Abstract
Background
Coronavirus disease 2019 (COVID-19) has exposed haemodialysis (HD) patients and kidney transplant (KT) recipients to an unprecedented life-threatening infectious disease, raising concerns about kidney replacement therapy (KRT) strategy during the pandemic. This study investigated the association of the type of KRT with COVID-19 severity, adjusting for differences in individual characteristics.
Methods
Data on KT recipients and HD patients diagnosed with COVID-19 between 1 February 2020 and 1 December 2020 were retrieved from the European Renal Association COVID-19 Database. Cox regression models adjusted for age, sex, frailty and comorbidities were used to estimate hazard ratios (HRs) for 28-day mortality risk in all patients and in the subsets that were tested because of symptoms.
Results
A total of 1670 patients (496 functional KT and 1174 HD) were included; 16.9% of KT and 23.9% of HD patients died within 28 days of presentation. The unadjusted 28-day mortality risk was 33% lower in KT recipients compared with HD patients {HR 0.67 [95% confidence interval (CI) 0.52–0.85]}. In a fully adjusted model, the risk was 78% higher in KT recipients [HR 1.78 (95% CI 1.22–2.61)] compared with HD patients. This association was similar in patients tested because of symptoms [fully adjusted model HR 2.00 (95% CI 1.31–3.06)]. This risk was dramatically increased during the first post-transplant year. Results were similar for other endpoints (e.g. hospitalization, intensive care unit admission and mortality >28 days) and across subgroups.
Conclusions
KT recipients had a greater risk of a more severe course of COVID-19 compared with HD patients, therefore they require specific infection mitigation strategies.
Keywords: COVID-19, dialysis, kidney, mortality, transplantation
INTRODUCTION
KEY LEARNING POINTS
What is already known about this subject?
Kidney failure patients represent a vulnerable population for coronavirus disease 2019 (COVID-19), raising concerns about kidney replacement therapy strategies during the pandemic.
What this study adds?
This study demonstrates that the mortality risk is higher in kidney transplant (KT) after adjustment for comorbidities compared with haemodialysis patients. The mortality risk is dramatically increased during the first post-transplant year.
What impact this may have on practice or policy?
KT recipients with COVID-19 should be closely monitored; they need intensification of infection mitigation strategies and prioritization in effective and safe COVID vaccine distribution. Postponing transplantations may be justified in some instances, especially during the highly active phase of the pandemic.
Since the start of the pandemic in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has exposed patients treated with kidney replacement therapy (KRT) to an unprecedented life-threatening infectious disease, coronavirus disease 2019 (COVID-19) [1, 2]. In-centre haemodialysis (HD) patients are at higher risk for COVID-19-related mortality, independent from known risk factors such as obesity, ischaemic heart disease and lung disease [2]. Kidney transplant (KT) recipients also represent a vulnerable population for viral diseases, because of their immunosuppressive agents, and specifically are at high risk for COVID-19-related mortality [3].
Reported case fatality rates for COVID-19 vary greatly by country, owing to differences in public health policy, case ascertainment and testing capacity [4, 5]. In the European Renal Association COVID-19 Database (ERACODA), which included 4298 kidney failure patients, 28-day mortality was 20.0% in 3285 patients receiving dialysis and 19.9% in 1013 recipients of a transplant [6]. The ERACODA database (1073 patients) reported a 28-day case fatality rate of 25.0% in 768 dialysis patients and 21.3% in 305 KT recipients during the first wave [7]. Other reports based on regional or national registries have also suggested lower mortality in KT versus HD patients [8, 9]. These data raise the question of whether there is an effect of the type of KRT in COVID-19-related outcomes. It should be noted that dialysis patients in general are older and have a higher prevalence of comorbid conditions than KT recipients. When analysing the association of the type of KRT with outcome, analyses should be adjusted for such differences in baseline characteristics.
When comparing COVID-19 mortality rates in dialysis patients versus KT recipients, another complicating factor is how patients were diagnosed to have COVID-19. In HD units, screening for COVID-19 may rely not only on symptoms, but also as part of routine surveillance or screening after contact, whereas transplant patients are most often tested because they present with symptoms.
Given these considerations, we aimed in this study to determine whether HD or kidney transplantation is associated with mortality in kidney failure patients with COVID-19 while accounting for comorbidities and the reason for COVID-19 testing.
MATERIALS AND METHODS
Study design and participants
For this study we used data from the ERACODA [10]. This database was established in March 2020 and currently involves cooperation of ∼200 physicians representing >130 centres in 31 countries, mostly in Europe. Data were collected on adult (>18 years of age) patients with kidney failure, either on HD or living with a functioning kidney allograft, who were diagnosed with COVID-19 based on a positive result on a real-time polymerase chain reaction assay of nasal and/or pharyngeal swab specimens and/or compatible findings on a computed tomography scan or chest X-ray of the lungs. Data are voluntarily reported from outpatients and hospitalized patients by physicians responsible for the care of these patients.
The ERACODA is hosted at the University Medical Centre Groningen, Groningen, The Netherlands. Data are recorded using REDCap software (Research Electronic Data Capture, Vanderbilt University Medical Centre, Nashville, TN, USA) for data collection [11]. Patient information is stored pseudonymized. The study was approved by the Institutional Review Board of the University Medical Centre Groningen, who deemed the collection and analysis of data exempt from ethics review in accordance with the Medical Research Involving Human Subjects Act.
Data collection
Detailed information was collected on patient (age, sex, ethnicity, frailty, comorbidities, hospitalization and medication use) and COVID-19-related characteristics (reason for COVID-19 screening, symptoms, vital signs and laboratory test results) at presentation. Frailty was assessed using the Clinical Frailty Scale developed by Rockwood et al. [12]. This score is widely used in non-COVID-19 epidemiological studies and ranges from a score of 1, representing very fit, to 9, representing terminally ill. Comorbidities were recorded from patient charts and obesity was defined as a body mass index >30 kg/m2. Kidney function was assessed by the estimated glomerular filtration rate (eGFR) from serum creatinine at presentation using the Chronic Kidney Disease Epidemiology Collaboration equation. Among dialysis patients, eGFR was assumed to be 0 for those with residual diuresis ≤200 mL/day and 5 mL/min/1.73 m2 for those with residual diuresis >200 mL/day. The primary outcome was vital status at Day 28 and the secondary outcomes were hospitalization, intensive care unit (ICU) admission and in-hospital mortality within 28 days after presentation.
Statistical analysis
Baseline characteristics are presented for the total population, for transplant recipients and dialysis patients separately and by reason for COVID-19 testing, i.e. the presence of symptoms versus contact with a COVID-19-positive person/routine screening of patients who may have been asymptomatic. Continuous data are presented as mean [standard deviation (SD)] or as median and interquartile range (IQR) in case of a non-normal distribution of data. Categorical data are presented as percentages. Characteristics were compared between groups using Student’s t-test for continuous variables (Mann–Whitney U-test for non-normally distributed data) and Pearson chi-square test for categorical variables.
The associations of the type of KRT (kidney transplantation versus HD) with the primary and secondary outcomes were examined in the total population and by reason for COVID-19 testing. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression models. To account for the competing risk of mortality, cause-specific hazards were calculated for hospitalization and ICU admission.
Multiple models were constructed to account for potential confounders in a stepwise manner. Model 1 is a crude (unadjusted) model. In Model 2 we adjusted for age (continuous) and sex (male/female) and in Model 3 we additionally adjusted for clinical frailty score. In Model 4 we additionally adjusted for factors known to be associated with COVID-19 outcome, i.e. smoking (never, current and former), obesity (yes/no), hypertension (yes/no), diabetes (yes/no), heart failure (yes/no) and chronic lung disease (yes/no). In the final model (Model 5) we further adjusted for time since the start of any KRT (continuous) and kidney function. The proportional hazards assumption was investigated by testing the interaction of log(time) with individual covariates.
All multivariable models were based on datasets constructed using multiple imputations to account for missing data. For 210 patients (13%), information was missing on the clinical frailty score, for 235 (14%) on obesity, for 249 (15%) on serum creatinine (required to calculate eGFR) and for 73 (4%) on duration of kidney failure. The proportion of missingness for the clinical frailty score (13.1% versus 11.3%; P = 0.30) and obesity (13.0% versus 16.5%; P = 0.06) was similar for HD patients and KT recipients, while serum creatinine was missing more often in HD patients (16.5% versus 11.1%; P = 0.004) and duration of kidney disease was missing more often in transplant recipients (2.5% versus 8.9%; P < 0.001). To account for missing information, multiple imputations were performed with the method of chained equations using all variables included in Model 4. Fully conditional specification was employed, meaning that data from available cases were used and that each variable with missing values was imputed using a regression model conditional on all of the other variables specified in the imputation model [13, 14]. In total, 10 imputed datasets were created with 100 interactions. For the pooled coefficient and standard error, Rubin’s rule was used [15].
To examine the robustness of our findings, we performed several sensitivity analyses, which are described in the Supplementary material.
All analyses were performed using Stata version 14.0 (StataCorp, College Station, TX, USA). Two-sided P-values ˂0.05 indicated statistical significance.
RESULTS
A total of 2575 KRT patients who were diagnosed with COVID-19 between 1 February 2020 and 1 December 2020 were included. After excluding patients on peritoneal dialysis due to their low number (n = 99) and patients with missing information on the type of KRT (n = 83), Day 28 vital status (115 KT recipients and 221 dialysis patients) and/or the reason for COVID-19 screening (156 KT recipients and 433 dialysis patients), there were 1670 patients left for the analyses. Of these, 496 were KT recipients and 1174 HD patients (Supplementary data, Figure S1).
Patient characteristics
The mean age of KT recipients was 56 years, which was 10 years younger than those on HD (Table 1). Overall, 62% were male and 85% were white/Caucasian, which was not different for the two groups. The prevalence of diabetes mellitus, coronary artery disease, heart failure, chronic lung disease and current or former smoking was higher in HD patients, who also had a higher clinical frailty score. Eighty-five percent of the KT recipients were diagnosed with COVID-19 after being tested because of symptoms, whereas this was the case in 61% of the HD patients. HD patients were more frequently diagnosed with COVID-19 as a result of a routine screening or because of a COVID-19 contact while being asymptomatic. Consequently, more KT recipients than HD patients presented with symptoms like cough, shortness of breath, headache, nausea or vomiting, diarrhoea, myalgia or arthralgia and fever. Although their oxygen (O2) saturation at presentation was similar to that of HD patients, KT recipients had a higher temperature, respiration rate, pulse rate and C-reactive protein (CRP) levels. KT recipients more often used renin–angiotensin–aldosterone system (RAAS) inhibitors than HD patients and all of them were on immunosuppression (Table 1).
Table 1.
Baseline characteristics of the study population by type of KRT
| Type of KRT |
||||
|---|---|---|---|---|
| Characteristics | Overall (N = 1670) | KT (n = 496) | HD (n = 1174) | P-value |
| Sex (male), % | 62 | 59 | 63 | 0.16 |
| Age (years), mean ± SD | 63 ± 15 | 56 ± 14 | 66 ± 15 | <0.001 |
| BMI (kg/m2), mean ± SD | 26.6 ± 5.5 | 26.9 ± 5.0 | 26.6 ± 5.7 | 0.34 |
| Race, % | 0.24 | |||
| Asian | 3 | 3 | 3 | |
| Black or African descent | 6 | 7 | 5 | |
| White or Caucasian | 85 | 85 | 85 | |
| Other or unknown | 6 | 5 | 7 | |
| Tobacco use, % | 0.03 | |||
| Current | 7 | 5 | 8 | |
| Prior | 24 | 20 | 25 | |
| Never | 46 | 50 | 45 | |
| Unknown | 23 | 25 | 22 | |
| Clinical frailty score (AU), mean ± SD | 3.6 ± 1.8 | 2.9 ± 1.6 | 3.9 ± 1.7 | <0.001 |
| Comorbidities, % | ||||
| Obesity | 22 | 23 | 22 | 0.69 |
| Hypertension | 84 | 86 | 83 | 0.14 |
| Diabetes mellitus | 39 | 29 | 43 | <0.001 |
| Coronary artery disease | 29 | 17 | 35 | <0.001 |
| Heart failure | 21 | 8 | 26 | <0.001 |
| Chronic lung disease | 13 | 9 | 15 | 0.003 |
| Active malignancy | 6 | 5 | 7 | 0.12 |
| Autoimmune disease | 4 | 5 | 4 | 0.73 |
| Primary kidney disease, % | ||||
| Primary glomerulonephritis | 18 | 20 | 17 | 0.14 |
| Pyelonephritis | 2 | 4 | 1 | 0.003 |
| Interstitial nephritis | 3 | 4 | 3 | 0.37 |
| Hereditary kidney disease | 9 | 14 | 7 | <0.001 |
| Congenital diseases | 2 | 4 | 2 | 0.002 |
| Vascular diseases | 12 | 8 | 14 | 0.001 |
| Secondary glomerular disease | 5 | 4 | 6 | 0.05 |
| Diabetic kidney disease | 21 | 9 | 26 | <0.001 |
| Other | 17 | 16 | 17 | 0.70 |
| Unknown | 11 | 18 | 8 | <0.001 |
| Residual diuresis ≥200 mL/day, % | 32 | – | 32 | – |
| Transplant waiting list status, % | – | |||
| Active on waiting list | 10 | NA | 10 | – |
| In preparation | 10 | NA | 10 | – |
| Temporarily not on list | 9 | NA | 9 | – |
| Not transplantable | 67 | NA | 67 | – |
| Unknown | 4 | NA | 4 | – |
| Time since transplantation (years), % | – | |||
| <1 | 6 | 6 | NA | – |
| 1–5 | 31 | 31 | NA | – |
| >5 | 63 | 63 | NA | – |
| Medication | ||||
| Use of RAAS inhibition, % | ||||
| ACE inhibitors | 16 | 24 | 14 | <0.001 |
| ARB | 15 | 20 | 12 | <0.001 |
| Use of immunosuppressive medication, % | ||||
| Prednisone | 31 | 87 | 7 | <0.001 |
| Tacrolimus | 24 | 79 | 1 | <0.001 |
| Cyclosporine | 3 | 10 | 0.7 | <0.001 |
| Mycophenolate | 21 | 70 | 0.5 | <0.001 |
| Azathioprine | 2 | 6 | 0.3 | <0.001 |
| mTOR inhibitor | 4 | 12 | 0.2 | <0.001 |
| Disease characteristics | ||||
| Reason for COVID-19 screening, % | <0.001 | |||
| Symptoms | 69 | 85 | 61 | – |
| Due to contact | 23 | 12 | 28 | – |
| Routine | 8 | 3 | 11 | – |
| Presenting symptoms, % | ||||
| Sore throat | 13 | 16 | 12 | 0.02 |
| Cough | 52 | 63 | 47 | <0.001 |
| Shortness of breath | 35 | 41 | 33 | 0.002 |
| Fever | 60 | 70 | 56 | <0.001 |
| Headache | 12 | 19 | 9 | <0.001 |
| Nausea or vomiting | 11 | 15 | 9 | <0.001 |
| Diarrhoea | 16 | 28 | 11 | <0.001 |
| Myalgia or arthralgia | 24 | 34 | 20 | <0.001 |
| Vital signs, mean ± SD | ||||
| Temperature (°C) | 37.5 ± 1.1 | 37.6 ± 1.1 | 37.4 ± 1.0 | 0.01 |
| Respiration rate/min | 19 ± 6 | 21 ± 7 | 19 ± 5 | <0.001 |
| O2 saturation room air (%) | 94 ± 6 | 94 ± 7 | 94 ± 5 | 0.41 |
| Systolic BP (mmHg) | 135 ± 24 | 132 ± 22 | 136 ± 25 | 0.01 |
| Diastolic BP (mmHg) | 76 ± 15 | 78 ± 14 | 75 ± 15 | <0.001 |
| Pulse rate (bpm) | 83 ± 16 | 87 ± 17 | 82 ± 15 | <0.001 |
| Laboratory test results | ||||
| Creatinine increase (>25%), % | – | 26 | – | – |
| Lymphocytes (×1000/µL), median (IQR) | 0.9 (0.6–1.3) | 0.8 (0.5–1.4) | 0.9 (0.6–1.3) | 0.52 |
| CRP (mg/L), median (IQR) | 25 (6–76) | 40 (8–88) | 22 (6–67) | 0.001 |
KT/dialysis groups were compared using one-wayanalysis of variance, Kruskal–Wallis test or chi-square test as appropriate. Obesity is defined as BMI >30 kg/m2. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; mTOR, mammalian target of rapamycin; BMI, body mass index; BP, blood pressure.
Among patients tested because of symptoms, transplant recipients generally had a higher prevalence of COVID-19-related symptoms compared with HD patients (Table 2). Compared with those identified by routine screening, HD patients with COVID-19 identified by testing because of symptoms were older and had more comorbidities, whereas symptomatic KT recipients had similar comorbidities. In both patient groups, those with symptoms had higher CRP levels (Supplementary data, Table S1).
Table 2.
Baseline characteristics of study population by reason for COVID-19 screening and type of KRT, i.e. KT or HD
| Symptoms (n = 1145) |
Contact/routine screening(n = 525) |
|||||
|---|---|---|---|---|---|---|
| Characteristics | KT [37% (n = 424)] | HD [63% (n = 721)] | P-value | KT [14% (n = 72)] | HD [86% (n = 453)] | P-value |
| Sex (male), % | 58 | 64 | 0.03 | 68 | 61 | 0.25 |
| Age (years), mean ± SD | 57 ± 14 | 68 ± 15 | <0.001 | 56 ± 15 | 64 ± 14 | <0.001 |
| BMI (kg/m2), mean ± SD | 26.9 ± 4.9 | 26.9 ± 6.2 | 0.99 | 26.4 ± 5.6 | 26.0 ± 4.8 | 0.48 |
| Race, % | 0.31 | 0.76 | ||||
| Asian | 3 | 3 | 1 | 2 | ||
| Black or African descent | 7 | 6 | 7 | 5 | ||
| White or Caucasian | 85 | 84 | 85 | 87 | ||
| Other or unknown | 4 | 7 | 7 | 6 | ||
| Tobacco use, % | 0.01 | 0.53 | ||||
| Current | 5 | 7 | 6 | 10 | ||
| Prior | 20 | 26 | 21 | 23 | ||
| Never | 50 | 41 | 51 | 50 | ||
| Unknown | 25 | 25 | 22 | 17 | ||
| Clinical frailty score (AU), mean ± SD | 2.9 ± 1.5 | 4.1 ± 1.8 | <0.001 | 3.0 ± 1.6 | 3.8 ± 1.7 | 0.001 |
| Comorbidities, % | ||||||
| Obesity | 23 | 25 | 0.53 | 22 | 17 | 0.35 |
| Hypertension | 86 | 83 | 0.18 | 83 | 82 | 0.84 |
| Diabetes mellitus | 28 | 47 | <0.001 | 32 | 36 | 0.55 |
| Coronary artery disease | 17 | 39 | <0.001 | 17 | 29 | 0.03 |
| Heart failure | 8 | 28 | <0.001 | 10 | 22 | 0.02 |
| Chronic lung disease | 10 | 16 | 0.002 | 7 | 12 | 0.20 |
| Active malignancy | 4 | 8 | 0.02 | 8 | 5 | 0.26 |
| Autoimmune disease | 5 | 4 | 0.53 | 3 | 4 | 0.52 |
| Primary kidney disease, % | ||||||
| Primary glomerulonephritis | 20 | 9 | <0.001 | 22 | 29 | 0.23 |
| Pyelonephritis | 4 | 2 | 0.06 | 3 | 1 | 0.04 |
| Interstitial nephritis | 4 | 3 | 0.58 | 3 | 2 | 0.78 |
| Hereditary kidney disease | 13 | 6 | <0.001 | 18 | 7 | 0.003 |
| Congenital diseases | 4 | 1 | 0.001 | 4 | 2 | 0.33 |
| Vascular diseases | 8 | 15 | <0.001 | 10 | 13 | 0.50 |
| Secondary glomerular disease | 3 | 6 | 0.05 | 6 | 6 | 0.88 |
| Diabetic kidney disease | 8 | 25 | <0.001 | 15 | 28 | 0.03 |
| Other | 18 | 25 | 0.01 | 6 | 4 | 0.62 |
| Unknown | 18 | 7 | <0.001 | 14 | 8 | 0.12 |
| Residual diuresis ≥200 mL/day, % | NA | 30 | – | NA | 35 | – |
| Transplant waiting list status, % | – | – | ||||
| Active on waiting list | NA | 11 | – | NA | 9 | – |
| In preparation | NA | 12 | – | NA | 9 | – |
| Temporarily not on list | NA | 7 | – | NA | 11 | – |
| Not transplantable | NA | 68 | – | NA | 65 | – |
| Unknown | NA | 3 | – | NA | 6 | – |
| Time since transplantation (years), % | – | – | ||||
| <1 | 5 | NA | – | 14 | NA | – |
| 1–5 | 32 | NA | – | 31 | NA | – |
| >5 | 64 | NA | – | 56 | NA | – |
| Medication use | ||||||
| Use of RAAS inhibition, % | ||||||
| ACE inhibitors | 24 | 12 | <0.001 | 24 | 14 | 0.05 |
| ARB | 20 | 15 | 0.01 | 22 | 8 | <0.001 |
| Use of immunosuppressive medication, % | ||||||
| Prednisone | 88 | 9 | <0.001 | 82 | 4 | <0.001 |
| Tacrolimus | 78 | 2 | <0.001 | 88 | 1 | <0.001 |
| Cyclosporine | 11 | 1 | <0.001 | 3 | 0 | <0.001 |
| Mycophenolate | 70 | 1 | <0.001 | 69 | 0.2 | <0.001 |
| Azathioprine | 6 | 0.1 | <0.001 | 3 | 1 | 0.09 |
| mTOR inhibitor | 13 | 0.1 | <0.001 | 10 | 0.2 | <0.001 |
| Disease-related characteristics | ||||||
| Presenting symptoms, % | ||||||
| Sore throat | 17 | 16 | 0.70 | 15 | 6 | 0.01 |
| Cough | 68 | 61 | 0.03 | 36 | 25 | 0.07 |
| Shortness of breath | 44 | 41 | 0.23 | 19 | 20 | 0.80 |
| Fever | 74 | 69 | 0.09 | 48 | 36 | 0.06 |
| Headache | 20 | 9 | <0.001 | 13 | 10 | 0.32 |
| Nausea or vomiting | 17 | 13 | 0.05 | 3 | 3 | 0.91 |
| Diarrhoea | 29 | 14 | <0.001 | 25 | 7 | <0.001 |
| Myalgia or arthralgia | 36 | 23 | <0.001 | 18 | 14 | 0.40 |
| Vital signs, mean ± SD | ||||||
| Temperature (°C) | 37.6 ± 1.1 | 37.7 ± 1.0 | 0.20 | 37.2 ± 0.9 | 37.0 ± 1.0 | 0.17 |
| Respiration rate/min | 21 ± 7 | 20 ± 5 | <0.001 | 17 ± 4 | 18 ± 3 | 0.23 |
| O2 saturation room air, % | 94 ± 7 | 93 ± 6 | 0.02 | 96 ± 4 | 95 ± 4 | 0.37 |
| Systolic BP (mmHg) | 131 ± 21 | 136 ± 26 | 0.02 | 133 ± 23 | 136 ± 23 | 0.38 |
| Diastolic BP (mmHg) | 79 ± 14 | 73 ± 15 | <0.001 | 76 ± 15 | 77 ± 14 | 0.59 |
| Pulse rate (bpm) | 88 ± 17 | 83 ± 17 | <0.001 | 82 ± 14 | 80 ± 12 | 0.37 |
| Laboratory test results | ||||||
| Creatinine increase (>25%), % | 27 | – | – | 15 | – | – |
| Lymphocytes (×1000/µL), mendian (IQR) | 0.8 (0.5–1.3) | 0.8 (0.5–1.3) | 0.20 | 0.9 (0.6–1.4) | 1.0 (0.7–1.4) | 0.38 |
| CRP (mg/L), median (IQR) | 45 (10–91) | 30 (10–94) | 0.35 | 10 (3–39) | 12 (3–37) | 0.83 |
KT/dialysis groups were compared using one-way analysis of variance, Kruskal–Wallis test or chi-square test as appropriate. Obesity is defined as BMI >30 kg/m2. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; mTOR, mammalian target of rapamycin.
Patient survival
The 28-day probability of death in KT recipients was 16.9% (95% CI 13.9–20.5), which was lower than the 23.9% (95% CI 21.6–26.5) in the HD patients. Supplementary data, Figure S2 shows the survival for the overall group and for the subgroup of patients identified by screening because of symptoms.
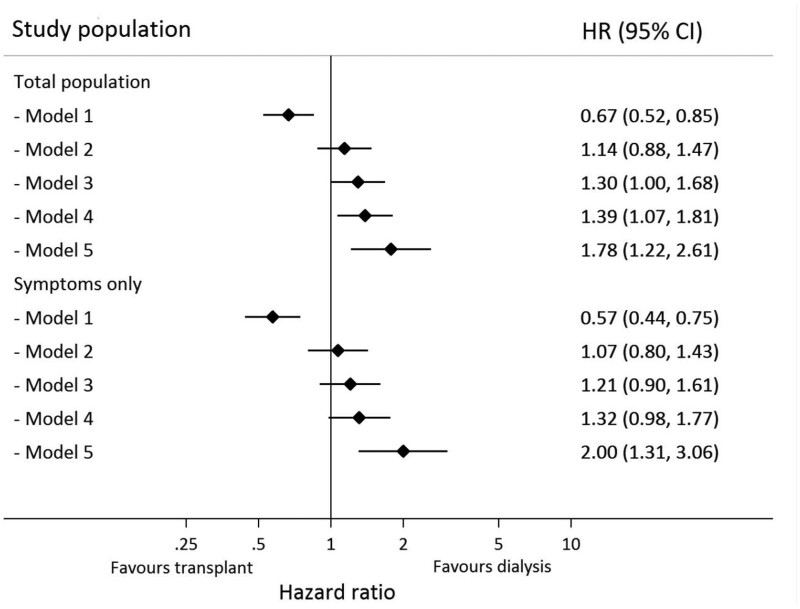
Table 3 and the upper part of Figure 1 show that after adjustment for age and sex, the risk for COVID-19-related mortality was higher in KT recipients compared with dialysis patients. When additionally adjusting for differences in clinical frailty score, ‘classical’ determinants for COVID-19 mortality (obesity, hypertension, diabetes, heart failure, chronic lung disease and smoking) and duration of kidney failure, mortality risk in KT recipients was significantly higher when compared with patients on HD [fully adjusted HR (aHR) 1.78 (95% CI 1.22–2.61)]. In the subgroup of patients who were diagnosed with COVID-19 because they were tested based on having symptoms, the risk of death was similarly higher in KT recipients [aHR 2.00 (95% CI 1.31–3.06)]. Analysis of complete data without multiple imputation confirmed the main results [aHR 2.42 (95% CI 1.52–3.86)] for transplant recipients compared with dialysis patients (Supplementary data, Table S2).
Table 3.
Association of type of KRT (KT versus HD) with incidence of 28-day COVID-19-related mortality, overall and by reason for COVID-19 screening, presented as HRs with 95% CIs
| Total, events (n) |
Symptoms, events (n) |
Contact/routine, events (n) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | HD 281 (1174) | KT 84 (496) | P-value | HD 205 (721) | KT 75 (424) | P-value | HD 76 (453) | KT 9 (72) | P-value |
| Model 1 | Ref. | 0.67 (0.52–0.85) | 0.001 | Ref. | 0.57 (0.44–0.75) | <0.001 | Ref. | 0.71 (0.36–1.43) | 0.34 |
| Model 2 | Ref. | 1.14 (0.88–1.47) | 0.33 | Ref. | 1.07 (0.80–1.43) | 0.65 | Ref. | 0.99 (0.49–1.99) | 0.97 |
| Model 3 | Ref. | 1.30 (1.00–1.68) | 0.05 | Ref. | 1.21 (0.90–1.61) | 0.21 | Ref. | 1.15 (0.57–2.34) | 0.69 |
| Model 4 | Ref. | 1.39 (1.07–1.81) | 0.02 | Ref. | 1.32 (0.98–1.77) | 0.07 | Ref. | 1.19 (0.58–2.46) | 0.63 |
| Model 5 | Ref. | 1.78 (1.22–2.61) | 0.003 | Ref. | 2.00 (1.31–3.06) | 0.001 | Ref. | 0.57 (0.17–1.91) | 0.36 |
Model 1: crude; Model 2: age and sex; Model 3: Model 2 + frailty; Model 4: Model 3 + obesity, hypertension, diabetes, heart failure, chronic lung disease and smoking; Model 5: Model 4 + duration of kidney failure and kidney function.
FIGURE 1.
Forest plot showing HRs for association of type of KRT (KT versus HD) with 28-day mortality in the total study population and in the subgroup of those patients tested for COVID-19 based on symptoms only. Model 1: crude; Model 2: age, sex; Model 3: Model 2 + frailty; Model 4: Model 3 + obesity, hypertension, diabetes, heart failure, chronic lung disease and smoking; Model 5: Model 4 + duration of kidney failure and kidney function.
Risk of hospitalization, ICU admission and in-hospital mortality
The risk of hospitalization was higher in KT recipients compared with HD patients in the total population [aHR 1.20 (95% CI 1.00–1.47)] as well as in the subgroup of symptomatic patients [aHR 1.28 (95% CI 1.03–1.60)] (Supplementary data, Table S3). Similarly, the adjusted risk of ICU admission was higher in KT recipients compared with HD patients in the total population [aHR 2.09 (95% CI 1.33–3.29)] and in symptomatic patients [aHR 2.10 (95% CI 1.28–3.45)] (Supplementary data, Table S4). For the association of KT versus HD with in-hospital mortality, the aHR was 1.81 (95% CI 1.19–2.75) in the total population and 2.16 (95% CI 1.37–3.43) in symptomatic patients (Supplementary data, Table S5). Only ∼6% of the deaths (n = 23) were reported not to be caused by COVID-19. An analysis restricted to COVID-19-related deaths showed results similar to the main results.
Sensitivity analyses
After excluding HD patients using immunosuppressive agents and/or who previously received a KT, the aHR for the association of being a KT recipient (versus HD patient) with 28-day mortality was 1.62 (95% CI 1.09–2.41) in the total population (n = 1541) and 1.68 (95% CI 1.06–2.65) in the symptomatic group (n = 1051) (Supplementary data, Table S6). In an analysis comparing KT recipients with HD patients on the KT waiting list or in preparation to be listed, the aHR for KT recipients was 4.81 (95% CI 2.25–10.29) in the total population (n = 703) and 5.48 (95% CI 2.26–13.27) in the symptomatic group (n = 553; Supplementary data, Table S7). Taking into account kidney recipients within their first post-transplant year, the HR for 28-day mortality was 10.27 (95% CI 2.40–43.94) compared with dialysis patients who were on the waiting list or in preparation for KT (Supplementary data, Table S8). The HR for kidney recipients in the first year of transplantation was 2.17 (95% CI 0.98–4.82) and was 1.72 (95% CI 1.17–2.53) after the first post-transplant year compared with all HD patients (Supplementary data, Table S9). Results were also similar to the overall results when analysing hospitalized patients only [aHR 1.82 (95% CI 1.20–2.74) in the total population and 2.20 (95% CI 1.39–3.47) in the symptomatic group] or when including those with missing information on the reason for COVID-19 screening [aHR 1.74 (95% CI 1.26–2.42) in the total population and 1.89 (95% CI 1.24–2.89) in the symptomatic group] (Supplementary data, Table S11).
Overall, crude mortality (i.e. mortality not limited to the 28-day window) was 19.9% and 27.7% in KT recipients and HD patients, respectively. For the risk of overall mortality in KT recipients (versus HD patients), the aHR was 1.87 (95% CI 1.31–2.67) in the total population and 2.04 (95% CI 1.37–3.06) in the symptomatic group (Supplementary data, Table S12). No significant interactions in the risk associated with KT compared with being on HD were found between subgroups except for subgroups defined by age (<65 and ≥65 years) (P = 0.02) (Supplementary data, Table S13).
DISCUSSION
Since the start of the COVID-19 outbreak, it has been a topic of discussion in the nephrology community whether HD or KT patients are more affected by COVID-19-related mortality. Here we present analyses derived from the ERACODA, the largest database with comprehensive individual-level data of patients on dialysis or living with a KT infected by SARS-CoV-2 in Europe. Our data indicate that 28-day mortality is higher for KT recipients compared with HD patients after adequate adjustment for age, sex, frailty and comorbidities. Stratified analyses limiting the dataset to patients tested because of symptoms confirmed that KT recipients have a higher mortality risk. Finally, we show that only very few HD patients were admitted to the ICU, less than patients with a KT.
COVID-19 is particularly life-threatening in patients on KRT, suggesting that kidney failure creates an environment for a more severe course of disease [3]. In our study, the unadjusted COVID-19-related 28-day mortality rate was 16.9% in transplant recipients and 23.9% in HD patients. These findings are in line with data from other regional and national registries [6, 9, 16–18]. These studies also show lower, or a trend towards lower, mortality in KT patients in a crude analysis compared with HD patients. This finding is not unexpected, because in our study HD patients were on average 10 years older and had more comorbidities. In a region-wide registry study from the Flanders region of Belgium, cumulative mortality was 29.6% versus 14.0% among patients on HD and KT recipients, respectively. Adjustment for age decreased cumulative mortality for dialysis patients (19.9%) but increased mortality for transplant patients (23%) [19]. This study demonstrates that adjustment for age is the first step towards obtaining a more reliable comparison of the mortality burden in patients on various modalities of kidney function replacement. In addition, it should be accounted for in burden differences of comorbidities. Diabetes, obesity, respiratory and cardiovascular disease are all well-identified predictors of COVID-19-related death in the general population [2, 5] as well as in kidney failure patients [8, 16, 18], albeit to a lesser extent in these latter patient groups [20]. In a recent study, Hilbrands et al. [7] showed that the clinical frailty score at presentation was the strongest predictor of COVID-19-related mortality in dialysis patients, an important patient characteristic that is unfortunately underreported in most studies.
Taking all these data into account, multivariable analysis for mortality was performed in our study with adjustment for age, sex, frailty and other classic determinants of COVID-19 mortality (diabetes, obesity, heart failure, chronic obstructive pulmonary disease, smoking, hypertension, kidney function and duration of kidney disease). Doing so, we documented a significantly higher risk of mortality [HR 1.78 (95% CI 1.22–2.61)] in KT recipients compared with HD patients. Several previous studies showed similar outcomes in transplant [21] and dialysis patients [22] compared with non-transplant/non-dialysis patients, but these were small studies. In this study, the main finding was robust in the various sensitivity analyses that we performed. Analysis of overall instead of Day 28 mortality showed similar results, as did exclusion of the very few dialysis patients using immunosuppressive treatments and/or who had a KT previously. To account for potential selection bias, we provide an analysis of symptomatic and asymptomatic (contact or routine testing) patients separately; however, due to their small number, we were not confident that we could draw statistically well-founded conclusions for the latter.
We observed that the risk of death associated with being a transplant recipient was considerably higher in patients >65 years of age. This may reflect longer kidney disease duration and longer chronic exposure to immunosuppressive agents. However, adjustment for the duration of kidney failure did not materially affect our results. It may therefore well be that elderly subjects are more vulnerable to the risk-enhancing effect of immunosuppressive agents, just as in the general population.
Under non-COVID-19 circumstances, KT recipients exhibit lower mortality rates than waitlisted dialysis patients after adjustment for age, sex, race and cause of renal disease [23]. Similarly, a report of 56 waitlisted dialysis patients and 80 KT recipients in New York found that waitlist status was independently associated with COVID-19-related mortality [odds ratio 2.65 (95% CI 1.18–5.96); P = 0.02] and that waitlisted dialysis patients were more likely to require hospitalization (82% versus 65%) than KT recipients [8]. The French nationwide registry noticed similar findings [24]. The risk of contracting SARS-CoV-2 infection is higher in HD patients, consistent with the difficulties in achieving social distancing while travelling to and from dialysis units. It should therefore be noted that the overall risk of mortality could be the same or lower in KT recipients if the risk of getting infected is lower in these patients. Previous studies have also reported that although the incidence of COVID-19 was higher in waitlisted dialysis patients as compared with KT recipients, the mortality rate remained lower [24–26]. This can be accounted for by the fact that many COVID-19 cases in dialysis patients are detected by a general screening policy, even in asymptomatic patients. In this study, adequate adjusted analysis documents that KT recipients exhibit a higher mortality rate compared with waitlisted patients.
KRT patients have a frail immunological system. Chronic exposure to uraemia alters the immune response in dialysis and KT recipients. In addition, a KT recipient requires lifelong immunosuppression, which affects both innate and adaptive immunity. Therefore, in KT recipients, viral infections are frequent [27] and lead to higher morbidity and mortality when compared with dialysis patients [28]. A role for immunosuppressive agents as the cause for the higher risk for COVID-19 mortality in KT recipients may be suspected. Indeed, when further examined in our data, patients during the first post-transplant year, i.e. when they are given the strongest immunosuppression, experience dramatically increased COVID-19-related mortality compared with waitlisted patients, a finding consistent with other studies [7, 29]. We also investigated the association of intensity and type of immunosuppressant use with mortality in KT recipients. Patients on dual therapy (versus triple therapy) and immunosuppression excluding corticosteroids (versus immunosuppression including corticosteroids) tended to have a lower risk of mortality, but these associations were not statistically significant (Supplementary data, Tables S14 and S15, respectively). Therefore we are unable to confirm the role of immunosuppressant use in the observed excess risk of mortality in KT recipients. However, future studies with larger sample sizes should examine the impact of immunosuppressive treatments on mortality in KT recipients.
Remarkably, the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial showed that corticosteroids would decrease COVID-19 mortality [30]. However, this concerns a relatively high dose to mitigate the cytokine storm related to a late stage of COVID-19 when ventilator support is needed [30], whereas it has been shown that chronic low-dose corticosteroid exposure is associated with higher influenza-related mortality in patients not only with various comorbidities [31], but also with COVID-19-related mortality in patients with rheumatoid arthritis [32–34].
Supportive care remains the mainstay of treatment for COVID-19. Despite hospitalization and ICU admission thresholds consistent across the centres, KT recipients were more often hospitalized and admitted to an ICU than HD patients. HD allows facilities to monitor these patients three times a week and may delay or avoid hospitalization. Nevertheless, patients frequently develop symptoms that require admission and intensive care management. In our study, substantial mortality occurred in dialysis patients who were not offered ICU admission compared with KT recipients (71.1% versus 56.1%; P = 0.01). This finding could be explained by advanced care being more frequently planned in HD patients, but speculatively could also be explained by an expected high risk of death in these patients leading to a restrictive policy for ICU admission. Such a policy of limiting care may have disserved the group of dialysis patients.
The strengths of this study are the completeness of data retrieved from the largest European database of KRT patients with COVID-19. This database encompasses detailed information on demographics, disease characteristics, hospital and ICU admission and mortality. The ERACODA also includes information on comorbidities, frailty, time from onset of kidney failure, reason for COVID-19 screening and mode of COVID-19 diagnosis, all factors important in determining whether a specific KRT impacts mortality risk in kidney failure patients with COVID-19.
Our study also has limitations. First, due to our study design, we may not have captured enough asymptomatic cases of COVID-19, especially among KT recipients. Moreover, the database might not include all patients in all participating centres. Testing policies may vary between centres and over time, but in general leads to reporting fewer cases in asymptomatic patients. Despite the rapid implementation of telemedicine or scheduled calls in most centres during the pandemic, underreporting of cases is still possible, particularly for transplant recipients. For these reasons, we also present the results of sensitivity analyses limited to the subset of transplant and HD patients who were identified in a similar manner (testing because of symptoms or hospitalization). We additionally accounted for any potential centre effect. These analyses confirm our main findings. Second, the ERACODA does not gather information on in-hospital patient management >48 h after admission. Change over time in recommendations for supportive care, immunosuppressive treatment and availability of specific therapies may have impacted outcome. Also, analysis taking into account a potential centre effect did not show discordant results. Differences in in-hospital patient management between groups are therefore not likely to have had a major impact on our comparative results.
In conclusion, this large European study demonstrates that kidney transplantation dramatically impacts mortality in kidney failure patients with COVID-19. Thus KT recipients with COVID-19 should be closely monitored and need intensification of infection mitigation strategies and prioritization of effective and safe COVID-19 vaccine distribution. In addition, these findings suggest that postponing transplantations may be justified, especially during the highly active phase of the pandemic. Investigation of the potential effect of immunosuppression on outcomes is also urgently needed. Finally, our data indicate that intensive care management should be considered for kidney failure patients based on demographics, comorbidities and disease characteristics and that the mere fact of being an HD patient should not discourage physicians from admitting such patients to an ICU.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The ERACODA collaboration is an initiative to study prognosis and risk factors for mortality due to COVID-19 in patients with a KT or on dialysis that is endorsed by the ERA-EDTA. The organizational structure contains a working group assisted by a management team and advisory board. The ERACODA Working Group members: C.F.M. Franssen, R.T. Gansevoort (coordinator), M.H. Hemmelder, L.B. Hilbrands and K.J. Jager. The ERACODA Management Team members: R. Duivenvoorden, M. Noordzij, P. Vart. The ERACODA Advisory Board members: D. Abramowicz, C. Basile, A. Covic, M. Crespo, Z.A. Massy, S. Mitra, E. Petridou, J.E. Sanchez and C. White. We thank all the people who entered information in the ERACODA database for their participation and especially all healthcare workers who have taken care of the included COVID-19 patients.
DATA AVAILABILITY STATEMENT
Collaborators that entered data in the ERACODA remain owner of these data. The database can therefore not be disclosed to any third party without the prior written consent of all data providers, but the database will be made available to the editorial offices of medical journals when requested. Research proposals can be submitted to the Working Group via COVID.19.KRT@umcg.nl. If deemed of interest and methodologically sound by the Working Group and Advisory Board, the analyses needed for the proposal will be carried out by the Management Team.
FUNDING
ERACODA received unrestricted research grants from the ERA-EDTA, the Dutch Kidney Foundation, Baxter and Sandoz.
AUTHORS’ CONTRIBUTIONS
All authors contributed to data collection, study design, data analysis, interpretation and drafting of this article.
CONFLICT OF INTEREST STATEMENT
Unrestricted research grants were obtained from the ERA-EDTA, the Dutch Kidney Foundation, Baxter and Sandoz. Organizations had no role in the design of the study, interpretation of results or writing of the manuscript.
APPENDIX
ERACODA Collaborators
Albert Schweitzer Hospital, Dordrecht, the Netherlands: Jeroen B. van der Net; Ambroise Pare Hospital, APHP Paris-Saclay University, Boulogne Billancourt, France: Marie Essig; Amphia Hospital, Breda, The Netherlands: Peggy W.G. du Buf-Vereijken, Betty van Ginneken and Nanda Maas; Amsterdam UMC, Amsterdam, The Netherlands: Liffert Vogt, Brigit C. van Jaarsveld, Frederike J. Bemelman, Farah Klingenberg-Salahova, Frederiek Heenan-Vos, Marc G. Vervloet and Azam Nurmohamed;Antwerp University Hospital, Antwerp, Belgium: Daniel Abramowicz and Sabine Verhofstede; Faculty of Medicine, Avicennes Military Hospital, Cadi Ayyad University, Marrakech, Morocco: Omar Maoujoud; AZ Delta, Roeselare, Belgium: Thomas Malfait; B. Braun Avitum, Litomerice, Czech Republic: Jana Fialova; Bellvitge University Hospital, Hospitalet de Llobregat, Barcelona, Spain: Edoardo Melilli, Alexandre Favà, Josep M. Cruzado and Nuria Montero Perez; Bernhoven Hospital, Uden, The Netherlands: Joy Lips; Bravis Hospital, Roosendaal/Bergen op Zoom, The Netherlands: Harmen Krepel; Cantonal Hospital Zenica, Zenica, Bosnia and Herzegovina: Harun Adilovic; Catharina Hospital, Eindhoven, The Netherlands: Maaike Hengst; Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland: Andrzej Rydzewski; Centre Hospitalier du Nord, Ettelbruck, Luxembourg and Centre of Postgraduate Medical Education, Warsaw, Poland: Ryszard Gellert; Centrodial, São João da Madeira, São João da Madeira, Portugal: João Oliveira; Centro Hospitalar Vila Nova de Gaia/Espinho, Vila Nova de Gaia, Portugal: Daniela G. Alferes; City Hospital n.a. S.P. Botkin, Moscow, Russia: Elena V. Zakharova; City Hospital Waid, Zürich, Switzerland: Patrice Max Ambuehl, Andrea Walker and Rebecca Winzeler; Claude Galien Hospital Ramsay santé, Quincy-sous-Sénart, France: Fanny Lepeytre, Clémentine Rabaté and Guy Rostoker; Clínica de Hemodiálise de Felgueiras, Felgueiras, Portugal: Sofia Marques; Clinical Centre of Vojvodina, Novi Sad, Serbia: Tijana Azasevac; Croatian Society of Nephrology, Dialysis and Transplantation, Zagreb, Croatia: Dajana Katicic; CWZ Nijmegen, Nijmegen, The Netherlands: Marc ten Dam; DaVita Geilenkirchen, Geilenkirchen, Germany: Thilo Krüger; DaVita, Wrocław, Poland: Szymon Brzosko; Deventer Ziekenhuis, Deventer, The Netherlands: Adriaan L. Zanen; Dianet Dialysis Center, Utrecht, The Netherlands: Susan J.J. Logtenberg; Dialysis Center Bochum, Bochum, Germany: Lutz Fricke; Elyse Klinieken voor Nierzorg, Kerkrade, The Netherlands: Jeroen J.P. Slebe; Erasme Hospital, Brussels, Belgium: Delphine Kemlin; Erasmus Medical Center, Rotterdam, The Netherlands: Jacqueline van de Wetering and Marlies E.J. Reinders; Faculty of Medicine in Pilsen, Charles University, Pilsen, Czech Republic: Jaromir Eiselt and Lukas Kielberger; Faculty of Medicine-Alexandria University, Alexandria, Egypt: Hala S. El-Wakil; Franciscus Gasthuis & Vlietland, Rotterdam, Schiedam, The Netherlands: Martine A.M. Verhoeven; Fundació Puigvert, Barcelona, Spain: Cristina Canal and Carme Facundo; Fundación Jiménez Díaz, Madrid, Spain: Ana M. Ramos; Gdansk Medical University, Gdansk, Poland: Alicja Debska-Slizien; Gelre Hospital, Apeldoorn, The Netherlands: Nicoline M.H. Veldhuizen; General Hospital of Athens ‘G. Gennimatas’, Athens, Greece: Eirini Tigka; General Hospital of Serres, Serres, Greece: Maria Anna Polyzou Konsta; General University Hospital of Alexandroupolis, Alexandroupolis, Greece: Stylianos Panagoutsos; Grande Ospedale Metropolitano and CNR, Reggio Calabria, Italy: Francesca Mallamaci; Grigore T. Popa University of Medicine and Pharmacy and Dr Ci Parhon Hospital, Iasi, Romania: Irina Matceac, Ionut Nistor and Monica Cordos; Haaglanden Medisch Centrum, The Hague, The Netherlands: J.H.M Groeneveld and Jolanda Jousma; Haga Hospital, The Hague, The Netherlands: Marjolijn van Buren; High Institute of Public Health-Alexandria University, Alexandria, Egypt: Samar Abd ElHafeez; Hospital Clínic de Barcelona, Barcelona, Spain: Fritz Diekmann; Hospital Curry Cabral–Central Lisbon University Hospital Center, Lisbon, Portugal: Tiago Assis Pereira; HC-UFMG, CMN Contagem-MG, Unimed-BH, Belo Horizonte, Brazil: Augusto Cesar S. Santos Jr.; Hospital del Mar, Barcelona, Spain: Carlos Arias-Cabrales, Marta Crespo, Laura Llinàs-Mallol, Anna Buxeda, Carla Burballa Tàrrega, Dolores Redondo-Pachon and Maria Dolores Arenas Jimenez; Hospital Gelderse Vallei, Ede, The Netherlands: Julia M. Hofstra; Hospital General of Alicante, Alicante, Spain: Antonio Franco; Hospital General Universitario Gregorio Marañón, Madrid, Spain: David Arroyo and Maria Luisa Rodríguez-Ferrero; Hospital Obispo Polanco, Salud Aragón, Spain: Sagrario Balda Manzanos; Hospital Universitario Ramón y Cajal, Madrid, Spain: R. Haridian Sosa Barrios; Hospital de Santa Cruz, Centro Hospitalar de Lisboa Ocidental, Lisboa, Portugal: Gonçalo Ávila, Ivo Laranjinha and Catarina Mateus; Imelda Hospital, Bonheiden, Belgium: Wim Lemahieu; Isala, Zwolle, The Netherlands: Karlijn Bartelet; Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey: Ahmet Burak Dirim, Erol Demir, Seda Şafak and Aydin Turkmen; Jeroen Bosch Ziekenhuis, Den Bosch, The Netherlands: Daan A.M.J. Hollander; Klinikum Aschaffenburg-Alzenau, Aschaffenburg, Germany: Stefan Büttner; Leiden University Medical Center, Leiden, The Netherlands: Aiko P.J. de Vries, Soufian Meziyerh, Danny van der Helm, Marko Mallat and Hanneke Bouwsma; Lister Hospital, Stevenage, UK: Sivakumar Sridharan; Lithuanian University of Health Sciences, Kaunas, Lithuania: Kristina Petruliene; Luzerner Kantonsspital, Luzern, Switzerland: Sharon-Rose Maloney; Maasstad Ziekenhuis, Rotterdam, The Netherlands: Iris Verberk; Maastricht University Medical Center, Maastricht, The Netherlands: Frank M. van der Sande, Maarten H.L Christiaans and Marc Hemmelder; Manipal Hospital, Bangalore, India: Mohan Kumar; Marche Nord Hospital, Pesaro, Italy: Marina Di Luca; Marmara University School of Medicine, Istanbul, Turkey: Serhan Z. Tuğlular; Martini Ziekenhuis, Groningen, The Netherlands: Andrea Kramer; Maxima Medisch Centrum, Veldhoven, The Netherlands: Charles Beerenhout; Meander Medisch Centrum, Amersfoort, The Netherlands: Peter T. Luik; Medical University Innsbruck, Innsbruck, Austria: Julia Kerschbaum; Austrian Dialysis and Transplant Registry, Rohr, Austria: Martin Tiefenthaler; Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands: Aaltje Y. Adema; Moscow Regional Research and Clinical Institute, Moscow, Russia: Vadim A. Stepanov and Alexey B. Zulkarnaev; Necmettin Erbakan University Meram School of Medicine, Konya, Turkey: Kultigin Turkmen; Nierenzentrum Reutlingen-Tübingen, Reutlingen, Germany: Anselm Fliedner; Norwegian Renal Registry, Oslo University Hospital, Rikshospitalet, Oslo, Norway: Anders Åsberg and Geir Mjoen; Okinawa Chubu Hospital, Okinawa, Japan: Hitoshi Miyasato; OLVG, Amsterdam, The Netherlands: Carola W.H. de Fijter; Ospedale S. Maurizio Bolzano, Bolzano, Italy: Nicola Mongera; Padua University Hospital, Padua, Italy: Stefano Pini; Radboud University Medical Center, Nijmegen, The Netherlands: Consuelo de Biase, Raphaël Duivenvoorden, Luuk Hilbrands, Angele Kerckhoffs and Rutger Maas; Regional Clinical Hospital, Yaroslavl, Russia: Olga Lebedeva; Regional Hospital of Malaga, Malaga, Spain: Veronica Lopez; Rijnstate Hospital, Arnhem, The Netherlands: Jacobien Verhave; RUDN University, Russia: Denis Titov; Saint Petersburg State University Hospital, Saint Petersburg, Russia: Ekaterina V. Parshina; San Marco Hospital, University of Catania, Catania, Italy: Luca Zanoli and Carmelita Marcantoni; Sint Antonius Ziekenhuis, Nieuwegein, The Netherlands: Liesbeth E.A. van Gils-Verrij; Southern Health and Social Care Trust, Newry, Northern Ireland: John C. Harty; Spaarne Gasthuis, Haarlem, The Netherlands: Marleen Meurs; SPWSZ Hospital, Szczecinie, Poland: Marek Myslak; St Anna University Hospital, Ferrara, Italy: Yuri Battaglia; Streekziekenhuis Koningin Beatrix, Winterswijk, The Netherlands: Edwin den Deurwaarder; Swedish Renal Registry, Jönköping, Sweden: Maria Stendahl; Tehran University of Medical Sciences, Tehran, Iran: Hormat Rahimzadeh; Tergooi, Hilversum, The Netherlands: Marcel Schouten; Third Faculty of Medicine, Charles University and Faculty Hospital Kralovske Vinohrady, Prague, Czech Republic: Ivan Rychlik; Toledo University Hospital, Toledo, Spain: Carlos J. Cabezas-Reina and Ana Maria Roca; Treant/Scheper Ziekenhuis, Emmen, The Netherlands: Ferdau Nauta; Université catholique de Louvain, Cliniques universitaires St Luc, Brussels, Belgium: Nada Kanaan, Laura Labriola and Arnaud Devresse; University Clinical Hospital of Santiago de Compostela, Santiago de Compostela, Spain: Anabel Diaz-Mareque; University Clinical Hospital of Valladolid, Valladolid, Spain: Armando Coca; University Hospital Leuven, Leuven, Belgium: Björn K.I. Meijers, Maarten Naesens, Dirk Kuypers and Bruno Desschans; University Hospital Brussels, Brussels, Belgium: Annelies Tonnelier and Karl M. Wissing; Universitary Hospital of Guadalajara, Guadalajara, Spain: Gabriel de Arriba; University Hospital Martin and Jessenius Faculty of Medicine Comenius University, Martin, Slovakia: Ivana Dedinska; University Hospital Medical Center Verona, Verona, Italy: Giuseppina Pessolano; University Hospital Parma, Parma, Italy: Ilaria Gandolfini and Umberto Maggiore; University Hospital of Patras, Patras, Greece: Evangelos Papachristou; University Medical Center Groningen, Groningen, The Netherlands: Casper F.M. Franssen, Stefan P. Berger, Esther Meijer, Akin Özyilmaz, MD, PhD (Dialysis Center Groningen) and Jan Stephan F. Sanders; University Medical Center Ljubljana, Ljubljana, Slovenia: Jadranka Buturović Ponikvar, Andreja Marn Pernat and Damjan Kovac; University Medical Centre Maribor, Maribor, Slovenia: Robert Ekart; University Medical Center Utrecht, Utrecht, The Netherlands: Alferso C. Abrahams, Femke M. Molenaar, Arjan D. van Zuilen, Sabine C.A. Meijvis and Helma Dolmans; University of California Irvine School of Medicine, Orange, CA, USA: Ekamol Tantisattamo; University of Genoa, Genoa, Italy: Pasquale Esposito; University of Liège, Liège, Belgium: Jean-Marie Krzesinski and Jean Damacène Barahira; University of Milano, Milan, Italy: Maurizio Gallieni and Gianmarco Sabiu; University of Navarra Clinic, Pamplona, Spain: Paloma Leticia Martin-Moreno; University of Piemonte Orientale, Novara, Italy: Gabriele Guglielmetti; Valais Hospital, Sion, Switzerland: Gabriella Guzzo; Vall d’Hebron University Hospital, Barcelona, Spain: Nestor Toapanta; VieCuri Medical Centre, Venlo, The Netherlands: Antinus J. Luik, Willi H.M. van Kuijk, Lonneke W.H. Stikkelbroeck and Marc M.H. Hermans; Vilnius University, Vilnius, Lithuania: Laurynas Rimsevicius; Vimercate Hospital, Vimercate, Italy: Marco Righetti; Zonguldak Ataturk State Hospital, Zonguldak, Turkey: Mahmud Islam; Zuyderland Medical Center, Geleen and Heerlen, The Netherlands: Nicole Heitink-ter Braak
Contributor Information
Eric Goffin, Department of Nephrology, Cliniques Universitaires Saint-Luc, Institute of Experimental and Clinical Research, Université Catholique de Louvain, Brussels, Belgium.
Alexandre Candellier, Department of Nephrology, Cliniques Universitaires Saint-Luc, Institute of Experimental and Clinical Research, Université Catholique de Louvain, Brussels, Belgium; Department of Nephrology, Centre Hospitalier Universitaire Amiens-Picardie, Amiens, France.
Priya Vart, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Marlies Noordzij, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Miha Arnol, Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia.
Adrian Covic, Nephrology Clinic, Dialysis and Renal Transplant Center, ‘C.I. PARHON’ University Hospital, ‘Grigore T. Popa’ University of Medicine, Iasi, Romania.
Paolo Lentini, Nephrology and Dialysis Unit, San Bassiano Hospital, Vicenza, Italy.
Shafi Malik, Department of Renal and Transplant, University Hospital of Coventry and Warwickshire and University of Leicester, Coventry, UK.
Louis J Reichert, Department of Nephrology, Rijnstate Hospital, Arnhem, The Netherlands.
Mehmet S Sever, Department of Nephrology, Istanbul School of Medicine, Istanbul University, Istanbul, Turkey.
Bruno Watschinger, Department of Nephrology, Medical University of Vienna, Vienna, Austria.
Kitty J Jager, Department of Medical Informatics, ERA-EDTA Registry, Amsterdam University Medical Center, University of Amsterdam, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Ron T Gansevoort, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
ERACODA Collaborators:
Jeroen B van der Net, Marie Essig, Peggy W G du Buf-Vereijken, Betty van Ginneken, Liffert Vogt, Brigit C van Jaarsveld, Frederike J Bemelman, Farah Klingenberg-Salahova, Frederiek Heenan-Vos, Marc G Vervloet, Azam Nurmohamed, Daniel Abramowicz, Sabine Verhofstede, Omar Maoujoud, Thomas Malfait, B Braun Avitum, Jana Fialova, Edoardo Melilli, Alexandre Favà, Josep M Cruzado, Nuria Montero Perez, Joy Lips, Harmen Krepel, Harun Adilovic, Maaike Hengst, Andrzej Rydzewski, Ryszard Gellert, João Oliveira, Daniela G Alferes, Elena V Zakharova, Patrice Max Ambuehl, Andrea Walker, Rebecca Winzeler, Fanny Lepeytre, Clémentine Rabaté, Guy Rostoker, Sofia Marques, Tijana Azasevac, Dajana Katicic, Marc ten Dam, Thilo Krüger, Szymon Brzosko, Adriaan L Zanen, Susan J J Logtenberg, Lutz Fricke, Jeroen J P Slebe, Delphine Kemlin, Jacqueline van de Wetering, Marlies E J Reinders, Jaromir Eiselt, Lukas Kielberger, Hala S El-Wakil, Martine A M Verhoeven, Cristina Canal, Carme Facundo, Ana M Ramos, Alicja Debska-Slizien, Nicoline M H Veldhuizen, Eirini Tigka, Maria Anna Polyzou Konsta, Stylianos Panagoutsos, Francesca Mallamaci, Irina Matceac, Ionut Nistor, Monica Cordos, J H M Groeneveld, Jolanda Jousma, Marjolijn van Buren, Samar Abd ElHafeez, Fritz Diekmann, Tiago Assis Pereira, Augusto Cesar S Santos, Jr., Carlos Arias-Cabrales, Marta Crespo, Laura Llinàs-Mallol, Anna Buxeda, Carla Burballa Tàrrega, Dolores Redondo-Pachon, Maria Dolores Arenas Jimenez, Julia M Hofstra, Antonio Franco, David Arroyo, Maria Luisa Rodríguez-Ferrero, Sagrario Balda Manzanos, R Haridian Sosa Barrios, Gonçalo Ávila, Ivo Laranjinha, Catarina Mateus, Wim Lemahieu, Ahmet Burak Dirim, Erol Demir, Seda Şafak, Aydin Turkmen, Daan A M J Hollander, Stefan Büttner, Aiko P J de Vries, Soufian Meziyerh, Danny van der Helm, Marko Mallat, Hanneke Bouwsma, Sivakumar Sridharan, Kristina Petruliene, Sharon-Rose Maloney, Iris Verberk, Frank M van der Sande, Maarten H L Christiaans, Marc Hemmelder, Mohan Kumar N, Marina Di Luca, Serhan Z Tuğlular, Andrea Kramer, Charles Beerenhout, Peter T Luik, Julia Kerschbaum, Martin Tiefenthaler, Aaltje Y Adema, Vadim A Stepanov, Alexey B Zulkarnaev, Kultigin Turkmen, Anselm Fliedner, Anders Åsberg, Geir Mjoen, Hitoshi Miyasato, Carola W H de Fijter, Nicola Mongera, Stefano Pini, Consuelo de Biase, Raphaël Duivenvoorden, Luuk Hilbrands, Angele Kerckhoffs, Rutger Maas, Olga Lebedeva, Veronica Lopez, Jacobien Verhave, Denis Titov, Ekaterina V Parshina, Luca Zanoli, Carmelita Marcantoni, Liesbeth E A van Gils-Verrij, John C Harty, Marleen Meurs, Marek Myslak, Yuri Battaglia, Edwin den Deurwaarder, Maria Stendahl, Hormat Rahimzadeh, Marcel Schouten, Ivan Rychlik, Carlos J Cabezas-Reina, Ana Maria Roca, Ferdau Nauta, Nada Kanaan, Laura Labriola, Arnaud Devresse, Anabel Diaz-Mareque, Armando Coca, Björn K I Meijers, Maarten Naesens, Dirk Kuypers, Bruno Desschans, Annelies Tonnelier, Karl M Wissing, Gabriel de Arriba, Ivana Dedinska, Giuseppina Pessolano, Ilaria Gandolfini, Umberto Maggiore, Evangelos Papachristou, Casper F M Franssen, Stefan P Berger, Esther Meijer, Akin Özyilmaz, Jan Stephan F Sanders, Jadranka Buturović Ponikvar, Andreja Marn Pernat, Damjan Kovac, Robert Ekart, Alferso C Abrahams, Femke M Molenaar, Arjan D van Zuilen, Sabine C A Meijvis, Helma Dolmans, Ekamol Tantisattamos, Pasquale Esposito, Jean-Marie Krzesinski, Jean Damacène Barahira, Maurizio Gallieni, Gianmarco Sabiu, Paloma Leticia Martin-Moreno, Gabriele Guglielmetti, Gabriella Guzzo, Nestor Toapanta, Antinus J Luik, Willi H M van Kuijk, Lonneke W H Stikkelbroeck, Marc M H Hermans, Laurynas Rimsevicius, Marco Righetti, Mahmud Islam, and Nicole Heitink-ter Braak
REFERENCES
- 1. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gansevoort RT, Hilbrands LB. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol 2020; 16: 705–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Centre for Disease Prevention and Control. COVID-19 situation update for the EU/EEA. https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea (19 March 2021, date last accessed)
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242 [DOI] [PubMed] [Google Scholar]
- 6. Jager KJ, Kramer A, Chesnaye NC et al. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020; 98: 1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hilbrands LB, Duivenvoorden R, Vart P et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 2020; 35: 1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Craig-Schapiro R, Salinas T, Lubetzky M et al. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant 2021; 21: 1576–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sánchez-Álvarez JE, Pérez Fontán M, Jiménez Martín C et al. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN). Nefrologia 2020; 40: 272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noordzij M, Duivenvoorden R, Pena MJ et al. ERACODA: the European database collecting clinical information of patients on kidney replacement therapy with COVID-19. Nephrol Dial Transplant 2020; 35: 2023–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Minor BL et al. The REDCap consortium: building an international community of software partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005; 173: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res 2015; 4: 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol 2010; 171: 624–632 [DOI] [PubMed] [Google Scholar]
- 15. Little R, Rubin D. Statistical Analysis with Missing Data. 1st edn. New York: John Wiley, 1987 [Google Scholar]
- 16. Couchoud C, Bayer F, Ayav C et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 2020; 98: 1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cravedi P, Mothi SS, Azzi Y et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant 2020; 20: 3140–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elias M, Pievani D, Randoux C et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol 2020; 31: 2413–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Meester J, De Bacquer D, Naesens M et al. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J Am Soc Nephrol 2021; 32: 385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ERA-EDTA Council, ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021; 36: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma P, Chen V, Fung CM et al. COVID-19 outcomes among solid organ transplant recipients: a case-control study. Transplantation 2021; 105: 128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan L, Jaladanki SK, Somani S et al. Outcomes of patients on maintenance dialysis hospitalized with COVID-19. Clin J Am Soc Nephrol 2021; 16: 452–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tonelli M, Wiebe N, Knoll G et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11: 2093–2109 [DOI] [PubMed] [Google Scholar]
- 24. Thaunat O, Legeai C, Anglicheau D et al. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int 2020; 98: 1568–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ravanan R, Callaghan CJ, Mumford L et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant 2020; 20: 3008–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohamed IH, Chowdary PB, Shetty S et al. Outcomes of renal transplant recipients with SARS-CoV-2 infection in the eye of the storm: a comparative study with waitlisted patients. Transplant 2021; 105: 115–120 [DOI] [PubMed] [Google Scholar]
- 27. Fishman JA. Infection in organ transplantation. Am J Transplant 2017; 17: 856–879 [DOI] [PubMed] [Google Scholar]
- 28. Manuel O, López-Medrano F, Keiser L. E et al. Influenza and other respiratory virus infections in solid organ transplant recipients. Clin Microbiol Infect 2014; 20(Suppl 7): 102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clarke C, Lucisano G, Prendecki M et al. Informing the risk of kidney transplantation versus remaining on the waitlist in the coronavirus disease 2019 era. Kidney Int Rep 2021; 6: 46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horby P, Lim WS, Emberson JR et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med 2021; 25: 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee N, Leo YS, Cao B et al. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur Respir J 2015; 45: 1642–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akiyama S, Hamdeh S, Micic D, et al. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2021; 80: 384–391 [DOI] [PubMed] [Google Scholar]
- 33. Gianfrancesco M, Hyrich KL, Al-Adely S et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020; 79: 859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brenner EJ, Ungaro RC, Gearry RB et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterol 2020; 159: 481–491.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Collaborators that entered data in the ERACODA remain owner of these data. The database can therefore not be disclosed to any third party without the prior written consent of all data providers, but the database will be made available to the editorial offices of medical journals when requested. Research proposals can be submitted to the Working Group via COVID.19.KRT@umcg.nl. If deemed of interest and methodologically sound by the Working Group and Advisory Board, the analyses needed for the proposal will be carried out by the Management Team.