Abstract
Background
Syncope is not a common manifestation of COVID-19, but it may occur in this context and it can be the presenting symptom in some cases. Different mechanisms may explain the pathophysiology behind COVID-19 related syncope. In this report, we aimed to examine the current frequency and etiology of syncope in COVID-19.
Methods
A systematic review across PubMed, ISI Web of Knowledge and SCOPUS was performed, according to PRISMA guidelines, in order to identify all relevant articles regarding both COVID-19 and syncope.
Results
We identified 136 publications, of which 99 were excluded. The frequency of syncope and pre-syncope across the selected studies was 4.2% (604/14,437). Unexplained syncope was the most common type (87.9% of the episodes), followed by reflex syncope (7.8% of the cases). Orthostatic hypotension was responsible for 2.2% of the cases and syncope of presumable cardiac cause also accounted for 2.2% of cases. Arterial hypertension was present in 52.0% of syncope patients. The use of angiotensin receptor blockers or angiotensin converting enzyme inhibitors were not associated with an increased incidence of syncope (chi-square test 1.07, p 0.30), unlike the use of beta-blockers (chi-square test 12.48, p < 0.01).
Conclusion
Syncope, although not considered a typical symptom of COVID-19, can be associated with it, particularly in early stages. Different causes of syncope were seen in this context. A reevaluation of blood pressure in patients with COVID-19 is suggested, including reassessment of antihypertensive therapy, especially in the case of beta-blockers.
Keywords: Covid-19, syncope, Arterial hypertension
1. Introduction
The ongoing Coronavirus pandemic has proved to be a challenging setback to the health of the world population ever since its first cases were announced in the city of Wuhan, China, around December 2019. As of the 1st of July 2021, there have been a total of approximately 181 million confirmed cases of COVID-19 (Coronavirus disease 2019) worldwide and 3.9 million deaths, translating to a fatality rate of 2% (Organization, 2021).
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is a novel betacoronavirus and COVID-19 is the infectious disease caused by this novel virus. Its spike protein (glycoprotein S) determines the specificity of the virus for epithelial cells of the respiratory tract (Santacroce et al., 2020). It is composed of a receptor binding domain that recognizes the ACE-2 (type 2 angiotensin converting enzyme) receptor specifically, allowing the entrance of the virus into its target cells (Wang et al., 2020). The ACE-2 receptor can be found on the surface of epithelial cells in the lungs, intestines, kidneys and blood vessels (Santacroce et al., 2020). It is currently known that, although the novel SARS-CoV-2 virus can lead to significant disease in the respiratory system, it can also negatively affect several other vital organ systems. Significant damage, namely, to the cardiovascular, nervous and hematopoietic systems has been outlined and an impact in hemostasis has also been thoroughly discussed as blood hypercoagulability is common among hospitalized COVID-19 patients (Terpos et al., 2020). Regarding the cardiovascular manifestations, heart failure, thromboembolism, myocarditis, arrhythmias, pericarditis and acute coronary syndromes have been described in this context (Shafi et al., 2020; Oates et al., 2020). On the other hand, the most common neurological symptoms reported in COVID-19 patients have been smell and taste disturbances, headache, myalgia, and altered mental status (Favas et al., 2020).
Syncope is largely defined as a transient loss of consciousness due to cerebral hypoperfusion (Cardiology ESo, 2018). It is characterized by a rapid onset, short duration and spontaneous, complete recovery (Cardiology ESo, 2018). Presyncope, on the other hand, is the state that resembles the prodrome of syncope (with all its signs and symptoms such as pallor, sweating, nausea, palpitations) without being followed by a loss of consciousness (Cardiology ESo, 2018).
In the light of a severe systemic disease, non-traumatic transient loss of consciousness can have distinct etiologies, varying from the benign reflex syncope and syncope due to orthostatic hypotension to the increasingly serious cardiac syncope (Brignole et al., 2018). Apart from unexplained syncope, these three main groups stem from different mechanisms and, therefore, may require specialized treatment. Consequently, an accurate diagnosis becomes imperative.
Recently, some case reports and case-series have emerged reporting syncope as a possible symptom of COVID-19, whether it had developed at the onset or during the course of the disease (Oates et al., 2020). It is important to mention that some of these reports outline its occurrence days before the main respiratory symptoms, or even as an isolated phenomenon (Ebrille et al., 2020). If a valid relationship between COVID-19 and syncope is established, a number of patients could be isolated in a timely manner, minimizing the contagious phase.
In the present report, we aimed to systematically review the recent published literature that describes syncope or presyncope as a symptom of COVID-19, having it been observed in the days before or after the diagnosis. We aimed to calculate its frequency and divide it into each different type of syncope observed.
As a secondary aim of the review, the investigation of the relationship between syncope and use of angiotensin receptor inhibitor drugs (ACEi), angiotensin receptor blockers (ARBs) and/or beta-blockers in the context of COVID-19 was carried out. This seemed to be important to investigate since arterial hypertension is a common comorbidity among COVID-19 patients (Tadic et al., 2020), and the use of standard anti-hypertensive agents could influence the incidence of this symptom.
2. Methods
2.1. Eligibility criteria
Regarding our population of interest, we were in the search for studies that simultaneously described COVID-19 and syncope or presyncope presented as a possible symptom of the acute infection or occuring in a post-acute COVID-19 setting. Articles were excluded if they described falls in the context of COVID-19 that were not stated to be of syncopal origin; episodes of syncope not temporally related with SARS-CoV-2 infection (for example, occurring throughout the year prior to the infection) and episodes of syncope with another possible underlying cause mentioned in the study as relevant apart from COVID-19. We included case-series, case-reports, cross-sectional studies with prospective data collection, retrospective analyses and letters published in 2020 or 2021 for which it was possible to extract an exact number of patients with COVID-19 exhibiting syncope/presyncope.
We did not restrict articles to witnessed syncope nor exclude articles that did not describe the specific comorbidities, clinical characteristics or evolution exhibited by the pre/syncope cohort. This was because our primary outcome measure was to quantify the number of COVID-19 related pre/syncopal episodes published in the literature thus far.
We considered articles written in English, Spanish, French, Italian, or Portuguese. Articles written in German, Hungarian or Mandarin were excluded (since the authors are not familiar with these languages).
2.2. Search strategy
A comprehensive literature search was carried out with the purpose of identifying all reported articles relating syncope to COVID-19, according to the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses ( Page et al., 2021 ). This search was conducted on the databases Medline (PUBMED), ISI Web of Knowledge and SCOPUS.
The search query, which took place on the 9th of March 2021, included the following MeSH terms and keywords: “(“COVID-19” OR “COVID 19” OR “SARS-COV-2” OR “coronavirus” OR “2019 novel coronavirus”) AND (“syncope” OR “presyncope” OR “syncopal”). Additionally, we scanned the list of references from the included studies in this analysis and of systematic reviews pertaining to neurological symptoms in the context of COVID-19.
2.3. Selection process
Two investigators independently assessed whether the studies addressed the topic in question and if all the inclusion/exclusion criteria were met. Initially, this was done according to the “screening phase”, where only the title and the abstract were analyzed. After this process, 52 articles were considered eligible. This was followed by the “inclusion phase”, where the integral text was fully evaluated. Any doubtful situation was solved by consensus between the authors, after which, concerning study eligibility, 100% agreement between authors was seen in each step of the study assessment.
2.4. Data collection process and data items
From the selected articles, two authors worked independently to retrieve the following data: location, number of patients (with and without pre/syncope), age, sex and ethnicity when available, comorbidities (from patients with and without pre/syncope), chronic medications the patients were on regarding treatment of arterial hypertension and the description of the clinical course, including relevant laboratory findings and any auxiliary exams performed, such as computerized tomography scans and cardiac magnetic resonances. Any doubtful situation was solved by consensus between the authors.
2.5. Study quality assessment
Quality of the observational cohorts and cross-sectional studies and case-series was evaluated using the National Heart, Lung and Blood Institute study quality assessment tools (National Heart LaBI, 2021) and is presented in Table 1, Table 2 . Any disagreements between the two main reviewers were discussed with a third evaluator.
Table 1.
Quality assessment tool for observational cohort and cross-sectional studies. Y - Yes; NR - Not Reported; NA - Not Applicable.
| Oates et al. | Chen et al. | Canetta et al. | Radmanesh et al. | Chachkhiani et al. | García-Moncó et al. | Xiong et al. | Romero-Sánchez et al. | Chuang et al. | Mizrahi et al | Martin-Sanchez et al. | Travi et al. | Chou et al. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Was the research question or OBJECTIVE in this paper clearly stated? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the study population clearly specified and defined? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the participation rate of eligible persons at least 50%? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was a sample size justification, power description, or variance and effect estimates provided? | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the exposure(s) assessed more than once over time? | NR | NR | NR | NR | NR | NR | NR | NR | NR | Y | Y | NR | NR |
| Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were the outcome assessors blinded to the exposure status of participants? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Was loss to follow-up after baseline 20% or less? | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | NR | NR | NR | NR | Y | NR | NR | NR | NR | Y | Y | Y | Y |
| Quality rating | Good | Fair | Good | Good | Good | Fair | Fair | Fair | Fair | Good | Good | Good | Good |
Table 2.
Quality assessment tool for case-series studies. Y - Yes; NR - Not reported; NA - Not applicable.
| Ebrille et al. | Birlutiu et al. | Argenziano et al. | Espinoza et al. | Gonfiotti et al. | |
|---|---|---|---|---|---|
| Was the study question or objective clearly stated? | Y | Y | Y | Y | Y |
| Was the study population clearly and fully described, including a case definition? | Y | Y | Y | Y | Y |
| Were the cases consecutive? | NR | NR | Y | NR | NR |
| Were the subjects comparable? | Y | Y | Y | Y | Y |
| Was the intervention clearly described? | Y | Y | Y | Y | Y |
| Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants? | Y | Y | Y | Y | Y |
| Was the length of follow-up adequate? | NR | Y | Y | NR | Y |
| Were the statistical methods well-described? | NA | NA | Y | NA | NA |
| Were the results well-described? | Y | Y | Y | Y | Y |
| Quality Rating | Fair | Good | Good | Fair | Good |
2.6. Outcome measures
The primary outcome measures assessed were the occurrence of syncope or presyncope either in the days prior or subsequent to a COVID-19 diagnosis and its relative frequency, divided into each type of syncope experienced.
We also assessed the association between the usage of ARBs or ACEi and beta blockers with the occurence of syncope as well as the association of syncope with mortality.
2.7. Effect measures
Concerning these latter data, a chi-square test was used, with a level of significance of 0.05. Statistical analysis was done using Stata, version 17.0, StataCorp, Texas, USA.
3. Results
3.1. Study selection
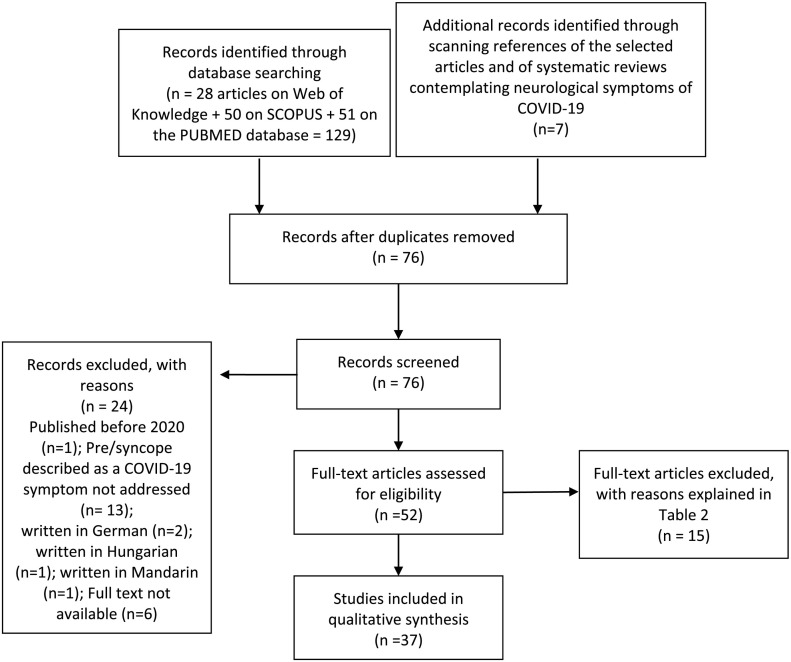
With the use of our keywords, we obtained 51 results from Medline (PUBMED), 28 from ISI Web of Knowledge, 50 from SCOPUS and 7 from scanning the references of the selected articles and adequate systematic reviews (Fig. 1 ) - with a total number of 37 articles selected for the purpose of the present study (Fig. 1). The complete set of selected studies is presented in Table 3 . SARS-CoV-2 infection was diagnosed by real-time reverse transcriptase polymerase chain reaction (RT-PCR) or a chest X-ray or CT scan showing the characteristic bilateral interstitial pneumonia of COVID-19 in all cases, except in the report by Romero-Sánchez et al., in which a minority of patients were diagnosed by means of serological testing (Romero-Sánchez et al., 2020).
Fig. 1.
Flowchart showing literature search method. n = number of articles.
Table 3.
Summary of included articles. Pts - patients; ARBs - angiotensin receptor blockers; PPM - permanent pacemaker implantation; ECG - electrocardiogram; ICD - implantable cardioverter-defibrillator; AV - atrioventricular; ACE-I - angiotensin-converting-enzyme inhibitors; CMR - cardiac magnetic resonance; CSF - cerebrospinal fluid; CT -computed tomography; MRI - magnetic resonance imaging; RT-PCR - real time polymerase chain reaction; CRP - C-Reactive Protein, NT-proBNP - N-terminal type B natriuretic peptide; POTS - Postural Orthostatic Tachycardia Syndrome; BP – blood pressure. For references see text.
| Source | Study type | Patients (n) | Patient characteristics | Comorbidities | Main findings | Syncope type |
|---|---|---|---|---|---|---|
| Oates et al. (U.S.A.) |
Retrospective study | 77 (Syncope cohort: 37 pts. (32 with syncope and 5 with presyncope); control group: 40 pts. without syncope/presyncope) | Syncope cohort: Median age: 69 51% males 24% Caucasian |
Syncope cohort: Arterial Hypertension (68%); Obesity (42%); Diabetes mellitus (32%); Coronary Artery Disease (27%); Chronic Kidney Disease (11%); Atrial Fibrillation (8%) | Incidence of syncope/presyncope was 3.7%; Syncope cohort: greater use of ARBs (p = 0.03); Systolic blood pressure lower in the syncope cohort (p = 0.01); Pulse rate lower in syncope cohort (p < 0.0001); Compared with the “control” group, there were no significant differences in both admission and peak blood levels of D-dimer, troponin-I, and CRP in the syncope cohort; Syncope cohort required less intensive care unit admissions & had lower need for mechanical ventilation | −59.4% Unexplained −15.6% Neurocardiogenic/Reflex −12.5% Hypotensive −3.1% Cardiopulmonary |
| Chen et al. (U.S.A) |
Prospective study | 102; (syncope cohort: 24 pts. with syncope, near syncope or nonmechanical fall) | Syncope cohort: Mean age: 61 |
Syncope cohort: Cardiovascular disease history in 29%. | More pts. from the syncope cohort required oxygen, had gastrointestinal symptoms and elevated troponin levels compared to the rest of the pts. (p > 0.05). | Unexplained |
| Ebrille et al. (Italy) |
Case-series | 5; (2 pts. with syncope and 3 with presyncope) | Patient 1: 71 year old male Patient 2: 65 year old female Patient 3: 79 year old male Patient 4: 75 year old male Patient 5: 75 year old male |
Patient 1: Arterial Hypertension, Coronary Artery Disease and PPM; Patient 2: Mitral Valve stenosis, PPM for AV block, Atrial Fibrillation; Patient 3: Arterial Hypertension, Diabetes Mellitus, Transient Ischemic Attack, 3rd degree AV block; Patient 4: Chagas disease, PPM due to AV block, ICD because of ventricular tachycardia; Patient 5: Dilated Cardiomyopathy, PPM. | All patients had an episode of syncope as the only initial symptom of COVID-19 infection; 4/5 patients were on ACE-I chronic therapy; Syncope due to arrhythmia, structural cardiac disease or pulmonary embolism was excluded. | Reflex Autonomic Dysfunction, either primary or secondary |
| Chang et al. (U.S.A) |
Case report | 1 | 49 year old Male |
No significant medical history. | Syncope and fever; ECG with Brugada pattern, uncovered during fever; Brugada Syndrome diagnosis. | Presumable cardiac cause |
| Pasquetto et al. (Italy) |
Case report | 1 | 52 year old Male |
– | Dyspnea and fever; 2 episodes of syncope during high fever; CRP was 160.7 mg/L and the troponin level was normal; The ECG presented a “coved-type” aspect in leads V1 and V2 and a first-degree AV block; Brugada Syndrome diagnosis. | Presumable cardiac cause |
| Luetkens et al. (Germany) |
Case report | 1 | 79 year old Male |
Asthma | Elevated CRP, troponin T and NT-proBNP; CMR showed diffuse interstitial myocardial edema with mild systolic dysfunction and mild pericardial effusion; COVID-19 associated myocarditis diagnosis. | Presumable cardiac cause |
| Logmin et al. (Germany) |
Case report | 1 | 70 year old Female History of syncopes over the years but not lately |
Psoriatic Arthritis; Neuropathic Pain; Paroxysmal Atrial Fibrillation. | 3 episodes of syncope, one of which convulsive; Respiratory symptoms emerged later; Normal long-term ECG, blood pressure monitoring and Schellong test; Initially, levels of D-dimer and LDH were normal, but both increased in the following days; Brain MRI without acute alterations (signs of minimal previous ischemic events); Normal Electroencephalogram and CSF analysis; Pathological Sympathetic Skin Response. | Autonomic dysfunction |
| Tapé et al. (U.S.A) |
Case report | 1 | 79 year old Female |
Coronary Artery Disease with multiple stents; Arterial Hypertension; Congestive Heart Failure. | Syncopal episode, myalgia, cough and fever; Initially admitted in the Emergency Department for a syncopal workup; BP of 116/62 mmHg sitting & 85/50 mmHg standing; Normal ECG and telemetry monitoring; Fever later prompted SARS-CoV-2 testing; Developed respiratory failure later | Orthostatic hypotension |
| Canetta et al. (Italy) |
Retrospective analysis | 103 (35 pts. with syncope and 68 pts. without) |
Mean age With syncope: 74 Without syncope: 72 69% of the pts. with syncope were male |
Syncope cohort: Arterial Hypertension (45.7%); Dyslipidemia (17%); Renal insufficiency (20%); Hypothyroidism (5.7%); Dementia (11.4%); Cancer (11.4%); Atrial Fibrillation (5.7%). | Hypocapnic Hypoxemia in most patients; Mean heart rate was significantly lower in pts. who experienced syncope; Patients reporting syncope had a normal cardiac assessment | Reflex |
| Singhania et al. (U.S.A) |
Case report | 1 | 71 year old Female |
Arterial Hypertension | Syncope and later altered mental status as the only complaints that ultimately led to COVID-19 diagnosis; Tachycardia, normal ECG. | Orthostatic hypotension |
| Sang et al. (U.S.A) |
Case Report | 1 | 62 year old Male |
Arterial Hypertension Dyslipidemia |
Patient with syncope, ventricular fibrillation and shock secondary to a massive pulmonary embolism in the setting of SARS-CoV-2 infection; Elevated troponin I, BNP, and D-dimer | Cardiopulmonary |
| Birlutiu et al. (Romania) |
Case-series | 4 | Patient 1: 67 year old Caucasian male; Patient 2: 65 year old Caucasian female; Patient 3: 61 year old Caucasian male; Patient 4: 48 year old Caucasian female | Patient 1: Arterial Hypertension, Stroke, Diabetes mellitus; Patient 2: Diabetes Mellitus, arterial hypertension, 3rd degree AV block with pacemaker; Patient 3: -; Patient 4: -. | All 4 patients presented with syncope after micturition; 2 of them had associated intense, persistent headaches either preceding or post syncope and 1 of them had diffuse abdominal pain and nausea as warning signs; One patient had an elevated D-dimer and 3 of them had an elevated CRP; Time of syncope varied from the 2nd day to the 11th day of hospitalization and 2 patients had repeated syncope over a 2-min interval and suffered acute traumatic brain injury as a consequence; Cardiologic investigation was normal in all 4 patients and there was no evidence of hypotension. | Reflex |
| Huang et al. (U.S.A) |
Case report | 1 | 40 year old Female |
Diabetes mellitus; Obesity | Patient presented with fever & syncope and was admitted for encephalitis; CSF was later found to be positive for SARS-COV-2 on rtPCR. | Probable autonomic dysfunction |
| Radmanesh et al. (U.S.A) |
Retrospective study | 242 (79 of whom presented with syncope) |
Mean age of 68.7 62% male 38% female |
– | 242 patients underwent at least 1 brain imaging (CT and/or MRI) examination within 2 weeks of testing positive for COVID-19; Syncope/fall was one of the most common clinical indications for imaging (79 patients, 32.6%); Of the 13 patients with acute/subacute infarcts, 2 were imaged due to syncope/fall; Among the 7 patients with acute hemorrhage, the clinical indication was syncope in 4 patients. | Unexplained |
| Chachkhiani et al. (U.S.A) |
Retrospective study | 250 (6 of whom had syncope) |
Mean age of 60 45% male 80% African American |
Group with Neurological chief complaint: Arterial Hypertension (71%); Diabetes mellitus (44%). | 34 (14%) patients had a neurological chief complaint at presentation, and syncope was one of the most common complaints (2%); Neurological complaints at presentation and during the hospital stay are associated with a higher risk of death, prolonged hospital stay, and intubation. |
Unexplained |
| García-Moncó et al. (Spain) |
Cross-sectional study with prospective data | 35 (2 of whom had syncope) |
Median Age: 66 71% male |
Arterial Hypertension (57%) Dyslipidemia (60%); Diabetes mellitus (17%) Smoking (23%); Obesity (6%). | Patients who presented with or developed a neurological disorder and were diagnosed with COVID-19 were analyzed. Of the 35 patients, 2 had syncope. CRP was elevated in most of the patients, especially in those who suffered from stroke. D-dimer was also frankly elevated, more so in the patients with stroke and in patients with encephalopathy. The CSF of one of these patients with syncope was tested for the presence of SARS-Cov-2 using the RT-PCR assay and was negative. | Unexplained |
| Xiong et al. (China) |
Retrospective cohort study | 917 (3 of whom had syncope) |
Mean age: 48.7 55% male |
44% had non-neurologic comorbidities 3% had neurologic comorbidities |
- Of the 917 people with COVID-19, 39 had new-onset neurologic events (3 with syncope). - The pts. with syncope were three women aged between 52 and 61 years without a previous history of neurologic or systemic disorders.ECGs recorded afterwards were normal. - Brain CT conducted in one of the syncope pts. did not reveal any new lesions. |
Unexplained |
| Romero-Sánchez et al. (Spain) |
Retrospective, observational study | 841 (5 of whom had syncope) |
Mean age: 66.42 56.2% male |
Arterial Hypertension; Dyslipidemia; Obesity; Heart disease; Diabetes mellitus; Chronic kidney disease. | Of 841 patients hospitalized with COVID-19, 57.4% developed some form of neurologic symptom. 5 patients suffered from a syncopal episode, all of which had non-severe COVID-19 disease. Elevated CRP and D-dimer, with higher values in severe disease. In 21 patients (2.5%), a neurologic manifestation was the reason that prompted the visit to the emergency department. 2 of those patients presented with syncope. | Unexplained |
| Argenziano et al. (U.S.A) |
Retrospective case-series | 1000 (48 of whom had syncope) |
Median age: 63.0 59.6% male |
Arterial Hypertension (60%); Diabetes mellitus (37%). | Syncope was the presenting symptom in: 4% of patients in whom the highest level of care was the emergency department; 5.7% of pts. who needed hospital care; 3% of pts. who needed care in the intensive care unit. Elevated CRP and D-dimer. | Unexplained |
| Chuang et al. (U.S.A) |
Retrospective study | 56 patients with COVID-19 exhibiting neurological symptoms (5 of which had a syncopal event) |
Mean age: 69.2 57% female |
Arterial Hypertension (82.1%); Dyslipidemia (60.7%); Diabetes mellitus (50%); History of stroke (29%); Coronary Artery Disease (27%); Chronic Kidney Disease (21%); Obesity (21%); COPD/Asthma (7%) | A syncopal event occurred in 12% of patients with both neurological and typical COVID-19 symptoms (fever, cough and dyspnea) on presentation. Of the patients with neurological symptoms but without the typical COVID-19 symptoms, a syncopal event occurred in 4%. | Unexplained |
| Espinoza et al. (U.S.A) |
Case series | 3 (2 of whom experienced presyncope) |
Patient 1: 80 year old female Patient 2: 54 year old male Patient 3: 64 year old female |
Patient 1: Coronary Artery Disease, Ischemic Cardiomyopathy, 30% ejection fraction, Atrial Fibrillation, Arterial Hypertension and Type 2 Diabetes Mellitus; Patient 2: Dyslipidemia; Patient 3: Hypothyroidism | Patient 1: Complaints of presyncope with no other associated symptoms at the time of admission. Chest X Ray showed moderate cardiomegaly. The ECG revealed non-specific T-wave abnormalities in anteropseptal leads. Transthoracic echocardiogram showed a drop in ejection fraction to 20% from baseline (30%) with new anteroseptal and apical akinesis. During the admission, the patient also suffered from an episode of nonsustained ventricular tachycardia. Elevated troponin, D-dimer and CRP. Patient 2: Near syncopal episode with no other complaints. ECG showed an incomplete right bundle branch block. Elevated troponin. During admission, the patient developed a transient episode of sinus bradycardia (40 bpm) and hypotension (90/50 mmHg). Both patients developed fever during their hospitalization which led to the diagnosis of COVID-19. | Presumable cardiac cause |
| Omotosho et al. (U.S.A) |
Case report | 1 | 45 year old Hispanic female | Non-insulin dependent type 2 Diabetes Mellitus (6 years earlier) medicated with metformin and gestational diabetes | Symptoms of progressively worsening fatigue, nonproductive cough and presyncope during the course of one week prior to admission. Associated symptoms included intermittent fever, chills, myalgia and generalized weakness. Laboratory data showed a high anion gap metabolic acidosis, blood glucose of 344 mg/dL and glycated hemoglobin of 13.7%. Inflammatory markers were all negative. Chest X-Ray was compatible with COVID-19 pneumonia (confirmed by rtPCTR). During the hospital stay, the patient did not require oxygen supplementation or treatment for COVID-19, having only her diabetic ketoacidosis reversed. - Patient was diagnosed with Latent Autoimmune Diabetes in Adults after performing the test for islet antibodies and C-peptide levels. | Orthosthatic hypotension (secondary autonomic failure) |
| Mizrahi et al. (Israel) |
Retrospective study and survey analysis | PRIMARY CARE VISITS 4066 COVID-19 positive adults (24 of whom experienced syncope) 862 COVID-19 positive children (3 of whom experienced syncope) SURVEY RESPONDERS: 499 COVID-19 positive adults. |
PRIMARY CARE VISITS Adults: Mean age: 43.03; 56% male Children: Mean age: 10.69; 51% male SURVEY RESPONDERS: Adults: Mean age: 42.15; 46% male |
Cardiovascular diseases; Diabetes mellitus; Arterial Hypertension; Obesity; Underweight; Malignancy; Cystic Fibrosis; Chronic Renal Failure; COPD; Depression; Inflammatory Bowel Disease; Blood disorders. | The study cohort was composed of 2 parts: 1) individuals with primary care visits and 2) survey responders. In adults who received primary care, syncope was significantly associated with COVID-19 diagnosis (OR = 2.09). In children, the OR was higher (2.45) but not statistically significant. Nonetheless, the overall prevalence of this symptom was very low in the cohort (0.6% and 0.3% of positive adults and children, respectively). |
Unexplained |
| Khan et al. (U.S.A) |
Case report | 1 | 54 year old male | No previous medical history. | 1 week history of fever, cough, progressive dyspnea and one episode of syncope. On admission the patient's oxygen saturation was 80%. Laboratory data showed mild lymphopenia elevated D-dimer and troponin-I. ECG had an S1Q3T3 consistent with pulmonary embolism. In one hour, the patient developed acute respiratory distress syndrome and shock. Bedside transthoracic echocardiogram revealed a large right heart thrombus-in-transit through the tricuspid valve into the right ventricle. A limited transthoracic echocardiogram was performed 1 week later, which showed preserved RV systolic function and no evidence of thrombus. | Cardiopulmonary |
| Chalela et al. (Spain) |
Case report | 1 | 63 year old male | Myotonic Dystrophy Disease (Steiner disease); Hiatal hernia. | Patient presented a transient loss of consciousness and muscle strength with spontaneous recovery while walking in the street; Electrocardiography demonstrated sinus tachycardia and complete right bundle branch block; D-dimer was elevated. A CT pulmonary angiography was performed and detected an obstruction of the posterior-basal and anterior-basal segmental arterial branches of the right lower lobe. | Cardiopulmonary |
| Pérez et al. (Spain) |
Case report | 1 | 78 year old male | Arterial Hypertension; Dyslipidemia; Hyperuricemia; Chronic bronchitis; Pulmonary fibrosis | Patient resorted to the emergency room for complaints of a syncopal episode without prodromes, abnormal body movements or automatisms. Recovery was observed in 2 min. No other symptoms were mentioned. Laboratory data showed a decreased glomerular filtration rate and an elevated D-dimer. Chronic medication with ACE-i. An angio-CT was performed in order to exclude pulmonary embolism, which it did. Instead, it revealed parenchymal alveolar infiltrates in the upper left lobule. COVID-19 test came back positive. | Unexplained |
| Chibane et al. (Canada) |
Case report | 1 | 66 year old female | Symptomatic Junctional Bradycardia and a permanent pacemaker implanted 6 years earlier; Immune thrombocytopenia; Breast cancer; Hodgkin's lymphoma; Splenectomy; Raynaud's phenomenon; Coronary Artery Disease. | Generalized malaise preceding pre-syncopal episode with no mention of infectious symptoms. Presence of mild livedo. Laboratory data showed elevated white blood cell count, creatinine and hs-troponin T levels. Initial COVID-19 rtPCR was negative. Transthoracic echocardiography showed very unusual hyperdense smoke-like haze in the right cardiac chambers and the inferior vena cava, suggesting pulmonary embolism. On day 2, the patient was found with aphasia and right-hand hemiparesis. Skin showed diffuse livedo racemosa, involving the whole body. Head CT documented loss of left frontal lobe gray-white matter differentiation, consistent with a hyperacute infarct. D-dimer was frankly elevated. Within minutes of starting heparin without bolus, there was hemodynamic collapse (mean arterial pressure of 60 mmHg). Post-mortem COVID-19 rtPCR returned positive. | Cardiopulmonary |
| Martín-Sánchez et al. (Spain) |
Retrospective observational study | 1379 (68 of whom experienced a syncopal event) |
Mean age: 62 years old 53.5% male |
Arterial Hypertension (40.5%); Dyslipidemia (37.9%); Diabetes mellitus (9.2%). | The population aged 65 and older showed a higher percentage of patients with a syncopal event (p < 0.001); 45–54 years: 4.5% of patients experienced a syncopal event; 55–64 years: 2.8%; 65–74 years: 5.9%; 75–84 years: 8.5%; ≥ 85 years: 9.8%; In the 18–44 age group, no patient exhibited signs of syncope. | Unexplained |
| Pendower et al. (United Kingdom) |
Case report | 1 | 64 year old female | Asthma; Hypothyroidism; Depression; Unprovoked deep vein thrombosis 10 years earlier. | 2 episodes of syncope prompted a visit to the emergency department. The patient had felt generally unwell with some breathlessness for the preceding 2 weeks. Oxygen saturations were 96% on room air, but while in hospital acutely desaturated to 76%. D-dimer, NT-proBNP and troponin T were elevated. CT pulmonary angiogram showed extensive bilateral lobar, segmental and subsegmental pulmonary emboli with a large saddle embolus, with dilatation of the right ventricle. Bedside echocardiogram confirmed right heart strain; Thrombolysis was conducted and the patient had a very good clinical response. | Cardiopulmonary |
| Aoi et al. (U.S.A) |
Case report | 1 | 70 year old Hispanic female | Arterial Hypertension; Dyslipidemia; Diabetes mellitus; supraventricular tachycardia treated successfully with ablation. | Witnessed syncopal episode upon standing following a week of progressive weakness, polyuria and hyperglycemia. On admission, blood pressure was 84/55 mmHg and oxygen saturation was 88% on room air. ECG showed the S1Q3T3 pattern, prolonged QTc and T-wave inversions. D-dimer, PCR and pro-BNP were elevated. Bedside echocardiogram revealed a moderately dilated right ventricle without thrombus. CT pulmonary angiogram showed a saddle pulmonary embolism with notable thrombus in the right atrium extending into the right ventricle. Despite treatment, the patient did not survive. | Cardiopulmonary |
| Doodnauth et al. (U.S.A) |
Case report | 1 | 84 year old female | Arterial Hypertension; Heart failure with preserved ejection fraction; Coronary Artery Disease; Chronic Kidney Disease; Type 1 Diabetes mellitus; Mild Aortic Stenosis. | Witnessed syncopal episode. Prior to the syncopal event, the patient complained of abdominal discomfort. Mildly elevated inflammatory markers. Chest X-ray revealed a small left-sided pleural effusion. rtPCR was positive for COVID-19. ECG showed sinus bradycardia. On day 3 the patient developed renal failure and hypoxemia. An electrophysiology study revealed a delayed sinus node recovery time, confirming sinus node dysfunction. | Presumable cardiac cause |
| Travi et al. (Italy) |
Retrospective study | 901 (81 of whom experienced syncope) |
Median age of 64 years 51.7% male 81.7% caucasian |
Arterial Hypertension (44%); Obesity (24.4%); Diabetes mellitus (19.5%); Ischemic Heart Disease (12.2%). | Patients were stratified according to the presence of respiratory and/or neurologic disturbances at admittance in the Emergency Department. Patients underwent cerebral CT scan in case of stroke, seizure or syncope. The majority presented only respiratory symptoms (69.8%) while 30.2% displayed at least one neurological complaint. The most common neurological complaints were dysgeusia/anosmia (9.1%) and syncope (9%). The presence of any neurologic involvement was higher in patients with moderate disease when compared to those with critical or severe disease. However, no significant difference was found when it came to the occurrence of syncope (10.1% versus 7.6%, p = 0.193). | Unexplained |
| Gonfiotti et al. (Italy) |
Case series | 5 (2 of whom experienced syncope) |
Patient 1: 67 year old male Patient 2: 74 year old male Patient 3: 70 year old male Patient 4: 80 year old male Patient 5: 79 year old male |
Patient 1: Rheumatoid Arthritis and squamous laryngeal tumour. Patient 2: Chronic Obstructive Pulmonary Disease. Patient 3: Chronic Obstructive Pulmonary Disease, hypothyroidism, Lung cancer. Patient 4: Chronic Obstructive Pulmonary Disease, Arterial Hypertension and Peripheral Arterial Occlusive Disease, Lung cancer | Patient 3: 10 days after the patient underwent surgery for an adenocarcinoma of the right upper lung lobe, fever, cough, diarrhea, syncope/hypotension and anemia without bleeding occured. PCR for COVID-19 was positive. On day 25, the patient exhibited acute renal failure. He was on the Intensive Care Unit for 25 days, underwent mechanical ventilation for 22 and died on day 32. Patient 4: 16 days after the patient underwent robotic-assisted middle lobectomy, a sudden episode of marked hypotension/syncope (blood pressure 85/50 mmHg) and tachycardia was observed. Anemia and an elevated white blood count were also present. The patient had no respiratory symptoms. His COVID-19 test came back positive. He was discharged on day 30. | Unexplained |
| Lam et al. (Australia) |
Letter | 18 (1 with presyncope) |
Mean age: 59 years 61% male |
Obesity; Ischemic heart disease; Diabetes mellitus; Arterial Hypertension. | 5% of patients exhibited presyncope | Unexplained |
| Kanjwal et al. (U.S.A) |
Case report | 1 | 36 year old female | No previous medical history. | Patient experienced only mild symptoms during her SARS-COV-2 infection. Three to four weeks after the COVID-19 diagnosis, the patient experienced fatigue, headaches, dizziness, chest pain, palpitations and presyncope. ECG, stress test and a transthoracic echocardiogram were normal. She had a sitting heart rate of 86 bpm and blood pressure of 115/65 mmHg. Upon standing, heart rate increased to 115 bpm and blood pressure 105/70 mmHg. Findings of Tilt Test were suggestive of POTS. | Autonomic dysfunction (POTS) |
| Miglis et al. (U.S.A) |
Case report | 1 | 26 year old female | Exercise-induced asthma; obsessive compulsive disorder resolved at age 18. | 3 weeks after the patient's COVID-19 diagnosis, she developed orthosthatic lightheadedness and presyncope; On day 24, episodic sensations of “adrenaline surges” began. On day 45, episodic facial fushing, dermatographia, and non-pruritic hives. 3 months after symptom onset, the patient underwent autonomic reflex testing. Exaggerated postural tachycardia with a heart rate increase of 65 bpm on Head-Up-Tilt test. Episodic hypertensive systolic BP surges to 170 mmHg in an oscillatory pattern. Robust blood pressure responses to Valsalva maneuver pattern in the absence of hyperventilation, suggestive of a hyperadrenergic state. | Autonomic dysfunction (Hyperadrenergic POTS) |
| Chou et al. | Retrospective and prospective cohort study | 3055 (152 with syncope) |
Mean age: 59.9 years 57% male overall 63% male in the syncope cohort |
Preexisting neurological disorders; diabetes; coronary artery disease; hypertension; cerebrovascular disease | Syncope was the least common symptom, with an incidence of 152 of 3054 (5%) in the all COVID-19 cohort. 8% of the >80 age group suffered from an episode of syncope, being the age group with the biggest incidence of syncope. 5% of the patients who had an episode of syncope died, whereas in the non-syncope group, 14% of patients died. |
Unexplained |
The excluded full-text articles are presented in Table 4 , with the corresponding reasons.
Table 4.
Articles excluded with reasons.
| Source | Reason for exclusion |
|---|---|
| Sapp et al. https://pubmed.ncbi.nlm.nih.gov/32299753/ |
Did not describe cases of pre/syncope as a symptom of the SARS-CoV-2 infection. |
| Pasqualetto et al. https://pubmed.ncbi.nlm.nih.gov/32665941/ |
The patient did not present with syncope. |
| Babapoor-Farrokhran et al. https://pubmed.ncbi.nlm.nih.gov/32989427/ |
The patient did not present with syncope. |
| Bogaert et al. https://pubmed.ncbi.nlm.nih.gov/32933917/ |
The patient did not test positive for COVID-19. |
| Favas et al. https://pubmed.ncbi.nlm.nih.gov/33089477/ |
Different study design. |
| Mahajan et al. https://pubmed.ncbi.nlm.nih.gov/32701540/ |
Did not describe cases of pre/syncope as a symptom of the SARS-CoV-2 infection. |
| Russo et al. https://pubmed.ncbi.nlm.nih.gov/33090884/ |
Did not describe cases of pre/syncope in patients with COVID-19. |
| Filippo Crea https://pubmed.ncbi.nlm.nih.gov/33532851/ |
Did not describe cases of pre/syncope in patients with COVID-19. |
| Nissan et al. https://pubmed.ncbi.nlm.nih.gov/33236588/ |
The episode of pre-syncope experienced by the patient occured during the year prior to the patient's SARS-CoV-2 infection. |
| Santacroce et al. https://pubmed.ncbi.nlm.nih.gov/33269412/ |
Did not describe cases of pre/syncope in patients with COVID-19. |
| Alkeridy et al. https://pubmed.ncbi.nlm.nih.gov/32383778/ |
Although the patient suffered from a fall, it was never stated that it had a syncopal origin. |
| Townsend et al. https://pubmed.ncbi.nlm.nih.gov/33630906/ |
Syncope was not addressed. |
| Gur et al. https://dergipark.org.tr/en/download/article-file/1173885 |
The syncope experienced in this context was attributed to carbon monoxide poisoning. |
| Hussain et al. https://pubmed.ncbi.nlm.nih.gov/32531128/ |
The syncope experienced by one patient in this case-series was likely associated with the patient's severe aortic stenosis. |
| Motiejunaite et al. https://pubmed.ncbi.nlm.nih.gov/33536937/ |
The 2 cases of syncope during exertion in COVID-19 survivors were attributed to hypocapnic hyperventilation, which is one of the conditions that is commonly misdiagnosed as syncope. |
3.2. Study characteristics
Of the 37 included articles, 18 were case reports (Tapé et al., 2013, Sang et al., 2020, Pasquetto et al., 2020, Luetkens et al., 2020, Logmin et al., 2020, Chang et al., 2020, Singhania et al., 2020, Huang et al., 2020, Omotosho et al., 2021, Khan et al., 2020, Chalela et al., 2020, Pérez et al., 2020, Chibane et al., 2020, Pendower et al., 2020, Aoi et al., 2020, Doodnauth et al., 2021, Kanjwal et al., 2020, Miglis et al., 2020), 10 were retrospective analyses (Oates et al., 2020, Romero-Sánchez et al., 2020, Chachkhiani et al., 2020, Xiong et al., 2020, Radmanesh et al., 2020, Canetta et al., 2020, Chuang et al., 2021, Mizrahi et al., 2020, Martín-Sánchez et al., 2020, Travi et al., 2020), 5 were case-series (Ebrille et al., 2020, Birlutiu et al., 2020, Argenziano et al., 2020, Espinoza et al., 2021, Gonfiotti et al., 2020) and 2 were prospective studies (García-Moncó et al., 2020, Chen et al., 2020). One was a letter (Lam et al., 2020). The study by Chou et al. (2021), included both retrospective and prospective data collection. Given the nature of this topic, every article was published in either 2020 or 2021. The United States of America contributed with most of the studies, with a total of 18 articles. 15 articles were conducted in Europe, 1 was from Canada, 1 from Australia and 1 from China. The study by Chou et al. (2021) represented 13 different countries and 4 continents.
The sample size included in the studies varied from 1 to 5427 patients. The mean age ranged from 26 to 84 years of age. Considering the studies in which it was possible to extract the sex of COVID-19 patients who experienced syncope, we found that 61.2% were male. The overall male percentage (including COVID-19 patients with syncope and without syncope) was 55.9%. Ethnicity data, when available, are presented in Table 3.
Information regarding number of participants, mean age, sex and usage of ARBs/ACEi as well as usage of beta-blockers in the syncope versus non-syncope cohort was available for 5 studies and is presented in Table 5 .
Table 5.
Demographic data of 5 of the included studies.
| Source | Syncope cohort | Non-syncope cohort |
|---|---|---|
| Oates et al. (U.S.A) |
- Number of patients: 37 - Mean age: 69 - Sex: 19 males (51%) - Beta blockers: 14 (38%) - ACE-I/ARBS: 15 (41%) - Number of deaths: 5 (14%) |
- Number of patients: 40 - Mean age: 68 - Sex: 23 males (58%) - Beta blockers: 9 (22%) - ACE-I/ARBS: 13 (33%) - Number of deaths: 9 (23%) |
| Chen et al. (U.S.A) |
- Number of patients: 24 - Mean age: 61 |
- Number of patients: 78 - Mean age: 57 |
| Canetta et al. (Italy) |
- Number of patients: 35 - Mean age: 74 - Sex: 24 males (69%) - Beta Blockers: 11 (31%) - ACE-I/ARBS: 14 (40%) |
- Number of patients: 68 - Mean age: 72 - Sex: 51 males (75%) - Beta Blockers: 18 (26%) - ACE-I/ARBS: 30 (44%) |
| Martín-Sanchez et al. (Spain) |
- Number of patients: 68 - Beta blockers: 14 (21%) - ACE-I/ARBS: 23 (34%) |
- Number of patients: 1311 - Beta blockers: 54 (4%) - ACE-I/ARBS: 45 (3%) |
| Chou et al. | - Number of patients: 152 - Number of deaths: 7 (5%) |
- Number of patients: 2902 - Number of deaths: 410 (14%) |
3.3. Synthesis of results
There were 604 cases of syncope and pre-syncope related to COVID-19 out of a total of 14,438 patients contemplated in this review, comprising an overall frequency of 4.2%.
The majority of studies, 16, described syncopal episodes of unexplained etiology (Romero-Sánchez et al., 2020, Pérez et al., 2020, Chachkhiani et al., 2020, Xiong et al., 2020, Radmanesh et al., 2020, Chuang et al., 2021, Pérez et al., 2020; Mizrahi et al., 2020, Martín-Sánchez et al., 2020, Travi et al., 2020, Argenziano et al., 2020, Gonfiotti et al., 2020, García-Moncó et al., 2020, Chen et al., 2020, Lam et al., 2020, Chou et al., 2021). 10 case reports alluded to possible cardiac etiology for syncope (Sang et al., 2020, Pasquetto et al., 2020, Luetkens et al., 2020, Chang et al., 2020, Khan et al., 2020, Chalela et al., 2020, Chibane et al., 2020, Pendower et al., 2020, Aoi et al., 2020, Doodnauth et al., 2021) as well as 1 case series (Espinoza et al., 2021), while another 6 studies attributed the cause of syncope to orthostatic hypotension (Ebrille et al., 2020, Tapé et al., 2013, Logmin et al., 2020, Singhania et al., 2020, Huang et al., 2020, Omotosho et al., 2021). In turn, reflex syncope was highlighted in only 3 of the studies (Ebrille et al., 2020, Canetta et al., 2020, Birlutiu et al., 2020). The retrospective study by Oates et al. (2020) contemplated all of the different types of syncope.
Two studies (Kanjwal et al., 2020, Miglis et al., 2020) described the occurrence of syncope in COVID-19 survivors, 3 to 4 weeks after diagnosis, in a post-acute COVID-19 phase. This raises the possibility of syncope playing a role not only during the acute phase of the disease, but also as a long-term consequence. In both these cases, the patients were diagnosed with a Postural Orthostatic Tachycardia Syndrome, included in the autonomic dysfunctions.
As stated before, unexplained pre/syncope was the most common cause of the transient loss of consciousness, being 87.9% (531/604) of the reported episodes. The overall frequency of reflex pre/syncope was 7.8% (47/604). Orthostatic hypotension accounted for 2.2% (13/604) and presumable cardiac pre/syncope was responsible for 2.2% (13/604) of the cases as well.
Regarding the comorbidities of patients with COVID-19 and syncope, arterial hypertension was heavily represented in 21 studies (Oates et al., 2020, Ebrille et al., 2020, Romero-Sánchez et al., 2020, Tapé et al., 2013, Sang et al., 2020, Singhania et al., 2020, Pérez et al., 2020, Aoi et al., 2020, Doodnauth et al., 2021, Chachkhiani et al., 2020, Canetta et al., 2020, Chuang et al., 2021, Mizrahi et al., 2020, Martín-Sánchez et al., 2020, Travi et al., 2020, Birlutiu et al., 2020, Argenziano et al., 2020, Espinoza et al., 2021, Gonfiotti et al., 2020, García-Moncó et al., 2020, Lam et al., 2020), making it the most prevalent comorbid condition exhibited by the participants. This was followed by Diabetes mellitus, obesity, dyslipidemia and heart disease. As shown in Table 6 , detailed data concerning comorbidities were possible to evaluate for 102 patients, and data concerning drug usage were then obtained for the 53 hypertensive patients. Arterial hypertension was present in 52.0% of patients and either ARBs or ACEi were used by 60.4% of hypertensive patients with COVID-19 and syncope.
Table 6.
Associated clinical conditions described in 102 patients with COVID-19 and syncope and drug usage in 53 hypertensive patients with COVID-19 and syncope.
| Associated conditions | Medications | N (%) |
|---|---|---|
| Arterial hypertension | 52.0% (53/102) | |
| Angiotensin receptor blockers OR angiotensin converting enzyme inhibitors | 60.4% (32/53) | |
| Diuretics | – | |
| Beta blockers | 49.1% (26/53) | |
| Calcium channel blockers | 22.6% (12/53) | |
| Diabetes mellitus | 19.6% (20/102) |
|
| Obesity | 15.7% (16/102) | |
| Coronary heart disease | 14.7% (15/102) | |
| Renal insufficiency | 12.7% (13/102) |
|
| Dyslipidemia | 9.8% (10/102) | |
| Atrial fibrillation | 7.8% (8/102) |
Out of the 18 case reports, syncope was the reason that prompted the visit to the Emergency Department in 13 of them (72.2%) (Tapé et al., 2013, Pasquetto et al., 2020, Luetkens et al., 2020, Logmin et al., 2020, Chang et al., 2020, Singhania et al., 2020, Huang et al., 2020, Chalela et al., 2020, Pérez et al., 2020, Chibane et al., 2020, Pendower et al., 2020, Aoi et al., 2020, Doodnauth et al., 2021). Furthermore, Ebrille et al. (2020) , Canetta et al. (2020) and Espinoza et al. (2021) also described syncope as the presenting symptom of the infection. From these studies, we calculated that, on average, syncope occurred 3.16 ± 1.40 days before a positive COVID-19 test.
On the other hand, Argenziano et al. (2020) stated that syncope was the presenting symptom in only 4% of patients in whom the highest level of care was the emergency department, 5.7% of patients who required hospital care and 3% of patients who needed care in the ICU. In the retrospective study by Martín-Sánchez et al. (2020) , no signs of syncope were exhibited by the 18–44 age group, while in the >85 age group, syncope was observed in approximately 10% of the patients. Similarly, in Chou et al. (2021) , the highest percentage of syncope patients was also observed in the >80 age group (8%).
Considering confirmed or presumable cardiac syncope, the 13 reported cases included myocarditis (Luetkens et al., 2020), pulmonary embolisms (Khan et al., 2020, Chalela et al., 2020, Chibane et al., 2020, Pendower et al., 2020, Aoi et al., 2020) and ventricular fibrillation (Sang et al., 2020), Brugada Syndrome (Chang et al., 2020, Pasquetto et al., 2020), new onset atrial fibrillation together with anterior wall ST elevation myocardial infarction (Oates et al., 2020), sinus node dysfunction (Doodnauth et al., 2021), non-sustained tachycardia (Espinoza et al., 2021) and sinus bradycardia (Espinoza et al., 2021) as the underlying conditions.
The most common laboratory findings in COVID-19 patients presenting with syncope included elevated C-reactive protein, elevated D-dimer and elevated troponin. However, the elevation of C-reactive protein and D-dimer was more frequently observed than the elevation of troponin. From the studies that discriminated laboratory data on admission, we found that the elevation of D-dimer and C-reactive protein was present in 32% of the patients that presented with COVID-19 and syncope (Sang et al., 2020, Luetkens et al., 2020, Logmin et al., 2020, Khan et al., 2020, Chalela et al., 2020, Pérez et al., 2020, Chibane et al., 2020, Pendower et al., 2020, Aoi et al., 2020, Birlutiu et al., 2020, Espinoza et al., 2021). On the other hand, the elevation of troponin was observed in 23% of these patients (Pasquetto et al., 2020, Luetkens et al., 2020, Birlutiu et al., 2020, Espinoza et al., 2021). Troponin T was the most prevalent subtype studied among COVID-19 patients with syncope (Luetkens et al., 2020, Chibane et al., 2020, Pendower et al., 2020). Lymphocytopenia was also characteristic.
Data concerning the occurrence of syncope in patients either using or not using ACEi and/or ARBs, as well as beta blockers, were available for three studies (Oates et al., 2020, Canetta et al., 2020, Martín-Sánchez et al., 2020). There was a significant increase in episodes of syncope in patients under beta blocker treatment (39/236 cases versus 101/1231 control cases; chi-square test 12.48, p < 0.01). However, this was not the case with ACEi and/or ARBs use (52/512 cases versus 88/1047 control cases, chi-square test 1.07, p = 0.30).
Data concerning mortality were available for two studies (Oates et al., 2020, Chou et al., 2021) and statistical analysis showed mortality to be significantly less common in patients with syncope, when compared with those without this symptom (12/188 cases versus 419/2938 control cases, chi-square test 7.45, p < 0.01).
4. Discussion
In the present report, the association of syncope with COVID-19 was under review. The results of our review suggest that syncope is a relatively uncommon manifestation of COVID-19, with an overall frequency of 4.2% in the reports under analysis. Different types of mechanisms leading to syncope in COVID-19 patients were observed, including reflex syncope, orthostatic hypotension and cardiac syncope. No single mechanism was identified that could explain all cases of this finding in the context under study.
Syncope may be the presenting symptom of COVID-19. Out of 18 case reports reviewed, syncope was the reason that prompted a visit to the Emergency Department in 72.2% of patients. This loss of consciousness occurred on average 3.16 ± 1.40 days before a positive COVID-19 test, which suggests that if syncope could be seen as a warning sign of a possible SARS-CoV-2 infection, this timely association could, in some cases, offer an opportunity to mitigate the dissemination of the disease by means of an early diagnosis.
Regarding the age of the patients, in the report by Martín Sánchez et al. (Martín-Sánchez et al., 2020), syncope showed a higher frequency in the >85 years age group (approximately 10%) and was not present at all within the 18–44 years old population. Data in the same direction were presented by Chou et al. (2021). Most of the reports included in our review included patients with a mean age of 60 years of age or older, with a few exceptions lying in the 40–60 age group (Table 3). This suggests that syncope, as a symptom of COVID-19, is more frequent in an elderly population. Because this population is particularly vulnerable to this novel virus, special attention should be paid.
Considering the post-acute COVID-19 scenario, two recurring pre-syncopal episodes were described, and these happened in 26 and 36 year olds (Kanjwal et al., 2020, Miglis et al., 2020), which offers a great contrast to the age of people who suffered from syncope during the active infection. Long-term COVID-19 sequelae are still in need of further investigation in order to grasp the future impact that the infection might have on its survivors, especially after a longer period of time.
Arterial hypertension was present in more than half of the patients with COVID-19 and syncope for whom detailed data were available (Table 6). We did not find a significant association between the use of either ACEi or ARBs and the occurrence of syncope. Concerning the use of beta blockers, a significant difference was observed, with syncope being more frequent in patients taking this type of drugs. This may indicate that an adequate compensatory response of the heart rate is important in this inflammatory setting. The present results suggest a careful reevaluation of blood pressure whenever a hypertensive patient develops COVID-19, especially if beta-blockers are used.
The presence of lower heart rate and lower blood pressure at admission that was observed in patients presenting with syncope did not necessarily correlate with severe COVID-19, as it was noted that these patients required less intensive care than the non-syncope cohort (Oates et al., 2020).
Patients with self reported syncope had lower in-hospital mortality than those without this symptom, in the report by Chou et al. (2021) (and also in our results). This may not represent the situation concerning the whole universe of COVID-19 patients, but may be due to the fact that patients with alternative and serious neurological conditions were represented heavily in the control group. One may speculate that syncope was drug-associated in some cases, and that therefore, by having a reversible nature, may also contribute to this finding.
Different authors suggested several types of dysfunction of the cardiovascular system, of the neurological system, or of both, in the context under evaluation. ACE2 is believed to be involved in the regulation of cardiovascular function at the central nervous system level (Xia and Lazartigues, 2010). Perhaps the affinity of SARS-Cov-2 to the ACE-2 receptors could play a role in affecting the baroreflex response (Canetta et al., 2020). Following this reasoning, the inappropriate baroreflex response could lead to a partial inhibition of the compensatory increase of heart rate during fever and acute hypoxemia. An inadequate response of heart rate was suggested in some cases (Ebrille et al., 2020, Canetta et al., 2020), a situation perhaps worsened in the context of beta-blocker use.
In the present review, elevated D-dimers were one of the most frequent abnormal laboratory findings (32%), supporting the concept that blood hypercoagulability, which is common among COVID-19 patients, is also present in patients who experience syncope while infected with SARS-CoV-2. A rise in troponins was also noted across some studies, underlining the impact of the virus in provoking a cardio-inflammatory response (Gaze, 2020).
4.1. Limitations of this systematic review
The large majority of studies in the present review are case reports, hence, the limitations include those intrinsic to this kind of studies, including the inability to generalize the results, mainly due to the fact that these studies do not show a representative population. In addition, case reports and case series have a retrospective nature, which means they sometimes lack relevant information. For example, some case reports did not mention the chronic medications the patients were on, although they described the respective comorbidities.
The population sample size is 14,437 which, when compared to the millions of COVID-19 cases worldwide, may be the subject of selection bias. We also analyzed a substantial amount of retrospective studies, most of which were not primarily designed to assess the frequency of syncope in the context of COVID-19 and therefore provide insufficient information regarding the syncope cohort. Given the nature of this topic, the number of published studies focusing on comparing syncope cohorts with non-syncope cohorts in a COVID-19 setting is still limited, hindering the ability to reach certain conclusions. We attempted at making a distinction between the different types of syncope, however, in some studies, we found it impossible to do so. Furthermore, our inclusion criteria limited the articles to those written in the English, Spanish, French, Italian and Portuguese languages, which may have omitted significant studies.
5. Conclusions
Syncope is a relatively uncommon manifestation of COVID-19, with an overall frequency of 4.2% in the reports under analysis. Different types of mechanisms leading to syncope in this context were observed, including reflex syncope, orthostatic hypotension and cardiac syncope. Syncope may be the presenting symptom of COVID-19 and its adequate recognition as such might be useful in allowing for an earlier diagnosis. The presence of this symptom does not necessarily correlate with severe COVID-19 and may be associated to lower in-hospital mortality. Arterial hypertension was particularly prevalent in patients with COVID-19 and syncope, which may indicate that maintaining the usual antihypertensive medication may be inadequate in some cases of COVID-19. This is particularly important in the case of beta-blockers use, drugs that were shown to be associated with an increased incidence of syncope. Further studies may be of interest in order to further evaluate the frequency, etiology and impact of syncope in this type of patients.
References
- Aoi S., Kakkar A.M., Golowa Y., Grushko M., Coyle C.M., Elrafei T., et al. Saddle pulmonary embolism and clot in transit in COVID-19 infection: a case report of catastrophic venous thromboembolism. Eur. Heart J. 2020 doi: 10.1093/ehjcr/ytaa437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020:369. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birlutiu V., Birlutiu R.M., Feiereisz A.I. SARS-CoV-2 infection associated with micturition syncope: our experience with 4 case reports. Medicine. 2020;99(31) doi: 10.1097/MD.0000000000021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignole M., Moya A., de Lange F.J., Deharo J.-C., Elliott P.M., Fanciulli A., et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018;39(21):1883–1948. doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- Canetta C., Accordino S., Buscarini E., Benelli G., La Piana G., Scartabellati A., et al. Syncope at SARS-CoV-2 onset. Auton. Neurosci. 2020;229 doi: 10.1016/j.autneu.2020.102734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiology ESo ESC guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018 doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- Chachkhiani D., Soliman M.Y., Barua D., Isakadze M., Villemarette-Pittman N.R., Devier D.J., et al. Neurological complications in a predominantly African American sample of COVID-19 predict worse outcomes during hospitalization. Clin. Neurol. Neurosurg. 2020;197 doi: 10.1016/j.clineuro.2020.106173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalela R., Caguana O., Zuccarino F., Khilzi K., Rodríguez-Chiaradía D.A. Case report on a patient with Steinert disease complicated by COVID-19. Vasc. Health Risk Manag. 2020;16:463. doi: 10.2147/VHRM.S266659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Saleh M., Garcia-Bengo Y., Choi E., Epstein L., Willner J. COVID-19 infection unmasking brugada syndrome. HeartRhythm Case Reports. 2020;6(5):237–240. doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Hanna J., Walsh E.E., Falsey A.R., Laguio-Vila M., Lesho E. Syncope, near-syncope, or non-mechanical falls as a presenting feature of COVID-19. Ann. Emerg. Med. 2020 doi: 10.1016/j.annemergmed.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibane S., Gibeau G., Poulin F., Tessier P., Goulet M., Carrier M., et al. Hyperacute multi-organ thromboembolic storm in COVID-19: a case report. J. Thromb. Thrombolysis. 2020;1–4 doi: 10.1007/s11239-020-02173-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S.H.-Y., Beghi E., Helbok R., Moro E., Sampson J., Altamirano V., et al. Registry Collaborative. 2021. Global prevalence of neurological manifestations among patients hospitalized with COVID-19: a report of the global consortium study of neurologic dysfunction in COVID-19 (GCS-NeuroCOVID) and the European Academy of Neurology [ENERGY] [Google Scholar]
- Chuang D.T., Aydemir S., Magda P., Thomas C., Zarnegar R. Neurological manifestations as primary presentation of COVID-19 in hospitalized patients. Acta Neurol. Scand. 2021 doi: 10.1111/ane.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doodnauth A.V., Jallad A., Rizk D., Valery E., McFarlane S.I. Syncope associated with sinus nodal dysfunction in a COVID-19 patient: a case report and review of the literature. Am. J. Med. Case Rep. 2021;9(4):263. [Google Scholar]
- Ebrille E., Lucciola M.T., Amellone C., Ballocca F., Orlando F., Giammaria M. HeartRhythm Case Reports. 2020. Syncope as the presenting symptom of COVID-19 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza D., Jasti M., Malwi U.H., Junia C. Cardiovascular disease risk and outcomes in patients infected with SARS-CoV-2. Cureus. 2021;13(1) doi: 10.7759/cureus.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favas T., Dev P., Chaurasia R.N., Chakravarty K., Mishra R., Joshi D., et al. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol. Sci. 2020;1–34 doi: 10.1007/s10072-020-04801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Moncó J.C., Cabrera-Muras A., Collía-Fernández A., Erburu-Iriarte M., Rodrigo-Armenteros P., Oyarzun-Irazu I., et al. Neurological reasons for consultation and hospitalization during the COVID-19 pandemic. Neurol. Sci. 2020;41(11):3031–3038. doi: 10.1007/s10072-020-04714-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze D.C. Clinical utility of cardiac troponin measurement in COVID-19 infection. Ann. Clin. Biochem. 2020;57(3):202–205. doi: 10.1177/0004563220921888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonfiotti A., Gatteschi L., Salvicchi A., Bongiolatti S., Lavorini F., Voltolini L. Clinical courses and outcomes of five patients with primary lung cancer surgically treated while affected by severe acute respiratory syndrome coronavirus 2. Eur. J. Cardiothorac. Surg. 2020;58(3):598–604. doi: 10.1093/ejcts/ezaa233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H., Jiang D., Huang J.T. A case of COVID-19 encephalitis. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjwal K., Jamal S., Kichloo A., Grubb B.P. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J. Innov. Cardiac Rhythm Manag. 2020;11(11):4302. doi: 10.19102/icrm.2020.111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H.M.W., Khan M.R., Munir A., Moughrabieh A., Changezi H.U. A Giant right-heart thrombus-in-transit in a patient with COVID-19 pneumonia. Am. J. Case Rep. 2020;21:e927380–e927381. doi: 10.12659/AJCR.927380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K., McClelland S., Dallo M.J. ECG: essential in care of patients with COVID-19. Med. J. Aust. 2020;213(10)):476. doi: 10.5694/mja2.50841. e1. [DOI] [PubMed] [Google Scholar]
- Logmin K., Karam M., Schichel T., Harmel J., Wojtecki L. Non-epileptic seizures in autonomic dysfunction as the initial symptom of COVID-19. J. Neurol. 2020;1 doi: 10.1007/s00415-020-09904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetkens J.A., Isaak A., Zimmer S., Nattermann J., Sprinkart A.M., Boesecke C., et al. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ. Cardiovasc. Imaging. 2020;13(5) doi: 10.1161/CIRCIMAGING.120.010897. [DOI] [PubMed] [Google Scholar]
- Martín-Sánchez F.J., Del Toro E., Cardassay E., Carbó A.V., Cuesta F., Vigara M., et al. Clinical presentation and outcome across age categories among patients with COVID-19 admitted to a Spanish emergency department. Euro. Geriatr. Med. 2020;11(5):829–841. doi: 10.1007/s41999-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglis M.G., Prieto T., Shaik R., Muppidi S., Sinn D.-I., Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 2020;30(5):449–451. doi: 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi B., Shilo S., Rossman H., Kalkstein N., Marcus K., Barer Y., et al. Longitudinal symptom dynamics of COVID-19 infection. Nat. Commun. 2020;11(1):1–10. doi: 10.1038/s41467-020-20053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart LaBI Study Quality Assessment Tools. 2021. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Available from:
- Oates C.P., Turagam M.K., Musikantow D., Chu E., Shivamurthy P., Lampert J., et al. Syncope and presyncope in patients with COVID-19. Pacing Clin. Electrophysiol. 2020 doi: 10.1111/pace.14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omotosho Y.B., Ying G.W., Stolar M., Mallari A.J.P. COVID-19-induced diabetic ketoacidosis in an adult with latent autoimmune diabetes. Cureus. 2021;13(1) doi: 10.7759/cureus.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH. WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/ [July 1, 2021]. Available from:
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- Pasquetto G., Conti G.B., Susana A., Leone L.A., Bertaglia E. Syncope, Brugada syndrome, and COVID-19 lung disease. J. Arrhythmia. 2020;36(4):768–770. doi: 10.1002/joa3.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendower L., Benedetti G., Breen K., Karunanithy N. Catheter-directed thrombolysis to treat acute pulmonary thrombosis in a patient with COVID-19 pneumonia. BMJ Case Rep. CP. 2020;13(8) doi: 10.1136/bcr-2020-237046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez I.H., de la Esperanza B.T., Peñacoba G.V., Azorin D.G. Isolated syncope as a form of presentation of COVID-19 infection. Neurología (English Edition) 2020 [Google Scholar]
- Radmanesh A., Raz E., Zan E., Derman A., Kaminetzky M. Brain imaging use and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. Am. J. Neuroradiol. 2020;41(7):1179–1183. doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang C.J., Heindl B., Von Mering G., Rajapreyar I. Massive pulmonary embolism in a COVID-19 patient: a case report. European Heart Journal-Case Reports. 2020 doi: 10.1093/ehjcr/ytaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacroce L., Charitos I.A., Carretta D.M., De Nitto E., Lovero R. The human coronaviruses (HCoVs) and the molecular mechanisms of SARS-CoV-2 infection. J. Mol. Med. 2020;1–14 doi: 10.1007/s00109-020-02012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi A.M., Shaikh S.A., Shirke M.M., Iddawela S., Harky A. Cardiac manifestations in COVID-19 patients—a systematic review. J. Card. Surg. 2020;35(8):1988–2008. doi: 10.1111/jocs.14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhania N., Bansal S., Singhania G. An atypical presentation of novel coronavirus disease 2019 (COVID-19) Am. J. Med. 2020 doi: 10.1016/j.amjmed.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic M., Cuspidi C., Grassi G., Mancia G. COVID-19 and arterial hypertension: hypothesis or evidence? J. Clin. Hypertens. 2020;22(7):1120–1126. doi: 10.1111/jch.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapé C., Byrd K.M., Aung S., Lonks J.R., Flanigan T.P., Rybak N.R. COVID-19 in a patient presenting with syncope and a normal chest X-ray. Rhode Island Med. J. 2013;103:50. 2020. [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020 doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travi G., Rossotti R., Merli M., D'Amico F., Chiappetta S., Panariello A., et al. 2020. Neurological Manifestations in Patients Hospitalized With COVID-19: A Retrospective Analysis From a Large Cohort in Northern Italy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.-Y., Zhao R., Gao L.-J., Gao X.-F., Wang D.-P., Cao J.-M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front. Cell. Infect. Microbiol. 2020:10. doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr. Hypertens. Rep. 2010;12(3):170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Mu J., Guo J., Lu L., Liu D., Luo J., et al. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95(11):e1479–e1487. doi: 10.1212/WNL.0000000000010034. [DOI] [PubMed] [Google Scholar]