Key Messages.
-
•
The dichotomy of the COVID-19 gestational diabetes screening criteria does not address the diversity of cases and the effects on health-care systems in Canada.
-
•
In this report, we propose a middle-ground screening criteria that can be used if there are moderate disruptions to testing for and treatment of gestational diabetes.
-
•
This suggests screening only high-risk women with a 50 g challenge followed by a 75 g oral glucose tolerance test using the same glucose thresholds recommended in the Diabetes Canada 2018 clinical practice guidelines.
The COVID-19 pandemic continues to have a significant impact on Canadians, including pregnant women and diabetes-in-pregnancy care teams. The “Temporary Alternative Screening Strategy for Gestational Diabetes Screening During the COVID-19 Pandemic” recommendations were developed early in the pandemic, when the degree of impact that the COVID-19 pandemic would have on Canadians was largely unknown (1). An alternative screening strategy was recommended if the “pandemic caused severe disruptions to laboratory testing and treatment, and/or patient refusal.” However, as the pandemic has progressed, there has been a wide range and varying incidence of COVID-19 across Canada, diverse effects on health-care systems and differences in the ability of various health-care infrastructures to adapt. The dichotomy of the current gestational diabetes (GDM) screening criteria does not address the diversity of cases and the effects on the health-care system across Canada. Although the number of COVID-19 cases in Canada has largely declined with public health measures and vaccinations, given the increasing number of COVID-19 variants and the continued uncertainty of the pandemic, we summarize the COVID-19 screening criteria and propose a middle-ground screening pathway for GDM during COVID-19 surges or other situations that may arise and lead to variable disruptions to health-care delivery.
Early Pregnancy Screening for Overt Diabetes
We continue to highlight the importance of screening for overt diabetes in high-risk women in early pregnancy. In accordance with the Diabetes Canada 2018 clinical practice guidelines (2), this can be done by assessment of glycated hemoglobin (A1C), or fasting plasma glucose if the A1C is unreliable.
Regular Screening (24 to 28 Weeks’ Gestation)
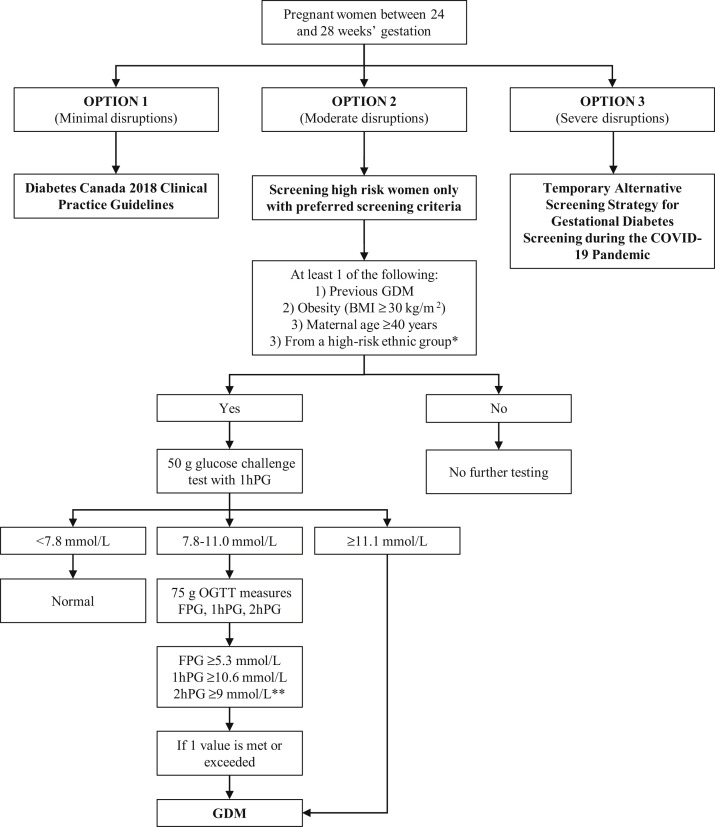
The COVID-19 pandemic has affected different regions at different times throughout the past year. Given this substantial variability, we have proposed an additional “middle-ground” screening criterion (Figure 1 ). The choice of screening procedures should be centre-based, depending on each centre’s clinical capacity, its capacity for adhering to safety measures and the burden of COVID-19 in the community. In addition to the screening recommendations given below, we suggest glucose testing at any time if there is high clinical suspicion of diabetes.
Figure 1.
Summary of screening pathways for gestational diabetes screening. ∗ High-risk ethnic groups include African, Arab, Asian, Hispanic, Indigenous or South Asian. ∗∗ Those requiring a 75 g OGTT can have only a fasting and 1-hour plasma glucose (no 2-hour plasma glucose) if the centre is able to accommodate this change. 1hPG, 1-hour plasma glucose; 2hPG, 2-hour plasma glucose; BMI, body mass index; FPG, fasting plasma glucose; GDM, gestational diabetes; OGTT, oral glucose tolerance test.
Option 1: No change to GDM screening
Option 1 assumes that there are minimal disruptions to testing for and treatment of GDM. Given the lower sensitivity of the other proposed options, the current Diabetes Canada clinical practice guidelines should be followed unless disruptions affect clinical care and/or laboratory testing capacity, for which a change in care delivery is required (2).
Option 2: Screen only high-risk women
Option 2 assumes that there are moderate disruptions to the testing for and treatment of GDM (Figure 1). Moderate disruptions are defined as anything in between minimal and severe disruptions. This change suggests screening only high-risk women. High risk is defined as having at least 1 of the following risk factors:
-
1.
Previous diagnosis of GDM.
-
2.
Prepregnancy or early pregnancy body mass index ≥30 kg/m2.
-
3.
Maternal age ≥40 years.
-
4.
Being in a high-risk ethnic group (African, Arab, Asian, Hispanic, Indigenous or South Asian).
In this scenario, we suggest screening women with at least 1 of the risk factors just defined with a nonfasting 50 g glucose challenge using the same existing glucose thresholds recommended in the Diabetes Canada 2018 clinical practice guidelines; that is, women with a 1-hour plasma glucose of ≥11.1 mmol/L are assumed to have a diagnosis of GDM and women with a 1-hour plasma glucose of 7.8 to 11.0 mmol/L should have a 75 g oral glucose tolerance test (OGTT) (2). The inclusion of the high-risk factors defined were chosen based on the strongest association with GDM and in the context of our Canadian population (3, 4, 5). Based on European data, it can be anticipated that this strategy could result in >29% of women having these risk factors, thus requiring screening (6). This strategy could hypothetically miss almost half of the women with GDM assuming universal testing with a 75 g OGTT and use of the 2013 International Association of Diabetes and Pregnancy Study Group diagnostic criteria (World Health Organization) (6). The actual performance of this strategy in our diverse Canadian population and without a universal confirmatory OGTT is difficult to predict with accuracy.
In health-care and laboratory systems that are able, we suggest considering the elimination of the 2-hour blood draw on the 75 g OGTT. The correlation between the 1-hour and 2-hour 75 g OGTT results in pregnancy is strong (0.68; p<0.001), so few women with GDM would be missed by performing a 75 g OGTT that only collects glucose samples fasting and 1 hour after 75 g glucose load (7). This reduces maternal exposure time in the laboratory. In addition, it decreases use of laboratory resources.
Option 3: Screen with an A1C and random plasma glucose only
Option 3 assumes severe disruptions to laboratory testing and treatment, and/or patient refusal. Severe disruptions would encompass the most extreme interruption to health-care delivery such as those seen at the height of the pandemic, the cessation of dynamic glucose testing or redeployment of a large number of staff. This option is the COVID-19 “Alternative Screening Strategy” guidelines and suggests the following:
-
•
All pregnant women without pre-existing diabetes will be screened with A1C and nonfasting, random plasma glucose.
-
•
Women with an A1C of <5.7% and a random plasma glucose of <11.1 mmol/L require no further testing or treatment.
-
•
Those with an A1C of ≥5.7% or a random plasma glucose of ≥11.1 mmol/L are identified as having GDM and should be referred to the interprofessional diabetes and pregnancy health-care team.
We again highlight that option 3 will miss many women with GDM, given its very low sensitivity (sensitivity=26% and specificity=96%, using an A1C cutoff of ≥5.7%) (8,9). However, it may still be required for systems that lack the laboratory capacity and resources to perform dynamic glucose testing in a safe manner. In addition, it may be an option for women who refuse other testing because of the concern for exposure to COVID-19 during testing.
In conclusion, it is important that we continue to provide high-quality care for our pregnant population during the COVID-19 pandemic. These suggestions are temporary in nature and the GDM screening pathway should be chosen based on the local burden of COVID-19 and be changed quickly as cases increase or decrease. Whenever the cases of COVID-19 and its strain on the health-care system are minimal, GDM screening should revert to the Diabetes Canada 2018 clinical practice guidelines recommendations (2).
Author Disclosures
Conflicts of interest: None.
Author Contributions
J.M.Y., L.E.D., D.S.F. and H.B. conceived the idea and formulated the recommendations. J.M.Y. wrote the first draft of the manuscript with input from all authors. All authors participated in the critical revision of the manuscript and approved the final version. J.M.Y. is the guarantor of this work.
References
- 1.Yamamoto J., Donovan L., Feig D., Berger H. temporary alternative screening strategy for gestational diabetes screening during the COVID-19 pandemic. A joint consensus statement from the Diabetes Canada Clinical Practice Guidelines Steering Committee and the Society of Obstetricians and Gynecologists of Canada. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/health%20advance/journals/jcjd/jcjd_covid_guidelines_020420.pdf November 5, 2020. Accessed February 1, 2021.
- 2.Diabetes Canada Clinical Practice Guidelines Expert Committee Diabetes and pregnancy. Can J Diabetes. 2018;42(Suppl. 1):S255–S282. doi: 10.1016/j.jcjd.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Teh W.T., Teede H.J., Paul E., Harrison C.L., Wallace E.M., Allan C. Risk factors for gestational diabetes mellitus: Implications for the application of screening guidelines. Aust NZ J Obstet Gynaecol. 2011;51:26–30. doi: 10.1111/j.1479-828X.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 4.Aljohani N., Rempel B.M., Ludwig S., et al. Impact of diabetes on maternal–fetal outcomes in Manitoba: Relationship with ethnic and environmental factors. Clin Invest Med. 2008;31:E338–E345. doi: 10.25011/cim.v31i6.4919. [DOI] [PubMed] [Google Scholar]
- 5.Read S.H., Rosella L.C., Berger H., et al. BMI and risk of gestational diabetes among women of South Asian and Chinese ethnicity: A population-based study. Diabetologia. 2021;64:805–813. doi: 10.1007/s00125-020-05356-5. [DOI] [PubMed] [Google Scholar]
- 6.Benhalima K., Van Crombrugge P., Moyson C., et al. Risk factor screening for gestational diabetes mellitus based on the 2013 WHO criteria. Eur J Endocrinol. 2019;180:353–363. doi: 10.1530/EJE-19-0117. [DOI] [PubMed] [Google Scholar]
- 7.Hapo Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 8.Meek C.L., Lindsay R.S., Scott E.M., et al. Approaches to screening for hyperglycaemia in pregnant women during and after the COVID-19 pandemic. Diabet Med. 2021;38 doi: 10.1111/dme.14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIntyre H.D., Gibbons K.S., Ma R.C.W., et al. Testing for gestational diabetes during the COVID-19 pandemic. An evaluation of proposed protocols for the United Kingdom, Canada and Australia. Diabetes Res Clin Pract. 2020;167 doi: 10.1016/j.diabres.2020.108353. [DOI] [PMC free article] [PubMed] [Google Scholar]