Abstract
Stroke is followed by an intricate immune interaction involving the engagement of multiple immune cells, including neutrophils. As one of the first responders recruited to the brain, the crucial roles of neutrophils in the ischemic brain damage are receiving increasing attention in recent years. Notably, neutrophils are not homogenous, and yet there is still a lack of full knowledge about the extent and impact of neutrophil heterogeneity. The biological understanding of the neutrophil response to both innate and pathological conditions is rapidly evolving as single-cell-RNA sequencing uncovers overall neutrophil profiling across maturation and differentiation contexts. In this review, we scrutinize the latest research that points to the multifaceted role of neutrophils in different conditions and summarize the regulatory signals that may determine neutrophil diversity. In addition, we list several potential targets or therapeutic strategies targeting neutrophils to limit brain damage following ischemic stroke.
Keywords: Ischemic stroke, neutrophil, neutrophil extracellular trap, polymorphonuclear granulocyte, neurovascular unit, blood brain barrier
Introduction
Ischemic stroke is a common neurological disease with devastating outcomes and the top leading cause of death and disability in China1 and worldwide,2,3 thus posing a tremendous burden to both individuals and society. The conventional belief that stroke is simply a neurovascular and thrombotic disease has been increasingly challenged by newly emerged experimental and clinical studies, which have begun to reveal a complex interplay between the central nervous system (CNS) and the immune system after stroke that requires the actions of various immune cells including neutrophils.4–8 Ischemic stroke triggers the brain to release a variety of neuronal “help me” signals, such as ATP, high mobility group box 1 (HMGB1), hypoxia‐inducible factor 1α (HIF‐1α), S100β, and brain‐derived antigens, thereby activating immune cells both within the CNS and in the periphery.9 Among the diverse types of immune cells associated with the innate immune response after stroke, neutrophils are first responders infiltrating into the ischemic brain and contributing to acute post-ischemic brain injury.10,11
Under physiological conditions, the blood-brain barrier (BBB) serves as a homeostatic control gate of the nervous system that limits the entry of potentially neurotoxic plasma components, blood cells, and pathogens into the brain.12–14 Different cell types of the neurovascular unit (NVU) including neurons, astrocytes, pericytes, maintain key CNS functionality by coordinating with each other.15–17 The components of the NVU tightly interact and participate in the formation of the BBB, thereby contributing to the regulation of BBB permeability, neurovascular coupling, cell-matrix interactions, neurotransmitter turnover, and angiogenesis and neurogenesis.15,16,18,19 After stroke, activated immune cells reach the ischemic area sequentially by way of the breakdown of BBB and can function as a double edged sword by either disrupting or protecting the integrity of BBB.20,21 By releasing matrix metalloproteinase‐9 (MMP‐9), neutrophils may cause the disintegration of the BBB and lead to the exacerbation of neuroinflammation.22 Not only in animal models of stroke has the infiltration of neutrophils been shown in the injured ischemic brain, but the number of circulating neutrophils also rise in patients with stroke, which is associated with stroke severity, infarct volume and worse functional outcomes.23 A high neutrophil–lymphocyte ratio has been associated with poor neurological recovery after ischemic stroke, suggesting a detrimental role of neutrophils in this setting.24 Taken together, these studies indicate that neutrophil infiltration and accumulation may contribute to post-ischemic brain injury, thus neutrophils may represent a potential therapeutic target for stroke.
Temporal and spatial distribution of neutrophils after stroke
Although the precise role of neutrophils in ischemic stroke still remains controversial,25 abundant experimental and clinical research have confirmed the importance of neutrophil infiltration in the pathogenesis of ischemic stroke.20,26,27 Neutrophils are among the first responders that are recruited to the injured brain.28,29 The recruitment of neutrophil is initiated within 30 min to a few hours in animal models of focal cerebral ischemia/reperfusion, peaks early in the first days, and then disappears or decreases rapidly with time.30 Interestingly, results derived from other researches in transient middle cerebral artery occlusion (tMCAo) model challenge the previous view by demonstrating that the infiltration of other inflammatory cells such as macrophages and microglia may precede neutrophil influx.31 Neutrophils were found to appear in small amounts 12 and 24 hours after reperfusion onset and increased steeply at day 3 after reperfusion then noticeably declined at day 7 after stroke.31 Similarly, in patients with ischemic stroke, the number of circulating neutrophils rise within the first few hours of stroke onset and the neutrophils are recruited to the ischemic brain within 24 hours of symptom onset.32
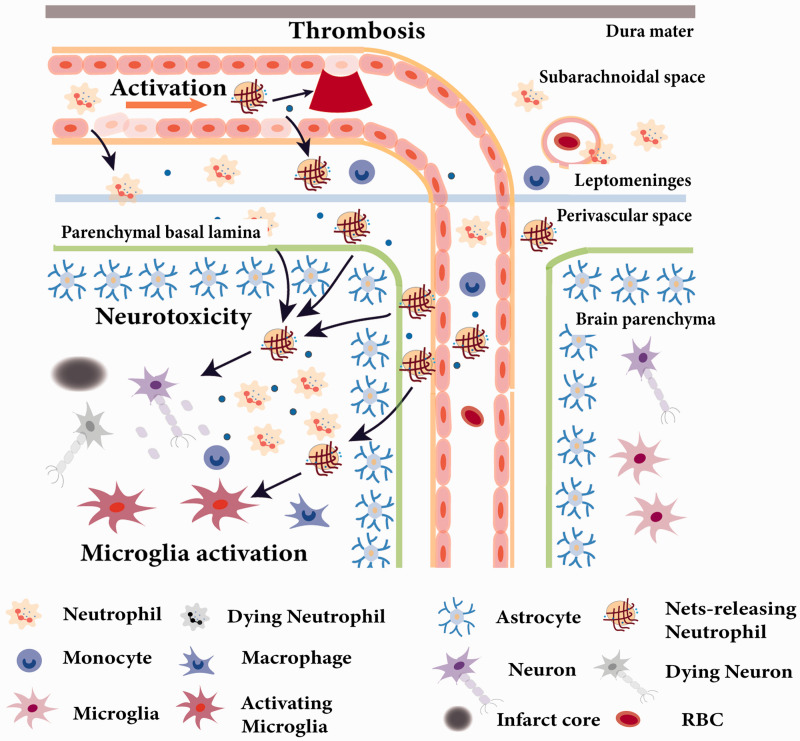
The exact anatomic location of neutrophil infiltration within the neurovascular unit and brain parenchyma remains controversial.33,34 Based on both a mouse model and human data, neutrophils do not gain access to brain parenchyma early after ischemic stroke, indicating that the NVU is a selective barrier to the infiltration of neutrophils into the brain.10 However, another recent study based on models of permanent ischemia induced by either cauterization of the distal portion of the MCA (c-MCAo) or intraluminal MCA occlusion (il-MCAo) argues that activated neutrophils can be seen within the leptomeninges as early as 6 hours, within the perivascular space by 15 hours, and within the CNS parenchyma by 24 hours.11 Neutrophils reach cortical brain territories devoid of blood flow after extravasating from leptomeningeal vessels surrounding the infarcted brain tissue and reaching the cortical parenchymal basal lamina as well as the perivascular spaces of cortical arterioles. After prolonged ischemic insults, neutrophils are activated showing signs of neutrophil extracellular traps (NETs) formation inside the vascular cavity and perivascular space, suggesting that the NVU is a target of neutrophil activity after stroke. Similarly, neutrophils were found in the leptomeninges surrounding the infarcted regions in postmortem human brain tissue of stroke patients, supporting the concept that neutrophil recruitment may be relevant to human ischemic stroke (Figure 1).11
Figure 1.
Neutrophil activation and recruitment in ischemic stroke. Neutrophils respond to ischemic stroke soon after ischemia and were found in leptomeninges as early as 6 h post reperfusion. Neutrophils release ROS and exacerbate BBB damage to extravasate from blood vessels to brain. After 24 hours of ischemic brain injury, neutrophils can be activated to form NETS in the vascular cavity, contributing to thrombosis and no reflow of blood post reperfusion. A large number of neutrophils are recruited to the perivascular space and brain parenchyma where they execute neurotoxic function(s) and peak at 24h after stroke. At 3 days after stroke, neutrophils show signs of apoptosis, are phagocytosed by microglia, and decrease with time.
Taken together, neutrophils represent one of the initial immune cells recruited to the ischemic tissue, primarily in perivascular spaces, and they target the NVU after stroke, according to studies both in animal model and stroke patients. There have been abundant experimental studies showing that inhibition of the neutrophil infiltration can be a protective approach,35 thus supporting the concept that neutrophils can be a prospective target for novel therapies for stroke. However, in a recent clinical trial (ACTION II), a trial of natalizumab, which neutralized neutrophils on the functional outcomes in patients with acute ischemic stroke, and it turned out as failed.36,37 The failure of the trial and preclinical data suggests that only animals with permanent middle cerebral artery occlusion and relatively small lesions benefit from the intervention. The clinical study populations instead was heterogeneous and also contained patients with relatively large strokes as only those >70 mL were excluded. Hence, the clinical population did not match the profile of the animals in which the therapy was effective. A subgroup analysis has not been performed in ACTION II. Moreover, patients receiving recanalization therapies were also included although the treatment was not effective after tMCAO in animals. All these discrepancies might explain the failure. Therefore, the difference between animals and human patients should be taken into consideration when designing the clinical trial. In addition, the heterogeneity of clinical study populations should also be taken into account in the clinical trial. The distinct roles of subpopulations of neutrophils are also highly warranted to be examined in order to fully characterize the multifaced roles of the neutrophils after ischemic stroke in the pursuit of discovering a more finely tuned therapeutic approach.
Neutrophils are powerful immune cells with highly functional plasticity after ischemic stroke
Neutrophils are short-lived but powerful immune cells which can provide an early and vigorous inflammatory response after tissue injury, including stroke.38 Neutrophils are one of the most abundant cell population recruited to the injured brain, with a peak influx between from 1 to 3 days following the ischemic stroke.31,32,39 Neutrophils are considered to worsen the stroke outcome via several mechanisms, including physical blockade within the microvascular network that may further reducing cerebral blood flow, as well as direct entry into the brain parenchyma followed by the release of granules containing antimicrobial enzymes and chemical components that could further injury brain tissue, such as MMP9.20,34,40 Since neutrophils are assumed to be a detrimental factor poststroke, preventing neutrophils from entering the brain could serve as a therapeutic strategy.
Though the negative impact of neutrophils after stroke has been well-established, recent evidence suggests that neutrophils may also exhibit the property of “functional plasticity”38 similar to other immune cells like macrophages, wherein they may possess a two-faced functional heterogeneity and shift between pro-inflammatory and anti-inflammatory phenotypes, called N1 neutrophils, which are induced by IFN-γ (alone or in combination with lipopolysaccharide(LPS) and an alternative N2 phenotype, which could be induced after transforming growth factor-β (TGF-β) stimulation.41–43 The so-called N1 neutrophil phenotype refers to the neutrophils with strong pro-inflammatory properties mainly engaging in antimicrobial activities and possessing antitumorigenic characteristics. On the contrary, the N2 phenotype displays enhanced anti-inflammatory properties and produces a higher content of beneficial molecules.38 Given that, the ratio of N1 over N2 may lead to the diversity of stroke outcome, prompting the pursuit of innovative therapeutic strategies aimed at reducing the detrimental properties of neutrophil infiltration. For example, based on a clinically relevant animal model of intracerebral hemorrhage (ICH), interleukin-27 (IL-27), a known negative regulator of reactive oxygen species and cytotoxic granule component production,44 can push neutrophil maturation toward a protective phenotype. Specifically, neutrophils deliver cytoprotective lactoferrin (LTF) to the ICH-affected brain, neutralizing iron and blocking its toxicity, thereby reducing brain edema and improving neurological deficits caused by ICH, a devastating form of stroke.45 This could happen due to the direct secretion of beneficial factors. In addition, neutrophils may impose a self-limitation of proinflammatory factors, such as IL-1RA.46 TGFβ is also a cytokine that acts as an inducer of N2 polarization of neutrophils and has been shown to reduce neuroinflammation and improve recovery after ICH.41,47
Thus, neutrophils are no longer simply viewed as damaging components in stroke pathology. The fact that neutrophils can be favorable participants in anti-neuroinflammation is absolutely inspiring, making neutrophils a potential candidate for resolution of brain injury poststroke.
Heterogeneity of neutrophils revealed by rapidly evolving sc-RNA sequencing techniques
Knowing that neutrophil populations are not homogenous across differentiation and maturation,48–50 enormous efforts have been made to catalogue and name neutrophils subsets according to their proliferative capacity, maturation status, phenotypic profile, site of origin, effector function, and so on for further research (Table 1).51 The concept of neutrophil heterogeneity first emerged in cancer studies.3,52,53 Nevertheless, taking transcriptional and epigenetic profiles of neutrophils into consideration when investigating them in any context may provide more reliable core characteristics for neutrophil distinction, which still remains unknown.51 Single-cell RNA sequencing (sc-RNA seq) revealed that neutrophils showed great diversity in both homeostasis and disease conditions.54,55 Depletion of neutrophils is detrimental at early disease stages but becomes protective at late stages in cancer,56,57 which means neutrophils undergo a reprogramming process to allow their functional switch and heterogeneous behavior.51 Consistent with the notion of an immune switch, scRNA-seq identified a neutrophil subset in peripheral blood named PMNb, which are discrete and definable neutrophils expressing interferon-stimulated genes (ISGs).55 Interestingly, while a group of ISG-expressing tumor-infiltrating neutrophils were recently identified in human and mouse lung cancers,58 their transcriptomes were significantly different from that of PMNb neutrophils, indicating significant neutrophil reprogramming in the tumor microenvironment. In chronic inflammation, neutrophils can perpetuate damage to organs if the instigating stimulus persists.59 Studies of neutrophils in various disease models including cardiovascular disease and neurodegenerative disorders54,60,61 suggest that neutrophils can be rapidly reprogrammed in the bone marrow towards a phenotype that antagonizes inflammation, yet the signals and the exact cell populations targeted remain unclear. Intriguingly, scRNA-seq also reveals that bacterial infection reprograms the neutrophil population and the dynamic transition between each subpopulation.55
Table 1.
Heterogeneity of neutrophils in different contexts.
| Subsets | Maturation status | Phenotypic profiles | Conditions | Functions | Reference |
|---|---|---|---|---|---|
| CD14+Ly6Glo immature neutrophils | With immature features | CD14+Ly6Glo | Optic nerve and spinal cord injury | Neuroprotective and axonogenic properties; in part via secretion of the growth factors NGF and IGF-1 | 112 |
| PMNb | Mature | Express a set of interferon-stimulated genes | Homeostasis and Infection; cancer | Exist in both humans and mice and combat invading pathogens during infection; reprogramme in tumor microenvironment | 55,58 |
| Low-density neutrophils (LDNs) | Immature | Mixed population with cells with band, lobular, or myelocyte-like nuclei | SLE and other autoimmune diseases | Pro-inflammatory, produce inflammatory cytokines and kill endothelial cells; enhanced NETs production | 113–115 |
| Tumor-associated neutrophils (TANs) | Immature | N1 TAN: Met+; hypersegmented nucleiN2 TAN: Rounded nuclei | Cancer | N1 TANs: inhibit tumor developmentN2 TANs: promote tumor development | 41,50,116,117 |
Thus, the rapidly evolving sc-RNA sequencing techniques bring a comprehensive reference map of differentiating and mature neutrophil transcriptional states, making it more hopeful to find biomarkers and therapeutic targets in neutrophil-related disease, including stroke. However, sc-RNA sequencing studies in stroke are still absent as of yet, and research in this regard is highly warranted to reveal the overall phenotypes of neutrophils present throughout the ongoing pathology of stroke.
Regulatory signals that determine neutrophil development and heterogeneity
Although intrinsic heterogeneity exists in bone marrow and blood, exposure to extrinsic factors typically modify neutrophil properties and have influence on highly coordinated transcriptional dynamics.51 Neutrophils exhibit diverse chromatin and transcriptional landscapes at different stages of maturation across mobilization and migration.62 Lineage-determining transcription factors (LDTFs), such as PU.1, enhancer-binding protein-α (C/EBPα), and C/EBPβ are highly expressed across neutrophil maturation. LDTFs may control neutrophil epigenomic organization in collaboration with other transcription factors.63 To be specific, it has been found that PU.1 (encoded by the SPI1 gene) plays a critical role in the development of lymphoid and myeloid lineages, with higher PU.1 levels in the myeloid cell types than in B cells.64 However, there is still a PU.1 dose-dependent differentiation within the myeloid lineage. For instance, neutrophil development requires relative lower levels of PU.1 expression when compared to macrophage development due to existence of C/EBPα, a transcription factor that is opposed to PU.1 in the final differentiation decision towards neutrophils.65 C/EBPɛ, another member in C/EBP family and two other transcription factors, lymphoid enhancer-binding factor 1 (LEF1) and growth factor-independent protein 1 (GFI1) have all been proved to be critical in neutrophil development.66,67 In addition, the transcriptional repressor zinc-finger protein GFI1 and interferon regulatory factor 8 (IRF8) are antagonistic and crucial in neutrophil maturation, as IRF8 is the main driver in monocyte lineage commitment, counterbalancing the effect of GFI1 in neutrophil development.68,69
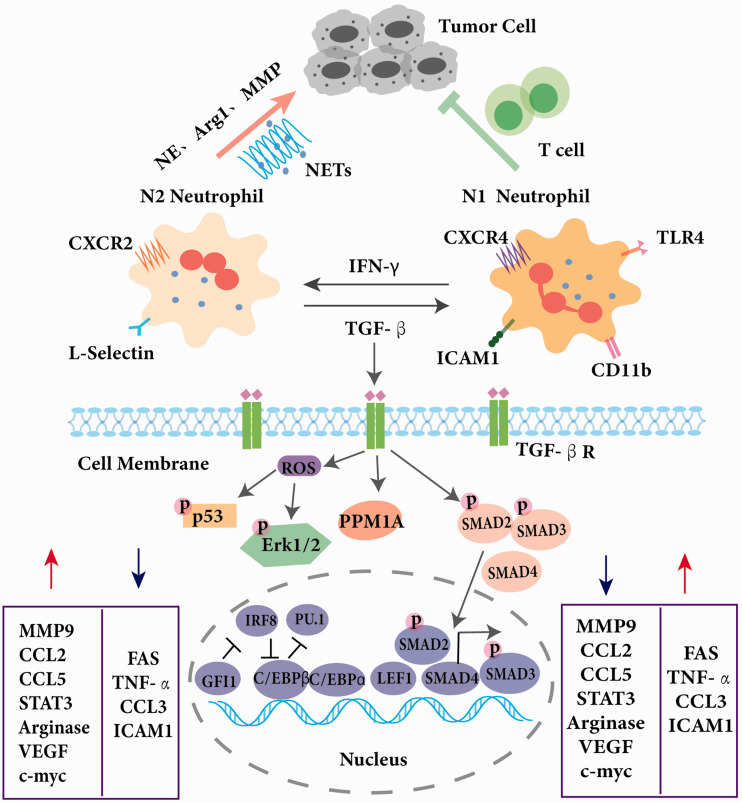
Although it’s becoming well-accepted that neutrophil populations are heterogeneous, the mechanisms underlying neutrophil diversity remain unclear. TGFβ is of particular interest as a neutrophil reprogramming factor in tumor progression, which results in activated transcription factors such as SMAD binding to different sites and diverse transcriptional outputs.63,70 Opposing the actions of TGFβ, interferon signaling instructs antitumoral properties in neutrophils. Type I interferon (IFN) treatment results in N1 polarization in vivo, exhibiting an increased tumor cell cytotoxicity and an immunoactivating ability (Figure 2).71 With the rapid development of analytical technologies such as genomic analyses at the single cell resolution, more candidates that contribute to neutrophil diversity will be identified for further exploration.
Figure 2.
Characterization of different neutrophil subsets. Neutrophils can be divided into N1 phenotype and N2 phenotype in a tumor environment. N2 neutrophils can be polarized into N1 neutrophils under the induction of TGF-β while N1 neutrophils can be transformed to be immature under the induction of IFN-γ. Compared with N2, N1 neutrophils downregulate FAS, TNF-α, CCL3, and ICAM1 and up-regulate MMP9, CCL2, CCL5, STAT3, Arginase, VEGF, and c-myc. N2 neutrophils could promote development of tumors while N1 neutrophils inhibit tumor progression. Multiple transcription factors determine maturation or reprogramming of neutrophil. TGF-β binds to the TGF-β receptor (TGF-βR), stimulating the generation of reactive oxygen species (ROS), which activates p53, Erk1/2, and the Smad family, and inducing downstream target gene transcription. Neutrophil development requires the expression of C/EBP which antagonizes PU.1 transcriptional activity. LEF and the transcriptional repressor GFI1 have a crucial role in neutrophil development. C/EBP, CCAAT/enhancer-binding protein; GFI1, growth factor-independent protein 1; LEF1, lymphoid enhancer-binding factor 1; STAT3, signal transducer and activator of transcription 3; VEGF, vascular endothelial growth factor; TGF-β, transforming growth factor β.
Potential interactions between neutrophils and brain microglia
Resident microglia are specialized macrophages in CNS and serve as regulators in homeostasis.72,73 In response to acute brain injuries such as stroke, microglia are the first immune cells being activated as CNS‐resident cells,31,74 thus joining in the complex immune network post-stroke. However, the activation of microglia is not invariable, and the diversity of microglia has been exhibited in the concept of M1 and M2 microglia polarization and other activation states, which is similar to neutrophils.75,76 Notably, interactions between neutrophils and brain microglia have been discovered, which may be potential mechanisms underlying the pathological role of neutrophils in ischemic stroke. Microglia phagocytose neutrophils in the ischemic brain.77,78 To be specific, brain resident microglia recognize the endothelial activation and the ensuing neutrophil invasion rapidly after stroke.79,80 Then neutrophils induce morphological alterations of microglia and together they formed cytoplasmic processes to defense activated endothelia and trap infiltrating neutrophils.77 In addition, microglia degenerate in the ischemic core and then neutrophils accumulate first in the perivascular spaces and later in parenchyma. When microglia function is reduced or microglia are eliminated by targeting colony stimulating factor 1 receptor (CSF1R), the number of neutrophils are increased and brain lesions are enlarged.81,82 Hence, targeting microglial phagocytic function may be a strategy to defense against the vascular and tissue damaging of neutrophils in ischemic stroke.
Potential targets or therapeutic strategies targeting neutrophils to limit brain damage following ischemic stroke
Though there has been abundant proof that neutrophils play an important role in stroke, the complex mechanisms underlying the role of neutrophils in brain damage and recovery still remain unclear due to their heterogeneity, both in phenotype and function.83 Regardless of the unknown mechanisms, the concept that neutrophils can be a potential target for stroke intervention is widely accepted and of great interest. According to the increasing research on stroke-related immune changes, neutrophils are primarily considered to be detrimental after stroke.27 For example, very-late-antigen-4 (VLA-4)-mediated brain invasion of neutrophils leads to interactions with microglia, increased ischemic injury, and impaired behavior in experimental stroke.77 Acutely depleting T cells84 and inhibiting brain infiltration of neutrophils might, therefore, be a powerful early stroke treatment. In addition, carcinoembryonic antigen–related cell adhesion molecule 1 (CEACAM1) inhibited MMP-9–mediated BBB breakdown in a mouse model for ischemic stroke, and thus may be an important inhibitory regulator of neutrophil-mediated tissue damage and BBB breakdown in focal cerebral ischemia.85 Alternatively, application of an IL-17A–blocking antibody within 3 hours after stroke induction decreased infarct size and improved neurologic outcome in a murine stroke model, so selective targeting of IL-17A signaling might provide a new therapeutic option for the treatment of stroke (Table 2).86 Furthermore, despite the unknowns raised above, the latest identified mechanisms related to the harmful effects of neutrophils and the development of possible therapeutic strategies are discussed below.
Table 2.
Potential therapeutic strategies targeting neutrophils to limit brain damage following ischemic stroke.
| Treatment | Targets | cFunction on stroke | References |
|---|---|---|---|
| PAD inhibition | NETs | Mouse-increased neovascularization and vascular repair; improved functional recovery | 96 |
| CypD depletion in platelets | CypD | Mouse-enhanced cerebral blood flow, improved neurological and motor functions, and reduced infarct volume | 102 |
| BR therapy | MMP-9 | Mouse-a reduced level of MMP-9 and improved stroke outcomes | 118 |
| Blockade of VLA-4 | VLA-4 | Mouse-reduced the ischemic tissue injury and improved behavioral impairment | 77 |
| CEACAM1 knock out | CEACAM1 | Mouse-inhibited MMP-9–mediated BBB breakdown | 85 |
| IL-17A–blocking antibody | IL-17A | Mouse- decreased infarct size and improved neurologic outcome | 86 |
Neutrophil extracellular traps
Recent evidence has suggested that neovascularization after brain injury is functionally important for an endogenous repair process in that the blockade of angiogenic responses leads to worsened outcomes after cerebral ischemia.87,88 In addition to their well-known function to kill bacteria, activated neutrophils also release nuclear and granular contents to form extensive web-like structures of DNA (NETs),89 which contain double-stranded DNA, histone, and granule proteins including neutrophil elastase, cathepsin G, and myeloperoxidase (MPO).90 NETs have been associated with autoimmune disorders,91 cardiovascular and pulmonary diseases,92,93 inflammation,94 and thrombosis.95 Recently, neutrophils have been described as producing intravascular and intraparenchymal NETs peaking at 3–5 days; neutrophil depletion reduces BBB breakdown and enhances neovascularization at 14 days.96 Furthermore, peptidylarginine deiminase 4 (PAD4),97,98 a key enzyme in NET formation, was markedly upregulated in the peri-ischemic cortex; overexpression of PAD4 resulted in amplified vascular damage and reduced neovascularization by releasing more NETs. Consistently, when PAD was pharmacologically inhibited or NET formation was inhibited, increased neovascularization and vascular repair and functional recovery improvement can be observed. Collectively, these results suggest that NETs are key targets for promoting stroke-mediated neovascularization and the resulting functional recovery.
Cyclophilin D-mediated platelet necrosis
Antiplatelet agents are used to prevent ischemic stroke but remain incompletely effective, with evidence indicating that platelets contribute to ischemic stroke through mechanisms which remain unclear.99 Both preclinical and clinical studies have shown that in addition to the accumulation of neutrophils in vessels in the brain, platelet-neutrophil interactions also drive inflammatory signaling and exacerbate brain injury after stroke.11,100,101 To explore the mechanism of how platelets interact with neutrophil in stroke, a novel study targeted cyclophilin D (CypD), a mediator regulating procoagulant platelet formation and platelet necrosis.102,103 Mice with a platelet-specific deletion of CypD (CypDplt–/–mice) exhibited significantly enhanced cerebral blood flow, improved neurological and motor functions, and reduced infarct volume after cerebral ischemia-reperfusion injury, which can at least partly be attributable to platelet-neutrophil interactions, since more circulating platelet-neutrophil aggregates (PNAs) were found in CypDplt+/+ mice 24 hours after stroke. Accordingly, the number of neutrophils and PNAs recruitment to the brain in mice with CypD-deficient platelets after stroke was reduced. Moreover, ex vivo-formed PNAs also displayed a tendency for necrotic platelets to interact with neutrophils. All these results promote the concept that targeting platelet CypD may be a potential therapeutic strategy to improve brain damage post stroke, especially reperfusion injury.
MMP-9 blockade and blood substitution therapy
Stroke is known as not only a disruption of cerebral blood flow, but also a dynamic breach of BBB observed both in animal stroke models and stroke patients.104 Following stroke and the disruption of BBB, a hyperinflammatory reaction is initiated, which is characteristic with an increase in inflammatory cells, cytokines, and chemokines in circulating blood.105 MMP-9, one of the proteinases secreted by activated neutrophils, may cause BBB leakage, extracellular matrix degeneration, and evolution of cerebral ischemia.106 Although MMP-9 is no longer a novel target for ischemic stroke, MMP-9 blockade or inhibition can be of therapeutic importance in ischemic stroke and improve stroke outcomes.107 It has been investigated that MMP-9 inhibitors DP-b9938, KB-R778539, SB-3CT40, and BB-9437 protect stroke outcomes. Besides, Mir-212 is a potential regulating mRNA of MMP9, which can promote recovery function and vascular regeneration of EPCs via downregulating MMP9 expression in ischemic stroke.108 Furthermore, icariside II (ICS II) has been found to significantly ameliorate I/R-induced BBB disruption and neuronal apoptosis in MCAO rats by regulating the MMP-9/TIMP1 balance.109
A recent study investigated a therapeutic strategy for stroke: substituting stroke mouse blood with whole blood obtained from naïve, healthy donor mice, called blood replacement (BR) therapy. This paradigm reduces infarct volume and improves neurological deficits.110 In addition, BR therapy robustly decreases neutrophils in blood according to analyses of immune cell subsets, as well as reduces levels of MMP-9 in the plasma and brains as determined by electrochemiluminescence detection. On the other side, the addition of MMP-9 in blood diminishes therapeutic effects of BR therapy. Taken together, BR therapy leads to profoundly improved stroke outcomes in mice, and a reduced level of MMP-9 could be one of the mechanisms by which BR achieves these outcomes. Currently, blood-based therapies are emerging as therapeutic to combat aging and fight neurodegenerative diseases. Researchers are increasingly aware that it is not likely that the complex pathological changes following a stroke can be treated by single medication. Although BR therapy is still an early experimental therapeutic approach with a long way to go to treat stroke patients in the clinic, the proposed therapy demonstrates a strategy that targets the pathological systemic responses to stroke,110 offering new insights into the mechanisms of stroke damage.
Given the highly successful rate of thrombectomy in patients with large vessel occlusions nowadays, however, nearly half of the patients do not experience clinical improvement.111 This lower-than-expected efficacy has been attributed to subsequent arterial re-occlusion or to the “no-reflow phenomenon, when microcirculatory flow is not restored in small arterioles and capillaries, despite full recanalization of the large artery.111 Stroke Treatment Academic Industry Roundtable X meeting in Washington, DC, in October 2017 developed consensus recommendations to facilitate the successful translation of cerebroprotective therapies in the new era of highly effective reperfusion. In the era of increasing recanalization rates, there are new opportunities to restudy and repurpose previous neuroprotective agents as well as to develop new neuroprotective agents as adjunctive treatments to reperfusion therapy. Therefore, those neutrophil targeted therapies might also be revisited and hold the promise for adjunctive treatments for stroke.
Concluding remarks and future perspectives
The convoluted role of immune responses in the field of stroke is of ballooning attention, accompanied by increased understanding of the involvement of neutrophils in both animal models and stroke patients. The neutrophil population has been divided into two opposing subsets, namely protumoral/pro-inflammatory and antitumoral/anti-inflammatory subsets. However, neutrophil populations are heterogeneous, and this kind of simple classification based on the traditional criteria such as localization and function is limited according to results derived from the swiftly emerging techniques including sc-RNA sequencing. To achieve a precise and all-around palette of neutrophil population, their phenotypic, transcriptional and functional profiles should be all taken into consideration. As one of the protagonists in stroke, the role of neutrophils in stroke is relatively unknown compared to their more established roles in cancer or inflammation, making further research an urgent priority. Although clarifying neutrophil subpopulations may require tremendous efforts, it might also be an excellent opportunity to identify a specific subgroup as a marker for diagnosis or prognosis rather than reaching a consensus on categorization itself. Meanwhile, dissection of plausible mechanisms of neutrophil heterogeneity and regulatory signals in neutrophil maturation and differentiation may facilitate the investigation of therapeutic targets for stroke. In the era of highly effective reperfusion, new term “brain cytoprotection” was raised instead of “neuroprotection” to more accurately describe the intended goal of protecting all brain components affected in stroke. Finally, since there have been potential targets for stroke discovered recently, particularly in the immune system, it is indeed necessary to take a step in translational research to fulfill the blueprint of novel therapeutic strategies.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: P. L. is supported by the National Natural Science Foundation of China (NSFC, 91, 957, 111, 819, 710, 968, 172,201, 782, 061, 130, 224), New Frontier Technology Joint Research sponsored by Shanghai Shenkang Hospital Development Center (SHDC12019102), and Shanghai Municipal Education Commission-Gaofeng Clinical Medical Grant Support (20,181,805), “Shuguang Program” (20SG17) supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission and the “Outstanding Academic Leaders Plan” (20XD1422400) supported by Shanghai Municipal Science and Technology Committee of Shanghai. P. L. is also supported by a Newton Advanced Fellowship grant provided by the UK Academy of Medical Sciences (NAF\R11\1010).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the authorship, and/or publication of this article.
References
- 1.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet (London, England) 2019; 394: 1145–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global, regional and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019; 18: 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu L, Wu D, Yang T, et al. Hypothermic neuroprotection against acute ischemic stroke: the 2019 update. J Cereb Blood Flow Metab 2020; 40: 461–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Nuñez G.Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamorro Á, Meisel A, Planas A, et al. The immunology of acute stroke. Nat Rev Neurol 2012; 8: 401–410. [DOI] [PubMed] [Google Scholar]
- 6.Iadecola C, Anrather J.The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Shen J, Zhao H. Ischemic postconditioning for stroke treatment: current experimental advances and future directions. Cond Med 2020; 3: 104–115. [PMC free article] [PubMed]
- 8.Yang J, Shakil F, Cho S.Peripheral mechanisms of remote ischemic conditioning. Cond Med 2019; 2: 61–68. [PMC free article] [PubMed] [Google Scholar]
- 9.An C, Shi Y, Li P, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol 2014; 115: 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enzmann G, Mysiorek C, Gorina R, et al. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol 2013; 125: 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 2015; 129: 239–257. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney M, Zhao Z, Montagne A, et al. Blood-brain barrier: from physiology to disease and back. Physiol Rev 2019; 99: 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Z, Nelson A, Betsholtz C, et al. Establishment and dysfunction of the blood-brain barrier. Cell 2015; 163: 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Profaci C, Munji R, Pulido R, et al. The blood-brain barrier in health and disease: important unanswered questions. The J Exp Med 2020; 217: e20190062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlokovic B.Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurgur H, Pinteaux E.Microglia in the neurovascular unit: blood-brain barrier-microglia interactions after central nervous system disorders. Neuroscience 2019; 405: 55–67. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Xiong X, Zhang L, et al. Neurovascular unit: a critical role in ischemic stroke. CNS Neurosci Ther 2021; 27: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iadecola C.The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zlokovic B.The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57: 178–201. [DOI] [PubMed] [Google Scholar]
- 20.Jickling G, Liu D, Ander B, et al. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 2015; 35: 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Zhu Z, Huang T, et al. The peripheral immune response after stroke—a double edge sword for blood-brain barrier integrity. CNS Neurosci Ther 2018; 24: 1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amantea D, Nappi G, Bernardi G, et al. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. Febs J 2009; 276: 13–26. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Song T, Park J, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis 2012; 222: 464–467. [DOI] [PubMed] [Google Scholar]
- 24.Silvestre-Roig C, Braster Q, Ortega-Gomez A, et al. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol 2020; 17: 327–340. [DOI] [PubMed] [Google Scholar]
- 25.Weston RM, Jones NM, Jarrott B, et al. Inflammatory cell infiltration after endothelin-1-induced cerebral ischemia: histochemical and myeloperoxidase correlation with temporal changes in brain injury. J Cereb Blood Flow Metab 2007; 27: 100–114. [DOI] [PubMed] [Google Scholar]
- 26.Cui LL, Zhang Y, Chen ZY, et al. Early neutrophil count relates to infarct size and fatal outcome after large hemispheric infarction. CNS Neurosci Ther 2020; 26: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdener SE, Tang J, Kilic K, et al. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: a hyperacute role for neutrophils in persistent traffic jams. J Cereb Blood Flow Metab 2021; 41: 236–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin R, Yang G, Li G.Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai W, Liu S, Hu M, et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res 2020; 11: 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yilmaz G, Granger D.Cell adhesion molecules and ischemic stroke. Neurol Res 2008; 30: 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009; 40: 1849–1857. [DOI] [PubMed] [Google Scholar]
- 32.Price C, Menon D, Peters A, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke 2004; 35: 1659–1664. [DOI] [PubMed] [Google Scholar]
- 33.Barone F, Hillegass L, Price W, et al. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res 1991; 29: 336–345. [DOI] [PubMed] [Google Scholar]
- 34.del Zoppo G, Schmid-Schönbein G, Mori E, et al. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991; 22: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 35.Doycheva D, Hadley T, Li L, et al. Anti-neutrophil antibody enhances the neuroprotective effects of G-CSF by decreasing number of neutrophils in hypoxic ischemic neonatal rat model. Neurobiol Dis 2014; 69: 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkind M, Veltkamp R, Montaner J, et al. Natalizumab in acute ischemic stroke (ACTION II): a randomized, placebo-controlled trial. Neurology 2020; 95: e1091–e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llovera G, Hofmann K, Roth S, et al. Results of a preclinical randomized controlled multicenter trial (pRCT): anti-CD49d treatment for acute brain ischemia. Sci Transl Med 2015; 7: 299ra121. [DOI] [PubMed] [Google Scholar]
- 38.Aronowski J, Roy-O'Reilly M.Neutrophils, the felons of the brain. Stroke 2019; 50: e42–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grønberg N, Johansen F, Kristiansen U, et al. Leukocyte infiltration in experimental stroke. J Neuroinflammation 2013; 10: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalimo H, del Zoppo G, Paetau A, et al. Polymorphonuclear neutrophil infiltration into ischemic infarctions: myth or truth? Acta Neuropathol 2013; 125: 313–316. [DOI] [PubMed] [Google Scholar]
- 41.Fridlender Z, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009; 16: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giese M, Hind L, Huttenlocher A.Neutrophil plasticity in the tumor microenvironment. Blood 2019; 133: 2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagiv J, Michaeli J, Assi S, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 2015; 10: 562–573. [DOI] [PubMed] [Google Scholar]
- 44.Wirtz S, Tubbe I, Galle P, et al. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med 2006; 203: 1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao X, Ting S, Liu C, et al. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat Commun 2017; 8: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McColl S, Paquin R, Ménard C, et al. Human neutrophils produce high levels of the interleukin 1 receptor antagonist in response to granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha. J Exp Med 1992; 176: 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor R, Chang C, Goods B, et al. TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest 2017; 127: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silvestre-Roig C, Hidalgo A, Soehnlein O.Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 2016; 127: 2173–2181. [DOI] [PubMed] [Google Scholar]
- 49.Yvan-Charvet L, Ng L.Granulopoiesis and neutrophil homeostasis: a metabolic, daily balancing act. Trends Immunol 2019; 40: 598–612. [DOI] [PubMed] [Google Scholar]
- 50.Jaillon S, Ponzetta A, Di Mitri D, et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer 2020; 20: 485–503. [DOI] [PubMed] [Google Scholar]
- 51.Ng L, Ostuni R, Hidalgo A.Heterogeneity of neutrophils. Nat Rev Immunol 2019; 19: 255–265. [DOI] [PubMed] [Google Scholar]
- 52.Coffelt S, Wellenstein M, de Visser K.Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016; 16: 431–446. [DOI] [PubMed] [Google Scholar]
- 53.Silvestre-Roig C, Fridlender Z, Glogauer M, et al. Neutrophil diversity in health and disease. Trends Immunol 2019; 40: 565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vafadarnejad E, Rizzo G, Krampert L, et al. Dynamics of cardiac neutrophil diversity in murine myocardial infarction. Circ Res 2020; 127: e232–e249. [DOI] [PubMed] [Google Scholar]
- 55.Xie X, Shi Q, Wu P, et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol 2020; 21: 1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishalian I, Bayuh R, Levy L, et al. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother 2013; 62: 1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galdiero M, Varricchi G, Loffredo S, et al. Roles of neutrophils in cancer growth and progression. J Leukoc Biol 2018; 103: 457–464. [DOI] [PubMed] [Google Scholar]
- 58.Zilionis R, Engblom C, Pfirschke C, et al. Single-Cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 2019; 50: 1317–1334.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soehnlein O, Steffens S, Hidalgo A, et al. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 2017; 17: 248–261. [DOI] [PubMed] [Google Scholar]
- 60.Zenaro E, Pietronigro E, Della Bianca V, et al. Neutrophils promote alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 2015; 21: 880–886. [DOI] [PubMed] [Google Scholar]
- 61.Fabene P, Navarro Mora G, Martinello M, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med 2008; 14: 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ecker S, Chen L, Pancaldi V, et al. BLUEPRINT Consortium. Genome-wide analysis of differential transcriptional and epigenetic variability across human immune cell types. Genome Biol 2017; 18: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monticelli S, Natoli G.Transcriptional determination and functional specificity of myeloid cells: making sense of diversity. Nat Rev Immunol 2017; 17: 595–607. [DOI] [PubMed] [Google Scholar]
- 64.Kueh H, Champhekar A, Champhekhar A, et al. Positive feedback between PU.1 and the cell cycle controls myeloid differentiation. Science (New York, N.Y.) 2013; 341: 670–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ostuni R, Natoli G, Cassatella M, et al. Epigenetic regulation of neutrophil development and function. Semin Immunol 2016; 28: 83–93. [DOI] [PubMed] [Google Scholar]
- 66.Person R, Li F, Duan Z, et al. Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nat Genet 2003; 34: 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skokowa J, Cario G, Uenalan M, et al. LEF-1 is crucial for neutrophil granulocytopoiesis and its expression is severely reduced in congenital neutropenia. Nat Med 2006; 12: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 68.Karsunky H, Zeng H, Schmidt T, et al. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat Genet 2002; 30: 295–300. [DOI] [PubMed] [Google Scholar]
- 69.Yáñez A, Ng M, Hassanzadeh-Kiabi N, et al. IRF8 acts in lineage-committed rather than oligopotent progenitors to control neutrophil vs monocyte production. Blood 2015; 125: 1452–1459. [DOI] [PubMed] [Google Scholar]
- 70.Mullen A, Orlando D, Newman J, et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell 2011; 147: 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohms M, Möller S, Laskay T.In vitroAn attempt to polarize human neutrophils toward N1 and N2 phenotypes. Front Immunol 2020; 11: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saijo K, Glass C.Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 2011; 11: 775–787. [DOI] [PubMed] [Google Scholar]
- 73.Li L, Huang Y, Yang Z, et al. Potential microglia-based interventions for stroke. CNS Neurosci Ther 2020; 26: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berchtold D, Priller J, Meisel C, et al. Interaction of microglia with infiltrating immune cells in the different phases of stroke. Brain Pathol )2020; 30: e12911–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernier L, York E, Kamyabi A, et al. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat Commun 2020; 11: 1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sankowski R, Böttcher C, Masuda T, et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci 2019; 22: 2098–2110. [DOI] [PubMed] [Google Scholar]
- 77.Neumann J, Riek-Burchardt M, Herz J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol 2015; 129: 259–277. [DOI] [PubMed] [Google Scholar]
- 78.Neumann J, Henneberg S, von Kenne S, et al. Beware the intruder: Real time observation of infiltrated neutrophils and neutrophil-microglia interaction during stroke in vivo. PLoS One 2018; 13: e0193970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faustino J, Chip S, Derugin N, et al. CX3CR1-CCR2-dependent monocyte-microglial signaling modulates neurovascular leakage and acute injury in a mouse model of childhood stroke. J Cereb Blood Flow Metab 2019; 39: 1919–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang X, Xuan W, Zhu ZY, et al. The evolving role of neuro-immune interaction in brain repair after cerebral ischemic stroke. CNS Neurosci Ther 2018; 24: 1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Otxoa-de-Amezaga A, Miró-Mur F, Pedragosa J, et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol 2019; 137: 321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Green K, Crapser J, Hohsfield L.To kill a microglia: a case for CSF1R inhibitors. Trends Immunol 2020; 41: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garcia-Culebras A, Duran-Laforet V, Pena-Martinez C, et al. Myeloid cells as therapeutic targets in neuroinflammation after stroke: specific roles of neutrophils and neutrophil-platelet interactions. J Cereb Blood Flow Metab 2018; 38: 2150–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou YX, Wang X, Tang D, et al. IL-2mAb reduces demyelination after focal cerebral ischemia by suppressing CD8(+) T cells. CNS Neurosci Ther 2019; 25: 532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ludewig P, Sedlacik J, Gelderblom M, et al. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits MMP-9-mediated blood-brain-barrier breakdown in a mouse model for ischemic stroke. Circ Res 2013; 113: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 86.Gelderblom M, Weymar A, Bernreuther C, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 2012; 120: 3793–3802. [DOI] [PubMed] [Google Scholar]
- 87.Horie N, Pereira M, Niizuma K, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells ( Cells 2011; 29: 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Z, Chopp M.Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 2009; 8: 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nathan C.Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006; 6: 173–182. [DOI] [PubMed] [Google Scholar]
- 90.Urban C, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 2009; 5: e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dwivedi N, Radic M.Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Ann Rheum Dis 2014; 73: 483–491. [DOI] [PubMed] [Google Scholar]
- 92.Mangold A, Alias S, Scherz T, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 2015; 116: 1182–1192. [DOI] [PubMed] [Google Scholar]
- 93.Ebrahimi F, Giaglis S, Hahn S, et al. Markers of neutrophil extracellular traps predict adverse outcome in community-acquired pneumonia: secondary analysis of a randomised controlled trial. Eur Respir J 2018; 51: 1701389. [DOI] [PubMed] [Google Scholar]
- 94.Schauer C, Janko C, Munoz L, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 2014; 20: 511–517. [DOI] [PubMed] [Google Scholar]
- 95.Martinod K, Wagner D.Thrombosis: tangled up in NETs. Blood 2014; 123: 2768–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang L, Yu H, Yang X, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun 2020; 11: 2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tanikawa C, Ueda K, Suzuki A, et al. Citrullination of RGG motifs in FET proteins by PAD4 regulates protein aggregation and ALS susceptibility. Cell Rep 2018; 22: 1473–1483. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hackam D, Spence J.Antiplatelet therapy in ischemic stroke and transient ischemic attack. Stroke 2019; 50: 773–778. [DOI] [PubMed] [Google Scholar]
- 100.Ritzel R, Lai Y, Crapser J, et al. Aging alters the immunological response to ischemic stroke. Acta Neuropathol 2018; 136: 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sreeramkumar V, Adrover J, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science (New York, NY) 2014; 346: 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Denorme F, Manne B, Portier I, et al. Platelet necrosis mediates ischemic stroke outcome in mice. Blood 2020; 135: 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hua V, Abeynaike L, Glaros E, et al. Necrotic platelets provide a procoagulant surface during thrombosis. Blood 2015; 126: 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sarvari S, Moakedi F, Hone E, et al. Mechanisms in blood-brain barrier opening and metabolism-challenged cerebrovascular ischemia with emphasis on ischemic stroke. Metab Brain Dis 2020; 35: 851–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan T, Chen Z, Chopp M, et al. Inflammatory responses mediate brain-heart interaction after ischemic stroke in adult mice. J Cereb Blood Flow Metab 2020; 40: 1213–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.del Zoppo G.The neurovascular unit, matrix proteases, and innate inflammation. Ann N Y Acad Sci 2010; 1207: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asahi M, Asahi K, Jung J, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab 2000; 20: 1681–1689. [DOI] [PubMed] [Google Scholar]
- 108.Hu C, Dong ZL.MicroRNA-212 promotes the recovery function and vascular regeneration of endothelial progenitor cells in mice with ischemic stroke through inactivation of the notch signaling pathway via downregulating MMP9 expression. J Cell Physiol 2019; 234: 7090–7103. [DOI] [PubMed] [Google Scholar]
- 109.Liu M, Wang W, Gao J, et al. Icariside II attenuates cerebral ischemia/reperfusion-induced blood-brain barrier dysfunction in rats via regulating the balance of MMP9/TIMP1. Acta Pharmacol Sin 2020; 41: 1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ren X, Hu H, Farooqi I, et al. Blood substitution therapy rescues the brain of mice from ischemic damage. Nat Commun 2020; 11: 4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erdener Ş, Tang J, Kılıç K, Postnov D, et al. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: a hyperacute role for neutrophils in persistent traffic jams. J Cereb Blood Flow Metab 2021; 41: 236--252. [DOI] [PMC free article] [PubMed]
- 112.Sas A, Carbajal K, Jerome A, et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol 2020; 21: 1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Denny M, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 1950; 184: 3284–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gupta S, Kaplan M.The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 2016; 12: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Villanueva E, Yalavarthi S, Berthier C, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011; 187: 538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coffelt S, Kersten K, Doornebal C, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015; 522: 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Finisguerra V, Di Conza G, Di Matteo M, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 2015; 522: 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol 2020; 19: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]