Abstract
[11C]UCB-J PET for synaptic vesicle glycoprotein 2 A (SV2A) has been proposed as a suitable marker for synaptic density in Alzheimer’s disease (AD). We compared [11C]UCB-J binding for synaptic density and [18F]FDG uptake for metabolism (correlated with neuronal activity) in 14 AD and 11 cognitively normal (CN) participants. We assessed both absolute and relative outcome measures in brain regions of interest, i.e., K1 or R1 for [11C]UCB-J perfusion, VT (volume of distribution) or DVR to cerebellum for [11C]UCB-J binding to SV2A; and Ki or KiR to cerebellum for [18F]FDG metabolism. [11C]UCB-J binding and [18F]FDG metabolism showed a similar magnitude of reduction in the medial temporal lobe of AD –compared to CN participants. However, the magnitude of reduction of [11C]UCB-J binding in neocortical regions was less than that observed with [18F]FDG metabolism. Inter-tracer correlations were also higher in the medial temporal regions between synaptic density and metabolism, with lower correlations in neocortical regions. [11C]UCB-J perfusion showed a similar pattern to [18F]FDG metabolism, with high inter-tracer regional correlations. In summary, we conducted the first in vivo PET imaging of synaptic density and metabolism in the same AD participants and reported a concordant reduction in medial temporal regions but a discordant reduction in neocortical regions.
Keywords: SV2A, PET, [18F]FDG, Synaptic Density, Alzheimer’s disease
Introduction
Positron emission tomography (PET) brain imaging with specific radiotracers has been used to evaluate biomarkers for Alzheimer’s disease (AD) under the current AT(N) research framework,1,2 including [11C]-PiB and F-18 labeled amyloid PET for β-amyloid,3,4 tau PET for neurofibrillary tangles,5,6 and 18F-fluorodeoxyglucose ([18F]FDG) for glucose metabolism (correlated with neuronal activity) and neurodegeneration.7 Structural MRI for gray matter volume has also been widely used as a biomarker of neurodegeneration in AD.1,2 Although the relationship between structural gray matter volume and [18F]FDG metabolism for functional neuronal activity has been well investigated in AD,8–10 direct in vivo PET imaging and quantification of synaptic density in comparison with neuronal activity has never been reported. PET imaging for quantification of synaptic density was recently developed as another potential biomarker for neurodegeneration in AD.11,12 Importantly, the ability to quantify synaptic density and neuronal activity in the same participants has the potential to provide a better understanding of AD pathophysiology. Here we conducted the first PET study with biomarkers for both synaptic density and glucose metabolism in the same AD participants. Because a discrepancy exists between regional brain atrophy and hypometabolism in AD,8–10 we hypothesized that the patterns of synaptic density loss and neuronal function in the susceptible regions of AD, including medial temporal lobe and posterior neocortical regions, would not be identical.
[18F]FDG brain PET is widely used for clinical differential diagnosis of dementia,13,14 and for tracking AD progression.15,16 [18F]FDG PET has also been used as a biomarker outcome measure for evaluation of treatment responses in many clinical trials of AD.17,18 Despite its frequent use as a surrogate marker of neuronal activity for neurodegeneration,7 [18F]FDG PET has its limitations. Because brain [18F]FDG uptake is often affected by sensory stimulation, medications, and blood glucose,19–22 the reproducibility of [18F]FDG PET quantification within and between participants can be affected by the difference of fasting status, current medications, and surrounding environmental stimulation of the participants.23–25 The appropriate preparation for the subjects before and during PET scanning is critical. Inadequate fasting could result in false positives in AD scans.21,22 These limitations in precision may ultimately hinder accurate assessment of disease progression or therapeutic effects in longitudinal clinical trials using [18F]FDG PET.
A potential imaging biomarker for synaptic density is the synaptic vesicle glycoprotein 2 A (SV2A), located in synaptic vesicles in presynaptic terminals and expressed in virtually all synapses.26,27 SV2A has a conserved expression pattern of about five copies per vesicle.28 [11C]UCB-J PET has been recently demonstrated as an excellent imaging agent for SV2A and has been applied in AD11,12 and other neuropsychiatric disorders.29–31 We have previously reported a low test-retest variability (3-9%) of [11C]UCB-J VT across all brain regions in the same human participants,32 and have also observed that [11C]UCB-J PET VT for synaptic density is stable in the presence of blood flow changes as with those accompanying visual stimulation.33 This excellent reproducibility and non-stimulation-dependent quantification of [11C]UCB-J PET could potentially be advantageous for use in longitudinal studies and clinical trials. Of note, most of the previous analyses for [11C]UCB-J PET studies including our first study in early AD11 were conducted using white matter (WM) in the centrum semiovale (CS) as the reference region.34–36 WM has the lowest total [11C]UCB-J binding with minimal SV2A specific binding and could therefore be a suitable reference region. However, the degree of [11C]UCB-J uptake in WM could be variable, especially in those elderly subjects with WM pathology. Also, larger inter-subject variability from the use of a WM reference region could be a potential disadvantage. Using the regional ratios of distribution volume (DVRs) with the cerebellum as an alternative reference region, our substantially larger follow-up study of AD demonstrated widespread statistically significant reductions of SV2A binding in broader neocortical regions in addition to the hippocampus and entorhinal cortex in AD compared to cognitive normal (CN) participants.12 These findings better reflect the pathologic findings of reduced cortical synaptic density in AD post mortem studies.37 Therefore, we will continue using regional DVRs to Cb as primary outcome measures of [11C]UCB-J binding.
In the present study, we conducted [11C]UCB-J dynamic PET scans with arterial input function for kinetic modeling and [18F]FDG PET in the same AD and CN participants. We computed multiple outcome measures from both [11C]UCB-J PET and [18F]FDG PET to compare absolute and relative measures for better differentiating AD from CN, both in terms of regional percentage differences (PD) and standardized effect sizes with Cohen’s d. Because absolute measures often have higher inter-subject variability, normalized measures could provide higher sensitivity for detecting group differences. We also explored the intra-regional associations between [11C]UCB-J binding for synaptic density and [18F]FDG metabolism in the same participants to better understand their relationships in AD pathophysiology. In addition, our previous exploratory K1 (indicating blood flow and tracer extraction) parametric imaging of [11C]UCB-J demonstrated broad regional differences in temporoparietal cortices of AD relative to CN11 that are similar to the known hypometabolic pattern of AD. Therefore, we also investigated whether K1 parametric imaging of [11C]UCB-J could serve as a surrogate for brain perfusion and provide similar information to [18F]FDG metabolism.
Materials and methods
Human participants
Fourteen participants with AD dementia or amnestic MCI due to AD and 11 CN participants enrolled in the study using the same methods as described previously11 with more comprehensive details provided in the supplemental material. All participants received a PET scan with [11C]Pittsburgh Compound B ([11C]PiB) first to determine the presence of brain amyloid-β (Aβ) accumulation as previously described.11 CN participants who were negative for amyloid, and AD dementia or amnestic MCI due to AD participants who were positive for amyloid were included in the study. The study was approved by the Yale University Human Investigation Committee and the Radioactive Drug Research Committee and was conducted in accordance with the World Medical Association Declaration of Helsinki. All participants provided written informed consent prior to participating in the study.
PET imaging experiments
[11C]UCB-J was synthesized according to previously described procedures.35 All participants received [11C]UCB-J and [18F]FDG dynamic PET measurement with the Siemens High Resolution Research Tomograph (HRRT; Siemens, Medical Solutions, Knoxville, TN, USA). The [11C]UCB-J PET was performed as described previously11 with details provided in the supplemental material. The [18F]FDG dynamic PET was also performed up to 90 min using the same acquisition protocol.
Quantitative analysis
For [11C]UCB-J, kinetic analysis was performed voxel-by-voxel using the one-tissue compartment model (1TC)32 and the metabolite-corrected arterial plasma curve to generate a parametric image of distribution volume (VT). VT is the tissue:plasma concentration ratio at equilibrium and serves as an index of SV2A density and is not dependent on blood flow. Parametric images of the delivery rate constant, K1, which is proportional to blood flow (K1 = flow × extraction fraction), as well as R1, the ratio of regional K1 values normalized to cerebellum (Cb) K1 (i.e., K1 ROI/K1 Cb) were also produced. R1 has lower inter-subject variability than K1 and could serve as an important outcome measure for flow. Regions of interest (ROIs) were applied to the parametric images using the combined transformations from AAL template to PET space according to previous methods.11 Additionally, distribution volume ratios (DVRs) for the ROIs using Cb as reference region were also calculated (i.e., VT,ROI/VT,Cb), derived directly from the 1TC parameters using arterial input function (AIF) as the gold standards, not from reference-region based analysis e.g. simplified reference tissue model 2 (SRTM2). We have previously demonstrated an excellent correlation between DVR values derived from 1-TC parameters with AIF and from SRTM2 (see supplemental materials from our recent paper).12 Therefore, the DVR findings in the current study can be extended to reference region methods.
For [18F]FDG, Patlak analysis38 was performed according to the methods described previously with modification, with t* set to 60 min and a 30-min scan duration was used to measure the influx rate constant Ki for each ROI. The net influx constant Ki is a commonly used outcome measure for cerebral glucose metabolism.38 A population-based input function (PBIF) was used for the analysis,39 which has been validated recently as a suitable alternative to the AIF. Briefly, the PBIF was generated from the averages of arterial plasma curves (in SUV units) from 40 participants (not included in this study). To scale the PBIF, venous plasma samples were acquired for each participant in this study. The PBIF was scaled by the ratio of the mean venous plasma SUV value to the mean PBIF between 40 and 60 minutes. The ratio of regional Ki values (KiR) for the ROIs using Cb as reference region were also calculated (i.e., KiR =Ki ROI/Ki Cb).
Gray matter tissue loss can affect PET measures. To minimize this, we masked the ROIs with a segmented MRI (gray matter masking)40 and performed full partial volume correction (PVC) using Muller-Gardner method (M-G).41,42 DVR, R1 and KiR after PVC were calculated using non-PVC Cb as reference region.
Statistical analysis
Statistical calculations were performed in Microsoft Excel 2016, IBM SPSS (version 26) and GraphPad PRISM (version 8). When comparing the group differences between tracers, the % difference of the means and Cohen’s d are listed as (PD:x%/d:x). Separate linear mixed models were used to compare each parameter of [11C]UCB-J (K1, R1, VT, DVR) or [18F]FDG (Ki, KiR) before or after PVC in 16 regions (within-subject factor) between AD and CN groups. A random intercept was included and the best-fitting variance-covariance structure was compound symmetry as determined by the Bayesian information criterion. Since the goal of this study is the comparison of [11C]UCB-J and [18F]FDG, we chose to assess the ability to detect group differences without including the effects of age, sex and education. Post-hoc comparisons utilized unpaired t-tests (2-tailed and P < 0.05 for significance). In addition, the Benjamini-Hochberg procedure was used to control the false discovery rate for multiple comparisons. Standardized effect sizes with Cohen’s d were also calculated for the binding parameters of both tracers in each ROI between AD and CN groups. Correlation between the binding parameters of [11C]UCB-J and [18F]FDG within each region was performed with Pearson correlation coefficients. The modified version of the Pearson–Filon statistical test,43 which accounts for non-independent data, was used to compare inter-tracer correlations between regions and the differences of correlations before and after PVC; significance was based on a two-tailed test, with P < 0.05.
Results
Participants characteristics
Fourteen individuals with amnestic MCI or dementia due to AD and 11 CN individuals participated in the study. Diagnostic groups were well balanced for age and education (Table 1). AD participants had clinical characteristics typical of amnestic MCI and mild dementia with MMSE = 23.9 ± 4.6 and CDR = 0.6 ± 0.2. All participants received one injection of [11C]UCB-J (604 ± 188 MBq and mass 1.60 ± 0.97 µg) and one injection of [18F]FDG (181 ± 6 MBqwith no significant difference in injection doses and masses between groups. The average time differences between the two scans were 6 ± 27 days for AD and 114 ± 136 days for CN participants, respectively. For [11C]UCB-J, the arterial input functions were successfully measured in all participants to measure K1, R1(to Cb), VT, and DVR (to Cb). For [18F]FDG, the PBIF was used to measure Ki and KiR (to Cb).
Table 1.
Demographic information and test results of included participants.
| Cognitively normal (CN) | Alzheimer’s disease (AD) | |
|---|---|---|
| Participants | 11 | 14 (mild AD:4, MCI: 10) |
| Sex (M/F) | 3/8 | 7/7 |
| Age (years) | 69.4 (9.0) (59–81) | 69.6 (5.7) (58–79) |
| Education (years) | 17.5 (2.5) (12–20) | 16.9(1.8) (13–19) |
| CDR-global | 0 (0) | 0.6 (0.2) (0.5–1) |
| CDR-sb | 0 (0) | 2.9 (1.5) (0.5–4.5) |
| MMSE | 29.0 (1.2) (27–30) | 23.9 (4.6) (14–29) |
| LMII | 13.1 (5.5) (5–19) | 2.4 (2.8) (0–8) |
| RAVLT | 11.1(3.0) (6–14) | 2.2 (2.6) (0–9) |
Data are n, or mean (SD) (range).
CDR-global: clinical dementia rating global score; CDR-sb: clinical dementia rating sum of boxes; MMSE: Mini-Mental State Examination; LMII: Logical Memory II score; RAVLT: Rey Auditory Verbal Learning Test delayed recall.
Regional synaptic loss and hypometabolism in AD
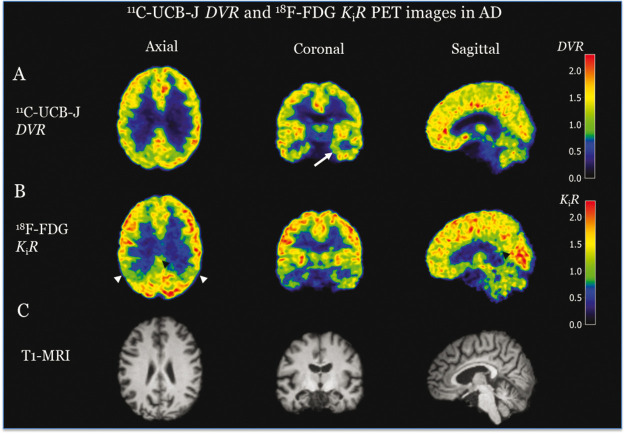
When comparing [11C]UCB-J binding and [18F]FDG metabolism, the regional pattern of lower [11C]UCB-J binding (VT, DVR) was different from that of hypometabolism (Ki, KiR) observed in AD participants. Typical AD images are shown in Figure 1. Our visual inspection of images revealed lower uptake of both [11C]UCB-J and [18F]FDG in the medial temporal lobe for the same AD participants, but we observed more pronounced reductions of [18F]FDG uptake in the lateral temporal lobe, the parietal lobe, and the posterior cingulate. Since there were no disease-specific effects in the cerebellum (Table S1), [11C]UCB-J VT and [18F]FDG Ki were normalized to cerebellum to yield DVR and KiR, respectively. We observed a similar pattern of lower [11C]UCB-J DVR and [18F]FDG KiR in AD participants with larger effect sizes (Table 2) when compared to the unnormalized values (Table S1). We found similar between-group differences and increased effect sizes in [11C]UCB-J DVR and [18F]FDG KiR in the medial temporal regions (Table 2). Significant between-group differences of [11C]UCB-J DVR were also present in the pulvinar with slightly larger effect sizes than [18F]FDG KiR (Table 2). With the improved effect sizes after cerebellum normalization, we were able to detect small between-group differences of [11C]UCB-J DVR in susceptible neocortical regions that were less than the PD of [18F]FDG KiR (Table 2). The regional relationships are plotted showing the percent differences in mean value (AD vs. CN) in Figure 2(c) and the Cohen’s d for differentiation between AD and CN in Figure 2(d). Comparing [11C]UCB-J DVR and [18F]FDG KiR demonstrated overall similar PD in medial temporal regions, but lower PD of [11C]UCB-J DVR in the neocortical regions. ROIs to the lower right of the identity line reflect greater difference/Cohen’s d for [11C]UCB-J, whereas those to the upper left indicate greater difference/Cohen’s d for [18F]FDG.
Figure 1.
Representative (A) [11C]UCB-J DVR and (B) [18F]FDG PET KiR images and (C) MRI images in AD. Evident lower [11C]UCB-J binding in the hippocampus of AD was noted (arrow denotes the left hippocampus). Evident hypometabolism was also noted in the hippocampus of AD. In addition, hypometabolism was also noted in lateral temporal and parietal cortices of AD (white arrows denote the parietal cortices) as well as posterior cingulate (black arrow heads).
Table 2.
Comparison of [11C]UCB-J DVR and [18F]FDG KiR in the ROIs of AD and CN.
|
[11C]UCB-J DVR |
[18F]FDG KiR |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | CN(n = 11)Mean (%SD) | AD (n = 14)Mean (%SD) | PD | P | Cohen’s d | CN (n = 11)Mean (%SD) | AD (n = 14)Mean (%SD) | PD | P | Cohen’s d | |||||
| Hippocampus | 1.01 | (7%) | 0.76 | (21%) | 24.8% | <0.0001*† | 1.93 | 0.91 | (10%) | 0.70 | (19%) | 23.4% | <0.0001*† | 1.89 | |
| Entorhinal | 1.16 | (8%) | 0.89 | (23%) | 23.2% | 0.0003*† | 1.62 | 0.80 | (15%) | 0.60 | (23%) | 24.4% | 0.001*† | 1.50 | |
| Amygdala | 1.38 | (9%) | 1.15 | (20%) | 17.1% | 0.003*† | 1.25 | 0.87 | (13%) | 0.69 | (20%) | 20.2% | 0.002*† | 1.39 | |
| Parahippocampus | 1.10 | (8%) | 0.94 | (14%) | 15.0% | 0.001*† | 1.44 | 0.88 | (10%) | 0.74 | (15%) | 15.4% | 0.003*† | 1.29 | |
| Precuneus | 1.45 | (8%) | 1.33 | (13%) | 8.3% | 0.044* | 0.82 | 1.66 | (9%) | 1.34 | (21%) | 19.2% | 0.002*† | 1.36 | |
| Post. Cingulate | 1.01 | (18%) | 0.90 | (25%) | 10.8% | 0.188 | 0.53 | 1.36 | (16%) | 1.05 | (21%) | 22.7% | 0.002*† | 1.39 | |
| Angular | 1.46 | (6%) | 1.33 | (13%) | 8.7% | 0.025*† | 0.91 | 1.59 | (8%) | 1.20 | (27%) | 24.6% | 0.001*† | 1.53 | |
| Parietal | 1.41 | (8%) | 1.29 | (13%) | 8.5% | 0.040*† | 0.84 | 1.55 | (11%) | 1.28 | (21%) | 17.4% | 0.005*† | 1.18 | |
| Temporal | 1.44 | (5%) | 1.33 | (9%) | 7.6% | 0.012*† | 1.04 | 1.33 | (8%) | 1.10 | (18%) | 16.8% | 0.002*† | 1.31 | |
| Ant. Cingulate | 1.34 | (9%) | 1.23 | (18%) | 8.6% | 0.106 | 0.64 | 1.16 | (11%) | 1.02 | (18%) | 12.2% | 0.033*† | 0.88 | |
| Caudate | 1.09 | (16%) | 0.96 | (14%) | 12.3% | 0.052 | 0.87 | 1.11 | (16%) | 0.93 | (16%) | 16.5% | 0.014*† | 1.12 | |
| Frontal | 1.35 | (7%) | 1.27 | (9%) | 5.4% | 0.084 | 0.71 | 1.54 | (8%) | 1.30 | (15%) | 15.2% | 0.001*† | 1.39 | |
| Occipital | 1.38 | (8%) | 1.33 | (10%) | 3.3% | 0.335 | 0.39 | 1.49 | (10%) | 1.33 | (19%) | 10.6% | 0.063 | 0.74 | |
| Pulvinar | 1.14 | (11%) | 0.93 | (15%) | 18.3% | 0.001*† | 1.55 | 1.14 | (14%) | 0.94 | (16%) | 17.3% | 0.005*† | 1.26 | |
| Putamen | 1.60 | (9%) | 1.58 | (8%) | 1.3% | 0.716 | 0.15 | 1.67 | (11%) | 1.54 | (11%) | 8.2% | 0.062 | 0.80 | |
| Thalamus | 1.03 | (10%) | 0.90 | (11%) | 12.4% | 0.005*† | 1.28 | 1.26 | (11%) | 1.13 | (12%) | 10.5% | 0.028*† | 0.95 | |
Data are mean (%SD/covariance) of ROI values normalized to cerebellum. P-values are for post hoc two-tailed, unpaired t-tests (uncorrected for multiplicity) performed after a linear mixed model analysis of [11C]UCB-J DVR and [18F]FDG KiR in multiple regions (within-subject factor) between CN and AD groups. PD is the percentage difference in the group means; 100(CN-AD)/CN.
*P < 0.05 prior to false discovery rate correction for multiple comparisons. †P < 0.05 after false discovery rate correction.
Please note the regions in this table as well as the supplement tables are partitioned in the same fashion, including medial temporal regions (Hippocampus, Entorhinal, Amygdala, Parahippocampus), susceptible neocortical regions (Precuneus, Post, Cingulate, Angular, Parietal, and Temporal), other brain regions (Ant. Cingulate, Caudate, Frontal, Occipital, Pulvinar, Putamen, Thalamus).
CN: cognitively normal; AD: Alzheimer’s disease.
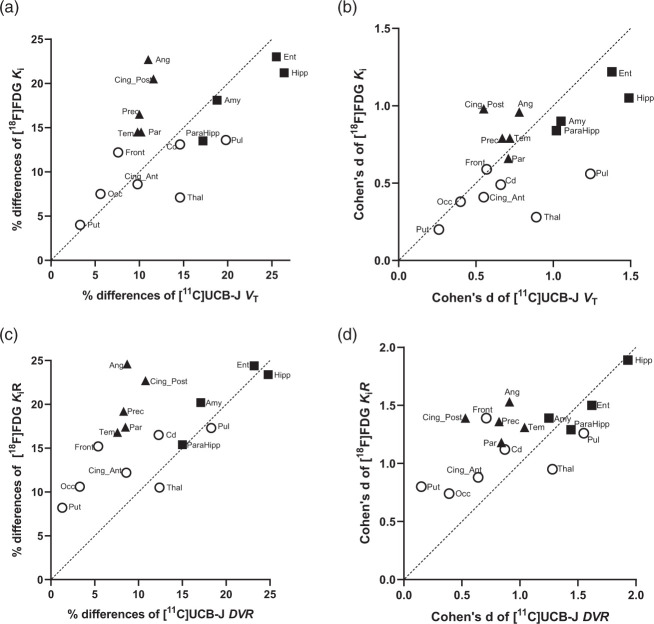
Figure 2.
Scatter plots of regional percentage differences of means between CN and AD (a, c) and Cohen’s d (b, d) for [11C]UCB-J (VT and DVR on x-axis) versus [18F]FDG (Ki and KiR on y-axis). (a) Comparing [11C]UCB-J VT and [18F]FDG Ki demonstrated distinct patterns, including slightly higher % differences of [11C]UCB-J VT in medial temporal regions (■ Hipp, Ent, Amy, and ParaHipp) and thalamic regions, but smaller % differences in susceptible neocortical regions (▲Ang, Cing_Post, Prec, Tem, Par). (b) There were larger Cohen’s d values for [11C]UCB-J VT vs. [18F]FDG Ki in medial temporal regions and thalamic regions (O Thal and Pul), but slightly lower Cohen’s d values in susceptible neocortical regions. (c) Comparing [11C]UCB-J DVR and [18F]FDG KiR demonstrated overall similar % differences in medial temporal regions, but lower % differences of [11C]UCB-J DVR in the susceptible neocortical and other examined regions (symbol O). (d) Higher Cohen’s d values for [11C]UCB-J DVR vs [18F]FDG KiR in some medial temporal regions (■ Hipp and Ent) and thalamic regions, but overall lower Cohen’s d values in [11C]UCB-J DVR in other examined regions.
Amy: Amygdala; Ang: angular; CS: centrum semiovale; Cd: Caudate; Cing_Ant: Ant. Cingulate; Cing_Post: Post. Cingulate; Ent: Entorhinal; Front: Frontal; Hipp: Hippocampus; Occ: Occipital, ParaHipp: ParaHippocampus; Par: Parietal; Prec: Precuneus; Pul: Pulvinar; Put: Putamen; Tem: Temporal; Thal: Thalamus.
The linear mixed model analysis (LMMA) demonstrated a significant group effect (F(1,23) = 15.4, p = 0.001) and group*region (F(15,345) = 2.2, p = 0.007) for [11C]UCB-J DVR. Post-hoc comparisons showed significantly lower [11C]UCB-J DVR in medial temporal and thalamic regions, and some neocortical regions after adjustment for multiple comparisons (Table 2). The LMMA also demonstrated a significant group effect (F(1,23) = 18.9, p < 0.001) and group*region (F(15,345) = 1.9, p = 0.019) for [18F]FDG KiR. Post-hoc comparisons revealed significantly lower [18F]FDG KiR in all medial temporal, thalamic and neocortical regions after adjustment for multiple comparisons (Table 2).
When comparing absolute kinetic values, i.e., [11C]UCB-J VT with [18F]FDG Ki, we detected slightly larger between-group differences for [11C]UCB-J VT in the medial temporal regions with higher effect sizes (Supplemental Table S1). We also found slightly larger between-group differences of [11C]UCB-J VT in the thalamic regions (thalamus and pulvinar). However, unlike [18F]FDG, [11C]UCB-J VT demonstrated no significant between-group differences in susceptible neocortical regions(Table S1). These regional values for the two tracers are also plotted, showing the between-group PD in Figure 2(a) and the Cohen’s d in Figure 2(b).
The LMMA demonstrated a significant group effect (F(1,23) = 5.6, p = 0.027) and group*region interaction (F(15,345) = 2.1, p = 0.008) for [11C]UCB-J VT. Post-hoc comparisons revealed significant group differences of [11C]UCB-J VT in the hippocampus, entorhinal, amygdala, and pulvinar after adjustment for multiple comparisons (Table S1). Note that LMMA demonstrated no significant effect of group (F(1,23) = 3.5, p = 0.07) or group*region interaction (F(15,345) = 1.5, p = 0.09) for [18F]FDG Ki and no significant between-group differences in the examined regions after adjustment for multiple comparisons (Table S1).
Regional hypoperfusion and hypometabolism in AD
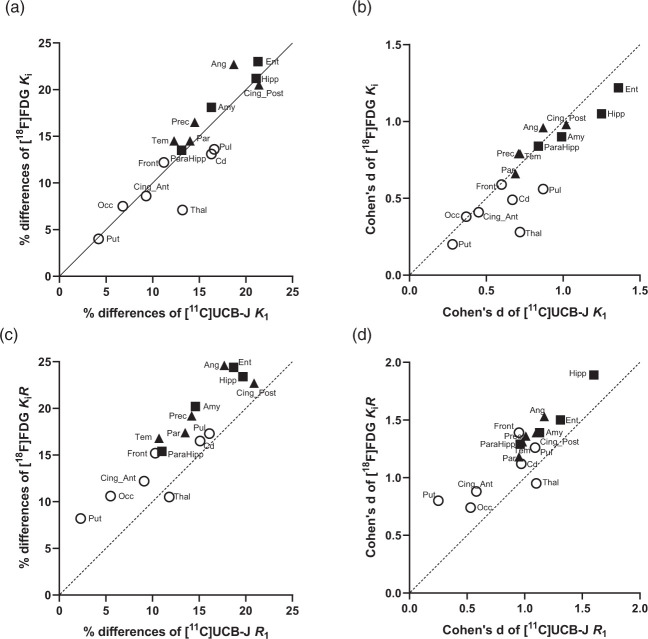
Comparing [11C]UCB-J K1 for perfusion and [18F]FDG Ki for metabolism, as potential surrogate measures of neuronal activity, we found a similar regional pattern and degree of group differences for both parameters, consistent with lower perfusion and metabolism in AD (Tables S1 and S2). The regional relationships are plotted showing the PDs in mean value (AD vs. CN) in Figure 3(a) and the Cohen’s d in Figure 3(b). Note that LMMA demonstrated close to significant group effect (F(1,23) = 4.2, p = 0.05) but no group*region interaction (F(15,345) = 1.3, P = 0.2) for [11C]UCB-J K1. Post-hoc comparisons only revealed significant [11C]UCB-J K1 differences in the hippocampus and entorhinal cortex of AD compared to CN after adjustment for multiple comparisons (Table S3).
Figure 3.
Scatter plots of regional percentage differences between CN and AD (a, c) and Cohen’s d (b, d) for [11C]UCB-J (K1 and R1 on x-axis) versus [18F]FDG (Ki and KiR on y-axis). (a) Comparing [11C]UCB-J K1 and [18F]FDG Ki demonstrated very similar % differences in most examined regions including in medial temporal regions (■ Hipp, Ent, Amy, and ParaHipp), susceptible neocortical regions (▲Ang, Cing_Post, Prec, Tem, Par), and other examined regions (o), which appeared well aligned along the identity line. (b) There were similar Cohen’s d values of [11C]UCB-J K1 vs [18F]FDG Ki in most examined regions. (c) Comparing [11C]UCB-J R1 and [18F]FDG KiR demonstrated generally lower % differences of [11C]UCB-J R1 in the examined regions. (d) Overall, there were slightly lower Cohen’s d values of [11C]UCB-J R1 vs [18F]FDG KiR in the examined regions.
Amy: Amygdala; Ang: angular; CS: centrum semiovale; Cd: Caudate; Cing_Ant: Ant. Cingulate; Cing_Post: Post. Cingulate; Ent: Entorhinal; Front: Frontal; Hipp: Hippocampus; Occ: Occipital, ParaHipp: ParaHippocampus; Par: Parietal; Prec: Precuneus; Pul: Pulvinar; Put: Putamen; Tem: Temporal; Thal: Thalamus.
With no group differences in cerebellum, [11C]UCB-J K1 and [18F]FDG Ki were normalized to cerebellum to yield R1 and KiR, respectively. We observed a similar pattern of lower values of both [11C]UCB-J R1 (Table S2) and [18F]FDG KiR (Table 2) in AD participants with higher effect sizes and statistical significance than the unnormalized values. These findings are illustrated in Figures 3(c) and (d) with the shift of the examined regions . The shift of PD values is probably due to the slight differences in reference cerebellum values. The LMMA for [11C]UCB-J R1 demonstrated a significant effect of group (F(1,23) = 10.5, p = 0.004) but no significant group*region interaction (F(15,345) = 1.6, P = 0.066). Post-hoc comparisons revealed significantly lower [11C]UCB-J R1 in AD compared to CN participants in all medial temporal, thalamic and neocortical regions after adjustment for multiple comparisons (Table S2).
Partial volume correction
We also performed PVC for correction of brain atrophy and gray-white spill-in. PVC was associated with an overall decrease in statistical significance and the effect sizes for most regions (Tables S3–5). Statistically significant group differences remained for [11C]UCB-J DVR in the hippocampus and the pulvinar after PVC (Table S4). Using the KiR of [18F]FDG after PVC, there were larger and significant between-group differences and more statistically significant regions than using [11C]UCB-J DVR after PVC(Table S4).
Regional correlations between synaptic density and metabolism
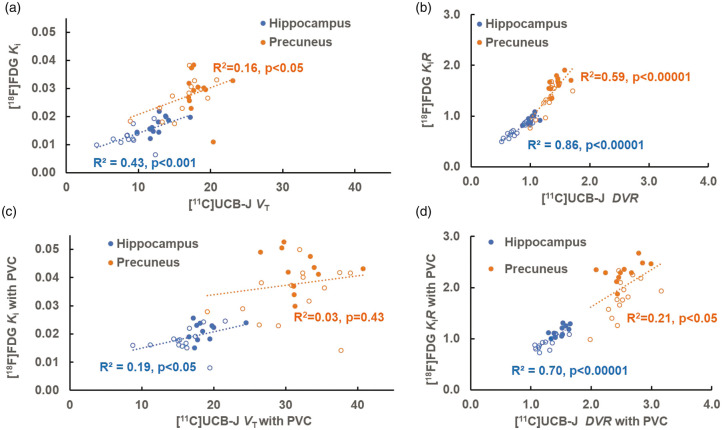
We explored inter-tracer correlations between the regional measures of [11C]UCB-J (K1, R1, VT, DVR,) and [18F]FDG (Ki and KiR) across participants. For the relationship between [11C]UCB-J binding (VT, DVR) and [18F]FDG metabolism (Ki and KiR), various degrees of correlation emerged between the two tracers among the examined regions, particularly in medial temporal and susceptible neocortical regions (Table S6). We selected hippocampus as the representative of medial temporal regions for demonstration purpose because we found the highest inter-group PD and Cohen’s d of [11C]UCB-J bindings within this region (Figure 2). We also selected precuneus as the representative of susceptible neocortical regions for demonstration because it is considered a vulnerable region of hypometabolism in AD and is characterized by less inter-subject variability than posterior cingulate (Table 2). Higher correlations were noted between [11C]UCB-J DVR and [18F]FDG KiR compared to those between [11C]UCB-J VT and [18F]FDG Ki in the same regions (Figure 4(a) and (b)). Overall, the correlations between [11C]UCB-J DVR and [18F]FDG KiR in the medial temporal regions were significantly higher than those in the susceptible neocortical regions. For example, the correlation in the hippocampus (R2 = 0.86) was higher than that in the precuneus (R2 = 0.59) (p = 0.022) (Figure 4(b)). We further performed inter-tracer correlations after PVC and found an overall decrease in correlations, as shown in Figure 4(c) and (d).
Figure 4.
Scatter plots of regional [11C]UCB-J (VT and DVR on x-axis) versus [18F]FDG (Ki and KiR on y-axis) in the hippocampus and precuneus before (a & b) and after PVC (c & d). The solid circles indicate CN participants and the open circles indicate AD participants. (a) There were statistically significant correlations in the hippocampus (R2=0.43) and in the precuneus (R2=0.16) between [11C]UCB-J VT and [18F]FDG Ki. (b) After normalization to cerebellum, the correlations between [11C]UCB-J DVR and [18F]FDG KiR increased with higher values in the hippocampus (R2=0.86) and in the precuneus (R2=0.59). (c) After PVC, the correlations between [11C]UCB-J VT and [18F]FDG Ki were significantly lower in the hippocampus (R2=0.19) and in the precuneus (R2=0.03). (d) After normalization to cerebellum, the correlations between [11C]UCB-J DVR and [18F]FDG KiR after PVC increased in the hippocampus (R2=0.70) and in the precuneus (R2=0.21).
Regional correlations between perfusion and metabolism
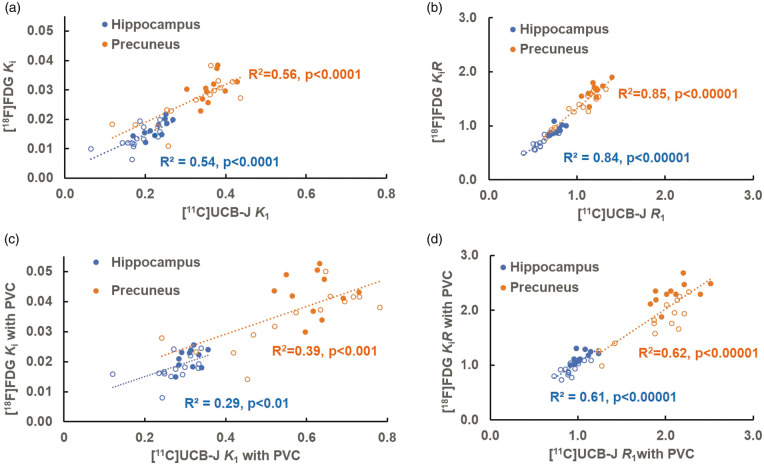
For perfusion (K1 and R1) and metabolism (Ki and KiR), the correlations between normalized measures ([11C]UCB-J R1 and [18F]FDG KiR) were overall very strong in the affected regions (medial temporal and neocortical regions) (R2 = 0.72-0.91) and were higher than those between the absolute measures ([11C]UCB-J K1 and [18F]FDG Ki) in the same regions (R2 = 0.21-0.66) (Table S6 and Figure 5(a) and (b)). We observed no significant regional differences in the correlations ([11C]UCB-J R1 vs [18F]FDG KiR) for the medial temporal and neocortical regions (e.g. R2 = 0.84 in the hippocampus versus R2 = 0.85 in the precuneus, p = 0.87). We further performed inter-tracer correlations after PVC and found an overall decrease in correlations (Table S6 and Figure 5(c) and (d)).
Figure 5.
Scatter plots of regional [11C]UCB-J (K1 and R1 on x-axis) versus [18F]FDG (Ki and KiR on y-axis) in the hippocampus and precuneus before (a & b) and after PVC (c & d). The solid circles indicate CN participants and the open circles indicate AD participants. (a) There were similar statistically significant correlations between [11C]UCB-J K1 and [18F]FDG Ki in the hippocampus (R2=0.54) and in the precuneus (R2=0.56). (b) After normalization to cerebellum, the correlations between [11C]UCB-J R1 and [18F]FDG KiR increased with higher values in the hippocampus (R2=0.84) and in the precuneus (R2=0.85). (c) After PVC, the correlations between [11C]UCB-J K1 and [18F]FDG Ki were significantly lower in the hippocampus (R2=0.29) and in the precuneus (R2=0.39). (d) After normalization to cerebellum, the correlations between normalized [11C]UCB-J R1 and [18F]FDG KiR after PVC increased in the hippocampus (R2=0.61) and in the precuneus (R2=0.62).
Discussion
We conducted the first comparison study of absolute and normalized regional measures of [11C]UCB-J binding for synaptic density and [18F]FDG uptake for metabolism in the same AD and CN participants. The most important findings from this study are the distinct regional patterns of synaptic loss and hypometabolism in AD. Overall, we found similar degrees of synaptic loss and hypometabolism in medial temporal regions but a greater degree of hypometabolism than synaptic loss in the susceptible neocortical regions of AD (Table 2 and Figure 2). We also demonstrated overall significant correlations between [11C]UCB-J binding and [18F]FDG metabolism within the affected regions, with higher inter-tracer correlations in the medial temporal regions (e.g. hippocampus) than in the neocortical regions (e.g. precuneus) (Figure 4 and Table S6). These findings strongly indicate that synaptic loss and hypometabolism were concordant in the medial temporal regions but were discordant in the susceptible neocortical regions.
Divergence of synaptic density and metabolism in AD
Hypometabolism in the temporoparietal lobes, precuneus, and posterior cingulate gyrus is the classic signature of [18F]FDG PET in early AD,44,45 although this posterior neocortical pattern differs somewhat from known pathological findings of neurodegeneration predominantly in medial temporal regions (entorhinal cortex and hippocampus) in early AD.9,46–48 In the current study, we did observe significantly lower [11C]UCB-J binding for synaptic density in the neocortical regions of AD (Table 2) consistent with our previous study12 and known postmortem AD pathology.37 However, the magnitude of AD hypometabolism exceeded that of synaptic loss in the association neocortices. Reasons for this divergence of synaptic density and metabolism in these regions are likely multifactorial and may include: incipient neurodegeneration within association neocortices, distant effects of neurodegeneration in the medial temporal lobes, and bioenergetic effects.
One possible explanation is disproportionate hypometabolism related to developing tau pathogenesis and neurodegeneration within the association neocortices that precedes synaptic loss in the direct projections to these regions. Although we did not perform tau PET imaging in the present study, a number of studies have reported strong correlations between regional tau deposition and hypometabolism49–52 and hypoperfusion.53 Moreover, early tau accumulation with associated hypometabolism in neocortical regions may precede synaptic loss and SV2A reductions in the direct projections to these regions. Further multi-tracer studies, incorporating tau PET will be valuable to investigate this possibility.
The divergence of synaptic density and metabolism in association neocortex may also pertain to distant effects of medial temporal lobe degeneration. Discrepant patterns of hypometabolism and atrophy in AD have previously been noted9,46–48 and have been attributed partly to diaschisis9,46–48 (i.e. the occurrence of neurophysiological changes distant from a focal brain lesion54) and/or indirect disconnection of association neocortices.9 Because neurodegeneration in entorhinal cortex and hippocampus is an early event in AD pathogenesis,55,56 it may be associated with hypometabolism in a number of projection regions, including temporoparietal cortex, posterior cingulate gyrus-precuneus, and orbito-medial frontal areas.9,46–48 Additional credence for this explanation is provided by studies in a nonhuman primate model of AD, which reported hypometabolism in several posterior association cortices and posterior hippocampus following neurotoxic lesions to entorhinal and perirhinal cortices.57 Early degeneration of the entorhinal and perirhinal cortices in AD likely produces a similar reduction in metabolism in these same structures. Although these posterior cortices receive multiple projections from other brain regions, the loss of excitatory inputs from medial temporal lobe may be sufficient to induce substantial hypometabolism without commensurate loss of synaptic density.
Yet another possible explanation for greater hypometabolism than synaptic loss in the neocortices of early AD is based on bioenergetics, whereby decreased glucose metabolism is an upstream event that precedes and results in the neuropathologic features of AD, including synaptic loss due to insufficient energy supply7,58 Although [18F]FDG PET is sometimes used as a surrogate for synaptic activity, it also reflects energy utilization by neurons and glia for a variety of functions.59–61 The direct measures of Ki simply indicate the influx of glucose for subsequent glycolysis. Moreover, impairments in astroglial transfer of glucose from the vasculature to neurons may contribute to metabolic deficits in AD59–61 and may be particularly harmful in watershed zones where blood supply is most limited. In this regard, it is noteworthy that the susceptible neocortical regions that show hypoperfusion and hypometabolism in AD lie in vulnerable watershed zones between the territories of the middle and posterior cerebral arteries,62 which could also explain the disproportionate hypometabolism relative to synaptic loss in those regions.
Lastly, the discrepancy between hypometabolism and SV2A reductions in the neocortical regions could be partly explained by compensatory synaptic reorganization in early stage of AD (see review Arendt 2009)63 or increased synaptic contact size64 (with increased SV2A per remaining synapse). Accumulations of SV2 has also been reported in dystrophic neurites within neuritic plaques that might obscure synaptic loss in amyloid-rich regions.65 Nevertheless, the current study provides the first in vivo analysis of the relationship between synaptic density and glucose metabolism in the same AD participants. Further studies incorporating tau PET or postmortem pathology may provide a better understanding of these issues.
Positive association of synaptic density and metabolism in AD
[18F]FDG brain PET for glucose metabolism is often utilized as a biomarker of neuronal and synaptic activity,7 presumed to be closely related to synaptic density, although direct in vivo evidence has been lacking. A previous study in a baboon demonstrated significant positive correlations between resting [18F]FDG PET and in vitro synaptophysin, a marker of synaptic density, across all the examined regions66 and supported the hypothesis that glucose metabolism reflects integrated synaptic activity.66 The current study provided the first evidence of significant positive correlations between in vivo SV2A PET for synaptic density and [18F]FDG PET for metabolism across susceptible regions for AD with higher inter-tracer correlations in the medial temporal regions and lower inter-tracer correlations in the neocortices (Figure 4 and Table S6). Although these inter-tracer correlations in the susceptible regions could be falsely amplified by brain atrophy, they persisted after PVC in the medial temporal regions. We also performed correlational analyses in CN and AD groups separately and found similar high inter-tracer correlations in the AD group, but not in the CN (data not shown), supporting the idea of concordant synaptic loss and decreased neuronal function in the medial temporal lobe; although the lack of significant correlation in the CN group could also be due to the smaller dynamic range of values in this group. In contrast, lower or no significant inter-tracer correlations in the neocortices after PVC is likely due to discordant synaptic loss and decreased neuronal function as discussed above. While revising our manuscript, a recently published study of FDG and SV2A PET in 20 female young healthy subjects (age 29.6 ± 9.9 yrs) also showed an overall statistically significant correlation (r > 0.47, p < 0.001) between global measures of glucose metabolism at rest and synaptic density.67 Interestingly, a substantial regional variation between glucose metabolism and synaptic density was also reported among the healthy young subjects,67 although this may not be directly applicable to the current study with elderly and AD participants. Future investigation in a larger study is needed for better understanding of the relationships between regional glucose metabolism and synaptic density in AD.
Cerebral perfusion and metabolism in AD
The current study further supports the notion that K1 and R1 from dynamic [11C]UCB-J PET imaging could serve as a surrogate for cerebral perfusion and could predict the pattern of [18F]FDG metabolism. Our recent study in healthy control participants showed that [11C]UCB-J K1 is highly sensitive to increased blood flow induced by visual stimulation, with no effects on synaptic density measures (VT).33 Our previous study in AD demonstrated broad regional differences in K1 in temporoparietal cortices relative to CN,11 similar to the pattern of known cortical hypometabolism in AD. Similar concepts have been investigated previously in the early dynamic flow images of PET with amyloid tracers.68–70 The high correlations between relative perfusion (R1) and metabolism (KiR) across all the susceptible regions further supported the similarity between those measures (Figure 5 and Table S6). We may be able to take advantage of this feature to gather useful information of both neuronal activity from K1 or R1 perfusion imaging and synaptic density from VT or DVR parametric images within a single [11C]UCB-J PET study.
Absolute and normalized outcome measures
In this study, we evaluated both absolute measures (K1, VT for [11C]UCB-J and Ki for [18F]FDG) and normalized measures to reference regions (R1, DVR, for [11C]UCB-J and KiR for [18F]FDG) for differentiation between AD and CN. It is critical to understand the pros and cons of absolute and normalized measures. Absolute measures are directly derived from kinetic modeling methods using input functions (i.e., arterial sampling for [11C]UCB-J and PBIF for [18F]FDG). Normalized measures require the availability of a suitable reference tissue/regions, usually devoid of specific binding (e.g., white matter) or having no effect from disease (e.g. cerebellum for AD). The absolute measures provide direct estimation of physiology but require arterial blood sampling and often have higher inter-subject variability (e.g., due to noise in the measurement of the input function) and therefore less sensitivity for detecting group differences. In contrast, normalized measures provide a relative estimate of physiology and usually have less inter-subject variability and higher sensitivity for detecting group differences. It is important to recognize that normalized measures could artifactually contribute to higher, positive correlations between [11C]UCB-J and [18F]FDG simply due to using the same cerebellum reference. Also, small numerical differences in reference region values could contribute inappropriately to the detection of group differences. Here, we observed lower, albeit nonsignificant, [11C]UCB-J K1 (PD:2.3%) and VT (PD:2.7%) in the cerebellum of AD compared to CN in this study, which was more than our previous studies (<1% differences),11,12 which potentially underestimated the between-group differences of [11C]UCB-J R1 and DVR after normalization. In contrast, we observed a slightly higher (again nonsignificant) [18F]FDG Ki (4.2%) in the cerebellum of AD compared to CN, which potentially overestimated the PD in regional hypometabolism (KiR) post normalization.
Limitations
This study has a number of limitations. First, it has a moderate sample size (n = 25) with small differences of sex and education years between AD and CN groups. In addition, we did not include sex, education level, or age variates in the linear mixed model analysis due to both the small sample size and the fact that we focused on the within-subject comparison between SV2A and [18F]FDG PET. As we recruit more AD participants for studies with multiple tracers, we hope to provide a better comparison between SV2A and [18F]FDG PET in the future. Methodologically, M-G PVC may not be an ideal method of correcting partial volume effects for both SV2A PET and [18F]FDG and might introduce uncertainty in the data of the current study. We are currently exploring other PVC methods including the iterative Yang approach for SV2A.71 Arterial blood sampling for SV2A PET is a cumbersome procedure for participants and limits the utility of this method for multi-center or longitudinal studies. Importantly, we have recently demonstrated that simpler SUVR measures are valid for SV2A-PET.72
Conclusion
We have presented the first comparison study for [11C]UCB-J for synaptic density and [18F]FDG metabolism for functional neuronal activity and have demonstrated distinct regional patterns in the same AD participants. The critical measures of synaptic loss and hypoperfusion/hypometabolism are likely to be complementary for understanding the pathophysiology of AD, and both can be obtained with a single dynamic [11C]UCB-J PET scan.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211004312 for Comparison of [11C]UCB-J and [18F]FDG PET in Alzheimer’s disease: A tracer kinetic modeling study by Ming-Kai Chen, Adam P Mecca, Mika Naganawa, Jean-Dominique Gallezot, Takuya Toyonaga, Jayanta Mondal, Sjoerd J Finnema, Shu-fei Lin, Ryan S O’Dell, Julia W McDonald, Hannah R Michalak, Brent Vander Wyk, Nabeel B Nabulsi, Yiyun Huang, Amy FT Arnsten, Christopher H van Dyck and Richard E Carson in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We thank all study participants, as well as the staff from the Yale Alzheimer’s Disease Research Unit and the Yale PET Center for their expert assistance. We thank UCB Pharma in Brussels Belgium for providing the [11C]UCB-J radiolabeling precursor and reference standard.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Dana Foundation David Mahoney Neuroimaging Grant, Pilot grant of Yale Alzheimer’s Disease Research Center NIH P50AG047270, NIH R01AG52560, NIH R01AG062276, NIH K23AG057784, NIH T32MH019961-21A1, Research grant from Eli Lilly.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MKC reports research support from the Dana Foundation and Eli Lilly for the conduct of the study. APM, REC, and CHvD report grants from National Institutes of Health for the conduct of the study. MKC reports consulting fees from Eisai and Actinium and clinical trial of Merck outside the submitted work. APM reports grants for clinical trials from Genentech and Eisai outside the submitted work. YH reports research grants from the UCB and Eli Lilly outside the submitted work. YH, NBN, and REC have a patent for a newer version of the tracer. REC is a consultant for Rodin Therapeutics and has received research funding from UCB. REC reports having received grants from AstraZeneca, Astellas, Eli Lilly, Pfizer, Taisho, and UCB, outside the submitted work. CHvD reports consulting fees from Kyowa Kirin, Roche, Merck, Eli Lilly, and Janssen and grants for clinical trials from Biogen, Novartis, Eli Lilly, Merck, Eisai, Janssen, Roche, Genentech, Toyama, and Biohaven, outside the submitted work. No other disclosures are reported.
Authors’ contributions: M-KC, REC, and CHV were responsible for the concept and design of the study. M-KC, APM, AFTA, REC, and CHV were responsible for literature search, data and imaging interpretation. M-KC was responsible for drafting of the original report, figures and tables., which was reviewed and revised by all coauthors. APM, JWM, HRM, and CHV were responsible for recruiting participants, acquisition of MRI scans, and clinical assessments. NBN, and YH were responsible for the synthesis of UCB-J radiotracers. M-KC, APM, REC, and CHV were responsible for acquisition of PET data. S-FL was responsible for metabolite analysis and arterial input function. APM, MN, SJF, TT, RO and JM were responsible for PET imaging analyses. M-KC, APM, and BVW were responsible for statistical analysis. Funding was obtained by M-KC, REC, and CHV. M-KC the first and corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary material: Supplemental material for this article is available online.
ORCID iDs: Mika Naganawa https://orcid.org/0000-0002-4408-2621
Jayanta Mondal https://orcid.org/0000-0002-2387-7054
Sjoerd J Finnema https://orcid.org/0000-0002-4972-3627
Nabeel B Nabulsi https://orcid.org/0000-0001-8129-0051
References
- 1.Jack CR, Jr, Bennett DA, Blennow K, et al.; Contributors. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018; 14: 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016; 87: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306–319. [DOI] [PubMed] [Google Scholar]
- 4.Barthel H, Sabri O.Clinical use and utility of amyloid imaging. J Nucl Med 2017; 58: 1711–1717. [DOI] [PubMed] [Google Scholar]
- 5.Betthauser TJ, Cody KA, Zammit MD, et al. In vivo characterization and quantification of neurofibrillary tau PET radioligand [(18)F]MK-6240 in humans from Alzheimer's disease dementia to young controls. J Nucl Med 2019; 60: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 2016; 79: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosconi L.Glucose metabolism in normal aging and Alzheimer's disease: methodological and physiological considerations for PET studies. Clin Transl Imaging 2013; 1: 217–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailly M, Destrieux C, Hommet C, et al. Precuneus and cingulate cortex atrophy and hypometabolism in patients with Alzheimer's disease and mild cognitive impairment: MRI and (18)F-FDG PET quantitative analysis using FreeSurfer. Biomed Res Int 2015; 2015: 1–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chetelat G, Desgranges B, Landeau B, et al. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer's disease. Brain 2008; 131: 60–71. [DOI] [PubMed] [Google Scholar]
- 10.Ishii K, Sasaki H, Kono AK, et al. Comparison of gray matter and metabolic reduction in mild Alzheimer's disease using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging 2005; 32: 959–963. [DOI] [PubMed] [Google Scholar]
- 11.Chen MK, Mecca AP, Naganawa M, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol 2018; 75: 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mecca AP, Chen MK, O'Dell RS, et al. In vivo measurement of widespread synaptic loss in Alzheimer's disease with SV2A PET. Alzheimers Dement 2020; 16: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbizu J, Festari C, Altomare D, et al.; EANM-EAN Task Force for the Prescription of FDG-PET for Dementing Neurodegenerative Disorders. Clinical utility of FDG-PET for the clinical diagnosis in MCI. Eur J Nucl Med Mol Imaging 2018; 45: 1497–1508. [DOI] [PubMed] [Google Scholar]
- 14.Drzezga A, Altomare D, Festari C, et al.; EANM-EAN Task Force for the Prescription of FDG-PET for Dementing Neurodegenerative Disorders. Diagnostic utility of 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) in asymptomatic subjects at increased risk for Alzheimer's disease. Eur J Nucl Med Mol Imaging 2018; 45: 1487–1496. [DOI] [PubMed] [Google Scholar]
- 15.Landau SM, Harvey D, Madison CM, et al.; Alzheimer's Disease Neuroimaging Initiative. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011; 32: 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nestor PJ, Altomare D, Festari C, et al.; EANM-EAN Task Force for the Prescription of FDG-PET for Dementing Neurodegenerative Disorders. Clinical utility of FDG-PET for the differential diagnosis among the main forms of dementia. Eur J Nucl Med Mol Imaging 2018; 45: 1509–1525. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain S, Gabriel H, Strittmatter W, et al. An exploratory phase IIa study of the PPAR Delta/gamma agonist T3D-959 assessing metabolic and cognitive function in subjects with mild to moderate Alzheimer's disease. J Alzheimers Dis 2020; 73: 1085–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dyck CH, Nygaard HB, Chen K, et al. Effect of AZD0530 on cerebral metabolic decline in Alzheimer disease: a randomized clinical trial. JAMA Neurol 2019; 76: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiers CE, Shokri-Kojori E, Wong CT, et al. Cannabis abusers show hypofrontality and blunted brain responses to a stimulant challenge in females but not in males. Neuropsychopharmacology 2016; 41: 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teipel SJ, Drzezga A, Bartenstein P, et al. Effects of donepezil on cortical metabolic response to activation during (18)FDG-PET in Alzheimer's disease: a double-blind cross-over trial. Psychopharmacology (Berl) 2006; 187: 86–94. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi K, Onishi A, Fujiwara Y, et al. Relationship between Alzheimer disease-like pattern of 18F-FDG and fasting plasma glucose levels in cognitively normal volunteers. J Nucl Med 2015; 56: 229–233. [DOI] [PubMed] [Google Scholar]
- 22.Burns CM, Chen K, Kaszniak AW, et al. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology 2013; 80: 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks RA, Di Chiro G, Zukerberg BW, et al. Test-retest studies of cerebral glucose metabolism using fluorine-18 deoxyglucose: validation of method. J Nucl Med 1987; 28: 53–59. [PubMed] [Google Scholar]
- 24.Schmidt ME, Ernst M, Matochik JA, et al. Cerebral glucose metabolism during pharmacologic studies: test-retest under placebo conditions. J Nucl Med 1996; 37: 1142–1149. [PubMed] [Google Scholar]
- 25.Shiyam Sundar LK, Muzik O, Rischka L, et al. Promise of fully integrated PET/MRI: Noninvasive clinical quantification of cerebral glucose metabolism. J Nucl Med 2020; 61: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajjalieh SM, Frantz GD, Weimann JM, et al. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci 1994; 14: 5223–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajjalieh SM, Peterson K, Linial M, et al. Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci USA 1993; 90: 2150–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutch SA, Kensel-Hammes P, Gadd JC, et al. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci 2011; 31: 1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes SE, Scheinost D, Finnema SJ, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun 2019; 10: 1529–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onwordi EC, Halff EF, Whitehurst T, et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun 2020; 11: 246–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matuskey D, Tinaz S, Wilcox KC, et al. Synaptic changes in Parkinson disease assessed with in vivo imaging. Ann Neurol 2020; 87: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finnema SJ, Nabulsi NB, Mercier J, et al. Kinetic evaluation and test-retest reproducibility of [(11)C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab 2018; 38: 2041–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smart K, Liu H, Matuskey D, et al. Binding of the synaptic vesicle radiotracer [(11)C]UCB-J is unchanged during functional brain activation using a visual stimulation task. J Cereb Blood Flow Metab 2020. Epub ahead of print 8 August 2020. DOI: 10.1177/0271678x20946198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finnema SJ, Rossano S, Naganawa M, et al. A single-center, open-label positron emission tomography study to evaluate brivaracetam and levetiracetam synaptic vesicle glycoprotein 2A binding in healthy volunteers. Epilepsia 2019; 60: 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nabulsi NB, Mercier J, Holden D, et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med 2016; 57: 777–784. [DOI] [PubMed] [Google Scholar]
- 36.Rossano S, Toyonaga T, Finnema SJ, et al. Assessment of a white matter reference region for (11)C-UCB-J PET quantification. J Cereb Blood Flow Metab 2020; 40: 1890–1901. [DOI] [PMC free article] [PubMed]
- 37.de Wilde MC, Overk CR, Sijben JW, et al. Meta-analysis of synaptic pathology in Alzheimer's disease reveals selective molecular vesicular machinery vulnerability. Alzheimers Dement 2016; 12: 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patlak CS, Blasberg RG, Fenstermacher JD.Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983; 3: 1–7. [DOI] [PubMed] [Google Scholar]
- 39.Naganawa M, Gallezot JD, Shah V, et al. Assessment of population-based input functions for patlak imaging of whole body dynamic (18)F-FDG PET. EJNMMI Phys 2020; 7: 67–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malone IB, Leung KK, Clegg S, et al. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage 2015; 104: 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mecca AP, Barcelos NM, Wang S, et al. Cortical beta-amyloid burden, gray matter, and memory in adults at varying APOE epsilon4 risk for Alzheimer's disease. Neurobiol Aging 2018; 61: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992; 12: 571–583. [DOI] [PubMed] [Google Scholar]
- 43.Weaver B, Wuensch KL.SPSS and SAS programs for comparing Pearson correlations and OLS regression coefficients. Behav Res Methods 2013; 45: 880–895. [DOI] [PubMed] [Google Scholar]
- 44.Minoshima S.A diagnostic approach in alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1978; 36: 1238–1248. [PubMed] [Google Scholar]
- 45.Minoshima S, Foster NL, Kuhl DE.Posterior cingulate cortex in Alzheimer's disease. Lancet 1994; 344: 895. [DOI] [PubMed] [Google Scholar]
- 46.Villain N, Desgranges B, Viader F, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. J Neurosci 2008; 28: 6174–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Wolk DA, Reddin JS, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology 2011; 77: 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yakushev I, Schreckenberger M, Müller MJ, et al. Functional implications of hippocampal degeneration in early Alzheimer's disease: a combined DTI and PET study. Eur J Nucl Med Mol Imaging 2011; 38: 2219–2227. [DOI] [PubMed] [Google Scholar]
- 49.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain 2016; 139: 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanseeuw BJ, Betensky RA, Schultz AP, et al. Fluorodeoxyglucose metabolism associated with tau-amyloid interaction predicts memory decline. Ann Neurol 2017; 81: 583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams JN, Lockhart SN, Li L, et al. Relationships between tau and glucose metabolism reflect Alzheimer's disease pathology in cognitively normal older adults. Cereb Cortex 2019; 29: 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bischof GN, Jessen F, Fliessbach K, et al.; Alzheimer's Disease Neuroimaging Initiative. Impact of tau and amyloid burden on glucose metabolism in Alzheimer's disease. Ann Clin Transl Neurol 2016; 3: 934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albrecht D, Isenberg AL, Stradford J, et al. Associations between vascular function and tau PET are associated with global cognition and amyloid. J Neurosci 2020; 40: 8573–8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrera E, Tononi G.Diaschisis: past, present, future. Brain 2014; 137: 2408–2422. [DOI] [PubMed] [Google Scholar]
- 55.Braak H, Thal DR, Ghebremedhin E, et al. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011; 70: 960–969. [DOI] [PubMed] [Google Scholar]
- 56.Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and pathological correlates of apolipoprotein E e4 in Alzheimer's disease. Ann Neurol 1996; 39: 62–70. [DOI] [PubMed] [Google Scholar]
- 57.Meguro K, Blaizot X, Kondoh Y, et al. Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non-human primate as shown by PET: implications for Alzheimer's disease. Brain 1999; 122: 1519–1531. [DOI] [PubMed] [Google Scholar]
- 58.Cunnane S, Nugent S, Roy M, et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 2011; 27: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merlini M, Meyer EP, Ulmann-Schuler A, et al. Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAβ mice. Acta Neuropathol 2011; 122: 293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morita M, Ikeshima-Kataoka H, Kreft M, et al. Metabolic plasticity of astrocytes and aging of the brain. Ijms 2019; 20: 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmer ER, Parent MJ, Souza DG, et al. ( 18)F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci 2017; 20: 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang CW, Hsu SW, Chang YT, et al. Cerebral perfusion insufficiency and relationships with cognitive deficits in Alzheimer's disease: a multiparametric neuroimaging study. Sci Rep 2018; 8: 1541–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arendt T.Synaptic degeneration in alzheimer's disease. Acta Neuropathol 2009; 118: 167–179. 2009/04/25. DOI: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- 64.DeKosky ST, Scheff SW.Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol 1990; 27: 457–464. [DOI] [PubMed] [Google Scholar]
- 65.Snow AD, Nochlin D, Sekiguichi R, et al. Identification and immunolocalization of a new class of proteoglycan (keratan sulfate) to the neuritic plaques of Alzheimer's disease. Exp Neurol 1996; 138: 305–317. [DOI] [PubMed] [Google Scholar]
- 66.Rocher AB, Chapon F, Blaizot X, et al. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage 2003; 20: 1894–1898. [DOI] [PubMed] [Google Scholar]
- 67.Aalst JV, Ceccarini J, Sunaert S, et al. In vivo synaptic density relates to glucose metabolism at rest in healthy subjects, but is strongly modulated by regional differences. J Cereb Blood Flow Metab. Epub ahead of print. DOI: 10.1177/0271678x20981502. [DOI] [PMC free article] [PubMed]
- 68.Kadir A, Almkvist O, Forsberg A, et al. Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer's disease. Neurobiol Aging 2012; 33: 198.e191–114. [DOI] [PubMed] [Google Scholar]
- 69.Meyer PT, Hellwig S, Amtage F, et al. Dual-biomarker imaging of regional cerebral amyloid load and neuronal activity in dementia with PET and 11C-labeled Pittsburgh compound B. J Nucl Med 2011; 52: 393–400. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Vieitez E, Carter SF, Chiotis K, et al. Comparison of early-phase 11C-deuterium-l-deprenyl and 11C-Pittsburgh compound B PET for assessing brain perfusion in Alzheimer disease. J Nucl Med 2016; 57: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 71.Erlandsson K, Buvat I, Pretorius PH, et al. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys Med Biol 2012; 57: R119–159. [DOI] [PubMed] [Google Scholar]
- 72.Naganawa M, Gallezot JD, Finnema S, et al. Simplified quantification of (11)C-UCB-J PET evaluated in a large human cohort. J Nucl Med 2021; 62: 418–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211004312 for Comparison of [11C]UCB-J and [18F]FDG PET in Alzheimer’s disease: A tracer kinetic modeling study by Ming-Kai Chen, Adam P Mecca, Mika Naganawa, Jean-Dominique Gallezot, Takuya Toyonaga, Jayanta Mondal, Sjoerd J Finnema, Shu-fei Lin, Ryan S O’Dell, Julia W McDonald, Hannah R Michalak, Brent Vander Wyk, Nabeel B Nabulsi, Yiyun Huang, Amy FT Arnsten, Christopher H van Dyck and Richard E Carson in Journal of Cerebral Blood Flow & Metabolism