Abstract
Background
The extent of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure and transmission in Mali and the surrounding region is not well understood. We aimed to estimate the cumulative incidence of SARS-CoV-2 in 3 communities and understand factors associated with infection.
Methods
Between July 2020 and January 2021, we collected blood samples and demographic, social, medical, and self-reported symptoms information from residents aged 6 months and older over 2 study visits. SARS-CoV-2 antibodies were measured using a highly specific 2-antigen enzyme-linked immunosorbent assay optimized for use in Mali. We calculated cumulative adjusted seroprevalence for each community and evaluated factors associated with serostatus at each visit by univariate and multivariate analysis.
Results
Overall, 94.8% (2533/2672) of participants completed both study visits. A total of 31.3% (837/2672) were aged <10 years, 27.6% (737/2672) were aged 10–17 years, and 41.1% (1098/2572) were aged ≥18 years. The cumulative SARS-CoV-2 exposure rate was 58.5% (95% confidence interval, 47.5–69.4). This varied between sites and was 73.4% in the urban community of Sotuba, 53.2% in the rural town of Bancoumana, and 37.1% in the rural village of Donéguébougou. Study site and increased age were associated with serostatus at both study visits. There was minimal difference in reported symptoms based on serostatus.
Conclusions
The true extent of SARS-CoV-2 exposure in Mali is greater than previously reported and may now approach hypothetical “herd immunity” in urban areas. The epidemiology of the pandemic in the region may be primarily subclinical and within background illness rates.
Keywords: SARS-CoV-2, COVID-19, Mali, West Africa, seroprevalence
This study demonstrates a large, previously unquantified burden of SARS-COV-2 infection in the community in West Africa. In this young study population, there was limited evidence of severe illness and seropositivity rates that may approach hypothetical “herd immunity.”
Many African nations have seemingly been spared the overwhelming burden of disease seen in other countries during the first waves of the coronavirus disease 2019 (COVID-19) pandemic. This may be related to a younger population age structure and other hypothetical but undefined host or virological factors [1, 2]. The true extent of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in many African nations is likely to be greater than previously reported. Understanding the extent of infection and burden of disease is critical to allocate limited public health resources, including vaccines. Case numbers may be underestimated because of paucisymptomatic infections as well as restrictions in healthcare access and diagnostic capacity.
Serosurveillance is a convenient and potentially powerful tool for population-based monitoring of SARS-CoV-2 infection. Despite the numerous seroassays available, many have not been qualified for use in populations under study nor reporting methods standardized. This is particularly relevant in sub-Saharan Africa, where the high infectious disease burden may affect serology interpretation [3–6] and laboratory infrastructure is often limited.
Using commercial point-of-care tests, serosurveillance throughout 2020 has identified gradually increasing seroprevalence rates in several West African countries [7–9]. Surveys elsewhere on the continent using laboratory-based single antigen ELISA have found similarly increasing seroprevalence [10–12]. These results suggest that SARS-CoV-2 is circulating throughout Africa in a largely unquantified community reservoir of transmission.
We sought to determine the age-specific cumulative incidence of SARS-CoV-2 infection in longitudinal cohorts at urban and rural sites in Mali, using a two-antigen enzyme-linked immunosorbent assay (ELISA) previously optimized for the local population [6]. In addition, we examined the association of social and medical factors with serostatus, compared seropositivity with seroconversion episodes to further assess assay performance, and characterized the longitudinal dynamics of the antibody response to each of the target antigens.
METHODS
Study Design and Population
This prospective cohort study was adapted from the World Health Organization population-based, age-stratified seroepidemiological investigation protocol for COVID-19 virus infection, version 1.1 [13]. We assessed individuals aged 6 months or older from 3 communities in Mali for anti-SARS-CoV-2 antibodies. The participating communities were Sotuba (urban), Bancoumana (rural town), and Donéguébougou (rural village) (Supplementary Figure 1, Supplementary Text 1). Each site has an existing Malaria Research and Training Center/National Institutes of Health study facility engaged with the local community. Community members including those enrolled in existing malaria studies were invited to participate.
Ethics Statement
The study was conducted as a public health surveillance activity under the Malian Ministry of Health and was approved by the ethics committee of Facultes de Medicine/d’Odonto-Stomatologie et de Pharmacie (2020/114/CE/FMOS/FAPH) and the Malian COVID-19 Scientific Review Committee. Written informed consent or assent was obtained from all participants.
Procedures
Participants were invited to 2 study visits. Visit 1 occurred at enrollment commencing 28 July 2020 and visit 2 occurred commencing 14 December 2020. Demographic, medical comorbidity, social, and symptoms information were collected (Supplementary Text 2). Infants aged 6–12 months were coenrolled with participating mothers. At each visit, a venous blood sample was collected. Data were collected and stored using REDCap. Participants received a reusable mask supplied by the Ministry of Health and were requested to observe site infection control procedures.
Sera were tested for immunoglobulin G antibodies to HEK293-expressed SARS-CoV-2 spike protein (VRC-SARS-CoV-2 S-2P-3C-His8-Strep2x2) and receptor binding domain (RBD) protein (Ragon-SARS-CoV-2 S-RBD(319–529)-3C-His8-SBP) at the Malaria Research and Training Center/Department of Epidemiology of Parasitic Diseases Immunology Laboratory using a reference ELISA [14]. The ELISA was optimized for use in Mali by testing 312 negative control samples, 23 positive control samples, assessing for cross-recognition with other betacoronaviruses, and establishing population-specific cutoffs as previously described [6]. Seropositivity was defined as spike protein and RBD assay absorbance values (optical density) above antigen cutoffs. The estimated sensitivity and specificity of these cutoffs was 73.9% (51.6–89.8) and 99.4% (97.7–99.9), respectively [6]. All samples were tested in duplicate alongside plate negative (pooled prepandemic Malian sera) and positive controls (monoclonal antibody CR3022) by trained laboratory staff. Samples with discordant duplicate results (>20%) or results around assay cutoffs were repeated and concordant results included.
Statistical Analysis
The sample size was based on pragmatic factors, with a provisional target of 500–1000 participants per site to allow age stratification [13]. Seroprevalence and 95% confidence intervals (CIs) for each site were calculated for each visit. Seroprevalence estimates were adjusted using two methods. First, results for each site were stratified by age group (<10 years, 10–17 years, ≥18 years) and weighted for community age structure and size using available census data (Supplementary Figure 2). Second, results were adjusted for test sensitivity and specificity [15]. The cumulative adjusted SARS-CoV-2 exposure prevalence at visit 2 was estimated by including seropositive cases from visit 1 that had seroreverted at visit 2 before applying adjustments. The daily rate of infection was estimated by calculating the adjusted incidence of new cases between visit 1 and visit 2 and dividing this number by the median number of days between visits. Chi-square tests were used to test for differences between site seroprevalence estimates.
Exploratory Analyses
To assess assay performance, seroconversion episodes were assessed in participants with prepandemic blood samples available and compared with seropositivity using antigen cutoffs (Supplementary Text 3, Supplementary Figure 4).
Effects of selected covariates on serostatus were modeled by multiple logistic regression. Site, age, sex, and self-reported symptoms (by category) were included a priori. Other covariates were selected based on univariate analysis of seronegative and seropositive groups at each visit, using a P value threshold of .05. Two-sided Fisher exact tests were used for categorical variables, and unpaired 2-tailed t tests for continuous variables. Similar covariates were grouped by category (i.e., nausea/vomiting, abdominal pain, and diarrhea were grouped as gastrointestinal symptoms).
In participants confirmed seropositive at visit 1, the proportion of seroreversions at visit 2, and the rate of change of SARS-CoV-2 spike and RBD absorbance values (optical density/100 days) were also calculated (Supplementary Text 4, Supplementary Tables 5 and 6).
In a subset of adult participants coenrolled in clinical trials, adverse events recorded during the study period were analyzed to discern clinical presentations of COVID-19 (Supplementary Text 5, Supplementary Tables 7 to 10).
Statistical analysis was performed with Microsoft Excel and GraphPad Prism 9 software.
RESULTS
Study Population
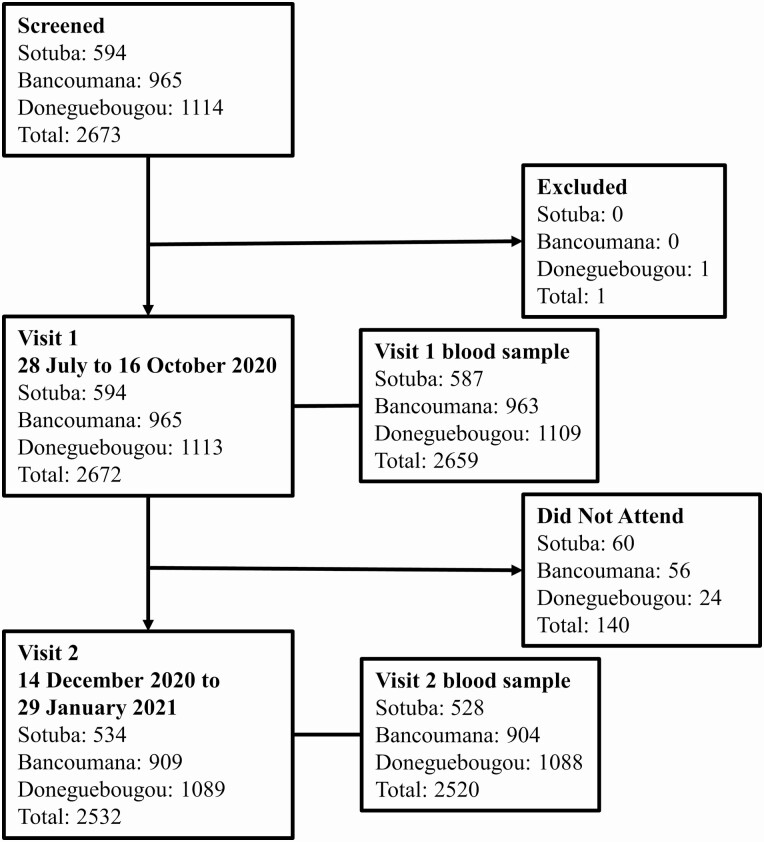
Among 2673 individuals screened, 2672 were enrolled at 3 study sites (Figure 1). The study population was relatively young with very few comorbidities (Table 1). At each site, a large proportion of participants were children, reflecting the age structure of the overall Malian population (Supplementary Figure 2). The median age was 14 years (interquartile range 8–31 years). No participant reported a personal history of COVID-19 diagnosis or household member with COVID-19 diagnosis at enrollment.
Figure 1.
Study flow chart.
Visit 1 was completed between 29 July and 16 October 2020 at the Sotuba site, 29 July and 24 September 2020 at the Bancoumana site, and 28 July and 27 August 2020 at the Donéguébougou site. Visit 2 was completed between 21 December 2020 and 26 January 2021 at the Sotuba site, 28 December 2020 and 29 January 2021 at the Bancoumana site, and 14 December 2020 and 15 January 2020 at the Donéguébougou site. A total of 94.7% (2532/2672) of participants completed visit 2.
Table 1.
Study population characteristics at visit 1 (July–October 2020)
| Sotuba | Bancoumana | Donéguébougou | Overall | |
|---|---|---|---|---|
| Sample size (n) | 594 | 965 | 1113 | 2672 |
| Individuals | 585 | 959 | 1113 | 2657 |
| Co-enrolled infantsa | 9 | 6 | 0 | 15 |
| Demographics (%, n) | ||||
| Sex, male | 43.3% 257/594 |
51.9% 501/965 |
52.3% 582/1113 |
50.3% 1343/2672 |
| Age, years (median, IQR) | 14 (8-25) | 15 (8-33) | 14 (6-35) | 14 (8-31) |
| Age, years (%, n) | ||||
| < 10 | 34.0% 202/594 |
28.9% 279/965 |
32.0% 356/1113 |
31.3% 837/2672 |
| 10 - 17 | 27.4% 163/594 |
29.4% 284/965 |
26.1% 290/1113 |
27.6% 737/2672 |
| ≥ 18 | 38.5% 229/594 |
41.7% 402/965 |
41.9% 467/1113 |
41.1% 1098/2672 |
| Medical factors (%, n) | ||||
| No co-morbid conditions | 96.4% 564/585 |
99.2% 951/959 |
99.3% 1105/1113 |
98.6% 2620/2657 |
| Co-morbid conditions | 3.6% 21/585 |
0.8% 8/959 |
0.7% 8/1113 |
1.4% 37/2657 |
| Obesity | 0.9% 5/585 |
0% 0/959 |
0.4% 5/1113 |
0.4%10/2657 |
| Diabetes | 0.9% 5/585 |
0% 0/959 |
0% 0/1113 |
0.2% 5/2657 |
| HIV/other immune deficiency | 0% 0/585 |
0% 0/959 |
0% 0/1113 |
0% 0/2657 |
| Hypertension | 2.7% 16/585 |
1.4% 13/965 |
0.2% 2/1113 |
1.2%31/2657 |
| Cardiovascular disease | 0.2% 1/585 |
0% 0/959 |
0.1% 1/1113 |
0.1% 2/2657 |
| Chronic pulmonary condition | 0% 0/585 |
0% 0/959 |
0%0/1113 | 0% 0/2657 |
| Chronic hepatic condition | 0% 0/585 |
0% 0/959 |
0% 0/1113 |
0% 0/2657 |
| Chronic hematological condition | 0% 0/585 |
0.1% 1/959 |
0% 0/1113 |
<0.1%1/2657 |
| Chronic kidney disease | 0% 0/585 |
0% 0/959 |
0% 0/1113 | 0% 0/2657 |
| Chronic neurological impairment/disease | 0% 0/585 |
0% 0/959 |
0% 0/1113 |
0% 0/2657 |
| Malignancy | 0% 0/585 |
0% 0/959 |
0.1% 1/1113 |
<0.1%1/2657 |
| BCG vaccination | 88.9% 520/585 |
70.2% 673/959 |
85.2% 948/1113 |
80.6% 2141/2657 |
| Antimalarial use (within 4 weeks of visit 1) | 3.6% 21/585 |
3.9% 37/959 |
0.1% 1/1113 |
2.2% 59/2657 |
| Smokerb | 2.9% 15/526 |
0.8% 7/904 |
4.4% 48/1089 |
2.8% 70/2519 |
| Pregnancy | 1.0% 6/585 |
1.6% 15/959 |
0.2% 2/1113 |
0.9% 23/2657 |
| Trimester 1 | 0.2% 1/585 |
0.1% 1/959 |
0.2% 2/1113 |
0.2% 4/2657 |
| Trimester 2 | 0.3% 2/585 |
0.9% 9/959 |
0% 0/1113 |
0.4% 11/2657 |
| Trimester 3 | 0.5% 3/585 |
0.5% 5/959 |
0% 0/1113 |
0.3% 8/2657 |
| Post-partum (< 6 weeks) | 0.2% 1/585 |
0.2% 2/959 |
0% 0/1113 |
0.1% 3/2657 |
| Social factors (%, n) | ||||
| Employed in healthcare setting | 1.2% 7/585 |
1.8% 17/959 |
4.9% 55/1113 |
3.0% 79/2657 |
| Household member employed in healthcare setting | 22.6% 132/585 |
21.2% 203/959 |
1.5% 17/1113 |
13.2% 352/2657 |
| Household member previously diagnosed with COVID-19 | 0% 0/585 |
0% 0/959 |
0% 0/1113 |
0% 0/2657 |
| Household size (mean, SD) | 10.2 (5.7) | 7.1 (4.1) | 6.8 (2.9) | 7.7 (4.3) |
Abbreviations: COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation.
aInfants aged 6–12 months were co-enrolled with their mother, and a limited history was collected.
bSmoking status collected at visit 2.
Seroprevalence of SARS-CoV-2 Antibodies
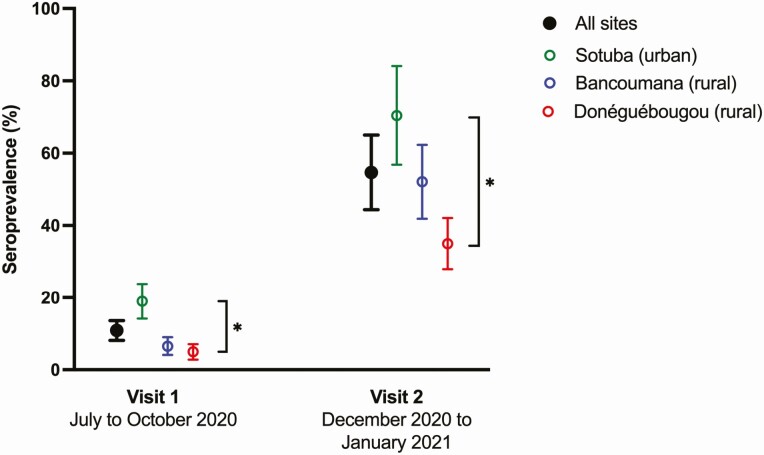
The adjusted seroprevalence of SARS-CoV-2 antibodies across all sites at visit 1 (July–October 2020) was 10.9% (95% CI, 8.1–13.6) and increased markedly to 54.7% (95% CI, 44.4–65.0) at visit 2 (December 2020–January 2021) (Figure 2). Accounting for seroreversions between visits, the cumulative adjusted SARS-CoV-2 exposure prevalence across all sites at visit 2 was 58.5% (95% CI, 47.5–69.4) and the estimated infection rate was 0.41%/d. This represents an approximately 1% population infection rate every 3 days between July 2020 and January 2021 (Table 2). The rate of SARS-CoV-2 antibody detection was highest in the urban site of Sotuba, versus the more rural township of Bancoumana, and the rural village of Donéguébougou, where it was lowest (visit 1: P < .0001, visit 2: P < .0001, Figure 2, Figure 3, Supplementary Table 1, Supplementary Figure 3). At each site, seroprevalence increased with age group (Supplementary Table 2). In Sotuba, visit 2 seroprevalence was 77.2% (95% CI, 61.5–92.9) in participants aged 18 years or older. Although children aged < 10 years had the lowest rate of SARS-CoV-2 antibody detection, there was evidence of increasing exposure over time in this age group at all sites.
Figure 2.
Seroprevalence of SARS-CoV-2 antibodies in Mali.
Seroprevalence adjusted for population age distribution and assay sensitivity and specificity [15].
Error bars represent 95% confidence intervals. Asterisk represents P < .0001 in comparison between sites. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Cumulative SARS-CoV-2 Exposure Prevalence and Rate of Infection at Sotuba (Urban), Bancoumana (Rural Town), and Donéguébougou (Rural Village) Sites
| Site | Visit 1 (Median)a | Visit 2 (Median) | Cumulative Exposure Prevalence Visit 2 (95% CI)b | Rate of Infection (% Population Infected/d)c |
|---|---|---|---|---|
| Sotuba | 6 August 2020 (29 July–16 October 2020) | 24 December 2020 (21 December 2020–26 January 2021) | 73.4% (59.2–87.5) | 0.45 |
| Bancoumana | 11 September 2020 (29 July–24 September 2020) | 6 January 2021 (28 December 2020–29 January 2021) | 53.2% (42.8–63.6) | 0.42 |
| Donéguébougou | 13 August 2020 (28 July–27 August 2020) | 19 December 2020 (14 December 2020–15 January 2021) | 37.1% (29.6–44.5) | 0.19 |
Abbreviations: 95% CI, 95% confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aMedian visit date defined as date half of all sample collections were completed.
bCumulative seropositivity rate calculated by adding seropositive cases from visit 1 that were seronegative at visit 2 before calculating adjusted seroprevalence.
cAdjusted incidence of new cases between visit 1 and visit 2 divided by median number of days between visits.
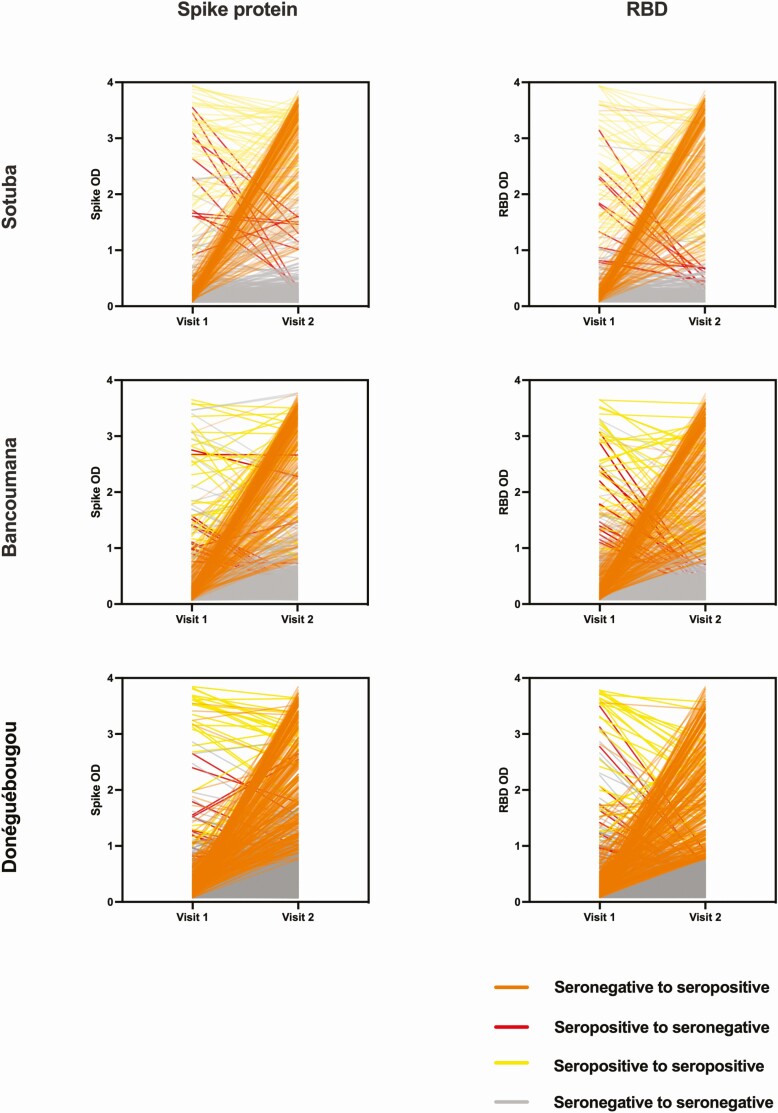
Figure 3.
Longitudinal SARS-CoV-2 antibody reactivity spike protein and RBD at study sites: Sotuba (top row), Bancoumana (middle row), and Donéguébougou (bottom row). Visit 1: 28 July–16 October 2020. Visit 2: 14 December 2020–29 January 2021. OD, optical density; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Factors Associated With SARS-CoV-2 Serostatus
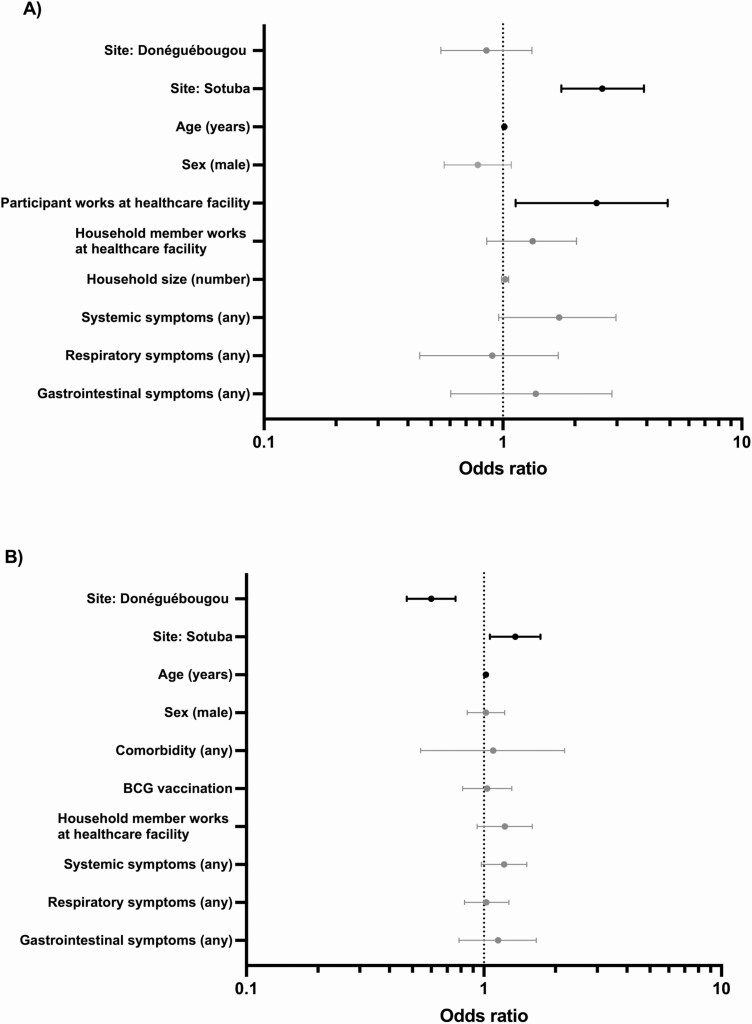
Factors associated with SARS-CoV-2 serostatus were evaluated by univariate analysis followed by multiple logistic regression for both visits. Covariates associated with serostatus at visit 1 by univariate analysis were female sex, age, participant employment at a healthcare facility, household member employment at a healthcare facility, household size, and self-reporting of any symptoms, systemic symptoms, fever, chills, myalgia, respiratory symptoms, or gastrointestinal symptoms since March 2020 (Supplementary Table 3). Following regression, age in years (odds ratio [OR] 1.01; 95% CI, 1.00–1.02), study site Sotuba (OR 2.61; 95% CI, 1.76–3.89), and participant employment at a healthcare facility (OR 2.47; 95% CI, 1.13–4.90), remained associated with seropositivity (Figure 4). Covariates associated with serostatus at visit 2 (assessing new seropositive cases only) by univariate analysis were age, household member employment at a healthcare facility, any medical comorbidity, self-reported systemic symptoms, chills, fatigue, and respiratory symptoms since visit 1 (Supplementary Table 4). Following regression, age in years (OR 1.02; 95% CI, 1.01–1.02), study site Sotuba (OR 1.35; 95% CI, 1.06–1.73), and study site Donéguébougou (OR 0.60; 95% CI, .47–.76) remained associated with serostatus (Figure 4). Among participants reporting symptoms, seeking medical attention for symptoms was associated with seropositivity (OR 1.76; 95% CI, 1.30–2.39) at visit 2, but not at visit 1.
Figure 4.
Odds ratio and 95% confidence intervals for covariates associated with serostatus at (A) visit 1 and (B) visit 2. Odds ratios following multiple logistic regression. Covariates with 95% confidence intervals crossing 1 in gray, not crossing 1 in black. Visit 1: 28 July–16 October 2020. Visit 2: 14 December 2020–29 January 2021.
Clinical Presentation in SARS-CoV-2 Seroconverters
Symptoms since the first cases of COVID-19 were detected in Mali in March 2020 were reported infrequently by seropositive participants (n = 173) at visit 1 (July–October 2020). Several systemic symptoms including fever (8.7% [17/173] vs 4.0% [100/2473], P = .010), chills (1.7% [3/173] vs 0.4% (9/2473), P = .039), myalgia (3.5% [6/173] vs 0.7% [18/2473], P = .004), and headache (11.0% [19/173] vs 3.7% [91/2473], P < .0001) were more frequently reported by seropositive participants compared to seronegative participants (Supplementary Table 3). Grouped systemic symptoms (any) were almost independently associated with serostatus following multiple logistic regression (OR 1.72; 95% CI, .96–2.98, Figure 4).
Among the larger group of newly seropositive participants at visit 2 (n = 724), symptoms occurring since visit 1 were reported with a greater frequency compared with the prior reporting period (Supplementary Tables 3 and 4). The most common symptoms were rhinorrhea (26.1% [189/724]), headache (22.7% [164/724]), cough (19.1% [138/724]), and fever (9.9% [72/724]). The remaining symptoms were reported in less than 5% of individuals, including loss of smell or taste (2.2% [16/724]). Among seropositive participants, 48.6% (352/724) reported a history of any symptoms, compared with 49.3% (803/1629) in seronegative participants. Given high background illness rates, it is difficult to establish which symptoms are associated with COVID-19 in the study population. Chills (3.7% [27/724] vs 2.1% [34/1619], P = .025) and fatigue (4.3% [31/724] vs 2.5% [41/1619], P = .028) were more frequently reported by seropositive participants. Similar to visit 1 analysis, grouped systemic symptoms (any) were almost independently associated with serostatus by multiple logistic regression (OR 1.22; 95% CI, .97–1.52; Figure 3). Among seropositive participants reporting symptoms, 15.6% (55/352) reported absenteeism from work or school, 63.4% (223/352) reported seeking medical attention, and 0.9% (3/352) reported hospitalization. The 3 seropositive participants reporting hospitalization included a 2-year-old male with fever, cough, and rhinorrhea; a 12-year-old male with headache; and a 30-year-old male with fever, headache, and rhinorrhea. Among participants reporting symptoms at visit 2, seropositive individuals were more likely to have sought medical attention for symptoms compared with seronegative individuals (63.4% [223/352] vs 45.9% [366/797], P < .0001, Supplementary Table 4).
Discussion
In this prospective cohort study of 3 sites in urban and rural Mali, we provide evidence of marked SARS-CoV-2 community transmission between July 2020 and January 2021 using population serosurveillance. The cumulative SARS-CoV-2 exposure rate was 58.5% (95% CI, 47.5–69.4), equating to an infection rate of approximately 1% of the population every 3 days between visits. Previously, serosurveillance in the United States has estimated a case detection ratio of 20%, whereas in Zambia, combined reverse transcriptase-polymerase chain reaction/serosurveillance estimated a 1% case detection ratio [10, 16]. The findings of our study would suggest a case detection ratio of approximately 0.1%–0.2% in Mali based on the number of previously reported cases nationwide [17]. This highlights the need for improved access to diagnostic testing in the community, although targeting these diagnostics will remain a challenge in the presence of limited disease.
Based on the estimated seroprevalence in our study, the hypothetical “herd immunity” threshold of 70%–80% may have been reached among adults in Sotuba. It is unclear if the evidence of natural infection provided by serosurveillance can be used to approximate population protection, although the presence of SARS-COV-2 antibodies has been associated with a lower risk of infection compared with seronegative individuals in adult populations [18, 19]. The clinical significance of our assay is uncertain; however, we have previously shown strong correlation between assay absorbance and pseudovirus neutralization activity in US samples [6]. In our study, RBD absorbance values waned more quickly than spike protein values. Despite the relatively mild illness reported, the durability of spike protein immunoglobulin G antibodies suggests a relatively long-lasted humoral response in our study population, and seropositivity may be a surrogate marker for longer term cellular immunity [20]. Taken together, the rapid increase in SARS-CoV-2 antibody seroprevalence and limited attributable severe illness during the study period may reflect a degree of protection in the community.
Conversely, the widespread SARS-CoV-2 transmission suggested by this study could promote the emergence of new variants that may escape any natural herd immunity. In a similar high seroprevalence scenario in Manaus, Brazil, a large resurgence in cases was reported following introduction of the B.1.1.248 variant, suggesting limited cross-protection from prior infection [21, 22]. We are uncertain of the locally circulating variants in Mali and whether our study may have coincided with emergence of a new variant. The South African variant B.1.351 was reported in Ghana in early January 2021 [23] and became the dominant circulating strain in South Africa over the study period [24].
In our cohort, seropositivity was associated with increasing age, employment in a healthcare setting, and residence in the urban community of Sotuba. Residence in the rural village of Donéguébougou was associated with seronegativity. This is consistent with other reports that show marked regional variation, lower seropositivity rates in children versus adults, and higher seropositivity rates in healthcare workers [8, 10]. Symptom history was not reliably associated with serostatus, highlighting the relatively limited clinical burden of the pandemic in the study population and the challenge of detecting cases by passive surveillance.
The frequency of reported symptoms in our population is in keeping with smaller SARS-CoV-2 serosurveys conducted in West Africa [8, 9]. Between July 2020 and January 2021, the background illness was high, with 49.1% (1155/2353) of participants reporting symptoms irrespective of serostatus. This period coincides with local seasonal malaria and emphasizes the importance of readily available and reliable diagnostics, particularly in regions where the differential diagnosis for nonspecific symptoms is broad and may include malaria and viral hemorrhagic fevers. The lack of excess clinical illness in the SARS-CoV-2 seroconverting group, including in a subpopulation intensively followed up for adverse events, suggests that the rate of COVID-19 attributable symptoms is low in our population, especially during the malaria transmission season. In an active surveillance study in Zambia, 23.8% of polymerase chain reaction-confirmed COVID-19 cases reported symptoms [10], which falls within the background rate of symptoms in our study.
Although our study is not designed to determine if severe COVID-19 is less common in sub-Saharan compared with other settings, we found minimal difference in reported symptoms, hospitalization, or absenteeism based on serostatus. Using estimated COVID-19 hospitalization rates from the United States adjusted for the age structure of our study population, we would expect a 2% hospitalization rate, or approximately 30 hospitalization events among the nearly 1500 SARS-CoV-2 infections estimated in our study [25]. In total, 6 hospitalizations were reported among the study population (3 seropositive participants and 3 seronegative participants).
These data provide valuable information for use in regional Public Health efforts. In this study, 2 visits were completed by 94.8% (2533/2672) of participants, including a large number of children, which reflects the local population age structure. The two-antigen ELISA used in this study has been used for population seroprevalence in the United States [16] and has been optimized to improve specificity in Mali [6]. Although single-antigen SARS-CoV-2 antibody cross-reactivity has been associated with malaria [26, 27], we did not observe an increased frequency of microscopy-confirmed malaria infection in seropositive participants (Supplementary Tables 7 and 9). Further studies are under way to better understand the possible relationship between serostatus and malaria in our population. The strong concordance between two-antigen seropositivity and 2-antigen 4-fold seroconversion compared to prepandemic baseline, provides further reassurance of assay performance.
Our study has several limitations, including the lack of prospective power calculations because of a lack of preexisting data, and the risk of recall bias in reported symptoms history. In a post hoc analysis, our study had >80% power to discriminate seroprevalence differences between age groups and study sites. In our study, reported symptoms history was similar in the overall study population to adverse events recorded for a subpopulation coenrolled in parallel clinical trials. Furthermore, we would expect recall bias to be similar between seropositive and seronegative participants. The study population was not selected randomly, and therefore there is also a risk of selection bias. Notably, no participant reported a personal history of prior COVID-19 diagnosis or household member with a diagnosis at enrollment, and there was a high proportion of community participation, particularly at the Donéguébougou site. By using an assay optimized to improve specificity, we have a reduced ability to detect low level SARS-CoV-2 antibody reactivity in crude seroprevalence estimates. In the setting of high background reactivity to SARS-CoV-2 antigens in our study population [6], a two-antigen assay with mathematical adjustment for test characteristics was considered the most appropriate approach to estimate the community burden of SARS-CoV-2 exposure and avoid the effect of possible cross-reactivity.
This study provides further evidence that Africa has not been spared by SARS-CoV-2, and that the epidemiology of disease in Malian communities with a young age structure may be primarily subclinical and within background illness rates. Although the Ministry of Health has implemented mitigation strategies, it is challenging to integrate measures such as physical distancing in day-to-day life in Mali. In this setting, community mitigation strategies may differ to other regions, and ongoing surveillance and augmentation of diagnostics, including characterizing locally circulating variants, will be critical to implement and monitor an effective vaccination program.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors gratefully acknowledge the support of Dr Thayne Dickey (LMIV/National Institute of Allergy and Infectious Diseases [NIAID]) for providing positive control monoclonal neutralizing antibody CR3022; Rathy Mohan (LMIV/NIAID) for database support; Dr David Cook and Alemush Imeru for curation of NCT03952650 MEDDra coded adverse event data; Dr Rebecca Prevots (LCIM/NIAID) and Dr Dean Follman (BRB/NIAID) for assistance designing collection and analysis of study data; J. Patrick Gorres (LMIV/NIAID) for editorial assistance; the Malian COVID-19 Coordinator; and Ministry of Health for permission to partner in developing serosurveillance capacity in Mali. Deidentified data collected for this study may be made available to others after approval of and with a signed data access agreement.
Financial support. This work was supported by the Intramural Research Program of the National Institutes of Health including the National Institute of Allergy and Infectious Diseases and the National Institute of Biomedical Imaging and Bioengineering; and the National Cancer Institute, National Institutes of Health, under contract number HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. Disclaimer: The NIH, its officers, and employees do not recommend or endorse any company, product, or service.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Njenga MK, Dawa J, Nanyingi M, et al. Why is there low morbidity and mortality of COVID-19 in Africa? Am J Trop Med Hyg 2020; 103:564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rice BL, Annapragada A, Baker RE, et al. Variation in SARS-CoV-2 outbreaks across sub-Saharan Africa. Nat Med 2021; 27:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tso FY, Lidenge SJ, Peña PB, et al. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int J Infect Dis 2021; 102:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yadouleton A, Sander AL, Moreira-Soto A, et al. Limited specificity of serologic tests for SARS-CoV-2 antibody detection, Benin. Emerg Infect Dis 2021; 27:233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nkuba Ndaye A, Hoxha A, Madinga J, et al. Challenges in interpreting SARS-CoV-2 serological results in African countries. Lancet Glob Health 2021; 9:e588–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sagara I, Woodford J, Dicko A, et al. SARS-CoV-2 seroassay optimization and performance in a population with high background reactivity in Mali. MedRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halatoko WA, Konu YR, Gbeasor-Komlanvi FA, et al. Prevalence of SARS-CoV-2 among high-risk populations in Lomé (Togo) in 2020. PLoS One 2020; 15:e0242124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Majiya H, Aliyu-Paiko M, Balogu VT, et al. Seroprevalence of COVID-19 in Niger State. MedRxiv 2020. [Google Scholar]
- 9. Milleliri JM, Coulibaly D, Nyobe B, et al. SARS-CoV-2 infection in ivory coast: a serosurveillance survey among gold mine workers. Am J Trop Med Hyg 2021; 104:1709–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulenga LB, Hines JZ, Fwoloshi S, et al. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: a cross-sectional cluster sample survey. Lancet Glob Health 2021; 9:e773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uyoga S, Adetifa IMO, Karanja HK, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science 2021; 371:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sykes W, Mhlanga L, Swanevelder R, et al. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021. Res Sq 2021. doi:10.21203/rs.3.rs-233375/v1. [Google Scholar]
- 13. WHO. Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection (v1.1). 2020. Available at: https://apps.who.int/iris/handle/10665/331656. Accessed 1 April 2020. [Google Scholar]
- 14. Klumpp-Thomas C, Kalish H, Drew M, et al. Standardization of ELISA protocols for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling. Nat Commun 2021; 12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang Z, Reiczigel J. Confidence limits for prevalence of disease adjusted for estimated sensitivity and specificity. Prev Vet Med 2014; 113:13–22. [DOI] [PubMed] [Google Scholar]
- 16. Kalish H, Klumpp-Thomas C, Hunsberger S, et al. Mapping a pandemic: SARS-CoV-2 seropositivity in the United States. medRxiv 2021; 13: eabh3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO. COVID-19 dashboard. Available at: https://covid19.who.int/. Accessed 27 March 2021.
- 18. Hall VJ, Foulkes S, Saei A, et al. ; SIREN Study Group. . COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 2021; 397:1725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harvey RA, Rassen JA, Kabelac CA, et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med 2021; 181:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371: eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sabino EC, Buss LF, Carvalho MPS, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet 2021; 397:452–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buss LF, Prete CA Jr, Abrahim CMM, et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science 2021; 371:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adu B, Egyir B, Kumordjie S, et al. GISAID: tracking of variants (B.1.351). Available at: https://www.gisaid.org/hcov19-variants/. Accessed 26 March.
- 24. Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N Engl J Med 2021; 384:1866–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reese H, Iuliano AD, Patel NN, et al. Estimated incidence of COVID-19 illness and hospitalization—United States, February–September, 2020. Clin Infect Dis 2020; 72:e1010–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinhardt LC, Ige F, Iriemenam NC, et al. Cross-reactivity of Two SARS-CoV-2 serological assays in a setting where malaria is endemic. J Clin Microbiol 2021; 59:e0051421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lapidus S, Liu F, Casanovas-Massana A, et al. Plasmodium infection induces cross-reactive antibodies to carbohydrate epitopes on the SARS-CoV-2 Spike protein. medRxiv 2021. doi: 10.1101/2021.05.10.21256855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.