Abstract
The coronavirus disease 2019 (COVID-19) pandemic has had a considerable impact on the work of physicians and surgeons. The connection between the patient and the surgeon cannot be replaced by telemedicine. For example, the surgical staff faces more serious difficulties compared to non-surgical specialists during the COVID-19 pandemic. The primary concerns include the safest solutions for protecting healthcare staff and patients and the ability to provide adequate surgical care. Additionally, the adverse effects of any surgery delays and the financial consequences complicate the picture. Therefore, patients' admission during the COVID-19 pandemic should be taken into consideration, as well as preoperative measures. The COVID-19 situation brings particular risk to patients during surgery, where preoperative morbidity and mortality rise in either asymptomatic or symptomatic COVID-19 patients. This review discusses the recent factors associated with surgical complications, mortality rates, outcomes, and experience in COVID-19 surgical patients.
Keywords: COVID-19, SARS-CoV-2, Surgery, Surgery complications, Mortality rate, Acute respiratory distress syndrome, Thrombosis
Core Tip: The clinical outcomes of coronavirus disease 2019 (COVID-19) patients undergoing urgent or emergent surgeries have shown that the estimated duration of hospitalization is 10.55 d, with 15% admission in the intensive care unit and up to 15% in the postoperative period. Several complications are described in patients with COVID-19 who underwent surgical procedures, including acute respiratory distress syndrome and other pulmonary complications (e.g., pneumonia, shortness of breath, dyspnea, fever, cough). These complications lead to respiratory and cardiovascular system failure (i.e. heart attack, arrhythmia, and infarction), secondary infection, fatigue or myalgia, severe lymphopenia, sepsis/shock, acute kidney injury, etc. Pulmonary complications are considered the primary cause for the low survival rate and prolonged immobility in surgical COVID-19 patients. Lung involvement is also associated with high postoperative mortality, contributing to a 30-d mortality rate of 38%. Furthermore, cancer patients are more likely to contract COVID-19 than non-cancer patients. Therefore, they are more vulnerable to complications and have a higher mortality rate postoperatively. Thus, the impact of the COVID-19 pandemic on surgical patients in terms of delays or complications is noteworthy, and is expected to continue after the end of the pandemic.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has had a considerable impact on the work of physicians and surgeons. The treatment for patients with surgical illness involves a special and personal connection between the patient and the surgeon. This connection cannot be replaced by telemedicine. For example, the surgical staff faces more serious difficulties compared to non-surgical specialists during the COVID-19 pandemic. The primary concerns include implementing the safest solutions for protecting healthcare staff and patients, and providing adequate surgical care. Additionally, the adverse effects of any surgery delays on people with surgical illness and the financial consequences complicate the picture. In addition, the economic effects of the pandemic on the patient care system, surgical staff management shortage, educational, scientific, professional growth ramifications, and the emotional tribute increase the impact of these issues[1].
Another big obstacle is the need to cease non-urgent and non-emergency surgical activities. As personal protective equipment availability improves and the research develops, resuming and extending surgical procedures have become essential. Unfortunately, many surgical patients have limited access to surgical care due to probable COVID-19 infections or lack of "COVID-free" facilities in the hospitals or physicians' offices[2]. As a result, non-urgent and non-emergency services have been deferred, and a considerable backlog of surgery patients has accumulated. Furthermore, the impact of the COVID-19 pandemic on patients with cancer or chronic illnesses and those anticipating organ donation has yet to be revealed. Therefore, it is necessary to create an algorithm for expanding surgical facilities in a practical and working manner[1].
PREADMISSION AND ADMISSION CONSIDERATIONS DURING THE COVID-19 PANDEMIC
The novel coronavirus outbreak has affected surgery all over the world. Elective operations are postponed from this point of view, and surgeons are called upon to perform only emergency and urgent (i.e. cancer) surgery[3-5]. Therefore, there is a need for preadmission and admission considerations for the patients indicated for such types of surgery. The main target is to categorize patients into two groups (based on the possibility of having COVID-19): Non-infected and infected. Infected people should be further divided into asymptomatic or symptomatic[6]. Other authors have proposed the classification of potentially severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected people for surgery into three categories (i.e. non-infected people, asymptomatic carriers, and symptomatic patients).
In addition, preadmission history involving the general health status of the patient, active or signs of recent respiratory or gastrointestinal disorders, the history of recent visits to endemic regions in the last 14 d, or history of contact with a person at risk for COVID-19 should be carefully assessed[3]. The presence of signs and symptoms (i.e. anosmia, loss of taste, cough, headache, nausea, vomiting, fever) should also be evaluated. Symptomatic or suspected infected patients need to be classified carefully to confirm the diagnosis of COVID-19. Detection of viral RNA using real-time reverse transcriptase-polymerase chain reaction (PCR) of the clinical diagnosis is used[7]. In addition, an antigen test for SARS-CoV-2 for asymptomatic patients and a screening test to detect or exclude COVID-19 should be done[8]. In cases where PCR and antigen testing is not available, all patients elected for admission due to emergency and urgent surgery should be considered infected and approached similarly to confirmed patients.
Many hospitals monitor all patients in the preoperative stage. The internal database of the University of North Carolina indicates that the positive test rate for pre-operational COVID-19 is about 0.86% (61 of 7100)[3].
Since the rates of pre-operational positive test results differ by area based on the prevalence of COVID-19 in the population, asymptomatic SARS-CoV-2 patients must be detected, and surgery must be postponed. Thus, this approach benefits the healthcare providers and patients by eliminating excessive exposure to SARS-CoV-2[3].
PERIOPERATIVE MEASURES IN COVID-19 PATIENTS
Firstly, stable and functional operational personnel are essential to provide surgical services. Both healthcare workers must also be adequately protected. At the onset of the pandemic, many healthcare services faced problems due to the lack of proper personal protective equipment. The capacity to defend the medical staff increased with the supply and availability of personal protective equipment. Universal pandemic preparations are in everybody's best interest[9,10]. Protection involves keeping physical distance, wearing a well-fitting mask over the nose and mouth, strict washing, and disinfection of the hands.
Additionally, beneficial practices include face and eye protection in all patient interactions and frequent surface disinfection[9]. These steps limit the propagation of COVID-19 from those that are sick and reduce the risk of obtaining COVID-19 from infected individuals.
In the operating room, the universal use of smoke evacuators is necessary to absorb any droplets produced by electrocautery. This approach decreases the risk of personnel exposure to aerosols[10]. In addition, special measures should be taken for all procedures affecting airways and the gastrointestinal tract. In general, well-established and accepted uniform pandemic measures must be followed[1].
Both healthcare providers in the COVID-19 pandemic must follow the uniform key perioperative acts, including the use of personal protective equipment, to monitor the spread of disease and prevent unintended complications. Patients must be treated as COVID-19-infected in lifesaving operations before verification. Elective treatment should be omitted. Only lifesaving and oncological surgery, whose delay is related to bad or fatal outcomes, should be considered. Laparoscopy is also a legitimate alternative to cope with pneumoperitoneum, cardiopulmonary physiology, and gas deflation for additional security[3].
PERIOPERATIVE MORTALITY RATE DUE TO UNEXPECTED COVID-19
There are limited data reporting the mortality rate due to unexpected COVID-19 in preoperative patients. However, according to a recently published study by Nahshon et al[11], the postoperative mortality rate of patients with COVID-19 during the perioperative period was 27.5%, caused essentially by severe pulmonic complications.
COVID-19 itself carries particular risks in surgical patients. The risk of perioperative morbidity and mortality is raised in both asymptomatic or symptomatic COVID-19 patients. Doglietto et al[12] observed that the 30-d mortality rate for COVID-19 patients undergoing surgery (n = 41) was slightly higher compared with non-COVID-19 patients (n = 82), according to an Italian case-control study (19.51% vs 2.44%; odds ratio [OR]: 9.5; 95% confidence interval [CI]: 1.8-96.5). There were also slightly higher risks for perioperative pulmonary complications (OR: 35.6; 95%CI: 9.3-205.6), such as the chances of thrombotic complications (OR: 13.2; 95%CI: 1.5-∞)[12].
On the other hand, Lei et al[13] reported 20% postoperative deaths and 100% postoperative pneumonia among 34 asymptomatic COVID-19 patients who underwent various surgeries. The average age of the described 34 cases was 55 years, 58.8% of whom were women. All patients developed COVID-19 pneumonia shortly after surgery, shock, arrhythmia, and acute cardiac injury. All patients had more than one accompanying medical condition, with the most common being cardiovascular disease, malignancy, and hypertension. The average duration from the first symptom to death was 9 d. According to the authors, the patients developed the first symptoms of COVID-19 infection very early (in general, 2-6 d) after surgery. Twenty percent of patients died due to COVID-19-related complications. Thus, the mortality rate was reportedly much higher than the reported overall mortality rate of 2-3% in patients without COVID-19, including patients without COVID-19 infection accepted in the intensive care unit due to other severe medical conditions[13].
In their systematic review and meta-analysis, Abate et al[14] showed a high mortality rate of 20% postoperatively among COVID-19 patients and a postoperative intensive care unit admission rate of 15%. In another meta-analysis by Wang et al[15] involving 269 patients from 47 studies, the average age of surgery COVID-19 patients was 50.91 years, with 49% being women. The analysis showed that 28 of the 269 operative patients died perioperatively or because of COVID-19 complications. Thus, the estimated overall mortality rate was 6%. Additionally, most of the patients rapidly developed respiratory failure/acute respiratory distress syndrome (ARDS). These results are consistent with other authors' findings[16,17].
Nevertheless, the outcomes of patients with unexpected coronavirus infection undergoing surgery vary broadly. Studies have shown that in-hospital mortality in patients with COVID-19 is very high, reaching 52% of hospitalized patients.
FACTORS ASSOCIATED WITH SURGICAL COMPLICATIONS AND MORTALITY RATE
In their meta-analysis, Wang et al[15] revealed the most common surgical complications in COVID-19 patients before, during, and after surgery. Postoperative and COVID-19 complications included one or more signs of respiratory failure, ARDS, shortness of breath, dyspnoea, fever, fatigue, weakness or myalgia, cardiopulmonary failure, acute kidney damage, and extreme lymphopenia. These symptoms were associated with significantly higher mortality (r = 0.891, P < 0.001). On the other hand, symptoms such as fever, cough, fatigue, or myalgia demonstrated a lighter impact on the mortality rate (r = 0.675, P = 0.023).
Twenty studies have documented infections in medical professionals and technicians. However, personnel who utilized biosafety level-3 protection was not contaminated. Nevertheless, the authors concluded that COVID-19 patients might have high postoperative mortality, especially those with severe respiratory complications. Therefore, medical personnel directly exposed to infectious patients should take high-level personal protection equipment[15].
In their study, Doglietto et al[12], using different models (cumulative link model and classification tree), demonstrated the risk factors for presurgical complications and death. In total, 33 (80.5%) of 41 patients were tested positive for COVID-19 preoperative, and 8 (19.5%) obtained positive results within 5 d after the surgery. The 30-d mortality of those with COVID-19 was slightly higher among the 123 patients in the combined cohorts (women, at average age 76.6 (14.4) years) compared to control patients without COVID-19 (OR: 9.5; 95%CI: 1.77-96.53). Age was considered a significant factor for developing complications. Each additional year contributed to a 1.04 higher OR for complications. The adjusted analysis demonstrated that 30-d mortality was associated with male sex (OR, 1.75; P < 0,0001), 70 years or older compared to younger patients (OR, 2.30; P < 0.0001), American Society of Anesthesiologists grades 3–5 vs grades 1–2 (OR, 2.35; P < 0.0001), malignant vs benign or obstetric diagnosis (OR, 1.55; P = 0.046), emergency vs elective surgery (OR, 1.67; P = 0.026), and major vs minor surgery (OR, 1.52; P = 0.047)[18], as well as pre-existing co-morbidities (cardiac and pulmonary, hypertension, diabetes). As discussed above, pulmonary and thrombotic complications were considered a significant attributing factor to high mortality. Therefore, surgery and major surgical procedures during the COVID-19, especially for men age 70 years and older, should be more limited in standard practice[18].
Surgery-related death and complications in COVID-19 patients were higher than in non-infected patients. Moreover, these results indicate that operations in patients with COVID-19 should be delayed whenever possible since COVID-19 is the primary factor for complications occurrence[12]. Indeed, the world guidelines have introduced the principle of limiting surgery to only severe and urgent (i.e. cancer).
SURGICAL COMPLICATIONS AND OUTCOMES IN COVID-19 PATIENTS
According to the modest data available in the literature, the most common surgical complications in patients with COVID-19 infection are as follows: ARDS (with a prevalence of about 71.5%)[19], other pulmonary complications leading to respiratory failure (such as pneumonia, shortness of breath, dyspnea, fever, severe cough) in 27.3%[20], cardiovascular complications (i.e. cardiac arrest, arrhythmia, and infarction), secondary infection, fatigue or myalgia, severe lymphopenia, sepsis/shock, and acute kidney injury[17,21,22].
As stated above, the detailed meta-analysis of Wang et al[15] revealed the most common causes of postoperative death in patients with COVID-19, including ARDS. In their scientific report, 28 of the 269 operated patients died from surgery or COVID-19-related complications; thus, the overall mortality rate was estimated as 6%. They also point out that the patient's immune system is a significant determinant of the disease's severity. The stress of surgery can impair immune function and cause a systemic inflammatory response[23].
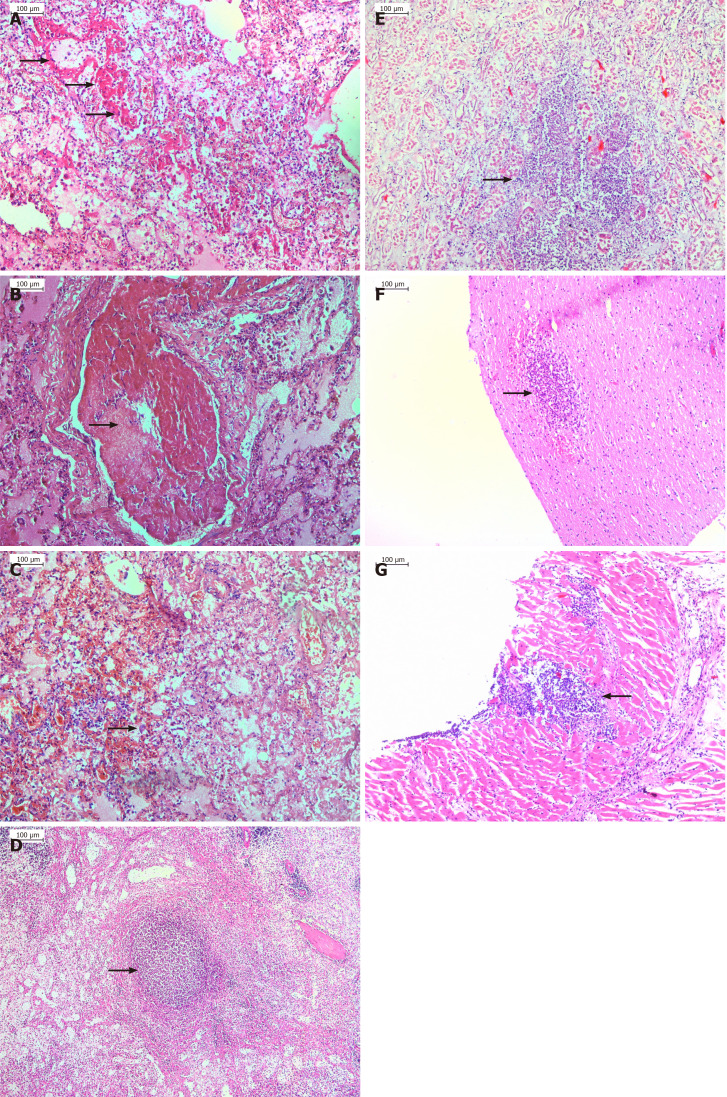
From our experience, the autopsy of patients with postoperatively developed ARDS shows inflammation-mediated damages (necrosis) of the alveolar-capillary endothelium and epithelium and interstitial and intra-alveolar edema (Figure 1A). Additionally, in most patients, histology examination revealed hyaline membranes on alveolar walls or sacs (Figure 1A), micro-thromboembolism (Figure 1B), and areas of hemorrhages (Figure 1C), as well as virus-associated intracellular inclusions in some of the cases. Furthermore, the morphological changes of the organs in sepsis cases are also considered to be a consequence of pathophysiologic changes cause by ARDS (Figure 1A-G). Similarly, the autopsy of patients with myocardial infarction showed typical histological changes expressed as coagulative/ischemic necrosis of the cardiomyocytes, well-developed neutrophilic infiltration, and in some areas, stretching and waviness of the myocardial fibers due to severe ischemia.
Figure 1.
Histology examination. A: Hyaline membranes (marked with an arrow) covering the alveolar walls in a case of septic acute respiratory distress syndrome originating from gangrenous appendicitis in coronavirus disease-2019 patient; B: Microthrombosis resulted in almost complete obstruction of the pulmonary vessel; C: Diffuse alveolar damage with interalveolar hemorrhages and inflammatory cell infiltration, as well as type II hyperplastic pneumocyte (marked with an arrow); D-G: Septicopyemic abscess in the spleen (D, marked with an arrow), kidney (E, marked with an arrow), brain (F, marked with an arrow) and myocardium (G, marked with an arrow); E: Acute tubular necrosis found.
The research article analyses of surgical outcomes allow evaluating the clinical outcomes of patients undergoing urgent or serious surgeries during the COVID-19 pandemic. It was established that the average duration of hospitalization among surgical COVID-19 patients was 10.55 d[24,25]. Once again, the main question in this pandemic situation remains the surgical outcomes in patients with COVID-19 infection[26]. The data indicate a very high rate of intensive care unit admission among surgical COVID-19 patients in the postoperative period - up to 15%[14,23]. Several complications in patients with COVID-19 who underwent surgical procedures are at a rate of about 14%[14]. Pulmonary complications[12,25,27] were responsible for low survival rate and prolonged immobility in surgical COVID-19 patients, contributing to a 30-d mortality rate of 38%[16,18,23,24]. Additionally, cancer patients are more likely to contract COVID-19 than non-cancer patients. They are more vulnerable and have a higher mortality postoperative rate[28].
CONCLUSION
During the COVID-19 pandemic, the surgery disciplines face significant difficulties. The impact of the pandemic on patients for surgery in terms of delay or complications is noteworthy. Additionally, health services have to cope with financial and other pressures without precedent. However, any surgical procedure on COVID-19 patients is related to a substantial elevation in the risk of complications and death. Simultaneously, operations tightened, education and experience of surgeons have improved. Although the future is unpredictable and hard to determine how long this pandemic will last, we can implement the surgical pre-pandemic approaches and be aware of the possible postsurgery outcomes. Many of the improvements adopted after the COVID-19 pandemic will become the current truth that has to be learned and embraced.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: February 7, 2021
First decision: March 16, 2021
Article in press: June 16, 2021
Specialty type: Surgery
Country/Territory of origin: Bulgaria
Peer-review report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rojas A, Xue F S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Milena Gulinac, Department of General and Clinical Pathology, Medical University, University Hospital "St George," Plovdiv 6000, Bulgaria.
Ivan P Novakov, Department of Thoraco-abdominal Surgery, Medical University, Plovdiv 6000, Bulgaria.
Svetozar Antovic, University Clinic for Digestive Surgery, Medical Faculty, Skopje 1000, Macedonia.
Tsvetelina Velikova, Department of Clinical Immunology, University Hospital Lozenetz, Sofia 1407, Bulgaria; Medical Faculty, Sofia University St. Kliment Ohridski, Sofia 1407, Bulgaria. tsvelikova@medfac.mu-sofia.bg.
References
- 1.Kibbe MR. Surgery and COVID-19. JAMA. 2020;324:1151–1152. doi: 10.1001/jama.2020.15191. [DOI] [PubMed] [Google Scholar]
- 2.Dahiya DS, Kichloo A, Albosta M, Pagad S, Wani F. Gastrointestinal implications in COVID-19. J Investig Med. 2020;68:1397–1401. doi: 10.1136/jim-2020-001559. [DOI] [PubMed] [Google Scholar]
- 3.Al-Balas M, Al-Balas HI, Al-Balas H. Surgery during the COVID-19 pandemic: A comprehensive overview and perioperative care. Am J Surg. 2020;219:903–906. doi: 10.1016/j.amjsurg.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brindle ME, Gawande A. Managing COVID-19 in Surgical Systems. Ann Surg. 2020;272:e1–e2. doi: 10.1097/SLA.0000000000003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng MH, Boni L, Fingerhut A. Minimally Invasive Surgery and the Novel Coronavirus Outbreak: Lessons Learned in China and Italy. Ann Surg. 2020;272:e5–e6. doi: 10.1097/SLA.0000000000003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J, Yoo SY, Ko JH, Lee SM, Chung YJ, Lee JH, Peck KR, Min JJ. Infection Prevention Measures for Surgical Procedures during a Middle East Respiratory Syndrome Outbreak in a Tertiary Care Hospital in South Korea. Sci Rep. 2020;10:325. doi: 10.1038/s41598-019-57216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HN, Lan KF, Nkyekyer E, Neme S, Pierre-Louis M, Chew L, Duber HC. Assessment of Disparities in COVID-19 Testing and Infection Across Language Groups in Seattle, Washington. JAMA Netw Open. 2020;3:e2021213. doi: 10.1001/jamanetworkopen.2020.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber DJ, Babcock H, Hayden MK, Wright SB, Murthy AR, Guzman-Cottrill J, Haessler S, Rock C, Van Schooneveld T, Forde CA, Logan LK, Malani A, Henderson DK SHEA Board of Trustees. Universal pandemic precautions-An idea ripe for the times. Infect Control Hosp Epidemiol. 2020;41:1321–1322. doi: 10.1017/ice.2020.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston EH. Surgery in a Time of Uncertainty: A Need for Universal Respiratory Precautions in the Operating Room. JAMA. 2020;323:2254–2255. doi: 10.1001/jama.2020.7903. [DOI] [PubMed] [Google Scholar]
- 11.Nahshon C, Bitterman A, Haddad R, Hazzan D, Lavie O. Hazardous Postoperative Outcomes of Unexpected COVID-19 Infected Patients: A Call for Global Consideration of Sampling all Asymptomatic Patients Before Surgical Treatment. World J Surg. 2020;44:2477–2481. doi: 10.1007/s00268-020-05575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doglietto F, Vezzoli M, Gheza F, Lussardi GL, Domenicucci M, Vecchiarelli L, Zanin L, Saraceno G, Signorini L, Panciani PP, Castelli F, Maroldi R, Rasulo FA, Benvenuti MR, Portolani N, Bonardelli S, Milano G, Casiraghi A, Calza S, Fontanella MM. Factors Associated With Surgical Mortality and Complications Among Patients With and Without Coronavirus Disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155:691–702. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei S, Jiang F, Su W, Chen C, Chen J, Mei W, Zhan LY, Jia Y, Zhang L, Liu D, Xia ZY, Xia Z. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abate SM, Mantefardo B, Basu B. Postoperative mortality among surgical patients with COVID-19: a systematic review and meta-analysis. Patient Saf Surg. 2020;14:37. doi: 10.1186/s13037-020-00262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Wu C, Xu J, Zhang B, Zhang X, Gao Z, Xia Z. Factors affecting the mortality of patients with COVID-19 undergoing surgery and the safety of medical staff: A systematic review and meta-analysis. EClinicalMedicine. 2020;29:100612. doi: 10.1016/j.eclinm.2020.100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeliger B, Philouze G, Cherkaoui Z, Felli E, Mutter D, Pessaux P. Acute abdomen in patients with SARS-CoV-2 infection or co-infection. Langenbecks Arch Surg. 2020;405:861–866. doi: 10.1007/s00423-020-01948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myles PS, Maswime S. Mitigating the risks of surgery during the COVID-19 pandemic. Lancet. 2020;396:2–3. doi: 10.1016/S0140-6736(20)31256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng S, Huang L, Zhao B, Zhou S, Braithwaite I, Zhang N, Fu X. Clinical course of coronavirus disease 2019 in 11 patients after thoracic surgery and challenges in diagnosis. J Thorac Cardiovasc Surg. 2020;160:585–592.e2. doi: 10.1016/j.jtcvs.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knisely A, Zhou ZN, Wu J, Huang Y, Holcomb K, Melamed A, Advincula AP, Lalwani A, Khoury-Collado F, Tergas AI, St Clair CM, Hou JY, Hershman DL, D'Alton ME, Huang YY, Wright JD. Perioperative Morbidity and Mortality of Patients With COVID-19 Who Undergo Urgent and Emergent Surgical Procedures. Ann Surg. 2021;273:34–40. doi: 10.1097/SLA.0000000000004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111–115. doi: 10.4103/0019-5049.79879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amodeo G, Bugada D, Franchi S, Moschetti G, Grimaldi S, Panerai A, Allegri M, Sacerdote P. Immune function after major surgical interventions: the effect of postoperative pain treatment. J Pain Res. 2018;11:1297–1305. doi: 10.2147/JPR.S158230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casanova J, Pissarra D, Costa R, Salgueiro E, Pinho P. Cardiothoracic surgery during the Covid-19 pandemic: Perioperative care, safety, and surgical results. J Card Surg. 2020;35:2605–2610. doi: 10.1111/jocs.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S, Zhang Y, Cai J, Wang Z. Clinical Characteristics of COVID-19 After Gynecologic Oncology Surgery in Three Women: A Retrospective Review of Medical Records. Oncologist. 2020;25:e982–e985. doi: 10.1634/theoncologist.2020-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilmans G, Chenevas-Paule Q, Muller X, Breton A, Mohkam K, Ducerf C, Mabrut JY, Lesurtel M. Surgical outcomes after systematic preoperative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) screening. Surgery. 2020;168:209–211. doi: 10.1016/j.surg.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YK, Peng S, Li LQ, Wang Q, Ping W, Zhang N, Fu XN. Clinical and Transmission Characteristics of Covid-19 - A Retrospective Study of 25 Cases from a Single Thoracic Surgery Department. Curr Med Sci. 2020;40:295–300. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelps DL, Saso S, Ghaem-Maghami S. Analysis of worldwide surgical outcomes in COVID-19-infected patients: a gynecological oncology perspective. Future Sci OA. 2020;6:FS0629. doi: 10.2144/fsoa-2020-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]