Abstract
Background:
Despite frequent PTEN loss and Akt/mTOR signaling in prostate cancer, the disease is insensitive to single-agent mTOR inhibition. IGF-1R inhibition may mitigate the feedback inhibition by Torc1 inhibitors, suppressing downstream Akt activation and thus potentiating the antitumor activity of mTOR inhibition.
Patients and Methods:
In this phase I study, patients with metastatic castration-resistant prostate cancer received weekly 6 mg/kg IV cixutumumab and 25 mg IV temsirolimus. The primary objective was safety and tolerability. Temsirolimus was decreased if 2 or more dose-limiting toxicities (DLTs) were observed in 6 patients. Correlative analyses included circulating tumor cells, FDG-PET, FDHT-PET, and tumor biopsies.
Results:
A total of 16 patients were enrolled across 3 cohorts (1, −1, −2). Two DLTs (Grade 3 oral mucositis) were observed in cohort 1 (temsirolimus 25 mg), and 1 DLT (Gr 3 lipase) in cohort −1 (temsirolimus 20 mg). The most common adverse events included hyperglycemia (100%; 31% Gr3), oral mucositis (63%; 19% Gr3), and diarrhea (44%; 0 Gr3). Low-grade pneumonitis occurred in 7/11 patients (44%; 0 Gr3), prompting the opening of a 3-weekly cohort (temsirolimus 20 mg/kg), without pneumonitis events. No patient had a >50% decline in PSA from baseline. Best radiographic response was stable disease, with median time on study of 22 weeks (range, 7–63).
Conclusions:
Despite a strong scientific rationale for the combination, temsirolimus plus cixutumumab demonstrated limited antitumor activity and a higher than expected incidence of toxicity such as low-grade pneumonitis and hyperglycemia. Hence, the trial was stopped in favor of alternative AR/PI3K-directed combinatorial therapies.
Keywords: mTOR, IGF-1R, PTEN, Akt
MicroAbstract
In metastatic CRPC, poor single agent response to Torc1 inhibition is postulated in part due to release of negative feedback on the PI3k/Akt/mTOR pathway. This phase I study of 16 patients aimed to overcome resistance to Torc1 inhibition monotherapy with concurrent IGF-1R inhibition. Results showed limited antitumor activity and higher than anticipated toxicity. The trial was stopped in favor of alternative AR/PI3K-directed combinatorial therapies.
INTRODUCTION
Prostate cancer is the most common malignancy (excluding nonmelanoma skin cancer) and the second leading cause of cancer mortality for men in the United States, with approximately 31,620 deaths from the disease expected in 2019 (1). Androgen deprivation therapy (ADT) provides the core of first-line treatment in advanced disease, achieving control for an average of 18–24 months. However, there is an inevitable transition to castration-resistant disease, evidenced in most patients by a rising prostate-specific antigen (PSA) level and/or regrowth of tumor. In addition to continuous ADT, five novel therapies have demonstrated survival benefit in this setting, including abiraterone, enzalutamide, and radium-223 (2–6). While these agents have established that androgen synthesis, androgen receptor (AR) signaling, and the bone microenvironment are therapeutic targets with proven clinical benefit, castration-resistant prostate cancer (CRPC) still carries a poor prognosis with a median survival of less than 2 years (7).
In addition to AR-related mechanisms, genetic dysregulation is a key factor in the development and progression of CRPC. Loss of PTEN (phosphatase and tensin homologue), which is associated with ~40% of primary prostate tumors and 100% of metastatic CRPCs, is the most common mechanism of phosphatidylinositol 3-kinase (PI3K) pathway activation (8). The PTEN protein catalyzes PIP3 de-phosphorylation, with downstream inactivation of Akt and mammalian target of rapamycin (mTOR) signaling. Despite the high rate of PI3K pathway dysregulation in CRPC, inhibition of downstream mTOR using rapalogs has been disappointing in the clinic when trialed as monotherapy (9, 10). A phase 2 study of single-agent weekly temsirolimus in 11 patients with metastatic CRPC and ≥5 circulating tumor cells (CTCs) per 7.5 mL of blood at baseline was stopped prematurely because of lack of efficacy (9). Only 3 patients had a decline to <5 CTCs, and median progression-free survival (PFS) for the cohort was 1.9 months. Another phase 2 study, with single-agent everolimus in patients with CRPC, found a better response in patients with PTEN deletion, but only 13 of 37 patients met the primary endpoint of PFS at 12 weeks and only 16% had PSA declines ≥30% (10).
Specific downstream targets of mTOR include eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) and ribosomal protein S6 kinase beta-1 (S6K1). mTOR complex 1 (mTORC1)-S6K1 induced phosphorylation of insulin receptor substrate 1 (IRS1) provides a negative feedback mechanism to downregulate the insulin-like growth factor 1 receptor (IGF-1R) (11). Release of this negative feedback with mTOR inhibition leads to increased IRS1 levels and allows upregulation of IGF-1R. Akt is one of the key proteins phosphorylated by PI3K downstream of IGF-1R; activation of IGF-1R activates PI3K, which triggers Akt phosphorylation and activation in tumor tissue. This potentially explains resistance to single-agent mTOR inhibition. In vitro models have shown that combination therapy with rapalogs and IGF-1R inhibitors can block rapamycin-induced Akt signaling, thereby sensitizing cells to mTOR inhibition, resulting in inhibition of cell growth (11–13).
Cixutumumab, a recombinant human monoclonal antibody directed at IGF-1R, has demonstrated a manageable toxicity profile with preliminary evidence of antitumor activity as monotherapy in CRPC (14). Temsirolimus specifically inhibits mTORC1 and is FDA approved for treatment of renal cell carcinoma and mantle cell lymphoma (15). A recommended phase 2 regimen—6 mg/kg IV of cixutumumab and 25 mg IV of temsirolimus—has been examined in several phase 1 and 2 trials in refractory Ewing’s sarcoma, adrenocortical carcinoma, metastatic breast cancer, and bone and soft tissue sarcoma (16–18).
We hypothesized that blocking the downstream effects of mTORC1 and the release of negative inhibition of IGF1R would synergistically inhibit tumor growth in metastatic CRPC. In this study, we evaluated the safety and tolerability of simultaneous IGF-1R and mTORC1 pathway inhibition using cixutumumab and temsirolimus as combination therapy in patients with metastatic CRPC.
PATIENTS AND METHODS
This phase 1, open label study (ClinicalTrials.gov identifier: NCT01026623) was conducted at the Sidney Kimmel Center for Prostate and Urologic Cancers at Memorial Sloan Kettering Cancer Center (MSK), between November 2009 and April 2012, and was performed in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by MSK’s institutional review board, and patients provided written informed consent prior to participation.
Study Population
Patients with progressive, metastatic CRPC were eligible. Inclusion criteria included histologically confirmed adenocarcinoma of the prostate, as well as evidence of progressive disease based on either 1) a minimum of 3 rising PSA levels at least 1 week apart, with the last result being at least 2 ng/mL, or 2) new or progressive soft tissue and/or bone disease, confirmed on CT/MRI or bone scans. Patients who had not undergone orchiectomy were required to maintain castrate levels of testosterone (<50 ng/dL) with ADT. Other eligibility criteria included Eastern Cooperative Oncology Group (ECOG) Performance Status 0 or 1; controlled diabetes mellitus, if present; and adequate cardiac, renal, hepatic, and bone marrow function. Patients were excluded if they had received prior treatment with any anti-insulin-like growth factor receptor (IGFR) agents, mTOR inhibitors, or chemotherapy in the metastatic setting. Due to pulmonary toxicities seen in the first patient cohort, the protocol was amended during the accrual period to also require adequate baseline lung function, as determined by a pulmonary function test with rest/exercise oximetry, defined as ≥70% for diffusing capacity of the lungs for carbon monoxide. This amendment was applied to all subsequent cohorts.
Study Design
Patients were accrued to the study with the objective of confirming the safety and tolerability of combined cixutumumab and temsirolimus, using the recommended phase 2 dose level for advanced solid tumors (16). Patients received weekly infusions of cixutumumab at 6 mg/kg IV over 60 minutes and 25 mg IV temsirolimus over 30 minutes. A de-escalation cohort (cohort −1; 20 mg temsirolimus weekly) was added based on the occurrence of 2 dose-limiting toxicities (DLTs) in the first 5 patients in cohort 1. Given difficulties with weekly scheduling and 8 of 11 patients (72%) experiencing low-grade pneumonitis, an expansion cohort of 5 patients (cohort −2) was explored with infusions of 20 mg/kg IV cixutumumab and 20 mg IV temsirolimus every 3 weeks. Patients were treated until disease progression, unacceptable toxicity, or withdrawal of consent.
Safety Assessments
Clinical and laboratory assessments were performed before treatment administration and 28 days after discontinuation of study drug. Laboratory assessments included routine hematology and chemistry panels, and urinalysis.
Adverse events were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0. DLTs were defined as any grade 3 or 4 non-hematologic toxicity or any grade 4 hematologic toxicity that occurred within the first 8 weeks of treatment, as defined by the NCI CTCAE v4.0. Exceptions included well-controlled toxicities, as follows: 1) grade 3 or 4 hyperglycemia that could be stably controlled and did not result in dose delay for >7 days or dose reduction within the first 4 weeks of treatment; 2) grade 3 hyperlipidemia that was without symptoms or significant medical consequences; 3) grade 3 nausea/vomiting that could be controlled with medication.
Pharmacodynamic Biomarkers
FDG- and FDHT-PET/CT imaging
All patients in our study were imaged with [18F]-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) imaging. All patients were offered participation in an ongoing study at MSK assessing 16β-[18F]-fluoro-α-dihydrotestosterone (FDHT)-PET/CT scans under a dedicated molecular imaging protocol approved by our institutional review board (19–22). Eight patients from cohorts 1 and −2 elected to enroll; no patients enrolled from cohort −1.
The uptake of FDHT, an analog of endogenous dihydrotestosterone, reflects AR expression and binding capacity; therefore, reductions in FDHT uptake indicate effective targeting of the drug to the AR (23). FDHT-PET/CT scans were obtained prior to initiation of therapy and after 4 weeks of therapy. Patients were imaged from mid-skull to upper thighs on a Discovery STE PET/CT scanner (GE Healthcare, Waukesha, Wisconsin) approximately 30–40 minutes after injection of ~333 MBq FDHT. An experienced nuclear medicine physician evaluated the reconstructed PET, CT, and fused PET/CT images using a PET-VCAR (volume computer-assisted reading) software package (GE Healthcare). The nuclear medicine physician was not blinded to dose. AR-positive lesions were determined by qualitative inspection of the FDHT-PET images (24). Any focus of activity visually higher than local background and not attributable to physiologic tracer distribution (eg, blood pool, excretion) was considered a true lesion. A volume of interest was semi-automatically placed around each lesion and the calculated maximum standard uptake value (SUVmax) was recorded. SUVmax values were adjusted for the contribution of stromal signal by subtracting a previously published population-based background threshold. The adjusted SUV data were then binned and averaged (SUVmax-avg) according to cohort and time point (baseline, 1 week, 4 weeks). Patients in cohort 1 were imaged at baseline and after 4 weeks, 12 weeks, and 24 weeks. Patients in cohort −2 were imaged at baseline, during the first week of treatment, and after 12 weeks. The percent change in SUVmax-avg between time points was calculated separately for each cohort.
Circulating tumor cells
Circulating tumor cells (CTCs) were isolated, analyzed, and enumerated using the validated CellSearch System (Menarini Silicon Biosystems Inc, Huntington Valley, PA) and reported as the number of CTCs per 7.5 mL of blood, as previously described (25). Samples were collected at baseline, 4 weeks, 12 weeks, and/or study termination, and analyzed in a CLIA-certified laboratory at MSK.
Antitumor Activity
Antitumor activity was assessed based on changes in individual disease manifestations using the Prostate Cancer Clinical Trials Working Group 2 (PCWG2) criteria (26). For PSA, the maximum decline that occurred at any point after treatment was reported using waterfall plots. Pretreatment PSA velocity was calculated using 3 pretreatment PSA values >7 days apart, and posttreatment PSA velocity was calculated using the first 3 on-study PSA values >7 days apart. The change in PSA velocity posttreatment was reported using waterfall plots. Imaging was performed at baseline, at 12-week intervals, and at the end of treatment. Changes in soft tissue disease were based on CT imaging using modified Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, and changes in osseous disease were reported as improved, stable, or progression, based on radionuclide bone scan per PCWG2 criteria (26, 27).
Statistical Analysis
The primary objective of this phase 1 study was to confirm the safety and tolerability of combined cixutumumab and temsirolimus using the recommended phase 2 dose level for advanced solid tumors. The primary objective of the phase 1 extension was to confirm the safety and tolerability of cixutumumab and temsirolimus given every 3 weeks.
All patients who received at least 1 dose of cixutumumab and temsirolimus were included in the analyses. During dose de-escalation, the number of patients enrolled in each dose de-escalation cohort was dependent upon the observed safety profile. Descriptive statistics were used to summarize patient characteristics, safety, and antitumor activity.
RESULTS
Sixteen patients were enrolled across 3 cohorts. Table 1 summarizes baseline clinical characteristics and previous treatments for all participating patients. Eight patients stopped temsirolimus alone at ≤12 weeks of treatment and continued cixutumumab. All patients had stopped study treatment at time of data analysis due to disease progression (n=11; 8 patients for radiographic disease progression and 3 patients for biochemical (PSA) progression, patient discretion (n=4), or toxicity (n=1; grade 3 neutropenia).
Table 1.
Patient Characteristics at Baseline (N = 16)
| Variable | No. of Patients | % | Median | Range |
|---|---|---|---|---|
| Age, y | 69 | 58–80 | ||
| Gleason score | 8 | 7–9 | ||
| PSA level, ng/mL | 88.2 | 2.4–545.7 | ||
| Extent of disease | ||||
| Soft tissue only | 4 | 25.0 | ||
| Bone only | 7 | 43.8 | ||
| Both soft tissue and bone | 5 | 31.3 | ||
| No. prior hormonal treatments | ||||
| 1 | 1 | 6.3 | ||
| 2 | 3 | 18.8 | ||
| 3 | 4 | 25.0 | ||
| >3 | 8 | 50.0 | ||
| Previous therapy | ||||
| Radical prostatectomy alone | 5 | 31.3 | ||
| Radiation therapy alone | 5 | 31.3 | ||
| Salvage radiation therapy | 2 | 12.5 | ||
| No prior prostatecto my or radiation therapy | 6 | 37.5 |
Abbreviation: PSA, prostate-specific antigen.
Safety
The most frequently reported adverse event (any cause) was hyperglycemia (100%), which was limited to grade 3 or lower (Table 3). Otherwise, grade 3 events occurring in more than 1 patient were oral mucositis or increases in serum amylase, alkaline phosphatase, or lipase. There were no grade 4 events or deaths. Of the non-hematologic adverse events, there were two DLTs of grade 3 oral mucositis in cohort 1 and one DLT of grade 3 lipase increase in cohort −1. Low-grade incidental pneumonitis (Supplemental figure 1) was found among 73% of patients in cohorts 1 and −1, prompting early discontinuation of temsirolimus and a change in treatment schedule for cohort −2. No DLTs or pneumonitis were seen in cohort −2.
Antitumor Response
No patient had a ≥50% decline in PSA as compared to baseline (Supplemental figure 2). Eleven patients (68.8%) had an increase in PSA velocity posttherapy.
Radiographic Response
Four patients (25%) presented with measurable soft tissue disease at baseline, 3 of whom developed radiographic progression. Seven patients (44%) presented with bone disease at baseline, 6 of whom developed disease progression based on PCWG2 criteria. Five patients (31%) presented with both soft tissue and bone disease at baseline, 3 of whom had disease progression in both bone and soft tissue; 2 patients progressed in soft tissue only. Of note, 3 patients with disease limited to soft tissue maintained stable disease responses for longer than 6 months.
Duration on Study
Median time on study was 22 weeks (range, 4–60; Figure 1). Eight patients discontinued temsirolimus treatment early; 8 patients because of low-grade pneumonitis and 1 patient because of recurrent thrombocytopenia. These patients continued with cixutumumab monotherapy for a median of 16 weeks after discontinuation of temsirolimus (range, 4–48).
Fig. 1.
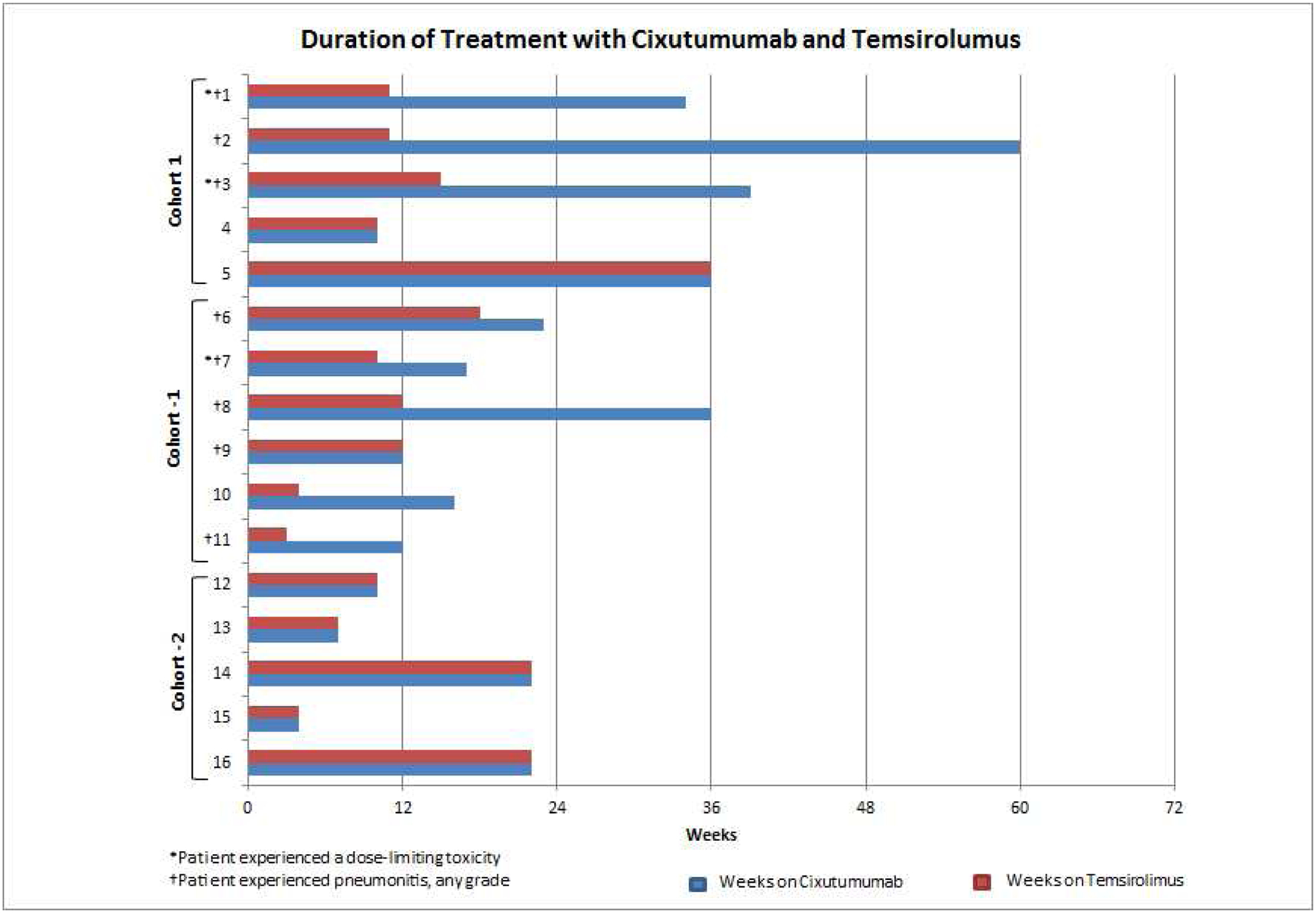
Duration of treatment with cixutumumab and temsirolimus, in weeks. Treatment was discontinued at patient’s discretion (#1, 3, 7, and 11), because of radiographic evidence of disease progression (#2, 5, 8, 9, 10, 12, 13, and 14), toxicity (neutropenia; #4), or biochemical evidence of disease progression (#6, 15, and 16).
FDG- and FDHT-PET/CT Analysis
All patients were imaged with FDG-PET/CT imaging. Eight patients among cohorts 1 and −2 were imaged with FDHT-PET/CT (Figure 2). Median FDG SUVmax-avg decreased 29%, 20.5%, and 15% for cohorts 1, −1, and −2, respectively. Median FDHT SUVmax-avg increased 13% and 15% for cohorts 1 and −2, respectively.
Fig. 2.
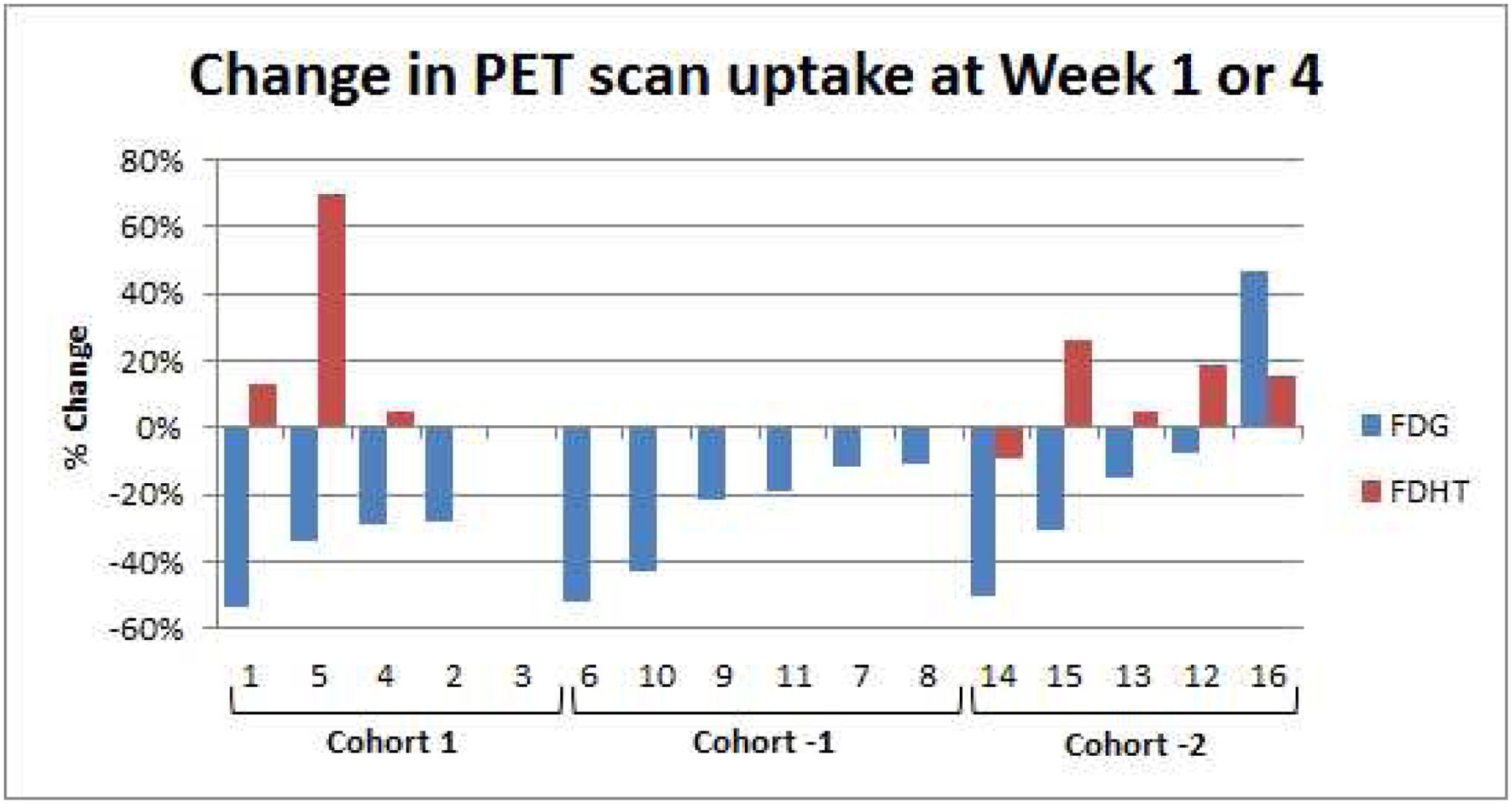
Changes in uptake of FDG or FDHT on PET scans at week 1 or 4
CTC Enumeration
At baseline, 5 of 16 patients (31%) had unfavorable CTC counts (ie, ≥5 cells/7.5 mL of blood). Of these, 2 showed a conversion from unfavorable to favorable status (<5 cells/7.5 mL of blood) after 4 weeks of treatment with cixutumumab and temsirolimus. Because of low CTC numbers, IGF-1R staining could not be performed.
DISCUSSION
Based on preclinical studies examining negative feedback inhibition as well as promising results in other solid tumors, there was a strong scientific rationale for this combination therapy in patients with CRPC. However, the results of this study showed limited antitumor activity with no objective responses or PSA decreases ≥50%. Although five patients remained on treatment for more than six months, overall, the combination of cixutumumab and temsirolimus was unable to effectively inhibit tumor growth in metastatic CRPC.
In addition, the incidence of DLTs and low-grade pneumonitis was higher than expected, prompting dose modification and discontinuation of weekly temsirolimus for some patients. Although most patients with pneumonitis were asymptomatic (grade 1), these precautions were taken to mitigate risk in this predominantly elderly, heavily pretreated patient population. Interestingly, phase 1 and 2 studies with this combination in other advanced malignancies did not encounter pneumonitis (16–18, 28–30). It is possible that the molecular imaging incorporated into this study detected low-grade pneumonitis early that may otherwise have remained clinically silent. The protocol was updated with pneumonitis guidelines from the RECORD-1 trial and the inclusion of an expansion cohort using 20 mg/kg IV cixutumumab and 20 mg IV temsirolimus infused every 3 weeks (31). This dosing schedule was found to be safe and tolerable, but antitumor activity remained limited.
We believe that comparison of FDG- and FDHT-PET/CT imaging before and after treatment with cixutumumab and temsirolimus could provide an early pharmacodynamic assessment of whether there is treatment-related activity in tumor cells, independent of overall effects on tumor growth that may be evident weeks to months later. Fourteen of 16 patients (87.5%) had some degree of FDG SUVmax-avg decline from baseline during assessment at either week 1 or week 4 (depending on each cohort’s PET imaging schedule), which may have been partly related to the phenomenon of Glut1 downregulation in response to mTOR inhibition (32).
Since the inception of this clinical trial, preclinical work has demonstrated that PI3K pathway activation is associated with repressed AR signaling, which may in part explain the castration-resistant phenotype observed in these patients (33). Importantly, this resistance is reversible, as evidenced by the restoration of AR signaling with PI3K pathway inhibition in PTEN-deficient prostate cells. Conversely, AR blockade relieves feedback inhibition of Akt. The result of this crosstalk is eloquently demonstrated in a representative patient on this study (Figure 3): Temsirolimus relieves mTORC1/2-mediated negative feedback on human epidermal growth factor receptor 2 (HER2)/HER3 signaling, which is upstream of AR. This leads to AR activation and increases in PSA and FDHT-PET SUVmax-avg in this patient. Upon stopping temsirolimus, there is a precipitous decline in PSA and FDHT-PET SUVmax-avg, as restoration of the PI3K pathways negative feedback on HER2/HER3 results in repressed AR activity. This reciprocal feedback regulation of the PI3K and AR pathways provides a compelling explanation in our study for the paradoxical rise in both FDHT-PET SUVmax-avg and PSA in response to temsirolimus, followed by a decline when the drug was subsequently stopped.
Fig. 3.

Percent changes in PSA levels and uptake of FDG and FDHT in one representative subject
Additionally, IGF1R and AR may not be the only pathways upregulated by mTOR inhibition resulting in the lack of response to treatment with rapalogs. The inhibition of S6K, with loss of the IRS negative feedback loop and lack of inhibition of mTORC2, causes hyperactivation of Akt and feedback activation of PI3K (15, 34). Dual mTOR and PI3K/mTOR inhibitors may have stronger anticancer activity than rapalogs, as a consequence of the more complete inhibition of mTOR signaling, also warranting clinical exploration (35).
CONCLUSION
We elected to stop this trial because of the unexpected toxicities associated with this combination and the minimal antitumor activity. Future success of trials in CRPC evaluating PI3K pathway inhibition could be improved with combinatorial PI3K pathway and AR-directed therapies, given the bidirectional crosstalk between these two critical CRPC survival pathways. Indeed, studies examining combinatorial AR and PI3K–Akt–mTOR inhibition have shown efficacy. In a recent phase 1b/2 trial, ipatasertib—a potent, novel, selective ATP-competitive small molecule inhibitor of all 3 isoforms of Akt—was studied at two different doses with abiraterone and demonstrated superiority over abiraterone alone in patients with PTEN-loss tumors treated at the higher dose (400 mg), with delayed radiographic PFS (36). This combination is currently being studied in an ongoing phase 3 international trial (ClinicalTrials.gov Identifier: NCT03072238).
Clinical Practice Points
Despite the high rate of PI3K pathway dysregulation in CRPC, trials of downstream mTOR inhibition as monotherapy have been disappointing in part due to relief of feedback on members receptor tyrosine kinase signaling. Downstream targets of mTOR provide negative feedback on IGF-1R. Consequently, mTOR inhibition releases the negative feedback, leading to upregulation of IGF-1R and activation of the PI3K/Akt/mTOR pathway, potentially explaining resistance. We postulated that the IGF-1R antibody Cixutumumab, would potentiate the antitumor activity of the mTOR inhibitor Temsirolimus. Despite the strong scientific rationale, the combination showed limited antitumor activity in this phase 1 study, with higher than anticipated incidence of DLTs and low-grade pneumonitis resulting in dose modifications and discontinuation of weekly temsirolimus in some patients.
Although this combination was ineffective, we postulate that single agent mTOR inhibition combined with upregulation of AR signaling contributed to the lack of efficacy seen in this study. Future success of trials in metastatic CRPC evaluating PI3K/Akt/mTOR pathway inhibition could be improved through combinatorial approaches using selective inhibition of the PI3K pathway upstream of mTOR and also targeting the AR-pathway, given the more recent finding of bidirectional crosstalk between these two CRPC survival pathways that are highly active in advanced disease. This approach has demonstrated efficacy in recent early phase studies and is now the subject of an ongoing international phase III trial (IPATential150; NCT03072238)
Supplementary Material
Supplemental fig. 1 Low-grade incidental pneumonitis
Supplemental fig. 2 PSA waterfall curve
Table 2.
Adverse Events (N = 16)
| Event | Grade 1 No. of Patients (%) | Grade 2 No. of Patients (%) | Grade 3 No. of Patients (%) | Grade 4 No. of Patients (%) | All No. of Patients (%) |
|---|---|---|---|---|---|
| Hyperglycemia | 8 (50%) | 3 (18.8) | 5 (31.3) | 0 | 16 (100) |
| Aspartate aminotransferas e increased | 13 (81.3) | 0 | 0 | 0 | 13 (81.3) |
| Hypertriglyceri demia | 7 (43.8) | 6 (37.5) | 0 | 0 | 13 (81.3) |
| Cholesterol high | 10 (62.5) | 2 (12.5) | 0 | 0 | 12 (75.0) |
| Platelet count decreased | 10 (62.5) | 1 (6.3) | 0 | 0 | 11 (68.8) |
| White blood cell decreased | 7 (43.8) | 4 (25.0) | 0 | 0 | 11 (68.8) |
| Anorexia | 10 (62.5) | 0 | 0 | 0 | 10 (62.5) |
| Fatigue | 5 (31.3) | 5 (31.3) | 0 | 0 | 10 (62.5) |
| Mucositis, oral | 6 (37.5) | 1 (6.3) | 3 (18.8) | 0 | 10 (62.5) |
| Creatinine increased | 8 (50.0) | 0 | 0 | 0 | 8 (50.0) |
| Hypoalbumine mia | 8 (50.0) | 0 | 0 | 0 | 8 (50.0) |
| Diarrhea | 7 (43.8) | 0 | 0 | 0 | 7 (43.8) |
| Pneumonitis | 4 (25.0) | 3 (18.8) | 0 | 0 | 7 (43.8) |
| Serum amylase increased | 3 (18.8) | 2 (12.5) | 2 (12.5) | 0 | 7 (43.8) |
| Urinary frequency | 7 (43.8) | 0 | 0 | 0 | 7 (43.8) |
| Alanine aminotransferas e increased | 5 (31.3) | 1 (6.3) | 0 | 0 | 6 (37.5) |
| Back pain | 5 (31.3) | 1 (6.3) | 0 | 0 | 6 (37.5) |
| Alkaline phosphatase increased | 3 (18.8) | 0 | 2 (12.5) | 0 | 5 (31.3) |
| Blood bilirubin increased | 5 (31.3) | 0 | 0 | 0 | 5 (31.3) |
| Cough | 5 (31.3) | 0 | 0 | 0 | 5 (31.3) |
| Dyspnea | 3 (18.8) | 2 (12.5) | 0 | 0 | 5 (31.3) |
| Hypocalcemia | 5 (31.3) | 0 | 0 | 0 | 5 (31.3) |
| Lipase increased | 2 (12.5) | 1 (6.3) | 2 (12.5) | 0 | 5 (31.3) |
| Pain in extremity | 5 (31.3) | 0 | 0 | 0 | 5 (31.3) |
| Rash, maculopapular | 5 (31.3) | 0 | 0 | 0 | 5 (31.3) |
| Skin & subcutaneous tissue disorders | 5 (31.3) | 0 | 0 | 0 | 5 (31.3) |
| Constipation | 3 (18.8) | 1 (6.3) | 0 | 0 | 4 (25.0) |
| Dysgeusia | 4 (25.0) | 0 | 0 | 0 | 4 (25.0) |
| Headache | 3 (18.8) | 1 (6.3) | 0 | 0 | 4 (25.0) |
| Hyperkalemia | 4 (25.0) | 0 | 0 | 0 | 4 (25.0) |
| Nausea | 3 (18.8) | 1 (6.3) | 0 | 0 | 4 (25.0) |
| Neutrophil count decreased | 2 (12.5) | 1 (6.3) | 1 (6.3) | 0 | 4 (25.0) |
| Pain | 4 (25.0) | 0 | 0 | 0 | 4 (25.0) |
| Vomiting | 4 (25.0) | 0 | 0 | 0 | 4 (25.0) |
| Dry skin | 3 (18.8) | 0 | 0 | 0 | 3 (18.8) |
| Edema limbs | 3 (18.8) | 0 | 0 | 0 | 3 (18.8) |
| Hypernatremia | 3 (18.8) | 0 | 0 | 0 | 3 (18.8) |
| Hyponatremia | 2 (12.5) | 0 | 1 (6.3) | 0 | 3 (18.8) |
| Oral pain | 3 (18.8) | 0 | 0 | 0 | 3 (18.8) |
| Peripheral sensory neuropathy | 2 (12.5) | 1 (6.3) | 0 | 0 | 3 (18.8) |
| Weight loss | 1 (6.3) | 2 (12.5) | 0 | 0 | 3 (18.8) |
Data are number (%).
All reported adverse events in >15% patients treated (N = 16)
Acknowledgments
We thank senior editor Margaret McPartland, who assisted in the writing and proof-reading of this manuscript.
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. This trial received ARRA funding (3U01CA069856-15S1) and is a DOD/PCF PCCTC trial sponsored by the Cancer Therapy Evaluation Program (CTEP) LOI #8147
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- 1.Siegel RL, Miller KD. Cancer statistics, 2019. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. The New England journal of medicine. 2014;371(5):424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364(21):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. The New England journal of medicine. 2013;369(3):213–23. [DOI] [PubMed] [Google Scholar]
- 5.Parker C, Sartor O. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;365(8):767. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine. 2012;367(13):1187–97. [DOI] [PubMed] [Google Scholar]
- 7.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. International journal of clinical practice. 2011;65(11):1180–92. [DOI] [PubMed] [Google Scholar]
- 8.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong AJ, Shen T, Halabi S, Kemeny G, Bitting RL, Kartcheske P, et al. A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer. Clinical genitourinary cancer. 2013;11(4):397–406. [DOI] [PubMed] [Google Scholar]
- 10.Templeton AJ, Dutoit V, Cathomas R, Rothermundt C, Bartschi D, Droge C, et al. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08). European urology. 2013;64(1):150–8. [DOI] [PubMed] [Google Scholar]
- 11.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66(3):1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer research. 2005;65(16):7052–8. [DOI] [PubMed] [Google Scholar]
- 13.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–40. [DOI] [PubMed] [Google Scholar]
- 14.Higano C, Alumkal J, Ryan C, Yu E, Beer T, Chandrawansa K, et al. A phase II study evaluating the efficacy and safety of single agent IMC A12, a monoclonal antibody (MAb), against the insulin-like growth factor-1 receptor (IGF-IR), as monotherapy in patients with metastastic, asymptomatic castration-resistant prostate cancer (CRPC). J Clin Oncol. 2009;27(15_suppl):5142-. [Google Scholar]
- 15.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nature reviews Cancer. 2006;6(9):729–34. [DOI] [PubMed] [Google Scholar]
- 16.Naing A, Kurzrock R, Burger A, Gupta S, Lei X, Busaidy N, et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(18):6052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(9):2625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naing A, Lorusso P, Fu S, Hong D, Chen HX, Doyle LA, et al. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. British journal of cancer. 2013;108(4):826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beattie BJ, Smith-Jones PM, Jhanwar YS, Schoder H, Schmidtlein CR, Morris MJ, et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51(2):183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehdashti F, Picus J, Michalski JM, Dence CS, Siegel BA, Katzenellenbogen JA, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. European journal of nuclear medicine and molecular imaging. 2005;32(3):344–50. [DOI] [PubMed] [Google Scholar]
- 21.Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2004;45(3):366–73. [PubMed] [Google Scholar]
- 22.Zanzonico PB, Finn R, Pentlow KS, Erdi Y, Beattie B, Akhurst T, et al. PET-based radiation dosimetry in man of 18F-fluorodihydrotestosterone, a new radiotracer for imaging prostate cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2004;45(11):1966–71. [PubMed] [Google Scholar]
- 23.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet (London, England). 2010;375(9724):1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox JJ, Autran-Blanc E, Morris MJ, Gavane S, Nehmeh S, Van Nuffel A, et al. Practical approach for comparative analysis of multilesion molecular imaging using a semiautomated program for PET/CT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52(11):1727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(23):7053–8. [DOI] [PubMed] [Google Scholar]
- 26.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(7):1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92(3):205–16. [DOI] [PubMed] [Google Scholar]
- 28.Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(18):5182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma CX, Suman VJ, Goetz M, Haluska P, Moynihan T, Nanda R, et al. A phase I trial of the IGF-1R antibody Cixutumumab in combination with temsirolimus in patients with metastatic breast cancer. Breast cancer research and treatment. 2013;139(1):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz GK, Tap WD, Qin LX, Livingston MB, Undevia SD, Chmielowski B, et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. The Lancet Oncology. 2013;14(4):371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White DA, Camus P, Endo M, Escudier B, Calvo E, Akaza H, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. American journal of respiratory and critical care medicine. 2010;182(3):396–403. [DOI] [PubMed] [Google Scholar]
- 32.Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, et al. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. American journal of physiology Cell physiology. 2008;295(3):C836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer cell. 2011;19(5):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends in molecular medicine. 2007;13(10):433–42. [DOI] [PubMed] [Google Scholar]
- 35.Fasolo A, Sessa C. Current and future directions in mammalian target of rapamycin inhibitors development. Expert opinion on investigational drugs. 2011;20(3):381–94. [DOI] [PubMed] [Google Scholar]
- 36.de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(3):928–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental fig. 1 Low-grade incidental pneumonitis
Supplemental fig. 2 PSA waterfall curve


