Abstract
Prediabetes and diabetes are important disease processes which have several perioperative implications. About one third of the United States population is considered to have prediabetes. The prevalence in surgical patients is even higher. This is due to the associated micro and macrovascular complications of diabetes that result in the need for subsequent surgical procedures. A careful preoperative evaluation of diabetic patients and patients at risk for prediabetes is essential to reduce perioperative mortality and morbidity. This preoperative evaluation involves an optimization of preoperative comorbidities. It also includes optimization of antidiabetic medication regimens, as the avoidance of unintentional hypoglycemic and hyperglycemic episodes during the perioperative period is crucial. The focus of the perioperative management is to ensure euglycemia and thus improve postoperative outcomes. Therefore, prolonged preoperative fasting should be avoided and close monitoring of blood glucose should be initiated and continued throughout surgery. This can be accomplished with either analysis in blood gas samples, venous phlebotomy or point-of-care testing. Although capillary and arterial whole blood glucose do not meet standard guidelines for glucose testing, they can still be used to guide insulin dosing in the operating room. Intraoperative glycemic control goals may vary slightly in different protocols but overall the guidelines suggest a glucose range in the operating room should be between 140 mg/dL to 180 mg/dL. When hyperglycemia is detected in the operating room, blood glucose management may be initiated with subcutaneous rapid-acting insulin, with intravenous infusion or boluses of regular insulin. Fluid and electrolyte management are other perioperative challenges. Notably diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic state are the two most serious acute metabolic complications of diabetes that must be recognized early and treated.
Keywords: Diabetes mellitus, Perioperative management, Insulin, Hyperglycaemia, Anaesthesia, Surgery
Core Tip: Diabetes is a common endocrine condition with multiple organ system involvement. Careful preoperative assessment and perioperative management is crucial in preventing perioperative morbidity and mortality in patients with diabetes presenting for surgery. The focus of preoperative risk assessment should be to assess and optimize the level of glycemic control, as well as identification of possible systemic comorbidities. Intraoperatively the focus is to ensure euglycemia, prevent metabolic and electrolyte derangements and maintain adequate organ perfusion. Postoperatively patients should be transitioned back to their home regimen once adequate oral intake is established.
INTRODUCTION
The prevalence of diabetes in the United States is about 10%. Additionally about one third of the United States population is considered to have prediabetes. The prevalence amongst surgical patients is most likely even higher, as prediabetic and diabetic patients are more likely to require surgical procedures due to the associated micro and macrovascular complications of diabetes. Surgical procedures are associated with an up-regulation of catecholamines, cortisol, and inflammatory cytokines, which subsequently decrease insulin sensitivity and increase secretion of glucagon and growth hormone[1]. These hormonal changes are enhanced in patients with insulin resistance. This leads to a catabolic state with increased gluconeogenesis, glycogenolysis, lipolysis, proteolysis and ketogenesis. This ultimately can result in hyperglycemia and potentially even to ketoacidosis[1]. Consequently, diabetic patients are at higher risk for postoperative morbidity and mortality after non-cardiac surgery, both short-term (21 d after surgery) and long-term (7 years), due to associated cardiac, renal, neurologic, metabolic, and endocrine complications[2,3]. Careful preoperative evaluation, optimization of preoperative comorbidities, and meticulous management of patients with diabetes during the perioperative period is essential to reduce perioperative mortality and morbidity. In this article we will discuss important aspects of preoperative evaluation and optimization, as well as the perioperative prevention and management of complications in diabetic patients presenting for non-cardiac surgery[4-9] (Figure 1).
Figure 1.
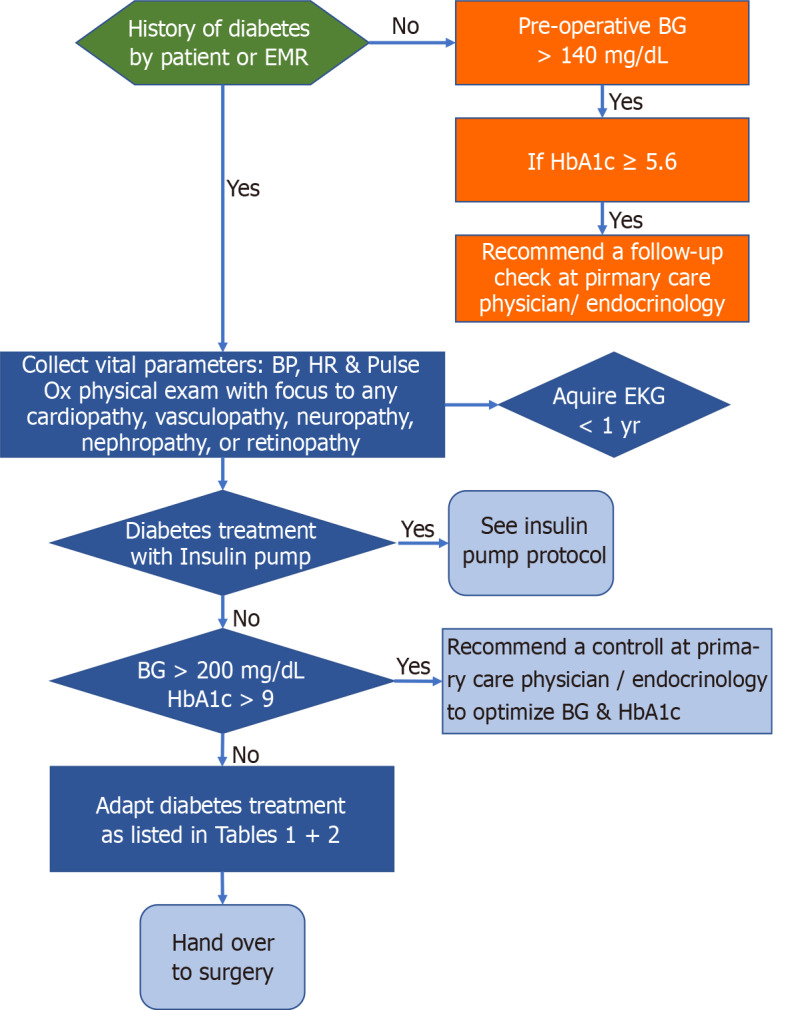
Preoperative management algorithm for diabetic patients undergoing non cardiac surgery. HbA1c: Hemoglobin A1C; BG: Blood glucose; EMR: Electronic medical record; EKG: Electrocardiogram; HR & Pulse Ox: Heart rate & Pulse Ox.
PRE-OPERATIVE ASSESSMENT AND MANAGEMENT
Diabetes is a common chronic endocrine condition which has an ever increasing prevalence. About half of diabetic patients are undiagnosed[10]. Diabetes is often first diagnosed during the preoperative workup in patients presenting for surgery[11,12]. A recent systematic review summarized, that there is currently insufficient evidence to support routine screening of blood glucose and/or hemoglobin A1C (HbA1c) in otherwise healthy patients undergoing elective surgery[13]. The American Diabetes Association recommends screening for diabetes in all overweight patients who have an additional risk factor. Risk factors include a history of cardiovascular disease, hypertension, hyperlipidemia, polycystic ovarian syndrome, or gestational diabetes, a first-degree relative with diabetes, or a higher risk race or ethnicity[14,15]. These guidelines are not specific for patients having surgery, however it makes intuitive sense to screen these patients, who are at risk for diabetes, before surgery.
Management of diabetic patients should involve a multidisciplinary approach. The main goals include a thorough pre-operative risk assessment and optimization, meticulous blood glucose control in the perioperative period and prevention and management of perioperative diabetic emergencies.
Preoperative assessment includes a thorough history and physical examination. History should include the type and duration of diabetes, degree of blood glucose control, current medications, presence of end organ damage and hospitalizations due to diabetic complications[12,16]. Glycated HbA1c and the blood glucose should be measured. The average home blood glucose levels should be assessed from history or review of charts[17]. Review of these lab measurements will indicate the degree of blood glucose control which will indicate the potential of hyper or hypo glycemic episodes in the perioperative period. Although HbA1c level exceeding 8% is associated with higher risk of postoperative complications, there is insufficient evidence to determine the upper threshold HbA1c level in which surgical cancellation or delay should be considered[3,18-21]. That being said, the etiology of the increased incidence of adverse perioperative events in diabetic patients may be related to presence of macro and microvascular complications due to long standing diabetes[3,21-26]. Consequently, the patient’s diabetic control should be optimized before surgery with a target HbA1c less than 8% and a regimen targeted towards consistent normoglycemia. If the HbA1c is more than 8% consideration should be given to postponement of elective surgery regardless of lack of established guidelines. Perioperative glucose levels (perioperative defined as day of surgery until third postoperative day) correlate more with surgical complications than the preoperative HbA1c-level[27]. Unsurprisingly, a high HbA1c is often associated with high perioperative blood glucose levels. Increased preoperative blood glucose levels are associated with increased risk of surgical complications after spinal, vascular, colorectal, trauma, breast, orthopedic and hepatobiliary[28,29] surgery[17,24]. Preoperative elevation of blood glucose exceeding 300 mL/dL, hyperosmolar hyperglycemic state, and diabetic ketoacidosis are further suggested indication for postponement of elective surgery.
Antidiabetic medication regimens need to be carefully reviewed and adjusted during the pre-surgical assessment to avoid unintentional hypoglycemic and hyperglycemic episodes. Adjustment and withholding of medications are crucial, as patient are fasting for several hours before surgery and therefore are at high risk of experiencing hypoglycemia. Generally, antidiabetic medications including metformin, glucagon-like peptide-1 agonists, sulfonylureas, and thiazolidinediones should be held on the day of surgery (Table 1)[30]. Sodium-glucose cotransporter (SGLT 2) inhibitors should be held for at least 3 d before surgery (Table 1).
Table 1.
Preoperative medication-management in diabetic patients (dosages may need to be individualized to the patient)
|
Drugs
|
Day before surgery
|
Day of surgery
|
| Metformin, Sulfonylurea (oral hypoglycemics) | Daily same dose | Hold on the day of surgery |
| Canaglifozin, Dapagliflozin, Empagliflozin (SGLT2 inhibitors) | Stop 3 d before surgery | Hold on the day of surgery |
| Ertuglozin (SGLT2 inhibitor) | Stop 4 d before surgery | Hold on the day of surgery |
| Injectable non-insulins | Normal dose the night before surgery | Hold on the day of surgery |
| Insulin pump | Depending on the insulin pump protocol | Depending on the insulin pump protocol |
| Levemir, Lantus, NPH (long-acting basal insulin) | Normal dose the night before surgery | Half the normal dose in the morning of surgery |
| Combination of long and short acting (mixed insulin) | Normal dose the night before surgery | Depending to the fasting blood glucose: If > 200 mg/dL: Take half of the normal dose; If < 200 mg/dL: No insulin |
SGLT2: Sodium-glucose cotransporter 2. NPH: Neutral protamine hagedorn.
In general, elective surgical procedures do not need to be cancelled or postponed, if the patient accidentally took an oral antidiabetic medication on the day of surgery. Cancellation of postponement of elective surgery should be considered if the patient accidentally took any SGLT 2 inhibitor or a sulfonylurea because of the risk of hypoglycemia or diabetic ketoacidosis[15].
Insulin dependent patients require careful preoperative evaluation and education on an individual basis. Home insulin regimens and fasting glucose measurements need to be reviewed. Patients with type 1 diabetes require insulin due to its absolute deficiency, while only a fraction of patients with type 2 diabetes need insulin. type 2 diabetic patients are also usually on higher individual doses of insulin, compared to type 1 diabetic patients. Insulin medications are classified according to their time to onset, time to peak, and duration of action. Insulin formulations may be rapid-acting, short-acting, intermediate-acting, or long-acting/basal insulins. Premixed insulins are also available.
Regimens for patients with type 1 diabetes typically cover the basal requirement, prandial and correctional insulin for anticipated nutritional intake or unanticipated accidental hyperglycemia[31]. Preoperatively, long-acting insulins are less likely to result in hypoglycemia and should therefore not be withheld. Half the normal dose of intermediate insulin should be taken on the day of surgery. Rapid-acting insulin should be withheld in patients with a blood glucose below 200 md/dL and carefully titrated in patients with blood glucose exceeding 200 ng/dL. General guidelines for insulin are summarized in Table 2. It may be reasonable to reduce the doses of insulin in patients with type 2 diabetes, although there is currently limited consensus. A reduction of basal insulin by 20%-25% the night before surgery was reported to be sufficient to achieve adequate blood glucose levels on the day of surgery[32]. Long-acting insulin should be reduced by 50% on the morning of surgery. Intermediate-acting insulin should be maintained the day before surgery, but a 25% or 50% reduction in doses is recommended the night before and the day of surgery. Short-acting insulin should generally be avoided on the day of surgery and only be administered according to actual blood glucose levels. It is useful to consult an endocrinologist for patients who have a subcutaneous insulin pump. Recommendations regarding potential changes in insulin regime may be required preoperatively. It is recommended that insulin pumps should be disconnected in emergency cases and surgeries lasting more than 3 h. A continuous infusion of insulin and titration according to actual blood glucose level is recommended[31,33].
Table 2.
Insulin treatment on the day of surgery
|
Insulin types
|
Regiment on the day of surgery
|
| Lantus/Toujeo, Tresiba, Levemir (long-acting insulins) | Half dose of normal in the morning of surgery |
| NPH-insulin (intermediate insulin) | Half dose of normal in the morning of surgery |
| Inulin aspart protamine, insulin aspart; Insulin lispro protamine, insulin lispro; Insulin neutral protamine hagedorn and insulin regular (all mixed insulin) | Depending to the blood glucose: If > 200 mg/dL: Take half of the normal dose in the morning; If < 200 mg/dL: No insulin |
| All models of insulin pumps: Endocrinology consult recommended, except patient ambulatory | Basal rate until operation, continue with iv insulin during surgery |
Specific assessment of the different organ systems
Cardiovascular: Cardiovascular death is a major source of morbidity and mortality in the diabetic patient. Up to 50% of the type 2 diabetic patients have unrecognized myocardial infarction events[17,34]. As well as coronary artery disease, patients with diabetes have a prevalence of heart failure due to diabetic cardiomyopathy that range from 19%-26%. This prevalence is higher than in age-matched individuals without diabetes[35,36]. These changes occur due to cardiac fibrosis with cell signaling abnormalities that lead to a stiff heart. This results in diastolic dysfunction and heart failure with preserved ejection fraction or sometimes heart failure with reduced ejection fraction[37]. Diabetic cardiomyopathy may be present without the existence of other cardiac risk factors.
Another major cardiovascular concern is cardiac autonomic neuropathy (CAN). This serious complication of diabetes mellitus is associated with a 5-fold increased risk of cardiovascular mortality[38,39] and cardiovascular events[40,41]. Patients with CAN often suffer from exercise intolerance, postural hypotension, dizziness and syncope[38,39]. A patient with CAN may present with sinus tachycardia, abnormal blood pressure regulation, intraoperative cardiovascular and hemodynamic instability and asymptomatic myocardial ischemia and infarction. Current recommendation suggests that CAN be evaluated preoperatively in patient with Type 1 diabetes. Tests such as Valsalva maneuver [heart rate (HR) change], electrocardiogram (ECG), long-term-ECG (HR variability), QT measurement, ambulatory BP-monitoring (dipping) may be performed[17]. These tests may confirm an imbalance in autonomic regulation. If autonomic dysregulation is found to exist, it may be prudent to avoid medications that could induce orthostatic hypotension. The ventilatory response to hypoxemia, hypercapnia and the patient’s response to hypothermia may also be affected[42,43].
Diabetes is a risk factor for myocardial injury after non-cardiac surgery[34]. Elective troponin screening on the day of surgery and the first three days after surgery should therefore be considered[34,44]. Cardiology should be consulted if troponin level is elevated, with or without clinical symptoms suggestive of an acute cardiac event[34]. Diabetes also increases the risk of peripheral artery and cerebrovascular disease. However, there are no specific considerations regarding perioperative anesthesia management of these disease states in the diabetic patient.
Airway and respiratory tract: Diabetic and obese patients are anecdotally considered to be at high risk for difficult intubation[45]. A large retrospective analysis of surgical patients indicate, that increasing BMI was associated with increasing odds of difficult intubation[46]. Obese patients are harder to intubate than lean patients, but difficult intubation is no more likely in morbidly obese patients than in those who are only slightly obese[46]. Well-known signs and tests such as the Mallampati-test, Wilson’s scale, “prayer sign”, and palm-print, help predict the possibility of a difficult airway. Stiff joints occur in diabetics due to glycosylation of tissue proteins. The abnormal collagen accumulates in the joints, including the atlanto-axial and temporomandibular joints. This leads to an impaired mobility of the neck and mouth which can result in difficult intubation[47,48].
Diabetic patients are also susceptible to postoperative respiratory infections. This is further exacerbated by obesity and history of smoking if these risk factors coexist. In these situations, adequate perioperative pulmonary testing and treatment should be initiated.
Renal: Diabetes is one of the most common causes of kidney disease and subsequently end-stage renal failure. Therefore, renal function should be assessed with serum creatinine concentration and GFR (glomerular filtration rate) in all patients having moderate-to major non-cardiac surgery[17].
Gastrointestinal: Gastroparesis is a major consideration for anesthesiologists. Gastroparesis is defined by a delayed emptying of the stomach, although there is often no anatomical outlet obstruction[49]. Gastroparesis can result in regurgitation and pulmonary aspiration of gastric content during the intubation process. Therefore, identification of patients at risk for gastroparesis is essential during the preoperative risk assessment. It may be prudent to perform a rapid sequence intubation and to premedicate with famotidine, metoclopramide and sodium citrate in diabetic patients with suspected gastroparesis.
Peripheral neuropathy: More than half of diabetic patients develop some type of peripheral neuropathy during their lifetime. It occurs due to a damage of peripheral nerves. Presentation is variable depending on which nerves are affected. It can affect the motor nerves (control of the movements), the sensory nerves (touch, temperature, pain) or the autonomic nerves (digestion, heartrate, breathing). Most diabetics with peripheral neuropathy suffer from polyneuropathy. An existing peripheral neuropathy must be adequately documented, especially if regional anesthesia, extreme positioning or placement of an arterial line is considered.
INTRAOPERATIVE MANAGEMENT OF DIABETES MELLITUS
Management of a potentially difficult airway, and prevention of renal failure and electrolyte disturbances are some of the important considerations when anesthetizing a diabetic patient. Meticulous monitoring of fluid and electrolyte balance is warranted as glucose has osmolar effects which can lead to serum electrolyte imbalance. Optimal blood glucose levels ranging between 120-180 mg/dL should be maintained. Simultaneously, hypoglycemic and hyperglycemic episodes should be strictly avoided.
Intraoperative glucose monitoring
Close monitoring of blood glucose during surgery is mandatory, as symptomatic hypoglycemia (tremor, anxiety, palpitations, or sweating) is masked by general anesthesia. Iatrogenic or accidental (over)administration of antidiabetic medications including preoperative oral medications and insulins are potential causes of intraoperative hypoglycemia. Other causes of intraoperative hypoglycemia include, inappropriate blood glucose monitoring, drug errors, and interruption of enteral or parental nutrition[50].
Hyperglycemia during surgery in a diabetic or non-diabetic patient might be mediated by the following: (1) Stress associated with the procedure, which alters the balance between hepatic glucose production and use[50]; (2) Inflammation which may interfere with peripheral glucose uptake promoting a state of relative insulin resistance[50]; (3) Use of dexamethasone for prevention of postoperative nausea and vomiting[50,51]; and (4) Volatile anesthetics leading to decreased insulin secretion[51].
Hyperglycemia is associated with poor outcomes such as postoperative infections, acute renal failure, acute myocardial infarction, increase 30-d mortality and longer intensive care unit (ICU) and hospital stays[52]. It has been reported that nearly 12%-30% of patients without previous history of diabetes may have hyperglycemia during surgery[3,51].
There are several options for measuring blood glucose intraoperatively. The most common in the operating room is the capillary blood. However, it is limited by a time constant for venous blood turnover which affects accuracy compared to a venous sample[51]. Peripheral intravenous catheter, arterial lines and central venous catheters are other alternatives for sampling. Central laboratory device is the gold standard glucose measurement. The limitation with central laboratory device is a turn-around-time of over an hour and the large sample volume needed, in comparison with blood gas analysis and point-of-care testing. Both have shorter turn-around-time and need smaller sample sizes. However, they but are less accurate, especially point-of-care testing[51]. Nevertheless, blood gas analysis, venous phlebotomy and point-of-care testing are the most frequently used devices to measure glucose in the perioperative setting despite their various limitations[50].
Neither arterial nor capillary whole blood glucose meet standard guidelines for blood glucose testing in acute and chronic care facilities. This suggests that caution must be exercised when using glucose meter values to dose intravenous insulin or when using more intensive glycemic control protocols[53]. Although point-of-care and arterial blood gas testing can be used to monitor glucose in the operating room, even for patients on an intravenous insulin infusion, we should be aware of the inaccuracy of these methods and the potential for significant harm[54].
Continuous glucose monitors (CGM) are not currently approved in perioperative setting[51] and have not been thoroughly evaluated in the intraoperative period[55]. In the future CGM might be a potential tool to monitor glucose level during surgery. CGM are usually minimally invasive and measure glucose in the interstitial fluid every 1 min to 5 min[50,55]. However, there is insufficient data on their accuracy during surgery. Common events during the course of surgery such as hypotension, hypothermia, hypoxia and electrical interference may also significantly affect their reliability[55]. Consensus statements[56] for inpatient use of CGM, including ICU, do exist, however these do not address monitoring issues in the operating room.
On the horizon, there are potential tools that might be useful for intraoperative glucose monitoring. One of these technologies involves a non-invasive monitoring of blood glucose using optical methods[57]. These devices have not been tested in the intraoperative setting, and many of them are prototypes. However, they could help to surmount the inconvenient frequent blood sampling of the point-of-care testing and the invasiveness of some CGM. Potential sampling sites include the skin (finger, lip, arm, hand, and ear lobe) or easily accessible body fluids (tear fluid, sweat, and saliva). With this technology the arterial vasculature can be accessed as with pulse oximetry. However, variations in signal amplitude and phase must be overcome to reach stable signals with an appropriate signal-to-noise ratio[57].
Intraoperative glucose management
As a general principle, prolonged preoperative fasting should be avoided and diabetic patients should have a surgical time scheduled for as early as possible in the morning.
The goals or target towards avoidance of hyperglycemia and hypoglycemia may change between different societal guidelines and institutional protocols. Intraoperative glycemic control goals are quite similar between most major guidelines[30]. Overall, the guidelines suggest a glucose target in the range of 140 mg/dL to 180 mg/dL intraoperatively[50]. The Association of Anesthetists of Great Britain and Ireland suggests a goal between 110 mg/dL and 180 mg/dL (6-10 mmol/L), with an upper limit of 216 mg/dL (12 mmol/L) in patients with previous poor control[21]. In general, the guidelines encourage initiation of insulin when glucose levels are greater than 180 mg/dL, and administration of intravenous glucose of levels below 70 mg/dL[21,30]. Tighter and more aggressive glycemic control have failed to show significant impact on clinical outcomes and are associated with a high risk of hypoglycemia[58]. Baseline blood glucose level should be assessed before surgery, preferably as close as possible to induction of general anesthesia. Thereafter, blood glucose level should be assessed regularly, and should not exceed 2 h between measurements[59].
When hyperglycemia is detected in the operating room, blood glucose management may be initiated with subcutaneous rapid-acting insulin, with intravenous insulin infusions or boluses of regular insulin[1,30,50]. The type of insulin and route of administration depends on different factors. Good candidates for subcutaneous insulin are patients undergoing ambulatory surgery or procedures of short duration (less than 4 h)[30]. Usually, an intravenous insulin infusion is recommended for patients undergoing procedures with anticipated hemodynamic changes, significant fluid shifts, expected changes in temperature, use of inotropes, or lengthy operative times (greater than 4 h)[30,60]. Some guidelines suggest that intravenous insulin infusions are overused in the perioperative setting and are associated with hypoglycemia, hyperglycemia, ketosis, and hyponatremia[21]. An insulin infusion may be considered in patients who will miss more than one meal and in type 1 diabetics who have not received background insulin or have poorly controlled diabetes (HbA1c > 69 mmol/mol or 8.5%)[21].
The dose of subcutaneous rapid-acting insulin can be calculated depending on the glucose measurement and the presence of insulin resistance. Correctional insulin dosing is intended to treat glucose measures greater than 180 mg/dL and can be calculated by measuring glucose minus 100/insulin sensitivity factor[30,61]. Insulin sensitivity factor is equal to 1800 divided by the patient’s total daily dose of insulin or 40 if the patient was previously taking oral medications[30].
With regard to intravenous insulin infusion, institutions should have a protocol that allows flexible rate adjustment based on current and previous glucose values[30]. Patients with type 1 diabetes generally begin the insulin infusion rates at 0.5-1 U/h, whereas infusion rates for type 2 diabetics generally begin at approximately 2-3 U/h or higher due to presence of insulin resistance[1].
Corticosteroids, specifically dexamethasone are often administered for the prevention of postoperative nausea and vomiting. They are also used to reduce postoperative stridor and airway edema. Current evidence suggests administration of low dose dexamethasone in these situations (4-5 mg) with close monitoring of glucose in diabetics after administration (hourly for at least 4 h)[21,50].
Insulin pump management
There is limited evidence on the use of a patient’s home insulin pump during surgery. There is also a wide variation in practice regarding continuing a patient’s home insulin pump intraoperatively. Some advocate continuing the pump during the procedure, others advocate discontinuing the pump and using an intravenous insulin infusion or using injectable subcutaneous insulin alone[50]. Recommendations rely on different factors such as the type of surgery, the ability of the provider to safely manage the pump, the fasting period, and the period of time until the patient can control the pump following surgery[50]. The Society for Ambulatory Anesthesia suggest that if the pump is not in the surgical field, and the patient and surgery meet the criteria for subcutaneous insulin use, a patient’s home pump can be continued in the operating room[59] using the preprogramed basal rate[30]. If use of the home insulin pump is not feasible, intravenousinsulin infusion can be substituted for the pump. In this situation, the pump is disconnected, and an insulin infusion started at the same basal rate used by the patient[30].
Fluid and electrolyte management
The aim of perioperative fluid therapy is to avoid dehydration and to maintain electrolyte balance. The recommendation in general is to avoid solutions with glucose, unless required for avoidance of hypoglycemia due to prolonged fasting[61]. Although 0.9% normal saline was traditionally the fluid of choice for patients with diabetes, balanced salt solutions are increasingly recommended in order to prevent hyperchloremic metabolic acidosis which can be associated with normal saline administration[61]. To avoid insulin-induced hypokalemia, potassium should be evaluated at least every 4 h during surgery[62].
POST-OPERATIVE MANAGEMENT
Postoperative hyperglycemia is associated with a higher risk of infections and prolonged hospital stay[63]. Diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic state are the two most serious acute metabolic complications of diabetes. In recent years, reports of hyperosmolar, hyperglycemic, nonketotic diabetic coma have proliferated greatly. The interaction of several commonly employed medications with hyperglycemic potential in the management of the critically ill surgical patient has been a major cause of hyperosmolar coma[64].
Transition to euglycemic state
Intravenous insulin is the preferred medication to maintain normoglycemia in patients in the operating room and ICU. The short half-life of intravenous insulin (35 min) allows easy titration and discontinuation in the event of unpredicted changes in patient’s health, concurrent medications, and nutrition. An insulin infusion should be initiated in the operating room or ICU when a patient’s blood glucose is ≥ 180 mg/dL. A variety of insulin protocols have been shown to be effective in achieving glycemic control, while minimizing hypoglycemic events and improving hospital outcomes[65]. The insulin infusion is transitioned to sliding scale insulin postoperatively. Blood glucose levels should be measured regularly, preferably every 6-8 h. Serum electrolytes should be measured daily. Sliding scale insulin should be transitioned to the regular home medications or a regimen familiar to the patient once the patient resumes their regular oral intake. During transition, patients should have more frequent routine glucose measurement during hospitalization. However, individual strategies depend on patient’s conditions, oral intake, and compliance.
CONCLUSION
Diabetes is a common endocrine condition which involves multiple organ systems. Careful preoperative assessment and perioperative management is crucial to the prevention of perioperative morbidity and mortality in patients with diabetes presenting for surgery. The focus of preoperative risk assessment should be to evaluate and optimize the level of glycemic control, as well to identify possible systemic comorbidities. Intraoperatively, the goal is to ensure euglycemia, prevent metabolic and electrolyte derangements and maintain adequate organ perfusion. Postoperatively patients should be transitioned back to their home regimen once adequate oral intake is established.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of authors contributed their efforts in this manuscript.
Manuscript source: Invited manuscript
Peer-review started: February 2, 2021
First decision: March 30, 2021
Article in press: July 9, 2021
Specialty type: Anesthesiology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Spartalis M, Wu QN S-Editor: Wu YXJ L-Editor: A P-Editor: Wang LYT
Contributor Information
Ursula Galway, Department of General Anesthesiology, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States.
Praveen Chahar, Department of General Anesthesiology, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States; Department of Intensive Care and Resuscitation, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States.
Marc T Schmidt, Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States.
Jorge A Araujo-Duran, Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States.
Jeevan Shivakumar, Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States.
Alparslan Turan, Department of General Anesthesiology, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States; Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States.
Kurt Ruetzler, Department of General Anesthesiology, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States; Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH 44195, United States. ruetzlk@ccf.org.
References
- 1.Sudhakaran S, Surani SR. Guidelines for perioperative management of the diabetic patient. Surg Res Pract . 2015;2015:284063. doi: 10.1155/2015/284063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krolikowska M, Kataja M, Pöyhiä R, Drzewoski J, Hynynen M. Mortality in diabetic patients undergoing non-cardiac surgery: a 7-year follow-up study. Acta Anaesthesiol Scand . 2009;53:749–758. doi: 10.1111/j.1399-6576.2009.01963.x. [DOI] [PubMed] [Google Scholar]
- 3.Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, Umpierrez GE. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care . 2010;33:1783–1788. doi: 10.2337/dc10-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Diabetes Basics. [cited 10 January 2021]. Available from: https://www.cdc.gov/diabetes/basics/diabetes.html .
- 5.Cruz NI, Santiago E, Abdul-Hadi A. Prevalence of diabetes mellitus in the surgical population of the university of puerto rico affiliated hospitals: a study using the surgery database. P R Health Sci J . 2016;35:160–164. [PubMed] [Google Scholar]
- 6.Lauruschkat AH, Arnrich B, Albert AA, Walter JA, Amann B, Rosendahl UP, Alexander T, Ennker J. Prevalence and risks of undiagnosed diabetes mellitus in patients undergoing coronary artery bypass grafting. Circulation . 2005;112:2397–2402. doi: 10.1161/CIRCULATIONAHA.105.534545. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub WS, Wenger NK, Jones EL, Craver JM, Guyton RA. Changing clinical characteristics of coronary surgery patients. Differences between men and women. Circulation . 1993;88:II79–II86. [PubMed] [Google Scholar]
- 8.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg . 1999;67:352–60; discussion 360. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 9.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, Guyton RA. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg . 1999;67:1045–1052. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 10.Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract . 2014;103:150–160. doi: 10.1016/j.diabres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Prevalence of diabetes and impaired fasting glucose in adults-United States, 1999-2000. MMWR Morb Mortal Wkly Rep . 2003;52:833–837. [PubMed] [Google Scholar]
- 12.Ruetzler K, Lin P, You J, Schacham Y, Naylor AJ, Sessler DI, Saager L. The association between timing of routine preoperative blood testing and a composite of 30-day postoperative morbidity and mortality. Anesth Analg . 2018;127:897–903. doi: 10.1213/ANE.0000000000003300. [DOI] [PubMed] [Google Scholar]
- 13.Bock M, Johansson T, Fritsch G, Flamm M, Hansbauer B, Mann E, Sönnichsen A. The impact of preoperative testing for blood glucose concentration and haemoglobin A1c on mortality, changes in management and complications in noncardiac elective surgery: a systematic review. Eur J Anaesthesiol . 2015;32:152–159. doi: 10.1097/EJA.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care . 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 15.Himes CP, Ganesh R, Wight EC, Simha V, Liebow M. Perioperative evaluation and management of endocrine disorders. Mayo Clin Proc . 2020;95:2760–2774. doi: 10.1016/j.mayocp.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Leung V, Ragbir-Toolsie K. Perioperative management of patients with diabetes. Health Serv Insights . 2017;10:1178632917735075. doi: 10.1177/1178632917735075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheisson G, Jacqueminet S, Cosson E, Ichai C, Leguerrier AM, Nicolescu-Catargi B, Ouattara A, Tauveron I, Valensi P, Benhamou D Working party approved by the French Society of Anaesthesia and Intensive Care Medicine (SFAR) the French Society for the study of Diabetes (SFD) Perioperative management of adult diabetic patients. Preoperative period. Anaesth Crit Care Pain Med . 2018;37 Suppl 1:S9–S19. doi: 10.1016/j.accpm.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Kallio PJ, Nolan J, Olsen AC, Breakwell S, Topp R, Pagel PS. Anesthesia preoperative clinic referral for elevated hba1c reduces complication rate in diabetic patients undergoing total joint arthroplasty. Anesth Pain Med . 2015;5:e24376. doi: 10.5812/aapm.5(3)2015.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab . 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 20.Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, Flum DR SCOAP-CERTAIN Collaborative. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg . 2015;261:97–103. doi: 10.1097/SLA.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Membership of the Working Party, Barker P, Creasey PE, Dhatariya K, Levy N, Lipp A, Nathanson MH, Penfold N, Watson B, Woodcock T. Peri-operative management of the surgical patient with diabetes 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia . 2015;70:1427–1440. doi: 10.1111/anae.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamblin PS, Topliss DJ, Chosich N, Lording DW, Stockigt JR. Deaths associated with diabetic ketoacidosis and hyperosmolar coma. 1973-1988. Med J Aust . 1989;151:439, 441–442, 444. doi: 10.5694/j.1326-5377.1989.tb101253.x. [DOI] [PubMed] [Google Scholar]
- 23.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care . 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 24.Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearse RM. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia . 2008;63:695–700. doi: 10.1111/j.1365-2044.2008.05560.x. [DOI] [PubMed] [Google Scholar]
- 25.Pearse RM, Harrison DA, James P, Watson D, Hinds C, Rhodes A, Grounds RM, Bennett ED. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care . 2006;10:R81. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George JT, Warriner D, McGrane DJ, Rozario KS, Price HC, Wilmot EG, Kar P, Stratton IM, Jude EB, McKay GA TOPDOC Diabetes Study Team. Lack of confidence among trainee doctors in the management of diabetes: the Trainees Own Perception of Delivery of Care (TOPDOC) Diabetes Study. QJM . 2011;104:761–766. doi: 10.1093/qjmed/hcr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care . 2018;41:782–788. doi: 10.2337/dc17-2232. [DOI] [PubMed] [Google Scholar]
- 28.Chuang SC, Lee KT, Chang WT, Wang SN, Kuo KK, Chen JS, Sheen PC. Risk factors for wound infection after cholecystectomy. J Formos Med Assoc . 2004;103:607–612. [PubMed] [Google Scholar]
- 29.Ambiru S, Kato A, Kimura F, Shimizu H, Yoshidome H, Otsuka M, Miyazaki M. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect . 2008;68:230–233. doi: 10.1016/j.jhin.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: an update. Anesthesiology . 2017;126:547–560. doi: 10.1097/ALN.0000000000001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sreedharan R, Abdelmalak B. Diabetes mellitus: preoperative concerns and evaluation. Anesthesiol Clin . 2018;36:581–597. doi: 10.1016/j.anclin.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth . 2017;36:184–188. doi: 10.1016/j.jclinane.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Partridge H, Perkins B, Mathieu S, Nicholls A, Adeniji K. Clinical recommendations in the management of the patient with type 1 diabetes on insulin pump therapy in the perioperative period: a primer for the anaesthetist. Br J Anaesth . 2016;116:18–26. doi: 10.1093/bja/aev347. [DOI] [PubMed] [Google Scholar]
- 34.Ruetzler K, Khanna AK, Sessler DI. Myocardial injury after noncardiac surgery: preoperative, intraoperative, and postoperative aspects, implications, and directions. Anesth Analg . 2020;131:173–186. doi: 10.1213/ANE.0000000000004567. [DOI] [PubMed] [Google Scholar]
- 35.Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, Pinkett T, Ghali JK, Wilson AC. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) trials and registry. Am J Cardiol . 1996;77:1017–1020. doi: 10.1016/s0002-9149(97)89163-1. [DOI] [PubMed] [Google Scholar]
- 36.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol . 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 37.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res . 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes . 2014;5:17–39. doi: 10.4239/wjd.v5.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhiyenko VA, Serhiyenko AA. Cardiac autonomic neuropathy: risk factors, diagnosis and treatment. World J Diabetes . 2018;9:1–24. doi: 10.4239/wjd.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes . 2002;51:3524–3531. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]
- 41.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care . 2010;33:1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGrane S, Atria NP, Barwise JA. Perioperative implications of the patient with autonomic dysfunction. Curr Opin Anaesthesiol . 2014;27:365–370. doi: 10.1097/ACO.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 43.Karayannis G, Giamouzis G, Cokkinos DV, Skoularigis J, Triposkiadis F. Diabetic cardiovascular autonomic neuropathy: clinical implications. Expert Rev Cardiovasc Ther . 2012;10:747–765. doi: 10.1586/erc.12.53. [DOI] [PubMed] [Google Scholar]
- 44.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators, Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, Srinathan S, Biccard BM, Chow CK, Abraham V, Tiboni M, Pettit S, Szczeklik W, Lurati Buse G, Botto F, Guyatt G, Heels-Ansdell D, Sessler DI, Thorlund K, Garg AX, Mrkobrada M, Thomas S, Rodseth RN, Pearse RM, Thabane L, McQueen MJ, VanHelder T, Bhandari M, Bosch J, Kurz A, Polanczyk C, Malaga G, Nagele P, Le Manach Y, Leuwer M, Yusuf S. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA . 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 45.Ruetzler K, Rivas E, Cohen B, Mosteller L, Martin A, Keebler A, Maheshwari K, Steckner K, Wang M, Praveen C, Khanna S, Makarova N, Sessler DI, Turan A. McGrath video laryngoscope versus macintosh direct laryngoscopy for intubation of morbidly obese patients: a randomized trial. Anesth Analg . 2020;131:586–593. doi: 10.1213/ANE.0000000000004747. [DOI] [PubMed] [Google Scholar]
- 46.Saasouh W, Laffey K, Turan A, Avitsian R, Zura A, You J, Zimmerman NM, Szarpak L, Sessler DI, Ruetzler K. Degree of obesity is not associated with more than one intubation attempt: a large centre experience. Br J Anaesth . 2018;120:1110–1116. doi: 10.1016/j.bja.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Salzarulo HH, Taylor LA. Diabetic "stiff joint syndrome" as a cause of difficult endotracheal intubation. Anesthesiology . 1986;64:366–368. doi: 10.1097/00000542-198603000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Kim RP. The musculoskeletal complications of diabetes. Curr Diab Rep . 2002;2:49–52. doi: 10.1007/s11892-002-0057-7. [DOI] [PubMed] [Google Scholar]
- 49.Camilleri M, Chedid V, Ford AC, Haruma K, Horowitz M, Jones KL, Low PA, Park SY, Parkman HP, Stanghellini V. Gastroparesis. Nat Rev Dis Primers . 2018;4:41. doi: 10.1038/s41572-018-0038-z. [DOI] [PubMed] [Google Scholar]
- 50.Duggan E, Chen Y. Glycemic management in the operating room: screening, monitoring, oral hypoglycemics, and insulin therapy. Curr Diab Rep . 2019;19:134. doi: 10.1007/s11892-019-1277-4. [DOI] [PubMed] [Google Scholar]
- 51.Miles ME, Rice MJ. Recent advances in perioperative glucose monitoring. Curr Opin Anaesthesiol . 2017;30:718–722. doi: 10.1097/ACO.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 52.Abdelmalak BB. Anesthesiologist’s guide to perioperative glycemic management. ASA Ref Courses Anesth . 2014;42 [Google Scholar]
- 53.Karon BS, Donato LJ, Larsen CM, Siebenaler LK, Wells AE, Wood-Wentz CM, Shirk-Marienau ME, Curry TB. Accuracy of capillary and arterial whole blood glucose measurements using a glucose meter in patients under general anesthesia in the operating room. Anesthesiology . 2017;127:466–474. doi: 10.1097/ALN.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice MJ, Pitkin AD, Coursin DB. Review article: glucose measurement in the operating room: more complicated than it seems. Anesth Analg . 2010;110:1056–1065. doi: 10.1213/ANE.0b013e3181cc07de. [DOI] [PubMed] [Google Scholar]
- 55.Rice MJ, Coursin DB. Continuous measurement of glucose: facts and challenges. Anesthesiology . 2012;116:199–204. doi: 10.1097/ALN.0b013e318236abf6. [DOI] [PubMed] [Google Scholar]
- 56.Wallia A, Umpierrez GE, Rushakoff RJ, Klonoff DC, Rubin DJ, Hill Golden S, Cook CB, Thompson B DTS continuous glucose monitoring in the hospital panel. consensus statement on inpatient use of continuous glucose monitoring. J Diabetes Sci Technol . 2017;11:1036–1044. doi: 10.1177/1932296817706151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delbeck S, Vahlsing T, Leonhardt S, Steiner G, Heise HM. Non-invasive monitoring of blood glucose using optical methods for skin spectroscopy-opportunities and recent advances. Anal Bioanal Chem . 2019;411:63–77. doi: 10.1007/s00216-018-1395-x. [DOI] [PubMed] [Google Scholar]
- 58.Palermo NE, Garg R. Perioperative management of diabetes mellitus: novel approaches. Curr Diab Rep . 2019;19:14. doi: 10.1007/s11892-019-1132-7. [DOI] [PubMed] [Google Scholar]
- 59.Joshi GP, Chung F, Vann MA, Ahmad S, Gan TJ, Goulson DT, Merrill DG, Twersky R Society for ambulatory anesthesia. society for ambulatory anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg . 2010;111:1378–1387. doi: 10.1213/ANE.0b013e3181f9c288. [DOI] [PubMed] [Google Scholar]
- 60.Thompson BM, Stearns JD, Apsey HA, Schlinkert RT, Cook CB. Perioperative management of patients with diabetes and hyperglycemia undergoing elective surgery. Curr Diab Rep . 2016;16:2. doi: 10.1007/s11892-015-0700-8. [DOI] [PubMed] [Google Scholar]
- 61.Moreira MR MH. Perioperative management of diabetes mellitus: a review. J Anesth Clin Res . 2019;10:5. [Google Scholar]
- 62.Cosson E, Catargi B, Cheisson G, Jacqueminet S, Ichai C, Leguerrier AM, Ouattara A, Tauveron I, Bismuth E, Benhamou D, Valensi P. Practical management of diabetes patients before, during and after surgery: a joint French diabetology and anaesthesiology position statement. Diabetes Metab . 2018;44:200–216. doi: 10.1016/j.diabet.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Vriesendorp TM, Morélis QJ, Devries JH, Legemate DA, Hoekstra JB. Early post-operative glucose levels are an independent risk factor for infection after peripheral vascular surgery: a retrospective study. Eur J Vasc Endovasc Surg . 2004;28:520–525. doi: 10.1016/j.ejvs.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Brenner WI, Lansky Z, Engelman RM, Stahl WM. Hyperosomolar coma in surgical patients: an latrogenic disease of increasing incidence. Ann Surg . 1973;178:651–654. doi: 10.1097/00000658-197311000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg . 2003;125:1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]


