Abstract
Examination of viability of trypomastigotes before and after single-dose pentamidine treatment demonstrated that single-dose pentamidine substantially affected motility of trypomastigotes, a proxy of drug efficacy. This suggests that single-dose pentamidine may be of added value to bridge time until suramin will be available for treatment of human East Africa trypanosomiasis.
Keywords: Trypanosoma brucei rhodesiense, African sleeping sickness, treatment, motility, trypomastigote, haemolymphatic phase, single-dose
East-African human trypanosomiasis is caused by Trypanosoma brucei rhodesiense and can run a potentially fulminant course.1 The majority of patients are diagnosed during the first, haemolymphatic, phase of the disease, for which suramin is the preferred treatment.1,2 In non-endemic countries suramin availability is complicated as the distribution of suramin is overseen by the World Health Organisation and is made available only after a case has been diagnosed.2 This may result in a substantial delay in suramin treatment in non-endemic areas.2,3 In many cases, pentamidine treatment (4 mg/kg intramuscular or intravenous, once daily) is therefore started to bridge the time until suramin will be delivered.1,4,5 Long-term pentamidine treatment has been used successfully and in line with this a substantial reduction of parasitemia has been reported after short-term treatment.2,6 However, it should be borne in mind that in naturally occurring, untreated infections the interplay between the immune response and antigenic variation of the parasite results in irregular fluctuations in parasitaemia.1 Hence, a decrease in parasitaemia over several days may not represent valid proof of treatment efficacy, and therefore, more supporting evidence for a potential beneficial effect of short-term pentamidine treatment in the haemolymphatic phase of T. b. rhodesiense infections is needed.1
A 66-year-old female presented at our clinic with complaints of cold shivers, headache and nausea, that started 2 days earlier when she returned to the Netherlands after a 2 week journey through Malawi. She had no relevant medical history, was properly vaccinated and used malaria-prophylaxis as prescribed. Physical examination demonstrated fever and a painful chancre on her back (Figure 1). She had noticed flies during her visit to a Wildlife Reserve, but could not recall being bitten. Additional laboratory results are listed in Supplementary Table S1, available as Supplementary data at JTM online. Malaria was excluded by microscopic examination of blood smears and Quantitative Buffy Coat (QBC) capillaries but highly motile T. b. rhodesiense parasites were seen with a parasitaemia of 80 trypomastigotes/μL (Supplementary movie 1, Supplementary data are available at JTM online).
Figure 1.
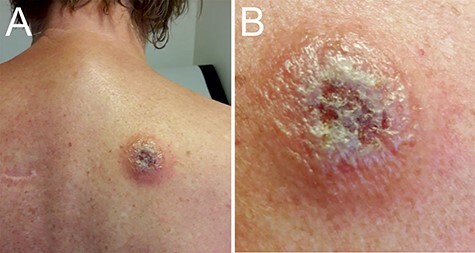
Trypanosomal chancre. Panel A shows the trypanosomal chancre on the back of the patient at presentation. The chancre had a diameter of ca. 5 cm with ulcerating and elevated edges. Panel B shows the swollen chancre at higher magnification.
Since East-African trypanosomiasis was diagnosed within 2 days after onset of symptoms, the disease was considered to be in its initial haemolymphatic phase. Therefore, no lumbar puncture was done to rule out the meningo-encephalitic involvement. Suramin was not immediately available in our hospital and had to be transported overnight by courier from Switzerland. To bridge the time between diagnosis and suramin treatment the patient was treated with a single-dose of intramuscular pentamidine (4 mg/kg). Approximately 12 h later and just before intravenous suramin treatment was started, a subsequent blood specimen was drawn to determine the parasitaemia and motility of the trypanosomes. Parasitaemia had dropped by 75% to 20 trypomastigotes/μL and the trypomastigote motility was severely affected into sluggish motility (Supplementary movie 2, Supplementary data are available at JTM online). The patient tolerated the subsequent standard course of intravenous suramin and fully recovered.
Since in-vitro studies have shown that reduced motility of trypomastigotes is a reliable proxy of drug efficacy,7 our observation of reduced trypomastigote motility after a single-dose of intramuscular pentamidine underlines that short-term bridging treatment with pentamidine is of added value in the clinical management of human East African trypanosomiasis and it is in agreement with the current guidelines for travellers in non-endemic countries.4,5
Supplementary Material
Acknowledgements
We thank the patient for informed consent. Dr Annemieke Bloem, Renske van Es-Bijl and Rob Koelewijn, are thanked for expert diagnostic analysis and the recording of the movies showing motile trypanosomes. The Swiss Tropical Institute, Basel, Switzerland, is thanked for providing and swiftly sending of suramin.
Contributor Information
Perry J J van Genderen, Department Medical Microbiology & Infectious Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands; Department Internal Medicine, Erasmus MC University Medical Center Rotterdam, the Netherlands; Institute for Tropical Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands.
Jan L Nouwen, Department Medical Microbiology & Infectious Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands; Department Internal Medicine, Erasmus MC University Medical Center Rotterdam, the Netherlands; Institute for Tropical Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands.
Mariana De Mendonça Melo, Department Medical Microbiology & Infectious Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands; Department Internal Medicine, Erasmus MC University Medical Center Rotterdam, the Netherlands; Institute for Tropical Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands.
Bart J A Rijnders, Department Medical Microbiology & Infectious Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands; Department Internal Medicine, Erasmus MC University Medical Center Rotterdam, the Netherlands; Institute for Tropical Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands.
Jaap J van Hellemond, Department Medical Microbiology & Infectious Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands; Institute for Tropical Diseases, Erasmus MC University Medical Center, Rotterdam, the Netherlands.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions
JJvH and PJJ wrote the first draft of the manuscript; all authors provided critical feedback on the draft. PJJvG, JLN, BJAR and MDMM were involved in clinical decisions and treatment of the patient. All authors contributed to the interpretation of the results, reviewed and approved the final version for publication.
Conflict of interest: None.
References
- 1. Buscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet 2017; 390:2397–409. [DOI] [PubMed] [Google Scholar]
- 2. Simarro PP, Franco JR, Cecchi G et al. Human African trypanosomiasis in non-endemic countries (2000-2010). J Travel Med 2012; 19:44–53. [DOI] [PubMed] [Google Scholar]
- 3. Clerinx J, Vlieghe E, Asselman V, Van de Casteele S, Maes MB, Lejon V. Human African trypanosomiasis in a Belgian traveller returning from the Masai Mara area, Kenya. February 2012 Euro Surveill 2012; 17:20111. [PubMed] [Google Scholar]
- 4. Neumayr A. Antiparasitic Treatment Recommendations, 2nd ed. Tredition Gmb H: Hamburg, 2018. [Google Scholar]
- 5. Urech K, Neumayr A, Blum J. Sleeping sickness in travelers–do they really sleep? PLoS Negl Trop Dis 2011; 5:e1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nadjm B, Van Tulleken C, Macdonald D, Chiodini PL. East African trypanosomiasis in a pregnant traveler. Emerg Infect Dis 2009; 15:1866–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hochstetter A, Stellamanns E, Deshpande S, Uppaluri S, Engstler M, Pfohl T. Microfluidics-based single cell analysis reveals drug-dependent motility changes in trypanosomes. Lab Chip 2015; 15:1961–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


