Abstract
The retinoblastoma tumor suppressor protein, Rb, interacts directly with the largest TATA-binding protein-associated factor, TAFII250, through multiple regions in each protein. To define the potential role(s) of this interaction, we examined whether Rb could regulate the intrinsic, bipartite kinase activity of TAFII250. Here, we report that Rb is able to inhibit the kinase activity of immunopurified and gel-purified recombinant TAFII250. Rb inhibits the autophosphorylation of TAFII250 as well as its phosphorylation of the RAP74 subunit of TFIIF in a dose-responsive manner. Inhibition of TAFII250 kinase activity involves the Rb pocket (amino acids 379 to 928) but not its amino terminus. In addition, Rb appears to specifically inhibit the amino-terminal kinase domain of TAFII250 through a direct protein-protein interaction. We further demonstrate that two different tumor-derived Rb pocket mutants, C706F and Δex22, are functionally defective for kinase inhibition, even though they are able to bind the amino terminus of TAFII250. Our results suggest a novel mechanism of transcriptional regulation by Rb, involving direct interaction with TAFII250 and inhibition of its ability to phosphorylate itself, RAP74, and possibly other targets.
The retinoblastoma protein, Rb, is a tumor suppressor whose mutational inactivation has been implicated in a variety of sporadic and familial human cancers (17). Rb is a key regulator of cell growth and differentiation (47), and although the exact mechanism by which it acts as a tumor suppressor is unclear, the ability of Rb to regulate transcription in a cell cycle-dependent manner is likely to be central to its function. Rb has been shown to either repress or stimulate the activity of specific promoters (23, 30), apparently through physical or functional interaction with transcription factors (15, 19, 22, 36, 44). Indeed, Rb can repress E2F-mediated transcription by binding directly to the E2F transcription factor (12), supposedly inhibiting transcription by blocking the transactivation domain of E2F (15). However, recent evidence from our laboratory and others has suggested that Rb can function as a general repressor of activated transcription, independent of an interaction with a specific transcription factor, when targeted to a promoter through fusion to a heterologous DNA-binding domain (1, 4, 35, 48). Consistent with this finding is the recent observation that Rb can repress transcription by recruiting the histone deacetylase protein HDAC1 (3, 26). Thus, it is likely that Rb, once recruited to a promoter, does not simply repress the function of a transcription factor but may regulate transcription via some alternative mechanism, perhaps through either deacetylase recruitment or interactions with the transcription initiation complex. Given that promoter-targeted Rb appears to repress activated rather than basal transcription, we examined the ability of Rb to interact with the TFIID coactivator proteins, TATA-binding protein (TBP)-associated factors (TAFs), as they are believed to be required predominantly for activated transcription, particularly of cell cycle-regulatory genes (40, 43). We have demonstrated a direct interaction between Rb and the largest TAF, TAFII250 (37), and have mapped the regions in each of the proteins important for their association (38).
Human TAFII250 is one of at least eight TAF subunits of TFIID (9, 42). It binds directly to TBP as well as several other TAFs, including hTAFII32 (dTAFII40) and hTAFII70 (dTAFII60) (5, 32), and has also been shown to bind the RAP74 subunit of TFIIF (31). TAFII250 is identical to CCG1, a cell cycle-regulatory protein thought to be important for progression through G1 phase (16). A temperature-sensitive mutant TAFII250 in the Syrian hamster cell line ts13 confers G1 arrest at the nonpermissive temperature (14), in part through the differential regulation of genes important for the cell cycle (33, 41). TAFII250 has been shown to possess both histone acetyltransferase (HAT) activity (27) and a protein kinase activity (7). The HAT activity of TAFII250 is conserved in Saccharomyces cerevisiae (yTAFII145), Drosophila melanogaster (dTAFII230), and humans and may play an important role in controlling access of the transcription machinery to nucleosome-bound promoter sequences. The kinase activity of TAFII250 is conserved in Drosophila and humans but not in yeast and thus may have functions specific to more complex organisms. Recent work has implicated TAFII250 kinase activity as being required for transcription of certain genes in vivo, including those for cyclin A and Cdc2 (29).
The TAFII250 kinase is bipartite, consisting of N- and C-terminal kinase (NTK and CTK) domains, and is capable of both autophosphorylation and specific transphosphorylation of the RAP74 subunit of TFIIF (7). The NTK domain of TAFII250 is required for the efficient rescue of ts13 cells at the nonpermissive temperature, and point mutations within regions of the NTK domain important for TAFII250 kinase activity decrease both its autophosphorylation and transphosphorylation activities (29). Interestingly, the Rb protein binds to TAFII250 at an amino-terminal site which overlaps the NTK domain (38).
In this report, we have examined the effect of Rb on the in vitro kinase activity of TAFII250. We demonstrate that the large pocket of Rb is able to inhibit amino-terminal TAFII250 kinase activity. In addition, we show that two tumor-associated Rb pocket mutants, C706F and Δex22, are unable to inhibit the kinase activity, even though they are able to bind efficiently to the amino terminus of TAFII250. Our results suggest that one function of the interaction of Rb with TAFII250 is to inhibit its intrinsic kinase activity, possibly repressing activated transcription from specific promoters which require TAFII250.
MATERIALS AND METHODS
Plasmid constructs.
The baculovirus transfer vector encoding hemagglutinin (HA)-tagged hTAFII250, pbHAX-hTAFII250, has been previously described (7, 31). The T7 expression vectors encoding either hTAFII250 (pTβ-STOP) or deletions within TAFII250 (N434 [pET-HAX] and ΔN700 [pTβ-HAX-STOP]) were provided by R. Tjian and S. Ruppert, respectively (31, 32). The glutathione S-transferase (GST)-TAFII250-N434 fusion construct was obtained from S. Ruppert. The GST fusion constructs for Rb(379–928), Rb-C706F(379–928), and Rb-Δex22(379–928) were provided by W. Kaelin (20). The GST fusion construct expressing Rb(10–330) was created by restriction digestion and ligation: GAL4-Rb(10–388), which was made as described for GAL4-Rb(10–308) (38), was digested with EagI/XbaI, and the ends were filled in with Klenow; the 960-bp fragment corresponding to amino acids 10 to 330 of Rb was ligated to SmaI-digested pGEX-2T (Pharmacia). The GST-TBP fusion protein construct was provided by S.-J. Kim (National Institutes of Health). The construct for expressing His-tagged RAP74 in bacteria for purification (pET23d-RAP74) was obtained from Z. F. Burton (11).
Protein purification.
The construct pET23d-RAP74 was used for IPTG (isopropyl-β-d-thiogalactopyranoside)-induced expression of His-tagged RAP74 in the BL21 strain of Escherichia coli. A large-scale purification was performed with Ni-nitrilotriacetic acid resin (Qiagen), following the manufacturer’s instructions for batch and column purification under denaturing conditions (6 M guanidine-HCl), renaturing on the column, and then elution with increasing concentrations of imidazole. The fractions were checked for protein content by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining, and those having peak elutions of RAP74 were pooled, concentrated, and analyzed by Western blotting with an anti-RAP74 antibody (C-18; Santa Cruz Biotechnology).
For GST and GST-tagged Rb proteins, expression was IPTG-induced in the DH5α strain of E. coli. A large-scale purification was performed, using glutathione-Sepharose 4B (Pharmacia) and following the manufacturer’s instructions for batch purification in phosphate-buffered saline (PBS). GST and fusion proteins were eluted from the beads with 10 to 15 mM reduced glutathione (Sigma) in 50 mM Tris-HCl (pH 8.0), and the fractions were checked by SDS–10% PAGE and Coomassie blue staining, as well as by spectrophotometric concentration determination (A595) with Bio-Rad protein assay dye reagent.
In vitro kinase assays with HA fusion proteins immobilized.
For in vitro kinase assays with full-length TAFII250 immobilized on beads (see Fig. 4 and 5), baculovirus-expressed human HA-tagged TAFII250 was immunoprecipitated from approximately 7.5 μg (per kinase reaction) of Sf9 cell extract, prepared as described previously (32), using a 1:1,000 dilution of purified monoclonal antibody 12CA5 (anti-HA; 1 mg/ml; BAbCo) at 4°C overnight. This was followed by coupling the TAFII250 to protein A-Sepharose beads (Sigma) ([per reaction] 20 μl of 50% [vol/vol] slurry in binding buffer 1 [10 mM HEPES, pH 7.4, 200 mM NaCl, 0.5 mM EDTA, 0.5 mM dithiothreitol, 0.1% Nonidet P-40]) at 4°C for 1 h. The beads were then washed four times with binding buffer 1, and immunocomplexes were incubated at room temperature (RT) for 45 min in 250 μl of binding buffer 1 with protease inhibitors (1 μg of aprotinin/ml, 1 μg of leupeptin/ml, 50 μg of phenylmethylsulfonyl fluoride/ml) and 3 mg of bovine serum albumin (BSA)/ml either with no added protein or with approximately 2 μg of the indicated purified GST-Rb protein. Following the binding reactions, the beads were washed once with binding buffer 1 and once with kinase buffer (25 mM HEPES [pH 7.9], 12.5 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, 0.1% Nonidet P-40) (7). The pelleted beads were then resuspended in 15 μl of kinase buffer with 0.5 to 0.7 μg of purified RAP74 and 10 μCi of [γ-32P]ATP (6,000 Ci/mmol; Amersham) and incubated at 30°C for 15 to 30 min. The reactions were stopped by the addition of Laemmli sample buffer, followed by SDS-PAGE and autoradiography for 5 to 60 min. The radioactive counts per minute were quantitated with an AMBiS radioanalytic imager. Titration kinase assays (see Fig. 6) were performed in the same manner, except that the binding reaction mixtures contained increasing amounts (0, 0.75, 1.5, and 2.25 μg) of the indicated purified GST-Rb protein.
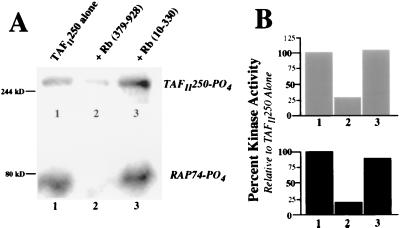
FIG. 4.
Inhibition of TAFII250 kinase activity is specific to the large pocket of Rb. (A) Phosphorylation of TAFII250 (top) and RAP74 (bottom). Anti-HA-immunoprecipitated hTAFII250, expressed from baculovirus in Sf9 cells, was immobilized on protein A-Sepharose and incubated either alone (lane 1) or with the purified recombinant large pocket of Rb (lane 2) or the amino terminus of Rb (lane 3). The bead-bound complexes were then tested for kinase activity, following addition of purified recombinant RAP74 and [γ-32P]ATP. The proteins were analyzed by SDS-PAGE and autoradiography. (B) Quantitation of kinase activity for samples in panel A. The radioactive counts per minute were measured, and the results are presented as the percentage of phosphorylation of TAFII250 (top) and RAP74 (bottom), relative to that seen for TAFII250 alone, which was set to 100% (lane 1). The column numbers correspond to the lane numbers in panel A.
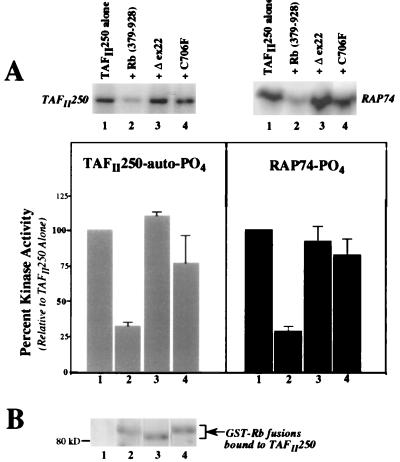
FIG. 5.
Tumor-derived Rb pocket mutants do not inhibit TAFII250 kinase activity. (A) Autoradiograph results (top) for TAFII250 autophosphorylation (left) and RAP74 transphosphorylation (right), representative of three separate assays. The reactions were carried out as described for Fig. 4, with kinase activity determined for TAFII250 alone (lane 1) or TAFII250 that had been incubated with various forms of the purified recombinant large pocket of Rb (wild-type pocket [lane 2]), pocket with exon 22 deleted [lane 3], and pocket with a Cys-to-Phe substitution at residue 706 [lane 4]). The radioactive counts per minute were measured and averaged with the results from two additional duplicate assays, and they are presented as the mean percentage of phosphorylation (bottom) relative to that seen for TAFII250 alone (set to 100%; bars 1). The bar numbers correspond to the lane numbers on the autoradiographs. The error bars indicate standard errors. (B) Western blot of samples from autoradiograph in panel A, detecting levels of GST-Rb fusion protein bound to immobilized HA-TAFII250 (see Materials and Methods for details). The blot was probed with a polyclonal anti-GST antibody. The lane numbers correspond to those in panel A.
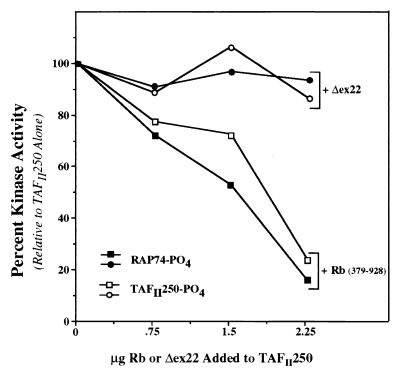
FIG. 6.
Rb inhibits TAFII250 kinase activity in a dose-responsive manner. Assays were performed as described for Fig. 4 and 5 with baculovirus-expressed HA-hTAFII250. A range of concentrations of Rb protein (wild-type pocket [squares] or Δex22 mutant [circles]) were tested for an effect on TAFII250 kinase activity. The reaction products were subjected to SDS-PAGE, and the counts per minute for phosphorylated TAFII250 (open symbols) and RAP74 (solid symbols) were measured on a radioanalytic imager. The results are plotted as the percentage of kinase activity relative to that seen for TAFII250 alone.
In preparation for Western analysis of TAFII250-bound GST-Rb proteins (see Fig. 5B), beads were pelleted following the kinase reaction, the supernatant (expected to contain RAP74 only) was transferred to a new tube, the beads (expected to contain bound TAFII250, with or without added Rb protein) were washed twice with binding buffer 1, Laemmli sample buffer was added to the beads and supernatant, and samples were subjected to SDS-PAGE. In the lanes with bead-bound protein, the gel section containing protein in a mass range greater than approximately 200 kDa (i.e., TAFII250) was excised for autoradiographic analysis, while the remainder of the lanes (<200 kDa) were subjected to Western analysis as described below.
For kinase assays with immobilized HA-TAFII250-N434 (NTK) or -ΔN700 (CTK) (see Fig. 8), the TAFII250 construct was either bacterially expressed in E. coli BL21 cells (CTK construct only) or in vitro expressed by using a TnT-T7 kit (Promega; described below) (both NTK and CTK constructs). Once expressed, the proteins were immunoprecipitated from either cell lysates (bacterial expression) or rabbit reticulocyte lysates (in vitro expression) with anti-HA antibody and protein A-Sepharose, as described above for baculovirus-expressed HA-hTAFII250, and subsequent binding and kinase reactions were also performed as described above. For mock reactions, reticulocyte lysates with no TAFII250 DNA added or, for mock bacterial expression, lysed cultures of untransformed BL21 cells were similarly incubated with anti-HA antibody and protein A-Sepharose and subjected to conditions identical to those for reactions with NTK or CTK alone (i.e., no added Rb protein).
FIG. 8.
Rb specifically inhibits only the NTK domain of TAFII250. (A) Bacterially expressed GST-TAFII250-N434 (NTK) or HA-TAFII250-ΔN700 (CTK) was immobilized on the appropriate beads and incubated either alone (lanes 2 and 5) or with purified GST-Rb pocket protein (lanes 3 and 6). For mock reactions (lanes 1 and 4) to determine the background levels of kinase activity, cell lysate from either untransformed E. coli DH5α (lane 1) or untransformed E. coli BL21 (lane 4) was treated identically to samples with kinase domain alone. The protein complexes were tested for kinase activity in the presence of RAP74, and samples were subjected to SDS-PAGE and autoradiography (top). The radioactive counts per minute were measured (bottom), and the results are presented as the percentage of RAP74 phosphorylation relative to that seen for the NTK or CTK domain alone (set to 100%; bars 2 and 5). The bar numbers correspond to the lane numbers above. (B) Reactions were repeated, exactly as described and labeled in panel A, except with in vitro-expressed, HA-tagged NTK or CTK domains. For mock reactions to determine the background levels of kinase activity (lanes 1 and 4), rabbit reticulocyte lysate (used for in vitro protein expression) without any added plasmid DNA was tested identically to the samples with kinase domain alone.
In vitro kinase assays with GST fusion proteins immobilized.
To analyze the kinase activity of TAFII250 bound to immobilized GST fusion proteins (see Fig. 2A), GST and GST-TBP or -Rb fusion proteins were expressed in E. coli (10 ml of culture per kinase reaction) by IPTG induction. The cells were lysed by sonication in 1× PBS, and Triton X-100 was then added to a final concentration of 1%. Cleared lysates were incubated with glutathione-Sepharose beads (Pharmacia) ([per reaction] 20 μl of 50% [vol/vol] slurry in 1× PBS) at 4°C for 30 min. The beads were then washed twice with 1% Triton-PBS and once with binding buffer 1. An aliquot of beads was set aside to determine the expression levels of the fusion proteins by SDS-PAGE and Coomassie blue staining. The remainder of the beads were then incubated in (per reaction) 250 μl of binding buffer 1 with protease inhibitors (1 μg [each] of aprotinin and leupeptin/ml, 50 μg of phenylmethylsulfonyl fluoride/ml), 3 mg of BSA/ml, and 10 μl of in vitro-expressed HA-hTAFII250 (produced as described below, but nonradioactive) at RT for 45 min. Duplicate reactions were performed for subsequent Western analysis of bound TAFII250 (see Fig. 2B). The beads were then washed three times with binding buffer 1 and once with kinase buffer, and the reactions to assay kinase activity were carried out as described above for the assays shown in Fig. 4 and 5. Samples for Western analysis were processed as described below.
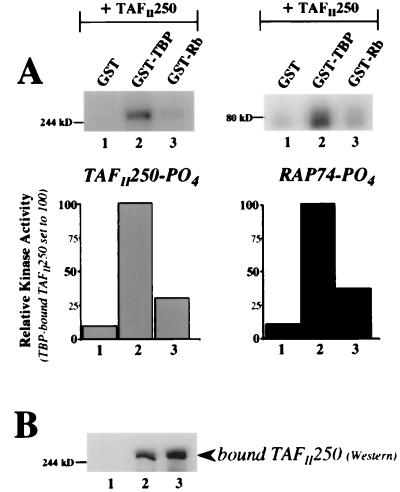
FIG. 2.
In vitro kinase activity of TAFII250 is inhibited when TAFII250 is bound to Rb. (A) Phosphorylation of TAFII250 (left) and RAP74 (right). GST, GST-TBP, and GST-Rb(379–928) were bacterially expressed, immobilized on glutathione-Sepharose, and incubated with in vitro-expressed hTAFII250. Washed protein complexes were then tested for kinase activity in the presence of purified recombinant RAP74. TBP-bound TAFII250 served as a positive control (lanes 2). The proteins were analyzed by SDS-PAGE and autoradiography (top). Radioactive counts per minute were measured on an AMBiS radioanalytic imager, and the results (bottom) are presented as the percentage of phosphorylation of TAFII250 (left) or RAP74 (right) relative to the levels seen for TBP-bound TAFII250, set to 100% (bar 2). The bar numbers correspond to the lane numbers on the autoradiographs. (B) Levels of in vitro-expressed TAFII250 bound to GST, TBP, or Rb, as detected by Western analysis (monoclonal anti-hTAFII250 antibody) of nonradioactive, duplicate kinase reactions performed concurrently with those shown in panel A. The lane numbers correspond to those in panel A.
Kinase assays with bacterially expressed and immobilized GST-TAFII250-N434 (NTK) (see Fig. 8) were performed as described above, with the following modification: the binding reactions (in 250 μl of buffer 1) immediately prior to the kinase reactions included, instead of in vitro-expressed TAFII250, either no added protein or approximately 2 μg of the indicated purified GST-Rb protein. Mock reactions were set up from cultures of untransformed DH5α cells, which were lysed, incubated with glutathione-Sepharose, and subjected to conditions identical to those for GST-NTK alone (i.e., no added Rb protein).
Denaturation-renaturation kinase assays.
To test the kinase activity of a more purified form of TAFII250 (see Fig. 3), a denaturation-renaturation assay was performed with gel-purified TAFII250, as described by Dikstein et al. (7), with slight modification. Briefly, immunopurified baculovirus-expressed HA-TAFII250 from either Sf9 or Sf21 cell lysates, obtained as described above, was resolved on SDS-PAGE (one lane per subsequent kinase reaction). As controls, two different mock immunoprecipitations were performed by incubating uninfected lysates from either Sf9 or Sf21 cells with anti-HA antibody and protein A-Sepharose, exactly as was done for TAFII250 lysate. The electrophoresed proteins were transferred onto a nitrocellulose membrane and stained with Ponceau S for visualization, and the region of the blot containing TAFII250 was excised (from the 244-kDa marker band to approximately 0.5 cm above this band, the width of one gel lane). The region that was excised aligned with the migration site of HA-hTAFII250 as detected by Western analysis (see Fig. 2B). For the Sf9 and Sf21 mock reactions, a slice of membrane was taken from this same region of each lane (244 kDa and above). As a third control, a section of “blank” membrane was also cut, again in the same >244-kDa region, from a lane that had only Laemmli buffer loaded. The membrane slices were placed into 1.5-ml Eppendorf tubes and incubated in denaturation solution (7 M guanidine-HCl, 50 mM Tris [pH 7.9], 2 mM EDTA, 10 mM dithiothreitol) for 1 h at RT, and then the protein was allowed to renature overnight at 4°C in kinase buffer. The slices were then washed once with binding buffer 1 and incubated either with no added protein (see Fig. 3) or with the indicated purified GST fusion protein (see Fig. 3B), carried out as described above for pre-kinase binding reactions (a 250-μl reaction volume of binding buffer 1 plus BSA and protease inhibitors). The slices were washed three times with kinase buffer and then incubated in 195 μl of kinase buffer plus approximately 0.75 μg of purified recombinant RAP74 and 15 μCi of [γ-32P]ATP (6,000 Ci/mmol; Amersham) for 30 min at 30°C. The entire volume of buffer (around 200 μl) was removed and concentrated to 10 to 15 μl with a Micro-Con 30 microconcentrating filter unit (Amicon). From this, phosphorylated RAP74 was detected by SDS-PAGE and autoradiography for 36 to 60 h.
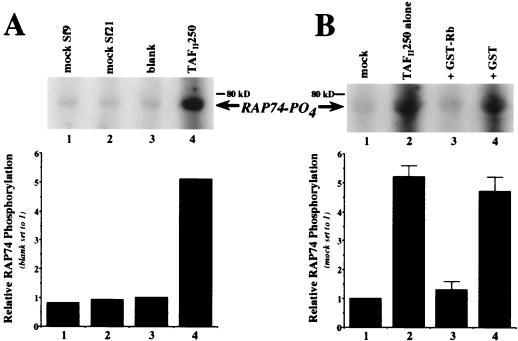
FIG. 3.
Rb specifically inhibits TAFII250 kinase and not a contaminating kinase. (A) Phosphorylation of recombinant RAP74 by immunopurified, baculovirus-expressed HA-hTAFII250 (lane 4) that had been subjected to gel purification, membrane immobilization, and denaturation-renaturation of the excised TAFII250 band (see Materials and Methods for details). Included as negative controls were mock-infected Sf9 (lane 1) and Sf21 (lane 2) cell lysates that had been subjected to procedures identical to those for the TAFII250 lysate, as well as a blank piece of nitrocellulose (lane 3). The membrane slices were tested for kinase activity in the presence of purified RAP74, and the reaction supernatants were analyzed by SDS-PAGE and autoradiography (top), followed by quantitation (bottom) of the radioactive counts per minute relative to the blank control (lane 3; set to a value of 1.0). The column numbers correspond to the lane numbers on the autoradiograph. (B) Inhibition of purified TAFII250 kinase by Rb (the autoradiograph is representative of five separate assays). An excised slice of nitrocellulose-bound hTAFII250, purified as for panel A from either Sf9 or Sf21 cell lysates, was incubated either alone (lane 2) or with purified GST-tagged Rb(379–928) (lane 3) or GST (lane 4), washed, and subsequently tested for kinase activity in the presence of RAP74. The mock reaction (lane 1) was a blank piece of nitrocellulose. The radioactive counts per minute were measured and averaged with the results from four additional duplicate assays (bottom), presented as mean relative RAP74 phosphorylation with “mock” set to 1.0 (lane 1). The column numbers correspond to the lane numbers on the autoradiograph. The error bars indicate standard errors.
In vitro binding assays.
For the TAFII250-Rb binding studies (see Fig. 7), 35S-labeled hTAFII250 ([35S]Met; 1,000 Ci/mmol; Amersham), both the full length protein and deletion mutations (TAFII250-N434 and TAFII250-ΔN700), was produced in vitro with a TnT-T7 protein expression kit, using 1 μg of input plasmid DNA per 50-μl kit reaction mixture. GST fusion proteins were expressed in E. coli DH5α and immobilized on beads, as described above, and binding reaction mixtures consisted of 250 μl of binding buffer 1 with protease inhibitors and BSA, as described above, and 10 μl of labeled TAFII250 product. After being incubated (with rotation) at RT for 45 min, the beads were washed four times with binding buffer 1, resuspended in Laemmli sample buffer, and subjected to SDS-PAGE and autoradiography. Ten percent (1 μl) of the input labeled TAFII250 protein was included on the gel for comparison, and the radioactive counts per minute were quantitated on an AMBiS radioanalytic imager.
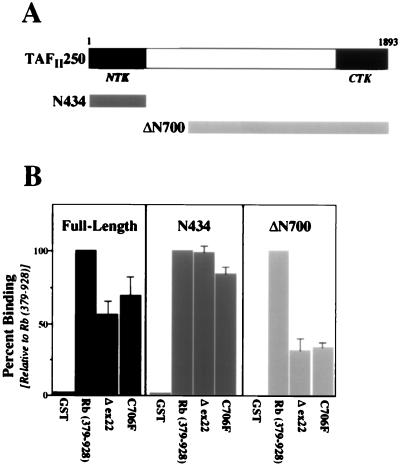
FIG. 7.
Rb pocket mutations do not affect binding to the amino terminus of TAFII250. (A) Schematic representation of full-length TAFII250 (top), as well as two deletion constructs (bottom) tested for binding to Rb constructs. N434 contains amino acids 1 to 434 of TAFII250, while ΔN700 contains amino acids 700 to 1893. (B) Quantitation of relative binding levels, averaged from three separate assays, of GST-Rb fusion proteins to either full-length TAFII250 (left), N434 (center), or ΔN700 (right). The indicated GST-Rb fusion proteins were bacterially expressed, immobilized on glutathione-Sepharose, and incubated with in vitro-expressed, 35S-labeled TAFII250 constructs. Following SDS-PAGE and autoradiography, the radioactive counts per minute were measured, and the results are presented as the mean percentage of binding relative to that seen for the wild-type Rb pocket (set to 100%; the second bar in each graph).
Western blot analyses.
For kinase assays with Rb incubated with bead-immobilized, full-length HA-TAFII250 (see Fig. 5), the levels of purified GST-Rb proteins bound to TAFII250 were analyzed by immunoblotting with polyclonal anti-GST antibody (Z-5; Santa Cruz Biotechnologies) at 1:3,000 dilution, followed by enhanced chemiluminescence detection (Amersham). For kinase assays with in vitro-expressed full-length TAFII250 incubated with immobilized GST-TBP or -Rb fusion proteins (see Fig. 2), the levels of in vitro-expressed TAFII250 bound to each fusion protein were analyzed by immunoblotting, using a monoclonal anti-hTAFII250 antibody (6B3; Santa Cruz Biotechnologies) at 1:1,000 dilution, followed by enhanced chemiluminescence detection. All Western analyses were carried out by following standard techniques (13).
RESULTS
Rb inhibits the intrinsic kinase activity of TAFII250 in vitro.
The interaction of Rb with TAFII250 is complex and occurs through multiple regions in each protein (Fig. 1) (38). Two nonoverlapping regions of Rb, the large pocket as well as the amino terminus, are able to bind to TAFII250 independently. Within TAFII250, one of the Rb-binding sites lies in a central region and may overlap with the binding site for the RAP74 subunit of TFIIF (31) while another site falls at the amino terminus of TAFII250, which contains a recently characterized NTK domain. This domain, together with a second, CTK domain, is capable of both TAFII250 autophosphorylation and specific phosphorylation of RAP74 (7). Thus, to define a functional role for the interaction of Rb with TAFII250, we examined whether Rb could affect the ability of TAFII250 to either phosphorylate itself or transphosphorylate RAP74.
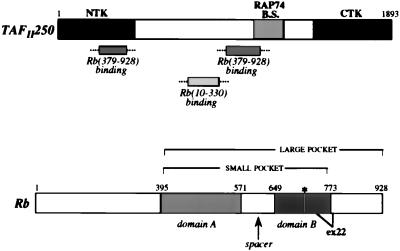
FIG. 1.
Rb and TAFII250 interact through multiple domains. Schematic representations of human TAFII250 (top) and Rb (bottom). RAP74 B.S. indicates the binding site (amino acids 1120 to 1270) for the RAP74 subunit of TFIIF. The mapped binding sites for the large pocket and amino terminus of Rb are indicated (note that the precise borders of these sites are uncertain). For Rb, the pocket region is labeled, with the amino acids at the borders of each domain indicated numerically. The asterisk denotes amino acid residue 706, whose mutation from Cys to Phe has been found to be associated with human tumors. The deletion of exon 22 (ex22; amino acids 738 to 775) is also tumor associated.
Bacterially expressed GST fusion proteins, including TBP (as a positive control) and the large pocket of Rb (amino acids 379 to 928), were immobilized on beads and incubated with in vitro-expressed hTAFII250. After extensive washing, purified RAP74 was added and the protein complexes were tested for kinase activity. As shown in Fig. 2A, the kinase activity of TAFII250 when bound to Rb was significantly reduced (lanes 3) compared to that of TBP-bound TAFII250 (lanes 2). Western analysis of duplicate nonradioactive kinase reactions demonstrated similar levels of TAFII250 binding for TBP and Rb (Fig. 2B, lanes 2 and 3). Comparable kinase inhibition was also seen with baculovirus-expressed full-length Rb protein which had been immunoprecipitated and incubated with TAFII250 (data not shown).
To verify that the kinase activity being monitored was indeed that of TAFII250 and not a contaminating kinase, recombinant TAFII250 was subjected to further purification and again tested for kinase activity with and without added Rb protein (Fig. 3). To do this, we performed a modified version of the denaturation-renaturation assay described by Dikstein et al. (7). Baculovirus-expressed HA-hTAFII250 was immunopurified from either Sf9 or Sf21 cell extract, separated by SDS-PAGE, and transferred to nitrocellulose. As controls, mock-infected Sf9 and Sf21 cell lysates were subjected to identical immunopurifications, separations, and membrane transfers. Following Ponceau S staining, the region of the membrane containing TAFII250 (see Materials and Methods for details) was excised, and the corresponding region was also cut from control lanes as well as from an empty lane. The immobilized TAFII250 protein and all controls were denatured in guanidine-HCl and then renatured. To test for kinase activity, all of the slices were incubated with purified RAP74 and radiolabeled ATP, and reaction supernatants were then concentrated and analyzed by SDS-PAGE and autoradiography. As shown in Fig. 3A, the results for both the Sf9 and Sf21 mock reactions (lanes 1 and 2) were identical to that seen for the blank control (lane 3), while the membrane-bound TAFII250 exhibited a significant level of RAP74 phosphorylation (lane 4). The faint bands seen in the control lanes most likely resulted from the nonspecific adherence of radiolabel to RAP74, especially since this band is present even in the blank control reaction, which did not contain any protein other than RAP74.
To test the effect of Rb on this membrane-bound TAFII250 (Fig. 3B), three identically processed slices of purified TAFII250 were incubated either alone or with recombinant GST-Rb(379–928) or GST purified from bacterial cells and were subsequently tested for kinase activity in the presence of purified RAP74. Compared to the significant levels of RAP74 phosphorylation observed with purified TAFII250 alone (Fig. 3B, lane 2), TAFII250 that had been incubated with Rb (Fig. 3B, lane 3) showed almost no kinase activity relative to the negative control (Fig. 3B, lane 1), while incubation with GST had no significant effect on TAFII250 kinase activity (Fig. 3B, lane 4). These results remained consistent throughout five separate but identical assays (the combined and averaged quantitation is shown in Fig. 3B). Note that a duplicate kinase reaction (TAFII250 alone) was subjected to Western blot analysis with a polyclonal anti-RAP74 antibody, which verified that the 75-kDa band present on the autoradiographs was phosphorylated RAP74 (data not shown). Thus, the inhibition of RAP74 phosphorylation by Rb is indeed due to a specific effect on TAFII250 kinase activity.
We next wanted to examine whether kinase inhibition could be seen with the amino terminus of Rb, which binds TAFII250 but not at the same site as the pocket of Rb (38). Baculovirus-expressed HA-hTAFII250 was immunopurified from an Sf9 cell extract and incubated either alone or with bacterially expressed, purified GST-Rb(379–928) (large pocket) or GST-Rb(10–330) (N terminus). Following the addition of purified RAP74, these immobilized protein complexes were assayed for kinase activity (Fig. 4). Relative to the phosphorylation levels seen for TAFII250 alone (Fig. 4, lane 1), the large pocket of Rb (Fig. 4, lane 2) was able to significantly inhibit both the phosphorylation of TAFII250 and of RAP74. The amino terminus of Rb (Fig. 4, lane 3) had no apparent effect on either of the kinase activities. These results correlate with prior mapping data (38) demonstrating that only the large pocket of Rb could bind to the amino terminus of TAFII250, which contains one of its two kinase domains. It is important to note that no phosphorylation of Rb by TAFII250 was detected (data not shown).
Tumor-associated Rb mutants are defective for kinase inhibition.
To determine if the ability of Rb to inhibit TAFII250 kinase activity is affected by naturally occurring mutations in Rb, two mutant Rb proteins associated with human tumors, C706F (21) and Δex22 (Δ738–775) (18), were used. Both the C706F (38) and Δex22 (39) Rb proteins are able to bind TAFII250, although usually at somewhat lower levels than wild-type Rb. Using the assay described above and shown in Fig. 4, bacterially expressed and purified GST-Rb fusion proteins, including GST-Rb(379–928)-C706F and GST-Rb(379–928)-Δex22, were bound to immobilized TAFII250, and the subsequent kinase activity was measured. As shown in Fig. 5A, neither of the Rb mutants (lanes 3 and 4) was able to inhibit the phosphorylation of TAFII250 or of RAP74. In contrast, the wild-type Rb pocket was able to reduce kinase activity fourfold (lane 2) relative to the activity seen for TAFII250 alone (lane 1). Western analysis of these reactions with an anti-GST antibody showed similar levels of each purified GST-Rb protein bound to the HA-immunopurified TAFII250 (Fig. 5B, lanes 2 to 4). Thus, while both the C706F and Δex22 Rb proteins are able to bind to TAFII250, neither is able to significantly inhibit TAFII250 kinase activity.
To determine if the effect of Rb on TAFII250 kinase activity is dose dependent, the assays described above and shown in Fig. 5 were repeated over a range of Rb concentrations. As shown in Fig. 6, the wild-type Rb pocket inhibited TAFII250 kinase activity in a linear, dose-responsive manner, while the Δex22 Rb pocket mutant failed to inhibit it at any concentration tested. The results demonstrate that the assays are being performed in the linear range and that higher doses of the mutant Rb protein would not result in the appearance of kinase inhibition.
Rb pocket mutations do not affect binding to the amino terminus of TAFII250.
To determine if the inability of the two Rb pocket mutants to inhibit kinase activity is due to specific differences in binding to TAFII250, particularly to the amino terminus of TAFII250, we performed a series of in vitro binding assays. The TAFII250 constructs tested are shown schematically in Fig. 7A, while a summarized quantitation of the binding assay results is presented in Fig. 7B. GST fusion proteins, including the wild-type Rb pocket as well as the C706F and Δex22 mutants, were bacterially expressed, immobilized on beads, and incubated with in vitro-expressed 35S-labeled TAFII250, either (i) the full-length protein, (ii) the amino terminus containing the NTK domain (N434; amino acids 1 to 434), or (iii) an N-terminal deletion (ΔN700; amino acids 700 to 1893). Both Rb pocket mutants were able to bind to full-length TAFII250, at a level approximately 75% of that of the wild-type Rb pocket for this particular assay (Fig. 7B). Interestingly, both Rb mutants were able to bind to the amino terminus of TAFII250 at or near the level of binding seen for the wild-type Rb pocket (Fig. 7B), but both displayed a greatly reduced ability to bind to the more central Rb-binding region in TAFII250 within ΔN700. Thus, although the C706 and Δex22 Rb mutants are functionally defective for inhibition of TAFII250 kinase activity, their ability to bind to the amino terminus of TAFII250 is unaffected. The decreased binding of the Rb mutants to TAFII250-ΔN700 does not directly explain their inability to inhibit TAFII250 kinase activity, as the Rb-binding site in this region of TAFII250 does not extend into the CTK domain (38), and although it does potentially overlap the RAP74-binding site, this site has been shown to be dispensible for phosphorylation of RAP74 by TAFII250 (7).
Rb specifically inhibits the NTK domain of TAFII250.
We next wanted to investigate whether the effect of Rb on TAFII250 kinase activity was specific to either the NTK or CTK domain of TAFII250. Each domain is independently capable of low-level autophosphorylation and RAP74 phosphorylation, although the efficiency of CTK activity is increased when the RAP74 interaction domain is present (7). The amino terminus of TAFII250 (N434; amino acids 1 to 434), containing the NTK domain, was bacterially expressed as a GST fusion protein, while the carboxy-terminal half of TAFII250 (ΔN700; amino acids 700 to 1893), containing both the RAP74-binding site and the CTK domain, was bacterially expressed as an HA fusion protein. These regions of TAFII250 were immobilized on beads, incubated either alone or with purified recombinant Rb pocket protein, and then tested for the ability to phosphorylate RAP74 (Fig. 8A). Rb was able to inhibit phosphorylation of RAP74 by the NTK domain of TAFII250 (Fig. 8A, lane 3), but was not able to inhibit the CTK domain (Fig. 8A, lane 6). Note that the kinase activity of the individual TAFII250 domains is reduced compared to that of full-length TAFII250, and thus autoradiographic visualization of phosphorylated products required much longer film exposures. To verify these results, we repeated the assays with in vitro-expressed, HA-tagged TAFII250 kinase domains. Again, the individual kinase domains were immobilized on beads, incubated either alone or with the purified Rb pocket protein, and then tested for the ability to phosphorylate RAP74 (Fig. 8B). The results were essentially the same as before: Rb inhibited the kinase activity of the NTK domain (Fig. 8B, lane 3) but not that of the CTK domain (Fig. 8B, lane 6). The C706F and Δex22 mutant Rb pocket proteins were also tested with both the in vitro and bacterially expressed kinase domains and failed to inhibit either domain (data not shown). Thus, using two different sources of the TAFII250 NTK and CTK domains, we have been able to demonstrate that Rb is able to specifically inhibit the NTK activity of TAFII250, apparently through a direct protein-protein interaction with the amino terminus of TAFII250.
DISCUSSION
We and others have shown that Rb can function as a general repressor of activated transcription when targeted to a promoter through fusion to a heterologous DNA-binding domain (1, 4, 35, 48). Interestingly, it has recently been demonstrated that Rb is able to repress transcription by recruiting the HDAC1 deacetylase protein (3, 26). These findings suggest that Rb might affect transcription through direct interaction with either chromatin remodeling factors, the transcription initiation complex, or additional transcription factors rather than by simply blocking the transactivation domain of a transcription factor such as E2F. In support of the hypothesis that E2F serves to recruit Rb to the promoter, where it can then actively repress transcription, E2F-1 knockout mice have a higher incidence of tumorigenesis (10, 49), which is presumed to be due to a loss of Rb-mediated transcriptional inhibition of cell cycle-regulatory genes (8). In addition, in vivo footprinting has shown that the E2F site in the B-myb promoter is only occupied when the promoter is repressed (50). Consistent with the idea that Rb is able to affect transcription through additional contacts at the promoter, we have demonstrated a direct, specific interaction of Rb with the TAFII250 subunit of TFIID (37). Mapping studies have established that the association of Rb with TAFII250 is complex and occurs through multiple domains in each protein (38). Interestingly, the large pocket of Rb interacts with the amino terminus of TAFII250 at a site overlapping a recently characterized NTK domain, one of two kinase domains in TAFII250 capable of both autophosphorylation and specific transphosphorylation of the RAP74 subunit of TFIIF (7). In addition, Rb also interacts with a more central region, near the RAP74-binding site on TAFII250.
To define a possible functional role for the physical association of Rb with TAFII250, we examined whether Rb can affect the kinase activity of TAFII250. Our results demonstrate that the large pocket of Rb is able to inhibit the kinase activity of both affinity-purified and gel-purified recombinant TAFII250. Rb inhibits the autophosphorylation of TAFII250 as well as the transphosphorylation of RAP74. Furthermore, the presence of either of two different tumor-associated mutations within domain B of the pocket region of Rb, a Cys-to-Phe amino acid substitution at residue 706 or deletion of exon 22, essentially abolished the ability of Rb to inhibit the kinase. These Rb pocket mutants are able to bind efficiently to TAFII250, with both Rb mutants retaining wild-type binding levels at the amino terminus of TAFII250; yet both display greatly reduced binding at the second, more central site within TAFII250. Although Rb can bind to multiple regions in TAFII250, the ability of Rb to inhibit kinase activity appears to involve only the amino terminus of TAFII250. We observed essentially the same inhibitory effect of Rb on the kinase activity of a truncated TAFII250 containing only the NTK domain (N434) but saw no effect on the activity of TAFII250 with the NTK domain deleted (ΔN700). Of note is the recent demonstration that a TAFII250 protein with specific point mutations in its NTK domain, which decrease both autophosphorylation and RAP74 phosphorylation, is greatly reduced in its ability to rescue ts13 cells expressing a temperature-sensitive TAFII250 and also results in impaired transcription from the cyclin A and Cdc2 promoters (29).
As the interaction between Rb and the central region of TAFII250 does not appear to affect kinase activity, perhaps instead it may regulate either binding of RAP74 or other TAFII250 activities. The central Rb-binding site on TAFII250 may overlap the mapped binding site for RAP74, and we have seen evidence of dose-responsive binding competition between Rb and RAP74 in vitro (39). It is conceivable that, by binding to this central region of TAFII250 and preventing interaction with RAP74, Rb could affect formation of the transcription preinitiation complex. We have also examined the possibility that Rb might affect the HAT activity of TAFII250, which has been mapped near the center of TAFII250 (27); however, preliminary results demonstrate that purified Rb has no apparent effect on histone acetylation in vitro and is not itself acetylated by TAFII250 (data not shown).
It is unclear how the binding of Rb to the amino terminus of TAFII250 is able to confer kinase inhibition. Apparently, binding alone is not sufficient for kinase inhibition, since the C706F and Δex22 Rb mutants are both able to bind the amino terminus of TAFII250 but are unable to inhibit its kinase activity. Though several models are plausible, such possibilities clearly represent a novel form of regulation by Rb, and the two Rb mutants may be defective for different reasons. It is possible that one or both of the mutant Rb proteins has an altered conformation such that it is unable to physically block access to the active site of the kinase after binding. Alternatively, the cysteine residue at amino acid 706 of Rb, which does not appear to be involved in binding the amino terminus of TAFII250, may be part of a domain important for directly inactivating the kinase activity. It is also possible that binding of the wild-type Rb pocket, but not the pocket mutants, alters the conformation of TAFII250 in a way that inactivates the kinase domain. Indeed, it has been shown that domains A and B of the Rb pocket interact with each other, and disruption of this interaction, such as through mutation, inhibits repressor activity (6). Additionally, recent structural studies of the Rb pocket provide evidence that the amino acid change in the C706F tumor-associated mutation, which occurs in domain B, would be expected to destabilize the folded state of domain B and disrupt a highly conserved extensive interface between domains A and B (24).
TAFII250 is an attractive target for a cell cycle-regulatory protein such as Rb, in that it is thought to be important for progression through G1 phase of the cell cycle. The temperature-sensitive cell line ts13 arrests in G1 at the nonpermissive temperature due to a point mutation in TAFII250 (14). Additionally, although TAFs do not appear to be required for general transcription activation in vivo (2, 28, 45), recent studies indicate that they have a specialized role in transcriptional regulation of genes important for cell cycle progression. Notably, yTAFII145, the yeast homolog of hTAFII250, has been shown to be required for transcription of G1- and S-phase cyclin genes, and its intracellular levels are regulated by the cellular growth state (46). Furthermore, we have demonstrated that hTAFII250 can directly or indirectly regulate the transcription of specific genes important for the cell cycle, such as cyclin D1 and p21/Waf-1 (33), and it has also been shown to regulate apoptosis (34). These observations demonstrate the importance of TAFII250 in regulating not only transcription but the cell cycle and cell death.
Recent work has suggested that the kinase activity of TAFII250 is required for the transcription of certain genes in vivo. Specifically, point mutations within the TAFII250 NTK domain which decrease kinase activity lead to an impaired ability to rescue ts13 cells at the nonpermissive temperature and also result in decreased transcription of cyclin A and Cdc2 (29). Because of the intimate association of TFIIF (through its RAP74 subunit) with RNA polymerase during transcription initiation, the covalent modification of RAP74, such as by phosphorylation, could serve either to influence the recruitment of RNA polymerase to the promoter or to modulate its elongation properties. Consistent with the significance of the functional interaction of TAFII250 with RAP74, the N-terminal globular domain of RAP74, important for binding to TAFII250 (31), appears both to fully support entry of RNA polymerase II into a preinitiation complex and to stimulate the elongation rate of the polymerase (25). In addition, the central region of RAP74, which is highly charged and contains many potential phosphorylation sites, has been shown to stimulate RNA polymerase II recycling and multiple-round transcription (25). The sites of phosphorylation by TAFII250 on RAP74 have not been mapped, and it is currently unknown how phosphorylation might affect the activities associated with the various functional domains of RAP74.
Taken together, our results suggest that the role of the interaction of Rb with TAFII250 is, in part, to inhibit the intrinsic kinase activity of TAFII250. Such a function could represent a novel means for Rb to regulate transcription, and this would not necessarily conflict with recent findings that Rb is able to repress transcription by recruiting the deacetylase HDAC1 (3, 26). The models are not mutually exclusive, and the effect of Rb on TAFII250 activity could be specific to only certain promoters. Clearly, additional studies are needed to further characterize the effect of Rb on the kinase activity of TAFII250 and to attempt to define the mechanism and biological role of this kinase inhibition. Similarly, it will be important to examine the effect of binding of both the amino terminus and the large pocket of Rb to the central region of TAFII250. It is likely that the multiple interactions of Rb with TAFII250 will regulate different, but important, TAFII250 functions.
ACKNOWLEDGMENTS
We gratefully thank W. Kaelin and W. Sellers for providing many of the recombinant Rb constructs, R. Tjian and S. Ruppert for providing the TAFII250 constructs, and Z. Burton and L. Lei for providing the recombinant RAP74 construct.
This work was supported by a public health service grant (55227) from the National Cancer Institute to P.D.R. and by a predoctoral research training grant from the United States Army to J.L.S.
REFERENCES
- 1.Adnane J, Shao Z, Robbins P D. The retinoblastoma susceptibility gene product represses transcription when directly bound to the promoter. J Biol Chem. 1995;270:8837–8843. doi: 10.1074/jbc.270.15.8837. [DOI] [PubMed] [Google Scholar]
- 2.Apone L M, Virbasius C A, Reese J C, Green M R. Yeast TAFII90 is required for cell cycle progression through G2/M but not for general transcription activation. Genes Dev. 1996;10:2368–2380. doi: 10.1101/gad.10.18.2368. [DOI] [PubMed] [Google Scholar]
- 3.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 4.Bremner R, Cohen B L, Spota M, Hamel P A, Ingles C J, Gallie B L, Phillips R A. Direct transcriptional repression by pRB and its reversal by specific cyclins. Mol Cell Biol. 1995;15:3256–3265. doi: 10.1128/mcb.15.6.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J-L, Attardi L D, Verrijzer C P, Yokomori K, Tjian R. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell. 1994;79:93–105. doi: 10.1016/0092-8674(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 6.Chow K N B, Dean D C. Domains A and B in the Rb pocket interact to form a transcriptional repressor motif. Mol Cell Biol. 1996;16:4862–4868. doi: 10.1128/mcb.16.9.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dikstein R, Ruppert S, Tjian R. TAFII250 is a bipartite protein kinase that phosphorylates the basal transcription factor RAP74. Cell. 1996;84:781–790. doi: 10.1016/s0092-8674(00)81055-7. [DOI] [PubMed] [Google Scholar]
- 8.Dynlacht B D. Regulation of transcription by proteins that control the cell cycle. Nature. 1997;389:149–152. doi: 10.1038/38225. [DOI] [PubMed] [Google Scholar]
- 9.Dynlacht B D, Hoey T, Tjian R. Coactivators associated with the TATA-binding protein mediate transcriptional activation in Drosophila. Cell. 1991;66:563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- 10.Field S J, Tsai F Y, Kuo F, Zubiaga A M, Kaelin W G, Jr, Livingston D M, Orkin S H, Greenberg M E. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein A, Kostrub C F, Li J, Chavez D P, Wang B Q, Fang S M, Greenblatt J, Burton Z F. A cDNA encoding RAP74, a general initiation factor for transcription by RNA polymerase II. Nature. 1992;355:464–467. doi: 10.1038/355464a0. [DOI] [PubMed] [Google Scholar]
- 12.Flemington E K, Speck S H, Kaelin W G., Jr E2F-1-mediated transactivation is inhibited by complex formation with the retinoblastoma susceptibility gene product. Proc Natl Acad Sci USA. 1993;90:6914–6918. doi: 10.1073/pnas.90.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 14.Hayashida T, Sekiguchi T, Noguchi E, Sunamoto H, Ohba T, Nishimoto T. The CCG1/TAFII250 gene is mutated in thermosensitive G1 mutants of the BHK21 cell line derived from golden hamster. Gene. 1994;141:267–270. doi: 10.1016/0378-1119(94)90583-5. [DOI] [PubMed] [Google Scholar]
- 15.Helin K, Harlow E, Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993;13:6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hisatake K, Hasagawa S, Takada R, Nakatani Y, Horikoshi M, Roeder R G. The p250 subunit of native TATA box-binding factor TFIID is the cell-cycle regulatory protein CCG1. Nature. 1993;362:179–181. doi: 10.1038/362179a0. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz J M, Park S-H, Bogenmann E, Cheng J-C, Yandell D W, Kaye F J, Minna J D, Dryja T P, Weinberg R A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci USA. 1990;87:2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horowitz J M, Yandell D W, Park S-H, Canning S, Whyte P, Buchkovich K, Harlow E, Weinberg R A, Dryja T P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989;243:937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- 19.Kaelin W G, Jr, Krek W, Sellers W R, DeCaprio J A, Ajchenbaum F, Fuchs C S, Chittenden T, Li Y, Farnham P J, Blanar M A, Livingston D M, Flemington E K. Expression and cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992;70:351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 20.Kaelin W G, Jr, Pallas D C, Decaprio J A, Kaye F J, Livingston D M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991;64:521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- 21.Kaye F J, Kratzke R A, Horowitz J M. A single amino acid substitution results in a retinoblastoma protein defective in phosphorylation and oncoprotein binding. Proc Natl Acad Sci USA. 1990;87:6922–6926. doi: 10.1073/pnas.87.17.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S-J, Onwuta U S, Lee Y I, Li R, Botchan M R, Robbins P D. The retinoblastoma gene product regulates Sp1-mediated transcription. Mol Cell Biol. 1992;12:2455–2463. doi: 10.1128/mcb.12.6.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S J, Lee H D, Robbins P D, Busam K, Sporn M B, Roberts A B. Regulation of transforming growth factor β1 gene expression by the product of the retinoblastoma susceptibility gene. Proc Natl Acad Sci USA. 1991;88:3052–3056. doi: 10.1073/pnas.88.8.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J-O, Russo A A, Pavletich N P. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 1998;391:859–865. doi: 10.1038/36038. [DOI] [PubMed] [Google Scholar]
- 25.Lei L, Ren D, Finkelstein A, Burton Z F. Functions of the N- and C-terminal domains of human RAP74 in transcriptional initiation, elongation, and recycling of RNA polymerase II. Mol Cell Biol. 1998;18:2130–2142. doi: 10.1128/mcb.18.4.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain J P, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–604. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 27.Mizzen C A, Yang X-J, Kokubo T, Brownell J E, Bannister A J, Owen-Hughes T, Workman J, Wang L, Berger S L, Kouzarides T, Nakatani Y, Allis C D. The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 28.Moqtaderi Z, Bai Y, Poon D, Weil P A, Struhl K. TBP-associated factors are not generally required for transcriptional activation in yeast. Nature. 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien T, Tjian R. Functional analysis of the human TAFII250 N-terminal kinase domain. Mol Cell. 1998;1:905–911. doi: 10.1016/s1097-2765(00)80089-1. [DOI] [PubMed] [Google Scholar]
- 30.Robbins P D, Horowitz J M, Mulligan R C. Negative regulation of c-fos expression by the retinoblastoma anti-oncogene. Nature. 1990;346:668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- 31.Ruppert S, Tjian R. Human TAFII250 interacts with RAP74: implications for RNA polymerase II initiation. Genes Dev. 1995;9:2747–2755. doi: 10.1101/gad.9.22.2747. [DOI] [PubMed] [Google Scholar]
- 32.Ruppert S, Wang E H, Tjian R. Cloning and expression of human TAFII250: a TBP-associated factor implicated in cell cycle regulation. Nature. 1993;362:175–179. doi: 10.1038/362175a0. [DOI] [PubMed] [Google Scholar]
- 33.Rushton J J, Steinman R A, Robbins P D. Differential regulation of transcription of p21 and cyclin D1 conferred by TAFII250. Cell Growth Differ. 1997;8:1099–1104. [PubMed] [Google Scholar]
- 34.Sekiguchi T, Nakashima T, Hayashida T, Kuraoka A, Hashimoto S, Tsuchida N, Shibata Y, Hunter T, Nishimoto T. Apoptosis is induced in BHK cells by the tsBN462/13 mutation in the CCG1/TAFII250 subunit of the TFIID basal transcription factor. Exp Cell Res. 1995;218:490–498. doi: 10.1006/excr.1995.1183. [DOI] [PubMed] [Google Scholar]
- 35.Sellers W R, Rodgers J W, Kaelin W G., Jr A potent transrepression domain in the retinoblastoma protein induces a cell cycle arrest when bound to E2F sites. Proc Natl Acad Sci USA. 1995;92:11544–11548. doi: 10.1073/pnas.92.25.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan B, Zhu X, Chen P-L, Durfee T, Yang Y, Sharp D, Lee W-H. Molecular cloning of cellular genes encoding retinoblastoma-associated proteins: identification of a gene with properties of the transcription factor E2F. Mol Cell Biol. 1992;12:5620–5631. doi: 10.1128/mcb.12.12.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao Z, Ruppert S, Robbins P D. The retinoblastoma-susceptibility gene product binds directly to the human TATA-binding protein-associated factor TAFII250. Proc Natl Acad Sci USA. 1995;92:3115–3119. doi: 10.1073/pnas.92.8.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao Z, Siegert J L, Ruppert S, Robbins P D. Rb interacts with TAFII250/TFIID through multiple domains. Oncogene. 1997;15:385–392. doi: 10.1038/sj.onc.1201204. [DOI] [PubMed] [Google Scholar]
- 39.Siegert, J. L., and P. D. Robbins. Unpublished data.
- 40.Struhl K. Selective roles for TATA-binding-protein-associated factors in vivo. Genes Funct. 1997;1:5–9. doi: 10.1046/j.1365-4624.1997.00004.x. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki-Yagawa Y, Guermah M, Roeder R G. The ts13 mutation in the TAFII250 subunit (CCG1) of TFIID directly affects transcription of D-type cyclin genes in cells arrested in G1 at the nonpermissive temperature. Mol Cell Biol. 1997;17:3284–3294. doi: 10.1128/mcb.17.6.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanese N, Pugh B F, Tjian R. Coactivators for a proline-rich activator purified from the multisubunit human TFIID complex. Genes Dev. 1991;5:2212–2224. doi: 10.1101/gad.5.12a.2212. [DOI] [PubMed] [Google Scholar]
- 43.Tansey W P, Herr W. TAFs: guilt by association? Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 44.Udvadia A J, Rogers K T, Higgins P D R, Murata Y, Martin K H, Humphrey P A, Horowitz J M. Sp-1 binds promoter elements regulated by the RB protein and Sp-1-mediated transcription is stimulated by RB coexpression. Proc Natl Acad Sci USA. 1993;90:3265–3269. doi: 10.1073/pnas.90.8.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker S S, Reese J C, Apone L M, Green M R. Transcription activation in cells lacking TAFIIs. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 46.Walker S S, Shen W-C, Reese J C, Apone L M, Green M R. Yeast TAFII145 required for transcription of G1/S cyclin genes and regulated by the cellular growth state. Cell. 1997;90:607–614. doi: 10.1016/s0092-8674(00)80522-x. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg R A. Tumor suppressor genes. Science. 1991;254:1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- 48.Weintraub S J, Chow K N B, Luo R X, Zhang S H, He S, Dean D C. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995;375:812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 49.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson N J. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 50.Zwicker J, Liu N, Engeland K, Lucibello F, Muller R. Cell cycle regulation of E2F site occupation in vivo. Science. 1996;271:1595–1597. doi: 10.1126/science.271.5255.1595. [DOI] [PubMed] [Google Scholar]