit-8 (CCK-8) assay
With the medium discarded, 150 μl freshly prepared α-MEM containing 10% cell-counting kit (CCK)-8 solution (96992, Sigma-Aldrich) was added to each well of 96-well plates. Meanwhile, a blank control well was set, in which only a mixture of α-MEM and CCK-8 was added. The plate was then incubated at 37°C for 2 h, and the optical density value was measured at a wavelength of 450 nm on the first, third, fifth, and seventh day, respectively.
Alizarin red S (ARS) staining
At the 14th day of osteogenesis induction, the calcium deposition of BMSCs was evaluated by ARS staining. In short, BMSCs were fixed with 4% paraformaldehyde at room temperature for 30 min and incubated with 1 ml of ARS staining solution (Cyagen, USA) for 30 min at room temperature. ARS staining solution was chelated with cell calcium to form alizarin red-calcium complex, showing bright red color. The images were then captured under an Olympus IX51 microscope. For quantitative analysis, 5% perchloric acid solution was added to each well to dissolve the stains on the calcified layer. A spectrophotometer was used to measure the absorbance at 420 nm to quantify the degree of mineralization.
ALP activity and staining
At the 14th day of osteogenesis induction, BMSCs were fixed in 4% paraformaldehyde for 20 min and washed three times with distilled water. Next, the cells were stained with 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitro blue tetrazolium (NBT) ALP Detection kit (Shanghai Beyotime Biotechnology Co. Ltd., Shanghai, China). To determine the activity of ALP, the cells on 96-well plates were lysed using lysis buffer (20 mmol/l Tris-HCl, pH 7.5, 150 mmol/l NaCl and 1% Triton X-100), followed by the addition of substrate and p-nitrophenol. ALP activity was then quantified by determining the absorbance at a wavelength of 650 nm.
Micro-computed tomography (CT) analysis of bone microstructure
The microstructural characteristics of the distal femur were examined using the Skyscan 1176 Micro-CT imaging system (Skyscan 1176, Kontich, Belgium). Bones were scanned at high resolution (18 μm) at 65 kV and 385 μA energy. A 3D reconstruction was performed using the NRecon v1.6 and CTAn v1.13.8.1 software. Region of interest was defined as the cancellous bone of the distal femur. The microstructure of bone was evaluated by calculating six parameters including BMD (mg/cm3), bone volume/tissue volume ratio (BV/TV, %), bone surface/bone volume (BS/BV, μm−1), trabecular separation (Tb.Sp, μm), trabecular thickness (Tb.Th, μm), and trabecular number (Tb.N, μm−1) of distal femur cancellous. Micro-CT was analyzed back-to-back by two veterinary pathologists experienced in rodent skeletal analysis who were blinded to group allocation, and the analysis results were summarized for statistics.
Hematoxylin-eosin (HE) staining
Bone was fixed overnight in 4% paraformaldehyde and decalcified in 10% (wt/wt) EDTA (pH 7.4) for 6 weeks. Then the bone specimen was dehydrated in gradient alcohol solution, embedded in paraffin, and cut into 5 μm-thick sections. The sections were then subjected to HE staining to observe the bone structure of the distal femur. Images were finally captured under a microscope (Zeiss Axiovert 200, Obercochen, Germany).
TOPflash/FOPflash luciferase reporter assay
BMSCs were transfected with TOPflash/FOPflash luciferase reporter plasmids (12457, Addgene, Cambridge, MA, USA) to determine β-catenin/transcription factor (TCF) transcription activity. BMSCs were inoculated in 24-well plates at a density of 2 × 104 cells/well and cultured for 12 h. According to the manufacturer’s instructions, TOPflash (containing repeated sequences with three TCF binding sites) or FOPflash plasmids (containing repeated sequences with three mutated TCF binding sites) were transfected into the cells. After transfection for 48 h, the luciferase activity was measured by the luciferase assay system, with the renilla luciferase activity as internal control.
Enzyme-linked immunosorbent assay (ELISA)
Mice were fasted for 4 h and then the blood samples were collected by puncturing the cheek bag. Next, the enzyme-linked immunosorbent assay (ELISA) kit was adopted to measure the serum levels of bone formation marker serum BALP (ab83369, Abcam Inc., Cambridge, UK) and bone absorption marker tartrate-resistant acid phosphatase 5b (TRAP5b; RJ15907, Renjie Biology, Shanghai, China).
Immunofluorescence staining
BMSCs were fixed with 4% paraformaldehyde for 30 min, sealed with 0.3% Triton X-100 and 1% bovine serum albumin for 30 min, and washed. Next, the cells were incubated with antibody against β-catenin (1:1600; Cell signaling technology, USA) and fluorescence-conjugated secondary antibody (Shanghai Beyotime Biotechnology Co. Ltd., Shanghai, China) for 60 min. Subsequently, the cells were treated with 4′,6-diamidino-2-phenylindole and GFP (Keygen Biotech, Nanjing, China) for 5 min to stain the nucleus. The samples were finally visualized by a confocal laser scanning microscope (Leica, Wetzlar, Germany).
Western blot analysis
Total protein was extracted from tissues and cells with the mixture of radioimmunoprecipitation assay lysis buffer (1% Triton X-100, 10 mmol/l Tris, 1 mmol/l EDTA, 1 mmol/l ethylene glycol bis [β-aminoethyl ether]-N,N’-tetraacetic acid [EGTA], and 150 mmol/l NaCl) and protease inhibitor at a ratio of 1:100. Next, protein concentration was determined by a BioRad Bradford assay kit. After separation using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the protein was transferred onto polyvinylidene fluoride membranes. The membrane was blocked with TBST (25 mmol/l Tris, pH 7.5, 150 mmol/l NaCl and 0.1% Tween 20) with 5% bovine serum albumin, and then probed at 4°C overnight with the following primary rabbit antibodies purchased from Abcam Inc.: HGF (ab83760, 1:1000), BMP4 (ab39973, 1:1000), Runx2 (ab192256, 1:1000), Sp7 (ab22552, 1:1000), OCN (ab13418, 1:1000), OPN (ab8448, 1:1000), BMP2 (ab14933, 1:1000), PTEN (ab32199, 1:1000), β-catenin (ab32572, 1:1000), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; ab205719, 1:1000). The following day, the membrane was re-probed with horseradish peroxidase-labeled secondary antibody to goat anti-rabbit immunoglobulin G (IgG; ab205718, 1:20,000, Abcam Inc.) for 1 h. The immunocomplexes on the membrane were visualized using enhanced chemiluminescence (ECL) reagent (Thermo Fisher Scientific, Rockford, IL, USA). Chemi-Scope Mini Imaging System (Clinx Science) was used for photography. The band intensities were quantified using the ImageJ software (National Institutes of Health, Bethesda, Maryland, USA). Relative protein expression = gray value of target protein/gray value of GAPDH protein.20
Immunohistochemistry
The tissues were cut into 4 μm-thick sections, followed by dewaxing and dehydration. Then the tissue sections were immunostained with the following diluted antibodies purchased from Abcam Inc.: rabbit monoclonal antibody to PTEN (ab32199, 1:1000), rabbit polyclonal antibody to Wnt (ab15251, 1:1000), and rabbit monoclonal antibody to β-catenin (ab32572, 1:1000). Next, the tissue sections were incubated with biotin-labeled secondary goat anti-rabbit IgG (ab6721, 1:1000, Abcam Inc.) for 30 min. After staining with 3,3′-diaminobenzidine (DAB; DA1010, Beijing Solarbio Science and Technology Co. Ltd., Beijing, China), the sections were dehydrated, cleared, and mounted. Finally, five high-power visual fields were randomly selected from each section and 100 cells were counted in each field under a light microscope (XSP-36, Boshida Optical Instrument Co. Ltd., Shenzhen, China). Positive cells <5% are negative, and positive cells ⩾5% are positive. The results were scored by two people independently.
RNA isolation and quantitation
Total RNA content was extracted from tissues using TRIzol reagents (15596026, Invitrogen). The extracted RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA) using PrimerScript RT reagent Kit (Takara, Shiga, Japan) and SYBR Premix EX Taq Kit (RR420A, Takara). Real-time qualitative polymerase chain reaction (RT-qPCR) was conducted on an ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA). All investigations involved at least three wells, each repeated in triplicate. With β-actin gene as the internal reference, the fold changes were calculated using relative quantification (the 2−△△Ct method). The primers are shown in Table 1.
Table 1.
Primer sequences for RT-qPCR.
| Gene | Sequences (5′-3′) |
|---|---|
| HGF | F: AAACATATCTGCGGAGGATC |
| R: ACGATTTGGAATGGCACATC | |
| PTEN | F: TGGATTCGACTTAGACTTGACCT |
| R: TGCTTTGAATCCAAAAACCTTACT | |
| Oct4 | F: GAGGAGTCCCAGGACATGAA |
| R: AGATGGTGGTCTGGCTGAAC | |
| Cyclin D1 | F: TCAAGTGTGACCCGGACTG |
| R: CTCCAGAAGGGCTTCAATCTGT | |
| C-myc | F: CGAGGAGAATGTCAAGAGGCGAAC |
| R: GCTTGGACGGACAGGATGTATGC | |
| CD44 | F: TCAGAGGAGTAGGAGAGAGGAAAC |
| R: GAAAAGTCAAAGTAACAATAACAGTGG | |
| GAPDH | F: CATGAGAAGTATGACAACAGCCT |
| R: AGTCCTTCCACGATACCAAAGT |
GADPH, glyceraldehyde 3-phosphate dehydrogenase; RT-qPCR, real-time qualitative polymerase chain reaction.
Statistical analysis
Statistical analyses were performed using the SPSS 21.0 software (IBM Corp. Armonk, NY, USA). Measurement data were expressed as mean ± standard deviation. Data between two groups were compared using unpaired t test. Data among multiple groups were compared by one-way analysis of variance (ANOVA) with Tukey’s test while data at different time points were analyzed by two-way ANOVA with Bonferroni’s correction. A value of p < 0.05 was indicative of statistical significance.
Results
Melatonin prevents bone loss in OVX mice
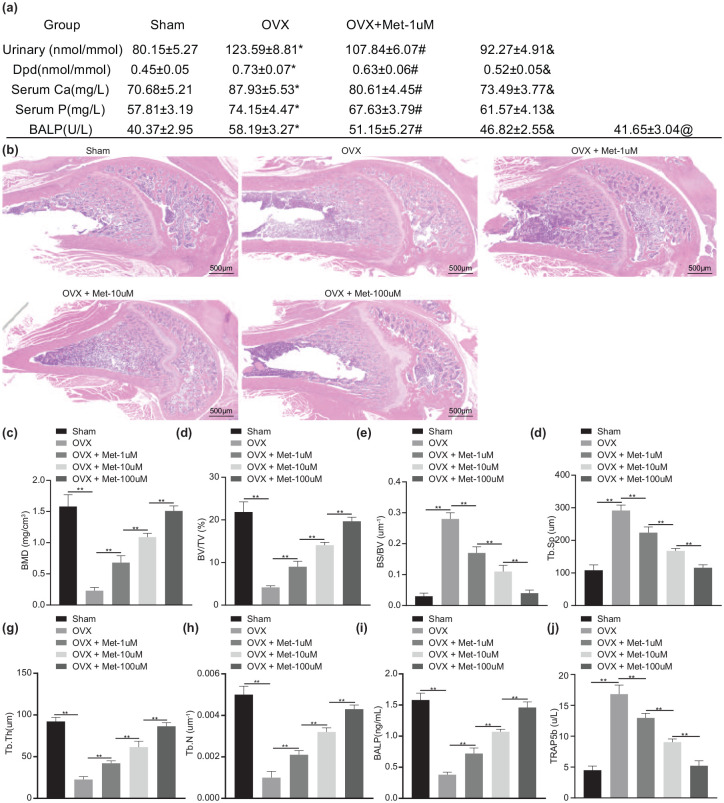
OVX mice showed obvious bone loss when compared to the sham-operated mice, while treatment of melatonin improved bone mass growth [Figure 1(b)]. Bone demineralization markers in each group were detected by biochemical analysis. As shown in Supplementary Table 1 or Figure 1(a), urinary Dpd, urinary calcium excretion, serum Ca, serum P, and BALP were significantly increased in OVX mice compared with sham-operated mice (p < 0.01). After melatonin treatment, urinary Dpd, urinary calcium excretion, serum Ca, serum P, and BALP were reduced in a dose-dependent manner (p < 0.01). In addition, a decline was found in the BMD, ratio of BV/TV, Tb.Th, and Tb.N, while BS/BV and Tb.Sp were increased in OVX mice (p < 0.01). However, treatment with melatonin enhanced the BMD, ratio of BV/TV, Tb.Th and Tb.N but decreased BS/BV ratio and Tb.Sp in a dose-dependent manner [p < 0.01; Figure 1(c)–(h)]. The quantitative analysis of the trabecular microstructure of the femur further verified the above results, indicating that melatonin could reduce the bone loss of OVX mice. Furthermore, ELISA detected downregulated BALP serum levels and increased TRAP5b serum levels in OVX mice relative to sham-operated mice (p < 0.01). Melatonin treatment resulted in dose-dependent increase in BALP serum levels while TRAP5b serum levels were decreased in a dose-dependent manner [p < 0.01; Figure 1(i) and (j)]. These results suggested that melatonin had the potential to mitigate the bone loss in OVX mice.
Figure 1.
Melatonin attenuates the bone loss in OVX mice.
OVX mice were treated with melatonin at different concentrations. (a) Bone demineralization markers in serum and urine as detected by biochemical analysis. (b) HE staining analysis in the bone structure of mouse distal femur (scale bar = 500 μm). (c) Micro-CT quantitative analysis of BMD in mouse femur. (d) Micro-CT quantitative analysis of BV/TV ratio in mouse femur. (e) Micro-CT quantitative analysis of BS/BV ratio in mouse femur. (f) Micro-CT quantitative analysis of Tb.Sp in mouse femur. (g) Micro-CT quantitative analysis of Tb.Th in mouse femur. (h) Micro-CT quantitative analysis of Tb.N in mouse femur. (i) Serum levels of BALP measured by ELISA. (j) Serum levels of TRAP5b measured by ELISA.
**p < 0.01, indicates statistical significance. Data (mean ± standard deviation) among multiple groups were compared by one-way ANOVA with Tukey’s test. n = 10 for mice following each treatment.
ANOVA, analysis of variance; BALP, bone-specific alkaline phosphatase; BS, bone surface; BV, bone volume; Ca, calcium; CT, computed tomography; Dpd, deoxypridinoline; ELISA, enzyme-linked immunosorbent assay; HE, hematoxylin eosin; Met, melatonin; P, phosphorus; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
Melatonin accelerates the osteogenic differentiation of BMSCs in vitro
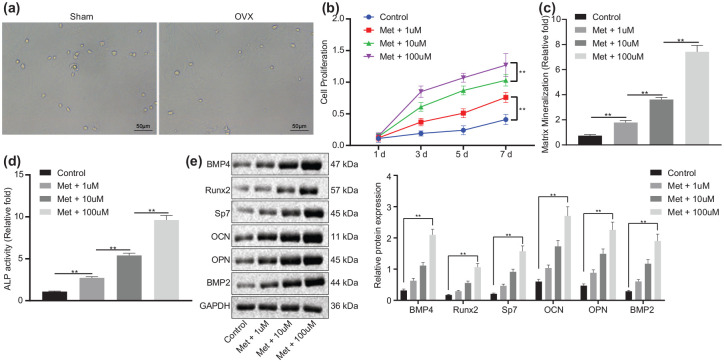
We then proceeded to examine the mechanism by which melatonin inhibits bone loss. After separation from mice, BMSCs in OVX mice showed similar cell morphologic characteristics to those of sham-operated mice [Figure 2(a)]. As shown in Figure 2(b), the proliferation of BMSCs was potentiated upon melatonin treatment, showing a dose-dependent manner (p < 0.01). The results of ARS and ALP [Figure 2(c) and (d)] revealed an increase in the stained area and density of mineralization and calcification of BMSCs following melatonin treatment in a dose-dependent manner (p < 0.01), indicating that melatonin might promote the differentiation of BMSCs into osteoblasts. Additionally, the expression of osteogenic-related factors was detected in BMSCs by Western blot analysis. As shown in Figure 2(e), the protein expression of BMP4, Runx2, Sp7, OCN, OPN and BMP2 was higher in BMSCs treated with melatonin than those in the control cells (p < 0.01), with a dose-dependent increase (p < 0.01). These data indicated that melatonin could promote osteogenic differentiation of BMSCs in vitro.
Figure 2.
Melatonin augments osteogenic differentiation of BMSCs in vitro.
(a) Morphologic characteristics of the isolated BMSCs from the OVX and sham-operated mice observed by optical microscope (scale bar = 50 μm). (b) Proliferation of BMSCs treated with melatonin at different concentrations measured by CCK-8 assay. (c) Mineralization of BMSCs treated with melatonin at different concentrations evaluated by ARS staining. (d) Calcification of BMSCs treated with melatonin at different concentrations evaluated by ALP activity and staining. (e) Western blot analysis of BMP4, Runx2, Sp7, OCN, OPN and BMP2 protein expression in BMSCs treated with melatonin at different concentrations.
**p < 0.01, indicates statistical significance. Data (mean ± standard deviation) among multiple groups were compared by one-way ANOVA with Tukey’s test while those at different time points were compared by two-way ANOVA with Bonferroni’s correction. Cell experiments were conducted in triplicate.
ALP, alkaline phosphatase; ANOVA, analysis of variance; ARS, alizarin red S; BMSCs, bone marrow mesenchymal stem cells; CCK, cell-counting kit; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Met, melatonin; OVX, ovariectomy.
Melatonin promotes the differentiation of BMSCs into osteoblasts by upregulating transcription factor HGF in BMSCs
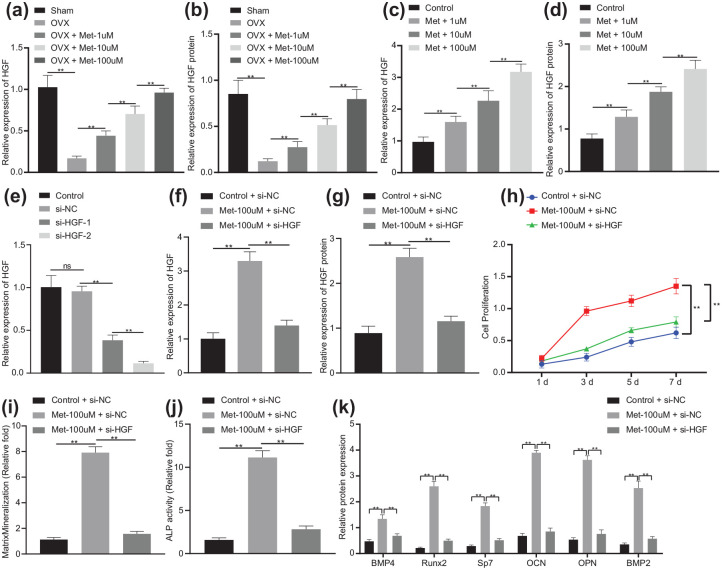
Next, we aimed to exploit the possible relationship between melatonin and transcription factor HGF. The results of RT-qPCR and Western blot analysis revealed that HGF expression was downregulated in the femur of OVX mice (p < 0.01), which was upregulated upon melatonin treatment in a dose-dependent manner [p < 0.01; Figure 3(a) and (b)]. The finding suggested the ability of melatonin to upregulate HGF in mice. Next, we intended to verify the regulatory effect of melatonin on HGF expression in vitro. As shown in Figure 3(c) and (d), melatonin treatment resulted in elevation in HGF expression in a dose-dependent manner (p < 0.01), which was consistent with the results observed in vivo. Therefore, melatonin elevated HGF expression both in vivo and in vitro.
Figure 3.
Melatonin facilitates the differentiation of BMSCs into osteoblasts via HGF upregulation in BMSCs.
(a) mRNA expression of HGF in the femur of OVX or OVX mice treated with melatonin at different concentrations determined by RT-qPCR (n = 10). (b) Western blot analysis of HGF protein expression in the femur of OVX or OVX mice treated with melatonin at different concentrations (n = 10). (c) mRNA expression of HGF in BMSCs treated with melatonin at different concentrations determined by RT-qPCR. (d) Western blot analysis of HGF protein expression in BMSCs treated with melatonin at different concentrations. (e) Silencing efficiency of HGF in BMSCs confirmed by RT-qPCR. (f) mRNA expression of HGF in BMSCs treated with Met-100 μmol/l or in combination with si-HGF determined by RT-qPCR. (g) Western blot analysis of HGF protein expression in BMSCs treated with Met-100 μmol/l or in combination with si-HGF. (h) Proliferation of BMSCs treated with Met-100 μmol/l or in combination with si-HGF measured by CCK-8 assay. (i) Mineralization of BMSCs treated with Met-100 μmol/l or in combination with si-HGF evaluated by ARS staining. (j) Calcification of BMSCs treated with Met-100 μmol/l or in combination with si-HGF evaluated by ALP activity and staining. (k) Western blot analysis of BMP4, Runx2, Sp7, OCN, OPN and BMP2 protein expression in BMSCs treated with Met-100 μmol/l or in combination with si-HGF.
**p < 0.01, indicates statistical significance. Data (mean ± standard deviation) among multiple groups were compared by one-way ANOVA with Tukey’s test while those at different time points were compared by two-way ANOVA with Bonferroni’s correction. Cell experiments were conducted in triplicate.
ALP, alkaline phosphatase; ANOVA, analysis of variance; ARS, alizarin red S; BMSCs, bone marrow mesenchymal stem cells; Met, melatonin; mRNA, messenger ribonucleic acid; OVX, ovariectomy; RT-qPCR, real-time qualitative polymerase chain reaction; si-, small interfering.
Subsequently, we performed a series of in vitro experiments to explore whether melatonin can promote the differentiation of BMSCs into osteoblasts by regulating transcription factor HGF. The silencing efficiency of HGF was confirmed by RT-qPCR, manifested by reduced expression of HGF in BMSCs treated with si-HGF-1 or si-HGF-2, of which si-HGF-2 showed a superior silencing efficiency (p < 0.01), and was thus selected for the following experiments [Figure 3(e)]. Moreover, HGF expression was elevated in Met-100 μmol/l-treated BMSCs (p < 0.01) while it was diminished in BMSCs treated with Met-100 μmol/l + si-HGF [p < 0.01; Figure 3(f) and (g)]. This indicated that knockdown of HGF reversed the upregulation of melatonin on HGF expression. The results of CCK-8 assay demonstrated that Met-100 μmol/l increased the proliferation of BMSCs while treatment with Met-100 μmol/l + si-HGF led to opposite results [p < 0.01; Figure 3(h)], which revealed that knockdown of HGF could reverse the promoting effect of melatonin on the proliferation of BMSCs. Figure 3(i) and (j) clarified that the mineralization and calcification of BMSCs were enhanced in response to Met-100 μmol/l treatment (p < 0.01), which was negated by treatment with Met-100 μmol/l + si-HGF (p < 0.01), indicating that knockdown HGF inhibited the osteogenic differentiation of BMSCs. Besides, the protein levels of BMP4, Runx2, Sp7, OCN, OPN and BMP2 were observed to be increased following Met-100 μmol/l treatment (p < 0.01), while treatment with Met-100 μmol/l + si-HGF counteracted the results [p < 0.01; Figure 3(k)]. These results suggested that melatonin promoted the differentiation of BMSCs into osteoblasts by upregulating HGF in vitro.
Melatonin regulates the differentiation of BMSCs into osteoblasts via HGF-dependent PTEN expression in BMSCs
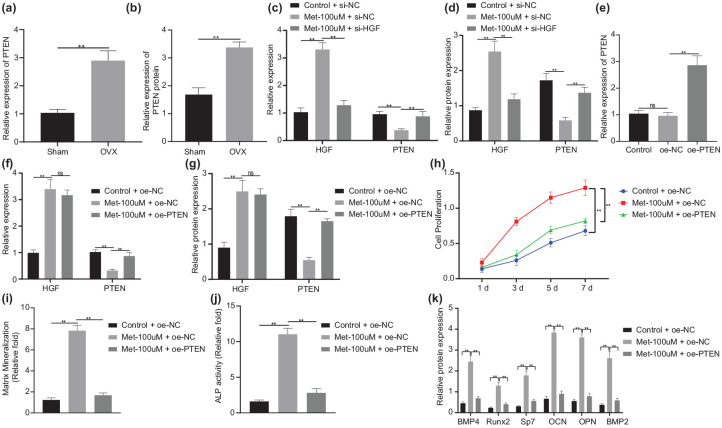
After uncovering the regulatory role of melatonin in the HGF expression, we next aimed to investigate the possible downstream mechanism of the HGF transcription factor. As illustrated in Figure 4(a) and (b), PTEN expression was augmented in OVX mice (p < 0.01). Besides, HGF expression was found to be upregulated while PTEN expression was downregulated in BMSCs following Met-100 μmol/l treatment (p < 0.01), which was neutralized upon treatment with Met-100 μmol/l + si-HGF [p < 0.01; Figure 4(c) and (d)], which implied that knockdown of HGF reversed the inhibition of melatonin on PTEN.
Figure 4.
Melatonin affects osteogenic differentiation of BMSCs via the HGF/PTEN axis in BMSCs.
(a) mRNA expression of PTEN in the femur of OVX or sham-operated mice determined by RT-qPCR (n = 10). (b) Western blot analysis of PTEN protein expression in the femur of OVX or sham-operated mice (n = 10). (c) mRNA expression of HGF and PTEN in BMSCs treated with Met-100 μmol/l or in combination with si-HGF determined by RT-qPCR. (d) Western blot analysis of HGF and PTEN protein expression in BMSCs treated with Met-100 μmol/l or in combination with si-HGF. (e) Overexpression efficiency of PTEN in BMSCs confirmed by RT-qPCR. (f) mRNA expression of HGF and PTEN in BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN determined by RT-qPCR. (g) Western blot analysis of HGF and PTEN protein expression in BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN. (h) Proliferation of BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN measured by CCK-8 assay. (i) Mineralization of BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN evaluated by ARS staining. (j) Calcification of BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN evaluated by ALP activity and staining. (k) Western blot analysis of BMP4, Runx2, Sp7, OCN, OPN and BMP2 protein expression in BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN.
**p < 0.01, indicates statistical significance. Data (mean ± standard deviation) among multiple groups were compared by one-way ANOVA with Tukey’s test while those at different time points were compared by two-way ANOVA with Bonferroni’s correction. Cell experiments were conducted in triplicate.
ALP, alkaline phosphatase; ANOVA, analysis of variance; BMSCs, bone marrow mesenchymal stem cells; CCK, cell-counting kit; Met, melatonin; mRNA, messenger ribonucleic acid; oe-, overexpression; OVX, ovariectomy; RT-qPCR, real-time qualitative polymerase chain reaction.
Subsequently, RT-qPCR verified the overexpression efficiency of PTEN, manifested by elevated PTEN expression in BMSCs transfected with oe-PTEN [p < 0.01; Figure 4(e)]. HGF expression showed an enhancement in Met-100 μmol/l-treated cells (p < 0.01), while that of PTEN presented a decline (p < 0.01). However, there was no significant difference in the mRNA and protein expression of HGF (p > 0.01) but that of PTEN was increased in cells treated with Met-100 μmol/l + oe-PTEN [p < 0.01; Figure 4(f) and (g)]. The results of CCK-8, as shown in Figure 4(h), revealed ascending cell proliferation trend in response to Met-100 μmol/l (p < 0.01), while it was impaired upon Met-100 μmol/l + oe-PTEN treatment (p < 0.01), indicating that PTEN disrupted the promoting effect of melatonin on BMSC proliferation. Moreover, there were increased mineralization and calcification of Met-100 μmol/l-treated cells (p < 0.01), while Met-100 μmol/l + oe-PTEN treatment undermined the trend [p < 0.01; Figure 4(i) and (j)], indicating that PTEN inhibited the osteogenic differentiation of BMSCs. In addition, Figure 4(k) presents enforced protein expression of BMP4, Runx2, Sp7, OCN, OPN and BMP2 in response to Met-100 μmol/l (p < 0.01), while Met-100 μmol/l + oe-PTEN treatment led to opposite results (p < 0.01). Based on the results obtained, melatonin could regulate the differentiation of BMSCs into osteoblasts through the HGF/PTEN axis in BMSCs.
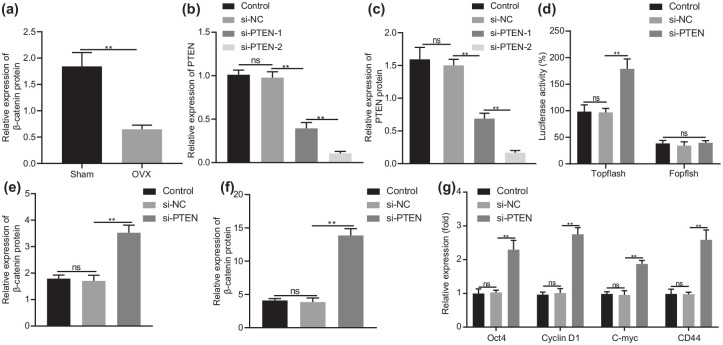
Knockdown of PTEN facilitates activation of the Wnt/β-catenin pathway in BMSCs
A downward inclination was observed in the protein expression of β-catenin in the femur of OVX mice [p < 0.01; Figure 5(a)], which was negatively correlated with PTEN expression and therefore, we speculated that PTEN might inhibit the Wnt/β-catenin pathway. To address this hypothesis, we first performed RT-qPCR and Western blot analysis to detect the silencing efficiency of PTEN in cells. The results displayed reduced PTEN expression in cells treated with si-PTEN-1 or si-PTEN-2 (p < 0.01), with a more pronounced decline in response to si-PTEN-2 [Figure 5(b) and (c)]. si-PTEN-2 was thus used in subsequent experiments. As shown in Figure 5(d), the activity of TOPflash was increased in si-PTEN-treated cells (p < 0.01). In addition, β-catenin protein was upregulated in si-PTEN-treated cells [p < 0.01; Figure 5(e)]. The nuclear accumulation of β-catenin was found to be increased in cells following PTEN knockdown [p < 0.01; Figure 5(f)]. Furthermore, Oct4, Cyclin D1, C-myc and CD44 exhibited amplified expression in si-PTEN-treated cells [p < 0.01; Figure 5(g)]. The data supported that inhibition of PTEN could activate the Wnt/β-catenin pathway in BMSCs.
Figure 5.
PTEN knockdown induces activation of the Wnt/β-catenin pathway in BMSCs.
(a) Western blot analysis of β-catenin protein expression in the femur of OVX or sham-operated mice (n = 10). (b) mRNA expression of PTEN in cells treated with si-PTEN-1 or si-PTEN-2 determined by RT-qPCR. (c) Western blot analysis of PTEN protein expression in cells treated with si-PTEN-1 or si-PTEN-2. (d) Activity of TOPflash in cells treated with si-PTEN detected by dual-luciferase reporter. (e) Western blot analysis of β-catenin protein expression in BMSCs treated with si-PTEN. (f) Nuclear accumulation of β-catenin in BMSCs treated with si-PTEN determined by immunofluorescence staining. (g) mRNA expression of Oct4, Cyclin D1, C-myc and CD44 in BMSCs treated with si-PTEN determined by RT-qPCR.
**p < 0.01, indicates statistical significance. Data (mean ± standard deviation) between two groups were compared using unpaired t test while those among multiple groups were compared by one-way ANOVA with Tukey’s test. Cell experiments were conducted in triplicate.
ANOVA, analysis of variance; BMSCs, bone marrow mesenchymal stem cells; mRNA, messenger ribonucleic acid; OVX, ovariectomy; RT-qPCR, real-time qualitative polymerase chain reaction; si-, small interfering.
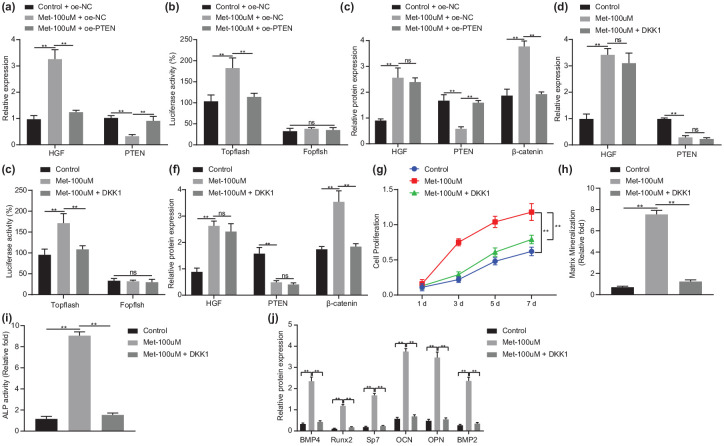
Melatonin elicits osteogenic differentiation of BMSCs via the HGF/PTEN/Wnt/β-catenin pathway in BMSCs
Since PTEN knockdown can activate the Wnt/β-catenin pathway in BMSCs, we aimed to explore the regulatory mechanism by which melatonin regulates the Wnt/β-catenin pathway through PTEN. As shown in Figure 6(a) to (c), an elevation was found in HGF expression, TOPflash activity, and β-catenin protein expression (p < 0.01) while PTEN expression was decreased in Met-100 μmol/l-treated cells (p < 0.01), which demonstrated that melatonin could promote the Wnt/β-catenin pathway. In response to Met-100 μmol/l + oe-PTEN, TOPflash activity and β-catenin protein expression were decreased (p < 0.01), while PTEN was upregulated in cells (p < 0.01), indicating that PTEN reversed the promoting effect of melatonin on the Wnt/β-catenin pathway.
Figure 6.
Melatonin stimulates osteogenic differentiation of BMSCs via the HGF/PTEN/Wnt/β-catenin axis in BMSCs.
(a) mRNA expression of HGF and PTEN in BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN determined by RT-qPCR. (b) Activity of TOPflash in BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN detected by dual-luciferase reporter. (c) Western blot analysis of HGF, PTEN and β-catenin protein expression in BMSCs treated with Met-100 μmol/l or in combination with oe-PTEN. (d) mRNA expression of HGF and PTEN in BMSCs treated with Met-100 μmol/l or in combination with DKK1 determined by RT-qPCR. (e) Activity of TOPflash in BMSCs treated with Met-100 μmol/l or in combination with DKK1 detected by dual-luciferase reporter. (f) Western blot analysis of HGF, PTEN and β-catenin protein expression in BMSCs treated with Met-100 μmol/l or in combination with DKK1. (g) Proliferation of BMSCs treated with Met-100 μmol/l or in combination with DKK1 measured by CCK-8 assay. (h) Mineralization of BMSCs treated with Met-100 μmol/l or in combination with DKK1 evaluated by ARS staining. (i) Calcification of BMSCs treated with Met-100 μmol/l or in combination with DKK1 evaluated by ALP activity and staining. (j) Western blot analysis of BMP4, Runx2, Sp7, OCN, OPN and BMP2 protein expression in BMSCs treated with Met-100 μmol/l or in combination with DKK1. **p < 0.01, indicates statistical significance. Data (mean ± standard deviation) among multiple groups were compared by one-way ANOVA with Tukey’s test while those at different time points were compared by two-way ANOVA with Bonferroni’s correction. Cell experiments were conducted in triplicate.
ALP, alkaline phosphatase; ANOVA, analysis of variance; ARS, alizarin red S; BMSCs, bone marrow mesenchymal stem cells; CCK, cell-counting kit; Met, melatonin; mRNA, messenger ribonucleic acid; oe-, overexpression; OVX, ovariectomy; RT-qPCR, real-time qualitative polymerase chain reaction.
Treatment with Met-100 μmol/l led to higher HGF expression, TOPflash activity, and β-catenin protein expression while lower PTEN expression in BMSCs than control BMSCs. By contrast, Met-100 μmol/l + DKK1 treatment downregulated TOPflash activity and β-catenin protein expression [p < 0.01; Figure 6(d)–(f)], indicating that DKK1 inhibited the activity of the Wnt/β-catenin pathway. In addition, the enhanced proliferation of BMSCs by Met-100 μmol/l treatment (p < 0.01) was reversed by DKK1 treatment [p < 0.01; Figure 6(g)], indicating that inhibition of Wnt/β-catenin pathway inhibited the proliferation of BMSCs. Furthermore, there were increased mineralization and calcification in cells treated with Met-100 μmol/l (p < 0.01), which was negated by DKK1 treatment [p < 0.01; Figure 6(h) and (i)], indicating that inhibited Wnt/β-catenin undermined the melatonin-induced osteogenic differentiation of BMSCs. In addition, Figure 6(j) exhibits augmented protein expression of BMP4, Runx2, Sp7, OCN, OPN, and BMP2 in response to Met-100 μmol/l (p < 0.01), while DKK1 treatment led to opposite results (p < 0.01). These results suggested that melatonin inhibited PTEN expression and then activated the Wnt/β-catenin pathway, thus promoting osteogenic differentiation of BMSCs in BMSCs.
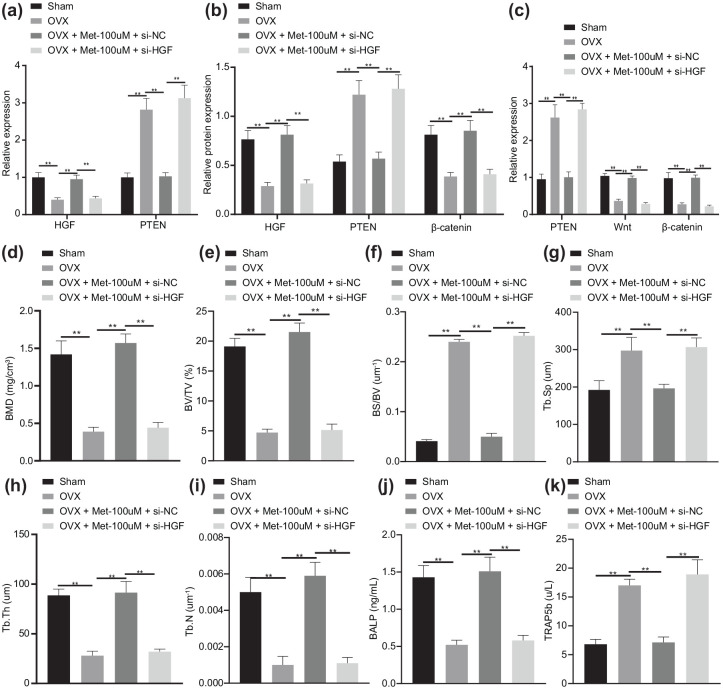
Melatonin ameliorates osteoporosis through the HGF/PTEN/Wnt/β-catenin axis in OVX mice
We then intended to investigate the effect of melatonin-mediated HGF/PTEN/Wnt/β-catenin axis on osteoporosis in OVX mice. As shown in Figure 7(a) and (b), there were higher HGF expression and β-catenin protein expression, while lower PTEN expression in Met-100 μmol/l-treated mice than OVX mice (p < 0.01). In response to Met-100 μmol/l + si-HGF, the β-catenin protein was downregulated (p < 0.01), while PTEN was upregulated (p < 0.01). Knockdown of HGF reversed the inhibition of melatonin on PTEN expression and the promotion on the Wnt and β-catenin expression [p < 0.01; Figure 7(c), Supplementary Figure 1(a)].
Figure 7.
Melatonin delays bone loss and relieves osteoporosis in OVX mice via the HGF/PTEN/Wnt/β-catenin axis.
OVX mice were treated with Met-100 μmol/l or in combination with si-HGF. (a) mRNA expression of HGF and PTEN determined by RT-qPCR. (b) Western blot analysis of HGF, PTEN and β-catenin protein expression in OVX mice. (c) Immunohistochemistry analysis of HGF, PTEN and β-catenin proteins. (d) Micro-CT quantitative analysis of BMD in mouse femur. (e) Micro-CT quantitative analysis of BV/TV ratio in mouse femur. (f) Micro-CT quantitative analysis of BS/BV ratio in mouse femur. (g) Micro-CT quantitative analysis of Tb.Sp in mouse femur. (h) Micro-CT quantitative analysis of Tb.Th in mouse femur. (i) Micro-CT quantitative analysis of Tb.N in mouse femur. (j) Serum levels of BALP measured by ELISA. (k) Serum levels of TRAP5b measured by ELISA. **p < 0.01, indicates statistical significance. Data (mean ± standard deviation) among multiple groups were compared by one-way ANOVA with Tukey’s test. n = 10 for mice following each treatment.
ANOVA, analysis of variance; ARS, alizarin red S; BALP, bone-specific alkaline phosphatase; BMD, bone mineral density; BMSCs, bone marrow mesenchymal stem cells; BS, bone surface; BV, bone volume; Ca, calcium; CCK, cell-counting kit; CT, computed tomography; Dpd, deoxypridinoline; ELISA, enzyme-linked immunosorbent assay; Met, melatonin; mRNA, messenger ribonucleic acid; OVX, ovariectomy; P, phosphorus; RT-qPCR, real-time qualitative polymerase chain reaction; si-, small interfering; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; TV, tissue volume.
OVX mice showed obvious bone loss which was alleviated by treatment of Met-100 μmol/l. However, further treatment with si-HGF led to reduced bone mass [Supplementary Figure 1(b)]. In addition, biochemical analysis (Supplementary Table 2), urinary Dpd, urinary calcium excretion, serum Ca, serum P, and BALP were increased in OVX mice in contrast to the sham-operated mice (p < 0.01). After Met-100 μmol/l treatment, urinary Dpd, urinary Ca, serum Ca, serum P, BALP in femoral bone of OVX mice were diminished (p < 0.01). In the presence of Met-100 μmol/l, urinary Dpd, urinary Ca, serum Ca, serum P, and BALP in OVX mice were augmented by silencing HGF (p < 0.01). A reduction was found in the BMD, ratio of BV/TV, Tb.Th, and Tb.N, while BS/BV and Tb.Sp were increased in OVX mice (p < 0.01). Treatment with Met-100 μmol/l enhanced the BMD, ratio of BV/TV, Tb.Th, and Tb.N but decreased BS/BV ratio and Tb.Sp [p < 0.01; Figure 7(d)–(i)]. The quantitative analysis of the trabecular microstructure of the femur further verified the above results, indicating that melatonin could reduce the bone loss of OVX mice via mediating the HGF/PTEN/Wnt/β-catenin axis. Furthermore, ELISA detected downregulated BALP serum levels and increased TRAP5b serum levels in OVX mice relative to sham-operated mice (p < 0.01). Met-100 μmol/l treatment led to elevated BALP serum levels and reduced TRAP5b serum levels (p < 0.01), which was abolished by subsequent combination with si-HGF [p < 0.01; Figure 7(j) and (k)]. The findings suggested that melatonin retarded bone loss and improved osteoporosis in OVX mice via the HGF/PTEN/Wnt/β-catenin axis.
Discussion
Osteoporosis results from the imbalance between bone resorption and bone formation, and thus restoring the balance of bone remodeling might be of highest clinical significance in finding treatment options for the disease.21 Melatonin has been largely reported to participate in the regulation of bone metabolism by controlling the lineage commitment and differentiation pathways of MSCs.5 Therefore, the current study aimed to explore the potential roles of melatonin in osteogenic differentiation of BMSCs and bone loss following osteoporosis. Both in vitro and in vivo findings suggested that melatonin could potentially enhance osteogenic differentiation of BMSCs and relieve bone loss via the HGF/PTEN/Wnt/β-catenin axis.
We initially found that melatonin attenuated the bone loss in OVX mice. A recent study has revealed the potential of melatonin to prevent the bone loss during space flight, as it significantly stimulates calcitonin (an osteoclast-inhibiting hormone) expression and decreases the expression of receptor activator of nuclear factor κB ligand (a promoter of osteoclastogenesis), alongside suppressed gene expression for osteoclast functions.22 Moreover, melatonin can alleviate bone loss in the retinoic-acid-induced osteoporosis mouse model, repair the trabecular microstructure, and promote bone formation by reducing oxidation levels in vivo and in vitro via the ERK/SMAD and NF-κB pathways.12 Additionally, we uncovered that melatonin could enhance the osteogenic differentiation of BMSCs in vitro. Similarly, administration of melatonin stimulates osteoblast differentiation of BMSCs by preserving silent information regulator type 1 (SIRT1)-mediated intracellular antioxidant properties.23 In addition, the research performed by Zhou et al.11 unveiled that melatonin augmented BMSC osteogenic action via MT2-inactivated NF-κB pathway. Also, another study uncovered that melatonin enhanced ALP activity and the expression of genes related to osteogenic and chondrogenic differentiation including ALP, osteopontin, osteocalcin, and runt-related transcription factor 2 in human MSCs via MT2.24 Moreover, research conducted by Amstrup et al.25 unraveled that melatonin actions on the skeleton were through effects on calcium excretion and no actions on bone-marker turnover, which warranted further research to explore the potential mechanisms of action of melatonin in bone in the future.
Recently, melatonin has been found to possess atheroprotective effects via the upregulation of anti-inflammatory HGF in an atherosclerosis rabbit model.26 HGF is a survival factor for BMSCs and the recombinant adenovirus-carrying HGF gene can promote human BMSC proliferation and osteogenic differentiation.27 Additionally, the results from the current study revealed that HGF expression was increased in OVX-induced mice. In accordance with our results, serum HGF levels are much higher in the ankylosing spondylitis patients compared with the healthy controls, and HGF is associated with lower BMD.28 Taken together, melatonin may have the potency to promote the differentiation of BMSCs into osteoblasts by upregulating transcription factor HGF.
In BMSCs exposed to hypoxia, miR-486 elevates HGF mRNA and protein levels while inhibiting the PTEN expression,29 suggesting a possible adverse correlation of HGF expression with the PTEN expression. In addition, HGF attenuates transforming growth factor-β-angiotensin II crosstalk through inhibition of the PTEN/Akt pathway in mice with renal injury.30 Furthermore, inhibiting the expression of PTEN by lncRNA GHET1 contributes to the promoted osteoblast proliferation and differentiation.31 Treatment with melatonin has the capacity to downregulate the levels of PTEN in primary neurons upon Aβ-induced neurotoxicity.32 Therefore, we concluded that melatonin might enhance the differentiation of BMSCs into osteoblasts via HGF-dependent PTEN downregulation.
Another important finding in the present study was that knockdown of PTEN facilitated activation of the Wnt/β-catenin pathway in BMSCs. Consistently, β-catenin activation is revealed to cooperate with PTEN deficiency to elicit androgen-receptor-independent castration-resistant prostate cancer.33 Promoted activity of PTEN could suppress the activation of the Wnt/β-catenin pathway in HeLa and SW620 cell lines.34 The augmented Wnt/β-catenin pathway activation induced by overexpression of miR-141 can inhibit the osteoporosis of jawbones in OVX-treated rats.35 LiCl, an activator of the Wnt/β-catenin pathway, restores the inhibited osteogenic differentiation by HOXA10 overexpression in human periodontal ligament stem cells.36 Activated Wnt/β-catenin pathway results in an increase in the osteogenic differentiation in BMSCs and significantly reduces bone loss by enhancing bone formation in OVX mice.18 Notably, melatonin promotes osteogenic differentiation in C3H10T1/2 cells partially by activating the β-catenin pathway.5 On the basis of aforementioned evidence, it can be concluded that melatonin has the potential to elicit osteogenic differentiation in BMSCs, as well as relieving osteoporosis in OVX mice via the HGF/PTEN/Wnt/β-catenin pathway.
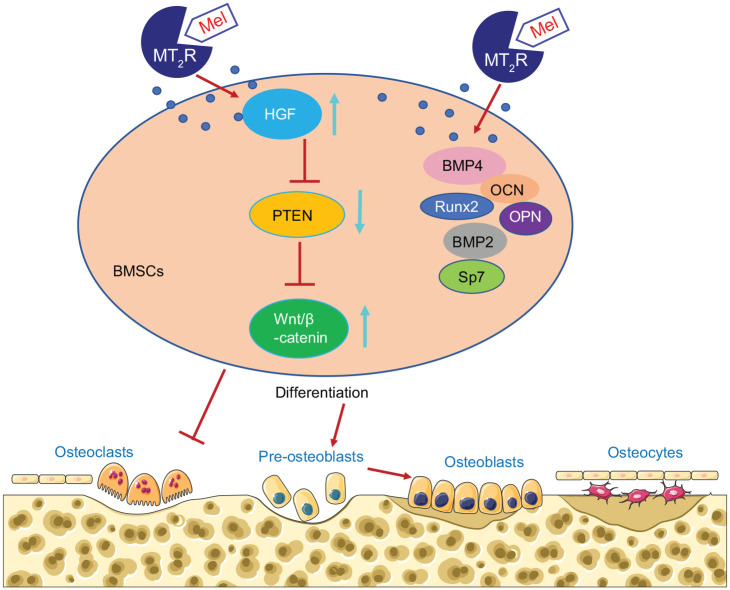
Conclusion
To conclude, we have demonstrated that melatonin can potentiate osteogenic differentiation and attenuate bone loss via the HGF/PTEN/Wnt/β-catenin axis. Melatonin could orchestrate osteogenic differentiation of BMSCs in a receptor-dependent and -independent manner. On the one hand, melatonin upregulates HGF and inhibits the expression of PTEN, which then activates the Wnt/β-catenin signaling pathway. On the other hand, melatonin promotes the osteogenic differentiation of BMSCs by promoting the expression of BMP4, Runx2, Sp7, OCN, OPN and BMP2 to prevent bone loss (Figure 8). Our data suggest that administration of melatonin is a promising strategy for treating patients with osteoporosis and other related bone-related diseases. However, future studies are still warranted on the specimens from osteoporosis-diagnosed patients for in-depth analysis of the melatonin/HGF/PTEN/Wnt/β-catenin signaling in osteoporosis.
Figure 8.
Molecular mechanism underling that melatonin induces osteogenic differentiation of BMSCs and alleviates bone loss via the HGF/PTEN/Wnt/β-catenin pathway.
BMSCs, bone marrow mesenchymal stem cells; Mel, melatonin.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_2040622321995685 for Melatonin restores osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells and alleviates bone loss through the HGF/PTEN/Wnt/β-catenin axis by Jun Zhang, Guoliang Jia, Pan Xue and Zhengwei Li in Therapeutic Advances in Chronic Disease
Supplemental material, sj-eps-2-taj-10.1177_2040622321995685 for Melatonin restores osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells and alleviates bone loss through the HGF/PTEN/Wnt/β-catenin axis by Jun Zhang, Guoliang Jia, Pan Xue and Zhengwei Li in Therapeutic Advances in Chronic Disease
Acknowledgments
We would like to give our sincere appreciation to the reviewers for their helpful comments on this article.
Footnotes
Author contributions: Jun Zhang, Guoliang Jia, Pan Xue and Zhengwei Li designed the study. Jun Zhang and Guoliang Jia collated the data, carried out data analyses and produced the initial draft of the manuscript. Pan Xue and Zhengwei Li contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Zhengwei Li  https://orcid.org/0000-0002-3795-1016
https://orcid.org/0000-0002-3795-1016
Availability of data and material: The datasets generated/analyzed during the current study are available.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jun Zhang, Department of Orthopedics, The Second Hospital of Jilin University, Changchun, P.R. China.
Guoliang Jia, Department of Orthopedics, The Second Hospital of Jilin University, Changchun, P.R. China.
Pan Xue, Department of Orthopedics, The Second Hospital of Jilin University, Changchun, P.R. China.
Zhengwei Li, Department of Orthopedics, The Second Hospital of Jilin University, No. 218, Ziqiang Road, Changchun, Jilin Province 130041, P.R. China.
References
- 1.Ensrud KE, Crandall CJ.Osteoporosis. Ann Intern Med 2017; 167: ITC17–ITC32. [DOI] [PubMed] [Google Scholar]
- 2.Yang HY, Huang JH, Chiu HW, et al. Vitamin D and bisphosphonates therapies for osteoporosis are associated with different risks of atrial fibrillation in women: a nationwide population-based analysis. Medicine (Baltimore) 2018; 97: e12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Dai Z, Lau EHY, et al. Prevalence of bone mineral density loss and potential risk factors for osteopenia and osteoporosis in rheumatic patients in China: logistic regression and random forest analysis. Ann Transl Med 2020; 8: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang DZ, Hou W, Zhou Q, et al. Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-BMP signaling. J Bone Miner Res 2010; 25: 1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang T, Xia C, Chen X, et al. Melatonin promotes the BMP9-induced osteogenic differentiation of mesenchymal stem cells by activating the AMPK/β-catenin signalling pathway. Stem Cell Res Ther 2019; 10: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Meng H, Wang X, et al. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med Sci Monit 2016; 22: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baltatu OC, Amaral FG, Campos LA, et al. Melatonin, mitochondria and hypertension. Cell Mol Life Sci 2017; 74: 3955–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastings MH, Maywood ES, Brancaccio M.Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci 2018; 19: 453–469. [DOI] [PubMed] [Google Scholar]
- 9.Sharan K, Lewis K, Furukawa T, et al. Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J Pineal Res 2017; 63: e12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maria S, Samsonraj RM, Munmun F, et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J Pineal Res 2018; 64: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Wang C, Si J, et al. Melatonin up-regulates bone marrow mesenchymal stem cells osteogenic action but suppresses their mediated osteoclastogenesis via MT2-inactivated NF-κB pathway. Br J Pharmacol 2020; 177: 2106–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Liang T, Zhu Y, et al. Melatonin prevents bone destruction in mice with retinoic acid-induced osteoporosis. Mol Med 2019; 25: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu P, Liu J, Shi J, et al. Melatonin protects ADSCs from ROS and enhances their therapeutic potency in a rat model of myocardial infarction. J Cell Mol Med 2015; 19: 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong F, Shi X, Xiao F, et al. Transplantation of hepatocyte growth factor-modified dental pulp stem cells prevents bone loss in the early phase of ovariectomy-induced osteoporosis. Hum Gene Ther 2018; 29: 271–282. [DOI] [PubMed] [Google Scholar]
- 15.Zhu A, Kang N, He L, et al. MiR-221 and miR-26b regulate chemotactic migration of MSCs toward HGF through activation of Akt and FAK. J Cell Biochem 2016; 117: 1370–1383. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Yu H, Zhu D, et al. miR-136-3p targets PTEN to regulate vascularization and bone formation and ameliorates alcohol-induced osteopenia. FASEB J 2020; 34: 5348–5362. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Yan X, Shi J, et al. Aberrantly expressed miR-188-5p promotes gastric cancer metastasis by activating Wnt/β-catenin signaling. BMC Cancer 2019; 19: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen G, Ren H, Shang Q, et al. Foxf1 knockdown promotes BMSC osteogenesis in part by activating the Wnt/β-catenin signalling pathway and prevents ovariectomy-induced bone loss. EBioMedicine 2020; 52: 102626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng X, Wang Y, Dong Q, et al. DLX2 activates Wnt1 transcription and mediates Wnt/β-catenin signal to promote osteogenic differentiation of hBMSCs. Gene 2020; 744: 144564. [DOI] [PubMed] [Google Scholar]
- 20.Li R, Shen Y.An old method facing a new challenge: re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sci 2013; 92: 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao XL, Chen JJ, Zhang GN, et al. Small molecule T63 suppresses osteoporosis by modulating osteoblast differentiation via BMP and WNT signaling pathways. Sci Rep 2017; 7: 10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikegame M, Hattori A, Tabata MJ, et al. Melatonin is a potential drug for the prevention of bone loss during space flight. J Pineal Res 2019; 67: e12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Chen X, Chen AC, et al. Melatonin restores the osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells by preserving SIRT1-mediated intracellular antioxidant properties. Free Radic Biol Med 2020; 146: 92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C, Xu C, Zhou T, et al. Abnormal osteogenic and chondrogenic differentiation of human mesenchymal stem cells from patients with adolescent idiopathic scoliosis in response to melatonin. Mol Med Rep 2016; 14: 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amstrup AK, Sikjaer T, Mosekilde L, et al. Melatonin and the skeleton. Osteoporos Int 2013; 24: 2919–2927. [DOI] [PubMed] [Google Scholar]
- 26.Hu ZP, Fang XL, Sheng B, et al. Melatonin inhibits macrophage infiltration and promotes plaque stabilization by upregulating anti-inflammatory HGF/c-Met system in the atherosclerotic rabbit: USPIO-enhanced MRI assessment. Vascul Pharmacol 2020; 127: 106659. [DOI] [PubMed] [Google Scholar]
- 27.Wen Q, Zhang S, Du X, et al. The multiplicity of infection-dependent effects of recombinant adenovirus carrying HGF gene on the proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells. Int J Mol Sci 2018; 19: 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres L, Klingberg E, Nurkkala M, et al. Hepatocyte growth factor is a potential biomarker for osteoproliferation and osteoporosis in ankylosing spondylitis. Osteoporos Int 2019; 30: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi XF, Wang H, Xiao FJ, et al. MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem Biophys Res Commun 2016; 470: 670–677. [DOI] [PubMed] [Google Scholar]
- 30.Iekushi K, Taniyama Y, Kusunoki H, et al. Hepatocyte growth factor attenuates transforming growth factor-β-angiotensin II crosstalk through inhibition of the PTEN/Akt pathway. Hypertension 2011; 58: 190–196. [DOI] [PubMed] [Google Scholar]
- 31.Li D, Li L, Chen X, et al. LncRNA GHET1 promotes osteoblast proliferation and differentiation by inhibiting PTEN. Panminerva Med. Epub ahead of print 29 July 2019. DOI: 10.23736/S0031-0808.19.03701-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Zhao R, Wu J, et al. Melatonin protects against Aβ-induced neurotoxicity in primary neurons via miR-132/PTEN/AKT/FOXO3a pathway. Biofactors 2018; 44: 609–618. [DOI] [PubMed] [Google Scholar]
- 33.Patel R, Brzezinska EA, Repiscak P, et al. Activation of β-catenin cooperates with loss of Pten to drive AR-independent castration-resistant prostate cancer. Cancer Res 2020; 80: 576–590. [DOI] [PubMed] [Google Scholar]
- 34.Lu D, Zhou Y, Li Q, et al. Synthesis, in vitro antitumor activity and molecular mechanism of novel furan derivatives and their precursors. Anticancer Agents Med Chem 2020; 20: 1475–1486. [DOI] [PubMed] [Google Scholar]
- 35.Liu TJ, Guo JL.Overexpression of microRNA-141 inhibits osteoporosis in the jawbones of ovariectomized rats by regulating the Wnt/β-catenin pathway. Arch Oral Biol 2020; 113: 104713. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Li Y, Yu K, et al. HOXA10 inhibit the osteogenic differentiation of periodontal ligament stem cells by regulating β-catenin localization and DKK1 expression. Connect Tissue Res. Epub ahead of print 27 April 2020. DOI: 10.1080/03008207.2020.17562711-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_2040622321995685 for Melatonin restores osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells and alleviates bone loss through the HGF/PTEN/Wnt/β-catenin axis by Jun Zhang, Guoliang Jia, Pan Xue and Zhengwei Li in Therapeutic Advances in Chronic Disease
Supplemental material, sj-eps-2-taj-10.1177_2040622321995685 for Melatonin restores osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells and alleviates bone loss through the HGF/PTEN/Wnt/β-catenin axis by Jun Zhang, Guoliang Jia, Pan Xue and Zhengwei Li in Therapeutic Advances in Chronic Disease