Abstract
Ileal Atresia is noted to be the commonest cause of intestinal obstruction in neonates worldwide but still under diagnosed in Sub-Saharan countries with scarce data reported on its occurrence. It is likely under diagnosed due to low index of suspicion. Advancement in foetal ultrasound during prenatal period will increase index of suspicion and hence early diagnosis and correction. This is the first case report in our setup highlighting this condition. We present a case of a 10-day-old male baby referred to us due to vomiting since birth. Clinically was in distress with a palpable supraumbilical mass that was firm and non-tender. Abdominal ultrasound scan showed poor peristalsis and minimal dilation of bowels suggestive of partial intestinal obstruction. Abdominal X-ray showed dilated bowels with multiple air-fluid levels, empty rectum with features suggestive of intestinal obstruction. Patient was successfully operated but unfortunately on day 3 post-surgery succumbed.
Keywords: Ileal atresia, neonatal intestinal obstruction, Tanzania
Background
Intestinal atresia refers to a congenital obstruction with complete occlusion of the intestinal lumen.1 This is one of the most frequent causes of bowel obstruction in the newborn and can occur at any point in the gastrointestinal tract.2 Among the intestinal atresias, Jejuno-ileal atresia (JIA) remains the most common form of intestinal obstruction in the paediatric group.3
JIA is caused by an in-utero vascular accident during foetal development involving branches of mesenteric vessels in the midgut, resulting to ischaemic necrosis leaving a blind proximal loop with distal being atretic.4
We report a case of a 10-day-old male baby who was diagnosed with ileal atresia pointing out as rare condition with difficulties in early diagnosis and post-operative care.
Case presentation
A 10-day-old male baby was referred to our centre due to vomiting since birth. The baby was born at home by vaginal delivery at term hence birth weight and Apgar score were unknown. The baby was nursed at home for 9 days when the mother decided to take him to a nearby health centre because of post-prandial vomiting and the baby was lethargic. Due to the critical condition, the baby was referred straight away for paediatric and surgical care.
Examination on admission, the baby was sick looking, wasted, dehydrated, in respiratory distress, pink in room air and saturating at 98%. His temperature was 37°C, respiratory rate was 80 breaths/minute and random blood glucose of 9.2 mmol/L. On respiratory examination, he had marked lower chest in drawing, nasal flaring, grunting with transmitted sounds. His abdomen was not distended, soft with a palpable mass in the supraumbilical region. Digital rectal exam was normal with normal male external genitalia.
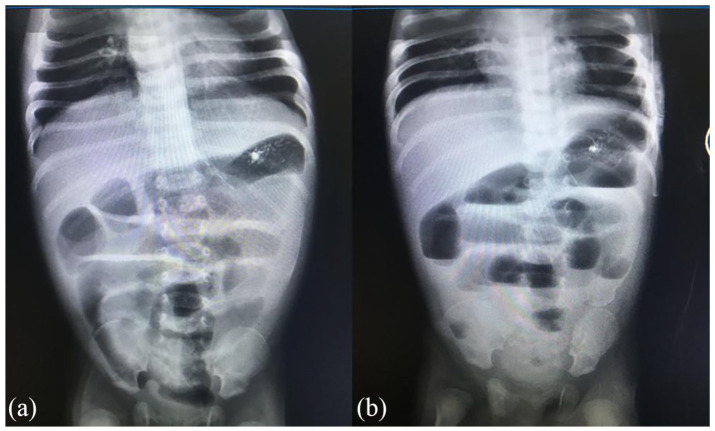
His abdominal ultrasound showed poor peristalsis and minimal dilation of bowel suggestive of intestinal obstruction. Abdominal X-ray showed dilated bowels with multiple air-fluid levels, empty rectum features suggestive of intestinal obstruction (Figure 1). His blood work-up showed a low serum sodium level of 117.82 mmol/L, potassium of 5.87 mmol/L, urea of 34.92 mmol/L, serum creatinine of 317 µmol/L with normal liver enzymes. Serology for Human Immunodeficiency Virus (HIV) was negative. We reached a diagnosis of IO, Late Onset Neonatal Sepsis and Acute Kidney Injury secondary to dehydration. The baby was kept nil orally and started on intravenous (IV) fluids 30 mL bolus for 30 min for shock management and was thereafter kept on maintenance Dextrose Normal Saline 500 mL with 3% hypertonic saline to correct the hyponatremia. Urethral catheter was inserted to monitor urine output and IV Ceftriaxone 255 mg once daily.
Figure 1.
Plain abdominal X-ray. (a) Supine view showing dilated small bowel loops. (b) Erect view showing multiple air-fluid levels.
After stabilizing and correcting of electrolytes (serum sodium and potassium), on day 3 post admission, laparotomy was done and intra-operatively there was distended loop of small intestine about 70 cm from Treitz Ligamentum with blind end, the distal loop was collapsed with a meconium plug at its blind loop (Figure 2). Patency was established by an end-to-end ileo-ileal anastomosis was done of the blind loops by increasing the distal calibre using a 2 cm slit on the antimesenteric side. Distal loop patency was confirmed by injecting saline. The mesentery vent was closed, one drain inserted and abdomen closed in layers after thorough abdominal lavage with saline.
Figure 2.
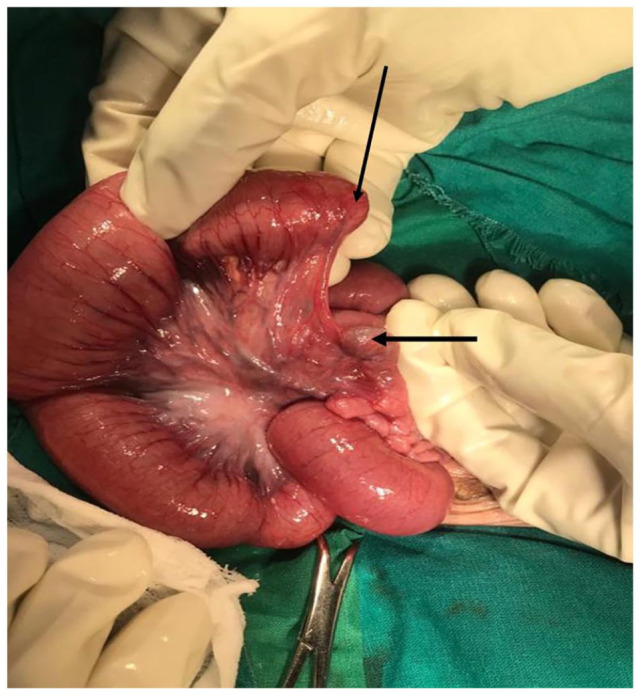
Intraoperative photograph showing type IIIa jejuno-ileal atresia (arrows showing blind ends).
On the second day post laparotomy, the baby was still very critical on maintenance IV fluids. Control laboratory investigations showed elevated serum creatinine of 244 µmol/L, serum sodium of 160.06 mmol/L, serum potassium of 2.87 mmol/L, serum urea of 34.33 mmol/L, total protein of 27.7 g/L and Albumin 15.94 g/L. He sustained multiple cardiac arrests over a period of 3 days. On all these occasions, he was resuscitated and responded yet remained in very critical condition. On day 3 post-surgery, he succumbed.
Discussion
The estimated prevalence of JIA ranges from 1 in 5000 to 1 in 14,000 live births.5,6 JIA is less common with other congenital anomalies but associated conditions are cystic fibrosis, intestine malrotation and gastroschisis, all of which are present in about 10% of cases.7,8
In 29%–50% of JIA cases, it can be prenatally detected on foetal ultrasound as polyhydramnios, ascites, dilated bowel loops and enhanced bowel echogenicity.5 Ultrasonography in the postnatal period is of limited value particularly in distal obstruction due to introduction of air to the gut which absorbs the ultrasound beam.9 This was also true in our patient where a postnatal ultrasound showed features suggestive of partial IO with dilated bowel loops. Postnatal JIA presents with signs and symptoms of intestinal obstruction such as abdominal distension, emesis, and in some cases, delayed passage of meconium. Sometimes, the meconium can appear normal, but most often, light-coloured plugs are passed from the rectum. In cases of distal bowel ischaemia, as seen in type IIIb (Table 1), blood may be seen in the rectum.5
Table 1.
Types of ileal atresia.10
| Types | Description |
|---|---|
| I | A thin diaphragm that occludes the lumen |
| II | Two blind ends are connected with a fibrous cord of atretic bowel |
| IIIa | (Most common), two blind ends terminate with a V-shaped mesenteric defect |
| IIIb | (Apple-peel or Christmas-tree atresia), involves a large, V-shaped mesenteric defect in which a blind-ended bowel distal to the atresia is wrapped around its collateral blood supply |
| IV | Defined as multiple atresia |
Prior to surgery, preoperative management includes decompression with nasogastric tube, fluid and electrolyte resuscitation, and intravenous broad-spectrum antibiotics in the event of perforation or evidence of infection. The type of surgical procedure used to repair intestinal atresias will depend on the lesion (type of atresia), but mostly involves resection of the atretic segment(s) with primary end-to-end anastomosis as done in the index case, or an intercurrent enterostomy with a take-down at a later date.11 Significant luminal discrepancies between the proximal and distal ends are addressed by amputation of large blind pouches, tapering enteroplasty and/or end-to-oblique anastomosis at the time of the primary surgery or more commonly on take-down of the enterostomy. The discrepancy was not significant in our case hence tapering enteroplasty was not needed, otherwise the surgeon can decide on the table on the means of surgical options. A certain degree of functional obstruction may still be encountered post-operatively, especially in proximal atresia.11
The outcome of neonates born with JIA has improved considerably in recent years due to many new acquisitions in neonatal intensive care and anaesthesia, operative procedures and use of total parenteral nutrition (TPN).12 As oppose to our setting, we were unable to provide ideal supportive care, due to financial constraints and unavailability of TPN we were reliant on nil per-oral protocol and IV fluids, which renders difficulties towards nutritional support and electrolyte corrections post-surgery. In addition, due to the lack of neonatal intensive care unit (NICU) and economic constrains pre- and post-operative investigations were partially carried out as the patient did not have medical insurance covered. Having medical insurance (which a lot of patients in our setting lack) can aid in prompt medical treatment including investigations without delays as this was evident in the index case. Due to lack of education on the importance of antenatal visits and hospital delivery along with some cultural beliefs among some groups causes delay in medical attention, therefore providing education through schools and media can sensitize such issues and health seeking behaviours.
Conclusion
JIA with reported data have good prognosis with low mortality. Despite atresia being common as reported, lack of skilful continuum of care from prenatal diagnosis towards post-operative care still marks the condition as fatal in our setting. Incorporating of prenatal screening specifically foetal ultrasound and with availability of parenteral nutrition post-operatively will change the prognostic image. Aggressive pre-operative stabilization of patients from initial diagnosis by referring doctors may reduce time to cutting hence improve post-operative outcomes. Such case reports will increase awareness among the medical personnel to increase their level of suspicion and improve the clinical acumen.
Acknowledgments
The authors would like to thank the child’s mother for permission to share her child’s medical history for educational purposes and publication.
Footnotes
Author Contributions: P.E.M., J.L. and D.N.M. conceptualized and prepared the manuscript. M.M., E.R.M. and E.K. reviewed the patient medical records, and all authors have read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Jay Lodhia  https://orcid.org/0000-0002-3373-5762
https://orcid.org/0000-0002-3373-5762
References
- 1.Shalkow J, Kim ES.Small intestial atresia and stenosis. Mexico: Anahuac University, 2019. [Google Scholar]
- 2.Wesson DE, Garcia-Prats JA, Heyma MB, et al. Intestinal atresia, https://www.uptodate.com/contents/intestinal-atresia (2020, accessed 18 June 2021).
- 3.Mangray H, Ghimenton F, Aldous C.Jejuno-ileal atresia: its characteristics and peculiarities concerning apple peel atresia, focused on its treatment and outcomes as experienced in one of the leading South African academic centres. Pediatr Surg Int 2020; 36(2): 201–207. [DOI] [PubMed] [Google Scholar]
- 4.Puri P, Fujimoto T.New observations on the pathogenesis of multiple intestinal atresias. J Pediatr Surg 1988; 23(3): 221–225. [DOI] [PubMed] [Google Scholar]
- 5.Osuchukwu OO, Rentea RM.Ileal atresia. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 6.Lupo PJ, Isenburg JL, Salemi JL, et al.; The National Birth Defects Prevention Network. Population-based birth defects data in the United States, 2010-2014: a focus on gastrointestinal defects. Birth Defects Res 2017; 109(18): 1504–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochran WJ. Jejunoileal atresia – pediatrics. MSD Manual Professional Edition, https://en.wikipedia.org/wiki/Intestinal_atresia (2021, accessed 16 June 2021). [Google Scholar]
- 8.Wisbach GG, David Vazquez W.Ileal atresia, malrotation and Hirschsprung’s disease: a case report. J Pediatr Surg Case Rep 2013; 1(1): e3–e5. [Google Scholar]
- 9.Neal MR, Seibert JJ, Vanderzalm T, et al. Neonatal ultrasonography to distinguish between meconium ileus and ileal atresia. J Ultrasound Med 1997; 16(4): 263–236; quiz 267. [DOI] [PubMed] [Google Scholar]
- 10.Martin LW, Zerella JT.Jejunoileal atresia: a proposed classification. J Pediatr Surg 1976; 11(3): 399–403. [DOI] [PubMed] [Google Scholar]
- 11.Song C, Niklas V.Structural anomalies of the gastrointestinal tract. In: Gleason C, Devaskar S. (eds) Avery’s diseases of the newborn. 9th ed. Amsterdam: Elsevier, 2012, pp. 979–993. [Google Scholar]
- 12.Wilmore DW.Factors correlating with a successful outcome following extensive intestinal resection in newborn infants. J Pediatr 1972; 80(1): 88–95. [DOI] [PubMed] [Google Scholar]