Abstract
Background
We systematically assessed benefits and harms of the use of ivermectin (IVM) in patients with coronavirus disease 2019 (COVID-19).
Methods
Published and preprint randomized controlled trials (RCTs) assessing the effects of IVM on adult patients with COVID-19 were searched until 22 March 2021 in 5 engines. Primary outcomes were all-cause mortality rate, length of hospital stay (LOS), and adverse events (AEs). Secondary outcomes included viral clearance and severe AEs (SAEs). The risk of bias (RoB) was evaluated using the Cochrane Risk of Bias 2.0 tool. Inverse variance random effect meta-analyses were performed, with quality of evidence (QoE) evaluated using GRADE methods.
Results
Ten RCTs (n = 1173) were included. The controls were the standard of care in 5 RCTs and placebo in 5. COVID-19 disease severity was mild in 8 RCTs, moderate in 1, and mild and moderate in 1. IVM did not reduce all-cause mortality rates compared with controls (relative risk [RR], 0.37 [95% confidence interval, .12–1.13]; very low QoE) or LOS compared with controls (mean difference, 0.72 days [95% confidence interval, −.86 to 2.29 days]; very low QoE). AEs, SAEs, and viral clearance were similar between IVM and control groups (low QoE for all outcomes). Subgroups by severity of COVID-19 or RoB were mostly consistent with main analyses; all-cause mortality rates in 3 RCTs at high RoB were reduced with IVM.
Conclusions
Compared with the standard of care or placebo, IVM did not reduce all-cause mortality, LOS, or viral clearance in RCTs in patients with mostly mild COVID-19. IVM did not have an effect on AEs or SAEs and is not a viable option to treat patients with COVID-19.
Keywords: ivermectin, SARS-CoV-2, COVID-19, mortality, meta-analysis
Our systematic review of randomized controlled trials showed that ivermectin (vs control) did not reduce all-cause mortality, hospital stays, or viral clearance in patients with mostly mild coronavirus disease 2019 (COVID-19). Ivermectin is not a viable treatment option for COVID-19.
The coronavirus disease 2019 (COVID-19) pandemic represents a global sanitary, social, and economic challenge. However, scientific advances have also amplified deficiencies and misinformation [1]. Biological plausibility, pathophysiological considerations, in vitro research, observational studies, and/or clinical trials with heterogeneous quality were used to evaluate several repurposed drugs repurposed for indications different from the approved ones. Some policy makers and regulatory institutions authorized emergency use of unproven COVID-19 treatments; the use of some of these treatments has been heavily politicized in some regions [2, 3].
Ivermectin (IVM) is a semisynthetic, anthelmintic agent for oral administration, and derived from the avermectins of Streptomyces avermitilis. IVM and its analogues selectively open inhibitory glutamate-gated chloride ion channels in the cell membranes of nematodes. In addition, IVM prevents the filarial ability to release inhibitors of the host immune response [4]. In tissue cultures, at concentrations higher than anthelmintic concentrations, IVM showed antiviral (eg, in dengue), antiparasitic (eg, in malaria), and anticancer (eg, in epithelial ovarian cancer) effects. However, these in vitro results have not been clinically demonstrated [4].
In March 2020, researchers from Australia showed IVM to be active against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cell cultures by drastically reducing viral RNA at 48 hours [5]. Concentrations were equivalent to >50-fold the normal maximum concentration achieved with a standard single dose of IVM 200 μg/kg, raising concerns about the efficacious dose of IVM for treating or preventing SARS-CoV-2 infection in humans and its tolerability [6]. However, theoretical considerations, experimental and observational evidence, misinformation, self-medication, and the wide availability of IVM led to its use as treatment of COVID-19 in low- and middle-income countries, assuming a priori efficacy and safety.
IVM is currently approved by the Food and Drug Administration (FDA) to treat people with intestinal strongyloidiasis and onchocerciasis. The European Medicines Agency [7] and the FDA [8] have not approved IVM for the treatment of COVID-19. World Health Organization (WHO) [9] and Infectious Diseases Society of America [10] guidelines do not recommend IVM for treatment of COVID-19 outside randomized controlled trials (RCTs).
Three systematic reviews on the effect of IVM on clinical outcomes have been published [11–13]. Padhy et al [11] included only 3 small observational studies. Siemieniuk et al [12] conducted a living systematic review of all treatments for COVID-19, but details were scarce, and the quality of evidence (QoE) was very low. Finally, Kow et al [13] evaluated 6 RCTs, 5 from Asia and none from Latin America. Other systematic reviews or narrative reviews of IVM effects have been disseminated only as preprints [14–16] or on Web sites [17–19]. We conducted a systematic review and meta-analysis to evaluate treatment effects of IVM on clinical outcomes and adverse events (AEs) in people with COVID-19.
METHODS
Sources and Searches
Two investigators (V. P. and A. V. H.) developed the search strategy, which was approved by the other investigators. Until 22 March 2021, we searched 5 databases: PubMed-MEDLINE, EMBASE-OVID, Scopus, Web of Science, the Cochrane Library; and preprints from www.medrxiv.org, www.preprints.org, and www.ssrn.com. The PubMed search strategy is shown in the Supplement.
Selection of Studies
We included RCTs in any language reporting benefit or harm outcomes of IVM as treatment in patients with COVID-19, both nonhospitalized and hospitalized, irrespective of COVID-19 severity. We excluded studies assessing prophylaxis for COVID-19 infection. Controls were the standard of care (SOC) or placebo. Two investigators (Y. M. R. and P. A. B.) independently screened titles and abstracts and then assessed full texts of selected abstracts. Discrepancies were resolved through discussion or by a third investigator (A. V. H.).
Outcomes
Primary outcomes were all-cause mortality rate, length of hospital stay (LOS), and AEs. Secondary outcomes were SARS-CoV-2 clearance on respiratory samples, clinical improvement, need for mechanical ventilation, and severe AEs (SAEs). AEs and SAEs were extracted as defined by authors.
Data Extraction
Two investigators (Y. M. R. and P. A. B.) independently extracted the following data: country, sample size, dose and duration of IVM treatment, type of control group (SOC vs placebo), COVID-19 severity, percentage of reverse-transcription polymerase chain reaction (RT-PCR) results positive for SARS-CoV-2, study setting (hospitalized vs nonhospitalized), mean age, proportions of female patients and patients with hypertension, diabetes mellitus, or cardiovascular disease, outcomes, and duration of follow-up. COVID-19 disease severity was defined as mild, moderate, or severe according to the WHO classification [20]. Discrepancies were resolved through discussion or by 2 other investigators (A. P. and A. V. H.).
Risk of Bias Assessment
Two investigators (Y. M. R. and P. A. B.) independently assessed the risk of bias (RoB) using the Cochrane Risk of Bias 2.0 tool for RCTs [21]; disagreements were resolved by discussion with a third investigator (A. P.). This tool evaluates 5 domains of bias: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. The RoB for each of the 5 domains and overall was described as low, some concerns, or high.
Statistical Analyses
We reported our systematic review according to 2009 PRISMA guidelines [22]. Inverse variance random effect meta-analyses were performed to evaluate the effects of IVM versus control on outcomes. Effects of meta-analyses were reported as relative risk (RR) for dichotomous outcomes and as mean difference for continuous outcomes. The between-study variance (τ 2) was calculated using the Paule-Mandel method [23], and the 95% confidence intervals (CIs) of effects were adjusted using the Hartung-Knapp method [24]. We adjusted for zero events in 1 or 2 RCT arms, using the continuity correction method [25].
The heterogeneity of effects among studies was quantified using the I2 statistic (I2 >60% indicates high heterogeneity). We prespecified subgroup analyses by severity of COVID-19 disease and RoB; P for interaction values <.1 indicated effect modification by subgroup. Sensitivity analyses excluding RCTs with shorter follow-up (ie, <21 days) were planned for the primary outcomes. The meta package of R 3.5.1 software (www.r-project.org) was used for meta-analyses. The certainty or quality of evidence (QoE) was evaluated using GRADE methods, which cover RoB, inconsistency, indirectness, imprecision, and publication bias [26]. The QoE was evaluated for each outcome, and described in the summary of findings tables; GRADEpro GDT software (version 2020) was used to create summary of findings tables [27].
RESULTS
Selection of Studies
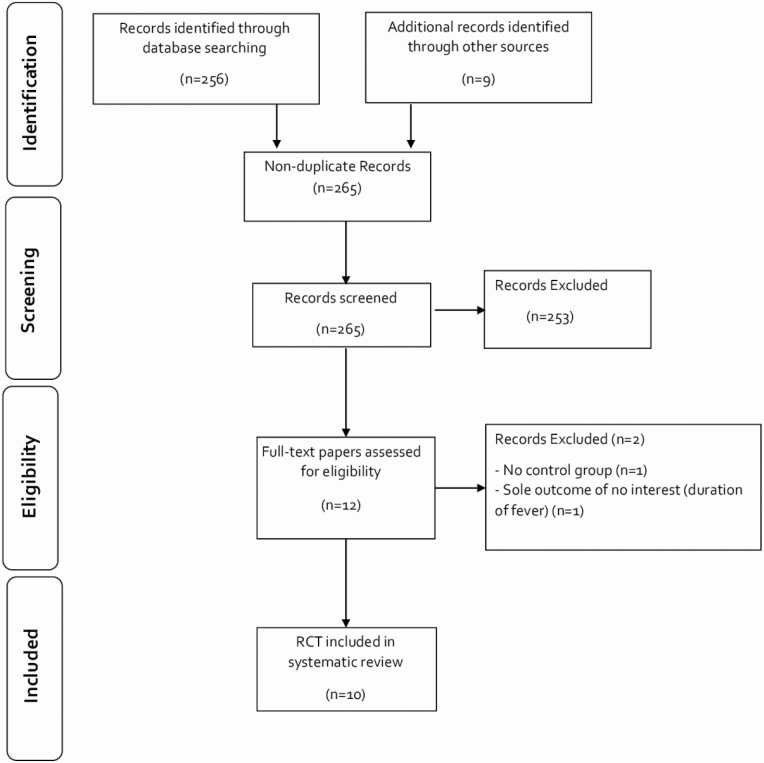
Our search yielded 256 citations with an additional 9 citations identified in preprint Web pages; 253 records were excluded. After assessing 12 full texts, we identified 10 RCTs [28–37] (n = 1173) (Figure 1). Two full texts were excluded; there was no control group in one of these studies, and an outcome of no interest (duration of fever) was the only outcome reported in the other.
Figure 1.
PRISMA flowchart diagram. Abbreviation: RCT, randomized controlled trial.
Characteristics of RCTs
One RCT was conducted in Spain [34], and the other 9 were conducted in low- and middle-income countries. Sample sizes for RCTs ranged from 24 [34] to 398 [36] patients. IVM doses were heterogeneous in terms of total doses (ranging from 12 mg [35] to 210 mg [29]) and duration (ranging from 1 [28, 31, 33–35] to 5 [29, 30, 32, 36] days). Controls were the SOC in 5 RCTs [28–31, 35] and placebo in 5 [32–34, 36, 37]. Most RCTs were conducted in patients with mild COVID-19: mild in all or most patients in 8 RCTs [28, 29, 31, 32, 34–37], moderate in 1 [33], and mild and moderate in 1 [30] (Table 1).
Table 1.
Study Characteristics of Included Randomized Controlled Trials
| Patients, % | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Authors (Year) | Country (Sample Size) | IVM Dose and Duration | Control Group | COVID-19 Severity by WHO Classificationa | Patient Age, Mean (SD) or Median (IQR), y | RT-PCR Positive for SARS-CoV-2 | Hospitalized | Female Sex | CVD or CHD | DM | HTN | Evaluated outcomes | Duration of Follow-up, d |
| Chachar et al (2020) [28] | Pakistan (n = 50) | 12 mg, 12 mg at 12 h, and 12 mg at 24 h | SOC | Mild in 100% | 42 (16) | 100 | 0 | 38 | 8 | 40 | 26 | Asymptomatic at d 7 | 7 |
| Krolewiecki et al (2020) [29] | Argentina (n = 45) | 0.6 mg/kg once daily for 5 d | SOC | Mild in 87%, moderate in 13% | 41 (12) | 100 | 100 | 44 | NR | 16 | 13 | Viral load at d 5, IVM plasma level | 30 |
| Niaee et al (2020) [30] | Iran (n = 180) | 4 doses: from 200 μg/kg single dose to 800 μg/kg over 5 d | SOC | Mild or moderate (unclear distribution) | 56 (45–67) | 71 | 100 | 50 | NR | NR | NR | All-cause mortality rate, time until remission of symptoms, LOS | 5 |
| Podder et al (2020) [31] | Bangladesh (n = 62) | Single dose: 200 μg/kg | SOC | Mild in 81%, moderate in 19% | 39 (12) | 100 | NR | 29 | NR | NR | NR | Time to full recovery, viral clearance | 10 |
| Ahmed et al (2021) [32] | Bangladesh (n = 48) | 12 mg once daily for 5 d | Placebo | Mild in 100% | 42 (NR) | 100 | 100 | 54 | 0 | 0 | 0 | Remission of symptoms, LOS, SAEs, oxygen requirement, time to viral clearance | 14 |
| Beltrán-Gonzalez et al (2021) [33] | Mexico (n = 73) | Single dose: 12 mg if <80 kg; 18 mg if >80 kg | Placebo | Moderate in 74% with PaO2/FiO2 ratio 100 to 300) | 53 (17) | 100 | 100 | 38 | NR | 34 | 32 | All-cause mortality rate, clinical recovery, LOS, AEs, respiratory deterioration | 28 |
| Chaccour et al (2021) [34] | Spain (n = 24) | Single dose 400 μg/kg | Placebo | Mild in 100% | 26 (19–36) | 100 | 0 | 50 | 0 | 0 | 0 | All-cause mortality rate, AEs, PCR at d 7 | 28 |
| Bukhari et al (2021) [35] | Pakistan (n = 86) | Single dose: 12 mg | SOC | Mild in most patients (percentage unclear) | 39 (42) | 100 | 100 | 15 | 5.8 | 12 | 14 | Time to viral clearance, AEs | 28 |
| López-Medina et al (2021) [36] | Colombia (n = 398) | 300 μg/kg once daily for 5 d | Placebo | Mild in 100% | 37 (29–48) | 100 | 1 | 78 | 1.7 | 6 | 13 | All-cause mortality rate, time to complete resolution, AEs, SAEs, escalation of care | 21 |
| Ravikirti et al (2021) [37] | India (n = 115) | 12 mg/d for 2 d | Placebo | Mild in 79%, moderate in 21% | 53 (15) | Positive RT-PCR or RAT results | 100 | 28 | 11 | 36 | 35 | All-cause mortality rate, admission to ICU, requirement for MV, viral clearance at d 6 | 10 |
Abbreviations: AEs, adverse events; CHD, coronary heart disease; COVID-19, coronavirus disease 2019; CVD, cardiovascular disease; d, days; DM, diabetes mellitus; HTN, hypertension; ICU, intensive care unit; IQR, interquartile range; IVM, ivermectin; LOS, length of hospital stay in days; MV, mechanical ventilation; NR, not reported; PaO2/FiO2, ratio of arterial oxygen partial pressure (PaO2; in millimeters of mercury) to fractional inspired oxygen (FiO2; expressed as fraction, not percentage); RAT, rapid antigen test; RT-PCR, reverse-transcription polymerase chain reaction; SAEs, severe AEs; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; SOC, standard of care; WHO, World Health Organization.
aWHO classification per reference 20.
All patients had RT-PCR results positive for SARS-CoV-2 at baseline, except in 2 RCTs: Niaee et al [30] reported 71% positivity, and Ravikirti et al [37] reported positive RT-PCR or rapid antigen test results. The RCTs by Chachar et al [28], Chaccour et al [34], and López-Medina et al [36] were conducted in nonhospitalized patients. Mean or median ages ranged from 26 to 56 years, and the percentage of female patients from 15% [35] to 78% [36], and most patients did not have hypertension, diabetes mellitus, or cardiovascular disease. Evaluated outcomes were also heterogeneous across RCTs, and the duration of follow-up ranged from 5 days [30] to 30 days [29].
RoB Assessment of Included RCTs
Eight RCTs had a high RoB [28–32, 35–37], the one reported by Beltrán-Gonzalez et al [33] had some concerns of bias in the randomization process, and the one reported by Chaccour et al [34] had a low RoB (see Supplementary Figure 1 for details).
Meta-analyses
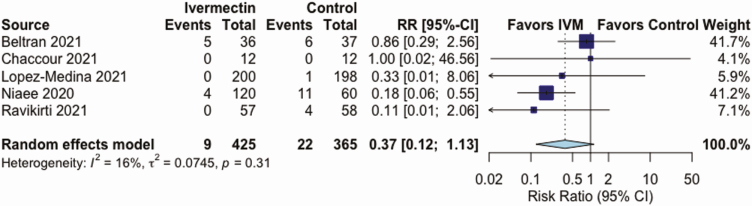
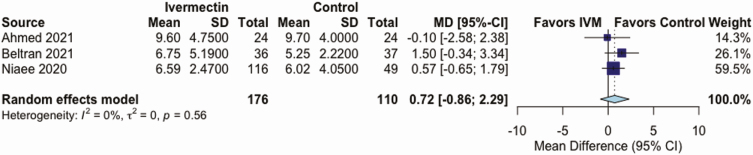
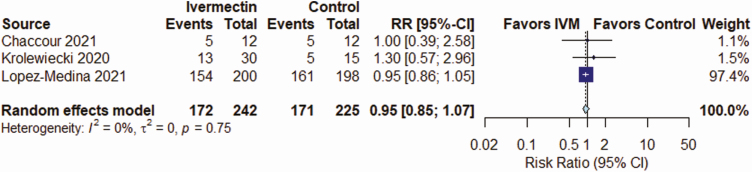
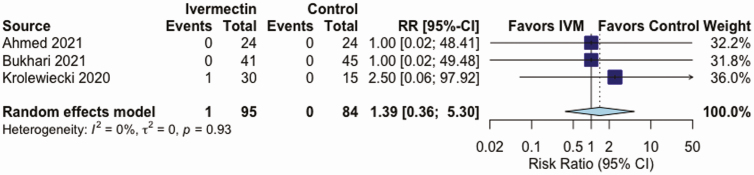
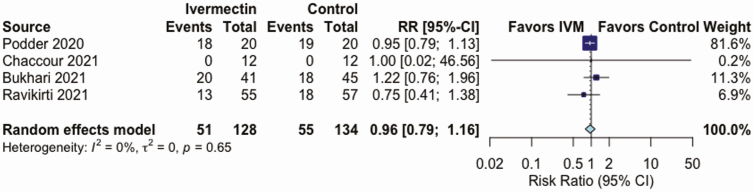
IVM, compared with control treatment, did not have an effect on the all-cause mortality rate in 5 RCTs (RR, 0.37 [95% CI, .12–1.13]; I2 = 16%; very low QoE) (Figure 2 and Table 2), on LOS in 3 RCTs (mean difference, 0.72 days [−0.86 to 2.29 days]; I2 = 0%; very low QoE) (Figure 3 and Table 2), or on AEs in 3 RCTs (RR, .95 [.85–1.07]; I2 = 0%; low QoE) (Figure 4 and Table 2). Compared with control treatment, IVM had no effect on SAEs in 3 RCTs (RR, 1.39 [95% CI, .36–5.30]; I2 = 0%; low QoE) (Figure 5 and Table 2) or on viral clearance in 4 RCTs (RR, 0.96, [.79–1.16]; I2 = 0%; low QoE) (Figure 6 and Table 2).
Figure 2.
Effect of ivermectin (IVM) on all-cause mortality rates in randomized controlled trials in patients with coronavirus disease 2019. Abbreviations: CI, confidence interval; RR, relative risk.
Table 2.
Summary of Findings on the Effect of Ivermectin Compared With Standard of Care or Placebo in Patients With Coronavirus Disease 2019
| Anticipated Absolute Effect (95% CI)a | |||||
|---|---|---|---|---|---|
| Outcome (Duration of Follow-up) | Risk With Control | Risk With Ivermectin | Relative Effect (95% CI) | No. of Participants (No. of RCTs) | Certainty of Evidenceb |
| All-cause mortality rate (5–28 d) | 6 per 100 | 2 per 100 (1 to 7) | RR, 0.37 (.12 to 1.13) | 787 (5) | ⊕〇〇〇 Very lowc,d |
| LOS (5–28 d) | Mean LOS, 10 d | MD, 0.72 d (−.86 to 2.29 d) | … | 286 (3) | ⊕〇〇〇 Very lowe,f |
| AEs (5–28 d) | 76 per 100 | 72 per 100 (65–81) | RR, 0.95 (.85–1.07) | 467 (3) | ⊕⊕〇〇Lowg |
| SAEs (5– 28 d) | 0 per 100 | 0 per 100 (0–0) | RR, 1.39 (.36–5.30) | 179 (3) | ⊕⊕〇〇 Lowh |
| Viral clearance (5–28 d) | 410 per 1000 | 394 per 1000 (312–472) | RR, 0.96 (.76–1.15) | 262 (4) | ⊕⊕〇〇 Lowi |
Abbreviations: CI, confidence interval; d, days; LOS, length of stay; MD, mean difference; RCTs, randomized controlled trials; RR, relative risk.
aThe risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
bGRADE Working Group grade of evidence. High certainty indicates confidence that the true effect lies close to that of the estimate of the effect. Moderate certainty indicates moderate confidence in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty indicates limited confidence in the effect estimate; the true effect may be substantially different from the estimate of the effect. Finally, very low certainty indicates very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect. ⊕○○○ means very low certainty of evidence; ⊕⊕○○ means low certainty of evidence.
cThe studies reported by López-Medina et al [36], Niaee et al [30], and Ravikirti et al [37] had a high risk of bias (RoB); that of Beltrán-Gonzalez et al [33] had some concerns; and that of Chaccour et al [34] had a low RoB.
dImprecision: 95% CI, .12–1.13.
eThe studies reported by Ahmed et al [32] and Niaee et al [30] had high RoB, and that of Beltrán-Gonzalez et al [33] had some concerns.
fImprecision: 95% CI, −2.03 to 4.25.
gThe studies reported by Krolewiecki et al [29] and López-Medina et al [36] had high RoB, and that of Chaccour et al [34], low RoB.
Figure 3.
Effect of ivermectin (IVM) on length of stay in days in randomized controlled trials in patients with coronavirus disease 2019. Abbreviations: CI, confidence interval; MD, mean difference; SD, standard deviation.
Figure 4.
Effect of ivermectin (IVM) on adverse events in randomized controlled trials in patients with coronavirus disease 2019. Abbreviations: CI, confidence interval; RR, relative risk.
Figure 5.
Effect of ivermectin on (IVM) severe adverse events in randomized controlled trials in patients with coronavirus disease 2019. Abbreviations: CI, confidence interval; RR, relative risk.
Figure 6.
Effect of ivermectin (IVM) on viral clearance in randomized controlled trials in patients with coronavirus disease 2019. Abbreviations: CI, confidence interval; RR, relative risk.
Subgroup and Sensitivity Analyses
Subgroup analyses by severity of COVID-19 disease or RoB were consistent with main analyses (see Supplementary Figures 2.1–2.5), except the subgroup analysis of all-cause mortality rate by RoB; 3 studies [30, 36, 37] with a high RoB showed a significant reduction in all-cause mortality (RR, 0.18 [95% CI, .07–.49]; RoB P for interaction = .1). Sensitivity analyses excluding studies with follow-up <21 days showed similar effects as primary analyses for all-cause mortality rate and LOS (see Supplementary Figures 3.1 and 3.2). The statistical heterogeneity of effects for all-cause mortality was 0% in sensitivity analysis.
DISCUSSION
We found in our systematic review that, compared with SOC or placebo, IVM did not reduce primary outcomes (all-cause mortality rate, LOS, and AEs) or secondary outcomes (SARS-CoV-2 clearance in respiratory samples, and SAEs) in RCTs of patients with mostly mild COVID-19 disease. The QoE was low or very low for all outcomes. Results of subgroup analyses by severity of COVID-19 disease or RoB were mostly consistent with those of the main analyses, except for a significant effect on all-cause mortality rate in 3 RCTs with high RoB.
Two conventional systematic review and meta-analyses and 2 living systematic review and meta-analyses were published [9, 11–13] (see Supplementary Table 1). Padhy et al [11] published the first systematic review about IVM in patients with COVID-19, and their primary outcome was all-cause mortality rate. This study included only 4 observational studies (n = 629). IVM showed reduction of all-cause mortality rate (odds ratio [OR], 0.53 [95% CI, .09–.36]). However, the authors express caution. as the QoE was very low [11]. Kow et al [13] published a systematic review of IVM effects on all-cause mortality rates in patients with COVID-19. Their study included only 6 RCTs (n = 1255), and it showed a reduction of all-cause mortality rate with IVM (OR, 0.21 [95% CI, .11–.42]).The authors reported a high RoB in most of the RCTs, described their findings as preliminary, and suggested that IVM should preferably be administered within RCTs [13].
The WHO published a living systematic review about IVM in patients with COVID-19, with all-cause mortality rate as the primary outcome [9]. Sixteen RCTs were evaluated, but only 5 directly compared IVM with SOC and reported mortality rates (n = 915); IVM reduced all-cause mortality rates (OR, 0.19 [95% CI, .09–.36]). However, the QoE was very low for mortality, and the panel concluded that the effect of IVM on mortality rates was uncertain. Other outcomes (ie, mechanical ventilation, hospital admission, and duration of hospitalization) also had very low QoE. WHO recommended using IVM only in RCTs [9]. Siemieniuk et al [12] published a living systematic review of IVM in patients with COVID-19, with mortality rate as the primary outcome and 10 other outcomes, including hospitalization and time to viral clearance [12]. Seven RCTs contributed to the mortality assessment (n = 751). IVM was associated with a reduced mortality rate (risk difference per 1000 vs SOC, −103 [95% CI, −117 to −78]), but the QoE was very low. For other critical outcomes the QoE was low. This study concluded that the effects of IVM were highly uncertain, without definitive evidence of important benefits and harms [12]. Taken together, the results of these 4 studies suggested that IVM should not be used in patients with COVID-19. Living systematic reviews allow authors to update the evidence regularly, which is particularly important in a pandemic scenario [38].
We also found 3 preprints of systematic reviews [14–16] (see Supplementary Table 2). Castañeda-Sabogal et al [14] evaluated 12 studies (6 RCTs, 5 retrospective cohorts, and 1 case series; n = 7412) without description of COVID-19 severity. IVM did not reduce the mortality rate (RR, 0.70 [95% CI, .31–2.28]) or increase the rate of recovery (1.37 [.61–3.07]). The authors concluded that there was insufficient certainty and low QoE. Hill et al [15] evaluated 18 RCTs (n = 2282) with mostly mild to moderate disease severity. In 6 RCTs (4 preprints and 2 trial registry Web records; n = 1255), IVM reduced the all-cause mortality rate (RR, 0.25 [95% CI, .12–.52]) but did not increase the recovery rate (1.37; .61–3.07). The RCT quality was classified as limited in 4 studies, fair in 1, and good in 1. Hill et al concluded that IVM should be evaluated in well-designed, large RCTs [15]. Finally, Bryant et al [16] evaluated 19 RCTs (n = 2003). In 13 of the 19 (3 published RCTs, 9 preprints, and 1 trial registry Web registry; n = 1892) with mostly mild to moderate disease severity, IVM reduced the mortality rate (adjusted RR, 0.32 [95% CI, .14–.72]) [16]; the QoE was low to moderate. Bryant et al recommended the use of IVM in COVID-19, particularly in early disease, without supporting data. The last 2 studies [15, 16] used very flexible research strategies and included 0% and 13% of peer-reviewed studies, respectively. In consequence, they were subject to selection bias, which may explain the reported effects of IVM on mortality rates.
Several Web sites published systematic reviews and meta-analyses about IVM in patients with COVID-19 with unclear or absent details on methods and reporting guidelines [17–19] (see Supplementary Table 2). These Web sites did not include protocol registration and have relevant omissions, such as inclusion criteria [19], searched databases [18, 19], study quality assessment [17, 19], meta-analysis methods [19], and the definition of heterogeneity [17, 19]. Arbitrarily broad inclusion criteria (ie, studies submitted directly to the Web sites, more preprints than peer-reviewed studies) led to a high number of RCTs and participants. For example, a “real time meta-analysis” reported by ivmmeta.com included 46 studies, 24 of them RCTs, and 15 480 participants [17]. Coincidentally, these 3 studies showed beneficial outcome effects with IVM [17–19]. In the context of a misinformation infodemic, the dissemination of these results caused confusion for patients, clinicians (in particular those without training in critical reading of scientific literature), and decision makers, who may manipulate the information with political interests [39].
The use of IVM to treat COVID-19 has shown several limitations in management strategies: lack of transparency by some political leaders or media to support drug use without evidence of efficacy and safety; lack of leadership in implementing therapeutic science-based guidelines; and misuse of effective scientific communication [40, 41]. Similar issues were previously experienced with hydroxychloroquine and will probably be repeated with other repurposed drugs. Therefore, there is an urgent need to establish collaborative efforts among scientists, practitioners, communicators, and policy makers. A large, well-designed, and well-reported RCT provides the most reliable information on efficacy in the specific target population from which the sample was drawn. Well-designed and well-reported meta-analyses can provide valuable and confirmatory information [42].
IVM is generally safe at conventional doses for approved indications [4, 5]. However, its safety became a concern owing to longer use and/or higher doses in patients with COVID-19. IVM was found to be similar to placebo in safety and tolerability, even at 10 times the highest FDA-approved dose of 200 μg/kg in healthy volunteers [43], but not in patients with COVID-19. In addition, the use of IVM needs further analysis when IVM is combined with other agents for COVID-19 [44, 45]. In several settings, it was wrongly assumed that the potential benefits of using repurposed drugs outweigh their potential harms [46]. Well-designed RCTs with longer treatment and higher doses are necessary to further evaluate the safety of IVM in patients with COVID-19.
Our study has several strengths. First, we performed a recent and comprehensive systematic search in 5 engines and unpublished studies without language restriction. Second, we evaluated only RCTs; several previous studies included all types of designs, and their findings may have been biased and confounded. Third, we evaluated outcomes with information from at least 2 RCTs; no data were available on clinical improvement or the need for mechanical ventilation. Fourth, we described the severity of COVID-19 disease in each RCT carefully, using the WHO classification [19]; our findings do not support the use of IVM in mild disease. Fifth, we performed subgroup analyses by RoB and severity of disease, the results of which were mostly similar to those of the main analyses; however, we found that 3 RCTs with a high RoB [30, 36, 37] had significant reductions in all-cause mortality rates. Sixth, we also performed sensitivity analysis by excluding studies with short follow-up times; the effects were similar. Finally, we evaluated the QoE using GRADE methods.
Our study also has some limitations. First, the QoE was low or very low for all outcomes. However, our study evaluated the best current available evidence, and all IVM effects were negative. Second, we included only 10 RCTs, 5 of which used placebo treatment as the control, and studies included relatively small numbers of participants. However, included RCTs are the studies available through 22 March 2021. Third, all selected RCTs evaluated patients with mild or mild to moderate COVID-19. However, the supposed benefit of IVM has been positioned precisely for mild disease, but we did not find differential IVM effects between these 2 severity categories. Fourth, some outcomes were scarce, in particular all-cause mortality rates and SAEs; we adjusted for zero events in one or both RCT arms in our analyses of these outcomes. Finally, analyses of primary outcomes excluding studies with short follow-up (5–10 days) showed similar IVM effects.
In conclusion, compared with SOC or placebo, IVM did not reduce all-cause mortality rate, LOS, respiratory viral clearance, AEs, or SAEs in RCTs of patients with mild to moderate COVID-19. We did not find data about IVM effects on clinical improvement or the need for mechanical ventilation. Additional ongoing RCTs should be completed to update our analyses. In the meanwhile, IVM is not a viable option for treating patients with COVID-19, and should be used only within clinical trials.
Supplementary Material
Note
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1.Science during COVID-19: where do we go from here? Lancet 2021; 396:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA 2020; 324:2161–2. [DOI] [PubMed] [Google Scholar]
- 3.Barberia LG, Gómez EJ. Political and institutional perils of Brazil’s COVID-19 crisis. Lancet 2020; 396:367–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin RJ, Robertson AP, Choudhary S. Ivermectin: an anthelmintic, an insecticide, and much more. Trends Parasitol 2021; 37:48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaccour C, Hammann F, Ramón-García S, Rabinovich NR. Ivermectin and COVID-19: keeping rigor in times of urgency. Am J Trop Med Hyg 2020; 102:1156–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency. EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials. Available at: https://www.ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-treatment-covid-19-outside-randomised-clinical-trials. Accessed 23 March 2021.
- 8.US Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. Available at: https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19. Accessed 5 May 2021.
- 9.World Health Organization. Therapeutics and COVID-19: living guideline. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1. Accessed 7 May 2021. [PubMed] [Google Scholar]
- 10.Bhimraj A, Morgan RL, Shumaker AH, et al. . Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America, 2021; version 4.2.0. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 15 April 2021. [DOI] [PMC free article] [PubMed]
- 11.Padhy BM, Mohanty RR, Das S, Meher BR. Therapeutic potential of ivermectin as add on treatment in COVID 19: a systematic review and meta-analysis. J Pharm Pharm Sci 2020; 23:462–9. [DOI] [PubMed] [Google Scholar]
- 12.Siemieniuk RA, Bartoszko JJ, Ge L, et al. . Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ 2020; 370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kow CS, Merchant HA, Mustafa ZU, Hasan SS. The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis. Pharmacol Rep. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castañeda-Sabogal A, Chambergo-Michilot D, Toro-Huamanchumo CJ, et al. . Outcomes of ivermectin in the treatment of COVID-19: a systematic review and meta-analysis. medRxiv [Preprint: not peer reviewed]. January 27, 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.01.26.21250420v1. [Google Scholar]
- 15.Hill A; International Ivermectin Project Team . Preliminary meta-analysis of randomized trials of ivermectin to treat SARSCoV-2 infection. Res Sq [Preprint: not peer reviewed]. January 19, 2021. Available from: https://www.researchsquare.com/article/rs-148845/v1. [Google Scholar]
- 16.Bryant A, Lawrie TA, Dowswell T, et al. . Ivermectin for prevention and treatment of COVID-19 infection: a systematic review and meta-analysis. Res Sq [Preprint: not peer reviewed]. March 18, 2021. Available from: https://www.researchsquare.com/article/rs-317485/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anonymous. Ivermectin for COVID-19: real-time meta-analysis of 54 studies. Available at: https://ivmmeta.com. Accessed 9 May 2021.
- 18.Lawrie T. Ivermectin reduces the risk of death from COVID-19—a rapid review and meta-analysis in support of the recommendation of the Front Line COVID-19 Critical Care Alliance. Available at: https://www.researchgate.net/publication/348230894_Ivermectin_reduces_the_risk_of_death_from_COVID-19_-a_rapid_review_and_meta-analysis_in_support_of_the_recommendation_of_the_Front_Line_COVID-19_Critical_Care_Alliance. Accessed 8 May 2021.
- 19.Kory P, Meduri GU, Iglesias J, et al. . Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. FLCCC Alliance. Available at: https://covid19criticalcare.com. Accessed 8 May 2021. [DOI] [PMC free article] [PubMed]
- 20.WHO Working Group on the Clinical Characterization and Management of COVID-19 Infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veroniki AA, Jackson D, Viechtbauer W, et al. . Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016; 7:55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med 2001; 20:3875–89. [DOI] [PubMed] [Google Scholar]
- 25.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23:1351–75. [DOI] [PubMed] [Google Scholar]
- 26.Balshem H, Helfand M, Schünemann HJ, et al. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401–6. [DOI] [PubMed] [Google Scholar]
- 27.GRADEpro GDT: GRADEpro guideline development tool [software]. Hamilton, ON, Canada: McMaster; University, 2020;. developed by Evidence Prime. Available at: http://www.gradepro.org. Accessed 7 May 2021. [Google Scholar]
- 28.Chachar AZK, Khan KA, Asif M, Tanveer K, Khaqan A, Basri R. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. Int J Sci 2020; 9:31–5. [Google Scholar]
- 29.Krolewiecki A, Lifschitz A, Moragas M, et al. . Antiviral effect of high-dose ivermectin in adults with COVID-19: a pilot randomized, controlled, open label, multicentre trial. SSRN [Preprint: not peer reviewed]. November 11, 2020. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3714649. [Google Scholar]
- 30.Niaee MS, Gheibi N, Namdar P, et al. . Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial. Res Sq [Preprint: not peer reviewed]. November 24, 2020. Available from: https://www.researchsquare.com/article/rs-109670/v1. [Google Scholar]
- 31.Podder C, Chowdhury N, Sina M, Haque W. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomized controlled study. IMC J Med Sci 2020; 14:11–8. [Google Scholar]
- 32.Ahmed S, Karim MM, Ross AG, et al. . A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis 2021; 103:214–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beltrán-Gonzalez JL, Gámez MG, Enciso EAM, et al. . Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial. medRxiv [Preprint: not peer reviewed]. February 23, 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.02.18.21252037v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaccour C, Casellas A, Blanco-Di Matteo A, et al. . The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine 2021; 32:100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bukhari KHS, Asghar A, Perveen N, et al. . Efficacy of ivermectin in COVID-19 patients with mild to moderate disease. medRxiv [Preprint: not peer reviewed].February 5, 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.02.02.21250840v1. [Google Scholar]
- 36.López-Medina E, López P, Hurtado IC, et al. . Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA 2021; 325:1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravikirti RR, Pattadar C, Raj R, et al. . Ivermectin as a potential treatment for mild to moderate COVID-19—a double blind randomized placebo-controlled trial. medRxiv [Preprint: not peer reviewed]. January 9, 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.01.05.21249310v1. [DOI] [PubMed] [Google Scholar]
- 38.Macdonald H, Loder E, Abbasi K. Living systematic reviews at the BMJ. BMJ 2020; 370:m2925. [DOI] [PubMed] [Google Scholar]
- 39.Garegnani LI, Madrid E, Meza N. Misleading clinical evidence and systematic reviews on ivermectin for COVID-19. BMJ Evid Based Med 2021; 1–3. [DOI] [PubMed] [Google Scholar]
- 40.Al Saidi AMO, Nur FA, Al-Mandhari AS, El Rabbat M, Hafeez A, Abubakar A. Decisive leadership is a necessity in the COVID-19 response. Lancet 2020; 396:295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheufele DA, Hoffman AJ, Neeley L, Reid CM. Misinformation about science in the public sphere. Proc Natl Acad Sci U S A 2021; 118:e2104068118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker E, Hernandez AV, Kattan MW. Meta-analysis: its strengths and limitations. Cleve Clin J Med 2008; 75:431–9. [DOI] [PubMed] [Google Scholar]
- 43.Guzzo CA, Furtek CI, Porras AG, et al. . Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol 2002; 42:1122–33. [DOI] [PubMed] [Google Scholar]
- 44.Amsden GW, Gregory TB, Michalak CA, Glue P, Knirsch CA. Pharmacokinetics of azithromycin and the combination of ivermectin and albendazole when administered alone and concurrently in healthy volunteers. Am J Trop Med Hyg 2007; 76:1153–7. [PubMed] [Google Scholar]
- 45.Banerjee K, Nandy M, Dalai CK, Ahmed SN. The battle against COVID 19 pandemic: what we need to know before we “test fire” ivermectin. Drug Res 2020; 70:337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalil AC. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 2020; 323:1897–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.