Abstract
Chinese kale (Brassica oleracea var. alboglabra) is rich in carotenoids, and neoxanthin is one of the most important carotenoids in Chinese kale. In this study, the function of the neoxanthin synthase gene (BoaNXS) in Chinese kale was investigated. BoaNXS, which had a 699-bp coding sequence, was cloned from the white flower cultivar of Chinese kale and was expressed in all developmental stages and organs of Chinese kale; its expression was highest in young seeds. The subcellular localization indicated that BoaNXS was localized in the chloroplast. BoaNXS-overexpressed plants were obtained via Agrobacterium-mediated transient overexpression methodology, and the gene overexpression efficiencies ranged from 2.10- to 4.24-fold. The color in the leaves of BoaNXS-overexpressed plants changed from green to yellow-green; the content of total and individual carotenoids, such as neoxanthin, violaxanthin, and lutein, was significantly increased, and the expression levels of most carotenoid biosynthetic genes were notably increased. These findings indicated that BoaNXS is of vital importance in carotenoid biosynthesis in Chinese kale and could be used as a candidate gene for enriching the carotenoid accumulation and color of Chinese kale and other Brassica vegetables.
Keywords: Chinese kale, BoaNXS, carotenoid biosynthesis, subcellular localization, gene transient overexpression
1. Introduction
Carotenoids are a major group of pigments that are distributed abundantly in a variety of plants [1]. They typically contain 40 carbons in their polyene backbones with conjugated double bonds and rings at the ends [2]. They play significant roles in plant development, including light harvesting, photoprotection against excess light, and the synthesis of plant hormones [3]. Most animals obtain necessary carotenoids through their diet [4]. The accumulation of carotenoids enhances both the sensory and nutritional quality of fruits, flowers, and vegetables; carotenoids are also beneficial to human health [5]. Furthermore, they are essential precursors to several phytohormones, such as abscisic acid and strigolactones [6]. In humans, carotenoids can help prevent several major diseases, including certain cancers and eye diseases [7]. The carotenoid biosynthesis pathway in higher plants consists of the condensation of small molecular substances into C40 compounds and a series of reactions, including dehydrogenation, isomerization, cyclization, hydroxylation, and epoxidation, to produce different carotenoids [8].
Neoxanthin, which is present in light-harvesting complexes, is a precursor of abscisic acid and a xanthophyll that is the last product of carotenoid biosynthesis in green plants [9,10]. The neoxanthin synthase gene (NXS) encodes a 56-kDa plastid-targeted protein that, when expressed in Escherichia coli, catalyzes the conversion of violaxanthin to neoxanthin [9]. In plants, one of the possible pathways that is thought to underlie this conversion is as follows: violaxanthin → neoxanthin → abscisic acid [11]. Previous studies have identified NXS from Arabidopsis thaliana [12], tomato (Solanum lycopersicum) [9], and potato (Solanum tuberosum) [13]. However, little research has been conducted on NXS compared with other carotenoid biosynthetic genes.
Chinese kale (Brassica oleracea var. alboglabra) is a vegetable within the Cruciferae family native to South China, where it is distributed widely [14]. The tender leaves and bolting stems are the most commonly used edible parts because of their high content of nutrients, such as glucosinolates, vitamin C, and carotenoids [15,16]. In our previous study, four carotenoids (lutein, neoxanthin, violaxanthin, and β-carotene) were observed in Chinese kale, among which the content of neoxanthin is second only to lutein [16]. However, the synthesis of the xanthophyll neoxanthin in Chinese kale remains unclear. In this study, the full-length coding sequence (CDS) of BoaNXS was cloned. Sequence analysis and subcellular location were performed. The role of BoaNXS in carotenoid biosynthesis was characterized by phenotypic analysis, gene expression, pigment composition, and content analysis in BoaNXS overexpression plants.
2. Materials and Methods
2.1. Plant Materials
The cultivar ‘Sijicutiao’ of white flower Chinese kale was used in this study. The plants were grown in trays containing a mixture of peat, perlite, and vermiculite (3:1:1) in an artificial climate chamber with a light intensity of 160 μmol m−2 s−1, a temperature of 25/20 °C (day/night), a 12/12 h (day/night) light cycle, and relative humidity maintained at approximately 70%. Fertilizer and water were applied as needed. Chinese kale materials were sampled at different developmental stages (germinating seeds, cotyledons, fifth to sixth true leaves, and mature leaves), various organs were sampled at the maturity stage (roots, bolting stems, leaves, petioles, inflorescences, seed pods, and young seeds), and floral organs were sampled at the flower bud stage and the opening flower stage (sepals, petals, stamens, and pistils) [17]. The samples were then quickly frozen in liquid nitrogen and were stored in a refrigerator at −80 °C for subsequent studies.
2.2. Molecular Cloning and Sequence Analysis
Specific primers for the BoaNXS gene were designed according to the sequences of NXS of homologous species such as cabbage and Chinese cabbage obtained from Brassica database (BRAD) (http://brassicadb.org accessed on 1 May 2019) (Supplementary Table S1). The primers were synthesized by Sangon Biotech Co. Ltd. (Sangon, Shanghai, China). The cDNA of fifth to sixth true leaves was used as the template, and PCR amplification was performed using TransStart FastPfu fly DNA polymerase Taq (TransGene, Beijing, China). The method of gene cloning refers to Sun et al. [18]. The NXS amino acid sequences of other species were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/ accessed on 31 July 2019) and Brassica database (BRAD) (http://brassicadb.org accessed on 31 July 2019) and then subjected to multiple sequence alignment using DNAMAN software (Lynnon Biosoft, Foster City, CA, USA). Subcellular localization was predicted by WoLF PSORT (http://www.genscript.com/wolf-psort.html accessed on 1 June 2019).
2.3. Subcellular Localization
The complete CDS of BoaNXS was amplified by primers NXS-GFP-F and NXS-GFP-R (Table S1) containing BamH I and Sal I restriction sites. Then, BoaNXS and pC2300-35S-eGFP plasmid digested with BamH I and Sal I were mixed to construct the pC2300-35S-NXS-eGFP plasmid. The recombinant plasmid pC2300-35S-NXS-eGFP and empty vector pC2300-35S-eGFP were transformed into Chinese kale mesophyll protoplasts, respectively. After being cultured in the dark at 23 °C for 24 h, the protoplasts were observed using a BX51 fluorescence microscope equipped with a DP70 camera (Olympus, Tokyo, Japan) [19].
2.4. Transient Overexpression of BoaNXS
Transient overexpression assay was conducted using the methods of a previous study [20]. The complete CDS of BoaNXS was amplified by primers NXS-pCAM-F and NXS-pCAM-R (Supplementary Table S1) containing BamH I and Sal I restriction sites. Then, BoaNXS and pCAMBIA1301-35S-Nos overexpression vector (kindly supplied by Associate Professor Chen Qing, Sichuan Agricultural University, China) digested with BamH I and Sal I were mixed to construct the pCAMBIA1301-BoaNXS plasmid. The recombinant plasmid pCAMBIA1301-BoaNXS and empty vector pCAMBIA3101 were transferred into Agrobacterium GV3101 by freeze-thaw method, and positive clones were screened under kanamycin, gentamicin, and rifampicin resistance conditions.
The Agrobacterium strains were washed with the infiltration buffer (10 mmol L−1 MES, 0.2 mmol L−1 acetosyringone, 10 mmol L−1 MgCl2) and were then cultured to OD600 = 0.6~0.8. The 4-week-old plants of Chinese kale cultivar ‘Sijicutiao’ were treated by using the infiltration buffer containing pCAMBIA1301-BoaNXS construct or pCAMBIA1301 empty vector, and ddH2O was used as the control. The second and third true leaves were injected until the liquid could fully penetrate the leaf back, and were then injected by using a needleless syringe to infiltrate the Agrobacterium every week, four times in total. The second day after the last injection, the leaves were sampled to detect the gene expression and carotenoid contents.
2.5. Color Analysis
Color analysis was performed on the leaves of overexpressed and control plants using an NR110 chromameter (3nh, Shenzhen, China). Three positions were randomly selected on the sampled leaves of each plant, and the values of L*, a*, and b* were statistically analyzed. L* values represented the lightness of the color, ranging between black (L* = 0) and white (L* = 100), a* values represented the color’s position between green (negative a*) and red (positive a*), and b* values represented the color’s position between blue (negative b*) and yellow (positive b*).
2.6. Determination of Carotenoid and Chlorophyll Composition and Contents
Carotenoid and chlorophyll concentrations were determined using the methods of a previous study [16]. Two hundred milligrams of leaves were ground and extracted with 25 mL of acetone, and were sonicated for 20 min and then centrifuged at 4000× g at room temperature for 5 min. The supernatant was filtered through 0.22 μm cellulose acetate filters and then analyzed by high-performance liquid chromatography (HPLC). HPLC analysis of carotenoids was carried out using an Agilent 1260 instrument equipped with a variable wavelength detector (VWD). Samples (10 μL) were separated at 30 °C on a Waters Nova-Pak C18 column (150 × 3.9 mm id; 3 μm particle size) using isopropanol and 80% acetonitrile-water at a flow rate of 0.5 mL min−1; the absorbance was measured at 448 and 428 nm (Supplementary Figure S1). Carotenoids (neoxanthin, violaxanthin, lutein, and β-carotene) and chlorophyll (chlorophyll a and chlorophyll b) were quantified according to the respective standard calibration curves, and their standards were obtained from Solarbio Science & Technology Co., Ltd. (Beijing, China).
2.7. RNA Extraction and qPCR Analysis
Total RNA was extracted using an alternative CTAB method. RNA concentration and quality were determined by photometric measurements (General Electric Company, Schenectady, NJ, USA) and gel electrophoresis. Intact total RNA was used for cDNA synthesis using the PrimeScript™ 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China).
The quantitative real-time PCR (qPCR) primers for carotenoid biosynthetic genes in Chinese kale were synthesized according to a previous study [16], and β-actin was used as the reference gene (Table S1). The expression of BoaNXS and other carotenoid biosynthetic genes (PSYs, PDSs, ZISO, ZDS, CRTISO, LCYb, LCYes, β-OHase, ε-OHase, VDE, ZEPs) [21] of overexpressed plants and control plants were performed by using the Bio-Rad iCycler thermocycler (Bio-Rad, Hercules, CA, USA), and the 2−ΔΔCT method was used to calculate the gene expression levels [22].
2.8. Data Analysis
The results were shown as the mean ± standard deviation (SD) of the three technical replicates. Statistical analysis was performed with SPSS software package 18 (SPSS Inc., Chicago, IL, USA). One way analysis of variance (ANOVA) was used to analyze the data. The least significant difference (LSD) test was used to compare the differences at the significance level of 0.05.
3. Results
3.1. Isolation and Characterization of BoaNXS
The CDS of BoaNXS was cloned from Chinese kale leaves and had a sequence length of 699 bp (GenBank accession MW284390), which encoded a 232-amino acid protein and belonged to the DUF4281 superfamily. The multiple sequence alignment results showed that the changes in the amino acid sequences of NXS among different species were mainly located at the N-terminus (Figure 1).
Figure 1.
Multiple sequence alignment of NXS from different plants. Alignment of the protein sequence of BoaNXS with selected homologs. The alignment was performed using DNAMAN software. The amino acids with 100% identity are shown with a black background, those with ≥75% identity are shown in red, and those with ≥50% identity are shown in blue. The species and their accession numbers in GenBank (Brassica oleracea var. alboglabra (Boa): BoaNXS (MW284390), Arabidopsis thaliana (At): AtNXS (VYS50248.1), Brassica juncea (Bju): BjuNXS (BjuA007212), Brassica napus (Bn): BnNXS (XP_013676374.1), Brassica oleracea var. oleracea (Boo): BooNXS (XP_013618475.1), Brassica rapa (Br): BrNXS (XP_009127521.1) are listed here.
3.2. Temporal and Spatial Expression of BoaNXS
The temporal and spatial expression results showed that BoaNXS was detected at all developmental stages and organs in Chinese kale (Figure 2). During the development of Chinese kale, the highest level of BoaNXS expression was in the germination stage, followed by the true leaf stage; BoaNXS expression was lowest in the cotyledon and maturity stages (Figure 2a). Among different organs at the maturity stage, the highest expression level of BoaNXS was observed in young seeds, where BoaNXS expression was twice that observed in other organs, followed by inflorescences and seed pods; relatively low expression was observed in the roots, bolting stems, petioles, and leaves (Figure 2b). Among different flower tissues, the lowest expression level of BoaNXS was observed in stamens. Furthermore, the expression levels of BoaNXS in all flower tissues decreased from the flower bud stage to the opening flower stage (Figure 2c,d).
Figure 2.
Relative expression levels of BoaNXS in different developmental stages (a), organs (b), and flower tissues in flower bud stage (c) and opening flower stage (d) of Chinese kale. The expression level of BoaNXS in germinating seeds was set as 1.
3.3. BoaNXS Was Localized in the Chloroplast
WoLF PSORT software predicted that BoaNXS was most likely to be located in the chloroplast. A clear GFP fluorescence signal of BoaNXS was only detected in the chloroplast (Figure 3), and the expression of the GFP protein was detected in the entire protoplast with green fluorescence signal in the control. These results indicated that BoaNXS was specifically localized in the chloroplast, which was consistent with the software prediction.
Figure 3.
Subcellular localization of BoaNXS in Chinese kale. Free green fluorescing protein served as a control. The upper column represents transient expression of GFP-BoaNXS fusion protein in Chinese kale protoplasts, and the lower column represents transient expression of GFP protein in Chinese kale protoplasts. Merge represents the merged images of GFP (green) and chloroplast autofluorescence (red). Bars = 30 μm.
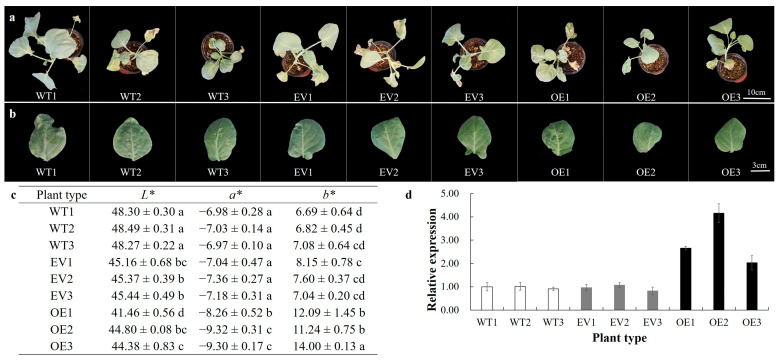
3.4. BoaNXS Overexpression Affected the Color of Chinese Kale
BoaNXS-overexpressed plants were yellow-green compared to the dark-green of the control (the wild-type (WT) plants) and agroinfiltrated plants with empty vector (EV) (Figure 4a,b). The red-green value a* was significantly lower for BoaNXS-overexpressed plants than for control and EV plants (Figure 4c), indicating that the leaves of the overexpressed plants were greener. Moreover, the yellow-blue value b* was significantly lower for BoaNXS-overexpressed plants than for control and EV plants (Figure 4c), indicating that the leaves of the overexpressed plants were yellowing. The BoaNXS gene expression levels of OE plants were 2.10- to 4.24-fold higher than those in WT and EV plants, especially in OE2 (Figure 4d).
Figure 4.
The phenotypes and expression of BoaNXS in wild-type (WT), the agroinfiltrated plants with empty vector (EV), and the overexpressed plants (OE) of Chinese kale: (a) Top view of the control (WT), EV, and OE plants. Bar = 10 cm; (b) The leaf front of sampling leaves. Bar = 3 cm; (c) The color parameters of the control (WT), EV, and OE plants at the second day after the last injection. L* values represent the lightness of the color, a* values represent the color’s position between green and red, and b* values represent the color’s position between blue and yellow. Data are expressed as a mean ± standard deviation (SD) of three replicates. Different lowercase letters in the same column indicate significant differences among values (p < 0.05) according to a Least Significant Difference (LSD) test; (d) The relative expression levels of BoaNXS in the control (WT), EV, and OE plants at the second day after the last injection.
3.5. Overexpression of BoaNXS Increased Carotenoid Accumulation in Chinese Kale
The content of total carotenoids was significantly increased in all three overexpressed plants (OE1, OE2, and OE3) (Figure 5a). The average content of total carotenoids in overexpressed plants was 4.99 mg g−1 DW, which was 1.26-fold higher compared with the average content of the control plants. Four carotenoids were detected in Chinese kale leaves: neoxanthin, violaxanthin, lutein, and β-carotene. The content of neoxanthin and lutein was significantly higher in all three overexpressed plants than in both WT and EV plants (Figure 5b,c), whereas violaxanthin content in OE1 and OE2 leaf tissues (Figure 5d), as well as β-carotene content in OE2 leaf tissue (Figure 5e), was significantly higher compared with that in WT plants. Although there were significant differences in the content of several individual carotenoids (violaxanthin, lutein, and β-carotene) between WT and EV plants, the content of total carotenoids did not significantly differ between WT and EV plants. Among the overexpressed plants, OE2 leaf tissue had the highest total and individual carotenoids (neoxanthin, violaxanthin, lutein, and β-carotene), which were up to 1.38, 1.40, 1.38, 1.37, and 1.32-fold higher compared with the average level of WT plants, respectively. The ratio of total carotenoids to total chlorophyll is also shown in Figure 5f, and the results are basically consistent with the color analysis results (Figure 4).
Figure 5.
Individual and total carotenoid content and ratio of total carotenoids to total chlorophyll of wild-type (WT), the agroinfiltrated plants with empty vector (EV), and the BoaNXS-overexpressed plants (OE) in Chinese kale. (a) content of total carotenoids; (b) neoxanthin content; (c) lutein content; (d) violaxanthin content; (e) β-carotene content; (f) ratio of total carotenoids to total chlorophyll. Samples of leaves were taken from the control (WT), EV, and OE plants at the second day after the last injection. Data are expressed as mean ± standard deviation. Different letters above the bars indicate significantly different values (p < 0.05) according to a Least Significant Difference (LSD) test.
3.6. Overexpression of BoaNXS Induced the Expression of Carotenoid Biosynthetic Genes
To determine whether BoaNXS affects carotenoid accumulation by regulating the transcription of other carotenoid biosynthetic genes, its expression in WT, EV, and OE leaf tissues were analyzed using qPCR (Figure 6). The average expression levels of all carotenoid biosynthetic genes in OE leaf tissues were notably increased, except for β-OHase and ε-OHase, which showed a slight decline. With the exception of BoaNXS, the average expression level of ZDS in OE leaf tissues was the highest among carotenoid biosynthetic genes in Chinese kale, which was 2.57-fold higher compared with WT plants. In addition, the average expression levels of most carotenoid biosynthetic genes in EV plants were lower compared with WT plants. These findings suggest that the up-regulation of BoaNXS expression promotes the entire carotenoid biosynthetic pathway in Chinese kale.
Figure 6.
Heat map of carotenoid biosynthetic gene expression in wild-type (WT), the agroinfiltrated plants with empty vector (EV), and the BoaNXS-overexpressed plants (OE) in Chinese kale. Samples of leaves were taken from the control (WT), EV, and OE plants at the second day after the last injection. Abbreviations: GGPP, geranylgeranyl diphosphate; PSY, phytoene synthase; PDS, phytoene desaturase; ZDS, ζ-carotene desaturase; Z-ISO, ζ-carotene isomerase; CRTISO, carotenoid isomerase; LCYe, lycopene ε-cyclase; LCYb, lycopene β-cyclase; ε-OHase, ε-carotene hydroxylase; β-OHase, β-carotene hydroxylase; VDE, violaxanthin de-epoxidase; ZEP, zeaxanthin epoxidase; NXS, neoxanthin synthase.
4. Discussion
By bringing Agrobacterium into contact with the recipient material through vacuum infiltration, floral dip, or clinical syringe injection methods, Agrobacterium-mediated transient expression is an effective tool for exploring the function of candidate genes in plants [23]. Transient expression technology has several advantages over stable transformation, such as being rapid and simple to perform [24]. In this study, we successfully overexpressed BoaNXS in Chinese kale via transient expression technology, which has been rarely used in research on Brassica vegetables. We found that the expression levels of BoaNXS in overexpressed plants were higher than those in WT plants and agroinfiltrated plants with empty vector. Thus, the Agrobacterium-mediated transient overexpression technology in this study could facilitate the study of gene function in Chinese kale and other Brassica vegetables. In previous studies, transient overexpression efficiencies of rice (Oryza sativa L. subspecies indica), banana (Musa sp.), and Salvia miltiorrhiza were 4.1~7.8 [25], 2.12~4.93 [26], and 3.77~8.39-fold [27], respectively. The gene overexpression efficiencies ranged from 2.10- to 4.24-fold in our study, which is lower compared with some of the above values. In future experiments, we plan to improve the efficiency of transient overexpression by increasing the number of injections, adjusting the sampling time, and changing the strain of Agrobacterium. Simultaneously, stable transformation could be used to verify the results.
The leaves of Chinese kale turned yellow, and the b* value increased in overexpressed plants. This result was similar to previous results in other carotenoid synthetic genes. For example, potato tubers overexpressing StLCYb appeared yellow; in contrast, the control plant was light-white [28]. Canola seeds (Brassica napus) overexpressing crtB (a bacterial phytoene synthase gene) were visibly orange [29]. In addition to leaves, tubers, and seeds, color changes have also been observed in floral organs. For example, Iris germanica L. ‘Fire Bride’ ectopically expressing crtB exhibited pronounced color changes in the ovaries (green to orange), flower stalk (green to orange), and anthers (white to pink) [30]. In our study, the expression of BoaNXS was clearly higher in the flower bud stage of Chinese kale than in the opening flower stage, and this finding was similar to the result of a previous study of Tiger Lily, in which the transcript levels of LllcyB decreased as flower buds developed [31].
NXS catalyzes the formation of neoxanthin from violaxanthin. When T.NXS was transiently overexpressed in tobacco leaves, the neoxanthin content increased as the content of violaxanthin decreased, and there was no significant change in the total pigment content [9]. Our result was inconsistent with this previous study but consistent with a previous study of Arabidopsis [12], in which the overexpression of BoaNXS resulted in consistent increases in both the neoxanthin and violaxanthin content compared with the control. This increase might stem from feedback control on an isomerase, which acts on both trans-neoxanthin and trans-violaxanthin [12], based on the increased levels of trans-neoxanthin generated from higher BoaNXS activity. This self-perpetuating state can promote increased carotenoid synthesis, so we speculate it could be regulated by a positive feedback mechanism [32]. Similarly, overexpression of Dclcyb1 in tobacco increased β-carotene, lutein, and total carotenoids [33]; overexpression of the IbZDS gene in agroinfiltrated sweet potato increased β-carotene and lutein content [34]; and up-regulation of an endogenous PSY gene in Arabidopsis increased β-carotene, lutein, violaxanthin, and the total carotenoid levels [35]. In our study, overexpression of BoaNXS increased the content of individual and total carotenoids, which indicated that this is an effective method for improving the variety of Chinese kale. This further indicates that BoaNXS plays an important role in the regulation of carotenoid content in Chinese kale. Furthermore, the expression of BoaNXS greatly increased the accumulation of transcripts of most carotenoid biosynthetic genes in OE leaf tissues (Figure 6). The same effect was observed in carrot where transcripts of endogenous Dcpsy1, Dcpsy2, and Dclcyb2 genes were increased when Dclcyb1 was overexpressed [36]. These results indicated that overexpression of BoaNXS induced a positive feedback loop affecting the expression of genes involved in carotenoid biosynthesis. Some carotenoids contents and pathway genes were downregulated in EV plants compared with the control in our results, which means the infection of Agrobacterium might affects the carotenoid biosynthesis. Nevertheless, its specific mechanism is still unclear, and the cause of this phenomenon needs further study.
Carotenoid enzymes are found in plastids (especially in chloroplasts and chromoplasts) of plant cells [37], where they exert their functions. BoaNXS translation forms neoxanthin synthase. Neoxanthin synthase is a class of important carotenoid enzymes that participate in carotenoid biosynthesis, ABA synthesis, and photosynthesis [38,39]. Abscisic acid (ABA) is derived from the cleavage of 9-cis isomers of violaxanthin and neoxanthin [38] and functions in embryogenesis and seed germination [40]. This could explain why the expression levels of BoaNXS were the highest in young seeds compared with other organs in our study and indicates that BoaNXS might be related to reproductive growth. In our following experiments, we will also focus on the effects of BoaNXS on ABA biosynthesis and measure some ABA biosynthetic genes to further explore the relationship between BoaNXS and ABA biosynthesis. Dall’Osto et al. found that neoxanthin played a specific role in the protection of Lhc proteins, the photosystem II (PSII) reaction center, and thylakoids from photooxidative stress and that its action was effective against the damaging effect of reactive oxygen species, particularly superoxide anion [39].
5. Conclusions
In summary, the neoxanthin synthase gene BoaNXS was cloned, and BoaNXS was located in the chloroplast. The expression of BoaNXS was detected in all development stages and organs of Chinese kale. BoaNXS-overexpressed agroinfiltrated plants were obtained through Agrobacterium-mediated transient expression technology. The induced expression of BoaNXS and most other carotenoid biosynthetic genes, as well as the increased carotenoid content, indicated that BoaNXS plays an important role in carotenoid biosynthesis in Chinese kale.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12081122/s1, Figure S1: High-performance liquid chromatography profile of carotenoids and chlorophyll in Chinese kale leaves. Table S1: Primers used in this study.
Author Contributions
Conceptualization, Q.W. and B.S.; investigation, Y.J., C.Z., W.H., M.J. and H.Z.; data curation, Y.W., Z.L., J.C., W.Z., H.M. and M.L.; writing—original draft preparation, Y.J., C.Z. and Y.W.; writing—review and editing, F.Z., H.L., Q.W. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32072586, 31500247), the Zhejiang Provincial Ten-thousand Program for Leading Talents of Science and Technology Innovation (2018R52026), the Sichuan Science and Technology Program (2018NZ0081), the Project of New Varieties Breeding of Sichuan Vegetable Innovation Team (sccxtd-2020-05), and the Technology Project of Zhishi Supply Chain Technology Co., Ltd. (2020008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the manuscript and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang J., Yuan H., Fei Z., Pogson B.J., Zhang L., Li L. Molecular characterization and transcriptome analysis of orange head Chinese cabbage (Brassica rapa L. ssp. pekinensis) Planta. 2015;241:1381–1394. doi: 10.1007/s00425-015-2262-z. [DOI] [PubMed] [Google Scholar]
- 2.Yuan H., Zhang J., Nageswaran D., Li L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015;2:15036. doi: 10.1038/hortres.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domonkos I., Kis M., Gombos Z., Ughy B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid Res. 2013;52:539–561. doi: 10.1016/j.plipres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Ma J., Li J., Xu Z., Wang F., Xiong A. Transcriptome profiling of genes involving in carotenoid biosynthesis and accumulation between leaf and root of carrot (Daucus carota L.) Acta Biochim. Biophys. Sin. 2018;50:481–490. doi: 10.1093/abbs/gmy027. [DOI] [PubMed] [Google Scholar]
- 5.Ye J., Hu T., Yang C., Li H., Yang M., Ijaz R., Ye Z., Zhang Y. Transcriptome Profiling of Tomato Fruit Development Reveals Transcription Factors Associated with Ascorbic Acid, Carotenoid and Flavonoid Biosynthesis. PLoS ONE. 2015;10:e0130885. doi: 10.1371/journal.pone.0130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazzonelli C.I. Carotenoids in nature: Insights from plants and beyond. Funct. Plant Biol. 2011;38:833–847. doi: 10.1071/FP11192. [DOI] [PubMed] [Google Scholar]
- 7.Krinsky N.I., Johnson E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Nisar N., Li L., Lu S., Khin N.C., Pogson B.J. Carotenoid Metabolism in Plants. Mol. Plant. 2015;8:68–82. doi: 10.1016/j.molp.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Bouvier F., D’Harlingue A., Backhaus R.A., Kumagai M.H., Camara B. Identification of neoxanthin synthase as a carotenoid cyclase paralog. JBIC J. Biol. Inorg. Chem. 2000;267:6346–6352. doi: 10.1046/j.1432-1327.2000.01722.x. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz S.H., Tan B.C., Gage D.A., Zeevaart J.A.D., Mccarty D.R. Specific Oxidative Cleavage of Carotenoids by VP14 of Maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 11.Parry A., Babiano M., Horgan R. The role of cis-carotenoids in abscisic acid biosynthesis. Planta. 1990;182:118–128. doi: 10.1007/BF00239993. [DOI] [PubMed] [Google Scholar]
- 12.North H.M., De Almeida A., Boutin J.-P., Frey A., To A., Botran L., Sotta B., Marion-Poll A. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J. 2007;50:810–824. doi: 10.1111/j.1365-313X.2007.03094.x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Babili S., Hugueney P., Schledz M., Welsch R., Frohnmeyer H., Laule O., Beyer P. Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS Lett. 2000;485:168–172. doi: 10.1016/S0014-5793(00)02193-1. [DOI] [PubMed] [Google Scholar]
- 14.Miao H.-Y., Wang M.-Y., Chang J.-Q., Tao H., Sun B., Wang Q.-M. Effects of glucose and gibberellic acid on glucosinolate content and antioxidant properties of Chinese kale sprouts. J. Zhejiang Univ. Sci. B. 2017;18:1093–1100. doi: 10.1631/jzus.B1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun B., Yan H., Zhang F., Wang Q. Effects of plant hormones on main health-promoting compounds and antioxidant capacity of Chinese kale. Food Res. Int. 2012;48:359–366. doi: 10.1016/j.foodres.2012.04.021. [DOI] [Google Scholar]
- 16.Sun B., Jiang M., Zheng H., Jian Y., Huang W.-L., Yuan Q., Zheng A.-H., Chen Q., Zhang Y.-T., Lin Y.-X., et al. Color-related chlorophyll and carotenoid concentrations of Chinese kale can be altered through CRISPR/Cas9 targeted editing of the carotenoid isomerase gene BoaCRTISO. Hortic. Res. 2020;7:161. doi: 10.1038/s41438-020-00379-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun B., Jiang M., Liang S., Zheng H., Chen Q., Wang Y., Lin Y.-X., Liu Z.-J., Wang X.-R., Zhang F., et al. Functional differences of BaPDS1 and BaPDS2 genes in Chinese kale. R. Soc. Open Sci. 2019;6:190260. doi: 10.1098/rsos.190260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun B., Zhang F., Xue S., Chang J., Zheng A., Jiang M., Miao H., Wang Q., Tang H. Molecular Cloning and Expression Analysis of the ζ-Carotene Desaturase Gene in Chinese kale (Brassica oleracea var. alboglabra Bailey). Hortic. Plant J. 2018;4:94–102. doi: 10.1016/j.hpj.2018.03.005. [DOI] [Google Scholar]
- 19.Sun B., Zhang F., Xiao N., Jiang M., Yuan Q., Xue S., Miao H., Chen Q., Li M., Wang X., et al. An efficient mesophyll protoplast isolation, purification and PEG-mediated transient gene expression for subcellular localization in Chinese kale. Sci. Hortic. 2018;241:187–193. doi: 10.1016/j.scienta.2018.07.001. [DOI] [Google Scholar]
- 20.Zheng H., Zhang F., Jian Y., Huang W.L., Liang S., Jiang M., Yuan Q., Wang Q.M., Sun B. Cloning and function identifi-cation of dihydroflavonol 4-reductase gene BoaDFR in Chinese kale. Acta Hortic. Sin. 2021;48:73–82. doi: 10.16420/j.issn.0513-353x.2020-0378. [DOI] [Google Scholar]
- 21.Jiang M., Zhang F., Yuan Q., Lin P., Zheng H., Liang S., Jian Y., Miao H., Li H., Wang Q., et al. Characterization of BoaCRTISO Reveals Its Role in Carotenoid Biosynthesis in Chinese Kale. Front. Plant Sci. 2021;12:662684. doi: 10.3389/fpls.2021.662684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Kanneganti T.-D., Huitema E., Kamoun S. In planta Expression of Oomycete and Fungal Genes. Methods Mol. Biol. 2007;354:35–43. doi: 10.1385/1-59259-966-4:35. [DOI] [PubMed] [Google Scholar]
- 24.Leuzinger K., Dent M., Hurtado J., Stahnke J., Lai H., Zhou X., Chen Q. Efficient Agroinfiltration of Plants for High-level Transient Expression of Recombinant Proteins. J. Vis. Exp. 2013;77:e50521. doi: 10.3791/50521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul S., Ali N., Gayen D., Datta S.K., Datta K. Molecular breeding of Osfer2 gene to increase iron nutrition in rice grain. GM Crop. Food. 2012;3:310–316. doi: 10.4161/gmcr.22104. [DOI] [PubMed] [Google Scholar]
- 26.Yadav K., Patel P., Srivastava A.K., Ganapathi T.R. Overexpression of native ferritin gene MusaFer1 enhances iron content and oxidative stress tolerance in transgenic banana plants. PLoS ONE. 2017;12:e0188933. doi: 10.1371/journal.pone.0188933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang N., Zhou W., Su J., Wang X., Li L., Wang L., Cao X., Wang Z. Overexpression of SmMYC2 Increases the Production of Phenolic Acids in Salvia miltiorrhiza. Front. Plant Sci. 2017;8:1804. doi: 10.3389/fpls.2017.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X.-Y., Zhu W.-J., Tang R.-M., Cai J.-H., Chen M., Yang Q. Over-expression of StLCYb increases β-carotene accumulation in potato tubers. Plant Biotechnol. Rep. 2016;10:95–104. doi: 10.1007/s11816-016-0390-y. [DOI] [Google Scholar]
- 29.Shewmaker C.K., Sheehy J.A., Daley M., Colburn S., Ke D.Y. Seed-specific overexpression of phytoene synthase: Increase in carotenoids and other metabolic effects. Plant J. 1999;20:401–412. doi: 10.1046/j.1365-313x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 30.Jeknić Z., Jeknić S., Jevremović S., Subotic A., Chen T.H.H., Jevremović S. Alteration of flower color in Iris germanica L. ‘Fire Bride’ through ectopic expression of phytoene synthase gene (crtB) from Pantoea agglomerans. Plant Cell Rep. 2014;33:1307–1321. doi: 10.1007/s00299-014-1617-4. [DOI] [PubMed] [Google Scholar]
- 31.Jeknić Z., Morré J.T., Jeknić S., Jevremović S., Subotić A., Chen T.H. Cloning and Functional Characterization of a Gene for Capsanthin-Capsorubin Synthase from Tiger Lily (Lilium lancifolium Thunb. ‘Splendens’) Plant Cell Physiol. 2012;53:1899–1912. doi: 10.1093/pcp/pcs128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrell J.E. Self-perpetuating states in signal transduction: Positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 2002;14:140–148. doi: 10.1016/S0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 33.Moreno J., Cerda A., Simpson K., Diaz I.L., Carrera E., Handford M., Stange C. Increased Nicotiana tabacumfitness through positive regulation of carotenoid, gibberellin and chlorophyll pathways promoted by Daucus carotalycopene β-cyclase (Dclcyb1) expression. J. Exp. Bot. 2016;67:2325–2338. doi: 10.1093/jxb/erw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R., Kang C., Song X., Yu L., Liu D., He S., Zhai H., Liu Q. A ζ-carotene desaturase gene, IbZDS, increases β-carotene and lutein contents and enhances salt tolerance in transgenic sweetpotato. Plant Sci. 2017;262:39–51. doi: 10.1016/j.plantsci.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Lindgren L.O., Stålberg K.G., Höglund A.S. Seed-Specific Overexpression of an Endogenous Arabidopsis Phytoene Synthase Gene Results in Delayed Germination and Increased Levels of Carotenoids, Chlorophyll, and Abscisic Acid. Plant Physiol. 2003;132:779–785. doi: 10.1104/pp.102.017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno J.C., Pizarro L., Fuentes P., Handford M., Cifuentes V., Stange C. Levels of Lycopene β-Cyclase 1 Modulate Carotenoid Gene Expression and Accumulation in Daucus carota. PLoS ONE. 2013;8:e58144. doi: 10.1371/journal.pone.0058144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong H., Deng Y., Mu J., Lu Q., Wang Y., Xu Y., Chu C., Chong K., Lu C., Zuo J. The Arabidopsis Spontaneous Cell Death1 gene, encoding a ζ-carotene desaturase essential for carotenoid biosynthesis, is involved in chloroplast development, photoprotection and retrograde signalling. Cell Res. 2007;17:458–470. doi: 10.1038/cr.2007.37. [DOI] [PubMed] [Google Scholar]
- 38.Perreau F., Frey A., Effroy-Cuzzi D., Savane P., Berger A., Gissot L., Marion-Poll A. Abscisic acid-deficient4 Has an Essential Function in Both cis-Violaxanthin and cis-Neoxanthin Synthesis. Plant Physiol. 2020;184:1303–1316. doi: 10.1104/pp.20.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dall’Osto L., Cazzaniga S., North H., Marion-Poll A., Bassi R. The Arabidopsis aba4-1 Mutant Reveals a Specific Function for Neoxanthin in Protection against Photooxidative Stress. Plant Cell. 2007;19:1048–1064. doi: 10.1105/tpc.106.049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nambara E., Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the manuscript and supplementary material.