Abstract
Diabetic macular edema (DME) is a very important and well-known cause of visual loss in diabetics. Blood–retina barrier disruption and consequent intraretinal fluid accumulation may lead to retinal thickening at the posterior pole namely DME. Even though it is not clearly understood, current evidence suggests that chronic low-grade inflammation characterized with various cytokines has a major role in the occurrence of DME. Clinical trials are continuously shaping our treatment approaches for the eyes with DME. Today, vascular endothelial growth factor (VEGF) inhibitor and steroid administrations are the main alternatives in DME treatment. Dexamethasone (DEX) implant (Ozurdex®; Allergan, Inc., Irvine, CA, United States) was approved by the United States Food & Drug Administration in 2014 for DME treatment. The implant is made up of a biodegradable solid copolymer that is broken down by releasing its active ingredient into the vitreous cavity over time. Biphasic release feature of this sustained-release drug delivery system ensures its efficacy for up to 6 mo with an acceptable and manageable safety profile. DEX implant provides a favorable anatomical and functional outcome in DME as shown in several randomized-controlled studies but has a relatively higher ocular side-effect profile such as increased risk of cataract formation and raised intraocular pressure when compared to the gold standard anti-VEGF agents. Thus, DEX implant becomes the second-line treatment option demonstrating inadequate clinical response to anti-VEGF therapy. However, it can be preferred as the first-line treatment in vitrectomized and pseudophakic eyes. Even in some selected conditions DEX implant is favored over anti-VEGF agents where the use of VEGF-inhibitors is either inappropriate or contraindicated such as the patients with a recent history of a major cardiovascular or cerebrovascular event, pregnancy and noncompliant to frequent visits. This mini-review briefly overviews the efficacy, safety profile and complications of DEX implant and summarizes the outcome of DEX implant administration in major clinical studies on DME treatment.
Keywords: Dexamethasone implant, Diabetic macular edema, Diabetic retinopathy, Drug-delivery system, Glaucoma, Pharmacotherapy
Core Tip: Administration of dexamethasone (DEX) implant is among the main therapeutic alternatives for treating the diabetic macular edema (DME). Though DEX implant provides a long-standing anatomic and visual improvement, implant induced cataract progression and intraocular pressure elevation limit its clinical use as the first-line treatment but DEX implant can sometimes be the preferred option in previously vitrectomized eyes, pseudophakic eyes, and in some specific conditions where the use of vascular endothelial growth factor inhibitors is either contraindicated or suboptimal. In this mini-review, we overviewed the randomized-controlled trials, real-life clinical experiences, and meta-analyses on DEX implant treatment in DME.
INTRODUCTION
Diabetic macular edema (DME) is a common cause of vision loss in the working-age population suffering from diabetes mellitus (DM) and characterized by retinal thickening at the posterior pole, intraretinal and/or subretinal fluid accumulation due to hyperpermeable retinal vasculature and microenvironmental alterations[1-3]. DME may occur at any stage of diabetic retinopathy (DR) varying from mild non-proliferative to proliferative DR and is often closely associated with systemic and ocular risk factors. Long disease duration, elevated systolic blood pressure and high HbA1c level are among the systemic risk factors whereas main ocular risk factor is the severity of DR[3]. Prevalence of DME varies between 2.7% and 11.1% among the patients having DR and the prevalence is affected by the type of DM, race, ethnicity and the disease duration[4-9]. Patients with a longer duration of diabetes have a higher prevalence of DME. It is estimated that 20% of patients who have diabetes for more than 10 years and 30% of patients having diabetes for more than 25 years will likely develop DME[10].
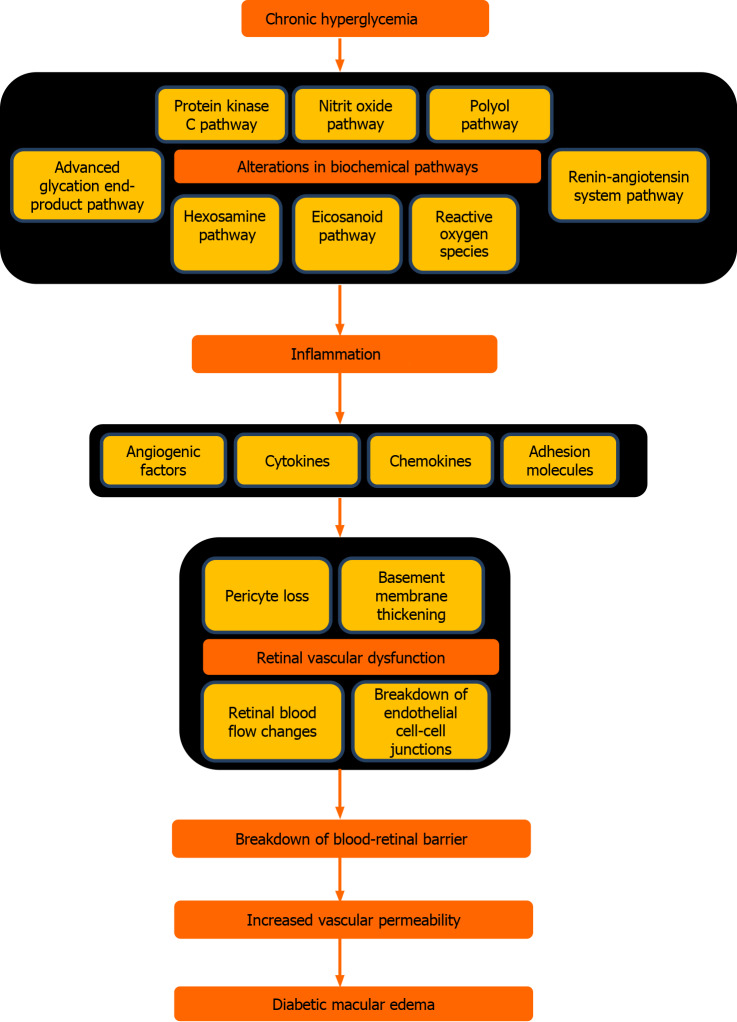
Although the exact molecular mechanisms remain unclear, building evidence has indicated the role of inflammation and angiogenesis in its pathogenesis. However, the sequence of the pathophysiological events is quite complex and not still fully understood. Chronic hyperglycemia is the main culprit initiating the pathologic process by triggering the endothelial dysfunction and subsequent blood-retina barrier disruption[11,12]. Capillary basement membrane thickening, pericyte loss, capillary dilation, and increased vascular permeability are the early retinal microvascular abnormalities that stimulate the pro-inflammatory and pro-angiogenic processes in DME[12]. Although vascular endothelial growth factor (VEGF), a potent cytokine and vasopermeability factor, seems to be the key player in the DME, it is definitely not the sole responsible inflammatory cytokine. Moreover, it is still obscure whether increased VEGF production is the cause or consequence of the inflammation or not[11]. Chronic low-grade inflammatory response involves leukostasis, macrophage accumulation and elevation of pro-inflammatory factors (cytokines, chemokines, adhesion molecules and angiogenic factors) such as interleukin (IL)-1β, IL-6, IL-8, interferon gamma-induced protein 10, stromal cell-derived factor 1, monocyte chemotactic protein 1, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, VEGF, platelet-derived growth factor, transforming growth factor-beta, placental growth factor, tumor necrosis factor-α, matrix metalloproteinase 1 and 9 and results in blood-retina barrier disruption characterized with damaged endothelial cell junctions and increased vasopermeability. Pathophysiological process and biochemical pathways of DME are illustrated in Figure 1[3,9-16].
Figure 1.
Pathophysiology and biochemical pathways of diabetic macular edema.
Currently, focal or grid laser photocoagulation, intravitreal VEGF-inhibitors, intravitreal steroids and pars plana vitrectomy are among the treatment options for the DME treatment[12]. However, their sequencing is still a matter of debate. Earlier studies have reported that focal laser, where laser beam is directed mainly at the leaking microaneurysms selectively and grid laser photocoagulation where laser beam is administered over the diffuse leakage areas generally reduces the risk of moderate vision loss in patients with fovea-involved DME, but does not usually provide any visual gain but laser treatment does not target at the molecular pathways underlying the formation of DME[10,17-19]. Nowadays, grid laser photocoagulation is very rarely used and focal laser photocoagulation is used only in a minority of patients due to success story of intravitreal therapies. Currently, pharmacotherapy (VEGF-inhibitors and steroids) is considered as a better therapeutic option than the laser therapy and widely employed especially for the center-involved DME as it targets the underlying molecular pathways[10,20,21]. The efficacy of VEGF-inhibitors has already been proven in many major randomized-controlled clinical trials as they provided a better anatomic and visual outcome. For the time being, European society of retina specialists (EURETINA) guideline is recommending the intravitreal anti-VEGF agents as the first-line therapy for the center involved DME[22]. However, the necessity of frequent even monthly intravitreal injections creates a great inconvenience for the majority of patients especially with persistent or recurrent DME due to their relatively short vitreous half-life and thereby anti-VEGF treatment causes a tremendous burden not only for the patients but also to the caregivers[20]. Physicians generally prefer to switch from one anti-VEGF agent to other or to a DEX implant in patients who do not respond well or show an inadequate response to ongoing anti-VEGF therapy or add laser treatment[21,22].
Intravitreal corticosteroid administration is utilized in eyes with DME as the paramount role of inflammation is evident. Steroids inhibit phospholipase A2 and arachidonic acid pathways and display their effects not only by reducing the VEGF expression but also suppressing the other inflammatory cytokines and thereby blocking the leukostasis and improving the vascular permeability[9,10]. Intravitreal drug administration is very efficacious as higher intraocular drug concentrations can be achieved with infrequent systemic side-effects[10]. Dexamethasone (DEX) phosphate, triamcinolone acetonide (TA), and fluocinolone acetonide (FA) are the three currently available synthetic steroids administered intravitreally and have different glucocorticoid-receptor binding activity and lipophilicity with contrasting relative potencies[9]. DEX, a steroid with potent anti-inflammatory effect, is five-times more effective than the TA, but has a shorter half-life in the vitreous cavity. TA has a lower anti-inflammatory effect but a longer half-life than DEX. Due to short half-life of DEX, TA has long been used for the management of DME intravitreally[9,17].
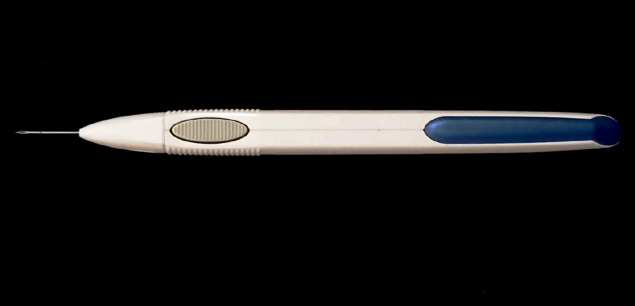
The sustained-release steroid implants have been developed in conjunction with the technological advances and innovations. Besides their long-acting anti-inflammatory effect, the implants look more advantageous as satisfactory anatomical and functional outcomes can be achieved with significantly fewer number of intravitreal injections[9,20,22,23]. DEX implant (Ozurdex®; Allergan, Inc., Irvine, CA, United States) is a biodegradable solid polymer drug-delivery system and contains 0.7 mg of DEX. The implant is administered intravitreally by a single-use injection device with a 23-gauge needle[23]. An example of Ozurdex intravitreal injection device is displayed in Figure 2. The drug-delivery system is based on the diffusion principle by releasing DEX into the vitreous cavity in two phases: an initial high-concentration phase, followed by a second low-concentration phase. Biphasic release fashion of the implant can extend its treatment effect up to 6 mo according to its label. However, the maximum effect of DEX implant occurs approximately 2 mo after the injection and then treatment effect slowly wanes[9,20,22,24]. The implant is initially metabolized into lactic and glycolic acid due to its biodegradable property, and then subsequently cleared from the vitreous by being metabolized into water and carbon dioxide[17]. Its effect is also predictable in vitrectomized eyes as vitreous surgery does not affect the pharmacokinetics of the implant. The appearance of a DEX implant in an eye with DME is illustrated in Figure 3.
Figure 2.
Ozurdex single-use intravitreal injection device with a 23-gauge needle.
Figure 3.
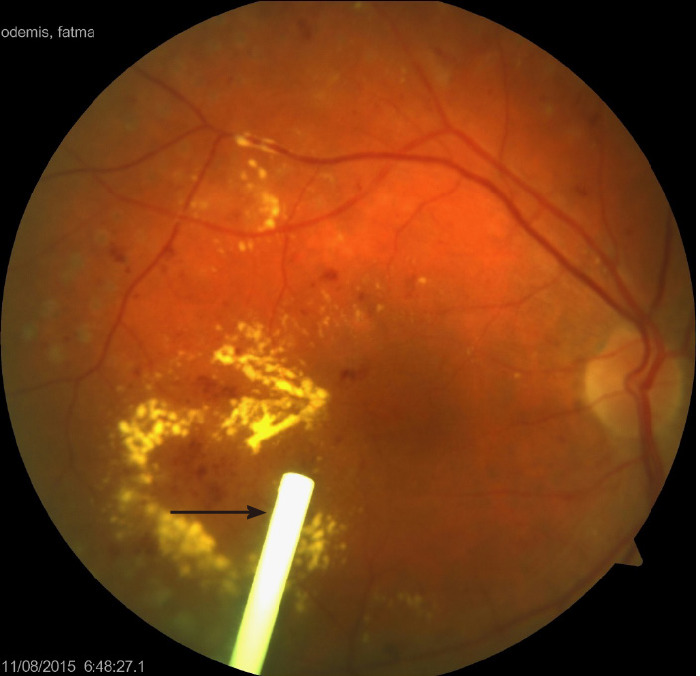
Color fundus picture of a right eye showing the diabetic macular edema related circinate hard exudates, intraretinal hemorrhages and old laser burns together with a Dexamethasone implant located in the vitreous cavity (black-arrow).
According to the EURETINA guidelines, steroids have been recommended as a second-line treatment for the DME treatment as they are fraught with a relatively higher ocular side effect profile than the VEGF-inhibitors, and generally preferred in patients with persistent DME who do not respond despite having 3-6 consecutive anti-VEGFs injections[22]. However, steroids can sometimes be preferred as the first-line treatment on some special occasions where VEGF inhibitors are either contraindicated (such as the patients with a history of major cardiovascular event and pregnancy) or expected to achieve suboptimal treatment outcome such as in vitrectomized and non-compliant patients[9,20,22].
While DEX implant is currently the most popular and commonly used corticosteroid implant, FA implant has also been employed for the treatment of chronic DME with a similar anti-inflammatory potency. Iluvien (Alimera Sciences, Aldershot, United Kingdom) is a commercially available, non-biodegradable steroid implant loaded with 0.19 mg FA. The implant is injected into the vitreous cavity with a 25-gauge needle and its effect may extend up to 36 mo. Iluvien is approved by United States Food & Drug Administration (FDA) for the treatment of chronic DME in patients who do not experience steroid induced intraocular pressure (IOP) rise previously based on the fluocinolone acetate for macular edema studies in September 2014. EURETINA guidelines have suggested that DEX implant should be preferred over FA implant. However, the guidelines have also suggested that FA implant may be considered for nonsteroid responder (patients who do not develop steroid-induced IOP elevation) with chronic DME who is unresponsive to other treatments[20-22]. Since TA is off-label and associated with more cataract formation and IOP elevation, EURETINA guidelines have recommended that TA should only be administered when patients cannot reach the FDA approved steroid agents[22].
MAJOR DEX IMPLANT TRIALS FOR THE TREATMENT OF DME
DEX implant was first approved for retinal vein occlusion treatment by FDA in 2009 and then was approved for the management of DME in 2014[20]. Table 1 summarizes the major clinical studies about DEX implant administration in patients with DME[24-33]. Most of these studies investigated the efficacy and safety profile of DEX implant in phakic and/or pseudophakic patients including previously vitrectomized eyes either as a monotherapy or in combination with laser photocoagulation or VEGF-inhibitors.
Table 1.
Summary of major clinical trials of dexamethasone implant in patients with diabetic macular edema
| Ref. | Study design, number of patients, mean or median follow-up period after the first injection | Treatment indications | Anatomical and functional outcomes |
| Boyer et al[24], Ozurdex MEAD study group | Multicenter, randomized, sham-controlled, phase III clinical study | DME | Statistically significantly higher mean CMT reduction from baseline during the study in both DEX implant groups than the sham group |
| 1048 patients | DEX implant 0.7 mg: -111.6 µm | ||
| DEX implant 0.7 mg (351) | DEX implant 0.35 mg: -107.9 µm | ||
| DEX implant 0.35 mg (347) | Sham group: - 41.9 µm | ||
| Sham (350) | Statistically significant improvement in BVCA with DEX implant at year 3. A significantly greater percentage of patients with a ≥ 15-letter improvement in BCVA from baseline in both DEX implant groups than sham at year 3 | ||
| 3 yr | DEX implant 0.7 mg: 22.2% | ||
| DEX implant 0.35 mg: 18.4% | |||
| Sham group: 12% | |||
| Haller et al[25] | Multicenter, randomized-controlled clinical trial | DME | A statistically significant improvement in CMT and fluorescein leakage after DEX implant compared with the observation group. Change in CMT from baseline at month 3: 0.7 mg DEX implant: -132 µm, observation group: +30 µm |
| 315 patients | A statistically significantly higher proportion of eyes improving in BCVA (≥ 10 letters) in 0.7 mg DEX implant (26% and 33%) than in observation group (9% and 12%) at months 2 and 3. The difference between the 0.7 mg DEX implant (30%) and observation group (23%) was maintained through month 6, but not statistically significant after month 3 | ||
| DEX implant 0.7 mg (105) | |||
| DEX implant 0.35 mg (105) | |||
| Observation group (105) | |||
| 6 m | |||
| Boyer et al[26], Ozurdex CHAMPLAIN study group | Multicenter, prospective, open-label, phase II clinical study | DME | Significant decrease in CMT at 2 mo and 6 mo after the first injection. The mean change from baseline CMT (403 µm): -156 µm at month 2 and -39 µm month 6 |
| 55 patients | Significant improvement in BCVA at month 2 and 6. The mean increase in BCVA from baseline (54.5 letters): 6.0 letters at month 2 and 3.0 letters at month 6. 30.4% of patients gained ≥ 10 letters in BCVA at month 2 | ||
| 6 mo | |||
| Callanan et al[27], Ozurdex PLACID study group | Multicenter, randomized, sham-controlled, double-masked, phase II clinical study | DME | Significant decrease in CMT and area of leakage in the combination of DEX implant with the laser than in the laser alone |
| 253 patients | The percentage of patients who gained ≥ 10 letters in BVCA in the combination of DEX implant with laser was significantly higher at week 1 and months 1, 4, and 9 (ranged from 22.2%-30.3%), but not month 12 | ||
| DEX implant plus laser (126 patients) | |||
| Laser alone (127 patients) | |||
| 12 mo | |||
| Gillies et al[28], BEVORDEX Study Group | Multicenter, prospective, randomized, phase II study | DME | Greater mean CMT reduction in the DEX group than in bevacizumab |
| 88 eyes of 61 patients | DEX implant: -187 µm | ||
| Bevacizumab (42 eyes) | Bevacizumab: -122 µm | ||
| DEX implant (46 eyes) | The percentage of patients with a ≥ 10-letter improvement in BCVA | ||
| 12 mo | Bevacizumab: 40% (17 eyes of 42) | ||
| DEX implant: 41% (19 eyes of 46) | |||
| Guigou et al[29], MOZART study | Multicenter, retrospective study | DME | Decrease in mean CMT: 239 μm at month 2 and 135 μm at month 6 |
| 74 patients | Improvement in mean BCVA from the baseline: 8.5 letters at month 2 and 7.6 letters at month 6. The percentage of patients who gained greater than 15 letters in BVCA at month 6 was 27% | ||
| 6 mo | |||
| Singer et al[30], REINFORCE Study | Multicenter, prospective, observational, phase IV clinical study | DME | Statistically significant decrease in CMT (from -121.2 μm to -140.3 μm) from the baseline after the first three DEX injections at all months through month 12 |
| 177 patients (180 eyes) | Statistically significant improvement in BCVA (ranged from +7.0 approximate ETDRS letters to +9.1 letters from baseline) after the first three DEX injections. The percentage of patients who gained greater than 15 approximate EDTRS letters in BVCA: 36% | ||
| 12 m | |||
| Mello Filho et al[31] | Multicenter, retrospective observational clinical study | DME | Statistically significant reduction in median CMT |
| 282 patients (329 eyes) | Baseline: 425 μm | ||
| After DEX implant: 270 μm | |||
| Statistically significant improvement in median BCVA. | |||
| Baseline: 0.7 log-MAR/50 letters | |||
| After DEX implant: 0.3 logMAR/70 letters | |||
| Malclès et al[32] RELDEX study | 128 eyes of 89 patients | DME | A statistically significant improvement in CMT |
| 16 mo | Baseline: 451 μm, month 12: 370 μm, month 24: 377 μm, and month 36: 280 μm | ||
| Statistically significant improvement in mean BCVA from the baseline (50.5 letters): 54 letters at month 2, 54.7 letters at month 12, 56 letters at month 24 and | |||
| 60.6 letters at month 36. The percentage of eyes achieving at least a 15-letter improvement from the baseline at month 36: 25.4% | |||
| Rosenblatt et al[33], ARTES study group | Multicenter, retrospective, cohort study | DME | Statistically significant CMT reduction was obtained throughout the first 6 mo. This effect diminished after month 3 but maintained until month 6. Mean change in CMT: -174 μm |
| 287 patients (340 eyes) | Significant improvement in BCVA during the first six months. The percentage of eyes with a ≥ 10-letter improvement in BCVA after DEX implant injections: 37.8% | ||
| 1.7 yr |
BCVA: Best-corrected visual acuity; CMT: Central macular thickness; DEX: Dexamethasone phosphate; DME: Diabetic macular edema; EDTRS: Early treatment diabetic retinopathy study.
MEAD (two randomized, multicenter, masked, sham-controlled, phase III trials with identical protocols) is the registering trial evaluating the safety and efficacy of DEX implant in patients with DME[24]. 1048 patients with center-involved DME were randomized for the administration of 0.7 mg and 0.35 mg of DEX implant or a sham procedure and followed up for 3 years with an average of 4-5 injections. MEAD trials elucidated that the percentage of patients with ≥ 15-letter improvement in best-corrected visual acuity (BCVA) and mean average reduction in central macular thickness (CMT) at the end of the 3-year follow-up from the baseline was higher in patients treated with 0.7 mg or 0.35 mg of DEX implants than the sham procedure with a favorable safety profile. 0.7 mg DEX implant was approved by the FDA based on the MEAD trials[24].
In a prospective, multicenter, phase II clinical study conducted by Ozurdex CHAMPLAIN study group[26], the efficacy and safety of 0.7 mg DEX implant were evaluated in difficult to treat previously vitrectomized eyes with DME. Statistically significant improvement in BCVA and reduction in CMT was reported following the first injection at 2 and 6 mo[26].
Multicenter Ozurdex® assessment for DME (MOZART)[29] study retrospectively investigated the efficacy and safety of 0.7 mg DEX implant in DME patients with visual impairment. The authors concluded that favorable anatomical and functional outcomes were obtained with an acceptable and manageable side effect profile as previously published studies[29].
In another study conducted by Ozurdex PLACID group[27], authors evaluated the efficacy of a combination of DEX implant with laser photocoagulation and compared it with the laser photocoagulation alone for the treatment of diffuse DME. They found that the percentage of patients who gained ≥ 10 letters in BVCA was significantly greater in the combination group at 1 and 9 mo, but no significant difference was noted between the two treatment arms at 12 mo. Similarly, the reduction in CMT and the leakage area were found significantly greater in the combination group than the group treated with laser photocoagulation alone. They suggested that the combination of DEX implant with laser reduced the vascular leakage and retinal edema and improved the BCVA than the laser treatment in eyes with diffuse DME[27].
The BEVORDEX study[28] compared DEX implant with bevacizumab administration for the center-involving DME and reported the clinical outcome at 12 mo. Although similar rates of improvement in BCVA were achieved in both treatment groups, the anatomical improvement was better in the DEX implant group. Also, improvement in anatomic and visual outcomes was attained with fewer injections in the DEX implant group than the bevacizumab group[28].
REINFORCE[30], a multicenter prospective phase IV clinical study, evaluated the real-world efficacy and safety of 0.7 mg DEX implant in patients with treatment-naïve and previously treated patients. This study reported that DEX implant was effective in improving BCVA and CMT in real-world clinical practice with similar administration frequency and safety profile as in previous reports[30].
In RELDEX study conducted by Malclès et al[32], the efficacy and safety profile of DEX implant were evaluated retrospectively in patients with DME. The authors reported that DEX implant administered in real-life situations provided favorable anatomical and visual acuity outcomes with a good safety profile over the 3-year follow-up[32].
In a very recent multicenter study conducted by ARTES study group[33], the real-life efficacy and safety of DEX implant in DME patients were evaluated. Patients were divided into groups as early (< 6 mo) and late (≥ 6 mo) stage DME, naïve and previously treated eyes, and controlled and uncontrolled DM. As a result of this retrospective cohort study comprising of 340 eyes of 287 patients, a statistically significant improvement in BCVA and reduction in CMT was reported after the DEX implant injections. However, its effect started to diminish after the 3 mo but still maintained till 6 mo. In addition, the authors reported that vision improvement was greater in treatment naïve patients than the previously treated patients. CMT reduction was more in patients with controlled DM than in uncontrolled DM. More eyes with early-stage DME gained ≥ 10 letters and lost ≥ 10 letters than the late-stage DME patients[33].
Castro-Navarro et al[34], in another study comparing the naïve and previous treated DME patients demonstrated a significant improvement in BCVA in both naïve and previous treated eyes with the DEX implant treatment. However, the proportion of DME patients who gained ≥ 10 letters was significantly greater in the naïve group. Mean CMT was decreased significantly in both groups but a greater mean CMT reduction was reported in the naïve group. The authors also reported favorable outcomes with the DEX implant even in the refractory patients who failed to respond to previous treatments[34].
As mentioned above, VEGF-inhibitors are generally the first-choice in center-involved DME management. However, DME may persist despite continuous anti-VEGF therapy in some patients. A Diabetic Retinopathy Clinical Research Network study Protocol U, a phase II multicenter randomized clinical trial, investigated the efficacy of DEX implant addition into the treatment regimen for the patients with persistent DME who have previously received ranibizumab injections without any clinical improvement. The study included 129 eyes of 116 patients who received at least three injections of ranibizumab before a run-in phase and had persistent DME with a BCVA ≤ 20/32. After the run-in phase, patients with persistent DME and poor vision was randomized into one of the two treatment group; a combination group (ranibizumab plus DEX implant, 64 eyes) and a ranibizumab only group (64 eyes). Then the patients were followed up monthly and received continued ranibizumab re-treatment in both groups every 4 wk based on a structured re-treatment protocol. This study demonstrated that there was no significant difference in BCVA between the combination (+ 2.7 letters) and ranibizumab only groups (+ 3 letters) at 6 mo. However, a significant difference between the two groups in terms of anatomical outcome was noted in favor of combination group (-110 μm vs -62 μm). In addition, the percentage of DME resolution was 52% in the combination group vs 31% in the ranibizumab only group. The authors stated that adding DEX implant to the ongoing ranibizumab treatment did not improve BCVA at 6 mo in patients with persistent DME, but CMT was reduced significantly[35].
In a retrospective study, Busch et al[36] have assessed the efficacy of DEX implant administered after three consecutive anti-VEGF injections in patients with persistent DME when compared to patients with continuous mono anti-VEGF treatment. The authors found that anatomical outcome was better in the group of patients switched early to DEX implant following three anti-VEGF injections at 12 mo than those patients kept on anti-VEGF injections[36].
Besides the studies reporting the efficacy of DEX implant either alone or sequential use with the VEGF-inhibitors, there are a few studies evaluating the simultaneous use of the DEX implant with an anti-VEGF agent for the DME treatment. These studies suggested that simultaneous intravitreal injections of anti-VEGF and DEX implant might be a superior treatment option than the anti-VEGF treatment alone with a favorable anatomic and visual outcome and an acceptable safety profile[37-39]. However, the size of these studies was small.
META-ANALYSES ON THE DME TREATMENT WITH DEX IMPLANT
In a recent meta-analysis conducted by He et al[20] comparing the effectiveness and safety of DEX implant and VEGF-inhibitors in DME treatment, visual improvement is comparable in both DEX implant and VEGF-inhibitor groups. However, better anatomic outcomes were obtained with the DEX implant at 6 mo with fewer injections. The researchers reported that there was no significant difference between the two groups in terms of reduction in CMT at 12 mo and added that the visual benefit was not fully reflected in patients who received DEX implants due to cataract progression. Because of its ocular side effects, the authors suggested that DEX implant can only be recommended as the first-line treatment in selected cases including patients unresponsive to anti-VEGF agents, pseudophakic patients and reluctant patients who did not want to have frequent visits and intravitreal injections[20].
In a current meta-analysis study conducted by Khan et al[40], 3859 patients from 15 studies were analyzed to evaluate the efficacy of DEX implant in patients with recalcitrant DME who do not respond despite having at least six previous anti-VEGFs injections. The mean follow-up period of the patients was 6 mo, ranging from 3-36 mo. Significant improvement in mean BCVA (four lines or 20 ETDRS letters) was reported with DEX implant. The authors suggested that clinicians fortunately have a chance to employ many treatment alternatives for DME management nowadays and they concluded that DME patients resistant to anti-VEGF therapy should be recognized and steroid therapy should be considered to reach better anatomic and visual outcomes. Authors noted that the efficacy of the DEX implant will decrease over time and repeated injections may be required to maintain the visual gain[40].
Kodjikian et al[41], have reported the efficacy of pharmacotherapy in patients with DME by assessing real-life observational studies and compared anti-VEGF agents with DEX implant. Overall, they have analyzed 63 studies evaluating the efficacy of VEGF-inhibitors (n: 32) and DEX implant (n: 31). The final BCVA value was 61.2 letters in the DEX implant group and 62 letters in the anti-VEGF group. Additionally, BCVA gains from the baseline were reported as + 9.6 letters in DEX implant group and + 4.7 letters anti-VEGF group. Although the final BVCA was similar in both groups, BCVA gains from the baseline were higher in DEX implant group. This meta-analyses has suggested that gain of more letters with the DEX implant might be partially explained by the lower baseline BCVA in patients receiving the DEX implant or less frequent anti-VEGF injections administered in observational studies than in the interventional studies[41].
EXPERT RECOMMENDATIONS FOR THE MANAGEMENT OF DME WITH DEX IMPLANT IN REAL-LIFE PRACTICE
Recommendations of EURETINA guidelines were based on the randomized-controlled trials, which do not fully reflect the real-life scenarios due to their designs. Experts of several countries have prepared their own consensus documents using a Delphi approach regarding the use of DEX implant for the management of DME in daily practice.
Spanish MOMENTUM-D study group has concluded that DEX implant is particularly beneficial in the DME treatment in patients with a history of major cardiovascular event, vitrectomized patients, pseudophakic patients, non-compliant patients, patients requiring cataract surgery, and patients with substantial inflammatory component. The consensus paper has also recommended that switching to DEX implant should be considered after three anti-VEGF injections[42].
Italian experts have stated that DEX implant has the best ocular tolerance among the other intravitreally administered steroids and should be considered first over TA and FA especially in pseudophakic and vitrectomized patients. According to them, switching to DEX implant should be employed after the loading-phase of anti-VEGF therapy in resistant DME patients. Experts have concluded that pro-re-nata is an appropriate treatment regimen for further DEX implant administrations and the clinicians should not wait for 6 mo for re-treatment[43].
German consensus paper suggested that switching to DEX implant should be performed in patients with inadequate treatment response following 3-6 monthly anti-VEGF injections. They also suggested that the implant might be useful in patients with long-term DME characterized with massive lipid exudates. BCVA, CMT and IOP have been stated to be the major parameters for the re-treatment decision. Similar to other consensus documents and EURETINA guidelines, DEX implant is recommended as the first-line therapy in pseudophakic patients, reluctant patients who do not want to attend frequent visits and receive frequent injections, and patients with known vascular diseases. They also stated that the implant should not be implemented in both eyes on same day[44].
SAFETY OF DEX IMPLANT ADMINISTRATIONS
DEX implant may cause some undesirable ocular complications during or after the administration[22-50]. Foreign body sensation, eye pain and pruritus, conjunctival hyperemia, conjunctival edema, conjunctival hemorrhage, anterior chamber cell and flare, increased IOP, cataract, vitreous hemorrhage, and myodesopsia are among the reported ocular adverse events[12,24-26]. Retinal tear, retinal detachment, vitreous loss, intralenticular injection of the implant and endophthalmitis have been reported as the rare ocular adverse events associated with any type of intravitreal injection[12,50]. In addition, DEX implant may migrate into the anterior chamber in aphakic eyes, vitrectomized eyes, eyes with sutured intraocular lens implantation, patients with weak lens zonules or a posterior capsule defect related to a previous complicated cataract surgery[48,49] and cause further ocular complications. Cataract progression and steroid-induced ocular hypertension or glaucoma are the most frequent and important ocular side-effects associated with the implant[47] DEX binds less to the trabecular meshwork and lens as it is less lipophilic than the TA and FA. Understandably, DEX implant is associated with a lower risk of glaucoma and cataract formation. Although the precise mechanism of IOP rise is not fully understood increased aqueous outflow resistance due to structural and biochemical changes in the trabecular meshwork is thought to be the cause for IOP elevation[9,12]. Many studies have been reported different rates of IOP elevation[24-33,46]. An IOP increase of ≥ 10 mmHg was reported between 6.8%-27.7% of the patients[24,25,27,28,30-33,46]. IOP of ≥ 25 mmHg was reported in 6.6%-32% of patients[24-33,46]. Also, IOP of ≥ 35 mmHg was reported in 0.9%-6.6% of patients[24,26,27,30-33,46]. IOP-lowering medication was required in 6.3% and 41.5% of the patients[24,26,27,30,32,33,46]. While 0.9% of the patients in the MEAD study required glaucoma surgery[24,46] some studies reported no need for glaucoma surgery[25-33]. Cataract formation or progression is the most common ocular complication of DEX implant leading to decrease in visual acuity and the need for cataract surgery usually increases after the second year of treatment[12]. This almost inevitable complication is reported between 4% and 67.9% of patients in several manuscripts[24,26,27,28,31,33,46]. Besides ocular adverse events, systemic adverse events such as worsening of hypertension, chest pain, angina, and renal failure have been rarely reported[28].
CONCLUSION
DEX implant can provide visual and anatomical improvement with a fewer number of intravitreal injections in eyes with DME as briefly summarized above. It has a well- accepted efficacy profile in DME treatment but is generally preferred as the second-line treatment option due to its less favorable safety profile than the anti-VEGF agents. On the other hand, it can be a primary option in a selected group of patients such as vitrectomized patients, pseudophakic patients, patients with a recent cerebrovascular or cardiovascular event history and patients who experienced suboptimal treatment benefit with anti-VEGF therapy. Cataract and increased IOP are the most common implant-related ocular adverse events but in most instances they are clinically acceptable and well- manageable. Although combined utilization of the anti-VEGF agents and DEX implant either consecutively or simultaneously is possible it is still not proven whether there is an additional treatment benefit in administering them in combination.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Turkish Society of Ophthalmology.
Peer-review started: December 27, 2020
First decision: April 20, 2021
Article in press: July 7, 2021
Specialty type: Ophthalmology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Sayyad HI, Lee SJ, Nicoara SD S-Editor: Liu M L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Omer Karti, Department of Ophthalmology, İzmir Democracy University, İzmir 35330, Turkey.
Ali Osman Saatci, Department of Ophthalmology, Dokuz Eylul University, İzmir 35330, Turkey. osman.saatci@yahoo.com.
References
- 1.Bandello F, Battaglia Parodi M, Lanzetta P, Loewenstein A, Massin P, Menchini F, Veritti D. Diabetic Macular Edema. Dev Ophthalmol. 2017;58:102–138. doi: 10.1159/000455277. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarthy U, Yang Y, Lotery A, Ghanchi F, Bailey C, Holz FG, Downey L, Weber M, Eter N, Dugel PU. Clinical evidence of the multifactorial nature of diabetic macular edema. Retina. 2018;38:343–351. doi: 10.1097/IAE.0000000000001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning DJ, Stewart MW, Lee C. Diabetic macular edema: Evidence-based management. Indian J Ophthalmol. 2018;66:1736–1750. doi: 10.4103/ijo.IJO_1240_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and associated factors of diabetic retinopathy. The Beijing Eye Study 2006. Graefes Arch Clin Exp Ophthalmol. 2008;246:1519–1526. doi: 10.1007/s00417-008-0884-6. [DOI] [PubMed] [Google Scholar]
- 5.Rubino A, Rousculp MD, Davis K, Wang J, Girach A. Diagnosed diabetic retinopathy in France, Italy, Spain, and the United Kingdom. Prim Care Diabetes. 2007;1:75–80. doi: 10.1016/j.pcd.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma R, Torres M, Peña F, Klein R, Azen SP Los Angeles Latino Eye Study Group. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111:1298–1306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Eldem B, Ozdek S, Saatci AO, Ozmert E, Ulay E, Nomak G. Clinical Characteristics of Patients with Newly Diagnosed Diabetic Macular Edema in Turkey: A Real-Life Registry Study-TURK-DEM. J Ophthalmol. 2017;2017:3596817. doi: 10.1155/2017/3596817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbančič M, Gardašević Topčić I. Dexamethasone implant in the management of diabetic macular edema from clinician's perspective. Clin Ophthalmol. 2019;13:829–840. doi: 10.2147/OPTH.S206769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: A systematic review of real-world studies. J Pharmacol Sci. 2018;138:219–232. doi: 10.1016/j.jphs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J Diabetes Res. 2016;2016:2156273. doi: 10.1155/2016/2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dugel PU, Bandello F, Loewenstein A. Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol. 2015;9:1321–1335. doi: 10.2147/OPTH.S79948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734–1746. [PMC free article] [PubMed] [Google Scholar]
- 14.Jonas JB, Jonas RA, Neumaier M, Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina. 2012;32:2150–2157. doi: 10.1097/IAE.0b013e3182576d07. [DOI] [PubMed] [Google Scholar]
- 15.Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013;4:151–169. doi: 10.1177/2042018813512360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tripathy K, Sharma YR, R K, Chawla R, Gogia V, Singh SK, Venkatesh P, Vohra R. Recent advances in management of diabetic macular edema. Curr Diabetes Rev. 2015;11:79–97. doi: 10.2174/1573399811999150324120640. [DOI] [PubMed] [Google Scholar]
- 17.Augustin AJ, Kuppermann BD, Lanzetta P, Loewenstein A, Li XY, Cui H, Hashad Y, Whitcup SM Ozurdex MEAD Study Group. Dexamethasone intravitreal implant in previously treated patients with diabetic macular edema: subgroup analysis of the MEAD study. BMC Ophthalmol. 2015;15:150. doi: 10.1186/s12886-015-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 19.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–1449. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Ren XJ, Hu BJ, Lam WC, Li XR. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol. 2018;18:121. doi: 10.1186/s12886-018-0779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banaee T, Ashraf M, Conti FF, Singh RP. Switching Anti-VEGF Drugs in the Treatment of Diabetic Macular Edema. Ophthalmic Surg Lasers Imaging Retina. 2017;48:748–754. doi: 10.3928/23258160-20170829-10. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, Jonas J, Larsen M, Tadayoni R, Loewenstein A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA) Ophthalmologica. 2017;237:185–222. doi: 10.1159/000458539. [DOI] [PubMed] [Google Scholar]
- 23.Demirel S, Argo C, Agarwal A, Parriott J, Sepah YJ, Do DV, Nguyen QD. Updates on the Clinical Trials in Diabetic Macular Edema. Middle East Afr J Ophthalmol. 2016;23:3–12. doi: 10.4103/0974-9233.172293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, Whitcup SM Dexamethasone DDS Phase II Study Group. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 26.Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, Liu CC, Lou J, Whitcup SM Ozurdex CHAMPLAIN Study Group. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915–923. doi: 10.1097/IAE.0b013e318206d18c. [DOI] [PubMed] [Google Scholar]
- 27.Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, Liu CC, Li XY, Hollander DA, Schiffman RM, Whitcup SM Ozurdex PLACID Study Group. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120:1843–1851. doi: 10.1016/j.ophtha.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, Aroney C, McAllister IL, Fraser-Bell S. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121:2473–2481. doi: 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Guigou S, Hajjar C, Parrat E, Merite PY, Pommier S, Matonti F, Prost-Magnin O, Meyer F. [Multicenter Ozurdex® assessment for diabetic macular edema: MOZART study] J Fr Ophtalmol. 2014;37:480–485. doi: 10.1016/j.jfo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Singer MA, Dugel PU, Fine HF, Capone A, Jr, Maltman J. Real-World Assessment of Dexamethasone Intravitreal Implant in DME: Findings of the Prospective, Multicenter REINFORCE Study. Ophthalmic Surg Lasers Imaging Retina. 2018;49:425–435. doi: 10.3928/23258160-20180601-07. [DOI] [PubMed] [Google Scholar]
- 31.Mello Filho P, Andrade G, Maia A, Maia M, Biccas Neto L, Muralha Neto A, Moura Brasil O, Minelli E, Dalloul C, Iglicki M. Effectiveness and Safety of Intravitreal Dexamethasone Implant (Ozurdex) in Patients with Diabetic Macular Edema: A Real-World Experience. Ophthalmologica. 2019;241:9–16. doi: 10.1159/000492132. [DOI] [PubMed] [Google Scholar]
- 32.Malclès A, Dot C, Voirin N, Agard É, Vié AL, Bellocq D, Denis P, Kodjikian L. Real-life study in diabetic macular edema treated with dexamethasone implant: The Reldex Study. Retina. 2017;37:753–760. doi: 10.1097/IAE.0000000000001234. [DOI] [PubMed] [Google Scholar]
- 33.Rosenblatt A, Udaondo P, Cunha-Vaz J, Sivaprasad S, Bandello F, Lanzetta P, Kodjikian L, Goldstein M, Habot-Wilner Z, Loewenstein A ARTES Study Group. A Collaborative Retrospective Study on the Efficacy and Safety of Intravitreal Dexamethasone Implant (Ozurdex) in Patients with Diabetic Macular Edema: The European DME Registry Study. Ophthalmology. 2020;127:377–393. doi: 10.1016/j.ophtha.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Castro-Navarro V, Cervera-Taulet E, Navarro-Palop C, Monferrer-Adsuara C, Hernández-Bel L, Montero-Hernández J. Intravitreal dexamethasone implant Ozurdex® in naïve and refractory patients with different subtypes of diabetic macular edema. BMC Ophthalmol. 2019;19:15. doi: 10.1186/s12886-018-1022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maturi RK, Glassman AR, Liu D, Beck RW, Bhavsar AR, Bressler NM, Jampol LM, Melia M, Punjabi OS, Salehi-Had H, Sun JK Diabetic Retinopathy Clinical Research Network. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2018;136:29–38. doi: 10.1001/jamaophthalmol.2017.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busch C, Zur D, Fraser-Bell S, Laíns I, Santos AR, Lupidi M, Cagini C, Gabrielle PH, Couturier A, Mané-Tauty V, Giancipoli E, Ricci GD, Cebeci Z, Rodríguez-Valdés PJ, Chaikitmongkol V, Amphornphruet A, Hindi I, Agrawal K, Chhablani J, Loewenstein A, Iglicki M, Rehak M International Retina Group. Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol. 2018;55:789–796. doi: 10.1007/s00592-018-1151-x. [DOI] [PubMed] [Google Scholar]
- 37.Kaya M, Kocak N, Ozturk T, Bolluk V, Ayhan Z, Kaynak S. Intravitreal ranibizumab and dexamethasone implant injections as primary treatment of diabetic macular edema: simultaneously double protocol. Eye (Lond) 2021;35:777–785. doi: 10.1038/s41433-020-0949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Andrade FL, Lopes FS, de Andrade GC, Prata TS, Maia A. Simultaneous Therapy with Intravitreal Dexamethasone Implant and Bevacizumab for the Treatment of Macular Edema. Med Hypothesis Discov Innov Ophthalmol. 2016;5:4–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Saatci AO, Ayhan Z, Engin CD. Simultaneous Intravitreal Ranibizumab and Dexamethasone Implant Administration at the Same Setting in Eyes with Severe Diabetic Macular Edema. Open j ophthalmol. 2016;6:112–118. [Google Scholar]
- 40.Khan Z, Kuriakose RK, Khan M, Chin EK, Almeida DR. Efficacy of the Intravitreal Sustained-Release Dexamethasone Implant for Diabetic Macular Edema Refractory to Anti-Vascular Endothelial Growth Factor Therapy: Meta-Analysis and Clinical Implications. Ophthalmic Surg Lasers Imaging Retina. 2017;48:160–166. doi: 10.3928/23258160-20170130-10. [DOI] [PubMed] [Google Scholar]
- 41.Kodjikian L, Bellocq D, Mathis T. Pharmacological Management of Diabetic Macular Edema in Real-Life Observational Studies. Biomed Res Int. 2018;2018:8289253. doi: 10.1155/2018/8289253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García Layana A, Adán A, Ascaso FJ, Cabrera F, Donate J, Escobar Barranco JJ, Peralta G, Reyes García R, Rodríguez Maqueda M, Ruiz-Moreno JM, Vinagre I MOMENTUM-D Study Group. Use of intravitreal dexamethasone implants in the treatment of diabetic macular edema: Expert recommendations using a Delphi approach. Eur J Ophthalmol. 2020;30:1042–1052. doi: 10.1177/1120672119861623. [DOI] [PubMed] [Google Scholar]
- 43.Giovannini A, Parravano M, Ricci F, Bandello F. Management of diabetic macular edema with intravitreal dexamethasone implants: Expert recommendations using a Delphi-based approach. Eur J Ophthalmol. 2019;29:82–91. doi: 10.1177/1120672118781236. [DOI] [PubMed] [Google Scholar]
- 44.Augustin AJ, Feltgen N, Haritoglou C, Hoerauf H, Maier MM, Mardin CY, Schargus M. [Clinical Decision Making for Treatment of Diabetic Macular Oedema with DEX Implant: a Consensus Paper] Klin Monbl Augenheilkd. 2021;238:73–84. doi: 10.1055/a-1024-4089. [DOI] [PubMed] [Google Scholar]
- 45.Karti O, Saatci AO. Intraocular Pressure Changes Following the Administration of Intravitreal Dexamethasone Implant: A Mini -Review. Int J Ophthalmic Res. 2019;5:302–307. [Google Scholar]
- 46.Maturi RK, Pollack A, Uy HS, Varano M, Gomes AM, Li XY, Cui H, Lou J, Hashad Y, Whitcup SM Ozurdex MEAD Study Group. Intraocular pressure in patients with diabetic macular edema treated with dexamethasone intravitreal implant in the 3-year mead study. Retina. 2016;36:1143–1152. doi: 10.1097/IAE.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 47.Rajesh B, Zarranz-Ventura J, Fung AT, Busch C, Sahoo NK, Rodriguez-Valdes PJ, Sarao V, Mishra SK, Saatci AO, Udaondo Mirete P, Querques G, Farah ME, Lanzetta P, Arevalo JF, Kodjikian L, Chhablani J for International Ozurdex Study Group. Safety of 6000 intravitreal dexamethasone implants. Br J Ophthalmol. 2020;104:39–46. doi: 10.1136/bjophthalmol-2019-313991. [DOI] [PubMed] [Google Scholar]
- 48.Kayıkcıoğlu Ö, Doğruya S, Sarıgül C, Mayalı H, Kurt E. Anterior Chamber Migration of Ozurdex Implants. Turk J Ophthalmol. 2020;50:115–122. doi: 10.4274/tjo.galenos.2019.43778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Röck D, Bartz-Schmidt KU, Röck T. Risk factors for and management of anterior chamber intravitreal dexamethasone implant migration. BMC Ophthalmol. 2019;19:120. doi: 10.1186/s12886-019-1122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sekeroglu MA, Anayol MA, Koc F, Tirhis H, Ozkan SS, Yilmazbas P. Intralenticular Sustained-Release Dexamethasone Implant: Is It Still Effective on Macular Edema? Case Rep Ophthalmol. 2016;7:85–89. doi: 10.1159/000444163. [DOI] [PMC free article] [PubMed] [Google Scholar]