Abstract
Colony-stimulating factor 1 receptor (CSF1R) is a specific biomarker for microglia. In this study, we developed a novel PET radioligand for CSF1R, 11C-GW2580, and compared it to a reported CSF1R tracer, 11C-CPPC, in mouse models of acute and chronic neuroinflammation and a rhesus monkey. Dynamic 11C-GW2580- and 11C-CPPC-PET images were quantified by reference tissue-based models and standardized uptake value ratio. Both tracers exhibited increased uptake in the lesioned striata of lipopolysaccharide-injected mice and in the forebrains of AppNL-G-F/NL-G-F-knock-in mice, spatially in agreement with an increased 18-kDa translocator protein radioligand retention. Moreover, 11C-GW2580 captured changes in CSF1R availability more sensitively than 11C-CPPC, with a larger dynamic range and a smaller inter-individual variability, in these model animals. PET imaging of CSF1R in a rhesus monkey displayed moderate-to-high tracer retention in the brain at baseline. Homologous blocker (i. e. unlabeled tracer) treatment reduced the uptake of 11C-GW2580 by ∼30% in all examined brain regions except for centrum semi-ovale white matter, but did not affect the retention of 11C-CPPC. In summary, our results demonstrated that 11C-GW2580-PET captured inflammatory microgliosis in the mouse brain with higher sensitivity than a reported radioligand, and displayed saturable binding in the monkey brain, potentially providing an imaging-based quantitative biomarker for reactive microgliosis.
Keywords: colony-stimulating factor 1 receptor, positron emission tomography; PET; neuroinflammation; 11C-GW2580; microglia
Introduction
Neuroinflammation has been recently acknowledged as one of the key participants in a variety of CNS disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis, and schizophrenia.1 In support of this notion, state-of-art gene profiling techniques, as well as genome-wide association analyses, have identified many risk loci that are enriched, or exclusively expressed in myeloid cells in AD and PD.2,3 In addition, positron-emission tomography (PET) imaging of translocator protein 18 kDa (TSPO) revealed a dynamic involvement of gliosis in the disease continuum.4,5 These new findings highlight the importance of further understanding the roles played by neuroinflammation in the CNS pathogenesis and appropriate means to manipulate such a process against hitherto untreatable brain diseases. TSPO-PET has been widely used as an in-vivo imaging tool to study reactive microgliosis in nonclinical and clinical settings. However, several well-known caveats of this approach, exemplified by the influence of TSPO polymorphisms on the radioligand binding and expression of TSPO in non-microglial cell species, hamper the accurate interpretation of imaging findings.6 In addition, diverse markers of neuroglial activation, besides TSPO, are necessary to characterize alterations of microglial phenotypes during the course of the disease. Hence, a variety of PET tracers have been developed for imaging of other microglial components potentially reflecting these phenotypes, including cyclooxygenase-2,7 cannabinoid receptor type 2,8 and P2X purinoceptor 7.9
To date, colony-stimulating factor 1 receptor (CSF1R) has also drawn attention as a target molecule for imaging and therapeutics, since it is predominantly expressed by microglia in the CNS, regulating the survival, differentiation, and proliferation of these cells.10 Mounting evidence has suggested the involvement of CSF1R signaling in a wide range of brain diseases. In fact, altered levels of CSF1R have been found in amyotrophic lateral sclerosis,11 multiple sclerosis,12 and traumatic brain injury.13 CSF1R was also reported to increase in amyloid plaque-associated microglia in a transgenic mouse model of AD.14 In addition, pharmacological inhibition of CSF1R in these AD mice mitigated microgliosis and converted the microglial phenotype from a pro-inflammatory to an anti-inflammatory mode.14 Furthermore, depletion of microglia with a CSF1R inhibitor halted the propagation of tau pathologies in a mouse model of tauopathy.15
In line with the demand for in-vivo visualization of CSF1R, a PET radioligand for this receptor, 11C-CPPC (5-cyano-N-(4-(4-11C-methylpiperazin-1-yl)-2-(piperidin-1-yl)phenyl)furan-2-carboxamide), was developed by Horti et al.16 The tracer captured neuroinflammatory changes in animal models of acute inflammation induced by lipopolysaccharide (LPS) injection and chronic inflammation triggered by amyloid depositions. In the present study, we developed a novel PET ligand, 11C-GW2580, based on the chemical structure of a CSF1R inhibitor, GW2580 (IC50=10 nM),17 and conducted head-to-head comparisons of this radiocompound and 11C-CPPC in quantitative PET imaging of CSF1R in mice presenting acute and chronic neuroinflammation similar to the above-mentioned models. The kinetics and binding saturability of these radioligands were also evaluated in a rhesus monkey. Postmortem immunohistochemical assessments of the scanned mouse brains were also performed to correlate levels and localization of CSF1R with in-vivo observations.
Materials and methods
Radiochemistry
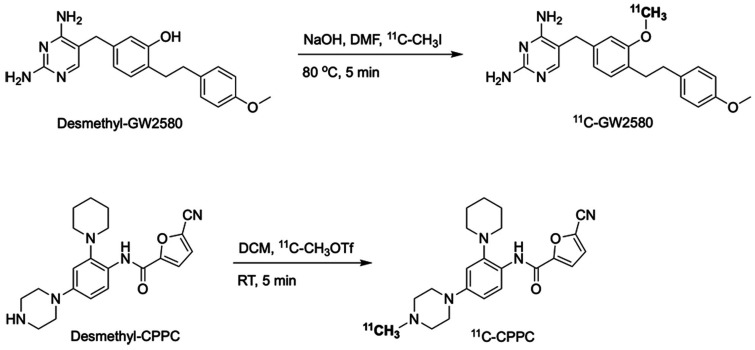
11C-GW2580 was synthesized by the reaction of its precursor (desmethyl-GW2580, 1 mg) with 11C-methyl iodide in the presence of sodium hydroxide (0.5 mol/L, 6 μL) in 300 μL of N,N-dimethylformamide for 5 min at 80 °C using a homemade automated synthesis system (Figure 1). The resulting radiolabeled product was purified by reversed-phase high-performance liquid chromatography (RP-HPLC) using Atlantis T3 OBD C18 column (5 μm, 10 × 250 mm) eluted with acetonitrile/50 mM ammonium acetate (45/55, v/v) at a flow rate of 3.5 mL/min and formulated in 3 mL of saline containing 100 μL of Tween 80 and 100 μL of 20% ascorbic acid.
Figure 1.
Radiosyntheses of 11C-GW2580 and 11C-CPPC. RT, room temperature; DMF, N,N-dimethylformamide; DCM, dichloromethane.
11C-CPPC was synthesized by the reaction of its precursor (desmethyl-CPPC, 0.5 mg) with 11C-methyl triflate in 300 μL of dichloromethane for 5 min at room temperature using a homemade automated synthesis system (Figure 1). The resulting radiolabeled product was purified by RP-HPLC using Phenomenex Luna C18 column (5 μm, 10 × 250 mm) eluted with acetonitrile/50 mM ammonium acetate (20/80, v/v) at a flow rate of 4 mL/min and formulated in 3 mL of saline containing 100 μL of Tween 80 and 100 μL of 25% ascorbic acid.
A TSPO radioligand, 11C-AC5216, was synthesized as described previously18 to track neuroinflammatory changes in our model animals.
Animals
All procedures involving animals and their care were conducted in accordance with Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of the National Institutes for Quantum and Radiological Science and Technology. All the animal study data were reported according to ARRIVE guidelines (Animal Research: Reporting in Vivo Experiment).
Mice were kept in a 12-h dark-light cycle. A rhesus monkey (#187; male; 7.5 kg; 12 years) was maintained in a 14-h light/10-h dark cycle. Food and water were provided ad libitum.
Acute inflammation model: FVB mice at 4–6 months of age (males, n = 13, CLEA Japan, Inc., Tokyo) were lesioned in the right striatum with 5 mg/mL LPS in 2 µL of saline using the following coordinate: anterior-posterior +1.75 mm, lateral +0.62 mm and dorsoventral −3.5 mm, relative to bregma. A 3.5-month-old male FVB mouse without surgery was used as a control mouse.
Chronic inflammation model: APP-KI mice (5 males/3 females, 16–22 months, n = 8) carrying Swedish K670N/M671L, the Arctic E693G, and the Iberian I716F mutations (AppNL-G-F/NL-G-F) on a C57BL/6J genetic background were included.19 Non-knock-in wild-type mice on a C57BL/6J genetic background (WT, 6 males/4 females, 16–21 months, n = 10, inhouse-bred) were used as control group.
Mice for whole body 11C-GW2580-PET/computed tomography (CT) imaging: mice with an FVB genetic background (males, 6–7 months of age, n = 3, inhouse-bred) were used to study the relationship between the radioactivity concentrations in the arterial blood and brain.
After the final scans, the brain tissues of several mice were collected for immunohistochemical studies and (E,E)-1-fluoro-2,5-bis(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (FSB, Dojindo Laboratories, Kumamoto) staining of amyloid-β plaques.
Analysis of 11C-GW2580 radiometabolites in monkey plasma
Arterial blood samples were collected from the monkey at 30 min and 90 min after injection of 11C-GW2580 during the baseline PET scan. The samples were centrifuged at 20,000 g for 3 min, and the supernatant was subsequently mixed with one volume of ice-cold acetonitrile followed by centrifugation at 20,000 g for 3 min. The resulting supernatant was injected into an HPLC system equipped with a highly sensitive detector for radioactivity. The radioactive components were eluted with a Phenomenex Luna C18 column (5 μm, 4.6 × 150 mm) and a mobile phase (methanol/50 mM ammonium acetate, 60/40, v/v) at a flow rate of 1 mL/min. The retention time for 11C-GW2580 was 6.6 min. Since the current study did not aim to perform a full kinetic analysis with arterial input function, no blood samples at additional time points were taken from the monkey.
PET measurements
Mice
Total 13 mice (#1 – #13) modeling acute neuroinflammation underwent a baseline PET scan with 11C-GW2580 (n = 7, #1 – #7), 11C-CPPC (n = 4, #8 – #11), or TSPO tracer 11C-AC5216 (n = 2, #12, #13) at 7 days after LPS treatment and with 11C-GW2580 (n = 3, #5 – #7) at 14 days. One day following the baseline scan, a PET scan with pretreatment of a homologous blocker was carried out for 11C-GW2580 (day 8, n = 7, #1 – #7; day 15, n = 3, #5 – #7) and 11C-CPPC (day 8, n = 4, #8 – #11). To compare the performance of 11C-GW2580 and 11C-CPPC in a head-to-head manner, PET imaging with 11C-GW2580 and 11C-CPPC was performed for the same individuals (n = 4, #8 – #11) at 12 days.
Ten WT control mice and eight APP-KI mice modeling chronic neuroinflammation received each of PET scans with 11C-GW2580 and 11C-CPPC for comparisons of these radioligands in the same individuals. In addition, respectively two of these APP-KI and WT mice also underwent 11C-AC5216-PET scans.
Animals were under isoflurane anesthesia (1.5-2%) during the PET scan period. The tail veins were catheterized for injection of 11C-GW2580 (51.5 ± 15.1 MBq, 0.7 ± 0.3 nmol), 11C-CPPC (48.9 ± 11.0 MBq, 0.4 ± 0.1 nmol), or 11C-AC5216 (27.7 ± 2.3 MBq, 0.2 ± 0.02 nmol). In the homologous blockade study, 1 mg/kg of the corresponding non-radioactive ligand was injected intravenously at 5 min prior to PET acquisitions. For all mice except for three animals undergoing the whole body PET/CT imaging, a 90- or 120-min PET acquisition using a microPET Focus220 system (Siemens Medical Solutions USA, Malvern, USA) was started immediately after the initiation of the radioligand injection. For the whole body 11C-GW2580-PET/CT imaging, one mouse underwent a baseline scan followed by a scan with the homologous blocker treatment. The other two mice had either a baseline scan or a scan with the homologous blocker treatment. A 90-min PET acquisition using a preclinical Inveon PET/CT scanner (Inveon®, Siemens Preclinical Solutions, Knoxville, TN) was acquired immediately after the injection of 11C-GW2580. A 2-min CT (Rigaku Co., Tokyo) scan was carried out after the PET scan.
Monkey
The monkey (#187) was initially anesthetized with an intramuscular injection of ketamine (10 mg/kg) and xylazine (0.2–0.5 mg/kg), and then intubated and kept anesthetized with 1–2% isoflurane. A 120-min dynamic PET acquisition using a microPET Focus220 system was started immediately after bolus injection of 11C-GW2580 (330.6 and 347.1 MBq, 2.47 nmol) or 11C-CPPC (364.5 and 377.8 MBq, 3.38 and 2.30 nmol). A transmission scan with a 68Ge-68Ga point source was carried out before PET acquisitions to generate an attenuation map. In the homologous blockade study, 1 mg/kg of the corresponding non-radioactive ligand was injected intravenously at 5 min prior to PET acquisitions.
PET data analysis
Mice
For the Focus220 system, list-mode emission data were sorted into 3 D sinograms and were then Fourier-rebinned into 2 D sinograms. The frames (90-min frames: 4 × 1 min, 8 × 2 min, 14 × 5 min; 120-min frames: 4 × 1 min, 8 × 2 min, 14 × 5 min, 3 × 10 min) were reconstructed by filtered back-projection. All images were sliced into a 128 × 128 × 95 matrix with a voxel size of 0.38 × 0.38 × 0.80 mm3.
After reconstruction, individual PET images were manually aligned to a T2-weighted magnetic resonance imaging (MRI) template of a mouse brain with predefined volumes of interest (VOIs) including the neocortex, striatum, thalamus, hippocampus, cerebellum, and entire forebrain to extract time-radioactivity curves (TACs) using PMOD software (version 3.8; PMOD Inc., Zurich, Switzerland). TACs were then normalized to body weight and injected dose to yield standardized uptake values (SUVs).
The uptake of 11C-GW2580 and 11C-CPPC was quantified using SUV ratio (SUVR) and distribution volume ratio (DVR) obtained by Ichise’s multilinear reference tissue model (MRTM) and reference-tissue Logan plot (RLogan) (PMOD version 3.8). The uptake of 11C-AC5216 was quantified using an averaged SUVR at 25–80 min (SUVR25–80min), as the SUVR became stable at 25 min after injection. Contralateral (non-lesioned) striatum was used as a pseudo-reference region for the lesioned striatum of the LPS-injected mice. The cerebellum was used as a pseudo-reference region for the chronic inflammation model.
For the Inveon system, the emission data were divided into 26 frames (4 × 1 min, 8 × 2 min, 14 × 5 min) and reconstructed by filtered-back projection with a 128 × 128 × 95 matrix and a voxel size of 0.78 × 0.78 × 0.80 mm3. VOIs were manually delineated in the brain and left cardiac ventricle on the PET-CT fusion images by a single observer, using PMOD software (Supplementary Fig. 1). TACs extracted from these VOIs were normalized to obtain SUVs. All analyses were performed by an analyzer who was blinded to the animal’s identity.
Monkey
PET data acquired from the monkey (#187) were reconstructed with corrections for attenuation and scatter. The resulting images were sliced into a 128 × 128 × 95 matrix with a voxel size of 0.95 × 0.95 × 0.80 mm3. After reconstruction, PET images were manually aligned to an individual T2-weighted MRI data with predefined VOIs, including the frontal cortex, caudate head, putamen, hippocampus, midbrain, pons, cerebellum, centrum semi-ovale white matter, and thalamus, to extract regional TACs using PMOD software. The uptake of 11C-GW2580 and 11C-CPPC was quantified as SUVR, DVR, and non-displaceable binding potential (BPND) derived from simplified reference tissue model (SRTM) with semi-ovale white matter as reference tissue.
Statistics
Results are expressed as mean ± standard deviation. Data were tested for normality using Kolmogorov-Smirnov test. As normality criteria was satisfied, unpaired two-tailed t-test with Bonferroni correction for multiple comparisons was used to assess the difference in DVRs and SUVRs between conditions. A probability value (p) <0.05 was considered statistically significant.
Histological examinations
The detailed procedures of immunohistochemistry and fluorescence microscopic imaging of brain slices derived from the scanned mice are described in the supplementary material.
Results
Radiochemistry
11C-GW2580 and 11C-CPPC were successfully radiolabeled within 35 min of the end of bombardment. 11C-GW2580 was obtained with a practical yield of 3.13 ± 0.55 GBq (n = 8), >99% radiochemical purity, and a molar activity of 102.8 ± 34.8 GBq/μmol (n = 9) at the time of injection. 11C-CPPC was obtained with a practical yield of 2.92 ± 0.99 GBq (n = 7), >98% radiochemical purity, and a molar activity of 122.9 ± 25.8 GBq/μmol (n = 9) at the time of injection. Both tracers were stable for at least 2 h after the end of syntheses.
PET imaging of CSF1R and TSPO in the LPS model
Representative PET images and TACs in the LPS-injected mouse brain after injection of 11C-GW2580 and 11C-CPPC are shown in Figure 2. TACs in a healthy 3.5-month-old FVB mouse are presented in Supplementary Fig. 2. 11C-GW2580 displayed slow kinetics, with uptake peaking at around 20 min after injection followed by a slow washout, whereas 11C-CPPC exhibited a fast clearance after the initial uptake peaking at around 2 min. SUVR for both tracers leveled off in the striatum ipsilateral to the LPS injection at 20 min (Figure 3(a)), and SUVR20-90min was in good agreement with DVR obtained by both MRTM (initial t (t*) = 4 min) and RLogan (t*=30 min), with less than 5% difference between these measures. Thus, SUVR20-90min was used as an outcome parameter denoting tracer binding in the lesioned striatum.
Figure 2.
(a) Representative coronal 11C-GW2580, 11C-CPPC, and 11C-AC5216 PET images of the mouse brain with lipopolysaccharide (LPS)-injection into the right striatum. The bottom row shows the PET images (presented in the top row) superimposed on an MRI template. The images were generated by averaging dynamic scan data at 15–90 min and are presented in neurological orientation. (b) Time-radioactivity curves of 11C-GW2580 and 11C-CPPC in the striata and cerebellum obtained from corresponding PET images from (a). The dotted circles delineate striata.
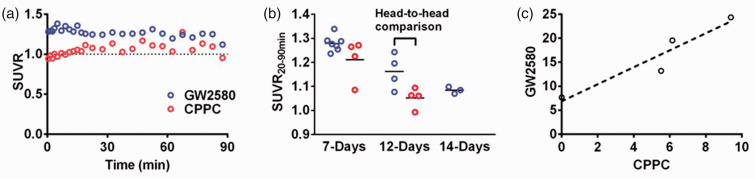
Figure 3.
(a) SUVRs for 11C-GW2580 and 11C-CPPC reached a steady state at 20 min in the lesioned striatum of a mouse at 12 days post LPS injection. (b) Time-course changes in average SUVR at 20–90 min (SUVR20–90min) in the LPS-injected striata. Each data point represents an individual animal. (c) Correlation of the radiotracer binding [calculated as (SUVR20–90min–1) × 100 (%)] between 11C-GW2580 and 11C-CPPC. The dashed line indicates linear regression (y = 1.8x +7). Each data point represents an individual animal.
As expected, a concomitant increase in SUVRs of radioligands for TSPO (106%, n = 2) and CSF1R (28 ± 3%, n = 7 for 11C-GW2580; 21 ± 9%, n = 4 for 11C-CPPC) was observed in the lesioned striatum at 7 days after LPS treatment (Figures 2 and 3). The self-blocking treatment in the following day reduced the tracer uptake in the lesioned striatum relative to the non-lesioned side (also noted as laterality, calculated as (SUVR20-90min-1)) by half for both 11C-GW2580 and 11C-CPPC (Supplementary Fig. 3). At 14 days post LPS treatment, 11C-GW2580 retention was 9 ± 2% higher in the injured striatum relative to the non-injured side (Figure 3(b)). This difference was completely abolished by pretreatment with unlabeled GW2580 in the self-blocking assay conducted at 15 days. In addition, a head-to-head comparison study has shown that 11C-GW2580 detected increased CSF1R availability in the lesioned striatum of all animals, and yielded larger ipsilateral-to-contralateral difference in radiosignal retention than 11C-CPPC (Supplementary Fig. 4, Figure 3(b) and (c)). Furthermore, a linear correlation was identified in laterality between 11C-GW2580 and 11C-CPPC retentions, with a 1.8-fold higher dynamic range and a lower detection threshold provided by 11C-GW2580 (Figure 3(c)).
PET imaging of CSF1R and TSPO in APP-KI mice
Representative SUVR30-90min images for 11C-GW2580, 11C-CPPC, and 11C-AC5216, and the corresponding TACs showing a head-to-head comparison of these three tracers in the same WT and APP-KI mice are presented in Supplementary Fig. 5. Tracer uptake in the cerebellum did not differ significantly across subjects (11C-GW2580 SUV60-90min: 0.56 ± 0.06 (WT) vs. 0.57 ± 0.08 (APP-KI), NS; 0.48 ± 0.18 (<20 month-old) vs. 0.58 ± 0.20 (>20 month-old), NS; 11C-CPPC SUV60-90min: 0.32 ± 0.05 (WT) vs. 0.32 ± 0.03 (APP-KI), NS; 0.30 ± 0.04 (<20 month-old) vs. 0.34 ± 0.03 (>20 month-old), NS), and the cerebellum was accordingly used as a reference region. The homologous blocker treatment in WT mice increased the overall brain uptake of 11C-GW2580, while the self-blocking affected neither the brain retention nor the target-to-cerebellum ratio of 11C-CPPC. To further investigate whether the increased 11C-GW2580 uptake in the brain was due to a higher tracer availability in arterial plasma, we acquired TACs in the brain and left cardiac ventricle by performing whole body PET/CT imaging (Supplementary Fig. 1). The homologous blocker treatment drastically enhanced the early phase uptake of 11C-GW2580 in the brain, and such effect persisted until around 65 min post tracer injection. Meanwhile, arterial blood SUV derived from the left cardiac ventricle was also elevated under the blocking condition, particularly between 0 to 3 min post tracer injection. Accordingly, the GW2580 treatment raised free tracer concentration in arterial plasma, possibly via systemic blockade of CSF1R in periphery, subsequently leading to an increased 11C-GW2580 uptake in the brain. It is also presumable that GW2580 mildly interacts with an efflux transporter at the blood-brain barrier (BBB), and a full blockade of this transporter by the excess non-radiolabeled compound leads to enhanced brain uptake of 11C-GW2580.
Using cerebellum as pseudo-reference tissue, the pharmacokinetics of 11C-GW2580 and 11C-CPPC in the brains of APP-KI and WT mice can be quantified by DVR estimated from both RLogan and MRTM. However, MRTM produced more variable data with a large t* (e.g. 15–40 min), and particularly poor fits to TACs for 11C-CPPC with a small t* (e.g. 4–10 min) in some cases (Supplementary Fig. 6). RLogan plot reached linearity at 30 min for 11C-GW2580, and at 40 min for 11C-CPPC (Supplementary Fig. 7). Therefore, the DVR of CSF1R tracers was quantified using RLogan with t* of 40 min. In addition, an excellent agreement between SUVR60-90min and DVR for 11C-GW2580 was observed, with a slope of 1.01, y-intercept of −0.004, and R2 of 0.97 (Figure 4). On the contrary, a less intimate correlation was observed between SUVR and DVR for 11C-CPPC, with SUVR30–60min being better correlated with DVR than SUVR60–90min, giving a slope of 0.96, y-intercept of 0.09, and R2 of 0.77 (Figure 4).
Figure 4.
Correlation between SUVR and DVR obtained by RLogan. 11C-GW2580 exhibited an excellent agreement between SUVR60–90min and DVR (linear regression, y = 1.01 × −0.004; R2 = 0.97). 11C-CPPC showed less intimate correlation between SUVR and DVR than 11C-GW2580; SUVR30–60min was better correlated with DVR than SUVR60–90min (linear regression for SUVR30-60min, y = 0.96x +0.09; R2 = 0.77). The dotted lines represent the lines of identity.
DVRs and SUVRs for 11C-GW2580 and 11C-CPPC were increased in the neocortex, hippocampus, striatum, and entire forebrain of APP-KI mice relative to WT mice, and 11C-CPPC yielded smaller inter-group difference and larger inter-subject variance in these parameters than 11C-GW2580 (Figure 5, Supplementary Figs. 8 and 9). Specifically, differences in SUVR60-90min between APP-KI and WT mice were 9–13% and 5–9% for 11C-GW2580 and 11C-CPPC, respectively, among target brain regions (Figure 5), and the group difference were 0–4% smaller in SUVR30–60min for both tracers. Elevated CSF1R availability was spatially overlapped with the upregulation of TSPO in APP-KI mice as compared with WT controls. However, SUVRs for 11C-AC5216 presented greater inter-group difference than those for CSF1R tracers (13–38% vs. 6–14% in a head-to-head comparison; n = 2 in each group). Albeit less sensitive than 11C-AC5216, 11C-GW2580 enabled detection of regional neuroinflammatory changes in APP-KI mice at an individual level (Figure 5, cut-off SUVR60–90min of 1.03 for hippocampus, 0.94 for forebrain), which was not allowed by 11C-CPPC. The inter-group difference in DVR for 11C-GW2580 and 11C-CPPC was also significant in most regions but was slightly smaller than the difference in SUVR60–90min (Supplementary Fig. 9).
Figure 5.
Comparison of 11C-GW2580 or 11C-CPPC uptakes in the neocortex (CTX), hippocampus (Hippo), entire forebrain (FB), and striatum (STR) between WT and APP-KI mice. 11C-GW2580 yielded greater inter-group difference and smaller inter-subject variability than 11C-CPPC. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Error bars indicate standard deviation.
PET imaging of CSF1R in a rhesus monkey
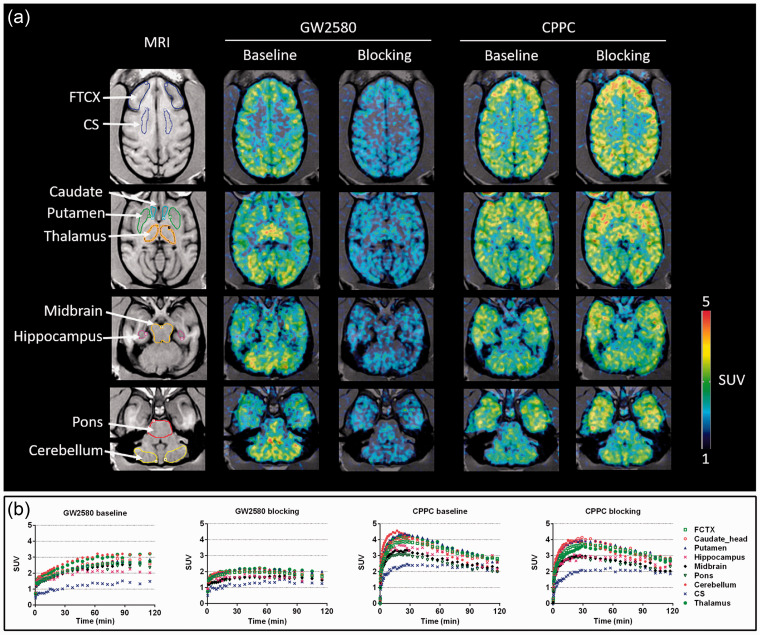
PET images presenting the uptakes of 11C-GW2580 and 11C-CPPC under baseline and self-blocking conditions and their corresponding TACs are shown in Figure 6. Similar to the observations in mice, 11C-GW2580 displayed slow kinetics. Under the baseline condition, the retention of this radioligand presented a gradual increase from the time of injection to around 60 min, and plateaued thereafter, whereas 11C-CPPC exhibited more rapid kinetics with its SUVs peaking at around 15–25 min postinjection followed by a gradual decline in most regions. The peak SUVs for 11C-GW2580 and 11C-CPPC at baseline were 1.5–3.1, and 2.4–4.6, respectively, in brain regions examined here. The centrum semi-ovale white matter displayed the lowest retentions for both tracers. Moreover, the 11C-GW2580 binding in this area was not displaced by the homologous blockade, in contrast to reduced radioligand retentions in other VOIs, allowing the use of the centrum semi-ovale as the reference region for the quantification of 11C-GW2580 kinetics without blood data. Unlike 11C-GW2580, the homologous blockade reduced the average SUV for 11C-CPPC at 60–120 min by 10% in the centrum semi-ovale, and by −3% – +6% in other regions, disqualifying the centrum semi-ovale as reference tissue. In addition, the use of other VOIs, as exemplified by the cerebellum, as a reference region was found to be inadequate for quantitative analysis of the 11C-CPPC binding since regional SUVRs to such a reference tissue did not reach a steady-state within the 90-min scan session (Supplementary Fig. 10(B) and (C)). Therefore, the specific binding of 11C-CPPC in the monkey brain could not be further assessed.
Figure 6.
(a) Representative transverse planes of 11C-GW2580 and 11C-CPPC SUV60-120min images of a monkey brain superimposed on the monkey’s own MR images at baseline and with a homologous blocker treatment. (b) Time-radioactivity curves of 11C-GW2580 and 11C-CPPC in various brain regions obtained from corresponding PET images from (a). FCTX, frontal cortex; CS, centrium semi-ovale white matter.
RLogan plot for 11C-GW2580 showed linearity beyond 30- and 60-min under baseline and blocking conditions, respectively, and the outcome DVRs with t* of 60-min were in good agreement with DVRs obtained from MRTM with t* of 60-min (Supplementary Fig. 11(A)). BPND values estimated by SRTM were smaller than DVR-1 (= BPND) obtained from RLogan and MRTM, particularly under the blocking condition, which led to underestimation of BPND by approximately 30%. The SUVRs for 11C-GW2580 became stable at 20–60 min (Supplementary Fig. 10(A)), and strong correlations were observed between SUVR60–90 min and DVRs (Supplementary Fig. 11(B)). The estimates of DVR and SUVR60–90min ranged between 1.6 in the hippocampus and 2.3 in the cerebellum and thalamus at baseline and reduced by 30% (range, 23–38%) upon the treatment with unlabeled GW2580 (Supplementary Fig. 10(A)).
Analysis of 11C-GW2580 radiometabolites in monkey plasma
A single peak of polar radioactive metabolite(s) was eluted at 2.3 min (Supplementary Fig. 12). Judging from the anticipated polarity, these metabolites were unlikely to efficiently cross the BBB. The 11C-GW2580 displayed a moderate rate of metabolism, with 60% intact tracer in plasma at 30 min, and this percentage was decreased to 15% at 90 min.
Immunostaining of CSF1R
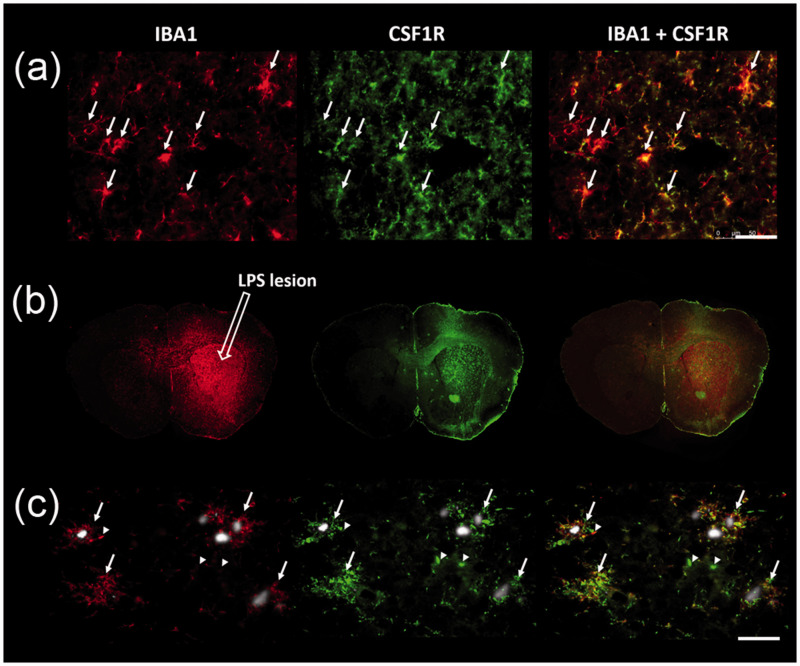
In agreement with previous gene profiling studies showing a predominant expression of csf1r in microglia in the CNS,20 CSF1R immunostaining was found to be co-localized with ionized calcium binding adaptor molecule 1 (IBA1)-positive microglia in mouse brain sections (Figure 7(a)). In addition, a significant increase in CSF1R immunofluorescence intensity was observed in the lesioned striatum relative to the non-lesioned side at 8 days post LPS injection, spatially matching the distribution of IBA1 immunoreactivity (Figure 7(b)), whereas no change in immunoactivity was detected in the non-lesioned striatum of LPS-injected animals as compared with untreated controls (Supplementary Fig. 13). Furthermore, APP-KI mice displayed increased IBA1 and CSF1R immunostaining in the neocortex, hippocampus, and less manifestly in other subcortical regions, as compared to the WT mice (Supplementary Fig. 14), which is in line with the regional distribution of CSF1R tracers. High-power magnification images showed that amyloid-β plaque-associated reactive microglia also abundantly expressed CSF1R (Figure 7(c), arrows). However, while spatially co-localized, a profound mismatch of IBA1 and CSF1R immunostaining intensities was noted in a small subset of putative microglia (Figure 7(c), arrowheads).
Figure 7.
Immunohistochemistry with anti-IBA1 (red, a microglial marker) and anti-CSF1R (green) antibodies in LPS-injected FVB (a, b) and APP-KI (c) mouse brain sections. (a) Representative images of costaining of CSF1R and IBA1 in the non-lesioned striatum (arrows). (b) In the lesioned striatum, LPS locally induced microglial activation accompanied by CSF1R overexpression. (c) Co-localization and intensive immunoreactivity of IBA1 and CSF1R in FSB-positive amyloid-β plaque (gray inclusions)-associated reactive microglia in the neocortex of a 17-month-old APP-KI mouse (arrows). Arrowheads show examples of IBA1 and CSF1R immunofluorescence with spatial co-localization but mismatched intensities. Scale bars represent 50 μm.
Discussion
In this study, we have documented the in-vivo performance of our newly developed and reported radioligands for CSF1R in a comparative manner as PET imaging agents for the detection of CSF1R under physiological and neuroinflammatory conditions in different species. Immunostaining results have demonstrated expressions of CSF1R, which were confined to microglia in the CNS, were enhanced in close association with LPS- and amyloid-β-provoked neuroinflammatory changes (Figure 7), justifying the utilization of CSF1R as a biomarker for reactive microgliosis. Correspondingly, PET imaging of CSF1R showed elevated retentions of 11C-GW2580 and 11C-CPPC in affected brain areas of the mice modeling acute and chronic inflammation. Notably, our study suggests the possibility of in-vivo detection of CSF1R with 11C-GW2580 with higher sensitivity than 11C-CPPC. Furthermore, a high brain uptake and displaceable binding of 11C-GW2580 in a non-human primate support the translatability of 11C-GW2580-PET to clinical neuroimaging of microgliosis.
Because both CSF1R tracers in the mouse brain presented slow kinetics and low concentrations, high doses of radioactivity (∼50 MBq) were administered to mice, resulting in mass doses of around 23 nmol/kg and 13 nmol/kg for 11C-GW2580 and 11C-CPPC, respectively. Nonetheless, these doses did not affect tracer retention in the brain, as neither SUVR60-90min nor SUV60-90min was correlated with mass doses (Supplementary Fig. 15).
The patterns of regional distribution of the two CSF1R radioligands were similar and fairly homogeneous in the brains of WT mice, except for the comparatively high 11C-GW2580 versus low 11C-CPPC uptake in the cerebellum (Supplementary Figs. 2 and 5). The cerebellar intensification of 11C-GW2580 radiosignals was also observed in the monkey (Figure 6) and is attributable to non-specific accumulations of this radioligand in view of a low density of immunohistochemical staining of CSF1R in the cerebellum (Supplementary Fig. 14). In contrast to mice, the uptake of 11C-GW2580 and 11C-CPPC in the monkey brain presented a larger regional difference, with the centrum semi-ovale being the region of the lowest retention, and several subcortical structures including the striatum and thalamus being the regions of the highest retention for both tracers.
The kinetics of 11C-GW2580 appeared slow in the brains of mice and the monkey, requiring a 90-min PET data acquisition to quantify the tracer retention, whereas an extension of the scan duration to 120 min did not improve the quality of kinetic assessments. Despite a slow washout from the brain, the RLogan plot for 11C-GW2580 became linear within 30 min in the mouse brains and 60 min in the monkey brain after the radioligand injection. Furthermore, the tracer uptake in the target brain regions relative to the reference region reached a steady-state within 60 min in most cases, except for a small (<5%) increase in SUVR from 30 to 90 min in APP-KI mice (Figure 3(a) and Supplementary Figs. 7 and 8). A close correlation between SUVR60-90min and DVR (Supplementary Fig. 11(B)) suggests the possibility of quantifying 11C-GW2580 uptake in the brain by performing a simple static scan at 60–90 min post tracer injection. Nonetheless, all these reference tissue-based approaches need to be validated against full kinetic analysis with an arterial plasma input function.
The binding kinetics of 11C-CPPC also seemed slow, as the RLogan plot reached linearity at 40 min in mice. Moreover, SUVR for this radioligand progressively reduced from 30 min in WT and APP-KI mice (Supplementary Fig. 8), likely reflecting an accumulation of radioactive metabolites in the brain.16 Similarly, neither centrum semi-ovale nor cerebellum as a reference region produced stable SUVRs in the monkey brain (Supplementary Fig. 10(B) and (C)). Furthermore, there was minimal (<10%) blockade of 11C-CPPC uptake by the corresponding unlabeled compound, implying a negligible binding of 11C-CPPC to CSF1R under a physiological condition. Despite this property, 11C-CPPC differentiated APP-KI mice from the WT controls, and Horti et al.16 reported a two-fold increase in total distribution volume of this tracer in an LPS-treated baboon, demonstrating the feasibility of 11C-CPPC for in-vivo detection of inflammatory microgliosis. A lack of blocking effect was also observed in healthy mouse brains for 11C-GW2580. Meanwhile, a 30% reduction in tracer binding upon the treatment with unlabeled GW2580 was noted in the monkey brain, indicating a species difference in the homeostatic expression levels of CSF1R.
In the aged APP-KI mice, increased neuroinflammation in amyloid-β-rich regions was captured by 11C-AC5216 and two CSF1R tracers. 11C-AC5216 yielded 1.8-3.7 folds higher contrasts for inflammatory responses than CSF1R tracers, but it should be noted that TSPO is also expressed by astroglia and endothelial cells. Indeed, amyloid plaques in APP transgenic and APP-KI mice are encompassed by TSPO-positive astrocytes and TSPO-negative microglia (Supplementary Fig. 16), unlike neuritic plaques surrounded by TSPO-positive microglia and TSPO-negative astrocytes in human AD brains.21,22 Furthermore, we found that LPS-triggered overexpression of TSPO co-localized with reactive astrocytes other than microglia (Supplementary Fig. 17). Collectively, our immunohistological findings demonstrate that TSPO-PET primarily measured reactive astrogliosis, whereas CSF1R-PET reflected microglial activation in LPS-lesioned mice and mouse models of amyloidosis. In light of these observations, in-vivo imaging of CSF1R could be more advantageous than imaging of TSPO for selective monitoring of microglial activities under diverse pathological conditions. It is yet to be clarified whether CSF1R is a marker of microglia with a specific phenotype, but it is conceivable that both homeostatic and reactive microglial cells express CSF1R at variable levels, in distinction from the enrichment of TSPO in detrimental microglia.
In both acute and chronic inflammatory conditions, 11C-GW2580 was found to be more sensitive than 11C-CPPC in detecting alternations in CSF1R density. The linear regression for the correlation between retentions of these two radioligands in LPS-injected mice demonstrated that 11C-GW2580 was capable of measuring LPS-induced CSF1R accumulations with a 1.8-fold higher dynamic range than 11C-CPPC and that 11C-CPPC failed to detect subtle inflammation eliciting an increase of the 11C-GW2580 retention by 8% (Figure 3(c)). In addition, 11C-GW2580 yielded an approximately 2-fold higher contrast for CSF1R along with less individual variability than 11C-CPPC in most of the affected brain regions in APP-KI mice relative to WT controls, leading to a greater statistical significance of the group differences (Figure 5). Hence, our data support the utility of 11C-GW2580-PET for the pursuit of rapid, transient as well as slow, persistent neuroinflammatory processes with reasonable reliability.
Along with technical merits in the use of CSF1R radioligands, our study implied several limitations of in-vivo assays using these probes. We observed an incomplete homologous blockage of 11C-GW2580 and 11C-CPPC uptakes 8 days after LPS treatment. This may indicate a remodeling of the local microenvironment as a result of LPS-induced neuroinflammatory changes or breakdown of the BBB, leading to increased tracer uptake in the lesioned striatum. Despite the promising potential of 11C-GW2580 as a probe for inflammatory microgliosis, it should be taken into account that CSF1R-expressing microglia exist across brain areas, precluding the definition of a proper reference region devoid of the radiotracer binding components. However, since the density of CSF1R in the cerebellum remains unaltered in the pathological condition and with aging (Supplementary Figs. 5 and 14), the utilization of cerebellum as a pseudo-reference region could be suited to detecting changes in microgliosis in pathological brain areas. Indeed, we have robustly observed increased DVR and SUVR in brain regions with amyloid depositions in APP-KI mice (Supplementary Fig. 9), further justifying the use of cerebellum as a pseudo-reference region. Another potential issue could be that a significant quantity of free and nonspecifically bound radioligands was present in the mouse brain, impeding visualization of CSF1R with a high signal-to-background ratio. The chemical structure of GW2580 would be modified to adjust its lipophilicity, which may lead to more rapid kinetics of the compound and reduced background noise. Such derivatization could be optimized by examining the structure-binding activity relationships of the chemicals, and the establishment of an in-vitro assay system to accurately measure the affinity and specificity of the candidates for CSF1R and their non-specific binding properties will be required for this purpose.
Conclusions
We have successfully developed 11C-GW2580, a new PET radioligand for CSF1R, which has offered more sensitive PET imaging of microglial responses to acute and chronic neuronal insults in comparison with an existing probe, 11C-CPPC. 11C-GW2580 and its future modifications will facilitate diagnostic and therapeutic approaches to neuroinflammatory pathologies in nonclinical and clinical settings, ranging from early post-ischemic changes to progressive neurodegenerative pathways in AD and PD.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211004146 for PET imaging of colony-stimulating factor 1 receptor: A head-to-head comparison of a novel radioligand, 11C-GW2580, and 11C-CPPC, in mouse models of acute and chronic neuroinflammation and a rhesus monkey by Xiaoyun Zhou, Bin Ji, Chie Seki, Yuji Nagai, Takafumi Minamimoto, Masayuki Fujinaga, Ming-Rong Zhang, Takashi Saito, Takaomi C Saido, Tetsuya Suhara, Yasuyuki Kimura and Makoto Higuchi in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present work was supported by JSPS KAKENHI (19K16274) to XZ, JSPS KAKENHI (19K08137) to BJ, Grants-in-Aid for Strategic Research Program for Brain Sciences (Integrated Research on Neuropsychiatric Disorders; 0207007) to TS. Grants-in-Aid for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS; 18dm0207018 and 19dm0207072) to MH and Research and Development Grants for Dementia (18dk0207026) to MH from the Japan Agency for Medical Research and Development and by Core Research for Evolutional Science and Technology (JPMJCR1652) to MH from Japan Science and Technology Agency.
Acknowledgements: We thank Nengaki Nobuki, Shoko Uchida, Takeharu Minamihisamastu, Kana Osawa, Kanami Ebata, Takashi Okauchi, Hidekatsu Wakizaka, Jun Kamei, Yuichi Matsuda, Yoshio Sugii, and Ryuji Yamaguchi for technical support, Dr. Masanori Ichise and Dr. Masafumi Shimojo for helpful comments on the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Xiaoyun Zhou, Bin Ji, and Makoto Higuchi conceptualized the study. Xiaoyun Zhou, Bin Ji, Chie Seki, Yuji Nagai, and Masayuki Fujinaga performed the experiments. Xiaoyun Zhou and Chie Seki analyzed the data. Xiaoyun Zhou, Bin Ji, Chie Seki, Yuji Nagai, Takafumi Minamimoto, Masayuki Fujinaga, Ming-Rong Zhang, Takashi Saito, Takaomi C Saido, Tetsuya Suhara, Yasuyuki Kimura and Makoto Higuchi interpreted the data. Xiaoyun Zhou and Makoto Higuchi wrote the manuscript. All authors commented on the draft. All authors approved the final manuscript.
ORCID iDs: Xiaoyun Zhou https://orcid.org/0000-0002-6894-7714
Takafumi Minamimoto https://orcid.org/0000-0003-4305-0174
Yasuyuki Kimura https://orcid.org/0000-0002-7927-9483
Supplementary material: Supplemental material for this article is available online.
References
- 1.Salter MW, Stevens B.Microglia emerge as central players in brain disease. Nat Med 2017; 23: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 2.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 2013; 45: 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalls MA, Pankratz N, Lill CM, et al. Large-scale Meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 2014; 46: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Z, Okello AA, Brooks DJ, et al. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain 2015; 138: 3685–3698. [DOI] [PubMed] [Google Scholar]
- 5.Edison P, Ahmed I, Fan Z, et al. Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology 2013; 38: 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perani D, Iaccarino L, Jacobs AH, et al. Application of advanced brain positron emission tomography-based molecular imaging for a biological framework in neurodegenerative proteinopathies. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2019; 11: 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim MJ, Shrestha SS, Cortes M, et al. Evaluation of two potent and selective PET radioligands to image COX-1 and COX-2 in rhesus monkeys. J Nucl Med 2018; 59: 1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamagishi S, Iga Y, Nakamura M, et al. Upregulation of cannabinoid receptor type 2, but not TSPO, in senescence-accelerated neuroinflammation in mice: a positron emission tomography study. J Neuroinflammation 2019; 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagens MHJ, Golla SSV, Janssen B, et al. The P2X7 receptor tracer [11C]SMW139 as an in vivo marker of neuroinflammation in multiple sclerosis: a first-in man study. Eur J Nucl Med Mol Imaging 2020; 47: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmore MRP, Najafi AR, Koike MA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014; 82: 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez-Muriana A, Mancuso R, Francos-Quijorna I, et al. CSF1R blockade slows the progression of amyotrophic lateral sclerosis by reducing microgliosis and invasion of macrophages into peripheral nerves. Sci Rep 2016; 6: 25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prieto-Morin C, Ayrignac X, Ellie E, et al. CSF1R-related leukoencephalopathy mimicking primary progressive multiple sclerosis. J Neurol 2016; 263: 1864–1865. [DOI] [PubMed] [Google Scholar]
- 13.Luo J, Elwood F, Britschgi M, et al. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med 2013; 210: 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olmos-Alonso A, Schetters STT, Sri S, et al. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 2016; 139: 891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asai H, Ikezu S, Tsunoda S, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 2015; 18: 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horti AG, Naik R, Foss CA, et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc Natl Acad Sci U S A 2019; 116: 1686–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Priceman SJ, Sung JL, Shaposhnik Z, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 2010; 115: 1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang MR, Kumata K, Maeda J, et al. 11C-AC-5216: a novel PET ligand for peripheral benzodiazepine receptors in the primate brain. J Nucl Med 2007; 48: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 19.Saito T, Matsuba Y, Mihira N, et al. Single app knock-in mouse models of Alzheimer’s disease. Nat Neurosci 2014; 17: 661–663. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014; 34: 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda J, Zhang M-R, Okauchi T, et al. In vivo positron emission tomographic imaging of glial responses to amyloid-beta and tau pathologies in mouse models of Alzheimer’s disease and related disorders. J Neurosci 2011; 31: 4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi M, Ji B, Maeda J, et al. In vivo molecular imaging of neuroinflammation in Alzheimer’s disease. Clin Exp Neuroimmunol 2016; 7: 139–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211004146 for PET imaging of colony-stimulating factor 1 receptor: A head-to-head comparison of a novel radioligand, 11C-GW2580, and 11C-CPPC, in mouse models of acute and chronic neuroinflammation and a rhesus monkey by Xiaoyun Zhou, Bin Ji, Chie Seki, Yuji Nagai, Takafumi Minamimoto, Masayuki Fujinaga, Ming-Rong Zhang, Takashi Saito, Takaomi C Saido, Tetsuya Suhara, Yasuyuki Kimura and Makoto Higuchi in Journal of Cerebral Blood Flow & Metabolism