Introduction
Plasma cell leukemia (PCL) is a rare and aggressive variant of multiple myeloma (MM) that could either develop de-novo (called primary PCL- pPCL) or as leukemic transformation of underlying MM (called secondary PCL- sPCL). Induction chemotherapy with an alkylator-based combination has shown modest responses in PCL with a median overall survival (OS) of less than a year1. In MM, proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs) followed by consolidation with an autologous hematopoietic stem cell transplantation (auto-HCT) has significantly improved outcomes. A similar benefit in treating pPCL with PIs and IMIDs in pre and post auto-HCT setting needs validation 2–4.
To assess progress in pPCL management, we performed a retrospective analysis of adult patients (≥18yrs, n=23) that received auto-HCT at MD Anderson Cancer Center between 2000–2016. pPCL was defined as the presence of absolute plasma cell count ≥ 2×109/l and/or ≥ 20% of peripheral white blood cell (WBC) count. Response to induction therapy was based on the International Myeloma Working Group (IMWG) criteria 5. Patients were deemed to have high-risk disease if conventional cytogenetics in at least 2 metaphases showed t (4;14), t (14;16), t (14;20), −17/del(17p), −13/del(13q), hypodiploidy (<45 chromosomes excluding −Y), or chromosome 1q amplification or 1p deletion. Furthermore, they were also considered high-risk if fluorescence in situ hybridization (FISH) detected t (4;14), t (14;16), t (14;20) or del 17p13, amplification 1q or del 1p at the time of diagnosis. Primary outcomes evaluated were progression-free survival (PFS) and overall survival (OS). Best response post auto-HCT and the impact of maintenance therapy were the secondary outcomes of interest. Kaplan-Meier product limit estimates were used to calculate the probabilities of OS and PFS. Statistical analyses were performed using Statistica software with a significance level of 0.05.
Results
Demographics and disease characteristics are summarized in Table 1. The median dose of CD34+ cells infused was 4.0 × 106/kg (Range 2.5–12.9). There were no graft failures post-transplant with a median time to neutrophil and platelet engraftment of 11 days (range 10–14 days) and 13 days (range 8–23 days) respectively.
Table 1:
Patient and Disease Characteristics
| Total | |
|---|---|
|
| |
| Number of Patients | 23 |
|
| |
| Median age at transplant (Range) | 56.5 years (42–71) |
|
| |
| Sex | |
| Male | 15 (65.2%) |
| Female | 8 (34.7%) |
|
| |
| Light Chain | |
| Kappa | 14 (60.8%) |
| Lambda | 9 (39.2%) |
|
| |
| Cytogenetic/FISH Group | |
| High risk | 16 (69.5%) |
| Standard risk | 7 (30.4%) |
|
| |
| International Staging System | |
| Stage 1 | 1(4.3%) |
| Stage 2 | 2 (8.6%) |
| Stage 3 | 14 (60.8%) |
| Unknown | 6 (26%) |
|
| |
| Disease status pre-transplant | |
| CR/sCR | 3 (13%) |
| nCR | 3 (13%) |
| VGPR | 4 (17.3%) |
| PR | 5 (21.7%) |
| Stable disease/Minimal | 3 (13%) |
| Progression/Relapse | 5 (21.7%) |
Abbreviations: FISH- Fluorescence in situ hybridization, CR- Complete Response, sCR- stringent Complete Response, nCR- near Complete Response, VGPR- Very Good Partial Response, PR- Partial Response
Induction, Conditioning and Maintenance Therapy:
All patients (n=23) received PI or IMiDs, either alone or in combination with steroids prior to auto-HCT. Four (17.3%) and 2 (11.7%) patients needed second and third-line therapies pre-transplant. Response to induction or latest salvage therapy was as follows: complete response (CR)/stringent CR (sCR) in 3 (13%), near CR (nCR) in 3 (13%), very good partial response (VGPR) in 4 (17%), partial response (PR) in 5 (22%), stable disease (SD) in 3 (13%) and progressive disease (PD) in 5 (22%) patients. Five (22%) received PI ± steroids, two (8%) IMiD ± steroids, seven (30%) triplets (PI ± IMiD ± Steroids) and 9 (39%) received PI or IMID in combination with chemotherapy as frontline induction therapy. Chemotherapy based regimens included VDT-PACE (bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide and etoposide referred as) or mCBAD (cyclophosphamide, bortezomib, doxorubicin, dexamethasone) or bortezomib, thalidomide/lenalidomide, cyclophosphamide ± steroids. Transplant conditioning was done with melphalan 200 mg/m2 in 19 (82%), combination of gemcitabine, busulfan and melphalan in 2 (8%), combination of melphalan, cyclophosphamide, and topotecan in 1 (4%), and melphalan with lenalidomide in 1 (4%) patient. Twelve (52%) patients received post auto-HCT maintenance therapy as a posttransplant consolidation strategy (Table 2). Median duration from the time of auto-HCT to the start of maintenance was 4.0 months (range 0.8–18.5). (Table 2).
Table 2:
Maintenance Drug(s) Use Post-Transplant
| Total (n-23) | |
|---|---|
| Maintenance (n) | |
| Proteasome Inhibitor (PI) | 1 |
| Immunomodulatory Drugs (IMiD) | 5 |
| PI + IMiD | 6 |
| None | 11 |
Post Transplantation Outcomes
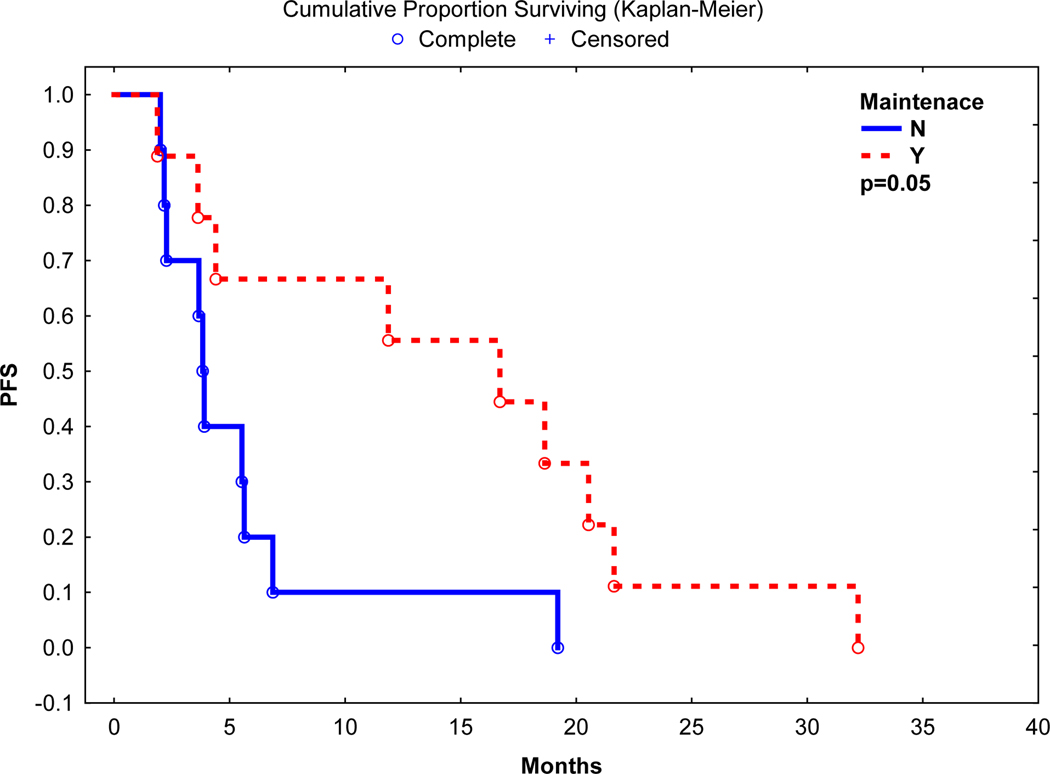
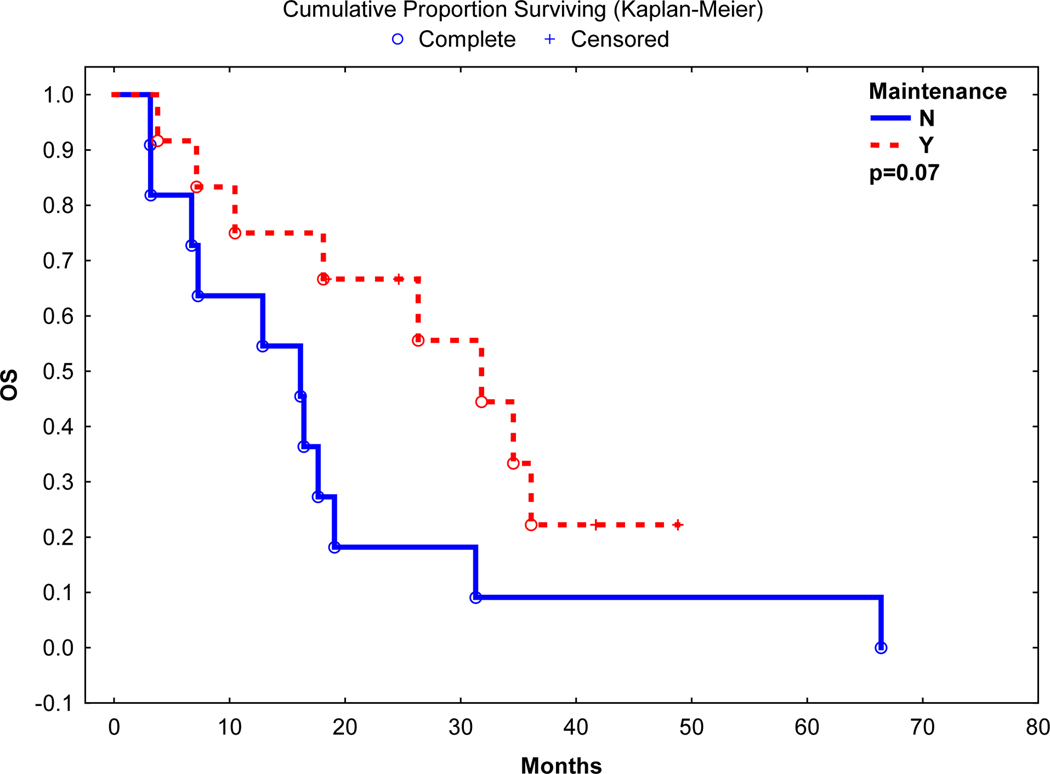
Best post auto-HCT response was CR in 6 (26%), nCR in 1 (4%), VGPR in 4 (17%) and PR in 6 (26%) with an overall response rate of 74%. After a median follow up of 18.1 months, the median progression-free survival (PFS) and overall survival (OS) was 5.5 and 18.1 months, respectively. Nineteen (95%) patients died of relapse, and 1 (5%) due to infectious complication. On univariate analyses, use of post-transplant maintenance was associated with a longer PFS (16.9 vs. 3.9 months, p=0.05) and a trend towards a longer OS (31.8 vs. 16.1 months, p=0.07) (Figure 1 and 2). Median PFS in patients with high-risk chromosomal abnormalities was 3.9 months vs. 19.2 months in patients without high-risk chromosomal abnormalities (p=0.01). However, there was no significant difference in OS between patients with or without high-risk chromosomal abnormalities (17.6 vs. 31.8 months, p=0.21).
Figure 1:
Progression Free Survival post Autologous Transplant
Median Progression Free Survival: 16.9 vs 3.9 months
Figure 2:
Overall Survival post Autologous Transplant
Median Overall Survival 31.8 vs 16.1 months
Our results suggest that auto-HCT for pPCL, particularly in combination with post transplant maintenance therapy, is an effective treatment and may prolong disease remission and survival. Outcomes for patients receiving PIs or IMiDs pre-transplant are superior to historical controls that received alkylator-based chemotherapy.
pPCL is marked by early relapse and mortality, mainly due to resistance to existing therapy 2. Historically, approximately 1 out of 4 pPCL patients died within a month of diagnosis, with a median OS of 7 months 3, 6. A prior study, which evaluated the efficacy of induction chemotherapy using conventional cytotoxic agents (vincristine, melphalan, cyclophosphamide, prednisone (VMCP) and adriamycin, or vincristine, adriamycin, and dexamethasone (VAD), or intermediate doses of melphalan) reported a an overall response rate (ORR) of 29% 7. However, the use of PIs and IMiDs in the last 15 years has been associated with an improved response rate of >70% 4, 6, 8–11. Consistent with these recent studies, the ORR in our study was 74%, again highlighting the impact of PIs and IMiDs in the induction therapy for pPCL.
High proliferation rates and adverse disease biology are the main drivers of poor outcomes in pPCL. To prevent rapid relapses, initial gains made with induction therapy need subsequent transplantation and maintenance strategies9. Addressing the question of an appropriate post-induction strategy, a large registry study showed superior outcomes with upfront auto-HCT over allo-HCT (3-year OS; 64% vs 39%)3. Although relapse rates were lower with allo-HCT, high treatment related mortality (TRM) (41% vs 5%) offset the benefits of allo-HCT compared with auto-HCT. Based on these observations, auto-HCT seems to be the preferred approach. The median PFS and OS in our study were 5.5 and 18 months, respectively, from the time of auto-HCT, with post-transplant relapse (95%) being the dominant cause of death. This warrants approaches to reduce relapse rate, perhaps with aggressive post-auto-HCT strategies. These approaches include either tandem auto-HCT, or auto followed by a reduced intensity allo-grafting. To address this question, a recent French study in a landmark analysis from the time of second transplant showed a superior OS (median not reached) for tandem-auto (n=7) vs 28.6 months with auto + allo (n=17) approach9. Alternatively, use of post auto-HCT consolidation and maintenance is another feasible option12. Nooka et al have shown promising outcome with post-transplant combination therapy in high-risk myeloma 13. Jakubowiak et al also demonstrated the potential benefit of post-transplant consolidation with a combination of carfilzomib, lenalidomide and dexamethasone14. Based on our retrospective experience, we showed better PFS (16.9 vs 3.9 months, p 0.05) and a trend towards improved OS (31.8 vs 16.1 months, p 0.07) in patients who received post-transplant maintenance. With the advent of newer PIs and monoclonal antibodies, we may see further improvement in PFS by using these newer approaches.
Our report is one of the larger single center experience for pPCL patients receiving auto-HCT with novel induction drugs. Despite encouraging findings, there are limitations to our study. We only included pPCL patients that received auto-HCT. Due to the nature of referral practice, we are unable to comment on patients who were not referred for transplant, hence our findings could not be generalized for non-transplant recipients. Use of maintenance drugs showed significant improvement in PFS in this study and warrants future study to address this question in a prospective design.
In conclusion, our study shows that auto-HCT is safe, feasible and can potentially improve the outcome for pPCL patients if given with PI or IMiD-based induction and post-transplant maintenance therapy. However, more work needs to be done to better understand the biology of this aggressive disease and to develop more effective treatment approaches.
References:
- 1.Ramsingh G, Mehan P, Luo J, Vij R, Morgensztern D. Primary plasma cell leukemia: a Surveillance, Epidemiology, and End Results database analysis between 1973 and 2004. Cancer 2009; 115(24): 5734–5739. doi: 10.1002/cncr.24700 [DOI] [PubMed] [Google Scholar]
- 2.Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, Van Wier SA et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia 2008; 22(5): 1044–1052. doi: 10.1038/leu.2008.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahindra A, Kalaycio ME, Vela-Ojeda J, Vesole DH, Zhang MJ, Li P et al. Hematopoietic cell transplantation for primary plasma cell leukemia: results from the Center for International Blood and Marrow Transplant Research. Leukemia 2012; 26(5): 1091–1097. doi: 10.1038/leu.2011.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musto P, Pagano L, Petrucci MT, Morabito F, Caravita T, Di Raimondo F et al. Primary plasma cell leukemia in the era of new drugs: has something changed? Crit Rev Oncol Hematol 2012; 82(2): 141–149. doi: 10.1016/j.critrevonc.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol 2014; 32(6): 587–600. doi: 10.1200/JCO.2013.48.7934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebovic D, Zhang L, Alsina M, Nishihori T, Shain KH, Sullivan D et al. Clinical outcomes of patients with plasma cell leukemia in the era of novel therapies and hematopoietic stem cell transplantation strategies: a single-institution experience. Clinical lymphoma, myeloma & leukemia 2011; 11(6): 507–511. doi: 10.1016/j.clml.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 7.Vela-Ojeda J, Garcia-Ruiz Esparza MA, Rosas-Cabral A, Padilla-Gonzalez Y, Garcia-Chavez J, Tripp-Villanueva F et al. Intermediate doses of melphalan and dexamethasone are better than vincristine, adriamycin, and dexamethasone (VAD) and polychemotherapy for the treatment of primary plasma cell leukemia. Ann Hematol 2002; 81(7): 362–367. doi: 10.1007/s00277-002-0480-5 [DOI] [PubMed] [Google Scholar]
- 8.D’Arena G, Valentini CG, Pietrantuono G, Guariglia R, Martorelli MC, Mansueto G et al. Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA Multiple Myeloma Working Party. Ann Oncol 2012; 23(6): 1499–1502. doi: 10.1093/annonc/mdr480 [DOI] [PubMed] [Google Scholar]
- 9.Royer B, Minvielle S, Diouf M, Roussel M, Karlin L, Hulin C et al. Bortezomib, Doxorubicin, Cyclophosphamide, Dexamethasone Induction Followed by Stem Cell Transplantation for Primary Plasma Cell Leukemia: A Prospective Phase II Study of the Intergroupe Francophone du Myelome. J Clin Oncol 2016; 34(18): 2125–2132. doi: 10.1200/JCO.2015.63.1929 [DOI] [PubMed] [Google Scholar]
- 10.Benson DM Jr., Smith MK. Effectiveness of lenalidomide (Revlimid) for the treatment of plasma cell leukemia. Leuk Lymphoma 2007; 48(7): 1423–1425. doi: 10.1080/10428190701361851 [DOI] [PubMed] [Google Scholar]
- 11.Guglielmelli T, Merlini R, Giugliano E, Saglio G. Lenalidomide, melphalan, and prednisone association is an effective salvage therapy in relapsed plasma cell leukaemia. J Oncol 2009; 2009: 867380. doi: 10.1155/2009/867380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366(19): 1770–1781. e-pub ahead of print 2012/05/11; doi: 10.1056/NEJMoa1114083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia 2014; 28(3): 690–693. e-pub ahead of print 2013/11/14; doi: 10.1038/leu.2013.335 [DOI] [PubMed] [Google Scholar]
- 14.Andrzej Jakubowiak KG, Jasielec Jagoda, Rosenbaum Cara, McDonnell Kathryn, Berdeja Jesus, Vij Ravi, Raje Noopur, Reece Donna, Dytfeld Dominik, Todd Zimmerman POST-TRANSPLANT CARFILZOMIB (KYPROLIS®), LENALIDOMIDE (REVLIMID®), AND DEXAMETHASONE (KRD) CONSOLIDATION IN NEWLY DIAGNOSED MULTIPLE MYELOMA (NDMM): EFFICACY AND TOLERABILITY OF EXTENDED TREATMENT. Hematologica 2015; 100: 312. [Google Scholar]