Abstract
Type 2 diabetes mellitus (T2DM) is among the most remarkable public health concerns globally. Accumulating research evidence documents that alteration of gut microbiota has an indispensable role in the onset and progression of obesity and T2DM. A reduced microbial diversity is linked to insulin resistance and energy metabolism, especially for the rise of the Firmicutes/Bacteroidetes ratio. Changes in metabolites followed by the gut dysbacteriosis are linked to the presence of T2DM. Moreover, endotoxin leakage and gut permeability caused by gut dysbacteriosis is more of a trigger for the onset and progression of T2DM. Research documents that natural products are remarkable arsenals of bioactive agents for the discovery of anti-T2DM drugs. Many studies have elucidated that the possible mechanisms of the anti-T2DM effects of natural products are remarkably linked to its regulation on the composition of gut microflora and the successive changes in metabolites directly or indirectly. This review presents a brief overview of the gut microbiota in T2DM and several relevant mechanisms, including short-chain fatty acids, biosynthesis and metabolism of branched-chain fatty acids, trimethylamine N-oxide, bile acid signaling, endotoxin leakage, and gut permeability, and describes how dietary natural products can improve T2DM via the gut microbiota.
Keywords: Diabetes, Obesity, Gut microbiota, Mechanisms, Dietary natural products, Metabolites
Core Tip: Numerous natural products possessing prebiotic effects like fruits, vegetables, and medicinal plants, have been found to ameliorate type 2 diabetes mellitus by modulating gut microbiota composition and abundance, reducing the gut permeability, and subsequently increasing the production of short-chain fatty acids and biosynthesis and metabolism of branched-chain fatty acids, decreasing the level of lipopolysaccharide, and inhibiting the inflammation.
INTRODUCTION
Diabetes mellitus (DM) is characterized by hyperglycemia and insufficient insulin secretion and/or dysfunction. Epidemiological studies have implied that the number of DM patients will rise from 422 million in 2018 to 592 million in 2035[1]. The dominant risk factor for DM is becoming more prevalent over time in both developed and developing regions[1,2].
Guidelines show that type 2 DM (T2DM) accounts for nearly 95% of DM types, which include T1DM, gestational DM, and so on[3,4]. T2DM has always been the focus and key point of research on DM. The incidence of T2DM is related with diminished secretion of insulin secretion along with insulin resistance (IR) caused by individual genetics and acquired environmental factors, for instance, air pollution, unhealthy lifestyle, and poor mental state, causing multiple organ injury and several complications[5,6]. Current studies are investigating disorders of energy metabolism, endoplasmic reticulum (ER) stress, oxidative stress, inflammatory response, mitochondrial dysfunction, as well as gut microbiota[7-9].
The Human Microbiome Project has been leveraged to explore how gut microbiota influences the development of the human diseases that have started to emerge[10]. The human gut harbors trillions of microorganisms, including > 1014 bacteria, which are mainly composed of six main phyla, i.e., Bacteroidetes, Verucomicrobia, Firmicutes, Proteobacteria, Fusobacteria and Actinobacteria[11]. Numerous studies have illustrated that the gut microflora modulates diverse cellular processes, e.g., micronutrient synthesis, bowel motility, and minerals and electrolytes absorption[12], and provides signals to activate the immune response, inflammation, and oxidative stress in many metabolic diseases, for instance, nonalcoholic fatty liver disease and T2DM[13,14]. However, the type of microbes that contribute to DM and mechanisms associated are still not fully understood. Therefore, a systematic search of various electronic databases, including Google Scholar, PubMed, Sciencedirect, and so on were performed with several keywords alone or in combination [diabetes, obesity, gut microbiota, short-chain fatty acids (SCFAs), branched-chain amino acids (BCAAs), inflammation, gut barrier, etc.]. Herein, we review the present studies on changes in the gut microflora and summarize the possible mechanism of the gut microbiota dysbiosis in T2DM as well as some relevant dietary natural products.
ALTERNATION OF GUT MICROBIOTA IN T2DM
It is estimated that > 80% of T2DM patients are overweight, which is recognized as the greatest risk factor for T2DM[15]. Apart from genetic and lifestyle factors, energy homeostasis disorder induced by gut microbial dysbiosis has an indispensible role in the onset and progression of T2DM. Many metagenome-wide association reports have shown remarkable correlations between variation of specific gut microbes, bacterial genes, and metabolic pathways in T2DM[16,17]. It has been verified that gut microflora is among the independent contributing factors for fat accumulation and IR, whereas germ-free (GF) mice, having no microbiota, had little weight gain and increase in body fat, and mild resistance to the diet relative to wild-type mice[18]. Obese mice-derived microbiota (FMT) increased weight gain in GF mice when transplanted unlike FMT derived from thin mice[19]. Moreover, FMT was also carried out in numerous studies in humans, including obesity, ulcerative colitis, and so on[20]. Several studies have shown that there is a high Firmicutes/Bacteroidetes ratio in obese mice or mice fed Western diets[21]. Larsen et al[22] found a low number of Firmicutes and increased Betaproteobacteria in DM, relative to nondiabetic patients, which were positively related with the plasma glucose contents, especially for Betaproteobacteria. Besides Bacteroidetes and Firmicutes, Prevotella spp., Clostridium coccoides, and Eubacterium rectale were more prevalent in individuals with diabetes and positively and remarkably linked to plasma glucose, rather than body mass index, indicating that these bacteria can directly influence the level of glucose tolerance[23]. The number of Clostridiales, Streptococcus mutans, and Lactobacillus gasseri is increased whereas that of Aecalibacterium prausnitzii (both butyrate-producing bacteria) and Roseburia intestinalis is decreased in various groups of T2DM patients. These findings suggest that gut microbiota dysbiosis is strongly linked to the onset and progression of T2DM.
METABOLITES OF GUT MICROBIOTA AFFECT ENERGY METABOLISM
SCFAs
The gut microbiota acts as a real organ, which can generate monosaccharides and SCFAs by hydrolyzing and fermenting dietary polysaccharides from the host, including acetate, propionate, and butyrate. The mechanism involving the production of adipose-tissue-derived satiety hormone leptin under the action of SCFAs is the most studied[24]. Nevertheless, recent studies have illustrated that SCFAs act against obesity by preventing fat accumulation. The production of SCFAs is different in obese and insulin-resistant subjects via regulation of metabolism in several organs and tissues, for instance, adipose tissue, the pancreas, and the brain[25,26]. The increased level of butyrate is linked to improved insulin sensitivity[27,28], highlighting the significance of butyrate-secreting bacteria in modulation of glycemia. By further analyzing stool samples from patients, Psichas et al[29] illustrated that high rates of colonic fermentation were closely related with high SCFA products in obese individuals. Therefore, the effect of SCFAs on energy metabolism is multidimensional, including involving lipid oxidation, appetite modulation, as well as glucose metabolism (Figure 1)[24].
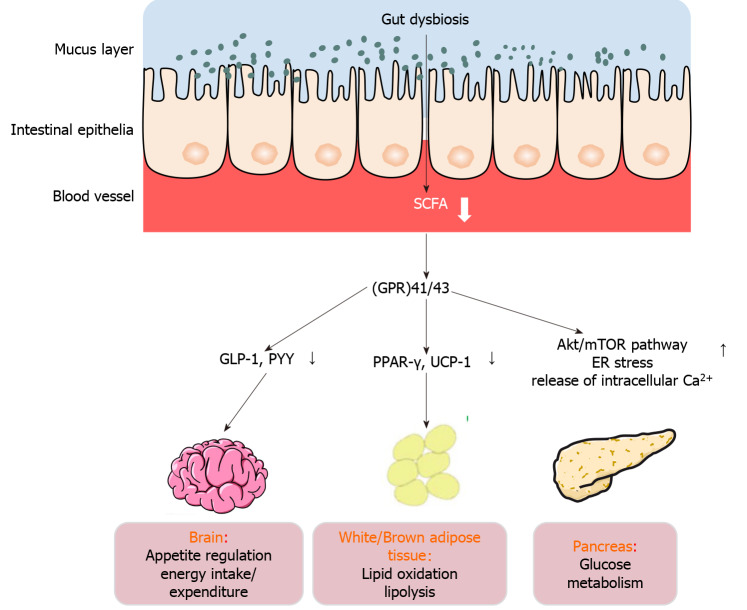
Figure 1.
Mechanisms of action of short-chain fatty acids in type 2 diabetes mellitus. The effect of short-chain fatty acids on energy metabolism is multidimensional, including appetite regulation, energy intake/expenditure, and lipid oxidation, as well as glucose metabolism. SCFA: Short-chain fatty acid; GLP-1: Glucagon-like peptide 1; PYY: Peptide YY; ER: Endoplasmic reticulum; UCP-1: Uncoupling protein-1; mTOR: Mammalian target of rapamycin.
Several studies have shown that SCFAs trigger the secretion of Peptide YY (PYY) along with glucagon-like peptide 1 (GLP-1), by recognizing and stimulating G-protein-coupled receptor (GPR)41/43 in rodent and human cell lines[30-32]. Similarly, the same effect and mechanisms have been documented and confirmed in vivo[33]. Moreover, numerous studies have demonstrated that exogenous GLP-1 and PYY acutely reduce food intake in humans, which has been widely used in the treatment of T2DM[34-38]. Therefore, SCFAs not only contribute 5%-10% of energy to the host, but are also recognized by endogenous ligands of GPR41/43, and act as signaling molecules to participate in adjustment of energy[39-41]. Kimura documented that GPR43-modulated adipose-insulin cascades and sympathetic activity were controlled by GPR41, which detected SCFAs released from gut microbiota[42]. Nevertheless, in GPR41/FFAR3-/- and GPR43/FFAR2-/- knockout cells, GLP-1 secretion activated by SCFAs was remarkably attenuated. However, unlike GPR41/ FFAR3-/- mice, GPR43/FFAR2-/- showed markedly downregulated GLP-1 in circulation, indicating that GPR43/FFAR2 plays a more important role in these effects. Confusingly, the level of satiety hormones at 24 wk following supplementation of inulin propionate ester at 10 g/d was not different from those in groups treated with 10 g/d inulin alone in overweight adults[43], emphasizing mechanisms of crosstalk between the gut microflora and host. Therefore, SCFAs, as metabolites of the gut microflora, are important signaling molecules in the regulation of host energy metabolism. Relevant research on the metabolic influences of SCFA delivery or production on the host is urgently needed, especially the influences on satiety-inducing hormones.
Besides, in rodent receiving acute and chronic oral supplementation of SCFAs, besides affecting the production of satiety hormones, SCFAs could also advantageously impact body weight via impacting energy expenditure[44]. Another study documented that a single oral administration of 1.5% AcOH with a stomach tube, in comparison to distilled water, elevated energy expenditure along with lipid oxidation[45]. Similar to the above results, injection with acetate (5.2 mg/kg) elevated whole-body oxygen consumption and decreased the body weight at 6 mo post-treatment in rats[46]. Moreover, butyrate (5% w/w) prevented high-fat diet (HFD)-induced obesity via enhanced lipid oxidation[47]. Studies on the mechanism showed that these effects were linked to the UCP-1 (uncoupling protein-1) and elevation of peroxisome proliferator-activated receptor-(PPAR)-γ co-activator 1α (PPARGC1A, coding for PGC1α) in brown adipose tissue[47]. The animal experimental data proved that SCFAs upregulate genes that modulate lipid oxidation and thermogenesis, thereby eliminating adiposity and weight gain[24]. Human studies have indicated that colonic infusion of SCFA mixtures, including acetate, butyrate, and propionate, reduces lipolysis and elevates energy expenditure and PYY, as well as fat oxidation in overweight/obese individuals[48]. In addition, acute oral sodium propionate ingestion elevated resting expenditure of energy along with lipid oxidation in 18 healthy volunteers in contrast with a sodium chloride control, and these effects were independent of insulin and glucose contents and sympathetic nervous system activity[49]. The result from a randomized double-blind crossover trial proved that sodium acetate infusion into the distal colon (180 mmol/L) enhanced lipid oxidation relative to sodium chloride placebo, and the resting energy expenditure between overnight-fasted overweight and obese individuals was similar[44]. Jocken et al[49] showed that SCFAs reduced lipolysis and promoted lipid oxidation in white adipose tissue (WAT), and that antilipolytic effect was orchestrated by FFAR3 and/or FFAR2 levels in WAT[50]. Therefore, these results strongly suggest that SCFAs are beneficial for weight control and influence energy expenditure. Research on the mechanism of action of SCFAs in DM has suggested that SCFAs induce GLP-1 and amylin secretion via FFA2 receptor, hence modulating glucose metabolism and insulin levels[51]. The Akt/mTOR pathway, ER stress, and release of intracellular Ca2+ play important roles in this process[51]. There is need to understand the pathways and regulators involved in a variety of cell models, for instance, human-derived adipocytes, hepatocytes, or skeletal muscle.
BCAAs
BCAAs have increasingly been studied as playing a role in diabetes[52]. BCAAs constitute nearly approximately 20% of the amino acids used to form proteins[53]. More studies have documented that plasma content elevations of BCAAs have been linked to obesity, as well as diabetes[54]. Previously, this phenomenon was thought as a consequence and not a cause of IR[55]. Nevertheless, recently, growing research evidence opines that BCAAs elevations contribute to IR: (1) Exogenous BCAAs remarkably diminish the sensitivity to insulin, as illustrated via hyperinsulinemic euglycemic clamps measurements[56,57]; and (2) coadministration of BCAAs with HFD generally worsens the ensuing IR in rodents[58]. The presence of HFD or lipids in all these rodents played a critical role in this effect, and BCAAs alone elicited insignificant or no impact, illustrating that BCAAs crosstalk with fatty acids (FAs) to promote IR[59]. Further studies have proved that FA oxidation disorders elevated BCAAs contents in plasma, and aggregation of the intermediate metabolites of BCAAs, for instance, C3 and C5 acylcarnitines along with acetyl-CoA can inhibit complete FA oxidation[60]. The crosstalk of BCAAs with FAs induces energy metabolism disorder, including ATP production, TCA (tricarboxylic acid cycle) cycle, as well as oxidative phosphorylation, and leads to mitochondrial dysfunction and inflammation, which are critical for the progression of DM[61-63]. Inflammatory factor signaling pathways, including the nuclear factor (NF)-κB pathway and mammalian target of rapamycin complex 1 (mTORC1), might be candidate therapeutic targets in this process[54,64]. Moreover, BCAAs supplementation could repress stimulation of Akt2 via the mTORC1- and mTORC2-dependent cascades and enhanced degradation that is dependent on Akt2 ubiquitin-proteasomes via mTORC2 signaling, indicating that mTORC2 might also play an important role in this process[65]. More importantly, by using metagenomics and metabonomics methods, increased concentrations of BCAAs were linked to a gut microbiome that has an abundant biosynthetic ability for BCAAs. Prevotella copri coupled with Bacteroides vulgatus are recognized as the primary species modulating the relationship of biosynthesis of BCAAs with IR[66], indicating that gut microbes affect host serum metabolome along with insulin sensitivity via regulating the level of BCAAs (Figure 2).
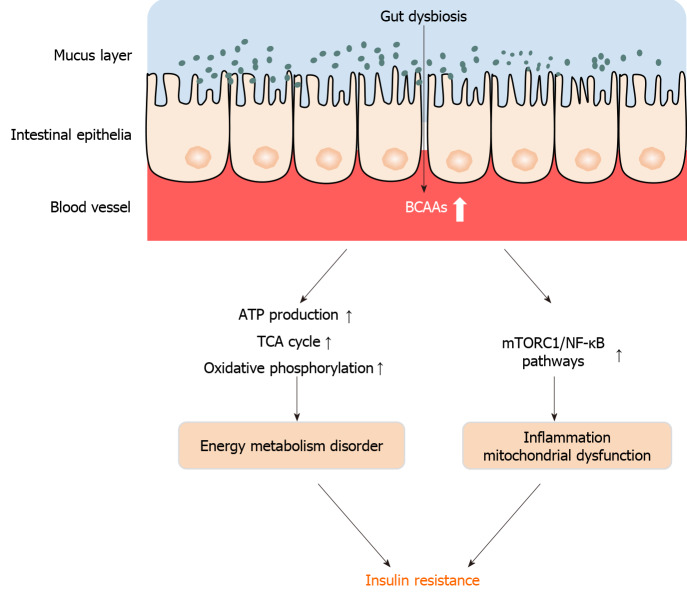
Figure 2.
Increased plasma levels of branched-chain amino acids induced by dysbiosis are closely associated with obesity and diabetes. The accumulation of branched-chain amino acids inhibits complete oxidation of fatty acids, induces energy metabolism disorder, including ATP production, tricarboxylic acid cycle, and oxidative phosphorylation, thereby causing energy metabolism disorder, and induces inflammation by targeting nuclear factor-κB and mammalian target of rapamycin complex 1. TCA: Tricarboxylic acid; NF-κB: Nuclear factor-κB; mTORC1: Mammalian target of rapamycin complex 1; BCAAs: Branched-chain amino acids.
Trimethylamine N-oxide
Terrestrial mammal trimethylamine N-oxide (TMAO) is derived from exogenous arsenals, with the TMA serving as the precursor, which is a metabolite of diverse other precursors, primarily choline, as well as carnitine originating from ingested foods[67]. Bacteria metabolize choline and L-carnitine into TMA, and flavin-containing monooxygenase (FMO)-3, a hepatic enzyme, oxidizes TMA into TMAO. There are two key steps for the generation of TMAO, illustrating that the gut microflora is an independent risk factor for DM[68,69]. Further analysis of the microbiota has shown that the primary bacterial phylum that degrades carnitine to TMA is Proteobacteria along with bacteria of the family Prevotellaceae, phylum Bacteroidetes. In contrast, the S24-7 family of Bacteroidetes (the family majorly involved in plant polysaccharides) is related with diminished TMAO contents[70-72].
The initial findings suggested a positive relationship of high plasma TMAO content with an elevated risk for major severe cardiovascular diseases including myocardial infarction, stroke, and atherosclerosis. More studies have documented that TMAO is opined to serve as a biomarker, as well as an independent predisposing factor for many diseases, for example, kidney failure, DM, and cancer[69]. Some research evidence opines that TMAO influences glucose metabolism, and remarkably higher median TMAO contents in plasma occur in individuals with diabetes in contrast with persons without DM[73,74]. A prospective mechanism connecting TMAO with IR is TMAO-dependent elevated concentrations of N-nitroso compounds and upregulated activity of FMO-3[75,76]. Research documents reduced TMAO and choline contents in the hepatic tissues of diabetic mice[77]. Metformin has been shown to decrease glucose and increase plasma TMAO[77]. TMAO measurements have low diagnostic significance in diabetic individuals who are obese due to the high variability of TMAO contents in plasma[78]. More importantly, treatment with TMAO promotes normal protein folding, counteracting ER stress in diabetic rats, illustrating a potentially beneficial impact of TMAO in DM[79]. Therefore, from the results so far, the characteristics of TMAO in DM are still controversial.
GUT PERMEABILITY GIVES A NOVEL INSIGHT INTO T2DM
Although chronic low inflammation induced by metabolic endotoxemia in serum is a risk factor for T2DM, gut permeability is more of a trigger for the onset and progression of T2DM. The intestinal mucosal lining functions as a barrier, preventing viruses, toxins, and pathogenic bacteria invading from the gut epithelium into the circulation[80]. Recent reports have chronicled that altered bowel function of the gut barrier is involved in DM pathogenesis[81]. Disruption of the gut barrier is documented in genetically obese mice, which promotes permeability of the intestinal mucosa, leading to lipopolysaccharide (LPS) leakage into the portal blood circulation, and increased metabolic endotoxemia, inflammatory cytokine concentrations, and pathogen colonization[82]. Tight junction protein expression reflects the disruption of the gut barrier, and tight junction proteins consist of zonula occludens (ZO)-1 and occludin, which are remarkably reduced in mice with HFD-induced obesity, thus resulting in inflammation, permeability of the intestines, increased metabolic endotoxemia, and more serious metabolic disorders[83]. Increases in endogenous GLP-2 production contribute to the enhancement of functions of the gut barrier during obesity and DM[84]. Pharmacological treatment with prebiotics or GLP-2 decreases gut permeability, which finally diminishes LPS contents in the plasma, as well as blunts the inflammatory state of ob/ob mice[84,85].
METABOLIC ENDOTOXEMIA-INDUCED CHRONIC LOW INFLAMMATION IN T2DM
At present, the mainstream view suggests that low-grade chronic systemic inflammation contributes to the onset and progression of IR, DM, and obesity. As a component of the cell wall of Gram-negative bacteria, LPS is defined as a metabolic endotoxin, which is a trigger for the maintenance of a low-grade chronic systemic inflammatory state in the host, responding to HFD[86]. Based on these results, many studies have documented that the circulating concentration of LPS is remarkably linked to some specific bacterial genera[87]. The amount of Bifidobacterium is remarkably and negatively correlated with high portal plasma measurements of LPS in HFD-induced models[88]. Similarly, bacterial community structural analysis shows that antibiotic treatment remarkably reduces the numbers of Lactobacillus, Bifidobacterium, as well as Bacteroides–Prevotella in ob/ob mice, indicating that metabolites of gut microflora are closely linked with the incidence and prevalence of DM[89]. Moreover, following exposure to 0.05% (wt/wt) aglycone quercetin by oral perfusion, metabolic endotoxemia and cecal content of LPS in HFD-induced mice are dramatically reduced, and then the fasting glycemia, inflammation, and body weight are also improved[64]. Growing in vitro along with in vivo research evidence suggests that Toll-like receptors (TLRs) are responsible for LPS-induced inflammatory responses[90]. TLR4 recognizes bacterial LPS, thereby triggering the expression of proinflammatory cytokines along with chemokines, including tumor necrosis factor (TNF)-α[91]. TNF-α is strongly linked to IR, promoting the onset and progression of DM[92]. Besides animal experimentation, clinical studies have also shown that TLR4 is a pivotal receptor of the natural immune system, with a core role of triggering the inflammatory response, including TNF-α, interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, and IL-1β[93]. Genetic variants in the TLR4 gene or IRAK1 and TIRAP genes might have an indispensable role in the onset and progression of IR and T2DM through disruption of the inflammatory reaction[92]. TLR5, as another member of the TLR family, which is mainly expressed in intestinal mucosa, has an indispensable role in the onset and progression of metabolic syndromes[94]. TLR5-deficient mice exhibit hyperphagia and develop metabolic diseases, for instance, hyperlipidemia, hypertension, IR, and obesity[95]. TLR5 knockout mice exhibited an increase in body mass and epididymal fat pad size in varying degrees compared to their wild-type counterparts, which was linked to increased contents of serum triglycerides and cholesterol, as well as increases in the proinflammatory proteins interferon-γ and IL-1β in adipose tissue[96]. Moreover, by transplanting the gut microflora from TLR5-deficient mice to their wild-type GF counterparts, the increased contents of proinflammatory cytokines and features of metabolic diseases, for instance, IR and obesity, have been documented[96]. The elevated inflammatory mediators in DM cause oxidative and ER stress in pancreatic islet β cells, then influence insulin sensitivity and glucose homeostasis, aggravating DM[97]. TLR2 can identify components of bacterial cell walls and lipid-containing molecules, thereby transducing inflammatory signaling by activating NF-κB and producing proinflammatory cytokines in cells[98]. More importantly, unlike TLR5-deficient mice, TLR2-deficient mice exhibit increased insulin sensitivity and faster clearance of glucose, accompanied by attenuated expression of inflammatory cytokines[99,100]. Therefore, TLRs have multiple effects on the expression of inflammatory cytokines in T2DM. The distribution of TLRs in tissues and organs may decide their role in the onset and progression of T2DM.
INTERACTIONS BETWEEN BILE ACIDS AND GUT MICROBIOTA
As the end-product of cholesterol metabolism, bile acids (BAs) are derived from cholesterol catabolism in the liver and are essential for the solubilization, absorption, and metabolism of lipid- and fat-soluble vitamins[101], as well as xenobiotics, including drugs and environmental contaminants[102]. However, BAs are now identified as key endogenous steroids that play critical roles in regulating and maintaining lipid, glucose, and energy metabolism, protecting against inflammation in the liver, intestine, and heart, and preventing DM and obesity (Figure 3)[103]. Clinical data prove that dysregulation of BA homeostasis and dysbiosis can induce metabolic disorder, for instance, disorders of lipid, glucose, and energy metabolism, as well as inflammatory cytokine generation, which are closely linked with T2DM[102]. BA synthesis occurs via two pathways. The rate-limiting enzyme cytochrome P450 cholesterol 7-ahydroxylase (CYP7A1) produces the majority of the BA pool, and is responsible for the classical pathway. There is also an alternative pathway (3%-18% of total BA synthesis in healthy humans), which is initiated by cytochrome P450 27-ahydroxylase (CYP27A1)[104]. All of the products of BAs are the primary BAs including cholic acid (CA) and chenodexycholic acid (CDCA), which are reabsorbed through the enterohepatic circulation. Around 95% of primary BAs are actively reabsorbed through the apical sodium-dependent BA transporter (ASBT/SLC10A2) and secreted at the basolateral membrane by the heterodimeric organic solute transporters α and β[104]. Only 5% of primary BAs reach the large intestine and are converted into secondary BAs by the gut microbiota, for instance, deoxycholic acid (DCA), lithocholic acid (LCA), and ursodesoxycholic acid (UDCA) in humans, and DCA, LCA, muricholic acid (MCA), hyodeoxycholic acid, and murideoxycholic acid in mice[105]. Further research has proved that these gut bacteria have bile salt hydrolase, including Lactobacillus, Bifidobacterium, Firmicutes, Enterococcus, Clostridium, and Bacteroides, which transform primary BAs into secondary BAs at millimolar concentrations in the intestine, and determine BA composition in the circulating pool and total BA pool size[104,106]. Thus, interactions between BAs and gut bacteria remarkably affect the health of the host and contribute to the pathogenesis of metabolic diseases, for instance, liver disease, obesity, and DM. However, it is still unclear how BA pool alterations affect DM.
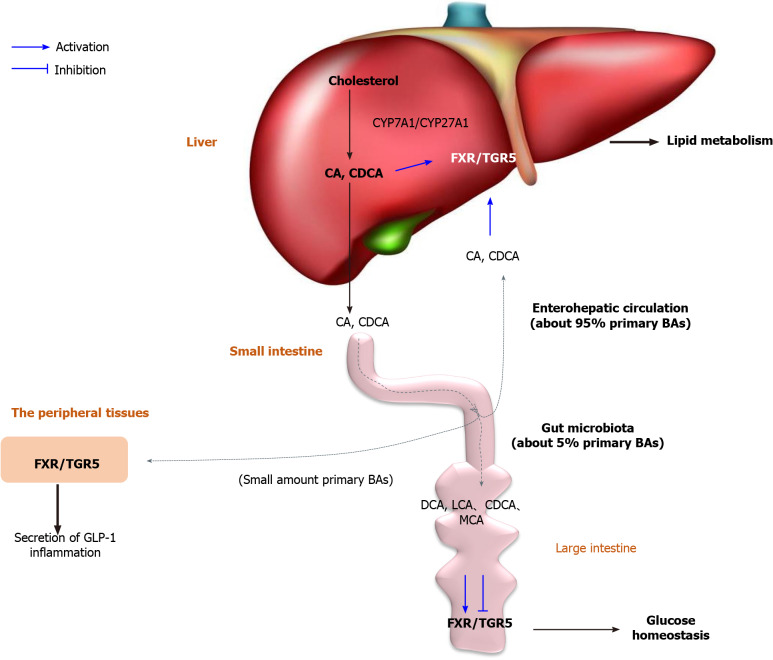
Figure 3.
The critical role of dysbiosis/bile acids/farnesoid X receptor/TGR5 axis in diabetes mellitus. Bile acids were synthesized by cytochrome P450 (CYP) 7A1/CYP27A1. Gut microbiota regulates the BA pool size and composition, thereby participating in energy metabolism, glucose homeostasis, lipid metabolism, and inflammation of the host by activating farnesoid X receptor/TGR5 in various tissues. BA: Bile acid; CYP: Cytochrome P450; FXR: Farnesoid X receptor; GPBAR1: G-protein-coupled bile acid receptor 1; CA: Cholic acid; CDCA: Chenodexycholic acid; GLP-1: Glucagon-like peptide 1; DCA: Deoxycholic acid; LCA: Lithocholic acid; MCA: Muricholic acid.
Farnesoid X receptor (FXR) and G-protein-coupled BA receptor 1 (GPBAR1, also known as TGR5) play critical roles in gut microbiota-mediated BA signaling. FXR and TGR5 are expressed in various tissues, including the liver, intestine, kidneys, adrenal glands, brown/white adipose tissue, and immune cells[105]. FXR also competes for other nuclear receptors, for instance, PPAR and NF-κB, thereby regulating lipid and glucose metabolism and the inflammatory response in (patho)physiological conditions in humans[104,107]. By raising FXR-deficient mice in GF conditions, Gonzalez et al[108] confirmed that the gut microflora regulates FXR signaling by acting on the conversion of primary BAs into secondary BAs, and by regulating their synthesis. BAs are natural ligands of FXR and TGR5, including agonists (CDCA, DCA, CA and LCA) and antagonists (MCA and possibly UDCA). Selectivity reduces the gut microflora production of β-MCA induced by dysbiosis in obese mice, a rodent-specific FXR antagonist, which reduces BA feedback regulation and increases BA synthesis in the liver by alleviating FXR repression in the ileum. This emphasizes that changes in the BA pool size and composition caused by dysbiosis are closely linked to obesity and DM[108,109]. Some studies have illustrated that: (1) In the intestine, FXR reduces postprandial glucose absorption, which is delayed in FXR-deficient mice; and (2) BAs regulate the production and secretion of GLP-1 via the activation of TGR5 and FXR in enteroendocrine L cells. BAs also control lipoprotein metabolism via hepatic FXR activation. FXR reduces lipogenesis by repressing hepatic sterol responsive element binding protein (SREBP)-1c expression in SHP-dependent and FGF15/19-dependent manners[110]. FXR represses microsomal triglyceride transfer protein and apolipoprotein B gene expression, thereby reducing very-low-density lipoprotein secretion[111]. FXR and TGR5 are expressed in several immune cell types, including monocytes, macrophages, and Kupffer cells, and human dendritic cells also have a critical role in the onset and progression of DM and related complications. For example, TGR5 activation reduces HFD-induced glucose intolerance, IR, and inflammation by inhibiting NLRP3 inflammasome activation via the TGR5-cyclic AMP-protein kinase A axis in mice[112]. These results indicate that the gut microflora regulates the BA pool size and composition, thereby participating in energy metabolism, glucose homeostasis, lipid metabolism, and inflammation of the host. The dysbiosis/BAs/FXR/TGR5 axis might play an important role in this process.
DIETARY NATURAL PRODUCTS AND GUT MICROBIOTA
Various synthetic drugs with antidiabetic effects are in current clinical use. However, the application of these drugs is usually limited by their various undesirable adverse effects, including weight gain, hypoglycemia, fluid retention, heart failure, urinary tract infection, and dyspepsia[2]. In contrast, numerous studies have indicated that herbal medicines and their active ingredients possess antidiabetic properties with few adverse effects, and are worthy of investigation for clinical application. An increasing number of studies have illustrated that the extracts of fruits, vegetables, herbs, and other plant foods alleviate T2DM by modulating the gut microbiota (Table 1)[113].
Table 1.
Main preclinical and human data reporting the effects of dietary natural products on gut microbiota and associated mechanisms in diabetes mellitus and related complications
| Name | Model | Key findings | Ref. |
| Pumpkin polysaccharide | HFD (mice) | Increases SCFAs production; selectively enhances the abundance of Bilophila and Prevotella | Liu et al[118] |
| Inulin | T2DM in rats induced by HFD and streptozotocin and clinic trial | Increases SCFA-producing bacteria including Lachnospiraceae, Phascolarctobacterium, Bacteroides, and Akkermansia muciniphila | Li et al[112] and Food and Drug Administration, HHS[113] |
| Lessonia nigrescens ethanolic extract | T2DM mice (streptozotocin injection) | Increases the ratio of Bacteroidetes/Firmicutes in the intestine | El Kaoutari et al[115] |
| Grape pomace extract | HFD (mice) | Reduces the abundance of Desulfovibrio and Lactococcus, and increases the abundance of Allobaculum and Roseburia; improves the gut barrier function | Bowey et al[123] |
| Resveratrol | NASH rat model | Ameliorates the intestinal barrier dysfunction and inflammation | Li et al[124] |
| Quercetin | HFD (mice) | Reverts gut microbiota imbalance and related endotoxemia-mediated TLR4 pathway induction | Solon-Biet et al[61] |
| Berberine | db/db mice High fat diet (mice and rats) FXR knockout (FXRint-/-) mice | Modulates the ratio of Firmicutes/Bacteroidetes; increases SCFA content in feces; regulates BCAAs biosynthesis and catabolism in liver and adipose tissue; reduces the increased expressions of inflammatory mediators and alleviates gut permeability by decreasing LPS level in plasma; modulates the bile acid cycle and subsequently the ileal FXR signaling pathway | Tesar and Kottke[129], He et al[130], Song et al[131], Wang et al[132], and Zhang et al[133] |
| Capsaicin | ob/ob mice | Increases the ratio of Firmicutes to Bacteroidetes and the number of Roseburia; decreases the levels of proinflammatory cytokines, including TNF-α and IL-6 | Christodoulou et al[128] |
HFD: High-fat diet; SCFAs: Short-chain fatty acids; T2DM: Type 2 diabetes mellitus; NASH: Nonalcoholic steatohepatitis; BCAAs: Branched-chain amino acids; FXR: Farnesoid X receptor; LPS: Lipopolysaccharide; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin 6.
Dietary fibers
Dietary fibers are compounds of natural origin present in plants. Chemically, these compounds are defined as nondigestible carbohydrates (with ≥ 3 monomeric units), for instance, polysaccharides and oligosaccharides[114]. Some prospective cohort studies have shown that individuals with high intake of dietary fiber are inversely linked to the risk of DM compared with low intake[115]. Although most dietary fibers belong to prebiotics, which are not digested and absorbed by the human gut and remain intact while passing through the gastrointestinal tract, they selectively arouse the growth and activity of potential beneficial bacteria in the gut. The human gut microflora encodes several types of carbohydrate-active enzymes, including glycoside hydrolases, polysaccharide lyases, glycosyltransferases, and carbohydrate esterases, which are capable of degrading dietary fiber and then generating small-molecular-weight metabolites (degradation products), which may display antidiabetic effects in T2DM[116]. Studies on anti-T2DM activity of fibers have shown that dietary fibers increase the abundance of some species, for instance, Eubacterium rectale, Roseburia, Prevotella, Ruminococcus bromii, Bacteroides, and Bifidobacterium, and decrease the number of some Gram-negative bacteria, for instance, Desulfovibrio and Enterobacteriaceae (LPS-producing bacteria)[117]. More importantly, these changes induced by dietary fiber intervention enhance the production of SCFAs, which can bind to the GPR and enhance the level of the enteroendocrine hormones PYY and GLP-1 in gut epithelial L-cells, thereby improving IR, appetite regulation, and energy intake/ expenditure, as well as lipid oxidation[118]. The alleviating effect of pumpkin polysaccharide in HFD-fed mice is linked with increased SCFA production and selective enhancement of some bacteria, for instance, Bacteroidetes, Prevotella, and Deltaproteobacteria[119]. Administration of inulin can reduce the fasting blood glucose level, increase GLP-1 level, and alleviate glucose intolerance as well as blood lipid contents in rats with T2DM induced by HFD and streptozotocin[120]. SCFA-producing bacteria have a key role in the process, including Lachnospiraceae, Phascolarctobacterium, and Bacteroides[120]. A double-blind, randomized, controlled clinical trial of 60 patients with T2DM found that supplementation with 10 g/d inulin powder promoted gut health by increasing the proportion of Akkermansia muciniphila[121].
Polyphenols
Dietary polyphenols are natural compounds that occur in many plant foods, such as fruits and vegetables. These compounds constitute a large heterogeneous collection of compounds, but with structural units common to all phenolic compounds (hydroxylated aromatic rings or phenol rings)[122]. Numerous studies have proved that the beneficial effects of dietary polyphenols may reduce the risk of T2DM and/or its complications[123]. However, it is proved that most polyphenols are not digested and absorbed by the small intestine, but remain in the colon, and are metabolized by gut microflora including demethylation, dihydroxylation, and decarboxylation[124]. Focusing on the changes of the intestinal microbiota, polyphenols improve intestinal health by promoting the growth of beneficial bacteria and inhibiting the pathogenic bacteria[117]. More importantly, the main antidiabetic actions of dietary polyphenols include: Protection of pancreatic β-cells against stimuli-induced oxidative stress; inhibition of the activities of various enzymes (for instance, α-amylases, α-glucosidases, and pancreatic lipase); promotion of β-cell proliferation and survival; and repression of advanced glycation end products formation[117]. An ethanolic extract of Lessonia nigrescens (rich in phenolics and flavonoids) displays its hypoglycemic effect by increasing the abundance of Bacteroidetes and decreasing Firmicutes in the intestines[118]. A recent study illustrated that the antidiabetic effects of polyphenols were also linked to changes in the markers of gut barrier function, for instance, ZO-1 and occludin[125]. Grape pomace extract (mixture of polyphenols consisting of anthocyanins, flavanols, and flavanol glycosides) improves fat mass gain, adipose tissue inflammation, impaired glucose tolerance, and IR by reducing the concentrations of Clostridium sensu stricto, Lactococcus, Desulfovibrionaceae, and Streptococcaceae, and increasing the abundance of Allobaculum, Prevotellaceae, Roseburia, and Erysipelotrichaceae, and improving gut barrier function[126]. Besides these extracting mixtures, the effects of several polyphenol monomeric compounds in T2DM/obesity are also closely linked to the alternation of gut microflora. Resveratrol attenuates HFD-induced nonalcoholic steatohepatitis and ameliorates the intestinal barrier dysfunction and inflammation in rats[127]. Quercetin reverted gut microflora imbalance and related endotoxemia-mediated TLR4 pathway induction, with subsequent repression of inflammasome response and reticulum stress pathway activation, leading to the blockage of lipid metabolism gene expression deregulation in obese mice[63].
Alkaloids
Alkaloids have antimalarial, antihyperglycemic, antiasthma, anticancer, and antibacterial activities[128]. Many of them have been utilized in traditional or modern medicines for drug discovery. Recent studies have illustrated that the pharmacological activity of alkaloids is mainly mediated by the gut microflora[129]. Because most alkaloids usually exhibit low oral bioavailability, their absorption into the bloodstream is difficult. The gut microbiota has a variety of enzymes, consisting of β-glucuronidase, β-glucosidase, β-galactase, nitroreductase, azoreductase, 7α-hydroxylase, and protease, and various carbohydrates, which can metabolize alkaloids into many different metabolites that are closely linked to DM[130]. Capsaicin improves glucose homeostasis and insulin tolerance in obese diabetic ob/ob mice by increasing the ratio of Firmicutes to Bacteroidetes and the number of Roseburia, which could decrease the contents of proinflammatory cytokines, for instance, TNF-α and IL-6[131]. Among the alkaloids, the most widely studied is berberine. As an isoquinoline alkaloid, berberine occurs in various medicinal plants, including Coptis chinensis Franch and Phellodendron chinense Schneid. Numerous experimental models have proved that the antiobesity and anti-hyperlipidemic effects of berberine are closely related to changes in the gut microbiome[128]. Researchers have shown that the blood level of BBR in hyperlipidemic patients was higher than that in healthy individuals owing to the differential microbiota composition[132]. Further studies have shown that the effects of berberine on DM are multidimensional: (1) Modulation of the ratio of Firmicutes to Bacteroidetes, thereby increasing SCFA content in feces[133]; (2) Regulation of BCAA biosynthesis and catabolism in liver and adipose tissue[134]; (3) Reduction of the increased expression of inflammatory mediators by decreasing LPS level in plasma and alleviation of gut permeability[135]; and (4) Modulation of the BA cycle and subsequently the ileal FXR signaling pathway[136].
In summary, numerous natural products, for instance, fruits, vegetables, and medicinal plants, possess prebiotic effects and have been illustrated to ameliorate T2DM by modulating gut microflora composition and abundance, reducing gut permeability, and subsequently increasing production of SCFAs and BCAAs, decreasing the level of LPS, and inhibiting inflammation. Current studies mainly focus on modulating the action of natural products and their bioactive components on the gut microbiota for preventing and managing T2DM. However, because the composition of natural products is so complex that the gut microflora may also influence host metabolism of natural products, further studies should focus on the metabolism of natural products and their bioactive components by the gut microbiota. This is important for the pharmacokinetic parameters, enhancing drug efficacy, and finding a novel lead compound via gut microflora-related mechanisms.
CONCLUSION
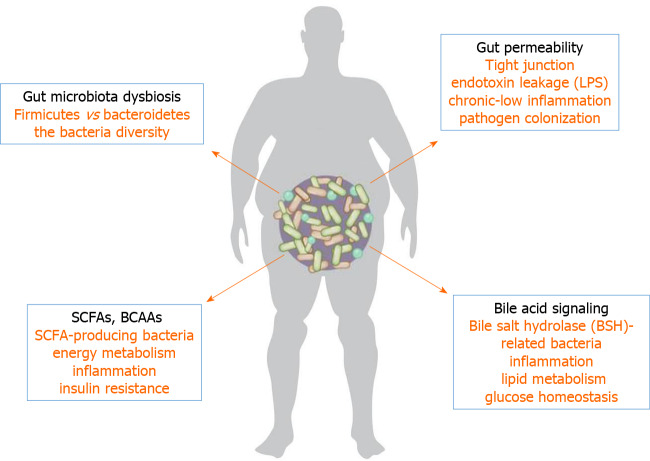
Trillions of microorganisms colonize the human gut, which are collectively termed the microbiome and provide us with genetic and metabolic attributes pertinent to the maintenance of our body homeostasis. Animal and epidemiological studies have demonstrated significant differences in the intestinal microbiota composition and abundance between diabetic and nondiabetic individuals. Moreover, by analyzing the metabolic product of the gut microbiota and their effects on host metabolism, SCFAs, BCAAs, endotoxin leakage, BA signaling, and gut permeability might be remarkably linked to initiation and aggravation of T2DM (Figure 4). The effects of some natural products on T2DM are also related to the regulation of the gut microflora and subsequent changes in metabolites. However, the available data in this field remain limited, for instance, most are small-sample clinical studies or rodent model studies. We conclude that the gut microbiota influences the onset and progression of diabetes through a variety of independent mechanisms. Moreover, by using isolating and culturing techniques, and the combination of multiomics, some new molecular markers of metabolites and mechanisms will be identified, which are related to the interaction of metabolites of the gut microflora and the host. This may provide a new insight into the role of the gut microflora and help us to make more accurate predictions for the future treatment of T2DM.
Figure 4.
Possible mechanisms of gut microbiota in type 2 diabetes mellitus. A variety of independent mechanisms that influence the development of diabetes mellitus via the gut microbiota are summarized. Short-chain fatty acids, branched-chain amino acids, endotoxin leakage, bile acid signaling, and gut permeability might be considered to participate in the process of type 2 diabetes mellitus. SCFAs: Short-chain fatty acids; BCAAs: Branched-chain amino acids; LPS: Lipopolysaccharide.
Footnotes
Conflict-of-interest statement: The authors have nothing to disclose.
Manuscript source: Unsolicited manuscript
Peer-review started: January 29, 2021
First decision: May 3, 2021
Article in press: July 5, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Arumugam VA, Heneberg P S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
Contributor Information
Fan Xia, Department of Pharmacy, The Seventh Affiliated Hospital of Sun Yat-Sen University, Shenzhen 518107, Guangdong Province, China.
Lu-Ping Wen, Department of Pharmacy, The Seventh Affiliated Hospital of Sun Yat-Sen University, Shenzhen 518107, Guangdong Province, China.
Bing-Chen Ge, Department of Pharmacy, The Seventh Affiliated Hospital of Sun Yat-Sen University, Shenzhen 518107, Guangdong Province, China.
Yu-Xin Li, Department of Pharmacology, Guangdong Medical University, Zhanjiang 524023, Guangdong Province, China.
Fang-Ping Li, Department of Endocrinology, The Seventh Affiliated Hospital, Sun Yat-Sen University, Shenzhen 518107, Guangdong Province, China.
Ben-Jie Zhou, Department of Pharmacy, The Seventh Affiliated Hospital of Sun Yat-Sen University, Shenzhen 518107, Guangdong Province, China. zhoubj163@163.com.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L, Li Y, Dai Y, Peng J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol Res. 2018;130:451–465. doi: 10.1016/j.phrs.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am. 2015;99:1–16. doi: 10.1016/j.mcna.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835, ix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 7.Lai N, Kummitha CM, Loy F, Isola R, Hoppel CL. Bioenergetic functions in subpopulations of heart mitochondria are preserved in a non-obese type 2 diabetes rat model (Goto-Kakizaki) Sci Rep. 2020;10:5444. doi: 10.1038/s41598-020-62370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heiss CN, Olofsson LE. Gut Microbiota-Dependent Modulation of Energy Metabolism. J Innate Immun. 2018;10:163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alokail MS, Sabico S, Al-Saleh Y, Al-Daghri NM, Alkharfy KM, Vanhoutte PM, McTernan PG. Effects of probiotics in patients with diabetes mellitus type 2: study protocol for a randomized, double-blind, placebo-controlled trial. Trials. 2013;14:195. doi: 10.1186/1745-6215-14-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Upadhyaya S, Banerjee G. Type 2 diabetes and gut microbiome: at the intersection of known and unknown. Gut Microbes. 2015;6:85–92. doi: 10.1080/19490976.2015.1024918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 12.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitocco D, Di Leo M, Tartaglione L, De Leva F, Petruzziello C, Saviano A, Pontecorvi A, Ojetti V. The role of gut microbiota in mediating obesity and diabetes mellitus. Eur Rev Med Pharmacol Sci. 2020;24:1548–1562. doi: 10.26355/eurrev_202002_20213. [DOI] [PubMed] [Google Scholar]
- 14.Saiyasit N, Chunchai T, Prus D, Suparan K, Pittayapong P, Apaijai N, Pratchayasakul W, Sripetchwandee J, Chattipakorn M D Ph D N, Chattipakorn SC. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition. 2020;69:110576. doi: 10.1016/j.nut.2019.110576. [DOI] [PubMed] [Google Scholar]
- 15.Singer-Englar T, Barlow G, Mathur R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev Gastroenterol Hepatol. 2019;13:3–15. doi: 10.1080/17474124.2019.1543023. [DOI] [PubMed] [Google Scholar]
- 16.Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, Stanton C. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016;92:286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 17.Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: Where are we now? Diabetes Res Clin Pract. 2018;146:111–118. doi: 10.1016/j.diabres.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 20.Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 21.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 22.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15:261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 24.Rastelli M, Knauf C, Cani PD. Gut Microbes and Health: A Focus on the Mechanisms Linking Microbes, Obesity, and Related Disorders. Obesity (Silver Spring) 2018;26:792–800. doi: 10.1002/oby.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ, Bakker BM. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 26.Blandino G, Inturri R, Lazzara F, Di Rosa M, Malaguarnera L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016;42:303–315. doi: 10.1016/j.diabet.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aroda VR. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20 Suppl 1:22–33. doi: 10.1111/dom.13162. [DOI] [PubMed] [Google Scholar]
- 31.Verdich C, Flint A, Gutzwiller JP, Näslund E, Beglinger C, Hellström PM, Long SJ, Morgan LM, Holst JJ, Astrup A. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 32.Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc. 2015;74:328–336. doi: 10.1017/S0029665114001657. [DOI] [PubMed] [Google Scholar]
- 33.McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39:338–342. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- 34.Shang J, Liu F, Zhang B, Dong K, Lu M, Jiang R, Xu Y, Diao L, Zhao J, Tang H. Liraglutide-induced structural modulation of the gut microbiota in patients with type 2 diabetes mellitus. PeerJ. 2021;9:e11128. doi: 10.7717/peerj.11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, Blottiere HM. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Small CJ, Bloom SR. The therapeutic potential of gut hormone peptide YY3-36 in the treatment of obesity. Expert Opin Investig Drugs. 2005;14:647–653. doi: 10.1517/13543784.14.5.647. [DOI] [PubMed] [Google Scholar]
- 38.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 39.Dentin R, Pégorier JP, Benhamed F, Foufelle F, Ferré P, Fauveau V, Magnuson MA, Girard J, Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 40.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 41.Kimura I. [Host energy regulation via SCFAs receptors, as dietary nutrition sensors, by gut microbiota] Yakugaku Zasshi. 2014;134:1037–1042. doi: 10.1248/yakushi.14-00169. [DOI] [PubMed] [Google Scholar]
- 42.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sukkar AH, Lett AM, Frost G, Chambers ES. Regulation of energy expenditure and substrate oxidation by short-chain fatty acids. J Endocrinol. 2019;242:R1–R8. doi: 10.1530/JOE-19-0098. [DOI] [PubMed] [Google Scholar]
- 44.Hattori M, Kondo T, Kishi M, Yamagami K. A single oral administration of acetic acid increased energy expenditure in C57BL/6J mice. Biosci Biotechnol Biochem. 2010;74:2158–2159. doi: 10.1271/bbb.100486. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita H, Maruta H, Jozuka M, Kimura R, Iwabuchi H, Yamato M, Saito T, Fujisawa K, Takahashi Y, Kimoto M, Hiemori M, Tsuji H. Effects of acetate on lipid metabolism in muscles and adipose tissues of type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2009;73:570–576. doi: 10.1271/bbb.80634. [DOI] [PubMed] [Google Scholar]
- 46.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Damink SWMO, Holst JJ, Masclee AAM, Dejong CHC, Blaak EE. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond) 2016;130:2073–2082. doi: 10.1042/CS20160263. [DOI] [PubMed] [Google Scholar]
- 48.Chambers ES, Byrne CS, Aspey K, Chen Y, Khan S, Morrison DJ, Frost G. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes Metab. 2018;20:1034–1039. doi: 10.1111/dom.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jocken JWE, González Hernández MA, Hoebers NTH, van der Beek CM, Essers YPG, Blaak EE, Canfora EE. Short-Chain Fatty Acids Differentially Affect Intracellular Lipolysis in a Human White Adipocyte Model. Front Endocrinol (Lausanne) 2017;8:372. doi: 10.3389/fendo.2017.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mandaliya DK, Seshadri S. Short Chain Fatty Acids, pancreatic dysfunction and type 2 diabetes. Pancreatology. 2019;19:280–284. doi: 10.1016/j.pan.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes. 2018;10:350–352. doi: 10.1111/1753-0407.12645. [DOI] [PubMed] [Google Scholar]
- 52.Arany Z, Neinast M. Branched Chain Amino Acids in Metabolic Disease. Curr Diab Rep. 2018;18:76. doi: 10.1007/s11892-018-1048-7. [DOI] [PubMed] [Google Scholar]
- 53.Saad MJ, Santos A, Prada PO. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda) 2016;31:283–293. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 54.Perng W, Gillman MW, Fleisch AF, Michalek RD, Watkins SM, Isganaitis E, Patti ME, Oken E. Metabolomic profiles and childhood obesity. Obesity (Silver Spring) 2014;22:2570–2578. doi: 10.1002/oby.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krebs M, Brehm A, Krssak M, Anderwald C, Bernroider E, Nowotny P, Roth E, Chandramouli V, Landau BR, Waldhäusl W, Roden M. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia. 2003;46:917–925. doi: 10.1007/s00125-003-1129-1. [DOI] [PubMed] [Google Scholar]
- 56.Harris LLS, Smith GI, Patterson BW, Ramaswamy RS, Okunade AL, Kelly SC, Porter LC, Klein S, Yoshino J, Mittendorfer B. Alterations in 3-Hydroxyisobutyrate and FGF21 Metabolism Are Associated With Protein Ingestion-Induced Insulin Resistance. Diabetes. 2017;66:1871–1878. doi: 10.2337/db16-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M, Viikari JS, Raitakari OT, Ala-Korpela M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648–655. doi: 10.2337/dc12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL, Arany Z. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–426. doi: 10.1038/nm.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye Z, Wang S, Zhang C, Zhao Y. Coordinated Modulation of Energy Metabolism and Inflammation by Branched-Chain Amino Acids and Fatty Acids. Front Endocrinol (Lausanne) 2020;11:617. doi: 10.3389/fendo.2020.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solon-Biet SM, Cogger VC, Pulpitel T, Wahl D, Clark X, Bagley E, Gregoriou GC, Senior AM, Wang QP, Brandon AE, Perks R, O'Sullivan J, Koay YC, Bell-Anderson K, Kebede M, Yau B, Atkinson C, Svineng G, Dodgson T, Wali JA, Piper MDW, Juricic P, Partridge L, Rose AJ, Raubenheimer D, Cooney GJ, Le Couteur DG, Simpson SJ. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab. 2019;1:532–545. doi: 10.1038/s42255-019-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhenyukh O, González-Amor M, Rodrigues-Diez RR, Esteban V, Ruiz-Ortega M, Salaices M, Mas S, Briones AM, Egido J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J Cell Mol Med. 2018;22:4948–4962. doi: 10.1111/jcmm.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porras D, Nistal E, Martínez-Flórez S, Pisonero-Vaquero S, Olcoz JL, Jover R, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 64.Zhao H, Zhang F, Sun D, Wang X, Zhang X, Zhang J, Yan F, Huang C, Xie H, Lin C, Liu Y, Fan M, Yan W, Chen Y, Lian K, Li Y, Zhang L, Wang S, Tao L. Branched-Chain Amino Acids Exacerbate Obesity-Related Hepatic Glucose and Lipid Metabolic Disorders via Attenuating Akt2 Signaling. Diabetes. 2020;69:1164–1177. doi: 10.2337/db19-0920. [DOI] [PubMed] [Google Scholar]
- 65.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K MetaHIT Consortium, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 66.Ufnal M, Zadlo A, Ostaszewski R. TMAO: A small molecule of great expectations. Nutrition. 2015;31:1317–1323. doi: 10.1016/j.nut.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Lundstrom RC, Racicot LD. Gas chromatographic determination of dimethylamine and trimethylamine in seafoods. J Assoc Off Anal Chem. 1983;66:1158–1163. [PubMed] [Google Scholar]
- 68.Oellgaard J, Winther SA, Hansen TS, Rossing P, von Scholten BJ. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr Pharm Des. 2017;23:3699–3712. doi: 10.2174/1381612823666170622095324. [DOI] [PubMed] [Google Scholar]
- 69.Zhu Y, Jameson E, Crosatti M, Schäfer H, Rajakumar K, Bugg TD, Chen Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci USA. 2014;111:4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ormerod KL, Wood DL, Lachner N, Gellatly SL, Daly JN, Parsons JD, Dal'Molin CG, Palfreyman RW, Nielsen LK, Cooper MA, Morrison M, Hansbro PM, Hugenholtz P. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4:36. doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, Chen Y, Liu J, Yang G, Zhao J, Liao G, Shi M, Yuan Y, He S, Lu Y, Cheng J. Serum metabolic variables associated with impaired glucose tolerance induced by high-fat-high-cholesterol diet in Macaca mulatta. Exp Biol Med (Maywood) 2012;237:1310–1321. doi: 10.1258/ebm.2012.012157. [DOI] [PubMed] [Google Scholar]
- 73.Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntyre CJ, Elmslie JL, Atkinson W, Molyneux SL, Troughton RW, Frampton CM, Richards AM, Chambers ST. Betaine and Trimethylamine-N-Oxide as Predictors of Cardiovascular Outcomes Show Different Patterns in Diabetes Mellitus: An Observational Study. PLoS One. 2014;9:e114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis. 2009;17:827–844. [PMC free article] [PubMed] [Google Scholar]
- 75.Seet RC, Loke WM, Khoo CM, Chew SE, Chong WL, Quek AM, Lim EC, Halliwell B. Acute effects of cigarette smoking on insulin resistance and arterial stiffness in young adults. Atherosclerosis. 2012;224:195–200. doi: 10.1016/j.atherosclerosis.2012.06.060. [DOI] [PubMed] [Google Scholar]
- 76.Huo T, Cai S, Lu X, Sha Y, Yu M, Li F. Metabonomic study of biochemical changes in the serum of type 2 diabetes mellitus patients after the treatment of metformin hydrochloride. J Pharm Biomed Anal. 2009;49:976–982. doi: 10.1016/j.jpba.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 77.McEntyre CJ, Lever M, Chambers ST, George PM, Slow S, Elmslie JL, Florkowski CM, Lunt H, Krebs JD. Variation of betaine, N,N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann Clin Biochem. 2015;52:352–360. doi: 10.1177/0004563214545346. [DOI] [PubMed] [Google Scholar]
- 78.Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62:944–952. doi: 10.2337/db12-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, Stärkel P, Belzer C, Hellerbrand C, Tsukamoto H, Ho SB, Schnabl B. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, Delzenne NM, Schrenzel J, François P, Cani PD. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8:2116–2130. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–G1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 82.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 83.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watson AJ, Duckworth CA. Gut microbiota control gut permeability through GLP-2. Gastroenterology. 2010;138:779–781. doi: 10.1053/j.gastro.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 85.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 87.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 88.Liang H, Hussey SE, Sanchez-Avila A, Tantiwong P, Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS One. 2013;8:e63983. doi: 10.1371/journal.pone.0063983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh A, Boden G, Rao AK. Tissue factor and Toll-like receptor (TLR)4 in hyperglycaemia-hyperinsulinaemia. Effects in healthy subjects, and type 1 and type 2 diabetes mellitus. Thromb Haemost. 2015;113:750–758. doi: 10.1160/TH14-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 91.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Degirmenci I, Ozbayer C, Kebapci MN, Kurt H, Colak E, Gunes HV. Common variants of genes encoding TLR4 and TLR4 pathway members TIRAP and IRAK1 are effective on MCP1, IL6, IL1β, and TNFα levels in type 2 diabetes and insulin resistance. Inflamm Res. 2019;68:801–814. doi: 10.1007/s00011-019-01263-7. [DOI] [PubMed] [Google Scholar]
- 93.Letran SE, Lee SJ, Atif SM, Uematsu S, Akira S, McSorley SJ. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur J Immunol. 2011;41:29–38. doi: 10.1002/eji.201040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chassaing B, Gewirtz AT. Mice harboring pathobiont-free microbiota do not develop intestinal inflammation that normally results from an innate immune deficiency. PLoS One. 2018;13:e0195310. doi: 10.1371/journal.pone.0195310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP, Das I, Wang R, Chen AC, Loudovaris T, Kay TW, Thomas HE, Whitehead JP, Forbes JM, Prins JB, McGuckin MA. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med. 2014;20:1417–1426. doi: 10.1038/nm.3705. [DOI] [PubMed] [Google Scholar]
- 97.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rütti S, Schuit FC, Lutz TA, Böni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53:1795–1806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 98.Davis JE, Braucher DR, Walker-Daniels J, Spurlock ME. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem. 2011;22:136–141. doi: 10.1016/j.jnutbio.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Kuo LH, Tsai PJ, Jiang MJ, Chuang YL, Yu L, Lai KT, Tsai YS. Toll-like receptor 2 deficiency improves insulin sensitivity and hepatic insulin signalling in the mouse. Diabetologia. 2011;54:168–179. doi: 10.1007/s00125-010-1931-5. [DOI] [PubMed] [Google Scholar]
- 100.Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med. 2015;21:702–714. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 101.Ferrell JM, Chiang JYL. Understanding Bile Acid Signaling in Diabetes: From Pathophysiology to Therapeutic Targets. Diabetes Metab J. 2019;43:257–272. doi: 10.4093/dmj.2019.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann Hepatol. 2017;16:s15–s20. doi: 10.5604/01.3001.0010.5494. [DOI] [PubMed] [Google Scholar]
- 103.Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:1679–1694.e3. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 104.Muñoz-Garach A, Diaz-Perdigones C, Tinahones FJ. Gut microbiota and type 2 diabetes mellitus. Endocrinol Nutr. 2016:560. doi: 10.1016/j.endonu.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 105.Ikegami T, Honda A. Reciprocal interactions between bile acids and gut microbiota in human liver diseases. Hepatol Res. 2018;48:15–27. doi: 10.1111/hepr.13001. [DOI] [PubMed] [Google Scholar]
- 106.Yu JH, Zheng JB, Qi J, Yang K, Wu YH, Wang K, Wang CB, Sun XJ. Bile acids promote gastric intestinal metaplasia by upregulating CDX2 and MUC2 expression via the FXR/NF-κB signalling pathway. Int J Oncol. 2019;54:879–892. doi: 10.3892/ijo.2019.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 108.Gonzalez FJ, Jiang C, Xie C, Patterson AD. Intestinal Farnesoid X Receptor Signaling Modulates Metabolic Disease. Dig Dis. 2017;35:178–184. doi: 10.1159/000450908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hirokane H, Nakahara M, Tachibana S, Shimizu M, Sato R. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J Biol Chem. 2004;279:45685–45692. doi: 10.1074/jbc.M404255200. [DOI] [PubMed] [Google Scholar]
- 111.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, Lu L, Wang D. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 112.Li BY, Xu XY, Gan RY, Sun QC, Meng JM, Shang A, Mao QQ, Li HB. Targeting Gut Microbiota for the Prevention and Management of Diabetes Mellitus by Dietary Natural Products. Foods. 2019:8. doi: 10.3390/foods8100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Food and Drug Administration, HHS. Food Labeling: Revision of the Nutrition and Supplement Facts Labels. Final rule. Fed Regist. 2016;81:33741–33999. [PubMed] [Google Scholar]
- 114.Salamone D, Rivellese AA, Vetrani C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta Diabetol. 2021 doi: 10.1007/s00592-021-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 116.Nie Q, Chen H, Hu J, Fan S, Nie S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit Rev Food Sci Nutr. 2019;59:848–863. doi: 10.1080/10408398.2018.1536646. [DOI] [PubMed] [Google Scholar]
- 117.Zhao C, Yang C, Chen M, Lv X, Liu B, Yi L, Cornara L, Wei MC, Yang YC, Tundis R, Xiao J. Regulatory Efficacy of Brown Seaweed Lessonia nigrescens Extract on the Gene Expression Profile and Intestinal Microflora in Type 2 Diabetic Mice. Mol Nutr Food Res. 2018:62. doi: 10.1002/mnfr.201700730. [DOI] [PubMed] [Google Scholar]
- 118.Liu G, Liang L, Yu G, Li Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int J Biol Macromol. 2018;115:711–717. doi: 10.1016/j.ijbiomac.2018.04.127. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Q, Yu H, Xiao X, Hu L, Xin F, Yu X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ. 2018;6:e4446. doi: 10.7717/peerj.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roshanravan N, Mahdavi R, Jafarabadi MA, Alizadeh E, Ghavami A, Saadat YR, Alamdari NM, Dastouri MR, Alipour S, Ostadrahimi A. The effects of sodium butyrate and high-performance inulin supplementation on the promotion of gut bacterium Akkermansia muciniphila growth and alterations in miR-375 and KLF5 expression in type 2 diabetic patients: A randomized, double-blind, placebo-cont. Eur J Integr Med. 2018:1–7. [Google Scholar]
- 121.Kinger M, Kumar S, Kumar V. Some Important Dietary Polyphenolic Compounds: An Anti-inflammatory and Immunoregulatory Perspective. Mini Rev Med Chem. 2018;18:1270–1282. doi: 10.2174/1389557517666170208143410. [DOI] [PubMed] [Google Scholar]
- 122.Landete JM. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr. 2012;52:936–948. doi: 10.1080/10408398.2010.513779. [DOI] [PubMed] [Google Scholar]
- 123.Bowey E, Adlercreutz H, Rowland I. Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol. 2003;41:631–636. doi: 10.1016/s0278-6915(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 124.Li W, Yang H, Zhao Q, Wang X, Zhang J, Zhao X. Polyphenol-Rich Loquat Fruit Extract Prevents Fructose-Induced Nonalcoholic Fatty Liver Disease by Modulating Glycometabolism, Lipometabolism, Oxidative Stress, Inflammation, Intestinal Barrier, and Gut Microbiota in Mice. J Agric Food Chem. 2019;67:7726–7737. doi: 10.1021/acs.jafc.9b02523. [DOI] [PubMed] [Google Scholar]
- 125.Van Hul M, Geurts L, Plovier H, Druart C, Everard A, Ståhlman M, Rhimi M, Chira K, Teissedre PL, Delzenne NM, Maguin E, Guilbot A, Brochot A, Gérard P, Bäckhed F, Cani PD. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Endocrinol Metab. 2018;314:E334–E352. doi: 10.1152/ajpendo.00107.2017. [DOI] [PubMed] [Google Scholar]
- 126.Wang P, Gao J, Ke W, Wang J, Li D, Liu R, Jia Y, Wang X, Chen X, Chen F, Hu X. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic Biol Med. 2020;156:83–98. doi: 10.1016/j.freeradbiomed.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 127.Habtemariam S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol Res. 2020;155:104722. doi: 10.1016/j.phrs.2020.104722. [DOI] [PubMed] [Google Scholar]
- 128.Christodoulou MI, Tchoumtchoua J, Skaltsounis AL, Scorilas A, Halabalaki M. Natural Alkaloids Intervening the Insulin Pathway: New Hopes for Anti-Diabetic Agents? Curr Med Chem. 2019;26:5982–6015. doi: 10.2174/0929867325666180430152618. [DOI] [PubMed] [Google Scholar]
- 129.Tesar GE, Kottke BA. Location and sequence of atherosclerotic plaque formation in white Carneau and show racer pigeons: reevaluation and redefinition. Arch Pathol Lab Med. 1978;102:581–586. [PubMed] [Google Scholar]
- 130.He CY, Fu J, Shou JW, Zhao ZX, Ren L, Wang Y, Jiang JD. In Vitro Study of the Metabolic Characteristics of Eight Isoquinoline Alkaloids from Natural Plants in Rat Gut Microbiota. Molecules. 2017:22. doi: 10.3390/molecules22060932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song JX, Ren H, Gao YF, Lee CY, Li SF, Zhang F, Li L, Chen H. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Front Physiol. 2017;8:602. doi: 10.3389/fphys.2017.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y, Tong Q, Shou JW, Zhao ZX, Li XY, Zhang XF, Ma SR, He CY, Lin Y, Wen BY, Guo F, Fu J, Jiang JD. Gut Microbiota-Mediated Personalized Treatment of Hyperlipidemia Using Berberine. Theranostics. 2017;7:2443–2451. doi: 10.7150/thno.18290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang W, Xu JH, Yu T, Chen QK. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed Pharmacother. 2019;118:109131. doi: 10.1016/j.biopha.2019.109131. [DOI] [PubMed] [Google Scholar]
- 134.Yue SJ, Liu J, Wang AT, Meng XT, Yang ZR, Peng C, Guan HS, Wang CY, Yan D. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am J Physiol Endocrinol Metab. 2019;316:E73–E85. doi: 10.1152/ajpendo.00256.2018. [DOI] [PubMed] [Google Scholar]
- 135.Liu D, Zhang Y, Liu Y, Hou L, Li S, Tian H, Zhao T. Berberine Modulates Gut Microbiota and Reduces Insulin Resistance via the TLR4 Signaling Pathway. Exp Clin Endocrinol Diabetes. 2018;126:513–520. doi: 10.1055/s-0043-125066. [DOI] [PubMed] [Google Scholar]
- 136.Sun R, Yang N, Kong B, Cao B, Feng D, Yu X, Ge C, Huang J, Shen J, Wang P, Feng S, Fei F, Guo J, He J, Aa N, Chen Q, Pan Y, Schumacher JD, Yang CS, Guo GL, Aa J, Wang G. Orally Administered Berberine Modulates Hepatic Lipid Metabolism by Altering Microbial Bile Acid Metabolism and the Intestinal FXR Signaling Pathway. Mol Pharmacol. 2017;91:110–122. doi: 10.1124/mol.116.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]