Abstract
BACKGROUND
Nowadays there is an increasing use of transcranial magnetic stimulation (TMS) both in neurological and psychiatric fields. After Food and Drug Administration approval of TMS for the therapy of treatment-resistant depression, TMS has been widely used in the context of mood disorders (MD). However, growing reports regarding the possibility of developing hypomanic/manic switch (HMS) have generated concern regarding its use in MDs.
AIM
To investigate the actual risk of developing HMS due to TMS in the treatment of MD.
METHODS
We led our research on PubMed, Scopus and Web of Science on March 22, 2020, in accordance to the PRISMA guidelines for systematic review. Only double blind/single blind studies, written in English and focused on the TMS treatment of MD, were included. A meta-analysis of repetitive TMS protocol studies including HMS was conducted using RevMan 5.4 software. The assessment of Risk of Bias was done using Cochrane risk of bias tool. This protocol was registered on PROSPERO with the CRD42020175811 code.
RESULTS
Twenty-five studies were included in our meta-analysis: Twenty-one double blind randomized controlled trials (RCT) and four single blind-RCT (no. of subjects involved in active stimulation = 576; no. of subjects involved in sham protocol = 487). The most frequently treated pathology was major depressive episode/major depressive disorder, followed by resistant depression, bipolar depression and other MD. The majority of the studies used a repetitive TMS protocol, and the left dorsolateral prefrontal cortex was the main target area. Side effects were reported in eight studies and HMS (described as greater energy, insomnia, irritability, anxiety, suicidal attempt) in four studies. When comparing active TMS vs sham treatment, the risk of developing HMS was not significantly different between conditions.
CONCLUSION
Applying the most usual protocols and the appropriate precautionary measures, TMS seems not to be related to HMS development.
Keywords: Hypomanic/manic switch, Transcranial magnetic stimulation, Active vs sham comparison, Mood disorders, Adverse event, Safety
Core Tip: Transcranial magnetic stimulation (TMS) has been widely used in the context of mood disorders. The purpose of this review/meta-analysis was to examine the risk of developing a hypomanic/manic switch (HMS) during active TMS treatment of mood disorders. Twenty-five double blind/single blind studies were included in the quantitative synthesis. When comparing active TMS vs sham treatment, we did not find any significant difference in the risk of developing HMS between conditions. So, we can conclude that, applying the appropriate precautionary measures, TMS seems not to be related to HMS development.
INTRODUCTION
Non-invasive brain stimulation is a complex of neuromodulation techniques that have a therapeutic effect by stimulating the nervous system[1]. Transcranial magnetic stimulation (TMS) uses the principle of magnetic induction to modulate the neural circuitry[2]. Some electrical currents pass through a coil to induce repetitive magnetic field pulses that, if applied on the scalp, can stimulate target brain regions depolarizing the underlying neurons[3]. Depending on the stimulation frequency, cortical activity can be inhibited [low frequencies (LF) ≤ 1 Hz] or improved [high frequencies (HF) ≥ 5 Hz][4].
There are different protocols based on the number of pulses administrated (single pulses or paired pulses TMS)[5] or specific intervals between trains [like intermittent theta burst stimulation (TBS)[6], but one of the most used protocol is repetitive TMS (rTMS) where the pulses are applied as repetitive stimulation trains set at a specific frequency[4].
The use of rTMS has found an application in the field of substance[7] and non-substance addiction[8] but also in major psychiatric disorders such as post-traumatic stress disorder, schizophrenia and obsessive–compulsive disorder and in suicide[9-12]. It has also been used in the treatment of bipolar disorder, with good results in the treatment of depressive states and controversial results in mania[13]. In particular, TMS was approved by Food and Drug Administration in 2007 as a therapy for the treatment resistant depression (TRD)[14]. The standard protocol used in the treatment of depression provides 75 trains per session for a total of 3000 pulses over about 35 min generated at 120% of resting motor threshold at 10 Hz, train duration of 4 s[14,15].
The role of the left dorsolateral prefrontal cortex (LDLPFC) in the pathophysiology of depression has been widely demonstrated with numerous evidences from functional imaging[16]. TMS, stimulating the DLPFC, is able to increase the neuronal excitability and to induce growth of the new connections having an antidepressant effect[17], both as a single treatment and as an add-on to antidepressants[18]. Some evidence, in fact, suggests that it may increase or decrease the neural excitability producing lasting changes in the efficiency of the synaptic transmission known as long-term potentiation and long-term depression[3,19].
When provided within recommended guidelines[20], rTMS is a very safe and well-tolerated technique both in the elderly[21] and in children[22] and has shown a favorable profile compared to antidepressant medications[23,24].
The most common side effects of TMS are headache, neck pain and local pain during the treatment at the site of stimulation[25]. The serious side effects are typically uncommon: Those reported were seizures, noted in less than 1% of healthy subjects and in patients with neurological morbidities and/or epilepsy[20,26], and hearing impairment, preventable with adequate protection[27]. Another potential side effect of TMS is the development of hypomanic/manic switch (HMS)[24,28].
HMS is one of the most critical events in bipolar disorder, affecting the severity of illness and being associated with an increased risk of suicide[29]. It can be linked to antidepressant treatments, and therefore antidepressants should be associated with mood stabilizers in bipolar patients[30]. Following the Diagnostic and Statistical Manual of Mental Disorders-5 criteria, hypomanic symptoms subsiding after stopping the antidepressants are called "antidepressant-induced hypomania", otherwise the episode can be defined as a true HMS[31,32].
During TMS treatment, cases of HMS were described in some reports[33,34], but to date the risk of TMS-induced HMS has not been yet extensively reported.
The purpose of this study was therefore to examine the actual risk of developing HMS due to TMS in the treatment of mood disorders, providing both qualitative and quantitative synthesis.
MATERIALS AND METHODS
The statistical methods of this study were reviewed by Gianna Sepede (GSe), who has qualified experience in Biomedical Statistics, Systematic Reviews and Meta-analysis.
Our systematic review was conducted to study the risk of developing HMS in a population of patients with mood disorders. We led our research on PubMed, Scopus and Web of Science (WoS) on March 22, 2020 using the following search strategy: (1) PubMed: (TMS OR Transcranial Magnetic Stimulation) AND (side effect OR adverse event) AND (depression OR manic OR bipolar OR hypomanic OR switch) NOT review NOT (animal OR rat OR mouse); (2) Scopus: [TITLE-ABS-KEY (tms) OR TITLE-ABS-KEY (transcranial AND magnetic AND stimulation) AND TITLE-ABS-KEY (side AND effect) OR TITLE-ABS-KEY (adverse AND event) AND TITLE-ABS-KEY (depression) OR TITLE-ABS-KEY (manic) OR TITLE-ABS-KEY (bipolar) OR TITLE-ABS-KEY (hypomanic) OR TITLE-ABS-KEY (switch) AND NOT TITLE-ABS-KEY (review) AND NOT TITLE-ABS-KEY (animal) OR TITLE-ABS-KEY (rat) OR TITLE-ABS-KEY (mouse)]; and (3) WoS: [(TMS OR Transcranial Magnetic Stimulation) AND (side effect OR adverse event) AND (depression OR manic OR bipolar OR hypomanic OR switch) NOT review NOT (animal OR rat OR mouse)]
All the procedures are in accordance with the PRISMA guidelines for systematic review[35]. Exclusion criteria, both for the first and the second phase of the screening, were: (1) Non-original research (e.g., review, commentary, editorial, book chapter); (2) Non full-text article (e.g., meeting abstract); (3) Language other than English; (4) Animal/in vitro studies; (5) Non double blind randomized controlled trial (DB-RCT) or single blind-RCT (SB-RCT) design; (6) Use of other neuromodulation techniques (e.g., tDCS, MST); (7) Treatment of other conditions unrelated to mood disorders and (8) Data not reported.
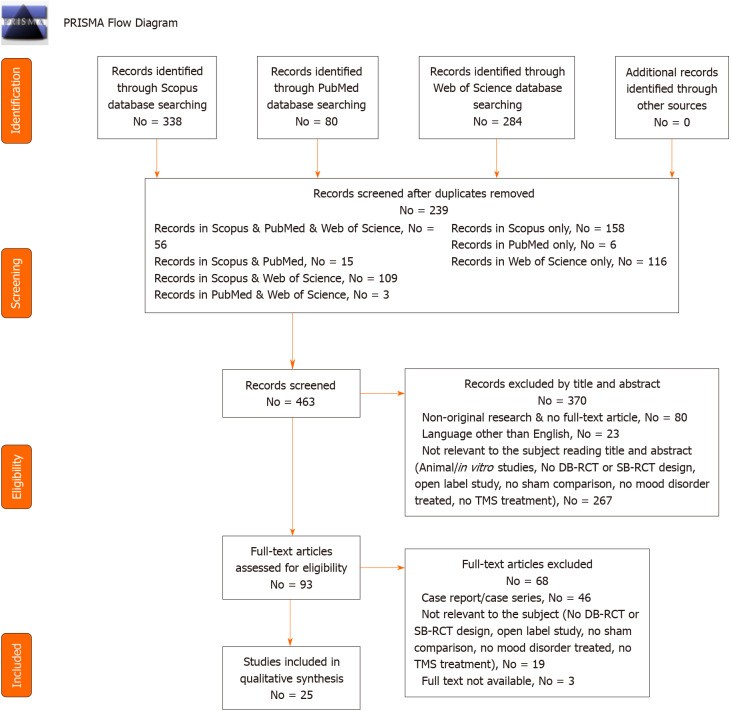
We found 702 articles (PubMed = 80; Scopus = 338; WoS = 284). After removing the duplicates (No = 239), we screened 463 records and, of all these, 80 were non-original articles (review, meta-analysis, commentary, letter to the editor without data available), 267 were not related to the focus of the review (animal/in vitro studies, no DB-RCT or SB-RCT design, open label study, no sham comparison, no mood disorder treated, no TMS treatment), and 23 were not written in English. Out of 93 articles assessed for eligibility, 46 were case report/series, 19 were not relevant to the subject (no DB-RCT or SB-RCT design, open label study, no sham comparison, no mood disorder treated, no TMS treatment) and three articles were not available. Twenty-five articles, finally, were taken into consideration for qualitative synthesis.
The process was conducted individually by AM, GS and Amo, creating an Excel database. For doubtful cases, the eligibility was discussed with GM, MP or MdG. These research methods were approved by PROSPERO (CRD42020175811 identification code). The method is summarized in Figure 1.
Figure 1.
PRISMA flow diagram: Studies included in qualitative and quantitative synthesis. DB-RCT: Double blind randomized controlled trials; SB-RCT: Single blind randomized controlled trials.
Risk of bias
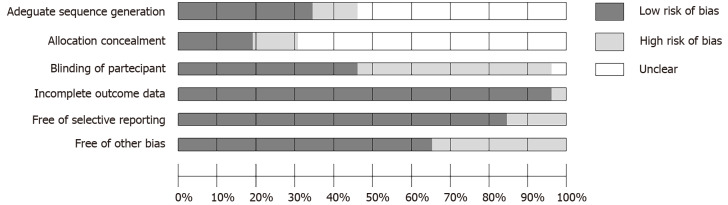
The assessment of risk of bias was measured independently by AM, GS and AMo using the Cochrane risk of bias tool (Figure 2)[36]. This result was discussed with GSe and evaluated by MP, GM and MdG.
Figure 2.
Risk of bias assessment.
Quantitative analysis
The main outcome was to calculate the risk of developing HMS with TMS therapy in a population with mood disorders, so an active vs sham treatment comparison was conducted. The meta-analysis was performed using Review Manager Software v 5.4[37]. Provided that HMS is an uncommon side effect and in order to include the studies with an event frequency of zero, a risk difference (RD) and not a risk ratio was applied[38,39].
The RD of the HSM for each individual article was calculated and, therefore, computed together obtaining a Fixed Effect with 95% confidence interval (CI). Statistical significance was set for values of P < 0.05.
We used I2 to calculate the heterogeneity of the studies: I2 < 30% low heterogeneity; 30% < I2 < 60% moderate heterogeneity; 60% < I2 < 75% substantial heterogeneity; I2 > 75% high heterogeneity[40].
In case of heterogeneous results, a meta-analysis per categorical variable level was performed to evaluate the influence of categorical moderators on study outcomes. The final result is shown in the Forest Plot.
In order to assess potential publication bias, a funnel plot of study effect sizes was visually inspected for asymmetry (Figure 3).
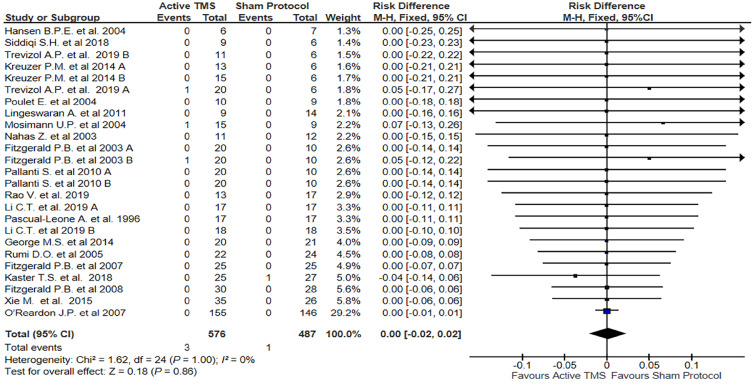
Figure 3.
Forest plot: Risk to develop a hypomanic/manic switch after a transcranial magnetic stimulation protocol. TMS: Transcranial magnetic stimulation.
RESULTS
All the characteristics of the included articles are described in Table 1.
Table 1.
Characteristics of the studies included in the systematic review
|
Ref.
|
Population (No total, No male, No subgroups, age ± SD of the subgroups)
|
Type of treatment
|
Protocol (type, Hz, No pulses/session, RMT, coil type, target area, treatment duration)
|
Mood Disorder Sub-type
|
Reported adverse events (yes, no, NS)
|
HMS (No tot)
|
Drop out due to HMS
|
| Trevizol et al[53], 2019-A | Active TMS, No = 20, age = 66.8 ± 5.8 (M = 13). Sham = 12, age = 64.1 ± 3.7 (M = 3) | Only TMS | rTMS, 1 Hz, 465 impulse/session, 120% RMT, B-65 figure-8 coil, RDLPFC, 15 session AND rTMS, 10 Hz, 750 impulse/session, 120% RMT, B-65figure-8 coil, LDLPFC, 15 session | Late-life TRD | No (light and not worth mentioning) | 0 | no |
| Trevizol et al[53], 2019-B | Active TMS, No = 11, age = 66.1 ± 8.5 (M = 4). Sham = 12, age = 64.1 ± 3.7 (M = 3) | Only TMS | rTMS, 10 Hz, 1450 impulse/session, 120% RMT, B-65 figure-8 coil, LDLPFC, 15 session | Late-life TRD | Yes (TMS, No = 2; SHAM, No = 0) | 1 | no |
| Rao et al[65], 2019 | Active TMS, No = 13, age = 39.8 ± 14.2 (M = 5). Sham = 17, age = 40.2 ± 14.6 (M = 11) | Only TMS | rTMS, 1 Hz, 12000 impulse/session, 110% RMT, B-65 figure-8 coil, RDLPFC, 20 session | MDE after traumatic Brain Injury | No (light and not worth mentioning) | 0 | no |
| Matsuda et al[64], 2020 | Active TMS, No = 20, age = 43.4 ± 5.5 (M = 18). Sham = 20, age = 45.2 ± 7 (M = 19) | Only TMS | dTMS, 18 Hz, 1980 impulse/session, 120% RMT, H1 coil, LDLPFC, 30 session | Depression NS | No (light and not worth mentioning) | 0 | no |
| Li et al[59], 2020-A | Active TMS, No = 35, age = 47.1 ± 13.8 (M = 11). Sham = 35, age = 47.1 ± 12.4 (M = 11) | Only TMS | rTMS, 10 Hz, 1600 impulse/session, 100% RMT, figure-8 coil, LDLPFC, 10 session | Recurrent major depression | Yes (TMS, No = 12; SHAM, No = 8) | 0 | no |
| Li et al[59], 2020-B | Active TMS, No = 35, age = 47.1 ± 14.2 (M = 12). Sham = 35, age = 47.1 ± 12.4 (M = 11) | Only TMS | piTMS, 50 Hz, 1800 impulse/session, 80% RMT, figure-8 coil, LDLPFC, 10 session | Recurrent major depression | Yes (TMS, No = 9; SHAM, No = 8) | 0 | no |
| Bulteau et al[56], 2019 | Active TMS, No = 12, age = 52.7 (M = 5). Sham = 14, age = 53.1 (M = 10) | Only TMS | iTBS, 50 Hz, 990 impulse/session, 80% RMT, figure-8 coil, LDLPFC, 15 session | BD | No | 0 | no |
| Siddiqi et al[63], 2019 | Active TMS, No = 9, age = 43 ± 13 (M = 7). Sham = 6, age = 50 ± 18 (M = 4) | Only TMS | Bilateral rTMS, 10 Hz, 4000 impulse/session, 120% RMT, B-65 figure-8 coil., LDLPFC, 25 session AND Bilateral rTMS, 1 Hz, 1000 impulse/session, 120% RMT, B-65figure-8 coil, RDLPFC, 25 session | MDE after traumatic Brain Injury | Yes (TMS, No = 9; SHAM, No = 0) | 0 | no |
| Kaster et al[52], 2018 | Active TMS, No = 25, age = 65.0 ± 5.5 (M = 17). Sham=27, age = 65.4 ± 5.5 (M = 15) | Only TMS | rTMS, 18 Hz, 6012 impulse/session, 120% RMT, H1 coil, LDLPFC, 20 session | Late-life MDE | No (light and not worth mentioning) | 1 | no |
| Xie et al[57], 2015 | Active TMS, No = 35, age=65.3 ± 5.1 (M = 12). Sham = 26, age = 64.7 ± 4.2 (M = 8) | rTMS + shuganjieyu | rTMS, 10 Hz, NS impulse/session, 30% RMT, B-65NS coil, LDLPFC, 5 session | MDD | Yes (TMS, No = 14; SHAM, No = 13) | 0 | no |
| Levkovitz et al[58], 2015 | Active TMS, No = 101, age = 45.1 ± 11.7 (M = 53). Sham = 111, age = 47.6 ± 11.6 (M = 58) | Only TMS | dTMS, 18 Hz, 1980 impulse/session, 120% RMT, H1 coil, LDLPFC, 20 session | MDD | No (light and not worth mentioning) | 0 | no |
| Kreuzer et al[42], 2015-A | Active TMS, No = 13, age = 43.5 ± 10.3 (M = 8). Sham = 12, age = 43.8 ± 10.5 (M = 4) | Only TMS | rTMS, 10 Hz, 2000 impulse/session, 110% RMT, B-65 double cone coil, Anterior Cingulate, 15 session | MDE | Yes (TMS, No = 7; SHAM, No = 8) | 0 | no |
| Kreuzer et al[42], 2015-B | Active TMS, No = 15, age = 46.1 ± 9.5 (M = 7). Sham = 12, age = 43.8 ± 10.5 (M = 4) | Only TMS | rTMS, 10 Hz, 2000 impulse/session, 110% RMT, B-65 figure-8 coil., LDLPFC, 15 session | MDE | Yes (TMS, No = 4; SHAM, No = 8) | 0 | no |
| George et al[62], 2014 | Active TMS, No = 20, age=38.7 ± 15 (M = 18). Sham = 21, age = 46.1 ± 15.9 (M = 17) | Only TMS | rTMS, 10 Hz, 6000 impulse/session, 120% RMT, B-65 figure-8 coil., LDLPFC, 9 session | MDE | Yes (TMS, No = 7; SHAM, No = 6) | 0 | no |
| Lingeswaran[51], 2011 | Active TMS, No = 9, age = 34 ± 10.5 (M = 3). Sham = 14, age = 37.26 ± 11.8 NS (M = 6) | Only TMS | rTMS, 10 Hz, 500 impulse/session, 100% RMT, B-65 figure-8 coil, LDLPFC, 10 session | MDE | No | 0 | no |
| Pallanti et al[54], 2010-A | Active TMS, No = 20, age=47.6 ± 12.33, (M = 9). Sham = 20, age = 47.85 ± 9.12 (M = 8) | Only TMS | Bilateral rTMS, 1 Hz, 420 impulse/session, 110% RMT, B-65 figure-8 coil., RDLPFC, 15 session AND rTMS, 10 Hz, 1000 impulse/session, 100% RMT, B-65 figure-8 coil., LDLPFC, 15 session | TRD | No (light and not worth mentioning) | 0 | no |
| Pallanti et al[54], 2010-B | Active TMS, No = 20, age = 51.2 ± 12.33, (M = 8). Sham = 20, age = 47.85 ± 9.12 (M = 8) | TMS + placebo | rTMS, 1 Hz, 420 impulse/session, 110% RMT, B-65 figure-8 coil., RDLPFC, 15 session | TRD | No (light and not worth mentioning) | 0 | no |
| Fitzgerald[55], 2008 | Active TMS, No = 30, age = 45.7 ± 10.8 (M = 10). Sham = 28, age = 44.8 ± 11.4 (M = 15) | Only TMS | LF rTMS, 1 Hz, 900 impulse/session, 110% RMT, B-65 figure-8 coil., rDLPFC, 10 session | Depression NS | No (light and not worth mentioning) | 0 | no |
| Fitzgerald et al[44], 2007 | Active TMS, No = 25, age = 46.8 ± 10.7 (M = 10). Sham = 25, age = 43.7 ± 10.2 (M = 9) | Only TMS | Bilateral rTMS, 10 Hz, 750 impulse/session, 100% RMT, B-65 figure-8 coil., LDLPFC, 30 session AND Bilateral rTMS, 1 Hz, 420 impulse/session, 110% RMT, B-65 figure-8 coil., RDLPFC, 30 session | TRD | No (light and not worth mentioning) | 0 | no |
| O’Reardon et al[50], 2007 | Active TMS, No = 155, age = 47.9 ± 11 (M = 69). Sham = 146, age = 48.7 ± 10.6 (M = 72) | Only TMS | rTMS, 10 Hz, 3000 impulse/session, 120% NS coil, LDLPFC, 25 session | MDE | No (light and not worth mentioning) | 0 | no |
| Fitzgerald et al[43], 2006 | Active TMS, No = 25, age = 46.8 ± 10.7 (M = 10). Sham = 25, age = 43.7 ± 10.2 (M = 9) | Only TMS | Bilateral rTMS, 10 Hz, 750 impulse/session, 100% RMT, B-65 figure-8 coil., LDLPFC, 30 session AND Bilateral rTMS, 1 Hz, 420 impulse/session, 110% RMT, B-65figure-8 coil., RDLPFC, 30 session | TRD | No (light and not worth mentioning) | 0 | no |
| Rumi et al[48], 2005 | Active TMS, No = 22, age = 39.3 ± 12.8 (M = 3). Sham = 24, age = 38.9 ± 8.8 (M = 4) | TMS + Amitriptyline | rTMS, 5 Hz, 1250 impulse/session, 120% RMT, B-65 figure-8 coil, LDLPFC, 20 session | MDE | No (light and not worth mentioning) | 0 | no |
| Boggio et al[49], 2005 | Active TMS, No = NS, age = NS (M = NS). Sham = NS, age = NS, (M = NS) | TMS + fluoxetine+ placebo | rTMS, 15 Hz, 3000 impulse/session, 110% RMT, B-65figure-8 coil, LDLPFC, 10 session | MDE in Parkinson’s disease | No | 0 | 0 |
| Poulet et al[46], 2004 | Active TMS, No = NS, age=NS (M = NS). Sham = NS, age = NS (M = NS) | TMS + paroxetine | rTMS, 10 Hz, 400 impulse/session, 80% RMT, B-65 figure-8 coil, LDLPFC, 10 session | MDE | No | 0 | no |
| Mosimann et al[47], 2004 | Active TMS, No = 15, age = 60 ± 13.4 (M = 10). Sham = 9, age = 64.4 ± 13 (M = 5) | Only TMS | rTMS, 20 Hz, 1600 impulse/session, 100% RMT, B-65 figure-8 coil, LDLPFC, 10 session | MDE | Yes (TMS, No = 7; SHAM, No = 5) | 1 | no |
| Hansen et al[45], 2004 | Active TMS, No = 6, age = 42.5 (M = 4). Sham = 7, age = 46, (M = 5) | TMS + antidepressant | rTMS, 10 Hz, 2000 impulse/session, 90% RMT, B-65 figure-8 coil, LDLPFC, 10 session | MDE | No | 0 | no |
| Nahas et al[41], 2003 | Active TMS, No = 11, age = 42.4 ± 7.3 (M = 4). Sham = 12, age = 43.4 ± 9.3 (M = 5) | Only TMS | rTMS, 5 Hz, 1600 impulse/session, 110% RMT, B-65 figure-8 coil, LDLPFC, 10 session | BD | No (light and not worth mentioning) | no | |
| Fitzgerald et al[66], 2003-A | Active TMS, No = 20, age = 42.4 ± 9.8 (M = 12). Sham = 20, age = 49C.15 ± 14.243 (M = 9) | Only TMS | rTMS, 1 Hz, 300 impulse/session, 100% RMT, B-65 figure-8 coil, RDLPFC, 10 session | BD,MDE, TRD | No (light and not worth mentioning) | 0 | no |
| Fitzgerald et al[66], 2003-B | Active TMS, No = 20, age = 45.55 ± 11.49 (M = 13). Sham = 20, age = 49.15 ± 14.243 (M = 9) | Only TMS | rTMS, 10 Hz, 1000 impulse/session, 100% RMT, B-65 figure-8 coil., LDLPFC, 10 session | BD,MDE, TRD | No (light and not worth mentioning) | 1 | no |
| Pascual-Leone et al[60], 1996 | Active TMS, No = 17, age = 48.6 ± NS (M = 6). Sham = 17, age = 48.6 ± NS (M = 6) | Only TMS | rTMS, 10 Hz, 2000 impulse/session, 90% RMT, B-65 figure-8 coil, LDLPFC, 5 session | Psychotic depression | Yes (TMS, No = 7; SHAM, No = 7) | 0 | no |
SD: Standard deviation; RMT: Resting motor thresholds; HMS: Hypomanic/manic switch; TMS: Transcranial magnetic stimulation; rTMS: Repetitive TMS; RDLPFC: Right dorsolateral prefrontal cortex; LDLPFC: Left dorsolateral prefrontal cortex; TRD: Treatment resistant depression; MDE: Major depressive episode; dTMS: Deep TMS; piTBS: Prolonged intermittent theta burst stimulation; iTBS: Intermittent theta-burst stimulation; BD: Bipolar depression; MDD: Major depressive disorder.
We obtained a total of 25 studies for our systematic review. Following our inclusion and exclusion criteria all of them were DB-RCTs except for four SB-RCTs[41-44].
Among the included studies, the most frequently treated pathology was major depressive episode (No = 10)[42,44-52] followed by resistant depression (No = 5)[43,44,53-55], bipolar depression (No = 4)[41,43,44,56], major depressive disorder (No = 3)[57-59] and other mood disorders (No = 6)[60-65].
We found four studies allowing the use of antidepressants: Fluoxetine[49], paroxetine[46], amitriptyline[48] and not specified molecules (tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors)[45].
The majority of the studies had LDLPFC as the main target area (No = 19)[41,42,44-53,56-60,62,64], although other areas have been stimulated, in particular: Right DLPFC (RDLPFC; No = 4)[44,53,55,65], bilateral (LDLPFC and RDLPFC; No = 4)[43,54,55,63] and other area (No = 1)[42], specifically the anterior cingulate.
The most used coil was the figure eight coil (No = 16)[41-49,51,53-56,59-63,65]. Other coils used were the double cone coil (No = 1)[42] and the H1 coil (No = 3)[52,58,64]. The number of studies where the type of coil was not specified were two[50,57].
The duration of the treatment also differed greatly in our sample: Most of our sample received 2 wk of acute treatment (No = 10)[41,43-47,49,51,59,61]. Other durations of acute treatment were 3 wk (No = 3)[42,54,56], 4 wk (No = 4)[48,52,58,65], 5 wk (No = 2)[50,63], 6 wk (No = 3)[53,55,64], only 1 wk (No = 2)[57,60] and less than 1 wk in just one study[62].
About the TMS technique used, most have used a rTMS protocol (No = 23)[41-55,57,59-63,65]. Other protocols were TBS at 50 Hz (No = 2)[56,59] and deep TMS (dTMS) at 18 Hz (No = 2)[58,64].
rTMS protocols
Based on the Hz set, the sample can be divided between those who have used LF treatment at 1 Hz (No = 7)[44,53-55,61,63,65] and those who have used a HF treatment (No = 21) divided in 5 Hz (No = 2)[41,48], 10 Hz (No = 15)[42-46,50,51,53-55,57,59,60,62,63], 15 Hz (No = 1)[49] and 20 Hz (No = 1)[47].
The number of pulses/session was very heterogeneous, more frequently 2000 pulses/session (No = 3)[42,45,60] and 3000 pulses/session (No = 2)[49,50].
Side effects
Only eight studies reported at least one side effect[42,47,53,59,60,62,63]. HMSs (described as greater energy, insomnia, irritability, anxiety and suicidal attempt in bipolar patients), were present in four studies[44,47,52,53] and, in particular, once in the sham group and three times in the active group. Only one episode of HMS was specifically reported[66], after stopping taking a mood stabilizer and after the end of the rTMS treatment. No drop-outs due to HMSs were reported. None of the studies that used antidepressants in addition to TMS treatment reported the onset of HMS[45,46,48,49].
Risk of bias
The results of the risk of bias assessment reveal a good quality of the reported data, as evidenced by the items “Incomplete Outcome Data” and “Free of Selective Report”. However, only a few of the included studies accurately indicated the blinding method and the allocation concealment (Figure 2).
Meta-analysis of rTMS protocols
The meta-analysis of the 25 studies included (No. of subjects involved in active stimulation = 576; No. of subjects involved in sham protocol = 487), showed no significant results about the risk of developing HMS after TMS stimulation (RD = 0.00; 95%CI = -0.02-0.02; P = 1.00; I2= 0%) for a fixed effect.
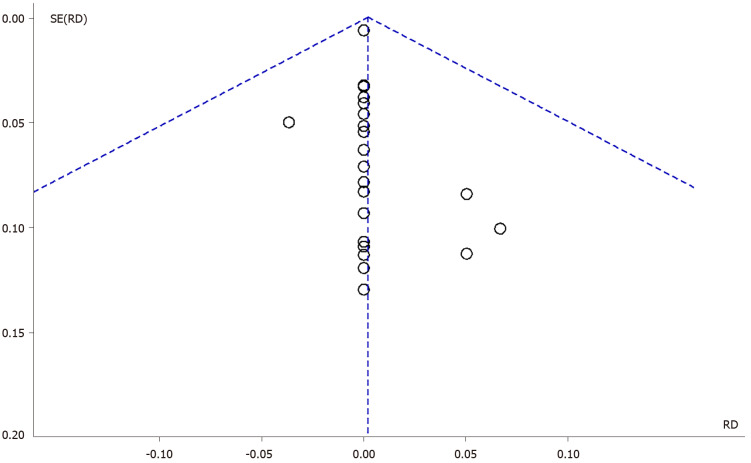
The inspection of the funnel plot of the RD of the included studies (Fixed Effect) suggested a symmetry of the studies, showing the same concentration of studies on the left and on the right of the mean RD (Figure 4).
Figure 4.
Funnel plot for publication bias. RD: Risk difference.
DISCUSSION
The mechanisms underlying HMSs in mood disorders are complex and still unknown, even if a main role of increased levels of dopamine and/or norepinephrine and of seasonal changes of neural activity were suggested[67]. Antidepressant treatments are well known triggers for HMSs, especially in bipolar disorders[68]. Due to its increasing use in the treatment of mood disorders, the same concern has been voiced about the safety of TMS. In particular, in a previous meta-analysis on the topic, it was hypothesized that HMS was induced by a too intensive treatment with TMS and an incorrect diagnosis of unipolar depression[69]. However, the most recent scientific literature regarding the use of TMS in mood disorders seems to deny the hypomanic effect of TMS. Nowadays, in fact, several TMS treatments (rTMS and TBS in particular)[6,70] are increasingly used not only for the therapy of TRD but also for the treatment of mood disorders in general, including bipolar disorders[71]. However, a critical role on the onset of HMS is probably played by the concomitant use of high-dosage antidepressant treatments[72,73], pointing out the importance of a deep and accurate anamnesis.
In our systematic review focused on RCTs using TMS to treat mood disorders, we found that only 4/25 studies reported a HMS as an adverse event, and the difference between sham and active treatment was not significant.
The most used protocol was rTMS, set at 10 Hz (16/25 studies), 120% RTM (9/25 studies) with a variable number of pulses (the most common included the 2,000 pulses/session present in 3/26 studies). The most investigated brain area was the DLPFC, (24/25 studies); only one study examined the AC with a deep-brain stimulation technique. However, in the context of the DLPFC, lateralization to the right seems preferred in the context of a LF stimulation (all eight studies in question stimulate the RDLPFC) while the left one in the context of a HF (all of the remaining 17 studies stimulate the LDLPFC). The application of the most widely used research protocols could therefore be a useful method to avoid the genesis of HMSs.
Another aspect highlighted by our results is the absence of drop-outs due to HMS. Considering the severity of HMS symptomatology, the absence of drop-outs is a further indication of the TMS safety in the treatment of MD.
CONCLUSION
Considering the still not understood and complex mechanisms underlying the development of HMS, from the results of our systematic review of the literature and from our meta-analysis (although in the context of a statistical non-significance), it seems clear that by applying the most usual protocols of rTMS and TBS and applying, where necessary, the appropriate precautionary measures (for example going on with mood stabilizers) TMS can be considered a safe technique also in the context of mood disorders.
ARTICLE HIGHLIGHTS
Research background
One of the most innovative and most investigated non-invasive brain stimulation techniques is Transcranial Magnetic Stimulation (TMS). This device has received Food and Drug Administration approval for the treatment of various neurological (headache) and psychiatric (treatment resistant depression) disorders. Several studies have been conducted to find new applications of TMS in conditions that do not respond or partially respond to standard psychopharmacological therapies.
Research motivation
TMS is an increasingly used technique in the neurological and psychiatric fields. One of the greatest concerns about its use is the possibility of developing severe side effects such as hypomanic/manic switches (HMS).
Research objectives
The aim of this meta-analysis is to quantify the risk of developing HMS after treatment with TMS in mood disorders and to evaluate the drop-out rate due to that adverse event.
Research methods
The search was conducted using PubMed, Scopus and Web of Science databases on March 22, 2020. All procedures were registered on PROSPERO and performed according to the PRISMA guidelines. Only double blind/single blind articles, written in English were included. RevMan 5.4 Software for Windows was used to perform the meta-analysis.
Research results
Of the 25 eligible studies, only four HMSs were described. No dropouts were reported due to symptoms severity.
Research conclusions
Our data confirm that, by applying appropriate psychopharmacological and anamnestic precautions, TMS is a safe technique for treating mood disorders.
Research perspectives
Greater uniformity of protocols, their online registration and the timely reporting of side effects on scientific papers could guarantee a more accurate analysis of the health risks induced by TMS in future meta-analyses.
ACKNOWLEDGEMENTS
We would like to thank Alessia Di Ferdinando for kindly reviewing the language and Gianluca Mancusi for his precious help in the revision of references.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Invited manuscript
Peer-review started: February 16, 2021
First decision: March 16, 2021
Article in press: July 20, 2021
Specialty type: Psychiatry
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tajiri K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Guo X
Contributor Information
Andrea Miuli, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy.
Gianna Sepede, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy.
Gianfranco Stigliano, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy.
Alessio Mosca, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy.
Francesco Di Carlo, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy. francesco.dic@hotmail.it.
Giacomo d’Andrea, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy.
Aliseo Lalli, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy.
Maria Chiara Spano, Department of Psychiatry Affective Neuropsychiatry, Sahlgrenska University Hospital, Göteborg 40530, Sweden.
Mauro Pettorruso, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy.
Giovanni Martinotti, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy; Department of Pharmacy, Clinical Science, University of Hertfordshire, Herts AL10 9AB, Italy.
Massimo di Giannantonio, Department of Neuroscience, Imaging and Clinical Sciences, “G. d'Annunzio” University of Chieti, Chieti 66100, Italy.
References
- 1.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 2.Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19:322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS) Ann Phys Rehabil Med. 2015;58:208–213. doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, Castelo-Branco L, Challet-Bouju G, Clark VP, Claus E, Dannon PN, Del Felice A, den Uyl T, Diana M, di Giannantonio M, Fedota JR, Fitzgerald P, Gallimberti L, Grall-Bronnec M, Herremans SC, Herrmann MJ, Jamil A, Khedr E, Kouimtsidis C, Kozak K, Krupitsky E, Lamm C, Lechner WV, Madeo G, Malmir N, Martinotti G, McDonald WM, Montemitro C, Nakamura-Palacios EM, Nasehi M, Noël X, Nosratabadi M, Paulus M, Pettorruso M, Pradhan B, Praharaj SK, Rafferty H, Sahlem G, Salmeron BJ, Sauvaget A, Schluter RS, Sergiou C, Shahbabaie A, Sheffer C, Spagnolo PA, Steele VR, Yuan TF, van Dongen JDM, Van Waes V, Venkatasubramanian G, Verdejo-García A, Verveer I, Welsh JW, Wesley MJ, Witkiewitz K, Yavari F, Zarrindast MR, Zawertailo L, Zhang X, Cha YH, George TP, Frohlich F, Goudriaan AE, Fecteau S, Daughters SB, Stein EA, Fregni F, Nitsche MA, Zangen A, Bikson M, Hanlon CA. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev. 2019;104:118–140. doi: 10.1016/j.neubiorev.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommer M, Tergau F, Wischer S, Paulus W. Paired-pulse repetitive transcranial magnetic stimulation of the human motor cortex. Exp Brain Res. 2001;139:465–472. doi: 10.1007/s002210100791. [DOI] [PubMed] [Google Scholar]
- 6.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, Knyahnytska Y, Kennedy SH, Lam RW, Daskalakis ZJ, Downar J. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–1692. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 7.Pettorruso M, Martinotti G, Santacroce R, Montemitro C, Fanella F, di Giannantonio M rTMS stimulation group. rTMS Reduces Psychopathological Burden and Cocaine Consumption in Treatment-Seeking Subjects With Cocaine Use Disorder: An Open Label, Feasibility Study. Front Psychiatry. 2019;10:621. doi: 10.3389/fpsyt.2019.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettorruso M, Martinotti G, Montemitro C, De Risio L, Spagnolo PA, Gallimberti L, Fanella F, Bonci A, Di Giannantonio M Brainswitch Study Group. Multiple Sessions of High-Frequency Repetitive Transcranial Magnetic Stimulation as a Potential Treatment for Gambling Addiction: A 3-Month, Feasibility Study. Eur Addict Res. 2020;26:52–56. doi: 10.1159/000504169. [DOI] [PubMed] [Google Scholar]
- 9.Koek RJ, Roach J, Athanasiou N, van 't Wout-Frank M, Philip NS. Neuromodulatory treatments for post-traumatic stress disorder (PTSD) Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:148–160. doi: 10.1016/j.pnpbp.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Osoegawa C, Gomes JS, Grigolon RB, Brietzke E, Gadelha A, Lacerda ALT, Dias ÁM, Cordeiro Q, Laranjeira R, de Jesus D, Daskalakis ZJ, Brunelin J, Cordes J, Trevizol AP. Non-invasive brain stimulation for negative symptoms in schizophrenia: An updated systematic review and meta-analysis. Schizophr Res. 2018;197:34–44. doi: 10.1016/j.schres.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi L, Zalesky A, Nott Z, Whybird G, Fitzgerald PB, Breakspear M. Transcranial magnetic stimulation in obsessive-compulsive disorder: A focus on network mechanisms and state dependence. Neuroimage Clin. 2018;19:661–674. doi: 10.1016/j.nicl.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozzay ML, Primack J, Barredo J, Philip NS. Transcranial magnetic stimulation to reduce suicidality - A review and naturalistic outcomes. J Psychiatr Res. 2020;125:106–112. doi: 10.1016/j.jpsychires.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold AK, Ornelas AC, Cirillo P, Caldieraro MA, Nardi AE, Nierenberg AA, Kinrys G. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9:e01419. doi: 10.1002/brb3.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath JC, Mathews J, Demitrack MA, Pascual-Leone A. The NeuroStar TMS device: conducting the FDA approved protocol for treatment of depression. J Vis Exp. 2010 doi: 10.3791/2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, Cook IA, O'Reardon J, Husain MM, Wall C, Krystal AD, Sampson SM, Morales O, Nelson BG, Latoussakis V, George MS, Lisanby SH National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J Clin Psychiatry. 2018;79 doi: 10.4088/JCP.16cs10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bersani FS, Minichino A, Enticott PG, Mazzarini L, Khan N, Antonacci G, Raccah RN, Salviati M, Delle Chiaie R, Bersani G, Fitzgerald PB, Biondi M. Deep transcranial magnetic stimulation as a treatment for psychiatric disorders: a comprehensive review. Eur Psychiatry. 2013;28:30–39. doi: 10.1016/j.eurpsy.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Zhu J, Xu L, Tang X, Cui H, Wei Y, Wang Y, Hu Q, Qian Z, Liu X, Tang Y, Li C, Wang J. Add-on rTMS for the acute treatment of depressive symptoms is probably more effective in adolescents than in adults: Evidence from real-world clinical practice. Brain Stimul. 2019;12:103–109. doi: 10.1016/j.brs.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Bologna M, Rocchi L, Paparella G, Nardella A, Li Voti P, Conte A, Kojovic M, Rothwell JC, Berardelli A. Reversal of Practice-related Effects on Corticospinal Excitability has no Immediate Effect on Behavioral Outcome. Brain Stimul. 2015;8:603–612. doi: 10.1016/j.brs.2015.01.405. [DOI] [PubMed] [Google Scholar]
- 20.Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, Cohen LG, Dowthwaite G, Ellrich J, Flöel A, Fregni F, George MS, Hamilton R, Haueisen J, Herrmann CS, Hummel FC, Lefaucheur JP, Liebetanz D, Loo CK, McCaig CD, Miniussi C, Miranda PC, Moliadze V, Nitsche MA, Nowak R, Padberg F, Pascual-Leone A, Poppendieck W, Priori A, Rossi S, Rossini PM, Rothwell J, Rueger MA, Ruffini G, Schellhorn K, Siebner HR, Ugawa Y, Wexler A, Ziemann U, Hallett M, Paulus W. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. 2017;128:1774–1809. doi: 10.1016/j.clinph.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iriarte IG, George MS. Transcranial Magnetic Stimulation (TMS) in the Elderly. Curr Psychiatry Rep. 2018;20:6. doi: 10.1007/s11920-018-0866-2. [DOI] [PubMed] [Google Scholar]
- 22.Zewdie E, Ciechanski P, Kuo HC, Giuffre A, Kahl C, King R, Cole L, Godfrey H, Seeger T, Swansburg R, Damji O, Rajapakse T, Hodge J, Nelson S, Selby B, Gan L, Jadavji Z, Larson JR, MacMaster F, Yang JF, Barlow K, Gorassini M, Brunton K, Kirton A. Safety and tolerability of transcranial magnetic and direct current stimulation in children: Prospective single center evidence from 3.5 million stimulations. Brain Stimul. 2020;13:565–575. doi: 10.1016/j.brs.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Jordan JM. Treating adolescents with cancer. Diss Abstr Int Sect B Sci Eng. 2013;16:33–35. [Google Scholar]
- 24.Loo CK, McFarquhar TF, Mitchell PB. A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. Int J Neuropsychopharmacol. 2008;11:131–147. doi: 10.1017/S1461145707007717. [DOI] [PubMed] [Google Scholar]
- 25.Bae EH, Schrader LM, Machii K, Alonso-Alonso M, Riviello JJ Jr, Pascual-Leone A, Rotenberg A. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav. 2007;10:521–528. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Cohen RB, Boggio PS, Fregni F. Risk factors for relapse after remission with repetitive transcranial magnetic stimulation for the treatment of depression. Depress Anxiety. 2009;26:682–688. doi: 10.1002/da.20486. [DOI] [PubMed] [Google Scholar]
- 27.Taylor R, Galvez V, Loo C. Transcranial magnetic stimulation (TMS) safety: a practical guide for psychiatrists. Australas Psychiatry. 2018;26:189–192. doi: 10.1177/1039856217748249. [DOI] [PubMed] [Google Scholar]
- 28.Zis P, Shafique F, Hadjivassiliou M, Blackburn D, Venneri A, Iliodromiti S, Mitsikostas DD, Sarrigiannis PG. Safety, Tolerability, and Nocebo Phenomena During Transcranial Magnetic Stimulation: A Systematic Review and Meta-Analysis of Placebo-Controlled Clinical Trials. Neuromodulation. 2020;23:291–300. doi: 10.1111/ner.12946. [DOI] [PubMed] [Google Scholar]
- 29.Niitsu T, Fabbri C, Serretti A. Predictors of switch from depression to mania in bipolar disorder. J Psychiatr Res. 2015;66-67:45–53. doi: 10.1016/j.jpsychires.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Viktorin A, Lichtenstein P, Thase ME, Larsson H, Lundholm C, Magnusson PK, Landén M. The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am J Psychiatry. 2014;171:1067–1073. doi: 10.1176/appi.ajp.2014.13111501. [DOI] [PubMed] [Google Scholar]
- 31.Gill N, Bayes A, Parker G. A Review of Antidepressant-Associated Hypomania in Those Diagnosed with Unipolar Depression-Risk Factors, Conceptual Models, and Management. Curr Psychiatry Rep. 2020;22:20. doi: 10.1007/s11920-020-01143-6. [DOI] [PubMed] [Google Scholar]
- 32.Barbuti M, Pacchiarotti I, Vieta E, Azorin JM, Angst J, Bowden CL, Mosolov S, Young AH, Perugi G BRIDGE-II-Mix Study Group. Antidepressant-induced hypomania/mania in patients with major depression: Evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187–192. doi: 10.1016/j.jad.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Dolberg OT, Schreiber S, Grunhaus L. Transcranial magnetic stimulation-induced switch into mania: a report of two cases. Biol Psychiatry. 2001;49:468–470. doi: 10.1016/s0006-3223(00)01086-6. [DOI] [PubMed] [Google Scholar]
- 34.Ozten E, Sayar GH, Karamustafalioglu O. Hypomanic shift observed during rTMS treatment of patients with unipolar depressive disorder: four case reports. Ann Gen Psychiatry. 2013;12:12. doi: 10.1186/1744-859X-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke M. Cochrane Collaboration. American Cancer Society, 2005. [Google Scholar]
- 38.Walter SD. Choice of effect measure for epidemiological data. J Clin Epidemiol. 2000;53:931–939. doi: 10.1016/s0895-4356(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 39.Messori A, Maratea D, Fadda V, Trippoli S. Using risk difference as opposed to odds-ratio in meta-analysis. Int J Cardiol. 2013;164:127. doi: 10.1016/j.ijcard.2012.06.078. [DOI] [PubMed] [Google Scholar]
- 40.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 41.Nahas Z, Kozel FA, Li X, Anderson B, George MS. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5:40–47. doi: 10.1034/j.1399-5618.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 42.Kreuzer PM, Schecklmann M, Lehner A, Wetter TC, Poeppl TB, Rupprecht R, de Ridder D, Landgrebe M, Langguth B. The ACDC pilot trial: targeting the anterior cingulate by double cone coil rTMS for the treatment of depression. Brain Stimul. 2015;8:240–246. doi: 10.1016/j.brs.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald PB, Benitez J, de Castella A, Daskalakis ZJ, Brown TL, Kulkarni J. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am J Psychiatry. 2006;163:88–94. doi: 10.1176/appi.ajp.163.1.88. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald PB, Sritharan A, Daskalakis ZJ, de Castella AR, Kulkarni J, Egan G. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol . 2007;27:488–492. doi: 10.1097/jcp.0b013e318151521c. [DOI] [PubMed] [Google Scholar]
- 45.Hansen PE, Videbech P, Clemmensen K, Sturlason R, Jensen HM, Vestergaard P. Repetitive transcranial magnetic stimulation as add-on antidepressant treatment. The applicability of the method in a clinical setting. Nord J Psychiatry. 2004;58:455–457. doi: 10.1080/08039480410011678. [DOI] [PubMed] [Google Scholar]
- 46.Poulet E, Brunelin J, Boeuve C, Lerond J, D'Amato T, Dalery J, Saoud M. Repetitive transcranial magnetic stimulation does not potentiate antidepressant treatment. Eur Psychiatry. 2004;19:382–383. doi: 10.1016/j.eurpsy.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Mosimann UP, Schmitt W, Greenberg BD, Kosel M, Müri RM, Berkhoff M, Hess CW, Fisch HU, Schlaepfer TE. Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patients. Psychiatry Res. 2004;126:123–133. doi: 10.1016/j.psychres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Rumi DO, Gattaz WF, Rigonatti SP, Rosa MA, Fregni F, Rosa MO, Mansur C, Myczkowski ML, Moreno RA, Marcolin MA. Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biol Psychiatry. 2005;57:162–166. doi: 10.1016/j.biopsych.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Boggio PS, Fregni F, Bermpohl F, Mansur CG, Rosa M, Rumi DO, Barbosa ER, Odebrecht Rosa M, Pascual-Leone A, Rigonatti SP, Marcolin MA, Araujo Silva MT. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson's disease and concurrent depression. Mov Disord. 2005;20:1178–1184. doi: 10.1002/mds.20508. [DOI] [PubMed] [Google Scholar]
- 50.O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 51.Lingeswaran A. Repetitive Transcranial Magnetic Stimulation in the Treatment of depression: A Randomized, Double-blind, Placebo-controlled Trial. Indian J Psychol Med. 2011;33:35–44. doi: 10.4103/0253-7176.85393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaster TS, Daskalakis ZJ, Noda Y, Knyahnytska Y, Downar J, Rajji TK, Levkovitz Y, Zangen A, Butters MA, Mulsant BH, Blumberger DM. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology. 2018;43:2231–2238. doi: 10.1038/s41386-018-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trevizol AP, Goldberger KW, Mulsant BH, Rajji TK, Downar J, Daskalakis ZJ, Blumberger DM. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Geriatr Psychiatry. 2019;34:822–827. doi: 10.1002/gps.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pallanti S, Bernardi S, Di Rollo A, Antonini S, Quercioli L. Unilateral low frequency versus sequential bilateral repetitive transcranial magnetic stimulation: is simpler better for treatment of resistant depression? Neuroscience. 2010;167:323–328. doi: 10.1016/j.neuroscience.2010.01.063. [DOI] [PubMed] [Google Scholar]
- 55.Fitzgerald PB. A randomized-controlled trial of bilateral rTMS for treatment-resistant depression. Prog Neurother Neuropsychopharmacol. 2008;3:211–226. [Google Scholar]
- 56.Bulteau S, Beynel L, Marendaz C, Dall'Igna G, Peré M, Harquel S, Chauvin A, Guyader N, Sauvaget A, Vanelle JM, Polosan M, Bougerol T, Brunelin J, Szekely D. Twice-daily neuronavigated intermittent theta burst stimulation for bipolar depression: A Randomized Sham-Controlled Pilot Study. Neurophysiol Clin. 2019;49:371–375. doi: 10.1016/j.neucli.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Xie M, Jiang W, Yang H. Efficacy and safety of the Chinese herbal medicine shuganjieyu with and without adjunctive repetitive transcranial magnetic stimulation (rTMS) for geriatric depression: a randomized controlled trial. Shanghai Arch Psychiatry. 2015;27:103–110. doi: 10.11919/j.issn.1002-0829.214151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, Tendler A, Daskalakis ZJ, Winston JL, Dannon P, Hafez HM, Reti IM, Morales OG, Schlaepfer TE, Hollander E, Berman JA, Husain MM, Sofer U, Stein A, Adler S, Deutsch L, Deutsch F, Roth Y, George MS, Zangen A. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14:64–73. doi: 10.1002/wps.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li CT, Cheng CM, Chen MH, Juan CH, Tu PC, Bai YM, Jeng JS, Lin WC, Tsai SJ, Su TP. Antidepressant Efficacy of Prolonged Intermittent Theta Burst Stimulation Monotherapy for Recurrent Depression and Comparison of Methods for Coil Positioning: A Randomized, Double-Blind, Sham-Controlled Study. Biol Psychiatry. 2020;87:443–450. doi: 10.1016/j.biopsych.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 61.Fitzgerald PB, Hoy K, McQueen S, Herring S, Segrave R, Been G, Kulkarni J, Daskalakis ZJ. Priming stimulation enhances the effectiveness of low-frequency right prefrontal cortex transcranial magnetic stimulation in major depression. J Clin Psychopharmacol. 2008;28:52–58. doi: 10.1097/jcp.0b013e3181603f7c. [DOI] [PubMed] [Google Scholar]
- 62.George MS, Raman R, Benedek DM, Pelic CG, Grammer GG, Stokes KT, Schmidt M, Spiegel C, Dealmeida N, Beaver KL, Borckardt JJ, Sun X, Jain S, Stein MB. A two-site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatients. Brain Stimul. 2014;7:421–431. doi: 10.1016/j.brs.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Siddiqi SH, Trapp NT, Hacker CD, Laumann TO, Kandala S, Hong X, Trillo L, Shahim P, Leuthardt EC, Carter AR, Brody DL. Repetitive Transcranial Magnetic Stimulation with Resting-State Network Targeting for Treatment-Resistant Depression in Traumatic Brain Injury: A Randomized, Controlled, Double-Blinded Pilot Study. J Neurotrauma. 2019;36:1361–1374. doi: 10.1089/neu.2018.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuda Y, Kito S, Igarashi Y, Shigeta M. Efficacy and Safety of Deep Transcranial Magnetic Stimulation in Office Workers with Treatment-Resistant Depression: A Randomized, Double-Blind, Sham-Controlled Trial. Neuropsychobiology. 2020;79:208–213. doi: 10.1159/000505405. [DOI] [PubMed] [Google Scholar]
- 65.Rao V, Bechtold K, McCann U, Roy D, Peters M, Vaishnavi S, Yousem D, Mori S, Yan H, Leoutsakos J, Tibbs M, Reti I. Low-Frequency Right Repetitive Transcranial Magnetic Stimulation for the Treatment of Depression After Traumatic Brain Injury: A Randomized Sham-Controlled Pilot Study. J Neuropsychiatry Clin Neurosci. 2019;31:306–318. doi: 10.1176/appi.neuropsych.17110338. [DOI] [PubMed] [Google Scholar]
- 66.Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, De Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–1008. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- 67.Young JW, Dulcis D. Investigating the mechanism(s) underlying switching between states in bipolar disorder. Eur J Pharmacol. 2015;759:151–162. doi: 10.1016/j.ejphar.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah N, Grover S, Rao GP. Clinical Practice Guidelines for Management of Bipolar Disorder. Indian J Psychiatry. 2017;59:S51–S66. doi: 10.4103/0019-5545.196974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia G, Gajwani P, Muzina DJ, Kemp DE, Gao K, Ganocy SJ, Calabrese JR. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11:119–130. doi: 10.1017/S1461145707007699. [DOI] [PubMed] [Google Scholar]
- 70.Somani A, Kar SK. Efficacy of repetitive transcranial magnetic stimulation in treatment-resistant depression: the evidence thus far. Gen Psychiatr. 2019;32:e100074. doi: 10.1136/gpsych-2019-100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voigt J, Carpenter L, Leuchter A. A systematic literature review of the clinical efficacy of repetitive transcranial magnetic stimulation (rTMS) in non-treatment resistant patients with major depressive disorder. BMC Psychiatry. 2019;19:13. doi: 10.1186/s12888-018-1989-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakkas P, Mihalopoulou P, Mourtzouhou P, Psarros C, Masdrakis V, Politis A, Christodoulou GN. Induction of mania by rTMS: report of two cases. Eur Psychiatry. 2003;18:196–198. doi: 10.1016/s0924-9338(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 73.Hausmann A, Kramer-Reinstadler K, Lechner-Schoner T, Walpoth M, Rupp CI, Hinterhuber H, Conca A. Can bilateral prefrontal repetitive transcranial magnetic stimulation (rTMS) induce mania? J Clin Psychiatry. 2004;65:1575–1576. doi: 10.4088/jcp.v65n1122a. [DOI] [PubMed] [Google Scholar]