Abstract
Recurrent pregnancy loss (RPL), pre-eclampsia (PE), fetal growth restriction (FGR), and preterm delivery are examples of ‘great obstetrical syndromes’ (GOS). Placental dysfunction is the most common pathogenesis of GOS. In human pregnancies, the effects of uterine natural killer cells involve angiogenesis, promoting the remodeling of uterine spiral artery, and improving the invasion of trophoblast cells. The uNK cells supply killer immunoglobulin-like receptors (KIRs), which come into contact with human leukocyte antigen-C (HLA-C) ligands expressed by extravillous trophoblast cells (EVTs). Numerous studies have investigated the association between GOS and KIR/HLA-C combination. However, the outcomes have not been conclusive. The present review aimed to reveal the association between GOS and KIR/HLA-C combination to screen out high-risk pregnancies, strengthen the treatment of pregnancy complications, and reduce the frequency of adverse maternal and fetal outcomes. It has been reported that a female with a KIR AA genotype and a neonate with a paternal HLA-C2 molecule is more prone to develop GOS and have a small fetus since less cytokines were secreted by uNK cells. Conversely, the combination of KIR BB haplotype (including the activating KIR2DS1) and HLA-C2 can induce the production of cytokines and increase trophoblast invasion, leading to the birth of a large fetus. KIR/HLA-C combinations may be applicable in selecting third-party gametes or surrogates. Detection of maternal KIR genes and HLA-C molecules from the couple could serve as useful markers for predicting and diagnosing GOS.
Keywords: KIR, HLA-C, recurrent pregnancy loss, pre-eclampsia, fetal growth restriction, great obstetrical syndromes
1. Introduction
Nearly 10% of all global diseases are due to pregnancy complications (1). Recurrent pregnancy loss (RPL), pre-eclampsia (PE), fetal growth restriction (FGR), and preterm delivery are examples of ‘great obstetrical syndromes’ (GOS) (2). Placental dysfunction is the most common pathogenesis of GOS (3). The placenta starts to form once implantation begins. The extravillous trophoblast cells (EVTs) form the endometrium and move into the decidua. The process of decidualization requires the morphological and functional changes of uterine stromal cells and involvement of immune cells. Immune cells account for nearly 40% of the decidual cells (4). The immune cells located in the myometrium and decidua come into contact with EVT cells. The removal of immune cells from the area of implantation or impeding their signaling pathway could lead to miscarriages (5,6).
From the window of implantation to the first trimester, most immune cells at the maternal-fetal interface are uterine natural killer (uNK) cells (70%) (1). An increasing number of studies have focused on the importance uNK cells in preserving the pregnancy (7-9). In normal human pregnancies, the effects of uNK cells involve angiogenesis, promoting the remodeling of uterine spiral artery, and improving the invasion of trophoblast cells (10-13). Moreover, uNK cells supply killer immunoglobulin-like receptors (KIRs), which come into contact with human leukocyte antigen-C (HLA-C) ligands expressed by EVTs. The parents influence the HLA-C genotypes in trophoblasts (14). Since KIR and HLA-C are both polymorphic, the bindings of KIR and HLA-C differ from one pregnancy to another. Numerous studies have investigated the association between GOS and KIR/HLA-C combination [for example, RPL (15-19), PE (20-23), and FGR (14)]. However, the outcomes have not been conclusive.
Studies have indicated the deficiency of activation as the reason for RPL, which is influenced by the improper binding of KIR/HLA-C (16.24,25). The KIR/HLA-C combination modulates the placental development physiologically. It contradicts the traditional idea that the mother has an immune defense response to the fetus (20). Specific KIR/HLA-C binding occurs between maternal NK cells and EVT cells in the decidua. This binding addresses activated tissue-remodeling issues occurring in implantation and placentation. The present review aimed to reveal the association between GOS and a combination of KIR/HLA-C, which may aid high-risk pregnancy screening, strengthen the treatment of pregnancy complications, and reduce the frequency of adverse maternal and fetal outcomes. Related articles were searched independently in PUBMED by two academics using the medical subject headings: ‘KIR’, ‘HLA-C’, ‘recurrent pregnancy loss’, ‘recurrent miscarriages’, ‘pre-eclampsia’, ‘fetal growth restriction’, or ‘pregnancy’. All articles were published from January 2003 to November 2020 in English since there are few related articles published before 2003. If a relationship exists between the HLA-C haplotype and KIR, it may be useful to use the KIR genotype and HLA-C molecule to select gametes from donors with favorable combinations.
2. uNK cells and their receptors
During the first trimester of pregnancy, the decrease of trophoblast invasion and poor recasting of uterine spiral artery affect the blood flow in placenta, resulting in pregnancy loss. NK cells are vital for the placental formation and fetal development during implantation and the initial stage of pregnancy. NK cells contain CD16 markers, which mediate antibody-dependent cell-mediated cytotoxicity (ADCC). In general, CD56dim NK cells belong to CD16+. CD16+CD56dim NK cells have cytotoxicity, while CD16-CD56bright NK cells exhibit immunomodulatory effects (26). Nearly 90% of uNK cells belong to the latter type. Hence, uNK cells do not harm the fetus (27). Numerous possibilities have been proposed about the origin of decidual NK (dNK) cells; i) peripheral NK cells flow into the uterus; ii) uterine stem cells differentiate into dNK cells; iii) from bone marrow; iv) endometrial NK cells in the lining form dNK cells by development and transformation (28). The dNK cells can produce numerous cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), chemokine C motif ligand (XCL) 1, angiopoietin (Ang) 1, Ang 2, vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-β1), pleiotrophin and osteoglycin, which play essential roles in sustaining uterine blood flow and fetal development (10,13,29-32).
NK cell receptors include the immunoglobulin-like transcripts (LILRB), C-type lectin heterodimer family (CD94:NKGs) and KIRs (33). The inhibiting NKG2A and activated NKG2C receptors are classified under the family of CD94:NKGs, which could distinguish and combine with HLA-E (34). HLA-G can discern the inhibiting receptor LILRB1(35). EVT cells supply class I HLA-C, non-classic HLA-G, and HLA-E antigens (36,37). However, only the polymorphic HLA-C ligands combine with KIRs expressed by uNK cells (33). The process of placentation is affected by KIRs and HLA-C allotypes (15).
3. NK education and KIR/HLA-C combination
Previously, studies involving mice (38) and humans (39-41) have indicated that NK cells may experience ‘education’ and ‘licensing’ with the combined specific KIR and cognate HLA-C allotypes to be effective. One likely scenario includes KIRs from the mother interacting with her own HLA-C allotypes during the growth of uNK cells, following this, ‘educating’ or ‘licensing’ the uNK cells of the mother and modifying the method of interaction with HLA-C allotypes of her child in the formation of the placenta (14,42). However, how dNK cells educate, license, or modulate in the uterus is yet to be established. Considering NK cell education experiments, it has been revealed that specific CD56bright NK cells react to interleukin (IL)-12 or IL-15, and the licensing function was not strong in resting NK cells, which implies that certain scenarios may neglect the licensing needs (39). The dNK cells exhibit an abundance of CD56(43), and release receptors and respond to IL-15(44). Evidently, invasive trophoblast cells are likely to increase the accumulation of dNK cells to the maternal-fetal interface via the production of different cytokines (13,45,46). Regarding the education mechanism of NK cells in the association between GOS and combined KIR/HLA-C, certain inhibiting KIRs and HLA-C molecules from the father may lead to pregnancy complications.
4. KIR family and HLA-C in pregnancy
The expression levels of KIR members partially influence the response of NK cells. The polymorphic KIRs include both activating and inhibitory receptors located on chromosome 19q13.4.11 in humans (47). KIRs are extraordinarily polymorphic, and the types of genes located in the KIR locus change prominently across different people and regions. The phenotype of dNK cells renders them more likely to express higher levels of KIRs specific for HLA-C molecules than what appears in peripheral blood for the same individual (48). For each pregnancy, the maternal KIR genes could be AA or Bx (47). KIR AA haplotype is short of the majority of activating KIRs, except KIR2DS4. However, the KIR Bx haplotype contains multiple activating KIRs (49,50). AB and BB haplotypes are both included in KIR Bx genotype (51). HLA-C molecules are ligands for KIRs, which are sorted into two types. HLA-C molecule with the C1 epitope (with asparagine at location 80) combines with inhibiting KIR2DL2 and KIR2DL3. However, HLA-C molecule with C2 epitope (with lysine at location 80) connects with inhibiting KIR2DL1 and activating KIR2DS1. C2 is a more potent ligand than C1(52). Despite both HLA-C1 and HLA-C2 combining with KIR genes, the degree of the downstream uNK activity is fundamentally affected through C1 and C2 zygosity in a specific pregnancy (53).
The KIR genotype appears comparatively later during the growth of NK cells. These NK cells are induced by IL-15, which is secreted by stromal cells to respond to progesterone in the uterus (38). The uNK cells may originate from the uterine progenitor cells instead of peripheral blood NK cells. Hence, the KIR gene is changeable through semaphores appearing in specific locations (54). The number of dNK cells during early pregnancy exhibits a higher percentage of NK cells delivering KIRs that can combine with HLA-C compared with the peripheral NK cells and with uNK cells that originated from the endometrium of non-pregnant females (55), implying that HLA-C molecules on EVTs may be crucial for NK cell education in the uterus.
Pregnancy is a unique physiological status, where the particular non-self HLA molecules of KIRs could encounter their cognate ligands. Polymorphic KIR/HLA-C binding has evolved partially due to birth pressures occurring with human evolution and the enlargement of brain tissues (42). Some bindings of KIR/HLA-C could be unfavorable for a normal pregnancy than others. Activating KIRs have protective effects during pregnancy complications, however, people with activating KIRs are susceptible to other autoimmune diseases (56). Insufficient activation of NK cells may cause adverse maternal and neonatal outcomes (57). If uNK cells gain an activating signal in a female having a KIR B haplotype (including KIR2DS1) and HLA-C2, the secretion of GM-CSF increases, which can enhance the ability of migration and invasion of trophoblast cells in cell lines (13).
5. KIR/HLA-C combination and GOS
KIR/HLA-C combination and RPL
RPL refers to the loss of embryos before twenty weeks of gestation in three or more pregnancies; however, the American Society for Reproductive Medicine (ASRM) defines it as two times or more (58). RPL is caused by numerous factors, such as chromosomal abnormalities, genetic factors (26), anatomical factors, endocrine abnormalities, and immune factors. Amidst these, the etiology of certain patients is unknown and may be related to autoimmune abnormalities. The correlation between KIR/HLA-C combination and RPL is listed in Table I, a few are inconsistent (14-17,19,25,26,59-65). Reportedly, RPL females had an elevated rate of maternal KIR AA haplotype and fetal HLA-C2 binding (14,15,60,66). However, other empirical results were different (16-19). A recent study reported a higher frequency of abortion after double embryo transfers (DETs) in KIR AA females compared with KIR AB or KIR BB (24). The genotype KIR AA has an inhibitory effect, indicating the essentialness of activation of uNK cells in early pregnancy. However, in a research from northern India, it was revealed that RPL females had an increased possibility of B haplotype rather than A haplotype (53). Likewise, it was reported that the KIR AA genotype has a protective effect on pregnant women (16). However, there were some shortcomings in this report; the sample size of the study was small (n=40), and the abortion group was defined as a single spontaneous abortion (16). Furthermore, Nowak et al (17) indicated that KIR AA and HLA-C1C2 females with HLA-C2C2 husbands had a higher probability of a normal pregnancy. Pregnancies with C1/C1 fetuses lead to a 2.5 times lower abortion rate relative to C2/C2 pregnancies (53). The higher rate of successful pregnancies of C1/C1 babies did not emerge when the transfer happened on an individual with a KIR B haplotype (53).
Table I.
Experiments on the association between KIR/HLA-C combination and recurrent pregnancy loss.
| Nationality | Authors (Refs.) | Year of publication | Experimental group | Control group | Samples | Findings |
|---|---|---|---|---|---|---|
| Greek | Varla-Leftherioti et al (59) | 2003 | RPL couples (n=26) | Fertile couples (n=26) | Peripheral blood | RPL women had decreased inhibiting KIR genes |
| Brazilian Caucasian | Witt et al (19) | 2004 | RPL women (n=51) | Fertile women (n=55) | NA | KIR genes did not change between RPL patients and controls |
| Northern Irish | Flores et al (60) | 2007 | RPL couples (n=88) | Fertile couples (n=30) and healthy controls (n=139) | NA | RPL patients had decreased KIR2DL2; the KIR AA genotype was more common in RPL women |
| Chinese | Wang et al (61) | 2007 | RPL couples (n=73) | Healthy couples (n=68) | Peripheral blood | RPL patients had increased numbers of activating KIR genes including KIR2DS1 |
| European | Hiby et al (15) | 2008 | RPL women (n=95) and their partners (n=67) | Fertile women (n=269) | Peripheral blood | RPL women had a higher frequency of KIR AA haplotype and decreased KIR2DS1 |
| Indian | Faridi et al (25) | 2009 | RPL women (n=205) | Healthy women (n=224) | Peripheral blood | RPL patients had more activating KIR genes and they tended to be BB genotypes |
| South Brazilian Caucasian | Vargas et al (62) | 2009 | RPL couples (n=68) | Fertile couples (n=68) | Peripheral blood | Females having more activating KIR genes were three times more likely to develop RPL |
| European | Hiby et al (14) | 2010 | RPL women (n=115) and their partners (n=81) | Fertile couples (n=592) | Placental tissues and maternal blood | RPL patients were less frequent in women with a KIR B haplotype |
| Polish | Nowak et al (17) | 2011 | RPL couples (n=125) | Fertile couples (n=117) | Peripheral blood | Among KIR AA females who had HLA-C2C2 partners, HLA-C1C2 females tended to have a normal pregnancy |
| Turkish | Ozturk et al (16) | 2012 | RPL women (n=40) | Fertile women (n=90) | Peripheral blood | RPL patients tended to be KIR Bx genotypes |
| Chinese | Wang et al (63) | 2014 | RPL women (n=30) | Fertile women (n=30) | Decidual tissues | There was decreased frequency of CD56+ CD16− natural killer cell staining for KIR2DL1/ S1 and KIR2DL2/S2/L3 in RPL women |
| American | Dambaeva et al (64) | 2016 | RPL women (n=139) and their partners (n=42) | HLA-C controls: 1,070 North American Caucasian Population (n=1,070) KIR controls: American Caucasian population (n=255) | Peripheral blood | KIR2DS1-positive RPL females had increased HLA-C2 |
| Turkey | Elbaşı et al (65) | 2020 | RPL couples (n=25) | Healthy couples (n=39) | Peripheral blood | HLA-C2C2 were more common in RPL partners; KIR2DL5 gene was more common in RPL couples |
| Caucasian | Yang et al (26) | 2020 | RPL patients (n=160) and their partners (n=99) | HLA-C controls: 1,070 North American Caucasian Population (n=1,070) KIR controls: American Caucasian population (n=255) | Women: peripheral blood; Partners: buccal cells | KIR2DL2-positive RPL females had lower HLA-C1C1, and their partners had increased HLA-C2C2 compared with KIR2DL2 negative RPL patients |
NA, non-analyzed; KIR, killer-cell immunoglobulin-like receptor; HLA, human leukocyte antigen; RPL, recurrent pregnancy loss.
A few empirical studies have indicated that RPL females may lack proper KIR genes (e.g., KIR2DL1 and KIR2DL2) that can combine with HLA-C in trophoblast cells and transfer inhibitory signals for NK cell activity (59,60,67-71). Compared with the normal controls, the levels of KIR2DL1/S1 on CD56+CD16− NK cells in the deciduae of RPL females were reduced (63). HLA-C1 is the ligand for KIR2DL2. Compared with healthy females, RPL patients exhibited higher KIR2DL2 and HLA-C2/C2 or HLA-C2/x combinations (65). Initially, the authors established that KIRs in partners could not directly result in a miscarriage; however, since the fetal KIR genotype is unclear, distributed fetal cells with potentially unfavorable KIRs can accelerate the ending course (65). It was recently reported that KIR2DL2-positive RPL females exhibited decreased molecules of HLA-C1 compared with KIR2DL2-negative RPL females (P<0.05) (26). Reduced ligands for inhibiting KIRs may cause inadequate inhibition of maternal uNK cells to the trophoblast, thus giving rise to RPL.
Some studies have revealed an apparent increase in the frequency of activating KIRs in RPL females (60-62,72). Patients with elevated activation of KIRs were nearly three times more susceptible to RPL (62). KIR2DS2 and KIR2DS3 are both activating KIRs, which are hazardous elements for RPL (73). The KIR genotypes in 205 RPL women and 224 normal subjects were analyzed (25). Accordingly, RPL patients had more activating KIRs than normal controls (25). Similar results were obtained in the Chinese Han population (61), the authors suggested that relative to fertile females, there was a lower rate of C2+ HLA-C molecules in RPL women (61). Reportedly, an elevated rate of KIR2DS2 in HLA-C1 homozygous parents was associated with RPL (16). Consequently, the physiological reason underlying the high frequency of activating KIR genotypes could be attributed to an extensive scope of diverse activating KIR receptors making NK cells to discriminate more activation ligands, which originated from fetal cells. This causes an induced percentage of cytotoxic and apoptotic signals; these fight the inhibiting function of receptors against semiallogeneic fetuses at the maternal-fetal interface and end in abortion.
It has been suggested that KIR2DS1 assumes a critical role in pregnancy, mainly because activation of dNK cells through KIR2DS1 induces the production of soluble mediators, such as GM-CSF. Reportedly, GM-CSF has been confirmed to be useful for placentation and promoting fetal growth (74). Additionally, C2+ HLA-C molecules were revealed to be more common among partners of KIR2DS1+ women in the Caucasian population (64). One possible reason is that NK cell education may lead to the hypo-responsiveness of KIR2DS1+ deciduae, and inhibit the positive effect of KIR2DS1 in a normal pregnancy. Since C2+ HLA-C molecules are supposed to combine with KIR2DS1, it is hard to conclusively elucidate the potential function of activating KIR2DS1 in the absence of its ligand.
On the other hand, in the Brazilian population, there was no difference between KIR genes and susceptibility to RPL (19). Therefore, future studies on the functionality of KIRs and their ligands should include a higher sample size of RPL couples in a specific area compared with fertile controls in the same region. Preferably, genotyping of embryos should also be included.
KIR/HLA-C combination and PE
PE is a severe pregnancy complication; it is characterized by hypertension (≧140/90 mmHg) and proteinuria following twenty weeks of pregnancy. Additionally, the prevalence of PE is 5-8% (75). Since the invasion ability of the trophoblast is affected, inappropriate combinations of KIR/HLA-C are reasons that cause PE. Reportedly, if a woman carries the KIR AA genotype and her baby has HLA-C2 from the father, then the possibility of PE in the pregnancy was increased (20,76) (Table II). This is because KIR AA/HLA-C2 binding may lead to inadequate activation of uNK cells, affecting uterine artery recasting and leading to PE. Furthermore, Hiby et al (14) revealed a higher PE incidence for females whose fetuses inherited another C2 from males. For women with the KIR AA genotype, the pregnancy with fetal HLA-C1C1 is more prone to PE than that of HLA-C1C2 or HLA-C2C2. According to the NK education theory, maternal HLA-C2 molecules educate KIR2DL1+ uNK cells to obtain functional properties during period of rest rather than during an immune response, and counteract the inhibitory input of fetal HLA-C2 molecules in pregnancy (14). A method for estimating KIR2DL1 allotypes coded by the diverse KIR genes has promoted the development of various anti-KIR antibodies and genotyping to indicate that KIR2DL1 on the KIR A instead of the KIR B haplotype is related to the higher probability of PE (77). Mexican researchers collected ten normal pregnant decidua tissues and nine PE decidua samples during cesarean section (22). It was revealed that there were more inhibitory KIRs in deciduae of PE patients than those in normal pregnancies (22). Due to the lack of activating KIRs, the activation signals cannot be provided concurrently. Thus, NK cells in these females exhibited a decreased physiological effect, without adequate support for placental development.
Table II.
Experiments on the relationship between KIR/HLA-C combination and PE.
| Nationality | Authors (Refs.) | Year of publication | Experimental group | Control group | Samples | Findings |
|---|---|---|---|---|---|---|
| British | Hiby et al (20) | 2004 | PE patients and their fetuses (n=200) | Normal pregnant women and their fetuses (n=201) | Maternal blood, cord samples or neonatal mouth swabs | The female with an AA genotype and a fetus with the HLA-C2 molecule tended to have PE |
| British | Hiby et al (14) | 2010 | PE couples (n=742) and their fetuses (n=733) | Normal pregnant couples (n=592) and their fetuses (n=423) | Maternal blood, cord samples or neonatal mouth swabs | There was a higher PE incidence for females whose fetuses inherited another C2 from males |
| Mexican | Sanchez- Rodriguez et al (22) | 2011 | PE patients (n=9) | Normal pregnant women (n=10) | Decidual samples | There were more inhibitory KIRs in deciduae of PE patients than those in normal pregnancies |
| Ugandan | Nakimuli et al (21) | 2015 | PE patients (n=254) | Normal pregnant women (n=484) | Maternal blood, cord samples | KIR2DS5 on Cen-B of Africans protected pregnancies from PE |
| Chinese Han population | Long et al (79) | 2015 | PE patients (n=271) | Normal pregnant women (n=295) | Maternal blood | Reduced KIR activation was observed in PE women |
| British | Huhn et al (77) | 2018 | PE patients (n=693) | Normal pregnant women (n=679) | Maternal blood | KIR2DL1A was associated with increased PE risk |
| Danish | Larsen et al (84) | 2019 | Severe PE patients (n=259) | Normal pregnant women (n=259) | Maternal and neonatal blood | A relationship was not found between maternal KIR genotypes and HLA-C in the fetuses |
| Ethiopian | Kelemu et al (76) | 2020 | PE patients (n=131) | Normal pregnant women (n=157) | Maternal blood, cord samples | There was a significant association between KIR AA genotype and PE |
KIR, killer-cell immunoglobulin-like receptor; HLA, human leukocyte antigen; PE, pre-eclampsia.
Reduced KIR activation was observed in PE women (78,79). In Europe and Ethiopia, the protective function of the maternal B haplotype is regulated by an activating C2 receptor, called KIR2DS1 (20,76,80). It stimulates uNK cells that increase the angiogenesis and immune reaction, which induces a successful pregnancy (81). KIR2DS4 and KIR2DS5 exhibited protective effects from PE development in Caucasians and Africans, respectively (21,30,82). Clinically, in some patients, the resistance index of the uterine artery increased, which indicated poor uterine artery recasting, and the levels of KIR2DL/S1, 3, and 5 were simultaneously reduced on the portion of dNK cells (78). The change of blood flow in the uterine artery reflects the clinical application of combined KIR/HLA-C in PE (83). In a Chinese study with a large sample size, it was revealed that PE women exhibited decreased activating receptors, including KIR2DS2, KIR2DS3, and KIR2DS5(83). Notably, the total number of activating KIRs in PE patients was less than that in controls (P=0.03) (83).
However, it has been established that Caucasian partners are more likely to have HLA-C2 molecules than Japanese men. Therefore, Japanese women having children with Caucasian partners are more likely to develop PE than those with Japanese men. Nevertheless, a previous study has reported a 1.54% chance of developing PE with a Caucasian partner, compared with 2.67% with a Japanese partner for a Japanese woman (78). A recent study in Denmark involved 259 patients with PE or eclampsia and 259 normal controls (84). Peripheral blood samples were obtained from both mothers and newborns (84). A relationship was not revealed between maternal KIR genotypes and HLA-C in the fetuses (84). Since the babies had additional HLA-C2 molecules compared with the mothers, there was no report of any difference in maternal KIR AA genotype between the experimental and control groups (84).
KIR/HLA-C combination and FGR
Hiby et al conducted a trial involving 118 FGR patients (fetal weight less than or equal to the 5th percentage) (14). Allegedly, the binding of maternal KIR AA genotype and fetal HLA-C2 was related to an induced possibility of developing FGR (14). This trend was consistent with PE previously discussed (14).
6. KIR/HLA-C combination and birth weight
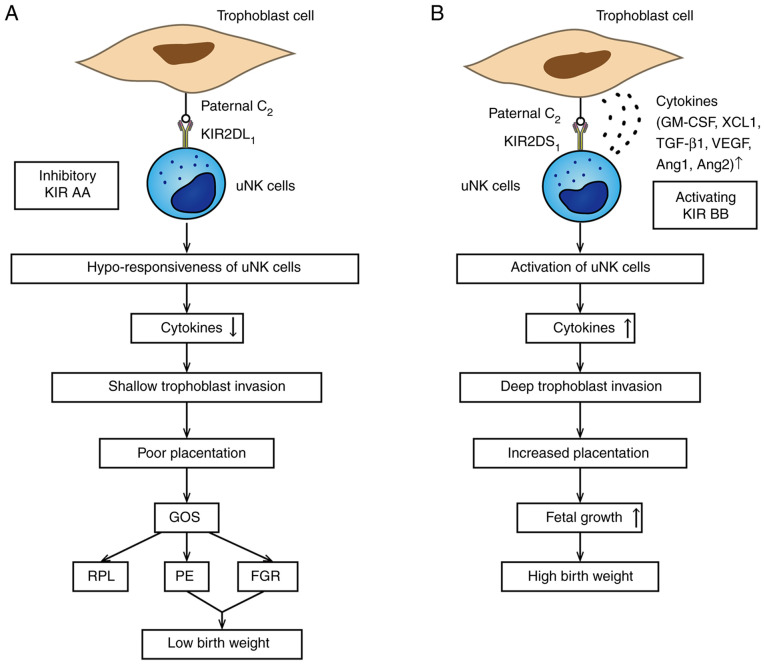
Human birth weight is influenced by stable selection (42). Macrosomia may cause obstructive dystocia, shoulder dystocia, and postpartum hemorrhage, affecting maternal and neonatal health (42). Often, a fetus that is too small will find it difficult to survive (42). An abundance of research has indicated that this process is regulated by the KIR genotype and their relevant HLA-C ligands on invasive trophoblast cells (42). Females having a KIR AA genotype and a baby with a paternal HLA-C2 molecule have restrained production of cytokines by uNK cells, which results in delivering a small fetus (85). Since the combination of KIR2DS1 and HLA-C2 can induce cytokine production and promote trophoblast invasion, the female having a KIR2DS1 gene and an HLA-C2 baby tends to have a large fetus (74,86). If the fetus is HLA-C1C1, the function of maternal KIR is invalid, and this correlation does not exist (14,74), which is attributable to the strict peculiarity and obvious restraining granted by KIR2DL1/HLA-C2 bindings relative to the weaker KIR2DL2/3 bindings with HLA-C alleles (87). In summary, in the placentation region, all effects of HLA-C in pregnancy are regulated by paternal HLA-C2, and the maternal KIR genotype decides the size of the baby (Fig. 1).
Figure 1.
Association between KIR/HLA-C combination and birth weight. (A) In this situation, the neonate inherits an HLA-C2 molecule from the father. The mother is KIR AA genotype; KIR2DL1 strongly combines to trophoblast HLA-C2 alleles leading to hypo-responsiveness of uNK cells, which is related to the poor placentation and leads to low birth weight in GOS. (B) Binding of KIR2DS1/HLA-C2 is associated with high birth weight. The KIR BB haplotype includes the activating KIR2DS1. Once this happens, uNK cells are activated to secrete plenty of cytokines, such as GM-CSF, which could enhance the placentation and lead to higher birth weight. KIR, killer-cell immunoglobulin-like receptor; HLA, human leukocyte antigen; uNK, uterine natural killer; GOS, great obstetrical syndromes; RPL, recurrent pregnancy loss; PE, preeclampsia; FGR, fetal growth restriction; GM-CSF, granulocyte-macrophage colony-stimulating factor; XCL1, chemokine C motif ligand 1; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor; Ang 1, angiopoietin 1.
There are significant differences in the trophoblasts of mice and human beings. Compared with human trophoblasts, the invasion of trophoblasts into deciduae is shallower in mice, and the mice do not express non-classical MHC (88). Murine NK receptors are part of the Ly49 family of receptors. In the mice experiment, when the additional MHC molecule H-2Dd was added, the vessel remodeling ability was decreased, compared with the same type of mice deficient only for H-2Dd (89). H-2Dd combines with the inhibitory receptor Ly49A and can suppress additional uNK cells. Noticeably, regardless of whether there was an H-2Dd molecule from the father or not, the fetal growth was affected. According to these results, some bindings of maternal NK receptors and paternal (or maternal) MHC molecules could affect trophoblast invasion and vascular recasting in mice. Therefore, it can be concluded that in both human and mice, the over inhibition of the function of uNK cells could result in fetal weight loss.
7. Future study directions
There are potentially critical clinical applications in these empirical studies. Considering the RPL woman with a KIR AA haplotype, the HLA-C molecules of the female and her partner are usable as a basis for selecting the donor egg or sperm. According to the epidemiology, donors homogeneous for HLA-C1 are considered to be safe; meanwhile, C2/C2 partners or oocyte donors may be harmful (14,90,91). HLA-C and the KIR genotype are applicable in selecting third-party gametes or surrogates (53). Subsequently, these immunological indicators can be detected through laboratory tests, which can be adopted to predict pregnancy outcomes. By detecting the KIR genotype and HLA-C molecule, the possible ligands can be inferred. The optimal binding will cause activation of uNK cells and angiogenesis in placentation, leading to a normal pregnancy. Females having an improper binding may experience difficulties during their pregnancies. The functionalities of these novel immune reactions have caused renewed comprehension of how to protect the fetus and may be useful to estimate and treat GOS. Detection of KIR genes in GOS patients could potentially assume a vital role in diagnosing the alloimmune etiology in these diseases. Studies conducted on a large-scale, employing modified statistical model sets, including the quantitative maternal-fetal genotype experiment, called a linear mixed-effect model (86), would enhance pregnancy outcomes. It entails statistically entering the KIR copy number from the SNP genotype (92) or utilizing a full-length transcript Smart-seq2 data (93).
Nevertheless, certain scholars claim that more conclusiveness is needed before clinical application despite the indications pointing towards credible evidence (94). For example, the binding of the KIR AA genotype and fetal HLA-C2 exists in roughly 10% of Caucasian pregnant females, making them twice as likely to have PE as normal women. Despite elevating the risk, there is a weak clinical application in this association. The only way to use this data predictively is to combine it with other clinical risk factors and serological indicators. KIR gene expression is highly polymorphic, and other factors that can regulate NK cells. Moreover, there are numerous ligands for activating receptors, and the mechanism of modulating NK cells is not clearly outlined. Furthermore, the exact manner of KIR/HLA-C combination in regulating NK cell education has not been established. Therefore, novel technologies are useful to distinguish the complex diversity of KIR and HLA-C. Sophisticated laboratory experiments can help determine the specific role of NK cells. Currently, studies detecting the function of uNK cells in vitro have not been conducted. The function of uNK cells can only be determined indirectly by detecting some cytokines, which are generally used to detect the functionality of peripheral blood NK cells. Mouse models can help understand the function of uNK cells. Some transgenic mice containing specific KIR and HLA-C molecules can help study the effect of distinct binding modes in vivo.
8. Conclusion
Medawar's theory states that immune cells in peripheral blood and the uterus need to be suppressed to form a successful pregnancy (95). However, KIR genotypes and HLA-C molecules reexamine the conditions for a successful pregnancy. Sufficient activation of uNK cells is crucial to maintain a normal pregnancy. Emphasis should be given on solving the following problems: Adopting accurate methods to determine KIR genotypes (e.g., by high-throughput genotyping), conforming with uniform clinical inclusion criteria, understanding the interaction between EVT and uNK cells. Moreover, the interaction between KIR/HLA-C and GOS should be studied by targeting women in a specific region since it is difficult to compare studies in different areas. Thus, a large sample-sized research in the same region, having a comparable control population, accurate detection of maternal KIRs, and both parents and fetal HLA-C molecules may determine the function of KIR and HLA-C in GOS.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported (grant no. 2020-MS-167) by the Natural Science Foundation of Liaoning Province (China).
Availability of data and materials
Not applicable.
Authors' contributions
XY and TM conceived the study, performed the literature search and analyzed the data. XY wrote the manuscript. TM revised the work for important intellectual content. XY and TM confirm the authenticity of all the raw data. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lopes MRU, Danowski A, Funke A, Rego J, Levy R, Andrade DCO. Update on antiphospholipid antibody syndrome. Rev Assoc Med Bras. 2017;63:994–999. doi: 10.1590/1806-9282.63.11.994. [DOI] [PubMed] [Google Scholar]
- 2.Egeland GM, Skurtveit S, Staff AC, Eide GE, Daltveit AK, Klungsøyr K, Trogstad L, Magnus PM, Brantsæter AL, Haugen M. Pregnancy-related risk factors are associated with a significant burden of treated hypertension within 10 years of delivery: Findings from a population-based norwegian cohort. J Am Heart Assoc. 2018;7(e008318) doi: 10.1161/JAHA.117.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The ‘Great Obstetrical Syndromes’ are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods. 1990;132:181–189. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- 5.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 6.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Gilman-Sachs A, Kwak-Kim J. Ovarian and endometrial immunity during the ovarian cycle. J Reprod Immunol. 2019;133:7–14. doi: 10.1016/j.jri.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Colucci F. The role of KIR and HLA interactions in pregnancy complications. Immunogenetics. 2017;69:557–565. doi: 10.1007/s00251-017-1003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moffett A, Colucci F. Uterine NK cells: Active regulators at the maternal-fetal interface. J Clin Invest. 2014;124:1872–1879. doi: 10.1172/JCI68107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulmer JN, Innes BA, Levey J, Robson SC, Lash GE. The role of vascular smooth muscle cell apoptosis and migration during uterine spiral artery remodeling in normal human pregnancy. FASEB J. 2012;26:2975–2985. doi: 10.1096/fj.12-203679. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci USA. 2009;106:5767–5772. doi: 10.1073/pnas.0901173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 14.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod. 2008;23:972–976. doi: 10.1093/humrep/den011. [DOI] [PubMed] [Google Scholar]
- 16.Ozturk OG, Sahin G, Karacor ED, Kucukgoz U. Evaluation of KIR genes in recurrent miscarriage. J Assist Reprod Genet. 2012;29:933–938. doi: 10.1007/s10815-012-9811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak I, Malinowski A, Tchorzewski H, Barcz E, Wilczynski JR, Banasik M, Grybos M, Kurpisz M, Luszczek W, Majorczyk E, et al. HLA-C C1C2 heterozygosity may protect women bearing the killer immunoglobulin-like receptor AA genotype from spontaneous abortion. J Reprod Immunol. 2011;88:32–37. doi: 10.1016/j.jri.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Faridi RM, Agrawal S. Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Hum Reprod. 2011;26:491–497. doi: 10.1093/humrep/deq341. [DOI] [PubMed] [Google Scholar]
- 19.Witt CS, Goodridge J, Gerbase-Delima MG, Daher S, Christiansen FT. Maternal KIR repertoire is not associated with recurrent spontaneous abortion. Hum Reprod. 2004;19:2653–2657. doi: 10.1093/humrep/deh483. [DOI] [PubMed] [Google Scholar]
- 20.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakimuli A, Chazara O, Hiby SE, Farrell L, Tukwasibwe S, Jayaraman J, Traherne JA, Trowsdale J, Colucci F, Lougee E, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci USA. 2015;112:845–850. doi: 10.1073/pnas.1413453112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Rodríguez EN, Nava-Salazar S, Mendoza-Rodríguez CA, Moran C, Romero-Arauz JF, Ortega E, Granados J, Cervantes-Peredo A, Cerbón M. Persistence of decidual NK cells and KIR genotypes in healthy pregnant and preeclamptic women: A case-control study in the third trimester of gestation. Reprod Biol Endocrinol. 2011;9(8) doi: 10.1186/1477-7827-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Yang Y, Yuan Y, Liu L, Meng T. The roles of uterine natural killer (NK) cells and KIR/HLA-C combination in the development of preeclampsia: A systematic review. Biomed Res Int. 2020;2020(4808072) doi: 10.1155/2020/4808072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alecsandru D, Barrio A, Garrido N, Aparicio P, Pellicer A, Moffett A, García-Velasco JA. Parental human leukocyte antigen-C allotypes are predictive of live birth rate and risk of poor placentation in assisted reproductive treatment. Fertil Steril. 2020;114:809–817. doi: 10.1016/j.fertnstert.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Faridi RM, Das V, Tripthi G, Talwar S, Parveen F, Agrawal S. Influence of activating and inhibitory killer immunoglobulin-like receptors on predisposition to recurrent miscarriages. Hum Reprod. 2009;24:1758–1764. doi: 10.1093/humrep/dep047. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Yang E, Wang WJ, He Q, Jubiz G, Katukurundage D, Dambaeva S, Beaman K, Kwak-Kim J. Decreased HLA-C1 alleles in couples of KIR2DL2 positive women with recurrent pregnancy loss. J Reprod Immunol. 2020;142(103186) doi: 10.1016/j.jri.2020.103186. [DOI] [PubMed] [Google Scholar]
- 27.Sacks G. Enough! Stop the arguments and get on with the science of natural killer cell testing. Hum Reprod. 2015;30:1526–1531. doi: 10.1093/humrep/dev096. [DOI] [PubMed] [Google Scholar]
- 28.Yagel S. The developmental role of natural killer cells at the fetal-maternal interface. Am J Obstet Gynecol. 2009;201:344–350. doi: 10.1016/j.ajog.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Abbas Y, Oefner CM, Polacheck WJ, Gardner L, Farrell L, Sharkey A, Kamm R, Moffett A, Oyen ML. A microfluidics assay to study invasion of human placental trophoblast cells. J R Soc Interface. 2017;14(20170131) doi: 10.1098/rsif.2017.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy PR, Chazara O, Gardner L, Ivarsson MA, Farrell LE, Xiong S, Hiby SE, Colucci F, Sharkey AM, Moffett A. Activating KIR2DS4 is expressed by uterine NK cells and contributes to successful pregnancy. J Immunol. 2016;197:4292–4300. doi: 10.4049/jimmunol.1601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z, Sun R, Tian Z, Wei H. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity. 2017;47:1100–1113.e6. doi: 10.1016/j.immuni.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 33.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 35.Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, Makadzange A, Rowland-Jones S, Willcox B, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King A, Hiby SE, Gardner L, Joseph S, Bowen JM, Verma S, Burrows TD, Loke YW. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors-a review. Placenta. 2000;21 (Suppl A):S81–S85. doi: 10.1053/plac.1999.0520. [DOI] [PubMed] [Google Scholar]
- 37.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 39.Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 41.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaëlsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115:1166–1174. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 42.Moffett A, Colucci F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol Rev. 2015;267:283–297. doi: 10.1111/imr.12323. [DOI] [PubMed] [Google Scholar]
- 43.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959–968. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- 45.Drake PM, Gunn MD, Charo IF, Tsou CL, Zhou Y, Huang L, Fisher SJ. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha. J Exp Med. 2001;193:1199–1212. doi: 10.1084/jem.193.10.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16-human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 47.Hsu KC, Chida S, Geraghty DE, Dupont B. The killer cell immunoglobulin-like receptor (KIR) genomic region: Gene-order, haplotypes and allelic polymorphism. Immunol Rev. 2002;190:40–52. doi: 10.1034/j.1600-065x.2002.19004.x. [DOI] [PubMed] [Google Scholar]
- 48.Alecsandru D, García-Velasco JA. Why natural killer cells are not enough: A further understanding of killer immunoglobulin-like receptor and human leukocyte antigen. Fertil Steril. 2017;107:1273–1278. doi: 10.1016/j.fertnstert.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Bashirova AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like receptor gene cluster: Tuning the genome for defense. Annu Rev Genomics Hum Genet. 2006;7:277–300. doi: 10.1146/annurev.genom.7.080505.115726. [DOI] [PubMed] [Google Scholar]
- 50.Robinson J, Halliwell JA, McWilliam H, Lopez R, Marsh SG. IPD-the immuno polymorphism database. Nucleic Acids Res. 2013;41(Database issue):D1234–D1240. doi: 10.1093/nar/gks1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: A database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2011;39(Database issue):D913–D919. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winter CC, Gumperz JE, Parham P, Long EO, Wagtmann N. Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J Immunol. 1998;161:571–577. [PubMed] [Google Scholar]
- 53.Morin SJ, Treff NR, Tao X, Scott RT III, Franasiak JM, Juneau CR, Maguire M, Scott RT. Combination of uterine natural killer cell immunoglobulin receptor haplotype and trophoblastic HLA-C ligand influences the risk of pregnancy loss: A retrospective cohort analysis of direct embryo genotyping data from euploid transfers. Fertil Steril. 2017;107:677–683.e2. doi: 10.1016/j.fertnstert.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010;185:3913–3918. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Male V, Sharkey A, Masters L, Kennedy PR, Farrell LE, Moffett A. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur J Immunol. 2011;41:3017–3027. doi: 10.1002/eji.201141445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hilton HG, Parham P. Missing or altered self: Human NK cell receptors that recognize HLA-C. Immunogenetics. 2017;69:567–579. doi: 10.1007/s00251-017-1001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong Y, Wang X, Lu P, Song Y, Lin Q. Killer immunoglobulin-like receptor repertoire on uterine natural killer cell subsets in women with recurrent spontaneous abortions. Eur J Obstet Gynecol Reprod Biol. 2008;140:218–223. doi: 10.1016/j.ejogrb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;89(1603) doi: 10.1016/j.fertnstert.2008.08.065. Practice Committee of the American Society for Reproductive Medicine. [DOI] [PubMed] [Google Scholar]
- 59.Varla-Leftherioti M, Spyropoulou-Vlachou M, Niokou D, Keramitsoglou T, Darlamitsou A, Tsekoura C, Papadimitropoulos M, Lepage V, Balafoutas C, Stavropoulos-Giokas C. Natural killer (NK) cell receptors' repertoire in couples with recurrent spontaneous abortions. Am J Reprod Immunol. 2003;49:183–191. doi: 10.1034/j.1600-0897.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 60.Flores AC, Marcos CY, Paladino N, Arruvito L, Williams F, Middleton D, Fainboim L. KIR receptors and HLA-C in the maintenance of pregnancy. Tissue Antigens. 2007;69 (Suppl 1):S112–S113. doi: 10.1111/j.1399-0039.2006.762_8.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Zhao YR, Jiao YL, Wang LC, Li JF, Cui B, Xu CY, Shi YH, Chen ZJ. Increased activating killer immunoglobulin-like receptor genes and decreased specific HLA-C alleles in couples with recurrent spontaneous abortion. Biochem Biophys Res Commun. 2007;360:696–701. doi: 10.1016/j.bbrc.2007.06.125. [DOI] [PubMed] [Google Scholar]
- 62.Vargas RG, Bompeixe EP, Franca PP, Marques de Moraes M, da Graca Bicalho M. Activating killer cell immunoglobulin-like receptor genes' association with recurrent miscarriage. Am J Reprod Immunol. 2009;62:34–43. doi: 10.1111/j.1600-0897.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 63.Wang S, Li YP, Ding B, Zhao YR, Chen ZJ, Xu CY, Fu YB, Wang XT. Recurrent miscarriage is associated with a decline of decidual natural killer cells expressing killer cell immunoglobulin-like receptors specific for human leukocyte antigen C. J Obstet Gynaecol Res. 2014;40:1288–1295. doi: 10.1111/jog.12329. [DOI] [PubMed] [Google Scholar]
- 64.Dambaeva SV, Lee DH, Sung N, Chen CY, Bao S, Gilman-Sachs A, Kwak-Kim J, Beaman KD. Recurrent pregnancy loss in women with killer cell immunoglobulin-like receptor KIR2DS1 is associated with an increased HLA-C2 allelic frequency. Am J Reprod Immunol. 2016;75:94–103. doi: 10.1111/aji.12453. [DOI] [PubMed] [Google Scholar]
- 65.Elbaşı MO, Tulunay A, Karagözoğlu H, Kahraman S, Ekşioğlu-Demiralp E. Maternal killer-cell immunoglobulin-like receptors and paternal human leukocyte antigen ligands in recurrent pregnancy loss cases in Turkey. Clin Exp Reprod Med. 2020;47:122–129. doi: 10.5653/cerm.2019.03223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alecsandru D, Garrido N, Vicario JL, Barrio A, Aparicio P, Requena A, García-Velasco JA. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum Reprod. 2014;29:2637–2643. doi: 10.1093/humrep/deu251. [DOI] [PubMed] [Google Scholar]
- 67.Varla-Leftherioti M, Spyropoulou-Vlachou M, Keramitsoglou T, Papadimitropoulos M, Tsekoura C, Graphou O, Papadopoulou C, Gerondi M, Stavropoulos-Giokas C. Lack of the appropriate natural killer cell inhibitory receptors in women with spontaneous abortion. Hum Immunol. 2005;66:65–71. doi: 10.1016/j.humimm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R. Tolerance mechanisms in pregnancy: A reappraisal of the role of class I paternal MHC antigens. Am J Reprod Immunol. 2010;63:93–103. doi: 10.1111/j.1600-0897.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 69.Nowak I, Malinowski A, Tchorzewski H, Barcz E, Wilczynski JR, Grybos M, Kurpisz M, Luszczek W, Banasik M, Reszczynska-Slezak D, et al. Frequencies of killer immunoglobulin-like receptor genotypes influence susceptibility to spontaneous abortion. J Appl Genet. 2009;50:391–398. doi: 10.1007/BF03195699. [DOI] [PubMed] [Google Scholar]
- 70.Yamada H, Shimada S, Kato EH, Morikawa M, Iwabuchi K, Kishi R, Onoé K, Minakami H. Decrease in a specific killer cell immunoglobulin-like receptor on peripheral natural killer cells in women with recurrent spontaneous abortion of unexplained etiology. Am J Reprod Immunol. 2004;51:241–247. doi: 10.1111/j.1600-0897.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 71.Varla-Leftherioti M. The significance of the women's repertoire of natural killer cell receptors in the maintenance of pregnancy. Chem Immunol Allergy. 2005;89:84–95. doi: 10.1159/000087915. [DOI] [PubMed] [Google Scholar]
- 72.Ntrivalas EI, Bowser CR, Kwak-Kim J, Beaman KD, Gilman-Sachs A. Expression of killer immunoglobulin-like receptors on peripheral blood NK cell subsets of women with recurrent spontaneous abortions or implantation failures. Am J Reprod Immunol. 2005;53:215–221. doi: 10.1111/j.1600-0897.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 73.Akbari S, Shahsavar F, Karami R, Yari F, Anbari K, Ahmadi SAY. Recurrent spontaneous abortion (RSA) and maternal KIR genes: A comprehensive meta-analysis. JBRA Assist Reprod. 2020;24:197–213. doi: 10.5935/1518-0557.20190067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hiby SE, Apps R, Chazara O, Farrell LE, Magnus P, Trogstad L, Gjessing HK, Carrington M, Moffett A. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J Immunol. 2014;192:5069–5073. doi: 10.4049/jimmunol.1400577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang X, Meng T. MiR-215-5p decreases migration and invasion of trophoblast cells through regulating CDC6 in preeclampsia. Cell Biochem Funct. 2020;38:472–479. doi: 10.1002/cbf.3492. [DOI] [PubMed] [Google Scholar]
- 76.Kelemu T, Erlandsson L, Seifu D, Hansson E, Abebe M, Teklu S, Girma S, Traherne JA, Moffett A, Hansson SR. Polymorphism in killer cell immunoglobulin-like receptors and human leukocyte antigen-c and predisposition to preeclampsia in Ethiopian pregnant women population. J Reprod Immunol. 2020;141(103169) doi: 10.1016/j.jri.2020.103169. [DOI] [PubMed] [Google Scholar]
- 77.Huhn O, Chazara O, Ivarsson MA, Retière C, Venkatesan TC, Norman PJ, Hilton HG, Jayaraman J, Traherne JA, Trowsdale J, et al. High-resolution genetic and phenotypic analysis of KIR2DL1 alleles and their association with pre-eclampsia. J Immunol. 2018;201:2593–2601. doi: 10.4049/jimmunol.1800860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saito S, Takeda Y, Sakai M, Nakabayahi M, Hayakawa S. The incidence of pre-eclampsia among couples consisting of Japanese women and Caucasian men. J Reprod Immunol. 2006;70:93–98. doi: 10.1016/j.jri.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Long W, Shi Z, Fan S, Liu L, Lu Y, Guo X, Rong C, Cui X, Ding H. Association of maternal KIR and fetal HLA-C genes with the risk of preeclampsia in the Chinese Han population. Placenta. 2015;36:433–437. doi: 10.1016/j.placenta.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 80.Gentle NL, Loubser S, Paximadis M, Puren A, Tiemessen CT. Killer-cell immunoglobulin-like receptor (KIR) and human leukocyte antigen (HLA) class I genetic diversity in four South African populations. Hum Immunol. 2017;78:503–509. doi: 10.1016/j.humimm.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 81.Higuma-Myojo S, Sasaki Y, Miyazaki S, Sakai M, Siozaki A, Miwa N, Saito S. Cytokine profile of natural killer cells in early human pregnancy. Am J Reprod Immunol. 2005;54:21–29. doi: 10.1111/j.1600-0897.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 82.Nakimuli A, Chazara O, Byamugisha J, Elliott AM, Kaleebu P, Mirembe F, Moffett A. Pregnancy, parturition and preeclampsia in women of African ancestry. Am J Obstet Gynecol. 2014;210:510–520.e1. doi: 10.1016/j.ajog.2013.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wallace AE, Whitley GS, Thilaganathan B, Cartwright JE. Decidual natural killer cell receptor expression is altered in pregnancies with impaired vascular remodeling and a higher risk of pre-eclampsia. J Leukoc Biol. 2015;97:79–86. doi: 10.1189/jlb.2A0614-282R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larsen TG, Hackmon R, Geraghty DE, Hviid TVF. Fetal human leukocyte antigen-C and maternal killer-cell immunoglobulin-like receptors in cases of severe preeclampsia. Placenta. 2019;75:27–33. doi: 10.1016/j.placenta.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Moffett A, Hiby SE, Sharkey AM. The role of the maternal immune system in the regulation of human birthweight. Philos Trans R Soc Lond B Biol Sci. 2015;370(20140071) doi: 10.1098/rstb.2014.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clark MM, Chazara O, Sobel EM, Gjessing HK, Magnus P, Moffett A, Sinsheimer JS. Human birth weight and reproductive immunology: Testing for interactions between maternal and offspring KIR and HLA-C genes. Hum Hered. 2016;81:181–193. doi: 10.1159/000456033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hilton HG, Vago L, Older Aguilar AM, Moesta AK, Graef T, Abi-Rached L, Norman PJ, Guethlein LA, Fleischhauer K, Parham P. Mutation at positively selected positions in the binding site for HLA-C shows that KIR2DL1 is a more refined but less adaptable NK cell receptor than KIR2DL3. J Immunol. 2012;189:1418–1430. doi: 10.4049/jimmunol.1100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, Moffett A, Colucci F, Hemberger M. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci USA. 2011;108:4012–4017. doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kieckbusch J, Gaynor LM, Moffett A, Colucci F. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodelling. Nat Commun. 2014;5(3359) doi: 10.1038/ncomms4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skjaerven R, Vatten LJ, Wilcox AJ, Rønning T, Irgens LM, Lie RT. Recurrence of pre-eclampsia across generations: Exploring fetal and maternal genetic components in a population based cohort. BMJ. 2005;331(877) doi: 10.1136/bmj.38555.462685.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hiby SE, Ashrafian-Bonab M, Farrell L, Single RM, Balloux F, Carrington M, Moffett A, Ebrahimi Z. Distribution of killer cell immunoglobulin-like receptors (KIR) and their HLA-C ligands in two Iranian populations. Immunogenetics. 2010;62:65–73. doi: 10.1007/s00251-009-0408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vukcevic D, Traherne JA, Næss S, Ellinghaus E, Kamatani Y, Dilthey A, Lathrop M, Karlsen TH, Franke A, Moffatt M, et al. Imputation of KIR types from SNP variation data. Am J Hum Genet. 2015;97:593–607. doi: 10.1016/j.ajhg.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polański K, Goncalves A, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563:347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moffett A, Chazara O, Colucci F, Johnson MH. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: Too early for clinical intervention. Reprod Biomed Online. 2016;33:763–769. doi: 10.1016/j.rbmo.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 95.Chaouat G. Reconsidering the Medawar paradigm placental viviparity existed for eons, even in vertebrates; without a ‘problem’: Why are Tregs important for preeclampsia in great apes? J Reprod Immunol. 2016;114:48–57. doi: 10.1016/j.jri.2015.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.