Abstract
What is already known about this topic?
Campylobacter genus bacteria are recognized as some of the leading causes of the bacterial diarrheal illness in both developing and developed countries. Recent pilot surveillance study revealed Campylobacter is the most common pathogen in the diarrheal cases using the enhanced filtration methods in Beijing. One outbreak caused by multi-drug resistant Campylobacter coli ( C. coli ) was identified in 2018.
What is added by this report?
This is the first identified gastroenteritis outbreak caused by local Campylobacter jejuni (C. jejuni) infection in Beijing. A total of 14 patients were identified from August 23 to 26, 2019. The epidemiological investigation indicated that all of the patients worked at the same factory and the diarrhea happened after the same meal supplied from one company which service the meal delivery. Fourteen C. jejuni isolates were obtained from 12 patients and 2 food workers. Whole Genome Sequencing (WGS) analysis indicated this outbreak was caused by one highly clonal C. jejuni.
What are the implications for public health practice?
Campylobacter is the major foodborne pathogen in the world. Surveillance and risk assessment for Campylobacter infection particularly for Guillain-Barré Syndrome (GBS) associated C. jejuni infection in China should closely monitored.
BACKGROUND
In the afternoon of August 26, 2019, the Shunyi District CDC of Beijing Municipality was informed that several acute gastroenteritis patients visited the Shunyi District Hospital and the Shunyi Chinese Medicine Hospital. The epidemiological investigation was performed by Shunyi CDC. The time of onset of the first patient and the final patient were the afternoon of August 24, 2019 (26 hours after the meal) and the morning of August 26, 2019 (66 hours after the shared meal), respectively.
Totally, 14 patients were identified. These 14 patients showed similar clinical symptoms, including high fever (over 38.5 ℃), diarrhea, abdominal pain, and headache. All of them had watery stool and diarrhea 2 to 10 times per day. According to the epidemic investigation, these 14 patients were workers at the same factory and the diarrhea happened after lunch supplied from a meal delivery company on August 23. Overall, 14 stool samples (7 fresh stool samples and 7 anal swabs) were collected from 14 individual patients, 7 anal swabs were collected from 7 individual workers of the food supplying company, and 18 suspected food samples and 6 samples from the environment of the kitchen were collected. All samples were screened for 10 major enteric pathogens based on the real-time polymerase chain reaction (PCR) methods published previously (1).
INVESTIGATION AND RESULTS
Real-time PCR was performed for 10 specific pathogens: Vibrio cholerae,Vibrio parahaemolyticus, C. jejuni, C. coli, Salmonella, Shigella, diarrheagenicE. coli, norovirus, rotavirus, and enteric adenovirus. Fourteen samples were positive for C. jejuni including 12 samples from 12 patients and 2 samples from 2 food workers, and the other samples were all negative. All samples were negative for other enteric pathogens except for C. jejuni.
Campylobacter isolation was performed for all collected samples and species identification was carried out for suspected colonies as previously described (2). In total, 80 colonies were obtained from 14 positive samples including 12 patients’ samples and 2 food workers’ samples. No isolate was obtained from other samples. For each positive sample, one isolate was selected for further investigation, and 14 isolates were picked in total.
Pulsed-field gel electrophoresis (PFGE) was performed for all 14 selected isolates (one isolate was selected from each positive patient and positive food worker) using SmaI as described previously (3). All of the selected isolates showed an identical PFGE profile (Figure 1).
Figure 1.
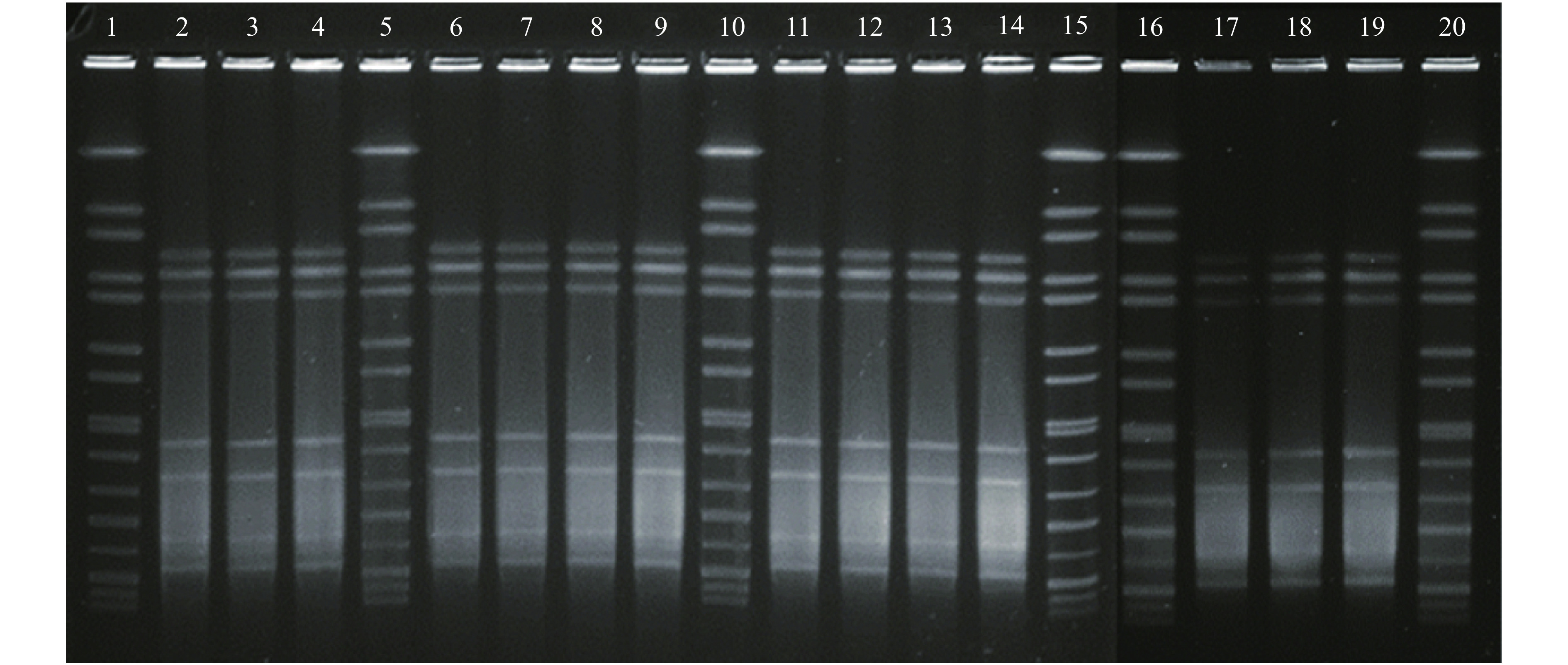
Pulsed-field gel electrophoresis (PFGE) analysis with SmaI for 14 Campylobacter jejuni isolates from 12 patients and 2 food workers. Lanes 1, 5, 10, 15, 16 and 20: refercnce standard H9812; Lanes 2, 3, 4, 6, 7, 8, 9, 11, 12, 13, 14, and 17: C. jejuni isolates from 12 patients (isolate 1, 2, 3, 4, 5, 6, 8, 9, 11, 12, 13, and 14); Lanes 18 and 19: C. jejuni isolates from 2 food workers (36 and 37). All of the 14 isolates had the same PFGE pattern.
Antibiotic susceptibility testing for 11 antimicrobials was performed with the agar dilution method as previous study (1). All of the selected isolates had the same susceptibility pattern: they were all resistant to nalidixic acid and ciprofloxacin and sensitive to other 9 drugs.
The draft genomes of 13 outbreak associated isolates (11 isolates from 11 individual patients and 2 isolates from 2 food workers) were sequenced. One isolate SF-18Cj008), which was isolated from a local sporadic diarrheal patient, and another isolate ARI1249, isolated from the diarrheal patients in UK, were selected to be enrolled in this study. The WGS of SF-18Cj008 was obtained from our previous study and the WGS of ARI1249 was obtained from the PubMLST database ( https://pubmlst.org/bigsdb?db=pubmlst_campylobacter_isolates&page=seqbin&isolate_id=43065). The Whole Genome Single Nucleotide Polymorphisms (wgSNP) was called among the 15 genomes and the Multilocus Sequence Typing (MLST) of the 15 isolates was determined using the online tool on PubMLST website ( http://pubmlst.org/campylobacter). The ad hoc whole-genome multilocus sequence typing (wgMLST) analysis was performed for 15 C. jejuni isolates with fast-GeP ( https://github.com/jizhang-nz/fast-GeP) using the annotated ARI1249 genome as reference (1). The Sequence Types (STs) of the entire selected 15 isolates all belong to ST-6959. The difference matrix of the allele loci among 15 C. jejuni WGSs were presented in Figure 2 and the neighbor-net phylogeny of 15 isolates using SplitsTree4 based on the shared loci was constructed and presented in Figure 3. The outbreak associated 13 isolates were genetically related (≤9 alleles difference). No mutation in 23S rRNA was found and the gyrA C257T mutant was identified in the entire 13 outbreak associated isolates which was consisting with the phenotype of drug resistance of the outbreak associated isolate.
Figure 2.
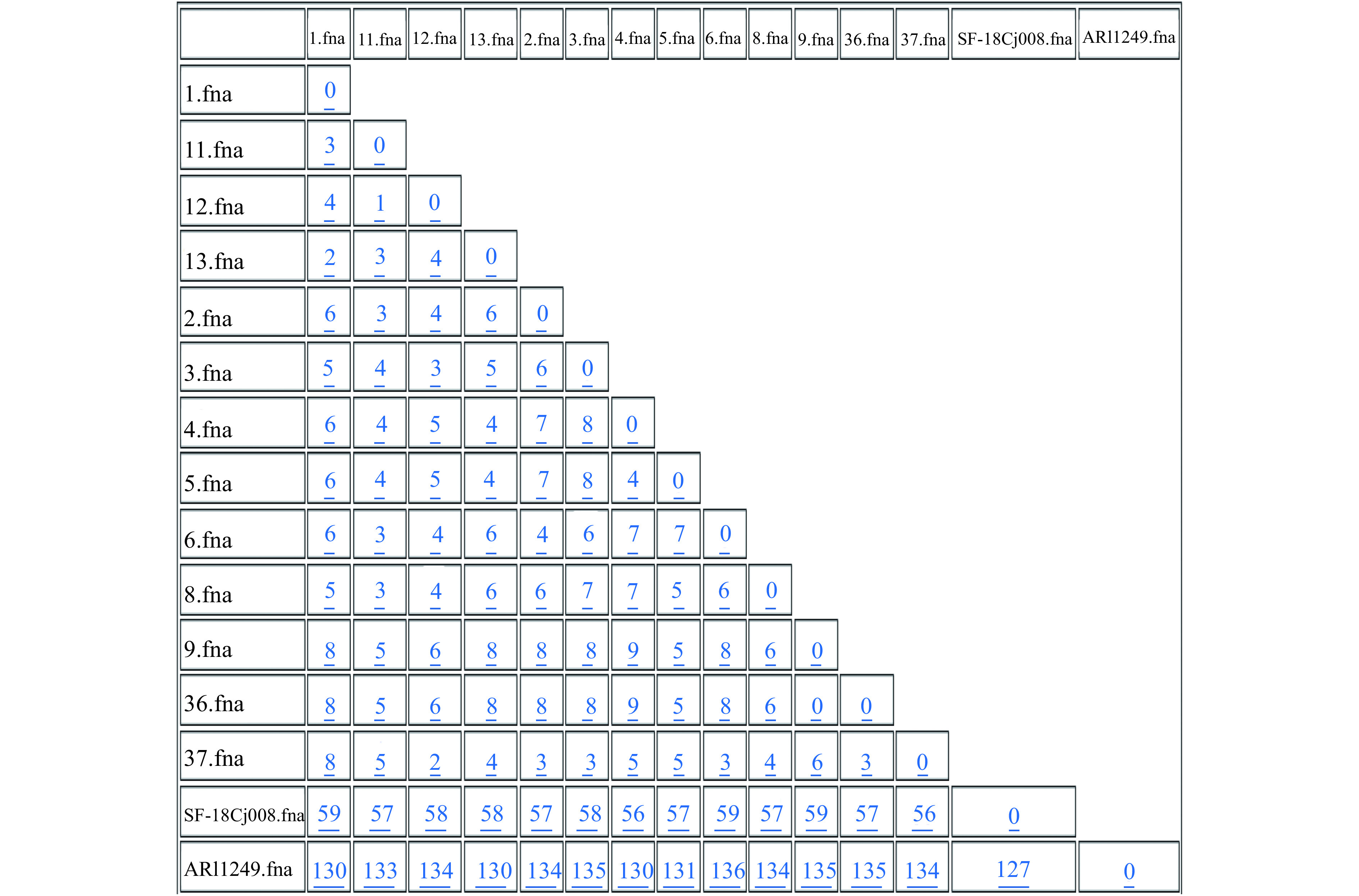
Difference matrix for alleles of the wgMLST with Fast-GeP analysis. Allele sequences were searched with BLAST+ (identity threshold ≥80). ARI1249 was used as the reference genome. The number of the loci in the reference genome was 1,667. The number of loci shared by the 15 genomic sequences was 1,649 and the number of the shared-loci that was found identical was 1,484. The shared-loci that was used to construct distance difference matrix was 1,644 (160 were polymorphic). Five shared-loci were excluded because of hypothetical gene duplication and 18 loci were excluded because of incomplete information (missing, truncation or containing nucleotide ambiguity). The horizontal and vertical columns of the matrix represent the isolates name. The number in the matrix indicated the different alleles numbers between the isolates in the horizontal and vertical. columns. The horizontal and vertical columns of the matrix represent the isolates name. The number in the matrix indicated the different alleles numbers between the isolates in the horizontal and vertical columns.
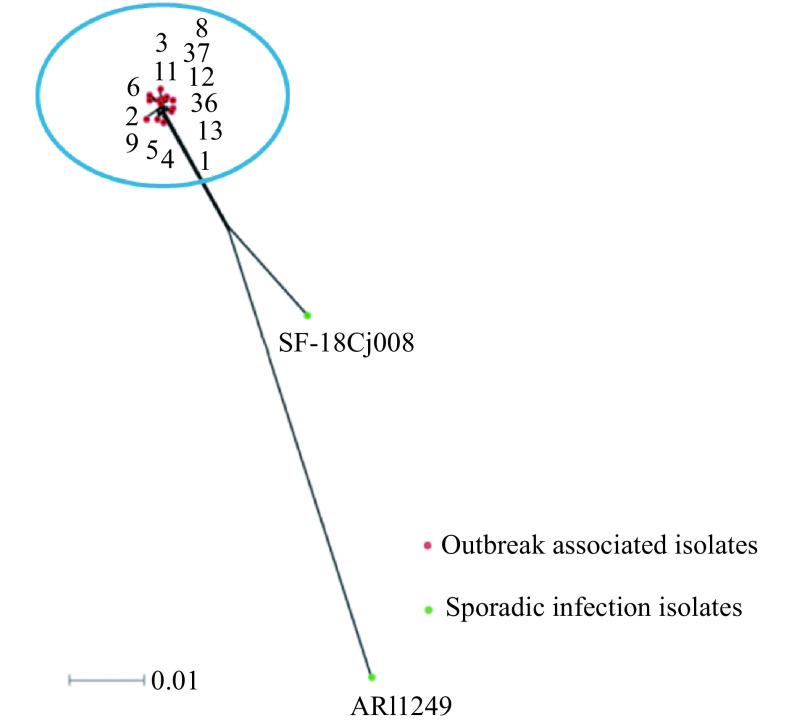
Figure 3.
Neighbor-net phylogeny for alleles of cgMLST loci of 15 C. jejuni isolates. All of these 15 isolates were belonging to ST-6959. Red points representing the outbreak associated 13 isolates (isolate 1, 2, 3, 4, 5, 6, 8, 9, 11, 12, 13, 36 and 37) and green points representing two sporadic isolates (SF-18Cj008 and ARI1249), one from local diarrheal patient, and another one from a diarrheal patient in UK. The blue circle representing the outbreak cluster.
DISCUSSION
In this study, 12 C. jejuni isolates were obtained from 12 patients. The real-time PCR screening results were consistent with the bacteria culture results. Unfortunately, no isolates were cultured from the two patients of whom the samples were anal swabs and PCR results were also negative. This might be due to an inadequate number of pathogens in the samples. Bacteria culture is time intensive, rapid multi-targets screening using molecular methods is helpful for pathogen identification during the outbreak investigation. This study confirmed that direct real-time PCR examination for Campylobacter from the stool sample of diarrheal patients is useful (4).
PFGE is useful for bacteria outbreak investigation (5). Recently, the WGS for bacterial pathogens become cheaper and faster. The bioinformatics’ analysis based on the WGS is crucial for both the epidemic and outbreak investigation (6). The ST of the isolate could be reached in real-time with the WGS using the in silico MLST analysis. WgSNP and wgMLST analysis were useful to recognize the genetic distance between the isolates. In this study, the fast-GeP analysis was an effective tool to identify the very closely related Campylobacter isolates; it is useful for Campylobacter outbreak investigation based on the WGS (7). Both the genotyping and antibiotics analysis results indicated this gastroenteritis outbreak was caused by one highly clonal C. jejuni.
The isolates from two food workers were of the same genotype as the patients. According to their health history, they did not have significant clinical symptoms. We do not know if they ate the same food as the patients on August 23 or if the bacteria they carried contaminated the foods they cooked. There was a report that C. jejuni could colonize in the human gut for extended periods of time (8), and there is chance the food workers may contaminate the food during preparation. Unfortunately, we did not get any more samples from any of the food workers for continued bacteria culture.
Recently, the accelerated pace of life may dramatically increase use of meal delivery in major cities and may subsequently increase the risk of infection or food poisoning caused by foodborne pathogens. With an enhanced filtration method, Campylobacter was recognized as the leading causes of bacterial diarrhea in Beijing (1,2,4). One gastroenteritis outbreak caused by C. coli infection was identified last year (1), and this was the first identified gastroenteritis outbreak caused by local C. jejuni infection in Beijing.
In addition to enteritis, C. jejuni infection can also cause GBS. Recently, 36 GBS patients outbreak caused by preceding C. jejuni infection were reported (9-10). According to our previous study, the serotype (HS:41) of this GBS outbreak associated C. jejuni strain was also identified from the sporadic diarrheal patient in Beijing. Pathogen surveillance and the risk assessment for Campylobacter infection particularly for GBS associated C. jejuni infection should be closely monitored.
Acknowledgements
We thank Shunyi CDC colleagues participating in the outbreak investigation; we are grateful to the help from Dr. Ji Zhang in New Zealand for the bioinformatics’ analysis.
Funding Statement
This work was supported by National Key Scientific and Technology Project (2018ZX10305409 and 2018ZX10712-001).
References
- 1.Li Y, Gu YX, Lv JC, Liang H, Zhang J, Zhang S, et al Laboratory study on the gastroenteritis outbreak caused by a multidrug-resistant Campylobacter coli in China . Foodborne Pathog Dis. 2020;17(3):187–93. doi: 10.1089/fpd.2019.2681. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Zhang S, He M, Zhang YC, Fu YY, Liang H, et al Prevalence and molecular characterization of Campylobacter spp. isolated from patients with diarrhea in Shunyi, Beijing . Front Microbiol. 2018;9:52. doi: 10.3389/fmicb.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang MJ, Gu YX, He LH, Ran L, Xia SL, Han XS, et al Molecular typing and antimicrobial susceptibility profiles of Campylobacter jejuni isolates from North China . J Med Microbiol. 2010;59(10):1171–7. doi: 10.1099/jmm.0.022418-0. [DOI] [PubMed] [Google Scholar]
- 4.Liang H, Wen ZY, Li Y, Duan YX, Gu YX, Zhang MJ Comparison of the filtration culture and multiple real-time PCR examination for Campylobacter spp. from stool specimens in diarrheal patients . Front Microbiol. 2018;9:2995. doi: 10.3389/fmicb.2018.02995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis KR, Dunn AC, Burnett C, McCullough L, Dimond M, Wagner J, et al. Campylobacter jejuni infections associated with raw milk consumption--Utah, 2014. MMWR Morb Mortal Wkly Rep 2016;65(12):301-5. https://www.cdc.gov/mmwr/volumes/65/wr/mm6512a1.htm.
- 6.Oakeson KF, Wagner JM, Rohrwasser A, Atkinson-Dunn R. Whole-genome sequencing and bioinformatic analysis of isolates from foodborne illness outbreaks of Campylobacter jejuni and Salmonella enterica. J Clin Microbiol 2018;56(11):e00161-18. https://jcm.asm.org/content/56/11/e00161-18.
- 7.Zhang J, Xiong YW, Rogers L, Carter GP, French N Genome-by-genome approach for fast bacterial genealogical relationship evaluation. Bioinformatics. 2018;34(17):3025–7. doi: 10.1093/bioinformatics/bty195. [DOI] [PubMed] [Google Scholar]
- 8.Bloomfield SJ, Midwinter AC, Biggs PJ, French NP, Marshall JC, Hayman DTS, et al Long-term colonization by Campylobacter jejuni within a human host: evolution, antimicrobial resistance, and adaptation . J Infect Dis. 2018;217(1):103–11. doi: 10.1093/infdis/jix561. [DOI] [PubMed] [Google Scholar]
- 9.Zhang MJ, Li Q, He LH, Meng FL, Gu YX, Zheng MH, et al Association study between an outbreak of Guillain-Barre syndrome in Jilin, China, and preceding Campylobacter jejuni infection . Foodborne Pathog Dis. 2010;7(8):913–9. doi: 10.1089/fpd.2009.0493. [DOI] [PubMed] [Google Scholar]
- 10.Zhang MJ, Gilbert M, Yuki N, Cao FF, Li JJ, Liu HY, et al Association of Anti-GT1a Antibodies with an outbreak of Guillain-Barré syndrome and analysis of ganglioside mimicry in an associated Campylobacter jejuni strain . PLoS One. 2015;10(7):e0131730. doi: 10.1371/journal.pone.0131730. [DOI] [PMC free article] [PubMed] [Google Scholar]