Abstract
The pancreatic islets of Langerhans secrete several hormones critical for glucose homeostasis. The β-cells, the major cellular component of the pancreatic islets, secrete insulin, the only hormone capable of lowering the plasma glucose concentration. The counter-regulatory hormone glucagon is secreted by the α-cells while δ-cells secrete somatostatin that via paracrine mechanisms regulates the α- and β-cell activity. These three peptide hormones are packed into secretory granules that are released through exocytosis following a local increase in intracellular Ca2+ concentration. The high voltage-gated Ca2+ channels (HVCCs) occupy a central role in pancreatic hormone release both as a source of Ca2+ required for excitation-secretion coupling as well as a scaffold for the release machinery. HVCCs are multi-protein complexes composed of the main pore-forming transmembrane α1 and the auxiliary intracellular β, extracellular α2δ, and transmembrane γ subunits. Here, we review the current understanding regarding the role of all HVCC subunits expressed in pancreatic β-cell on electrical activity, excitation-secretion coupling, and β-cell mass. The evidence we review was obtained from many seminal studies employing pharmacological approaches as well as genetically modified mouse models. The significance for diabetes in humans is discussed in the context of genetic variations in the genes encoding for the HVCC subunits.
Keywords: voltage gated calcium channel, beta cell, insulin, diabetes
1. Introduction
Plasma glucose concentration is controlled by several hormones produced and secreted by the cells of the pancreatic islets of Langerhans. The β-cells are the major cellular component (~70%) of the pancreatic islet and secrete insulin, the only hormone capable of lowering plasma glucose concentration [1,2]. The insulin counter-regulatory hormone, glucagon, is secreted by the pancreatic α-cells, which account for ~20% of the total islet cells. Intercalated between α- and β-cells and in close functional connectivity with the β-cells, the pancreatic δ-cells (5%) secrete somatostatin, a hormone critical for islet paracrine modulation [3,4]. While human islets display a mosaic pattern of α-, β-, and δ-cells, mouse islets contain a core of β-cells surrounded by an α-cell mantle [5]. Despite these anatomical differences, which have been discussed in detail elsewhere [6,7,8], numerous studies have shown that the molecular mechanisms of glucose-induced insulin secretion (GIIS) are fairly well conserved between mouse and men. In low plasma glucose concentrations, the K+ equilibrium potential sets the mouse β-cell resting membrane potential between −80 mV and −70 mV [6]. Following the rise in plasma glucose concentration, glucose uptake in the pancreatic β-cells and subsequent mitochondrial metabolism result in increased ATP/ADP ratio. This inhibits the activity of the ATP-sensitive K+ channels (KATP) [9,10,11,12,13]. It has been postulated that β-cells must be equipped with a “background inward current”, which depolarizes the membrane when KATP channel activity is low. Cl−-permeable leucine-rich repeat containing protein Swell1 [14] and various members of the nonselective cation channels transient receptor potential (TRP) [6] are in this regard the most promising candidates. As soon as the membrane potential reaches the depolarization threshold of the voltage-gated Na+ and Ca2+ channels (between −60 mV and −50 mV), their rapid activation leads to an increase in the electrical activity in a glucose-dependent manner [6]. In glucose concentrations >6 mM the β-cell electrical activity is characterized by trains of action potentials (AP) on top of a depolarizing plateau alternating with electrically quiet and hyperpolarized intervals [6,15,16,17]. Raising the glucose concentration to ~10 mM increases the frequency and duration of the AP-trains as well as the AP frequency during a train. Above 15 mM glucose, the β-cells display an almost continuous electrical activity. Most excitable cells are capable of spontaneous or even induced electrical activity over a very narrow interval of the resting membrane potential. In contrast, β-cells express in the membrane a whole repertoire of voltage-gated ion channels perfectly suited for the generation and maintenance of spontaneous activity over a very wide range of membrane potentials. This is because the ion channel complement contributing to β-cell activity changes rapidly with the change in plasma glucose concentration. The voltage-gated ion channels reside in three main conformational states dependent on the resting membrane potential, e.g., resting, activated, and inactivated state [18,19,20,21,22,23,24]. The transition between the three states as well as the state in which each channel finds itself at any given time is voltage- and time-dependent. Normally, when the resting membrane potential sits at -80mV, most ion channels are in the resting, ready-to-open state. The increasing membrane potential induces conformational changes that lead to channel activation. During the membrane depolarization, most ion channels undergo intrinsic time-dependent conformational rearrangements that drive the channel into inactivation. The transition from inactivated to resting state (closed but ready to open again) occurs at resting membrane potentials and is time-dependent. These three important ion channel conformations and activity states dictate at any given time and in a membrane potential-dependent manner the percentage of ion channels available for the generation and maintenance of the electrical activity in any excitable cell. As mentioned above, in pancreatic β-cells, the resting membrane potential is glucose-dependent and therefore also the ion channel availability. Most mouse β-cells express voltage-gated Na+ channels (NaV) that inactivate at very hyperpolarized potentials [15,16]. In low glucose concentrations, the voltage-activated Na+ currents contribute to AP generation, a notion substantiated by the fact that tetrodotoxin (TTX) application reduces the AP frequency and amplitude as well as insulin release [6]. Under high glucose concentrations, the increasing ATP/ADP ratio leads to an almost complete KATP channel block increasing the membrane potential during an AP train above the steady state inactivation voltage of the NaV channels. In consequence, TTX application had minimal effects on AP properties. However, the application of L-type Ca2+ channel blockers completely inhibited β-cell electrical activity, demonstrating that in high glucose concentrations the AP generation relies only on L-type voltage-gated Ca2+ channels activity [25]. Besides its contribution to the electrical activity, the Ca2+ influx conducted by the high voltage-gated Ca2+ channel (HVCC) is also crucial for vesicle fusion with the plasma membrane and insulin secretion, gene transcription and regulation, cell survival and differentiation.
2. Glucose-Induced Insulin Secretion (GIIS)
During a step increase in extracellular glucose concentration (usually from 3 mM to 15–20 mM glucose), insulin release follows a biphasic time course. The first phase is characterized by a fast rise and decay and relatively high amplitude. The second phase is characterized by a continuous steady release but smaller in amplitude [26,27,28]. First phase insulin release begins within 2 min after an increase of blood glucose levels, displays a distinct peak, and ends approximately 10 min later [29]. In the first phase of GIIS, the rise in intracellular Ca2+ concentration leads to the fusion of the pre-docked and primed insulin secretory granules from the readily releasable pool with the plasma membrane [30,31,32]. The second phase of secretion requires the recruitment, tethering, and docking of new insulin granules from a reserve pool [33] and lasts as long as glucose remains elevated with a concentration staying about two- to fivefold above the basal secretion level [34]. The insulin release lacks these two distinguishable phases and shows a progressive and monotonous increase during a ramp in extracellular glucose concentration [35]. Independent of the stimulation method, the dynamics and amplitudes of GIIS are controlled by the expression levels and biophysical properties of different HVCC α1 isoforms and their auxiliary subunits.
3. High Voltage-Gated Ca2+ Channel Structure
HVCCs have a hetero-multimeric composition being formed by the transmembrane α1, the intracellular β, extracellular α2δ, and transmembrane γ subunits (Figure 1A) [36,37]. In a nutshell, the α1 subunit forms the channel pore and confers the HVCC complex its biophysical properties and pharmacological profile, while the auxiliary subunits are important for α1 membrane incorporation and correct localization as well as modulation of its biophysical properties [38,39]. Additionally, at least for α1, α2δ, and β subunits, it has been shown that they also act as important scaffolds for a multitude of other regulatory and effector proteins [40]. In the next paragraphs, we will discuss the role of each HVCC subunit isoform expressed in pancreatic β-cells on electrical activity and insulin release.
4. α1 Subunit
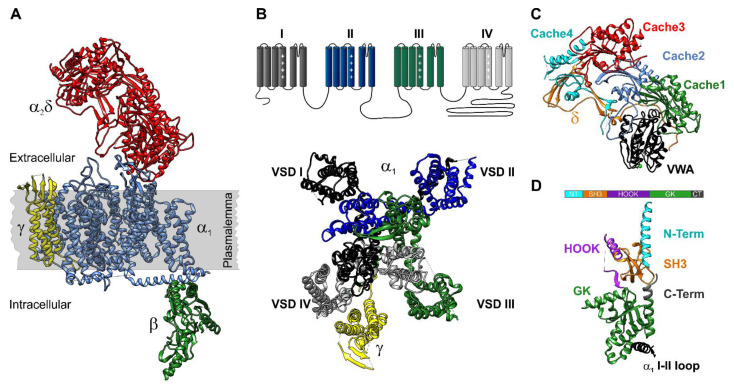
The α1 subunit is a polypeptide comprising of four homologous repeats (I–IV) with six transmembrane segments each (S1–S6) (Figure 1B). The S1 to S4 segments of each repeat form the voltage sensing domains (VSDs) while S5–S6 loop together with S5 of all repeats come together and form the actual channel pore [36,41]. The S4 segments contain four to five positively charged amino acids evenly spaced every three amino acids that serve as voltage sensors [42].
Figure 1.
Structure of the HVCC complex. (A). The HVCC complex consists of the transmembrane, pore-forming α1 and auxiliary intracellular β, extracellular α2δ and transmembrane γ subunits (PDB access code 5GJV [36]). (B). The α1 subunit is formed by four homologous repeats with six transmembrane domains each. The S1 to S4 segments of each repeat form the voltage sensing domain (VSD). S4 carrying four to five positively charged amino acids lysine or arginine serve as actual voltage sensors. The S5 and S6 segments of each repeat form the channel pore. The transmembrane γ subunit interacts with the fourth VSD. (C). The extracellular α2δ subunit consists of four Cache and one von Willebrand factor A (VWA) domain. The VWA domain contains the MIDAS motif (metal ion adhesion site) that coordinates a metal ion (Ca2+) together with residues in the extracellular linker between S1 and S2 segments of the first VSD. (D). Topology of the β2a subunit in complex with the CaV1.2-AID (α interaction domain) located in the intracellular loop between repeats I and II (PDB access code 5V2P [43]). The β subunits are organized in 5 regions: N-terminus, the SH3 domain, HOOK region, and the GK domain.
Following the membrane depolarization, the S4 segments rotate and slide through the membrane [44,45,46], pulling on the intracellular S4–S5 linker that induces a conformational change of the S5 and S6 domains. The concerted conformational changes in all four repeats result in the opening of the channel pore [42], with each of the four VSDs having an uneven and isoform-specific contribution [23,44,46,47,48,49,50,51,52,53]. Seven genes encode for the HVCC α1 subunit isoforms. Based on their biophysical and pharmacological properties, the HVCC α1 subunits can be separated in L-type Ca2+ channels (CaV1.1, CaV1.2, CaV1.3 and CaV1.4), P-Q type (CaV2.1), N-type (CaV2.2) and R-type (CaV2.3). While CaV1.1 and CaV1.4 seem to be exclusively and alone expressed in skeletal muscle [51] and retina, respectively [54], other excitable cells express multiple HVCC α1 subunit isoforms. Accordingly, mRNA profiling has demonstrated that mouse and human pancreatic β-cells express CACNA1C gene encoding for CaV1.2-α1C subunit, CACNA1D encoding for CaV1.3-α1D, CACNA1A encoding for CaV2.1-α1A, CACNA1B encoding for CaV2.2-α1B, and CACNA1E encoding for CaV2.3-α1E subunit. Pharmacological dissection demonstrated that ~60–70% of β-cell Ca2+ influx is conducted by isradipine-sensitive L-type CaV1 channels, ~20% by SNX-482-sensitive R-type channels, and ~20% by ω-agatoxin IVA-sensitive P/Q-type channels [55,56,57,58]. In a very limited number of studies, it has been shown that ω-conotoxin GVIA, a CaV2.2 antagonist, blocks a very small percentage (~5%) of β-cell Ca2+ influx in both mouse and human islets [16,59]. However, since this had no effect on insulin secretion the contribution of CaV2.2 N-type channels to β-cell function will not be further discussed in this review.
5. CaV1.2 and CaV1.3 L-Type Ca2+ Channels
L-type Ca2+ channels take their name from their property to conduct long-lasting and slow inactivating Ca2+ currents. In muscle cells, L-type Ca2+ channels are responsible for excitation-contraction coupling [60,61], while in most neurons, L-type calcium channels are located on the cell body and proximal dendrites and are crucial for the synaptic signal transmission and regulation of gene expression [62]. Pharmacologically L-type channels are characterized by their high sensitivity to dihydropyridine (amlodipine, felidipine, nifedipine, isradipine), phenylalkylamine (verapamil), and benzothiazepine (diltiazem) [22]. Although the binding sites for the different L-type channel blockers overlap [63,64,65], their mechanism of action is different; the dihydropyridines (DHPs) prefer and stabilize the inactivated state of the channel [66,67,68,69,70], while binding of phenylalkylamines and benzothiazepines is favored by the open channel state [71]. Consequently, the DHPs are better at blocking the channels during prolonged membrane depolarization, even close to resting membrane potential, while phenylalkylamines and benzothiazepines lead to a pronounced frequency- or use-dependent inhibition of the AP firing [71]. Consistent with this notion, early pharmacological studies in rodents have demonstrated that nifedipine has a stronger effect on β-cell electrical activity compared to verapamil and increasing the glucose concentration augments the β-cell sensitivity to verapamil but not nifedipine [25]. Despite the different activity-induced drug sensitivity, both compounds reduced the β-cell spike frequency in a dose-dependent manner, and at high concentrations (nifedipine > 10−7 M, verapamil > 10−6 M), lead to the disappearance of spikes through a decrease in amplitude [25]. Consequently, in a dose-dependent manner, verapamil reduces both phase of GIIS triggered by high glucose, and at low µM range, almost completely blocked GIIS [72] (Figure 2B,C). Importantly, 10 µM isradipine completely abolished the electrical activity and insulin secretion induced by 20 mM glucose also in human pancreatic β-cells and islets [16]. In agreement with all these in vitro pharmacology studies, overdose of L-type channel blockers in humans leads to hyperglycemia due to hypoinsulinemia [73]. However, at therapeutic doses, L-type channel blockers show no evident block of insulin release but instead help preserve β-cell mass in adults with recent-onset type1 diabetes [74]. Whether this effect is caused only by reducing the hyperglycemia-induced β-cell electrical activity and subsequently the oxidative stress or it is a direct effect of the different L-type channel isoforms inhibition remains to be shown. As previously mentioned, at mRNA level pancreatic β-cells express two L-type Ca2+ channels—CaV1.2 and CaV1.3—and they seem to have very specific roles in β-cell function and survival. Despite the fact that several transgenic mouse models for both CaV1.2 [57,58,75] and CaV1.3 [30,76,77] have been previously investigated, the exact role of these two isoforms on β-cell electrical activity, insulin release and β-cell mass are still controversial. CaV1.2 β-cell-specific deletion reduced by ~45% Ca2+ influx but this has been reported to have only moderate effects on electrical activity induced by 10 mM glucose. Considering that in 10 mM glucose the β-cell membrane potential during a depolarizing plateau is ~−50 mV, the AP generation most probably relies on NaV channel activity. In higher glucose concentrations (15–20 mM), when the plateau potential reaches ~−40 mV (which drives the NaV channels into inactivation) the electrical activity in CaV1.2−/− β-cells might be initiated by another HVCC isoform. However, the P/Q-type channels activate at higher membrane potentials, making it very unlikely that they are capable of initiating the electrical activity [78,79]. While the CaV2.3 R-type channels activate at lower membrane potentials, they display a very strong steady-state inactivation and low availability already at −50 mV, making them a very unlikely candidate for pace-making [80]. In contrast, the CaV1.3 channels have a depolarization threshold at ~−50 mV [75,77,81,82,83], and it has been shown that they are involved in the initiation of the electrical activity in the sino-atrial node [84] and chromaffin cells of the adrenal gland medulla [85,86]. Two transgenic CaV1.3 knock-out mouse models have previously been independently generated producing contradictory results regarding the involvement of CaV1.3 in β-cell function. In one mouse model, CaV1.3 genetic ablation did not change the β-cell Ca2+ current biophysical properties and did not cause a diabetes phenotype [30,77]. Additionally, L-type channel blocker isradipine and activator BayK8644 failed to alter the HVCC Ca2+ currents recorded in β-cells isolated from mice expressing a CaV1.2 channel insensitive to dihydropyridines (CaV1.2DHP−/−) [75]. Corroborated, these studies made a very strong case that the CaV1.3 channel is not expressed in mouse β-cells. However, a second CaV1.3−/− mouse model did show a diabetes phenotype due to reduced postnatal β-cell generation and lower β-cell mass [76]. β-cell Ca2+ influx was also not reduced in amplitude, but the voltage dependence of activation was shifted towards more positive potentials by ~10 mV (Figure 2B). Given that CaV1.3 L-type channels have an ~20mV more negative half maximal activation compared to CaV1.2 channels, the similar whole-cell Ca2+ current, but a rightwards shift in the voltage dependence of activation in CaV1.3−/− β-cells compared to wild-type could be indicative of a compensatory upregulation of CaV1.2 channels. Of note is the observation that CaV1.3 deletion reduced the insulin secretion only in lower glucose concentrations, supporting the notion that CaV1.3 is involved in β-cell pace-making at lower resting membrane potentials (Figure 2C) [76]. One possible explanation for the observed discrepancies between the two mouse models is that the β-cells are an inhomogeneous population with only a subset of cells expressing CaV1.3 channels. In fact, a recent study has demonstrated using single channel recordings that only ~20% of β-cells express CaV1.3 channels [87]. Additionally, it has been shown that CaV1.3 channels have a lower dihydropyridine sensitivity compared to CaV1.2 [88]; therefore, some of the pharmacology experiments might have underestimated the contribution of CaV1.3 to the total β-cell Ca2+ influx.
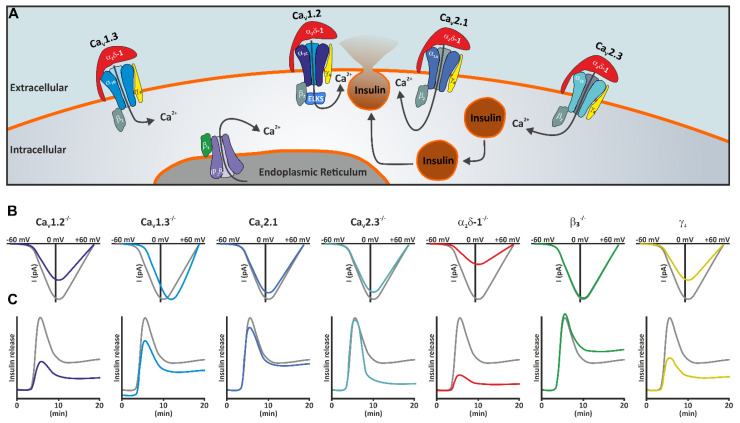
Figure 2.
Role of the HVCC subunits on pancreatic β-cell insulin release. (A). Localization of the HVCC isoforms in pancreatic β-cells with respect to insulin vesicles. At least in mouse, CaV1.2 channels are coupled to vesicle release, while evidence suggests that in human β-cells, CaV2.1 takes the central role. The localization of CaV1.2 channels at the release sites is controlled by the interaction between β2 subunit and ELKS. CaV2.3 channels are necessary for sustained insulin release, while the role of CaV1.3 is very controversial. β3 isoform does not come in complex with any HVCC but interacts with the IP3R. (B) Effect of genetic deletion or pharmacological block of each HVCC subunit isoform on total β-cell Ca2+ current. CaV1.2 contribute with ~45% of the whole-cell Ca2+ influx while CaV2.1 and CaV2.3 with ~20% each. CaV1.3 deletion only shifts the voltage dependence of activation. α2δ-1 deletion reduces all HVCC Ca2+ currents by ~70% while γ4 altered expression reduces only L-type Ca2+ currents (~70%). (C) Role of the HVCC subunits on GIIS. CaV1.2 deletion has the strongest effect as it is coupled to insulin vesicle release. CaV1.3 deletion reduces insulin secretion at lower glucose concentration while lack of CaV2.3 Ca2+ currents reduces second-phase GIIS. α2δ-1 deletion strongly reduces GIIS, while β3 deletion increases insulin release. γ4 reduced expression is expected to have similar effects as CaV1.2 deletion.
Besides their contribution to electrical activity and increase in cytosolic Ca2+ concentration, HVCCs are also critical for vesicle fusion with the plasma membrane. At the central and peripheral nervous system synapses, CaV2.1 and CaV2.2 channels in particular mediate neurotransmitter vesicle release [40], whereas ribbon synapses of the auditory system and the retina utilize CaV1.3 and CaV1.4 L-type Ca2+ channels, respectively [89,90]. In neurons, exocytosis of synaptic vesicles is generally regulated by the interaction of the active zone proteins Rab3, RIM (Rab3-interacting molecules) and RBP (RIM binding proteins), and the SNARE proteins syntaxin, SNAP25 and synaptobrevin [91]. Ca2+ influx through the CaV2 family triggers vesicle fusion with the plasma membrane through interaction with synaptotagmin and the synprint site of the II-III loop of the CaV2.1 and CaV2.2 channel [37,40,91]. While accumulating evidence shows that the release machinery in pancreatic β-cells has largely the same components as in the neuronal synapses, the HVCC isoform central for insulin vesicle exocytosis is CaV1.2 L-type Ca2+ channel (Figure 2A) [30,57,92,93]. CaV1.2 deletion in mice strongly reduced and delayed the first rapid phase vesicle exocytosis, indicating that CaV1.2 is directly coupled to vesicle release machinery [57]. Importantly, this association is disrupted in β-cells from Type 2 Diabetes Mellitus (T2DM) patients and INS-1 cells cultured in fatty acids that mimic the diabetic state. This dissociation leads to a dramatic reduction in rapid Ca2+ influx-dependent exocytosis, explaining why the first phase insulin release is missing in T2DM patients [92].
Despite several decades of research, the exact molecular mechanisms for how L-type channels control β-cell function, differentiation, and survival still need further investigation. Nevertheless, it is clear that both CaV1.2 and CaV1.3 are intimately involved in GIIS in both mice and men. CaV1.2-α1C and CaV1.3-α1D main subunit deletion in mice led to impaired glucose tolerance and diabetes [57,76] while L-type pharmacological block showed strong effects on β-cell activity [25,72,73]. Strong evidence regarding the involvement of both L-type channel isoforms in human β-cell function comes from recent identification of genetic alteration in CACNA1C and CACNA1D genes. CaV1.2 gain-of-function mutations lead to hyperinsulinism accompanied by intermittent hypoglycemia leading to death [94]. Similarly, CACNA1D gain-of-function mutations lead to hypoglycemic events [95,96] while genetic polymorphisms, suspected to lead to CaV1.3 loss-of-function, correlate with a higher T2DM incidence [97]. However, the effect of CaV1.2 and CaV1.3 mutations on glucose metabolism is probably more complex and expands beyond their role in pancreatic hormone release. Both channel isoforms are expressed in many endocrine cells and are responsible for the secretion of other hormones intimately involved in glucose homeostasis such as catecholamines [98] and glucocorticoids [99,100,101].
6. CaV2.3 R-Type Calcium Channels
Besides CaV1.2 and CaV1.3 L-type channels, mouse pancreatic β-cells also express CaV2.3 R-type channels. Initially, CaV2.3 channels have been described as having biophysical properties very similar to low voltage-activated Ca2+ channels (LVCC) [79,102]. CaV2.3 display transient currents with fast activation and inactivation kinetics and, similar to LVCCs, show a larger Sr+ single-channel conductance compared to Ca2+ or Ba2+. Initially it has also been proposed that CaV2.3 channel’s membrane incorporation and biophysical properties are less modulated by auxiliary β and α2δ HVCC subunits [79], another LVCC characteristic. However, several later studies have disproved these observations. In heterologous cells, the presence of an α2δ subunit does not seem to be required for CaV2.3 membrane incorporation but it accelerates the activation kinetics and shifts the voltage dependence of activation towards more positive potentials [80,103,104]. By contrast, the presence of a β subunit dramatically increases CaV2.3 Ca2+ influx by increasing the number of channels functionally incorporated in the membrane, enhancing the open probability as well as by shifting the voltage dependence of activation towards more negative potentials [80]. However, perhaps the most physiologically relevant effect of β subunits on CaV2.3 function is the modulation of CaV2.3 inactivation properties in a β isoform-specific manner. Depending on the co-expressed β isoform, the CaV2.3 Ca2+ current half maximal steady-state inactivation varies from ~−50 mV to ~−80 mV [80]. What does this mean for β-cell function? At low glucose concentrations, when the membrane potential is close to −80 mV, most of the CaV2.3 channels should be available to open and contribute to electrical activity and cytosolic Ca2+ concentration. In higher glucose concentrations (20 mM), when the resting membrane potential can reach ~−40 mV, the number of CaV2.3 channels available to open will be dramatically reduced (potentially zero) depending on which β subunit they complex with. Indeed, insulin release stimulated by 20 mM glucose was completely unaffected by SNX-482 [16], a CaV2.3 channel blocker [105], supporting this notion. However, SNX-482 also had minimal effects on human β-cell Ca2+ influx when the resting membrane potential was −70 mV [16], indicating either that CaV2.3 channel expression in human β-cells is very low or that a large CaV2.3 channel population is already in the inactivated state at resting membrane potential. Despite the observation that CaV2.3 channels have a marginal contribution to β-cell functions in humans, several polymorphisms in CACNA1E gene encoding for CaV2.3-α1E subunit have been reported to associate with an increased incidence of T2DM [106,107]. This could be caused by effects of CaV2.3 on insulin release in low glucose concentrations or functions outside the pancreas. Supporting the extra-pancreatic hypothesis regarding the role of CaV2.3 channels in glucose homeostasis come the observations that mice with a global genetic deletion of CaV2.3 channel [108] develop a non-insulin-dependent form of diabetes with increased body weight and fasting glucose levels, and impaired insulin sensitivity accompanied by higher basal insulin secretion [109]. A second independently generated mouse model also showed impaired glucose tolerance but, in this case, caused by reduced insulin secretion [110]. Electrophysiological analysis of pancreatic β-cells isolated from CaV2.3−/− mice showed an ~25% reduction in whole-cell Ca2+ influx [55], similar to the pharmacological effect of SNX-482 application observed in other studies (Figure 2B) [56,57]. This reduction in Ca2+ influx did not affect the peak islet Ca2+ transients induced by 15mM glucose [55]. However, after the initial peak, the Ca2+ transients showed a reduced amplitude and oscillation frequency in CaV2.3−/− mice compared to control. Similarly, the first-phase GIIS in response to 16.7 mM glucose was neither affected by CaV2.3 genetic ablation nor SNX-482 application but the second phase was strongly reduced (Figure 2C). Interestingly, capacitance measurements showed that the initial Ca2+ influx-induced exocytosis was not altered by CaV2.3 deletion, in good agreement with the observations that insulin vesicle release in mouse pancreatic β-cells is primarily controlled by CaV1.2 channels [57,92]. However, the same depolarization train showed a reduced exocytosis in CaV2.3−/− β-cells towards the end of the train, indicating that CaV2.3-mediated Ca2+ influx is required for sustained vesicle release. The reduced exocytosis during prolonged depolarizing trains in combination with reduced electrical activity (as indicated by reduced oscillation frequency of the islet Ca2+ transients) result in the diminished second phase GIIS and ultimately glucose intolerance. However, CaV2.3 deletion could also alter glucose metabolism by controlling the release of the other islet hormones. Cav2.3-deficient mice showed an impaired glucose suppression of glucagon release [111]. However, this is not caused by a direct effect on α-cell glucagon release [55] but indirectly through a reduced somatostatin secretion [112,113], a hormone known to have an inhibitory paracrine effect on insulin and mostly glucagon secretion at high plasma glucose concentrations [3,4,114,115].
7. CaV2.1 P/Q- Type Ca2+ Channels
Although the primary role in insulin release is taken by the L- and R-type Ca2+ channels, pharmacological dissection shows that approximately 20% of Ca2+ influx in pancreatic β-cells of both mouse [57] and human [16,116] is conducted by ω-agatoxin IVA-sensitive CaV2.1, P/Q-type channels. The P/Q-type Ca2+ currents activate fast, with a depolarization threshold of ~−30 mV, and reach their maximum at +10 mV. Although experimental evidence is missing, the biophysical properties CaV2.1 channels suggest that they do not directly account for the electrical activity but contribute to the increase in cytosolic Ca2+ concentration during the depolarization train. In agreement with this hypothesis, ω-agatoxin IVA application reduced insulin release stimulated by 6 mM and 20 mM glucose albeit to a lot lower extent compared to L-type channel blockers [16,116]. It has been proposed that CaV2.1 channels play a much more important role in human β-cells compared to mouse. While in mouse it has been shown that CaV1.2 channels are coupled to insulin vesicle exocytosis [57], in human β-cells ω-agatoxin IVA resulted in a much stronger reduction in exocytosis of immediately releasable pool of granules compared to isradipine [16]. This suggests that in human β-cells the vesicle release machinery is centered around CaV2.1 channel (Figure 2A). However, a very elegant recent study contradicted these observations [92]. Using high-resolution microscopy, Gandasi and colleagues [92] showed that CaV1.2 L-type Ca2+ channels cluster with the insulin vesicles both in the INS-1 rat insulin secreting cell line as well as in human β-cells. They showed that the interaction occurs via the CaV1.2 II–III loop with the Munc13 synaptic protein and, importantly, the disruption of this interaction abolished the fast exocytosis. Interestingly, T2DM-mimicking conditions also disturb the association of CaV1.2 L-type channels with the insulin vesicles underlying the blunted first phase insulin release observed in T2DM patients. Additionally, many loss- and gain-of-function mutations in the CACNA1A gene encoding for CaV2.1-α1A subunit have been identified in humans as a cause of epilepsy, episodic ataxia, and migraine [117]. However, to our knowledge, none of these mutations have been associated to hyperglycemia or hyperinsulinism, cementing the notion that CaV2.1 is not critical for human β-cell function and insulin release.
8. β-Subunits
The HVCC complex cannot be formed by α1 subunits alone as proper membrane trafficking and localization depends on the auxiliary subunits, among which the intracellular β play a central role. It has been well established that the β subunit promotes the membrane surface expression of CaV1 and CaV2 channels. Initially, it was postulated that the β-subunits enhance channel trafficking by masking an endoplasmic reticulum retention signal at the α1 I–II intracellular linker [118]. Such a mechanism could not be confirmed in subsequent experiments [119,120,121]. Regardless of the mechanism, the notion that a β subunit is required for α1 membrane incorporation was cemented by many studies performed in heterologous expression systems, where proper Ca2+ current densities could not be achieved in the absence of a co-expressed β subunit [80,118,122,123]. In congruency with these observations, the genetic ablation of the β1 and β2 subunits led to a dramatically reduced HVCC current amplitude in skeletal [124,125] and cardiac [126] muscle, neurons [127], retina [128], and inner hair cells (IHC) [129]. Conversely, β subunit overexpression increases HVCC currents in native cardiac myocytes, suggesting that HVCC membrane expression is limited by β subunit abundance [130]. The dogma that the presence of a β subunit is absolutely necessary for the membrane incorporation of the HVCC complex was, however, challenged when cardiomyocyte-specific conditional deletion of the CACNB2 gene in adult mice reduced β2 protein by 96% but caused only a modest 29% reduction in Ca2+ current, with no obvious cardiac impairment [131]. A possible explanation for this controversial result might be that the presence of other β subunit isoforms compensates for the loss of β2 [132]. Alternatively, owing to its high affinity binding to α1 subunit and their close proximity in the ER, the remnant 4% β2 are sufficient to inhibit the α1 subunit ER retention signal and release it into the cytosol, where other α1 interacting proteins are responsible for proper membrane incorporation. Additional to their role in promoting α1 membrane incorporation, the β subunits modulate in an isoform and splice variant-specific manner the channel’s voltage dependence [80,122,124,126] and time course [122,130] of activation and inactivation. The β subunits alter the whole cell HVCC Ca2+ current kinetics by modulating the single-channel open probability properties [133,134,135,136,137].
The β subunits are encoded by four genes (CACNB1-CACNB4) with broad tissue distributions [138]. While some tissues show a very restrictive expression pattern (e.g., β1 in skeletal and β2 in cardiac muscle) other cell types such as endocrine cells and neurons express at least two different β isoforms, with the β2 showing the widest expression pattern. Pancreatic islet cells express both β2 and β3 subunits [139]. In contradiction with the known role of β subunits in promoting α1 membrane incorporation, β3 deletion [140] in pancreatic β-cells showed no change in HVCC Ca2+ current properties [139]. Instead, β3−/− mice showed a higher insulin release and glucose tolerance due to increased glucose-stimulated intracellular Ca2+ oscillation frequency [139]. Under normal conditions in pancreatic β-cells, β3 isoform negatively modulates inositol triphosphate receptor (InsP3R) activity and therefore its genetic deletion enhanced InsP3R function, resulting in increased Ca2+ mobilization from internal stores (Figure 2) [139]. In agreement with those earlier findings and using a second independently generated β3−/− mouse model [141], recently, it has been shown that β3 deletion protects mice against high-fat-diet-induced diabetes, while β3 overexpression in isolated human islets impaired insulin secretion [142]. Importantly, the InsP3R-β3 interaction is not restricted to pancreatic β-cells and it works in a similar manner in controlling intracellular Ca2+ signals in fibroblasts [143]. β3 deletion enhanced fibroblast InsP3R store Ca2+ release and consequently enhanced fibroblast migration in vitro and wound healing in vivo [143]. The observation that in pancreatic β-cells β3 isoform has HVCC independent functions suggests that β2 isoform must be responsible for promoting HVCC membrane incorporation and biophysical properties. Knock-out of β2 HVCC isoform is embryonically lethal [126]. However, adult mice harboring only an extracardiac tissue deletion of β2 show altered retina development [128] and impaired hearing due to impaired inner hair cell development and reduced Ca2+ influx [129]. However, β2 deletion did not lead to any metabolic dysfunctions. This effect could be explained by a functional compensation by the existing β3 subunits. While in the presence of the β2 subunit, β3 takes HVCC-independent functions, it is possible that, upon β2 genetic deletion, β3 assumes its role as a HVCC subunit, as it does in other cell types [138,141,144].
As members of the membrane-associated guanylate kinase (MAGUK) protein family [138], β subunits are comprised of two highly conserved central domains—GK (guanylate kinase) and SH3 (Src-homology 3)—separated by a less conserved HOOK domain and flanked and interspersed by more variable N- and C-termini (Figure 1D). The β2 subunit is subject to extensive alternative splicing of the N-terminus and within the central HOOK domain [138]. To date, there are nine known β2 splice variants that confer the α1 subunits’ very distinct biophysical properties [138,145]. Functionally, perhaps the most interesting ones are the β2a and β2e variants that, besides binding to the α1 subunits, also interact with the plasma membrane through palmitoyl groups or electrostatic and hydrophobic interactions, respectively [138,146]. RNAseq data showed that human pancreatic islets highly express β2a splice variant. β2a overexpression in INS-1 rat insulinoma cells or higher palmitoylation results in an increased amount of HVCC in the membrane, leading to higher intracellular Ca2+ concentration that promotes apoptotic cell death [147].
The β subunits do not control only the HVCC density at the plasma membrane but also the subcellular localization. In central and sensory neurons, the neurotransmitter release is restricted to a specialized presynaptic compartment called the active zone (AZ) [91]. The AZ proteins play a critical organizational role in defining the presynaptic sites of synaptic vesicle docking and fusion. Several proteins important for the AZ organization have been characterized, including ELKS (also known as ERC1) and CAST (ERC2) [148], and both have been shown to directly interact with other AZ proteins, including RIM1, Piccolo and Bassoon, and indirectly with Munc13-1. This leads to the formation of a large molecular complex that regulates the architecture of the presynaptic compartment. Importantly, it has been shown that both CAST and ELKS physically and functionally interact with β4 subunit in neurons [149,150]. Although pancreatic β-cells lack defined active zones, it was shown that the insulin vesicles are polarized towards the venous vasculature [151]. ELKS binding to β2 subunit in pancreatic β-cells enhanced L-type Ca2+ current at the vascular side of the β-cell plasma membrane and regulated the initial polarized Ca2+ influx and rapid insulin vesicle exocytosis [152]. Another important mechanism that dynamically modulates the HVCC membrane incorporation and function is the interaction of the β and α1 subunits with the small GTPases RGK protein family members (Rem, Rem2, Rad, Gem/Kir) [153]. Initially it has been shown that co-expression of Gem with CaV1.2 or CaV1.3 channels resulted in Ca2+ influx inhibition, and these findings were later extended to the other RGK family members (Rad [154], Rem and Rem2 [155,156], Gem [157,158]) and HVCC isoforms [156,159]. RGK proteins have been reported to inhibit HVCC activity by distinct mechanisms, including a reduction of the number of channels in the membrane and lowering the single channel open probability [156,157]. Functionally, the different RGK family members exert their effects in a β-binding-dependent and -independent manner. Rem and Rad use both β-binding-dependent and independent mechanisms, whereas Gem and Rem2 solely utilize a β-binding-dependent mode of inhibition [160]. Importantly, the RGKs expression is activity-dependent as it has been shown that high electrical activity in response to elevated glucose levels strongly induced Rem2 expression in the mouse insulinoma MIN6 cell line [155], while in neurons, KCl treatment upregulates Rem2 and Gem mRNA levels [161]. Similarly, mice deficient for Gem GTPase show abnormal glucose tolerance and reduced intracellular Ca2+ transients [158]. This evidence demonstrates that the interaction between RGKs and β2 subunit in pancreatic β-cells and RGK-dependent modulation of HVCC function is controlled by the glucose levels.
9. α2δ Subunits
A total of four genes (CACNA2D1-4) encode for α2δ subunits (α2δ-1 to α2δ-4), with distinct tissue distribution [162]. Each α2δ subunit is a product of a single gene that is post-translationally cleaved into α2 and δ peptides, which remain associated via disulfide bonds [163]. Until recently, it had been considered that the δ subunit constitutes a single-pass membrane, and the α2 subunit a glycosylated extracellular protein. However, this classical view has been challenged by the observation that α2δ subunits can be GPI-anchored to the membrane and that this posttranslational modification may be crucial for α2δ function [164]. Few attempts have previously been made to identify the interaction site between α2δ and α1 subunit. Using coimmunoprecipitation assays of α2δ-1 together with trypsinized skeletal muscle α1S subunit, it has been shown that the first extracellular loop of the α1S IIIS5–IIIS6 linker bind to α2δ-1 subunit [165]. It has also been proposed that the putative transmembrane segment of the δ subunit interacts with α1 [166]; however, if α2δ subunits are GPI-anchored proteins [164], this notion needs to be reconsidered. Another interaction between α2δ and α1 subunit occurs via the common coordination of an ion in the metal ion adhesion site (MIDAS) (Figure 1C). While all α2δ subunits contain a putative MIDAS, only α2δ-1 and α2δ-2 incorporate the “perfect” motif, where five amino acids coordinate one metal ion [167]. The 3.6 Å resolution cryo-EM structure of the CaV1.1 channel complex [36] allowed for the first time to visualize, predict, and validate the amino acids responsible for the interaction between α1 and α2δ subunits. The structure shows that α1S has four possible interaction sites with α2δ-1 subunit located in the first three repeats confirming the previously published studies [165,166,167,168]. Functional mutagenesis confirmed [169,170] that many of these potential interactions are important for conferring the two-fold role of α2δ in the HVCC complex: (a) to increase the number of channels functionally incorporated in the membrane and (b) to modify the Ca2+ current biophysical properties. However, the effects of α2δ are α1 isoform- and tissue-specific. Due to the large number of interaction sites between the two proteins, α1 and α2δ isoform-specific sequence variations could be responsible for preferential interaction between certain isoforms as well as differential modulation. It has been shown that the α2δ subunit is required for membrane incorporation of CaV1.2-α1C, CaV1.3-α1D, CaV2.3-α1F, CaV2.1-α1A, and CaV2.2-α1B subunits expressed in human embryonic kidney (HEK) cells, Xenopus oocytes, or neurons [83,171,172]. However, we have shown that when expressed in muscle cells, the CaV1.1-α1S and CaV1.2-α1C channels do not require an α2δ for membrane incorporation [38,39,50,173,174]. This effect is most probably caused by supplementary membrane targeting interactions present in muscle cells such as the newly identified Stac3 adaptor proteins [175,176,177,178,179,180,181,182,183]. Functional evidence suggests that membrane expression of CaV2.3- α1E channels is also less dependent on the presence of a α2δ subunit. Expression of CaV2.3-α1E alone in Xenopus oocytes produced large currents [102,104] and co-expression of α2δ-1 failed to further increase CaV2.3 currents [104]. When expressed in HEK-293 cells, α2δ-1 produced a twofold increase in the CaV2.3-α1E current density if β subunit was not present [80]. Nevertheless, if a β subunit was expressed, α2δ-1 failed to further increase the CaV2.3 currents. This evidence strongly suggests that the CaV2.3-α1E subunit has weaker interactions with α2δ compared to the other HVCCs and might be less if at all modulated in native cells. Regarding the role of α2δs in modulating the Ca2+ current biophysical properties, we and others have shown that the CaV1.2-α1C channel is the only HVCC where α2δ induces a shift in the voltage dependence of activation towards more hyperpolarizing potentials [168,174,184,185]. We have also shown that the lack of α2δ-1 slows down the activation and inactivation kinetics of α1C [174], and this result has been confirmed in the α2δ-1-/- mouse model [186]. Conversely, we demonstrated that the shRNA knock-down of α2δ-1 in skeletal muscle cells has the opposite effects on α1S current kinetics, increasing the speed of current activation and inactivation [173]. While α2δs do not seem to alter the voltage dependence of α1A [187], α1B [188], and α1E [80], there is evidence that different α2δ isoforms alter the inactivation kinetics of α1B channels in an α2δ isoform-specific manner [189] and α2δ-1 alters the activation kinetics of CaV2.3 channels [104]. Therefore, genetic alterations of α2δ subunit isoforms will lead to complex changes of Ca2+ influx biophysical properties and subsequently a complex disease phenotype. However, the initial characterization showed that global deletion of α2δ-1 (the most widely expressed α2δ isoform [190]) does not lead to an obvious phenotype [186]. Mice looked normal, were viable, and without gross anatomical alterations. However, functionally, they had diminished cardiac contractility due to reduced CaV1.2 Ca2+ influx in cardiac myocytes. In agreement with previous studies performed in heterologous expression systems [168,184,185] or muscle cells [39,174], deletion of α2δ-1 reduced the Ca2+ influx by >40%, slowed down the activation and inactivation kinetics and led to a positive shift in the voltage dependence of activation and inactivation [186]. Additionally, male α2δ-1−/− mice displayed a tendency for bladder dilation [186] and enlarged kidneys [56]. A closer analysis indicated that α2δ-1 deletion in mice led to polyuria, polydipsia, and reduced survival in a sex-specific manner. Male mice displayed threefold higher basal non-fasting blood glucose levels at one month and approximately ninefold higher at five months of age. Female mice showed minimal alteration of their basal glucose levels. An intraperitoneal glucose tolerance test also showed that male mice are more affected. Two hours after the glucose challenge, male α2δ-1−/− mice failed to reduce the plasma glucose concentration back to base levels, whereas α2δ-1−/− females, albeit with a slower kinetic compared to wild types, did so. At the single-cell level, α2δ-1 deletion (the main α2δ isoform expressed in mouse pancreatic β-cells) equally reduced the β-cell Ca2+ influx by ~70% and slowed down the activation and inactivation kinetics in both males and females. Pharmacological dissection showed that all HVCC isoforms expressed in pancreatic β-cells are equally reduced, confirming the role of α2δ in membrane targeting of all HVCCs in a native cell system. However, despite an equal effect of α2δ-1 deletion on β-cell Ca2+ currents in both sexes, the islet Ca2+ transients, insulin release and β-cell mass were more reduced in males compared to females demonstrating the need for a better understanding of the role of sex-dimorphism in β-cell function and diabetes etiology [191].
Additional evidence regarding the critical role of α2δ-1 in glucose homeostasis comes from recent genome-wide association studies where it has been shown that variants in TCF7L2 transcription factor confer the strongest risk of T2D among common genetic variants [192]. Indirectly via ISL1, TCF7L2 regulates proinsulin production and processing via MAFA, PDX1, NKX6.1, PCSK1, PCSK2, and SLC30A8 [192]. However, TCF7L2 is also a critical regulator of α2δ-1 expression in pancreatic β-cells [193]. Silencing the TCF7L2 activity results in reduced α2δ-1 protein levels causing reduced CaV1.2 membrane expression due to retention of the channel in recycling endosomes. The altered Ca2+ influx reduces the cytosolic Ca2+ transients as well as insulin release [193], as seen in the murine α2δ-1−/− β-cells [56]. Overexpression of α2δ-1 in TCF7L2-silenced cells rescued the TCF7L2-dependent impairment of Ca2+ signaling, but not the reduced insulin secretion. This is understandable as TCF7L2 transcription factor activity is responsible also for the expression of many proteins involved in insulin exocytosis, such as Synaptotagmin-14, syntaxin binding protein 1 (Munc 18-1), and Vamp2 [193]. Therefore, TCF7L2 activity controls both insulin synthesis and release.
Although several patients carrying loss-of-function mutations in CACNA2D1 gene encoding for α2δ-1 have been identified with epilepsy and intellectual disability [194], short QT syndromes [195,196] and cardiac arrhythmias [197], no metabolic alterations have been reported for these patients. Metabolic disorders and increase in body weight have been reported in patients prescribed gabapentinoids drugs (inhibiting α2δ-1 and α2δ-2 function [198]) against neuropathic pain and seizure disorders [199,200]; however, it is difficult to speculate on the mechanism of action. In vivo, gabapentin (GBP) and pregabalin (PG) application has been shown to have hardly any acute analgesic effects but to be efficient in releasing neuropathic pain upon chronic application (reviewed in [201,202]). One likely explanation for their mode of action is that chronic treatment lowers the amount of α2δ-1 and α2δ-2 on the cell surface by inhibiting rab11-dependent recycling [203], thereby decreasing the trafficking of the channel complex to the membrane and reducing Ca2+ influx [204,205]. However, in vitro, gabapentinoid drug application resulted in rather inconsistent effects depending on the cell system studied. Long-term incubation caused reduced Ca2+ currents in dorsal root ganglion neurons [205] but resulted in decreased catecholamine secretion from adrenal chromaffin cells without affecting the Ca2+ influx [206]. Studies using acute gabapentinoids application either report no impact at all on HVCC Ca2+ current density [205] or show inhibitory effects on transmitter release and/or Ca2+ currents [207,208]. To date, no information regarding the effect of gabapentinoid drugs application on pancreatic β-cell function has been published. Due to its very broad tissue expression pattern, alteration in α2δ-1 expression level and function (due to loss- and gain-of-function mutations or gabapentinoid drugs treatment) will also affect glucose metabolism independent of β-cell insulin release. It has been shown that reduced surface expression of α2δ-1 in the ventromedial hypothalamus (VMH) neurons leads to hyperphagia, obesity, and metabolic disturbances [209]. Interestingly, α2δ-1 restricted deletion in SF1+ (steroidogenic factor-1) VMH neurons showed a sex-specific incidence of the metabolic alterations [210]. While male mice exhibit glucose intolerance, altered lipolysis, and decreased cholesterol content in adipose tissue [211], α2δ-1−/− females showed only a modest increase in body weight and enhanced glycemic control when fed a chow diet [210,211]. However, the glucose tolerance was altered to a lot smaller extent compared to global α2δ-1 deletion [56]. Mechanistically, α2δ-1 seems to control the activity of SF1+ neurons independent of its role as an HVCC auxiliary subunit. α2δ-1 deletion did not reduce the whole-cell Ca2+ influx in SF1+ neurons but led to a membrane hyperpolarization and dramatically reduced electrical activity and synaptic transmission [211]. In fact, several recent studies demonstrate the role of α2δ proteins in synapse formation and function or neuronal excitability independent of its role as an HVCC subunit [202,212,213] but due to its interactions with NMDA [214] and GABAA receptors [215], thrombospondins [216] or BK potassium channels [217]. In pancreatic β-cells, it has been shown that the interaction of α2δ-1 with RalA GTPase is important for binding α2δ-1 on insulin granules and tethering these granules to the HVCCs in the plasma membrane [218]. Therefore, α2δ-1 shines out as a very important modulator of glucose metabolism though its pancreatic and extra-pancreatic roles acting as an HVCC subunit or through HVCC-independent interactions.
10. γ Subunit
The initial purification of the skeletal muscle HVCC complex showed that besides the intracellular β1 and extracellular α2δ-1, the HVCC complex also contains the γ1 subunit [219,220]. Recent Cryo-EM data confirmed that γ1 subunit is a transmembrane protein consisting of four helices and interacts with the fourth VSD of the α1 subunit (Figure 1B) [36,41]. In skeletal muscle, the γ1 subunit has a dual role: to limit the Ca2+ influx by stabilizing the inactivated state of CaV1.1 channel [221,222] and to reduce the depolarization-induced sarcoplasmic Ca2+ release [223]. γ1 belongs to a family of eight genes (γ1 to γ8) predominantly expressed in the brain. They are known as transmembrane AMPA receptor regulatory proteins (TARPs) [37] as they play an important role in targeting and anchoring of these ionotropic glutamate receptors in the postsynaptic membrane of neurons and glia cells [220]. However, heterologous expression systems have demonstrated that several γ isoforms can modulate HVCC function [224,225,226,227,228]. Coexpression of the γ1 subunit together with CaV1.2 L-type Ca2+ channels in Xenopus laevis oocytes [225] or tsA201 cells [227] increased the current density and led a shift of the voltage dependence of activation towards more negative potentials. The expression of CaV1.2 complex (α1C + β1 or α1C + β2 + α2δ − 1) in HEK293 cells showed that γ4, γ6, γ7 and γ8 subunits interact with the channel and modulate Ca2+ current properties in a γ isoform-specific manner. In the presence of β1 and α2δ-1 subunits γ4, γ7 and γ8 shift the voltage dependence of inactivation towards more positive potentials without affecting the voltage dependence of activation [229]. Therefore, contradictory to the effect of γ1 on CaV1.1 L-type skeletal muscle channel, γ1 [225], γ4, γ7 and γ8 [229] seem to have a gain-of-function effect on CaV1.2 L-type Ca2+ currents. However, all these effects were gone in the presence of the β2 subunit [229]. Furthermore, the results were obtained in heterologous expression systems, thereby omitting other potentially critical modulatory factors. Recently, it has been shown that γ4 subunits modulate HVCC currents in pancreatic β-cells [230]. This is the first evidence that a γ subunit can modulate an HVCC function in a native cell system besides γ1 and CaV1.1 in skeletal muscle cells. In good agreement with the heterologous expression systems [225,227,229], reduced γ4 expression in pancreatic β-cells leads to decreased L-type Ca2+ currents and therefore glucose-stimulated insulin secretion. Interestingly, γ4 expression is reduced in islets from human donors with diabetes, hyperglycemic and T2D animal models Goto-Kakizaki rats, and db/db mice, as well as in islets cultured in conditions simulating diabetes such as high glucose and palmitate [230].
11. Conclusions
The crucial role of HVCC Ca2+ influx on excitability, vesicle exocytosis, and gene transcription and regulation has been extensively demonstrated in many excitable cells such as neurons, cardiac myocytes, skeletal muscle, neuro endocrine cells, etc. Critical information regarding the biophysical and pharmacological properties of all HVCC subunit isoforms has been obtained in simplified heterologous co-expression systems such as Xenopus laevis oocytes or HEK293 cells. In this review article, we summarized the knowledge regarding the structure and function of the HVCCs, how this impacts on pancreatic β-cell insulin release and glucose metabolism and, whenever we saw fit, compared these findings with data obtained from research performed in other cell types. The physiological role of Ca2+ channels subunits in pancreatic hormone release and glucose metabolism has been obtained mostly from research performed on transgenic mouse models. Critical experiments have also been performed in isolated human pancreatic islets and cells from healthy or diabetes donors. This provides a very important translational aspect to the research performed in animal models.
Genetic ablation of most HVCC subunits in pancreatic β-cells led to reduced β-cell Ca2+ influx and therefore altered excitability [56,57], reduced intracellular Ca2+ concentration and oscillations [55,56], reduced insulin secretion [55,56,57,230], and also altered β-cell differentiation and survival [55,56,76]. However, the severity of the effects induced by HVCC subunit deletion varied greatly. CaV1.2 genetic deletion or pharmacological block reduced the β-cell Ca2+ influx by ~60% (Figure 2C, dark blue) compared to controls (grey) and, because CaV1.2-α1C subunit is coupled to vesicle release (Figure 2A) it almost completely abolished the first phase insulin release and strongly reduced second-phase release (Figure 2C, dark blue). CaV1.3 deletion did not affect the β-cell peak Ca2+ influx but resulted in a shift of the voltage dependence of activation towards more positive potentials. CaV1.3 deletion reduced insulin release at lower glucose concentration and, due to smaller β-cell mass, also reduced the total insulin release, thereby leading to glucose intolerance in mice. CaV2.1 pharmacological block resulted in an ~20% reduction in β-cell Ca2+ currents. Although its role on GIIS has been demonstrated only in static experiments, based on its potential contribution to the peak of APs, it is expected that CaV2.1 loss-of-function would slightly reduce both phases of GIIS. If in human β-cells CaV2.1 is coupled to secretory machinery, then its genetic deletion or pharmacological block will reduce both phases of GIIS to a similar extent as CaV1.2 does in mouse β-cells. Similar to CaV2.1, CaV2.3 channels also contribute with ~20% to total β-cell Ca2+ influx. CaV2.3 deletion or SNX-482 application did not alter the first phase but strongly reduced second-phase GIIS due to its proposed role in promoting vesicle trafficking to the release sites (Figure 2, cyan). α2δ-1, the main α2δ isoforms expressed in pancreatic β-cells, forms a complex with all HVCC isoforms. Consequently, its genetic deletion resulted in ~70% decreased β-cell Ca2+ influx and a strong reduction of both phases GIIS (Figure 2, red). γ4 subunit seems to promote only L-type channel membrane incorporation; therefore, its reduced expression is expected to have a similar effect on GIIS as L-type channel block (Figure 2, yellow). An exception from the rule applies. In pancreatic β-cell, the β3 subunit has HVCC-independent functions and its genetic deletion increases IP3R Ca2+ release from intracellular stores, thereby most probably increasing insulin secretion only in the second phase since the first phase is limited by the size of the readily releasable pool of vesicles (Figure 2, green).
During the preparation of Figure 2, we intended to incorporate the information regarding the role of all HVCC subunits on glucose-induced electrical activity and intracellular Ca2+ transients. However, such a figure would be mostly speculative since the experiments have either never been performed or the data have been obtained under very different experimental settings. We are confident that these gaps in our knowledge will soon be filled and complemented by additional RNAseq and proteomics data. This will shed light on the known and hitherto unknown roles of the HVCC subunits in pancreatic hormone release and glucose homeostasis in health and diabetes.
Author Contributions
Conceptualization P.T.; Writing-original draft and review, P.T., T.T., N.J.-P., S.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Austrian Science Fund (FWF) P31434 and DOC30-B30 to P.T. and TWF F18863 to S.M.G. Open Access Funding by the Austrian Science Fund (FWF).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bonnevie-Nielsen V., Steffes M.W., Lernmark A. A major loss in islet mass and b-cell function precedes hyperglycemia in mice given multiple low doses of streptozotocin. Diabetes. 1981;30:424–429. doi: 10.2337/diab.30.5.424. [DOI] [PubMed] [Google Scholar]
- 2.Hollander P.M., Asplin C.M., Palmer J.P. Glucose modulation of insulin and glucagon secretion in nondiabetic and diabetic man. Diabetes. 1982;31:489–495. doi: 10.2337/diab.31.6.489. [DOI] [PubMed] [Google Scholar]
- 3.Vergari E., Denwood G., Salehi A., Zhang Q., Adam J., Alrifaiy A., Wernstedt Asterholm I., Benrick A., Chibalina M.V., Eliasson L., et al. Somatostatin secretion by Na+-dependent Ca2+-induced Ca2+ release in pancreatic delta-cells. Nat. Metab. 2020;2:32–40. doi: 10.1038/s42255-019-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rorsman P., Huising M.O. The somatostatin-secreting pancreatic delta-cell in health and disease. Natnat. Rev. Endocrinol. 2018;14:404–414. doi: 10.1038/s41574-018-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Diaz R., Dando R., Jacques-Silva M.C., Fachado A., Molina J., Abdulreda M.H., Ricordi C., Roper S.D., Berggren P.O., Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rorsman P., Ashcroft F.M. Pancreatic beta-cell electrical activity and insulin secretion: Of mice and men. Physiol. Rev. 2018;98:117–214. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rorsman P., Braun M. Regulation of insulin secretion in human pancreatic islets. Annu. Rev. Physiol. 2013;75:155–179. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 8.Rorsman P., Eliasson L., Renstrom E., Gromada J., Barg S., Gopel S. The cell physiology of biphasic insulin secretion. News Physiol. Sci. 2000;15:72–77. doi: 10.1152/physiologyonline.2000.15.2.72. [DOI] [PubMed] [Google Scholar]
- 9.Hattersley A.T., Ashcroft F.M. Activating mutations in kir6.2 and neonatal diabetes: New clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 10.Ellard S., Flanagan S.E., Girard C.A., Patch A.M., Harries L.W., Parrish A., Edghill E.L., Mackay D.J., Proks P., Shimomura K., et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous sur1 mutations with opposite functional effects. Am. J. Hum. Genet. 2007;81:375–382. doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard C.A., Wunderlich F.T., Shimomura K., Collins S., Kaizik S., Proks P., Abdulkader F., Clark A., Ball V., Zubcevic L., et al. Expression of an activating mutation in the gene encoding the katp channel subunit kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J. Clin. Investig. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashcroft F.M., Rorsman P. K(atp) channels and islet hormone secretion: New insights and controversies. Nat. Rev. Endocrinol. 2013;9:660–669. doi: 10.1038/nrendo.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashcroft F.M., Harrison D.E., Ashcroft S.J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 14.Kang C., Xie L., Gunasekar S.K., Mishra A., Zhang Y., Pai S., Gao Y., Kumar A., Norris A.W., Stephens S.B., et al. Swell1 is a glucose sensor regulating beta-cell excitability and systemic glycaemia. Nat. Commun. 2018;9:367. doi: 10.1038/s41467-017-02664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopel S., Kanno T., Barg S., Galvanovskis J., Rorsman P. Voltage-gated and resting membrane currents recorded from b-cells in intact mouse pancreatic islets. J. Physiol. 1999;521:717–728. doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun M., Ramracheya R., Bengtsson M., Zhang Q., Karanauskaite J., Partridge C., Johnson P.R., Rorsman P. Voltage-gated ion channels in human pancreatic beta-cells: Electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 17.Rorsman P., Eliasson L., Kanno T., Zhang Q., Gopel S. Electrophysiology of pancreatic beta-cells in intact mouse islets of langerhans. Prog. Biophys. Mol. Biol. 2011;107:224–235. doi: 10.1016/j.pbiomolbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Tsien R.W. Calcium channels in excitable cell membranes. Annu. Rev. Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- 19.Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X., Yan N. The conformational shifts of the voltage sensing domains between na(v)rh and na(v)ab. Cell Res. 2013;23:444–447. doi: 10.1038/cr.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papazian D.M., Shao X.M., Seoh S.A., Mock A.F., Huang Y., Wainstock D.H. Electrostatic interactions of s4 voltage sensor in shaker k+ channel. Neuron. 1995;14:1293–1301. doi: 10.1016/0896-6273(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 22.Zamponi G.W., Striessnig J., Koschak A., Dolphin A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharm. Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flucher B.E. Specific contributions of the four voltage-sensing domains in l-type calcium channels to gating and modulation. J. Gen. Physiol. 2016;148:91–95. doi: 10.1085/jgp.201611663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andranovits S., Beyl S., Hohaus A., Zangerl-Plessl E.M., Timin E., Hering S. Key role of segment is4 in cav1.2 inactivation: Link between activation and inactivation. Pflug. Arch. 2017;469:1485–1493. doi: 10.1007/s00424-017-2038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasseur M., Debuyser A., Joffre M. Sensitivity of pancreatic beta cell to calcium channel blockers. An electrophysiologic study of verapamil and nifedipine. Fundam. Clin. Pharm. 1987;1:95–113. doi: 10.1111/j.1472-8206.1987.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 26.Henquin J.C., Ishiyama N., Nenquin M., Ravier M.A., Jonas J.C. Signals and pools underlying biphasic insulin secretion. Diabetes. 2002;51(Suppl. S1):S60–S67. doi: 10.2337/diabetes.51.2007.S60. [DOI] [PubMed] [Google Scholar]
- 27.Henquin J.C., Nenquin M., Ravier M.A., Szollosi A. Shortcomings of current models of glucose-induced insulin secretion. Diabetesobes. Metab. 2009;11:168–179. doi: 10.1111/j.1463-1326.2009.01109.x. [DOI] [PubMed] [Google Scholar]
- 28.Henquin J.C. Glucose-induced insulin secretion in isolated human islets: Does it truly reflect beta-cell function in vivo? Mol. Metab. 2021;48:101212. doi: 10.1016/j.molmet.2021.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curry D.L., Bennett L.L., Grodsky G.M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83:572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- 30.Barg S., Ma X., Eliasson L., Galvanovskis J., Gopel S.O., Obermuller S., Platzer J., Renstrom E., Trus M., Atlas D., et al. Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic b cells. Biophys. J. 2001;81:3308–3323. doi: 10.1016/S0006-3495(01)75964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barg S., Eliasson L., Renstrom E., Rorsman P. A subset of 50 secretory granules in close contact with l-type Ca2+ channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes. 2002;51:S74–S82. doi: 10.2337/diabetes.51.2007.S74. [DOI] [PubMed] [Google Scholar]
- 32.Olofsson C.S., Gopel S.O., Barg S., Galvanovskis J., Ma X., Salehi A., Rorsman P., Eliasson L. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic b-cells. Pflug. Arch. 2002;444:43–51. doi: 10.1007/s00424-002-0781-5. [DOI] [PubMed] [Google Scholar]
- 33.Gaisano H.Y. Recent new insights into the role of snare and associated proteins in insulin granule exocytosis. Diabetesobes. Metab. 2017;19:115–123. doi: 10.1111/dom.13001. [DOI] [PubMed] [Google Scholar]
- 34.Porte D., Jr., Pupo A.A. Insulin responses to glucose: Evidence for a two pool system in man. J. Clin. Investig. 1969;48:2309–2319. doi: 10.1172/JCI106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doliba N.M., Qin W., Najafi H., Liu C., Buettger C.W., Sotiris J., Collins H.W., Li C., Stanley C.A., Wilson D.F., et al. Glucokinase activation repairs defective bioenergetics of islets of langerhans isolated from type 2 diabetics. Am. J. Physiol. Endocrinol. Metab. 2012;302:E87–E102. doi: 10.1152/ajpendo.00218.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J., Yan Z., Li Z., Qian X., Lu S., Dong M., Zhou Q., Yan N. Structure of the voltage-gated calcium channel cav1.1 at 3.6 a resolution. Nature. 2016;537:191–196. doi: 10.1038/nature19321. [DOI] [PubMed] [Google Scholar]
- 37.Catterall W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flucher B.E., Obermair G.J., Tuluc P., Schredelseker J., Kern G., Grabner M. The role of auxiliary dihydropyridine receptor subunits in muscle. J. Muscle Res. Cell Motil. 2005;26:1–6. doi: 10.1007/s10974-005-9000-2. [DOI] [PubMed] [Google Scholar]
- 39.Obermair G.J., Tuluc P., Flucher B.E. Auxiliary Ca(2+) channel subunits: Lessons learned from muscle. Curr. Opin. Pharm. 2008;8:311–318. doi: 10.1016/j.coph.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Dolphin A.C., Lee A. Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 2020;21:213–229. doi: 10.1038/s41583-020-0278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J., Yan Z., Li Z., Yan C., Lu S., Dong M., Yan N. Structure of the voltage-gated calcium channel cav1.1 complex. Science. 2015;350:aad2395. doi: 10.1126/science.aad2395. [DOI] [PubMed] [Google Scholar]
- 42.Catterall W.A. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Findeisen F., Campiglio M., Jo H., Abderemane-Ali F., Rumpf C.H., Pope L., Rossen N.D., Flucher B.E., De Grado W.F., Minor D.L., Jr. Stapled voltage-gated calcium channel (cav) alpha-interaction domain (aid) peptides act as selective protein-protein interaction inhibitors of cav function. ACS Chem. Neurosci. 2017;8:1313–1326. doi: 10.1021/acschemneuro.6b00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuluc P., Yarov-Yarovoy V., Benedetti B., Flucher B.E. Molecular interactions in the voltage sensor controlling gating properties of cav calcium channels. Structure. 2016;24:261–271. doi: 10.1016/j.str.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coste de Bagneaux P., Campiglio M., Benedetti B., Tuluc P., Flucher B.E. Role of putative voltage-sensor countercharge d4 in regulating gating properties of cav1.2 and cav1.3 calcium channels. Channels. 2018;12:249–261. doi: 10.1080/19336950.2018.1482183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez-Quintero M.L., El Ghaleb Y., Tuluc P., Campiglio M., Liedl K.R., Flucher B.E. Structural determinants of voltage-gating properties in calcium channels. eLife. 2021;10:e64087. doi: 10.7554/eLife.64087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pantazis A., Savalli N., Sigg D., Neely A., Olcese R. Functional heterogeneity of the four voltage sensors of a human l-type calcium channel. Proc. Natl. Acad. Sci. USA. 2014;111:18381–18386. doi: 10.1073/pnas.1411127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beyl S., Hohaus A., Andranovits S., Timin E., Hering S. Upward movement of is4 and iiis4 is a rate-limiting stage in cav1.2 activation. Pflug. Arch. 2016;468:1895–1907. doi: 10.1007/s00424-016-1895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savalli N., Pantazis A., Sigg D., Weiss J.N., Neely A., Olcese R. The alpha2delta-1 subunit remodels cav1.2 voltage sensors and allows Ca2+ influx at physiological membrane potentials. J. Gen. Physiol. 2016;148:147–159. doi: 10.1085/jgp.201611586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuluc P., Molenda N., Schlick B., Obermair G.J., Flucher B.E., Jurkat-Rott K. A cav1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters ec coupling in developing skeletal muscle. Biophys. J. 2009;96:35–44. doi: 10.1016/j.bpj.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuluc P., Flucher B.E. Divergent biophysical properties, gating mechanisms, and possible functions of the two skeletal muscle ca(v)1.1 calcium channel splice variants. J. Muscle Res. Cell Motil. 2011;32:249–256. doi: 10.1007/s10974-011-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuluc P., Benedetti B., Coste de Bagneaux P., Grabner M., Flucher B.E. Two distinct voltage-sensing domains control voltage sensitivity and kinetics of current activation in cav1.1 calcium channels. J. Gen. Physiol. 2016;147:437–449. doi: 10.1085/jgp.201611568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flucher B.E., Tuluc P. How and why are calcium currents curtailed in the skeletal muscle voltage-gated calcium channels? J. Physiol. 2017;595:1451–1463. doi: 10.1113/JP273423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pangrsic T., Singer J.H., Koschak A. Voltage-gated calcium channels: Key players in sensory coding in the retina and the inner ear. Physiol Rev. 2018;98:2063–2096. doi: 10.1152/physrev.00030.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jing X., Li D.Q., Olofsson C.S., Salehi A., Surve V.V., Caballero J., Ivarsson R., Lundquist I., Pereverzev A., Schneider T., et al. Cav2.3 calcium channels control second-phase insulin release. J. Clin. Investig. 2005;115:146–154. doi: 10.1172/JCI200522518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mastrolia V., Flucher S.M., Obermair G.J., Drach M., Hofer H., Renstrom E., Schwartz A., Striessnig J., Flucher B.E., Tuluc P. Loss of alpha2delta-1 calcium channel subunit function increases the susceptibility for diabetes. Diabetes. 2017;66:897–907. doi: 10.2337/db16-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulla V., Renstrom E., Feil R., Feil S., Franklin I., Gjinovci A., Jing X.J., Laux D., Lundquist I., Magnuson M.A., et al. Impaired insulin secretion and glucose tolerance in beta cell-selective ca(v)1.2 Ca2+ channel null mice. Embo. J. 2003;22:3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vignali S., Leiss V., Karl R., Hofmann F., Welling A. Characterization of voltage-dependent sodium and calcium channels in mouse pancreatic a- and b-cells. J. Physiol. 2006;572:691–706. doi: 10.1113/jphysiol.2005.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang S.N., Berggren P.O. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr. Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- 60.Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- 61.Bers D.M., Perez-Reyes E. Ca channels in cardiac myocytes: Structure and function in ca influx and intracellular ca release. Cardiovasc. Res. 1999;42:339–360. doi: 10.1016/S0008-6363(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 62.Cohen S.M., Ma H., Kuchibhotla K.V., Watson B.O., Buzsaki G., Froemke R.C., Tsien R.W. Excitation-transcription coupling in parvalbumin-positive interneurons employs a novel cam kinase-dependent pathway distinct from excitatory neurons. Neuron. 2016;90:292–307. doi: 10.1016/j.neuron.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hockerman G.H., Peterson B.Z., Johnson B.D., Catterall W.A. Molecular determinants of drug binding and action on l-type calcium channels. Annu. Rev. Pharm. Toxicol. 1997;37:361–396. doi: 10.1146/annurev.pharmtox.37.1.361. [DOI] [PubMed] [Google Scholar]
- 64.Tikhonov D.B., Zhorov B.S. Structural model for dihydropyridine binding to l-type calcium channels. J. Biol. Chem. 2009;284:19006–19017. doi: 10.1074/jbc.M109.011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Catterall W.A., Swanson T.M. Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol. Pharm. 2015;88:141–150. doi: 10.1124/mol.114.097659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hess P., Lansman J.B., Tsien R.W. Different modes of ca channel gating behaviour favoured by dihydropyridine ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 67.Lee K.S., Tsien R.W. Mechanism of calcium channel blockade by verapamil, d600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983;302:790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- 68.Bean B.P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: Modulation by dihydropyridine drugs. Circ. Res. 1986;59:229–235. doi: 10.1161/01.RES.59.2.229. [DOI] [PubMed] [Google Scholar]
- 69.Hamilton S.L., Yatani A., Brush K., Schwartz A., Brown A.M. A comparison between the binding and electrophysiological effects of dihydropyridines on cardiac membranes. Mol. Pharm. 1987;31:221–231. [PubMed] [Google Scholar]
- 70.Berjukow S., Hering S. Voltage-dependent acceleration of ca(v)1.2 channel current decay by (+)- and (-)-isradipine. Br. J. Pharm. 2001;133:959–966. doi: 10.1038/sj.bjp.0704181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shabbir W., Beyl S., Timin E.N., Schellmann D., Erker T., Hohaus A., Hockerman G.H., Hering S. Interaction of diltiazem with an intracellularly accessible binding site on ca(v)1.2. Br. J. Pharm. 2011;162:1074–1082. doi: 10.1111/j.1476-5381.2010.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devis G., Somers G., Van Obberghen E., Malaisse W.J. Calcium antagonists and islet function. I. Inhibition of insulin release by verapamil. Diabetes. 1975;24:247–251. doi: 10.2337/diabetes.24.6.247. [DOI] [PubMed] [Google Scholar]
- 73.Levine M., Boyer E.W., Pozner C.N., Geib A.J., Thomsen T., Mick N., Thomas S.H. Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Crit. Care Med. 2007;35:2071–2075. doi: 10.1097/01.CCM.0000278916.04569.23. [DOI] [PubMed] [Google Scholar]
- 74.Ovalle F., Grimes T., Xu G., Patel A.J., Grayson T.B., Thielen L.A., Li P., Shalev A. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat. Med. 2018;24:1108–1112. doi: 10.1038/s41591-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinnegger-Brauns M.J., Hetzenauer A., Huber I.G., Renstrom E., Wietzorrek G., Berjukov S., Cavalli M., Walter D., Koschak A., Waldschutz R., et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by l-type Ca2+ channels. J. Clin. Investig. 2004;113:1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Namkung Y., Skrypnyk N., Jeong M.J., Lee T., Lee M.S., Kim H.L., Chin H., Suh P.G., Kim S.S., Shin H.S. Requirement for the l-type Ca(2+) channel alpha(1d) subunit in postnatal pancreatic beta cell generation. J. Clin. Investig. 2001;108:1015–1022. doi: 10.1172/JCI200113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Platzer J., Engel J., Schrott-Fischer A., Stephan K., Bova S., Chen H., Zheng H., Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class d l-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/S0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 78.Lipscombe D., Andrade A., Allen S.E. Alternative splicing: Functional diversity among voltage-gated calcium channels and behavioral consequences. Biochim. Biophys. Acta. 2013;1828:1522–1529. doi: 10.1016/j.bbamem.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bourinet E., Zamponi G.W., Stea A., Soong T.W., Lewis B.A., Jones L.P., Yue D.T., Snutch T.P. The alpha 1e calcium channel exhibits permeation properties similar to low-voltage-activated calcium channels. J. Neurosci. 1996;16:4983–4993. doi: 10.1523/JNEUROSCI.16-16-04983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones L.P., Wei S.K., Yue D.T. Mechanism of auxiliary subunit modulation of neuronal alpha1e calcium channels. J. Gen. Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matthes J., Yildirim L., Wietzorrek G., Reimer D., Striessnig J., Herzig S. Disturbed atrio-ventricular conduction and normal contractile function in isolated hearts from cav1.3-knockout mice. Naunyn-Schmiedeberg’s Arch. Pharm. 2004;369:554–562. doi: 10.1007/s00210-004-0940-7. [DOI] [PubMed] [Google Scholar]
- 82.Singh A., Gebhart M., Fritsch R., Sinnegger-Brauns M.J., Poggiani C., Hoda J.C., Engel J., Romanin C., Striessnig J., Koschak A. Modulation of voltage- and Ca2+-dependent gating of cav1.3 l-type calcium channels by alternative splicing of a c-terminal regulatory domain. J. Biol. Chem. 2008;283:20733–20744. doi: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koschak A., Reimer D., Huber I., Grabner M., Glossmann H., Engel J., Striessnig J. Alpha 1d (cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J. Biol. Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 84.Mangoni M.E., Couette B., Bourinet E., Platzer J., Reimer D., Striessnig J., Nargeot J. Functional role of l-type cav1.3 ca2+ channels in cardiac pacemaker activity. Proc. Natl. Acad. Sci. USA. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marcantoni A., Vandael D.H., Mahapatra S., Carabelli V., Sinnegger-Brauns M.J., Striessnig J., Carbone E. Loss of cav1.3 channels reveals the critical role of l-type and bk channel coupling in pacemaking mouse adrenal chromaffin cells. J. Neurosci. 2010;30:491–504. doi: 10.1523/JNEUROSCI.4961-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vandael D.H., Zuccotti A., Striessnig J., Carbone E. Ca(v)1.3-driven sk channel activation regulates pacemaking and spike frequency adaptation in mouse chromaffin cells. J. Neurosci. 2012;32:16345–16359. doi: 10.1523/JNEUROSCI.3715-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang G., Shi Y., Yu J., Li Y., Yu L., Welling A., Hofmann F., Striessnig J., Juntti-Berggren L., Berggren P.O., et al. Cav1.2 and cav1.3 channel hyperactivation in mouse islet beta cells exposed to type 1 diabetic serum. Cell. Mol. Life Sci. 2015;72:1197–1207. doi: 10.1007/s00018-014-1737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engel J., Michna M., Platzer J., Striessnig J. Calcium channels in mouse hair cells: Function, properties and pharmacology. Adv. Oto-Rhino-Laryngol. 2002;59:35–41. doi: 10.1159/000059243. [DOI] [PubMed] [Google Scholar]
- 89.Zampini V., Johnson S.L., Franz C., Lawrence N.D., Munkner S., Engel J., Knipper M., Magistretti J., Masetto S., Marcotti W. Elementary properties of cav1.3 Ca(2+) channels expressed in mouse cochlear inner hair cells. J. Physiol. 2010;588:187–199. doi: 10.1113/jphysiol.2009.181917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee A., Wang S., Williams B., Hagen J., Scheetz T.E., Haeseleer F. Characterization of cav1.4 complexes (alpha11.4, beta2, and alpha2delta4) in hek293t cells and in the retina. J. Biol. Chem. 2015;290:1505–1521. doi: 10.1074/jbc.M114.607465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sudhof T.C. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gandasi N.R., Yin P., Riz M., Chibalina M.V., Cortese G., Lund P.E., Matveev V., Rorsman P., Sherman A., Pedersen M.G., et al. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J. Clin. Investig. 2017;127:2353–2364. doi: 10.1172/JCI88491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bokvist K., Eliasson L., Ammala C., Renstrom E., Rorsman P. Co-localization of l-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic b-cells. EMBO J. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Splawski I., Timothy K.W., Sharpe L.M., Decher N., Kumar P., Bloise R., Napolitano C., Schwartz P.J., Joseph R.M., Condouris K., et al. Ca(v)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 95.Flanagan S.E., Vairo F., Johnson M.B., Caswell R., Laver T.W., Lango Allen H., Hussain K., Ellard S. A cacna1d mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatric Diabetes. 2017;18:320–323. doi: 10.1111/pedi.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Mingo Alemany M.C., Mifsud Grau L., Moreno Macian F., Ferrer Lorente B., Leon Carinena S. A de novo cacna1d missense mutation in a patient with congenital hyperinsulinism, primary hyperaldosteronism and hypotonia. Channels (Austin) 2020;14:175–180. doi: 10.1080/19336950.2020.1761171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reinbothe T.M., Alkayyali S., Ahlqvist E., Tuomi T., Isomaa B., Lyssenko V., Renstrom E. The human l-type calcium channel ca(v)1.3 regulates insulin release and polymorphisms in cacna1d associate with type 2 diabetes. Diabetologia. 2013;56:340–349. doi: 10.1007/s00125-012-2758-z. [DOI] [PubMed] [Google Scholar]
- 98.Calorio C., Gavello D., Guarina L., Salio C., Sassoe-Pognetto M., Riganti C., Bianchi F.T., Hofer N.T., Tuluc P., Obermair G.J., et al. Impaired chromaffin cell excitability and exocytosis in autistic timothy syndrome ts2-neo mouse rescued by l-type calcium channel blockers. J. Physiol. 2019;597:1705–1733. doi: 10.1113/JP277487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barbara J.G., Takeda K. Voltage-dependent currents and modulation of calcium channel expression in zona fasciculata cells from rat adrenal gland. J. Physiol. 1995;488:609–622. doi: 10.1113/jphysiol.1995.sp020994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Enyeart J.J., Enyeart J.A. Ca2+ and k+ channels of normal human adrenal zona fasciculata cells: Properties and modulation by acth and angii. J. Gen. Physiol. 2013;142:137–155. doi: 10.1085/jgp.201310964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Enyeart J.J., Enyeart J.A. Adrenal fasciculata cells express t-type and rapidly and slowly activating l-type Ca2+ channels that regulate cortisol secretion. Am. J. Physiol. 2015;308:C899–C918. doi: 10.1152/ajpcell.00002.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soong T.W., Stea A., Hodson C.D., Dubel S.J., Vincent S.R., Snutch T.P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 103.Stephens G.J., Page K.M., Burley J.R., Berrow N.S., Dolphin A.C. Functional expression of rat brain cloned alpha1e calcium channels in cos-7 cells. Pflug. Arch. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- 104.Qin N., Olcese R., Stefani E., Birnbaumer L. Modulation of human neuronal alpha 1e-type calcium channel by alpha 2 delta-subunit. Am. J. Physiol. 1998;274:C1324–C1331. doi: 10.1152/ajpcell.1998.274.5.C1324. [DOI] [PubMed] [Google Scholar]
- 105.Bourinet E., Stotz S.C., Spaetgens R.L., Dayanithi G., Lemos J., Nargeot J., Zamponi G.W. Interaction of snx482 with domains iii and iv inhibits activation gating of alpha(1e) (ca(v)2.3) calcium channels. Biophys. J. 2001;81:79–88. doi: 10.1016/S0006-3495(01)75681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holmkvist J., Tojjar D., Almgren P., Lyssenko V., Lindgren C.M., Isomaa B., Tuomi T., Berglund G., Renstrom E., Groop L. Polymorphisms in the gene encoding the voltage-dependent Ca(2+) channel ca (v)2.3 (cacna1e) are associated with type 2 diabetes and impaired insulin secretion. Diabetologia. 2007;50:2467–2475. doi: 10.1007/s00125-007-0846-2. [DOI] [PubMed] [Google Scholar]
- 107.Trombetta M., Bonetti S., Boselli M., Turrini F., Malerba G., Trabetti E., Pignatti P., Bonora E., Bonadonna R.C. Cacna1e variants affect beta cell function in patients with newly diagnosed type 2 diabetes. The verona newly diagnosed type 2 diabetes study (vnds) 3. PLoS ONE. 2012;7:e32755. doi: 10.1371/journal.pone.0032755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saegusa H., Kurihara T., Zong S., Minowa O., Kazuno A., Han W., Matsuda Y., Yamanaka H., Osanai M., Noda T., et al. Altered pain responses in mice lacking alpha 1e subunit of the voltage-dependent Ca2+ channel. Proc. Natl. Acad. Sci. USA. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsuda Y., Saegusa H., Zong S., Noda T., Tanabe T. Mice lacking ca(v)2.3 (alpha1e) calcium channel exhibit hyperglycemia. Biochem. Biophys. Res. Commun. 2001;289:791–795. doi: 10.1006/bbrc.2001.6051. [DOI] [PubMed] [Google Scholar]
- 110.Pereverzev A., Mikhna M., Vajna R., Gissel C., Henry M., Weiergraber M., Hescheler J., Smyth N., Schneider T. Disturbances in glucose-tolerance, insulin-release, and stress-induced hyperglycemia upon disruption of the ca(v)2.3 (alpha 1e) subunit of voltage-gated Ca(2+) channels. Mol. Endocrinol. 2002;16:884–895. doi: 10.1210/mend.16.4.0801. [DOI] [PubMed] [Google Scholar]
- 111.Pereverzev A., Salehi A., Mikhna M., Renstrom E., Hescheler J., Weiergraber M., Smyth N., Schneider T. The ablation of the ca(v)2.3/e-type voltage-gated Ca2+ channel causes a mild phenotype despite an altered glucose induced glucagon response in isolated islets of langerhans. Eur. J. Pharm. 2005;511:65–72. doi: 10.1016/j.ejphar.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Q., Bengtsson M., Partridge C., Salehi A., Braun M., Cox R., Eliasson L., Johnson P.R., Renstrom E., Schneider T., et al. R-type Ca(2+)-channel-evoked cicr regulates glucose-induced somatostatin secretion. Nat. Cell Biol. 2007;9:453–460. doi: 10.1038/ncb1563. [DOI] [PubMed] [Google Scholar]
- 113.Braun M., Ramracheya R., Amisten S., Bengtsson M., Moritoh Y., Zhang Q., Johnson P.R., Rorsman P. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia. 2009;52:1566–1578. doi: 10.1007/s00125-009-1382-z. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Q., Ramracheya R., Lahmann C., Tarasov A., Bengtsson M., Braha O., Braun M., Brereton M., Collins S., Galvanovskis J., et al. Role of katp channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell. Metab. 2013;18:871–882. doi: 10.1016/j.cmet.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Briant L.J.B., Reinbothe T.M., Spiliotis I., Miranda C., Rodriguez B., Rorsman P. Delta-cells and beta-cells are electrically coupled and regulate alpha-cell activity via somatostatin. J. Physiol. 2018;596:197–215. doi: 10.1113/JP274581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davalli A.M., Biancardi E., Pollo A., Socci C., Pontiroli A.E., Pozza G., Clementi F., Sher E., Carbone E. Dihydropyridine-sensitive and -insensitive voltage-operated calcium channels participate in the control of glucose-induced insulin release from human pancreatic beta cells. J. Endocrinol. 1996;150:195–203. doi: 10.1677/joe.0.1500195. [DOI] [PubMed] [Google Scholar]
- 117.Pietrobon D., Brennan K.C. Genetic mouse models of migraine. J. Headache Pain. 2019;20:79. doi: 10.1186/s10194-019-1029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bichet D., Cornet V., Geib S., Carlier E., Volsen S., Hoshi T., Mori Y., De Waard M. The i-ii loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron. 2000;25:177–190. doi: 10.1016/S0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 119.Cornet V., Bichet D., Sandoz G., Marty I., Brocard J., Bourinet E., Mori Y., Villaz M., De Waard M. Multiple determinants in voltage-dependent p/q calcium channels control their retention in the endoplasmic reticulum. Eur. J. Neurosci. 2002;16:883–895. doi: 10.1046/j.1460-9568.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 120.Altier C., Garcia-Caballero A., Simms B., You H., Chen L., Walcher J., Tedford H.W., Hermosilla T., Zamponi G.W. The cavbeta subunit prevents rfp2-mediated ubiquitination and proteasomal degradation of l-type channels. Nat. Neurosci. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 121.Fang K., Colecraft H.M. Mechanism of auxiliary beta-subunit-mediated membrane targeting of l-type (Ca(v)1.2) channels. J. Physiol. 2011;589:4437–4455. doi: 10.1113/jphysiol.2011.214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olcese R., Qin N., Schneider T., Neely A., Wei X., Stefani E., Birnbaumer L. The amino terminus of a calcium channel beta subunit sets rates of channel inactivation independently of the subunit’s effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 123.Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T.P., Campbell K.P. Calcium channel beta-subunit binds to a conserved motif in the i-ii cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 124.Gregg R.G., Messing A., Strube C., Beurg M., Moss R., Behan M., Sukhareva M., Haynes S., Powell J.A., Coronado R., et al. Absence of the beta subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the alpha 1 subunit and eliminates excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schredelseker J., Di Biase V., Obermair G.J., Felder E.T., Flucher B.E., Franzini-Armstrong C., Grabner M. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc. Natl. Acad. Sci. USA. 2005;102:17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weissgerber P., Held B., Bloch W., Kaestner L., Chien K.R., Fleischmann B.K., Lipp P., Flockerzi V., Freichel M. Reduced cardiac l-type Ca2+ current in ca(v)beta2−/− embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ. Res. 2006;99:749–757. doi: 10.1161/01.RES.0000243978.15182.c1. [DOI] [PubMed] [Google Scholar]
- 127.Berrow N.S., Campbell V., Fitzgerald E.M., Brickley K., Dolphin A.C. Antisense depletion of beta-subunits modulates the biophysical and pharmacological properties of neuronal calcium channels. J. Physiol. 1995;482:481–491. doi: 10.1113/jphysiol.1995.sp020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ball S.L., Powers P.A., Shin H.S., Morgans C.W., Peachey N.S., Gregg R.G. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Investig. Ophthalmol. Vis. Sci. 2002;43:1595–1603. [PubMed] [Google Scholar]
- 129.Neef J., Gehrt A., Bulankina A.V., Meyer A.C., Riedel D., Gregg R.G., Strenzke N., Moser T. The Ca2+ channel subunit beta2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing. J. Neurosci. 2009;29:10730–10740. doi: 10.1523/JNEUROSCI.1577-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wei S.K., Colecraft H.M., DeMaria C.D., Peterson B.Z., Zhang R., Kohout T.A., Rogers T.B., Yue D.T. Ca(2+) channel modulation by recombinant auxiliary beta subunits expressed in young adult heart cells. Circ. Res. 2000;86:175–184. doi: 10.1161/01.RES.86.2.175. [DOI] [PubMed] [Google Scholar]
- 131.Meissner M., Weissgerber P., Londono J.E., Prenen J., Link S., Ruppenthal S., Molkentin J.D., Lipp P., Nilius B., Freichel M., et al. Moderate calcium channel dysfunction in adult mice with inducible cardiomyocyte-specific excision of the cacnb2 gene. J. Biol. Chem. 2011;286:15875–15882. doi: 10.1074/jbc.M111.227819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buraei Z., Yang J. Structure and function of the beta subunit of voltage-gated Ca2+ channels. Biochim. Biophys. Acta. 2013;1828:1530–1540. doi: 10.1016/j.bbamem.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hullin R., Khan I.F., Wirtz S., Mohacsi P., Varadi G., Schwartz A., Herzig S. Cardiac l-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J. Biol. Chem. 2003;278:21623–21630. doi: 10.1074/jbc.M211164200. [DOI] [PubMed] [Google Scholar]
- 134.Herzig S., Khan I.F., Grundemann D., Matthes J., Ludwig A., Michels G., Hoppe U.C., Chaudhuri D., Schwartz A., Yue D.T., et al. Mechanism of ca(v)1.2 channel modulation by the amino terminus of cardiac beta2-subunits. FASEB J. 2007;21:1527–1538. doi: 10.1096/fj.06-7377com. [DOI] [PubMed] [Google Scholar]
- 135.Hullin R., Matthes J., von Vietinghoff S., Bodi I., Rubio M., D’Souza K., Friedrich Khan I., Rottlander D., Hoppe U.C., Mohacsi P., et al. Increased expression of the auxiliary beta(2)-subunit of ventricular l-type Ca(2)+ channels leads to single-channel activity characteristic of heart failure. PLoS ONE. 2007;2:e292. doi: 10.1371/journal.pone.0000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jangsangthong W., Kuzmenkina E., Bohnke A.K., Herzig S. Single-channel monitoring of reversible l-type Ca(2+) channel ca(v)alpha(1)-ca(v)beta subunit interaction. Biophys. J. 2011;101:2661–2670. doi: 10.1016/j.bpj.2011.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Despang P., Salamon S., Breitenkamp A.F., Kuzmenkina E., Herzig S., Matthes J. Autism-associated mutations in the cavbeta2 calcium-channel subunit increase Ba(2+)-currents and lead to differential modulation by the rgk-protein gem. Neurobiol. Dis. 2020;136:104721. doi: 10.1016/j.nbd.2019.104721. [DOI] [PubMed] [Google Scholar]
- 138.Buraei Z., Yang J. The ss subunit of voltage-gated Ca2+ channels. Physiol. Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Berggren P.O., Yang S.N., Murakami M., Efanov A.M., Uhles S., Kohler M., Moede T., Fernstrom A., Appelskog I.B., Aspinwall C.A., et al. Removal of Ca2+ channel beta3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 2004;119:273–284. doi: 10.1016/j.cell.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 140.Murakami M., Fleischmann B., De Felipe C., Freichel M., Trost C., Ludwig A., Wissenbach U., Schwegler H., Hofmann F., Hescheler J., et al. Pain perception in mice lacking the beta3 subunit of voltage-activated calcium channels. J. Biol. Chem. 2002;277:40342–40351. doi: 10.1074/jbc.M203425200. [DOI] [PubMed] [Google Scholar]
- 141.Namkung Y., Smith S.M., Lee S.B., Skrypnyk N.V., Kim H.L., Chin H., Scheller R.H., Tsien R.W., Shin H.S. Targeted disruption of the Ca2+ channel beta3 subunit reduces n- and l-type Ca2+ channel activity and alters the voltage-dependent activation of p/q-type Ca2+ channels in neurons. Proc. Natl. Acad. Sci. USA. 1998;95:12010–12015. doi: 10.1073/pnas.95.20.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee K., Kim J., Kohler M., Yu J., Shi Y., Yang S.N., Ryu S.H., Berggren P.O. Blocking Ca(2+) channel beta3 subunit reverses diabetes. Cell Rep. 2018;24:922–934. doi: 10.1016/j.celrep.2018.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Belkacemi A., Hui X., Wardas B., Laschke M.W., Wissenbach U., Menger M.D., Lipp P., Beck A., Flockerzi V. Ip3 receptor-dependent cytoplasmic Ca(2+) signals are tightly controlled by cavbeta3. Cell Rep. 2018;22:1339–1349. doi: 10.1016/j.celrep.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 144.He L.L., Zhang Y., Chen Y.H., Yamada Y., Yang J. Functional modularity of the beta-subunit of voltage-gated Ca2+ channels. Biophys. J. 2007;93:834–845. doi: 10.1529/biophysj.106.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takahashi S.X., Mittman S., Colecraft H.M. Distinctive modulatory effects of five human auxiliary beta2 subunit splice variants on l-type calcium channel gating. Biophys. J. 2003;84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miranda-Laferte E., Ewers D., Guzman R.E., Jordan N., Schmidt S., Hidalgo P. The n-terminal domain tethers the voltage-gated calcium channel beta2e-subunit to the plasma membrane via electrostatic and hydrophobic interactions. J. Biol. Chem. 2014;289:10387–10398. doi: 10.1074/jbc.M113.507244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kazim A.S., Storm P., Zhang E., Renstrom E. Palmitoylation of Ca(2+) channel subunit cavbeta2a induces pancreatic beta-cell toxicity via Ca(2+) overload. Biochem. Biophys. Res. Commun. 2017;491:740–746. doi: 10.1016/j.bbrc.2017.07.117. [DOI] [PubMed] [Google Scholar]
- 148.Hida Y., Ohtsuka T. Cast and elks proteins: Structural and functional determinants of the presynaptic active zone. J. Biochem. 2010;148:131–137. doi: 10.1093/jb/mvq065. [DOI] [PubMed] [Google Scholar]
- 149.Billings S.E., Clarke G.L., Nishimune H. Elks1 and Ca(2+) channel subunit beta4 interact and colocalize at cerebellar synapses. Neuroreport. 2012;23:49–54. doi: 10.1097/WNR.0b013e32834e7deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kiyonaka S., Nakajima H., Takada Y., Hida Y., Yoshioka T., Hagiwara A., Kitajima I., Mori Y., Ohtsuka T. Physical and functional interaction of the active zone protein cast/erc2 and the beta-subunit of the voltage-dependent Ca(2+) channel. J. Biochem. 2012;152:149–159. doi: 10.1093/jb/mvs054. [DOI] [PubMed] [Google Scholar]
- 151.Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of langerhans. Diabetes. 1988;37:616–621. doi: 10.2337/diab.37.5.616. [DOI] [PubMed] [Google Scholar]
- 152.Ohara-Imaizumi M., Aoyagi K., Yamauchi H., Yoshida M., Mori M.X., Hida Y., Tran H.N., Ohkura M., Abe M., Akimoto Y., et al. Elks/voltage-dependent Ca(2+) channel-beta subunit module regulates polarized Ca(2+) influx in pancreatic beta cells. Cell Rep. 2019;26:1213–1226. doi: 10.1016/j.celrep.2018.12.106. [DOI] [PubMed] [Google Scholar]
- 153.Colecraft H.M. Designer genetically encoded voltage-dependent calcium channel inhibitors inspired by rgk gtpases. J. Physiol. 2020;598:1683–1693. doi: 10.1113/JP276544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Finlin B.S., Crump S.M., Satin J., Andres D.A. Regulation of voltage-gated calcium channel activity by the rem and rad gtpases. Proc. Natl. Acad. Sci. USA. 2003;100:14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Finlin B.S., Mosley A.L., Crump S.M., Correll R.N., Ozcan S., Satin J., Andres D.A. Regulation of l-type Ca2+ channel activity and insulin secretion by the rem2 gtpase. J. Biol. Chem. 2005;280:41864–41871. doi: 10.1074/jbc.M414261200. [DOI] [PubMed] [Google Scholar]
- 156.Chen H., Puhl H.L., 3rd, Niu S.L., Mitchell D.C., Ikeda S.R. Expression of rem2, an rgk family small gtpase, reduces n-type calcium current without affecting channel surface density. J. Neurosci. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beguin P., Nagashima K., Gonoi T., Shibasaki T., Takahashi K., Kashima Y., Ozaki N., Geering K., Iwanaga T., Seino S. Regulation of Ca2+ channel expression at the cell surface by the small g-protein kir/gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 158.Gunton J.E., Sisavanh M., Stokes R.A., Satin J., Satin L.S., Zhang M., Liu S.M., Cai W., Cheng K., Cooney G.J., et al. Mice deficient in gem gtpase show abnormal glucose homeostasis due to defects in beta-cell calcium handling. PLoS ONE. 2012;7:e39462. doi: 10.1371/journal.pone.0039462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bannister R.A., Colecraft H.M., Beam K.G. Rem inhibits skeletal muscle ec coupling by reducing the number of functional l-type Ca2+ channels. Biophys. J. 2008;94:2631–2638. doi: 10.1529/biophysj.107.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang T., Puckerin A., Colecraft H.M. Distinct rgk gtpases differentially use alpha1- and auxiliary beta-binding-dependent mechanisms to inhibit cav1.2/cav2.2 channels. PLoS ONE. 2012;7:e37079. doi: 10.1371/journal.pone.0037079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ghiretti A.E., Paradis S. Molecular mechanisms of activity-dependent changes in dendritic morphology: Role of rgk proteins. Trends. Neurosci. 2014;37:399–407. doi: 10.1016/j.tins.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Arikkath J., Campbell K.P. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003;13:298–307. doi: 10.1016/S0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 163.Dolphin A.C. Calcium channel auxiliary alpha(2)delta and beta subunits: Trafficking and one step beyond. Nat. Rev. Neurosci. 2012;13:542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 164.Davies A., Kadurin I., Alvarez-Laviada A., Douglas L., Nieto-Rostro M., Bauer C.S., Pratt W.S., Dolphin A.C. The alpha2delta subunits of voltage-gated calcium channels form gpi-anchored proteins, a posttranslational modification essential for function. Proc. Natl. Acad. Sci. USA. 2010;107:1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gurnett C.A., Felix R., Campbell K.P. Extracellular interaction of the voltage-dependent Ca2+ channel alpha2delta and alpha1 subunits. J. Biol. Chem. 1997;272:18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- 166.Felix R., Gurnett C.A., De Waard M., Campbell K.P. Dissection of functional domains of the voltage-dependent ca2+ channel alpha2delta subunit. J. Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Canti C., Dolphin A.C. Cavbeta subunit-mediated up-regulation of cav2.2 currents triggered by d2 dopamine receptor activation. Neuropharmacology. 2003;45:814–827. doi: 10.1016/S0028-3908(03)00277-6. [DOI] [PubMed] [Google Scholar]
- 168.Gurnett C.A., De Waard M., Campbell K.P. Dual function of the voltage-dependent Ca2+ channel alpha 2 delta subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/S0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 169.Bourdin B., Briot J., Tetreault M.P., Sauve R., Parent L. Negatively charged residues in the first extracellular loop of the l-type cav1.2 channel anchor the interaction with the cavalpha2delta1 auxiliary subunit. J. Biol. Chem. 2017;292:17236–17249. doi: 10.1074/jbc.M117.806893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Briot J., Mailhot O., Bourdin B., Tetreault M.P., Najmanovich R., Parent L. A three-way inter-molecular network accounts for the cavalpha2delta1-induced functional modulation of the pore-forming cav1.2 subunit. J. Biol. Chem. 2018;293:7176–7188. doi: 10.1074/jbc.RA118.001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Luo Z.D., Calcutt N.A., Higuera E.S., Valder C.R., Song Y.H., Svensson C.I., Myers R.R. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharm. Exp. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 172.Dolphin A.C. The alpha(2)delta subunits of voltage-gated calcium channels. Biochim. Biophys. Acta. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 173.Obermair G.J., Kugler G., Baumgartner S., Tuluc P., Grabner M., Flucher B.E. The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1s or excitation-contraction coupling. J. Biol. Chem. 2005;280:2229–2237. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- 174.Tuluc P., Kern G., Obermair G.J., Flucher B.E. Computer modeling of sirna knockdown effects indicates an essential role of the Ca2+ channel alpha2delta-1 subunit in cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 2007;104:11091–11096. doi: 10.1073/pnas.0700577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Polster A., Perni S., Bichraoui H., Beam K.G. Stac adaptor proteins regulate trafficking and function of muscle and neuronal l-type Ca2+ channels. Proc. Natl. Acad. Sci. USA. 2015;112:602–606. doi: 10.1073/pnas.1423113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Campiglio M., Coste de Bagneaux P., Ortner N.J., Tuluc P., Van Petegem F., Flucher B.E. Stac proteins associate to the iq domain of cav1.2 and inhibit calcium-dependent inactivation. Proc. Natl. Acad. Sci. USA. 2018;115:1376–1381. doi: 10.1073/pnas.1715997115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Horstick E.J., Linsley J.W., Dowling J.J., Hauser M.A., McDonald K.K., Ashley-Koch A., Saint-Amant L., Satish A., Cui W.W., Zhou W., et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in native american myopathy. Nat. Commun. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nelson B.R., Wu F., Liu Y., Anderson D.M., McAnally J., Lin W., Cannon S.C., Bassel-Duby R., Olson E.N. Skeletal muscle-specific t-tubule protein stac3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA. 2013;110:11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Reinholt B.M., Ge X., Cong X., Gerrard D.E., Jiang H. Stac3 is a novel regulator of skeletal muscle development in mice. PLoS ONE. 2013;8:e62760. doi: 10.1371/journal.pone.0062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang Y., Cong X., Wang A., Jiang H. Identification of the stac3 gene as a skeletal muscle-specifically expressed gene and a novel regulator of satellite cell differentiation in cattle. J. Anim. Sci. 2014;92:3284–3290. doi: 10.2527/jas.2014-7656. [DOI] [PubMed] [Google Scholar]
- 181.Polster A., Nelson B.R., Olson E.N., Beam K.G. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation. Proc. Natl. Acad. Sci. USA. 2016;113:10986–10991. doi: 10.1073/pnas.1612441113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wong King Yuen S.M., Campiglio M., Tung C.C., Flucher B.E., Van Petegem F. Structural insights into binding of stac proteins to voltage-gated calcium channels. Proc. Natl. Acad. Sci. USA. 2017;114:E9520–E9528. doi: 10.1073/pnas.1708852114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Flucher B.E., Campiglio M. Stac proteins: The missing link in skeletal muscle ec coupling and new regulators of calcium channel function. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:1101–1110. doi: 10.1016/j.bbamcr.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 184.Dai S., Klugbauer N., Zong X., Seisenberger C., Hofmann F. The role of subunit composition on prepulse facilitation of the cardiac l-type calcium channel. FEBS Lett. 1999;442:70–74. doi: 10.1016/S0014-5793(98)01632-9. [DOI] [PubMed] [Google Scholar]
- 185.Platano D., Qin N., Noceti F., Birnbaumer L., Stefani E., Olcese R. Expression of the alpha(2)delta subunit interferes with prepulse facilitation in cardiac l-type calcium channels. Biophys. J. 2000;78:2959–2972. doi: 10.1016/S0006-3495(00)76835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fuller-Bicer G.A., Varadi G., Koch S.E., Ishii M., Bodi I., Kadeer N., Muth J.N., Mikala G., Petrashevskaya N.N., Jordan M.A., et al. Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am. J. Physiol. 2009;297:H117–H124. doi: 10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brodbeck J., Davies A., Courtney J.M., Meir A., Balaguero N., Canti C., Moss F.J., Page K.M., Pratt W.S., Hunt S.P., et al. The ducky mutation in cacna2d2 results in altered purkinje cell morphology and is associated with the expression of a truncated alpha 2 delta-2 protein with abnormal function. J. Biol. Chem. 2002;277:7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- 188.Wakamori M., Mikala G., Mori Y. Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary n-type Ca2+ channel current in xenopus oocytes. J. Physiol. 1999;517:659–672. doi: 10.1111/j.1469-7793.1999.0659s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dolphin A.C. Voltage-gated calcium channels and their auxiliary subunits: Physiology and pathophysiology and pharmacology. J. Physiol. 2016;594:5369–5390. doi: 10.1113/JP272262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Catterall W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 191.Mauvais-Jarvis F. Role of sex steroids in beta cell function, growth, and survival. Trends Endocrinol. Metab. Tem. 2016;27:844–855. doi: 10.1016/j.tem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou Y., Park S.Y., Su J., Bailey K., Ottosson-Laakso E., Shcherbina L., Oskolkov N., Zhang E., Thevenin T., Fadista J., et al. Tcf7l2 is a master regulator of insulin production and processing. Hum. Mol. Genet. 2014;23:6419–6431. doi: 10.1093/hmg/ddu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ye Y., Barghouth M., Luan C., Kazim A., Zhou Y., Eliasson L., Zhang E., Hansson O., Thevenin T., Renstrom E. The tcf7l2-dependent high-voltage activated calcium channel subunit alpha2delta-1 controls calcium signaling in rodent pancreatic beta-cells. Mol. Cell. Endocrinol. 2020;502:110673. doi: 10.1016/j.mce.2019.110673. [DOI] [PubMed] [Google Scholar]
- 194.Vergult S., Dheedene A., Meurs A., Faes F., Isidor B., Janssens S., Gautier A., Le Caignec C., Menten B. Genomic aberrations of the cacna2d1 gene in three patients with epilepsy and intellectual disability. Eur. J. Hum. Genet. Ejhg. 2015;23:628–632. doi: 10.1038/ejhg.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Templin C., Ghadri J.R., Rougier J.S., Baumer A., Kaplan V., Albesa M., Sticht H., Rauch A., Puleo C., Hu D., et al. Identification of a novel loss-of-function calcium channel gene mutation in short qt syndrome (sqts6) Eur. Heart J. 2011;32:1077–1088. doi: 10.1093/eurheartj/ehr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Burashnikov E., Pfeiffer R., Barajas-Martinez H., Delpon E., Hu D., Desai M., Borggrefe M., Haissaguerre M., Kanter R., Pollevick G.D., et al. Mutations in the cardiac l-type calcium channel associated with inherited j-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bourdin B., Shakeri B., Tetreault M., Sauve R., Lesage S., Parent L. Functional characterization of cav alpha2delta mutations associated with sudden cardiac death. J. Biol. Chem. 2015;290:2854–2869. doi: 10.1074/jbc.M114.597930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gee N.S., Brown J.P., Dissanayake V.U., Offord J., Thurlow R., Woodruff G.N. The novel anticonvulsant drug, gabapentin (neurontin), binds to the alpha2delta subunit of a calcium channel. J. Biol. Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 199.DeToledo J.C., Toledo C., DeCerce J., Ramsay R.E. Changes in body weight with chronic, high-dose gabapentin therapy. Drug Monit. 1997;19:394–396. doi: 10.1097/00007691-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 200.Hoppe C., Rademacher M., Hoffmann J.M., Schmidt D., Elger C.E. Bodyweight gain under pregabalin therapy in epilepsy: Mitigation by counseling patients? Seizure. 2008;17:327–332. doi: 10.1016/j.seizure.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 201.Dolphin A.C. Voltage-gated calcium channel alpha 2delta subunits: An assessment of proposed novel roles. F1000Res. 2018;7:1830. doi: 10.12688/f1000research.16104.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Geisler S., Schopf C.L., Obermair G.J. Emerging evidence for specific neuronal functions of auxiliary calcium channel alpha(2)delta subunits. Gen. Physiol. Biophys. 2015;34:105–118. doi: 10.4149/gpb_2014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tran-Van-Minh A., Dolphin A.C. The alpha2delta ligand gabapentin inhibits the rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J. Neurosci. 2010;30:12856–12867. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bauer C.S., Nieto-Rostro M., Rahman W., Tran-Van-Minh A., Ferron L., Douglas L., Kadurin I., Sri Ranjan Y., Fernandez-Alacid L., Millar N.S., et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J. Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hendrich J., Van Minh A.T., Heblich F., Nieto-Rostro M., Watschinger K., Striessnig J., Wratten J., Davies A., Dolphin A.C. Pharmacological disruption of calcium channel trafficking by the α2β ligand gabapentin. Proc. Natl. Acad. Sci. USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Todd R.D., McDavid S.M., Brindley R.L., Jewell M.L., Currie K.P. Gabapentin inhibits catecholamine release from adrenal chromaffin cells. Anesthesiology. 2012;116:1013–1024. doi: 10.1097/ALN.0b013e31825153ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Davies A., Hendrich J., Van Minh A.T., Wratten J., Douglas L., Dolphin A.C. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 208.Micheva K.D., Taylor C.P., Smith S.J. Pregabalin reduces the release of synaptic vesicles from cultured hippocampal neurons. Mol. Pharm. 2006;70:467–476. doi: 10.1124/mol.106.023309. [DOI] [PubMed] [Google Scholar]
- 209.Cordeira J.W., Felsted J.A., Teillon S., Daftary S., Panessiti M., Wirth J., Sena-Esteves M., Rios M. Hypothalamic dysfunction of the thrombospondin receptor alpha2delta-1 underlies the overeating and obesity triggered by brain-derived neurotrophic factor deficiency. J. Neurosci. 2014;34:554–565. doi: 10.1523/JNEUROSCI.1572-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Felsted J.A., Meng A., Ameroso D., Rios M. Sex-specific effects of alpha2delta-1 in the ventromedial hypothalamus of female mice controlling glucose and lipid balance. Endocrinology. 2020;161:bqaa06. doi: 10.1210/endocr/bqaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Felsted J.A., Chien C.H., Wang D., Panessiti M., Ameroso D., Greenberg A., Feng G., Kong D., Rios M. Alpha2delta-1 in sf1(+) neurons of the ventromedial hypothalamus is an essential regulator of glucose and lipid homeostasis. Cell Rep. 2017;21:2737–2747. doi: 10.1016/j.celrep.2017.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kurshan P.T., Oztan A., Schwarz T.L. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat. Neurosci. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ablinger C., Geisler S.M., Stanika R.I., Klein C.T., Obermair G.J. Neuronal alpha2delta proteins and brain disorders. Pflug. Arch. 2020;472:845–863. doi: 10.1007/s00424-020-02420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen J., Li L., Chen S.R., Chen H., Xie J.D., Sirrieh R.E., MacLean D.M., Zhang Y., Zhou M.H., Jayaraman V., et al. The alpha2delta-1-nmda receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018;22:2307–2321. doi: 10.1016/j.celrep.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Geisler S., Schopf C.L., Stanika R., Kalb M., Campiglio M., Repetto D., Traxler L., Missler M., Obermair G.J. Presynaptic alpha2delta-2 calcium channel subunits regulate postsynaptic gabaa receptor abundance and axonal wiring. J. Neurosci. 2019;39:2581–2605. doi: 10.1523/JNEUROSCI.2234-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Eroglu C., Allen N.J., Susman M.W., O’Rourke N.A., Park C.Y., Ozkan E., Chakraborty C., Mulinyawe S.B., Annis D.S., Huberman A.D., et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory cns synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang F.X., Gadotti V.M., Souza I.A., Chen L., Zamponi G.W. Bk potassium channels suppress cavalpha2delta subunit function to reduce inflammatory and neuropathic pain. Cell Rep. 2018;22:1956–1964. doi: 10.1016/j.celrep.2018.01.073. [DOI] [PubMed] [Google Scholar]
- 218.Xie L., Kang Y., Liang T., Dolai S., Xie H., Parsaud L., Lopez J.A., Lam P.P., James D.E., Sugita S., et al. Rala gtpase tethers insulin granules to l- and r-type calcium channels through binding alpha(2) delta-1 subunit. Traffic. 2013;14:428–439. doi: 10.1111/tra.12047. [DOI] [PubMed] [Google Scholar]
- 219.Sharp A.H., Campbell K.P. Characterization of the 1,4-dihydropyridine receptor using subunit-specific polyclonal antibodies. Evidence for a 32,000-da subunit. J. Biol. Chem. 1989;264:2816–2825. doi: 10.1016/S0021-9258(19)81686-1. [DOI] [PubMed] [Google Scholar]
- 220.Chen R.S., Deng T.C., Garcia T., Sellers Z.M., Best P.M. Calcium channel gamma subunits: A functionally diverse protein family. Cell Biochem. Biophys. 2007;47:178–186. doi: 10.1007/s12013-007-0002-0. [DOI] [PubMed] [Google Scholar]
- 221.Andronache Z., Ursu D., Lehnert S., Freichel M., Flockerzi V., Melzer W. The auxiliary subunit gamma 1 of the skeletal muscle l-type Ca2+ channel is an endogenous Ca2+ antagonist. Proc. Natl. Acad. Sci. USA. 2007;104:17885–17890. doi: 10.1073/pnas.0704340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Freise D., Held B., Wissenbach U., Pfeifer A., Trost C., Himmerkus N., Schweig U., Freichel M., Biel M., Hofmann F., et al. Absence of the gamma subunit of the skeletal muscle dihydropyridine receptor increases l-type Ca2+ currents and alters channel inactivation properties. J. Biol. Chem. 2000;275:14476–14481. doi: 10.1074/jbc.275.19.14476. [DOI] [PubMed] [Google Scholar]
- 223.Ursu D., Schuhmeier R.P., Freichel M., Flockerzi V., Melzer W. Altered inactivation of Ca2+ current and Ca2+ release in mouse muscle fibers deficient in the dhp receptor gamma1 subunit. J. Gen. Physiol. 2004;124:605–618. doi: 10.1085/jgp.200409168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Singer D., Biel M., Lotan I., Flockerzi V., Hofmann F., Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 225.Wei X.Y., Perez-Reyes E., Lacerda A.E., Schuster G., Brown A.M., Birnbaumer L. Heterologous regulation of the cardiac Ca2+ channel alpha 1 subunit by skeletal muscle beta and gamma subunits. Implications for the structure of cardiac l-type Ca2+ channels. J. Biol. Chem. 1991;266:21943–21947. doi: 10.1016/S0021-9258(18)54728-1. [DOI] [PubMed] [Google Scholar]
- 226.Klugbauer N., Dai S., Specht V., Lacinova L., Marais E., Bohn G., Hofmann F. A family of gamma-like calcium channel subunits. FEBS Lett. 2000;470:189–197. doi: 10.1016/S0014-5793(00)01306-5. [DOI] [PubMed] [Google Scholar]
- 227.Sipos I., Pika-Hartlaub U., Hofmann F., Flucher B.E., Melzer W. Effects of the dihydropyridine receptor subunits gamma and alpha2delta on the kinetics of heterologously expressed l-type Ca2+ channels. Pflug. Arch. 2000;439:691–699. doi: 10.1007/s004249900201. [DOI] [PubMed] [Google Scholar]
- 228.Rousset M., Cens T., Restituito S., Barrere C., Black J.L., 3rd, McEnery M.W., Charnet P. Functional roles of gamma2, gamma3 and gamma4, three new Ca2+ channel subunits, in p/q-type Ca2+ channel expressed in xenopus oocytes. J. Physiol. 2001;532:583–593. doi: 10.1111/j.1469-7793.2001.0583e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang L., Katchman A., Morrow J.P., Doshi D., Marx S.O. Cardiac l-type calcium channel (cav1.2) associates with gamma subunits. FASEB J. 2011;25:928–936. doi: 10.1096/fj.10-172353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Luan C., Ye Y., Singh T., Barghouth M., Eliasson L., Artner I., Zhang E., Renstrom E. The calcium channel subunit gamma-4 is regulated by mafa and necessary for pancreatic beta-cell specification. Commun. Biol. 2019;2:106. doi: 10.1038/s42003-019-0351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]