Abstract
Background:
The Vesical Imaging-Reporting and Data System (VI-RADS) score is a novel standardized approach to image and report bladder cancer (BC) with multiparametric MRI (mpMRI).
Objectives:
To describe and evaluate the performance of the VI-RADS score using mpMRI and assess its potential clinical applications and limitations.
Methods:
A systematic review was conducted using the MEDLINE and EMBASE electronic bibliographic databases between June 2020 and December 2020. All reports deemed relevant to describe the VI-RADS score and assess its performance and applications were retrieved. Results presentation stands as narrative, purely descriptive synthesis based on aggregate studies data.
Results:
A total of 20 relevant studies were retrieved: three meta-analyses, five prospective studies, and twelve retrospective studies. The retrospective studies covered 1676 patients, while the prospective studies included a total number of 468 patients. Pooled sensitivity, specificity to differentiate muscle-invasive from non-muscle-invasive bladder cancer, ranged from 74.1% to 97.3%, and 77% to 100%, respectively. The chosen VI-RADS score thresholds for this discrimination varied across studies. The interreader agreement ranged from 0.73 to 0.95. Currently, the potential clinical applications of VI-RADS consist of initial BC risk stratification, assessment of neoadjuvant therapies response, and bladder sparing approaches, although further validation is required.
Conclusions:
The VI-RADS score helps to discriminate muscle invasive from non-muscle invasive BC with good performance and reproducibility. A simple algorithm based on four basic questions may enhance its popularization. Further studies are required to validate the clinical applications.
Keywords: magnetic resonance imaging, multimodal therapy, multiparametric, neoadjuvant therapy, urinary bladder neoplasms, urinary bladder neoplasms/diagnosis, urinary bladder neoplasms/diagnostic imaging, staging
Introduction
Bladder cancer (BC) represents a real public health challenge. About 550,00 bladder cancer cases were diagnosed in 2018, worldwide.1 One of the main challenges in bladder cancer management lies in the need to quickly distinguish between patients with non-muscle invasive BC (NMIBC) and patients with muscle invasive BC (MIBC). In Europe, the 5-year age-standardized relative survival rate of all BC was approximately 70%, ranging on average from 60% to 80% between countries.2
Current imaging modalities such as ultrasound or computerized tomography urogram (CTU) have shown limited performance to assess muscle invasion. Despite this, the good overall sensitivity (about 90%) to diagnose BC with CT urography, NMIBC/MIBC discrimination remains challenging.3–5 Therefore, the use of complementary investigations, especially with transurethral resection of bladder tumors (TURBT) is needed. To achieve such a goal, other imaging modalities should be further investigated. Magnetic resonance imaging (MRI) was largely investigated in many cancers but not BC. The Prostate Imaging-Reporting And Data System (PI-RADS) and Breast Imaging-Reporting And Data System (BI-RADS) are already validated and broadly used.6,7 As of mid-2019, they have been 19 ‘reporting and data system’ reported. Four of them included the use of MRI (Liver Imaging and Reporting and Data System, Neck Imaging and Reporting and Data System, BI-RADS, and PI-RADS). Panebianco et al.8 in 2018 provided a novel standardized approach to the imaging and reporting of BC with multiparametric MRI (mpMRI). They developed a Vesical Imaging-Reporting and Data System (VI-RADS) score. After performing a non-systematic literature review in BC and MRI imaging, they proposed the use of T2-weighted (T2W) images, diffusion-weighted magnetic resonance imaging (DWI), dynamic contrast-enhanced image (DCE) tumors appearances to constitute the VI-RADS score. This new score was designed for untreated bladders and does not yet have validated clinical applications. This may explain a relatively low uptake among the radiology and urology communities so far.
Our aims were first to conduct a systematic review of the literature to describe, outreach, and evaluate the performance of the VI-RADS score and secondly to report its potential applications, future outlooks, and limitations.
Methodology
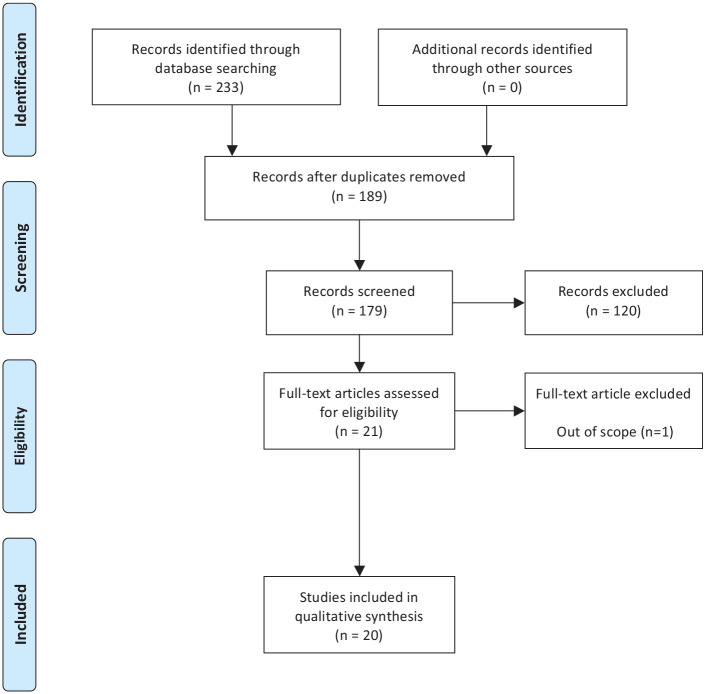
Between June 2020 and December 2020, we conducted a systematic review using the MEDLINE and EMBASE electronic bibliographic databases. An overall PICO assessment for the review was performed (Supplemental Table 1).9 The search strategy included a mix of the following terms: ‘Magnetic Resonance Imaging’, ‘VI-RADS’, ‘Non-muscle invasive bladder cancer’, ‘muscle invasive bladder cancer’, ‘urinary bladder/neoplasm’, ‘urinary bladder/diagnosis’, and ‘urinary bladder/imaging’. The search strategy followed the PRISMA guidelines.10 Only studies written in English or French were accepted. Studies published between January 2018 and December 2020 were sought. The searches were re-run just before the final submission and one additional study was retrieved for inclusion. We included all reports to assess the VI-RADS score, its performance, and its applications. All selected abstracts were implemented in RAYYAN.11 Two authors (DS and GM) independently identified studies that potentially met the inclusion criteria using titles and/or abstracts, followed by a full text analysis also performed independently. The full text of these potentially eligible studies was retrieved and independently assessed. No disagreements between the readers were noted. A total of 20 relevant studies: three meta-analyses, five prospective studies, and twelve retrospective studies were retrieved (Figure 1). Extracted information included: study type, population studied, definition of the VI-RADS score, how performance was assessed, control for accuracy of the scoring system, interobserver agreement, sensitivity, specificity, and accuracy for each score. The specificity and sensitivity we reported for each threshold evaluated regarded the likelihood of muscle invasive disease. A risk bias assessment was performed following the Cochrane Handbook for systematic review (Supplemental Table 2).8,12 Aggregate studies data was used and a purely descriptive narrative synthesis was planned.
Figure 1.
Prisma flow diagram.
VI-RADS score description
Panebianco et al.8 in 2018 provided a standardized approach to imaging and reporting mpMRI for bladder cancer; as a result, they developed the VI-RADS score. This score was designed to assess the likelihood of muscle invasion on pathology (<pT2 versus ⩾pT2) and is based on a high-resolution T2 weighted image (T2WI) acquisition and two functional MRI techniques: diffusion-weighted imaging (DWI) and dynamic contrast enhanced (DCE). These sequences were chosen for the combined advantages they provided; T2W allowed the assessment of the detrusor, while DWI allowed the muscularis propria assessment and the DCE sequence provided accurate information concerning the inner layer. After staging the normal and pathological aspect of each bladder wall layers in each MRI sequences, they offered a five-stage score ranging from 1 (highly unlikely muscle invasion) to 5 (invasion of muscle and beyond the bladder is very likely). Stage 3 corresponds to an equivocal aspect of muscular invasion. It should be noted that spin-Echo T2 weighted image (T1W) acquisition is also required to diagnose eventual hemorrhage and blood clots in the bladder and bone metastasis; however, its findings do not contribute to the score.
Patient preparation
As stated in the original VI-RADS publication,8 antispasmodic agents should be administered to the patient, priory to image acquisition, to minimize artifacts resulting from bowel peristalsis. The authors advised that one apply saturation bands on the anterior abdominal wall to reduce respiratory influences. They also stressed the major importance of adequate distension of the bladder. As a result, patients are instructed to void 1–2 hours before imaging and to drink 500–1000 ml of water 30 minutes before the examination. MRI can be used as a real-time imaging technique to determine bladder filling. They stated the optimal bladder volume for imaging is around 300 ml. In case of under distended bladder, the examination should be delayed by 30–60 minutes. For patients with a urethral catheter, 250–400 ml of sterile saline can be instilled to distend the bladder.
Acquisition modalities
With regards to T2WI acquisition, three planar (axial, coronal, sagittal) images without fat suppression are required. This sequence is the first that radiologists will be looking at. To gain appropriate spatial resolution, 2D or 3D, fast-spin-echo (FSE) or turbo-spin-echo sequences are acquired with a slice thickness of 3–4 mm and a small field of view (FOV). 3D sequences can also be used, with thinner slices and the ability to reconstruct images in the 3 planes.
DWI acquisition is a type of functional acquisition and based on the study of Brownian movements of water molecules. It is possible to carry out acquisitions in DWI sequences on the coronal and/or sagittal planes, particularly for lesions at the bladder dome. The DW sequences have multiple b-values, typically 800–1000 s/mm2 b-values. Contrary to prostate MRI, care must be taken not to produce a too high b-value, as it could harm the signal-to-noise ratio.13 The resolution of the DWI sequence, especially in its anatomical interpretation, is limited due to the signal suppression of the background, making confrontation with T2W images therefore essential.
Dynamic, contrast-enhanced MRI provides accurate information concerning the inner layer of the bladder wall and tumor enhancement. A gadolinium-based contrast agent is administered using a power-injector system at a dose of 0.1 mmol/kg of body weight at a rate of 1.5–2.0 ml/s; it is followed by a saline flush.
Scoring methodology
Each acquisition sequence generates a result for a bladder lesion that will be ranked from one to five, in three categories. T2WI acquisition allows for the analysis of the structural category (SC), while DWI and ADC map are the support of the DW category (DW) and DCE is the support of the contrast-enhanced category (CE).
Firstly, T2WI images are analyzed to evaluate the integrity of the muscularis propria (detrusor) and proceed with the structural category (SC) ranging. The normal bladder mucosa (inner layer) should show high T2W signal intensity (SI) and the detrusor should appear as a low SI regular line on T2W images. Pathological findings appear as an exophytic tumor, with a stalk and/or high SI thickened inner layer, or as a sessile/broad-based tumor with high SI thickened inner layer (score 2/3). An interruption of the low SI line suggests extension of the intermediate SI tumor tissue to muscularis propria (score 4), while extension of the intermediate SI tumor to the perivesical fat represents the invasion of the entire bladder wall and extravesical tissues (score 5). Each corresponding structural category definition (1 to 5) is defined in detailed in Supplemental Table 3.
Following this, DW and CE categories help to further stratify the lesion. On DWI/ADC, muscularis propria should present an intermediate SI, while the stalk and inner layer have low SI on DWI. For high scores, the tumor appears hyperintense on DWI and hypointense on the ADC map with extension to the muscularis propria. On DCE, muscularis propria should maintain no enhancement in the early phase; it is recognizable as a low SI line under the tumor. Tumor and inner layers early enhancement are associated with a higher score. The definition of both DW and DCE categories are detailed in the Supplemental Table 3.
The final VI-RADS score levels correspond to a determined combination of each level defined per category.
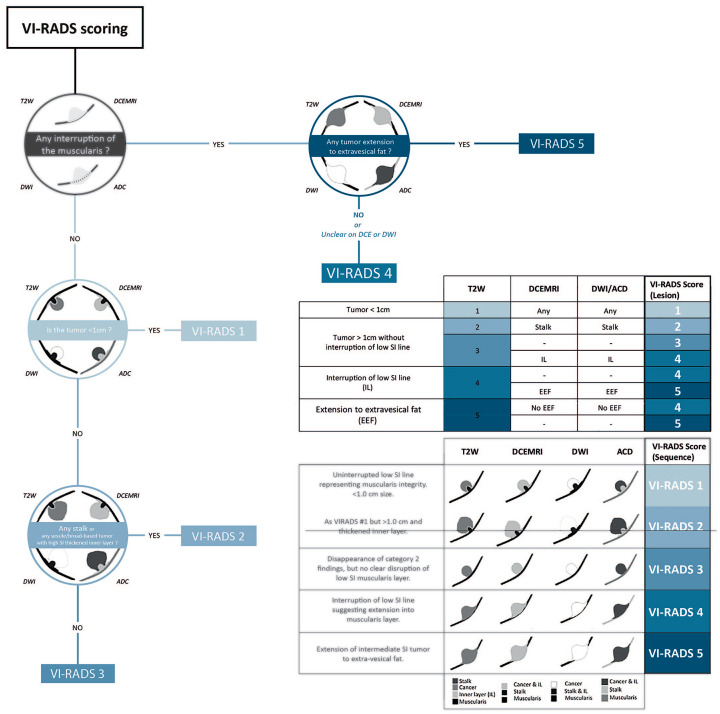
From our early experience, the VI-RADS score can be assess using the following 4 questions: (i) Is there any interruption of the muscularis? (ii) Is there any extension of the intermediate SI tumor to extravesical fat? (iii) What is the lesion size (<1 cm or >1 cm)?(iv) Is there any stalk or sessile/broad-based tumor with high SI thickened inner layer? (Figure 2). The combination of these questions may ease mpMRI scoring and control. To illustrate this simple VI-RADS scoring technique, we provide 2 examples of mpMRI, with their corresponding VI-RADS scores (Figure 3). This algorithm serves as a surrogate to simplify the scoring method for clinicians. This algorithm is not meant to replace a thorough radiological examination. In addition, since tumor location drives unequal likelihood of muscle invasion,14 a systematic use of bladder diagrams is recommended. Such diagram also enhanced the way mpMRI is reported, enhancing communication between radiologists and urologists.
Figure 2.
Step by step method for VI-RADS scoring system.
Adapted with permission from Panebianco et al.8
VI-RADS, vesical imaging-reporting and data systems.
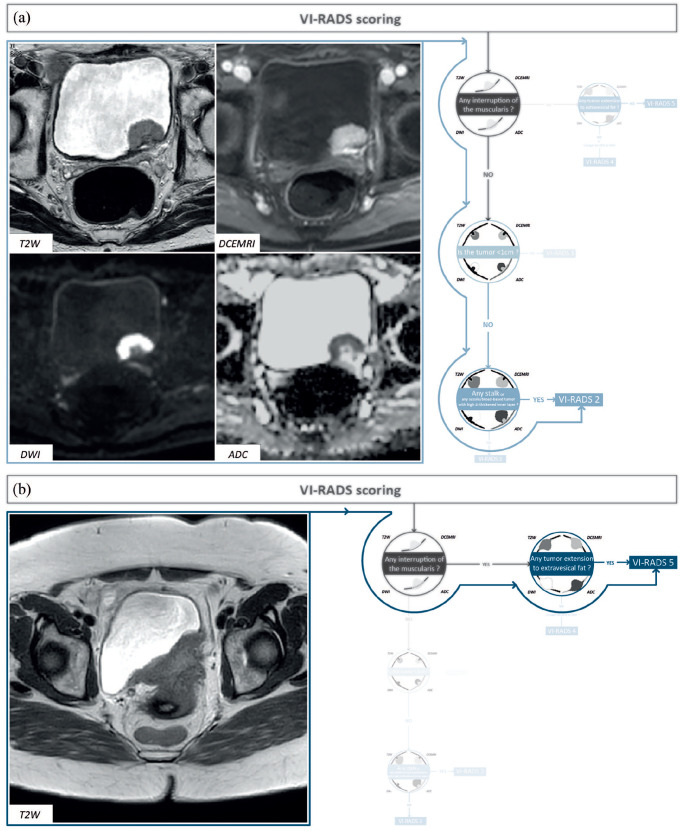
Figure 3.
Examples for VI-RADS scoring using our algorithm. (a) A 70-year-old male with a posterior bladder wall lesion. Following our algorithm, an interruption of low SI line which was not identified, the tumor size was 2 cm, and a stalk was identified thus classifying this lesion as a VI-RADS 2. Pathological analysis revealed a high-grade papillary urothelial carcinoma with no carcinoma in situ associated. (b) A 79-year-old male with prior history of NMIBC. Following our algorithm, an interruption of low SI line was identified, with extension to extravesical fat classifying this lesion as a VI-RADS 5. Pathological analysis revealed a muscle-invasive high-grade urothelial carcinoma.
NMIBC, non-muscle invasive bladder cancer; SI, sacroiliac; VI-RADS, vesical imaging-reporting and data systems.
VI-RADS score performance
Retrospective studies
From our review, 12 retrospective studies performed between 2019 and 2020 were retrieved (Table 1).15–26 These studies covered a total of 1676 patients. Authors of those studies investigated various thresholds to discriminate MIBC from NMIBC. For a VI-RADS 2 threshold, the estimated sensitivity was 100% and the specificity ranged from 21% to 81% (based on 3 studies including 61 patients).21,22,26 For a VI-RADS 3 threshold, all 12 studies provided data. The sensitivity ranged from 78% to 100%, specificity from 44% to 100%.15–24 For a VI-RADS 4 threshold, sensitivity ranged from 63 to 100%, specificity from 76% to 96% (including 7 studies with 88 patients).15,16,19,21–23 Finally, for a VI-RADS 5 threshold, the sensitivity ranged from 68% to 86%, and the specificity from 95% to 99%.15,17,20–23,26 As outlined here, the number of patients used to assess the VI-RADS score performance for each given threshold is limited. There is an unmet need for larger studies.
Table 1.
Results of systematic review analysis on VI-RADS score performance and reproducibility.
| Authors | Study type | Number of patients included | VI-RADS threshold evaluated | Sensitivity per threshold (%) | Specificity per threshold (%) | AUC for chosen threshold | Reference standard | Interreader agreement |
|---|---|---|---|---|---|---|---|---|
| Ueno et al.15 | Retrospective | 74 | ⩾3 ⩾4 |
88 76 |
77 93 |
0.90 (pooled) | TURBT | ICC 0.85 |
| Yoshida et al.25 | Retrospective | 115 | ⩾2 | NA* | 0.83 (T2WI), 0.88 (DWI), 0.84 (DCE) | |||
| Wang et al.17 | Retrospective | 340 | ⩾3 | 87 | 96.5 | 0.94 | TURBT, PC, RC | K 0.92 |
| Kim19 | Retrospective | 297 | ⩾3 | 95 | 44 | NA | TURBT, PC, RC | K 0.89 (T2WI), 0.82 (DWI), 0.85 (DCE) |
| Barchetti et al.20 | Retrospective | 75 | ⩾3 | R1: 90.9, R2: 81.8† | R1: 88.7, R2:84.9† | R1: 0.926, R2: 0.873† | TURBT | K 0.731 |
| Hong et al.26 | Retrospective | 66 | ⩾3 | 90 | 100 | 0.95 | TURBT, RC | ICC 0.979 |
| Liu et al.21 | Retrospective | 126 | ⩾4 | 94 | 92.1 | 0.966 | TURBT, RC | NA |
| Ueno et al.16 | Retrospective | 91 | ⩾4 | 74.1 | 94.1 | 0.87 (pooled Exp.) | TURBT, RC | K 0.67 (Inexp), K 0.75 (Exp versus Inexp) |
| Vaz and Zaparolli22 | Retrospective | 30 | ⩾3 | 100 | 77.2% | 0.9675 (pooled) | TURBT | NA |
| Wang et al.18 | Retrospective | 220 | ⩾3 ⩾4 |
97.3 82.3 |
77.6 95.3 |
0.960 (pooled) | TURBT, PC, RC | NA |
| Sakamoto et al.23 | Retrospective | 176 | ⩾3 ⩾4 |
78 63 |
70 96 |
0.86 (pooled) | TURBT | K 0.43 |
| Arita et al.24 | Retrospective | 66 | ⩾3 | (82.4–94.1) | (83.7, 89.8) | (0.89–0.94) | TURBT | K (0.81–0.91) |
| Makboul et al.27 | Prospective | 50 | ⩾3 | 78 | 88 | 0.83 | TURBT | K 0.87 |
| Del Giudice et al.28 | Prospective | 231 | ⩾3 | 91.9 | 91.1 | 0.94 | TURBT, RC | K 0.92 |
| Marchioni et al.29 | Prospective | 38 (68) | ⩾4 | 85.7 | 86.9 | 0.90 (pooled) | TURBT | ICC 0.76 |
| Del Giudice et al.30 | Prospective | 149 | ⩾4 | 90.2 | 98.1 | 0.94 | TURBT, RC | K 0.89 |
| Metwally et al.31 | Prospective | 331 | >3 | 84.1 | 92.3 | 87.9 | TURBT | K 0.93 |
(Pooled): AUC provided were the result of a calculation of the overall score performance and not for the designated threshold.
Sensibility and specificity where not available, however negative predictive values for subsequent acquisition were 95% for T2W, 95% for DWI, and 94% for DCE.
R1 Reader 1; R2 Reader 2
AUC, area under the curve; Exp, experienced; ICC, intraclass correlation; Inexp, inexperienced; K, kappa; NA, Non-available; PC, Partial cystectomy; RC, Radical cystectomy; TURBT, Trans-urethral resection of bladder tumour; VI-RADS, vesical imaging-reporting and data system.
The estimated area under the curve (AUC) in these studies, the proposed results ranged from 0.83 (in a specified estimated AUC for a T2WI acquisition) to 0.97 (a pooled AUC on every threshold, according to Vaz and Zaparolli22). When available, the overall interreader agreement was rated from fair to excellent, across studies based on the Kappa score ranging widely from 0.43 to 0.92. In the majority of the studies, a dedicated reader, specialized in uro-radiology, conducted the scoring. Only one study included a urologist as a reader.24
Prospective studies
From our systematic review, five prospective studies were retrieved.27–31 These studies included a total number of 799 patients. Del Guidice et al.28,30 studies included a total of 380 patients, Metwally et al.31 included 331 patients; while the two other studies included 50 and 38 patients respectively.27,29
Three studies used a VI-RADS 3 threshold.27,28,31 The reported sensitivity was similar to retrospective studies and ranged from 78% to 92%, while the specificity ranged from 88% to 91%. Only Marchioni et al.29 have specifically chosen a VI-RADS 4 threshold to discriminate NMIBC from MIBC. Their sensitivity was estimated at 85.7% and their specificity 86.9%; however, only 7 patients were classified at this score level (for a total of 38 patients included in the study and 68 tumors evaluated).
The latest report from Del Guidice et al.30 included 149 patients, also using the VI-RADS 4 threshold for NMIBC/MIBC discrimination, although the primary endpoint of this study was to assess the likelihood of locally advanced disease in the pre-TURBT setting. For this endpoint, they chose a VIRADS 5 threshold, which resulted in an estimated sensitivity of 90.2% and a specificity of 98.1%.
These prospective studies reinforced an overall good-to-excellent interreader agreement, with a Kappa score ranging from 0.87 to 0.92 and demonstrating a high AUC ranging from 0.83 to 0.94.
Meta-analysis
Two meta-analyses provided an overall synthesis of VI-RADS performance based on the studies aforementioned.32,33
Woo et al.32,34 included a total number of 1170 of patients from six different studies, with an overall sensitivity of 83% and a specificity of 90%. It should be noted that this meta-analysis pooled different thresholds for the NMIBC/MIBC discrimination, therefore limiting data extrapolation. They reported an AUC of 0.94 and a good-to-excellent overall interreader agreement, ranging from 0.81 to 0.92 (Kappa score). They also reported significant heterogeneity between the studies based on the Cochran’s Q test (Q = 29, p < 0.01) and considerable heterogeneity based on the Higgins I2 statistics (I2 for sensitivity = 88% and I2 for specificity = 91%).
Luo et al.33 included a total of 1064 patients from six different studies (5 studies were included in the previously described meta-analysis). They calculated with pooled data an overall sensitivity of 90% and a specificity of 86%, with the same limitations previously explained. They also reported an AUC of 0.93. Using VI-RADS 4 as the cutoff value, a 77% sensitivity, 97% sensibility, and an AUC of 0.92 were reported. They stressed that the meta-regression analysis revealed that the study design (p = 0.01) and the surgical pattern of reference standard (p = 0.02) were a source of significant heterogeneity of the pooled sensitivity. The interreader agreement was not assessed in this study.
One additional meta-analysis from Del Guidice et al.35 focused on the specifically of the estimation of inter-reader agreement. They selected 8 studies, for a total of 1016 patients, analyzed by 21 uro-radiologists. They were able to confirm an excellent inter-reader agreement of the VI-RADS score to discriminate MIBCs from NMIBCs. The pooled weighted mean kappa estimate was 0.83 [95% confidence interval (CI): 0.78–0.88].
VI-RADS score and bladder mpMRI potential clinical applications - future outlooks
As outlined in this manuscript, MRI and the VI-RADS score are promising. In fact, as of January 2021, 23 ongoing clinical trials are evaluating the role of MRI in all stages of bladder cancer (Table 2). These trials aim to either evaluate mpMRI and the VI-RADS score or each one separately, in all BC stages and to establish the global interest of this imaging modality. Results are eagerly awaited.
Table 2.
Ongoing bladder cancer clinical trials synthesis using mpMRI.
| Clinicialtrial.gov identifier | Phase | Status | Expected Nbr of patients | Eligibility | Imaging | Intervention | Outcomes |
|---|---|---|---|---|---|---|---|
| NCT03914001 | Unknown | Recruiting | 100 | BC post-first TURBT | mpMRI | TURBT | mpMRI performance diagnostic residual malignancy after 1st TURBT |
| NCT02662166 | Unknown | Completed | 50 | Suspected BC based on cystoscopical evaluation | mpMRI | TURBT | Accuracy mpMRI staging BC |
| NCT00938145 | Unknown | Recruiting | 180 | MIBC | 3TMRI/EX-VIVO | Cystectomy + Lymph Node dissection | Agreement in tumor staging: pathology versus 3T-MRI |
| NCT01918592 (ACEBIB) | Unknown | Completed | 25 | BC (non-metastatic) | mpMRI | TURBT | Staging accuracy of PET/MRI in bladder cancer |
| NCT00612326 | Unknown | Completed | 26 | MIBC | mpMRI/11C acetate-PET | SOC | Nodal metastases detection |
| NCT04167631 | Unknown | Active, not recruiting | 150 | BC | mpMRI | En-bloc transurethral resection of bladder tumors (ERBT) | Discriminating NMIBC from MIBC using VIRADS |
| NCT00622973 | III | Completed | 130 | Histologically proven BC scheduled for cystectomy | mpMRI USPIO-enhanced | Radical prostatectomy/cystectomy | Discrimination N+ versus N0 |
| NCT02141490 | II | Completed | 43 | BC with LNI | Ferumoxytol Enhanced MRI | None | Percentage change (from baseline to 24 hours) between metastatic and benign nodes |
| NCT03138837 | Unknown | Unknown | 385 | BC | 3T mpMRI | Possible cystectomy | Assess 3T diagnostic performance |
| NCT04369560 | I | Recruiting | 42 | MIBC/papillary tumor scheduled for TURBT | mpMRI | TURBT | Accuracy MRI to predict pathologic stage at tumor resection |
| NCT02662166 | Unknown | Completed | 50 | Suspected BC | mpMRI | TURBT/cystectomy | Accuracy mpMRI staging BC |
| NCT04533672 | Unknown | Not yet recruiting | 182 | Confirmed or suspected primary BC | mpMRI | TURBT/cystectomy | Presence tumor infiltration into the muscular bladder wall, as per pathology report |
| NCT02203136 | Observational | Completed | 16 | BC | mpMRI | Unspecified bladder cancer surgery | Detection improvement of cancer outside of the bladder |
| NCT03007719 | II | Terminated | 4 | MIBC | PET/MRI | Chemotherapy, immunotherapy, surgery | Assess change in ([18F]F-AraG) uptake in primary and/or M+ tumor(s) on whole-body [18F]F-AraG PET/MR imaging associated with neoadjuvant atezolizumab and (SOC) anti-PD-1 or anti-PD-L1 treatment. |
| NCT01616875 (Bristol Bladder Trial) | II | Unknown | 28 | BC | mpMRI | Chemotherapy, surgery | Overall pathological response rate |
| NCT02662309 (ABACUS) | II | Active, not recruiting | 96 | MIBC | mpMRI | Cystectomy | Efficacy to assess pCR |
| NCT03534492 (NEODURVARIB) | II | Completed | 29 | MIBC | mpMRI | Cystectomy | Radiological response of durvalumab + olaparib as presurgical treatment |
| NCT03327883 | Observational | Completed | 16 | Metastatic BC | FDGPET_MRI | None | Evaluate use of MRI/PET diagnosing/treating M+ BC |
| NCT02287701 | Unknown | Completed | 24 | BC | PET/MRI | None | Tumor SUV from 18-fluorodeoxyglucose PET/MRI scan |
| Bladder Path | II | Recruiting | 950 | BC | mpMRI | Cystectomy, TUBRT | Clinical progression-free survival |
| NCT04588168 | Unknown | Recruiting | 100 | BC | mpMRI | Chemotherapy | Changes from baseline tumor mp-MRI parameters at immediate mp-MRI evaluation |
| NCT04442724 | NA | Recruiting | 60 | BC | mpMRI | Chemo-radiotherapy | Change in bladder volumes targeted for high dose radiation |
| NCT02736266 (PURE-O1) | II | Recruiting | 90 | BC | mpMRI-PET-CT | Immunotherapy | Association between individual and combined MRI sequences and the pathologic response |
3TMRI, 3 Tesla MRI; BC, bladder cancer; CT, computed tomography; ERBC, en-bloc transurethral resection of bladder tumor; FDGPET-MRI, fluoro-deoxy-glucose positron emission tomography-magnetic resonance imaging; (N)MIBC, (non)-muscle invasive bladder cancer; mpMRI, multi-parametric magnetic resonance imaging; MRI, magnetic resonance imaging; pCR, pathological complete response; PET, positron emission tomography; SUV, standardized uptake value; TURBT, trans-urethral resection of bladder tumor; VIRADS, vesical imaging-reporting and data system.
Initial BC risk stratification
Interestingly, the ability of urologists to discriminate stage and grade using only cystoscopy findings is relatively good. A prospective trial, including 224 patients, reported that the positive predictive values for predicting low- and high-grade cancers through cystoscopy were 85.8% and 71.3%, respectively.36 NMIBC and MIBC were predicted accurately in 93.4% and 85.2% patients, respectively. Only 6 out of 161 tumors predicted to be NMIBC were actually MIBC in final TURBT histology. Adding MRI to cystoscopy findings has the ability to unravel a new era in BC diagnosis. In fact, James et al.37 recently presented a phase II/III trial at ASCO 2020 called the BladderPath trial. Based on cystoscopy findings, patients are randomized between the standard of care versus risk-stratified mpMRI-directed care pathways. In the latest guidelines, patients with probable NMIBC undergo TURBT, and those with probable MIBC undergo mpMRI; if MRI confirms the MIBC suspicion, patients undergo RC without TURBT. The endpoints are feasibility, time to correct MIBC therapy, and clinical progression-free survival. They reported the feasibility of randomizing possible MIBC patients to TURBT or mpMRI for staging, based on their preliminary findings.37 The final results are still to be released; however, to our knowledge, the VI-RADS score is not implemented in this clinical trial design.
Response to neoadjuvant therapies
A complete response post NAC (ypT0) is associated with improved overall survival, since about 90% of ypT0 patients were alive at 5 years in the landmark SWOG 8710 trial.38 In this trial, patients were randomized between RC alone or NAC plus RC, and complete response (ypT0) was reported in 38% of the patients in the NAC arm, versus 15% for patients undergoing RC alone. This notion raised the question of potential bladder sparing therapies for this subset of patients.
A recent retrospective study, from a prospectively-maintained single institution database, investigated the reliability of staging post NAC.39 In this study, 114 patients underwent TURBT after NAC and prior to RC. They showed that post-NAC TURBT failed to reliably predict ypT stage at RC since up to 32% of patients were falsely downstaged to NMIBC on post-NAC TURBT. In fact, 53 patients had no evidence of disease (rT0) on post-NAC TUR; however, of these, only 25 (47%) were ultimately ypT0 on final RC pathology. The remaining 28 (53%) had residual disease at RC, including 13 (25%) with residual CIS and 12 (23%) with residual ⩾ypT2 disease, highlighting the challenges in accurately-predicting tumor stage after NAC without definitive RC. This is an area where mpMRI, in addition to TURBT, might improve outcomes by better selecting patients to bladder sparing therapies. This field of research is highly relevant, since multiple trials are currently investigating the ability of bladder sparing approaches post NAC for MIBC, notably RETAIN (Clinicialtrial.gov identifier: NCT02710734), A031701 (Clinicialtrial.gov identifier: NCT03609216), and Hoosier (Clinicialtrial.gov identifier: NCT03558087) trials. However, care needs to be taken when staging with MRI post NAC as well due to inflammatory infiltrations and/or fibrous changes caused by the chemotherapy that can make precise staging more challenging.40 Regarding this question, a useful insight was provided by a study of Ngyuen et al.41 in 2017. They studied 20 patients with pT2 bladder cancer who underwent NAC. Fifteen patients were defined as responders. They demonstrated that a high level of ADC heterogeneity is associated with tumor resistance. Compared to responders, resistant cases had significantly higher entropy (p < 0.01) and lower uniformity (the criteria assessing the image homogeneity) (p = 0.01).
More recently, Ahmed et al.42 evaluated in a prospective study including 90 patients the diagnostic performance of DCE MRI and DWI in assessing pathologic complete response (pCR) after neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer. They specially analyzed the correlation between pCR and semi-quantitative parameters (wash-in rate, wash-out rate) and ADC value. They reported confirmation of 43.3% pCR on cystectomy specimens. Regarding imaging analysis, they demonstrated that pCR had negative correlation with the wash-out rate (r = −0.701, p = 0.01), ADC value (r = −0.621, p = 0.01) and had no significant correlation with wash-in rate (r = 0.187, p = 0.14). They concluded that the semi-quantitative parameter (wash-out) derived from DCE MRI and ADC could assess the tumor’s complete pathologic response. They reported the potential of those parameters in assessing tumor response to NAC in MIBC for the combination of wash-out rate and ADC value. Despite this, like other authors, they also report the difficulty induced by the lack of standardization of ADC measurement technique, representing an inherent limitation of DWI.
The performance of MRI to assess tumor response to neoadjuvant pembrolizumab in MIBC was also recently reported in an ancillary study of PURE-01 (open-label, single-arm, phase II study assessing the activity of pembrolizumab as neoadjuvant therapy before RC in patients with MIBC).43,44 In PURE-01, 143 T2-T4aN0M0 patients received 200 mg pembrolizumab intravenously for three cycles before radical cystectomy. The reported pCR was 42%. Out of the 143 included patients, 136 patients underwent pre- and post-pembrolizumab mpMRI.45,46 Nine patients received additional neoadjuvant chemotherapy; four could not receive intravenous gadolinium contrast due to severe renal function impairment. This ancillary study’s objective was to evaluate the association between bladder mpMRI findings and the complete pathological response (pT0). In their first publication, they reported that mpMRI rightfully diagnosed a pT0 stage in 23/37 (62%) and 19/26 (73%) patients in internal and external review, respectively.45 These assessments were only qualitative; in the first exploratory analysis, the mean ADC values in post-therapy regions of interest was not associate with pathological findings. In the second publication specifically focused on this question, they reported an overall estimated accuracy of 0.72 in predicting ypT0N0 response for mpMRI. In addition, they evaluated the performance of each MRI sequences alone and the performance of biparametric MRI. The prediction of pathologic-downstaging to non-muscle-invasive cancer was accurate in 95% of patients in either assessment method.46
As a result, those publications paved the way to support mpMRI as a non-invasive tool in the neoadjuvant setting; despite this, to our knowledge, the previously mentioned study did not use the VI-RADS score.
Bladder sparing approaches
MRI and the VI-RADS score may also play a role in MIBC trimodal therapy (TMT), one of the most promising bladder-sparing approaches. In fact, the largest retrospective single center cohort so far, with a median follow-up of 7 years, reported comparable oncological outcomes to RC: the 5- and 10-yr overall survival rates were 57% and 39%, respectively.47 The rate of salvage cystectomy at 5 years was 29%. MRI and VI-RADS score may help to improve patient selection to TMT since the overall MRI performance to discriminate T2 tumors from T3-4 seems promising. Daneshmand et al.48 evaluated the accuracy of mpMRI to detect extravesical BC and lymph node-positive disease in patients with MIBC in a prospective study. They included a total number of 122 patients with 15 patients (12.3%) pT4, 27 (22.1%) pT3, and 38 (31.1%) pT2. They found that mpMRI (with DCE) correctly differentiated organ-confined MIBC (pT2 pN0) from locally advanced (pT3-4 pN0) MIBC in 74% of lymph node-negative cases. The overall sensitivity and specificity for such differentiation were 87.5% and 47.6% respectively, with an accuracy to detect lymph node positive disease was 80% (40% sensitivity and 91% specificity).
Moreover, enhancing post radiation-based bladder sparing staging is of paramount importance. In fact, a recent single-center retrospective study on patients with MIBC (cT2-4aN0-2M0) treated with curative intent RT assessed the role of tumor bed biopsy.49 Although the large majority of patients were correctly staged with cystoscopy, the authors reported a 5% rate of residual MIBC in optically normal bladders post TMT. In fact, Yoshida et al.50 in 2010 included in a small prospective study (n = 20) T2-4aN0M0 MIBC patients who underwent induction ‘low-dose’ chemoradiotherapy (40 Gy with concomitant 2 cycles of cisplatin) followed by partial (n = 13) or radical cystectomy (n = 7). They reported that all macroscopic residual tumors were depicted with the use of mpMRI. The use of DWI alone was associated with a high sensitivity of 92% (12 of 13 patients concerned) to predict pathologic residual BC. As a result, a standardized use of DWI, which was not the case at time of this study, may help improve the detection of residual tumor after radiation-based therapies for MIBC.
Futures outlooks
The ability of mpMRI to determine the tumor aggressiveness was previously explored. Takeuchi et al stated that the presence of a low ADC (<1 × 10−3 mm2/sec) could indicate that the tumor is more likely to be high grade.13 They included 40 patients in their final analysis (52 tumors analyzed). They generated ADC maps of the 41 tumors large enough (>5 mm) to contain regions of interest. The differences in ADC were significant between G1 and G3 tumors and between G2 and G3 tumors (both p < 0.01), but not between G1 and G2. To nuance their results, the authors mentioned that because the histological grade and ADC are each influenced by other factors than cellular density, the correlation between them could be limited. Moreover, Kobayashi et al.51 provided further interesting data and nuances regarding the link between ADC values and tumor aggressiveness. They stated that ADC values were significantly lower in high-grade (versus low-grade, p < 0.0001) and higher stage (T2 versus T1 versus Ta, p < 0.0001) tumors. As a result, an ADC value cut-off of 0.86 × 10–3 mm2/s best differentiated clinically aggressive disease from less aggressive disease (low-grade Ta, high- grade Ta, or low-grade T1) with a sensitivity of 88%, a specificity of 85%, and an accuracy of 87%. Despite this, the cutoff provided in the aforementioned study depends largely on the system and sequences used to generate and calculate the ADC values, therefore limiting reproducibility. Implementing ADC values to assess tumor aggressiveness could further enhance the standardization provided by the VI-RADS score.
Regarding future technical outcomes, recent literature also provides examples of how specific modalities can help further develop the exploitation of mpMRI for discrimination of muscle-invasive bladder cancer. Meng et al.52 designed a study which aimed to evaluate the image quality of a reduced filed-of-view (rFOV) compared to the full field-of-view (fFOV) for bladder cancer assessment and further evaluate the superiority of a bi-planar rFOV DWI over single planar rFOV DWI in predicting muscle-invasiveness of bladder cancer. They notably made the hypothesis that since the bladder has a spherical shape, the single conventional plane could not identify the invasiveness depth. As a result, a bi-planar (axial combined with sagittal) could improve the BC characterization. In their study, they retrospectively included 61 patients with a total number of 89 lesions, 42 patients were classified as NMIBC, and 19 patients were carriers of MIBC. Their analysis demonstrated a significantly lower ADC value of the MIBC group than the one of NMIBC group in both rFOV DWI and fFOV DWI sequences. They assumed that rFOV DWI could help improve the quality of scoring and better anatomic description in comparison of the fFOV DWI. They also exploited the VI-RADS score based on a bi-planar rFOV DWI and demonstrated that it allows a high predictive performance (AUC = 0.946) for predicting muscle-invasiveness.
Furthermore, Li et al.53 compared the diagnostic performance of conventional DWI and diffusion kurtosis imaging (DKI) for MIBC/NMIBC discrimination. They assumed that, since DKI provides better information regarding the irregularity and heterogeneity of cell microstructure and tissue components, it could demonstrate a valuable diagnostic performance for predicting bladder muscle invasion. They included 51 patients with 19 cases of MIBC and 32 cases of NMIBC. They reported that both ADC and DKI values differed significantly between MIBC and NMIBC. It should be noted that the apparent diffusional kurtosis values were higher in the MIBC group than those of the MIBC group. Those two publications, among others, also tend to demonstrate the vast potentiality of both mpMRI and VI-RADS scoring development in bladder cancer management.
Limitations of the VI-RADS score
Aside of the previously outlined limitations regarding the VI-RADS score and our risk of bias analysis, there are several other shortcomings that should be pointed out. As the VI-RADS developers have recognized, the aim of this system was to create a score for untreated patients to differentiate NMIBC from MIBC.54 Therefore, the potential clinical applications outside of this scope and outlined in this manuscript should be validated by dedicated studies. Moreover, the current state of the score does not take into account several validated predictors of muscle invasion such as ureteral infiltration (responsible of hydronephrosis) or the number and location of lesions (including diverticular tumors). In addition, there is currently no reliable way to depict several adverse pathological features commonly use in patient risk stratification with the use of mpMRI such as CIS, lymphovascular invasion, and variant histology. Currently, the VI-RADS score should serve as an adjunction of pathological assessment.
Several reports on MRI and VI-RADS score had also offered the possibility of using MRI in NMIBC surveillance. However, such use of mpMRI will require serial acquisitions over time, which is associated with a heavy cost-related burden compared to cystoscopy.55 In addition, systematic use of mpMRI will require multiple contrast injection that are associated with side effects.56,57 Some authors also argued that the cost-reduction induced by the use of the VI-RADS in the staging settings might be beneficial; however, dedicated cost-analysis studies are warranted to address this point. MRI accessibility is another challenge: the use of mpMRI should not delay treatment, especially in MIBC.58,59 Several reports have shown the negative effect on survival associated with a delay in radical treatment administration.60,61 Moreover, the reliability in scoring among non-specialized radiologists is unknown. The data outlined in this report are mainly from academic centers. Future, larger studies should validate the inter-reader agreement.
Conclusion
The VI-RADS score was designed to assess the likelihood of muscle invasion using mpMRI. This score helps to discriminate MIBC from NMIBC, with good performance and reproducibility in a non-invasive manner. A simple algorithm based on four basic questions may enhance its popularization. Besides initial BC risk stratification, response to post neoadjuvant therapies and bladder sparing approaches are other potential clinical applications of the score.
Supplemental Material
Supplemental material, sj-docx-1-tau-10.1177_17562872211039583 for Multiparametric magnetic resonance imaging for bladder cancer: a comprehensive systematic review of the Vesical Imaging-Reporting and Data System (VI-RADS) performance and potential clinical applications by Denis Séguier, Philippe Puech, Ronald Kool, Léa Dernis, Héléna Gabert, Wassim Kassouf, Arnauld Villers and Gautier Marcq in Therapeutic Advances in Urology
Footnotes
Author contributions: (I) Conception and design: GM, DS, PP
(II) Administrative support: None
(III) Provision of study materials or patients: Non applicable
(IV) Collection and assembly of data: GM, DS, LD, HG
(V) Data analysis and interpretation: GM, DS, PP, RK, WK
(VI) Manuscript writing: All authors
(VII) Final approval of manuscript: All authors
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Ronald Kool  https://orcid.org/0000-0001-7471-6456
https://orcid.org/0000-0001-7471-6456
Gautier Marcq  https://orcid.org/0000-0002-1806-1448
https://orcid.org/0000-0002-1806-1448
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Denis Séguier, Urology Department, Claude Huriez Hospital, CHU Lille, Lille, Hauts-de-France, France.
Philippe Puech, Univ. Lille, Inserm, CHU Lille, Department of Radiology, U1189 - ONCO-THAI - Image Assisted Laser Therapy for Oncology, Lille, France.
Ronald Kool, Division of Urology, McGill University Health Centre, McGill University, Montreal, QC, Canada.
Léa Dernis, Department of Radiology, U1189 - ONCO-THAI - Image Assisted Laser Therapy for Oncology, Lille, France.
Héléna Gabert, Urology Department, Claude Huriez Hospital, CHU Lille, Lille, Hauts-de-France, France.
Wassim Kassouf, Division of Urology, McGill University Health Centre, McGill University, Montreal, QC, Canada.
Arnauld Villers, Urology Department, Claude Huriez Hospital, CHU Lille, Lille, Hauts-de-France, France; Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, UMR9020-U1277 – CANTHER - Cancer Heterogeneity Plasticity and Resistance to Therapies, Lille, France.
Gautier Marcq, Lille University, School of Medicine, Urology Department, Claude Huriez Hospital, CHRU Lille, LILLE Cedex, France Researcher - PhD Candidate, Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, UMR9020-U1277 – CANTHER - Cancer Heterogeneity Plasticity and Resistance to Therapies, Lille, France; Lille University, School of Medicine, Urology Department, Claude Huriez Hospital, CHRU Lille, LILLE Cedex, France; Researcher - PhD Candidate, Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, UMR9020-U1277 – CANTHER - Cancer Heterogeneity Plasticity and Resistance to Therapies, Lille, France.
References
- 1.Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol 2020; 38: 1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babjuk M.Trends in bladder cancer incidence and mortality: success or disappointment? Eur Urol 2017; 71: 109–110. [DOI] [PubMed] [Google Scholar]
- 3.Hilton S, Jones LP.Recent advances in imaging cancer of the kidney and urinary tract. Surg Oncol Clin N Am 2014; 23: 863–910. [DOI] [PubMed] [Google Scholar]
- 4.Lee CH, Tan CH, Faria SDC, et al. Role of imaging in the local staging of urothelial carcinoma of the bladder. Am J Roentgenol 2017; 208: 1193–1205. [DOI] [PubMed] [Google Scholar]
- 5.Galgano SJ, Porter KK, Burgan C, et al. The role of imaging in bladder cancer diagnosis and staging. Diagnostics 2020; 10: 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balleyguier C, Ayadi S, Van Nguyen K, et al. BIRADS classification in mammography. Eur J Radiol 2007; 61: 192–194. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol 2016; 69: 16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panebianco V, Narumi Y, Altun E, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur Urol 2018; 74: 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochrane Training. Chapter 9: summarizing study characteristics and preparing for synthesis [Internet], https://training.cochrane.org/handbook/current/chapter-09 (accessed 18 June 2021).
- 10.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochrane Training. Chapter 25: assessing risk of bias in a non-randomized study [Internet], https://training.cochrane.org/handbook/current/chapter-25 (accessed 18 June 2021).
- 13.Takeuchi M, Sasaki S, Ito M, et al. Urinary bladder cancer: diffusion-weighted MR imaging—accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009; 251: 112–121. [DOI] [PubMed] [Google Scholar]
- 14.Xiao G-Q, Rashid H.Bladder neck urothelial carcinoma: a urinary bladder subsite carcinoma with distinct clinicopathology. Int J Surg Pathol 2015; 23: 517–523. [DOI] [PubMed] [Google Scholar]
- 15.Ueno Y, Takeuchi M, Tamada T, et al. Diagnostic accuracy and interobserver agreement for the vesical imaging-reporting and data system for muscle-invasive bladder cancer: a multireader validation study. Eur Urol 2019; 76: 54–56. [DOI] [PubMed] [Google Scholar]
- 16.Ueno Y, Tamada T, Takeuchi M, et al. VI-RADS: multiinstitutional multireader diagnostic accuracy and interobserver agreement study. AJR Am J Roentgenol. Epub ahead of print 29 July 2020. DOI: 10.2214/AJR.20.23604. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Luo C, Zhang F, et al. Multiparametric MRI for bladder cancer: validation of VI-RADS for the detection of detrusor muscle invasion. Radiology 2019; 291: 668–674. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Shang Y, Luan T, et al. Evaluation of the value of the VI-RADS scoring system in assessing muscle infiltration by bladder cancer. Cancer Imaging 2020; 20: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SH.Validation of vesical imaging reporting and data system for assessing muscle invasion in bladder tumor. Abdom Radiol (NY) 2020; 45: 491–498. [DOI] [PubMed] [Google Scholar]
- 20.Barchetti G, Simone G, Ceravolo I, et al. Multiparametric MRI of the bladder: inter-observer agreement and accuracy with the Vesical Imaging-Reporting and Data System (VI-RADS) at a single reference center. Eur Radiol 2019; 29: 5498–5506. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Xu F, Xu T, et al. Evaluation of Vesical Imaging-Reporting and Data System (VI-RADS) scoring system in predicting muscle invasion of bladder cancer. Transl Androl Urol 2020; 9: 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaz A, Zaparolli M.Diagnostic accuracy of retrospective application of the vesical imaging-reporting and data system: preliminary results. Radiol Bras 2020; 53: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto K, Ito M, Ikuta S, et al. Detection of muscle-invasive bladder cancer on biparametric MRI using Vesical Imaging-Reporting and Data System and Apparent Diffusion Coefficient values (VI-RADS/ADC). Bladder Cancer 2020; 6: 161–169. [Google Scholar]
- 24.Arita Y, Shigeta K, Akita H, et al. Clinical utility of the vesical imaging-reporting and data system for muscle-invasive bladder cancer between radiologists and urologists based on multiparametric MRI including 3D FSE T2-weighted acquisitions. Eur Radiol. Epub ahead of print 23 August 2020. DOI: 10.1007/s00330-020-07153-5. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida* S, Tanaka H, Kijima T, et al. MP80-03 staging accuracy of Vesical Imaging-Reporting and Data System (VI-RADS) for bladder cancer. J Urol 2019; 201(Suppl 4): e1161–e1161. [Google Scholar]
- 26.Hong SB, Lee NK, Kim S, et al. Vesical imaging-reporting and data system for multiparametric MRI to predict the presence of muscle invasion for bladder cancer. J Magn Reson Imaging 2020; 52: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 27.Makboul M, Farghaly S, Abdelkawi IF.Multiparametric MRI in differentiation between muscle invasive and non-muscle invasive urinary bladder cancer with Vesical Imaging Reporting and Data System (VI-RADS) application. Br J Radiol 2019; 92: 20190401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Giudice F, Barchetti G, De Berardinis E, et al. Prospective assessment of Vesical Imaging Reporting and Data System (VI-RADS) and its clinical impact on the management of high-risk non-muscle-invasive bladder cancer patients candidate for repeated transurethral resection. Eur Urol. Epub ahead of print 5 November 2019. DOI: 10.1016/j.eururo.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Marchioni M, Primiceri G, Delli Pizzi A, et al. Could bladder multiparametric MRI be introduced in routine clinical practice? Role of the new VI-RADS score: results from a prospective study. Clin Genitourin Cancer 2020; 18: 409–415.e1. [DOI] [PubMed] [Google Scholar]
- 30.Del Giudice F, Leonardo C, Simone G, et al. Preoperative detection of VI-RADS (Vesical Imaging-Reporting and Data System) score 5 reliably identifies extravesical extension of urothelial carcinoma of the urinary bladder and predicts significant delayed time-to-cystectomy: time to reconsider the need for primary deep trans-urethral resection of bladder tumor in case of locally advanced disease? BJU Int 2020; 126: 610–619. [DOI] [PubMed] [Google Scholar]
- 31.Metwally MI, Zeed NA, Hamed EM, et al. The validity, reliability, and reviewer acceptance of VI-RADS in assessing muscle invasion by bladder cancer: a multicenter prospective study. Eur Radiol. Epub ahead of print 19 February 2021. DOI: 10.1007/s00330-021-07765-5. [DOI] [PubMed] [Google Scholar]
- 32.Woo S, Panebianco V, Narumi Y, et al. Diagnostic performance of vesical imaging reporting and data system for the prediction of muscle-invasive bladder cancer: a systematic review and meta-analysis. Eur Urol Oncol 2020; 3: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo C, Huang B, Wu Y, et al. Use of Vesical Imaging-Reporting and Data System (VI-RADS) for detecting the muscle invasion of bladder cancer: a diagnostic meta-analysis. Eur Radiol 2020; 30: 4606–4614. [DOI] [PubMed] [Google Scholar]
- 34.Woo S, Panebianco V, Narumi Y, et al. Corrigendum to ‘Diagnostic performance of vesical imaging reporting and data system for the prediction of muscle-invasive bladder cancer: a systematic review and meta-analysis’ [European Urology Oncology 3 (2020) 306-315]. Eur Urol Oncol 2020; 3: 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Giudice F, Pecoraro M, Vargas HA, et al. Systematic review and meta-analysis of Vesical Imaging-Reporting and Data System (VI-RADS) inter-observer reliability: an added value for muscle invasive bladder cancer detection. Cancers 2020; 12: 2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariappan P, Lavin V, Phua CQ, et al. Predicting grade and stage at cystoscopy in newly presenting bladder cancers-a prospective double-blind clinical study. Urology 2017; 109: 134–139. [DOI] [PubMed] [Google Scholar]
- 37.James ND, Pirrie S, Liu W, et al. Replacing TURBT with mpMRI for staging MIBC: pilot data from the BladderPath study. J Clin Oncol 2020; 38: 446–446. [Google Scholar]
- 38.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. New Engl J Med 2003; 49: 859–866. [DOI] [PubMed] [Google Scholar]
- 39.Becker REN, Meyer AR, Brant A, et al. Clinical restaging and tumor sequencing are inaccurate indicators of response to Neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. Epub ahead of print 17 August 2020. DOI: 10.1016/j.eururo.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura K, Fujiyama C, Nakashima K, et al. The effects of neoadjuvant chemotherapy and chemo-radiation therapy on MRI staging in invasive bladder cancer: comparative study based on the pathological examination of whole layer bladder wall. Int Urol Nephrol 2009; 41: 869–875. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen HT, Mortazavi A, Pohar KS, et al. Quantitative assessment of heterogeneity in bladder tumor MRI diffusivity: can response be predicted prior to Neoadjuvant chemotherapy? Bladder Cancer 2017; 3: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed SA, Taher MGA, Ali WA, et al. Diagnostic performance of contrast-enhanced dynamic and diffusion-weighted MR imaging in the assessment of tumor response to neoadjuvant therapy in muscle-invasive bladder cancer. Abdom Radiol (NY) 2021; 46: 2712–2721. [DOI] [PubMed] [Google Scholar]
- 43.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol 2018; 36: 3353–3360. [DOI] [PubMed] [Google Scholar]
- 44.Necchi A, Raggi D, Gallina A, et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol 2020; 77: 439–446. [DOI] [PubMed] [Google Scholar]
- 45.Necchi A, Bandini M, Calareso G, et al. Multiparametric magnetic resonance imaging as a noninvasive assessment of tumor response to neoadjuvant pembrolizumab in muscle-invasive bladder cancer: preliminary findings from the PURE-01 study. Eur Urol 2020; 77: 636–643. [DOI] [PubMed] [Google Scholar]
- 46.Bandini M, Calareso G, Raggi D, et al. The value of multiparametric magnetic resonance imaging sequences to assist in the decision making of muscle-invasive bladder cancer. Eur Urol Oncol. Epub ahead of print 27 June 2020. DOI: 10.1016/j.euo.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Giacalone NJ, Shipley WU, Clayman RH, et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the Massachusetts General Hospital experience. Eur Urol 2017; 71: 952–960. [DOI] [PubMed] [Google Scholar]
- 48.Daneshmand S, Ahmadi H, Huynh LN, et al. Preoperative staging of invasive bladder cancer with dynamic gadolinium-enhanced magnetic resonance imaging: results from a prospective study. Urology 2012; 80: 1313–1318. [DOI] [PubMed] [Google Scholar]
- 49.Kool R, Marcq G, El-Achkar A, et al. Refining assessment of response to radiation-based therapy for muscle-invasive bladder cancer: is post-treatment tumor bed biopsy always necessary? Urol Oncol. Epub ahead of print 23 October 2020. DOI: 10.1016/j.urolonc.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida S, Koga F, Kawakami S, et al. Initial experience of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology 2010; 75: 387–391. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi S, Koga F, Yoshida S, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol 2011; 21: 2178–2186. [DOI] [PubMed] [Google Scholar]
- 52.Meng X, Hu H, Wang Y, et al. Application of bi-planar reduced field-of-view DWI (rFOV DWI) in the assessment of muscle-invasiveness of bladder cancer. Eur J Radiol 2021; 136: 109486. [DOI] [PubMed] [Google Scholar]
- 53.Li Q, Cao B, Tan Q, et al. Prediction of muscle invasion of bladder cancer: a comparison between DKI and conventional DWI. Eur J Radiol 2021; 136: 109522. [DOI] [PubMed] [Google Scholar]
- 54.Panebianco V, Barentsz J, Narumi Y, et al. Reply to Andrea Necchi, Antonella Messina, and Alberto Briganti’s letter to the editor re: Valeria Panebianco, Yoshifumi Narumi, Ersan Altun, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur Urol 2018;74:294–306. Eur Urol 2018; 74: e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svatek RS, Hollenbeck BK, Holmäng S, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 2014; 66: 253–262. [DOI] [PubMed] [Google Scholar]
- 56.Guo BJ, Yang ZL, Zhang LJ.Gadolinium deposition in brain: current scientific evidence and future perspectives. Front Mol Neurosci 2018; 11: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adin ME, Yousem DM.Disappearance of T1-weighted MRI hyperintensity in dentate nuclei of individuals with a history of repeat gadolinium administration. Radiology 2018; 288: 911–912. [DOI] [PubMed] [Google Scholar]
- 58.Pecoraro M, Takeuchi M, Vargas HA, et al. Overview of VI-RADS in bladder cancer. AJR Am J Roentgenol 2020; 214: 1259–1268. [DOI] [PubMed] [Google Scholar]
- 59.Panebianco V, Del Giudice F, Leonardo C, et al. VI-RADS scoring criteria for alternative risk-adapted strategies in the management of bladder cancer during the COVID-19 pandemic. Eur Urol 2020; 78: e18–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sánchez-ortiz RF, Huang WC, Mick R, et al. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol 2003; 169: 110–115; discussion 115. [DOI] [PubMed] [Google Scholar]
- 61.Stein JP.Contemporary concepts of radical cystectomy and the treatment of bladder cancer. J Urol 2003; 169: 116–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tau-10.1177_17562872211039583 for Multiparametric magnetic resonance imaging for bladder cancer: a comprehensive systematic review of the Vesical Imaging-Reporting and Data System (VI-RADS) performance and potential clinical applications by Denis Séguier, Philippe Puech, Ronald Kool, Léa Dernis, Héléna Gabert, Wassim Kassouf, Arnauld Villers and Gautier Marcq in Therapeutic Advances in Urology