Abstract
Lymphocytes play an important role in the immune response after stroke. However, our knowledge of the circulating lymphocytes in ischemic stroke is limited. Herein, we collected the blood samples of clinical ischemic stroke patients to detect the change of lymphocytes from admission to 3 months after ischemic stroke by flow cytometry. A total of 87 healthy controls and 210 patients were enrolled, and the percentages of circulating T cells, CD4+ T cells, CD8+ T cells, double negative T cells (DNTs), CD4+ regulatory T cells (Tregs), CD8+ Tregs, B cells and regulatory B cells (Bregs) were measured. Among patients, B cells, Bregs and CD8+ Tregs increased significantly, while CD4+ Tregs dropped and soon reversed after ischemic stroke. CD4+ Tregs, CD8+ Tregs, and DNTs also showed high correlations with the infarct volume and neurological scores of patients. Moreover, these lymphocytes enhanced the predictive ability of long-term prognosis of neurological scores when added to basic clinical information. The percentage of CD4+ Tregs within lymphocytes showed high correlations with both acute and long-term neurological outcomes, which exhibited a great independent predictive ability. These findings suggest that CD4+ Tregs can be a biomarker to predict stroke outcomes and improve existing therapeutic strategies of immunoregulatory lymphocytes.
Keywords: Circulating lymphocytes, flow cytometry, ischemic stroke, regulatory B cells, regulatory T cells
Introduction
Ischemic stroke is a leading cause of mortality and morbidity in adults worldwide.1 Novel and effective therapeutic strategies for stroke is urgently needed because of the limited pharmacological treatment.2–4 The development of biomarkers is also important because biomarkers of ischemic stroke can improve the care of patients.5
The immune response plays a critical role in the pathophysiological process of ischemic stroke, and inflammation is an important therapeutic target in stroke.6,7 Clinical studies suggest the inflammatory response after stroke correlates with the risk of clinical worsening the long-term functional outcome. Neutrophils are thought to be the first immune cell-type to enter the ischemic brain, meanwhile peripheral neutrophils also rise in the early stage after stroke.8,9 Then lymphocytes are elevated in the ischemic brain and play an active role in ischemic brain pathogenesis, modulating brain inflammation by producing pro-inflammatory cytokines and cytotoxic post-stroke.10 Interestingly, clinical studies also show dynamic changes in circulating lymphocyte subpopulations of stroke patients.11 Immune activation in the peripheral blood is found after stroke, and lymphocytes are likely to be present in peripheral blood before entering the brain.12 Moreover, the change of peripheral blood lymphocyte subpopulations can reflect the local inflammation of the central nervous system.13 Increased lymphocyte subpopulations are observed with an increased risk of stroke recurrence and death.14
Lymphocytes consist of several subtypes with different functions.15,16 T lymphocytes are found to participate in both innate and adaptive immune responses after stroke. They have been found to infiltrate into brain parenchyma and contribute to brain damage as well as functional recovery due to their pro-inflammatory and anti-inflammatory functions.17,18 Clinical studies also suggest some stroke patients may develop a B lymphocytes response to stroke that contributes to dementia.19 Moreover, regulatory lymphocytes, regulatory T cells (Tregs), and B cells (Bregs) have been characterized as protective cells after stroke.20–22 In stroke patients, the number of Tregs decrease soon and then increase persistently for several weeks after stroke.12 Tregs are associated with infarct volume and outcomes. Regulatory B cells have been demonstrated to play an anti-inflammatory role by secreting IL-10, which modulates the immune response in experimental stroke.23 Besides, double-negative T cells (DNTs), accounting for a small percentage of T cells, increasing in the brain and peripheral blood of stroke patients and promote neuroinflammation in the animal model of ischemic stroke.24
Biomarkers are indicators found in the blood, CSF, or tissues that predict disease states or drug response and outcomes.25 There is an urgent need for biomarkers to detect or predict stroke outcomes, which can help identify patients at risk of disease, improve clinical management, and monitor the effects of therapy.26 Moreover, in stroke patients, the neurological outcome depends on multiple factors such as the duration and severity of ischemia, age and sex differences, and admission clinical characteristics.27–29 Here we investigated the relationships among the proportions of circulating lymphocytes, clinical information, and stroke outcomes. Immunoregulatory lymphocytes, especially the CD4+ Tregs, exhibiting a high predictive ability with multiple admission clinical information of stroke patients. These data suggest specific lymphocyte subsets can be an attractive target for the treatment of ischemic stroke or be a new biomarker and indicator that can predict stroke outcomes, helping to improve existing clinical neuroprotective strategies.
Materials and methods
Ethics
The clinical study was approved by the ethical review board of Minhang District Central Hospital, Shanghai, China. (“Approval of ethics committee, document No.28 (2017)”, Minhang District Central Hospital, Shanghai, China. June 28, 2017. Supplementary ethics approved document). Procedures were followed in accordance with the ethical standards of the Helsinki Declaration.
Subjects
210 patients with a clinical diagnosis of stroke were included by following rules: (1) Age > 18 years; (2) Cerebral infarction or transient ischemic attack (TIA) confirmed by head CT or MRI (excluded intracranial hemorrhage); (3) First time of onset, or recurrence without neurofunctional deficits; (4) NIHSS score < 20 and without aphasia; (5) Onset time ≤ 7 days; (6) Informed consent of patients or family members. Patients with tuberculosis, neoplasms, acute infections after stroke, and other conditions that may affect the immune environment were excluded. Clinical information and characteristics included age, sex, risk factors (hypertension, diabetes, a disorder of lipid metabolism, atrial fibrillation, Coronary artery disease, smoking, drinking, and cerebral infarction history) of patients were collected at admission. National Institute of Health Stroke Scale (NIHSS) score, Modified Rankin Score (mRS), and Glasgow Come Scale (GCS) were measured at admission, discharge, and 3 months after admission. Infarct volume was measured by MRI scanning, and tPA treatment was considered.
Our study is double-blind and randomized. 210, 100, 49, and 66 patients at admission, discharge, 1 month, and 3 months, respectively, were included in our study. Control subjects were 87 healthy volunteers of age, gender ratio comparable to the ischemic stroke patients, and without a history of stroke or other vascular diseases. All participants voluntarily participated and gave written informed consent for this study.
Isolation of human peripheral mononuclear cells (PBMCs)
Peripheral blood samples of patients were collected within 24 h of admission day, discharge day, 1 month, and 3 months after onset. Peripheral blood samples of controls were collected when they attended the physical examination. Peripheral blood samples were collected through forearm veins from the patients and healthy controls and keep in the EDTA vacutainer blood collection tube after obtaining informed consent from them or their legal representatives. After collecting peripheral blood, PBMCs were isolated by density gradient centrifugation within 3 h. The blood samples were diluted 1:1 with PBS buffer and then layered over Ficoll-Paque (GE Healthcare Bio-Sciences) in a 15 ml conical tube. After centrifuging at 400 g and 20 °C for 30 minutes, PBMCs were collected from the plasma-Ficoll interphase.
Flow cytometry analysis
PBMCs were quantified by trypan blue exclusion method, then 106-107 cells were labeled with fluorochrome-conjugated antibodies specific for FITC Mouse Anti-Human CD3, APC-H7 Mouse Anti-Human CD4, APC Mouse Anti-Human CD25, PE Mouse Anti-Human CD127, Percp-cy5.5 Mouse Anti-Human CD19, PE Mouse Anti-Human CD24, APC Mouse Anti-Human CD27, Percp-cy5.5 Mouse Anti-Human CD8, PE Mouse Anti-Human CD45RA, APC Mouse Anti-Human CD62L, PE-cy7 Mouse Anti-Human CD183, which were purchased from BD Pharmingen. Samples were run on the LSRFortessa (BD Biosciences) using FACSDiva software version 8.0 and analyzed using FlowJo version 9.9.6.
Statistics
The sample size in this study was determined by power analyses to provide 80% power to detect a difference of expected effect sizes of 1.2 estimated from pilot studies. In sample size calculations, we assumed an α error of 5% with Bonferroni adjustment according to the number of pairwise comparisons.
All data were assessed by the Shapiro-Wilk normality test to test normal distribution. If the data pass the normal distribution test, a parametric test was employed for significance analysis. Otherwise, a nonparametric test was employed. Patient characteristics and experimental data are presented as means ± standard deviations (SDs) or numbers (%) in tables, medians, 25 and 75% quartiles in violin plots and means ± SDs in line charts. Correlations between cell proportion and multiple characteristics were analyzed using Spearman's correlation analysis. The statistical significance of differences between experimental groups was assessed with the two-sided Mann-Whitney U test or two-sided unpaired T test if data satisfied normal distribution, Kruskal-Wallis test with Dunn’s multiple comparisons test, and Two-way ANOVA with Bonferroni's multiple comparisons test as appropriate.
All the above statistical analyses were performed with GraphPad Prism software (version 8.0). Logistic regression, multiple linear regression analyses were performed with SPSS 25.0 software package (SPSS Inc., Chicago, Illinois). The heatmap of linear correlation was visualized with R-3.5.2 software (https://www.r-project.org/) using R package pheatmap.
The ROC curve and AUC were analyzed by R package rattle 5.4.0 (Togaware Pty Ltd.). The whole data were divided into 60% as a training dataset and 40% as a test dataset to evaluate the prediction ability. Four classification models: Logistic regression, Support Vector Machine (SVM), Decision Tree, and Random Forest were applied and compared.
Results
Characteristics of patients and controls enrolled in the study
A total of 210 acute ischemic stroke patients and 87 healthy volunteer controls were enrolled in our study, whose characteristics were described in Supplementary Table 1 and Table 1. No statistically significant difference was observed in either age or sex between patients and controls (Supplementary Table 1). All patients’ neurological scores were tracked and recorded at admission, discharge (approximately seven days after admission) and 3 months after onset. Of 210 patients included in this study, 113 (53.81%) patients had fairly good functional stroke outcomes (mRS < 2), and none of them died in 3 months.
Table 1.
Baseline characteristics of patients.
| Patients (n = 210) | |
|---|---|
| Onset to admission time, days, mean ± SD | 2.469 ± 1.37 |
| Infarct volume, cm3, mean ± SD | 6.238 ± 24.90 |
| tPA treatment, n (%) | 27 (12.86%) |
| Admission | |
| NIHSS, mean ± SD | 3.938 ± 3.520 |
| mRS, mean ± SD | 2.086 ± 1.179 |
| GCS, mean ± SD | 14.61 ± 1.233 |
| systolic blood pressure, mmHg mean ± SD | 140.8 ± 19.63 |
| diastolic blood pressure, mmHg mean ± SD | 81.83 ± 10.69 |
| Blood LDL, mmol/l, mean ± SD | 2.936 ± 0.9711 |
| Discharge | |
| NIHSS, mean ± SD | 2.776 ± 2.647 |
| mRS, mean ± SD | 1.890 ± 1.242 |
| GCS, mean ± SD | 14.84 ± 0.6206 |
| systolic blood pressure, mmHg mean ± SD | 128 ± 15.02 |
| diastolic blood pressure, mmHg mean ± SD | 81.03 ± 8.949 |
| Blood LDL, mmol/l, mean ± SD | 2.671 ± 0.9622 |
| 3 months | |
| NIHSS, mean ± SD | 1.038 ± 0.3506 |
| mRS, mean ± SD | 1.438 ± 0.5607 |
| GCS, mean ± SD | 14.95 ± 0.2233 |
| Risk factors | |
| Hypertension, n (%) | 144 (68.57%) |
| Diabetes, n (%) | 70 (33.33%) |
| Disorder of lipid metabolism, n (%) | 33 (15.71%) |
| Atrial fibrillation, n (%) | 20 (9.52%) |
| Coronary artery disease, n (%) | 10 (4.76%) |
| Smoking, n (%) | 41 (19.52%) |
| Drinking, n (%) | 24 (11.43%) |
SD: standard deviation; tPA: tissue plasminogen activator; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale; GCS: Glasgow Coma Scale; LDL: low density lipoprotein.
Changes of lymphocyte subsets in acute ischemic stroke
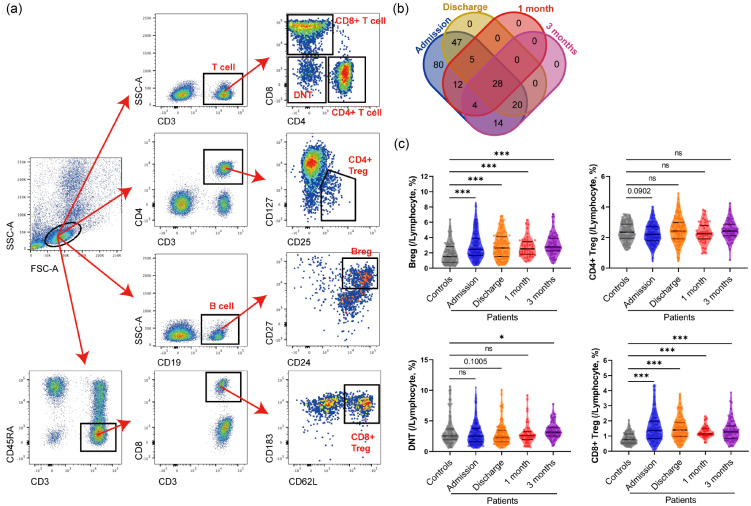
As failing to follow up and missing data accounted for incomplete data, only 210, 100, 49, and 66 patients’ data at admission, discharge, 1 month and 3 months, respectively, were adopted for further analysis, whose specific distributions were illustrated by the Venn diagram (Figure 1(b)). Different lymphocyte subsets were analyzed, including CD19+ cells (B cells), CD3+ cells (T cells), CD3+ CD4+ cells (CD4+ T cells), CD3+CD8+ cells (CD8+ T cells), CD3+CD4-CD8- cells (Double Negative T cells, DNTs), and immunoregulatory lymphocytes: CD19+CD24+CD27+ cells (Bregs), CD3+CD4+CD25+CD127– cells (CD4+ Tregs) and CD3+CD45RA–CD8+CD183+CD62L+ cells (CD8+ Tregs) among all controls and ischemic stroke patients at different time points (Figure 1(a) and Supplementary Figure 1). Our study revealed a dramatic increase in the percentage of B cells within lymphocytes after acute ischemic stroke (11.49 ± 5.276%), compared with control’s level (6.547 ± 3.736%), which remained steadily high to 3 months (10.70 ± 3.777%). While T cells kept stable at healthy control’s level (64.27 ± 12.82%) from admission (64.16 ± 11.44%) to 3 months (65.60 ± 11.26%) after admission (Supplementary Figure 2).
Figure 1.
Different clusters of regulating-cells in lymphocytes between control and different time points after acute ischemic stroke. (a) Representative flow cytometry analyses of total lymphocytes, B cells, T cells, CD4+ T cells, CD8+ T cells, Bregs, CD4+ Tregs, CD8+ Tregs, and DNTs. (b) Venn diagram of data at different time points. (c) Violin plots with median, 25 and 75% quartiles of the percentage of Bregs, DNTs, CD4+ Tregs, and CD8+ Tregs within lymphocytes of Controls (grey, n = 87) and different time points after acute ischemic stroke (Admission: blue, n = 210; Discharge: orange, n = 100; 1 month: red, n = 49; 3 months: purple, n = 66). Mann-Whitney test was employed to examine the difference between Controls and each time point. Decimals represent p-value. *: p < 0.05, ***: p < 0.001, ns: no significance.
T cells play an important role in the immune response. Two main types of T cells, CD4+ helper T cells and CD8+ cytotoxic T cells, both are considered involved in ischemic stroke.11 CD4+ T cells secrete complex immune cytokines while CD8+ T cells may induce brain injury through the cytotoxic mechanism. Although the percentage of T cells within lymphocytes is not changed after stroke, the CD4+ T cells were tended to increasing (p = 0.0978) at admission (39.12 ± 9.916%) and then fluctuated to control’s level (37.23 ± 9.195%), while no significant dynamic changes were observed in CD8+ T cells (control: 22.03 ± 8.203%) (Supplementary Figure 2).
T cells’ subsets, including DNTs, CD4+ Tregs and CD8+ Tregs, are complicated and controversial ischemic stroke participants20 that attracted our concerns. DNTs are a small subpopulation of T cells regulating immunological homeostasis.24 Though DNTs’ level had not a significant tendency of falling after stroke compared with control’s level (3.291 ± 2.270%), its ascending at 3 months (3.437 ± 1.312%) was significant (Figure 1(c) and Supplementary Figure 2). CD4+ Tregs regulate immune homeostasis by lymphocyte activation and inhibiting antigen-presenting cells, while CD8+ Tregs also have a potentially neuroprotective function of regulatory IL-10-producing B-cells.30 In our study, the percentage of CD4+ Tregs within lymphocytes tended to decrease (p = 0.0902) at admission (2.243 ± 0.6628%) than healthy controls (2.380 ± 0.6012%) (Figure 1(c)), but significantly reversed to even higher at discharge (2.485 ± 0.8346%), recovered to healthy control’s level at 1 month (2.300 ± 0.6648%) and then rose again at 3 months (2.491 ± 0.6314%) (Supplementary Figure 2). On the contrary, the percentage of CD8+ Tregs within lymphocytes increased significantly after stroke (admission: 1.532 ± 0.8931%) and stayed at a high level in the following 3 months, compared with control’s level (0.8323 ± 0.3736%) (Figure 1(c) and Supplementary Figure 2). As observed, these special immunoregulatory T cells were more sensitive to ischemic stroke than regular T cell subsets, suggesting tighter relationships they embraced with the pathology of stroke.
Moreover, regulatory B cells (Bregs), a regulatory subpopulation of B cells, is thought to be potentially beneficial for stroke patients.23 In this study, the percentage of Bregs within lymphocytes dramatically increased after stroke (admission: 2.942 ± 1.750%), compared with controls (1.910 ± 1.357%), insistent with the rise of total B cells after the stroke (Figure 1(c), Supplementary Figure 2).
Relationships between the regulatory lymphocytes and severity of neurofunctional deficits in the acute stage
Infarct volume and NIHSS can indicate stroke severity and determine the post-stroke immune change.31 Recent studies suggest acute ischemic lesion severity, regions, and the degree of reperfusion that might affect the pathogenic contribution of leukocytes.32 The changes in immunoregulatory lymphocytes were significant and impressive. Thus, to reveal the relationships between regulatory lymphocytes and neurofunctional deficits at the acute stage, the percentages of regulatory lymphocytes within lymphocytes at admission, discharge, 1 month, and 3 months were analyzed respectively, by using linear regression analyses with admission infarct volume, NIHSS, and mRS (Supplementary Table 2), which were also summarized in heat maps (Figure 2(a)).
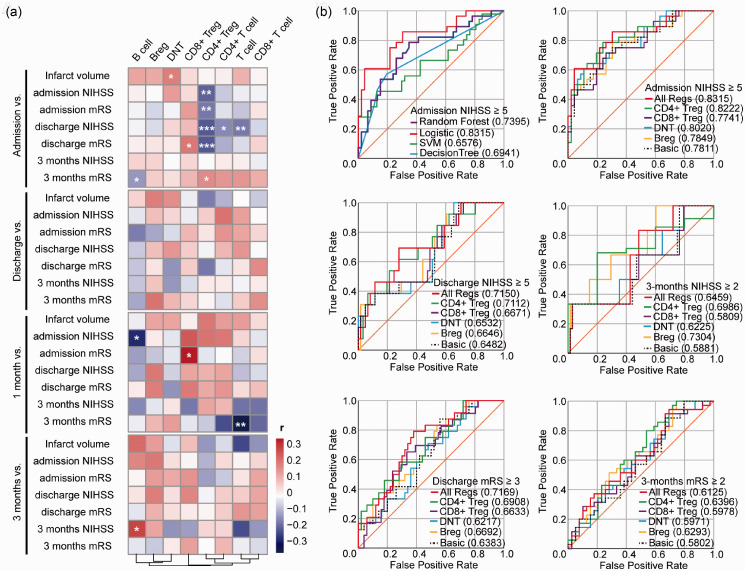
Figure 2.
Correlation matrix between the percentage of lymphocyte subsets and neurological outcomes and Receiver operating characteristic (ROC) curves for predicting stroke severity and prognosis. (a) Heat map used to visualize correlations between the percentage of different clusters of lymphocytes at different time points and infarct volume, NIHSS and mRS score at admission, discharge, and 3 months. The color bar represents Spearman r value and star symbols (*) represents for statistical significance. Heatmap was drawn by R package pheatmap, clustered by columns. *: p < 0.05, **: p < 0.01, ***: p < 0.001, no symbol: no statistical significance. (b) ROC curves for prediction of poor admission NIHSS (NIHSS ≥5) using Random Forest (purple line), Logistic regression (red line), Support Vector Machine (SVM) (green line) and Decision Tree (blue line) models (Top left); for prediction of poor admission (Top right), discharge NIHSS (NIHSS ≥5) (Middle left), 3-month NIHSS (NIHSS ≥2) (Middle right), discharge mRS (mRS ≥3) (Bottom left) and poor 3-month mRS (mRS ≥2) (Bottom right) using Logistic regression model. “Basic” (dotted black line): factors including age, sex, past history of hypertension, diabetes and disorder of lipid metabolism, and habits of drinking and smoking, tPA treatment and infarct volume. “CD4+ Treg”(green line), “CD8+ Treg” (purple line), “DNT” (blue line), “Breg” (yellow line): “Basic” adding CD4+ Tregs/lymphocytes, CD8+ Tregs/lymphocytes, DNTs/lymphocytes, or Bregs/lymphocytes respectively; “All Regs” (red line): “Basic” adding Bregs/lymphocytes, CD4+ Tregs/lymphocytes, CD8+ Tregs/lymphocytes, and DNTs/lymphocytes. Decimals in brackets: area under the curve (AUC). NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale.
The percentage of CD4+ Tregs within lymphocytes at admission indicated the most significant negative correlations with NIHSS or mRS among all these markers (Supplementary Table 2, Spearman r=–0.21 & –0.19, p = 0.002 & 0.01). While its parent subsets, CD4+ T cells or T cells, showed little correlation with short-term neurological outcomes (Supplementary Table 2). It suggested that a lower level of CD4+ Tregs was an evident reflection of worse neurofunctional deficits at admission.
At 1 month, the percentages of CD8+ Tregs within lymphocytes also showed a significant positive relationship with mRS score at admission (Supplementary Table 2, Spearman r = 0.31, p = 0.03). These results suggested worse severity at the acute stage could result in a greater increase of CD8+ Tregs level, which kept stable in 1 month after onset. Besides, the percentage of B cells within lymphocytes at 1 month, but not at admission, was also negatively related to admission NIHSS score (Supplementary Table 2, Spearman r=–0.29, p = 0.04).
As for infarct volumes, the percentage of DNTs within lymphocytes was the only element correlated to infarct volume at admission (Supplementary Table 2, Spearman r = 0.16 p = 0.03). A higher level of DNTs was observed in patients with larger infarct volumes.
To explore the existing administration of stroke patients, it is eager for exploring a biomarker that can predict the severity and prognosis of stroke. Thus, to further explore the potential of regulatory lymphocytes assessing stroke severity at the acute stage, regulatory lymphocytes at admission were analyzed using multivariate logistic regression analyses with larger infarct volume (≥3 cm3) and unfavorable neurofunctional scores (NIHSS ≥5 or mRS ≥3) (Table 2). However, none of these variables showed a strong association with the infarct volume at admission. The percentage of DNTs within lymphocytes exhibited a significant positive relationship with NIHSS score[OR (95%CI) = 1.27 (1.07–1.49), p = 0.004], though the level of DNTs at admission was not changed. Only the percentage of CD4+ Tregs within lymphocytes had a negative correlation with both NIHSS and mRS scores [OR (95%CI) = 0.48 (0.28–0.81) & 0.55 (0.33–0.89), p = 0.006 & 0.016]. These results suggested that the lower level of CD4+ Tregs or the higher level of DNTs at admission, the worse of neurofunction after stroke.
Table 2.
Logistic regression of acute stage characteristics.
| Admission (/Lyphocytes, %) |
Infarct volume ≥ 3 cm3 |
NIHSS ≥ 5 |
mRS ≥ 3 |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Bregs | 1.09 (0.89-1.34) | 0.396 | 1.11 (0.92-1.32) | 0.279 | 1.07 (0.90-1.28) | 0.445 |
| CD4+ Tregs | 1.02 (0.57-1.83) | 0.954 | 0.48 (0.28-0.81) | 0.006 | 0.55 (0.33-0.89) | 0.016 |
| CD8+ Tregs | 1.26 (0.86-1.86) | 0.239 | 0.90 (0.63-1.27) | 0.533 | 0.95 (0.68-1.33) | 0.762 |
| DNTs | 1.16 (0.98-1.38) | 0.095 | 1.27 (1.07-1.49) | 0.004 | 0.93 (0.79-1.10) | 0.399 |
Data were analyzed using binary logistic regression. OR: odds rate; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale.
In addition, to evaluate the prediction ability of lymphocytes in predicting stroke outcomes, we used lymphocytes’ percentages along with the clinical features to calculate receiver operating characteristic (ROC) curves and the area under the curve (AUC) by applying and comparing four classification models: Logistic regression, Support Vector Machine (SVM), Decision Tree, and Random Forest. Among them, Logistic regression showed the greatest predictive ability and was considered for further prediction analysis. The addition of all investigated immunoregulatory lymphocytes at the admission to the logistic regression model that included factors known to affect the stroke outcomes improved the AUC for the prediction of NIHSS at admission, especially CD4+ Tregs and DNTs (Figure 2(b)). It was suggested regulatory lymphocytes, especially CD4+ Tregs and DNTs, provided more accuracy to assess the acute stage severity of ischemic stroke.
Relationships between the regulatory lymphocytes and stroke outcomes
Infiltration of lymphocytes persists during the late-stage and is involved in the resolution phase of brain injury in animal models of stroke.33 Clinical studies suggested that the changes in lymphocytes may impact the long-term outcome after brain injuries.34,35 And the frequencies of circulating B- and T-lymphocytes can be indicators for stroke outcomes.36 Next, similar linear regression analyses were also conducted (Supplementary Table 3) and summarized (Figure 2(a)) to assess the correlations between lymphocytes and stroke outcomes at discharge and 3 months. As a result, at discharge, no elements showed a significant relationship with same-time neurological scores.
At 3 months, only the percentage of B cells within lymphocytes had a significant relationship with neurological scores at the same time-point (Supplementary Table 3, NIHSS: Spearman r = 0.25, p = 0.04). Only the percentage of T cells within lymphocytes at 1 month had statistically significant correlations with the mRS score at 3 months (Supplementary Table 3, Spearman r=-0.37, p = 0.01).
The greatest predictive ability of neurological outcomes at the sub-acute stage was manifested in the level of CD4+ Tregs at admission, too. A lower level of CD4+ Tregs at admission suggested poorer NIHSS and mRS scores at discharge in our present study (Supplementary Table 3, Spearman r=–0.23 & –0.23, p = 0.001 & 0.001). The percentages of CD4+ T cells and T cells within lymphocytes at admission were also negatively related to NIHSS score at discharge (Supplementary Table 3, Spearman r=–0.17 & –0.19, p = 0.01 & 0.006). While the percentage of CD8+ Tregs at admission were positively related to mRS score at discharge (Supplementary Table 3, Spearman r = 0.18, p = 0.01), which provided evidence on higher CD8+ Tregs’ potential of predicting unfavored short-term stroke outcomes.
Excitingly, our study suggested that the percentage of CD4+ Tregs within lymphocytes at admission had a great predictive ability of mRS score at 3 months after stroke (Supplementary Table 3, Spearman r = 0.14, p = 0.04), as well as the percentage of B cells within lymphocytes (Supplementary Table 3, Spearman r=–0.14, p = 0.04).
Furthermore, the abilities of regulatory lymphocytes to predict the ischemic stroke outcomes were further evaluated by logistic regression analyses (Table 3). Multivariate logistic regression analyses suggested patients with higher percentage of CD4+ Tregs within lymphocytes at admission had lower risks of unfavorable outcomes at discharge [NIHSS: OR (95%CI) = 0.47 (0.26–0.85), p = 0.012; mRS: OR (95%CI) = 0.44 (0.25–0.75), p = 0.003]. Meanwhile, higher percentage of Bregs within lymphocytes at admission suggested worse outcomes at discharge [NIHSS: OR (95%CI) = 1.19 (0.98–1.46), p = 0.085; mRS: OR (95%CI) = 1.21 (1.00–1.45), p = 0.047], though no significance was observed in linear regression. For long-term outcomes, however, due to the excellent functional recoveries obtained, the defines of unfavorable outcomes were adjusted as NIHSS ≥2 or mRS ≥2. the percentage of CD4+ Tregs within lymphocytes at admission was also a potential predictor of mRS score at 3 months [OR (95%CI) = 1.59 (1.01–2.50), p = 0.043].
Table 3.
Logistic regression of discharge and 3 months characteristics.
| Admission (/Lyphocytes, %) |
Discharge |
3 months |
||||||
|---|---|---|---|---|---|---|---|---|
|
NIHSS ≥ 5 |
mRS ≥ 3 |
NIHSS ≥ 2 |
mRS ≥ 2 |
|||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Bregs | 1.19 (0.98–1.46) | 0.085 | 1.21 (1.00–1.45) | 0.047 | 1.14 (0.87–1.50) | 0.354 | 0.97 (0.82–1.14) | 0.698 |
| CD4+ Tregs | 0.47 (0.26–0.85) | 0.012 | 0.44 (0.25–0.75) | 0.003 | 0.74 (0.33–1.65) | 0.459 | 1.59 (1.01–2.50) | 0.043 |
| CD8+ Tregs | 0.75 (0.48–1.15) | 0.180 | 1.07 (0.75–1.51) | 0.720 | 0.91 (0.51–1.63) | 0.740 | 1.20 (0.87–1.64) | 0.268 |
| DNTs | 1.02 (0.85–1.23) | 0.808 | 0.97 (0.81–1.15) | 0.708 | 1.03 (0.80–1.34) | 0.803 | 1.04 (0.90–1.21) | 0.575 |
Data were analyzed using binary logistic regression. OR: odds rate; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale.
Although the changes in the percentages of lymphocyte subsets were significant until 3 months, the correlations’ significance was mostly exhibited at admission but not discharge and long-term (Figure 2). It’s suggested that acute-stage regulatory lymphocytes’ changes were crucial to not only short-term but also long-term outcomes, but their later changes barely mattered.
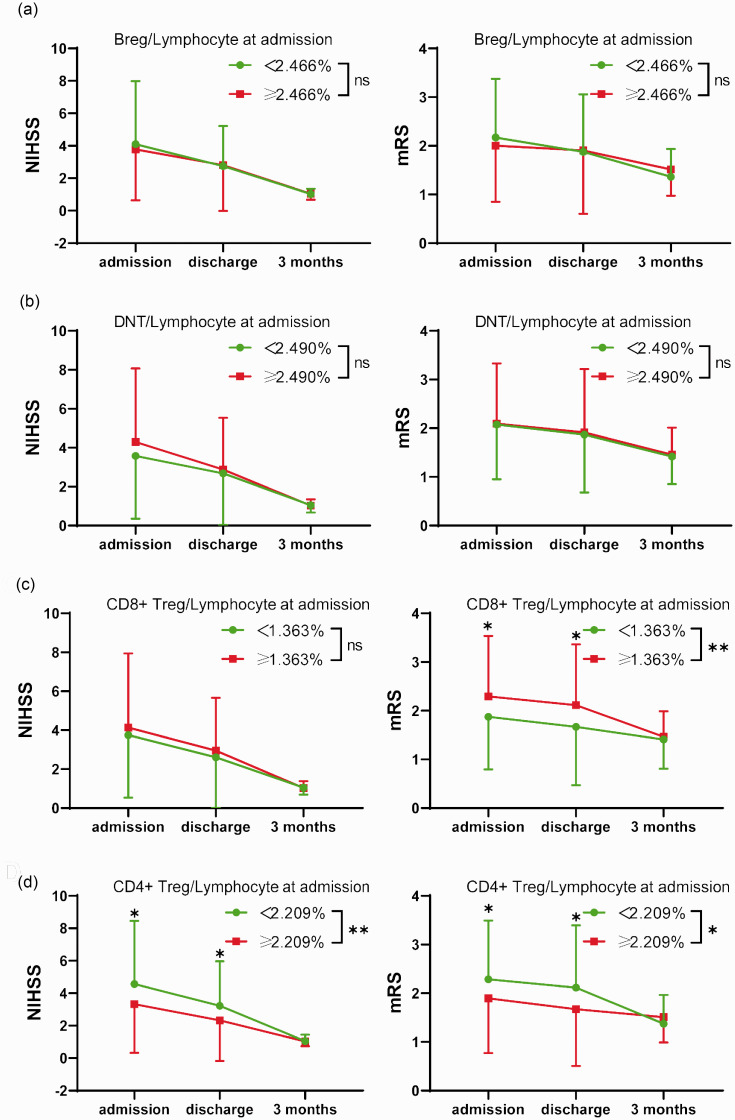
Dividing patients into two groups by the median of the percentage of these regulatory lymphocytes within total lymphocytes at admission (Bregs: 2.466%; DNTs: 2.490%; CD8+ Tregs: 1.363%; CD4+ Tregs: 2.209%), significant differences were observed in the severity process of stroke between higher and lower CD4+ Tregs and in mRS changes between higher and lower CD8+ Tregs, but not in Bregs and DNTs (Figure 3). Furthermore, the time-score lineal fitting curves suggested patients with a lower percentage of CD4+ Tregs within lymphocytes at admission had worse neurological outcomes at the early stage but recovered more quickly than those with a high percentage (Supplementary Figure 3(d)). For CD8+ Tregs, lower CD8+ Tregs at admission suggested favored mRS outcomes from admission to discharge (Supplementary Figure 3(c)).
Figure 3.
Changes of neurological score from admission to 3 months in different levels of regulatory lymphocytes. Variation of NIHSS (left) or mRS score (right) from admission to 3 months grouped by the median of the percentage of regulatory lymphocytes within total lymphocytes at admission ((a): Breg: 2.466%; (b): DNT: 2.490%; (c); CD8+Treg: 1.363%; (d): CD4+Treg: 2.209%). Green circle symbols: patients with the percentage of regulatory lymphocytes lower than the median; red square symbols: patients with the percentage of regulatory lymphocytes higher than the median. Data are presented as mean ± SD. Two-way ANOVA and Bonferroni's multiple comparisons tests were employed to test significance. *: p < 0.05, **: p < 0.01, ns: no significance.
The combination of these analyses indicated that a higher percentage of CD4+ Tregs within lymphocytes at admission predicted favored short-term outcomes, but worse mRS long-term outcomes. While in some level, higher CD8+ Tregs paralleled with unfavored short-term mRS outcomes, higher DNTs with unfavored acute-stage NIHSS outcomes, and higher Bregs with unfavored discharge neurological outcomes.
ROC curves also demonstrated that the addition of immunoregulatory lymphocytes at admission to the Logistic regression model improved the AUC for the prediction of neurological outcomes at discharge and 3 months (Figure 2(b)). For sub-acute stage predictions, CD4+ Tregs exhibited the best predictive ability, while the predictive ability of CD8+ Tregs was not further reflected; for long-term predictions, CD4+ Tregs and Bregs enhanced the model’s predictive ability. In a word, these results provided further evidence on immunoregulatory lymphocytes enhancing the model’s predictive ability of short-term and long-term outcomes.
Independency of the regulatory lymphocytes with clinical risks of stroke
Patient history and neurological exam are important for stroke diagnosis.37 In stroke patients, the neurological outcome depends on multiple factors including age, sex differences, and admission clinical characteristics27–29 Blood leukocytes are also known to be influenced by a series of diseases or habits. Thus, the relationships between the regulatory lymphocytes and clinical risks of stroke were further assessed. It was revealed that in the healthy controls, Bregs and DNTs’ percentages were negatively related to age, but not related to sex difference. While the levels of CD4+ and CD8+ Tregs did not correspond to either age or sex (Supplementary Table 4).
After stroke, admission Bregs and DNTs’ percentages were still negatively related to age. However, CD4+ and CD8+ Tregs’ percentages also showed relationships with patients’ age at admission (Supplementary Table 4). Older patients had lower CD4+ Tregs and higher CD8+ Tregs, which was also consistent with the regulatory lymphocytes’ changes after stroke. Only CD4+ Tregs exhibited a significant difference in sex (Supplementary Table 4). Females had significantly lower CD4+ Tregs than males at admission as we observed.
All the investigated regulatory lymphocytes presented weak relationships with clinical risks including drinking, smoking, hypertension, and diabetes. Only admission Bregs showed positive relationships with admission LDL’s level (Supplementary Table 4). It is revealed that admission regulatory lymphocytes, especially CD4+ and CD8+ Tregs, were independent with most clinical risks, which provided them more evidence on their dependability to predict stroke outcomes.
Due to the low proportions of patients with atrial fibrillation and coronary artery disease (9.52% & 4.76%), the influences of these comorbidities were not further evaluated.
Independent correlation and predictive ability of the CD4+ Tregs in acute ischemic stroke
Our data have indicated that CD4+ Tregs were an impressive marker with the ability to not only reflect severity after acute ischemic stroke but also predict long-term outcomes, meanwhile embracing considerable independence with stroke risk factors. Thus CD4+ Tregs’ characteristics and dynamic changes were further concentrated.
Further assessments involving clinical and demographic determinants were provided to detect whether CD4+ Tregs are an independent predicting factor of ischemic stroke outcomes. Among these patients enrolled in our study, the admission CD4+ Tregs was significantly associated with age and sex (Supplementary Table 4). Also, tPA treatment is a known factor related to stroke outcomes and post-stroke CD4+ Tregs change [8]. However, among age, sex, and tPA treatment, multivariable linear regression suggested a lower percentage of CD4+ Tregs within lymphocytes at admission was independently associated with poorer mRS at admission or discharge, but greater mRS at 3 months (Supplementary Table 5). Meanwhile, a lower percentage of CD4+ Tregs within lymphocytes at admission was independently associated with poorer NIHSS at admission and discharge (Supplementary Table 5).
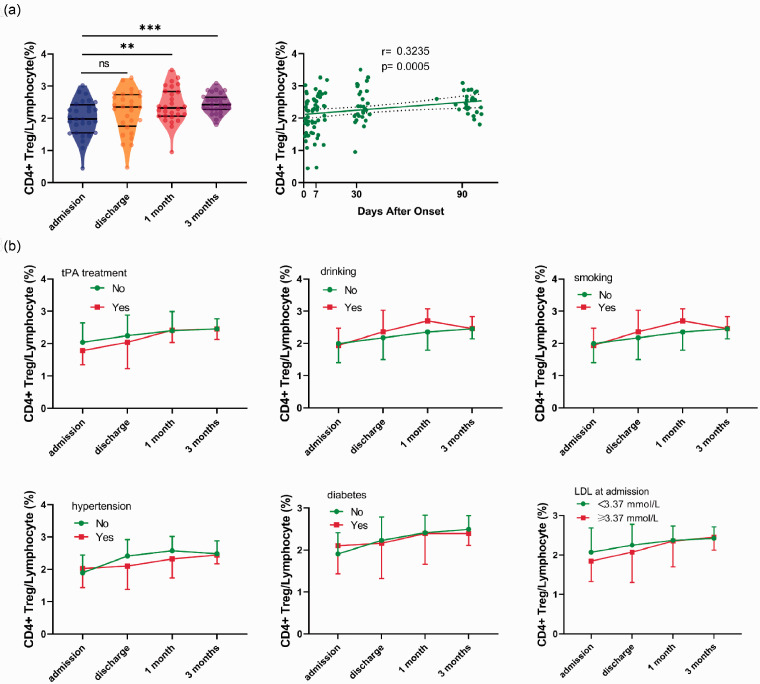
Finally, the data of the 28 patients with complete blood sample collection at 4 time-points were focused on tracking the CD4+ Tregs’ dynamic changes. The percentage of CD4+ Tregs within lymphocytes had an excellent correlation with the time after onset (Figure 4(a)). No significant difference in the percentage of CD4+ Tregs within lymphocytes changes was observed between patients with or without tPA treatment. Meanwhile, the dynamic changes of CD4+ Tregs were independent of smoking, drinking, hypertension, diabetes, and LDL level (Figure 4(b)). However, no statistically significant difference was observed in the continuous changes of CD4+ Tregs between poorer and greater neurological outcomes and in their time-percentage linear fitting curves, except between the linear fitting curves of NIHSS at 3 months (Supplementary Figure 4). The fitting curves demonstrated patients who had unfavorable long-term NIHSS outcomes tended to have a higher level of CD4+ Tregs all the time, consistent with the previous discovery that a lower level of CD4+ Treg at admission predicted a favored long-term prognosis.
Figure 4.
Continuous changes in the percentage of CD4+ Tregs within lymphocytes in 28 patients. (a) Left: Violin plots with median, 25 and 75% quartiles of the percentage of CD4+ Tregs within lymphocytes of different time points after acute ischemic stroke (Admission: blue, Discharge: orange, 1 month: red, 3 months: purple) in the 28 patients with complete data (RM one-way ANOVA, p = 0.0005). Bonferroni's multiple comparisons tests were employed to examine the differences between time-points. Right: Spearman correlation between the percentage of CD4+ Tregs within lymphocytes and time after onset. The dotted line represents 95% confidence bands of the best-fit line. (b) Variation of the percentage of CD4+ Tregs within lymphocytes from admission to 3 months in patients with tissue plasminogen activator (tPA) treatment (n = 6), habits of drinking (n = 5), smoking (n = 4), history of hypertension (n = 19), diabetes (n = 11), blood low-density lipoprotein (LDL) higher than 3.37 mmol/L (n = 13) or not. All data are presented as mean ± SD. Two-way ANOVA and Bonferroni's multiple comparisons tests were employed to test significance. **: p < 0.01, ***: p < 0.001, “ns” or no symbol: no significance.
Discussion
In both animal models of stroke and stroke patients, the change of quantity or function in peripheral blood lymphocytes can be observed.38–40 The dynamic change of circulating lymphocytes is suggested because of the activation of the sympathetic nervous system and the release of multiple molecular patterns from the injured brain.41,42 Stroke, while traditionally considered as an "acute" neurological disorder, has been recognized as accompanied by chronic pathological outcomes, it will also be necessary to monitor lymphocyte levels acutely and chronically. Moreover, lymphocytes not only contribute to early inflammation and brain injury, but some subsets of them can also contribute to the repair and regeneration of the brain at later stages.43 It is challenging so far as to identify a cost-effective and sensitive biomarker for stroke. Peripheral blood lymphocytes embrace its convenience to collect and analyze, which provides the potential to be a hopeful clinical biomarker for stroke diagnosis and prediction.
Our present study demonstrated long-term dramatic changes in the lymphocyte subsets among acute ischemic stroke patients, including B cells, CD4+ T cells, Bregs, CD4+ Tregs, CD8+ Tregs, and DNTs. Immunity plays various roles in stroke pathology and is regarded as a key target of stroke therapy.44 Regulatory lymphocytes perform a factual and controversial role in stroke.20 Thus, the dynamic changes of these lymphocytes set off our interests. B cells extraordinarily increased after stroke and maintained for the long term. This proliferation may be interpreted into the B lymphocytes’ activation response to antigen release after stroke.45 While total T cells’ quantity kept stable after stroke. CD4+ helper T cells slightly and shortly increased after stroke. CD8+ cytotoxic T cells, which is thought detrimental to stroke-related demyelination,46 remained steady after stroke.
Attractingly, the changes in immunoregulatory lymphocytes were significant and noteworthy. CD4+ Tregs are highly attention-attracting immunoregulatory cells involved in stroke, though their role is yet considered to be controversial.20 The dynamic change of CD4+ Tregs enrolled in our study was consistent with previous researches,47,48 which means they dropped significantly within three days after stroke but rose reversely at about seven days, then quickly returned normal. CD4+ Tregs are cerebroprotective immunomodulators of inflammatory brain damage that happens after stroke. The change of CD4+ Tregs may due to endogenous adaptive immune response and multiple inflammatory pathways after brain injury.49 Bregs are considered to be potentially beneficial for stroke patients,23 which were also found significantly increased in our study. Another regulatory T cells–CD8+ Tregs, which are also found reacting to stroke,30 increased dramatically and stayed high after stroke. The change of CD8+ Tregs can be induced by multiple cytokine and post-stroke neuroinflammation. A previous study suggested IL-10-secreting Bregs involve in a potential neuroprotection function by inducing CD8+ Tregs and ameliorating neuroinflammation after stroke.30 It is interesting to investigate CD8+ Tregs considering the differences in natural Treg function. Differently from a previous study,24 DNTs were observed to up-regulated at 3 months after stroke only.
Long-lasting changes in the frequencies of circulating lymphocytes are associated with cellular and humoral immune status. Our further statistical analyses had revealed great relativity with stroke outcomes among these lymphocyte subsets. Although in the long-term, the lymphocytes’ proportion lacks correlations with neurological scores, possibly due to most lymphocytes converted back to normal levels swiftly. Another considerable reason is the long-term blood lymphocytes level is easily influenced by various environmental factors like inflammation, nutrition conditions, etc. Besides, a limitation of our study is that most patients had a favored recovery making their 3-months neurological score somehow indistinguishable. Anyhow, a higher level of B cells at 3 months was the only biomarker accompanied by poorer neurological outcomes, which supported the hypothesis of autoantibody induces dementia after stroke.19
Excitingly, correlation analysis revealed high correlations between lymphocytic proportions and neurological assessment at the early stage, especially at admission, but not infarct volume. ROC curves suggested that lymphocytic proportions exhibit extraordinary predictive abilities for admission, discharge, and 3-months neurofunctional outcomes.
Linear regression analyses suggested B cells showed a weak correlation to admission severity of stroke at admission, but admission severity could influence B cells level at discharge and 1 month. While higher B cells’ proportion within lymphocytes was positively related to favored long-term neurofunctional outcomes which is consistent with the previous research.11 However, it is hard to explain why a high level of B cells at admission was associated with a great 3-months prognosis, but a high level of B cells at 3 months was on the contrary, leaving a complex mechanism of the humoral immune response to ischemic stroke to be solved. Interestingly, the level of Bregs at admission predicted poorer neurological outcomes at discharge in logistic regressions, which provides little evidence on Bregs being a good immunomodulator in stroke.23
DNTs were the only marker reflecting the severity of infarct volume at admission. Infarct volume showed a weak correlation with blood lymphocyte subsets’ proportions in our study. DNTs showed poor correlations with neurological outcomes in the linear regression. However, DNTs showed positive relativity with NIHSS score at admission in logistic regression, suggesting a delicate predictive ability for short-term severity. Thus, our research provides some evidence for previous research that believes DNTs exacerbated ischemic brain injury.24
CD8+ Tregs is a unique subgroup of regulatory T cells, which was had little knowledge of. In our study, a higher percentage of CD8+ Tregs at admission predicted poorer mRS neurological outcomes at admission and discharge. CD8+ Tregs’ level stayed stable that was still related to admission mRS score at 1 month, which is unique in all regulatory lymphocytes we investigated. It is inferred that CD8+ Tregs were a potential reflection of short-term stroke severity, though it remained ambiguous whether it played a damage-aggravating or a pro-restore role in stroke.
As a mostly researched regulatory lymphocytes, CD4+ Tregs showed extraordinary predictive abilities not only to reflect short-term NIHSS and mRS neurological severity but also to predict long-term mRS prognosis in our study. Unlike other regulatory lymphocytes, the percentage of CD4+ Tregs dropped at admission. It is notice-worthy that a lower level of CD4+ Tregs at admission stood for greater severity at admission and discharge, but for greater prognosis at 3 months. A reasonable explanation is that the lack of Tregs augments the activation of inflammatory responses at the early stage, thus exacerbating secondary brain damage.49 Regarding its promotion for long-term restoration, further researches are required to fully reveal the function of CD4+ Tregs as the role it plays in stroke is so controversial. Our results proved that immunoregulatory lymphocytes, especially CD4+ Tregs, were more sensitive biomarkers of stroke severity and outcomes than regular lymphocyte subsets.
Human stroke outcomes depend on age, gender, and comorbid conditions.50 Considering circulating lymphocytes are sensitive to a series of clinical diseases and conditions, the independence of regulatory lymphocytes was assessed in further concern. All regulatory lymphocytes succeeded in the examination of independence with risk factors including habits of drinking, smoking and hypertension, and diabetes. Besides, none of them was distinguishable between sex in healthy controls. However, female witnessed lower CD4+ Tregs at admission. According to our previous finding that lower admission CD4+ Tregs predicted worse neurological outcomes, the sex difference in CD4+ Tregs is consistent with the previous report that women witnessed poorer stroke outcomes than men.28 In our study, age was found to be a determination of the circulating Bregs and DNTs’ levels, which has not been reported yet to our knowledge. While after stroke, all investigated regulatory lymphocytes were found to be influenced by age. The older patients were, the lower levels of Bregs, DNTs, CD4+ Tregs, but the higher level of CD8+ Tregs they had. As reported and we observed, older patients are more vulnerable to ischemia stroke.27 It is inferred that there should be some age-related mechanism affecting regulatory lymphocytes’ activation. Cardiovascular comorbidities are also reported to be associated with post-stroke neurological deficiency;29 however, the influences of history of atrial fibrillation and coronary artery disease were not further concerned because of the small proportions. Conclusively, it was revealed that regulatory lymphocyte subsets were relatively independent biomarkers predicting stroke outcomes.
The comprehensive correlation and independence of CD4+ Tregs attracted our further attention. More detailed analyses reveal that little relativities were observed of CD4+ Tregs with clinical risks of stroke. Moreover, multivariant linear regressions evidenced that CD4+ Tregs’ predictive ability was independent with age, sex, and tPA treatment.
Overall, our data evidence that the proportions of regulatory lymphocytes in PBMCs may be potentially clinical biomarkers for stroke outcomes prediction as they enhanced the predictive abilities of the model including traditional risk factors. CD4+ Tregs are a highlight in immune-associated stroke research, and its importance was proved again because of its great correlation and predictive ability not only in the acute stage but also in long-term outcomes. We are the first to comprehensively investigate the role of regulatory lymphocytes, including less focused regulatory lymphocytes, Bregs, CD8+ Tregs and DNTs, in long-term stroke outcomes. They also showed less but nonnegligible degrees of correlation and predictive ability in acute severity of ischemic stroke. Further studies are needed to understand the roles of these regulatory lymphocytes and the cooperation or antagonism among them in ischemic stroke patients.
Our study evaluates the correlations between the number of circulating lymphocytes and the long-term outcomes in stroke patients. The change of immunoregulatory lymphocytes overtime after stroke will be useful to predict stroke outcomes or the effectiveness of stroke treatment. The novelty of our study includes the comprehensive investigations of several known but unclear regulatory lymphocytes, the long-term follow-ups of these immunological members, the multifaceted analyses of clinical assessments, and evaluation of the regulatory lymphocytes as stroke biomarkers. Some limitations of our study should be noted. First, our study is restricted to the peripheral blood as lymphocyte levels in the brain are not readily accessible in patients. Second, the neurological assessments at 1 month after stroke were ignored, though patients’ blood was collected at that time-point. And then, at 3 months, most patients enrolled in this study had near and favored outcomes, which narrows the discrimination between them. Third, the clinical information on stroke risk factors among the controls was not recorded because they were from the healthy volunteers who lacked intact information. Fourth, although the long-term follow-up of neurological scores by telephones or visits was successful, the follow-up of blood collection of these subjects was considerably missing because some patients refused to come back to the hospital to collect blood samples for personal reasons. The 28 patients with complete data from admission to 3 months, which is limited for tracking CD4+ Tregs’ dynamic changes. Last, besides CD4+ Tregs, CD8+ Tregs also had a nonnegligible potential of reflecting and predicting stroke severity and prognosis, as well as DNTs and Bregs, which deserves our further concentration.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X21995694 for Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke by Sicheng Li, Yichen Huang, Yang Liu, Marcelo Rocha, Xiaofan Li, Pengju Wei, Dilidaer Misilimu, Yunhe Luo, Jing Zhao and Yanqin Gao in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Chinese Key R&D Plan of the State Ministry of Science and Technology 2017YFC1308403 (to Y.G), and Chinese Natural Science Foundation grants 8,187,097,181,571,285 (to Y.G) , Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZJ Lab. We thank the patients and healthy volunteers for the donation of blood samples.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: YG designed the study. JZ designed the clinical information of patients. SL, YH, PW and MD performed the experiments. SL, YL, XL and YL collected the clinical information of patients. YH, YG, SL and MR analyzed the data. YH, SL and YG wrote the manuscript. YG critically edited the manuscript.
ORCID iDs: Marcelo Rocha https://orcid.org/0000-0002-4503-5827
Yanqin Gao https://orcid.org/0000-0002-4915-9819
Supplementary material: Supplemental material for this article is available online.
References
- 1.Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet 2008; 371: 1612–1623. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 3.Jickling GC, Sharp FR.Improving the translation of animal ischemic stroke studies to humans. Metab Brain Dis 2015; 30: 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo EH, Dalkara T, Moskowitz MA.Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003; 4: 399–415. [DOI] [PubMed] [Google Scholar]
- 5.Jickling GC, Sharp FR.Blood biomarkers of ischemic stroke. Neurotherapeutics 2011; 8: 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 2011; 10: 471–480. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Zhu Z-Y, Huang T-T, et al. The peripheral immune response after stroke – a double edge sword for blood-brain barrier integrity. CNS Neurosci Ther 2018; 24: 1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao HW, Kuan CY.Early neutrophil infiltration is critical for inflammation-sensitized hypoxic-ischemic brain injury in newborns. J Cereb Blood Flow Metab 2020; 40: 2188–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui LL, Zhang Y, Chen ZY, et al. Early neutrophil count relates to infarct size and fatal outcome after large hemispheric infarction. CNS Neurosci Ther 2020; 26: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawabori M, Yenari MA.Inflammatory responses in brain ischemia. Curr Med Chem 2015; 22: 1258–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urra X, Cervera A, Villamor N, et al. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience 2009; 158: 1174–1183. [DOI] [PubMed] [Google Scholar]
- 12.Yan J, Greer JM, Etherington K, et al. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol 2009; 206: 112–117. [DOI] [PubMed] [Google Scholar]
- 13.Guoping P, Wei W, Xiaoyan L, et al. Characteristics of the peripheral T cell immune response of patients at different stages of vascular cognitive impairment. Immunol Lett 2015; 168: 120–125. [DOI] [PubMed] [Google Scholar]
- 14.ZG N, H L, V W et al. Elevated pro-inflammatory CD4+CD28− lymphocytes and stroke recurrence and death. Neurology 2004; 63: 1446–1451. [DOI] [PubMed] [Google Scholar]
- 15.Liesz A, Kleinschnitz C.Regulatory T cells in post-stroke immune homeostasis. Transl Stroke Res 2016; 7: 313–321. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10: 490–500. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Zhang H, Xu Y.Crosstalk between microglia and T cells contributes to brain damage and recovery after ischemic stroke. Neurol Res 2016; 38: 495–503. [DOI] [PubMed] [Google Scholar]
- 18.Xie L, Li W, Hersh J, et al. Experimental ischemic stroke induces long-term T cell activation in the brain. J Cereb Blood Flow Metab 2019; 39: 2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle KP, Quach LN, Sole M, et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci 2015; 35: 2133–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liesz A, Hu X, Kleinschnitz C, et al. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke 2015; 46: 1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Wing K, Onishi Y, et al. Regulatory T cells: how do they suppress immune responses? Int Immunol 2009; 21: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 22.Neal EG, Acosta SA, Kaneko Y, et al. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J Cereb Blood Flow Metab 2019; 39: 1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seifert HA, Vandenbark AA, Offner H.Regulatory B cells in experimental stroke. Immunology 2018; 154: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng H, Zhao H, Cao X, et al. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc Natl Acad Sci U S A 2019; 116: 5558–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SJ, Moon GJ, Bang OY.Biomarkers for stroke. J Stroke 2013; 15: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saenger AK, Christenson RH.Stroke biomarkers: progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin Chem 2010; 56: 21–33. [DOI] [PubMed] [Google Scholar]
- 27.Sommer CJ.Ischemic stroke: experimental models and reality. Acta Neuropathol 2017; 133: 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008; 7: 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao X, Zhang J, Peng Y, et al. Admission clinical characteristics and early clinical outcomes among acute ischemic stroke patients. J Biomed Res 2012; 26: 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodhankar S, Chen Y, Lapato A, et al. Regulatory CD8(+)CD122 (+) T-cells predominate in CNS after treatment of experimental stroke in male mice with IL-10-secreting B-cells. Metab Brain Dis 2015; 30: 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hug A, Dalpke A, Wieczorek N, et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke 2009; 40: 3226–3232. [DOI] [PubMed] [Google Scholar]
- 32.Planas AM.Role of immune cells migrating to the ischemic brain. Stroke 2018; 49: 2261–2267. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y, Liao S, Wei C, et al. Infiltration and persistence of lymphocytes during late-stage cerebral ischemia in Middle cerebral artery occlusion and photothrombotic stroke models. J Neuroinflammation 2017; 14: 248–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Lin YP, Chen JL, et al. Role of regulatory T cell in clinical outcome of traumatic brain injury. Chin Med J 2015; 128: 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laginha I, Kopp MA, Druschel C, et al. Natural killer (NK) cell functionality after human spinal cord injury (SCI): protocol of a prospective, longitudinal study. BMC Neurol 2016; 16: 170–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Liu J, Wang X, et al. Frequencies of circulating B- and T-lymphocytes as indicators for stroke outcomes. Neuropsychiatr Dis Treat 2017; 13: 2509–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamova B, Ander BP, Jickling G, et al. The intracerebral hemorrhage blood transcriptome in humans differs from the ischemic stroke and vascular risk factor control blood transcriptomes. J Cereb Blood Flow Metab 2019; 39: 1818–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill D, Veltkamp R.Dynamics of T cell responses after stroke. Curr Opin Pharmacol 2016; 26: 26–32. [DOI] [PubMed] [Google Scholar]
- 39.Stubbe T, Ebner F, Richter D, et al. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab 2013; 33: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulze J, Zierath D, Tanzi P, et al. Severe stroke induces long-lasting alterations of high-mobility group box 1. Stroke 2013; 44: 246–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q, Jin WN, Liu Y, et al. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity 2017; 46: 474–487. [DOI] [PubMed] [Google Scholar]
- 42.Liesz A, Dalpke A, Mracsko E, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci 2015; 35: 583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brait VH, Arumugam TV, Drummond GR, et al. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metsab 2012; 32: 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iadecola C, Anrather J.The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doyle KP, Buckwalter MS.Does B lymphocyte-mediated autoimmunity contribute to post-stroke dementia? Brain Behav Immun 2017; 64: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y-X, Wang X, Tang D, et al. IL-2mAb reduces demyelination after focal cerebral ischemia by suppressing CD8 T cells. CNS Neurosci Ther 2019; 25: 532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao L, Li P, Zhu W, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain 2017; 140: 1914–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang X, Qian W.Changes in regulatory T-cell levels in acute cerebral ischemia. J Neurol Surg A Cent Eur Neurosurg 2017; 78: 374–379. [DOI] [PubMed] [Google Scholar]
- 49.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009; 15: 192–199. [DOI] [PubMed] [Google Scholar]
- 50.Balkaya M, Cho S.Optimizing functional outcome endpoints for stroke recovery studies. J Cereb Blood Flow Metab 2019; 39: 2323–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X21995694 for Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke by Sicheng Li, Yichen Huang, Yang Liu, Marcelo Rocha, Xiaofan Li, Pengju Wei, Dilidaer Misilimu, Yunhe Luo, Jing Zhao and Yanqin Gao in Journal of Cerebral Blood Flow & Metabolism