Abstract
The use of tunneled dialysis catheters (TDCs) for patients in need of hemodialysis treatments (HDs) cause a significant number of blood stream infections (BSIs), with very few viable preventative/treatment methods. Use of antibiotics are relatively ineffective due to the development of multi-drug resistant bacterial strains and their inability to penetrate bacterial biofilms. Nitric oxide (NO) is an endogenous gas molecule that has broad spectrum antimicrobial/anti-biofilm activity. In this study, the potential of creating a NO releasing insert device that is attached onto the hub region cap of TDCs and locally releases NO within the TDC hub is evaluated for its antimicrobial/anti-biofilm effectiveness. The NO releasing insert contains the natural NO donor S-nitrosoglutathione (GSNO) along with zinc oxide (ZnO) nanoparticles to accelerate NO release from the GSNO, within a short silicone tube that is sealed at both ends and attached to the catheter cap. An in vitro 3 d long antimicrobial study using catheter hubs yielded >6.6 log reductions of both P. aeruginosa and S. aureus for the NO releasing insert device compared to controls. Two 14 d long sheep studies demonstrated that the NO releasing insert devices are exceptionally potent at preventing bacteria/biofilm growth on the inner lumen walls of TDCs compared to controls that have no preventative treatment devices as well as implanted TDCs that have commercially available chlorhexidine treated insert devices placed within the hub regions.
Keywords: catheters, nitric oxide, antimicrobial, anti-biofilm, S-nitrosoglutathione, zinc oxide nanoparticles
Graphical Abstract

1. Introduction
Tunneled dialysis catheters (TDCs) are commonly utilized for patients with End Stage Renal Disease (ESRD) that require hemodialysis (HD) treatments. Even though it is generally accepted that TDCs are the least desirable form of dialysis vascular access, in 2010 approximately 80% of patients started HD treatment using a TDC and this percentage has remained relatively unchanged since then.1 One of the major complications associated with TDCs is blood stream infections (BSIs). BSIs caused by TDCs leaves patients with substantial morbidity, mortality, expense, and a decrease in their quality of life.2
For long-term TDCs, BSIs predominately occur from bacteria/biofilm growth via the intraluminal route because of hub region contamination.3 The most favored treatment of foreign body-related infection is removal of the foreign body (TDC); however, this may not be practical for the patient. Thus, methods and devices have been developed to treat/prevent BSIs related to TDCs. A few notable treatment/prevention methods include antimicrobial lock therapy, Tego Needlefree Hemodialysis Connector plus a Curos Disinfecting Cap for Tego, and a ClearGuard HD Antimicrobial Barrier Cap.
Antimicrobial lock therapy (ALT) involves infusing a highly concentrated antibiotic solution into the catheter lumen for a minimum of 2-4 h.2, 4 Although there is no Food and Drug Administration (FDA) approved ALT, ALT practice is still common.2 However, there are limitations to the use of ALTs such as generation of antibiotic resistant organisms and the inability of ALTs to penetrate pre-existing biofilm. Certain chemicals such as citrate and ethylenediaminetetraacetic acid (EDTA) have been added to help disrupt the biofilm, but high rates of ALT failure still occur with Staphylococcus aureus and Pseudomonas aeruginosa.2, 5, 6
A recent study compared the performance of two FDA approved devices in relation to the rate of BSIs. These devices included the combination of Tego Needlefree Hemodialysis Connector (Tego) plus Curos Disinfecting Cap for Tego (Curos) and the ClearGuard HD Antimicrobial Barrier Cap (ClearGuard).7 Tego is a device that attaches to the hub and aims to prevent infection by using a “closed” system to reduce catheter hub manipulation.7, 8 Curos is a port protector that utilizes 70% isopropanol to kill bacteria on the surface of Tego.7, 9 ClearGuard is a device with a protruding rod and threads that are coated with chlorhexidine.7, 10 Chlorohexidine is a well-known, broad spectrum antimicrobial agent with minimal risk of developing resistant bacteria strains.7, 10 After a 13 month long study, the ClearGuard had a significantly lower BSI rate of 0.28 positive blood cultures (PBC) per 1000 TDC-days versus 0.75 PBC per 1000 TDC-days for Tego + Curos.7 The potential downfall of the Tego + Curos device is that only the outer surface of the catheter hub is targeted. A potential limitation of the ClearGuard product is that chlorhexidine has trouble dispersing and killing bacterial biofilms.11
A device that retains the positive characteristics (effective broad spectrum antimicrobial agent, minimal risk of developing resistant bacteria strains) to prevent BSIs but also solves prior device short-comings (effectiveness against biofilms) is one that involves the use of nitric oxide. Nitric oxide (NO) is an endogenously produced, free radical gas molecule that is synthesized by the enzyme nitric oxide synthase (NOS).12-15 The body naturally produces NO when fighting an infection via immune system macrophages.13, 14 NO is highly effective against a broad spectrum of bacteria including antibiotic-resistant strains.12, 13, 16
Nitric oxide is also effective at dispersing/displacing biofilms.17-20 For example, NO releasing S-nitroso-N-acetyl-D-penicillamine (SNAP) doped catheters were found to reduce S. aureus biofilms significantly over a 7-day test period in a bioreactor, with a 5 log unit reduction in bacterial counts compared to control catheters without NO release.20 Therefore, a NO releasing insert device that could disinfect the inner lumen of TDCs has great potential for preventing BSIs because of its inherent antimicrobial non-specificity, as well as biofilm dispersal and killing capabilities.
Herein, the NO releasing characteristics of various NO releasing insert devices are evaluated along with their antimicrobial efficacy against S. aureus in a simulated catheter hub model. The most promising NO releasing insert is also evaluated for stability of the NO-donor molecule after being subjected to different sterilization methods. The antimicrobial efficacy of the most promising NO releasing insert formulation is further tested in commercially available hemodialysis catheter hubs against both gram-negative (P. aeruginosa) and gram-positive (S. aureus) bacteria strains in an in vitro model. Finally, two 14-d long sheep studies compare the antimicrobial/anti-biofilm capabilities of the chosen NO releasing insert versus a control normal hemodialysis catheter cap and as well as versus a commercially available cap that utilizes the antimicrobial agent chlorhexidine. It will be shown that the new NO release inserts provide superior antimicrobial/anti-biofilm activity compared to the existing commercial chlorhexidine TDC insert technology.
2. Materials and Methods
2.1. Materials
L-Glutathione reduced (GSH), hydrochloric acid (HCl), sodium nitrite, and polyethylene glycol (MW = 3,350) were purchased from Sigma-Aldrich (St. Louis, MO). Acetone; DOWSIL 3140, MIL-A-46146 RTV Silicone Coating; Dow Corning Silastic Laboratory Tubing (ID 0.058" × OD 0.077", 2415542); and Helix Medical Inc. Silicone Tubing (0.125" × 0.250", 6001121) were purchased from Fisher Scientific Inc. (Pittsburgh, PA). Male Luer Lock Injection Site caps (80149) were purchased from Qosina (Ronkonkoma, NY). Luria-Bertani (LB) agar broth and 10 mM phosphate buffered saline (PBS) (pH 7.2) were purchased from ThermoFisher Scientific (Grand Island, NY). Zinc oxide nanoparticles (APS 30 nm in diameter) were purchased from EPRUI Biotech Co. Ltd. (ShangHai, China). All aqueous solutions were prepared with 18.2 M Ω deionized water using a Milli-Q filter (Milli-q purified water) from EMD Millipore (Billerica, MA). Staphylococcus aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 27853 were obtained from the American Type Culture Collection (Manassas, VA).
2.2. S-Nitrosoglutathione (GSNO) synthesis
GSNO was synthesized as previously reported. In brief, an adapted method of the procedure reported initially by Hart et al. was used to synthesize GSNO.21 Synthesis was completed in the absence of light. Reduced glutathione (GSH) was dissolved in aqueous HCl, cooled to 0°C using an ice bath, and continuously purged under nitrogen. An equal molar amount of sodium nitrite was added directly to the GSH solution. After stirring for 40 min, ice-cold acetone was added into the reaction mixture to precipitate the GSNO. After stirring for another 10 min, the pink precipitate was separated by vacuum filtration. The resulting pink powder was washed by ice-cold water (3 × 10 mL) and acetone (3 × 10 mL), respectively. Finally, the desired GSNO product was obtained after drying under vacuum at room temperature for 24 h. GSNO was stored at freezer temperature (−20°C) in the dark for any further use.
2.3. Fabrication of NO releasing inserts
The entire procedure involving GSNO was completed in the absence of direct light. Silicone tubing (ID 0.058″, OD 0.077″) was cut to ~3 cm and sealed at one end using an adhesive glue (DOWSIL 3140, MIL-A-46146 RTV Coating) and allowed to dry for 24 h. Then, 12 ± 0.2 mg of a desired dry powder formulation was dispensed into the tubing using a glass funnel pipet. The dry powder formulations used included (a) 75% GSNO : 25% 30 nm size ZnO nanoparticles; (b) 25% GSNO : 75% 30 nm size ZnO nanoparticles; (c) 60% GSNO : 20% 30 nm size ZnO nanoparticles : 20% solid polyethylene glycol (MW = 3,350) (PEG); (d) 75% GSNO : 25% fumed silica. Before use, the GSNO was crushed into a fine powder using a mortar and pestle and mixed with the other components by vortexing for 1 min to achieve a homogeneous dry powder mixture. After filling the tubing with the desired powdered formulation via a glass funnel (~12 mg = ~1.8 cm fill length), the end that was employed to fill the tubing was cut to achieve ~0.2 cm head space above the fill powder. Lastly, adhesive glue was used to seal the open end and allowed to dry for 24 h. The final length of the inserts are ~2.0 cm.
2.4. Measuring real-time NO release from inserts
Nitric oxide release from the NO releasing inserts was measured using a Sievers Chemiluminescence Nitric Oxide Analyzer (NOA) 280i (Boulder, CO). The NOA was calibrated before via a two-point calibration of N2 gas passed through a NOA zero air filter and a standard of 44.3 ppm NO in N2 gas. Saline solution (0.9% sodium chloride) was made using 18.2 M Ω deionized water using a Milli-Q filter (Milli-q purified water) from EMD Millipore (Billerica, MA). The NOA sample cell was filled with 11 mL of saline solution and the NO releasing insert was placed below a floating polypropylene barrier to keep the insert fully submerged at all times. The saline solution reservoir was bubbled with N2 gas at a rate of 50 mL/min to allow the NO generated from GSNO to escape from the solution and be carried into the NOA by the N2 sweep gas. All NOA sample cells were wrapped in aluminum foil to shield the samples from light exposure. NO release was continuously monitored for 72 h at room temperature (24°C).
2.5. In vitro simulated catheter hub antimicrobial assay
To simulate the hub region of a catheter, 3 cm of silicone tubing (ID 0.125″, OD 0.250″) was employed. A volume of 0.3 mL of overnight grown bacteria cultures (1 × 108 CFU/mL) in 10% LB broth was transferred into the simulated hub clamped at one end. Then, a NO releasing insert was placed inside of the simulated hub and the other end clamped shut. For control samples, no NO releasing insert was added. Each sample was incubated at room temperature (24°C) in the dark, for 72 h, on a shaker at low speed. After 72 h of incubation, 20 μL of bacteria culture liquid was retrieved from each simulated hub and 10-fold serially diluted. Fifty μL of each dilution was spread on LB agar plates and incubated at 37°C overnight for colony-forming unit (CFU) counting. The simulated hub was also sliced into small pieces and the inside was stained with BacLight Live/Dead staining kit in the dark for 15 min to assess the degree of biofilm. Microscopic images were obtained by using a fluorescent microscope with appropriate filter sets (488/520 nm for SYTO-9 and 493/636 nm for propidium iodide).
2.6. Sterilization studies: Ethylene oxide (EO) vs. hydrogen peroxide (H2O2)
Nitric oxide releasing inserts (formulation (a)) were prepared as described in Section 2.3, individually packaged into separate pouches, and sent to the University of Michigan hospital sterilization facility for ethylene oxide (EO) or hydrogen peroxide (H2O2) treatment. For EO treatment, the NO releasing inserts undergo a 1 h preconditioning and humidification process (54°C, 40-80% humidity), followed by 3 h of exposure to ethylene oxide gas under the same temperature and humidity. Then, a 2 h ethylene oxide gas evacuation process occurs, followed by 12 h of air washes. For H2O2 treatment or STERRAD®, the process takes approximately 45 min total. Under vacuum, 59% (nominal) aqueous H2O2 is vaporized to cover the NO releasing inserts. Diffusion of the gaseous H2O2 occurs while the pressure is reduced, forming low-temperature H2O2 gas plasma after radio frequency (RF) energy is applied. The H2O2 gas plasma generated sterilizes the NO releasing inserts. Control NO releasing inserts were prepared as described in Section 2.3, but not sterilized. The amount/stability of GSNO within each insert was measured by releasing all of the NO from GSNO via shining UV light on them and detecting/quantitating the total amount of NO released using a NOA. Specifically, 2 mL of Milli-q purified water was added to an NOA sample cell. After a steady baseline was achieved, a NO releasing insert was cut open and the powder filling was transferred into the sample cell using another 2 mL of Milli-q purified water. An additional 1 mL of Milli-q purified water was used to rinse all remaining powder on the NOA sample cell walls, down into the bulk solution (total 5 mL of Milli-q purified water). The GSNO/ZnO containing solution was bubbled with N2 gas at a rate of 50 mL/min to escape from the solution and be carried into the NOA by the N2 sweep gas. UV light was used to irradiate the sample until NO release from GSNO was exhausted, marked by a return to the original baseline. The amount of NO released from each NO releasing insert was directly converted to GSNO because the mole ratio is 1:1. The highest amount of GSNO measured from the three NO releasing control inserts (not sterilized) was assumed to be 100% recovery of GSNO; therefore. all other samples (sterile and non-sterile) were normalized to this value. Thus, >100% GSNO recovery was possible.
2.7. Stability Study
Nitric oxide releasing inserts using formulation (a) (75% GSNO : 25% 30 nm size ZnO nanoparticles) were prepared. Three NO releasing inserts were measured for their amount of GSNO on Day 0 without any sterilization processes. On Day 0 the remaining NO releasing inserts were sterilized using the H2O2 sterilization method described above. After sterilization was completed on Day 0, each sterile insert remained in their individual sterilization pouches and were stored in a sealed glass jar with desiccant, in the dark, at room temperature (24°C) until further use. The %GSNO recovery from a NO releasing insert on any given day was achieved using the method outlined in Section 2.6. Briefly, the NO releasing inserts were cut open and the powder washed into a NOA sample cell. All the NO was exhausted from GSNO using UV light. The NO was quantitated and measured by a NOA. All recovery values obtained were normalized to the highest amount of GSNO obtained from one of the three control NO releasing inserts (not sterilized).
2.8. In vitro catheter hub antimicrobial assay
The catheters utilized for this assay were 28 cm long Permcath™ Pediatric Silicone Chronic Dual Lumen Oval Catheters, Covidien/Medtronic (Ref 8815543001, Lot 1611800146). The clamp on the catheter’s hub region was clamped shut and 0.3 mL of overnight grown bacteria cultures (1 × 108 CFU/mL) in 10% LB broth was added. A NO releasing insert (pre-sterilized by the H2O2 sterilization method mentioned above) was inserted inside of the catheter hub and sealed by a cap. For control samples, no NO releasing insert was added. Each catheter was incubated at room temperature (24°C) in the dark, for 72 h, on a shaker at low speed. After 72 h of incubation, 20 μL of bacteria culture liquid was retrieved from each hub region and 10-fold serially diluted. Fifty μL of each dilution was spread on LB agar plates and incubated at 37°C overnight for colony-forming unit (CFU) counting.
2.9. Sheep studies - general procedure
This study including animal handling and surgical procedures that were approved by the University of Michigan Committee on Use and Care of Animals (24 h fasting and pre-surgical analgesia with Fentanyl transdermal patch 100 μg/h) in accordance with university and federal regulations. Adult sheep weighing 45-50 kg were utilized. Under general anesthesia, 28 cm long (13 cm cuff to proximal tip) Permcath™ Pediatric Silicone Chronic Dual Lumen Oval Catheters, Covidien/Medtronic (8815543001) were placed using the Seldinger wire technique in the right and left jugular veins (3-5 cm above the subclavian), aiming to place the proximal tip in the RA-SVC junction. Caution was taken not to expose or manipulate the vessels. Catheters were tunneled (5cm), secured to the skin, sterile gauze was placed at the insertion site, and the neck was covered with elastic wrapping and a neck collar. After catheter placements, the sheep were recovered from anesthesia and housed in a barn (non-sterile conditions) for the remainder of the experiment.
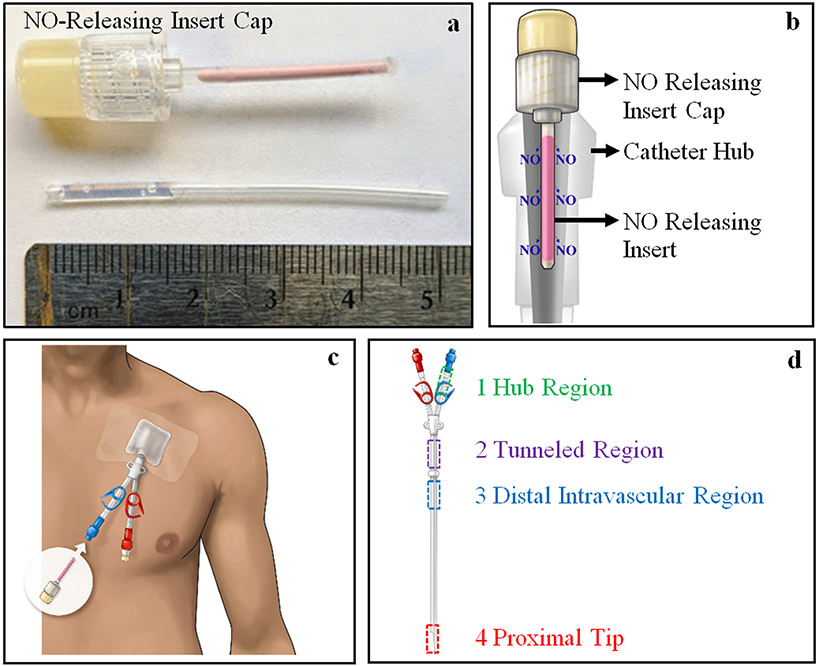
All catheters were capped and filled with 2,000 U heparinized saline solution (2 mL) (West-Ward, Eatontown, NJ) injected via the distal end of the lumen. Each sheep was evaluated for redness and swelling at the sight of catheter placement as well as for fever and eating habits during each study. The NO releasing inserts used for both sheep studies were made using formulation (a) 75% GSNO : 25% 30 nm size ZnO nanoparticles. The NO releasing inserts were attached to male luer lock injection site caps, Qosina (80149), using the same adhesive glue used in Section 2.3, and allowed to dry for 24 h (Fig. 1a). Each NO releasing insert cap was individually packaged and sterilized using the H2O2 sterilization method described previously. The sterile NO releasing insert caps were stored at room temperature (24°C) and shielded from light until further use.
Figure 1.
(a) (top) NO releasing insert cap used in Sheep Study #1 and #2 and (bottom) the silicone tubing filled partially with adhesive glue prior to gluing it to cap (pre-glued section is inside of cap). (b) Schematic of NO releasing insert cap attached to a catheter hub. (c) Image showing placement of the NO releasing insert cap on the hub region of catheters and outside of the body. (d) Regions of catheter studied in Sheep Study #1 and #2.
Prior to necropsy (day 14), 10,000 U bolus of heparin was given via a cephalic vein angiocath, followed by FatalPlus IV (Vortech Pharmaceutical, Dearborn, MI) injection. Each catheter was procured using sterile techniques. The external surface of each catheter was sterilized by wiping with a 70% ethanol solution. One cm length sections were cut from each section of catheter as shown in Figure 1b. Each section was homogenized in 2 mL of 1 × PBS (10 mM, pH 7.2) in a 15-mL tube using a homogenizer (OMNI TH, OMNI International, Kennesaw, GA) at full speed to remove all bacteria/biofilm adhered to the inner lumen walls, and the resulting solution was 10-fold serially diluted. Fifty μL of each dilution was spread on LB agar plates and incubated at 37°C overnight for CFU counting. Additionally, >0.5 cm length sections were cut from each section of catheter described in Figure 1d, and the inner lumen surfaces were stained with BacLight Live/Dead staining kit in the dark for 15 min. Microscopic images were obtained by using a fluorescent microscope with appropriate filter sets (488/520 nm for SYTO-9 and 493/636 nm for propidium iodide).
2.10. Sheep Study #1: Hemodialysis catheters with NO releasing insert caps vs control (without NO)
Two adult sheep, were randomly assigned to have either inserts without NO release (controls) or NO releasing insert caps (experimental). Control sheep (n = 4 catheter hubs total, without NO releasing inserts) and experimental animal (n = 4 catheter hubs total, using NO releasing insert caps). On postoperative days 0, 2, 4, 7, 9, 11, and 14, 50 μL of liquid from the hub region of each catheter lumen was taken for CFU counting. Then, 3.5 mL of blood was drawn from each catheter lumen, the lumens were then locked with 2,000 U heparinized saline solution (2 mL), and both NO releasing insert caps and control caps were replaced with new caps, respectively (Fig. 1b).
2.11. Sheep Study #2: Hemodialysis catheters with chlorhexidine insert caps vs NO releasing insert caps
In a similar fashion as the study described above, two adult sheep were assigned to have commercially available antimicrobial caps with chlorhexidine as the antimicrobial agent or NO releasing insert caps. For one sheep, the catheter implanted in the right jugular vein was designated for the commercial chlorhexidine caps and the catheter implanted in the left jugular vein was designated for the NO release insert caps. For the second sheep, experimental catheters were placed in the contralateral side. In total, for chlorhexidine caps there were n = 4 catheter hubs and for the NO releasing insert caps there were also n = 4 catheter hubs. On postoperative days 0, 2, 3, 6, 8, 10, 12, and 14, 50 μL of liquid from the hub region of each catheter lumen was taken for CFU counting. Then, 3.5 mL of blood were drawn from each lumen, locked with 2,000 U heparinized saline solution (2 mL), and both NO releasing insert caps and chlorhexidine caps were replaced with new caps, respectively (Fig. 1b).
3. Results and Discussion
3.1. Design of NO releasing inserts
The dimensions of the NO releasing insert were designed based on the hub dimensions of commonly used hemodialysis catheters (see Fig. S1 for a schematic of the NO releasing insert). S-nitrosoglutathione (GSNO) was chosen as the NO donor molecule because it is endogenously produced and fairly stable when stored as a powder in the absence of moisture.22, 23 ZnO nanoparticles (30 nm diameter) were chosen to enhance the NO release from GSNO.24 This combination was demonstrated via the development of a topical antimicrobial cream. Therefore, the combination of using GSNO and ZnO together to create the NO release insert devices for TDCs was chosen to add another application of this new NO releasing combination. Silicone rubber tubing was chosen because the high diffusivity of NO through silicone rubber compared to other biomedical grade polymers and its relatively low hardness allows moisture to pass through its walls to initiate NO release from GSNO.25
3.2. Characterizing real-time NO release of NO releasing insert formulations
The real-time NO release of four different NO releasing insert formulations was evaluated to determine each formulation’s NO release characteristics. Nitric oxide release was measured from each NO releasing insert over a 72 h period while submerged in saline solution in the dark at room temperature (24°C). The measurement conditions were as close to “real-world” conditions of hemodialysis catheter hubs as possible. Measuring over a 72 h period was chosen because hemodialysis treatments normally occur every 2-3 days, enabling the NO release insert to be changed at each dialysis session. The other conditions were chosen because catheter hubs are located outside of the body, opaque, and locked with a saline lock solution (Fig 1c).
Four different NO releasing insert formulations were tested for their NO release characteristics under real world conditions (Fig. 2). The dry powder NO releasing formulations studied include (a) 75% GSNO : 25% 30 nm size ZnO nanoparticles; (b) 25% GSNO : 75% 30 nm size ZnO nanoparticles; (c) 60% GSNO : 20% 30 nm size ZnO nanoparticles : 20% solid polyethylene glycol (MW = 3,350) (PEG); and (d) 75% GSNO : 25% fumed silica. Formulation (a) yielded a large burst of NO over the first 24 h and tapered off until the 72 h mark was reached (Fig. 2). For formulation (b), the percentages of GSNO and ZnO were reversed compared to formulation (a). Formulation (b) demonstrated a similar NO release profile compared to formulation (a), however the initial burst lasted only 12 h and tapered off significantly afterwards due to the lower amount of GSNO initially present (Fig. 2). In attempt to level/smooth out the NO release profile of formulation (a), polyethylene glycol (MW=3,350) (PEG) was added to increase the viscosity of the insert’s internal components (GSNO and ZnO). The addition of PEG should increase the internal viscosity upon moisture absorption, imposing a cage effect on the thiyl and NO radical pair such that they recombine to form GSNO and slow the rate of NO release.26, 27 The addition of PEG led to formulation (c) and still includes GSNO and ZnO. The expected leveling/smoothing effect was achieved, leading to a more consistent NO release rate over 72 h (Fig. 2). To prove that ZnO was necessary to enhance NO release in each formulation, fumed silica particles were substituted in place of ZnO as an inert agent that does not react with GSNO. The NO release profile of formulation (d) shows minimal NO release over 72 h (Fig. 2). This data definitively proves that ZnO is needed to achieve significant NO release from GSNO contained within the silicone tubing.
Figure 2.
Real-time NO flux of inserts prepared with different formulations a-d in the dark at 24°C over a 72 h period. Data represents the mean ± SD (n = 3).
3.3. Antimicrobial efficacy of NO releasing formulations (a-c) inserts in simulated catheter hubs
Formulations (a-c) displayed unique NO releasing profiles over 72 h. Therefore, each was tested for their bactericidal effects using a “Simulated Hub” antimicrobial experiment. This experiment was designed to mimic the conditions of a real hemodialysis catheter hub. Silicone tubing with a similar inner diameter to that of an actual hemodialysis catheter hub was utilized as the “Simulate Hub”. A detailed description of this study can be found in Section 2.5. Briefly, a given concentration of Staphylococcus aureus (S. aureus) in 10% LB broth was put inside of the silicone tubing that was sealed at one end. The NO releasing inserts were placed inside of the simulated hub and the opposite end closed. The controls contained no NO releasing insert. The prepared simulated hubs were subjected to real world ambient conditions as stated previously (24°C, dark, 72 h, and on a shaker). After 72 h, the amount of bacteria in the liquid broth was enumerated for each sample and the inner lumen wall of the simulated hub was imaged for bacteria/biofilm adhered to its surface.
To confirm that no ZnO or zinc ions leached out of the NO releasing insert over the 72 h soaking period and potentially contribute to the antimicrobial effects observed, we measured the concentration of zinc in the soaking solution after 72 h using inductively coupled plasma mass spectrometry (ICP-MS). Our results showed that no ZnO or zinc ions leached out of the NO releasing insert over the 72 h soaking period (data not shown). For each NO releasing insert formulation (a-c), the antimicrobial assay detected no bacteria present in the liquid broth of each simulated hub, leading to a 6.4 log reduction of bacteria compared to the control (no NO releasing insert) (see Fig. S2). This data indicates that formulations (a-c) are capable of killing S. aureus bacterial cells in the liquid broth of a simulated catheter hub region. This data also demonstrates how potent/effective local NO release can be by minimizing the opportunity NO has to be consumed by reacting with other molecules. Fluorescent microscopic images were taken of the inner lumen wall of each simulated hub and representative images are pictured in Figure 3. The control as well as formulations (b) and (c) displayed evidence of S aureus bacteria/biofilm adhered to the inner lumen wall of the simulated hubs (Fig. 3). However, formulation (a) showed no evidence of significant S aureus bacteria/biofilm adhesion (Fig. 3). The data from formulation (a) suggests that having a large burst of NO over the first 24 h is needed to prevent biofilm formation. Therefore, formulation (a), 75% GSNO : 25% 30 nm ZnO nanoparticles, was chosen as the formulation to continue testing because of its excellent antimicrobial/anti-biofilm characteristics.
Figure 3.
Fluorescent microscopic images of S. aureus bacteria/biofilm adhered to the inner lumen wall of the simulated hub after exposure to no insert (control) or NO releasing inserts with formulations (a-c) for 72 h in the dark at 24°C. Live/Dead dye stain was used where green = alive cells and red = dead cells.
3.4. Sterilization and Stability Testing
Sterilization of the NO releasing inserts would be needed for animal testing. GSNO naturally reacts to release NO in the presence of light, heat, metal ions, and water.22, 23, 26, 28-32 Thus, different sterilization methods were tested to see if they had any negative effects on GSNO stability. Again, the NO releasing formulation (a) insert was utilized in these studies. Two common sterilization methods were tested as described in Section 2.6 (above), ethylene oxide (EO) gas and hydrogen peroxide (H2O2) plasma. EO sterilization is a traditional sterilization method that takes place at a temperature of 54°C and requires several hours to complete. H2O2 sterilization is a relatively new method of sterilization that occurs at a temperature of 40°C and takes ~45 min to complete.
To test if the sterilization methods effected the stability of GSNO inside of the silicone insert tubing, the amount of GSNO (%recovery of GSNO) was measured for NO releasing formulation (a) inserts subjected to no sterilization (control), H2O2 sterilization, and EO sterilization. Each NO releasing insert was made by hand, therefore the amount of GSNO inevitably varies slightly. Thus, the trial that yielded the highest amount of GSNO measured from the three NO releasing control inserts (not sterilized) was assumed to be 100% recovery of GSNO and after normalization, all three trials were averaged to yield 98.4 ± 1.6% (Table 1). All other NO releasing inserts tested were normalized to this value, and hence >100% recovery values were possible. The results of this study are shown in Table 1. Ultimately, H2O2 sterilization was chosen as the best method compared to EO sterilization because of lower standard deviation and quicker turn-around period.
Table 1.
%Recovery of GSNO after no sterilization (control), hydrogen peroxide (H2O2) sterilization, or ethylene oxide (EO) sterilization processes. Values are normalized to the highest amount of GSNO recovered from the control (no sterilization) trials. Data represents the mean ± SD (n = 3).
| Control (no sterilization) | H2O2 Sterilization | EO Sterilization |
|---|---|---|
| 98.4 ± 1.6% | 100.2 ± 2.4% | 95.3 ± 10.1% |
After the sterilization method was selected, it was important to test the long-term stability of the GSNO inside of the NO releasing inserts. For this study, NO releasing formulation (a) inserts were fabricated and sterilized via H2O2. However, three NO releasing inserts were not sterilized (control) and the amount of GSNO in each insert was measured on Day 0 (93.8 ± 5.5%) using the method described in Section 2.7. Note, again, that the trial that yielded the highest amount of GSNO measured from the three NO releasing control inserts (not sterilized) was assumed to be 100% recovery of GSNO and all other values gathered afterwards were normalized to this value. After H2O2 sterilization was completed on Day 0 for the remaining inserts, they remained in their individual sterilization pouches and stored in a sealed glass jar with desiccant, in the dark, at room temperature (24°C) until further use. After nearly 2 months of storage (56 d), the GSNO recovered from inside of the NO releasing formulation (a) inserts was 89.4 ± 2.9% (~4.3% degradation) (Fig. S3). Therefore, GSNO is relatively stable when stored dry with ZnO nanoparticles inside of the silicone insert devices at room temperature.
3.5. Antimicrobial efficacy of NO releasing formulation (a) inserts in hemodialysis catheter hubs
The antimicrobial efficacy of NO releasing formulation (a) inserts in real hemodialysis catheter hubs were tested against gram-positive and gram-negative strains, S. aureus, and P. aeruginosa, respectively. A detailed procedure can be found in Section 2.8, above. Briefly, a given concentration of bacteria in 10% LB broth was added to a real hemodialysis catheter hub region and the hub region was closed at the proximal end of the hub region using the pre-existing clamp. H2O2 sterilized NO releasing inserts were then placed inside of the catheter hubs and closed via the attached cap. The control trials contained no NO releasing inserts, just a normal hub cap. The hemodialysis catheter hubs were then subjected to real world conditions as stated previously (24°C, dark, 72 h, and on a shaker). Upon completions of the 72 h study, bacteria counts were obtained from the liquid broth inside of the catheter hubs containing the NO releasing formulation (a) inserts versus the controls with no inserts. This led to a log reduction of 6.6 and 6.7 (compared to controls) against S. aureus and P. aeruginosa, respectively. This data suggests that the NO releasing inserts containing formulation (a) are extremely effective at killing both gram-positive and gram-negative strains.
3.6. Sheep Study #1: Hemodialysis catheters with NO releasing insert caps vs control (without NO)
The purpose of this study was to evaluate the antimicrobial/anti-biofilm performance of the NO releasing formulation (a) insert caps versus normal hemodialysis catheter caps by replacing each cap every 2-3 d over a 14 d long period. Detailed procedures for Sheep Study #1 can be found in Sections 2.9 and 2.10. Briefly, two sheep were studied during Sheep Study #1 with one sheep designated as a control (without NO release inserts) and the other sheep experimental (NO releasing inserts). Each sheep had two surgically implanted dual-lumen TDC catheters for a total of four catheter hubs each. The NO releasing formulation (a) inserts were glued to catheter caps for convenience (Fig. 1a). During the 14 d study the sheep were housed in a barn at the U of M farm. Control and experimental caps were changed every 2-3 d and blood was drawn through each lumen to simulate the average time between dialysis treatments and blood exposure. Bacteria cultures from the liquid in each hub region were taken prior to replacing the caps. After 14 d, the study was terminated and each hemodialysis catheter was evaluated for the amount of bacteria/biofilm present on the inside wall of four separate regions of the catheter (Fig. 1b).
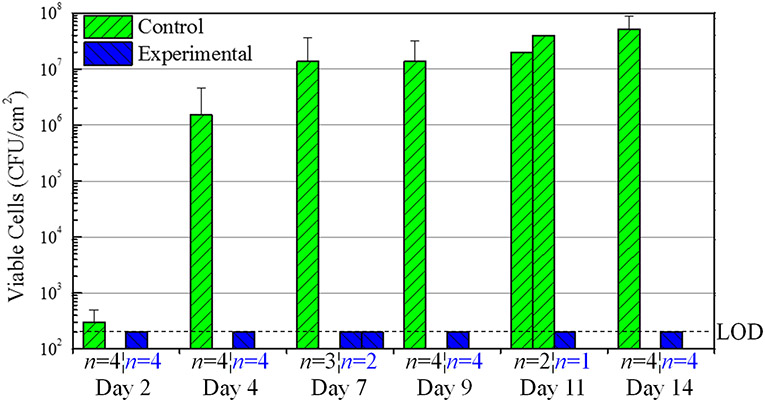
The results of the bacteria counts taken from the liquid within the hub region every 2-3 days are summarized in Figure 4. On particular days, clotting/occlusion prevented obtaining a proper liquid sample from each hub region (Day 7, and 11) (Fig. 4). However, the data remains consistent where the control sheep displayed significant bacteria counts after Day 4 and the experimental sheep (NO releasing insert caps) showed no bacteria on any day, therefore reaching the limit of detection (220 CFU/mL) after each day of testing (Fig. 4). A log reduction of 3.88 was already observed by Day 4 and increased to 5.42 by Day 14 for experimental sheep versus control (Fig. 4). This data suggests that the NO releasing formulation (a) insert caps have a significant antimicrobial effect against bacteria present in the liquid of the hub region under real world conditions.
Figure 4.
Sheep Study #1 bacteria counts from the hub solution on days 2, 4, 7, 9, 11, and 14 using normal catheter caps (Control) and NO releasing insert caps (Experimental). Dashed line is the limit of detection (200 CFU/mL). Log reduction values are given for Experimental vs. Control. The number of samples (n = X) is specified for each day. It should be noted that on Days 7 and 11 for the Experimental Insert Caps and Day 11 for the Control Caps, less than n = 3 trials were collected, and therefore each trial is represented by one bar on the graph with no error bar. Data with an error bar represents the mean ± SD.
Upon completion of the 14 d study, four regions of each catheter were tested for bacteria/biofilm adhered to the inner lumen walls (Fig. 1b). The purpose of this test was to gauge the ability of NO to act as a barrier not only to ingress but also to colonization of bacteria from the catheter hub region. This test was conducted by first sterilizing the outside of the catheter, cutting out the specific sections, and using a homogenizer to remove all of the adhered bacteria/biofilm from the inner lumen wall for bacteria enumeration. The results of this study are summarized in Figure 5. For the control catheters, all four regions of the catheters had a significant amount of bacteria/biofilm present. For catheters with the experimental NO releasing insert caps, no bacteria were detected in any of the four regions (Fig. 5). Interestingly, bacteria/biofilm prevention was observed in all regions of the catheter and not just the hub region (where the NO is locally released). This data quantitatively suggests that bacteria can migrate to the proximal regions of the catheter from the hub region and that the NO releasing formulation (a) insert caps have significant antibacterial/anti-biofilm potential throughout all regions of a catheter during a real world situation. The clinical implications of this data are that the NO releasing insert caps not only prevent ingress but also diminish colonization of bacteria, which is essential to reduce risk of clinical infection.
Figure 5.
Sheep Study #1 bacteria/biofilm adhered to the inner lumen wall of the hub region, tunneled region, distal intravascular region, and proximal tip of catheters using normal catheter caps (Control) and NO releasing insert caps (Experimental). Dashed line is the limit of detection (400 CFU/segment). Log reduction values are given for Experimental vs. Control. The number of samples (n = X) are specified for each section of catheter. Data with an error bar represents the mean ± SD. * p < 0.01, Hub Region Control vs. Experimental.
Fluorescent microscopic images were taken of the inner lumen walls of each region of the catheters using Live/Dead dye stain upon termination of the study (see Fig. S4). Experimental catheters (NO releasing insert caps) displayed minimal to no bacteria adhered to the inner lumen walls in all regions. The control catheters displayed significant bacteria/biofilm adhesion in all four regions. This data qualitatively suggests that the NO releasing formulation (a) insert cap prevents bacteria/biofilm formation in each catheter region during large animal studies.
3.7. Sheep Study #2: Hemodialysis catheters with chlorhexidine insert caps vs NO releasing insert caps
The purpose of this study was to compare the antimicrobial/anti-biofilm performance the NO releasing formulation (a) insert cap versus a commercially available antimicrobial cap that utilizes chlorhexidine as the antimicrobial agent. For both type of inserts, each cap was replaced every 2-3 d over a 14 d long period. Detailed procedures for Sheep Study #2 can be found in Sections 2.9 and 2.11. Briefly, two sheep were studied and for one sheep the dual-lumen catheter was surgically implanted in the right jugular vein and it was designated for use with the chlorhexidine caps, while the dual-lumen catheter implanted in the left jugular vein was designated for the NO release insert caps. For the second sheep, the cap designations were reversed to the opposite jugular veins. Both sheep were housed in a barn during the 14 d study. The same procedures were performed in Sheep Study #1 as were performed in Sheep Study #2. Chlorhexidine and experimental NO release caps were changed every 2-3 d and blood was drawn through each lumen to simulate the average time between dialysis treatments and blood exposure. Bacteria cultures were taken from the liquid in each hub region prior to replacing the caps. After the study was terminated on Day 14, each hemodialysis catheter was evaluated for the amount of bacteria/biofilm present in four different regions of the catheter (Fig. 1b).
The bacteria counts taken from the liquid of the hub region every 2-3 days yielded no bacteria detected on any day for both chlorhexidine and experimental catheters. This data suggests that chlorhexidine caps display significant antimicrobial effects against bacteria present in the liquid phase of the hub region. This data also confirms the results obtained in Sheep Study #1 for the NO releasing formulation (a) insert cap because the same results (no bacteria detected on any day) were obtained in Sheep Study #2.
After the 14 d study was completed, four regions of each catheter were tested for bacteria/biofilm adhered to the inner lumen walls (Fig. 1b). Again, the purpose of this test was to gauge the ability of NO and chlorhexidine to act as a barrier not only to ingress but also to reduce colonization of bacteria with the catheter hub and elsewhere along the inner walls of the entire catheter. The outside of each catheter was sterilized, then the specific sections of the catheter were cut out and bacteria/biofilm adhered to the inner lumen walls were removed using a homogenizer for bacteria enumeration. The results of this study are summarized in Figure 6. For the experimental catheters, minimal bacteria were detected in the hub and tunneled regions and no bacteria were detected in the distal intravascular region and proximal tip (Fig. 6). For the chlorhexidine catheters, bacteria/biofilm was detected in all four regions (Fig. 6). The tunneled region of the catheter had the largest log reduction of bacteria (3.82) for the experimental versus chlorhexidine catheters (Fig. 6). Overall, this data suggests that the NO releasing formulation (a) insert caps are much more capable at preventing bacteria/biofilm formation in all four regions of a hemodialysis catheter compared to commercially available chlorhexidine caps.
Figure 6.
Sheep Study #2 bacteria/biofilm adhered to the inner lumen wall of the hub region, tunneled region, distal intravascular region, and proximal tip of catheters using normal catheter caps (Control) and NO releasing insert caps (Experimental). Dashed line is the limit of detection (400 CFU/segment). Log reduction values are given for Experimental vs. Control. The number of samples (n = X) are specified for each section of catheter. Data with an error bar represents the mean ± SD. * p < 0.01, Hub Region Chlorhexidine vs. Experimental.
Fluorescent microscopic images were taken of the inner lumen walls of each region of the catheters using Live/Dead dye stain after study termination (see Fig. S5). The chlorhexidine catheters displayed significant bacteria/biofilm adhered to the inner lumen wall of the tunneled region, which corresponds to the elevated bacteria/biofilm counts obtained from the tunneled region (Fig. 6, Fig. S5). The experimental catheters (NO releasing insert caps) displayed minimal to no bacteria adhered to the inner lumen walls of all four regions. This data indicates that the NO releasing formulation (a) insert cap prevents more bacteria/biofilm formation in each catheter region compared to the commercially available chlorhexidine cap.
Despite the NO releasing insert cap significantly reducing the colonization of bacteria compared to control caps and chlorohexidine caps, the redness and swelling at each catheter placement site was no different between each sheep. Also, no changes in the eating habits or temperature, which are common first signs of clinical infection, were noted. Therefore, to potentially observe common signs of clinical infection and comment on the potential lifespan of each sheep, the health of each sheep would need to be observed for much longer than the two week period of the studies reported here.
4. Conclusion
The real-time NO releasing characteristics of four different NO releasing insert formulations were determined under real world conditions (24°C, dark, 72 h, submerged in saline). Formulation (a) demonstrated high initial NO flux levels compared to the other formulations. Formulation (b) demonstrated similar characteristics to formulation (a), however the NO fluxes were significantly lower. Formulation (c) contained PEG to smooth/level out the NO flux over the 72 h period. Formulation (d) contained no ZnO and displayed minimal NO release, therefore further proving that ZnO is needed in the formulation to display significant NO release capability. Testing the antimicrobial/anti-biofilm efficacy of formulations (a-c) using a simulated catheter hub assay against S. aureus revealed that formulation (a) was the best at preventing biofilm formation because of the large initial burst of NO that formulation (a) provides. Testing the %recovery of GSNO from inside of the NO releasing formulation (a) inserts after different sterilization techniques revealed that minimal to no GSNO decomposition occurs during the H2O2 sterilization process. Thus, due to minimal GSNO decomposition and faster sample turn-around time, H2O2 sterilization was chosen as the main sterilization procedure. A preliminary shelf-life stability study of H2O2 sterilized NO releasing formulation (a) inserts showed minimal degradation of GSNO (4.3%) after 56 d of being stored at 24°C, in the dark, and in the presence of desiccant. It was also discovered that H2O2 sterilized NO releasing formulation (a) inserts are highly effective at killing both gram-negative (P. aeruginosa) and gram-positive (S. aureus) strains (6.7 and 6.6 log reduction, respectively) from the liquid present in actual catheter hubs under real world conditions (24°C, dark, 72 h). An ovine 14 d study, revealed that the NO releasing formulation (a) insert caps are highly effective at killing/preventing bacteria and biofilm formation at all regions of a hemodialysis catheter compared to the control that had a normal catheter cap (not releasing NO). In our second animal study, the findings of the first study were confirmed for the NO releasing formulation (a) insert cap. In the second ovine study, data suggests that the NO releasing formulation (a) insert cap prevents bacteria growth and biofilm formation better than a commercially available antimicrobial cap that utilizes chlorhexidine. Overall, this research has clearly demonstrated that the NO releasing formulation (a) insert cap demonstrates significant antimicrobial/anti-biofilm effects and should be useful in significantly decreasing the risk of infection for dialysis patients that have TDCs in place.
Supplementary Material
Acknowledgment
We gratefully acknowledge the National Institutes of Health (grant # HL128337) for supporting this research.
Footnotes
Declaration of interest
The authors J. Doverspike, S. Mack, A. Lou, B. Stringer, S. Reno, M. Cornell, A. Rojas-Pena, J. Wu, and Prof. C. Xi have no conflict of interest to report. Prof. A. Yevzlin and Prof. M. Meyerhoff are shareholders of a new start-up company, NitriCap LLC, that is in the process of negotiating a license from the University of Michigan for the patent-pending NO release insert technology described in this publication.
Appendix A. Supplementary data
The Supplementary Data file contains a schematic of the NO releasing insert (Figure S1), a figure showing bacteria counts from the liquid broth of the in vitro simulated catheter hub antimicrobial assay study (Figure S2), a figure showing the %recovery of GSNO on days 1, 7, and 56 for the NO releasing insert shelf-life stability study (Figure S3), and figures illustrating fluorescent microscopic images of bacteria/biofilm adhered to the inner lumen walls of four regions of the TDCs used in both sheep studies (Figures S4 and S5).
References
- (1).Lok CE; Foley R Vascular Access Morbidity and Mortality: Trends of the Last Decade. Clin J Am Soc Nephrol 2013, 8 (7), 1213–1219. [DOI] [PubMed] [Google Scholar]
- (2).Rupp ME; Karnatak R Intravascular Catheter–Related Bloodstream Infections. Infect Dis Clin North Am 2018, 32 (4), 765–787. [DOI] [PubMed] [Google Scholar]
- (3).Buetti N; Timsit JF Management and Prevention of Central Venous Catheter-Related Infections in the ICU. Semin Respir Crit Care Med 2019, 40 (4), 508–523. [DOI] [PubMed] [Google Scholar]
- (4).Justo JA; Bookstaver PB Antibiotic Lock Therapy: Review of Technique and Logistical Challenges. Infect Drug Resist 2014, 7, 343–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Banin E; Brady KM; Greenberg EP Chelator-Induced Dispersal and Killing of Pseudomonas Aeruginosa Cells in a Biofilm. Appl Environ Microbiol 2006, 72 (3), 2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Raad II; Fang X; Keutgen XM; Jiang Y; Sherertz R; Hachem R The Role of Chelators in Preventing Biofilm Formation and Catheter-Related Bloodstream Infections. Curr Opin Infect Dis 2008, 21 (4), 385–392. [DOI] [PubMed] [Google Scholar]
- (7).Brunelli SM; Van Wyck DB; Njord L; Ziebol RJ; Lynch LE; Killion DP Cluster-Randomized Trial of Devices to Prevent Catheter-Related Bloodstream Infection. J Am Soc Nephrol 2018, 29 (4), 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Brunelli SM; Njord L; Hunt AE; Sibbel SP Use of the Tego Needlefree Connector is Associated with Reduced Incidence of Catheter-Related Bloodstream Infections in Hemodialysis Patients. Int J Nephrol Renovasc Dis 2014, 7, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sweet MA; Cumpston A; Briggs F; Craig M; Hamadani M Impact of Alcohol-Impregnated Port Protectors and Needleless Neutral Pressure Connectors on Central Line–Associated Bloodstream Infections and Contamination of Blood Cultures in an Inpatient Oncology Unit. Am J Infect Control 2012, 40 (10), 931–934. [DOI] [PubMed] [Google Scholar]
- (10).Hymes JL; Mooney A; Van Zandt C; Lynch L; Ziebol R; Killion D Dialysis Catheter-Related Bloodstream Infections: A Cluster-Randomized Trial of the ClearGuard HD Antimicrobial Barrier Cap. Am J Kidney Dis 2017, 69 (2), 220–227. [DOI] [PubMed] [Google Scholar]
- (11).Bonez PC; dos Santos Alves CF; Dalmolin TV; Agertt VA; Mizdal CR; Flores V; Marques JB; Santos RCV; Anraku de Campos MM Chlorhexidine Activity Against Bacterial Biofilms. Am J Infect Control 2013, 41 (12), e119–e122. [DOI] [PubMed] [Google Scholar]
- (12).Hetrick EM; Shin JH; Stasko NA; Johnson CB; Wespe DA; Holmuhamedov E; Schoenfisch MH Bactericidal Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. ACS Nano 2008, 2 (2), 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wo Y; Brisbois EJ; Bartlett RH; Meyerhoff ME Recent Advances in Thromboresistant and Antimicrobial Polymers for Biomedical Applications: Just Say Yes to Nitric Oxide (NO). Biomater Sci 2016, 4 (8), 1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ghaffari A; Miller CC; McMullin B; Ghahary A Potential Application of Gaseous Nitric Oxide as a Topical Antimicrobial Agent. Nitric Oxide 2006, 14 (1), 21–29. [DOI] [PubMed] [Google Scholar]
- (15).Loscalzo J; Welch G Nitric Oxide and its role in the Cardiovascular System. Prog Cardiovasc Dis 1995, 38 (2), 87–104. [DOI] [PubMed] [Google Scholar]
- (16).Fang FC Perspectives Series: Host/Pathogen Interactions. Mechanisms of Nitric Oxide-Related Antimicrobial Activity. J Clin Invest 1997, 99 (12), 2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Barraud N; Hassett DJ; Hwang S-H; Rice SA; Kjelleberg S; Webb JS Involvement of Nitric Oxide in Biofilm Dispersal of Pseudomonas Aeruginosa. J Bacteriol 2006, 188 (21), 7344–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Barraud N; Storey MV; Moore ZP; Webb JS; Rice SA; Kjelleberg S Nitric Oxide-Mediated Dispersal in Single- and Multi-Species Biofilms of Clinically and Industrially Relevant Microorganisms. Microb Biotechnol 2009, 2 (3), 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Xu L-C; Wo Y; Meyerhoff ME; Siedlecki CA Inhibition of Bacterial Adhesion and Biofilm Formation by Dual Functional Textured and Nitric Oxide Releasing Surfaces. Acta Biomater 2017, 51, 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wo Y; Li Z; Brisbois EJ; Colletta A; Wu J; Major TC; Xi C; Bartlett RH; Matzger AJ; Meyerhoff ME Origin of Long-Term Storage Stability and Nitric Oxide Release Behavior of CarboSil Polymer doped with S-nitroso-N-acetyl-D-penicillamine. ACS Appl Mater Interfaces 2015, 7 (40), 22218–22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hart TW Some Observations Concerning the S-nitroso and S-phenylsulphonyl Derivatives of L-cysteine and Glutathione. Tetrahedron Lett 1985, 26 (16), 2013–2016. [Google Scholar]
- (22).Broniowska KA; Diers AR; Hogg N S-nitrosoglutathione. Biochim Biophys Acta 2013, 1830 (5), 3173–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lautner G; Stringer B; Brisbois EJ; Meyerhoff ME; Schwendeman SP Controlled Light-Induced Gas Phase Nitric Oxide Release from S-nitrosothiol-Doped Silicone Rubber Films. Nitric Oxide 2019, 86, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Doverspike JC; Zhou Y; Wu J; Tan X; Xi C; Meyerhoff ME Nitric Oxide Releasing Two-Part Creams containing S-nitrosoglutathione and Zinc Oxide for Potential Topical Antimicrobial Applications. Nitric Oxide 2019, 90, 1–9. [DOI] [PubMed] [Google Scholar]
- (25).Ren H; Bull JL; Meyerhoff ME Transport of Nitric Oxide (NO) in various Biomedical Grade Polyurethanes: Measurements and Modeling Impact on NO Release Properties of Medical Devices. ACS Biomater Sci Eng 2016, 2 (9), 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Shishido SM; Oliveira MG Polyethylene Glycol Matrix reduces the rates of Photochemical and Thermal Release of Nitric Oxide from S-nitroso-N-acetylcysteine. Photochem Photobiol 2000, 71 (3), 273–280. [DOI] [PubMed] [Google Scholar]
- (27).Rabinowitch E; Wood WC The Collison Mechanism and the Primary Photochemical Process in Solutions. Trans Faraday Soc 1936, 32, 1381–1387. [Google Scholar]
- (28).Dicks AP; Swift HR; Williams DLH; Butler AR; Al-Sa'doni HH; Cox BG Identification of Cu+ as the Effective Reagent in Nitric Oxide formation from S-nitrosothiols (RSNO). J Chem Soc, Perkin Trans 21996, (4), 481–487. [Google Scholar]
- (29).Singh SP; Wishnok JS; Keshive M; Deen WM; Tannenbaum SR The Chemistry of the S-Nitrosoglutathione/Glutathione System. Proc Natl Acad Sci 1996, 93 (25), 14428–14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Williams DLH The Chemistry of S-nitrosothiols. Acc Chem Res 1999, 32 (10), 869–876. [Google Scholar]
- (31).Wood PD; Mutus B; Redmond RW The Mechanism of Photochemical Release of Nitric Oxide from S-nitrosoglutathione. Photochem Photobiol 1996, 64 (3), 518–524. [Google Scholar]
- (32).de Souza GFP; Denadai JP; Picheth GF; de Oliveira MG Long-Term Decomposition of Aqueous S-nitrosoglutathione and S-nitroso-N-acetylcysteine: Influence of Concentration, Temperature, pH and Light. Nitric Oxide 2019, 84, 30–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.