Abstract
Simple Summary
Inflammation plays a major role in cancer development and progression and has the potential to be used as a prognostic marker in cancer. Previous studies have attempted to evaluate PLR, NLR and MLR as indicators of inflammation/prognostic markers in cancer, but there is no common consensus on its application in clinical practice. The aim of this systematic review and meta-analysis (a) assess the prognostic efficacy of all three prognostic markers in comparison to each other and, (b) investigate the prognostic potential of these three markers in HNC. The study followed PRISMA guidelines, with literature being collated from multiple bibliographic databases. Preliminary and secondary screening were carried out using stringent inclusion/exclusion criteria.
Abstract
Inflammation plays a major role in cancer development and progression and has the potential to be used as a prognostic marker in cancer. Previous studies have attempted to evaluate Platelet-to-lymphocyte ratio (PLR), neutrophil–lymphocyte ratio (NLR) or monocyte–lymphocyte ratio (MLR) as indicators of inflammation/prognostic markers in cancer, but there is no common consensus on their application in clinical practice. The aim of this systematic review and meta-analysis is to (a) assess the prognostic efficacy of all three prognostic markers in comparison to each other and (b) investigate the prognostic potential of these three markers in HNC. The study followed PRISMA guidelines, with the literature being collated from multiple bibliographic databases. Preliminary and secondary screening were carried out using stringent inclusion/exclusion criteria. Meta-analysis was carried out on selected studies using CMA software and HR as the pooled effect size metric. A total of 49 studies were included in the study. The pooled HR values of PLR, NLR and MLR indicated that they were significantly correlated with poorer OS. The pooled effect estimates for PLR, NLR and MLR were 1.461 (95% CI 1.329–1.674), 1.639 (95% CI 1.429–1.880) and 1.002 (95% CI 0.720–1.396), respectively. Significant between-study heterogeneity was observed in the meta-analysis of all three. The results of this study suggest that PLR, NLR and MLR ratios can be powerful prognostic markers in head and neck cancers that can guide treatment. Further evidence from large-scale clinical studies on patient cohorts are required before they can be incorporated as a part of the clinical method. PROSPERO Registration ID: CRD42019121008
Keywords: PLR, NLR, MLR, HNC, prognosis, meta-analysis, systematic review
1. Introduction
It is an established fact that cancer pathophysiology relies heavily on the manipulation of the immune system involved in cancer cell growth, proliferation and tumorigenesis [1]. Immunological involvement, or, more specifically, inflammation, has been defined as one of the hallmarks of cancer and plays a role in malignancy, angiogenesis and genomic instability [2]. Studies have shown that the presence of inflammation and inflammatory markers in cancer is associated with a change in prognosis [3]. Therefore, an assessment of the magnitude of inflammation in cancer patients could be used to determine disease prognosis. The magnitude of inflammation could be indirectly explored via the measurement of malnutrition and systemic inflammation-based indicators such as neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR) and monocyte–lymphocyte ratio (MLR). NLR can act as a proxy measurement of the degree of inflammation in cancers, as inflammation leads to systemic alterations in the levels of peripheral blood leukocytes [4,5]. Similarly, platelets release pro-inflammatory mediators, such as cytokines and chemokines, which intensify the inflammatory microenvironment in tumors, resulting in PLR being another viable measure of inflammation [6,7]. In addition, monocytes also have a key role in inflammation, as seen in their presence in atherosclerosis [8]. Individually, or in combination, these measures could be used as prognostic markers in cancer. The dense immune cell influx may explain the robust immune response in HPV-driven head and neck cancers and its potential to eliminate some of the tumor cells. An assessment of the prognosis of patients with cancer is significant, since a precise prediction of prognosis will permit appropriate treatment, improving patient outcomes [9]. The systemic inflammatory response elicited by the immune cells seen in cancer is interconnected with the nutritional depletion seen in cancer patients and affects the prognosis and course of the disease independently of tumor stage [10]. The host immune system is involved in cancer initiation, progression and metastasis. In addition, the immune–inflammatory retort to the toxic chemoradiation therapy adds to its clinical benefits. Lymphocytes represent one third of the total white blood cells, play a significant role in cellular immunity and may stimulate the clearance of malignant cells [11]. Antagonizing the action of lymphocytes, the monocytes enable tumor progression by promoting angiogenesis and immunosuppression [12]. An assessment of the MLR at different stages pretreatment or post-treatment can help in anticipating the prognosis of the disease and improving treatment decisions [13].
The relative ease of assessment, swift detection and minimal cost using NLR, PLR and MLR offers a lucrative option to gauge cancer prognosis and guide treatment. Furthermore, the need for only peripheral blood samples with little to no patient discomfort or pain increases the patient compliance [14]. Current methods for cancer prognosis in patients involve the use of molecular markers (such as BRCA1 in breast cancer and EGFR in NSCLC) [15,16], which require complex and expensive assays for measurement and quantification (immunohistochemistry, q-RT PCR) [17,18], while also generating a greater degree of patient discomfort (biopsy), with an additional risk of seeding of tumor cells. Interpretation of tumor markers with complex assays does offer vital information on cancer prognosis and tumor-specific immunotherapy but they are not ideal for the constant and real-time monitoring of prognosis, as they are expensive, cumbersome and time-consuming. Therefore, significant research interest has been directed towards the use of PLR, NLR and MLR as additional or supplementary biomarkers for cancer prognosis.
Clinical studies have further explored the use of PLR, NLR and MLR as pre-operative prognostic biomarkers in addition to assessing their utility as diagnostic cancer biomarkers [19,20]. Further, the vast literature on these serum biomarkers has provided an impetus to conduct systematic review and meta-analysis studies [21,22]. However, a knowledge gap that exists, despite the presence of previous reviews, is that no systematic review or meta-analysis study published to date has investigated either (a) the prognostic efficacy of all three prognostic markers, PLR, NLR and MLR, in comparison to each other, or (b) the comparative utility of these markers with regard to HNC cancers. Previous systematic review and meta-analysis studies have either only focused on a single cancer type, with no study focusing on assessing the effectiveness of all three proposed biomarkers in HNC, and/or comparing and contrasting all three biomarkers against each other. While Mellor et al. recently published a systematic review and meta-analysis study, including all cancer types, the study only focused on NLR alone as the prognostic marker of choice [23]. Similarly, Zhu et al. focused on multiple inflammatory markers, with emphasis on PLR and NLR, but limited their study to ovarian cancer [24], while another study by Zhang et al. limited their analysis to only colorectal cancer [21].
This comprehensive systematic review and meta-analysis study seeks to amend the aforementioned knowledge gap and attempts to provide a better understanding of the utility of PLR, NLR and MLR as prognostic markers in cancer. In addition, it seeks to highlight the comparative efficacy of PLR, NLR and MLR in each studied cancer type.
2. Methods
2.1. Search Strategy
This systematic review and meta-analysis study was conducted based on the standard review guidelines and methodology as detailed in the Preferred Reporting Items for Systematic Review and Meta-Analyses Protocol (PRISMA-P) guidelines [25,26]. The PRISMA compliance has been delineated in the PRISMA checklist table provided in Supplementary Data Table S1. The search strategy was expansive and exhaustive, with multiple bibliographic databases being searched. These databases included EMBASE, MEDLINE, Science Direct, Scopus and Web of Science. These databases were used to scope out relevant studies published within a ten-year span of time, i.e., from July 1999 to July 2019. This time constraint was placed to the search strategy in order to keep the results of the search relevant to the current developments in cancer prognosis and treatment. The search strategy also allowed for scoping of the reference lists of review articles and other publications for additional relevant studies for inclusion in the review. The bibliographic search was conducted via the construction of a logic grid and subsequent identification of specific ‘keywords’. These ‘keywords’ were then used to construct ‘search strings’, which allowed for a thorough and robust search of the bibliographic databases.
Keywords: Platelet-to-Lymphocyte Ratio (PLR), Neutrophil-to-Lymphocyte Ratio (NLR), Monocyte-to-Lymphocyte Ratio (MLR), Biomarker, Head and Neck Cancer, Prognosis, Survival (Overall Survival, Disease Free Survival, Disease Specific Survival), Patient Study, Cohort, Blood, Systematic-review, Meta-analysis.
The search was conducted individually by two independent reviewers (CK and RJ), in order to eliminate the likelihood of selection bias occurring. Primary article and study screening was based on the pertinence of the title and abstract of each publication to the topic of the systematic review and meta-analysis being undertaken. The screening was conducted simultaneously alongside the initial search, under the discretion of the two reviewers (CK and RJ). Additional studies from the reference lists of reviews and other included publications were screened for and included after primary screening. Any disputes and differences in opinion arising during initial screening were settled through the inclusion of the third reviewer (VT).
2.2. Study Selection
After the primary screening procedure, the full texts of articles were subject to selection based on specific, predefined inclusion and exclusion criteria (secondary screening). The inclusion and exclusion criteria were based on previous similar systematic review and meta-analysis studies, adapted to the parameters of this study.
2.3. Inclusion Criteria
The studies must discuss the survival outcome of HNC cancer patients based on PLR, NLR and MLR levels.
The survival outcome must be presented in the form of HR (hazard ratios) and 95% CI (confidence intervals).
The survival outcome must be presented in the form of Kaplan–Meier curves, along with patient cohort information, for each treatment arm represented in the KM Curves. (This is required only if the HR and 95% CI values have not been presented in the manuscript, as the above information is required to extract approximated HR values.)
2.4. Exclusion Criteria
Conference abstracts, reviews, letters to the editor and other non-clinical literature will not be considered for either systematic review or meta-analysis.
Included studies must be clinical studies or involve patient samples. (In vitro, in silico and animal studies will be excluded.)
Unpublished or non-peer-reviewed literature will be excluded.
Studies that do not focus on survival outcomes and prognosis aspects of PLR, NLR and MLR in cancer patients will not be considered.
If the sample size of each individual study is of low power (sample size < 10), they will be excluded.
No limitations were placed on the types of patients involved, their clinicopathological characteristics or their demographic characteristics. No restrictions were placed based on age, sex, ethnicity, location, follow-up period, duration of treatment or method of treatment.
2.5. Data Extraction and Recording
After the secondary screening procedure, based on the predefined inclusion and exclusion criteria, the full-text formats of the selected studies were collated and subjected to a data extraction process. Data extraction followed a top-down approach, with the selected full-text studies being examined for relevant patient and study data individually by three reviewers, a process designed to generate redundancy, while reducing individual error. A standardized data extraction form, containing all the required data items, was prepared using Microsoft Excel and utilized by the reviewers to extract the data. After individual data extraction was performed by all reviewers, duplicated information/studies were removed. The resulting dataset of study information was collated into a single database for further analysis. The data items extracted from full-text versions of individual studies included:
Author names;
Year of publication;
Marker studied (PLR, NLR or MLR);
Size of patient cohort;
Diagnostic methods;
Follow-up period;
Gender split of cohort;
TNM staging split of cohort;
Survival endpoint of each study (overall survival, disease-free survival, disease-specific survival);
General features of each study (will be presented as short qualitative opinions/observations of reviewers, for each study being included).
2.6. Quality Assessment
The quality assessment of the studies was based on the standard Newcastle–Ottawa Scale (NOS) for the quality assessment of nonrandomized studies in meta-analysis [27]. This scale presents a ‘star system’, which assigns each of the 3 broad parameters of the study (1–4 stars range) in increasing order of quality. The 3 broad perspectives being assessed include the selection of study groups in each individual study, the comparability of groups and the ascertainment of exposure/outcome of interest for case–control/cohort studies [28,29,30,31,32].
2.7. Meta-Analysis
The meta-analysis was conducted using the Comprehensive Meta-Analysis (CMA) software (version 3.3.070; Biostat, Englewood, NJ, USA) [33]. The hazard ratio was chosen as the appropriate effect size metric for this study, and overall survival (OS) was selected as the survival endpoint for the meta-analysis. The HR indicates the probability of survival of the patient cohort in each included study and was pooled across all included studies to determine the likelihood of overall survival (OS) of patients, across all studies. The pooled results were represented visually using forest plots. Meta-analyses on PLR, NLR and MLR were performed independently, to determine the prognostic effectiveness of each, as a cancer marker. Statistical significance (p-values) of each of the three aforementioned prognostic markers was also calculated. The meta-analysis was conducted based on the random-effects model to account for inherent heterogeneity between each of the included studies [34].
2.8. Assessment of Heterogeneity
Assessment of heterogeneity was conducted using 3 parameters in order to increase the robustness of analysis [35,36,37,38,39]. The Higgins I2 statistic was used as the primary method to determine heterogeneity, as it has a high power of detection of heterogeneity [40]. However, as I2 is not an infallible metric of heterogeneity, and can provide biased results in small meta-analyses [41], we also used the Cochran’ Q and Tau2 parameters to assess heterogeneity alongside the I2 statistic [42,43].
2.9. Subgroup Analysis
The major meta-analysis subgroups selected for this study were cohorts of studies that assessed PLR, NLR and MLR. As sufficient data were not available, only the survival endpoint of OS was assessed. Additionally, no subgroup analysis was carried out based on the demographic or clinicopathological characteristics, due to a lack of sufficient high-quality studies with comparable data, which would lead to a lack of power in the subsequent statistical analysis.
2.10. Publication Bias
Publication bias assessment was conducted as per PRISMA guidelines. The Egger’s graphical bias indicator test was used to construct a funnel plot [44]. The funnel plot symmetry along the regression line was used to assess the existence of publication bias, wherein the symmetry of the funnel plot inversely correlated to the degree of publication bias in the meta-analysis study. Adjustment for small and missing studies was carried out via imputation of possible small studies using the Orwin’s Fail-Safe N test [45]. The Begg and Mazumdar’s Rank Correlation test was used to check correlation between ranks of effect sizes and variances [46], wherein a positive result indicated accurate publication bias assessment.
3. Results
The search strategy yielded a total of 28,716 studies across all databases. The majority of these studies were screened out by reviewers during the initial primary screening process, due to a lack of relevance to the topic of review. After primary screening, full texts of 120 articles were obtained, which were then further assessed for duplicates (which were removed) and adherence to pre-defined inclusion and exclusion criteria. This secondary screening process eliminated 71 studies, which left 49 studies suitable for inclusion in the systematic review and meta-analysis. However, due to a lack of sufficient quality data in all screened studies, only 34 publications that had the appropriate effect size data (HR and 95% CI values for OS) were included in the final meta-analysis. The data were subsequently extracted from the selected studies as per the defined data extraction procedure and used to construct a systematic review table and perform the meta-analysis. The entire process was monitored by the second and third reviewer (RJ and VT) at all stages. The selection process is delineated in Figure 1.
Figure 1.
Search Strategy.
Study Characteristics: The 49 studies included in this systematic review and meta-analysis study were conducted across nine countries across the globe, as described in Table 1. The largest number of published studies were from China (n = 28). Other countries that featured in multiple publications on this topic included Japan (n = 9), the United Kingdom (n = 3), Taiwan (n = 3) and the Republic of Korea (n = 2), while Italy (n = 1), Switzerland (n = 1), Thailand (n = 1) and Turkey (n = 1) each only featured in a single publication.
Table 1.
Study characteristics of included studies for systematic review.
| Author Name | Year of Publication | Prognostic Parameter (PLR/NLR/MLR) | Cohort Size | Anatomic Location of Cancer | Country of Study | Type of Study | Gender | Stage of Cancer | Metastasis | Risk Factors | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wen et al. [47] | 2018 | PLR NLR |
723 | Esophageal (65%) Gastric (35%) |
UK | retrospective study | male (75.5%) female (24.5%) |
T0 (4.6%) T1 (14.4%) T2 (21.0%) T3 (51.9%) T4 (8.2%) |
M1 2.5% | NA | 66.1 ± 10.5 |
| Kawakita et al. [48] | 2016 | PLR NLR |
140 | Parotid gland (78%) Submandibular gland (20%) Others (2%) |
Japan | retrospective study | male (86%) female (14%) |
T1 (9%) T2 (26%) T3 (20%) 4a (44%) 4b (1%) |
M0 (93%) M1 (7%) |
NA | 64 (26–84) |
| Chen et al. [49] | 2014 | NLR PLR |
211 | Nasopharynx (100%) | China | retrospective study | male (85.8%) female (14.2%) |
NA | NA | NA | 46 (14–72) |
| Zhang et al. [50] | 2015 | NLR PLR |
468 | Esophagus (100%) | China | retrospective study | male (80.3%) female (19.7%) |
stage I (9.8 %) stage II (42.6 %) stage III (47.6 %) |
NA | Smoking Alcohol consumption |
59.5 ± 9.0 |
| Jiang et al. [51] | 2017 | PLR NLR LMR |
78 | Thyroid (100%) | China | retrospective study | male (43.6%) female (56.4%) |
stage I (23.1%) stage II (20.5%) stage III (20.5%) stage IV (35.9%) |
M1 (2.6%) | NA | 47.3 ± 13.8 |
| Li et al. [52] | 2016 | PLR NLR LMR |
388 | Nasopharynx (100%) | China | retrospective study | NA | NA | M0 (100%) | NA | |
| Feng et al. [53] | 2014 | NLR PLR |
483 | Esophagus (100%) | China | retrospective study | male (85.1%) female (14.9%) |
T1 (18.0%) T2 (16.6%) T3 (54.9%) T4 (10.5%) |
M0 (56.7%) M1 (43.3%) |
NA | 59.1 ± 8.0 |
| Jiang et al. [54] | 2015 | PLR | 1261 | Nasopharynx (100%) | China | retrospective study | male (72.9%) female (27.1%) |
Clinical stage I (3.25) II (14.8%) III (52.7%) IV (29.4%) Tumor stage T1 (8.6%) T2 (23.6%) T3 (46.2%) T4 (21.5%) Node stage N0 (16.8%) N1 (36.6%) N2 (37.7%) N3 (9.0%) |
M1 (12.4) M0 (87.6) |
Smoking Chronic HBV infection Cardiovascular disease Diabetes mellitus Family history of NPC |
46 (39–55) |
| Jung et al. [55] | 2015 | NLR PLR |
119 | Esophagus (100%) | Korea | retrospective study | male (94.1%) female (5.9%) |
Pathological stage I (31.1%) II 33 (27.7%) III 49 (41.2) |
M0 (100%) | NA | 63.64 ± 8.42 |
| He et al. [56] | 2016 | NLR PLR |
317 | Esophagus (100%) | China | retrospective study | male (84.5%) female (15.5%) |
TNM I–II (68.5%) III–IV (31.5%) |
M0 (53.9%) M1 (46.1%) |
Smoking Alcohol consumption |
60 (37–77) |
| Jiang et al. [57] | 2016 | NLR PLR |
70 | Thyroid (100%) | China | retrospective study | male (40%) female (60%) |
Stage III or IV (50.0%) | M1 (2.9%) | NA | 47.7 ± 13.9 |
| Hirahara et al. [58] | 2017 | LMR NLR PLR |
147 | Esophagus (100%) | Japan | retrospective study | male (89.8%) female (10.2% |
Pathological stage Ia–1b (40.1%) 2a–2b (22.4%) 3a–3c (37.4%) |
NA | NA | NA |
| Ong et al. [59] | 2016 | LMR NLR PLR |
133 | Tongue (100%) | China | retrospective study | male (53.4%) female (46.6%) |
pT classification, n (%) T1 (39.1%) T2 (60.9%) |
M0 (96.2%) M1 (3.8%) |
NA | 51.92 (24–74) |
| Mao et al. [60] | 2017 | PLR | 899 | Larynx (100%) | China | retrospective study | male (97.1%) female (2.9%) |
T 1 (22.9%) 2 (28.9%) 3 (29.1%) 4 (19.0%) N 0 (81.2%) 1 (9.7%) 2 (8.6%) 3 (0.6%) |
M0 (100%) | Smoking Alcohol consumption |
60 (22–87) |
| Dutta et al. [61] | 2011 | NLR PLR |
112 | Esophagus (100%) | UK | retrospective study | male (75.9%) female (24.1%) |
TNM stage I 17.9% II 34.8% III 46.4% IV 0.9% |
NA | NA | <65 (60.7%) 65–74 (33.9%) ≥75 (5.4%) |
| Li et al. [62] | 2016 | NLR PLR |
409 | Nasopharynx (100%) | China | retrospective study | male (70.4%) female (29.6%) |
(I–II) (18.8%) (III–IV) (81.2%) |
M0 (84.4%) M1 (15.6%) |
NA | 45 (18–77) |
| Messager et al. [63] | 2015 | PLR | 153 | Esophagus (100%) | UK | live patient samples | male (83.7%) female (16.3%) |
||||
| Hirahara et al. [64] | 2016 | LMR NLR PLR |
147 | Esophagus (100%) | Japan | retrospective study | male (89.8%) female (10.2%) |
Pathological stage Ia–Ib 40.1% IIa–Iib 22.5% IIIa–IIIc 37.4% |
NA | NA | NA |
| Moon et al. [65] | 2015 | NLR PLR |
153 | Oropharynx 33.3% Nasopharynx 31.4% Larynx 18.3% Hypopharynx 17.0% |
Korea | live patient samples | male (84.3%) female (15.7%) |
Clinical TNM stage T1/T2 17.6%/36.6% T3/T4 14.4%/31.4% N0/N1 21.6%/20.9% N2/N3 49.7%/7.8% Overall I/II 4.6%/17.0% Overall III/IV 13.7%/64.75% |
M0 (100%) | Smoking Alcohol consumption |
57 (16–78) |
| Hirahara et al. [66] | 2016 | LMR NLR PLR |
147 | Esophagus (100%) | Japan | retrospective study | male (89.8%) female (10.2%) |
pathological stage 1a–1b (40.2%) 2a–2b (22.4%) 3a–3c (37.4%) |
NA | NA | |
| Turri–Zanoni et al. [67] | 2016 | NLR PLR |
215 | Paranasal sinus (100%) | Italy | retrospective study | male (34%) female (66%) |
pT classification pT1 19% pT2 18% pT3 22% pT4a 16% pT4b 25% |
M0 (100%) | NA | 65 (8–87) |
| Xie et al. [68] | 2014 | NLR PLR |
317 | Esophagus (100%) | China | retrospective study | male (77%) female (23%) |
Tumor stage Stage I 88.4 Stage II 69.7 Stage III 42.4 |
M0 (100%) | NA | 58.1 ± 8.9 |
| Bojaxhiu et al. [69] | 2018 | NLR PLR |
186 | Oral cavity (28%) Oropharynx (45%) Hypopharynx (15%) Larynx (13%) |
Switzerland | retrospective study | male (79%) female (22%) |
UICC stage, N (%) I (3%) II (6%) III (24%) IV (68%) |
M0 (100%) | Smoking Alcohol consumption |
61 (41–88) |
| Sun et al. [70] | 2017 | NLR PLR |
148 | Nasopharynx (100%) | China | retrospective study | male (83.8%) female (16.2%) |
M0 (100%) | Smoking | 45 (24–72) | |
| Sun et al. [71] | 2015 | NLR PLR |
251 | Nasopharynx (100%) | China | retrospective study | male (71.7%) female (28.3%) |
UICC/AJCC stage I 2.4% II 15.9% III 47.4% IV 34.3% |
M0 (100%) | NA | 46 (15–76) |
| Tangthongkum et al. [72] | 2017 | PLR | 274 | Oral (100%) | Thailand | retrospective study | males (64.4%) females (35.6%) |
NA | NA | NA | 60 (21–92) |
| Ozturk et al. [73] | 2016 | NLR PLR |
57 | Tongue (100%) | Turkey | retrospective study | male (38.6%) female (61.4%) |
stage I (64.9 %) stage II (35.1 %) |
M0 (100%) | NA | 57.8 (23–88) |
| Toyokawa et al. [74] | 2016 | NLR PLR |
185 | Esophagus (100%) | Japan | retrospective study | male (82.2 %) female (17.8%) |
Clinical TNM stage I 36.2% II 42.2% III/IV 21.6% |
M0 (100%) | NA | <65 51.4% ≥65 48.6% |
| Urabe et al. [75] | 2017 | LMR NLR PLR |
1363 | Resectable Gastric and Esophagogastric Junction | Japan | retrospective study | male (71.5%) female (28.5%) |
T stage T1 58% T2 11.8% T3 17.6% T4 12.6% |
M0 (100%) | NA | NA |
| Wang et al. [76] | 2014 | PLR | 252 | Upper aerodigestive tract | China | retrospective study | male (68.7%) female (31.3%) |
Ann Arbor stage IE 61.5% IIE 38.5 % |
NA | NA | 41 (9–80) |
| Wei et al. [77] | 2015 | NLR PLR |
423 | Esophagus (100%) | China | retrospective study | male (80.6%) female (19.4) |
TNM stage (AJCC, 7th) I (12.8%) II (39.7%) III (33.6%) IV (13.9%) |
M0 (86.1) M1 (13.9) |
NA | 58 (24–88) |
| Xu et al. [78] | 2015 | NLR PLR |
468 | Esophagus (100%) | China | retrospective study | male (88.9%) female (11.1%) |
Clinical stage I II IIIA IIIB + IIIC |
NA | Smoking Alcohol consumption |
58 |
| Yang et al. [79] | 2018 | NLR PLR |
515 | Esophagus (100%) | China | retrospective study | male (81.2%) female (18.8%) |
TNM Stage I II III |
M0 (100%) | NA | 61(33–92) |
| Ye et al. [80] | 2018 | NLR PLR |
427 | Nasopharynx (100%) | China | retrospective study | male (71.9%) female (28.1%) |
TNM stage I 2.1 II 18.7% III 48.7% IV 30.5% |
yes | NA | 48 (17–82) |
| Yuan et al. [81] | 2014 | NLR PLR |
327 | Esophagogastric junction (100%) | China | retrospective study | male (86.2%) female(13.8%) |
pTNM stage I and II (45.9%) III and IV (54.1%) |
yes | NA | 63.1± 9.7 (39–77) |
| Zhang et al. [82] | 2017 | NLR PLR |
355 | Esophagogastric junction (100%) | China | retrospective study | male (79.2%) female (20.8%) |
TNM stage I, II (43.4%) III, IV (56.6%) |
NA | NA | 64 (34–82) |
| Chen et al. [20] | 2018 | NLR PLR MLR |
361 | Larynx (100%) | China | retrospective study | male (97.8%) female (2.2%) |
TNM stage I 31.6% II 32.7% III 19.7% IV 16.0% |
yes | NA | 60 (35–87) |
| Hsu et al. [83] | 2009 | MLR | 1069 | Esophagus (100%) | Taiwan | retrospective study | male (94.5%) female (5.5%) |
stage I 53 (10.9%) II 197 (40.4%) III 138 (28.3%) IV 100 (20.5%) |
yes | NA | 63.8 (34–88) |
| Chien et al. [84] | 2016 | MLR | 2025 | Esophagus (100%) | Taiwan | retrospective study | male (94.1%) female (5.9%) |
cStage T1–2N0 T3–4N0 T1–2N (+) T3–4N (+) Unknown |
yes | NA | 55.2 ± 9.8 |
| Furukawa et al. [85] | 2019 | LMR | 103 | Tongue (100%) | Japan | retrospective study | male (53.8%) female (46.2%) |
stage I, II 84.5% III, IV 15.5% |
yes | Smoking Alcohol consumption |
63 (26–92) |
| Hsueh et al. [86] | 2017 | NLR PLR LMR |
979 | Larynx (100%) | China | retrospective study | male (97.5%) female (2.5%) |
stage I 23.7% II 36.4% III 26.8% IV 13.1% |
yes | NA | 60.81 ± 9.68 |
| Huang et al. [87] | 2015 | LMR | 348 | Esophagus (100%) | China | retrospective study | male (87.1%) female (12.9%) |
NA | yes | NA | 59.2 ±7.8 |
| Kano et al. [88] | 2016 | NLR PLR LMR |
285 | Larynx (23.5%) Oropharynx (40.7%) Hypopharynx (35.8%) |
Japan | retrospective study | male (88.4%) female (11.6%) |
Clinical stage I, II (22.1%) III, IV (77.9%) |
no | NA | 61 (37–80) |
| Li et al. [89] | 2013 | LMR | 1547 | Nasopharynx (100%) | China | retrospective study | male 72.7% female 27.3% |
Overall stage I-II (21.6%) III-IV (78.4%) |
yes | NA | 51 (6–87) |
| Li et al. [52] | 2017 | NLR PLR LMR |
249 | Nasopharynx (100%) | China | prospective study | male (73.9%) female (26.1%) |
Clinical stage I-II 26.1 III-IV 73.9 |
yes | NA | ≤50 (65.9%) >50 (34.1%) |
| Liu et al. [90] | 2015 | NLR PLR LMR |
326 | Esophagus (100%) | China | retrospective study | male (86.8%) female (13.2%) |
T stage T1 (18.1%) T2 (18.4%) T3 (53.7%) T4 (9.8%) |
NA | NA | 59.2 ± 7.9 (38–80) |
| Oya et al. [91] | 2018 | NLR PLR LMR |
441 | Oral cavity 44% Larynx 28% Oropharynx 10% Hypopharynx 13 % Other 5% |
Japan | retrospective study | male (73%) female (27%) |
Stage I 32% II 18% III 15% IV 35% |
no | NA | 68 (27–92) |
| Yang et al. [92] | 2018 | NLR LMR |
197 | Hypopharyngeal (100%) | China | retrospective study | male (99.0%) female (0.01%) |
Clinical stage I (2.0%) II (13.2%) III (27.5%) IV (57.4%) |
yes | Smoking Drinking |
<59 (50.8%) ≥59 (49.2%) |
Most of these studies were large-scale retrospective studies, representing a total pooled patient cohort of 20,739 patients. The collated data across these 49 studies suggest that nearly all studies in this field of PLR-, NLR- and MLR-based cancer prognostics have focused on head and neck cancers (HNC), with the main anatomical locations described being the oral cavity, nasopharyngeal, oropharyngeal, hypopharyngeal, laryngealnasopharyngeal, laryngeal and tongue regions. Further observation of the demographic data also suggests that most studies included in this review involved a higher percentage of men, in comparison to women (n = 45), with only a few studies reporting a higher percentage of women in their studies (n = 4). Not all studies assessed all three prognostic indicators of PLR, NLR and MLR. A large proportion of the studies assessed PLR and NLR in conjunction (n = 24), with a smaller proportion assessing all three simultaneously (n = 13). A few studies were also noted to have assessed each of the prognostic markers individually, with PLR (n = 6) and MLR (n = 3) both being assessed equally, while no study assessed NLR individually (n = 0). While most studies did not present any information regarding underlying risk factors (n = 39), the studies that did provide this information highlighted smoking (n = 10) and drinking (n = 8) as the primary risk factors, while one study also detailed additional risk factors such as chronic HPV infection, CVD, diabetes and family history.
4. Meta-Analysis
As described in the methodology and protocol of the study, a meta-analysis of pooled HR and 95% CI values was carried out under three subgroups, for the survival endpoint of OS. The subgroups analyzed were based on the three potential prognostic markers PLR, NLR and MLR. Each individual cohort of each study focusing uniquely on each of the three aforementioned prognostic markers was assessed as an independent study, contributing to the respective subgroup being analyzed. The pooled results were graphically represented in the form of forest plots.
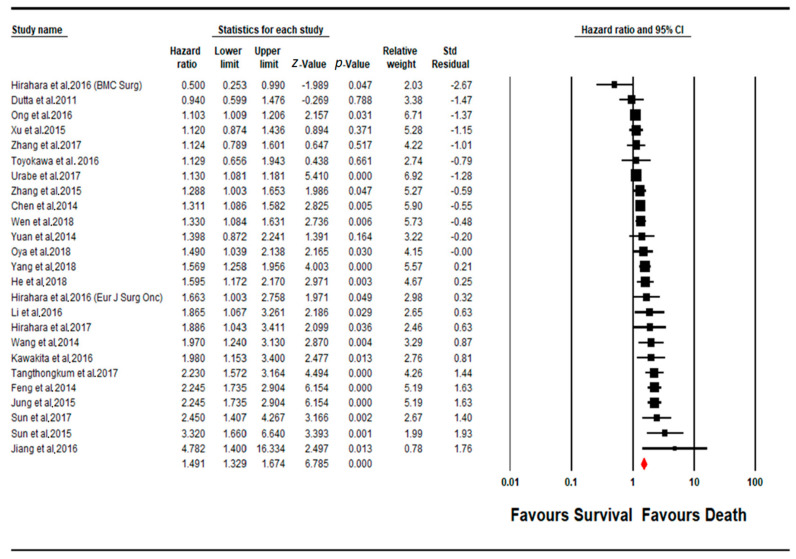
4.1. Meta-Analysis PLR Subgroup
A total of 25 cohorts of studies were pooled for determining the prognostic impact of a change (increase) in PLR levels upon the overall survival of the patient cohort (Figure 2). Twenty-three studies showed a positive correlation between increased PLR levels and a worse disease outcome (poorer prognosis), out of which 18 studies showed a significant level of said correlation (p < 0.05). Interestingly, two other additional studies contradicted the rest, suggesting an inverse correlation between PLR levels and patient survival, thereby suggesting a better prognosis for OS. The pooled effect estimate (HR) was found to be statistically significant, at a value of 1.461 (95% CI 1.329–1.674; p = 0.0001). Assessment of heterogeneity suggests that there is substantial heterogeneity between the studies included in the subgroup meta-analysis for PLR (I2 = 80.320; Tau2 = 0.050; Cochran’s Q = 121.949).
Figure 2.
Forest plot of PLR subgroup.
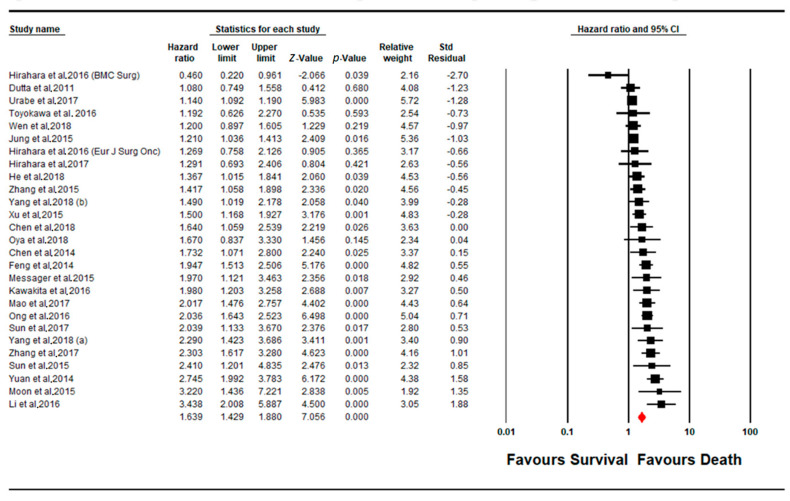
4.2. Meta-Analysis NLR Subgroup
A total of 27 cohorts of studies were pooled for determining the prognostic impact of a change (increase) in NLR levels upon the overall survival of the patient cohort (Figure 3). The majority of the studies (n = 23) showed a positive correlation between increased NLR levels and a worse disease outcome (poorer prognosis), out of which 20 studies showed a significant level of said correlation (p < 0.05). Interestingly, only one other study contradicted the majority, suggesting an inverse correlation between NLR levels and patient survival, indicating a better prognosis for OS. The pooled effect estimate (HR) was found to be statistically significant, at a value of 1.639 (95% CI 1.429–1.880; p = 0.001). Assessment of heterogeneity suggests that there is substantial heterogeneity between the studies included in the subgroup meta-analysis for NLR (I2 = 82.152; Tau2 = 0.085; Cochran’s Q = 145.674).
Figure 3.
Forest plot of NLR subgroup.
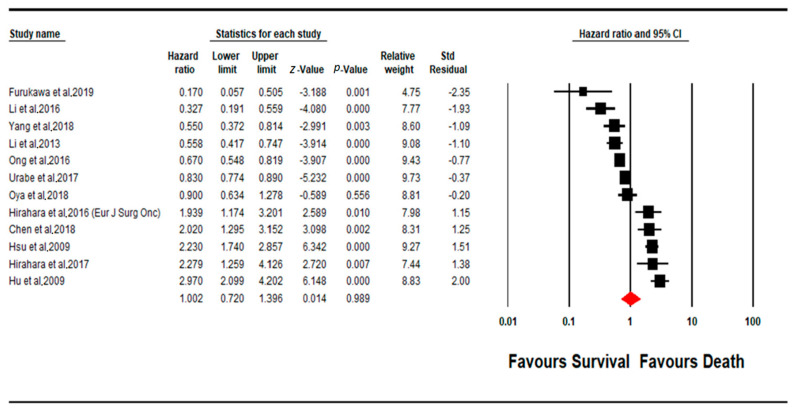
4.3. Meta-Analysis MLR Subgroup
Fewer of the included studies focused on MLR as a prognostic marker, in comparison to PLR and NLR, with a total of only 12 studies being available for inclusion in the assessment of the prognostic efficacy of MLR (Figure 4). In contrast to PLR and NLR, in MLR, the majority of the studies (n = 7) showed a negative correlation between increased MLR levels and a worse disease outcome (poorer prognosis), which indicates an increase in patient survival (only 1 study out of the aforementioned 7 had a non-significant effect size, p < 0.05). However, the rest of the included studies (n = 5) instead showed a contradictory result, where all five studies indicated a statistically significant effect of MLR levels of poorer patient prognosis (p < 0.05). Overall, the pooled effect estimate (HR) was found to not be statistically significant, with a pooled HR value of 1.002 (95% CI 0.720–1.396; p = 0.989). Assessment of heterogeneity suggests that there is substantial heterogeneity between the studies included in the subgroup meta-analysis for PLR (I2 =93.902; Tau2 = 0.292; Cochran’s Q = 180.381).
Figure 4.
Forest plot of MLR subgroup.
4.4. Publication Bias
The Eggers’ graphical test was used to assess possible publication bias. The funnel plot was constructed using the same software used to conduct the meta-analysis, CMA (Ver 3.3.070; Biostat, Englewood, NJ, USA). The funnel plots are visualized in Figures (X), (Y) and (Z) for the subgroups, PLR, NLR and MLR, respectively. Orwin’s fail-safe N test was applied the publication bias assessment for the PLR and NLR subgroups, for the imputation of multiple missing studies. The funnel plot indicates the presence of publication bias in the PLR and NLR subgroups, which is observed in the significant skew in the distribution of studies along the line of mean effect. However, no significant publication bias was observed in the MLR subgroup.
4.5. Quality Assessment
The Newcastle–Ottawa scale (NOS) was used to assess the quality of all included studies. All included studies were found to have quality greater than 2 stars on the assessment scale, which was deemed to be satisfactory for the inclusion of said studies in the meta-analysis. Regardless, the main requirement of inclusion in the study was the availability of good-quality, extractable statistical data from each individual study.
5. Discussion
The current systematic review and meta-analysis were conducted to scrutinize and explore the prognostic potential of PLR, NLR and MLR ratios as prognostic markers in cancer. The use of these prognostic markers has progressed due to the existing established evidence that inflammation can drive cancer growth and progression [3]. In particular, the ease of accessibility, low cost and patient comfort associated with this analysis provide significant benefits if PLR, NLR and MLR are established as clinically reliable cancer prognosticators. Earlier patient cohort studies have investigated the possibility of the use of PLR, NLR and MLR as prognostic markers in cancer [21,22]. However, no comprehensive systematic review or meta-analysis currently exists detailing the comparative efficacy between the three prognostic markers.
A previously published meta-analysis study focused on NLR as a cancer prognostic marker in solid tumors, but did not explore or investigate MLR and PLR as potential prognostic markers in head and neck cancers [23]. Furthermore, while other meta-analysis studies have discussed the efficacy of PLR, NLR and MLR as inflammatory indicators in other chronic conditions, such as infectious diseases and psychosis, their effect in cancer still requires further exploration. While this study looked only at these parameters as predictors of overall outcomes, there remains the possibility that dynamic changes in these parameters during treatment may predict response to treatment. This is especially useful in a tumor such as HNC, especially human papilloma virus (HPV)-negative HNC, because there is currently no universally approved non-invasive and affordable biomarker that serves as a surrogate for tumor burden or treatment response. For HPV-positive cancers, serum levels of HPV DNA show promise as reliable surrogates for treatment response and tumor burden.
This systematic review and meta-analysis study that assessed 49 studies, across nine countries and involving 20,729 patients, investigated the effectiveness of peripheral blood PLR, NLR and MLR ratios in head and neck cancer prognosis. All three of the aforementioned prognostic markers were assessed separately during the meta-analysis as three individual subgroups. It was observed that, overall, more studies had evaluated the prognostic potential of NLR and PLR on patient survival, compared to MLR. The systematic review and meta-analysis study by Mellor et al. serves to validate the findings of this study with regard to PLR as a prognostic marker. While Mellor et al.’s study had a much smaller sample size of included studies (n = 5), for assessing patient OS when compared to this study (n = 25), the results obtained corroborate and lay a foundation for the findings garnered by Mellor et al., where an increase in PLR levels was found to be significantly associated with poor survival [23]. Furthermore, in the meta-analysis study conducted by Zhu et al., they assessed the effectiveness of PLR and NLR as prognostic markers in ovarian cancer [24]. Despite the difference in the types of cancer being investigated, with the current study assessing the prognostic potential of PLR and NLR in HNC and Zhu et al.’s study investigating ovarian cancer, the results of these twin studies indicated poor OS in cancer patients with higher PLR and NLR levels. A study similar to Zhu et al.’s was conducted by Zhang et al., wherein they focused on highlighting the prognostic potential of PLR and NLR in colorectal cancer. Zhang et al.’s study also reached a similar conclusion, with elevated NLR levels being indicative of poor overall survival in their study [21]. Previous studies, such as the systematic review and meta-analysis by Tham et al., indicate that MLR, unlike PLR and NLR, may have a positive prognostic effect, with elevated MLR levels being correlated with an improved OS [93]. The results of another study by Kano et al. also reflect this statement [88]. However, it is important to note that Tham et al.’s study had a small sample size, pooling five studies for OS, while Kano et al.’s study was a singular retrospective study. Interestingly, the studies used by Tham et al. overlap with the seven studies in our meta-analysis, where we observed a similar pattern of MLR leading to an improved OS. However, when considering the larger sample size of our meta-analysis for MLR, with a near equal number of studies (n = 5) showing a statistically significant negative effect of increased MLR levels on patient survival, MLR’s overall effect on OS remains inconclusive.
This study does have a few limitations that warrant being highlighted. The limited quantity of homogeneous, high-quality literature published in this field impeded detailed subgroup analysis based on clinicopathological and demographic criteria. Additionally, not all studies reported the HR and 95% CI values in a numerical form, which required HR and 95% CI values to be extracted from Kaplan–Meier curves for OS presented in these publications. As extracting numerical data from graphical representations involves estimation, there may be some degree of error introduced into the study, which must be considered when applying the results of this study in the clinical sphere.
Overall, this systematic review and meta-analysis study is validated by previous results seen in other studies in the published literature, while simultaneously bringing into question the results of other studies, by building upon them with a much broader scope of approach and a larger pool of literature incorporated into the meta-analysis. Therefore, the results presented in this paper provide evidence to suggest that PLR and NLR ratios may be capable of being used clinically, as prognostic markers in HNCs, and could, in conjunction with the traditional prognostic indicators of cancer, provide a more robust clinical analysis of patient survival and prognosis in HNC. With regard to MLR, however, there appears to be some contention on its effect on survival in HNC, indicating that further research is needed before a conclusive statement on its clinical utility as a prognostic marker in HNC is presented.
6. Conclusions
The study indicates that while PLR and NLR ratios have evidenced potential as prognostic markers for clinical use, particularly in HNC, MLR cannot be currently recommended for clinical use as a prognostic marker. Furthermore, we would like to highlight that the results presented here were obtained from the pooling of multiple individual studies, which each had their own study design, parameters and analysis methods. Therefore, despite the results presented here and previous studies validating these results, the inherent heterogeneity between individual studies being pooled requires that further large-scale clinical studies with a large sample size and a homogeneous approach are conducted before bringing these prognostic markers into clinical practice. Therefore, large-scale, longitudinal patient studies focusing on PLR and NLR as prognostic markers are necessary before they can be incorporated into standard practice as complementary biomarkers to currently existing prognostic markers in cancer. Until then, the results of this systematic review and meta-analysis serve to aid in both clinical decision-making and ongoing and future research in this field.
Acknowledgments
All contributors have been acknowledged in authorship.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13164166/s1, Table S1: PRISMA checklist for systematic reviews and meta-analyses detailing the compliance of study with PRISMA method.
Author Contributions
Conceptualization, C.K. and R.J.; Methodology, C.K.; Software R.J.; Validation, K.S., D.S., S.S., G.K.M., S.B.; Formal Analysis, C.K., R.J.; Investigation, C.K., V.T.; Resources, V.T.; Data Curation, C.K., V.T.; writing—original draft preparation, C.K.; writing—review and editing, K.S., D.S., S.S., G.K.M., S.B.; visualization, R.J.; Supervision, R.J.; project administration, R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was not supported by any external funding.
Conflicts of Interest
All authors involved in the manuscript declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang M., Zhao J., Zhang L., Wei F., Lian Y., Wu Y., Gong Z., Zhang S., Zhou J., Cao K. Role of tumor microenvironment in tumorigenesis. J. Cancer. 2017;8:761. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 3.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y., Wang W.J., Zhi Q., Shen M., Jiang M., Bian X., Gong F.R., Zhou C., Lian L., Wu M.Y., et al. Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget. 2017;8:88835–88844. doi: 10.18632/oncotarget.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akinci Ozyurek B., Sahin Ozdemirel T., Buyukyaylaci Ozden S., Erdogan Y., Kaplan B., Kaplan T. Prognostic Value of the Neutrophil to Lymphocyte Ratio (NLR) in Lung Cancer Cases. Asian Pac. J. Cancer Prev. 2017;18:1417–1421. doi: 10.22034/APJCP.2017.18.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krenn-Pilko S., Langsenlehner U., Thurner E.M., Stojakovic T., Pichler M., Gerger A., Kapp K.S., Langsenlehner T. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br. J. Cancer. 2014;110:2524. doi: 10.1038/bjc.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan M., Jurasz P. The role of platelets in the tumor microenvironment: From solid tumors to leukemia. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016;1863:392–400. doi: 10.1016/j.bbamcr.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Reviews. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMillan D.C. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer: Nutrition Society and BAPEN Medical Symposium on ‘Nutrition support in cancer therapy’. Proc. Nutr. Soc. 2008;67:257–262. doi: 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- 10.Fearon K.C.H. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer. 2008;44:1124–1132. doi: 10.1016/j.ejca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Pardoll D.M., Topalian S.L. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 1998;10:588–594. doi: 10.1016/S0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 12.Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi L., Qin X., Wang H., Xia Y., Li Y., Chen X., Shang L., Tai Y.T., Feng X., Acharya P., et al. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget. 2017;8:18792–18801. doi: 10.18632/oncotarget.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X., Cui F., Yin H., Wu D., Wang N., Yuan M., Fei Y., Wang Q. Association between EGFR mutation and expression of BRCA1 and RAP80 in non-small cell lung cancer. Oncol. Lett. 2018;16:2201–2206. doi: 10.3892/ol.2018.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen E.M., Fan S., Pestell R.G., Goldberg I.D. BRCA1 gene in breast cancer. J. Cell. Physiol. 2003;196:19–41. doi: 10.1002/jcp.10257. [DOI] [PubMed] [Google Scholar]
- 17.Brennan D.J., Gallagher W.M. Prognostic ability of a panel of immunohistochemistry markers—retailoring of an ‘old solution’. Breast Cancer Res. BCR. 2008;10:102. doi: 10.1186/bcr1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mocellin S., Rossi C.R., Pilati P., Nitti D., Marincola F.M. Quantitative real-time PCR: A powerful ally in cancer research. Trends Mol. Med. 2003;9:189–195. doi: 10.1016/S1471-4914(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 19.Ying H.-Q., Deng Q.-W., He B.-S., Pan Y.-Q., Wang F., Sun H.-L., Chen J., Liu X., Wang S.-K. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen L., Zeng H., Yang J., Lu Y., Zhang D., Wang J., Kuang C., Zhu S., Wang M., Ma X. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer. 2018;18:816. doi: 10.1186/s12885-018-4730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J., Zhang H.Y., Li J., Shao X.Y., Zhang C.X. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: A systematic review and meta-analysis. Oncotarget. 2017;8:68837–68846. doi: 10.18632/oncotarget.18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solmaz S., Uzun O., Acar C., Sevindik O.G., Piskin O., Ozsan H.G., Demirkan F., Undar B., Alacacioglu A., Ozcan M.A. Is the platelet-to-lymphocyte ratio a new prognostic marker in multiple myeloma? J. Lab. Physicians. 2018;10:363. doi: 10.4103/JLP.JLP_36_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellor K.L., Powell A.G.M.T., Lewis W.G. Systematic Review and Meta-Analysis of the Prognostic Significance of Neutrophil-Lymphocyte Ratio (NLR) After R0 Gastrectomy for Cancer. J. Gastrointest. Cancer. 2018;49:237–244. doi: 10.1007/s12029-018-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y., Zhou S., Liu Y., Zhai L., Sun X. Prognostic value of systemic inflammatory markers in ovarian Cancer: A PRISMA-compliant meta-analysis and systematic review. BMC Cancer. 2018;18:443. doi: 10.1186/s12885-018-4318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumarasamy C., Sabarimurugan S., Madurantakam R.M., Lakhotiya K., Samiappan S., Baxi S., Nachimuthu R., Gothandam K.M., Jayaraj R. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer—A protocol for systematic review and meta-analysis. Medicine. 2019;98:e14834. doi: 10.1097/MD.0000000000014834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells G.A., Shea B., O’connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute; Ottawa, ON, Canada: 2009. [Google Scholar]
- 28.Jayaraj R., Kumarasamy C., Madhav M.R., Pandey V., Sabarimurugan S., Ramesh N., Gothandam K.M., Baxi S. Comment on “Systematic Review and Meta-Analysis of Diagnostic Accuracy of miRNAs in Patients with Pancreatic Cancer”. Dis. Markers. 2018;2018:2. doi: 10.1155/2018/6904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayaraj R., Kumarasamy C. Comment on ‘Prognostic biomarkers for oral tongue squamous cell carcinoma: A systematic review and meta-analysis’. Br. J. Cancer. 2018;118:e11. doi: 10.1038/bjc.2017.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayaraj R., Kumarasamy C.J.O.O. Comment on, “Survival for HPV-positive oropharyngeal squamous cell carcinoma with surgical versus non-surgical treatment approach: A systematic review and meta-analysis”. Oral. Oncol. 2018;90:137–138. doi: 10.1016/j.oraloncology.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Jayaraj R., Kumarasamy C., Sabarimurugan S., Baxi S.J.F.i.P. Commentary: Blood-Derived microRNAs for Pancreatic Cancer Diagnosis: A Narrative Review and Meta-Analysis. Front. Physiol. 2018;9:1896. doi: 10.3389/fphys.2018.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayaraj R., Kumarasamy C.J.O.O. Conceptual interpretation of analysing and reporting of results on systematic review and meta-analysis of optimal extent of lateral neck dissection for well-differentiated thyroid carcinoma with metastatic lateral neck lymph nodes. Oral. Oncol. 2019;89:153–154. doi: 10.1016/j.oraloncology.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Borenstein M., Rothstein H. Comprehensive Meta-Analysis. Biostat; 1999. [(accessed on 17 August 2021)]. Available online: https://www.meta-analysis.com/downloads/Meta-Analysis%20Manual%20V3.pdf. [Google Scholar]
- 34.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 35.Jayaraj R., Kumarasamy C., Gothandam K.M. Letter to the editor “Prognostic value of microRNAs in colorectal cancer: A meta-analysis”. Cancer Manag. Res. 2018;10:3501–3503. doi: 10.2147/CMAR.S177875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayaraj R., Kumarasamy C.J.O.o. Letter to the Editor about the Article: “Performance of different imaging techniques in the diagnosis of head and neck cancer mandibular invasion: A systematic review and meta-analysis”. Oral. Oncol. 2018;89:159–160. doi: 10.1016/j.oraloncology.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Jayaraj R., Kumarasamy C., Sabarimurugan S., Baxi S. Letter to the Editor in response to the article,” The epidemiology of oral human papillomavirus infection in healthy populations: A systematic review and meta-analysis”. Oral. Oncol. 2018;84:121–122. doi: 10.1016/j.oraloncology.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Jayaraj R., Kumarasamy C., Samiappan S., Swaminathan P. Letter to the Editor regarding, “The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis”. Oral. Oncol. 2018 doi: 10.1016/j.oraloncology.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Jayaraj R., Kumarasamy C., Madurantakam Royam M., Devi A., Baxi S. Letter to the editor: Is HIF-1α a viable prognostic indicator in OSCC? A critical review of a meta-analysis study. World J. Surg. Oncol. 2018;16:111. doi: 10.1186/s12957-018-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ Br. Med. J. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Hippel P.T. The heterogeneity statistic I (2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015;15:35. doi: 10.1186/s12874-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11:193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 43.Deeks J.J., Higgins J.P.T., Altman D.G. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; Hoboken, NJ, USA: 2008. Analysing data and undertaking meta-analyses; pp. 243–296. (Cochrane Book Series). [Google Scholar]
- 44.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orwin R.G. A fail-safe N for effect size in meta-analysis. J. Educ. Stat. 1983;8:157–159. doi: 10.2307/1164923. [DOI] [Google Scholar]
- 46.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 47.Wen J., Bedford M., Begum R., Mitchell H., Hodson J., Whiting J., Griffiths E. The value of inflammation based prognostic scores in patients undergoing surgical resection for oesophageal and gastric carcinoma. J. Surg. Oncol. 2018;117:1697–1707. doi: 10.1002/jso.25057. [DOI] [PubMed] [Google Scholar]
- 48.Kawakita D., Tada Y., Imanishi Y., Beppu S., Tsukahara K., Kano S., Ozawa H., Okami K., Sato Y., Shimizu A. Impact of hematological inflammatory markers on clinical outcome in patients with salivary duct carcinoma: A multi-institutional study in Japan. Oncotarget. 2017;8:1083. doi: 10.18632/oncotarget.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C., Sun P., Dai Q.-s., Weng H.-w., Li H.-p., Ye S. The Glasgow Prognostic Score predicts poor survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. PLoS ONE. 2014;9:e112581. doi: 10.1371/journal.pone.0112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang F., Chen Z., Wang P., Hu X., Gao Y., He J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumor Biol. 2016;37:9323–9331. doi: 10.1007/s13277-015-4774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang K., Lei J., Li C., Shu K., Li W., Zhang Y., Li Z., Gong R., Zhu J. Comparison of the prognostic values of selected inflammation based scores in patients with medullary thyroid carcinoma: A pilot study. J. Surg. Oncol. 2017;116:281–287. doi: 10.1002/jso.24683. [DOI] [PubMed] [Google Scholar]
- 52.Li X.H., Chang H., Xu B.Q., Tao Y.L., Gao J., Chen C., Qu C., Zhou S., Liu S.R., Wang X.H. An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: An analysis of a prospective study. Cancer Med. 2017;6:310–319. doi: 10.1002/cam4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng J.-F., Huang Y., Chen Q.-X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J. Surg. Oncol. 2014;12:1–6. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang R., Zou X., Hu W., Fan Y.-Y., Yan Y., Zhang M.-X., You R., Sun R., Luo D.-H., Chen Q.-Y. The elevated pretreatment platelet-to-lymphocyte ratio predicts poor outcome in nasopharyngeal carcinoma patients. Tumor Biol. 2015;36:7775–7787. doi: 10.1007/s13277-015-3505-0. [DOI] [PubMed] [Google Scholar]
- 55.Jung J., Park S.Y., Park S.-j., Park J. Prognostic value of the neutrophil-to-lymphocyte ratio for overall and disease-free survival in patients with surgically treated esophageal squamous cell carcinoma. Tumor Biol. 2016;37:7149–7154. doi: 10.1007/s13277-015-4596-3. [DOI] [PubMed] [Google Scholar]
- 56.He Y.F., Luo H.Q., Wang W., Chen J., Yao Y.W., Yan Y., Wu S.S., Hu X.X., Ke L.H., Niu J.Y. Preoperative NLR and PLR in the middle or lower ESCC patients with radical operation. Eur. J. Cancer Care. 2017;26:e12445. doi: 10.1111/ecc.12445. [DOI] [PubMed] [Google Scholar]
- 57.Jiang K., Lei J., Chen W., Gong Y., Luo H., Li Z., Gong R., Zhu J. Association of the preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios with lymph node metastasis and recurrence in patients with medullary thyroid carcinoma. Medicine. 2016;95:e5079. doi: 10.1097/MD.0000000000005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirahara N., Tajima Y., Fujii Y., Yamamoto T., Hyakudomi R., Hirayama T., Taniura T., Ishitobi K., Kidani A., Kawabata Y. A novel prognostic scoring system using inflammatory response biomarkers for esophageal squamous cell carcinoma. World J. Surg. 2018;42:172–184. doi: 10.1007/s00268-017-4144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong H.S., Gokavarapu S., Wang L.Z., Tian Z., Zhang C.P. Low pretreatment lymphocyte-monocyte ratio and high platelet-lymphocyte ratio indicate poor cancer outcome in early tongue cancer. Int. J. Oral Maxillofac. Surg. 2017;75:1762–1774. doi: 10.1016/j.joms.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 60.Mao Y., Fu Y., Gao Y., Yang A., Zhang Q. Platelet-to-lymphocyte ratio predicts long-term survival in laryngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2018;275:553–559. doi: 10.1007/s00405-017-4849-4. [DOI] [PubMed] [Google Scholar]
- 61.Dutta S., Crumley A.B.C., Fullarton G.M., Horgan P.G., McMillan D.C. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. Am. J. Surg. 2012;204:294–299. doi: 10.1016/j.amjsurg.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Li J.-P., Chen S.-L., Liu X.-M., He X., Xing S., Liu Y.-J., Lin Y.-H., Liu W.-L. A novel inflammation-based stage (I Stage) predicts overall survival of patients with nasopharyngeal carcinoma. Int. J. Mol. Sci. 2016;17:1900. doi: 10.3390/ijms17111900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Messager M., Neofytou K., Chaudry M.A., Allum W.H. Prognostic impact of preoperative platelets to lymphocytes ratio (PLR) on survival for oesophageal and junctional carcinoma treated with neoadjuvant chemotherapy: A retrospective monocentric study on 153 patients. Eur. J. Surg. Oncol. 2015;41:1316–1323. doi: 10.1016/j.ejso.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Hirahara N., Matsubara T., Kawahara D., Nakada S., Ishibashi S., Tajima Y. Prognostic significance of preoperative inflammatory response biomarkers in patients undergoing curative thoracoscopic esophagectomy for esophageal squamous cell carcinoma. Eur. J. Surg. Oncol. 2017;43:493–501. doi: 10.1016/j.ejso.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Moon H., Roh J.-L., Lee S.-w., Kim S.-B., Choi S.-H., Nam S.Y., Kim S.Y. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother. Oncol. 2016;118:330–334. doi: 10.1016/j.radonc.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 66.Hirahara N., Matsubara T., Mizota Y., Ishibashi S., Tajima Y. Prognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomy. BMC Surg. 2016;16:1–12. doi: 10.1186/s12893-016-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turri–Zanoni M., Salzano G., Lambertoni A., Giovannardi M., Karligkiotis A., Battaglia P., Castelnuovo P. Prognostic value of pretreatment peripheral blood markers in paranasal sinus cancer: Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio. Head Neck. 2017;39:730–736. doi: 10.1002/hed.24681. [DOI] [PubMed] [Google Scholar]
- 68.Xie X., Luo K.J., Hu Y., Wang J.Y., Chen J. Prognostic value of preoperative platelet–lymphocyte and neutrophil–lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis. Esophagus. 2016;29:79–85. doi: 10.1111/dote.12296. [DOI] [PubMed] [Google Scholar]
- 69.Bojaxhiu B., Templeton A.J., Elicin O., Shelan M., Zaugg K., Walser M., Giger R., Aebersold D.M., Dal Pra A. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat. Oncol. 2018;13:1–9. doi: 10.1186/s13014-018-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun P., Chen C., Xia Y., Bi X., Liu P., Zhang F., Yang H., An X., Jiang W., Wang F. The ratio of C-reactive protein/albumin is a novel inflammatory predictor of overall survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. Dis. Markers. 2017;2017:6570808. doi: 10.1155/2017/6570808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun W., Zhang L., Luo M., Hu G., Mei Q., Liu D., Long G., Hu G. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck. 2016;38:E1332–E1340. doi: 10.1002/hed.24224. [DOI] [PubMed] [Google Scholar]
- 72.Tangthongkum M., Tiyanuchit S., Kirtsreesakul V., Supanimitjaroenporn P., Sinkitjaroenchai W. Platelet to lymphocyte ratio and red cell distribution width as prognostic factors for survival and recurrence in patients with oral cancer. Eur. Arch. Oto-Rhino-Laryngol. 2017;274:3985–3992. doi: 10.1007/s00405-017-4734-1. [DOI] [PubMed] [Google Scholar]
- 73.Ozturk K., Akyildiz N.S., Uslu M., Gode S., Uluoz U. The effect of preoperative neutrophil, platelet and lymphocyte counts on local recurrence and survival in early-stage tongue cancer. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:4425–4429. doi: 10.1007/s00405-016-4098-y. [DOI] [PubMed] [Google Scholar]
- 74.Toyokawa T., Kubo N., Tamura T., Sakurai K., Amano R., Tanaka H., Muguruma K., Yashiro M., Hirakawa K., Ohira M. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC Cancer. 2016;16:1–11. doi: 10.1186/s12885-016-2696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urabe M., Yamashita H., Watanabe T., Seto Y. Comparison of prognostic abilities among preoperative laboratory data indices in patients with resectable gastric and esophagogastric junction adenocarcinoma. World J. Surg. 2018;42:185–194. doi: 10.1007/s00268-017-4146-9. [DOI] [PubMed] [Google Scholar]
- 76.Wang K.-f., Chang B.-y., Chen X.-q., Liu P.-p., Wuxiao Z.-j., Wang Z.-h., Li S., Jiang W.-q., Xia Z.-j. A prognostic model based on pretreatment platelet lymphocyte ratio for stage IE/IIE upper aerodigestive tract extranodal NK/T cell lymphoma, nasal type. Med. Oncol. 2014;31:1–7. doi: 10.1007/s12032-014-0318-8. [DOI] [PubMed] [Google Scholar]
- 77.Wei X.-l., Wang F.-h., Zhang D.-s., Qiu M.-z., Ren C., Jin Y., Zhou Y.-x., Wang D.-s., He M.-m., Bai L. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: The C-reactive protein/albumin ratio. BMC Cancer. 2015;15:1–11. doi: 10.1186/s12885-015-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu X.-L., Yu H.-Q., Hu W., Song Q., Mao W.-M. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS ONE. 2015;10:e0138657. doi: 10.1371/journal.pone.0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y., Xu H., Zhou L., Deng T., Ning T., Liu R., Zhang L., Wang X., Ge S., Li H. Platelet to lymphocyte ratio is a predictive marker of prognosis and therapeutic effect of postoperative chemotherapy in non-metastatic esophageal squamous cell carcinoma. Clin. Chim. Acta. 2018;479:160–165. doi: 10.1016/j.cca.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 80.Ye L., Oei R.W., Kong F., Xu T., Shen C., Wang X., He X., Kong L., Hu C., Ying H. Prognostic values of hematological biomarkers in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Eur. Arch. Oto-Rhino-Laryngol. 2018;275:1309–1317. doi: 10.1007/s00405-018-4956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan D., Zhu K., Li K., Yan R., Jia Y., Dang C. The preoperative neutrophil–lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J. Surg. Oncol. 2014;110:333–340. doi: 10.1002/jso.23651. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L., Su Y., Chen Z., Wei Z., Han W., Xu A. The prognostic value of preoperative inflammation-based prognostic scores and nutritional status for overall survival in resected patients with nonmetastatic Siewert type II/III adenocarcinoma of esophagogastric junction. Medicine. 2017;96:e7647. doi: 10.1097/MD.0000000000007647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsu W.H., Hsu P.K., Hsieh C.C., Huang C.S., Wu Y.C. The metastatic lymph node number and ratio are independent prognostic factors in esophageal cancer. J. Gastrointest. Surg. 2009;13:1913–1920. doi: 10.1007/s11605-009-0982-8. [DOI] [PubMed] [Google Scholar]
- 84.Chien H.-C., Chen H.-S., Wu S.-C., Hsu P.-K., Liu C.-Y., Wang B.-Y., Shih C.-H., Liu C.-C. The prognostic value of metastatic lymph node number and ratio in oesophageal squamous cell carcinoma patients with or without neoadjuvant chemoradiation. Eur. J. Cardiothorac. Surg. 2016;50:337–343. doi: 10.1093/ejcts/ezw016. [DOI] [PubMed] [Google Scholar]
- 85.Furukawa K., Kawasaki G., Naruse T., Umeda M. Prognostic Significance of Pretreatment Lymphocyte–to–Monocyte Ratio in Patients with Tongue Cancer. Anticancer Res. 2019;39:405. doi: 10.21873/anticanres.13126. [DOI] [PubMed] [Google Scholar]
- 86.Hsueh C., Tao L., Zhang M., Cao W., Gong H., Zhou J., Zhou L. The prognostic value of preoperative neutrophils, platelets, lymphocytes, monocytes and calculated ratios in patients with laryngeal squamous cell cancer. Oncotarget. 2017;8:60514–60527. doi: 10.18632/oncotarget.16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Y., Feng J.-F. Low preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. OncoTargets Ther. 2015;8:137–145. doi: 10.2147/OTT.S73794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kano S., Homma A., Hatakeyama H., Mizumachi T., Sakashita T., Kakizaki T., Fukuda S. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck. 2017;39:247–253. doi: 10.1002/hed.24576. [DOI] [PubMed] [Google Scholar]
- 89.Li J., Jiang R., Liu W.-S., Liu Q., Xu M., Feng Q.-S., Chen L.-Z., Bei J.-X., Chen M.-Y., Zeng Y.-X. A Large Cohort Study Reveals the Association of Elevated Peripheral Blood Lymphocyte-to-Monocyte Ratio with Favorable Prognosis in Nasopharyngeal Carcinoma. PLoS ONE. 2013;8:e83069. doi: 10.1371/journal.pone.0083069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu J.-S., Huang Y., Yang X., Feng J.-F. A nomogram to predict prognostic values of various inflammatory biomarkers in patients with esophageal squamous cell carcinoma. Am. J. Cancer Res. 2015;5:2180–2189. [PMC free article] [PubMed] [Google Scholar]
- 91.Oya R., Takenaka Y., Aoki K., Hamaguchi H., Takemura K., Nozawa M., Kitamura T., Yamamoto Y., Uno A. Prognostic Significance of Hematologic Markers in Patients with Head and Neck Squamous Cell Carcinomas. Int. J. Otorhinolaryngol. Head Neck Surg. 2018;7:55–65. doi: 10.4236/ijohns.2018.72008. [DOI] [Google Scholar]
- 92.Yang J., Hsueh C.-Y., Cao W., Zhou L. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for hypopharyngeal squamous cell carcinoma. Acta Otolaryngol. 2018;138:734–740. doi: 10.1080/00016489.2018.1449965. [DOI] [PubMed] [Google Scholar]
- 93.Tham T., Olson C., Khaymovich J., Herman S.W., Costantino P.D. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: A systematic review and meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2018;275:1663–1670. doi: 10.1007/s00405-018-4972-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.