Abstract
What is already known about this topic?
Enterovirus 71 (EV-A71) is the main causative pathogen for severe and fatal patients with Hand, Foot, and Mouth Disease (HFMD) in mainland China from 2008 to 2017. Non-EV-A71 and non-CV-A16 (other enterovirus) serotypes were the major causative-serotypes for mild HFMD in years of 2013, 2015, and 2017.
What is added by this report?
In 2018, other enterovirus serotypes replaced EV-A71 for the first time as the major cause of severe HFMD with a proportion of 70.7%. However, at the national level, only a small proportion of the other enterovirus serotypes were further identified as CV-A6 and CV-A10.
What are the limitations for public health practice?
Further identification of other enterovirus serotypes is highly recommended for provincial CDCs, especially for severe HFMD. Studies contributing to a multivalent vaccine for HFMD should be prioritized.
Hand, foot, and mouth disease (HFMD) is a common pediatric disease affecting children’s health in China caused by various human enteroviruses (1). Enterovirus 71 (EV-A71) and Coxsackievirus A16 (CV-A16) are the most common causative serotypes (1-2). EV-A71 is the most frequently reported serotype responsible for severe and fatal HFMD (1), but other enterovirus serotypes, such as enterovirus A species, CV-A6, and CV-A10, can also lead to neurological complications (3-4). The results of surveillance data indicate that EV-A71 is the predominant serotype in 2008 to 2012 and 2014, and other enterovirus serotypes are predominant in 2013, 2015, and 2017 (5). The proportion of other enterovirus serotypes is dramatically increased in patients with mild conditions of HFMD after 2013 (5). This study aims to explore the spectrum of enterovirus serotypes by clinical severity in 2018, which will provide the scientific evidence for constructing HFMD control and prevention strategies in China.
The pathogen surveillance is a part of HFMD national surveillance routine work, which was launched in 31 provincial-level administrative divisions (PLADs) in China from 2008 (6). Pathogen surveillance data of HFMD is required to be reported monthly to the Chinese Center for Disease Control and Prevention (China CDC) via email in all 31 PLADs. The information includes basic demographic information (sex, age, and address), disease severity (mild or severe), specimen types, death status, date of symptoms onset, date of specimen collection, date of sample detection, sample detection methods, date of death, and the identified enterovirus serotypes.
To comply with the HFMD national surveillance guidelines, samples including throat swabs, anal swabs, or fecal samples were collected at the county-level from the first five patients with mild and probable HFMD who visited hospital outpatient departments every month, and specimens from all severe and fatal cases are required to be collected and tested. City-level CDC laboratories are responsible for identifying the enterovirus serotypes by real-time PCR or virus isolation (6). We classified the test results into four categories: enterovirus negative, EV-A71 positive, CV-A16 positive, and positive for other enterovirus (non-EV-A71 and non-CV-A16) without further serotype identification. The enterovirus serotypes of CV-A6 and CV-A10 were further identified in 18 PLADs①.
A total of 30 PLADs reported virological data of HFMD to China CDC in 2018. To avoid deviation in the study results, we excluded the incomplete data from two PLADs and unqualified data from one PLAD. The HFMD patients whose onset dates were from January 1, 2018 to December 31, 2018 of 27 PLADs were finally enrolled in the study. Descriptive epidemiological method was used to analyze the data, and all analyses were conducted in SAS (version 9.3, SAS Institute Inc.).
A total of 116,290 HFMD probable and laboratory confirmed HFMD cases were reported from January 1, 2018 to December 31, 2018 in 27 PLADs. The reported number of mild, severe, and fatal cases was 114,297, 1,780, and 7, respectively, and 206 cases were missing the disease severity variable. The epidemic peak was from May to June (Figure 1A, B). Children aged under 5 years were the mainly infected population with a proportion of 86.7%. Among the total cases, the male-to-female ratio was 1.5∶1.
Figure 1.
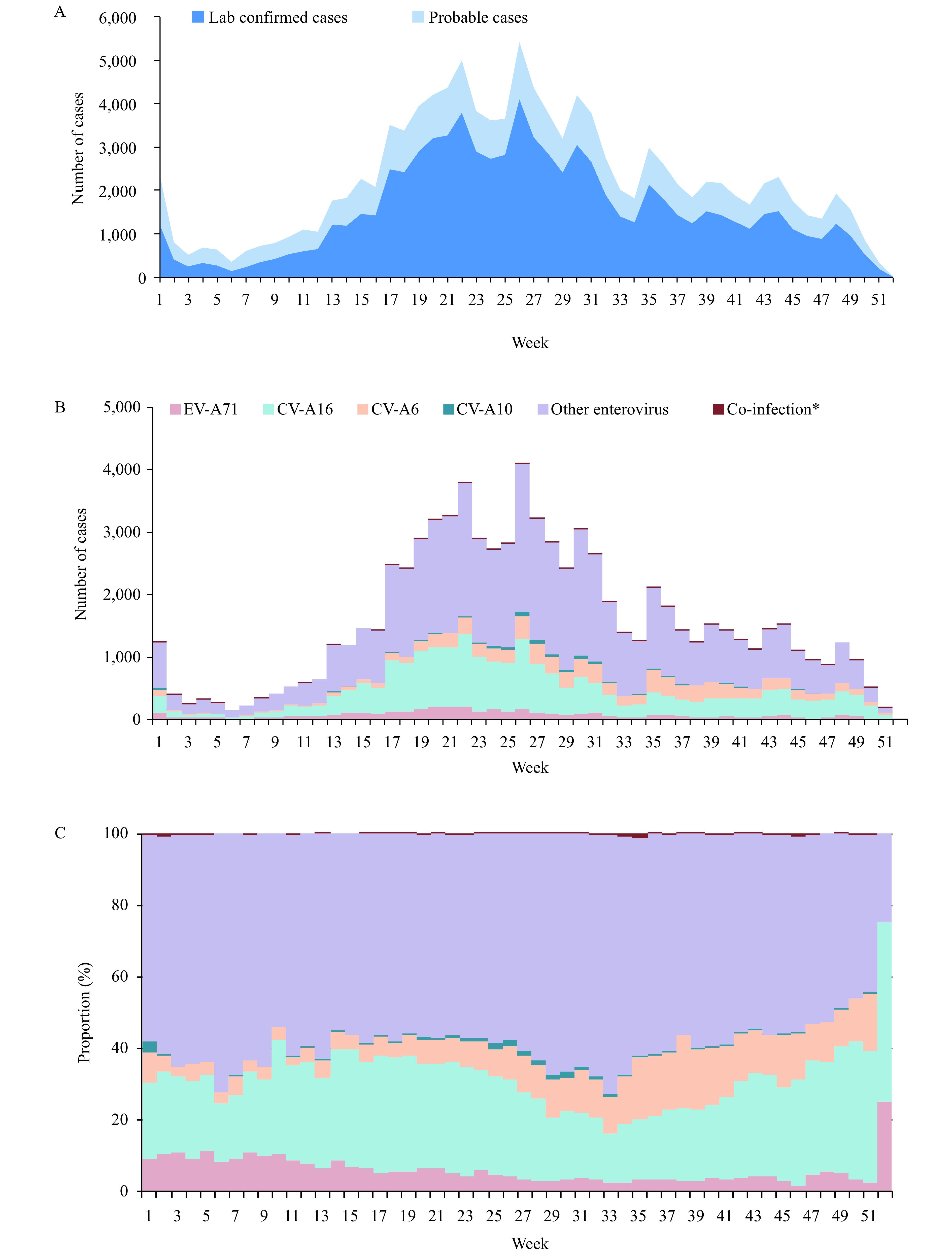
Enterovirus tested negative and laboratory-confirmed HFMD in mainland China, 2018. (A) Time series of weekly probable and laboratory-confirmed patients with HFMD. (B) Time series of weekly laboratory-confirmed patients with HFMD by enterovirus serotypes. (C) Weekly proportions of laboratory-confirmed patients with HFMD by enterovirus serotype. *Co-infection included EV-A71&CV-A16, CV-A16&CV-A10, CV-A6&CV-A10, CV-A6&EV-A71, and CV-A6&CV-A16.
The number of laboratory-confirmed cases was 80,793, and the whole enterovirus detection positive rate was 69.5%. The enterovirus positive rate of mild, severe, and fatal cases was 69.3%, 80.7%, and 71.4%, respectively. Excluding Tibet (37.3%), the enterovirus detection positive rate at the provincial level was higher than 50% in 26 PLADs.
Among laboratory-confirmed cases, the proportion of EV-A71, CV-16, and other enterovirus serotypes was 4.8% (3,913), 25.6% (20,709), and 69.3% (56,002), respectively (Figure 1B,C). In cases that were positive for other enterovirus serotypes, 13.5% and 1.2% were further identified as CV-A6 and CV-A10 infections. 0.2% of cases were identified as co-infections, including 112 as EV-A71&CV-A16, 2 of CV-A16&CV-A10, 20 of CV-A6&CV-A10, 7 of CV-A6&EV-A71, and 28 of CV-A6&CV-A16 infection (Figure 1B,C). At the provincial level, 2 PLADs were predominantly identified as CV-A16, 4 PLADs were predominantly identified as CV-A6, and 21 PLADs were predominantly identified as other enterovirus serotypes (for the enterovirus geographic distribution, Supplementary Figure S1 available in http://weekly.chinacdc.cn/).
Figure S1.
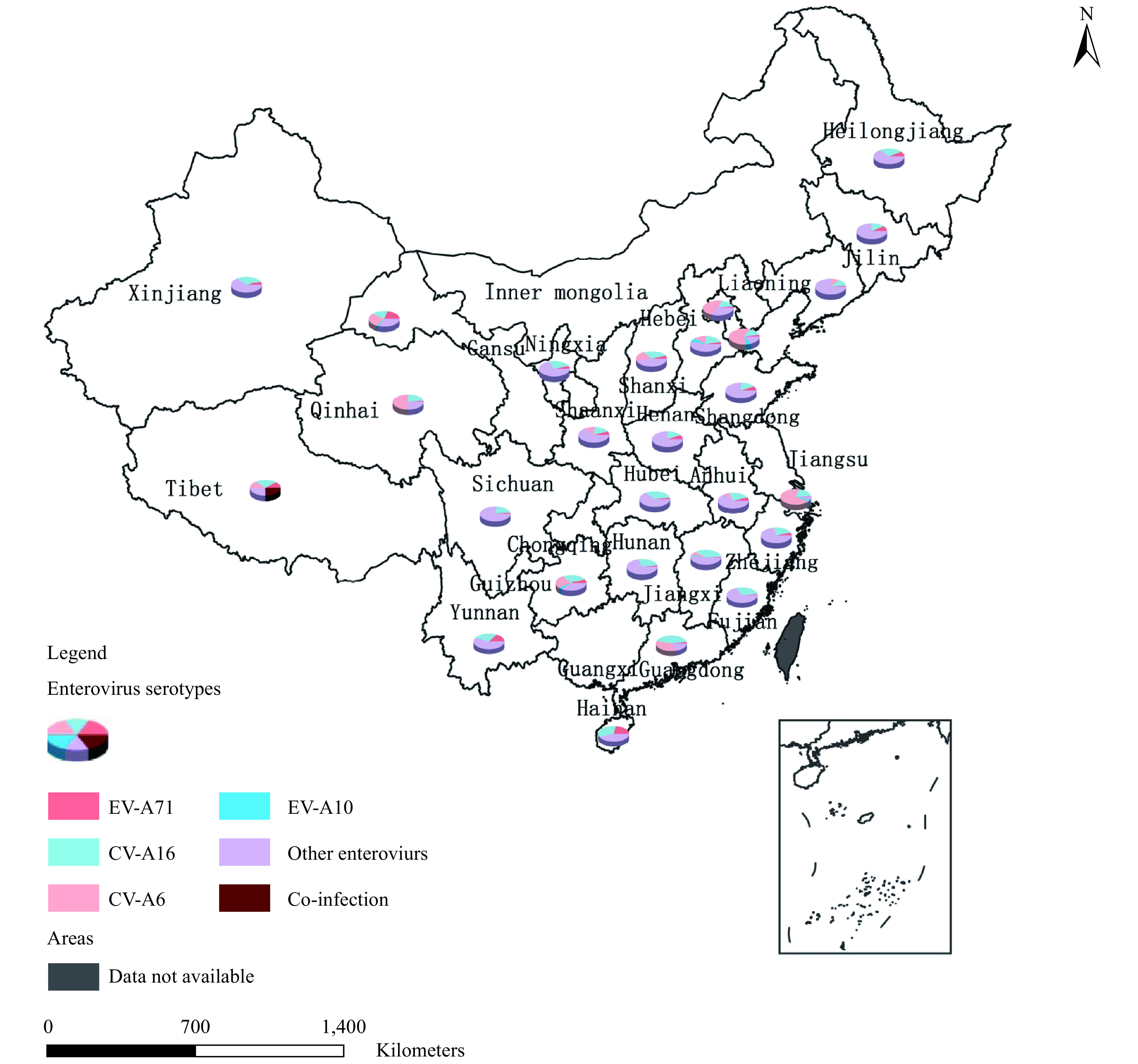
The enteroviruses geographic distribution of mainland China, 2018. The composition of enterovirus serotypes of Jiangsu Province, Inner Mongolia Autonomous Region, Guangxi Zhuang Autonomous Region and Chongqing Municipality were not showed in this map.
In mild cases, 69.3% of the cases were infected by other enterovirus serotypes, and the proportion of EV-A71, CV-A16, and co-infection was 4.6%, 25.9%, and 0.2%, respectively. In severe cases, 70.7% were infected by other enterovirus serotypes, while the proportion of EV-A71, which was previously the predominant serotype, was only 15.3%. The proportion of CV-A16 and co-infection was 13.7% and 0.3%, respectively.
The age profile of laboratory-confirmed cases indicated that both mild and severe cases were mainly distributed in age groups of 12–24 months and 25–59 months with proportions of 49.0%, 27.3%, for mild cases, respectively, and 63.8%, 19.8%, for severe cases, respectively. Fatal cases occurred in the age groups of 6–11 months (20.0%) and 12–24 months (80.0%). The age distribution of probable cases showed similar compositions with the laboratory-confirmed cases (Figure 2A, C, E).
Figure 2.
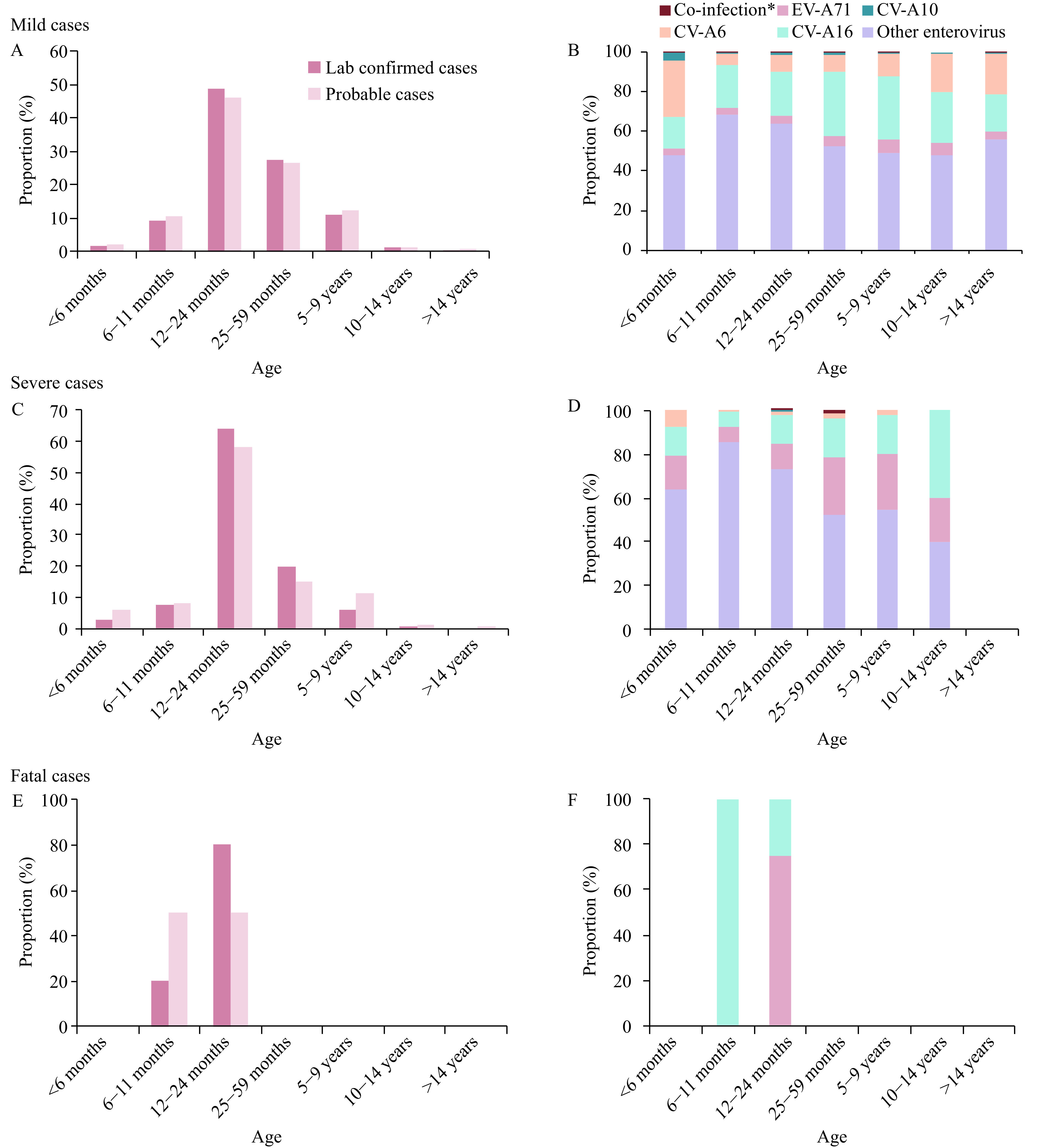
Age profile and enterovirus serotypes distribution in the laboratory-confirmed HFMD based on clinical severity in mainland China, 2018. (A)&(B), Mild cases; (C)&(D), Severe cases; (E)&(F), Fatal cases. *Co-infection included EV-A71&CV-A16, CV-A16&CV-A10, CV-A6&CV-A10, CV-A6&EV-A71 and CV-A6&CV-A16 in the mild cases. Among severe cases, Co-infection refers to EV-A71&CV-A16.
Other enterovirus serotypes were the predominant serotypes in both mild and severe cases in age groups aged under 5 years. In mild cases, CV-A6 was the second major serotype in patients aged less than 6 months, while CV-A16 was the second major serotype in age groups 6 months and older, accounting for 26.1% of cases (Figure 2B).
In severe cases, CV-A6 was mainly distributed in age groups of under 24 months years with a proportion of 69.7%. All CV-A10 infected cases were distributed in the age groups of 12–24 months. A total of 4 severe cases were identified as EV-A71&CV-A16 co-infection, including 1 patient aged 24 months and 3 patients aged 36 months (Figure 2D).
A total of 5 laboratory-confirmed case fatalities were reported. Of the 5, 3 patients had been infected by EV-A71 and were in the age groups of 12–24 months. 2 patients had been infected by CV-A16 and were in the age groups of 6–11 months and 12–24 months (Figure 2F).
Discussion
A total of 116,290 probable and laboratory-confirmed HFMD cases were reported from 27 provincial-level CDCs in 2018. The number of laboratory-confirmed cases was 80,793 and the enterovirus detection positive rate was 69.5%. Patients with HFMD were mainly aged under five years (87.6%). The epidemic peak was from May to June, and other enterovirus serotypes were the predominant serotype, mainly circulating in 21 PLADs and accounting for 69.3% of mild cases and 70.7% of severe cases in 2018. EV-A71 was still the predominant serotype in fatal cases.
The demographic characteristics of age and sex of HFMD in 2018 were similar with results we published in Lancet in 2014 (2). The temporal pattern also did not change in 2018 when compared to surveillance results of previous years (2). A remarkable change in 2018 was the replacement of enterovirus serotypes in patients with severe HFMD. EV-A71 was always the main causative serotype for severe and fatal HFMD, which has accounted for 70.0% of severe and 92.3% of fatal cases from 2008 to 2017 (5). In 2018, other enterovirus serotypes were the predominant serotypes with a proportion of 69.3%, which caused 69.3% of mild cases and 70.7% of severe cases. The epidemic activities of EV-A71 and CV-A16 were very low, especially for the EV-A71, which only accounted for a proportion of 4.8%. Although EV-71 being replaced by other enterovirus serotypes had been observed before, this marks the first time other enterovirus serotypes became the predominant serotype for severe HFMD since HFMD was classified as a notifiable disease in China in 2008.
CV-A6 and CV-A10 circulations have been reported from several PLADs in China, such as Hunan and Hebei (7-8). Four PLADs were affected by CV-A6 the most in 2018, however, at the national level, the CV-A6 and CV-10 were not required to be identified. Among positive cases due to other enterovirus serotypes, only 13.5% and 1.2% of the cases were identified as CV-A6 and CV-A10. Most of the other enterovirus serotype infected cases, especially for the severe cases (96.5%), were not further to be identified. Considering age as a risk factor for HFMD, we find that the severity risk is higher in younger age groups and decreases with increasing age (9). Our results indicate CV-A6 and CV-A10 are the main infection for children aged 2 years and under in the severe cases. In addition, CV-A6 and CV-A10 are also reported to potentially cause neurological complications such as encephalitis and meningitis (3-4). Therefore, including CV-A6 and CV-A10 into the routine detection serotypes could be largely beneficial.
A small proportion of co-infections was found both in mild and severe HFMD. The co-infection of severe HFMD was also reported from an outbreak analysis of Shandong province in 2012 (10). The results of co-infection were needed to be further validated, since the detection method was critical for co-infection identification. The relationship between co-infection and HFMD, especially the pathogenesis of HFMD severity, also requires further study.
This study is subject to at least a few limitations. Although we provided the enterovirus serotype spectrum in 2018, the spectrum was not complete at the national level due to a lack of pathogen data in four PLADs. Only a small part of the other enterovirus serotypes were further identified as CV-A6 or CV-A10, and a large proportion of other enterovirus serotypes were still unknown. Another limitation was that the proportion of EV-A 71 or other enterovirus serotypes might be underestimated because 0.2% of the data lacked disease severity information. Due to the limited information we collected, we cannot explain the shift in enterovirus serotypes in patients with severe HFMD.
Therefore, further identification of other enterovirus serotypes is highly recommended for provincial CDCs, especially for severe HFMD cases. The testing of CV-A6 and CV-A10 should be included in routine HFMD surveillance and researches contributing to a multivalent vaccine for HFMD should be prioritized.
Acknowledgments
We thank the hospitals, local health departments, and county, prefectural and provincial level CDCs for assistance in coordinating data collection.
Footnotes
18 PLADs including administrative divisions from Anhui, Beijing, Gansu, Guangdong, Guizhou, Hebei, Henan, Hunan, Jiangxi, Liaoning, Qinghai, Shanxi, ShaanXi, Shanghai, Tianjin, Tibet, Xinjiang, and Zhejiang.
References
- 1.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10(11):778–90. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 2.Xing WJ, Liao QH, Viboud C, Zhang J, Sun JL, Wu JT, et al Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis. 2014;14(4):308–18. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau SKP, Zhao PSH, Sridhar S, Yip CCY, Aw-Yong KL, Chow EYY, et al Molecular epidemiology of coxsackievirus A6 circulating in Hong Kong reveals common neurological manifestations and emergence of novel recombinant groups. J Clin Virol. 2018;108:43–9. doi: 10.1016/j.jcv.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Bian LL, Gao F, Mao QY, Sun SY, Wu X, Liu SY, et al Hand, foot, and mouth disease associated with coxsackievirus A10: more serious than it seems. Expert Rev Anti-Infect Ther. 2019;17(4):233–42. doi: 10.1080/14787210.2019.1585242. [DOI] [PubMed] [Google Scholar]
- 5.Ji TJ, Han TL, Tan XJ, Zhu SL, Yan DM, Yang Q, et al Surveillance, epidemiology, and pathogen spectrum of hand, foot, and mouth disease in mainland of China from 2008 to 2017. Biosafety Health. 2019;1(1):32–40. doi: 10.1016/j.bsheal.2019.02.005. [DOI] [Google Scholar]
- 6.China Ministry of Health. Guideline for HFMD public health response. http://www.gov.cn/gzdt/2009-06/04/content_1332078.htm. [2009-6-4]. (In Chinese)
- 7.Gao LD, Zou G, Liao QH, Zhou YH, Liu FF, Dai BB, et al Spectrum of enterovirus serotypes causing uncomplicated hand, foot, and mouth disease and enteroviral diagnostic yield of different clinical samples. Clin Infect Dis. 2018;67(11):1729–35. doi: 10.1093/cid/ciy341. [DOI] [PubMed] [Google Scholar]
- 8.Tian HF, Zhang Y, Sun Q, Zhu SL, Li XJ, Pan Z, et al Prevalence of multiple enteroviruses associated with hand, foot, and mouth disease in Shijiazhuang City, Hebei province, China: outbreaks of coxsackieviruses A10 and B3. PLoS One. 2014;9(1):e84233. doi: 10.1371/journal.pone.0084233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang BY, Liu FF, Liao QH, Wu P, Chang ZR, Huang J, et al Epidemiology of hand, foot and mouth disease in China, 2008 to 2015 prior to the introduction of EV-A71 vaccine. Euro Surveill. 2017;22(50):16–00824. doi: 10.2807/1560-7917.ES.2017.22.50.16-00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han JF, Zhang Y, Hou PQ, Zhu SY, Wu XY, Zhao H, et al Human enterovirus co-infection in severe HFMD patients in China. J Clin Virol. 2014;61(4):621–2. doi: 10.1016/j.jcv.2014.09.005. [DOI] [PubMed] [Google Scholar]


