Abstract
Globally, lung cancer is the most common cause of cancer-related deaths. After diagnosis at all stages, <7% of patients survive for 10 years. Thus, diagnosis at later stages and the lack of effective and personalized drugs reflect a significant need to better understand the mechanisms underpinning lung cancer progression. Metastasis should be responsible for the high lethality and recurrence rates seen in lung cancer. Metastasis depends on multiple crucial steps, including epithelial–mesenchymal transition, vascular remodeling, and colonization. Therefore, in-depth investigations of metastatic molecular mechanisms can provide valuable insights for lung cancer treatment. Recently, long noncoding RNAs (lncRNAs) have attracted considerable attention owing to their complex roles in cancer progression. In lung cancer, multiple lncRNAs have been reported to regulate metastasis. In this review, we highlight the major molecular mechanisms underlying lncRNA-mediated regulation of lung cancer metastasis, including (1) lncRNAs acting as competing endogenous RNAs, (2) lncRNAs regulating the transduction of several signal pathways, and (3) lncRNA coordination with enhancer of zeste homolog 2. Thus, lncRNAs appear to execute their functions on lung cancer metastasis by regulating angiogenesis, autophagy, aerobic glycolysis, and immune escape. However, more comprehensive studies are required to characterize these lncRNA regulatory networks in lung cancer metastasis, which can provide promising and innovative novel therapeutic strategies to combat this disease.
Keywords: microRNA, long noncoding RNA, lung cancer, metastasis, invasion, migration, immune escape
Introduction
Lung cancer is the most lethal malignancy accounting for 18% of total cancer deaths.1 Cancer statistics from 2021 indicated that the disease accounted for ∼22% of cancer-related deaths, with 235,760 new cases of bronchial and lung cancer diagnosed in the United States.2 In China, the age-standardized mortality rate for lung cancer is 18.1%, which ranks first in the cancer spectrum.3 After lung cancer diagnosis at all stages, <7% of patients survive for 10 years.3 The disease typically incorporates two pathological subtypes: nonsmall cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC is the main subtype and includes lung adenocarcinoma (LAD), lung squamous cell carcinoma (LSCC), and large-cell carcinoma. These cancers are induced by smoking, radon exposure, viral infection, and other harmful factors.4–6
Lung cancer metastasis is a key factor implicated in lung cancer recurrence and death.7 Generally, the metastatic cascade contains several discrete stages: nascent tumor cells invade surrounding tissues and intravasate into the vascular system where they survive in the circulatory system. They then extravasate through dysfunctional vascular walls into target tissues or organ sites where they expand and grow.8 During the local invasion, normal epithelial cells undergo depolarization, losing cell–cell interactions, transforming into mesenchymal cells, and acquiring a metastatic phenotype.9 This complicated process is termed epithelial–mesenchymal transition (EMT) and is the driving force of tumor cell invasion.10 Nascent tumor cells then break epithelial junctions by exploiting matrix-degrading enzymes induced by EMT to access the circulation via leaky and circuitous tumor vasculature induced by angiogenesis.11 However, after detaching from primary sites, disseminated tumor cells must resist programmed apoptosis termed anoikis.12 Finally, disseminated tumor cells colonize at distant sites.13 Metastasis is a complex and intricate cancer behavior; therefore, a better understanding of metastatic processes will help us overcome the heavy burden of lung cancer.13
The Encyclopedia of DNA Elements project indicates that the human genome is generally transcribed into multiple nonprotein-coding RNA molecules, commonly referred to as noncoding RNAs (ncRNAs).14 In general, based on molecular size, ncRNAs are categorized into two major types: short ncRNAs and long ncRNAs (lncRNAs).15 Short ncRNAs are composed of <200 nucleotides and include microRNAs (miRNAs), small interfering RNAs, and small nuclear RNAs.16 Conversely, lncRNAs are a novel group of RNA transcripts measuring >200 nucleotides, but they do not encode peptides.17 Many studies have reported that lncRNAs participate in multiple physiological processes, including cell proliferation, regulation of apoptosis, invasion, and cellular energy metabolism.18,19 Similarly, their aberrant expression is particularly related to several diseases, especially cancers.20 Several studies have demonstrated that the abnormal expression of lncRNAs exerts key functions in lung cancer tumorigenesis and metastasis.21–23 Emerging studies have also reported that lncRNAs are promising therapeutic targets for treating lung cancer metastasis.21,22 Therefore, exploring the regulatory mechanisms underpinning lncRNA expression in lung cancer metastasis is warranted.
In this review, we comprehensively explored lncRNAs in lung cancer metastasis and summarized key mechanisms behind their regulation with a view to advance our knowledge of lung cancer metastasis.
Overview of lncRNAs: Biogenesis, Classification, and Function
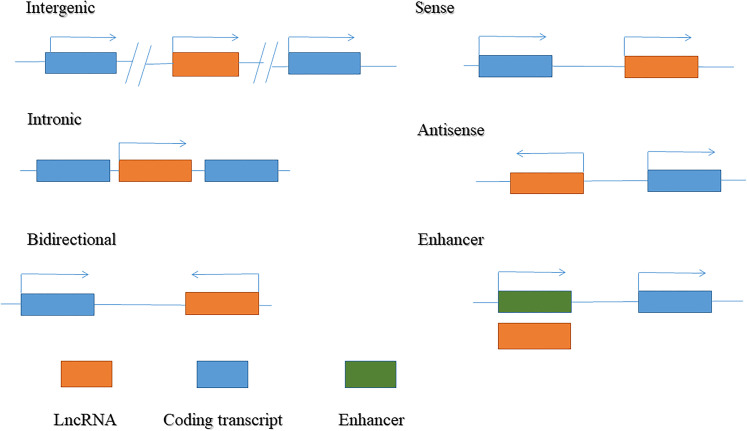
LncRNAs are endogenous transcripts transcribed by RNA polymerase II, with many lncRNAs harboring a 7mC cap and poly-A tail similar to messenger RNA (mRNA).17 lncRNAs are located in both coding and noncoding gene regions. Based on their relative proximity to neighboring genes and genomic origins, lncRNAs are broadly classified into six categories (Figure 1): (1) intergenic lncRNAs originating from intergenic regions of protein-coding genes; (2) intronic lncRNAs transcribed from intronic regions of protein-coding genes; (3) bidirectional lncRNAs produced from the promoter of a coding transcript, but in a divergent direction; (4) sense lncRNAs that cover exons of neighboring protein-coding genes in the same direction; (5) antisense lncRNAs transcribed in the opposite direction to a neighboring coding transcript; and (6) enhancer lncRNAs generated from enhancer regions.17,24
Figure 1.
Classification of long non-coding RNA (lncRNA).
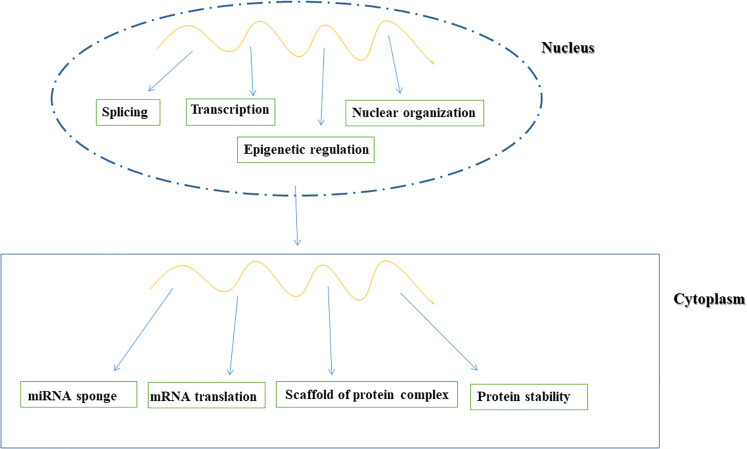
It was reported that lncRNA functions are strongly associated with their subcellular localization.25 Evidence from human cells using single-molecule RNA fluorescence in situ-hybridization revealed diverse lncRNA subcellular localization patterns, including specific nuclear locations and nonspecific localization to both the nucleus and cytoplasm.26 The wide-ranging subcellular localization of lncRNAs suggests diverse modes of action (Figure 2).
Figure 2.
Function of long non-coding RNA (lncRNA).
lncRNAs play important roles in nuclear architecture as they are involved in the creation and maintenance of nuclear paraspeckles.27 Additionally, lncRNAs modify gene expression by guiding chromatin modifiers to genomic loci.28 Other lncRNAs accumulate in the cytoplasm where they function as sponges for miRNAs to regulate gene expression.29
lncRNAs also play crucial roles in transcription as they interact with transcription factors to activate or interfere with these processes.30 Moreover, lncRNAs posttranscriptionally regulate gene expression by interacting with RNA-binding proteins to influence splicing and translation.31 Further, lncRNAs control protein stability by regulating proteasome-mediated degradation.32
Molecular Mechanisms of lncRNAs in Lung Cancer Metastasis
lncRNAs Regulate Lung Cancer Metastasis by Sponging miRNAs
miRNAs regulate gene expression by regulating mRNA at the posttranscriptional level.33 A previous study has reported that lncRNAs sponge certain miRNAs to alter miRNA abundance and suppress downstream target gene expression.34 This molecular model is termed competing endogenous RNA (ceRNA) that is essential for cancer progression.35 Here, we describe several lncRNAs that serve as ceRNAs to regulate lung cancer metastasis (Table 1).
Table 1.
LncRNAs Regulate Lung Cancer Metastasis Through Sponging miRNAs.
| LncRNA | Expression | Cancer type | Molecular mechanism | Function | Reference |
|---|---|---|---|---|---|
| LCAT1 | Up | LAD | Sponges miR-4715 to 5p to upregulate RAC1 | Promotes tumor metastasis in vivo. | Yang et al36 |
| MALAT1 | Up | LAD | Regulates miR-204/SLUG; miR-145 to 5p/NEDD9; and miR-200a-3p/PD-L1 | Promotes cell EMT and metastasis; promotes lung tumor
nodules formation Correlates with high metastatic risk in patients with early stage lung cancer |
Ji et al,37 Gutschner et al,38 Li et al,39 Yu et al,40 and Wei et al41 |
| UCA1 | Up | NSCLC | Regulates miR-193a/HMGB1 | Promotes cell invasion and migration Plasma UCA1 severs as a valuable biomarker for the diagnosis of NSCLC |
Wang et al42 and Wu and Zhou43 |
| RSF1-IT2 | Up | NSCLC | HMGB1 activates its transcription; regulates miR-129 to 5p/SNAIL1 | Promotes cell invasion, migration in vitro and lung metastasis in vivo | Wu et al44 |
| H19 | Up | NSCLC | Regulates miR-200a/ZEB1 and miR-200a/ZEB2 | Promotes cell EMT, invasion, and migration | Zhao et al45 |
| PVT1 | Up | NSCLC | Regulates miR-200a/MMP9 and miR-200b/MMP9 | Facilitates cell invasion and migration Correlates with lymph node metastasis |
Yang et al46 and Chen et al47 |
| HOTAIR | Up | NSCLC | Sponges miR-203 and miR-613; regulates miR-217/DACH1 | Facilitates cell migration and invasion | Zhang et al,48 Jiang et al,49 and Chen et al50 |
| SUMO1P3 | Up | NSCLC | Sponges miR-136 | Promotes cell invasion and migration | Zhang et al51 |
| DLX6-AS1 | Up | NSCLC | Regulates miR-27b-3p/GSPT1 and miR-144/ PRR11 | Promotes cell invasion and migration | Sun et al52 and Huang et al53 |
| DGCR5 | Down | NSCLC | Regulates miR-211 to 5p/EPHB6 and miR-873 to 5p/TUSC3 | Inhibits cell migration and invasion Relates with lymph node metastasis |
Kang et al54 and Luo et al55 |
| FOXD3-AS1 | Down | NSCLC | Regulates miR-150/ SRCIN1 | Inhibits cell invasion and associates with lymph node metastasis | Ji et al56 |
| LIFR-AS1 | Down | NSCLC | Regulates miR-942 to 5p/ZNF471 | Inhibits metastasis both in vitro and in vivo | Wang et al57 |
| GATA6-AS1 | Down | NSCLC | Regulates miR-543/RKIP axis | Inhibits cell invasion and migration | Gong et al58 |
| HCG11 | Down | NSCLC | Sponges miR-522 to 3p to upregulate SOCS5 | Inhibits tumor metastasis and growth both in vivo and in vitro | Fan et al59 |
Abbreviations: DACH1, Dachshund homolog 1; DGCR5, DiGeorge syndrome critical region gene 5; DLX6-AS1, distal-less homeobox 6 antisense 1; EMT, epithelial–mesenchymal transition; EPHB6, EPH receptor B6; FOXD3-AS1, Forkhead box D3 antisense RNA 1; GATA6-AS1, GATA6 antisense RNA 1; GSPT1, G1–S phase transition 1; HCG11, HLA complex group 11; HMGB1, high-mobility group protein B1; HOTAIR, HOX transcript antisense intergenic RNA; LAD, lung adenocarcinoma; LCAT1, lung cancer-associated transcript 1; LIFR-AS1, leukemia inhibitory factor receptor antisense RNA 1; MALAT1; metastasis-associated LAD transcript 1; miRNA, microRNA; lncRNA, long noncoding RNA; MMP9, matrix metalloproteinase 9; NEDD9, neural precursor cell-expressed developmentally downregulated 9; NSCLC, nonsmall cell lung cancer; PD-L1, programmed death ligand 1; PRR11, proline-rich 11; PVT1, plasmacytoma variant translocation 1; RAC1, Rac family small GTPase 1; RKIP, raf kinase inhibitor protein; RSF1-IT2, remodeling and spacing factor 1-intronic transcript 2; SLUG, zinc-finger transcription repressor SNAI2; SOCS5, suppressor of cytokine signaling 5; SRCIN1, SRC kinase signaling inhibitor 1; SUMO1P3, small ubiquitin-like modifier 1 pseudogene 3; TUSC3, tumor suppressor candidate 3 expression; UCA1, urogenital carcinoma antigen 1; ZEB1, zinc-finger e-box binding homeobox 1; ZEB2, zinc-finger e-box binding homeobox 2; ZNF471, zinc-finger protein 471.
Oncogenic lncRNAs act as ceRNAs
lncRNA lung cancer-associated transcript 1 (LCAT1) is upregulated in LAD tissues and is closely correlated with poor prognosis. LCAT1 directly sponges miR-4715 to 5p to increase the expression of the Rac family small GTPase 1 (RAC1), thus promoting tumor metastasis in vivo.36
Elevated metastasis-associated LAD transcript 1 (MALAT1) expression is related to a high metastatic risk in patients with early stage LAD.37 Gutschner et al38 reported that MALAT1 promotes lung tumor nodule formation in vivo; however, detailed mechanisms remain unclear. Recently, several studies indicated that MALAT1 acted as a ceRNA to increase the expression of metastasis-related genes, including zinc-finger transcription repressor SNAI2 (SLUG), neural precursor cell-expressed developmentally downregulated 9 (NEDD9), and programmed death ligand 1 (PD-L1), by competitively sponging miR-204, miR-145 to 5p, and miR-200a-3p.39–41
Wang et al42 revealed that urogenital carcinoma antigen 1 (UCA1) is upregulated during NSCLC progression. Using receiver operating characteristic curve analysis, these authors showed that plasma UCA1 demonstrates a considerable diagnostic value for detecting NSCLC. Moreover, UCA1 promoted NSCLC metastasis by sponging miRNA-193a to increase high-mobility group protein B1 (HMGB1) expression.43 HMGB1 is a highly conserved nuclear DNA-binding protein with an essential role in gene transcription,60 eg, remodeling and spacing factor 1-intronic transcript 2 (RSF1-IT2) is transcriptionally activated by HMGB1.44 Highly expressed RSF1-IT2 promoted NSCLC metastasis by sponging miR-129 to 5p and upregulating the expression of snail family transcriptional repressor 1. Importantly, upregulated RSF1-IT2 induced by HMGB1 was strongly associated with tumor metastasis in xenograft nude mice.44 These findings suggest that HMGB1 and its related lncRNAs may be potential therapeutic targets for NSCLC.
The miR-200 family suppresses EMT by directly targeting EMT markers, such as E-cadherin, zinc-finger e-box binding homeobox 1 (ZEB1), and ZEB2.61 Imprinted maternally expressed transcript (H19) is one such oncogenic lncRNA reported to promote NSCLC metastasis by acting as a ceRNA of miR-200a.45 Intriguingly, lncRNA plasmacytoma variant translocation 1 (PVT1), which is upregulated in NSCLC and related to positive lymph node metastasis, was shown to sponge miR-200a and miR-200b to increase matrix metalloproteinase 9 (MMP9) expression, thereby promoting NSCLC metastasis.46,47 These studies identified a novel mechanism whereby the miR-200 family serves as an upstream regulator of MMP9.
Similarly, HOX transcript antisense intergenic RNA (HOTAIR) was shown to facilitate NSCLC migration and metastasis by modulating miR-203 and miR-613 expression.48,49 Using bioinformatics, Chen et al50 revealed that HOTAIR shares conserved binding sites for miR-217 with Dachshund homolog 1 (DACH1). Moreover, silencing HOTAIR suppressed NSCLC cell migration and invasion by enhancing miR-217 expression, which was reversed by DACH1.50 Therefore, HOTAIR can function as a potential marker for NSCLC diagnosis and treatment.
Small ubiquitin-like modifier 1 pseudogene 3 (SUMO1P3) is upregulated in NSCLC and promotes cell invasion and migration by sponging miR-136.51 Intriguingly, SUMO1P3 expression was not associated with clinical prognosis in patients with NSCLC, suggesting that SUMO1P3 may be regulated by other oncogenes or tumor suppressor genes during NSCLC development.51 This association warrants further investigation.
Distal-less homeobox 6 antisense 1 (DLX6-AS1) is an oncogenic lncRNA known to facilitate NSCLC cell invasion and migration by regulating the miR-27b-3p/G1–S phase transition 1 (GSPT1) and miR-144/proline-rich 11 (PRR11) axis. These interactions may contribute to a comprehensive understanding of tumorigenesis in lung cancer.52,53
Tumor-Suppressive lncRNAs act as ceRNAs
DiGeorge syndrome critical region gene 5 (DGCR5) plays a crucial role in cell proliferation, invasion, and metastasis in different cancers.62 In NSCLC, DGCR5 inhibits tumor cell migration and metastasis by regulating miR-211 to 5p and EPH receptor B6 (EPHB6) expression.54 Luo et al55 reported that increased DGCR5 expression is related to a low incidence of lymph node metastasis in patients with LSCC. Their study also revealed that DGCR5 interacts with miR-873 to 5p and upregulates tumor suppressor candidate 3 expression (TUSC3), thereby suppressing A549 cell (human adenocarcinoma alveolar basal epithelial cells) migration and invasion.55 Collectively, these findings suggested that DGCR5 may function as a potential therapeutic target for lung cancer.
Forkhead box D3 antisense RNA 1 (FOXD3-AS1) is downregulated in NSCLC and associated with lymph node metastasis.56 Overexpressed FOXD3-AS1 restrains H1299 cell (human nonsmall cell lung carcinoma cell line) invasion by indirectly upregulating SRC kinase signaling inhibitor 1 (SRCIN1) expression by targeting miR-150.56 Therefore, FOXD3-AS1 is a potential target for improved NSCLC treatment.
lncRNA leukemia inhibitory factor receptor antisense RNA 1 (LIFR-AS1) displayed significantly lower expression in NSCLC tissues than in normal tissues. In vivo and in vitro studies by Wang et al57 demonstrated that LIFR-AS1 sponges miR-942 to 5p to increase zinc-finger protein 471 (ZNF471) expression. Notably, ZNF471 served as a tumor suppressor by inhibiting tumor growth and metastasis.57 Therefore, LIFR-AS1 inhibited NSCLC metastasis via the miR-942 to 5p/ZNF471 axis.
Gong et al58 determined a hitherto unknown role for the lncRNA GATA6 antisense RNA 1 (GATA6-AS1)/miR-543/raf kinase inhibitor protein (RKIP) regulatory axis. These authors discovered that the axis inhibits NSCLC metastasis and proposed it as a valuable therapeutic approach for patients with late-stage metastatic NSCLC.58 Moreover, in vivo and in vitro studies showed that the novel lncRNA, HLA complex group 11 (HCG11), which is downregulated in NSCLC, upregulated the expression and activity of suppressor of cytokine signaling 5 (SOCS5) by sponging miR-522 to 3p and suppressing tumor metastasis and growth.59
LncRNAs Regulate Lung Cancer Metastasis by Regulating Signaling Pathways
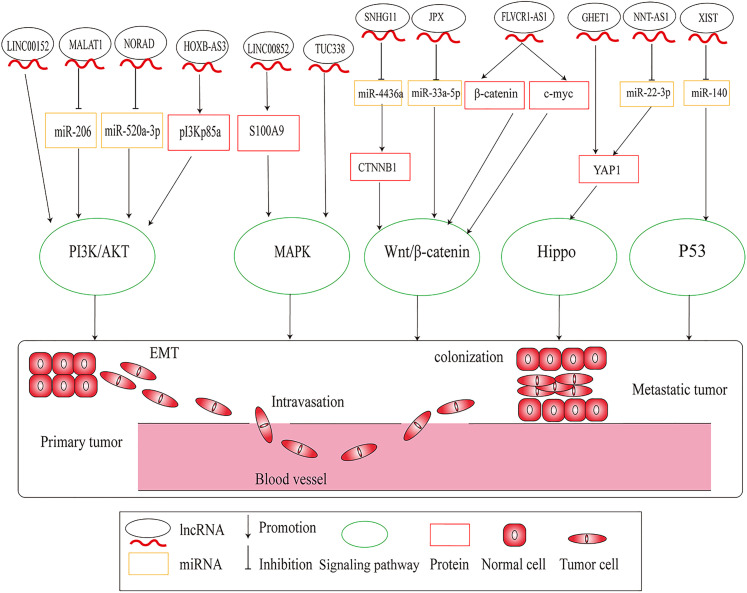
In recent years, cancer-related classical signaling pathways have been recognized as crucial factors in the complicated regulatory network of cancer progression and have provided key clues for overcoming cancer.63–65 Studies have revealed that lncRNAs regulate lung cancer metastasis via several diverse signaling pathways, such as phosphoinositide 3-kinase (PI3K),66 mitogen-activated protein kinase (MAPK),67 wingless/integrated (Wnt)/β-catenin,68 Hippo,69 and p5370 (Figure 3).
Figure 3.
Long noncoding RNAs (lncRNAs) regulate lung cancer metastasis through regulating signaling pathways.
PI3K Signaling Pathway
PI3K/protein kinase B (AKT) is one of the most important intercellular signaling pathways contributing to cancer progression, metastasis, and metabolism.71,72 For example, the knockdown of long intergenic noncoding RNA 00152 repressed NSCLC cell migration and invasion by reducing PI3K/AKT pathway activity.73 Liu et al74 reported that lncRNA tumor protein P73 antisense RNA 1 (TP73-AS1) expression is significantly increased in LAD tissues and is associated with poor prognosis. Moreover, TP73-AS1 contributed to cell migration and invasion by activating the PI3K/AKT signaling pathway.74 The novel lncRNA, Fer-1-like protein 4 (FER1L4) was downregulated in both in vivo and in vitro NSCLC models. Overexpressed FER1L4 suppressed cell metastasis by reducing PI3K and AKT expression.75 Additionally, lncRNA HOXB cluster antisense RNA 3 significantly increased PI3Kp85a protein expression and activating the PI3K/AKT pathway to exacerbate NSCLC invasion and migration both in vitro and in vivo.76
Vimentin-associated lncRNA (VAL) is a potent oncogenic molecule upregulated during AKT overexpression in LAD cells.77 Mechanistically, AKT overactivation induced VAL by enhancing the transcriptional activity of signal transducer and activator of transcription 3 (STAT3). This process promoted tumor invasion, anoikis resistance, and metastasis77 and indicated that VAL was an essential lncRNA in overactivated AKT-mediated LAD metastasis.
MAPK Signaling Pathway
MAPKs are classical serine/tyrosine-activated kinases that regulate cell proliferation, apoptosis, and differentiation.78 The MAPK signal transduction pathway is divided into four branches: (1) extracellular signal-regulated kinase (ERK), (2) c-Jun NH2-terminal kinase, (3) p38/MAPK, and (4) ERK5.78
lncRNAs are essential MAPK pathway regulators, eg, transcribed ultraconserved element 338 was reported to facilitate NSCLC cell invasion and migration by directly activating MAPK signaling.67 LINC00852 is another oncogenic lncRNA that targeted the S100 calcium-binding protein A9 to promote LAD spinal metastasis by activating the MAPK pathway.79 Therefore, LINC00852 is a promising target for early intervention against LAD spinal metastasis.
Additionally, Zhu and He80 reported that terminal differentiation-induced noncoding RNA (TINCR) is significantly elevated in NSCLC cell lines and tissues. Upregulated TINCR expression was related to enhanced NSCLC cell migration and viability by increasing the kinase activity of v-raf murine sarcoma viral oncogene homolog B (BRAF) to activate the MAPK signaling pathway. Furthermore, TINCR promoted xenograft tumor growth in mouse models.80 Therefore, the BRAF/MAPK pathway is essential for the oncogenic role of TINCR.
Wnt/β-Catenin Signaling Pathway
The Wnt/β-catenin signaling pathway is a major regulatory factor in cancer metastasis.81 Protein disulfide isomerase family A member 3 pseudogene 1 (PDIA3P) is a novel lncRNA that activates tumorigenesis in NSCLC cells.82 Enhanced PDIA3P expression significantly promoted cell metastasis and tumor growth in vivo. In addition, PDIA3P activated the Wnt/β-catenin signaling pathway during NSCLC development,82 suggesting that PDIA3P is an essential regulator of the Wnt/β-catenin pathway. Moreover, lncRNA feline leukemia virus subgroup C receptor 1 antisense RNA 1 (FLVCR1-AS1) was upregulated in NSCLC, with high FLVCR1-AS1 levels related to lymph node metastasis and advanced tumor histological grades. FLVCR1-AS1 silencing directly inhibited β-catenin and c-Myc expression, thereby inhibiting NSCLC cell invasion and migration.83
Brain cytoplasmic RNA 1 (BCYRN1) is another oncogenic lncRNA upregulated in several malignant tumors, including lung cancer, gastric cancer, and glioma.84 Wang et al85 demonstrated that BCYRN1 is upregulated in NSCLC and promotes cell proliferation and metastasis by increasing the expression of cell cycle regulatory proteins, such as cyclin D1, and stimulating the Wnt/β-catenin signaling pathway. This study showed that BCYRN1 facilitated NSCLC progression via the Wnt/β-catenin pathway, thus highlighting a promising therapeutic target for NSCLC treatment.
Hippo Signaling Pathway
The Hippo signaling pathway influences tissue growth and regeneration by inhibiting Yes-associated protein (YAP) and the transcriptional coactivator with PDZ-binding motif (TAZ). Dysregulated Hippo signaling facilitates uncontrolled cell proliferation and is associated with tumorigenesis.86,87 Gastric carcinoma highly expressed transcript 1 knockdown inhibited NSCLC cell EMT and invasion by decreasing YAP1 expression.88 Cadherin 1 (CDH1) is a cell–cell adhesion molecule that establishes cell polarity and regulates epithelial differentiation and proliferation.89 Evidence indicated that CDH1 downregulation promoted tumor metastasis.90 The oncogenic lncRNA nonsmall cell LCAT1 (NSCLCAT1) reportedly promoted NSCLC cell metastasis by regulating the Hippo signaling pathway by transcriptionally inhibiting CDH1.91 Therefore, the functional inhibition of NSCLCAT1 may prove to be a novel therapeutic strategy to control metastatic NSCLC.
P53 Signaling Pathway
As the guardian of the genome, p53 plays a central role in regulating genomic stability, DNA damage repair, cell proliferation, and apoptosis.92 p53 is deregulated in many cancers and regulates the expression of multiple target genes resulting in tumor growth inhibition.93 Generally, p53 levels are controlled by the ubiquitin–proteasome pathway.94 The E3 ubiquitin ligase, mouse double minute 2 (MDM2), acts as a p53 suppressor by promoting its ubiquitination.94 Notably, lncRNA maternally expressed gene 3 (MEG3) was reported to activate p53 by inhibiting MDM2, leading to suppressed cell invasion in NSCLC.70
Additionally, lncRNAs may function as downstream targets of p53, eg, NONMMUT015812 was remarkably increased in mice with LAD and promoted cell invasion and migration. Intriguingly, p53 negatively regulated NONMMUT015812 expression.95 PVT1b is a stress-specific PVT1 isoform activated by p53; activated PVT1b promoted transcriptional inhibition of Myc and inhibited cellular proliferation in LAD cell lines.96 Furthermore, PVT1b loss enhanced tumor growth that indicated a potential to control lung cancer development by regulating PVT1b activity.96
Overall, p53 signaling pathway functions in lung cancer are partially manifested by inhibiting tumor growth and metastasis, and as such, lncRNAs may play crucial roles in these processes.
Although lncRNAs regulate cancer-related signaling pathways, the current literature is not comprehensive. Notably, several pathways have not yet been characterized with respect to lung cancer metastasis, eg, Ras and mesenchymal–epithelial transition factor signaling pathways. Therefore, more research is required to explore downstream genes associated with these pathways during lung cancer metastasis.
LncRNAs Regulate Lung Cancer Metastasis by Coordinating With Enhancer of Zeste Homolog 2 (EZH2)
LncRNAs Bind to EZH2
EZH2 maps to chromosome 7q35 and is a histone–lysine methyltransferase subunit of polycomb repressive complex 2 (PRC2).97 PRC2 serves as a methyltransferase of histone H3 lysine 27 (H3K27) and facilitates transcriptional inhibition by modulating chromatin structures via posttranslational histone modification.98
EZH2 catalyzes the mono-, di-, and trimethylation of H3K27 and plays a crucial role in epigenetic gene silencing.99 Compelling evidence has suggested that EZH2 modulates many biological functions in lung cancer.100 Li et al101 showed that EZH2 functions as an anticancer molecule by directly binding to the nuclear factor erythroid 2 p45-related factor 2 (Nrf2) promoter, thus reducing Nrf2 expression and upregulating H3K27me3, leading to tumor growth reduction. Another study by Xia et al102 reported that EZH2 exerts positive effects on lung cancer cell migration and invasion by upregulating chemokine ligand 5 expression. However, detailed mechanisms remain unclear.
LncRNAs can interact with EZH2 to affect lung cancer metastasis,100 eg, lncRNA taurine upregulated gene 1 (TUG1) was overexpressed in SCLC and facilitated cell migration, invasion, and proliferation.103 The lncRNA affected cell metastasis by modulating Lin11, Isl-1, and Mec-3 kinase 2b (LIMK2b) expression via EZH2 binding.103 As a member of LIMK2, LIMK2b is mapped on 300 kp of TUG1 and participated in tumor growth and metastasis by encoding a kinase that regulated cofilin phosphorylation and promoted actin dynamics.104,105 Therefore, TUG1 promoted SCLC metastasis by inhibiting LIMK2b expression via EZH2 binding.
Small nucleolar RNA host gene 20 (SNHG20) served as an oncogenic molecule by enhancing NSCLC proliferation and migration.106 Notably, SNHG20 inhibited p21 transcription by recruiting EZH2 to its promoter.106 p21 belongs to the cyclin-dependent kinase inhibitor family, mediates the growth inhibitory effects of transforming growth factor β (TGF-β), and inhibits tumorigenesis.107 Therefore, EZH2-mediated p21 repression is essential for the oncogenic role of SNHG20. However, other mechanisms whereby SNHG20 functions in NSCLC development require more exploration.
Moreover, prostate cancer-associated transcript 6 (PCAT6) and UFC1 are other lncRNAs that promote NSCLC metastasis by interacting with EZH2.108,109
LncRNAs Serve as EZH2 Regulators or Effectors
In addition to repressing gene expression by binding to EZH2, lncRNAs also serve as EZH2 regulators or effectors,100 eg, lncRNA SOX2 overlapping transcript (Sox2ot) facilitated tumor metastasis and indicated poor prognosis in esophageal cancer, pancreatic ductal adenocarcinoma, osteosarcoma, and ovarian cancer.110 Sox2ot was also upregulated in NSCLC and predicted shorter patient survival.111,112 Upregulated Sox2ot also promoted NSCLC cell migration and invasion.113 Depleted Sox2ot downregulated EZH2 expression and precluded cell growth, and similarly, increased EZH2 expression reversed the effects induced by Sox2ot knockdown.111 These data suggested that Sox2ot mediated NSCLC progression partially by modulating EZH2 expression.
The SPRY4 intronic transcript 1 (SPRY4-IT1) was downregulated in NSCLC and related to poor prognosis.114 Overexpressed SPRY4-IT1 suppressed EMT and increased apoptosis in NSCLC cells.114 Furthermore, EZH2 inhibited SPRY4-IT1 expression by binding to its promoter region.114 In EZH2-depleted cells, which showed impaired metastasis, SPRY4-IT1 silencing rescued oncogenic phenotypes, suggesting that SPRY4-IT1 suppression played a key role in EZH2-mediated oncogenesis.114 Similarly, Wei et al115 reported that EZH2 induces the suppression of lncRNA SVUGP2, leading to enhanced NSCLC migration and invasion. Overall, these studies suggest that downregulated SPRY4-IT1 or SVUGP2 may predict a higher metastatic risk for patients with NSCLC.
Many lncRNAs regulate lung cancer metastasis by interacting with EZH2 (Table 2). Moreover, lncRNAs can be regulated by EZH2. Thus far, lncRNA–EZH2 regulatory network data implicated in epigenetic regulation are preliminary; however, the comprehensive investigation of these processes will undoubtedly identify a mass of as-yet untapped therapeutic targets for lung cancer.
Table 2.
LncRNAs Regulate Lung Cancer Metastasis Through Interacting with EZH2.
| LncRNA | Cancer type | Expression | Molecular mechanism | Function | Reference |
|---|---|---|---|---|---|
| TUG1 | SCLC | Up | Represses LIMK2b expression via binding to EZH2 | Promotes metastasis | Niu et al103 |
| SNHG20 | NSCLC | Up | Represses p21 expression via binding to EZH2 | Promotes metastasis | Chen et al106 |
| PCAT6 | NSCLC | Up | Represses LATS2 expression via binding to EZH2 | Promotes metastasis | Shi et al108 |
| UFC1 | NSCLC | Up | Represses PTEN expression via binding to EZH2 | Promotes metastasis | Zang et al109 |
| Sox2ot | NSCLC | Up | Promotes EZH2 expression | Promotes metastasis | Hou et al,111 Kamel et al,112 and Zhang et al113 |
| SPRY4-IT1 | NSCLC | Down | EZH2 reduces its expression | Inhibits metastasis | Sun et al114 |
| SVUGP2 | NSCLC | Down | EZH2 reduces its expression | Inhibits metastasis | Wei et al115 |
Abbreviations: EZH2, enhancer of zeste homolog 2; LATS2, large tumor suppressor kinase 2; LIMK2b, Lin11, Isl-1, and Mec-3 kinase 2b; lncRNA, long noncoding RNA; NSCLC, nonsmall cell lung cancer; PCAT6, prostate cancer-associated transcript 6; SCLC, small cell lung cancer; SNHG20, small nucleolar RNA host gene 20; Sox2ot, SOX2 overlapping transcript; SPRY4-IT1, SPRY4 intronic transcript 1; TUG1, taurine upregulated gene 1.
Regulatory Patterns of lncRNAs in Lung Cancer Metastasis
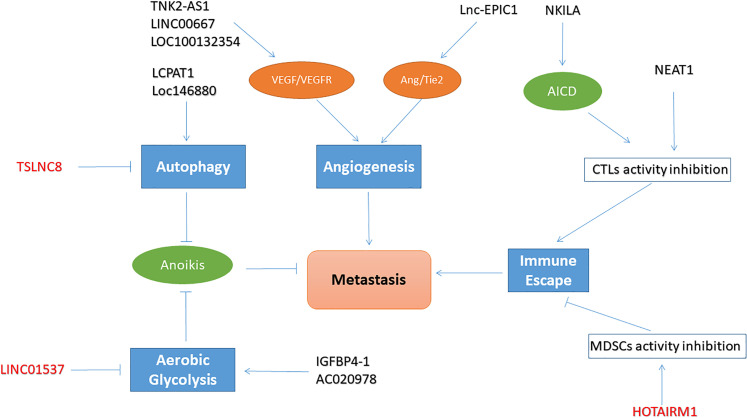
Metastasis is a characteristic cancer behavior116 and is reflected not only by tumor invasion and dissemination but also by a series of changes in physiological activities. While these changes are conducive to tumor metastasis, lncRNAs are now recognized as essential regulatory molecules implicated in these processes (Figure 4 and Table 3).
Figure 4.
The regulatory patterns of long noncoding RNAs (lncRNAs) in lung cancer metastasis.
Table 3.
The Regulatory Patterns of LncRNAs in Lung Cancer Metastasis.
| LncRNA | Cancer type | Expression | Model | Hallmark | Molecular mechanism | Function | Reference |
|---|---|---|---|---|---|---|---|
| TNK2-AS1 | NSCLC | Up | Vitro | Angiogenesis | Interacts with STAT3 to upregulate VEGFA expression | Promotes angiogenesis and metastasis | Wang et al117 |
| LOC100132354 | LAD | Up | Vitro | Angiogenesis | Activates VEGFA/VEGFR2 pathway | Promotes angiogenesis and metastasis | Wang et al118 |
| LINC00667 | NSCLC | Up | Vitro and vivo | Angiogenesis | Stabilizes VEGFA mRNA by EIF4A3 | Promotes angiogenesis and metastasis | Yang et al119 |
| Lnc-EPIC1 | NSCLC | Up | Vitro and vivo | Angiogenesis | Activates Ang2/Tie2 pathway | Promotes angiogenesis and metastasis | Hou et al120 |
| LCPAT1 | NSCLC | Up | Vitro | Autophagy | Promotes smoking and PM-2.5-joint induced autophagy | Promotes autophagy and metastasis | Lin et al121 |
| Loc146880 | LAD | Up | Vitro | Autophagy | PM-2.5 induce ROS production to elevate its
expression Promotes PM-2.5-induced autophagy |
Promotes autophagy and metastasis | Deng et al122 |
| TSLNC8 | NSCLC | Down | Vitro | Autophagy | Inhibits STAT3/HIF-1α pathway | Inhibits autophagy and metastasis | Fan et al123 |
| IGFBP4-1 | NSCLC | Up | Vitro | Aerobic glycolysis | Increasing the expression of HK2 | Promotes glycolysis and metastasis | Yang et al124 |
| AC020978 | NSCLC | Up | Vitro | Aerobic glycolysis | Prevents PKM2 from ubiquitin-mediated degradation and increases PKM2-enhanced HIF-1α transcription activity | Promotes glycolysis and metastasis | Hua et al125 |
| LINC01537 | NSCLC | Down | Vitro | Aerobic glycolysis | Inhibits PGK1 expression by targeting PDE2A | Inhibits glycolysis and metastasis | Gong et al126 |
| NEAT1 | NSCLC | Up | Vitro and vivo | Immune escape | Downregulates tumor-infiltration cytotoxic T cells through cGAS/STING/IFN pathway | Promotes immune escape and metastasis | Ma et al127 and Chen et al128 |
| NKILA | NSCLC | Up | Vitro and vivo | Immune escape | Promotes AICD of CTLs via NF-κB pathway | Promotes immune escape and metastasis | Huang et al129 |
| HOTAIRM1 | LAD | Down | Vitro and vivo | Immune escape | Inhibits immunosuppressive activity of MDSCs | Inhibits immune escape and metastasis | Tian et al130 and Chen et al131 |
Abbreviations: AICD, activation-induced cell death; Ang2, angiopoietin 2; cGAS, cyclic GMP-AMP synthase; CTL, cytotoxic T lymphocyte; EIF4A3, eukaryotic translation initiation factor 4A3; HIF-1α, hypoxia-inducible factor-1α; HK2, hexokinase 2; HOTAIR, HOX transcript antisense intergenic RNA; HOTAIRM1, HOTAIR myeloid-specific 1; IFN, interferon; IGFBP4-1, insulin-like growth factor binding protein 4 to 1; LAD, lung adenocarcinoma; LCPAT1, lung cancer progression-associated transcript 1; Lnc-EPIC1, long noncoding RNA EPIC1; MDSC, myeloid-derived suppressor cell; lncRNA, long noncoding RNA; mRNA, messenger RNA; NEAT1, nuclear paraspeckle assembly transcript 1; NF-κB, nuclear factor kappa B; NKILA, NF-κB interacting lncRNA; NSCLC, nonsmall cell lung cancer; PDE2A; phosphodiesterase 2A; PGK1, phosphoglycerate kinase 1; PKM2, M2 isoform of pyruvate kinase; PM-2.5, particulate matter-2.5; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription 3; STING, stimulator of interferon gene; TNK2-AS1, tyrosine kinase nonreceptor 2 antisense RNA 1; TSLNC8, tumor-suppressive role of lncRNA on chromosome 8p12; VEGFA, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2.
LncRNAs Involved in Angiogenesis
Angiogenesis is the generation of new vasculature from preexisting blood capillaries or postcapillary venules and is a key process supplying nutrients and removing metabolites to promote tumor growth.132 Tumor-induced neovascularization frequently exhibits morphological alterations characterized by premature and circuitous vascular structures.116 High permeability in new vasculature structures promotes the direct entry of tumor cells into the circulation.133 Moreover, the irregular and unstable structure of these neovascular systems lead to a dense vascular bed that increases contact surfaces between tumors and the bloodstream, promoting tumor access to the circulation and facilitating distant metastasis.133 Therefore, angiogenesis is instrumental in primary tumor metastasis and growth.
Generally, angiogenesis is regulated by proangiogenic signaling pathways, including the vascular endothelial growth factor/vascular endothelial growth factor receptor and angiopoietin (Ang)/Tie2 pathways.134 LncRNA tyrosine kinase nonreceptor 2 antisense RNA 1 (TNK2-AS1) is upregulated in NSCLC tissues.117 TNK2-AS1 enhances NSCLC metastasis and interacts with STAT3 to elevate its stability by inhibiting proteasome-mediated degradation. STAT3 also binds to the TNK2-AS1 promoter to activate its transcription. Moreover, this positive feedback mechanism elevates vascular endothelial growth factor A (VEGFA) expression to promote angiogenesis.117 Similarly, Wang et al118 reported that LOC100132354 promotes metastasis and induces angiogenesis in LAD by activating the VEGFA/VEGF receptor 2 (VEGFR2) pathway. Another study demonstrated that LINC00667 promoted cell migration and pathological angiogenesis in NSCLC by increasing VEGFA mRNA stability by recruiting eukaryotic translation initiation factor 4A3 (EIF4A3).119
Moreover, lncRNA EPIC1 (Lnc-EPIC1) reportedly facilitated cell viability and invasion in NSCLC.120 Elevated Lnc-EPIC1 expression stimulated angiogenesis by activating the Ang2/Tie2 pathway, and furthermore, Lnc-EPIC1 overexpression simultaneously enhanced new blood vessel formation in vivo.120
Unequivocally, metastasis and angiogenesis are central topics in cancer, with lncRNAs participating in lung cancer metastasis by regulating angiogenesis. Nevertheless, the precise molecular processes underpinning lncRNA-regulated angiogenesis in lung cancer metastasis are poorly characterized. Further research is warranted to uncover the clinical significance of lncRNAs in lung cancer angiogenesis.
LncRNAs Involved in Autophagy
Autophagy is an intricate biological process that maintains cellular homeostasis via lysosome-dependent degradation.135 The process is activated under specific conditions, such as cellular stress, nutrient deficiency, and hypoxia.136 As a highly conserved process, autophagy plays vital roles in cellular energy metabolism135,136 and is classified into three forms: microautophagy, macroautophagy, and chaperone-mediated autophagy.136 Macroautophagy, which is characterized by autophagosome engulfment of corrupted cytoplasmic elements and fusion with lysosomes to promote material degradation, is the most common autophagy type and displays contradictory effects during metastasis.135,137–139 During primary metastatic stages, autophagy inhibits metastasis by precluding tumor necrosis and reducing subsequent inflammatory cell infiltration.140 However, as metastasis invariably proceeds, autophagy promotes the process by protecting disseminating tumor cells from anoikis.140,141 Therefore, autophagy is essential for metastasis.
In lung cancer, smoking and particulate matter-2.5 (PM-2.5) are critical environmental carcinogens that impair pulmonary function and facilitate tumorigenesis by inducing cell autophagy.142,143 Recent studies have reported that lncRNAs play essential roles in autophagy induced by environmental carcinogens.121,122 For example, lung cancer progression-associated transcript 1 (LCPAT1) knockdown suppressed smoking and PM-2.5-induced autophagy, thereby inhibiting EMT in NSCLC.121
Interestingly, in PM-2.5-exposed LAD cells, lncRNA loc146880 expression was upregulated due to excessive reactive oxygen species (ROS) production.122 Elevated loc146880 enhanced autophagy, leading to increased cell invasion and EMT. However, loc146880 knockdown reversed these positive effects induced by PM-2.5.122 Therefore, these data suggested that loc146880 enhanced autophagy to promote lung cancer metastasis in cancer cells exposed to PM-2.5.
Hypoxia also induces autophagy and promotes tumorigenesis.144 Under hypoxia, the tumor-suppressive role of lncRNA on chromosome 8p12 (TSLNC8) reportedly suppressed the expression of autophagy-related proteins, including ATG14 and Beclin-1, leading to enhanced cell apoptosis and impaired NSCLC metastasis.123 Furthermore, overexpressed TSLNC8 decreased hypoxia-inducible factor-1α (HIF-1α) levels and inhibited STAT3 phosphorylation, suggesting that TSLNC8 regulated hypoxia-induced autophagy and NSCLC progression in a STAT3-dependent manner.123
Thus, autophagy induced by smoking, PM-2.5, or hypoxia is a crucial mechanism during lung cancer metastasis, with lncRNAs increasingly recognized as essential molecules regulating this process. Therefore, more research is required to explore the complex relationship between autophagy and metastasis with the hope of identifying new lncRNA therapies for lung cancer treatment.
LncRNAs Involved in Aerobic Glycolysis
Reprogrammed energy metabolism, which enhances glycolysis and glucose uptake to accelerate cell proliferation, survival, and migration, has been identified as a malignancy hallmark.116 Under normal physiological conditions, mitochondrial oxidative phosphorylation is the major energy source of the body that generates 30 or 32 adenosine triphosphate (ATP) molecules.145 However, cancer cells are inclined to obtain energy by enhancing glycolysis, which only generates two ATP molecules.146 To compensate for insufficient ATP production, cancer cells reprogram metabolism to enhance the rate of glucose uptake.147 This specific phenomenon was first observed by Warburg in the 1920s and termed the Warburg effect or aerobic glycolysis; thus, increased glucose uptake permits the synthesis of more metabolites, such as lactic acids, lipids, and nucleic acids, which favor tumor growth.146,148
Elevated glycolysis enhances glucose utilization, decreases ROS production, and elevates the antioxidant ability of cancer cells to resist anoikis and promote metastasis.149,150 Similarly, tumor cells were shown to alter metabolism in remote organs to accelerate implantation and metastasis.151
Emerging evidence has suggested that lncRNAs are key players in cancer metabolic remodeling processes by targeting metabolic enzymes, eg, UCA1 is upregulated in glioma cells and upregulates 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 expression, a critical glycolytic enzyme, to induce cell migration and invasion.152 In lung cancer, lncRNAs are also shown to participate in metastasis partially by regulating glycolytic enzymes.124
The first step of glycolysis involves glucose phosphorylation to glucose 6-phosphate by hexokinases (HKs) that are crucial glycolytic enzymes regulating glucose metabolic rates; thus, elevated HK expression maintains a rapid glycolytic rate in cancer tissues, facilitating cell metastasis.153 Geng et al154 reported that hexokinase 2 (HK2) modulates the expression of zonula occludens-1, E-cadherin, vimentin, and N-cadherin and accelerates metastasis in hepatocellular carcinoma cells. A high expression of the lncRNA insulin-like growth factor binding protein 4 to 1 (IGFBP4-1) was associated with greater lymph node metastasis and facilitated lung cancer cell migration and invasion via metabolic reprogramming mechanism by increasing HK2 expression.124
The M2 isoform of pyruvate kinase (PKM2) is preferentially expressed during developmental embryo stages and in various tumor cells.155 PKM2 transforms the final rate-limiting step of glycolysis and leads to cancer-specific glycolysis, thus promoting glucose uptake and lactic acid production.155 In the nucleus, the direct reciprocity of PKM2 with TGF-β-induced factor homeobox 2 resulted in the recruitment of histone deacetylase 3 to the E-cadherin promoter.156 This was followed by histone H3 deacetylation and the inhibition of E-cadherin transcription, thereby facilitating EMT in colon cancer cells.156 LncRNA-AC020978 was upregulated and promoted cell metastasis in NSCLC cells125; it stabilized PKM2 by inhibiting ubiquitin-mediated degradation, increasing PKM2-enhanced HIF-1α transcription activity, and facilitating glycolytic metabolism and lung cancer metastasis.125
Phosphoglycerate kinase 1 (PGK1) is an essential metabolic enzyme in glycolysis; it catalyzes 1,3-bisphosphate glycerate to 3-phosphoglycerate and generates the first ATP in the glycolysis.157 Several studies reported that PGK1 played an important role in cancer metastasis.158,159 Xie et al160 showed that PGK1 is upregulated in hepatocellular carcinoma tissues, and overexpressed PGK1 is correlated with poor prognosis. Moreover, PGK1 knockdown dramatically decreased cell proliferation, migration, and invasion of hepatocellular carcinoma cells, suggesting a positive role of PGK1 in hepatocellular carcinoma metastasis.160 Lowered expression of LINC01537 was associated with poor prognosis of lung cancer, whereas overexpressed LINC01537 limited aerobic glycolysis and inhibited metastasis in NSCLC cells.126 Further investigations showed that LINC01537 regulated PGK1 expression by directly targeting phosphodiesterase 2A (PDE2A), a critical member of the PDE family that modulates mitochondrial cyclic adenosine monophosphate expression and is highly associated with energy metabolism processes.126 Therefore, the LINC01537/PDE2A/PGK1 axis may be a potential target for inhibiting cellular energy metabolism and metastasis during NSCLC.
Since Weinberg identified metabolic reprogramming as an emerging cancer hallmark, several studies have validated the involvement of cancer metabolism during malignant processes.116,150 LncRNAs regulate cancer glycolysis by modulating the expression of several metabolic enzymes, resulting in lung cancer metastasis. Importantly, several reports have suggested that lncRNAs may alter the distribution of glycolytic enzymes in human cancer cells, thus participating in cancer progression.161,162 However, in lung cancer, these things are missing. Therefore, further research is required to comprehensively understand the complex interaction networks that lncRNAs operate in cancer metabolic and metastatic processes.
LncRNAs Involved in Immune escape
Tumorigenesis and metastatic cascades rely on the evolving molecular functions of cancer cells and interactions with the immune system.163 During each stage of the metastatic process, the immune system strives to block cancer cell dissemination.164 For instance, CD8+ T cells recognize tumor-related antigens and differentiate into cytotoxic T lymphocytes (CTLs), leading to the specific destructions of tumor cells.163,165 Accordingly, nascent tumor cells evolve evasive mechanisms to evade the immune system, including increasing the expression of immunosuppressive factors, enlisting immunosuppressive cells, and inhibiting the antitumor functions of CTLs.163,164 These processes decrease cancer cell immunogenicity, thereby establishing an immunosuppressive tumor microenvironment contributing to tumor metastasis.
Evidence has suggested that lncRNAs help lung tumor cells evade antitumor immune responses, thus facilitating tumor growth and metastasis. Notably, lncRNA regulation during lung cancer immune escape broadly covers two areas: (1) the influence of lncRNAs on T cell activity and function and (2) lncRNA regulation of myeloid-derived suppressor cell (MDSC) activity and function.127,129,130
Nuclear paraspeckle assembly transcript 1 (NEAT1) is an oncogenic lncRNA facilitating lung cancer progression128; however, its molecular mechanism is unclear. Ma et al127 reported that NEAT1 knockdown represses NSCLC cell viability and invasion and upregulates tumor-infiltration CTLs. Further investigations revealed that NEAT1 silencing upregulated cyclic GMP-AMP synthase (cGAS), a stimulator of interferon gene (STING) levels, and increased interferon-β (IFN-β) expression.127 cGAS is a sensitive DNA sensor that responds to cytosolic DNA and mediates immune responses.166 Moreover, activated cGAS promotes activation of the STING pathway to produce downstream factors, such as IFN-β.167 IFN-β belongs to the type 1 IFN family that exerts significant stimulatory effects toward T cell activation, thereby improving T cell immune responses and inhibiting cancer progression.168 Taken together, NEAT1 putatively promotes NSCLC metastasis by inhibiting cGAS-STING- IFN-β signaling to repress T cell activation and help tumor cells evade immunosurveillance.
Activation-induced cell death (AICD) is a specific mechanism regulating T cell activity and function.169 It is a vital immune protection process that activates Fas (cluster of diffraction 95)-Fas ligand signaling to restrict excessive T cell activation.169 However, AICD may be used to evade immunological assault to exert significant effects on cancer development.169 Evidence has revealed that nuclear factor kappa B (NF-κB) interacting lncRNA (NKILA) participates in cancer metastasis by regulating the NF-κB pathway.170,171 Huang et al129 suggested that NKILA knockdown suppresses the AICD sensitivity of lung cancer cells lysates-activated CTLs, which was abrogated by NF-κB downregulation, suggesting that NKILA regulated AICD via the NF-κB pathway. More importantly, in NKILA-deletion mice, tumor apoptosis was elevated, and tumor growth was inhibited.129 Collectively, NKILA silencing in tumor-specific CTLs may combat tumor immune escape by restricting AICD via NF-κB signaling, thereby inhibiting tumor development and probably metastasis. These findings underscore the importance of lncRNAs in tumor immune escape and suggest that engineered lncRNAs in adoptively transferred T cells may be a novel cancer immunotherapy.
MDSCs are immature immunosuppressive cells that differentiate into macrophages and monocytes.172 However, under pathological circumstances, particularly cancer, MDSCs differentiation is impeded; thus, they accumulate.173–175 MDSCs also facilitate cancer metastasis by suppressing CTL responses and inducing regulatory T cells by generating inhibitory molecules, such as arginase 1, myeloperoxidase, and ROS.176 Previous studies showed that lncRNAs played critical roles in regulating MDSCs suppressive activities.177,178 Notably, lncRNA HOTAIR myeloid-specific 1 (HOTAIRM1) was downregulated in LAD and associated with advanced lymph node metastasis.131 Elevated HOTAIRM1 inhibited MDSCs immunosuppressive activities that improved antitumor responses, retarded tumor growth, and delayed tumor metastasis.130 These findings indicated that HOTAIRM1 could be an immunotherapeutic target for lung cancer treatment.
These findings provide invaluable molecular clues on how lncRNAs influence immune responses in tumor cells by regulating T cell and MDSC activity, thus promoting metastasis. However, how lncRNAs modulate the immune microenvironment of lung cancer remains uncharacterized. More comprehensive research is required to explore these linkages.
Conclusion and Perspectives
Metastasis causes high lethality and recurrence rates in lung cancer. Emerging studies have increasingly identified lncRNAs as promising therapeutic targets for treating this disease. This review provided a comprehensive understanding of the main molecular mechanisms and regulatory roles of several lncRNAs and their impact on lung cancer metastasis. LncRNAs are an essential type of ncRNAs that regulate lung cancer metastasis via three main molecular mechanisms: (1) lncRNAs act as ceRNAs; (2) lncRNAs regulate the transduction of several signal pathways; and (3) lncRNAs coordinate with EZH2. Thus, lncRNAs exert multiple functions toward lung cancer metastasis by regulating angiogenesis, autophagy, aerobic glycolysis, and immune escape. Interestingly, the molecular mechanism of one particular lncRNA involves several aspects, eg, SNHG3 not only sponges miR-340 to 5p179 and miR-515 to 5p180 but also activates the TGF-β pathway to promote lung cancer metastasis.181 However, lncRNA research is still in its infancy; therefore, future investigations should explore the regulatory mechanisms of lncRNAs and their impact on lung cancer metastasis.
Moreover, dysregulated lncRNAs stably exist in internal environments, exhibit specific expression profiles in different lung cancer subtypes, and provide potential options for lung cancer treatment. Current research on lncRNAs as diagnostic and therapeutic biomarkers should focus on their application to clinical settings; however, this requires comprehensive verification and authentication in large-scale studies. Going forward, this research strategy will significantly improve lncRNA therapeutic outcomes in treating metastatic lung cancer.
Abbreviations
- AICD
activation-induced cell death
- AKT
protein kinase B
- Ang
angiopoietin
- ATP
adenosine triphosphate
- BCYRN1
brain cytoplasmic RNA 1
- CDH1
cadherin 1
- ceRNA
competing endogenous RNA
- cGAS
cyclic GMP-AMP synthase
- CTL
cytotoxic T lymphocyte
- DACH1
Dachshund homolog 1
- DGCR5
DiGeorge syndrome critical region gene 5
- DLX6-AS1
distal-less homeobox 6 antisense 1
- EIF4A3
eukaryotic translation initiation factor 4A3
- EMT
epithelial–mesenchymal transition
- EPHB6
EPH receptor B6
- ERK
extracellular signal-regulated kinase
- EZH2
enhancer of zeste homolog 2
- FER1L4
Fer-1-like protein 4
- FLVCR1-AS1
feline leukemia virus subgroup C receptor 1 antisense RNA 1
- FOXD3-AS1
forkhead box D3 antisense RNA 1
- GATA6-AS1
GATA6 antisense RNA 1
- GSPT1
G1–S phase transition 1
- H3K27
histone H3 lysine 27
- HCG11
HLA complex group 11
- HIF-1α
hypoxia-inducible factor-1α
- Hk
hexokinase
- HMGB1
high-mobility group protein B1
- HOTAIR
HOX transcript antisense intergenic RNA
- HOTAIRM1
HOTAIR myeloid-specific 1
- IFN-β
interferon-β
- IGFBP4-1
insulin-like growth factor binding protein 4 to 1
- LAD
lung adenocarcinoma
- LCAT1
lung cancer-associated transcript 1
- LCPAT1
lung cancer progression-associated transcript 1
- LIFR-AS1
leukemia inhibitory factor receptor antisense RNA 1
- LIMK
Lin11, Isl-1, and Mec-3 kinase
- Lnc-EPIC1
long noncoding RNA EPIC1
- lncRNAs
long noncoding RNA
- LSCC
lung squamous cell carcinoma
- MALAT1
metastasis-associated LAD transcript 1
- MAPK
mitogen-activated protein kinase
- MDM2
mouse double minute 2
- MDSC
myeloid-derived suppressor cell
- MEG3
maternally expressed gene 3
- miRNA
microRNA
- MMP9
matrix metalloproteinase 9
- mRNA
messenger RNA
- ncRNA
noncoding RNA
- NEAT1
nuclear paraspeckle assembly transcript 1
- NEDD9
neural precursor cell-expressed developmentally downregulated 9
- NF-κB
nuclear factor kappa B
- NKILA
NF-κB interacting lncRNA
- Nrf2
nuclear factor erythroid 2 p45-related factor 2
- NSCLC
nonsmall cell lung cancer
- NSCLCAT1
nonsmall cell LCAT1
- PCAT6
prostate cancer-associated transcript 6
- PDE2A
phosphodiesterase 2A
- PDIA3P
protein disulfide isomerase family A member 3 pseudogene 1
- PD-L1
programmed death ligand 1
- PGK1
phosphoglycerate kinase 1
- PI3K
phosphoinositide 3-kinase
- PKM2
M2 isoform of pyruvate kinase
- PM-2.5
particulate matter-2.5
- PRC2
polycomb repressive complex 2
- PRR11
proline-rich 11
- PVT1
plasmacytoma variant translocation 1
- RAC1
Rac family small GTPase 1
- RKIP
raf kinase inhibitor protein
- ROS
reactive oxygen species
- RSF1-IT2
remodeling and spacing factor 1-intronic transcript 2
- SCLC
small cell lung cancer
- SNHG20
small nucleolar RNA host gene 20
- SOCS5
suppressor of cytokine signaling 5
- Sox2ot
SOX2 overlapping transcript
- SPRY4-IT1
SPRY4 intronic transcript 1
- SRCIN1
SRC kinase signaling inhibitor 1
- STAT3
signal transducer and activator of transcription 3
- STING
stimulator of interferon gene
- SUMO1P3
small ubiquitin-like modifier 1 pseudogene 3
- TAZ
transcriptional coactivator with PDZ-binding motif
- TGF-β
transforming growth factor β
- TINCR
terminal differentiation-induced noncoding RNA
- TNK2-AS1
tyrosine kinase nonreceptor 2 antisense RNA 1
- TP73-AS1
tumor protein P73 antisense RNA 1
- TSLNC8
tumor-suppressive role of lncRNA on chromosome 8p12
- TUG1
taurine upregulated gene 1
- TUSC3
tumor suppressor candidate 3 expression
- UCA1
urogenital carcinoma antigen 1
- VAL
vimentin-associated lncRNA
- VEGFA
vascular endothelial growth factor A
- VEGFR2
VEGF receptor 2
- Wnt
wingless/integrated
- YAP
Yes-associated protein
- ZEB1
zinc-finger e-box binding homeobox 1
- ZNF471
zinc-finger protein 471.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Science and Technology of Sichuan Province (grant number 2020YJ0147).
ORCID iDs: Peng Huang https://orcid.org/0000-0003-4102-946X
Xin Liang https://orcid.org/0000-0003-2167-5368
Linjiang Song https://orcid.org/0000-0002-0512-0410
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Li M, Meng H, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62(5):640-647. doi: 10.1007/s11427-018-9461-5. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48(3):889-902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535-546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng M. Classification and pathology of lung cancer. Surg Oncol Clin N Am. 2016;25(3):447-468. doi: 10.1016/j.soc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35(1):75-91. doi: 10.1007/s10555-016-9618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274-284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 9.Diepenbruck M, Christofori G. Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43(12):7-13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Chaffer CL, San Juan BP, Lim E, et al. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35(4):645-654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417-427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 12.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272(2):177-185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16(4):201-218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore JE, Purcaro MJ, Pratt HE, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57-74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbastabar M, Sarfi M, Golestani A, et al. lncRNA involvement in hepatocellular carcinoma metastasis and prognosis. EXCLI J. 2018;17(9):900-913. doi: 10.17179/excli2018-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861-874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 17.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47-62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 18.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703-719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965-3981. doi: 10.1158/0008-5472.Can-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beermann J, Piccoli MT, Viereck J, et al. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96(4):1297-1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Zitello E, Guo R, et al. The function of LncRNAs and their role in the prediction, diagnosis, and prognosis of lung cancer. Clin Transl Med. 2021;11(4):e367. doi: 10.1002/ctm2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aftabi Y, Ansarin K, Shanehbandi D, et al. Long non-coding RNAs as potential biomarkers in the prognosis and diagnosis of lung cancer: a review and target analysis. IUBMB Life. 2021;73(2):307-327. doi: 10.1002/iub.2430. [DOI] [PubMed] [Google Scholar]
- 23.Ghafouri-Fard S, Shoorei H, Branicki W, et al. Non-coding RNA profile in lung cancer. Exp Mol Pathol. 2020;114(6):104411. doi: 10.1016/j.yexmp.2020.104411. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):925-933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41(9):761-772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Cabili MN, Dunagin MC, McClanahan PD, et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16(1):20. doi: 10.1186/s13059-015-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naganuma T, Hirose T. Paraspeckle formation during the biogenesis of long non-coding RNAs. RNA Biol. 2013;10(3):456-461. doi: 10.4161/rna.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinodoz S, Guttman M. Long noncoding RNAs: an emerging link between gene regulation and nuclear organization. Trends Cell Biol. 2014;24(11):651-663. doi: 10.1016/j.tcb.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3(10):1113-1121. doi: 10.1158/2159-8290.Cd-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonasio R, Shiekhattar R. Regulation of transcription by long noncoding RNAs. Annu Rev Genet. 2014;48:433-455. doi: 10.1146/annurev-genet-120213-092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonas K, Calin GA, Pichler M. RNA-binding proteins as important regulators of long non-coding RNAs in cancer. Int J Mol Sci. 2020;21(8):2969-2990. doi: 10.3390/ijms21082969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noh JH, Kim KM, McClusky WG, et al. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip Rev RNA. 2018;9(3):e1471. doi: 10.1002/wrna.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199-227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17(5):272-283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 35.Qi X, Zhang DH, Wu N, et al. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52(10):710-718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Qiu Q, Qian X, et al. Long noncoding RNA LCAT1 functions as a ceRNA to regulate RAC1 function by sponging miR-4715-5p in lung cancer. Mol Cancer. 2019;18(1):171. doi: 10.1186/s12943-019-1107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031-8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 38.Gutschner T, Hämmerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73(3):1180-1189. doi: 10.1158/0008-5472.Can-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Wang J, Chen Y, et al. lncRNA MALAT1 exerts oncogenic functions in lung adenocarcinoma by targeting miR-204. Am J Cancer Res. 2016;6(5):1099-1107. [PMC free article] [PubMed] [Google Scholar]
- 40.Yu W, Ding J, He M, et al. Estrogen receptor beta promotes the vasculogenic mimicry (VM) and cell invasion via altering the lncRNA-MALAT1/miR-145-5p/NEDD9 signals in lung cancer. Oncogene. 2019;38(8):1225-1238. doi: 10.1038/s41388-018-0463-1. [DOI] [PubMed] [Google Scholar]
- 41.Wei S, Wang K, Huang X, et al. lncRNA MALAT1 contributes to non-small cell lung cancer progression via modulating miR-200a-3p/programmed death-ligand 1 axis. Int J Immunopathol Pharmacol. 2019;33:2058738419859699. doi: 10.1177/2058738419859699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang HM, Lu JH, Chen WY, et al. Upregulated lncRNA-UCA1 contributes to progression of lung cancer and is closely related to clinical diagnosis as a predictive biomarker in plasma. Int J Clin Exp Med. 2015;8(7):11824-11830. [PMC free article] [PubMed] [Google Scholar]
- 43.Wu H, Zhou C. Long non-coding RNA UCA1 promotes lung cancer cell proliferation and migration via microRNA-193a/HMGB1 axis. Biochem Biophys Res Commun. 2018;496(2):738-745. doi: 10.1016/j.bbrc.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 44.Wu XJ, Chen YY, Guo WW, et al. HMGB1 regulates SNAI1 during NSCLC metastasis, both directly, through transcriptional activation, and indirectly, in a RSF1-IT2-dependent manner. Mol Oncol. 2020;14(6):1348-1364. doi: 10.1002/1878-0261.12691. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Zhao Y, Feng C, Li Y, et al. lncRNA H19 promotes lung cancer proliferation and metastasis by inhibiting miR-200a function. Mol Cell Biochem. 2019;460(1–2):1-8. doi: 10.1007/s11010-019-03564-1. [DOI] [PubMed] [Google Scholar]
- 46.Yang YR, Zang SZ, Zhong CL, et al. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7(10):6929-6935. [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W, Zhu H, Yin L, et al. lncRNA-PVT1 facilitates invasion through upregulation of MMP9 in nonsmall cell lung cancer cell. DNA Cell Biol. 2017;36(9):787-793. doi: 10.1089/dna.2017.3725. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Xu L, Deng G, et al. Exosomal HOTAIR promotes proliferation, migration and invasion of lung cancer by sponging miR-203. Sci China Life Sci. 2020;63(8):1265-1268. doi: 10.1007/s11427-019-1579-x. [DOI] [PubMed] [Google Scholar]
- 49.Jiang C, Yang Y, Yang Y, et al. Long noncoding RNA (lncRNA) HOTAIR affects tumorigenesis and metastasis of non-small cell lung cancer by upregulating miR-613. Oncol Res. 2018;26(5):725-734. doi: 10.3727/096504017X15119467381615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen SS, Peng M, Zhou GZ, et al. Long non-coding RNA HOTAIR regulates the development of non-small cell lung cancer through miR-217/DACH1 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(2):670-678. doi: 10.26355/eurrev_201901_16905. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Li Y, Han L, et al. SUMO1P3 Is associated clinical progression and facilitates cell migration and invasion through regulating miR-136 in non-small cell lung cancer. Biomed Pharmacother. 2019;113(5):108686. doi: 10.1016/j.biopha.2019.108686. [DOI] [PubMed] [Google Scholar]
- 52.Sun W, Zhang L, Yan R, et al. lncRNA DLX6-AS1 promotes the proliferation, invasion, and migration of non-small cell lung cancer cells by targeting the miR-27b-3p/GSPT1 axis. Onco Targets Ther. 2019;12(12):3945-3954. doi: 10.2147/OTT.S196865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Y, Ni R, Wang J, et al. Knockdown of lncRNA DLX6-AS1 inhibits cell proliferation, migration and invasion while promotes apoptosis by downregulating PRR11 expression and upregulating miR-144 in non-small cell lung cancer. Biomed Pharmacother. 2019;109(1):1851-1859. doi: 10.1016/j.biopha.2018.09.151. [DOI] [PubMed] [Google Scholar]
- 54.Kang M, Shi J, Li B, et al. lncRNA DGCR5 regulates the non-small cell lung cancer cell growth, migration, and invasion through regulating miR-211-5p/EPHB6 axis. Biofactors. 2019;45(5):788-794. doi: 10.1002/biof.1539. [DOI] [PubMed] [Google Scholar]
- 55.Luo J, Zhu H, Jiang H, et al. The effects of aberrant expression of LncRNA DGCR5/miR-873-5p/TUSC3 in lung cancer cell progression. Cancer Med. 2018;7(7):3331–3341. doi: 10.1002/cam4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji T, Zhang Y, Wang Z, et al. FOXD3-AS1 suppresses the progression of non-small cell lung cancer by regulating miR-150/SRCIN1axis. Cancer Biomark. 2020;29(3):417-427. doi: 10.3233/cbm-200059. [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, Wu J, Huang H, et al. lncRNA LIFR-AS1 suppresses invasion and metastasis of non-small cell lung cancer via the miR-942-5p/ZNF471 axis. Cancer Cell Int. 2020;20(5):180. doi: 10.1186/s12935-020-01228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong Z, Chen X, Zhang Y, et al. lncRNA GATA6-AS1 inhibits the progression of non-small cell lung cancer via repressing microRNA-543 to up-regulating RKIP. Cancer Manag Res. 2020;12(12):9327-9338. doi: 10.2147/cmar.S254184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan G, Jiao J, Shen F, et al. Long non-coding RNA HCG11 sponging miR-522-3p inhibits the tumorigenesis of non-small cell lung cancer by upregulating SOCS5. Thorac Cancer. 2020;11(10):2877-2886. doi: 10.1111/1759-7714.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xue J, Suarez JS, Minaai M, et al. HMGB1 as a therapeutic target in disease. J Cell Physiol. 2021;236(5):3406-3419. doi: 10.1002/jcp.30125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Georgakopoulos-Soares I, Chartoumpekis DV, Kyriazopoulou V, et al. EMT factors and metabolic pathways in cancer. Front Oncol. 2020;10(4):499. doi: 10.3389/fonc.2020.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue C, Chen C, Gu X, et al. Progress and assessment of lncRNA DGCR5 in malignant phenotype and immune infiltration of human cancers. Am J Cancer Res. 2021;11(1):1-13. [PMC free article] [PubMed] [Google Scholar]
- 63.Chatterjee S, Sil PC. Targeting the crosstalks of Wnt pathway with hedgehog and notch for cancer therapy. Pharmacol Res. 2019;142(2):251-261. doi: 10.1016/j.phrs.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 64.Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62(1):50-60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661-5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang Y, Xiao G, Chen Y, et al. lncRNA MALAT1 promotes migration and invasion of non-small-cell lung cancer by targeting miR-206 and activating Akt/mTOR signaling. Anticancer Drugs. 2018;29(8):725-735. doi: 10.1097/CAD.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 67.Zhang YX, Yuan J, Gao ZM, et al. lncRNA TUC338 promotes invasion of lung cancer by activating MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22(2):443-449. doi: 10.26355/eurrev_201801_14193. [DOI] [PubMed] [Google Scholar]
- 68.He R, Zhang FH, Shen N. LncRNA FEZF1-AS1 enhances epithelial–mesenchymal transition (EMT) through suppressing E-cadherin and regulating WNT pathway in non-small cell lung cancer (NSCLC). Biomed Pharmacother. 2017;95(11):331-338. doi: 10.1016/j.biopha.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 69.He W, Zhang Y, Xia S. LncRNA NNT-AS1 promotes non-small cell lung cancer progression through regulating miR-22-3p/YAP1 axis. Thorac Cancer. 2020;11(3):549-560. doi: 10.1111/1759-7714.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu KH, Li W, Liu XH, et al. Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC cancer. 2013;13(10):461. doi: 10.1186/1471-2407-13-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katso R, Okkenhaug K, Ahmadi K, et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615-675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 72.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606-619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Xiang C, Wang Y, et al. lncRNA LINC00152 knockdown had effects to suppress biological activity of lung cancer via EGFR/PI3K/AKT pathway. Biomed Pharmacother. 2017;94(10):644-651. doi: 10.1016/j.biopha.2017.07.120. [DOI] [PubMed] [Google Scholar]
- 74.Liu C, Ren L, Deng J, et al. lncRNA TP73-AS1 promoted the progression of lung adenocarcinoma via PI3K/AKT pathway. Biosci Rep. 2019;39(1):1-12. doi: 10.1042/bsr20180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao X, Wang N, Wu S, et al. Long non-coding RNA FER1L4 inhibits cell proliferation and metastasis through regulation of the PI3K/AKT signaling pathway in lung cancer cells. Mol Med Rep. 2019;20(1):182-190. doi: 10.3892/mmr.2019.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang W, Kai J, Li D, et al. lncRNA HOXB-AS3 exacerbates proliferation, migration, and invasion of lung cancer via activating the PI3K-AKT pathway. J Cell Physiol. 2020;235(10):7194-7203. doi: 10.1002/jcp.29618. [DOI] [PubMed] [Google Scholar]
- 77.Tian H, Lian R, Li Y, et al. AKT-induced lncRNA VAL promotes EMT-independent metastasis through diminishing Trim16-dependent vimentin degradation. Nat Commun. 2020;11(1):5127. doi: 10.1038/s41467-020-18929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaestel M. MAPK-activated protein kinases (MKs): novel insights and challenges. Front Cell Dev Biol. 2015;3:88):1-6. doi: 10.3389/fcell.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu P, Wang H, Liang Y, et al. LINC00852 promotes lung adenocarcinoma spinal metastasis by targeting S100A9. J Cancer. 2018;9(22):4139-4149. doi: 10.7150/jca.26897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu ZJ, He JK. TINCR facilitates non-small cell lung cancer progression through BRAF-activated MAPK pathway. Biochem Biophys Res Commun. 2018;497(4):971-977. doi: 10.1016/j.bbrc.2018.02.059. [DOI] [PubMed] [Google Scholar]
- 81.Zhou P, Li Y, Li B, et al. NMIIA promotes tumor growth and metastasis by activating the Wnt/β-catenin signaling pathway and EMT in pancreatic cancer. Oncogene. 2019;38(27):5500-5515. doi: 10.1038/s41388-019-0806-6. [DOI] [PubMed] [Google Scholar]
- 82.Yang X, Yang B. lncRNA PDIA3P regulates cell proliferation and invasion in non-small cell lung cancer. Exp Ther Med. 2019;18(4):3184-3190. doi: 10.3892/etm.2019.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin H, Shangguan Z, Zhu M, et al. lncRNA FLVCR1-AS1 silencing inhibits lung cancer cell proliferation, migration, and invasion by inhibiting the activity of the Wnt/beta-catenin signaling pathway. J Cell Biochem. 2019;120(6):10625-10632. doi: 10.1002/jcb.28352. [DOI] [PubMed] [Google Scholar]
- 84.Ghafouri-Fard S, Dashti S, Hussen BM, et al. BCYRN1: an oncogenic lncRNA in diverse cancers. Pathol Res Pract. 2021;220(4):153385. doi: 10.1016/j.prp.2021.153385. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Bai W, Wang M, et al. Long non-coding RNA brain cytoplasmic RNA 1 acts as an oncogene and regulates cell proliferation and metastasis in non-small cell lung cancer. J Nanosci Nanotechnol. 2019;19(4):1978-1985. doi: 10.1166/jnn.2019.16402. [DOI] [PubMed] [Google Scholar]
- 86.Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20(4):211-226. doi: 10.1038/s41580-018-0086-y. [DOI] [PubMed] [Google Scholar]
- 87.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246-257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 88.Guan ZB, Cao YS, Li Y, et al. Knockdown of lncRNA GHET1 suppresses cell proliferation, invasion and LATS1/YAP pathway in non small cell lung cancer. Cancer Biomark. 2018;21(3):557-563. doi: 10.3233/CBM-170431. [DOI] [PubMed] [Google Scholar]
- 89.Reardon SN, King ML, MacLean JA. II, et al. CDH1 Is essential for endometrial differentiation, gland development, and adult function in the mouse uterus. Biol Reprod. 2012;86(5):141, 141-110. doi: 10.1095/biolreprod.112.098871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang C, Lv GQ, Cui LF, et al. MicroRNA-572 targets CDH1 to promote metastasis of Wilms’ tumor. Eur Rev Med Pharmacol Sci. 2019;23(9):3709-3717. doi: 10.26355/eurrev_201905_17794. [DOI] [PubMed] [Google Scholar]
- 91.Zhao W, Zhang LN, Wang XL, et al. Long noncoding RNA NSCLCAT1 increases non-small cell lung cancer cell invasion and migration through the Hippo signaling pathway by interacting with CDH1. FASEB J. 2019;33(1):1151-1166. doi: 10.1096/fj.201800408R. [DOI] [PubMed] [Google Scholar]
- 92.Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 2014;12(1):3-13. doi: 10.1158/1541-7786.Mcr-13-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duffy MJ, Synnott NC, Crown J. Mutant p53 as a target for cancer treatment. Eur J Cancer. 2017;83(9):258-265. doi: 10.1016/j.ejca.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 94.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1(14):1001-1008. [PubMed] [Google Scholar]
- 95.Zhang M, Jiang N, Cui R, et al. Deregulated lncRNA expression profile in the mouse lung adenocarcinomas with KRAS-G12D mutation and P53 knockout. J Cell Mol Med. 2019;23(10):6978-6988. doi: 10.1111/jcmm.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olivero CE, Martínez-Terroba E, Zimmer J, et al. P53 activates the long noncoding RNA Pvt1b to inhibit Myc and suppress tumorigenesis. Mol Cell. 2020;77(4):761-774.e768. doi: 10.1016/j.molcel.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Czermin B, Melfi R, McCabe D, et al. Drosophila enhancer of zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal polycomb sites. Cell. 2002;111(2):185-196. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 98.Di Croce L, Helin K. Transcriptional regulation by polycomb group proteins. Nat Struct Mol Biol. 2013;20(10):1147-1155. doi: 10.1038/nsmb.2669. [DOI] [PubMed] [Google Scholar]
- 99.Gan L, Yang Y, Li Q, et al. Epigenetic regulation of cancer progression by EZH2: from biological insights to therapeutic potential. Biomark Res. 2018;6(3):10. doi: 10.1186/s40364-018-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Su M, Xiao Y, Tang J, et al. Role of lncRNA and EZH2 interaction/regulatory network in lung cancer. J Cancer. 2018;9(22):4156-4165. doi: 10.7150/jca.27098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Z, Xu L, Tang N, et al. The polycomb group protein EZH2 inhibits lung cancer cell growth by repressing the transcription factor Nrf2. FEBS Lett. 2014;588(17):3000-3007. doi: 10.1016/j.febslet.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 102.Xia L, Zhu X, Zhang L, et al. EZH2 Enhances expression of CCL5 to promote recruitment of macrophages and invasion in lung cancer. Biotechnol Appl Biochem. 2019;67(6):1011-1019. 10.1002/bab.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Niu Y, Ma F, Huang W, et al. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer. 2017;16(1):5. doi: 10.1186/s12943-016-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smolich B, Vo M, Buckley S, et al. Cloning and biochemical characterization of LIMK-2, a protein kinase containing two LIM domains. J Biochem. 1997;121(2):382-388. doi: 10.1093/oxfordjournals.jbchem.a021599. [DOI] [PubMed] [Google Scholar]
- 105.Suyama E, Wadhwa R, Kawasaki H, et al. LIM kinase-2 targeting as a possible anti-metastasis therapy. J Gene Med. 2004;6(3):357-363. doi: 10.1002/jgm.491. [DOI] [PubMed] [Google Scholar]
- 106.Chen Z, Chen X, Chen P, et al. Long non-coding RNA SNHG20 promotes non-small cell lung cancer cell proliferation and migration by epigenetically silencing of P21 expression. Cell Death Dis. 2017;8(10):e3092. doi: 10.1038/cddis.2017.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hou PF, Jiang T, Chen F, et al. KIF4A facilitates cell proliferation via induction of p21-mediated cell cycle progression and promotes metastasis in colorectal cancer. Cell Death Dis. 2018;9(5):477. doi: 10.1038/s41419-018-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi X, Liu Z, Liu Z, et al. Long noncoding RNA PCAT6 functions as an oncogene by binding to EZH2 and suppressing LATS2 in non-small-cell lung cancer. EBioMedicine. 2018;37(11):177-187. doi: 10.1016/j.ebiom.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zang X, Gu J, Zhang J, et al. Exosome-transmitted lncRNA UFC1 promotes non-small-cell lung cancer progression by EZH2-mediated epigenetic silencing of PTEN expression. Cell Death Dis. 2020;11(4):215. doi: 10.1038/s41419-020-2409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y, Wu N, Luo X, et al. SOX2OT, A novel tumor-related long non-coding RNA. Biomed Pharmacother. 2020;123(3):109725. doi: 10.1016/j.biopha.2019.109725. [DOI] [PubMed] [Google Scholar]
- 111.Hou Z, Zhao W, Zhou J, et al. A long noncoding RNA Sox2ot regulates lung cancer cell proliferation and is a prognostic indicator of poor survival. Int J Biochem Cell Biol. 2014;53(8):380-388. doi: 10.1016/j.biocel.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Kamel LM, Atef DM, Mackawy AMH, et al. Circulating long non-coding RNA GAS5 and SOX2OT as potential biomarkers for diagnosis and prognosis of non-small cell lung cancer. Biotechnol Appl Biochem. 2019;66(4):634-642. doi: 10.1002/bab.1764. [DOI] [PubMed] [Google Scholar]
- 113.Zhang K, Li Y, Qu L, et al. Long noncoding RNA Sox2 overlapping transcript (SOX2OT) promotes non-small-cell lung cancer migration and invasion via sponging microRNA 132 (miR-132). Onco Targets Ther. 2018;11(11):5269-5278. doi: 10.2147/ott.S168654. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Sun M, Liu XH, Lu KH, et al. EZH2-mediated Epigenetic suppression of long noncoding RNA SPRY4-IT1 promotes NSCLC cell proliferation and metastasis by affecting the epithelial–mesenchymal transition. Cell Death Dis. 2014;5(6):e1298. doi: 10.1038/cddis.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wei S, Liu J, Li X, et al. Repression of lncRNA-SVUGP2 mediated by EZH2 contributes to the development of non-small cell lung cancer via brisking Wnt/beta-catenin signal. Artif Cells Nanomed Biotechnol. 2019;47(1):3400-3409. doi: 10.1080/21691401.2019.1648279. [DOI] [PubMed] [Google Scholar]
- 116.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Han D, Pan L, et al. The positive feedback between lncRNA TNK2-AS1 and STAT3 enhances angiogenesis in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;507(1–4):185-192. doi: 10.1016/j.bbrc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, Zhang F, Wang J, et al. lncRNA LOC100132354 promotes angiogenesis through VEGFA/VEGFR2 signaling pathway in lung adenocarcinoma. Cancer Manag Res. 2018;10(10):4257-4266. doi: 10.2147/cmar.S177327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang H, Yang W, Dai W, et al. LINC00667 promotes the proliferation, migration, and pathological angiogenesis in non-small cell lung cancer through stabilizing VEGFA by EIF4A3. Cell Biol Int. 2020;44(8):1671-1680. doi: 10.1002/cbin.11361. [DOI] [PubMed] [Google Scholar]
- 120.Hou Y, Jia H, Cao Y, et al. lncRNA EPIC1 promotes tumor angiogenesis via activating the Ang2/Tie2 axis in non-small cell lung cancer. Life Sci. 2021;267(2):118933. doi: 10.1016/j.lfs.2020.118933. [DOI] [PubMed] [Google Scholar]
- 121.Lin H, Zhang X, Feng N, et al. lncRNA LCPAT1 mediates smoking/ particulate matter 2.5-induced cell autophagy and epithelial-mesenchymal transition in lung cancer cells via RCC2. Cell Physiol Biochem. 2018;47(3):1244-1258. doi: 10.1159/000490220. [DOI] [PubMed] [Google Scholar]
- 122.Deng X, Feng N, Zheng M, et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim Biophys Acta Gen Subj. 2017;1861(2):112-125. doi: 10.1016/j.bbagen.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 123.Fan H, Li J, Wang J, et al. Long non-coding RNAs (lncRNAs) tumor-suppressive role of lncRNA on chromosome 8p12 (TSLNC8) inhibits tumor metastasis and promotes apoptosis by regulating interleukin 6 (IL-6)/signal transducer and activator of transcription 3 (STAT3)/hypoxia-inducible factor 1-alpha (HIF-1alpha) signaling pathway in non-small cell lung cancer. Med Sci Monit. 2019;25(10):7624-7633. doi: 10.12659/MSM.917565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang B, Zhang L, Cao Y, et al. Overexpression of lncRNA IGFBP4-1 reprograms energy metabolism to promote lung cancer progression. Mol Cancer. 2017;16(1):154. doi: 10.1186/s12943-017-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hua Q, Mi B, Xu F, et al. Hypoxia-induced lncRNA-AC020978 promotes proliferation and glycolytic metabolism of non-small cell lung cancer by regulating PKM2/HIF-1α axis. Theranostics. 2020;10(11):4762-4778. doi: 10.7150/thno.43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gong W, Yang L, Wang Y, et al. Analysis of survival-related lncRNA landscape identifies a role for LINC01537 in energy metabolism and lung cancer progression. Int J Mol Sci. 2019;20(15):3713-3728. doi: 10.3390/ijms20153713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma F, Lei YY, Ding MG, et al. lncRNA NEAT1 interacted With DNMT1 to regulate malignant phenotype of cancer cell and cytotoxic T cell infiltration via epigenetic inhibition of p53, cGAS, and STING in lung cancer. Front Genet. 2020;11(11):250. doi: 10.3389/fgene.2020.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen LM, Niu YD, Xiao M, et al. lncRNA NEAT1 regulated cell proliferation, invasion, migration and apoptosis by targeting has-miR-376b-3p/SULF1 axis in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2020;24(9):4810-4821. doi: 10.26355/eurrev_202005_21170. [DOI] [PubMed] [Google Scholar]
- 129.Huang D, Chen J, Yang L, et al. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat Immunol. 2018;19(10):1112-1125. doi: 10.1038/s41590-018-0207-y. [DOI] [PubMed] [Google Scholar]
- 130.Tian X, Ma J, Wang T, et al. Long non-coding RNA HOXA transcript antisense RNA myeloid-specific 1-HOXA1 axis downregulates the immunosuppressive activity of myeloid-derived suppressor cells in lung cancer. Front Immunol. 2018;9(9):473. doi: 10.3389/fimmu.2018.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen TJ, Gao F, Yang T, et al. lncRNA HOTAIRM1 inhibits the proliferation and invasion of lung adenocarcinoma cells via the miR-498/WWOX axis. Cancer Manag Res. 2020;12(12):4379-4390. doi: 10.2147/cmar.S244573. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Stratigos M, Matikas A, Voutsina A, et al. Targeting angiogenesis in small cell lung cancer. Transl Lung Cancer Res. 2016;5(4):389-400. doi: 10.21037/tlcr.2016.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Treps L, Gavard J. Tumor angiogenesis: when the tree of life turns bad. Med Sci. 2015;31(11):989-995. doi: 10.1051/medsci/20153111013. [DOI] [PubMed] [Google Scholar]
- 134.Caporarello N, Lupo G, Olivieri M, et al. Classical VEGF, notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions (review). Mol Med Rep. 2017;16(4):4393-4402. doi: 10.3892/mmr.2017.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069-1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27-42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Onorati AV, Dyczynski M, Ojha R, et al. Targeting autophagy in cancer. Cancer. 2018;124(16):3307-3318. doi: 10.1002/cncr.31335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Münz C. The macroautophagy machinery in endo- and exocytosis. J Mol Biol. 2017;429(4):473-485. doi: 10.1016/j.jmb.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 139.Zhang J, Yang Z, Xie L, et al. Statins, autophagy and cancer metastasis. Int J Biochem Cell Biol. 2013;45(3):745-752. doi: 10.1016/j.biocel.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 140.Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol. 2010;22(2):241-245. doi: 10.1016/j.ceb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene. 2017;36(12):1619-1630. doi: 10.1038/onc.2016.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Deng X, Zhang F, Rui W, et al. PM2.5-induced Oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicology In Vitro. 2013;27(6):1762-1770. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 143.Hou HH, Pan HJ, Liao WY, et al. Autophagy in fibroblasts induced by cigarette smoke extract promotes invasion in lung cancer cells. Int J Cancer. 2020;147(9):2587-2596. doi: 10.1002/ijc.33127. [DOI] [PubMed] [Google Scholar]
- 144.Deng M, Zhang W, Yuan L, et al. HIF-1a regulates hypoxia-induced autophagy via translocation of ANKRD37 in colon cancer. Exp Cell Res. 2020;395(1):112175. doi: 10.1016/j.yexcr.2020.112175. [DOI] [PubMed] [Google Scholar]
- 145.Lin YH. Crosstalk of lncRNA and cellular metabolism and their regulatory mechanism in cancer. Int J Mol Sci. 2020;21(8):2947-2964. doi: 10.3390/ijms21082947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211-218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95(7):912-919. doi: 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 148.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356(2 Pt A):156-164. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu J. The Warburg metabolism fuels tumor metastasis. Cancer Metastasis Rev. 2019;38(1–2):157-164. doi: 10.1007/s10555-019-09794-5. [DOI] [PubMed] [Google Scholar]
- 151.Fong MY, Zhou W, Liu L, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183-194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.He Z, You C, Zhao D. Long non-coding RNA UCA1/miR-182/PFKFB2 axis modulates glioblastoma-associated stromal cells-mediated glycolysis and invasion of glioma cells. Biochem Biophys Res Commun. 2018;500(3):569-576. doi: 10.1016/j.bbrc.2018.04.091. [DOI] [PubMed] [Google Scholar]
- 153.Zhang J, Wang S, Jiang B, et al. c-Src phosphorylation and activation of hexokinase promotes tumorigenesis and metastasis. Nat Commun. 2017;8(1):13732. doi: 10.1038/ncomms13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Geng XL, Long WH, Hai J, et al. The role of hexokinase 2 in the metastasis of hepatocellular carcinoma cells. Zhonghua Zhong Liu Za Zhi. 2016;38(10):739-742. doi: 10.3760/cma.j.issn.0253-3766.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 155.Chaneton B, Gottlieb E. Rocking cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem Sci. 2012;37(8):309-316. doi: 10.1016/j.tibs.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 156.Hamabe A, Konno M, Tanuma N, et al. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial–mesenchymal transition. Proc Natl Acad Sci USA. 2014;111(43):15526-15531. doi: 10.1073/pnas.1407717111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.He Y, Luo Y, Zhang D, et al. PGK1-mediated cancer progression and drug resistance. Am J Cancer Res. 2019;9(11):2280-2302. [PMC free article] [PubMed] [Google Scholar]
- 158.Liang C, Shi S, Qin Y, et al. Localisation of PGK1 determines metabolic phenotype to balance metastasis and proliferation in patients with SMAD4-negative pancreatic cancer. Gut. 2020;69(5):888-900. doi: 10.1136/gutjnl-2018-317163. [DOI] [PubMed] [Google Scholar]
- 159.Yu T, Zhao Y, Hu Z, et al. Metalnc9 facilitates lung cancer metastasis via a PGK1-activated AKT/mTOR pathway. Cancer Res. 2017;77(21):5782-5794. doi: 10.1158/0008-5472.Can-17-0671. [DOI] [PubMed] [Google Scholar]
- 160.Xie H, Tong G, Zhang Y, et al. PGK1 drives hepatocellular carcinoma metastasis by enhancing metabolic process. Int J Mol Sci. 2017;18(8):1630-1640. doi: 10.3390/ijms18081630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang C, Li Y, Yan S, et al. Interactome analysis reveals that lncRNA HULC promotes aerobic glycolysis through LDHA and PKM2. Nat Commun. 2020;11(1):3162. doi: 10.1038/s41467-020-16966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zheng Q, Lin Z, Xu J, et al. Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting β-catenin by activating PKM2 and inactivating PTEN. Cell Death Dis. 2018;9(3):253. doi: 10.1038/s41419-018-0305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267-1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Janssen LME, Ramsay EE, Logsdon CD, et al. The immune system in cancer metastasis: friend or foe? J Immunother Cancer. 2017;5(1):79. doi: 10.1186/s40425-017-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749-761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xia P, Wang S, Gao P, et al. DNA sensor cGAS-mediated immune recognition. Protein Cell. 2016;7(11):777-791. doi: 10.1007/s13238-016-0320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ruangkiattikul N, Nerlich A, Abdissa K, et al. cGAS-STING-TBK1-IRF3/7 induced interferon-β contributes to the clearing of non tuberculous mycobacterial infection in mice. Virulence. 2017;8(7):1303-1315. doi: 10.1080/21505594.2017.1321191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zitvogel L, Galluzzi L, Kepp O, et al. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405-414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 169.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193(6):70-81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 170.Wang F, Jiang X, Wang P. NF-κB interaction long non-coding RNA inhibits migration, invasion and epithelial–mesenchymal transition of cervical cancer cells through inhibiting NF-κB signaling pathways. Exp Ther Med. 2020;20(2):1039-1047. doi: 10.3892/etm.2020.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen R, Cheng Q, Owusu-Ansah KG, et al. NKILA, a prognostic indicator, inhibits tumor metastasis by suppressing NF-κB/slug mediated epithelial–mesenchymal transition in hepatocellular carcinoma. Int J Biol Sci. 2020;16(3):495-503. doi: 10.7150/ijbs.39582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5(1):3-8. doi: 10.1158/2326-6066.Cir-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin Y, Yang X, Liu W, et al. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene. 2017;36(25):3599-3608. doi: 10.1038/onc.2016.516. [DOI] [PubMed] [Google Scholar]
- 174.Gao J, Wu Y, Su Z, et al. Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer. PLoS One. 2014;9(8):e104453. doi: 10.1371/journal.pone.0104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gabrilovich D, Nefedova Y. ROR1C regulates differentiation of myeloid-derived suppressor cells. Cancer Cell. 2015;28(2):147-149. doi: 10.1016/j.ccell.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Groth C, Hu X, Weber R, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120(1):16-25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou Q, Tang X, Tian X, et al. lncRNA MALAT1 negatively regulates MDSCs in patients with lung cancer. J Cancer. 2018;9(14):2436-2442. doi: 10.7150/jca.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tian X, Ma J, Wang T, et al. Long non-coding RNA RUNXOR accelerates MDSC-mediated immunosuppression in lung cancer. BMC cancer. 2018;18(1):660. doi: 10.1186/s12885-018-4564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.He WW, Ma HT, Guo X, et al. lncRNA SNHG3 accelerates the proliferation and invasion of non-small cell lung cancer by downregulating miR-340-5p. J Biol Regul Homeost Agents. 2020;34(6):2017-2027. doi: 10.23812/20-388-a. [DOI] [PubMed] [Google Scholar]
- 180.Li Y, Gao L, Zhang C, et al. lncRNA SNHG3 promotes proliferation and metastasis of non-small-cell lung cancer cells through miR-515-5p/SUMO2 axis. Technol Cancer Res Treat. 2021;20(20):15330338211019376. doi: 10.1177/15330338211019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shi J, Li J, Yang S, et al. lncRNA SNHG3 is activated by E2F1 and promotes proliferation and migration of non-small-cell lung cancer cells through activating TGF-β pathway and IL-6/JAK2/STAT3 pathway. J Cell Physiol. 2020;235(3):2891-2900. doi: 10.1002/jcp.29194. [DOI] [PubMed] [Google Scholar]