Abstract
Hyperbaric oxygen therapy (HBOT) is commonly used as treatment in several diseases, such as non-healing chronic wounds, late radiation injuries and carbon monoxide poisoning. Ongoing research into HBOT has shown that preconditioning for surgery is a potential new treatment application, which may reduce complication rates and hospital stay. In this review, the effect of HBOT on oxidative stress, inflammation and angiogenesis is investigated to better understand the potential mechanisms underlying preconditioning for surgery using HBOT. A systematic search was conducted to retrieve studies measuring markers of oxidative stress, inflammation, or angiogenesis in humans. Analysis of the included studies showed that HBOT-induced oxidative stress reduces the concentrations of pro-inflammatory acute phase proteins, interleukins and cytokines and increases growth factors and other pro-angiogenesis cytokines. Several articles only noted this surge after the first HBOT session or for a short duration after each session. The anti-inflammatory status following HBOT may be mediated by hyperoxia interfering with NF-κB and IκBα. Further research into the effect of HBOT on inflammation and angiogenesis is needed to determine the implications of these findings for clinical practice.
Keywords: hyperbaric oxygen therapy, hyperbaric oxygenation, oxidative stress, inflammation, angiogenesis, neovascularization
1. Introduction
Since the adjunctive use of hyperbaric oxygen therapy (HBOT) was first described in 1879 [1], it has been further explored and is nowadays a widely accepted treatment in several diseases, such as delayed radiation injury, diabetic foot ulcers, carbon monoxide poisoning, decompression sickness and arterial gas embolism [2]. The Undersea and Hyperbaric Medical Society (UHMS) describes HBOT as an intervention whereby patients breathe near 100% oxygen while being pressurized to at least 1.4 atmosphere absolute (ATA) in a hyperbaric chamber [1]. Currently, the UHMS has accepted 14 indications for HBOT [3], yet new applications of HBOT have been described, including preconditioning for surgery [4,5,6,7].
Several cohort studies and randomized controlled trials, executed in different surgical procedures (e.g., abdominoplasty and pancreaticoduodenectomy), reported lower postoperative complication rates and a reduced length of stay on the intensive care unit after preoperative HBOT [4,5,6,7]. As the occurrence of postoperative complications is associated with worse short-term and long-term outcomes [8], a decrease in psychosocial well-being [9] and higher healthcare costs [10], HBOT may prevent those adverse effects of surgery.
To realize this perioperative protective effect, HBOT must be able to prevent infection and increase wound healing. It is likely that oxidative stress, which has been confirmed to be the main effect of HBOT [11], plays an activating role in the mechanisms underlying the therapeutic pathway of preconditioning for surgery with HBOT. An increase in reactive oxygen species (ROS) levels is associated with enhanced pathogen clearance [12]. Furthermore, ROS induce the synthesis of several growth factors, such as vascular endothelial growth factor (VEGF), placental growth factor (PGF) and angiopoietin (Ang) 1 and 2 and recruit stem cells from the bone marrow, which are responsible for neovascularization [13]. However, a frequently mentioned argument against the use of HBOT revolves around the induction of oxidative stress as well, since higher levels of ROS and reactive nitrogen species (RNS) may lead to oxidative and nitrosative damage, mitochondrial aging, genotoxicity and maintenance of (chronic) inflammation [14,15,16].
The aim of this review is to gain more insight into the mechanisms of HBOT by assessing its effect on oxidative stress, inflammation and angiogenesis markers in humans. More insight into these effects of HBOT will predict and underpin the outcome of innovative uses of HBOT and balance its benefits against potential damage. No systematic overview of research into these parameters in human beings has yet been published.
2. Methods
A search of the literature was performed in MEDLINE and EMBASE on 2 November 2020. Key terms used in the search were ‘hyperbaric oxygen’ and ‘oxidative stress’, ‘inflammation’, or ‘wound healing’. The results were not restricted as no filters were applied. The detailed literature search can be found in Appendix A (see Table A1 and Table A2).
All studies found were screened on title and abstract by one reviewer (S.D.D.W.), who excluded those studies that met any of the following criteria: (1) absence of abstract, (2) congress abstract, errata or guideline, (3) case report (defined as five or less patients), (4) narrative review, (5) animal research, (6) no treatment with HBOT, or (7) one of the following outcome measures: cure, complication rate, or a disease-specific outcome parameter. The same reviewer assessed the full-text of the remaining studies. The following inclusion criteria were applied: (1) measurement of at least one marker of oxidative stress, inflammation, or angiogenesis before and after HBOT, (2) study in humans (or human material) and (3) English full-text available. EndNote X9 was used to keep track of the screening process.
The included studies were divided into an “in vivo” and “in vitro” group. In vivo studies were performed in a clinical setting in which all subjects were at least pressurized once, whereas in vitro studies obtained human material what was subsequently exposed to HBOT. Information on first author, publication year, investigated parameters and patient (in vivo)/sample (in vitro) characteristics and results (solely of the parameters of interest) were extracted. Outcomes of statistical tests with a p-value < 0.05 were considered significant. All information was extracted by hand and documented in Microsoft Excel (v16.0).
3. Results
3.1. Eligible Studies
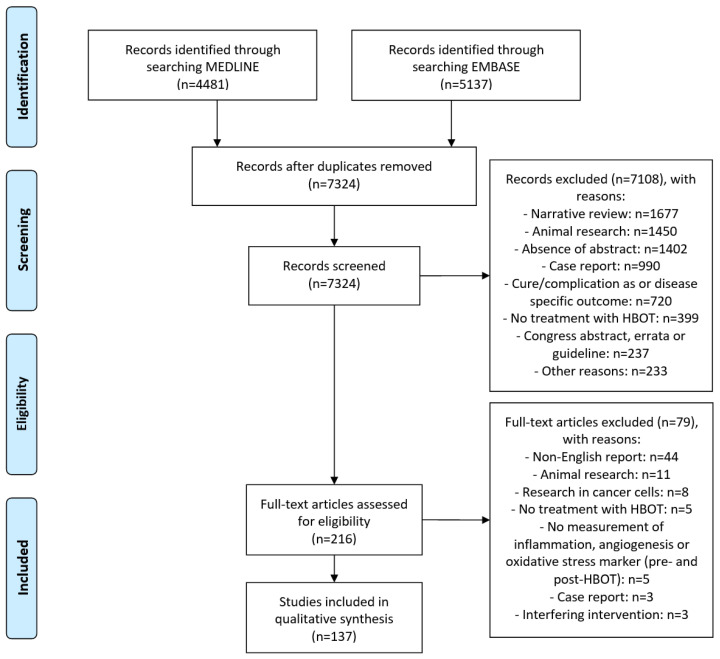
The search retrieved 9618 records. After removing duplicates and screening of title and abstract, 216 studies were screened full-text. Finally, 137 articles were included in this review (see Figure 1). Most of the included articles were clinical studies (n = 98) and performed in patients with diabetes mellitus and/or non-healing chronic wounds (n = 27). Furthermore, 27 articles describing the effect of HBOT in healthy volunteers (including divers) were found. Sixteen included studies reported on other biomarkers than described in Table 1, Table 2 and Table 3 (data not shown) [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
Figure 1.
PRISMA Flow Diagram of the selection progress.
Table 1.
The effect of HBOT on oxidative stress markers.
| Main Aspect | Associated Markers | Stimulating Effect |
No Effect | Inhibiting Effect |
|---|---|---|---|---|
| Causers of oxidative stress |
Reactive oxygen species (including superoxide-ion and hydrogen peroxide) | [33,34,35,36,37,38,39,40,41] | [33,37,38,42,43] | [33,42,43,44] |
| Nitric oxide synthase (NOS) (including endothelial NOS and inducible NOS) | [34,45,46,47,48] | [49,50,51] | [52,53,54] | |
| Reactive nitrogen species (including nitric oxygen, nitrite and nitrate) | [33,34,45,49,55,56,57,58,59,60] | [33,46,61,62,63,64,65,66,67,68] | [53,61,67,68,69,70,71,72,73] | |
| Hydrobenzoates | [74,75] | [74] | ||
| Free fatty acid | [53] | |||
| Myeloperoxidase | [34,62] | |||
| Lipid peroxidation | Isoprostanes | [76,77] | [78,79] | |
| Isofurans | [78] | |||
| Malondialdehyde | [56,77,80,81,82] | [14,34,49,55,76,83] | [62,84] | |
| Thiobarbituric acid reactive substances | [63,74,85,86,87] | [33,63,85,88,89,90] | ||
| Lipid hydroperoxides | [36] | [91] | [92] | |
| Oxidized low-density lipoprotein | [82] | |||
| Protein peroxidation |
Nitrotyrosine | [49] | [64] | |
| Advanced oxidation protein products | [77] | |||
| Carbohydrate peroxidation |
Protein carbonyls | [93] | ||
| Carbonyl group | [56] | |||
| Protein carbonyl derivates | [55] | |||
| Plasma carbonyl proteins | [83] | [82] | ||
| DNA/RNA damage |
8-hydroxydeoxyguanosine | [83] | [82] | |
| Tail moment | [38,94,95,96] | [76,97,98] | ||
| Sister chromatid exchange | [14] | |||
| Gene expression | Nuclear factor erythroid 2- related factor 2 | [45] | ||
| Other residues of oxidative stress | Reactive oxygen metabolites | [80] | ||
| Intracellular calcium concentration | [43] | |||
| Antioxidants | Total antioxidant capacity | [91,93,94,99] | ||
| Catalase | [33,34,43,45,55,62,81,100] | [43,55,62,63,76,83,89,94,97] | [63,80,85] | |
| Superoxide dismutase | [55,56,81,84,100,101] | [14,33,55,62,63,76,83,89,94,97,102] | [80,85,86] | |
| Glutathione | [92] | [76,80,83,94] | ||
| Glutathione disulfide | [76,101] | |||
| Glutathione reductase | [55,62] | |||
| Glutathione peroxidase | [34,63,82,85] | [14,55,62,63,76,80,83,89,94,100] | [63] | |
| Thiols | [88] | [93] | ||
| Vitamin A | [80,94] | |||
| Vitamin C | [94] | |||
| Vitamin E | [80,94] | |||
| Uric acid | [91,102] | [103] | ||
| Heme oxygenase-1 | [45,95,96,97] | |||
| NAD(P)H dehydrogenase [quinone] 1 | [45] |
Table 2.
The effect of HBOT on inflammation markers.
| Main Aspect | Associated Markers | Stimulating Effect | No Effect | Inhibiting Effect |
|---|---|---|---|---|
| Acute-phase proteins | (high-sensitivity) C-reactive protein | [88,101,104,105] | [45,53,58,59,60,84,101,103,106,107,108,109] | |
| Granulocyte-colony stimulating factor | [110] | [110,111,112] | ||
| Ferritin | [96] | |||
| Insulin-like growth factor-1 | [113] | [67,114,115] | [114,115] | |
| Albumin | [116] | [102,117] | ||
| Interleukins (IL) | IL-1α | [45] | [111,112] | |
| IL-1β | [35,87,111,112,118,119] | [71,72,73,120,121,122] | ||
| IL-1Ra | [111] | [123] | ||
| IL-1 | [4] | [124] | ||
| IL-4 | [111,112,118,125,126] | |||
| IL-6 | [62,123,125] | [87,104,105,111,112,118,123,127,128] | [4,35,122,124,129,130,131,132] | |
| IL-8 | [45] | [4,67,105,110,111,112,128] | [46,105,127] | |
| IL-10 | [45] | [106,107,113,114,120,127,128] | [4,105] | |
| Interferons (IFN) | IFN-α | [111,112] | ||
| IFN-γ | [118] | [111,112,125,126] | [45,133] | |
| Cytokines | Tumor necrosis factor-α | [87,127,134] | [4,45,111,112,128,135,136] | [35,84,105,119,120,121,123,124,129,131,132,133,137,138] |
| Nuclear factor kappa B | [139] | [125] | [52,53,125,132,137,140] | |
| Others | Erythrocyte sedimentation rate | [107] | [106,108] |
Table 3.
The effect of HBOT on angiogenesis markers.
| Main Aspect | Associated Markers | Stimulating Effect |
No Effect | Inhibiting Effect |
|---|---|---|---|---|
| Growth factors/ cytokines |
Vascular endothelial growth factor | [45,62,84,125,130,138,141,142] | [50,51,113,120,127,137,143,144,145,146] | [132] |
| (basic) Fibroblast growth factor | [45,141] | [102,111,147,148] | [141,143] | |
| Platelet-derived growth factor | [45,111] | [111] | ||
| Insulin-like growth factor-binding protein | [125] | [67,125] | [67] | |
| Epidermal growth factor | [45] | [50,111,135,136] | ||
| Insulin-like growth factor-2 | [67] | |||
| Hematopoietic growth factor | [130,141] | [136] | ||
| Keratinocyte growth factor | [141] | |||
| Placental growth factor | [40,141] | [141] | ||
| Tumor growth factor-α | [111] | |||
| Tumor growth factor-β | [112,147,148] | [115,136] | ||
| Angiopoietin | [84] | [144] | ||
| Erythropoietin | [145] | |||
| Granulocyte-macrophage colony-stimulating factor | [111,112] | |||
| Stromal cell-derived factor-1α | [130] | |||
| Cytokine receptors |
Tie-2 | [144] | ||
| Erythropoietin-receptor | [37] | |||
| Proteases | Matrix metalloproteinase-3 | [52,54,72] | ||
| Matrix metalloproteinase-9 | [52] | |||
| Matrix metalloproteinase-13 | [52] | |||
| Transcription factors | Hypoxia-inducible factor-1α | [125] | [47,130,132] | |
| Downstream effectors | Phosphatidylinositol-3 kinase (PI3K) | [48] | ||
| AKT | [48] | [53] | ||
| p38 mitogen-activated protein kinase (p38 MAPK) | [44,52,71,72] | |||
| Extracellular signal-regulated kinase (ERK) | [142] | [44] | [52,53,71] |
3.2. Oxidative Stress
In total, 74 articles reporting on the effect of HBOT on oxidative stress were found. Subjects mainly received one session of HBOT in a hyperbaric chamber pressurized to 2–2.5 ATA (203–253 kPa), yet in seven studies a wet exposure to hyperbaric oxygen (i.e., a dive) to up to 6 ATA (608 kPa) was employed. Nearly 40% (n = 21) of the clinical studies were conducted in healthy volunteers (see Table A3). Catalase, glutathione peroxidase (GPx), malondialdehyde (MDA), nitric oxide synthase (NOS), ROS, RNS, superoxide dismutase (SOD) and thiobarbituric acid reactive substances (TBARS) were the most frequent markers of interest (see Table 1).
A clear stimulating effect of HBOT on ROS (see Table 1) was found. Nonetheless, two out of the three studies assessing hydrogen peroxide described lower concentrations after HBOT [33,42] (see Table A3). NOS and RNS concentrations seem to increase after HBOT as well, although this effect was less pronounced, which can be explained by a repeatedly reported decrease in exhaled nitric oxygen [61,69,70]. Timing of sampling may also play a role, as several articles only noted an increase in inducible NOS or nitrite three hours after the end of an HBOT session [34,49,55].
Not only the presence of NOS, RNS and ROS has been investigated, but also their effects on lipids, proteins, carbohydrates and DNA/RNA (see Table 1). Little research has been done regarding protein and carbohydrate modifications following HBOT, but no effect or a stimulating effect on lipid peroxidation, resulting in MDA and other aldehydes (TBARS), has been reported in various studies. DNA-damaging effects of HBOT were not demonstrated employing the most commonly used DNA-lesion-marker 8-hydroxydeoxyguanosine [146].
Concerning the concentrations of anti-oxidative enzymes that protect against the potentially harmful effects of oxidative stress, such as catalase, SOD and GPx, conflicting results were found (see Table 1). In general, no effect or an indication for an increasing effect of HBOT on the enzyme activity of those antioxidants has been demonstrated. HBOT may have a uniform effect on SOD and catalase, as most of the studies reported increased, decreased, or stable SOD and catalase levels and, thus, no differences in effect of HBOT between these two enzymes [76,80,81,83,85,94,97,100]. However, a difference between SOD and/or catalase concentrations in respectively plasma and erythrocytes has been reported [55,62,63]. Benedetti et al. [80] and Dennog et al. [94] describe no effect of HBOT on the free radical trapping anti-oxidants with an exogenous origin, such as vitamin A, vitamin C and vitamin E [149].
3.3. Inflammation
Of the 140 studies included, 58 articles describing inflammatory markers were identified. Most of the research included at least three HBOT-sessions, yet study protocols consisting of 20–40 sessions were common, in particular in articles reporting acute-phase proteins (see Table A4). Popular variables of interest were interleukins (IL) (n = 31), acute-phase proteins (n = 26) and tumor necrosis factor-α (TNF-α) (n = 25) (see Table 2).
Concerning acute phase proteins, a decreasing effect of HBOT on (high-sensitivity) C-reactive protein ((hs-)CRP) was found as 75% (n = 12) of the studies investigating (hs-) CRP reported lower concentrations post-HBOT. Strikingly, HBOT may have a stimulating impact on granulocyte-colony stimulating factor and an inhibiting effect on insulin-like growth factor-1, both reflecting a pro-inflammatory state [150] (see Table 2).
No impact of HBOT on most interleukin concentrations (IL-2, IL-3, IL-5, IL-7, IL-9, IL-12p70, IL-13, IL-15, IL-17, IL-18 and IL-22) has been demonstrated, although Hao et al. [111] reported a decrease in IL-12p40 levels (see Table A4). Concerning the proinflammatory interleukins, a potentially inhibiting effect of HBOT on IL-1β, IL-6 and IL-8 was found, whereas Dhadmodharan et al. [45] suggested an increase in IL-1α levels. On the other hand, a rise in the anti-inflammatory IL-1Ra was reported, alongside a possible inhibiting effect of HBOT on IL-10 and no effect on IL-4. Both results support an anti-inflammatory state (see Table 2) [151].
In line with the outcomes regarding (hs-)CRP and interleukins, an anti-inflammatory effect of HBOT was also shown by decreasing levels of the pro-inflammatory cytokines interferon-γ (IFN-γ), nuclear factor kappa B (NF-κB) and TNF-α (see Table 2). However, HBOT may have an initial pro-inflammatory effect, as some studies described an increase in TNF-α during or shortly after HBOT [87,127,134].
3.4. Angiogenesis
Concerning the angiogenesis research, 34 studies were found in addition to the earlier mentioned studies reporting on interleukins, interferons, insulin-like growth factor 1 (IGF-1), NF- κB and TNF-α. Most of the articles described angiogenesis-inducing cytokines or growth factors and were performed in clinical setting (n = 20). However, five out of seven studies on downstream effectors of angiogenesis were conducted in vitro (see Table A5). Epidermal growth factor (EGF), extracellular signal-regulated kinase (ERK), (basic) fibroblast growth factor, tumor growth factor-β (TGF-β), VEGF, IFN-γ, IL-6, IL-8, NF-κB and TNF-α (see Table 2 and Table 3) were the only angiogenesis markers reported in at least five articles.
HBOT most likely has a stimulating effect on various growth factors involved in angiogenesis (i.e., EGF, hematopoietic growth factor, keratinocyte growth factor, PGF and VEGF). This effect may only be present shortly after the intervention, since several studies with repeated HBOT sessions described no differences in pre-HBOT values or only a raise after the first session (and not after following sessions) [62,141,147] (see Table A5). Whereas for some angiogenesis-stimulating cytokines, such as stromal cell-derived factor-1α, a similar increasing effect of HBOT was found, no or an inhibiting effect on TGF was seen. HBOT seems not to affect the cytokine receptors (see Table 3).
HBOT decreased matrix metalloproteinases (MMPs) [52,54,72]. According to Niu et al. [52,72], the effect on MMPs is delayed and only manifests after two or three HBOT sessions. Hypoxia-inducible factor-1α (HIF-1α) and NF-κB were inhibited by HBOT (see Table 2 and Table 3), although Anguinano-Hernandez et al. [125] described an increase in NF-κB in the cytosol.
As HBOT causes an increase in angiogenesis-promoting growth factors and cytokines, one would also expect a stimulating effect on the downstream effectors of blood vessel formation. However, inconsistent outcomes were reported (see Table 3). The phosphatidylinositol-3 kinase (PI3K)/AKT pathway was upregulated and the ERK and p38 mitogen-activated protein kinase (p38 MAPK) pathways were downregulated. Therefore, HBOT effects on downstream effectors of blood vessel formation seem to differ depending on the intracellular effector route.
4. Discussion
This review is the first to systematically summarize the effect of HBOT on oxidative stress, inflammation and angiogenesis markers in human beings. HBOT increases the levels of oxygen radicals, which induce oxidative stress. An anti-inflammatory action of HBOT was demonstrated by decreasing concentrations of several pro-inflammatory markers. Furthermore, HBOT seems to stimulate the release of angiogenesis-promoting cytokines, including growth factors.
In the light of previous research, reporting a link between oxidative stress and a pro-inflammatory state [152,153,154], it is remarkable that HBOT leads to a more anti-inflammatory state. However, these findings do correspond with studies into the effects of HBOT using thermal imaging, in which a decrease in wound temperature was found [155,156]. This temperature reduction could indicate a local decline in inflammation. This anti-inflammatory effect is likely mediated by the inhibition of NF-κB, a transcription factor for pro-inflammatory genes [157,158,159]. A direct anti-inflammatory action of HBOT seems less probable, since no differences in the concentrations of anti-inflammatory markers (except IL-1Ra) were noted. Although beyond the scope of this review, Yu et al. [160] have shown in an animal model that HBOT decreases the NF-κB concentrations by higher release of IκBα, which is an inhibitor of NF-κB and degrades under hypoxic circumstances [161]. An increase in IκBα along with a decrease in NF-κB after HBOT was also seen in the only study in the current review reporting on IκBα [52]. Therefore, hyperoxia generated during HBOT may stimulates the preservation of IκBα and thereby inhibits NF-κB release, resulting in less gene transcription of pro-inflammatory cytokines and, thus, an anti-inflammatory state despite oxidative stress.
NF-κB is not only a crucial transcription factor in inflammation, but also plays a role, together with HIF-1α, in the induction of angiogenesis. Growth factors and other angiogenesis-promoting cytokines induce new vessel formation by increased expression of pro-angiogenesis genes, which is mediated by NF-κB or (under hypoxia) HIF-1α [162,163]. Since the current review demonstrates an inhibiting effect of HBOT on both transcription factors and little research, with contradicting outcomes, into the downstream effectors of angiogenesis (i.e., PI3K, Akt, p38 MAPK, ERK) has been done, it is unclear how increased levels of pro-angiogenesis growth factors and cytokines actually induce increased tube formation, as shown by Anguiano-Hernandez et al. [125], Lin et al. [130] and Shyu et al. [40]. Thus, further research into the relation between NF-κB, HBOT and the angiogenesis pathways is needed.
Another striking finding concerning angiogenesis is that several articles reported an increase in growth factors only or particularly after the first HBOT session [40,141,147], while it is common to conduct 20–40 sessions for chronic non-healing wounds or radiation-induced tissue injury (indications strongly relying on the angiogenesis effects of HBOT) [2]. Furthermore, Sureda et al. [62] describe, in the only in vivo study assessing the effect of HBOT on growth factors at several time points during follow-up, an increase in VEGF immediately after each session, yet VEGF levels determined pre-session #5 and #20 were similar to the baseline (pre-session #1) value. Those findings possibly suggest a short pro-angiogenesis effect of HBOT. However, due to a shortage of studies reporting on angiogenesis markers on a daily or weekly basis during a treatment protocol including 20–40 sessions, it remains unclear which markers are involved in this short-term effect of HBOT and whether other factors play a role in this angiogenesis process.
The aim of this review was to gather a comprehensive overview of the effects of HBOT on oxidative stress, inflammation and angiogenesis. We must conclude that existing research does not allow for a complete understanding of the physiology underlying new promising treatment modalities for HBOT, such as preconditioning for surgery. Due to the heterogeneity of included patient populations and the inclusion of studies in healthy volunteers, it is difficult to extrapolate findings to the surgical patient in general. Furthermore, this review did not focus on clinical outcomes related to inflammation, angiogenesis and oxidative stress, making it impossible to determine the implications of the described findings in practice. In conclusion, hyperoxia and oxidative stress induced by HBOT affect inflammation and angiogenesis markers, but whether hyperoxia and oxidative stress induce a clinically relevant decrease in inflammation and increase in angiogenesis remains unclear and needs to be further investigated before innovative interventions can be widely applied.
Appendix A
Table A1.
Search strategy used in PubMed (MEDLINE).
| Key Terms | Mesh-Terms | Title/Abstract-Terms |
|---|---|---|
| Hyperbaric oxygen | hyperbaric oxygenation[MeSH] | hyperbaric oxygen[tiab] OR hyperbaric oxygenation[tiab] OR hyperbaric oxygen therapy[tiab] OR hyperbaric oxygen therapies[tiab] OR HBO[tiab] OR HBOT[tiab] OR hyperbaric medicine[tiab] |
| AND | ||
| Inflammation | inflammation[MeSH] OR inflammation mediators[MeSH] OR autacoids[MeSH] OR chemokines[MeSH] OR synthetic prostaglandins[MeSH] OR interleukin[MeSH] OR infection[MeSH] | inflammation[tiab] OR inflammations[tiab] OR inflammatory[tiab] OR inflammatory respons[tiab] OR inflammation mediators[tiab] OR mediators of inflammation[tiab] OR chemokines[tiab] OR slow reacting substances[tiab] OR chemotactic cytokines[tiab] OR synthetic prostaglandins[tiab] OR intercrines[tiab] OR PG analogs[tiab] OR prostaglandin analogues[tiab] OR prostaglandin analogs[tiab] OR interleukin[tiab] OR infection[tiab] OR infection and infestation[tiab] OR infections and infestations[tiab] OR infestation and infection[tiab] OR infestations and infections[tiab] |
| OR | ||
| Wound healing | wound healing[MeSH] OR re-epithelialization[MeSH] OR angiogenesis modulating agents[MeSH] OR neovascularization, physiologic[MeSH] OR cell proliferation[MeSH] | woundhealing[tiab] OR wound healing[tiab] OR cicatrization[tiab] OR re-epithelialization[tiab] OR wound epithelialization[tiab] OR angiogenesis[tiab] OR angiogenesis modulating agents[tiab] OR vasculogenesis[tiab] OR blood vessel formation[tiab] OR bloodvessel formation[tiab] OR neovascularization[tiab] OR neovascularisation[tiab] OR cell proliferation[tiab] OR endothelial proliferation[tiab] OR vascular proliferation[tiab] |
| OR | ||
| Oxidative stress | Oxidative stress[MeSH] OR nitrosative stress[MeSH] OR reactive oxygen species[MeSH] OR reactive nitrogen species[MeSH] | oxidative stress[tiab] OR nitrosative stress[tiab] OR reactive oxygen species[tiab] OR reactive nitrogen species[tiab] OR peroxide[tiab] OR peroxides[tiab] OR superoxide[tiab] OR superoxides[tiab] OR hydroxy radical[tiab] OR hydroxy radicals[tiab] OR singlet oxygen[tiab] OR alpha-oxygen[tiab] OR nitric oxide[tiab] OR peroxynitrite[tiab] OR nitrogen dioxide[tiab] OR oxidant stress[tiab] OR reactive oxygen metabolite[tiab] OR reactive oxygen metabolites[tiab] OR reactive nitrogen metabolite[tiab] OR reactive nitrogen metabolites[tiab] |
Table A2.
Search strategy used in Ovid (EMBASE).
| Key-Terms. | Emtree-Terms | Title/Abstract/Author Keywords-Terms |
|---|---|---|
| Hyperbaric oxygen | hyperbaric oxygen/ OR hyperbaric oxygen therapy/ | hyperbaric oxygen therapy.ti,ab,kw OR hyperbaric oxygen therapies.ti,ab,kw OR HBOT.ti,ab,kw OR hyperbaric oxygenation.ti,ab,kw OR hyperbaric oxygen.ti,ab,kw OR HBO.ti,ab,kw OR hyperbaric medicine.ti,ab,kw |
| AND | ||
| Inflammation | Inflammation/ OR inflammation autocoid/ OR chemokine/ OR chronic inflammation/ OR inflammation/ OR cytokine/ OR infection/ | inflammation.ti,ab,kw OR inflammations.ti,ab,kw OR inflammatory.ti,ab,kw OR inflammatory respons.ti,ab,kw OR inflammation mediators.ti,ab,kw OR mediators of inflammation.ti,ab,kw OR chemokines.ti,ab,kw OR slow reacting substances.ti,ab,kw OR chemotactic cytokines.ti,ab,kw OR synthetic prostaglandins.ti,ab,kw OR intercrines.ti,ab,kw OR PG analogs.ti,ab,kw OR prostaglandin analogues.ti,ab,kw OR prostaglandin analogs.ti,ab,kw OR interleukin.ti,ab,kw OR infection.ti,ab,kw OR (infection and infestation).ti,ab,kw OR (infections and infestations).ti,ab,kw OR (infestation and infection).ti,ab,kw OR (infestations and infections).ti,ab,kw |
| OR | ||
| Wound healing | wound healing/ OR epithelialization/ OR angiogenesis/ OR cell proliferation/ | woundhealing.ti,ab,kw OR wound healing.ti,ab,kw OR cicatrization.ti,ab,kw OR re-epithelialization.ti,ab,kw OR wound epithelialization.ti,ab,kw OR angiogenesis.ti,ab,kw OR neovascularization.ti,ab,kw OR neovascularisation.ti,ab,kw OR (angiogenesis modulating agents).ti,ab,kw OR vasculogenesis.ti,ab,kw OR blood vessel formation.ti,ab,kw OR bloodvessel formation.ti,ab,kw OR cell proliferation.ti,ab,kw OR endothelial proliferation.ti,ab,kw OR vascular proliferation.ti,ab,kw |
| OR | ||
| Oxidative stress | oxidative stress/ OR nitrosative stress/ OR reactive oxygen metabolite/ OR reactive nitrogen species/ | oxidative stress.ti,ab,kw OR nitrosative stress.ti,ab,kw OR reactive oxygen species.ti,ab,kw OR reactive nitrogen species.ti,ab,kw OR peroxide.ti,ab,kw OR peroxides.ti,ab,kw OR superoxide.ti,ab,kw OR superoxides.ti,ab,kw OR hydroxy radical.ti,ab,kw OR hydroxy radicals.ti,ab,kw OR singlet oxygen.ti,ab,kw OR alpha-oxygen.ti,ab,kw OR nitric oxide.ti,ab,kw OR peroxynitrite.ti,ab,kw OR nitrogen dioxide.ti,ab,kw OR oxidant stress.ti,ab,kw OR reactive oxygen metabolite.ti,ab,kw OR reactive oxygen metabolites.ti,ab,kw OR reactive nitrogen metabolite.ti,ab,kw OR reactive nitrogen metabolites.ti,ab,kw |
Table A3.
Retrieved results on oxidative stress markers in detail.
| Study | Year | Design | Subjects | Methods | Type oxidative Stress Marker | Outcome | Remarks | ||
|---|---|---|---|---|---|---|---|---|---|
| Amount of Sessions | Maximum Pressure (ATA) a | Moment of Sample Taking b | |||||||
| Ansari et al. [100] | 1986 | In vivo | 18 patients; multiple sclerosis | 20 | 2 | Within 30 min post-HBOT | Catalase, GPx, SOD | Catalase: increase SOD: increase GPx: no significant differences were found |
No effect of HBOT was seen in the group breathing chamber air at 2 ATA |
| Bearden et al. [86] | 1999 | In vivo | 10 divers | 1 | 1.4–1.5 (dive) | Within 15 min post-dive | SOD, TBARS | SOD: decrease TBARS: increase |
|
| Benedetti et al. [80] | 2004 | In vivo | 12 patients; several pathological conditions | 15 | 2.5 | Immediately post-session #1 and #15 | Catalase, glutathione, GPx, MDA, reactive oxygen metabolites, retinol (vitamin A), SOD, α-tocopherol (vitamin E) | Catalase: decrease MDA: increase Reactive oxygen metabolites: increase SOD: decrease Glutathione, GPx, retinol (vitamin A), a-tocopherol (vitamin E): no significant differences were found |
Reported outcomes are based on a comparison of the pre-session values of sessions #1 and #15. No significant differences in post-session values were found |
| Bosco et al. [35] | 2018 | In vivo | 23 patients; unilateral femoral head necrosis | 60 | 2.5 | Post-session #15, #30, and #60 and pre-session #31 | ROS | Increase at post-session #15, post-session #30 and pre-session #31 | |
| Boykin et al. [57] | 2007 | In vivo | 6 patients; chronic non-healing wound | 20 | 2 | Post-session #10, post-session #20, 1 week post-HBOT, and 4 weeks post-HBOT | Nitric oxygen | Increase at 1 week post-HBOT and 4 weeks post-HBOT | |
| Burgos et al. [91] | 2016 | In vivo | 12 young soccer players | 15 | 2 | Pre- and post-session #5, #10, and #15 | Antioxidant capacity, lipid hydroperoxides, uric acid | No significant differences were found | Results are possible influenced by exercising during HBOT |
| Chang et al. [102] | 2020 | In vivo | 10 healthy male volunteers | 1 | 2.8 | 30 min, 2 days, and 1 week post-HBOT | Uric acid | No significant differences were found | |
| Chen et al. [36] | 2011 | In vitro | Blood samples of healthy males | 1 | 1.5, 2, and 2.5 | ? | Lipid peroxides, superoxide-ion | Lipid peroxides: increase Superoxide-ion: increase |
|
| Chen et al. [88] | 2018 | In vivo | 50 patients; acute non-cardioembolic stroke | 1 | 2.5 | 1 month post-HBOT | TBARs, thiols | No significant differences were found | |
| Chen et al. [67] | 2007 | In vivo | 31 patients with diabetes mellitus type 2 and 29 healthy volunteers | 3 | 2.5 | Immediately post-session #1 and post-session #3 | Nitric oxygen | Decrease in diabetes mellitus group | |
| Cheung et al. [37] | 2018 | In vitro | Umbilical cord blood enriched with CD34-cells | 1 | 2.5 | 24 h post-HBOT | ROS | Increase in nucleus and mitochondria | No significant differences were found in the cytoplasm |
| Corcoran et al. [78] | 2017 | In vivo | 12 patients; osteonecrosis secondary to radiation therapy | 1 | 2.4 | During HBOT, immediately post-HBOT, and 30 min post-HBOT | Isofurans, isoprostanes | No significant differences were found | |
| Dejmek et al. [164] | 2018 | In vitro | Human fetal lung fibroblasts | 5 | 3 | ? | SOD | Increase | |
| Dennog et al. [94] | 1999 | In vivo | Healthy volunteers | 1 | 2.5 | Immediately and 24 h post-HBOT | Catalase, glutathione, GPx, SOD, tail moment, total antioxidant capacity, vitamin A, vitamin C, vitamin E | Tail moment: increase Catalase, glutathione, GPx, SOD, total antioxidant capacity, vitamin A, vitamin C, vitamin E: no significant differences were found |
|
| Dhamodharan et al. [45] | 2019 | In vivo | 37 patients; diabetic foot ulcer | 25 | 2.2 | 20 days after the first HBOT-session | Catalase, endothelial NOS, heme oxygenase-1, NAD(P)H dehydrogenase [quinone] 1, nitrite, nuclear factor erythroid-2 related factor 2 | Catalase: increase Endothelial NOS: increase Heme oxygenase-1: increase NAD(P)H dehydrogenase [quinone] 1: increase Nitrite: increase Nuclear factor erythroid-2 related factor 2: increase |
|
| Dise et al. [92] | 1987 | In vivo | Adult male volunteers | 1 | 3 | Within 60 min post-HBOT and 24 h post-HBOT | glutathione, lipid hydroperoxides | Glutathione: increase Lipid hydroperoxides: decrease |
|
| Dragic et al. [65] | 2020 | In vivo | 64 patients; peripherial arterial disease | 10 | 2.2 | Post-session #10 | Nitric oxygen | No significant differences were found | |
| Eken et al. [14] | 2005 | In vivo | 15 patients | 20 | 2.5 | Immediately post-session #1, #10, and #20 | GPx, MDA, sister chromatide exchange, SOD | Sister chromatide exchange: increase GPx, MAD, SOD: no significant differences were found |
|
| Ferrer et al. [34] | 2007 | In vivo | 7 male divers and 12 male physically active volunteers | 1 | 5 (dive)/2.2 | Immediately and 3 h post-dive/30 min post-HBOT | catalase, GPx, hydrogen peroxide, inducible NOS, MDA, myeloperoxidase, nitrite | Catalase: increase 3 h post-dive GPx: increase Hydrogen peroxide: increase 3 h post-dive and post-HBOT Inducible NOS: increase 3 h post-dive Myeloperoxidase: decrease 3 h post-dive Nitrite: increase 3 h post-dive MDA: no significant differences were found |
|
| Gasier et al. [63] | 2013 | In vivo | 12 healthy male divers | 3 | 1.5/2 | 15 min, 1 h, and 2 h after each session | Catalase, GPx, nitrite, SOD, TBARS | Catalase: decrease in erythrocytes post-HBOT at 2 ATA and 1 h post-HBOT at 1.5 ATA GPx: increase in erythrocytes post-HBOT at 1.5 ATA and a decrease in erythrocytes post-HBOT at 2 ATA TBARS: increase in erythrocytes 15 min post-HBOT at 1.5 ATA Nitrite, SOD: no significant differences were found |
No significant differences in catalase, GPX, and TBARS were found in the plasma |
| Grimberg-Peters et al. [44] | 2016 | In vitro | Neutrophils from severely injured patients and healthy volunteers | 1 | 2 | ? | ROS | Decrease | After 3 h stimulation with PMA |
| Gröger et al. [38] | 2009 | In vitro | Lymphocytes from combat swimmers, divers, and nondiving volunteers | 1 | 4 | Immediately, 1 h, and 2 h post-HBOT | Superoxide-ion, tail moment | Superoxide-ion: increase Tail moment: increase immediately post-HBOT |
No increase in superoxide radical was seen in the combat swimmers group, which had high baseline superoxide radical levels. Superoxide radical has been measured only once (at which measurement point is unknown) |
| Gronow et al. [74] | 2005 | In vivo | 28 divers and 10 volunteers | 1 | 1.7 (dive)/2.8 | ? | Hydrobenzoates, TBARS | Hydrobenzoates: increase TBARS: increase |
No significant differences were found concerning monohydrobenzoates |
| Gurdol et al. [68] | 2010 | In vivo | 18 patients; diabetic foot ulcers | 25/30 | 2.4 | Post session #25/#30 | Nitric oxygen | No significant differences were found | A decrease in NO levels post-HBOT was seen in the group with <50% wound healing, which had significantly higher baseline NO values |
| Gürdöl et al. [77] | 2008 | In vivo | 20 patients; type 2 diabetic with foot ulcers | 15 | 2.4 | 30 min post-session #1 and #15 | Advanced oxidation proteins products, isoprostanes, MDA | Advanced oxidation proteins products: decrease at pre-session #15 Isoprostanes: increase post-session #15 MDA: increase post-session #1 |
|
| Handy et al. [99] | 2005 | In vivo | 31 patients; non-healing wounds | 20 | 2.2 | Immediately post-session #1 and #20 | Total antioxidant capacity | No significant differences were found | |
| Kähler et al. [75] | 2013 | In vivo | 118 volunteers | 1 | 2.4/2.8 | ? | Dihydroxylated benzoate | Increase | Administration of 100% oxygen significantly increased the dihydroxylated benzoate levels, yet pressurization had no extra effect. |
| Karadurmus et al. [103] | 2010 | In vivo | 28 patients; diabetic foot ulcers | 30 | 2.4 | Post-session #10, #20, and #30 | Uric acid | Decrease | |
| Kendall et al. [46] | 2012 | In vitro | Human umbilical vein endothelial cells | 1 | 2.4 | Immediately, 5 h, and 22.5 h post-HBOT | Endothelial NOS, nitrate + nitrite, nitric oxygen | Endothelial NOS: increase Nitrate + nitrite, nitric oxygen: no significant differences were found |
|
| Kendall et al. [42] | 2013 | In vitro | Human umbilical vein endothelial cells | 1 | 2.4 | ? | Hydrogen peroxide, superoxide-ion | Hydrogen peroxide: decrease Superoxide-ion: no significant differences were found |
|
| Kot et al. [93] | 2003 | In vivo | 96 healthy volunteers | 1 | 2.8 | Immediately post-HBOT | Protein carbonyls, total antioxidant status, total thiol | Protein carbonyls: increase Total thiol: decrease Total antioxidant status: no significant differences were found |
|
| Kozakiewicz et al. [56] | 2018 | In vivo | 42 healthy volunteers | 1 | 3 | ? | Carbonyl group, MDA, nitrate/nitrite, SOD | Carbonylgroep: increase MDA: increase Nitrate/nitrite: increase SOD: increase |
The baseline values were significantly higher (carbonyl group) and lower (nitrate/nitrite and SOD-1) in the HBOT-group compared to the control group |
| Lambrechts et al. [64] | 2013 | In vivo | 10 military divers | 1 | 4 (dive)/1.7 | 1 h post-dive/1 h post-HBOT | Nitrotyrosine, nitric oxygen | No significant differences were found | |
| Li et al. [84] | 2017 | In vivo | 78 patients; chronic diabetic wounds | By average 48 | 2.4 | 30 days after the first HBOT-session | MDA, SOD | MDA: decrease SOD: increase |
|
| Li et al. [58] | 2018 | In vivo | 115 patients; coronary artery disease with drug-eluting stents | 24 | 2 | ? | Nitric oxygen | Increase | |
| Li et al. [59] | 2019 | In vivo | 115 patients; coronary artery disease with coronary stent implantation | 24 | 2 | ? | Nitric oxygen | Increase | |
| Li et al. [60] | 2018 | In vivo | 98 patients; slow coronary flow | 24 | 2 | ? | Nitric oxygen | Increase | |
| Lin et al. [39] | 2008 | In vitro | Detroit 551 normal human dermal fibroblasts | 3 | 2.5 | ? | ROS | Increase | |
| Ma et al. [81] | 2013 | In vivo | 36 patients; diabetic foot ulcers | 20 | 2.5 | 7 and 14 days after the first HBOT-session | Catalase, MDA, SOD | Catalase: increase post-session #14 MDA: increase post-session #14 SOD: increase post-session #14 |
|
| Matzi et al. [82] | 2015 | In vivo | 23 healthy volunteers | 1 | 2.2 | During HBOT and immediately post-HBOT | 8-hydroxy-deoxyguanosine, GPx, MDA, oxidized low-density lipoprotein, plasma carbonyl proteins | 8-hydroxydeoxyguanosine: decrease GPx: increase during HBOT MDA: increase during HBOT Plasma carbonyl proteins: decrease during HBOT Oxidized low-density lipoprotein: no significant differences were found |
|
| Morabito et al. [43] | 2011 | In vivo | 6 healthy male, well-trained recreational divers | 1 | 1.6 (dive)/2.2 (dive) | Immediately post-dive | Catalase, hydrogen peroxide, intracellular calcium concentration | Catalase: increase in 2.2 ATA group Hydrogen peroxide: decrease in 2.2 ATA group Intracellular calcium concentration: decrease |
No significant differences in catalase and hydrogen peroxide levels were found in the 1.6 ATA group |
| Muth et al. [76] | 2004 | In vivo | 17 healthy male volunteers | 1 | 2.5 | Immediately post-HBOT | Catalase, glutathione, glutathione disulfide, GPx, isoprostanes, MDA, SOD, tail moment | Isoprostanes: increase Catalase, glutathione, glutathione disulfide, GPx, MDA, SOD, tail moment: no significant differences were found |
|
| Niu et al. [71] | 2013 | In vitro | Disc tissue from degenerated lumbar intervertebral discs | 3 | 2.5 | 24 h after each session | Nitric oxygen | Decrease post-session #2 and post-session #3 | |
| Niu et al. [52] | 2019 | In vitro | Disc tissues from degenerated lumbar intervertebral discs | 3 | 2.5 | 12 h post-HBOT | Inducible NOS | Decrease | |
| Niu et al. [72] | 2011 | In vitro | Disc tissue from degenerated lumbar intervertebral discs | 3 | 2.5 | 24 h after each session | Nitric oxygen | Decrease post-session #2 and post-session #3 | |
| Paprocki et al. [89] | 2020 | In vivo | 23 patients; difficult-to heal skin wounds following mechanical injuries | 25 | 2.5 | Post-session #1 and #25 | Catalase, GPx, SOD, TBARS | No significant differences were found | |
| Paprocki et al. [85] | 2019 | In vivo | 40 patients; sudden sensorineural hearing loss | 14 | 2.5 | 5 min post-session #1 and post-session #14 | Catalase, GPx, SOD, TBARS | Catalase: decrease post-session #1 GPx: increase post-session #14 SOD: decrease post-session #14 TBARS: increase in the erythrocytes post-session #14 |
No significant differences in TBARS levels in the plasma were found |
| Puthucheary et al. [69] | 2006 | In vivo | 15 patients | 1 | 2.4 | Immediately post-HBOT | (exhaled) Nitric oxygen | Decrease | |
| Resanovic et al. [53] | 2019 | In vivo | 19 patients; type 1 diabetes mellitus | 10 | 2.4 | ? | Free fatty acid, inducible NOS, nitrate/nitrite | Free fatty acid: decrease Inducible NOS: decrease Nitrate/nitrite: decrease |
|
| Rocco et al. [87] | 2001 | In vivo | 15 healthy volunteers | 1 | 2/2.8 | During HBOT and 30 min post-HBOT | TBARS | Increase | Only pressurization (without breathing 100% O2) has approximately the same effect |
| Rockswold et al. [79] | 2010 | In vivo | 69 patients; severe traumatic brain injury | 1 | 1.5 | ? | Isoprostanes | No significant differences were found | |
| Rossignol et al. [101] | 2007 | In vivo | 18 patients; children with autism | 40 | 1.3/1.5 | Within 24 h post-HBOT | Glutathione disulfideSSG | No significant differences were found | |
| Rothfuss et al. [95] | 2001 | In vitro | Human lymphocytes | 1 | 3 | 1 h, 4 h, 8 h, 12 h, and 24 h post-HBOT | Heme oxygenase-1, tail moment | Heme oxygenase-1: increase as of 4 h post-HBOT Tail moment: increase |
|
| Rothfuss et al. [96] | 2002 | In vitro | Human lymphocytes | 1 | 1.5 | 1 h, 4 h, 8 h, 12 h, and 24 h post-HBOT | Heme oxygenase-1, tail moment | Heme oxygenase-1: increase as of 4 h post-HBOT Tail moment: increase |
|
| Shaw et al. [66] | 2009 | In vitro | Human platelets | 1 | 2.2 | ? | Nitrate, nitrite | No significant differences were found | The level of significance was not determined |
| Shyu et al. [40] | 2008 | In vitro | Bone marrow-derived human mesenchymal stem cells | 1 | 2.5 | ? | ROS | Increase | |
| Sinan et al. [165] | 2016 | In vivo | 33 patients; various disorders | 20 | 2.4 | Post-session #1 and #20 | SOD | No significant differences were found | |
| Speit et al. [97] | 2000 | In vivo | 14 healthy volunteers | 1 | 2.5 | Immediately or 1 day post-HBOT | catalase, heme oxygenase-1, SOD, tail moment | Heme oxygenase-1: increase Catalase, SOD, tail moment: no significant differences were found |
|
| Sureda et al. [49] | 2014 | In vivo | 9 mail professional divers | 1 | 6 (dive) | 30 min and 3 h post-dive | Inducible NOS, MDA, nitrite, nitrotyrosine, nitric oxide | Nitrite: increase 3 h post-dive Nitrotyrosine: increase Nitric oxide: increase Inducible NOS, MDA: no significant differences were found |
|
| Sureda et al. [62] | 2016 | In vivo | 14 patients; chronic non-healing wound | 20 | 2.2 | Pre- and 2 h post-session #1, #5, and #20 | Catalase, glutathione reductase, GPx, MDA, myeloperoxidase, nitrite, SOD | Catalase: increase post-session #1 and post-session #5 in plasma MDA: decrease pre-session #20 and post-session #20 Myeloperoxidase: decrease post-session #1, post-session #5, and post-session #20 Glutathione reductase, GPx, nitrite, SOD: no significant differences were found |
No significant differences were found in catalase levels in erythrocytes |
| Sureda et al. [55] | 2009 | In vivo | 7 male preprofessional divers | 1 | 5 (dive) | Immediately and 3 h post-dive | Catalase, glutathione reductase, GPx, MDA, nitrite, protein carbonyl derivates, SOD | Catalase: increase immediately post-dive in plasma Nitrite: increase 3 h post-dive SOD: increase 3 h post-dive in plasma Glutathione reductase, GPx, MDA, protein carbonyl derivates: no significant differences were found |
No significant differences were found in catalase and SOD levels in erythrocytes |
| Taraldsoy et al. [70] | 2007 | In vivo | 8 patients; chronic radiation-induced injury | 20 | 2.3 | Post-session #1 and #19 | (exhaled) Nitric oxide | Decrease | |
| Taylor et al. [90] | 2012 | In vivo | 6 healthy, recreationally active, non-smoking male volunteers | 1 | 2.8 | Within 1 h post-HBOT and 5 h post-HBOT | TBARS | No significant differences were found | |
| Teksam et al. [83] | 2019 | In vivo | 54 patients; children with CO poisoning | 1 | 5 | Within 1 h post-HBOT | 8-hydroxy-deoxyguanosine, catalase, glutathione, GPx, MDA, plasma carbonyl proteins, SOD | No significant differences were found | |
| Tepic et al. [33] | 2018 | In vivo | 50 patients; type 2 diabetes mellitus | 10 | 1.7 | Post-session #3, #5, #7, and #10 | Catalase, hydrogen peroxide, nitrite, SOD, superoxide-ion, TBARS | Catalase: increase post-session #3 in group without vascular complications and post-session #10 in group with vascular complications Hydrogen peroxide: decrease post-session #3 in group without vascular complications Nitrite: increase post-session #3 in group with vascular complications Superoxide-ion: increase post-session #3 and post-session #10 in group with vascular complications SOD, TBARS: no significant differences were found |
|
| Thom et al. [47] | 2011 | In vivo | 8 patients; diabetes mellitus | 20 | 2 | ? | NOS | Increase | |
| Tillmans et al. [98] | 2019 | In vitro | Peripheral blood mononuclear cells from 49 healthy male subjects | 1 | 4 | Immediately post-HBOT and 18 h post-HBOT | Tail moment | No significant differences were found | |
| Uusijärvi et al. [61] | 2015 | In vivo | 19 healthy volunteers | 1 | 2.5 | During HBOT, 5 min post-HBOT and 30 min post-HBOT | Nitrate, nitrite, nitric oxygen | Nitrite: decrease during HBOT and 5 min post-HBOT NO: decrease in exhaled values Nitrate: no significant differences were found |
No significant differences were found in the NO values in the plasma |
| Wang et al. [50] | 2011 | In vivo | 77 patients; diabetic foot ulcers | 20 | 2.5 | ? | Endothelial NOS | No significant differences were found | |
| Wang et al. [51] | 2009 | In vivo | 74 patients; diabetic foot ulcers | 30 | 2.5 | ? | Endothelial NOS | No significant differences were found | |
| Wang et al. [73] | 2011 | In vitro | Disc tissue from lumbar intervertebral discs | 3 | 2.5 | 24 h post-HBOT | Nitric oxygen | Decrease | |
| Wang et al. [48] | 2020 | In vivo | 78 patients; spinal cord injury | 30 | 2 | ? | Endothelial NOS | Increase | |
| Yuan et al. [54] | 2014 | In vitro | Atricular cartilage specimens | 1 | 2.5 | 24 h post-HBOT | Inducible NOS | Decrease | |
| Zhou et al. [41] | 2018 | In vitro | Human umbilical vein endothelial cells | 1 | 2.8 | ? | ROS | Increase | |
a All studies used a dry exposure in a hyperbaric chamber, unless ‘dive’ is specified. b In minutes (min), hours (h), days, weeks, or months post-HBOT. The baseline measurement point has not been included. ? No information on the moment of sample taking (or just ‘post-HBOT’) was noted in the study.
Table A4.
Retrieved results on inflammation markers in detail.
| Study | Year | Design | Subjects | Methods | Type Inflammation Marker | Outcome | Remarks | ||
|---|---|---|---|---|---|---|---|---|---|
| Amount of Sessions |
Maximum Pressure (ATA) a | Moment of Sample Taking b | |||||||
| Akcali et al. [104] | 2018 | In vivo | 40 patients; CO poisoning | 1 | 2.4 | 6 h post-HBOT | hs-CRP, IL-6, IL-10 | No significant differences were found | |
| Alex et al. [127] | 2005 | In vivo | 64 patients; on-pump coronary artery bypass grafting | 3 (within 24 h) | 1.5/2.4 | Preoperative (4 h post-HBOT), 2 h postoperative, and 24 h postoperative | IL-6, IL-8, TNF-α | IL-8: decrease preoperative TNF-α: increase 2 h postoperative IL-6: no significant differences were found |
On-pump coronary artery bypass grafting in the follow-up period |
| Anguiano-Hernandez et al. [125] | 2019 | In vivo | 18 patients; diabetic foot ulcers | 20 | 1.4 | Post-session #20 | IFN-γ, IL-4, IL-6, IL-10, NF-κB | IL-6: increase NF-κB: decrease in the nucleus IFN-γ, IL-4, IL-10: no significant differences were found |
No significant differences in NF-κB levels were seen in the cytosol. The levels of IL-4 were below detection limits. |
| Aydin et al. [113] | 2013 | In vivo | 48 patients; diabetic foot ulcers | 30 | 2.4 | ? | Insulin-like growth factor-1 | Increase | The level of significance was not determined |
| Baiula et al. [120] | 2020 | In vivo | 30 patients; chronic non-healing wound | 15 | 2.4 | Immediately post-session #4, #8, #12, and #15 and 1 month post-HBOT | IL-1β, TNF-α | IL-1β: decrease as of post-session #12 TNF-α: decrease as of post-session #12 |
|
| Benson et al. [121] | 2003 | In vitro | Peripheral blood mononuclear cells | 1 | 2.4 | ? | IL-1β, TNF-α | IL-1β: decrease TNF-α: decrease |
LPS-, lipid A- and PHA-induced IL-1β and TNF-α production was measured |
| Bent et al. [112] | 2012 | In vivo | 10 children; autism spectrum disorder | 80 | 1.5 | Post-session #40 and #80 | Granulocyte-colony stimulating factor, IFN-α, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, TNF-α | No significant differences were found | |
| Bosco et al. [4] | 2014 | In vivo | 21 patients; pancreactico-duodenectomy | 1 | 2.5 | Post-HBOT, 1 day postoperative, and 7 days postoperative | IL-1, IL-6, IL-8, IL-10, IL-12p70, TNF-α | IL-6: decrease IL-10: decrease IL-1, IL-8, IL-12p70, TNF-α: no significant differences were found |
Pancreaticoduodenectomy in the follow-up period |
| Bosco et al. [35] | 2018 | In vivo | 23 patients; unilateral femoral head necrosis | 60 | 2.5 | Post-session #15, #30, and #60 and pre-session #31 | IL-1β, IL-6, TNF-α | IL-6: decrease TNF-α: decrease IL-1β: no significant differences were found |
|
| Chang et al. [102] | 2020 | In vivo | 10 healthy male volunteers | 1 | 2.8 | 30 min, 2 days, and 1 week post-HBOT | Albumin | No significant differences were found | |
| Chen et al. [106] | 2017 | In vivo | 38 patients; diabetic foot ulcers | 20 | 2.5 | Post-session #10, post-session #20, and 2 weeks post-HBOT | CRP, erythrocyte sedimentation rate | CRP: decrease 2 weeks post-session Erythrocyte sedimentation rate: decrease 2 weeks post-session |
|
| Chen et al. [88] | 2018 | In vivo | 50 patients; acute non-cardioembolic stroke | 1 | 2.5 | 1 month post-HBOT | hs-CRP | No significant differences were found | |
| Chen et al. [67] | 2007 | In vivo | 61 patients; diabetes mellitus type 2 | 3 | 2.5 | Immediately post-session #1 and #3 | Insulin-like growth factor-1, IL-8 | No significant differences were found | |
| Chong et al. [118] | 2013 | In vivo | 17 patients; thermal burns | 2 | 2.4 | ? | IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, TNF-α | IFN-γ: increase IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13: no significant differences were found |
Outcome on TNF-α is not reported |
| Dhamodharan et al. [45] | 2019 | In vivo | 37 patients; diabetic foot ulcer | 25 | 2.2 | 20 days after the first HBOT-session | CRP, IFN-γ, IL-1α, IL-8, IL-10, TNF-α | CRP: decrease IFN-γ: decrease IL-1α: increase IL-8: increase IL-10: increase TNF-α: no significant differences were found |
|
| Fan et al. [122] | 2020 | In vivo | 122 patients; Parkinson’s disease dementia | 40 | 20 MPA | ? | IL-1β, IL-6 | IL-1β: decrease IL-6: decrease |
|
| Fildissis et al. [128] | 2004 | In vitro | Blood samples from 16 healthy volunteers | 1 | 2.4 | ? | IL-6, IL-8, TNF-α | No significant differences were found | |
| Guggino et al. [133] | 2019 | In vivo | 36 patients; primary fibromyalgia | 40 | 2 | 1 month post-HBOT | IFN-γ, IL-9, IL-17, IL-22, TNF-α | IFN-γ: decrease TNF-α: decrease IL-9, IL-17, IL-22: no significant differences were found |
|
| Hao et al. [111] | 2020 | In vivo | 30 patients; plastic surgery | 7 | 2 | 24 h post-HBOT | Granulocyte-colony stimulating factor, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-1Ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, TNF-α | IL-1Ra: increase IL-12p40: decrease Granulocyte-colony stimulating factor, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, TNF-α: no significant differences were found |
The samples were taken during surgery |
| Hedetoft et al. [114] | 2020 | In vivo | 39 patients; diabetes | 30 | 2.4 | Post-session #30 and after 90 days follow-up | Insuline-like growth factor-1 | Decrease after 90 days follow-up in the diabetic group | No significant differences in IGF-1 values were seen in the non-diabetic group |
| Hou et al. [129] | 2019 | In vivo | 132 patients; brain tumor | 10 | 1.8 | ? | IL-6, TNF-α | IL-6: decrease TNF-α: decrease |
|
| Irawan et al. [116] | 2018 | In vivo | 36 patients; diabetic foot ulcers | 20 | 2.4 | Post-session #20 | Albumin | Increase | |
| Irawan et al. [117] | 2018 | In vivo | 30 patients; diabetic foot ulcers | 10 | 2.4 | ? | Albumin | No differences were found | The level of significance was not determined |
| Karadurmus et al. [103] | 2010 | In vivo | 28 patients; diabetic foot ulcers | 30 | 2.4 | Post-session #10, #20, and #30 | hs-CRP | Decrease | |
| Kendall et al. [46] | 2012 | In vitro | Human umbilical vein endothelial cells | 1 | 2.4 | 5 h and 22.5 h post-HBOT | IL-8 | Decrease 5 h post-HBOT | |
| Li et al. [84] | 2017 | In vivo | 78 patients; chronic diabetic wounds | By average 48 | 2.4 | 30 days after the first session | CRP, TNF-α | CRP: decrease TNF-α: decrease |
|
| Li et al. [58] | 2018 | In vivo | 115 patients; coronary artery disease with drug-eluting stents | 24 | 2 | ? | hs-CRP | Decrease | |
| Li et al. [59] | 2019 | In vivo | 115 patients; coronary artery disease with coronary stent implantation | 24 | 2 | ? | hs-CRP | Decrease | |
| Li et al. [60] | 2018 | In vivo | 98 patients; slow coronary flow | 24 | 2 | ? | hs-CRP | Decrease | |
| Lin et al. [130] | 2018 | In vivo | 57 patients; peripheral arterial occlusive disease | 10–15 | 2.5 | Post-session #3 and #5 | IL-6 | Decrease | |
| Liu et al. [137] | 2020 | In vivo | 140 patients; unilateral idiopathic sudden sensorineural hearing loss | 15 | 2 | ? | NF-κB, TNF-α | NF-κB: decrease TNF-α: decrease |
|
| MacKenzie et al. [126] | 2000 | In vitro | Peripheral blood mononuclear cells | 1 (duration: 48 h) | 1.7 | 7 days post-HBOT | IFN-γ, IL-4, IL-10 | No significant differences were found | The IL-4 values were below detection limits |
| Madden et al. [139] | 2011 | In vivo | 10 healthy male volunteers | 1 | 2.8 | Immediately post-HBOT and 4 h post-HBOT | NF-κB | Increase 4 h post-HBOT | |
| Nasole et al. [135] | 2014 | In vivo | 27 patients; chronic leg wounds | 40 | 2.5 | 7 and 14 days after the first HBOT-session | TNF-α | No significant differences were found | |
| Niu et al. [71] | 2013 | In vitro | Disc tissue from degenerated lumbar intervertebral discs | 3 | 2.5 | 24 h after each session | IL-1β | Decrease | |
| Niu et al. [52] | 2019 | In vitro | Abnormal disc tissues from degenerated lumbar intervertebral discs | 3 | 2.5 | 1 h post-session #3 | NF-κB | Decrease in the nucleus | |
| Niu et al. [72] | 2001 | In vitro | Disc tissue from degenerated lumbar intervertebral discs | 3 | 2.5 | 24 h after each session | IL-1β | Decrease | |
| Resanovic et al. [53] | 2019 | In vivo | 19 patients; type 1 diabetes mellitus | 10 | 2.4 | ? | CRP, NF-κB | CRP: decrease NF-κB: decrease |
|
| Rocco et al. [87] | 2001 | In vivo | 15 healthy volunteers | 1 | 2.8 | During HBOT and 30 min post-HBOT | IL-1β, IL-6, TNF-α | TNF-α: increase during HBOT IL-1β, IL-6: no significant differences were found |
|
| Romero-valdovinos et al. [115] | 2011 | In vitro | Dermal fibroblasts | 30 | 3 | ? | Insulin-like growth factor-1 | Decrease in the keloid fibroblast group | The nonkeloid fibroblasts did not express IGF-1 |
| Rosario et al. [131] | 2018 | In vivo | 6 patients; ischemic stroke | 40 | 2 | ? | IL-6, TNF-α | IL-6: decrease TNF-α: decrease |
|
| Rossignol et al. [101] | 2007 | In vivo | 18 patients; children with autism | 40 | 1.3/1.5 | Within 24 h post-HBOT | CRP | Decrease | In the group with lower CRP baseline levels no decrease in CRP concentrations was seen |
| Rothfuss et al. [96] | 2002 | In vitro | Human lymphocytes | 2 | 1.5/3 | 4 h, 8 h, and 24 h post-HBOT | Ferritin | No significant differences were found | |
| Schnittger et al. [110] | 2004 | In vivo | 8 patients; CO poisoning | 3 (within 24 h) | 2.8 | Before and after each session | Granulocyte-colony stimulating factor, IL-8 | Granulocyte-colony stimulating factor: increase pre- and post-session #2 in the patient group IL-8: no significant differences were found |
No significant differences in granulocyte-colony stimulating factor values were seen in the control group |
| Semadi et al. [138] | 2019 | In vivo | 32 patients; diabetic foot ulcer | 20 | 2.4 | Post-session #20 | TNF-α | Decrease | |
| Song et al. [132] | 2018 | In vivo | 134 patients; keloid surgery and radiotherapy | 14 | 2 | Post-operation #2 | IL-6, NF-κB, TNF-α | IL-6: decrease NF-κB: decrease TNF-α: decrease |
Keloid surgery and radiotherapy in the follow-up period |
| Sun et al. [119] | 2018 | In vivo | 80 patients; brain injury | 20 | 2 | 8 h, 24 h, 48 h, and 72 h post-HBOT | IL-1β, TNF-α | TNF-α: decrease IL-1β: no significant differences were found |
Baseline values of TNF-α were significantly higher in the HBOT-group compared to the control group |
| Sun et al. [140] | 2019 | In vivo | 79 patients; acute spinal cord injury | 30 | 2 | Post session #1, #3, #7, #10, and #30 | NF-κB | Decrease post-session #3, #7, #10, and #30 | |
| Sureda et al. [62] | 2016 | In vivo | 14 patients; chronic non-healing wound | 20 | 2.2 | Pre- and 2 h post-session #1, #5, and #20 | IL-6 | Increase 2 h post-session #1, #5, and #20 | |
| Top et al. [107] | 2007 | In vivo | 38 patients; type 2 diabetes mellitus | ? | 2 | 2 weeks after the first HBOT-session | CRP, erythrocyte sedimentation rate | CRP: decrease Erythrocyte sedimentation rate: increase |
|
| Vezzani et al. [105] | 2016 | In vivo | 30 patients; CO poisoning | 1 | 2.5/2.8 | Immediately post-HBOT | CRP, IL-6, IL-8, IL-10, TNF-α | IL-8: decrease in the control group IL-10: decrease in the patient group TNF-α: decrease CRP, IL-6: no significant differences were found |
No significant differences regarding IL-8 and IL-10 were seen in respectively the patient group and the control group |
| Wang et al. [134] | 2011 | In vitro | Human coronary artery endothelial cells | 1 | 2.5 | ? | TNF-α | Increase during HBOT | The TNF-α values returned to the baseline values before the HBOT ended |
| Wang et al. [73] | 2011 | In vitro | Disc tissue from lumbar intervertebral discs | 1 | 2.5 | 48 h, 96 h, and 144 h post-HBOT | IL-1β | Decrease | |
| Weisz et al. [124] | 1997 | In vivo | 7 patients; perianal Crohn’s disease | 20/40 | 2.5 | Immediately post-session #1, 24 h post-session #1, 20 h post-session #2, and 20 h post-session #20 | IL-1, IL-6, TNF-α | IL-1: decrease immediately and 24 h post-session #1 and 20 h post-session #2 IL-6: decrease immediately and 24 h post-session #1 and 20 h post-session #2 TNF-α: decrease immediately post-session #1 and 20 h post-session #2 |
|
| Wilkinson et al. [123] | 2015 | In vivo | 19 male volunteers; overweight/obese | 4 | 2 | During HBOT, immediately post-session #4, and 24 h post-session #4 | IL-1Ra, IL-6, IL-18, TNF-α | IL-6: increase during HBOT and immediately post-session #4 in the non-diabetes group TNF-α: decrease 24 h post-session #4 IL-1Ra, IL-18: no significant differences were found |
|
| Xie et al. [109] | 2007 | In vivo | 60 patients; craniocerebral injury | 10 | 2.5 | Within 24 h post-HBOT | CRP | Decrease | |
| Yildiz et al. [108] | 2016 | In vivo | 43 patients; hidradenitis suppurativa | 20 | 2.4 | Post-session #20 and 6 weeks post-HBOT | CRP, erythrocyte sedimentation rate | CRP: decrease Erythrocyte sedimentation rate: decrease |
|
| Yoshinoya et al. [136] | 2020 | In vitro | Adipose-derived stem cells | 5 | 2/3 | 90 min before and immediately after each session | TNF-α | No significant differences were found | The TNF-α values were below detection limits |
a All studies used a hyperbaric chamber for pressurization. b In minutes (min), hours (h), days, weeks, or months post-HBOT. The baseline measurement point has not been included. ? No information on the moment of sample taking (or just ‘post-HBOT’) was noted in the study.
Table A5.
Retrieved results on angiogenesis markers in detail.
| Study | Year | Design | Subjects | Methods | Type Angiogenesis Marker | Outcomes | Remarks | ||
|---|---|---|---|---|---|---|---|---|---|
| Amount of Sessions |
Maximum Pressure (ATA) a | Moment of Sample Taking b | |||||||
| Anguiano-Hernandez et al. [125] | 2019 | In vivo | 18 patients; diabetic foot ulcers | 20 | 1.4 | Post-session #20 | HIF-1α, insulin-like growth factor binding protein-3, VEGF | Insulin-like growth factor binding protein-3: increase in nucleus and fibroblast VEGF: increase in the cytosol HIF-1α: no significant differences were found |
No significant differences in insulin-like growth factor binding protein-3 and VEGF levels were found in the cytoplasm and nucleus, respectively |
| Bent et al. [112] | 2012 | In vivo | 10 children; autism spectrum disorder | 80 | 1.5 | Post-session #40 and #80 | Granulocyte-macrophage colony-stimulating factor, TGF-β1, TGF-β2 | No significant differences were found | |
| Chang et al. [102] | 2020 | In vivo | 10 healthy male volunteers | 1 | 2.8 | 30 min, 2 days, and 1 week post-HBOT | FGF21 | No significant differences were found | |
| Chen et al. [67] | 2007 | In vivo | 61 patients; diabetes mellitus type 2 | 3 | 2.5 | Immediately post-session #1 and #3 | Insulin-like growth factor-2, insulin-like growth factor binding protein-1, insulin-like growth factor binding protein-3 | Insulin-like growth factor binding protein-1: decrease post-session #1 and (less prominent) post-session #3 Insulin-like growth factor-2, insulin-like growth factor binding protein-3: no differences were found |
No significance levels were determined |
| Cheung et al. [37] | 2018 | In vitro | Umbilical cord blood enriched with CD34-cells | 1 | 2.5 | 24 h post-HBOT | Erythropoietin-receptor | No significant differences were found | |
| Chong et al. [118] | 2013 | In vivo | 17 patients; thermal burns | 2 | 2.4 | ? | VEGF | No significant differences were found | |
| Dhamodharan et al. [45] | 2019 | In vivo | 37 patients; diabetic foot ulcer | 25 | 2.2 | 20 days after the first HBOT-session | EGF, FGF-2, platelet-derived growth factor, VEGF | EGF: increase FGF-2: increase VEGF: increase Platelet-derived growth factor: no significant differences were found |
|
| Grimberg-Peters et al. [44] | 2016 | In vitro | Neutrophils from severely injured patients and healthy volunteers | 1 | 2 | ? | ERK, p38 MAPK | p38 MAPK: decrease ERK: no significant differences were found |
The decrease in p38 MAPK levels was only found after 3h of stimulation with PMA |
| Hao et al. [111] | 2020 | In vivo | 30 patients; plastic surgery | 7 | 2 | 24 h post-HBOT | EGF, FGF-2, granulocyte-macrophage colony-stimulating factor, platelet-derived growth factor-AA, platelet-derived growth factor-BB, TGF-α, VEGF | Platelet-derived growth factor-BB: decrease EGF, FGF-2, granulocyte-macrophage colony-stimulating factor, platelet-derived growth factor-AA, TGF-α, VEGF: no significant differences were found |
The samples were taken during surgery. |
| Jung et al. [143] | 2010 | In vivo | 86 patients; acute hearing loss/tinnitus | 10 | 1.55 | 1, 2, 5, and 10 days after the first HBOT-session | bFGF, VEGF | bFGF: decrease VEGF: no significant differences were found |
|
| Kang et al. [147] | 2004 | In vitro | Fibroblast primary cell lines | 7 | 1.5/2/2.5/3 | 1 day, 3 days, 5 days, and 7 days after the first HBOT-session | bFGF, TGF-β1, VEGF | No significant differences were found | Administration of 100% oxygen significantly increased the bFGF levels at day 1, yet pressurization had no extra effect. The TGF-β1 values were below detection limits. |
| Kunnavatana et al. [148] | 2005 | In vitro | Fibroblast cell line | 7 | 2 | 1 day, 3 days, 5 days, and 7 days after the first HBOT-session | bFGF, TGF-β1, VEGF | No significant differences were found | |
| Lee et al. [142] | 2006 | In vitro | Human umbilical vein endothelial cells | 1 | 2.5 | ERK, VEGF | ERK: increase VEGF: increase |
||
| Li et al. [84] | 2017 | In vivo | 78 patients; chronic diabetic wounds | By average 48 | 2.4 | 30 days after the first HBOT-session | Ang-2, VEGF | Ang-2: increase VEGF: increase |
|
| Lin et al. [130] | 2018 | In vivo | 57 patients; peripheral arterial occlusive disease | 10 of 15 | 2.5 | Post-session #3 and #5 | Hematopoietic growth factor, HIF-1α, stromal cell-derived factor-1α, VEGF | Hematopoietic growth factor: increase HIF-1α: decrease Stromal cell-derived factor-1α: increase VEGF: increase |
|
| Lin et al. [144] | 2002 | In vitro | Human umbilical vein endothelial cells | 1 | 2.5 | ? | Ang-1, Ang-2, Tie-2, VEGF | No significant differences were found | Administration of 100% oxygen significantly increased the Ang-2 levels, yet pressurization had no extra effect |
| Mutzbauer et al. [145] | 2015 | In vivo | 16 divers | 3 | 1.4 (dive) | Within 1 h pre- and post-dive | Erythropoietin | Decrease post-dive #2 and #3 | |
| Nasole et al. [135] | 2014 | In vivo | 27 patients; chronic leg wounds | 40 | 2.5 | 7 and 14 days after the first HBOT-session | EGF, VEGF | No significant differences were found | |
| Niu et al. [71] | 2013 | In vitro | Disc tissue from degenerated lumbar intervertebral discs | 3 | 2.5 | 30 min and 60 min post-session #3 | ERK1/2, p38 MAPK | ERK1/2: decrease p38 MAPK: decrease |
Phosphorylation of p38 MAPK and ERK has been measured |
| Niu et al. [52] | 2019 | In vitro | Abnormal disc tissues from degenerated lumbar intervertebral discs | 3 | 2.5 | ERK1/2, p38 MAPK: 15 min and 30 min post-session #3 MMP-3, MPP-9, MMP-13: 12 h after each session |
ERK1/2, MMP-3, MMP-9, MMP-13, p38 MAPK | ERK1/2: decrease 30 min post-session #3 MMP-3: decrease post-session #2 and #3 MMP-9: decrease post-session #2 and #3 MMP-13: decrease post-session #2 and #3 p38 MAPK: decrease |
Phosphorylation of p38 MAPK and ERK has been measured |
| Niu et al. [72] | 2011 | In vitro | Disc tissue from degenerated lumbar intervertebral discs | 3 | 2.5 | MMP-3: 24 h after each session p38 MAPK: 15 min, 30 min, and 60 min post-session #3 |
MMP-3, p38 MAPK | MMP-3: decrease post-session #3 p38 MAPK: decrease |
Phosphorylation of p38 MAPK and ERK has been measured |
| Resanovic et al. [53] | 2019 | In vivo | 19 patients; type 1 diabetes mellitus | 10 | 2.4 | ? | Akt, ERK1/2 | Akt: decrease ERK1/2: decrease |
|
| Romero-valdovinos et al. [115] | 2011 | In vitro | Dermal fibroblasts | 30 | 3 | ? | TGF-β | TGF-β: decrease | |
| Semadi et al. [138] | 2019 | In vivo | 32 patients; diabetic foot ulcer | 20 | 2.4 | Post-session #20 | VEGF | Increase | |
| Shyu et al. [40] | 2008 | In vitro | Bone marrow-derived human mesenchymal stem cells | 1 | 2.5 | 1 h, 2 h, 4 h, and 6 h post-HBOT | PGF | Increase | The increase in PGF levels was higher at 1h and 2h post-HBOT compared to 4h and 6h post-HBOT |
| Song et al. [132] | 2018 | In vivo | 134 patients; keloid surgery and radiotherapy | 14 | 2 | Post-operation #2 | HIF-1α, VEGF | HIF-1α: decrease VEGF: decrease |
Keloid surgery and radiotherapy in the follow-up period |
| Sureda et al. [62] | 2016 | In vivo | 14 patients; chronic non-healing wound | 20 | 2.2 | Pre- and 2 h post-session #1, #5, and #20 | VEGF | Increase post-session #1, post-session #5, and post-session #20 | |
| Thom et al. [47] | 2011 | In vivo | 8 patients; diabetes mellitus | 20 | 2 | Pre- and post-session #1, #10, and #20 | HIF-1α | Decrease post-session #1, #10, and #20 | No significant differences in HIF-1α levels were found pre-session |
| Tra et al. [141] | 2014 | In vitro | Tissue-engineered mucosa and human umbilical vein endothelial cells | 1/3/5 | 2.4 | Immediately post-HBOT | bFGF, hematopoietic growth factor, keratinocyte growth factor, PGF, VEGF | bFGF: an increase in the one-session group and a decrease in the three- and five-session group Hematopoietic growth factor: increase in the one-session group Keratinocyte growth factor: increase in the one- and five-session group PGF: an increase in the one- and five-session group and a decrease in the three-session group VEGF: increase in the five-session group |
|
| Wang et al. [50] | 2011 | In vivo | 77 patients; diabetic foot ulcers | 20 | 2.5 | ? | EGF, VEGF | No significant differences were found | |
| Wang et al. [51] | 2009 | In vivo | 74 patients; diabetic foot ulcers | 30 | 2.5 | ? | VEGF | No significant differences were found | |
| Wang et al. [48] | 2020 | In vivo | 78 patients; spinal cord injury | 30 | 2 | ? | Akt, PI3K | Akt: increase PI3K: increase |
|
| Yoshinoya et al. [136] | 2020 | In vitro | Adipose-derived stem cells | 5 | 2/3 | 90 min before and immediately after each session | EGF, hematopoietic growth factor, TGF-β | TGF-β: decrease post-session #3 in the 2 ATA group and post-session #4 in the 3 ATA group EGF, hematopoietic growth factor: no significant differences were found |
The EGF values were below detection limits |
| Yuan et al. [54] | 2014 | In vitro | Atricular cartilage specimens | 1 | 2.5 | 24 h post-HBOT | MMP-3 | Decrease | |
a All studies used a dry exposure in a hyperbaric chamber, unless ‘dive’ is specified. b In minutes (min), hours (h), days, or months post-HBOT. The baseline measurement point has not been included. ? No information on the moment of sample taking (or just ‘post-HBOT’) was noted in the study.
Author Contributions
Conceptualization, R.P.W. and R.A.V.H.; methodology, S.D.D.W., R.P.W. and R.A.V.H.; validation, S.D.D.W., R.H.H., R.P.W., M.W.H. and R.A.V.H.; formal analysis, S.D.D.W.; investigation, S.D.D.W.; data curation, S.D.D.W.; writing—original draft preparation, S.D.D.W.; writing—review and editing, S.D.D.W., R.H.H., R.P.W., M.W.H. and R.A.V.H.; visualization, S.D.D.W.; supervision, R.P.W., M.W.H. and R.A.V.H.; project administration, R.P.W. and R.A.V.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Society U.H.M. In: Hyperbaric Oxygen Therapy Indications. 14th ed. Moon R., editor. Best Publishing Company; North Palm Beach, FL, USA: 2019. [Google Scholar]
- 2.Tibbles P.M., Edelsberg J.S. Hyperbaric-oxygen therapy. N. Engl. J. Med. 1996;334:1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- 3.Society U.A.H.M. UHMS Guidelines for credentialing, privileging and supervision of hyperbaric oxygen therapy in the U.S.A. Undersea Hyperb. Med. 2018;45:117–127. doi: 10.22462/01.02.2018.19. [DOI] [Google Scholar]
- 4.Bosco G., Casarotto A., Nasole E., Camporesi E., Salvia R., Giovinazzo F., Zanini S., Malleo G., Di Tano A., Rubini A., et al. Preconditioning with hyperbaric oxygen in pancreaticoduodenectomy: A randomized double-blind pilot study. Anticancer Res. 2014;34:2899–2906. [PubMed] [Google Scholar]
- 5.Friedman T., Menashe S., Landau G., Sherf M., Wiser I., Seligman Y., Friedman M., Hadanny A., Efrati S., Heller L. Hyperbaric oxygen preconditioning can reduce postabdominoplasty complications: A retrospective cohort study. Plast. Reconstr. Surg. Glob. Open. 2019;7:e2417. doi: 10.1097/GOX.0000000000002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Dong H., Chen M., Liu J., Yang L., Chen S., Xiong L. Preconditioning with repeated hyperbaric oxygen induces myocardial and cerebral protection in patients undergoing coronary artery bypass graft surgery: A prospective, randomized, controlled clinical trial. J. Cardiothorac. Vasc. Anesth. 2011;25:908–916. doi: 10.1053/j.jvca.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Yogaratnam J.Z., Laden G., Guvendik L., Cowen M., Cale A., Griffin S. Hyperbaric oxygen preconditioning improves myocardial function, reduces length of intensive care stay, and limits complications post coronary artery bypass graft surgery. Cardiovasc. Revascularization Med. 2010;11:8–19. doi: 10.1016/j.carrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Toner A., Hamilton M. The long-term effects of postoperative complications. Curr. Opin. Crit. Care. 2013;19:364–368. doi: 10.1097/MCC.0b013e3283632f77. [DOI] [PubMed] [Google Scholar]
- 9.Pinto A., Faiz O., Davis R., Almoudaris A., Vincent C. Surgical complications and their impact on patients’ psychosocial well-being: A systematic review and meta-analysis. BMJ Open. 2016;6:e007224. doi: 10.1136/bmjopen-2014-007224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey K., Stefos T., Zhao S., Borzecki A.M., Rosen A.K. Excess costs attributable to postoperative complications. Med. Care Res. Rev. 2011;68:490–503. doi: 10.1177/1077558710396378. [DOI] [PubMed] [Google Scholar]
- 11.Camporesi E.M., Bosco G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb. Med. 2014;41:247–252. [PubMed] [Google Scholar]
- 12.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fosen K.M., Thom S.R. Hyperbaric oxygen, vasculogenic stem cells, and wound healing. Antioxid. Redox Signal. 2014;21:1634–1647. doi: 10.1089/ars.2014.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eken A., Aydin A., Sayal A., Ustundag A., Duydu Y., Dundar K., Aydın A. The effects of hyperbaric oxygen treatment on oxidative stress and SCE frequencies in humans. Clin. Biochem. 2005;38:1133–1137. doi: 10.1016/j.clinbiochem.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Speit G., Dennog C., Radermacher P., Rothfuss A. Genotoxicity of hyperbaric oxygen. Mutat. Res. Mol. Mech. Mutagen. 2002;512:111–119. doi: 10.1016/S1383-5742(02)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Chelombitko M.A. Role of reactive oxygen species in inflammation: A minireview. Mosc. Univ. Biol. Sci. Bull. 2018;73:199–202. doi: 10.3103/S009639251804003X. [DOI] [Google Scholar]
- 17.Hehenberger K., Brismar K., Lind F., Kratz G. Dose-dependent hyperbaric oxygen stimulation of human fibroblast proliferation. Wound Repair Regen. 1997;5:147–150. doi: 10.1046/j.1524-475X.1997.50206.x. [DOI] [PubMed] [Google Scholar]
- 18.Chiang I.-H., Chen S.-G., Huang K.-L., Chou Y.-C., Dai N.-T., Peng C.-K. Adjunctive hyperbaric oxygen therapy in severe burns: Experience in Taiwan formosa water park dust explosion disaster. Burns. 2017;43:852–857. doi: 10.1016/j.burns.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Dimitrijevich S.D., Paranjape S., Wilson J.R., Gracy R.W., Mills J.G. Effect of hyperbaric oxygen on human skin cells in culture and in human dermal and skin equivalents. Wound Repair Regen. 1999;7:53–64. doi: 10.1046/j.1524-475x.1999.00053.x. [DOI] [PubMed] [Google Scholar]
- 20.Granowitz E.V., Tonomura N., Benson R.M., Katz D.M., Band V., Makari-Judson G.P., Osborne B.A. Hyperbaric oxygen inhibits benign and malignant human mammary epithelial cell proliferation. Anticancer Res. 2005;25:3833–3842. [PubMed] [Google Scholar]
- 21.Hibbs H.W., Harasym M.P., Bansal D., Stewart J. Effects of a single hyperbaric oxygen exposure on haematocrit, prothrombin time, serum calcium, and platelet count. Diving Hyperb. Med. South Pac. Underw. Med. Soc. 2007;37:143–145. [Google Scholar]
- 22.Hollander D.A., Hakimi M.Y., Hartmann A., Wilhelm K., Windolf J. The influence of hyperbaric oxygenation (HBO) on proliferation and differentiation of human keratinocyte cultures in vitro. Cell Tissue Bank. 2000;1:261–269. doi: 10.1023/A:1010145312698. [DOI] [PubMed] [Google Scholar]
- 23.Jüttner B., Scheinichen D., Bartsch S., Heine J., Ruschulte H., Elsner H.A., Franko W., Jaeger K. Lack of toxic side effects in neutrophils following hyperbaric oxygen. Undersea Hyperb. Med. 2003;30:305–311. [PubMed] [Google Scholar]
- 24.Kairuz E., Upton Z., Dawson R.A., Malda J. Hyperbaric oxygen stimulates epidermal reconstruction in human skin equivalents. Wound Repair Regen. 2007;15:266–274. doi: 10.1111/j.1524-475X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Zhao D., Diao M., Yang C., Zhang Y., Lv Y., Zhao J., Pan S. Hyperbaric oxygen treatments attenuate the Neutrophil-to-Lymphocyte ratio in patients with idiopathic sudden sensorineural hearing loss. Otolaryngol. Neck Surg. 2015;153:606–612. doi: 10.1177/0194599815589072. [DOI] [PubMed] [Google Scholar]
- 26.Melcher C., Sievers B., Höchsmann N., Düren F., Jansson V., Müller P.E. Effect of hyperbaric oxygen on proliferation and gene expression of human chondrocytes: An in vitro study. Cartilage. 2019;10:459–466. doi: 10.1177/1947603518764281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monaca E., Strelow H., Jüttner T., Hoffmann T., Potempa T., Windolf J., Winterhalter M. Assessment of hemostaseologic alterations induced by hyperbaric oxygen therapy using point-of-care analyzers. Undersea Hyperb. Med. 2014;41:17–26. [PubMed] [Google Scholar]
- 28.Passavanti G., Tanasi P., Brauzzi M., Pagni M., Norgini E., Aloisi A. can hyperbaric oxygenation therapy (HOT) modify the blood testosterone concentration? Urol. J. 2010;77:52–56. doi: 10.1177/039156031007700109. [DOI] [PubMed] [Google Scholar]
- 29.Ravaioli M., Baldassare M., Vasuri F., Pasquinelli G., Laggetta M., Valente S., De Pace V., Neri F., Siniscalchi A., Zanfi C., et al. Strategies to restore adenosine triphosphate (ATP) level after more than 20 hours of cold ischemia time in human marginal kidney grafts. Ann. Transplant. 2018;23:34–44. doi: 10.12659/AOT.905406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts G.P., Harding K.G. Stimulation of glycosaminoglycan synthesis in cultured fibroblasts by hyperbaric oxygen. Br. J. Dermatol. 1994;131:630–633. doi: 10.1111/j.1365-2133.1994.tb04973.x. [DOI] [PubMed] [Google Scholar]
- 31.Roberts G.P., Qu B., Harding K.G. The effects of hyperbaric oxygen on cultured fibroblasts. J. Wound Care. 1994;3:189–193. doi: 10.12968/jowc.1994.3.4.189. [DOI] [PubMed] [Google Scholar]
- 32.Vezzani G., Iezzi M., Rizzato A., Quartesan S., Mangar D., Camporesi E.M., Paganini M., Bosco G. Effects of hyperbaric oxygen exposure on mobilization of endothelial progenitor cells in healthy volunteers. Acta Med. Mediterr. 2017;33:801–805. doi: 10.19193/0393-6384_2017_5_118. [DOI] [Google Scholar]
- 33.Tepić S., Petković A., Srejović I., Jeremić N., Zivković V., Loncarević S., Bradić J., Jakovljević V., Zivkovć M. Impact of hyperbaric oxygenation on oxidative stress in diabetic patients. Undersea Hyperb. Med. Soc. 2018;45:9–17. doi: 10.22462/01.02.2018.2. [DOI] [PubMed] [Google Scholar]
- 34.Ferrer M.D., Sureda A., Batle J.M., Tauler P., Tur J.A., Pons A. Scuba diving enhances endogenous antioxidant defenses in lymphocytes and neutrophils. Free. Radic. Res. 2007;41:274–281. doi: 10.1080/10715760601080371. [DOI] [PubMed] [Google Scholar]
- 35.Bosco G., Vezzani G., Mrakic-Sposta S., Rizzato A., Enten G., Abou-Samra A., Malacrida S., Quartesan S., Vezzoli A., Camporesi E. Hyperbaric oxygen therapy ameliorates osteonecrosis in patients by modulating inflammation and oxidative stress. J. Enzym. Inhib. Med. Chem. 2018;33:1501–1505. doi: 10.1080/14756366.2018.1485149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C.-H., Chien M.-Y., Liang Y.-C., Liu D.-Z., Hu M.-L. An in vitro hyperbaric oxygen system for evaluation of free radical damage and protection by catechins on hemorheological parameters. Clin. Hemorheol. Microcirc. 2011;48:211–221. doi: 10.3233/CH-2011-1412. [DOI] [PubMed] [Google Scholar]
- 37.Cheung K.Y., Berry A., Li D., Aljitawi O.S. Hyperbaric oxygen treatment effects on in vitro cultured umbilical cord blood CD34 (+) cells. Cytotherapy. 2018;20:87–94. doi: 10.1016/j.jcyt.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Gröger M., Öter S., Simkova V., Bolten M., Koch A., Warninghoff V., Georgieff M., Muth C.-M., Speit G., Radermacher P. DNA damage after long-term repetitive hyperbaric oxygen exposure. J. Appl. Physiol. 2009;106:311–315. doi: 10.1152/japplphysiol.90737.2008. [DOI] [PubMed] [Google Scholar]
- 39.Lin H.-I., Chu S.-J., Perng W.-C., Wu C.-P., Lin Z.-Y., Huang K.-L. Hyperbaric oxygen attenuates cell growth in skin fibroblasts cultured in a high-glucose medium. Wound Repair Regen. 2008;16:513–519. doi: 10.1111/j.1524-475X.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- 40.Shyu K.-G., Hung H.-F., Wang B.-W., Chang H. Hyperbaric oxygen induces placental growth factor expression in bone marrow-derived mesenchymal stem cells. Life Sci. 2008;83:65–73. doi: 10.1016/j.lfs.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Q., Huang G., Yu X., Xu W. A novel approach to estimate ROS origination by hyperbaric oxygen exposure, targeted probes and specific inhibitors. Cell. Physiol. Biochem. 2018;47:1800–1808. doi: 10.1159/000491061. [DOI] [PubMed] [Google Scholar]
- 42.Kendall A.C., Whatmore J.L., Winyard P., Smerdon G.R., Eggleton P. Hyperbaric oxygen treatment reduces neutrophil-endothelial adhesion in chronic wound conditions through S-nitrosation. Wound Repair Regen. 2013;21:860–868. doi: 10.1111/wrr.12108. [DOI] [PubMed] [Google Scholar]
- 43.Morabito C., Bosco G., Pilla R., Corona C., Mancinelli R., Yang Z., Camporesi E.M., Fanò G., Mariggiò M.A. Effect of pre-breathing oxygen at different depth on oxidative status and calcium concentration in lymphocytes of scuba divers. Acta Physiol. 2010;202:69–78. doi: 10.1111/j.1748-1716.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- 44.Grimberg-Peters D., Büren C., Windolf J., Wahlers T., Paunel-Görgülü A. Hyperbaric oxygen reduces production of reactive oxygen species in neutrophils from polytraumatized patients yielding in the inhibition of p38 MAP kinase and downstream pathways. PLoS ONE. 2016;11:e0161343. doi: 10.1371/journal.pone.0161343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhamodharan U., Karan A., Sireesh D., Vaishnavi A., Somasundar A., Rajesh K., Ramkumar K.M. Tissue-specific role of Nrf2 in the treatment of diabetic foot ulcers during hyperbaric oxygen therapy. Free. Radic. Biol. Med. 2019;138:53–62. doi: 10.1016/j.freeradbiomed.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Kendall A.C., Whatmore J.L., Harries L., Winyard P.G., Smerdon G.R., Eggleton P. Changes in inflammatory gene expression induced by hyperbaric oxygen treatment in human endothelial cells under chronic wound conditions. Exp. Cell Res. 2012;318:207–216. doi: 10.1016/j.yexcr.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Thom S.R., Milovanova T.N., Yang M., Bhopale V.M., Sorokina E.M., Uzun G., Malay D.S., Dpm M.A.T., Hardy K.R., Lambert D.S., et al. Vasculogenic stem cell mobilization and wound recruitment in diabetic patients: Increased cell number and intracellular regulatory protein content associated with hyperbaric oxygen therapy. Wound Repair Regen. 2011;19:149–161. doi: 10.1111/j.1524-475X.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L., Sun H., Fu S., Shao S., Hou H., Liu X. The role of pi3k/akt/enos pathway in spinal cord injury and the recovery of motor function. Int. J. Clin. Exp. Med. 2020;13:4960–4965. [Google Scholar]
- 49.Sureda A., Batle J.M., Capó X., Martorell M., Córdova A., Tur J.A., Pons A. Scuba diving induces nitric oxide synthesis and the expression of inflammatory and regulatory genes of the immune response in neutrophils. Physiol. Genom. 2014;46:647–654. doi: 10.1152/physiolgenomics.00028.2014. [DOI] [PubMed] [Google Scholar]
- 50.Wang C.-J., Ko J.-Y., Kuo Y.-R., Yang Y.-J. Molecular changes in diabetic foot ulcers. Diabetes Res. Clin. Pract. 2011;94:105–110. doi: 10.1016/j.diabres.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 51.Wang C.-J., Kuo Y.-R., Wu R.-W., Liu R.-T., Hsu C.-S., Wang F.-S., Yang K.D. extracorporeal shockwave treatment for chronic diabetic foot ulcers. J. Surg. Res. 2009;152:96–103. doi: 10.1016/j.jss.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 52.Niu C.-C., Lin S.-S., Yuan L.-J., Lu M.-L., Ueng S.W.N., Yang C.-Y., Tsai T.-T., Lai P.-L. Upregulation of miR-107 expression following hyperbaric oxygen treatment suppresses HMGB1/RAGE signaling in degenerated human nucleus pulposus cells. Arthritis Res. Ther. 2019;21:1–14. doi: 10.1186/s13075-019-1830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resanovic I., Gluvic Z., Zaric B., Sudar-Milovanovic E., Jovanovic A., Milacic D., Isakovic R., Isenovic E.R. Early Effects of hyperbaric oxygen on inducible nitric oxide synthase activity/expression in lymphocytes of Type 1 diabetes patients: A prospective pilot study. Int. J. Endocrinol. 2019;2019:1–12. doi: 10.1155/2019/2328505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan L.-J., Niu C.-C., Lin S.-S., Yang C.-Y., Chan Y.-S., Chen W.-J., Ueng S.W. Effects of low-intensity pulsed ultrasound and hyperbaric oxygen on human osteoarthritic chondrocytes. J. Orthop. Surg. Res. 2014;9:5. doi: 10.1186/1749-799X-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sureda A., Ferrer M.D., Batle J.M., Tauler P., Tur J.A., Pons A. Scuba diving increases erythrocyte and plasma antioxidant defenses and spares no without oxidative damage. Med. Sci. Sports Exerc. 2009;41:1271–1276. doi: 10.1249/MSS.0b013e3181951069. [DOI] [PubMed] [Google Scholar]
- 56.Kozakiewicz M., Kedziora-Kornatowska K., Kaczerska D., Siermontowski P., Olszanski R., Krefft K. Influence of exposure in hyperbaric chambers on selected parametersof oxidative stress in professional divers. Undersea Hyperb. Med. 2018;45:49–54. doi: 10.22462/01.02.2018.7. [DOI] [PubMed] [Google Scholar]
- 57.Boykin J.V., Baylis C. Hyperbaric oxygen therapy mediates increased nitric oxide production associated with wound healing. Adv. Ski. Wound Care. 2007;20:382–389. doi: 10.1097/01.ASW.0000280198.81130.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Hao Y., Wang T., Wei L., Wang W., Liang Y., Guo X. The effect of hyperbaric oxygen therapy on myocardial perfusion after the implantation of drug-eluting stents. Ann. Clin. Lab. Sci. 2018;48:158–163. [PubMed] [Google Scholar]
- 59.Li Y., Hao Y.-F., Wang T., Zhang J.-F., Liang Y., Xiao W.-L., Guo X. Hyperbaric oxygen may improve vascular endothelial function in patients undergoing coronary stent implantation. Undersea Hyperb. Med. 2019;46:145–152. doi: 10.22462/04.06.2019.7. [DOI] [PubMed] [Google Scholar]
- 60.Li Y., Zhang H., Liang Y., Wang W., Xu T., Zhang J., Xiao W., Wang T. Effects of hyperbaric oxygen on vascular endothelial function in patients with slow coronary flow. Cardiol. J. 2018;25:106–112. doi: 10.5603/CJ.a2017.0132. [DOI] [PubMed] [Google Scholar]
- 61.Uusijärvi J., Eriksson K., Larsson A.C., Nihlén C., Schiffer T., Lindholm P., Weitzberg E. Effects of hyperbaric oxygen on nitric oxide generation in humans. Nitric Oxide. 2015;44:88–97. doi: 10.1016/j.niox.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Sureda A., Batle J.M., Martorell M., Capó X., Tejada S., Tur J.A., Pons A. Antioxidant response of chronic wounds to hyperbaric oxygen therapy. PLoS ONE. 2016;11:e0163371. doi: 10.1371/journal.pone.0163371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasier H.G., Fothergill D.M. Oxidative stress, antioxidant defenses and nitric oxide production following hyperoxic exposures. Undersea Hyperb. Med. 2013;40:125–134. [PubMed] [Google Scholar]
- 64.Lambrechts K., Pontier J.-M., Mazur A., Buzzacott P., Morin J., Wang Q., Théron M., Guerrero F. Effect of decompression-induced bubble formation on highly trained divers microvascular function. Physiol. Rep. 2013;1:e00142. doi: 10.1002/phy2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dragic S., Momcicevic D., Zlojutro B., Jandric M., Kovacevic T., Djajić V., Gajić A., Talić G., Kovacevic P. Serum levels of nitric oxide and endothelin-1 in vasculopathy managed with hyperbaric oxygen therapy. Clin. Hemorheol. Microcirc. 2020;75:233–241. doi: 10.3233/CH-190796. [DOI] [PubMed] [Google Scholar]
- 66.Shaw F.L., Winyard P., Smerdon G.R., Bryson P.J., Moody J., Eggleton P. Hyperbaric oxygen treatment induces platelet aggregation and protein release, without altering expression of activation molecules. Clin. Biochem. 2009;42:467–476. doi: 10.1016/j.clinbiochem.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Chen S.-J., Yu C.-T., Cheng Y.-L., Yu S.-Y., Lo H.-C. Effects of hyperbaric oxygen therapy on circulating interleukin-8, nitric oxide, and insulin-like growth factors in patients with type 2 diabetes mellitus. Clin. Biochem. 2007;40:30–36. doi: 10.1016/j.clinbiochem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Gurdol F., Cimsit M., Oner-Iyidogan Y., Kocak H., Sengun S., Yalcinkaya-Demirsoz S. Collagen synthesis, nitric oxide and asymmetric dimethylarginine in diabetic subjects undergoing hyperbaric oxygen therapy. Physiol. Res. 2010;59:423–429. doi: 10.33549/physiolres.931702. [DOI] [PubMed] [Google Scholar]
- 69.Puthucheary Z.A., Liu J., Bennett M., Trytko B., Chow S., Thomas P.S. Exhaled nitric oxide is decreased by exposure to the hyperbaric oxygen therapy environment. Mediat. Inflamm. 2006;2006:1–6. doi: 10.1155/MI/2006/72620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taraldsøy T., Bolann B.J., Thorsen E. Reduced nitric oxide concentration in exhaled gas after exposure to hyperbaric hyperoxia. Undersea Hyperb. Med. 2007;34:321–327. [PubMed] [Google Scholar]
- 71.Niu C.-C., Lin S.-S., Yuan L.-J., Chen L.-H., Wang I.-C., Tsai T.-T., Lai P.-L., Chen W.-J. Hyperbaric oxygen treatment suppresses MAPK signaling and mitochondrial apoptotic pathway in degenerated human intervertebral disc cells. J. Orthop. Res. 2012;31:204–209. doi: 10.1002/jor.22209. [DOI] [PubMed] [Google Scholar]
- 72.Niu C.-C., Yuan L.-J., Chen L.-H., Lin S.-S., Tsai T.-T., Liao J.-C., Lai P.-L., Chen W.-J. Beneficial effects of hyperbaric oxygen on human degenerated intervertebral disk cells via suppression of IL-1β and p38 MAPK signal. J. Orthop. Res. 2010;29:14–19. doi: 10.1002/jor.21195. [DOI] [PubMed] [Google Scholar]
- 73.Wang I.-C., Ueng S.W.N., Lin S.-S., Niu C.-C., Yuan L.-J., Su C.-I., Chen C.-H., Chen W.-J. Effect of Hyperbaric Oxygenation on intervertebral disc degeneration. Spine. 2011;36:1925–1931. doi: 10.1097/BRS.0b013e3181feebde. [DOI] [PubMed] [Google Scholar]
- 74.Gronow G., Kähler W., Koch A., Klause N. Benzoate hydroxylation. Oxygen Transport to Tissue XXVI. 2006;566:223–229. doi: 10.1007/0-387-26206-7_30. [DOI] [PubMed] [Google Scholar]
- 75.Kähler W., Koch I., Wohlrab C., Kowalski J., Witte J., Koch A. Influence of hyperoxia and physical exercise on *OH-radical stress in humans as measured by dihydroxylated benzoates (DHB) in urine. Undersea Hyperb. Med. 2013;40:231–238. [PubMed] [Google Scholar]
- 76.Muth C.M., Glenz Y., Klaus M., Radermacher P., Speit G., Leverve X. Influence of an orally effective SOD on hyperbaric oxygen-related cell damage. Free. Radic. Res. 2004;38:927–932. doi: 10.1080/10715760412331273197. [DOI] [PubMed] [Google Scholar]
- 77.Gürdöl F., Cimşit M., Öner-Iyidoğan Y., Körpinar Ş., Yalçinkaya S., Kocak H. Early and late effects of hyperbaric oxygen treatment on oxidative stress parameters in diabetic patients. Physiol. Res. 2008;57:41–47. doi: 10.33549/physiolres.931139. [DOI] [PubMed] [Google Scholar]
- 78.Corcoran T., Ting S., Mas E., Phillips M., O’Loughlin E., Barden A., Mori T.A. Hyperbaric oxygen therapy is not associated with oxidative stress assessed using plasma F2-isoprostanes and isofurans. Prostaglandins, Leukot. Essent. Fat. Acids. 2017;127:16–19. doi: 10.1016/j.plefa.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 79.Rockswold S.B., Rockswold G.L., Zaun D.A., Zhang X., Cerra C.E., Bergman T.A., Liu J. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J. Neurosurg. 2010;112:1080–1094. doi: 10.3171/2009.7.JNS09363. [DOI] [PubMed] [Google Scholar]
- 80.Benedetti S., Lamorgese A., Piersantelli M., Pagliarani S., Benvenuti F., Canestrari F. Oxidative stress and antioxidant status in patients undergoing prolonged exposure to hyperbaric oxygen. Clin. Biochem. 2004;37:312–317. doi: 10.1016/j.clinbiochem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 81.Ma L., Li P., Shi Z., Hou T., Chen X., Du J. A prospective, randomized, controlled study of hyperbaric oxygen therapy: Effects on healing and oxidative stress of ulcer tissue in patients with a diabetic foot ulcer. Ostomy Wound Manag. 2013;59:18–24. [PubMed] [Google Scholar]
- 82.Matzi V., Greilberger J.F., Lindenmann J., Neuboeck N., Nuhsbaumer S., Zelzer S., Tafeit E., Maier A., Smolle-Juettner F.-M. Application of hyperbaric oxygen reduce oxidative damage of plasmatic carbonyl proteins and 8-OHdG by activating glutathion peroxidase. Clin. Lab. 2015;61:587–593. doi: 10.7754/Clin.Lab.2014.140929. [DOI] [PubMed] [Google Scholar]
- 83.TekSam O., Sabuncuoğlu S., Girgin G., Özgüneş H. Evaluation of oxidative stress and antioxidant parameters in children with carbon monoxide poisoning. Hum. Exp. Toxicol. 2019;38:1235–1243. doi: 10.1177/0960327119867751. [DOI] [PubMed] [Google Scholar]
- 84.Li N., Meng X.E., Guo D.Z., Fan D.F., Pan S.Y. Wound healing process and related laboratory indexes in patients with type 2 diabetes mellitus after hyperbaric oxygen intervention. Biomed. Res. India. 2017;28:8838–8843. [Google Scholar]
- 85.Paprocki J., Sutkowy P., Piechocki J., Woźniak A. Markers of oxidant-antioxidant equilibrium in patients with sudden sensorineural hearing loss treated with hyperbaric oxygen therapy. Oxidative Med. Cell. Longev. 2019;2019:1–8. doi: 10.1155/2019/8472346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bearden S.E., Cheuvront S.N., Ringes E.M. Oxidative stress during a 3.5-hour exposure to 120 kPa(a) PO2 in human divers. Undersea Hyperb. Med. 1999;26:159–164. [PubMed] [Google Scholar]
- 87.Rocco M., Antonelli M., Letizia V., Alampi D., Spadetta G., Passariello M., Conti G., Serio P., Gasparetto A. Lipid peroxidation, circulating cytokine and endothelin 1 levels in healthy volunteers undergoing hyperbaric oxygenation. Minerva Anestesiol. 2001;67:393–400. [PubMed] [Google Scholar]
- 88.Chen C.-Y., Wu R.-W., Tsai N.-W., Lee M.S., Lin W.-C., Hsu M.-C., Huang C.-C., Lai Y.-R., Kung C.-T., Wang H.-C., et al. Increased circulating endothelial progenitor cells and improved short-term outcomes in acute non-cardioembolic stroke after hyperbaric oxygen therapy. J. Transl. Med. 2018;16:255. doi: 10.1186/s12967-018-1629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paprocki J., Pawłowska M., Sutkowy P., Piechocki J., Woźniak A. evaluation of oxidative stress in patients with difficult-to-heal skin wounds treated with hyperbaric oxygen. Oxidative Med. Cell. Longev. 2020;2020:1–8. doi: 10.1155/2020/1835352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taylor L., Midgley A.W., Sandstrom M.E., Chrismas B., McNaughton L.R. The effect of the hyperbaric environment on heat shock protein 72 expression in vivo. Res. Sports Med. 2012;20:142–153. doi: 10.1080/15438627.2012.660830. [DOI] [PubMed] [Google Scholar]
- 91.Burgos C., Henríquez-Olguín C., Andrade D.C., Ramírez-Campillo R., Araneda O.F., White A., Cerda-Kohler H. Effects of exercise training under hyperbaric oxygen on oxidative stress markers and endurance performance in young soccer players: A pilot study. J. Nutr. Metab. 2016;2016:1–8. doi: 10.1155/2016/5647407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dise C.A., Clark J.M., Lambersten C.J., Goodman D.B. Hyperbaric hyperoxia reversibly inhibits erythrocyte phospholipid fatty acid turnover. J. Appl. Physiol. 1987;62:533–538. doi: 10.1152/jappl.1987.62.2.533. [DOI] [PubMed] [Google Scholar]
- 93.Kot J., Sićko Z., Woźniak M. Oxidative stress during oxygen tolerance test. Int. Marit. Health. 2003;54:117–126. [PubMed] [Google Scholar]
- 94.Dennog C., Radermacher P., Barnett Y., Speit G. Antioxidant status in humans after exposure to hyperbaric oxygen. Mutat. Res. Mol. Mech. Mutagen. 1999;428:83–89. doi: 10.1016/S1383-5742(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 95.Rothfuss A., Radermacher P., Speit G. Involvement of heme oxygenase-1 (HO-1) in the adaptive protection of human lymphocytes after hyperbaric oxygen (HBO) treatment. Carcinogenesis. 2001;22:1979–1985. doi: 10.1093/carcin/22.12.1979. [DOI] [PubMed] [Google Scholar]
- 96.Rothfuss A., Speit G. Investigations on the mechanism of hyperbaric oxygen (HBO)-induced adaptive protection against oxidative stress. Mutat. Res. Mol. Mech. Mutagen. 2002;508:157–165. doi: 10.1016/S0027-5107(02)00213-0. [DOI] [PubMed] [Google Scholar]
- 97.Speit G., Dennog C., Eichhorn U., Rothfuβ A., Kaina B. Induction of heme oxygenase-1 and adaptive protection against the induction of DNA damage after hyperbaric oxygen treatment. Carcinogenesis. 2000;21:1795–1799. doi: 10.1093/carcin/21.10.1795. [DOI] [PubMed] [Google Scholar]
- 98.Tillmans F., Sharghi R., Noy T., Kähler W., Klapa S., Sartisohn S., Sebens S., Koch A. Effect of hyperoxia on the immune status of oxygen divers and endurance athletes. Free. Radic. Res. 2019;53:522–534. doi: 10.1080/10715762.2019.1612890. [DOI] [PubMed] [Google Scholar]
- 99.Handy R.D., Bryson P., Moody A.J., Handy L.M., Sneyd J.R. Oxidative metabolism in platelets, platelet aggregation, and hematology in patients undergoing multiple hyperbaric oxygen exposures. Undersea Hyperb. Med. 2006;32:327–340. [PubMed] [Google Scholar]
- 100.Ansari K., Wilson M., Slater G., Haglin J., Kaplan E. Hyperbaric oxygenation and erythrocyte antioxidant enzymes in multiple sclerosis patients. Acta Neurol. Scand. 1986;74:156–160. doi: 10.1111/j.1600-0404.1986.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 101.Rossignol D.A., Rossignol L.W., James S.J., Melnyk S., Mumper E. The effects of hyperbaric oxygen therapy on oxidative stress, inflammation, and symptoms in children with autism: An open-label pilot study. BMC Pediatr. 2007;7:36. doi: 10.1186/1471-2431-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang J.S., Chang E., Lee Y., Cha Y.S., Cha S.-K., Gil Cho W., Jeong Y., Kim H., Park K.-S. Hyperbaric oxygen exposure attenuates circulating stress biomarkers: A pilot interventional study. Int. J. Environ. Res. Public Health. 2020;17:7853. doi: 10.3390/ijerph17217853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karadurmus N., Sahin M., Tasci C., Naharci I., Ozturk C., Ilbasmis S., Dulkadir Z., Sen A., Sağlam K. Potential benefits of hyperbaric oxygen therapy on atherosclerosis and glycaemic control in patients with diabetic foot. Endokrynol. Pol. 2010;61:275–279. [PubMed] [Google Scholar]
- 104.Akcali G., Uzun G., Arziman I., Aydin I., Yildiz S. The relationship between intoxication severity and blood interleukin 6, interleukin 10 and CRP levels in carbon monoxide-poisoned patients. Undersea Hyperb. Med. 2019;45:646–652. [PubMed] [Google Scholar]
- 105.Vezzani G., Socias S., Bianco A., Paoli A., Caberti L., Cantadori L., Manelli D., Mordacci M., Mangar D., Camporesi E.M., et al. Inflammatory mediators and other biomarkers in co-intoxicated patients after hyperbaric oxygen therapy (HBO2) Acta Med. Mediterr. 2016;32:189–193. doi: 10.19193/0393-6384_2016_1_29. [DOI] [Google Scholar]
- 106.Chen C.-Y., Wu R.-W., Hsu M.-C., Hsieh C.-J., Chou M.-C. Adjunctive hyperbaric oxygen therapy for healing of chronic diabetic foot ulcers. J. Wound Ostomy Cont. Nurs. 2017;44:536–545. doi: 10.1097/WON.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 107.Top C., Yildiz S., Öncül O., Qydedi T., Çevikbaş A., Soyogul U.G., Çavuşlu S. Phagocytic activity of neutrophils improves over the course of therapy of diabetic foot infections. J. Infect. 2007;55:369–373. doi: 10.1016/j.jinf.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 108.Yildiz H., Senol L., Ercan E., Bilgili M.E., Abuaf O.K. A prospective randomized controlled trial assessing the efficacy of adjunctive hyperbaric oxygen therapy in the treatment of hidradenitis suppurativa. Int. J. Dermatol. 2015;55:232–237. doi: 10.1111/ijd.12936. [DOI] [PubMed] [Google Scholar]
- 109.Xie Z., Zhuang M., Lin L., Xu H., Chen L., Hu L. Changes of plasma C-reactive protein in patients with craniocerebral injury before and after hyperbaric oxygenation: A randomly controlled study. Neural Regen. Res. 2007;2:314–317. [Google Scholar]
- 110.Schnittger V., Rosendahl K., Lind F., Palmblad J. Effects of carbon monoxide poisoning on neutrophil responses in patients treated with hyperbaric oxygen. J. Investig. Med. 2004;52:523–530. doi: 10.1136/jim-52-08-24. [DOI] [PubMed] [Google Scholar]
- 111.Hao Y., Dong X., Zhang M., Liu H., Zhu L., Wang Y. Effects of hyperbaric oxygen therapy on the expression levels of the inflammatory factors interleukin-12p40, macrophage inflammatory protein-1β, platelet-derived growth factor-BB, and interleukin-1 receptor antagonist in keloids. Med. Baltim. 2020;99:e19857. doi: 10.1097/MD.0000000000019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bent S., Bertoglio K., Ashwood P., Nemeth E., Hendren R.L. Brief report: Hyperbaric oxygen therapy (HBOT) in children with autism spectrum disorder: A clinical trial. J. Autism Dev. Disord. 2011;42:1127–1132. doi: 10.1007/s10803-011-1337-3. [DOI] [PubMed] [Google Scholar]
- 113.Aydin F., Kaya A., Karapinar L., Kumbaraci M., Imerci A., Karapinar H., Karakuzu C., Incesu M. IGF-1 Increases with hyperbaric oxygen therapy and promotes wound healing in diabetic foot ulcers. J. Diabetes Res. 2013;2013:1–6. doi: 10.1155/2013/567834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hedetoft M., Olsen N.V., Smidt-Nielsen I.G., Wahl A.M., Bergström A., Juul A., Hyldegaard O. Measurement of peripheral arterial tonometry in patients with diabetic foot ulcers during courses of hyperbaric oxygen treatment. Diving Hyperb. Med. J. 2020;50:17–23. doi: 10.28920/dhm50.1.17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Romero-Valdovinos M., Cárdenas-Mejía A., Gutiérrez-Gómez C., Flisser A., Kawa-Karasik S., Ortiz-Monasterio F. Keloid skin scars: The influence of hyperbaric oxygenation on fibroblast growth and on the expression of messenger RNA for insulin like growth factor and for transforming growth factor. Vitr. Cell. Dev. Biol. Anim. 2011;47:421–424. doi: 10.1007/s11626-011-9418-3. [DOI] [PubMed] [Google Scholar]
- 116.Irawan H., Semadi I.N., Devi A. Effect of hyperbaric oxygen therapy to improve serum albumin for patients with diabetic foot ulcers. Biomed. Pharmacol. J. 2018;11:569–575. doi: 10.13005/bpj/1409. [DOI] [Google Scholar]
- 117.Irawan H., Semadi I.N., Widiana I.G.R. A pilot study of short-duration hyperbaric oxygen therapy to improve HbA1c, Leukocyte, and serum creatinine in patients with diabetic foot ulcer Wagner 3–4. Sci. World J. 2018;2018:1–6. doi: 10.1155/2018/6425857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chong S.J., Kan E.M., Song C., Soh C.R., Lu J. Characterization of early thermal burns and the effects of hyperbaric oxygen treatment: A pilot study. Diving Hyperb. Med. J. 2013;43:157–161. [PubMed] [Google Scholar]
- 119.Sun J., Zheng J., Wang F., Zhang G., Wu J. Effect of hyperbaric oxygen combined with nimodipine on treatment of diffuse brain injury. Exp. Ther. Med. 2018;15:4651–4658. doi: 10.3892/etm.2018.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baiula M., Greco R., Ferrazzano L., Caligiana A., Hoxha K., Bandini D., Longobardi P., Spampinato S., Tolomelli A. Integrin-mediated adhesive properties of neutrophils are reduced by hyperbaric oxygen therapy in patients with chronic non-healing wound. PLoS ONE. 2020;15:e0237746. doi: 10.1371/journal.pone.0237746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benson R.M., Minter L.M., Osborne B.A., Granowitz E.V. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin. Exp. Immunol. 2003;134:57–62. doi: 10.1046/j.1365-2249.2003.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fan A., Zhou J. Effect of the combination of donepezil with hyperbaric oxygen therapy and functional rehabilitation training on parkinson’s disease dementia and the neurological function system. Int. J. Clin. Exp. Med. 2020;13:5867–5875. [Google Scholar]
- 123.Wilkinson D., Nolting M., Mahadi M.K., Chapman I., Heilbronn L. Hyperbaric oxygen therapy increases insulin sensitivity in overweight men with and without type 2 diabetes. Diving Hyperb. Med. J. 2015;45:30–36. [PubMed] [Google Scholar]
- 124.Weisz G., Lavy A., Adir Y., Melamed Y., Rubin D., Eidelman S., Pollack S. Modification of in vivo and in vitro TNF-α, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal crohn’s disease. J. Clin. Immunol. 1997;17:154–159. doi: 10.1023/A:1027378532003. [DOI] [PubMed] [Google Scholar]
- 125.Anguiano-Hernandez Y.M., Contreras-Mendez L., Hernandez-Cueto M.D.L.A., Muñoz-Medina J.E., Santillan-Verde M.A., Barbosa-Cabrera R.E., Delgado-Quintana C.A., Trejo-Rosas S., Santacruz-Tinoco C.E., Gonzalez-Ibarra J., et al. Modification of HIF-1α, NF-κB, IGFBP-3, VEGF and adiponectin in diabetic foot ulcers treated with hyperbaric oxygen. Undersea Hyperb. Med. 2019;46:35–44. doi: 10.22462/01.03.2019.4. [DOI] [PubMed] [Google Scholar]
- 126.MacKenzie D.A., Sollinger H.W., Hullett D.A. Role of CD4+ regulatory T cells in hyperbaric oxygen-mediated immune nonresponsiveness. Hum. Immunol. 2000;61:1320–1331. doi: 10.1016/S0198-8859(00)00214-7. [DOI] [PubMed] [Google Scholar]
- 127.Alex J., Laden G., Cale A.R., Bennett S., Flowers K., Madden L., Gardiner E., McCollum P.T., Griffin S.C. Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: A prospective randomized double-blind trial. J. Thorac. Cardiovasc. Surg. 2005;130:1623–1630. doi: 10.1016/j.jtcvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 128.Fildissis G., Venetsanou K., Myrianthefs P., Karatzas S., Zidianakis V., Baltopoulos G. Whole blood pro-inflammatory cytokines and adhesion molecules post-lipopolysaccharides exposure in hyperbaric conditions. Eur. Cytokine Netw. 2004;15:217–221. [PubMed] [Google Scholar]
- 129.Hou S., Wu G., Liang J., Cheng H., Chen C. Hyperbaric oxygen on rehabilitation of brain tumors after surgery and effects on TNF-α and IL-6 levels. Oncol. Lett. 2019;17:3277–3282. doi: 10.3892/ol.2019.10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin P.-Y., Sung P.-H., Chung S.-Y., Hsu S.-L., Chung W.-J., Sheu J.-J., Hsueh S.-K., Chen K.-H., Wu R.-W., Yip H.-K. Hyperbaric oxygen therapy enhanced circulating levels of endothelial progenitor cells and angiogenesis biomarkers, blood flow, in ischemic areas in patients with peripheral arterial occlusive disease. J. Clin. Med. 2018;7:548. doi: 10.3390/jcm7120548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rosario E.R., Kaplan S.E., Khonsari S., Vazquez G., Solanki N., Lane M., Brownell H., Rosenberg S.S. The effect of hyperbaric oxygen therapy on functional impairments caused by ischemic stroke. Neurol. Res. Int. 2018;2018:1–12. doi: 10.1155/2018/3172679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Song K.-X., Liu S., Zhang M.-Z., Liang W.-Z., Liu H., Dong X.-H., Wang Y.-B., Wang X.-J. Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J. Zhejiang Univ. Sci. B. 2018;19:853–862. doi: 10.1631/jzus.B1800132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guggino G., Schinocca C., Pizzo M.L., Di Liberto D., Garbo D., Raimondo S., Alessandro R., Brighina F., Ruscitti P., Giacomelli R., et al. T helper 1 response is correlated with widespread pain, fatigue, sleeping disorders and the quality of life in patients with fibromyalgia and is modulated by hyperbaric oxygen therapy. Clin. Exp. Rheumatol. 2019;37:81–89. [PubMed] [Google Scholar]
- 134.Wang B.-W., Lin C.-M., Wu G.-J., Shyu K.-G. Tumor necrosis factor-α enhances hyperbaric oxygen-induced visfatin expression via JNK pathway in human coronary arterial endothelial cells. J. Biomed. Sci. 2011;18:27. doi: 10.1186/1423-0127-18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nasole E., Nicoletti C., Yang Z.-J., Girelli A., Rubini A., Giuffreda F., Di Tano A., Camporesi E., Bosco G. Effects of alpha lipoic acid and its R+ enantiomer supplemented to hyperbaric oxygen therapy on interleukin-6, TNF-α and EGF production in chronic leg wound healing. J. Enzym. Inhib. Med. Chem. 2013;29:297–302. doi: 10.3109/14756366.2012.759951. [DOI] [PubMed] [Google Scholar]
- 136.Yoshinoya Y., Böcker A.H., Ruhl T., Siekmann U., Pallua N., Beier J.P., Kim B.-S. The effect of hyperbaric oxygen therapy on human adipose-derived stem cells. Plast. Reconstr. Surg. 2020;146:309–320. doi: 10.1097/PRS.0000000000007029. [DOI] [PubMed] [Google Scholar]
- 137.Liu X.H., Liang F., Jia X.Y., Zhao L., Zhou Y., Yang J. Hyperbaric oxygen treatment improves hearing level via attenuating TLR4/NF-κB mediated inflammation in Sudden sensorineural hearing loss patients. Biomed. Environ. Sci. 2020;33:331–337. doi: 10.3967/bes2020.045. [DOI] [PubMed] [Google Scholar]
- 138.Semadi N.I. The role of VEGF and TNF-Alpha on epithelialization of diabetic foot ulcers after hyperbaric oxygen therapy. Open Access Maced. J. Med Sci. 2019;7:3177–3183. doi: 10.3889/oamjms.2019.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Madden L.A., Vince R.V., Laden G. The effect of acute hyperoxia in vivo on NF kappa B expression in human PBMC. Cell Biochem. Funct. 2010;29:71–73. doi: 10.1002/cbf.1712. [DOI] [PubMed] [Google Scholar]
- 140.Sun L., Zhao L., Li P., Liu X., Liang F., Jiang Y., Kang N., Gao C., Yang J. Effect of hyperbaric oxygen therapy on HMGB1/NF-κB expression and prognosis of acute spinal cord injury: A randomized clinical trial. Neurosci. Lett. 2019;692:47–52. doi: 10.1016/j.neulet.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 141.Tra W.M.W., Spiegelberg L., Tuk B., Hovius S.E.R., Perez-Amodio S. Hyperbaric oxygen treatment of tissue-engineered mucosa enhances secretion of angiogenic factors in vitro. Tissue Eng. Part A. 2014;20:1523–1530. doi: 10.1089/ten.tea.2012.0629. [DOI] [PubMed] [Google Scholar]
- 142.Lee C.-C., Chen S.-C., Tsai S.-C., Wang B.-W., Liu Y.-C., Lee H.-M., Shyu K.-G. Hyperbaric oxygen induces VEGF expression through ERK, JNK and c-Jun/AP-1 activation in human umbilical vein endothelial cells. J. Biomed. Sci. 2005;13:143–156. doi: 10.1007/s11373-005-9037-7. [DOI] [PubMed] [Google Scholar]
- 143.Jung S., Wermker K., Poetschik H., Ziebura T., Kleinheinz J. The impact of hyperbaric oxygen therapy on serological values of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) Head Face Med. 2010;6:29. doi: 10.1186/1746-160X-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin S., Shyu K.-G., Lee C.-C., Wang B.-W., Chang C.-C., Liu Y.-C., Huang F.-Y., Chang H. Hyperbaric oxygen selectively induces angiopoietin-2 in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2002;296:710–715. doi: 10.1016/S0006-291X(02)00924-5. [DOI] [PubMed] [Google Scholar]
- 145.Mutzbauer T., Schneider M., Neubauer B., Weiss M., Tetzlaff K. Antioxidants may attenuate plasma erythropoietin decline after hyperbaric oxygen diving. Int. J. Sports Med. 2015;36:1035–1040. doi: 10.1055/s-0035-1555782. [DOI] [PubMed] [Google Scholar]
- 146.Wu L.L., Chiou C.-C., Chang P.-Y., Wu J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 147.Kang T.S., Gorti G.K., Quan S.Y., Ho M., Koch R.J. Effect of hyperbaric oxygen on the growth factor profile of fibroblasts. Arch. Facial Plast. Surg. 2004;6:31–35. doi: 10.1001/archfaci.6.1.31. [DOI] [PubMed] [Google Scholar]
- 148.Kunnavatana S.S., Quan S.Y., Koch R.J. Combined effect of hyberbaric oxygen and n-acetylcysteine on fibroblast proliferation. Arch. Otolaryngol. Head Neck Surg. 2005;131:809–814. doi: 10.1001/archotol.131.9.809. [DOI] [PubMed] [Google Scholar]
- 149.Naito Y., Lee M., Kato Y., Nagai R., Yonei Y. Oxidative Stress Markers. Anti-Aging Med. 2010;7:36–44. doi: 10.3793/jaam.7.36. [DOI] [Google Scholar]
- 150.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 151.Ansar W., Ghosh S. Biology of C Reactive Protein in Health and Disease. Springer; New Delhi, India: 2016. Inflammation and Inflammatory Diseases, Markers, and Mediators: Role of CRP in Some Inflammatory Diseases; pp. 67–107. [Google Scholar]
- 152.Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016;2016:1–9. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vaziri N.D., Rodríguez-Iturbe B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 154.Chatterjee S. Oxidative Stress and Biomaterials. Academic Press; Cambridge, MA, USA: 2016. Oxidative Stress, Inflammation, and Disease; pp. 35–58. [Google Scholar]
- 155.Glik J., Cholewka A., Stanek A., Sieroń K., Mikuś-Zagórska K., Knefel G., Nowak M., Kawecki M., Englisz B. Thermal imaging and planimetry evaluation of the results of chronic wounds treatment with hyperbaric oxygen therapy. Adv. Clin. Exp. Med. 2019;28:229–236. doi: 10.17219/acem/92304. [DOI] [PubMed] [Google Scholar]
- 156.Englisz-Jurgielewicz B., Cholewka A., Firganek E., Knefel G., Kawecki M., Glik J., Nowak M., Sieroń K., Stanek A. Evaluation of hyperbaric oxygen therapy effects in hard-to-heal wounds using thermal imaging and planimetry. J. Therm. Anal. Calorim. 2019;141:1465–1475. doi: 10.1007/s10973-019-09129-0. [DOI] [Google Scholar]
- 157.Frangogiannis N., Smith C., Entman M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002;53:31–47. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 158.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lawrence T. The nuclear factor NF- B pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu X., Li Y., He X., Li X., Din B., Gan Y., Xu M. Hyperbaric oxygen reduces inflammatory response in acute pancreatitis by inhibiting NF-κB activation. Eur. Surg. Res. 2009;42:130–135. doi: 10.1159/000196164. [DOI] [PubMed] [Google Scholar]
- 161.Szade A., Grochot-Przeczek A., Florczyk U., Jozkowicz A., Dulak J. Cellular and molecular mechanisms of inflammation-induced angiogenesis. IUBMB Life. 2015;67:145–159. doi: 10.1002/iub.1358. [DOI] [PubMed] [Google Scholar]
- 162.Fallah A., Sadeghinia A., Kahroba H., Samadi A., Heidari H.R., Bradaran B., Zeinali S., Molavi O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019;110:775–785. doi: 10.1016/j.biopha.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 163.Maulik N. Redox signaling of angiogenesis. Antioxid. Redox Signal. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- 164.Dejmek J., Kohoutová M., Kripnerová M., Cedikova M., Tůma Z., Babuška V., Bolek L., Kuncová J. repeated exposure to hyperbaric hyperoxia affects mitochondrial functions of the lung fibroblasts. Physiol. Res. 2018;67:S633–S643. doi: 10.33549/physiolres.934046. [DOI] [PubMed] [Google Scholar]
- 165.Sinan M., Ertan N.Z., Mirasoglu B., Yalcin O., Atac N., Toklu A.S., Basaran-Kucukgergin C., Baskurt O.K. Acute and long-term effects of hyperbaric oxygen therapy on hemorheological parameters in patients with various disorders. Clin. Hemorheol. Microcirc. 2016;62:79–88. doi: 10.3233/CH-151952. [DOI] [PubMed] [Google Scholar]