Abstract
Endoscopically placed intragastric balloons (IGBs) have played a significant role in obesity treatment over the last 30 years, successfully bridging the gap between lifestyle modification/pharmacotherapy and bariatric surgery. Since they provide a continuous sensation of satiety that helps the ingestion of smaller portions of food, facilitating maintenance of a low-calorie diet, they have generally been considered an effective and reversible, less invasive, non-surgical procedure for weight loss. However, some studies indicate that balloons have limited sustainable effectiveness for the vast majority attempting such therapy, resulting in a return to the previous weight after balloon removal. In this review we try to summarize the pros and cons of various balloon types, to guide decision making for both the physician and the obese individual looking for effective treatment. We analyzed the six most commonly used IGBs, namely the liquid-filled balloons Orbera, Spatz3, ReShape Duo and Elipse, and the gas-filled Heliosphere and Obalon - also including comments on the adjustable Spatz3, and the swallowable Obalon and Elipse - to optimize the choice for maximum efficacy and safety.
Keywords: Obesity, Intragastric balloon, Fluid-filled balloons, Gas-filled balloons, Swallowable balloons
Core Tip: Intragastric balloons have played a significant role in the management of obesity. Their easy application, reversibility and good short-term results have led to the development of a wide variety of balloon types. However, long-term results are not as good, and concerns about complications have also arisen. We tried to analyze the characteristics and effectiveness of the 6 most popular balloon types, in order to provide guidance in choosing the most appropriate balloon for each patient.
INTRODUCTION
Obesity, defined as an excess of body weight, and particularly of body fat, and associated with an increased number of co-morbidities, remains a considerable threat to human health, due to the high prevalence of morbidity and mortality, both from the syndrome itself and the related co-morbidities. Lifestyle modification, covering the combination of energy restriction, physical exercise, and behavioral changes is widely recommended as a stepwise approach to control/treat obesity. However, this measure usually leads to a modest decrease in weight, with a short success time - somewhat similar results to that of pharmacotherapy[1-8].
Although the pathophysiology of obesity is complex, the excess in calorie intake lies at the root of the weight gain mechanism[9]. One of the factors associated with greater calorie intake is definitely the greater fasting gastric capacity[10]; thus, an obvious solution would be the reduction of gastric capacity: either by surgery (resection or bypass procedures) or by placing a space-occupying device, mimicking a bezoar[11].
Bariatric surgery is generally effective, but always carries the risk of complications as well as low patient acceptance. It is estimated that less than 1% of obese patients who qualify for bariatric surgery opt for this procedure, mainly for fear of perceived risks of postoperative complications and mortality and, among others, the high surgical costs, and the lack of access to surgery. Furthermore, surgery is not indicated for overweight and obese class I patients[12-17].
Therefore, endoscopic bariatric and metabolic therapies have emerged over the years, to provide less invasive options beyond lifestyle modifications, pharmacotherapy and surgery, for patients who have failed with conservative treatment and are not or not yet surgical candidates, or refuse surgery because of its invasiveness and fear of complications[12,18]. According to the Statements after the Brazilian Intragastric Balloon Consensus, held in Sao Paulo, Brazil, in June 2016, obese individuals who are candidates for balloon implantation must be over 12 years of age, with established puberty, while there is no maximum age limit, each patient being evaluated individually. The minimum body mass index (BMI) is 25 kg/m2, after failure of clinical treatment, with no influence of BMI on the choice of balloon type, this being at the discretion of the physician. It is common sense that the presence of an active gastric ulcer, or in any other location, of gastric or esophageal varices, of a hiatal hernia longer than 5 cm as well as previous gastric surgery, are all considered as absolute contraindications[19]. Intragastric balloons (IGBs)-based on the philosophy of restrictive surgical procedures – are space-occupying devices, first described by Niebeb in 1982[11]. They are the most extensively studied and the most commonly used endoscopic “therapies” for obesity, due to their great efficacy and safety. Five years later, in 1987, the consensus meeting of international experts in Tarpon Springs, Florida[20], defined a number of specifications for a balloon to be considered suitable for use and primarily safe: It must (1) have a smooth surface with low potential for causing erosions, ulcers or obstructions; (2) be constructed of durable materials that do not leak; (3) be filled with liquid and not air; (4) be marked with a radiopaque marker that allows proper follow up of the device in case of deflation; and (5) have the capability of being adjusted to various sizes.
Mathus-Vliegen et al[18] who have been studying their mode of action for more than a decade, consider IGBs to mediate satiety both peripherally, by being a physical impediment to food intake, by reducing the gastric capacity and by delaying gastric emptying, and centrally, by activating gastric stretch receptors that transmit signals via afferent vagal nerves, the solitary tract and paraventricular nuclei, to the ventromedial and lateral hypothalamus[21-23].
In the intervening decades these devices have evolved to become more functional, effective and safe and the whole procedure less invasive, while keeping the advantages of being reversible and not altering the gastrointestinal anatomy[12,24,25].
Currently, there are many IGB designs, with little variation between them, several of which are now available in clinical practice, but few of which have gained Food and Drug Administration (FDA) approval. They may differ in relation to the method of insertion and removal, the filling volume, adjustability and duration of implantation, while still adhering to the main idea of the artificial bezoar that occupies space in the stomach causing mechanical gastric distention, and providing a continuous sensation of satiety, and thus reduction in food intake, finally resulting in weight loss[12,26,27].
In an effort to facilitate physician choice, the present study attempts to describe the technical characteristics of FDA and European Community (CE)-approved balloons, providing information on their effectiveness and safety, based on the large-scale clinical studies of the last decade.
BALLOON DESCRIPTION
Orbera IGB
Orbera IGB (Apollo Endosurgery, Austin, TX, United States), formerly BioEnterics IGB (BIB, Inamed Corporation, Santa Barbara, CA, United States) was the first of the new generation of balloons which appeared in 1991, following the Tarpon Springs Consensus meeting[20]. To date, it is the most popular and most commonly used endoscopic device for weight loss, having also the most historical data supporting its use; all the other balloons, which follow chronologically, are practically based on the same idea and, unavoidably are comparable to it[4,5,27-29].
The FDA-approved Orbera (2005) is a single spherical silicone-made balloon of about 13 cm in diameter, arriving commercially compressed and impacted at the end of a filling tube attached to a radiopaque self-sealing valve (Figure 1). After an initial diagnostic endoscopy, the balloon placement assembly is inserted orally into the gastric fundus and a volume of 500 to 700 mL saline solution - at the discretion of the physician - is used for balloon inflation through a closed infusion circuit, the whole procedure being performed under direct endoscopic supervision[13,30,31]. After completion of inflation, the infusion system is closed, creating a sudden vacuum resulting in the valve self-sealing and allowing the easy release of the filling tube, which is then gently pulled out through the mouth, leaving the balloon in the fundus, but floating freely in the stomach[32,33].
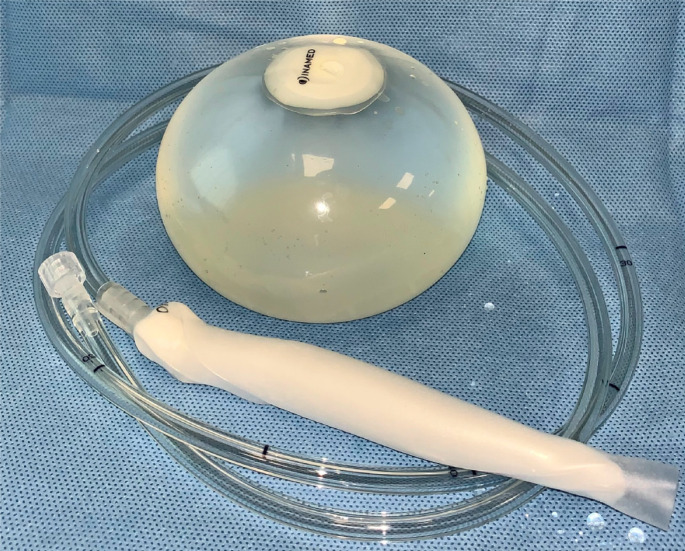
Figure 1.
Orbera balloon. (This photo is from our personal photo-archive).
According to manufacturer, the Orbera balloon could safety remain implanted for up to a maximum of 6 mo, because of the increasing risk of perforation and sudden emptying thereafter, which might allow the balloon to migrate towards the gut and possibly obstruct the bowel[5,13]. It requires sedation and endoscopy for deflation and removal; a double-channel endoscope and two long-jaw rat-tooth forceps may facilitate the procedure[4,13].
For the last two years a balloon which can remain in situ for 12 mo has also been available; the second generation “Orbera365”, having almost exactly the same characteristics[34].
Heliosphere balloon
Over the years, it has become obvious that the excess weight of a liquid-filled balloon is the cause of an increased rate of nausea, vomiting and epigastric pain in the days immediately following balloon placement; thus, the air-filled Heliosphere balloon, known as the Heliosphere bag (Helioscopie Medical implants, Vienne, France) was developed to circumvent this disadvantage, and was introduced into clinical practice in 2004[35,36].
It is a single spherical high-volume-capacity, air-filled, polyurethane balloon weighing less than 30 g and is enclosed in a silicone envelope. It requires endoscopy for positioning and is loaded with a simple inflation system, allowing 900-1000 mL of air, within a median time of 12 min[30,37-40]. The balloon is generally well-tolerated during the 6 mo implantation period. However, its use has raised several concerns about procedure-related complications due to technical difficulties in balloon passage through the cardia and the upper esophageal sphincter–large size, low pliability, high failure rates for positioning and spontaneous deflation[28,36,38]; similar difficulties have also been referred to during endoscopic removal, leading, in a few cases, to surgical removal or to the use of a rigid endoscope[35], thus, the use of a two-claw forceps for catching it in the valve is advised. The whole procedure generally takes longer than that for other balloons, including the Orbera, and results in more discomfort, making deep sedation a prerequisite for both patient and endoscopist[41]. A severe warning for those candidates for gas-filled-Helioshere balloons is to totally refrain from scuba diving and travelling in unpressurized airplane cabins[5].
Spatz3® balloon
Spatz3® balloon (Spatz3; Spatz FGIA, Great Neck, NY, United States) is the 3rd generation Spatz device manufactured with the first criterion of the Tarpon Springs Conference requirements in mind –i.e., its volume can be adjusted - increased or reduced - throughout the treatment period and not only initially at the time of inflation[20]. Additionally, it is the first balloon that can safely remain in the stomach for 360 d, thus facilitating sustained weight loss for one full year, as well as leaving more time for the patient to undergo feeding re-education and lifestyle modification. However, it has the serious disadvantage of not having a completely smooth surface, since the site for insertion of the filling valve forms a sort of ‘tail’[42,43]. On the other hand, according to the manufactures, this ‘tail’ may prevent or delay a deflated balloon from passing through the duodenum. To date it has received the European Union CE mark but not yet gained FDA approval[42,44,45].
It is a spherical silicone, saline-filled balloon, with the unique feature of an extractable, thin, filling catheter with a valve at the end, which enables saline to be added or removed in situ, thus adjusting the intragastric volume according to patient tolerance and the desired weight-loss outcome. The system consists of 3 parts: the balloon; a silicone covered anchor, with an internal network, to facilitate balloon insertion and removal and prevent migration; and the silicone filling tube, able to stretch to modify the fluid volume of the balloon and shrink back into the stomach[35,43,44] (Figure 2).
Figure 2.
Spatz-3 balloon. [Courtesy of Ms Ariel Nezry (VP Marketing, Spatz FGIA Inc)].
The Spatz3 is designed to be inserted with a well-lubricated endoscope. The balloon is mounted on the tip of the scope by the use of a type of ‘condom’. After visual confirmation that the whole balloon and its apparatus is fully within the gastric cavity - so avoiding the risk of inflation within the esophagus - balloon inflation is carried out under direct view, with 400-700 mL of saline. After inflation, the filling catheter is pulled up until its valve reaches the patient’s mouth. Then the catheter is disconnected from the valve, which is closed with its cap, which has a blue nylon loop. Holding the loop, the valve is gently pushed back towards the oropharynx and the gastroscope facilitates the correct positioning of the valve in the gastric fundus[45].
For balloon deflation, in the case of intolerance in the early days - excessive and/or persistent vomiting for more than 7 d - the blue nylon loop is grasped endoscopically by foreign body forceps and pulled up to the mouth. At this level the previous mentioned filling catheter is adjusted and, by aspiration of 100 to 300 mL, the balloon volume is appropriately reduced. The same process is followed, usually 3 mo after implantation when the patient stops or has minimized weight loss, or should he/she report a decrease in satiety, to increase the balloon volume by a standard volume of 250 mL[43,46]. At the end the 12-mo implantation period, the balloon must be removed endoscopically after emptying by standard balloon needle or deflation utilizing the valve, by the same process as for insertion. However, its size and the described irregular morphology make the endoscopic extraction more difficult and laborious, and thus anaesthesia is absolutely necessary[30,35,38,44].
ReShape Duo integrated dual balloon system
ReShape Duo integrated dual balloon system (ReShape Medical, Inc, San Clemente, CA, United States) consists of two independently filled silicone spheres joined by a central, short, non-communicating flexible silicone shaft. The main idea behind this system design is to decrease the chance of balloon intestinal migration should one of the balloons accidentally deflate. Additionally, this flexible configuration, according to the manufacturers, allows the balloons to conform to the natural contours of the stomach[5,30,47-49].
The ReShape Duo balloon, FDA-approved system is inserted transorally and advanced into the stomach by means of an endoscopic guidewire. Each is filled separately with up to 450 mL of saline (maximum total volume 900 mL), although a smaller volume is recommended for individuals less than 64.5 inches in height[47-51]. When inflated, it occupies a significant portion of the stomach (900 mL), while maintaining the natural gastric anatomy. For balloon system deflation and removal, after a maximum 6 mo period, anaesthesia and endoscopy are definitely required[5,49,52].
As of December 2018, Apollo Endosurgery (Apollo Endosurgery, Austin, TX, United States) purchased ReShape Medical and will focus exclusively on its own Orbera balloon going forward. With this transaction, the ReShape balloon will be phased out[53].
Obalon®
The Obalon® (Obalon Therapeutics Inc, Carlsbad, CA, United States) is a new thin-walled, 250 mL gas-filled, swallowable IGB, designed to allow easy gastric volume titration, by using additional balloons. It is an FDA-approved device, consisting of a series of three individual balloons, equating to a total volume of 750 mL that can be consequently swallowed one month apart, and is relatively well-tolerated by most patients[54].
Each balloon is compressed, folded, and fitted into a 6 g dissolvable gelatin capsule, which is swallowed under fluoroscopic visualization to verify that the entire capsule has entered the stomach[40,54,55]. A thin, 2 Fr catheter is attached to the balloon and once the capsule reaches the stomach the other end of the catheter, which extends outside the mouth, is used for remote, automated balloon inflation to a maximal volume of 250 mL, using a canister filled with a proprietary air mixture that is mostly nitrogen based. The procedure is relatively easy and executable by a single operator. After balloon inflation, the catheter is detached and removed, allowing the balloon valve to safely self-seal[5,54-56].
The balloons can remained implanted for up to 6 mo and require endoscopy only for deflation and removal of all 3 balloons at the same time. The balloons are punctured and then grasped by forceps for extraction under general anaesthesia[40,54,56].
Recently, the FDA also approved the Obalon navigation system. It utilizes magnetic resonance to provide a real-time image of the Obalon on a computer screen instead of fluoroscopy to confirm balloon positioning. This technology, besides minimizing the exposure of patient and personnel to radiation and decreasing the cost of radiography, makes the procedure itself relatively easier. The Obalon has been used in pediatrics with promising results[54-56]. In Europe, the Obalon, rather than other balloons, is indicated for use for individuals with a lower BMI (27 kg/m2)[5].
Elipse balloon
The Elipse balloon (Elipse; Allurion Technologies, Wellesley, MA, United States) is a non-FDA-approved IGB, similar in size, shape and function to the most widely used and endoscopically placed Orbera balloon. However, this is the first intragastric device not requiring anaesthesia, or an invasive endoscopic procedure, either for placement or removal[36,57,58]. It thus represents an innovative option for weight loss, minimizing the costs and the complication risks of the endoscopic procedure for insertion or removal and hence offers an option to obese individuals feeling uncomfortable with endoscopy and/or at risk for anesthesia[36,44]. However, by omitting the pre-implantation endoscopic surveillance of the stomach, the possibility of recognizing mucosal lesions (erosions or ulcers) or anatomical abnormalities (hiatus hernia), which could, theoretically, lead to unexpected complications at the time of balloon remaining in the stomach, is lost[57].
The balloon, made from a thin polymer film without rigid parts, is enclosed, well compressed, inside a small, swallowable vegetarian capsule attached to a thin catheter 75 cm long and 1.3 mm in diameter, via a self-sealing valve, and is designed to deploy spontaneously in the stomach. The capsule is as easily swollen with water as a pill, but in the case of difficulty, a stylet can be fed through the catheter to stiffen it, allowing the physician to gently push the capsule during swallowing. Once swallowed, its proper position in the stomach is confirmed through X-ray visualization of the balloon’s radiopaque ring-shape marker; after which, the balloon is filled with 550 mL of fluid, consisting of distilled water with potassium sorbate preservative, through the catheter which is then removed by simply pulling it back[58-60]. Placement is performed in a 20 min outpatient visit.
After a 4 mo period, the device is designed to spontaneously empty; the reabsorbable material, remaining closed inside the sealing balloon valve, completely degrades, leaving the device to self - deflate and then naturally pass - thanks to its construction from a thin film without rigid parts-through the gastrointestinal tract and be excreted[35,36,46,57,58].
The ease of insertion and self-removal enables many physicians who do not perform endoscopy to use the balloon and this is expected to lower the total cost of diet programs. However, this may lead to its inappropriate implantation in unsuitable individuals and thus to increased risks of intolerance. Another cause of increased intolerance may be the absence of endoscopic surveillance of the stomach for any pathology prior to its insertion (Table 1).
Table 1.
Summary of Intragastric balloon characteristics
|
|
|
FDA/CE approved
|
|
|
CE approved
|
|
|
Balloon type
|
Orbera
|
ReShape Duo
|
Obalon
|
Heliosphere
|
Spatz
|
Elipse
|
| Manufacturer | Apollo Endosurgery | ReShape Medical | Obalon Therapeutics | Helioscopie Medical Implants | Spatz FGIA | Allurion Technologies |
| Filled with | Saline | Saline | Nitrogen gas | Air | Saline | Liquid |
| Capacity (mL) | 400-700 | 450 × 2 | 250 × 3 | 900-1000 | 300-900 | 550 |
| Number of balloons | 1 | 2 | Up to 3 | 1 | 1 | 1 |
| Insertion | Endoscopy | Endoscopy | Swallowed | Endoscopy | Endoscopy | Swallowed |
| Removal | Endoscopy | Endoscopy | Endoscopy | Endoscopy | Endoscopy | Natural pass |
| Duration | 6 | 6 | 6 | 6 | 12 | 4 |
| Adjustable | No | No | No | No | Yes | No |
FDA: Food and Drug Administration; CE: European Community.
EFFECTIVENESS FOR BODY WEIGHT LOSS
The first balloon fulfilling the Tarpon Springs Consensus standards was the Bioenterics IGB (Inamed® Corporation, Santa Barbara, CA, United States) now available as Orbera commercially available since 1991. For more than a decade it remained unique in the market, and thus, inevitably, is the subject of many observational and randomized published studies, analyzing its effectiveness, which, in most studies, was impressive. Today, almost 30 years later, the idea of using a balloon as a space-occupying device in the stomach to give the feeling of fullness, still remains not only attractive, but also effective, as demonstrated by the multiple attempts to copy, with modifications, the original idea, many of which have been considered successful and become commercially available. This chapter aims to show in numbers - through meta-analysis and large series - studies published in recent years - the effectiveness in weight loss of the IGBs now in use in clinical practice. For comparison and homogeneity of expression the parameters of percentage total body weight loss (%TBWL), percentage excess weight loss (%EWL) and BMI are used[51,61] (Table 2).
Table 2.
Representative studies of the effectiveness of intragastric balloons
|
Ref.
|
Study type
|
Cases
|
Balloon type
|
Mo
|
Mean BMI loss kg/m2
|
Mean BWL kg
|
%TWL
|
%EWL
|
| Genco et al[64], 2005 | Observational | 2515 | Bioenterics | 6 | 4.9 ± 12.7 | |||
| Kotzampassi et al[13], 2012 | Observational | 500 | Bioenterics | 6 | 7.39 ± 3.57 | 21.19 ± 10.3 | 38.09 ± 20.18 | |
| Lopez-Nava et al[67], 2011 | Observational | 714 | Bioenterics | 6 | 6.5 ± 12.7 | 18.8 ± 9 | 41.6 ± 21.8 | |
| Fittipaldi-Fernandez et al[68], 2020 | Observational | 5874 | air-filled | 6 | 19.13 ± 8.86 | 18.42 ± 7.25 | 65.66 ± 36.24 | |
| Abu Dayyeh et al[71], 2019 | Observational | 187 | Spatz3 | 9 | 14.9 ± 7.2 plus 4.7* | |||
| Fittipaldi-Fernandez et al[45], 2020 | Observational | 180 | Spatz3 | 7.12 ± 1.63 | 6.18 ± 4.07 | 17.51 ± 11.67 | 16.22 ± 9.74 | 56.68 ± 40.12 |
| Schwaab et al[72], 2020 | Cross-sectional | 360/144 | Orbera/Spatz3 | 6 up to 12 | 15.4 ± 7/15.5 ± 9.6 | |||
| Sullivan et al[73], 2018 | RCT | 185/181 | Obalon/sham | 6 | 6.6 ± 5.1/3.4 ± 5.0 | |||
| Ienca et al[58], 2020 | Observational | 1770 | Elipse | 4 | 4.9 ± 2.0 | 13.5 ± 5.8 | 14.2 ± 5.0 | 67.0 ± 64.1 |
| Genco et al[59], 2018 | Observational | 38 | Elipse | 4 | 4.2 | 12.7 | 11.6 | 26 |
| Taha et al[77], 2020 | Observational | 96 | Elipse | 4 | 4.9 ± 2.0 | 11.2 ± 5.1 | 12.1 ± 5.2 | |
| Ponce et al[47], 2015 | RCT | 187/139 | ReShapeDuo diet/exercise | 6 | 25.1 ± 1.6/11.3 ± 1.9 | |||
| Agnihotri et al[50], 2018 | Observational | 202 | ReShapeDuo | 6 | 11.7 ± 7.3 | 11.4 ± 6.7 | 29.9 ± 18.2 |
BMI: Body mass index; %TWL: Percentage total weight loss; %EWL: Percentage excess weight loss; RCT: Randomised controlled trial.
Classical Orbera
In 2016 Moura et al[62] analyzed 9 out of 12 collected randomized controlled trials (RCTs), all between 1990 and 2014, in an effort to assess the effectiveness of the Orbera IGB-plus-diet against sham balloon-plus-diet. This meta-analysis found the balloon/diet treatment to be more effective than the sham/diet; the former obese patients experienced a higher BMI loss, with a mean difference of 1.41 kg/m2 (95%CI: -2.17 to –0.64, P = 0.0003) and a higher weight loss with a mean difference of 3.55 Kg (95%CI: -6.20 to -0.90, P = 0.009). Regarding %EWL, a higher %value was found by the Student’s t test in balloon groups, with a mean difference of 14.0% compared to the sham group; however, no significant difference was found between the groups by quantitative analysis, due to a significant heterogeneity of the studies. Furthermore, there are some serious limitations in the study: besides the long period of time covered by the collected RCTs, the main problem is that some of these studies were conducted in the early years of Orbera use; the second is the small number of patients (from 8 to 31 per study group) in all studies except one, which included 187 patients and 139 controls.
Since it is recommended that the Orbera IGB be filled with a volume, ranging between 400 and 700 mL of saline, Kumar et al[63] decided to correlate the balloon filling volume to clinically relevant endpoints, namely weight loss outcomes, balloon tolerability, and adverse events. This review, by the inclusion of 44 studies (5549 patients) demonstrating a low risk of publication bias, remains by far the largest meta-analysis of studies dealing with only Orbera balloons. Meta-analysis did not reveal any statistically significant association between filling volumes, between 400 and 700 mL, the percentage of TBWL being 13.2% (95%CI: 12.3–14.0) at 6 mo for all patients. The authors attributed the negative findings to the relationship between balloon size and volume: the diameter of a 400-mL saline-filled balloon is 9.14 cm, while those of a 700-mL is only 20% wider at 11.0 cm. Similarly, there was no association between balloon filling volume and early removal rates (P = 0.1), gastroesophageal reflux symptoms (P = 0.64), or gastric ulcer rates (P = 0.09). However, they recommend the balloon be inflated with a volume of 600–650 mL, since such a volume–inexplicably–reduces esophagitis: 9.4% vs 2.4% for a volume higher than 600 mL (P < 0.001), and migration rates: 2.26% vs 0.5% for a volume higher than 600 mL (P = 0.004).
Additionally, Yorke et al[64] reported, in their systematic review which included 26 studies (6101 patients), a reduction in body weight of 15.7 ± 5.3 kg and of BMI of 5.9 ± 1.0 kg/m2, although 25 of the 26 are case series and not RCTs. Furthermore, they presented a percentage of 23.3% of patients experiencing nausea and vomiting, and 19.9% epigastric pain; the incidence of mortality was 0.05%, the 0.1% attributed to gastric perforation.
Although meta-analyses are certainly considered more reliable because they provide cumulative information from RCTs well-controlled for their reliability, there are many serious problems in the subject analyzed: (1) randomized studies of balloon treatment against sham treatment are very few and with a small number of cases; (2) not all studies included in a meta-analysis provide the same information regarding weight loss assessment parameters; and (3) studies comparing balloon types are also few, for two reasons: there are even now no observational studies with a large number of patients and no follow-up for most of the new balloons. The Orbera balloon, on the other hand, has a long history of clinical application and is thus considered trustworthy and reliable by the clinician, deterring many clinicians from changing from the well-known and safe Orbera just for the sake of a study. Thus, observational studies with a large number of patients were unavoidably used in the present analysis.
The most populated retrospective study (2515 patients) from the data-base of the Italian Collaborative Study Group, Genco et al[65] in 2005 reported a mean BMI reduction of 4.9 ± 12.7 (range, 0–25 kg/m2) at 6 mo; from 44.4 ± 7.8 (range, 28–79.1 kg/m2) to 35.4 ± 11.8 (range, 24–73 kg/m2), and a mean EWL from 59.5 ± 29.8 (range, 16–210 kg) to 33.9 ± 18.7 (range, 0–87 kg), accompanied by a sign of resolution of diabetes and arterial hypertension in the majority of cases. Intolerance leading to early removal of the Bioenterics IGB was evidenced in 11 out of 2515 (0.44%) patients, while the overall complication rate was relatively low (2.8%).
A case series for 500 consecutive patients treated with the Bioenterics IGB, who were recruited from a single center and followed-up for a 5 year period was reported by Kotzampassi et al[13]. There was a mean body weight loss of 21.19 ± 10.3 kg or a 16.79% reduction, a mean BMI reduction of 7.39 ± 3.57 kg/m2 or 16.89%, and a percent EWL of 38.09 ± 20.18, meaning that a target of more than 20% EWL had been achieved in 83% of patients at the time of balloon removal. At the 60 mo follow-up, a total of 195 patients completed the study and were found to have retained a weight loss of 7.26 ± 5.41 kg, a BMI reduction of 2.53 ± 1.85 kg/m2, and a %EWL of 12.97 ± 8.54. At this time, 46 out of the 195 (23%) retained %EWL greater than 20%. The authors comment that those obese patients who lost 80% of their total weight loss during the first 3 mo of the 6-mo treatment, succeeded in maintaining a percent EWL of > 20 long-term after BIB removal: more precisely, this cutoff point was achieved in 83% at the time of removal and in 53%, 27%, and 23% at 12-, 24-, and 60-mo follow-up[13]. Quite similar were the results of a meta-analysis of 7 studies (409 patients) reporting a mean weight loss of 12.9 ± 0.8 kg at 3 mo and 16 ± 0.9 at 6 mo, meaning that 80% of the weight loss was achieved within the first 3 mo of treatment[66].
Similarly, in a large series of 714 consecutive Spanish patients treated with the BioEnterics IGB (now Orbera), Lopez-Nava et al[67] found their initial mean weight to be 106.3 ± 21.5 kg (range, 68–190), mean BMI 37.6 ± 5.7 kg/m2 (range, 31–57) and mean EW 56.3 ± 27.1 (range, 16–205 kg). After balloon removal at 6 mo, mean weight was 94.7 ± 22 (range, 52–160 kg); mean BMI 31.1 ± 7.2 (range, 24–48 kg/m2), mean %EWL 41.6 ± 21.8 (range, 0–77), mean weight loss 18.8 ± 9 (range, 0–45 kg); mean BMI loss 6.5 ± 12.7 (range, 0–21 kg/m2); and mean %EBL was 44.5 ± 22.6 (range, 0–81).
In 2015 American Society for Gastrointestinal Endoscopy (ASGE)[25] published a meta-analysis of 17 studies with 1638 patients which demonstrated a percentage of excess weight loss of 25.44% (95%CI: 21.47%-29.41%) with the Orbera balloon at 12 mo and a percentage of total weight loss of 11.27% (95%CI: 8.17%–14.36%) at 12 mo after implantation; thus they considered the Orbera balloon an appropriate treatment option since it exceeded the threshold of the preservation and incorporation of valuable endoscopic innovations of 5% TBWL.
In 2018, 39 Brazilian expert endoscopists[19] reached a consensus on guidelines on indications, patient selection, filling volume, techniques of insertion and removal and adverse events, based on their experience with 41.863 balloons-32.735 subjects with the non-adjustable fluid-filled Orbera (78.2%), another 16.9% with similar balloons, such as the Silimed, 1020 patients (2.4%) with the adjustable fluid-filled balloon Spatz and another 2.5% of cases with the Heliosphere air-filled balloon. The mean percentage total weight loss (%TWL) was 18.4% ± 2.9%, ranging from 13% to 25% and the mean BMI reduction was 7.2 ± 3.1 kg/m2, ranging from 3.5 to 18.0. The total early removal rate due to intolerance was 2.2% (928 cases)-more common with the adjustable balloon (2.5% in 1020 subjects), and rather uncommon (0.8%) with the Heliosphere air-filled balloon. The adverse event rate after the adaptation period was reported at 2.5%, the most common being 0.9% hyperinflation and 0.8% spontaneous deflation of the device. Finally, there were only 3 deaths; a gastric rupture due to overfeeding in a super-obese patient, a pulmonary aspiration with vomiting, and a pulmonary embolism, which may not have been directly attributable to the balloon.
The most recently published study was that from 5 private clinics in Brazil (2000-2017) by Fittipaldi-Fernandez et al[68], which included 5874 patients in whom a liquid-filled balloon not named, but having characteristics intimating the Orbera was placed (600-700 mL saline). After 6 to 7 mo, patients were found to have a weight loss of 19.13 ± 8.86 kg, and a %TWL of 18.42 ± 7.25%, treatment success rate, i.e. rate of patients achieving a %TWL over 10%, being 85%. The %EWL was 65.66 ± 36.24%, while BMI also decreased significantly, from 36.94 ± 5.67 to 30.08 ± 5.06 kg/m2, P < 0.0001.
Air-filled Heliosphere
Over time, new balloons have been designed, keeping the initial idea of the Orbera-space-occupation in the stomach-but looking to improve the characteristics responsible for the adverse events of nausea and vomiting early after implantation, i.e. the combination of large volume and weight of the saline filled balloon. Thus, in 2017 Saber et al[69] were the first to introduce the air-filled balloon in their meta-analysis. They analyzed a total of 20 RCTs (13 with the fluid-filled Orbera balloon and 7 with air-filled balloons) involving 1195 patients assessed prior to, at 3 mo after balloon placement, and upon its removal. Unfortunately, from the 7 studies – 190 cases only – relating to air-filled balloons, 6 concluded that the air-filled balloons were not effective. The overall meta-analysis, regardless of the balloon type, revealed a significant reduction of 1.59 and 1.34 kg/m2 for overall and for 3-mo BMI, respectively; a significant reduction of 14.25 and 11.16% for overall and > 3-mo percentage of excess weight loss, respectively; and a significant reduction of 2.81, 1.62, and 4.09 % for overall, 3-mo, and > 3-mo percent of weight loss, respectively. Overall a significant difference was calculated that favored the fluid-filled over air-filled IGBs; however, data was available only for a 3-mo study period comparison (P = 0.02). In general, due to the large heterogeneity within the studies (fluid and air-filled) the efficacy of all IGBs appears to be less impressive. However, generally speaking, the gas-filled balloons have better tolerance after implantation, but result in less weight loss in comparison to the fluid-filled[27].
Along the same line, Bazerbachi et al[52] analyzed 15 RCTs involving patients treated with FDA approved, fluid-filled (Orbera; 12 studies, ReShape Duo; 1 study) or air-filled balloons (Heliosphere; 1 study, Obalon; 1 study) for at least 6-mo compared with another balloon, sham-balloon, or open-label control groups, in an effort to assess the effectiveness and tolerability of each. In meta-analysis, the fluid-filled devices were found superior in achieving a significant change of %TBWL, in 96.8% and 96.6% of cases at 6 and 12 mo, respectively: the Orbera resulted in a 6.72% reduction of total body weight (95%CI: 5.55, 7.89); and the ReShape Duo 4% (95%CI: 2.69, 5.31) as opposed to the air-filled balloons Heliosphere and Obalon, which achieved 6.71% (95%CI: 0.82, 14.23) and 3.3% (95%CI: 2.30, 4.30), respectively. Although the fluid-filled balloons had the greater likelihood of being superior in achieving %TBWL, in the present meta-analysis the Orbera was finally associated with a non-significant difference in relation to the gas-filled Heliosphere 2.20% (-0.76, 5.16); the statistical findings probably relating both to the heterogeneity and small number of studies (Orbera n = 12 vs one for each other balloon type) for pair-wise comparisons. Finally, fluid-filled balloons were considered to be associated with a higher rate of intolerance; the combination of their high volume and weight have a profound impact on gastric motility, leading to a delay in gastric emptying of solids and thus to the increased sense of fullness and satiation, and as a result to body weight loss.
Adjustable Spatz
Another requirement in the Tarpon Springs Consensus meeting was that the balloon volume capacity be variable and adjustable, according to patient tolerance and success in losing weight. This was achieved with the Spatz adjustable balloon system by a rather complex and sophisticated mechanism which allows the filling volume to be adjusted, up or down, after implantation. Modifications ultimately resulted in the 3rd generation of adjustable balloons, the Spatz3.
One of the first available comparative studies carried out between 2010 and 2014, was that of Russo et al[70]. It comprised a small patient group: 20 elderly patients in whom the BioEnterics IGB was implanted and 10 patients given the Spatz Adjustable Balloon System. The two groups were compared in terms of weight loss, complications, and maintenance of weight after removal. They had a BMI ranging between 37 to 46 kg/m2 and a weight range of 103 to 165 kg. For both procedures, median BMI at the end of treatment was 32 ± 2 kg/m2 and the median weight loss was 20 ± 3 kg. At 6 mo follow-up, weight gains were 6 ± 1.5 kg for the 10 patients with the Bioenterics balloon vs 6 ± 2 kg for the five patients with the Spatz. In 2 out of each group the balloon was removed early, due to intolerance. In one additional BioEnterics balloon patient the balloon was removed due to deflation; and in 3 additional Spatz patients the balloon was adjusted due to intolerance, but finally two of the latter achieved no significant weight loss.
Abu Dayyeh et al[71], at 8 US centers, studied the efficacy and safety of the Spatz3 in 187 patients in relation to lifestyle modification alone for a 32-wk period. Percentage total weight loss was 14.9 ± 7.2% in the treatment group compared to 3.6 ± 5.8% in the control group; an additional 4.7% TBWL was achieved after upward volume adjustment between weeks 18 and 32 and more than 40% of the treatment group had maintained their weight loss at 56wks. Serious adverse events were reported at a rate of 5.3%, 4% of which were attributed to gastric ulcers.
Fittipaldi-Fernandez et al[45] presented 180 patients randomly divided into a Spatz3 balloon group in which the balloon was inflated with 600 mL of saline, the volume remaining stable throughout treatment, and a second Spatz3 balloon group in which the balloon volume was adjusted upward with 250 mL more saline. At removal, after 7.12 ± 1.63 mo, BMI was found decreased from 39.51 to 32.84 kg/m2 (P < 0.0001), body weight from 111.87 to 90.28 kg (P < 0.0001), and excess weight from 41.55 to 22.99 kg (P < 0.0001). The volume adjustment resulted in greater mean weight loss of only 4.35 kg, but no increased %TWL, %EWL, or decrease in BMI compared with the not-adjusted group. The authors conclude that the Spatz3 balloon seems to be an effective weight loss procedure, although it was found to be related to a higher morbidity (16.14%) in relation to traditional balloons.
Schwaab et al[72] 2020 published a cross-sectional study of 470 overweight or obese patients who were treated by either a non-adjustable IGB (Orbera), 326 subjects implanted for 6 mo; or an adjustable balloon (Spatz) in 144 subjects for up to 12 mo. A total of 414 out of 470 individuals completed the treatment period. The Orbera-treated patients achieved a %TBWL of 15.4 ± 7% and the Spatz-treated patients 15.5 ± 9.6%. Similarly, 264 Orbera-treated patients (88.6%) against 93 Spatz-treated patients (80.2%) achieved a %EWL over 25%, P = 0.038. However, the balloon volume adjustment seems not to have made a significant difference: within the Spatz group, 67 (85.9%) patients subjected to re-adjustment of balloon volume vs 27 (73%) not subjected to re-adjustment achieved a %EWL over 25%, P = 0.203.
Swallowable Obalon and Elipse
The Obalon, the gas-filled, swallowable IGB, designed to allow easy gastric volume titration by using additional balloons was studied against a lifestyle modification-alone group by Sullivan et al[73] (the SMART trial). A total of 387 patients were included from 15 centers in United States; 185 patients swallowed at least one Obalon capsule and 181 a sham capsule. After a 6 mo treatment period, the Obalon resulted in a %TBWL of 6.6 ± 5.1% in relation to 3.4 ± 5.0% in the control group, P = 0.0354, the difference being 3.2% (95%CI: 2.2, 4.2); the responder rate was 62.1% in the Obalon group, the end-point being 35% and 30.7% in control group, P < 0.0001. At 48 wk, subjects who had achieved a weight loss at week 24, maintained their loss at a rate of 88.5% (7.8 ± 4.4%TBWL at 24 wk and 6.9 ± 6.5% TBWL at 48 wk, n = 151). Finally, they presented 0.3% severe adverse events, including one bleeding gastric ulcer.
There are few previously published clinical studies, with only a small number of participants: Mion et al[54] in 2013 first reported a pilot study in 17 patients – 43 balloons - to assess the efficacy of the Obalon for weight loss over a 3mo study period. There was a median %EWL of 36.2 (range 0 to 118%) and a BMI reduction from 31.0 kg/m2 to 28.1 kg/m2, with no serious side-effects. Similarly, in 17 cases of pediatric/adolescent morbid obesity De Peppo et al[56] in 2017 reported a statistically significant decrease (P > 0.05) of mean BMI value from 35.27 ± 5.89kg/m2 to 32.25 ± 7.1 kg/m2; and a %EWL of 20.1 ± 9.8 (range 2.3 to 35.1) after 3 mo of treatment.
The Elipse IGB is a swallowable fluid-filled balloon, which is spontaneously deflated at week 16 and passes through the gut to be self-removed through the natural orifice; it can thus be considered the ‘evolution’ of the Obalon, since it is both placed and removed without the need of anesthesia and endoscopy. Recently, Ienca et al[58] published the largest trial comprising 1770 consecutive Elipse patients. After 4 mo treatment a weight loss of 13.5 ± 5.8 kg, a %EWL of 67.0 ± 64.1, a BMI reduction of 4.9 ± 2.0, and a %TBWL 14.2 ± 5.0 was reported. Eleven emptied balloons (0.6%) were vomited and another 52 (2.9%) were endoscopically removed due to patient intolerance. Three deflated balloons led to small bowel obstruction, requiring surgical intervention.
The difference in the reliability of the statistical results depends on the number of patients in the study sample, as well as the use of a multidisciplinary approach and counseling for these patients; thus Genco et al[59] presenting their early experience with the Elipse balloon in only 38 Italian patients who received a multidisciplinary approach, reported a mean weight loss of 12.7 kg, a %EWL of 26%, a mean BMI reduction of 4.2 kg/m2, and a %TBWL of 11.6%.
At the same time, Vantanasiri et al[74] 2020 published a systematic review and meta-analysis of six prospective studies of the Elipse balloon, involving 2013 patients. The largest study was that already discussed (Ienca et al[58]–1770 patients) and the other 5 were small cohort studies (30 to 135 patients) with high heterogeneity. The mean %TWL after completion of treatment (4 to 6 mo) was 12.8% (95%CI: 11.6%–13.9%; I2 = 83%) and at 12 mo 10.9% (95%CI: 5.0%–16.9%, I2 = 98%). However, the long-term effects after the Elipse balloon treatment still remain unclear. Additionally, there is no study comparing the Elipse balloon with any other IGB. A rate of 0.2% of serious adverse events was reported; three patients suffered small bowel obstruction due to a deflated balloon and one experienced gastric perforation, resolved surgically. Although it seems to be safe and easily handled, its application by an inexperienced bariatric endoscopist, as no endoscopy is needed, poses the risk of overlooking or misunderstanding a serious adverse event, as Angrisani et al[75] points out in his commentary entitled “the pitfalls of excessive simplicity”.
In the same year another meta-analysis of 7 Elipse balloon-studies, involving 2152 patients was conducted by Ramai et al[76], with the same disadvantage as the previous one: only Ienca’s study[58] had 1770 cases, while all other six studies ranged from 12 to 135 cases, with high heterogeneity. The results, however, were quite similar: %TBWL was 12.2% (95%CI: 10.1-14.3, I2 = 94%) and %EBWL was 49.1% (95%CI: 30.6-67.5, I2 = 97%). Pooled adverse events were 37.5% abdominal pain, 29.6% vomiting, 15.4% diarrhea and 0.5% small bowel obstruction.
Finally, a recent study of 96 patients from Egypt, not included in the previous meta-analyses, was published by Taha et al[77], 2020. After the 4 mo period following implantation the %TBWL was 12.1 ± 5.2%, the mean weight loss was 11.2 ± 5.1 kg, and the mean BMI reduction was 4.9 ± 2.0 kg/m2. The authors also reported 3.1% intolerance, resulting in early balloon removal; one (1.1%) balloon deflated early and was uneventfully passed, and, surprisingly, there were 11.5% attacks of diarrhea and 21.9% of colicky abdominal pain for a week around the time of balloon self-deflation.
Double balloon
Regarding the ReShape Duo IGB, Ponce et al[48], 2013 published the first results after its placement in 21 subjects vs 9 controls-diet only. These data belong to the phase 1 portion of the REDUCE study, which stopped prematurely to be redesigned, since its primary endpoints seemed to be unachieved. At 6mo these patients presented no significant difference in %EWL, although their findings were not negligible (31.8% ± 21.3% in the balloon group and 18.3% ± 20.9% in the controls, respectively, P = 0.1371); a percentage of 64% of balloon-treated maintained their weight loss 6mo after balloon removal.
Two years thereafter Ponce et al[47] presented the final results of the REDUCE pivotal trial: the ReShape balloon-treated patients (n = 187) had a 25.1 ± 1.6% (mean ± SE) %EWL, 48.8% of cases achieving a %EWL over 25% vs 11.3 ± 1.9% in the diet and exercise only control patients (n = 139), P = 0.0041; sudden balloon deflation occurred in 6% of cases, but no migrations; balloon intolerance led to early balloon removal in 9%. Gastric ulcers at the level of gastric incisura were initially observed in 35% of patients due to pressure of the distal tip of the device. After a minor modification to make it shorter, smoother and with a 50% reduced diameter, the frequency of ulcers dropped to 10%.
Another study with 202 patients in whom the Reshape Duo balloon had been placed was published in 2018 by Agnihotri et al[50]. At 6 mo they reported a statistically significant decrease (P < 0.001) in BMI values from 36.8 ± 8.4 kg/m2 in baseline to 32.8 ± 6.7 kg/m2, a %TBWL of 11.4 ± 6.7% and a %EWL of 29.9 ± 18.2%. The authors also referred to a high rate of nausea, vomiting and abdominal pain in the early days: 66.4%, 49% and 25.2%, respectively, leading to a 6.4% of early balloon removal. Finally, there was only one case of balloon migration, resulting in a small bowel obstruction and requiring surgical intervention.
Finally, Suchartlikitwong et al[49] in 2019 presented their experience in 35 cases using the Reshape Duo balloon. They reported a 7% decrease in BMI value, or 2.7 ± 2.9 kg/m2, P < 0.001. Nausea and vomiting presented in 23% of patients, requiring balloon removal in two. 3% of patients suffered gastric erosions, but one patient with a history of ulcer experienced gastric hemorrhage requiring blood transfusion. Finally, one patient required surgery for balloon removal after deflation and distal movement leading to bowel obstruction.
Efficacy and tolerability
Looking for comparative assessment of the efficacy of IGBs, Kotinda et al[12] performed a systematic review and meta-analysis of 13 randomized controlled trials (1523 overweight and obese adults) focusing on the efficacy of IGBs for weight loss. Eight studies used the Orbera, one the Orbera or Heliosphere (gas-filled), two the ReShape Duo, and one each the Spatz and theObalon (gas-filled). They found a highly significant difference in mean %EWL of 17.98% (95%CI: 8.37-27.58, P < 0.00001) in the balloon group in comparison to the sham/life-style modification group. In the subgroup analysis there was no significant difference between balloon types for this outcome. When assessing data in respect to %TWL, they also found a highly significant difference in mean %TWL of 4.40% (95%CI: 1.37-7.43, P < 0.00001), but, in subgroup analysis, this effect was mostly related to the Spatz balloon [11.30 (9.77, 12.83)], although other balloons (Obalon, Orbera, and ReShape Duo) also had favorable outcomes. However, on analysis of the data in relation to BMI loss, a significant difference of 2.13 Kg/m2 (95%CI: 0.57-3.68, P < 0.00001) was found in the balloon group, while in subgroup analysis it was mainly due to the Orbera balloon [2.49 (0.19, 4.80)], although the Obalon, Heliosphere, and ReShape Duo also showed favorable results. They finally analyzed the values of absolute weight loss, not commonly found as a study parameter. From a total of 7 studies (1005 participants), a mean difference of 6.12 kg (95%CI: 3.80 to 8.44, P < 0.00001), in favor of the balloon group was evident, mainly achieved by the Orbera balloon [7.88 (3.81-11.95)], although the Obalon and the ReShape Duo also had positive outcomes.
IGBs are space-occupying devices designed to induce satiety and thus reduce food intake, which ultimately results in weight loss; it is reasonable and obvious to expect that the sudden but permanent onset of fullness of the stomach by means of increasing the balloon volume, and, in the case of fluid-filled balloons, of the additional sensation of weight could be ‘translated’ by the obese as a sense of persistent nausea and/or tendency to vomit, as well as generalized abdominal pain and/or discomfort, back pain, and acid reflux. These accommodative symptoms are common after balloon placement, but are usually self-limiting. In terms of patient tolerance of the IGB, and especially during the first 1-2 wk of placement, Trang et al[78] in 2018 conducted a systematic review and meta-analysis of the incidence of nausea and vomiting after IGB placement in bariatric patients. In this review of 10 studies they focused on four types of balloons: the fluid-filled Orbera, the ReShape Duo, the Elipse, and the gas-filled Obalon, and calculated the meta-analytic rates of nausea and vomiting based on adverse event sample size. A total of 564 out of 938 patients reported nausea; 63.33% (95%CI: 61.49%–65.16%), and 507 patients reported vomiting; 55.29% (95%CI: 53.59%–56.99%). Fluid-filled balloons were placed in obese participants in 7 studies: 394 and 434 out of 575 patients experienced nausea and vomiting respectively; rates of 72.99% (95%CI: 69.54%–76.45%) and 76.95% (95%CI: 73.86%–80.05%), respectively. The gas-filled Obalon balloon, was used in 3 studies: 200 and 62 out of 363 patients reported nausea and vomiting, respectively; rates of 55.10% (95%CI: 50.00%–60.00%) and 16.20% (95%CI: 12.43%–19.96%), respectively. Further analysis of fluid-filled balloons, i.e. the Orbera, ReShape Duo, and Elipse, revealed that the Orbera balloon caused the highest rates of nausea and vomiting compared to all other balloons. Three studies using the Orbera reported nausea and vomiting in 195 and 177 out of 248 individuals respectively; rates of 81.97% (95%CI: 77.00%–87.00%) and 72.16% (95%CI: 66.65%–77.67%) respectively. Comparatively, 2 studies with the ReShape and another 2 with the Elipse balloons reported nausea and vomiting respectively in 178 and 246 out of 285 patients and in 21 and 23 out of 42 patients; rates of 63.18% (95%CI: 58.00%–69.00%) and 86.42% (95%CI: 82.44%–90.39%) for the ReShape and 51.42% (95%CI: 46.00%–57.00%) and 12.48% (95%CI: 8.51%–16.44%), for the Elipse, respectively. The authors comment that the large variation rate of symptoms, even that of vomiting, [a relatively objective parameter], apart from the type of balloon used, might be related to the type, the dosage and the frequency of medications prescribed during any specific study.
Gastric emptying and weight loss
Based on the general hypothesis that the rates of gastric emptying and the stomach accommodation volume regulate food intake, appetite, satiation and satiety, and are thus associated with postprandial fullness, bloating, and finally weight loss, Vargas et al[24] analyzed the changes in time of gastric emptying in 19 studies, after either IGB placement or bariatric surgery. Fluid-filled balloons (3 studies) increased gastric emptying time by 116 min (95%CI: 29.4–203.4 min) as opposed to air-filled balloons (2 studies) which did not result in a statistically significant difference in gastric emptying time [-2.9 min (95%CI: -21.7 to 15.9 min)]. When authors analyzed pooled data of 5 studies, the mean change in gastric emptying time was only 42.7 min, (non-significant); however, meta-regression revealed prolongation of gastric emptying time which was associated with a higher percentage of total body weight lost at 6 mo (P = 0.05). When the association between gastric emptying time and weight loss was analyzed in fluid-filled (Orbera) balloons, the significantly prolonged gastric emptying time led to a greater excess weight loss at 6 mo (P = 0.04), potentially explaining the difference in efficacy and tolerance found across air vs fluid-filled balloons[52].
Quality of life and mental health
Gadd et al[79] tried to analyze the impact of endoscopic bariatric procedures, IGBs included, in the improvement of quality of life (QoL) and mental health, assessed by using a validated tool. Twenty studies published between 2008 and 2019 with a total number of 876 participants (77% female) were included, evaluating five different endoscopic procedures. Fourteen out of 20 referred to IGBs and finally 9 (371 participants - 350 at 6 to 76-mo follow-up) were included via meta-analysis. IGB placement was associated with a significant improvement in QoL (SMD: 0.78; 95%CI: 0.56, 1.00; P = 0.05; I2: 48%). Following sensitivity analysis, IGB placement was associated with a large improvement in post-procedural QoL (SMD: 0.85; 95%CI: 0.69, 1.02; P < 0.00001; I2: 7%). Five studies (367 participants at 6 to 76 mo follow-up) out of the nine were analyzed in respect to mental health, depression, and anxiety, and IGBs revealed a significant improvement (SMD: 0.86; 95%CI: 0.29, 1.42; P = 0.003; I2 = 92%). All studies correlate improvement of quality of life, mental health, depression, and anxiety with significant improvement in obesity related parameters. The two studies (Guedes et al[80] and Deliopoulou et al[81]) with the largest improvements in mental health also had the greatest weight loss. However, the authors commented that all these patients received multidisciplinary support in the form of unlimited 24-h phone support, follow-up by a dietitian and nutrition counseling, cognitive behavioral therapy, and/or a lifestyle modification programme. The greater the support, the more significant the improvement in mental health and weight loss.
DISCUSSION
The IGB is a well-established therapeutic tool for the treatment of obesity, being the most popular technique of those included under the concept of endoscopic bariatric and metabolic therapies, which have emerged over the years, to provide alternative options beyond lifestyle modifications, pharmacotherapy, and surgery. It is actually a completely non-invasive endoscopic technique, in the absolute sense of the term, since its leading advantage is that it does not interfere permanently with the anatomy and volume-shaping of the stomach by means of interventions in the gastric wall, such as sutures, stomas, thermal destruction of the mucosa, etc., used by other modern endoscopic techniques.
Thus, IGB insertion represents a generally safe, easy to perform, adjustable, reversible, and reproducible endoscopic gastric restriction procedure, successfully applied for weight loss over the last 30 years. It covers a broad spectrum of indications from the overweight to the obese individual who does not fulfill the criteria for bariatric surgery, up to the morbidly obese, who qualifies for bariatric surgery but has uncontrolled co-morbidities causing her/him to be of high-risk for anesthesia and surgery or denied anesthesia and/or surgery, or its use as a bridge to bariatric surgery, and, finally, to anyone who just needs to achieve limited weight reduction, either prior to surgery of whatever kind and for whatever reason or merely for aesthetic purposes[51,82,83]. Generally speaking, the specific indications for balloon implantation for each candidate for such treatment must be built on the absolute judgment of the treating physician or the multidisciplinary working team; however, the positive response, that is the weight loss, is due exclusively to the responsibility of the patient to strictly adhere to a diet/exercise program and follow-up sessions throughout the treatment period, whatever type of balloon has been used.
To reconfirm the advantages of the procedure, we use the concepts formulated by Fobi and Baltasar to define quality indicators for bariatric surgery procedures which should also be somehow applicable to bariatric endoscopy[84]. According to these criteria any relevant procedure should be: (1) safe, exhibiting a mortality of less than 1%, and a morbidity of less than 10%; (2) effective and long-lasting, with excess weight loss of over 50% in more than 75% of patients at 5 year follow-up; (3) reproducible, so the results of different centers performing the procedure provide a similar, easy learning curve; (4) provide good quality of life; (5) require revisions less than 2%; (6) have minimal adverse effects; and (7) be easily reversible, from an anatomical or functional perspective.
However, IGB effectiveness, as a non-permanent intervention, remains debatable, as there is no consensus on the proportion of weight loss that should be achieved for an endoscopic procedure to be considered effective and thus be recommended for clinical use. The ASGE[25] defined a mean minimum threshold of 25% EWL, measured at 12 mo, for any endoscopic bariatric and metabolic therapy intended as a primary obesity intervention, and 5% of %TBWL as the absolute minimum threshold for any non-primary intervention, such as bridging therapy. It also recommended that the risk of serious adverse events related to the procedure be equal or less than 5%-most of the reported adverse events with IGBs (nausea, vomiting, abdominal pain) are classified as mild to moderate, according to ASGE Quality Task Force recommendations[25].
Today there are already six commercially available balloons, three of which are FDA-approved; some of them having one or more ‘clones’, available in different parts of the world. Chronologically, the first balloon designed and manufactured according to the Tarpon Springs Directives was the Bioenterics IGB (now available as the Orbera)[20]. Based on the advantages and disadvantages of this balloon, there have been many attempts to develop new balloons, incorporating technical improvements, but without compromising the baseline characteristics of the Orbera, which has long remained at the top of the field.
The main disadvantages of the Orbera balloon, which should be improved, are the following: (1) The balloon placement and removal must be performed by means of endoscopy, and at least the removal to be done under conscious sedation, which increases not only the overall cost of treatment, but also the potential risks of both endoscopy and anesthesia; (2) The first week after balloon placement patients experience some degree of discomfort, in the form of nausea, vomiting and epigastric pain, well-attributed to the 600-700 gr of saline with which the balloon is inflated. This etiology is true for all fluid-filled balloons. On the other hand, this is the feature which makes the fluid-filled devices more effective in weight loss, in comparison to gas-filled balloons; (3) The effectiveness of the Orbera and of other fluid-filled balloons is generally satisfactory, especially when combined with diet and exercise counseling and the patient is under a multidisciplinary assessment group, not excluding, occasionally, psychiatric supervision. After balloon removal, however, the maintenance of good results in weight loss varies in the long-term, depending on many subject-related and not balloon-related factors, as, exactly similarly, occurs in real life; ex-obese individuals must maintain the new habits and lifestyle, feeding re-education and physical exercise, but mainly the behavioral modification and positive psychological state resulting from the changes in their physical appearance (body shape), physical functioning through improvements in co-morbidities, and social functioning due to increased self-esteem[13,85,86].
Based on this, some argue that a long-lasting balloon such as one with 12 mo lifespan in the stomach (the Orbera365 and the Spatz3) may be more useful since it allows more time for life-style re-education to become habituated[87,88]. On the other hand, it is well known that the greatest weight loss, even up to 80% of the total %EWL, is achieved within the first 3 mo of balloon-life in the stomach; weight loss then continues, but at a reduced percent monthly[13,66,89]. Thus, a 12-mo lifespan balloon probably offers questionable benefits. It might also be suggested that long-term contact with gastric mucosa, especially if the balloon is not totally smooth and spherical (Spatz3), could be more traumatic, possibly resulting in gastric mucosal erosions and bleeding.
The counter-argument would be that the 4 mo life-span of the Elipse could be considered an inadequate time to achieve the desired results. Although the 6 mo balloons achieve the greatest weight loss within the first 3 mo, the additional 3 mo in the stomach is a time during which it works at very least as a space-occupying device preventing excessive food intake and consequently of early weight gain.
Unfortunately, there are no studies at all comparing the weight loss with the classical Orbera against the new Orbera365 - that is 6 mo vs 12 mo of the balloon remaining in the stomach. Theoretically, this could be an argument for inserting two consecutive balloons, but there is little evidence of success achieved by the second, which is why some authors recommend a time lapse between the first and second balloon[90,91]. In contrast, the application of the Spatz3 for 12 mo cannot be compared with the Orbera365, since the latter is designed as ‘adjustable’, meaning that at 3 mo, when the patient stops losing weight quickly, a volume of 250 mL of saline is added, changing both the volume and weight of the balloon, and thus the results. However, when compared, the weight loss between groups in which the Spatz3 balloons was adjusted or not, no significant difference was found[45].
Comparing the filling volume of the various liquid-filled balloons, it is clear that the volume of the balloon does not seem to directly determine weight loss. This was demonstrated in a study in which the Orbera balloon was filled with volumes of 400 mL to 700 mL[63], but also from the results of all studies with various balloons, with more or less the same volumes of saline. Furthermore, it is well known that short-term satiety is primarily affected by gastric distension and gastric volume; as we know from research that mechanical gastric balloon distension to a volume greater than 400 mL during meals significantly reduces oral intake[92,93]. However, it should emphasized that gastric distension and gastric volume are related to the weight and volume of the ‘food’, rather than its energy content, thus decisions regarding food ingredients has to rely on the patient's choice to comply with dietary rules[23,92].
For this reason all patients must undergo a psychological screening before entering the process of balloon implantation[61,86]. This does not in any way mean that obese patients with bipolar disorders or other psychiatric diseases under medication should be excluded from treatment. On the contrary, it seems that there is a clear improvement in depression status with weight loss and the improvement of their body image[13,85,94], called by Spirou et al[95] the “psychological honeymoon period”. In our opinion, a key component in their preliminary interview must be for the obese individuals to describe the social and psychological impact of obesity on their life, make a brief statement on their motivation to lose weight (for instance, to alleviate physical symptoms or to become more attractive/marriageable), and to recognize how they are affected by external factors, such as social support and reinforcement. This information – particularly the reason for strongly desiring to lose weight - should then be used at every follow-up session to inspire them to continue the effort towards weight loss or loss maintenance[13].
Another essential tool for achieving a significant and sustainable weight loss is the requirement for the patient to attend follow-up consultation sessions, which also bolster self-confidence. In a study analyzing 583 obese individuals treated with the Orbera balloon in respect to weight loss, the group of successful responders (%EWL more than 50%) and the group of poor responders (%EWL less than 20%) were compared. 85.2% of successful responders, n = 162, had attended the maximum of six interviews, whereas the 83.8% of the 105 poor responders attended fewer than four interviews[13,85,96]. Similar results were reported by Schwaab et al[72]: patients with more than four consultations achieved notably higher %EWL values (more than 18%, P < 0.001).
As has already been mentioned in the ‘drawbacks’ to the Orbera, the liquid-filled balloons have a higher rate of intolerance during the first week after implantation; which is why air-filled (Heliosphere bag) or gas-filled balloons (Obalon) were designed. The Heliosphere has a volume of 550 mL, but a weight of only 30 gr, thus allowing a soft transition to new nutritional status, without nausea and vomiting, but in exchange for less weight loss in some studies. Some difficulty in balloon placement through gastric cardia has also been reported[12]. To overcome the same problem of early intolerance, the Spatz3 was designed with the unique feature of post-implantation volume control, meaning its volume can be reduced in case of early intolerance and, when symptoms cease, the volume can be increased. These procedures do, however, presuppose anesthesia and endoscopy[44,45].
The improvements and advances made in the design of the other balloons (the ReShape Duo, the Obalon and the Elipse) modifying the classic Orbera configuration, could be summarized as follows: The ReShape Dual balloon system[30,47] has been re-designed as two smaller, independent silicone spheres of 450 mL each, joined by a central, short, non-communicating flexible silicone shaft. This flexible balloon configuration allows them to conform to the natural anatomy of the stomach, while decreasing the chance of balloon intestinal migration should one of the balloons accidentally deflate[5,47,56,57]. Unfortunately, Apollo Endosurgery discontinued this product line after purchasing ReShape Medical Inc, CA, in 2018.
The Obalon and the Elipse balloons have the advantage of not requiring endoscopy for insertion and, in the case of the Elipse, for removal too, both being easily swallowable. Nevertheless, fluoroscopy is mandatory for proper positioning, because although the total cost of treatment is significantly reduced, as is the theoretical danger of complications due to anaesthesia and endoscopy, there is still a risk[59]. However, the endoscopy-free insertion carries its own disadvantages: the balloon is placed in a stomach with unknown mucosal pathology, and unknown anatomy, thus all the ‘exclusions’ described for the other balloons remain obscure (huge hiatus hernia, gastric ulcer/erosions, prior gastric surgery). The Elipse has the additional advantage of being degradable after a 4mo period, when it freely passes through the rectum.
Major complications related to IGB placement include esophageal/ gastric ulcerations and tears due to permanent mucosal irritation by the balloon or iatrogenic trauma and/or perforation during balloon insertion and, mainly, removal; and bowel obstruction, due to balloon self-deflation and migration to the gut[97]. According to the Tarpon Springs directives[20] for “the safe and effective balloon” a balloon must have “a smooth surface having low potential for causing erosions, ulcers or obstructions”. The greatest conformity to this description is the Orbera. The early design flaw of the ReShape Duo, with the distal tip, was the cause of gastric ulceration in up to 35% of cases, which, however, dropped immediately to 10% after design modification[48]. Similarly, the Spatz3 balloon, although exactly meeting the criterion of being adjustable, has failed to fulfill the criterion of having a completely smooth surface, since it has a sort of ‘tail’ at the site of insertion of the filling valve[43]. This balloon has also been implicated in causing acute pancreatitis[98].
In a recent publication Stavrou et al[99] systematically reviewed PubMed and Scopus archived publications up to the end of 2018, describing Orbera-related life-threatening visceral complications, i.e. perforations and obstructions, and classified them according to blame: the device, the patient or the doctor. In a total of over 277000 balloons implanted worldwide by the end of September 2018, according to Apollo Endosurgery reports[100], 22 cases of gastric perforation, 2 cases of esophageal perforation and 10 cases of bowel obstruction were found. For the gastric perforation the endoscopist was responsible in 9 cases, the patient in 4, and the balloon itself in 9. For the 2 cases of esophageal perforation, the endoscopists were responsible, while for the 12 cases of bowel obstruction, the patient was responsible for 7 and the device for the other 5 cases.
CONCLUSION
As a final comment at the end of this analysis, we must underline that balloon placement, and even more balloon endoscopic removal should not be considered to be, in any way, a simple endoscopic procedure to be carried out by an inexperienced endoscopist. Individual doctors or even institutions without experience, accreditation, or the ability to resolve obesity-related or bariatric surgery-related complications must not undertake such procedures, if we do not want an increase in complications[95,101,102]. This danger increases with the increased availability of swallowable balloons on the market. Their advertising and the ease of use, as presented, can become a disastrous trap if an uncertified and inexperienced doctor dares to use them. The fact that endoscopy is not mandatory and becomes a matter of patient choice removes the necessity for a doctor with the appropriate training to be able to recognize and deal with any complication which might suddenly occur. This point is further emphasized in the latest published directives of the ASGE: “...training and skill acquisition with endoscopic bariatric techniques and technologies is mandatory before clinical application is undertaken, and should include didactic as well as hands-on practical education”. And, furthermore, “...importantly, any practitioner who is interested in performing an endoscopic bariatric procedure should also be educated in the clinical management of obese patients,” which means, have the ability to resolve complications[25].
From the above analyses, it is clear that: (1) There are no “good” and “bad” balloons, at first glance; all new balloons must be given an equal chance to be tested by experienced endoscopists before being judged; and (2) There is no special indication for the use of a particular balloon - all fit all stomachs. However, the use of one rather than another of the six balloons mentioned in this review, or between some others of lower cost, or of national manufacturers, relies on the absolute discretion of the physician, and not of the obese patient, and I personally never discuss it.
Footnotes
Conflict-of-interest statement: All authors declare no conflict of interest for this article
Manuscript source: Invited manuscript
Peer-review started: March 10, 2021
First decision: May 5, 2021
Article in press: July 13, 2021
Specialty type: Surgery
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rabago LR, Romano L S-Editor: Zhang H L-Editor: A P-Editor: Wang LYT
Contributor Information
George Stavrou, Department of Colorectal Surgery, Addenbrooke’s Hospital, Cambridge CB22QQ, United Kingdom. stavgd@gmail.com.
Anne Shrewsbury, Department of Surgery, Endoscopy Unit, Aristotle University of Thessaloniki, Thessaloniki 54636, Greece.
Katerina Kotzampassi, Department of Surgery, Endoscopy Unit, Aristotle University of Thessaloniki, Thessaloniki 54636, Greece.
References
- 1.Bazerbachi F, Vargas EJ, Abu Dayyeh BK. Endoscopic Bariatric Therapy: A Guide to the Intragastric Balloon. Am J Gastroenterol. 2019;114:1421–1431. doi: 10.14309/ajg.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 2.Pajot G, Calderon G, Acosta A. Endoscopic Treatments for Obesity. Curr Treat Options Gastroenterol. 2017;15:660–675. doi: 10.1007/s11938-017-0158-7. [DOI] [PubMed] [Google Scholar]
- 3.Ruban A, Doshi A, Lam E, Teare JP. Medical Devices in Obesity Treatment. Curr Diab Rep. 2019;19:90. doi: 10.1007/s11892-019-1217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikolić M, Boban M, Ljubicić N, Supanc V, Mirosević G, Nikolić BP, Zjacić-Rotkvić V, Gaćina P, Mirković M, Bekavac-Beslin M. Position of intragastric balloons in global initiative for obesity treatment. Coll Antropol. 2011;35:1353–1362. [PubMed] [Google Scholar]
- 5.Laing P, Pham T, Taylor LJ, Fang J. Filling the Void: A Review of Intragastric Balloons for Obesity. Dig Dis Sci. 2017;62:1399–1408. doi: 10.1007/s10620-017-4566-2. [DOI] [PubMed] [Google Scholar]
- 6.Farha J, Abbarh S, Haq Z, Itani MI, Oberbach A, Kumbhari V, Badurdeen D. Endobariatrics and Metabolic Endoscopy: Can We Solve the Obesity Epidemic with Our Scope? Curr Gastroenterol Rep. 2020;22:60. doi: 10.1007/s11894-020-00798-8. [DOI] [PubMed] [Google Scholar]
- 7.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD Endocrine Society. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 9.Sharma AM, Padwal R. Obesity is a sign - over-eating is a symptom: an aetiological framework for the assessment and management of obesity. Obes Rev. 2010;11:362–370. doi: 10.1111/j.1467-789X.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 10.Delgado-Aros S, Cremonini F, Castillo JE, Chial HJ, Burton DD, Ferber I, Camilleri M. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126:432–440. doi: 10.1053/j.gastro.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982;1:198–199. doi: 10.1016/s0140-6736(82)90762-0. [DOI] [PubMed] [Google Scholar]
- 12.Kotinda APST, de Moura DTH, Ribeiro IB, Singh S, da Ponte Neto AM, Proença IM, Flor MM, de Souza KL, Bernardo WM, de Moura EGH. Efficacy of Intragastric Balloons for Weight Loss in Overweight and Obese Adults: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes Surg. 2020;30:2743–2753. doi: 10.1007/s11695-020-04558-5. [DOI] [PubMed] [Google Scholar]
- 13.Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg. 2012;22:896–903. doi: 10.1007/s11695-012-0607-2. [DOI] [PubMed] [Google Scholar]
- 14.Doldi SB, Micheletto G, Perrini MN, Librenti MC, Rella S. Treatment of morbid obesity with intragastric balloon in association with diet. Obes Surg. 2002;12:583–587. doi: 10.1381/096089202762252398. [DOI] [PubMed] [Google Scholar]
- 15.Mathus-Vliegen EM, van Weeren M, van Eerten PV. Los function and obesity: the impact of untreated obesity, weight loss, and chronic gastric balloon distension. Digestion. 2003;68:161–168. doi: 10.1159/000075525. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 17.Funk LM, Jolles S, Fischer LE, Voils CI. Patient and Referring Practitioner Characteristics Associated With the Likelihood of Undergoing Bariatric Surgery: A Systematic Review. JAMA Surg. 2015;150:999–1005. doi: 10.1001/jamasurg.2015.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathus-Vliegen E, Spångeus A, Walter S, Ericson AC. Weight loss with or without intragastric balloon causes divergent effects on ghrelin cell expression. Obes Sci Pract. 2021;7:199–207. doi: 10.1002/osp4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neto MG, Silva LB, Grecco E, de Quadros LG, Teixeira A, Souza T, Scarparo J, Parada AA, Dib R, Moon R, Campos J. Brazilian Intragastric Balloon Consensus Statement (BIBC): practical guidelines based on experience of over 40,000 cases. Surg Obes Relat Dis. 2018;14:151–159. doi: 10.1016/j.soard.2017.09.528. [DOI] [PubMed] [Google Scholar]
- 20.Schapiro M, Benjamin S, Blackburn G, Frank B, Heber D, Kozarek R, Randall S, Stern W. Obesity and the gastric balloon: a comprehensive workshop. Tarpon Springs, Florida, March 19-21, 1987. Gastrointest Endosc. 1987;33:323–327. doi: 10.1016/s0016-5107(87)71611-3. [DOI] [PubMed] [Google Scholar]
- 21.Sam AH, Troke RC, Tan TM, Bewick GA. The role of the gut/brain axis in modulating food intake. Neuropharmacology. 2012;63:46–56. doi: 10.1016/j.neuropharm.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148:1219–1233. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ello-Martin JA, Ledikwe JH, Rolls BJ. The influence of food portion size and energy density on energy intake: implications for weight management. Am J Clin Nutr. 2005;82:236S–241S. doi: 10.1093/ajcn/82.1.236S. [DOI] [PubMed] [Google Scholar]
- 24.Vargas EJ, Bazerbachi F, Calderon G, Prokop LJ, Gomez V, Murad MH, Acosta A, Camilleri M, Abu Dayyeh BK. Changes in Time of Gastric Emptying After Surgical and Endoscopic Bariatrics and Weight Loss: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020; 18: 57-68. :e5. doi: 10.1016/j.cgh.2019.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee. Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, Sullivan S, Thompson CC, Banerjee S. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 2015; 82: 425-38. :e5. doi: 10.1016/j.gie.2015.03.1964. [DOI] [PubMed] [Google Scholar]
- 26.Gómez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: Results of a prospective study. Obesity (Silver Spring) 2016;24:1849–1853. doi: 10.1002/oby.21555. [DOI] [PubMed] [Google Scholar]
- 27.Štimac D, Klobučar Majanović S, Belančić A. Endoscopic Treatment of Obesity: From Past to Future. Dig Dis. 2020:1–13. doi: 10.1159/000505394. [DOI] [PubMed] [Google Scholar]
- 28.Tsesmeli N, Coumaros D. Review of endoscopic devices for weight reduction: old and new balloons and implantable prostheses. Endoscopy. 2009;41:1082–1089. doi: 10.1055/s-0029-1215269. [DOI] [PubMed] [Google Scholar]
- 29.Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis. 2016;12:430–435. doi: 10.1016/j.soard.2015.10.074. [DOI] [PubMed] [Google Scholar]
- 30.Giardiello C, Borrelli A, Silvestri E, Antognozzi V, Iodice G, Lorenzo M. Air-filled vs water-filled intragastric balloon: a prospective randomized study. Obes Surg. 2012;22:1916–1919. doi: 10.1007/s11695-012-0786-x. [DOI] [PubMed] [Google Scholar]
- 31.Wahlen CH, Bastens B, Herve J, Malmendier C, Dallemagne B, Jehaes C, Markiewicz S, Monami B, Weerts J. The BioEnterics Intragastric Balloon (BIB): how to use it. Obes Surg. 2001;11:524–527. doi: 10.1381/096089201321209468. [DOI] [PubMed] [Google Scholar]
- 32.Papavramidis TS, Grosomanidis V, Papakostas P, Penna S, Kotzampassi K. Intragastric balloon fundal or antral position affects weight loss and tolerability. Obes Surg. 2012;22:904–909. doi: 10.1007/s11695-012-0620-5. [DOI] [PubMed] [Google Scholar]
- 33.Papavramidis TS, Stavrou G, Papakostas P, Grosomanidis V, Kokkota S, Michalopoulos A, Kolios G, Kotzampassi K. Displacement of the Intragastric Balloon from the Fundus to the Antrum Results in Enhanced Weight Loss. Obes Surg. 2018;28:2374–2378. doi: 10.1007/s11695-018-3168-1. [DOI] [PubMed] [Google Scholar]
- 34.ORBERA365 What is ORBERA365™? A safe and non-surgical weight-loss program. Available from: https://www.orbera.com/uk-ireland/about-orbera .
- 35.Espinet-Coll E, Nebreda-Durán J, Gómez-Valero JA, Muñoz-Navas M, Pujol-Gebelli J, Vila-Lolo C, Martínez-Gómez A, Juan-Creix-Comamala A. Current endoscopic techniques in the treatment of obesity. Rev Esp Enferm Dig. 2012;104:72–87. doi: 10.4321/s1130-01082012000200006. [DOI] [PubMed] [Google Scholar]
- 36.Choi SJ, Choi HS. Various Intragastric Balloons Under Clinical Investigation. Clin Endosc. 2018;51:407–415. doi: 10.5946/ce.2018.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trande P, Mussetto A, Mirante VG, De Martinis E, Olivetti G, Conigliaro RL, De Micheli EA. Efficacy, tolerance and safety of new intragastric air-filled balloon (Heliosphere BAG) for obesity: the experience of 17 cases. Obes Surg. 2010;20:1227–1230. doi: 10.1007/s11695-008-9786-2. [DOI] [PubMed] [Google Scholar]
- 38.Forestieri P, De Palma GD, Formato A, Giuliano ME, Monda A, Pilone V, Romano A, Tramontano S. Heliosphere Bag in the treatment of severe obesity: preliminary experience. Obes Surg. 2006;16:635–637. doi: 10.1381/096089206776945156. [DOI] [PubMed] [Google Scholar]
- 39.Mion F, Gincul R, Roman S, Beorchia S, Hedelius F, Claudel N, Bory RM, Malvoisin E, Trepo F, Napoleon B. Tolerance and efficacy of an air-filled balloon in non-morbidly obese patients: results of a prospective multicenter study. Obes Surg. 2007;17:764–769. doi: 10.1007/s11695-007-9141-z. [DOI] [PubMed] [Google Scholar]
- 40.Kumar N. Endoscopic therapy for weight loss: Gastroplasty, duodenal sleeves, intragastric balloons, and aspiration. World J Gastrointest Endosc. 2015;7:847–859. doi: 10.4253/wjge.v7.i9.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lecumberri E, Krekshi W, Matía P, Hermida C, de la Torre NG, Cabrerizo L, Rubio MÁ. Effectiveness and safety of air-filled balloon Heliosphere BAG® in 82 consecutive obese patients. Obes Surg. 2011;21:1508–1512. doi: 10.1007/s11695-010-0314-9. [DOI] [PubMed] [Google Scholar]
- 42.Genco A, Dellepiane D, Baglio G, Cappelletti F, Frangella F, Maselli R, Dante MC, Camoirano R, Lorenzo M, Basso N. Adjustable intragastric balloon vs non-adjustable intragastric balloon: case-control study on complications, tolerance, and efficacy. Obes Surg. 2013;23:953–958. doi: 10.1007/s11695-013-0891-5. [DOI] [PubMed] [Google Scholar]
- 43.Machytka E, Klvana P, Kornbluth A, Peikin S, Mathus-Vliegen LE, Gostout C, Lopez-Nava G, Shikora S, Brooks J. Adjustable intragastric balloons: a 12-month pilot trial in endoscopic weight loss management. Obes Surg. 2011;21:1499–1507. doi: 10.1007/s11695-011-0424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Usuy E, Brooks J. Response Rates with the Spatz3 Adjustable Balloon. Obes Surg. 2018;28:1271–1276. doi: 10.1007/s11695-017-2994-x. [DOI] [PubMed] [Google Scholar]
- 45.Fittipaldi-Fernandez RJ, Zotarelli-Filho IJ, Diestel CF, Klein MRST, de Santana MF, de Lima JHF, Bastos FSS, Dos Santos NT. Randomized Prospective Clinical Study of Spatz3® Adjustable Intragastric Balloon Treatment with a Control Group: a Large-Scale Brazilian Experiment. Obes Surg. 2021;31:787–796. doi: 10.1007/s11695-020-05014-0. [DOI] [PubMed] [Google Scholar]
- 46.Espinet Coll E, Nebreda Durán J, López-Nava Breviere G, Ducóns García J, Rodríguez-Téllez M, Crespo García J, Marra-López Valenciano C. Multicenter study on the safety of bariatric endoscopy. Rev Esp Enferm Dig. 2017;109:350–357. doi: 10.17235/reed.2017.4499/2016. [DOI] [PubMed] [Google Scholar]
- 47.Ponce J, Woodman G, Swain J, Wilson E, English W, Ikramuddin S, Bour E, Edmundowicz S, Snyder B, Soto F, Sullivan S, Holcomb R, Lehmann J REDUCE Pivotal Trial Investigators. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11:874–881. doi: 10.1016/j.soard.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis. 2013;9:290–295. doi: 10.1016/j.soard.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Suchartlikitwong S, Laoveeravat P, Mingbunjerdsuk T, Vutthikraivit W, Ismail A, Islam S, Islam E. Usefulness of the ReShape intragastric balloon for obesity. Proc (Bayl Univ Med Cent) 2019;32:192–195. doi: 10.1080/08998280.2018.1559397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agnihotri A, Xie A, Bartalos C, Kushnir V, Sullivan S, Islam S, Islam E, Lamet M, Lamet A, Farboudmanesch R, Overholt BF, Altawil J, Early DS, Bennett M, Lowe A, Mullady DK, Adeyeri CS, El Zein M, Mishra P, Fayad L, Dunlap M, Oberbach A, Cheskin LJ, Kalloo AN, Khashab MA, Kumbhari V. Real-World Safety and Efficacy of Fluid-Filled Dual Intragastric Balloon for Weight Loss. Clin Gastroenterol Hepatol 2018; 16: 1081-1088. :e1. doi: 10.1016/j.cgh.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 51.Moore RL, Eaton L, Ellner J. Safety and Effectiveness of an Intragastric Balloon as an Adjunct to Weight Reduction in a Post-Marketing Clinical Setting. Obes Surg. 2020;30:4267–4274. doi: 10.1007/s11695-020-04798-5. [DOI] [PubMed] [Google Scholar]
- 52.Bazerbachi F, Haffar S, Sawas T, Vargas EJ, Kaur RJ, Wang Z, Prokop LJ, Murad MH, Abu Dayyeh BK. Fluid-Filled Versus Gas-Filled Intragastric Balloons as Obesity Interventions: a Network Meta-analysis of Randomized Trials. Obes Surg. 2018;28:2617–2625. doi: 10.1007/s11695-018-3227-7. [DOI] [PubMed] [Google Scholar]
- 53.ORBERA Apollo Endosurgery. News about ReShape ready. 2018. https://www.orbera.com/reshape/
- 54.Mion F, Ibrahim M, Marjoux S, Ponchon T, Dugardeyn S, Roman S, Deviere J. Swallowable Obalon® gastric balloons as an aid for weight loss: a pilot feasibility study. Obes Surg. 2013;23:730–733. doi: 10.1007/s11695-013-0927-x. [DOI] [PubMed] [Google Scholar]
- 55.Turkeltaub JA, Edmundowicz SA. Endoscopic Bariatric Therapies: Intragastric Balloons, Tissue Apposition, and Aspiration Therapy. Curr Treat Options Gastroenterol. 2019;17:187–201. doi: 10.1007/s11938-019-00232-7. [DOI] [PubMed] [Google Scholar]
- 56.De Peppo F, Caccamo R, Adorisio O, Ceriati E, Marchetti P, Contursi A, Alterio A, Della Corte C, Manco M, Nobili V. The Obalon swallowable intragastric balloon in pediatric and adolescent morbid obesity. Endosc Int Open. 2017;5:E59–E63. doi: 10.1055/s-0042-120413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jamal MH, Almutairi R, Elabd R, AlSabah SK, Alqattan H, Altaweel T. The Safety and Efficacy of Procedureless Gastric Balloon: a Study Examining the Effect of Elipse Intragastric Balloon Safety, Short and Medium Term Effects on Weight Loss with 1-Year Follow-Up Post-removal. Obes Surg. 2019;29:1236–1241. doi: 10.1007/s11695-018-03671-w. [DOI] [PubMed] [Google Scholar]
- 58.Ienca R, Al Jarallah M, Caballero A, Giardiello C, Rosa M, Kolmer S, Sebbag H, Hansoulle J, Quartararo G, Zouaghi SAS, Juneja G, Murcia S, Turro R, Pagan A, Badiuddin F, Dargent J, Urbain P, Paveliu S, di Cola RS, Selvaggio C, Al Kuwari M. The Procedureless Elipse Gastric Balloon Program: Multicenter Experience in 1770 Consecutive Patients. Obes Surg. 2020;30:3354–3362. doi: 10.1007/s11695-020-04539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genco A, Ernesti I, Ienca R, Casella G, Mariani S, Francomano D, Soricelli E, Lorenzo M, Monti M. Safety and Efficacy of a New Swallowable Intragastric Balloon Not Needing Endoscopy: Early Italian Experience. Obes Surg. 2018;28:405–409. doi: 10.1007/s11695-017-2877-1. [DOI] [PubMed] [Google Scholar]
- 60.Machytka E, Chuttani R, Bojkova M, Kupka T, Buzga M, Stecco K, Levy S, Gaur S. Elipse™, a Procedureless Gastric Balloon for Weight Loss: a Proof-of-Concept Pilot Study. Obes Surg. 2016;26:512–516. doi: 10.1007/s11695-015-1783-7. [DOI] [PubMed] [Google Scholar]
- 61.Espinet Coll E, López-Nava Breviere G, Nebreda Durán J, Marra-López Valenciano C, Turró Arau R, Esteban López-Jamar JM, Muñoz-Navas M. Spanish Consensus Document on Bariatric Endoscopy. Part 1. General considerations. Rev Esp Enferm Dig. 2018;110:386–399. doi: 10.17235/reed.2018.4503/2016. [DOI] [PubMed] [Google Scholar]
- 62.Moura D, Oliveira J, De Moura EG, Bernardo W, Galvão Neto M, Campos J, Popov VB, Thompson C. Effectiveness of intragastric balloon for obesity: A systematic review and meta-analysis based on randomized control trials. Surg Obes Relat Dis. 2016;12:420–429. doi: 10.1016/j.soard.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 63.Kumar N, Bazerbachi F, Rustagi T, McCarty TR, Thompson CC, Galvao Neto MP, Zundel N, Wilson EB, Gostout CJ, Abu Dayyeh BK. The Influence of the Orbera Intragastric Balloon Filling Volumes on Weight Loss, Tolerability, and Adverse Events: a Systematic Review and Meta-Analysis. Obes Surg. 2017;27:2272–2278. doi: 10.1007/s11695-017-2636-3. [DOI] [PubMed] [Google Scholar]
- 64.Yorke E, Switzer NJ, Reso A, Shi X, de Gara C, Birch D, Gill R, Karmali S. Intragastric Balloon for Management of Severe Obesity: a Systematic Review. Obes Surg. 2016;26:2248–2254. doi: 10.1007/s11695-016-2307-9. [DOI] [PubMed] [Google Scholar]
- 65.Genco A, Bruni T, Doldi SB, Forestieri P, Marino M, Busetto L, Giardiello C, Angrisani L, Pecchioli L, Stornelli P, Puglisi F, Alkilani M, Nigri A, Di Lorenzo N, Furbetta F, Cascardo A, Cipriano M, Lorenzo M, Basso N. BioEnterics Intragastric Balloon: The Italian Experience with 2,515 Patients. Obes Surg. 2005;15:1161–1164. doi: 10.1381/0960892055002202. [DOI] [PubMed] [Google Scholar]
- 66.Gaur S, Levy S, Mathus-Vliegen L, Chuttani R. Balancing risk and reward: a critical review of the intragastric balloon for weight loss. Gastrointest Endosc. 2015;81:1330–1336. doi: 10.1016/j.gie.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 67.Lopez-Nava G, Rubio MA, Prados S, Pastor G, Cruz MR, Companioni E, Lopez A. BioEnterics® intragastric balloon (BIB®). Single ambulatory center Spanish experience with 714 consecutive patients treated with one or two consecutive balloons. Obes Surg. 2011;21:5–9. doi: 10.1007/s11695-010-0093-3. [DOI] [PubMed] [Google Scholar]
- 68.Fittipaldi-Fernandez RJ, Zotarelli-Filho IJ, Diestel CF, Klein MRST, de Santana MF, de Lima JHF, Bastos FSS, Dos Santos NT. Intragastric Balloon: a Retrospective Evaluation of 5874 Patients on Tolerance, Complications, and Efficacy in Different Degrees of Overweight. Obes Surg. 2020;30:4892–4898. doi: 10.1007/s11695-020-04985-4. [DOI] [PubMed] [Google Scholar]
- 69.Saber AA, Shoar S, Almadani MW, Zundel N, Alkuwari MJ, Bashah MM, Rosenthal RJ. Efficacy of First-Time Intragastric Balloon in Weight Loss: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes Surg. 2017;27:277–287. doi: 10.1007/s11695-016-2296-8. [DOI] [PubMed] [Google Scholar]
- 70.Russo T, Aprea G, Formisano C, Ruggiero S, Quarto G, Serra R, Massa G, Sivero L. BioEnterics Intragastric Balloon (BIB) versus Spatz Adjustable Balloon System (ABS): Our experience in the elderly. Int J Surg. 2017;38:138–140. doi: 10.1016/j.ijsu.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 71.Abu Dayyeh BK, Noar MD, Lavin T, Hussan H, Chapman CG, Popov V, Acosta A, French MS, Rizk M, Huseini M, Grothe K, Clark M, Vargas EJ, Thompson CC. Pivotal randomized-controlled trial of the adjustable (Spatz-3) intragastric balloon system for weight loss. Gastrointest Endosc . 2019;89:AB58–AB59. [Google Scholar]
- 72.Schwaab ML, Usuy EN Jr, Albuquerque MM, Moreira DM, Derossi VO, Usuy RT. Assessment Of Weight Loss After Non-Adjustable And Adjustable Intragastric Balloon Use. Arq Gastroenterol. 2020;57:13–18. doi: 10.1590/S0004-2803.202000000-04. [DOI] [PubMed] [Google Scholar]
- 73.Sullivan S, Swain J, Woodman G, Edmundowicz S, Hassanein T, Shayani V, Fang JC, Noar M, Eid G, English WJ, Tariq N, Larsen M, Jonnalagadda SS, Riff DS, Ponce J, Early D, Volckmann E, Ibele AR, Spann MD, Krishnan K, Bucobo JC, Pryor A. Randomized sham-controlled trial of the 6-month swallowable gas-filled intragastric balloon system for weight loss. Surg Obes Relat Dis. 2018;14:1876–1889. doi: 10.1016/j.soard.2018.09.486. [DOI] [PubMed] [Google Scholar]
- 74.Vantanasiri K, Matar R, Beran A, Jaruvongvanich V. The Efficacy and Safety of a Procedureless Gastric Balloon for Weight Loss: a Systematic Review and Meta-Analysis. Obes Surg. 2020;30:3341–3346. doi: 10.1007/s11695-020-04522-3. [DOI] [PubMed] [Google Scholar]
- 75.Angrisani L, Santonicola A, Vitiello A, Belfiore MP, Belfiore G, Iovino P. Elipse Balloon: the Pitfalls of Excessive Simplicity. Obes Surg. 2018;28:1419–1421. doi: 10.1007/s11695-018-3148-5. [DOI] [PubMed] [Google Scholar]
- 76.Ramai D, Singh J, Mohan BP, Madedor O, Brooks OW, Barakat M, Ofosu A, Khan SR, Chandan S, Dhindsa B, Dhaliwal A, Facciorusso A, McDonough S, Adler DG. Influence of the Elipse Intragastric Balloon on Obesity and Metabolic Profile: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2020:Publish Ahead of Print. doi: 10.1097/MCG.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 77.Taha O, Abdelaal M, Asklany A, Alaa M, Belal S, El Assal I, Shahin M, Abubasha A, Elbanhawy D. Outcomes of a Swallowable Intragastric Balloon (Elipse™) on 96 Overweight and Obese Patients. Obes Surg. 2021;31:965–969. doi: 10.1007/s11695-020-05086-y. [DOI] [PubMed] [Google Scholar]
- 78.Trang J, Lee SS, Miller A, Cruz Pico CX, Postoev A, Ibikunle I, Ibikunle CA. Incidence of nausea and vomiting after intragastric balloon placement in bariatric patients - A systematic review and meta-analysis. Int J Surg. 2018;57:22–29. doi: 10.1016/j.ijsu.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 79.Gadd N, McIntosh A, Fear-Keen B, Hoult J, Maimone IR, Marshall S. Do Endoscopic Bariatric Procedures Improve Postprocedural Quality of Life and Mental Health? Obes Surg. 2020;30:4091–4100. doi: 10.1007/s11695-020-04860-2. [DOI] [PubMed] [Google Scholar]
- 80.Guedes MR, Fittipaldi-Fernandez RJ, Diestel CF, Klein MRST. Changes in Body Adiposity, Dietary Intake, Physical Activity and Quality of Life of Obese Individuals Submitted to Intragastric Balloon Therapy for 6 Months. Obes Surg. 2019;29:843–850. doi: 10.1007/s11695-018-3609-x. [DOI] [PubMed] [Google Scholar]
- 81.Deliopoulou K, Konsta A, Penna S, Papakostas P, Kotzampassi K. The impact of weight loss on depression status in obese individuals subjected to intragastric balloon treatment. Obes Surg. 2013;23:669–675. doi: 10.1007/s11695-012-0855-1. [DOI] [PubMed] [Google Scholar]
- 82.Ball W, Raza SS, Loy J, Riera M, Pattar J, Adjepong S, Rink J. Effectiveness of Intra-Gastric Balloon as a Bridge to Definitive Surgery in the Super Obese. Obes Surg. 2019;29:1932–1936. doi: 10.1007/s11695-019-03794-8. [DOI] [PubMed] [Google Scholar]
- 83.Kotzampassi K, Shrewsbury AD. Intragastric balloon: ethics, medical need and cosmetics. Dig Dis. 2008;26:45–48. doi: 10.1159/000109386. [DOI] [PubMed] [Google Scholar]
- 84.Larrad-Jimenez A, Sánchez-Cabezudo C. Quality indicators in bariatric surgery and criteria for long-term success. Cirugía Española. 2004;75:301–304. [Google Scholar]
- 85.Kotzampassi K, Shrewsbury AD, Papakostas P, Penna S, Tsaousi GG, Grosomanidis V. Looking into the profile of those who succeed in losing weight with an intragastric balloon. J Laparoendosc Adv Surg Tech A. 2014;24:295–301. doi: 10.1089/lap.2013.0439. [DOI] [PubMed] [Google Scholar]
- 86.Abu Dayyeh BK. Intragastric Balloons for Obesity Management. Gastroenterol Hepatol (N Y) 2017;13:737–739. [PMC free article] [PubMed] [Google Scholar]
- 87.Familiari P, Costamagna G, Bléro D, Le Moine O, Perri V, Boskoski I, Coppens E, Barea M, Iaconelli A, Mingrone G, Moreno C, Devière J. Transoral gastroplasty for morbid obesity: a multicenter trial with a 1-year outcome. Gastrointest Endosc. 2011;74:1248–1258. doi: 10.1016/j.gie.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 88.Guedes EP, Madeira E, Mafort TT, Madeira M, Moreira RO, Mendonça LM, Godoy-Matos AF, Lopes AJ, Farias ML. Impact of a 6-month treatment with intragastric balloon on body composition and psychopathological profile in obese individuals with metabolic syndrome. Diabetol Metab Syndr. 2016;8:81. doi: 10.1186/s13098-016-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lopez-Nava G, Jaruvongvanich V, Storm AC, Maselli DB, Bautista-Castaño I, Vargas EJ, Matar R, Acosta A, Abu Dayyeh BK. Personalization of Endoscopic Bariatric and Metabolic Therapies Based on Physiology: a Prospective Feasibility Study with a Single Fluid-Filled Intragastric Balloon. Obes Surg. 2020;30:3347–3353. doi: 10.1007/s11695-020-04581-6. [DOI] [PubMed] [Google Scholar]
- 90.Dumonceau JM, François E, Hittelet A, Mehdi AI, Barea M, Deviere J. Single vs repeated treatment with the intragastric balloon: a 5-year weight loss study. Obes Surg. 2010;20:692–697. doi: 10.1007/s11695-010-0127-x. [DOI] [PubMed] [Google Scholar]
- 91.Genco A, Cipriano M, Bacci V, Maselli R, Paone E, Lorenzo M, Basso N. Intragastric balloon followed by diet vs intragastric balloon followed by another balloon: a prospective study on 100 patients. Obes Surg. 2010;20:1496–1500. doi: 10.1007/s11695-010-0231-y. [DOI] [PubMed] [Google Scholar]
- 92.Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48:592–594. doi: 10.1093/ajcn/48.3.592. [DOI] [PubMed] [Google Scholar]
- 93.Geliebter A, Melton PM, McCray RS, Gage D, Heymsfield SB, Abiri M, Hashim SA. Clinical trial of silicone-rubber gastric balloon to treat obesity. Int J Obes. 1991;15:259–266. [PubMed] [Google Scholar]
- 94.Kotzampassi K, Shrewsbury A, Stavrou G. Depression and Intragastric Balloon Treatment. In: Preedy VR, Rajendram R, Martin CR. Metabolism and pathophysiology of bariatric surgery. Nutrition, procedures, outcomes and adverse effect. Elsevier Inc, 2017: 621-627. [Google Scholar]
- 95.Spirou D, Raman J, Smith E. Psychological outcomes following surgical and endoscopic bariatric procedures: A systematic review. Obes Rev. 2020;21:e12998. doi: 10.1111/obr.12998. [DOI] [PubMed] [Google Scholar]
- 96.Abu Dayyeh BK, Edmundowicz S, Thompson CC. Clinical Practice Update: Expert Review on Endoscopic Bariatric Therapies. Gastroenterology. 2017;152:716–729. doi: 10.1053/j.gastro.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 97.Abu-Dayyeh B. A randomized, multi-center study to evaluate the safety and effectiveness of the orbera intragastric balloon as an adjunct to a behavioral modification program. In: Comparison with a Behavioral Modification Program Alone in the Weight Management of Obese Subjects. Rochester, MN: Mayo Clinic; 2015. [Google Scholar]
- 98.Stavrou G, Rafailidis V, Kotzampassi K. No More Publications for Acute Pancreatitis : Letter to the Editor. Obes Surg. 2019;29:2972–2973. doi: 10.1007/s11695-019-03973-7. [DOI] [PubMed] [Google Scholar]
- 99.Stavrou G, Tsaousi G, Kotzampassi K. Life-threatening visceral complications after intragastric balloon insertion: Is the device, the patient or the doctor to blame? Endosc Int Open. 2019;7:E122–E129. doi: 10.1055/a-0809-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.ORBERA Orbera Endosurgery. [cited 26 Feb 2021]. Available from: https://apolloendo.com/orbera .
- 101.Runkel N, Colombo-Benkmann M, Hüttl TP, Tigges H, Mann O, Flade-Kuthe R, Shang E, Susewind M, Wolff S, Wunder R, Wirth A, Winckler K, Weimann A, de Zwaan M, Sauerland S. Evidence-based German guidelines for surgery for obesity. Int J Colorectal Dis. 2011;26:397–404. doi: 10.1007/s00384-011-1136-5. [DOI] [PubMed] [Google Scholar]
- 102.US Food and Drug Ministration Weight-Loss and Weight-Management Devices. [cited 27 Apr 2021]. Available from: https://www.fda.gov/medical-devices/products-and-medical-procedures/weight-loss-and-weight-management-devices . [Google Scholar]