Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) has evolved from a primarily diagnostic to therapeutic procedure in hepatobiliary and pancreatic disease. Most commonly, ERCPs are performed for choledocholithiasis with or without cholangitis, but improvements in technology and technique have allowed for management of pancreatic duct stones, benign and malignant strictures, and bile and pancreatic leaks. As an example of necessity driving innovation, the new disposable duodenoscopes have been introduced into practice. With the advantage of eliminating transmissible infections, they represent a paradigm shift in quality improvement within ERCP. With procedures becoming more complicated, the necessity for anesthesia involvement and safety of propofol use and general anesthesia has become better defined. The improvements in endoscopic ultrasound (EUS) have allowed for direct bile duct access and EUS facilitated bile duct access for ERCP. In patients with surgically altered anatomy, selective cannulation can be performed with overtube-assisted enteroscopy, laparoscopic surgery assistance, or the EUS-directed transgastric ERCP. Cholangioscopy and pancreatoscopy use has become ubiquitous with defined indications for large bile duct stones, indeterminate strictures, and hepatobiliary and pancreatic neoplasia. This review summarizes the recent advances in infection prevention, quality improvement, pancreaticobiliary access, and management of hepatobiliary and pancreatic diseases. Where appropriate, future research directions are included in each section.
Keywords: Cholangiopancreatography, Endoscopic retrograde, Cholangioscopy, Cannulation, Endoscopic ultrasound, Disposable duodenoscopes
Core Tip: Disposable duodenoscopes present a way to eliminate transmission of drug resistant infections. Access to single operator cholangioscopy and panreatoscopy has made complex intraductal assessment and therapy more ubiquitous. Future research will clarify the role of endoscopic ultrasound bile duct access for variant anatomy or failed endoscopic retrograde cholangiopancreatography (ERCP), photodynamic therapy, and indomethacin and pancreas duct (PD) stents in post ERCP pancreatitis prophylaxis.
INTRODUCTION
This coronavirus disease 2019 (COVID-19) pandemic has changed our collective understanding of infection transmission, vaccine development, and the challenges of providing continuity of care in a rapidly evolving health care crisis. The evolution in endoscopic retrograde cholangiopancreatography (ERCP) has been more gradual, but certainly there have been periods of innovation punctuated by rapid change. Given the global pandemic, an area of interest with accelerated focus is the use of disposable duodenoscopes to break the chain of infection in ERCP. With rising concerns over reusable duodenoscopes implicated in nosocomial outbreaks, the trend toward transitioning to disposable components and completely disposable duodenoscopes has begun.
As highlighted in previous reviews, ERCP has moved from a diagnostic to primarily therapeutic procedure[1]. The therapeutic indications for ERCP include stones in the biliary and pancreatic ducts, benign and malignant strictures, and bile and pancreatic leaks[1]. Despite the near ubiquitous access to advanced radiology and endoscopic ultrasound (EUS) in North America, ERCP still has diagnostic indications in patients with a solitary dilated duct, cholangiocarcinoma, primary sclerosing cholangitis, and autoimmune cholangitis. This article will focus on the current state of practice for diagnosing and managing hepatobiliary and pancreatic disease with ERCP in 2021.
As competency-based training programs have evolved to include EUS and ERCP, hybrid procedures have evolved. Any future textbooks will have to include both procedures given their complementary nature. In addition to the advances made in these hybrid procedures, our focus should remain on clinical success and mitigating risk independent of technical success during a single procedure. This article will review the progress made since the last review in this journal[2] and clarify future research directions in the field.
INFECTION PREVENTION AND QUALITY IMPROVEMENT
Disposable duodenoscopes
While some practice changes in ERCP have been adopted because of an enthusiasm for technologic advance and the opportunity to treat complex problems, this past year was a somber reminder of our oath to do no harm. At no point in our history has there been a greater focus on infection prevention in health care with the ever-present threat of COVID-19. The prevention of transmissible infections has added cost and complexity to the reprocessing of duodenoscopes. Duodenoscopes have a complex design with intricate moving parts, long working channels, and are heat labile which make them difficult devices to disinfect[3]. Contaminated duodenoscopes have been implicated in the spread of multidrug resistant organisms[4-7]. Several measures have been taken to improve the disinfection process to mitigate cross contamination[8]. Along with this, the Food and Drug Administration (FDA) recommended a transition to a newer design of duodenoscopes with disposable components which can simplify the disinfection process[9]. This has also led to innovations in duodenoscope design which include disposable parts and the development of a completely disposable duodenoscope.
Development of a single-use duodenoscope began in 2017. The challenge was manufacturing a scope comparable in performance and efficacy to a conventional reusable duodenoscope and eliminate the risk of any cross contamination[10]. Although there have been disposable bronchoscopes, nasopharyngoscopes, and ureteroscopes in clinical use, a disposable scope in gastroenterological clinical practice has been unprecedented[10]. In December 2019, the FDA cleared the first fully disposable duodenoscope — EXALT™ Model D Single-Use Duodenoscope (Figure 1), Boston Scientific Corporation (Marlborough, MA, United States)[11]. The endoscope has a 4.2 mm working channel, LED light, and conventional four-way steering. The current model D has a similar elevator lift angle and viewing angle when compared to the available reusable duodenoscopes. Subsequently in July 2020, a second disposable duodenoscope was cleared by the FDA-Duodenoscope model aScope™ Duodeno, Ambu A/S (Ballerup, Denmark)[5].
Figure 1.
The EXALT duodenoscope in use at our center.
Advantages of a single-use duodenoscope are that they are sterile with no risk of cross contamination between patients. There is no need for disinfection or reprocessing, and it also eliminates the cost of maintenance and repair. Initial studies with the use of disposable duodenoscopes in a bench model, real patients, and a randomized study comparing with conventional duodenoscopes have shown equivalent performance characteristics compared to reusable duodenoscopes[10,12,13]. The significant disadvantages of the adoption of disposable duodenoscopes are the increased costs and increased environmental waste[14]. Further studies on the safety, efficacy, costs, patient outcomes, and environmental impact will help navigate the transition toward these novel devices.
Periprocedural management: Anesthesia involvement and propofol use in ERCP
ERCP has become safer with better equipment, standardized training programs, and better periprocedural care. As ERCP applications have broadened to include other modalities like EUS, there has been a significant increase in the use of involvement of anesthesia services in endoscopy. The safety of anesthesia-directed sedation in endoscopy is complex to analyze, but now better understood.
Safe sedation is a dynamic process that allows for technical and clinical success. In a United Kingdom study of therapeutic procedures, sedation was deemed inappropriate in up to 14% of cases[15]. Prior to Propofol use and general anesthesia, intolerance of sedation with discomfort was noted in one third to one half of ERCPs[16]. Comorbid patients with higher American Society of Anesthesiologist scores are more likely to have anesthesiologist involvement[17]. The safety of anesthesia service in endoscopy was analysed in a large cross-sectional study using the National Anesthesia Clinical Outcomes Registry. A total of 27721 patients had an ERCP performed with 12 deaths and 1052 anesthesia-related complications reported[17]. In the unadjusted model, ERCP was associated with an elevated odds ratio (OR) of 8.83 [95% confidence interval (CI): 7.70-10.12] relative to colonoscopy, that was not significant in the multivariate analysis.
Propofol is a sedative and hypnotic medication with a shorter duration of action compared to midazolam and fentanyl. Benefits of propofol include improvements in patient satisfaction, procedural outcome, and quicker recovery when compared to procedural sedation[18-20]. Propofol can cause significant hypotension and rapid respiratory depression. Further study was required to clarify propofol’s safety in endoscopy. The ProSed 2 study[21] was a large multicenter prospective study reviewing sedation methods and associated complications of which 20967 procedures (6.7%) were ERCPs. The lowest rates of sedation-related complications were in patients receiving propofol monotherapy, and only 5 reported fatalities occurred during these ERCPs. An important point from the study is that their data collection focused on adverse events related to sedation alone, and delayed complications were not included. As with the Lieber study[17], delayed adverse events like post ERCP pancreatitis would not be captured by the author’s study design[22]. Respiratory complications are more common in upper endoscopies[17], and the decision to intubate a patient remains individualized to the nature of the intended procedure and the patient’s comorbidities. If anesthesia services are involved at our institution, any decision regarding the patient’s anesthesia and intubation is collaborative with shared care decision making.
Future directions: Reducing post ERCP pancreatitis
Guidewire cannulation[23], pancreatic duct stents[24], intensive intravenous hydration[25,26], and rectal indomethacin[27] are used to reduce post ERCP pancreatitis[28]. In the landmark trial published in the NEJM assessing the benefits indomethacin for post ERCP prophylaxis, more than 80% of patients also received a pancreatic duct stent[27]. The dose of rectal indomethacin used in the study was 100 mg. There was a reduction in post ERCP pancreatitis in both patients who received a stent (16.1% to 9.7% P = 0.04) and those who did not (20.6% to 6.3% P = 0.049). Post hoc analysis of this data suggested that the use of rectal indomethacin alone was better than a stent alone or the combination of stent and rectal indomethacin[29]. Despite data to support rectal indomethacin given before the procedure[30], and the double wire technique[31], the current state of practice remains individual to the practitioner. Side effects of long-term nonsteroidal anti-inflammatory drug use include renal impairment and peptic ulcer disease. A single dose of indomethacin did not result in a significant risk of acute renal impairment or clinically significant gastrointestinal bleeding[27]. The stent vs indomethacin for preventing post-ERCP pancreatitis (SVI) trial will clarify the value of a prophylactic pancreatic stent when added to rectally administered indomethacin[29] and should help further define standards of practice.
CANNULATION, BILIARY ACCESS, AND ALTERED ANATOMY
EUS assisted biliary access
Cannulation techniques have continued to evolve with advances in equipment[32]. Adding the EUS rendezvous may represent the last advance necessary to achieve 100% cannulation success during the index procedure. However, the additional risk of adding an EUS rendezvous to the index procedure needs to be evaluated prospectively in many centers. Failed cannulations are currently managed with a referral to interventional radiology for percutaneous transhepatic cholangiography (PTC). Biliary access and management would take the form of a combined PTC with ERCP, PTC with formation of an established tract, or antegrade stenting and stone removal[33]. EUS-guided rendezvous was first published in 2004[34]. Technical success has been reported with rates as high as 80% to 81%[35,36] with adverse event rates being 11%. A recent systematic review and meta-analysis reported a technical success of 86.1% (95%CI: 78.4-91) (12 studies reporting a total of 342 patients) and clinical success of 80.8% (95%CI: 64.1-90.8) (4 studies reporting a total of 94 patients)[37]. Consistent with previous reports, the pooled rate of adverse events was 14% (95%CI: 10.5-18.4) (12 studies; 42 events in 342 patients)[37]. At this time, the role of EUS rendezvous in ERCP is still not standardized and has not been compared to PTC in a comparative study[33]. In addition to EUS rendezvous, EUS directed transmural bile duct drainage is an alternate option. Transmural options for biliary drainage include hepaticogastrostomy (for proximal biliary obstruction) and choledochoduodenostomy (for distal biliary obstruction). While hepaticogastrostomy is performed using tubular metal stents, choledochoduodenostomy can be performed using tubular stents or LAMS based on bile duct size. A recent RCT compared EUS guided transmural biliary drainage vs ERCP for distal malignant obstruction and reported similar technical and clinical success[38].
Overtube-assisted enteroscopy and laparoscopic surgery-assisted ERCP
Given the burden of obesity and weight loss surgeries, expertise in altered surgical anatomy ERCP is necessary at tertiary referral centers. In a previous systematic review of overtube-assisted enteroscopy (OAE) and ERCP[39], patients with a Roux-en-Y with gastric bypass had a technically successful ERCP in just 70% of cases. Additionally, patients with a Roux-en-Y and either a hepaticojejunostomy (Figure 2) or pancreaticoduodenectomy undergoing ERCP had success in 76% of cases. A systematic review and meta-analysis[40] published in 2020 included 10 studies reporting a total of 398 procedures. The pooled rates of technical success of enteroscopy and OAE-ERCP were comparable at 75.3% (95%CI: 64.5-83.6) and 64.8% (95%CI: 53.1-74.9), respectively. The pooled rate of adverse events was 8.0% (95%CI: 5.2-12.2). The pooled rate of enteroscopy success with a double-balloon enteroscope in the 4 available studies was 83.5% (95%CI 68.3-92.2). Importantly, technical success of double-balloon enteroscopy ERCP (DBE-ERCP) was also higher at 72.5% (95%CI: 52.3-86.4). The pooled rate of adverse events with DBE-ERCP was 9.0% (95%CI: 5.4-14.5)[40].
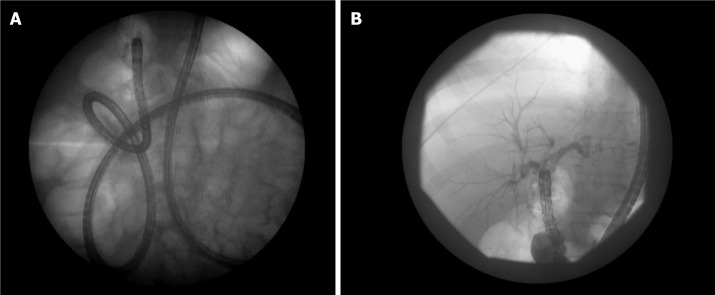
Figure 2.
An overtube assisted enteroscopy and endoscopic retrograde cholangiopancreatography performed for a stent exchange and stone extraction. The patient had a Roux-en-Y hepaticojejunostomy after a bile duct injury. A: Stent exchange; B: Stone extraction.
Another approach to altered anatomy is the laparoscopic surgery-assisted ERCP[41]. At our institution, this surgery involves 4 Laparoscopic ports placed under direct visualization, formation of a gastrotomy, and placement of a rigid 19 mm sigmoidoscope into the gastrotomy. The duodenoscope is advanced through the sigmoidoscope, pylorus, and into the duodenum[42]. A meta-analysis in 2020 found that laparoscopic assisted surgery is significantly more effective than enteroscopy-assisted ERCP[43]. Therapeutic success was defined as completion of the diagnostic or therapeutic indication of the ERCP. The pooled proportion of patients with therapeutic success was higher in the surgery group at 97.9% (95%CI: 96.7-98.7) compared to 73.2% (95%CI: 62.5-82.6) in the enteroscopy-assisted ERCP patients. The benefits were countered by a higher rate of adverse events (19%; 95%CI: 12.6-26.4 vs 6.5%; 95%CI: 3.9-9.6) and a longer procedural time (158.5 min SD ± 20 vs 100.5 min SD ± 19.2 min).
EUS-directed transgastric ERCP
Given the challenges in managing patients with altered anatomy, EUS-directed transgastric ERCP (EDGE) is a novel way to approach patients with Roux-en-Y gastric bypass (RYGB)[44,45] and avoids the previously described laparoscopic-assisted access into the disconnected portion of the stomach. Importantly, the procedure has gained popularity since 2015[46] because of the ability to use conventional cannulation techniques and equipment. A retrospective multicenter review[47] of 178 patients reported a technical success of 98% (175/178) countered by 4 severe adverse events (SAE) (2.2%) and 10% of patients having a documented persistent fistula (9/90). It has been proposed that the EDGE could be used in patients with a RYGB, of which the details like limb length are unknown, and in patients with a surgically absent gallbladder[48]. A meta-analysis showed comparable rates of success to the laparoscopic assisted ERCP[45]. The significantly higher rates of technical success justify future comparative study of OAE and DBE ERCP with the EDGE procedure. The challenge for any prospective multicenter comparison will be that the EDGE can be done in 2 sessions[45]. The EUS placement of a transluminal stent, and then a second procedure at a follow-up interval to perform the ERCP. Although an EDGE procedure can be done at the time of LAMS placement, stent migration and free perforation can occur and most endoscopists wait 4-6 weeks prior to proceeding to ERCP.
ERCP AND ITS ROLE IN THE DIAGNOSIS AND MANAGEMENT OF BILIARY DISEASE
ERCP in complex bile duct stones
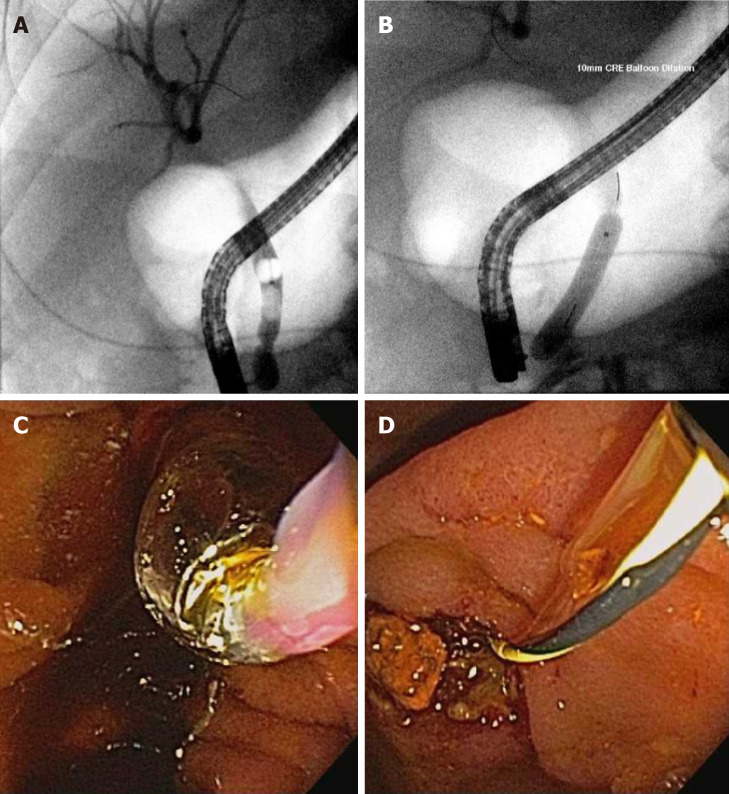
The main indication for ERCP is choledocholithiasis[49] which can cause cholangitis, biliary obstruction, and pancreatitis. For routine stones < 1 cm, a sphincterotomy with stone extraction using a balloon or basket is performed. Large bile duct stones present a particular challenge for safe and complete removal[50]. Recent guidelines have suggested performing a sphincterotomy and then a large balloon dilation over a sphincterotomy alone[51] for large stones. In a systematic review and meta-analysis, patients were more likely to have complete clearance of large stones (≥ 1 cm) OR 2.8, 95%CI: 1.4-5.7, I226% if a balloon dilation was performed after a sphincterotomy (Figure 3).
Figure 3.
Large bile duct stone extraction. A: Bile duct stone; B-D: Balloon sphincteroplasty performed (B and C) with extracted stone fragment (D).
Cholangioscopy is ideal for complex lithotripsy because of the ability to visualize the stone and introduce either a laser lithotripsy or electrohydraulic lithotripsy catheter[52]. Observational studies have reported procedural success in stone cases up to 92% with single operator cholangioscopy[53]. However, prior randomized controlled trials had not shown a significant difference between large balloon sphincteroplasty and cholangioscopy guided lithotripsy[54]. In a randomized comparison of large balloon sphincteroplasty with single-operator cholangioscopy guided lithotripsy, the proportion of ductal clearance was 72.7% and 93.9% in 1 session, respectively[55]. Treatment costs were higher in the cholangioscopy arm with no significant difference in complications. Future directions include standardized training in cholangioscopy and development of treatment algorithms for large bile duct stones[51].
ERCP in strictures and cholangiocarcinoma: Diagnosis and management
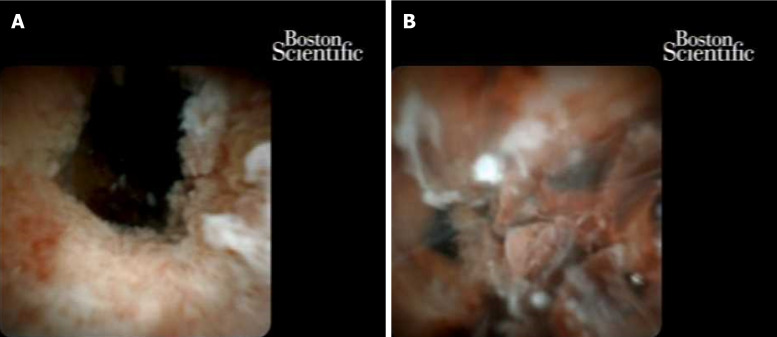
Cholangioscopy has progressed significantly since the transition from a dual-operator to a single-operator cholangioscope[52]. With the advent and proliferation of access to single-operator cholangioscopy, sensitivity for diagnosis of obstructive biliary pathology has improved. Cohort studies have shown adequate tissue for diagnostic assessment in 88% of patients with a biopsy performed with cholangioscopy[53]. A recent randomized multicenter trial confirmed higher first sample sensitivity with cholangioscopy compared to standard brushings (68.3% vs 21.4% P < 0.01) in patients with indeterminate biliary strictures[56]. Their data showed that the addition of the visual impression by digital single-operator cholangioscopy and direct biopsy had the highest likelihood of diagnosing malignancy in an indeterminate biliary stricture (Figure 4). For patients with primary sclerosing cholangitis, additional biopsies for fluorescence in situ hybridization (FISH) has been shown to improve sensitivity of indeterminate biliary strictures[57].
Figure 4.
Cholangioscopy: Multifocal intraductal papillary neoplasm of bile ducts with high-grade dysplasia, that became cholangiocarcinoma. A: High-grade dysplasia; B: Cholangiocarcinoma.
Management of unresectable cholangiocarcinoma has largely been limited to systemic chemotherapy and radiation. Currently, the main role of ERCP in cholangiocarcinoma is treating biliary obstructions with biliary stents. The advent of endoscopic options for unresectable cholangiocarcinoma has provided some hope in this field. Photodynamic therapy (PDT) and radiofrequency ablation (RFA) provide 2 available options for these patients. PDT works to ablate cancer tissue by using a photosensitizer that is activated by laser light. This results in tissue destruction by apoptosis and necrosis[58]. The main adverse event associated with PDT is photosensitivity. A sentinel study showed a survival benefit in patients receiving PDT[59]. A systematic review and meta-analysis published in 2017 by this journal[60] included 10 studies with 402 patients analyzed. The pooled OR for successful biliary drainage, defined as a reduction in bilirubin of 50% or greater at 7 d, was 4.39 (95%CI: 2.35-8.19) when comparing PDT and biliary stenting to biliary stenting alone. Future directions include targeted placement of the photsensitizer. Pullulan acetate-conjugated pheophorbide A is a photosensitizer that was successfully incorporated into self-expanding metal stent[61].
RFA is a local ablative therapy from a bipolar probe using high frequency current. A randomized trial from 2017 compared the outcomes of RFA with biliary stenting or biliary stenting alone[62]. The primary outcome of the study was mean survival time from the first RFA to time of death. In 21 months of follow-up, the mean survival time was significantly higher in the RFA and stent group (13.2 ± 0.6 mo) than if the patient received a biliary stent alone (8.3 ± 0.5 mo, P < 0.001). A previous retrospective comparative trial showed no difference between PDT and RFA in terms of survival rates[63]. Despite expected advances, the possible benefit of drug eluting stents remains untested in clinical trials. Vorinostat-eluting nanofiber membranes have showed antineoplastic effects against cholongiocarcinoma[64]. Stents with histone deacetylase inhibitors[65] and stents coated with gemcitabine and cisplatin have been fabricated[66], but neither have been tested in prospective studies.
PANCREATIC DISEASE: PANCREATIC STONES AND PANCREATIC LEAKS
ERCP in the management of pancreatic strictures
Radiological studies like CT and MRI/MRCP are the primary means of diagnosing chronic pancreatitis and strictures in 2021. However, in the early stages of chronic pancreatitis where the structural changes are limited, a combination of EUS, MRCP with secretin, and pancreatic function tests can be done in patients with high suspicion and risk factors[67]. ERCP is an important treatment option for patients with symptomatic chronic pancreatitis and strictures[68], with main pancreatic duct (MPD) strictures as the most likely to be intervened on. ERCP is recommended in patients with symptomatic, dominant strictures. These are defined as upstream MPD dilatation ≥ 6 mm in diameter, prevention of contrast medium outflow alongside a 6-Fr catheter inserted upstream from the stricture, or abdominal pain during continuous infusion of a nasopancreatic catheter inserted upstream from the stricture with 1 L saline over 12-24 h[69]. Stenting across the pancreatic duct stricture using ERCP decompresses the duct, helps relieve pain, and can result in improvement of exocrine pancreatic function[68]. Multiple studies have shown that stenting in chronic pancreatitis with strictures can improve pain[70-73]. A large multicenter study of more than 1000 patients followed up for a mean 4.9 years showed long-term success of endotherapy in 86% of patients but was lower at 65% in intention to treat analysis[68]. A large meta-analysis involving 16 studies and 1498 patients showed immediate pain relief in 88% and long-term pain relief in 67%. Complication rates for endotherapy were 7.85%[74]. More recently, rendezvous access using transgastric EUS puncture of the pancreatic duct and guidewire placement through a tight stenosis has allowed treatment of previously inaccessible strictures[75]. This is particularly effective in post Whipple patients with a stenotic pancreaticojejunostomy[76].
Commonly, a single plastic stent is used in pancreatic strictures. Multiple side-by-side plastic stents have also been used in treatment refractory strictures which did not respond to a single stent[77]. Newer stents like the fully covered self-expandable metal stents and a biodegradable noncovered self-expandable stents have been evaluated[78,79]. Preliminary studies with longitudinal follow-up of fully covered self-expanding metal stents (FCSEMSs) in symptomatic main duct pancreatic strictures[79] are promising. In patients with MPD strictures that remained symptomatic after a single plastic stent who were treated with a 6 mm or 8 mm Niti-S Bumpt Stent (Taewoong Medical, Gimpo-SI, South Korea), 89% of patients were asymptomatic after 3 years. Given the technical success of FCSEMS[80] and relative safety[81,82], larger studies with long-term data will be performed. An ongoing trial will look at the degree of pain reduction, SAE, and stricture resolution[83] in patients who received a FCSEMS. To date, SEMS in the pancreatic duct in the United States remains investigational.
Pancreatoscopy, pancreatic stones, and pancreatic leaks
The indications for pancreatoscopy include direct visualization of strictures, filling defects, and to differentiate benign from malignant intraductal pathology. Pancreatoscopy can be helpful in the management of suspected intraductal papillary mucinous neoplasms as it can diagnose and stage the disease prior to surgical resection[84-86]. Per oral pancreatoscopy was first demonstrated in 1970s by Kawai et al[87], but required a second operator, and the technology was limited[88-90]. The first digital SpyGlass™ direct visualization cholangiopancreatoscope (Boston Scientific Corporation, Marlborough, MA, United States) was introduced in 2007. This included a working channel for biopsies and allowed for irrigation[91,92]. Further iterations had improved digital image quality[93]. The most recent digital version was launched in 2018 and has increased resolution, improved lighting, a retrieval basket, and a retrieval snare. The primary therapeutic indication of pancreatoscopy is direct lithotripsy for pancreatic duct stones[94]. Complication rates post pancreatoscopy have ranged from 3.8% to 12% and mainly include mild pancreatitis[85,95-97].
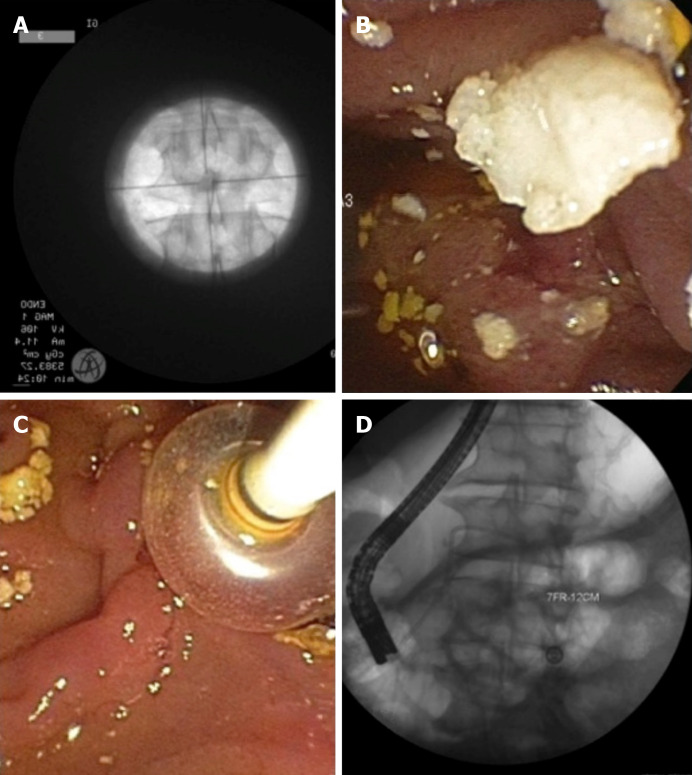
Chronic calcific pancreatitis is complicated by intraductal pancreatic stones which can be difficult to manage. In symptomatic patients, preprocedure imaging is mandatory to decide on adding extracorporeal shock wave lithotripsy (ESWL) before ERCP (Figure 5). ESWL is indicated if there are larger stones (≥ 5 mm) with ductal obstruction. Previous studies have shown that adding ESWL significantly decreases pain scores, yearly hospitalizations for pancreatitis, and opioid use[98]. A systematic review and meta-analyses of 22 ESWL ERCP studies noted high rates of complete stone fragmentation at 86.3% (95%CI: 76.0-94.0)[99]. The pooled percentage of patients with complete ductal clearance, however was 69.8% (95%CI: 63.8-75.5). This is a difficult patient population to manage and overall ESWL resulted in a moderate proportion of patients with complete absence of pain 64.2% (95%CI: 57.5-70.6). At our institution we perform an ESWL and ERCP in the same session (Figure 5). Repeat treatments are arranged based on post treatment symptom burden, interval imaging, and stone burden on repeat pancreatogram.
Figure 5.
Chronic pancreatitis with a large pancreatic stone. A: Extracorpeal shock wave lithotripsy with stone; B and C: Successful stone extraction; D: Placement of a plastic pancreatic stent.
Pancreatic inflammation can cause a pancreatic duct leak with the unfortunate consequences of peripancreatic fluid collection, pseudocyst, walled-off pancreatic necrosis, pancreatic ascites, and fistula formation[100]. Management of pancreatic duct leaks historically involved conservative management including TPN and octreotide as a bridge to surgery. ERCP allows for diagnosis of the leak, transpapillary stent placement, and avoidance of surgery. Fluid collections from a pancreatic leak can be managed with internal luminal drainage and percutaneous drains[101,102]. Transluminal pigtail stents placed for pancreatic fluid leak in disconnected duct syndrome can be left in indefinitely as removing stents leads to risk of recurrent fluid collection[103].
CONCLUSION
ERCPs are done for multiple important reasons[1]. Although the most common indication remains choledocholithiasis with or without cholangitis[49], evolving indications include cholangiopancreatoscopy with directed diagnostic and therapeutic procedures. Further training and improvements in practice have allowed for the use of over-tube, laparoscopic surgery-assisted, and EUS-facilitated ERCP[104] in patients who have undergone RYGB for morbid obesity. New developments in technology have allowed for the potential use of SEMS for refractory pancreatic duct strictures and the redesign of a duodenoscopes to include marketing of a disposable scopes to mitigate infectious complications from inadequately reprocessed devices. Despite the tumultuous last year and a half, there continues to be hope in the field of ERCP for managing complex disease.
ACKNOWLEDGEMENTS
Smith TD provided editing and administrative support in the production of this manuscript.
Footnotes
Conflict-of-interest statement: Dr. Sanders DJ and Dr. Bomman S has no conflicts of interest related to the nature or content of this article. Dr. Krishnamoorthi R receives research support from Boston Scientific. Dr. Kozarek RA receives research support from Boston Scientific Corporation and the National Institute of Health.
Manuscript source: Invited manuscript
Peer-review started: March 19, 2021
First decision: May 4, 2021
Article in press: July 9, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chow WK, Espinel J, Matsubara S, Sato H S-Editor: Gao CC L-Editor: A P-Editor: Li JH
Contributor Information
David J Sanders, Digestive Disease Institute, Virginia Mason Medical Center, Seattle, WA 98101, United States.
Shivanand Bomman, Digestive Disease Institute, Virginia Mason Medical Center, Seattle, WA 98101, United States.
Rajesh Krishnamoorthi, Digestive Disease Institute, Virginia Mason Medical Center, Seattle, WA 98101, United States.
Richard A Kozarek, Digestive Disease Institute, Virginia Mason Medical Center, Seattle, WA 98101, United States. richard.kozarek@virginiamason.org.
References
- 1.Kozarek RA. The Past, Present, and Future of Endoscopic Retrograde Cholangiopancreatography. Gastroenterol Hepatol (N Y) 2017;13:620–622. [PMC free article] [PubMed] [Google Scholar]
- 2.Salerno R, Mezzina N, Ardizzone S. Endoscopic retrograde cholangiopancreatography, lights and shadows: Handle with care. World J Gastrointest Endosc. 2019;11:219–230. doi: 10.4253/wjge.v11.i3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. Safety Communications > Design of Endoscopic Retrograde Cholangiopancreatography (ERCP) Duodenoscopes May Impede Effective Cleaning: FDA Safety Communication. [cited 22 October 2020] In: U.S. Food and Drug Administration [Internet]. Available from: https://wayback.archive-it.org/7993/20170722213105/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm434871.htm . [Google Scholar]
- 4.Aumeran C, Poincloux L, Souweine B, Robin F, Laurichesse H, Baud O, Bommelaer G, Traoré O. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy. 2010;42:895–899. doi: 10.1055/s-0030-1255647. [DOI] [PubMed] [Google Scholar]
- 5.Cryan EM, Falkiner FR, Mulvihill TE, Keane CT, Keeling PW. Pseudomonas aeruginosa cross-infection following endoscopic retrograde cholangiopancreatography. J Hosp Infect. 1984;5:371–376. doi: 10.1016/0195-6701(84)90004-5. [DOI] [PubMed] [Google Scholar]
- 6.Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S, Pacilli M, Moulton-Meissner H, Rasheed JK, Avillan JJ, Kitchel B, Limbago BM, MacCannell D, Lonsway D, Noble-Wang J, Conway J, Conover C, Vernon M, Kallen AJ. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312:1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verfaillie CJ, Bruno MJ, Voor in 't Holt AF, Buijs JG, Poley JW, Loeve AJ, Severin JA, Abel LF, Smit BJ, de Goeij I, Vos MC. Withdrawal of a novel-design duodenoscope ends outbreak of a VIM-2-producing Pseudomonas aeruginosa. Endoscopy. 2015;47:493–502. doi: 10.1055/s-0034-1391886. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. Safety Communications > Supplemental Measures to Enhance Duodenoscope Reprocessing: FDA Safety Communication. [cited 22 October 2020] In: U.S. Food and Drug Administration [Internet]. Available from: http://wayback.archive-it.org/7993/20170722150658/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm454766.htm . [Google Scholar]
- 9.U.S. Food and Drug Administration. The FDA is Recommending Transition to Duodenoscopes with Innovative Designs to Enhance Safety: FDA Safety Communication. [cited 22 October 2020] In: U.S. Food and Drug Administration [Internet]. Available from: https://www.fda.gov/medical-devices/safety-communications/fda-recommending-transition-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication . [Google Scholar]
- 10.Ross AS, Bruno MJ, Kozarek RA, Petersen BT, Pleskow DK, Sejpal DV, Slivka A, Moore D, Panduro K, Peetermans JA, Insull J, Rousseau MJ, Tirrell GP, Muthusamy VR. Novel single-use duodenoscope compared with 3 models of reusable duodenoscopes for ERCP: a randomized bench-model comparison. Gastrointest Endosc. 2020;91:396–403. doi: 10.1016/j.gie.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. FDA clears first fully disposable duodenoscope, eliminating the potential for infections caused by ineffective reprocessing. [cited 10 January 2021] In: U.S. Food and Drug Administration [Internet]. Available from: https://www.fda.gov/news-events/press-announcements/fda-clears-first-fully-disposable-duodenoscope-eliminating-potential-infections-caused-ineffective . [Google Scholar]
- 12.Muthusamy VR, Bruno MJ, Kozarek RA, Petersen BT, Pleskow DK, Sejpal DV, Slivka A, Peetermans JA, Rousseau MJ, Tirrell GP, Ross AS. Clinical Evaluation of a Single-Use Duodenoscope for Endoscopic Retrograde Cholangiopancreatography. Clin Gastroenterol Hepatol. 2020;18:2108–2117.e3. doi: 10.1016/j.cgh.2019.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Bang JY, Hawes R, Varadarajulu S. Equivalent performance of single-use and reusable duodenoscopes in a randomised trial. Gut. 2021;70:838–844. doi: 10.1136/gutjnl-2020-321836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elta GH, Law RJ. Great haste makes great waste: Do available data support the widespread adoption of disposable endoscopes? Gastrointest Endosc. 2020;91:404–405. doi: 10.1016/j.gie.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Cullinane M, Gray AJG, Hargraves CMK, Lucas S, Schubert M, Sherry KM, Wardle T. The 2004 report of the National Confidential Enquiry into Patient Outcome and Death. London: National Confidential Enquiry into Patient Outcome and Death, 2004: 1-20. [Google Scholar]
- 16.Jeurnink SM, Steyerberg E, Kuipers E, Siersema P. The burden of endoscopic retrograde cholangiopancreatography (ERCP) performed with the patient under conscious sedation. Surg Endosc. 2012;26:2213–2219. doi: 10.1007/s00464-012-2162-2. [DOI] [PubMed] [Google Scholar]
- 17.Lieber SR, Heller BJ, Martin CF, Howard CW, Crockett S. Complications of Anesthesia Services in Gastrointestinal Endoscopic Procedures. Clin Gastroenterol Hepatol. 2020;18:2118–2127.e4. doi: 10.1016/j.cgh.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67:910–923. doi: 10.1016/j.gie.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 19.Smith I, Durkin D, Lau KW, Hebbar S. Establishing an anaesthetist-delivered propofol sedation service for advanced endoscopic procedures: implementing the RCA/BSG guidelines. Frontline Gastroenterol. 2018;9:185–191. doi: 10.1136/flgastro-2017-100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, Clarke AC, Hillman LC, Horiuchi A, Cohen LB, Heuss LT, Peter S, Beglinger C, Sinnott JA, Welton T, Rofail M, Subei I, Sleven R, Jordan P, Goff J, Gerstenberger PD, Munnings H, Tagle M, Sipe BW, Wehrmann T, Di Palma JA, Occhipinti KE, Barbi E, Riphaus A, Amann ST, Tohda G, McClellan T, Thueson C, Morse J, Meah N. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137:1229–37; quiz 1518. doi: 10.1053/j.gastro.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 21.Behrens A, Kreuzmayr A, Manner H, Koop H, Lorenz A, Schaefer C, Plauth M, Jetschmann JU, von Tirpitz C, Ewald M, Sackmann M, Renner W, Krüger M, Schwab D, Hoffmann W, Engelke O, Pech O, Kullmann F, Pampuch S, Lenfers B, Weickert U, Schilling D, Boehm S, Beckebaum S, Cicinnati V, Erckenbrecht JF, Dumoulin FL, Benz C, Rabenstein T, Haltern G, Balsliemke M, de Mas C, Kleber G, Pehl C, Vogt C, Kiesslich R, Fischbach W, Koop I, Kuehne J, Breidert M, Sass NL, May A, Friedrich C, Veitt R, Porschen R, Ellrichmann M, Arlt A, Schmitt W, Dollhopf M, Schmidbaur W, Dignass A, Schmitz V, Labenz J, Kaiser G, Krannich A, Barteska N, Ell C. Acute sedation-associated complications in GI endoscopy (ProSed 2 Study): results from the prospective multicentre electronic registry of sedation-associated complications. Gut. 2019;68:445–452. doi: 10.1136/gutjnl-2015-311037. [DOI] [PubMed] [Google Scholar]
- 22.Kozarek R. Are Gastrointestinal Endoscopic Procedures Performed by Anesthesiologists Safer Than When Sedation is Given by the Endoscopist? Clin Gastroenterol Hepatol. 2020;18:1935–1938. doi: 10.1016/j.cgh.2019.11.055. [DOI] [PubMed] [Google Scholar]
- 23.Tse F, Yuan Y, Moayyedi P, Leontiadis GI. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2013;45:605–618. doi: 10.1055/s-0032-1326640. [DOI] [PubMed] [Google Scholar]
- 24.Mazaki T, Mado K, Masuda H, Shiono M. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: an updated meta-analysis. J Gastroenterol. 2014;49:343–355. doi: 10.1007/s00535-013-0806-1. [DOI] [PubMed] [Google Scholar]
- 25.Shaygan-Nejad A, Masjedizadeh AR, Ghavidel A, Ghojazadeh M, Khoshbaten M. Aggressive hydration with Lactated Ringer's solution as the prophylactic intervention for postendoscopic retrograde cholangiopancreatography pancreatitis: A randomized controlled double-blind clinical trial. J Res Med Sci. 2015;20:838–843. doi: 10.4103/1735-1995.170597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang RC, Jiang ZK, Xie YK, Chen JS. Aggressive hydration compared to standard hydration with lactated ringer's solution for prevention of post endoscopic retrograde cholangiopancreatography pancreatitis. Surg Endosc. 2021;35:1126–1137. doi: 10.1007/s00464-020-07477-9. [DOI] [PubMed] [Google Scholar]
- 27.Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, Waljee AK, Repaka A, Atkinson MR, Cote GA, Kwon RS, McHenry L, Piraka CR, Wamsteker EJ, Watkins JL, Korsnes SJ, Schmidt SE, Turner SM, Nicholson S, Fogel EL U. S. Cooperative for Outcomes Research in Endoscopy (USCORE). A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ASGE Standards of Practice Committee. Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32–47. doi: 10.1016/j.gie.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 29.Elmunzer BJ, Serrano J, Chak A, Edmundowicz SA, Papachristou GI, Scheiman JM, Singh VK, Varadarajulu S, Vargo JJ, Willingham FF, Baron TH, Coté GA, Romagnuolo J, Wood-Williams A, Depue EK, Spitzer RL, Spino C, Foster LD, Durkalski V SVI study group and the United States Cooperative for Outcomes Research in Endoscopy (USCORE) Rectal indomethacin alone vs indomethacin and prophylactic pancreatic stent placement for preventing pancreatitis after ERCP: study protocol for a randomized controlled trial. Trials. 2016;17:120. doi: 10.1186/s13063-016-1251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaghoobi M, Alzahrani MA, McNabb-Baltar J, Martel M, Barkun AN. Rectal Indomethacin Prevents Moderate to Severe Post-ERCP Pancreatitis and Death and Should Be Used Before the Procedure: A Meta-Analysis of Aggregate Subgroup Data. J Can Assoc Gastroenterol. 2018;1:67–75. doi: 10.1093/jcag/gwy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tse F, Yuan Y, Bukhari M, Leontiadis GI, Moayyedi P, Barkun A. Pancreatic duct guidewire placement for biliary cannulation for the prevention of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. Cochrane Database Syst Rev. 2016:CD010571. doi: 10.1002/14651858.CD010571.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ASGE Technology Committee. Kethu SR, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kwon RS, Mamula P, Pedrosa MC, Rodriguez SA, Tierney WM. ERCP cannulation and sphincterotomy devices. Gastrointest Endosc. 2010;71:435–445. doi: 10.1016/j.gie.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Itoi T, Dhir V. EUS-guided biliary rendezvous: slow, hesitant, baby steps forward. Gastrointest Endosc. 2016;83:401–403. doi: 10.1016/j.gie.2015.05.049. [DOI] [PubMed] [Google Scholar]
- 34.Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100–107. doi: 10.1016/s0016-5107(03)02300-9. [DOI] [PubMed] [Google Scholar]
- 35.Iwashita T, Yasuda I, Mukai T, Iwata K, Ando N, Doi S, Nakashima M, Uemura S, Mabuchi M, Shimizu M. EUS-guided rendezvous for difficult biliary cannulation using a standardized algorithm: a multicenter prospective pilot study (with videos) Gastrointest Endosc. 2016;83:394–400. doi: 10.1016/j.gie.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 36.Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol. 2014;7:94–102. doi: 10.1007/s12328-014-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klair JS, Zafar Y, Ashat M, Bomman S, Murali AR, Jayaraj M, Law J, Larsen M, Singh DP, Rustagi T, Irani S, Ross A, Kozarek R, Krishnamoorthi R. Effectiveness and Safety of EUS Rendezvous After Failed Biliary Cannulation With ERCP: A Systematic Review and Proportion Meta-analysis. J Clin Gastroenterol. 2021 doi: 10.1097/MCG.0000000000001543. [DOI] [PubMed] [Google Scholar]
- 38.Bang JY, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos) Gastrointest Endosc. 2018;88:9–17. doi: 10.1016/j.gie.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Skinner M, Popa D, Neumann H, Wilcox CM, Mönkemüller K. ERCP with the overtube-assisted enteroscopy technique: a systematic review. Endoscopy. 2014;46:560–572. doi: 10.1055/s-0034-1365698. [DOI] [PubMed] [Google Scholar]
- 40.Klair JS, Jayaraj M, Chandrasekar VT, Priyan H, Law J, Murali AR, Singh D, Larsen M, Irani S, Kozarek R, Ross A, Krishnamoorthi R. ERCP with overtube-assisted enteroscopy in patients with Roux-en-Y gastric bypass anatomy: a systematic review and meta-analysis. Endoscopy. 2020;52:824–832. doi: 10.1055/a-1178-9741. [DOI] [PubMed] [Google Scholar]
- 41.Krutsri C, Kida M, Yamauchi H, Iwai T, Imaizumi H, Koizumi W. Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy. World J Gastroenterol. 2019;25:3313–3333. doi: 10.3748/wjg.v25.i26.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schreiner MA, Chang L, Gluck M, Irani S, Gan SI, Brandabur JJ, Thirlby R, Moonka R, Kozarek RA, Ross AS. Laparoscopy-assisted vs balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748–756. doi: 10.1016/j.gie.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Ayoub F, Brar TS, Banerjee D, Abbas AM, Wang Y, Yang D, Draganov PV. Laparoscopy-assisted vs enteroscopy-assisted endoscopic retrograde cholangiopancreatography (ERCP) in Roux-en-Y gastric bypass: a meta-analysis. Endosc Int Open. 2020;8:E423–E436. doi: 10.1055/a-1070-9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kedia P, Tarnasky PR, Nieto J, Steele SL, Siddiqui A, Xu MM, Tyberg A, Gaidhane M, Kahaleh M. EUS-directed Transgastric ERCP (EDGE) Versus Laparoscopy-assisted ERCP (LA-ERCP) for Roux-en-Y Gastric Bypass (RYGB) Anatomy: A Multicenter Early Comparative Experience of Clinical Outcomes. J Clin Gastroenterol. 2019;53:304–308. doi: 10.1097/MCG.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 45.Dhindsa BS, Dhaliwal A, Mohan BP, Mashiana HS, Girotra M, Singh S, Ohning G, Bhat I, Adler DG. EDGE in Roux-en-Y gastric bypass: How does it compare to laparoscopy-assisted and balloon enteroscopy ERCP: a systematic review and meta-analysis. Endosc Int Open. 2020;8:E163–E171. doi: 10.1055/a-1067-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kedia P, Kumta NA, Widmer J, Sundararajan S, Cerefice M, Gaidhane M, Sharaiha R, Kahaleh M. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: a novel technique. Endoscopy. 2015;47:159–163. doi: 10.1055/s-0034-1390771. [DOI] [PubMed] [Google Scholar]
- 47.Runge TM, Chiang AL, Kowalski TE, James TW, Baron TH, Nieto J, Diehl DL, Krafft MR, Nasr JY, Kumar V, Khara HS, Irani S, Patel A, Law RJ, Loren DE, Schlachterman A, Hsueh W, Confer BD, Stevens TK, Chahal P, Al-Haddad MA, Mir FF, Pleskow DK, Huggett MT, Paranandi B, Trindade AJ, Brewer-Gutierrez OI, Ichkhanian Y, Dbouk M, Kumbhari V, Khashab MA. Endoscopic ultrasound-directed transgastric ERCP (EDGE): a retrospective multicenter study. Endoscopy. 2021;53:611–618. doi: 10.1055/a-1254-3942. [DOI] [PubMed] [Google Scholar]
- 48.Kochhar GS, Mohy-Ud-Din N, Grover A, Carleton N, Kulkarni A, Farah K, Dhawan M, Thakkar S. EUS-directed transgastric endoscopic retrograde cholangiopancreatography vs laparoscopic-assisted ERCP vs deep enteroscopy-assisted ERCP for patients with RYGB. Endosc Int Open. 2020;8:E877–E882. doi: 10.1055/a-1164-6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kröner PT, Bilal M, Samuel R, Umar S, Abougergi MS, Lukens FJ, Raimondo M, Carr-Locke DL. Use of ERCP in the United States over the past decade. Endosc Int Open. 2020;8:E761–E769. doi: 10.1055/a-1134-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McHenry L, Lehman G. Difficult bile duct stones. Curr Treat Options Gastroenterol. 2006;9:123–132. doi: 10.1007/s11938-006-0031-6. [DOI] [PubMed] [Google Scholar]
- 51.ASGE Standards of Practice Committee. Buxbaum JL, Abbas Fehmi SM, Sultan S, Fishman DS, Qumseya BJ, Cortessis VK, Schilperoort H, Kysh L, Matsuoka L, Yachimski P, Agrawal D, Gurudu SR, Jamil LH, Jue TL, Khashab MA, Law JK, Lee JK, Naveed M, Sawhney MS, Thosani N, Yang J, Wani SB. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc. 2019;89:1075–1105.e15. doi: 10.1016/j.gie.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ASGE Technology Committee. Komanduri S, Thosani N, Abu Dayyeh BK, Aslanian HR, Enestvedt BK, Manfredi M, Maple JT, Navaneethan U, Pannala R, Parsi MA, Smith ZL, Sullivan SA, Banerjee S. Cholangiopancreatoscopy. Gastrointest Endosc. 2016;84:209–221. doi: 10.1016/j.gie.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow DK, Slivka A, Haluszka O, Petersen BT, Sherman S, Devière J, Meisner S, Stevens PD, Costamagna G, Ponchon T, Peetermans JA, Neuhaus H. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos) Gastrointest Endosc. 2011;74:805–814. doi: 10.1016/j.gie.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 54.Franzini T, Moura RN, Bonifácio P, Luz GO, de Souza TF, Dos Santos MEL, Rodela GL, Ide E, Herman P, Montagnini AL, D'Albuquerque LAC, Sakai P, de Moura EGH. Complex biliary stones management: cholangioscopy vs papillary large balloon dilation - a randomized controlled trial. Endosc Int Open. 2018;6:E131–E138. doi: 10.1055/s-0043-122493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bang JY, Sutton B, Navaneethan U, Hawes R, Varadarajulu S. Efficacy of Single-Operator Cholangioscopy-Guided Lithotripsy Compared With Large Balloon Sphincteroplasty in Management of Difficult Bile Duct Stones in a Randomized Trial. Clin Gastroenterol Hepatol. 2020;18:2349–2356.e3. doi: 10.1016/j.cgh.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Gerges C, Beyna T, Tang RSY, Bahin F, Lau JYW, van Geenen E, Neuhaus H, Nageshwar Reddy D, Ramchandani M. Digital single-operator peroral cholangioscopy-guided biopsy sampling vs ERCP-guided brushing for indeterminate biliary strictures: a prospective, randomized, multicenter trial (with video) Gastrointest Endosc. 2020;91:1105–1113. doi: 10.1016/j.gie.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 57.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uppal DS, Wang AY. Cholangiocarcinoma: endoscopic therapies. Tech Gastrointest Endosc . 2016;18:83–90. [Google Scholar]
- 59.Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 60.Moole H, Tathireddy H, Dharmapuri S, Moole V, Boddireddy R, Yedama P, Uppu A, Bondalapati N, Duvvuri A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J Gastroenterol. 2017;23:1278–1288. doi: 10.3748/wjg.v23.i7.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bae BC, Yang SG, Jeong S, Lee DH, Na K, Kim JM, Costamagna G, Kozarek RA, Isayama H, Deviere J, Seo DW, Nageshwar Reddy D. Polymeric photosensitizer-embedded self-expanding metal stent for repeatable endoscopic photodynamic therapy of cholangiocarcinoma. Biomaterials. 2014;35:8487–8495. doi: 10.1016/j.biomaterials.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Wang J, Zhou H, Zhou Y, Wang Y, Jin H, Lou Q, Zhang X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50:751–760. doi: 10.1055/s-0043-124870. [DOI] [PubMed] [Google Scholar]
- 63.Strand DS, Cosgrove ND, Patrie JT, Cox DG, Bauer TW, Adams RB, Mann JA, Sauer BG, Shami VM, Wang AY. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80:794–804. doi: 10.1016/j.gie.2014.02.1030. [DOI] [PubMed] [Google Scholar]
- 64.Kwak TW, Lee HL, Song YH, Kim C, Kim J, Seo SJ, Jeong YI, Kang DH. Vorinostat-eluting poly(DL-lactide-co-glycolide) nanofiber-coated stent for inhibition of cholangiocarcinoma cells. Int J Nanomedicine. 2017;12:7669–7680. doi: 10.2147/IJN.S141920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nabi Z, Reddy DN. Endoscopic Palliation for Biliary and Pancreatic Malignancies: Recent Advances. Clin Endosc. 2019;52:226–234. doi: 10.5946/ce.2019.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao JB, Weng JY, Hu YY, Deng GL, Wan XJ. Feasibility and efficacy evaluation of metallic biliary stents eluting gemcitabine and cisplatin for extrahepatic cholangiocarcinoma. World J Gastroenterol. 2020;26:4589–4606. doi: 10.3748/wjg.v26.i31.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Majumder S, Chari ST. Chronic pancreatitis. Lancet. 2016;387:1957–1966. doi: 10.1016/S0140-6736(16)00097-0. [DOI] [PubMed] [Google Scholar]
- 68.Rösch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, Ell C, Haber G, Riemann JF, Jakobs R, Hintze R, Adler A, Neuhaus H, Zavoral M, Zavada F, Schusdziarra V, Soehendra N European Society of Gastrointestinal Endoscopy Research Group. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002;34:765–771. doi: 10.1055/s-2002-34256. [DOI] [PubMed] [Google Scholar]
- 69.Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51:179–193. doi: 10.1055/a-0822-0832. [DOI] [PubMed] [Google Scholar]
- 70.Binmoeller KF, Jue P, Seifert H, Nam WC, Izbicki J, Soehendra N. Endoscopic pancreatic stent drainage in chronic pancreatitis and a dominant stricture: long-term results. Endoscopy. 1995;27:638–644. doi: 10.1055/s-2007-1005780. [DOI] [PubMed] [Google Scholar]
- 71.Smits ME, Badiga SM, Rauws EA, Tytgat GN, Huibregtse K. Long-term results of pancreatic stents in chronic pancreatitis. Gastrointest Endosc. 1995;42:461–467. doi: 10.1016/s0016-5107(95)70051-x. [DOI] [PubMed] [Google Scholar]
- 72.Eleftherladis N, Dinu F, Delhaye M, Le Moine O, Baize M, Vandermeeren A, Hookey L, Devière J. Long-term outcome after pancreatic stenting in severe chronic pancreatitis. Endoscopy. 2005;37:223–230. doi: 10.1055/s-2005-860988. [DOI] [PubMed] [Google Scholar]
- 73.Cremer M, Devière J, Delhaye M, Baize M, Vandermeeren A. Stenting in severe chronic pancreatitis: results of medium-term follow-up in seventy-six patients. Endoscopy. 1991;23:171–176. doi: 10.1055/s-2007-1010649. [DOI] [PubMed] [Google Scholar]
- 74.Jafri M, Sachdev A, Sadiq J, Lee D, Taur T, Goodman A, Gress F. Efficacy of Endotherapy in the Treatment of Pain Associated With Chronic Pancreatitis: A Systematic Review and Meta-Analysis. JOP. 2017;18:125–132. [PMC free article] [PubMed] [Google Scholar]
- 75.Tyberg A, Sharaiha RZ, Kedia P, Kumta N, Gaidhane M, Artifon E, Giovannini M, Kahaleh M. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: a multicenter international collaborative study. Gastrointest Endosc. 2017;85:164–169. doi: 10.1016/j.gie.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 76.Chen YI, Levy MJ, Moreels TG, Hajijeva G, Will U, Artifon EL, Hara K, Kitano M, Topazian M, Abu Dayyeh B, Reichel A, Vilela T, Ngamruengphong S, Haito-Chavez Y, Bukhari M, Okolo P 3rd, Kumbhari V, Ismail A, Khashab MA. An international multicenter study comparing EUS-guided pancreatic duct drainage with enteroscopy-assisted endoscopic retrograde pancreatography after Whipple surgery. Gastrointest Endosc. 2017;85:170–177. doi: 10.1016/j.gie.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 77.Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, Petruzziello L, Familiari P, Mutignani M. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38:254–259. doi: 10.1055/s-2005-921069. [DOI] [PubMed] [Google Scholar]
- 78.Cahen DL, van der Merwe SW, Laleman W, Poley JW, Bruno MJ. A biodegradable non-covered self-expandable stent to treat pancreatic duct strictures in chronic pancreatitis: a proof of principle. Gastrointest Endosc. 2018;87:486–491. doi: 10.1016/j.gie.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 79.Tringali A, Vadalà di Prampero SF, Landi R, Bove V, Familiari P, Hamanaka J, Attili F, Costamagna G. Fully covered self-expandable metal stents to dilate persistent pancreatic strictures in chronic pancreatitis: long-term follow-up from a prospective study. Gastrointest Endosc. 2018;88:939–946. doi: 10.1016/j.gie.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 80.Lee YN, Moon JH, Park JK, Jo SJ, Lee TH, Cha SW, Cho YD, Park SH. Preliminary study of a modified, nonflared, short, fully covered metal stent for refractory benign pancreatic duct strictures (with videos) Gastrointest Endosc. 2020;91:826–833. doi: 10.1016/j.gie.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 81.Oh D, Lee JH, Song TJ, Park DH, Lee SK, Kim MH, Lee SS. Long-term outcomes of 6-mm diameter fully covered self-expandable metal stents in benign refractory pancreatic ductal stricture. Dig Endosc. 2018;30:508–515. doi: 10.1111/den.13041. [DOI] [PubMed] [Google Scholar]
- 82.Sharaiha RZ, Novikov A, Weaver K, Marfatia P, Buscaglia JM, DiMaio CJ, Diehl D, Gabr MM, Gaidhane M, Siddiqui A, Kahaleh M. Fully covered self-expanding metal stents for refractory pancreatic duct strictures in symptomatic chronic pancreatitis, US experience. Endosc Int Open. 2019;7:E1419–E1423. doi: 10.1055/a-0858-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deviere J. Fully Covered Self Expanding Metal Stents (FCSEMS) for Pancreatic Duct Strictures in Patients With Chronic Pancreatitis [accessed 2021 Mar 1]. In: ClinicalTrials.gov [Internet]. Aurora (CO): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02802020. ClinicalTrials.gov Identifier: NCT02802020.
- 84.Miura T, Igarashi Y, Okano N, Miki K, Okubo Y. Endoscopic diagnosis of intraductal papillary-mucinous neoplasm of the pancreas by means of peroral pancreatoscopy using a small-diameter videoscope and narrow-band imaging. Dig Endosc. 2010;22:119–123. doi: 10.1111/j.1443-1661.2010.00926.x. [DOI] [PubMed] [Google Scholar]
- 85.Yamao K, Ohashi K, Nakamura T, Suzuki T, Sawaki A, Hara K, Fukutomi A, Baba T, Okubo K, Tanaka K, Moriyama I, Fukuda K, Matsumoto K, Shimizu Y. Efficacy of peroral pancreatoscopy in the diagnosis of pancreatic diseases. Gastrointest Endosc. 2003;57:205–209. doi: 10.1067/mge.2003.72. [DOI] [PubMed] [Google Scholar]
- 86.Tyberg A, Raijman I, Siddiqui A, Arnelo U, Adler DG, Xu MM, Nassani N, Sejpal DV, Kedia P, Nah Lee Y, Gress FG, Ho S, Gaidhane M, Kahaleh M. Digital Pancreaticocholangioscopy for Mapping of Pancreaticobiliary Neoplasia: Can We Alter the Surgical Resection Margin? J Clin Gastroenterol. 2019;53:71–75. doi: 10.1097/MCG.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 87.Kawai K, Nakajima M, Akasaka Y, Shimamotu K, Murakami K. [A new endoscopic method: the peroral choledocho-pancreatoscopy (author's transl)] Leber Magen Darm. 1976;6:121–124. [PubMed] [Google Scholar]
- 88.Nguyen NQ, Binmoeller KF, Shah JN. Cholangioscopy and pancreatoscopy (with videos) Gastrointest Endosc. 2009;70:1200–1210. doi: 10.1016/j.gie.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 89.Howell DA, Dy RM, Hanson BL, Nezhad SF, Broaddus SB. Endoscopic treatment of pancreatic duct stones using a 10F pancreatoscope and electrohydraulic lithotripsy. Gastrointest Endosc. 1999;50:829–833. doi: 10.1016/s0016-5107(99)70168-9. [DOI] [PubMed] [Google Scholar]
- 90.Jung M, Zipf A, Schoonbroodt D, Herrmann G, Caspary WF. Is pancreatoscopy of any benefit in clarifying the diagnosis of pancreatic duct lesions? Endoscopy. 1998;30:273–280. doi: 10.1055/s-2007-1001254. [DOI] [PubMed] [Google Scholar]
- 91.Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video) Gastrointest Endosc. 2007;65:832–841. doi: 10.1016/j.gie.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 92.Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc. 2007;65:303–311. doi: 10.1016/j.gie.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 93.Navaneethan U, Hasan MK, Kommaraju K, Zhu X, Hebert-Magee S, Hawes RH, Vargo JJ, Varadarajulu S, Parsi MA. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video) Gastrointest Endosc. 2016;84:649–655. doi: 10.1016/j.gie.2016.03.789. [DOI] [PubMed] [Google Scholar]
- 94.Attwell AR, Brauer BC, Chen YK, Yen RD, Fukami N, Shah RJ. Endoscopic retrograde cholangiopancreatography with per oral pancreatoscopy for calcific chronic pancreatitis using endoscope and catheter-based pancreatoscopes: a 10-year single-center experience. Pancreas. 2014;43:268–274. doi: 10.1097/MPA.0b013e3182965d81. [DOI] [PubMed] [Google Scholar]
- 95.Hara T, Yamaguchi T, Ishihara T, Tsuyuguchi T, Kondo F, Kato K, Asano T, Saisho H. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology. 2002;122:34–43. doi: 10.1053/gast.2002.30337. [DOI] [PubMed] [Google Scholar]
- 96.Tajiri H, Kobayashi M, Ohtsu A, Ryu M, Yoshida S. Peroral pancreatoscopy for the diagnosis of pancreatic diseases. Pancreas. 1998;16:408–412. doi: 10.1097/00006676-199804000-00032. [DOI] [PubMed] [Google Scholar]
- 97.El Hajj II, Brauer BC, Wani S, Fukami N, Attwell AR, Shah RJ. Role of per-oral pancreatoscopy in the evaluation of suspected pancreatic duct neoplasia: a 13-year U.S. single-center experience. Gastrointest Endosc. 2017;85:737–745. doi: 10.1016/j.gie.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 98.Kozarek RA, Brandabur JJ, Ball TJ, Gluck M, Patterson DJ, Attia F, France R, Traverso LW, Koslowski P, Gibbons RP. Clinical outcomes in patients who undergo extracorporeal shock wave lithotripsy for chronic calcific pancreatitis. Gastrointest Endosc. 2002;56:496–500. doi: 10.1067/mge.2002.128105. [DOI] [PubMed] [Google Scholar]
- 99.van Huijgevoort NCM, Veld JV, Fockens P, Besselink MG, Boermeester MA, Arvanitakis M, van Hooft JE. Success of extracorporeal shock wave lithotripsy and ERCP in symptomatic pancreatic duct stones: a systematic review and meta-analysis. Endosc Int Open. 2020;8:E1070–E1085. doi: 10.1055/a-1171-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larsen M, Kozarek R. Management of pancreatic ductal leaks and fistulae. J Gastroenterol Hepatol. 2014;29:1360–1370. doi: 10.1111/jgh.12574. [DOI] [PubMed] [Google Scholar]
- 101.Devière J, Antaki F. Disconnected pancreatic tail syndrome: a plea for multidisciplinarity. Gastrointest Endosc. 2008;67:680–682. doi: 10.1016/j.gie.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 102.Tanaka T, Kuroki T, Kitasato A, Adachi T, Ono S, Hirabaru M, Matsushima H, Takatsuki M, Eguchi S. Endoscopic transpapillary pancreatic stenting for internal pancreatic fistula with the disruption of the pancreatic ductal system. Pancreatology. 2013;13:621–624. doi: 10.1016/j.pan.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 103.Arvanitakis M, Delhaye M, Bali MA, Matos C, De Maertelaer V, Le Moine O, Devière J. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65:609–619. doi: 10.1016/j.gie.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 104.Kozarek RA. The future of ERCP. Endosc Int Open. 2017;5:E272–E274. doi: 10.1055/s-0043-101697. [DOI] [PMC free article] [PubMed] [Google Scholar]