Abstract
Vascular calcification (VC) involves the deposition of calcium apatite in vascular intima or media. Individuals of advanced age, having diabetes mellitus or chronic kidney disease (CKD) are particularly at risk. The pathogenesis of CKD-associated VC evolves considerably. The core driver is the phenotypic change involving vascular wall constituent cells toward manifestations similar to that undergone by osteoblasts. Gender-related differences are observed regarding the expressions of osteogenesis-regulating effectors, and presumably the prevalence/risk of CKD-associated VC exhibits gender-related differences as well. Despite the wealth of data focusing on gender-related differences in the risk of atherosclerosis, few report whether gender modifies the risk of VC, especially CKD-associated cases. We systematically identified studies of CKD-associated VC or its regulators/modifiers reporting data about gender distributions, and extracted results from 167 articles. A significantly higher risk of CKD-associated VC was observed in males among the majority of original investigations. However, substantial heterogeneity exists, since multiple large-scale studies yielded neutral findings. Differences in gender-related VC risk may result from variations in VC assessment methods, the anatomical segments of interest, study sample size, and even the ethnic origins of participants. From a biological perspective, plausible mediators of gender-related VC differences include body composition discrepancies, alterations involving lipid profiles, inflammatory severity, diversities in matrix Gla protein (MGP), soluble Klotho, vitamin D, sclerostin, parathyroid hormone (PTH), fibroblast growth factor-23 (FGF-23), and osteoprotegerin levels. Based on our findings, it may be inappropriate to monotonously assume that male patients with CKD are at risk of VC compared to females, and we should consider more background in context before result interpretation.
Keywords: aortic calcification, chronic kidney disease, dialysis, fetuin-A, gender, end-stage renal disease, fibroblast growth factor-23, Klotho, mineral bone disorder, osteoprotegerin, sclerostin, sex, vascular calcification, valvular calcification, vitamin D
1. Vascular Calcification (VC): An Introduction
The ectopic deposition of calcium-containing crystals in vascular intima or media is termed VC and involves large conduit arteries and small ones as well. VC frequently affects individuals of advanced age and those with accelerated biological ageing related to diabetes mellitus (DM) or chronic kidney disease (CKD). Findings from large population-based cohorts identified a VC prevalence of 40% to 60% and 30% to 40% among older [1,2] and middle-aged adults [3]. Meta-analyses revealed that the VC prevalence rose to 60% among patients with CKD regardless of age [4] and also increased in those with DM [5]. Calcifications affecting conduit arteries lead to vessel stiffening, increased peripheral resistance and the risk of hypertension, cardiac hypertrophy, heart failure and cardiovascular mortality. Calcifications involving microcirculation aggravate tissue perfusion through microvascular dysfunction and they induce silent ischemic episodes [6], leading to a higher risk of cardiovascular events [7,8]. VC-related adverse outcome influences become more prominent among those with CKD [9]. Valvular calcification portends a similarly worse outcome among this population [10]. VC further elevates their risk of having rapid renal function decline [11], developing morbidities including malignancies, hip fractures, pneumonia, and detrimental phenotypes like frailty [12,13]. The understanding of pathogenesis related to CKD-associated VC evolves considerably but remains elusive. It is now believed that vascular smooth muscle cells (VSMCs) and/or their neighboring constituents adopt an osteoblast-like phenotype and these trans-differentiated cells secrete osteoid matrices under pervasive exposure, leading to VC [14]. This calcification process is further augmented by redox imbalance, epigenetic changes, uremic milieu, the reduction in endogenous anti-calcific defense machineries, extracellular vesicle secretion and the dysregulated microRNA network [15,16,17,18].
2. Risk Factors of CKD-Associated VC: Gender Matters?
VC is one of the CKD-mineral bone disorder (CKD-MBD) components, which encompass divalent ion dysregulation (dyscalcemia, hyperphosphatemia), osteodystrophy, and ectopic calcification involving soft tissues. There are two types of VC, intimal and medial calcification. The former resides within atherosclerotic plaques based on tunica intima, and shares atherosclerotic risk factors including an advanced age, genetic susceptibility such as polymorphisms involving ApoE and Lp(a), obesity, DM and/or insulin resistance, other cardiovascular morbidities, etc. The latter type is associated with atypical risk factors such as uremic toxin exposure, osteoporosis/sarcopenia, chronic inflammation, and elevated oxidative stress/glycation endproducts [19]. Medial calcification is proposed to be more characteristic of CKD-associated VC relative to intimal calcification, although the incidence of both disease categories rises in the CKD population.
Traditional belief maintains that males have a significantly higher prevalence of atherosclerosis, are more likely to have accelerated lesion progression, be symptomatic, and to have more severe/unstable plaque features compared to females [20]. There are also studies suggesting that the influence of beneficial lifestyle changes on the risk of atherosclerosis may have gender-related differences [21]. On the contrary, females tend to occupy a greater proportion of patients with atherosclerosis or ischemic heart disease than males after 75 years [22]. Gender-related differences in atherosclerosis are mainly attributed to estrogenic action, since younger females with primary ovarian insufficiency have a significantly elevated risk of ischemic heart events and overall mortality than those without [23]. Despite the wealth of data regarding gender differences in atherosclerotic risk, few address whether gender modifies VC risk, especially CKD-associated VC. A recent study showed that males had a 3-fold higher risk of coronary artery calcification (CAC) compared to females, and the former had a more diffuse distribution of calcification than the latter [24]. Consequently, we performed a scoping literature review aiming to summarize the existing knowledge regarding gender-related differences in VC prevalence, severity, risk, and potential mediators.
3. Literature Search Strategy
We adopted a systematic approach to identify articles focusing on VC in patients across all stages of CKD, using the following MeSH or Emtree keywords: ‘vascular calcification’, ‘male’ or ‘female’, and ‘renal insufficiency, chronic’ or ‘renal replacement therapy’, from databases including PubMed, MEDLINE, EMBASE, Google Scholar, and Cochrane Reports between 1968 and 6 May 2021. Inclusion criteria were original investigations involving human subjects, reporting data of the gender variable and any types of VC, pulse wave velocity (PWV), serum calcification propensity (T50), or potential functional regulators of VC, among populations with various stages of CKD. Identified studies were initially screened by two investigators (P.Y.W. and C.T.C.) independently. This was followed by the application of exclusion criteria, consisting of review articles, those without an abstract available for screening, those that did not provide data on gender-related differences in the prevalence/course/risk of VC or its related regulators, or those that focused on non-CKD patients. We subsequently reviewed the abstract of identified articles and their reference lists to uncover additional studies containing original data of similar topics. Discrepancies between the two reviewers regarding article eligibility were resolved by arbitration from a senior investigator (J.W.H.).
We extracted relevant parameters from the identified studies, including the year and authors of publication, the stages of participants’ CKD at baseline, methods for measuring VC status and severities, the distribution of gender and/or the gender as a predictor of VC or its regulators, and results from univariate and multivariate analyses of clinical features between males and females. We organized results according to the following categories: gender-related difference in the prevalence, severities and/or courses of VC; gender as a risk factor for incident/worsening VC; and gender as a modifier for VC risk.
4. Summary of Findings
A total of 893 studies were screened initially (Figure 1). After exclusion of articles that met our exclusion criteria following the title/abstract/full-text review, 167 articles were retained for summarization. In most studies of non-dialysis CKD patients, the estimated glomerular filtration rate (eGFR) was obtained based on the Modification of Diet in Renal Disease (MDRD) formula, while some earlier studies used elevated serum creatinine to identify those with CKD. Staging of CKD, when available, was done according to the Kidney Disease Improving Global Outcome (KDIGO) classification [25]. For the estimation of VC risk in multivariate analyses, factors that were adjusted for included at least age and gender and could further include interfering parameters such as body size, comorbidities, medications, and/or laboratory indicators.
Figure 1.
Literature selection algorithm of this review. CKD, chronic kidney disease.
4.1. Gender-Related Differences in the Prevalence and/or Severity of CKD-Associated VC
In total, 101 (60.5%) original investigations evaluated the differences in the prevalence and/or severity of CKD-associated VC between different genders [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126]. Among these articles, 27 (26.7%), 64 (63.4%) and 10 (9.9%) included patients with non-dialysis CKD, dialysis-dependent CKD, and renal transplant recipients, respectively (Supplementary Table S1). In those involving patients with non-dialysis CKD, one-third (n = 9) found no differences in VC prevalence/risk between genders, while two-thirds (n = 18) reported that males had a higher VC prevalence/severity than females. Interestingly, in reports involving patients with non-dialysis CKD, those directly assessing VC based on coronary computed tomography (CT) or plain radiography for aortas tended to derive results that suggested higher male VC prevalence/severity, when the case number was large. On the other hand, studies that involved the assessment of PWV or T50 tended to have neutral results (Supplementary Table S1).
In studies involving patients with dialysis-dependent CKD, 37 (57.8%) found no differences in VC prevalence/severity between genders, while 22 (34.4%) found that males had a higher VC prevalence than females (Supplementary Table S1). On the contrary, 5 (7.8%) reports identified that females had a higher VC prevalence than males. If coronary VC was the phenotype of interest in studies involving patients with dialysis-dependent CKD, males were more likely to have a higher prevalence/severity of VC than females (neutral vs. male higher, 5 out of 37 (13.5%) vs. 8 out of 22 (36.4%) studies using Agatston scores as surrogates). Reports that identified a higher VC prevalence/severity in dialysis-dependent CKD females than males tended to originate from Asian countries and involved carotid artery calcification (Supplementary Table S1).
In studies involving patients receiving renal transplant (CKD stage 5T), 6 (60%) found no differences in VC prevalence/severity between genders, while 4 (40%) reported more males with a higher VC prevalence/severity than females (Supplementary Table S1). Similar to studies involving dialysis-dependent CKD patients, if coronary VC was the phenotype of interest, males were more likely to be reported as having higher prevalence/severity of VC than females (neutral vs. male higher, 1 out of 6 (16.7%) vs. 2 out of 4 (50%) studies using Agatston scores as surrogates).
Few studies adopted a histopathological approach to identify gender-related differences in CKD-associated VC prevalence/severity (n = 5; 5.0%), and even fewer addressed gender-related differences in valvular calcification (n = 3, 3.0%) (Supplementary Table S1). There were reports suggesting neutral results or higher male prevalence/severity of VC using the histopathological approach, while none of the reports addressing valvular calcification identified gender-related differences in prevalence.
4.2. Gender-Related Differences in the Adjusted Risk of CKD-Associated VC Presence or Severity
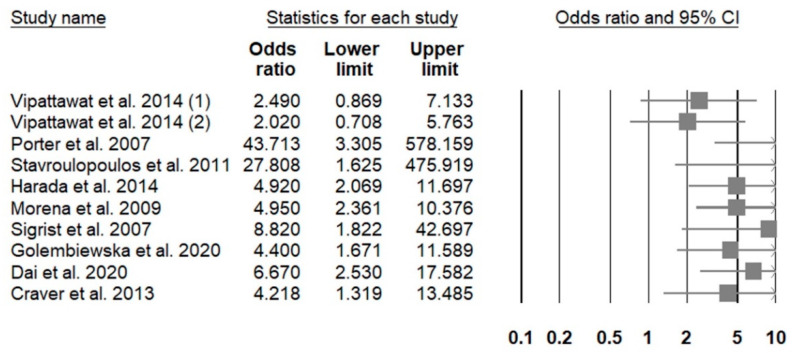
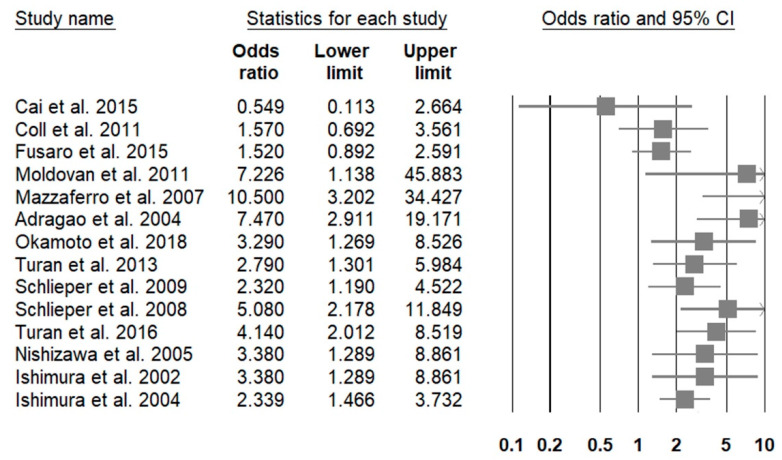
A total of 62 (37.1%) original investigations reported gender-associated adjusted risk of having higher prevalence/severity of VC among patients with CKD [40,42,44,47,48,58,63,64,65,67,68,84,86,88,90,93,95,100,101,102,106,112,113,116,117,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155]. Among these articles, 17 (27.4%), 40 (64.5%) and 5 (8.1%) included patients with non-dialysis CKD, dialysis-dependent CKD, and renal transplant recipients, respectively (Supplementary Table S2). In those involving patients with non-dialysis CKD, only 3 (17.6%) found no differences in VC risk between genders, while 14 (82.4%) reported that males had a higher adjusted risk of VC than females. A forest plot illustrating the risk of VC presence or greater severity associated with male gender in those with non-dialysis CKD is shown in Figure 2. Among studies enrolling more than 100 patients, most concluded that males exhibited a significantly higher risk of having VC involving coronary arteries, aortas or higher PWVs than females (Supplementary Table S2). The male-to-female odds ratio (ORs) of developing VC ranged between 4.2 and 43, depending on the cohort size and the combinations of adjusted variables, regardless of assessment methods for VC. In those involving patients with dialysis-dependent CKD, relatively few (n = 13; 32.5%) found no gender-related alterations in VC risk, and reports identifying a higher VC risk among males (n = 25; 62.5%) significantly outnumbered those identifying a higher VC risk among females (n = 2; 5%) (Supplementary Table S2). A forest plot illustrating the risk of VC presence or greater severity associated with male gender in those with dialysis-dependent CKD is shown in Figure 3. The male-to-female ORs or relative risks (RRs) of CKD-associated VC were between 1.9 and 10.5. It appears that the male-to-female OR/RRs for VC tend to be lower if aortic calcification was considered (between 2.2 and 3.3) and higher if CAC was studied (between 2.8 and 10.5) (Supplementary Table S2). Reports identifying a higher CKD-associated VC risk among females than males focused on aortic calcification and carotid artery calcification. Among studies involving patients receiving renal transplantation, most (n = 4; 80%) concluded that males had a significantly higher risk of VC than females, and 75% of them assess CAC (Supplementary Table S2).
Figure 2.
A forest plot showing the male to female risk among patients with non-dialysis chronic kidney disease.
Figure 3.
A forest plot showing the male to female risk among patients with dialysis-dependent chronic kidney disease.
4.3. Potential Mediators of Gender-Related Differences in CKD-Associated VC Risk
A total of 53 original investigations addressed potential modifiers of the gender-VC relationship among patients with CKD, based on our literature summary [36,40,43,58,59,61,66,86,106,107,110,115,129,130,134,152,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192]. We divided these mediators into 7 categories: genetic susceptibility, body composition and nutrition/inflammation status, medication, epigenetic mediators, divalent ion/electrolyte balances, osteogenesis regulators, and miscellaneous (Table 1). Studies involving polymorphisms of the vitamin K metabolism-related gene (VKORC1) and calcification inhibitors (matrix Gla protein; MGP) did not find gender-related differences in allelic distributions. In terms of body composition, males had significantly higher pericardial fat areas, low density lipoprotein (LDL) cholesterol, and angiotensin-converting enzyme 2 (ACE2) levels but lower total fat mass, adiponectin, and high density lipoprotein (HDL) cholesterol than females (Table 1). Fluid status, either in the form of extracellular fluid volume or serum copeptin levels, did not exhibit gender-related differences when CKD-associated VC risk was considered. There were studies addressing gender-related differences in CKD-associated VC risk modifying medication (statin) or microRNA levels, but the results were all neutral. Divalent ions and electrolytes such as serum phosphorus, calcium, and magnesium were reported to influence the risk of CKD-associated VC [193]; in our literature summary, females were found to have a higher probability of hyperphosphatemia than males (Table 1), but there was no difference in serum magnesium or calciuria levels. Thirty (56.6%) reports intended to examine gender-related differences in multiple osteogenesis regulator levels, including MGP (n = 2; 6.7%), fetuin-A (n = 4; 13.3%), fibroblast growth factor-23 (FGF-23) (n = 2; 6.7%), osteoprotegerin (n = 8; 26.7%), parathyroid hormone (PTH) (n = 2; 6.7%), sclerostin (n = 8; 26.7%), soluble Klotho (n = 3; 10%), and vitamin D (n = 2; 6.7%). No gender-related differences were observed regarding fetuin-A, while most studies concluded that males had significantly lower dephosphorylated uncarboxylated MGP and Klotho but higher sclerostin and vitamin D levels than females (Table 1). Studies focusing on gender-related differences in PTH, FGF-23, and osteoprotegerin levels among patients with CKD had controversial findings; those addressing PTH or FGF-23 had either neutral results or higher levels in females than males. Studies addressing osteoprotegerin yielded neutral, male higher, or female higher results. Interestingly, reports garnering higher patient numbers uniformly concluded that females had higher osteoprotegerin levels than males (Table 1). Finally, several studies focusing on VC identified gender-related differences in signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) and tissue advanced glycation (Table 1), which might bear potential influences on gender-related VC risk.
Table 1.
Potential gender-related modifiers of vascular calcification in existing studies.
| Authors | Country | Time | CKD Stages | Sample Size | Gender Effect | Data | Potential Modifiers | Ref. |
|---|---|---|---|---|---|---|---|---|
| Genetic susceptibility | ||||||||
| Holden et al. | Canada | 2014 | 3–5 | 167 | Neutral | CC vs. CG/GG, male 68% vs. 55%, p = 0.11 | VKORC1 (vitamin K epoxide reductase complex 1) polymorphism | [166] |
| Yoshikawa et al. | Japan | 2013 | 5D (HD) | 134 | Neutral | CC vs. CT vs. TT genotype, male 14% vs. 48% vs. 33%, p = 0.53 | MGP Genotype T-138C | [110] |
| Body composition and inflammatory status | ||||||||
| Axelsson et al. | Sweden | 2008 | 5 | 198 | Male lower | Male β = −1.68, p < 0.001 | Total fat mass | [157] |
| Park et al. | Korean | 2018 | 1–5 | 1741 | Neutral | Quartile 1 (lowest) vs. 2 vs. 3 vs. 4, male 80.6% vs. 59.0% vs. 39.7% vs. 39.6, p < 0.001 Male β = 0.095 (−0.421~0.231), p = 0.566 |
ECF excess | [179] |
| Choi et al. | Korea | 2019 | 5D (HD) | 97 | Neutral | Marker count 2 vs. 1 vs. 0, male 61.5% vs. 41.9% vs. 34.1%, p = 0.216 | Nutrition (albumin) and inflammation (CRP) markers | [43] |
| Golembiewska et al. | Sweden | 2020 | 5 | 149 | Neutral | High vs. middle vs. low levels, male 71% vs. 67% vs. 65%, p = 0.84 Regression model, male β −0.08, p = 0.31 |
Copeptin | [134] |
| Harada et al. | Brazil | 2014 | 2–5 | 117 | Male more severe | Area in males vs. females, 220.4 ± 110.5 vs. 135.4 ± 76.0, p < 0.001 | Pericardial fat | [58] |
| Turan et al. | Turkey | 2013 | 5D (HD) | 191 | Neutral | Low vs. middle vs. high, female 46% vs. 39% vs. 58%, p = 0.1 | Epicardial fat | [152] |
| Miyatake et al. | Japan | 2020 | 5T | 50 | Female higher HDL, adiponectin but lower LDL | For LDL-C, male vs. female 113.0 (97.0–132.5) vs. 90.0 (76.5–98.3), p < 0.01 For HDL-C, male vs. female 57.0 (51.0–67.0) vs. 78.0 (66.8–96.5), p < 0.01 For HMW-ADPN, male vs. female 2.48 (1.62–3.33) vs. 4.52 (3.02–6.79), p < 0.01 For LMW-ADPN, male vs. female 1.67 (1.14–1.89) vs. 2.26 (1.85–2.83), p < 0.01 For CTRP9, male vs. female 2.08 (2.01–2.13) vs. 2.03 (2.00–2.07), p > 0.05 |
Lipid profile, circulating C1q/TNF-a related protein-9 (CTRP9), and adiponectin | [86] |
| Zhang et al. | China | 2015 | 5D (HD) | 90 | Male higher | ≤30 vs. 30–60 vs. ≥ 60 U/L, male 29.4% vs. 46.1% vs. 85.7%, p < 0.001 Male correlation coefficient 0.362, p < 0.001 |
Serum ACE2 | [190] |
| Medications | ||||||||
| Chen et al. | Sweden | 2017 | 5D, 5T | 240 | Neutral | Users vs. non-users, male 65% vs. 62%, p = 0.678 | Statin use | [41] |
| Epigenetic mediators | ||||||||
| Chao et al. | Taiwan | 2019 | 5D | 223 | Neutral | High vs. low levels, female 63% vs. 51%, p = 0.14 | microRNA-125b | [160] |
| Divalent ion/electrolyte balance | ||||||||
| Block et al. | United States | 1998 | 5D (HD) | 6407 | Female higher | Male OR 0.774, p = 0.0001 | Serum phosphorus > 6.5 mg/dL | [158] |
| Zou et al. | China | 2016 | 1–5 | 296 | Neutral | Tertile 1 (lowest) vs. 2 vs. 3, male 60.2% vs. 50.0% vs. 61.2%, p = 0.21 | Serum phosphorus | [192] |
| Karsli Ceppioğlu et al. | Turkey | 2011 | 3–5 | 84 | Female higher | Female vs. male, higher in the former, p = 0.002 | Serum copper | [170] |
| Ikee et al. | Japan | 2016 | 5D (HD) | 86 | Neutral | Male vs. female, 2.51 ± 0.38 vs. 2.42 ± 0.33, p = 0.52 | Serum magnesium | [167] |
| Stolic et al. | Serbia | 2016 | 5D (HD) | 88 | Neutral | Male vs. female, mean 1.2 (0.7–1.6) vs. 1.2 (0.8–1.5) mmol/L, p = 0.896 | Serum magnesium | [185] |
| Ramalho et al. | Brazil | 2019 | 3–4 | 356 | Neutral | Tertile 1 (lowest) vs. 2 vs. 3, 59% vs. 64% vs.66%, p = 0.5 Male β = 0.22 (−0.02~0.45), p = 0.07 |
Urinary calcium excretion | [180] |
| Osteogenesis/mineralization regulators | ||||||||
| Aoun et al. | Lebanon | 2017 | 5D (HD) | 50 | Male lower | <5000 vs. >5000 pmol/L, male 68.3% vs. 22.2%, p = 0.02 | Dp-ucMGP | [156] |
| Schlieper et al. | Serbia | 2011 | 5 | 188 | Neutral | <6139 vs. >6139 pmol/L, female OR 0.62 (0.35–1.11), p = 0.11 | dp-cMGP | [182] |
| Okamoto et al. | Japan | 2018 | 5D (HD) | 230 | Neutral | <0.213 vs. ≥0.213 g/L, male 61% vs. 68%, p = 0.400 Male OR 0.48 (0.22–1.02), p = 0.056 |
Fetuin-A | [178] |
| Chen et al. | Taiwan | 2013 | 5D (HD) | 238 | Neutral | Tertile 1 (lowest) vs. 2 vs. 3, female 55% vs. 48% vs. 57%, p = 0.5 | Fetuin-A | [161] |
| Stenvinkel et al. | Sweden | 2005 | 5 | 258 | Neutral | Male vs. female, median 0.225 vs. 0.223 g/L, p > 0.05 | Fetuin-A | [184] |
| Metry et al. | Sweden | 2008 | 5D (HD) | 222 | Neutral | High Fetuin-A/Low CRP vs. High Fetuin-A/High CRP vs. Low Fetuin-A/Low CRP vs. Low Fetuin-A/High CRP, 60.9% vs. 52.5% vs. 44.4% vs. 58.8%, p > 0.05 | Fetuin-A and CRP | [174] |
| Turan et al. | Turkey | 2016 | 5D (HD) | 224 | Neutral | <35.5 vs. 35.5–123.4 vs. >123.4 pg/mL, male 56.6% vs. 46.5% vs. 50.0%, p = 0.38 | FGF-23 | [106] |
| Nishiura et al. | Japan | 2009 | 5D (HD) | 99 | Male higher | Low vs. high, male 56% vs. 75.5%, p = 0.057 Male HR 3.034 (1.028–8.948), p = 0.044 |
Osteoprotegerin | [177] |
| Hou et al. | Taiwan | 2019 | 5D (HD) | 120 | Female lower | 72.97–237.13 vs. 237.36–323.54 vs. 329.61–835.78 pg/L, female 62.5% vs. 55.0% vs. 32.5%, p = 0.008 | Osteoprotegerin | [61] |
| Sigrist et al. | United Kingdom | 2009 | 3–4 | 134 | Neutral | ≤25 vs. >25 pmol/L, male 63% vs. 70%, p = 0.47 | Osteoprotegerin | [183] |
| Nakashima et al. | Japan | 2011 | 5D (HD) | 151 | Neutral | Male β = 0.531, p = 0.51 | Osteoprotegerin | [175] |
| Scialla et al. | United States | 2011 | 1–5 | 351 | Female higher | 1.21–5.03 vs. 5.05–7.45 vs. 7.46–22.31 pmol/L, female 24.8% vs. 26.5% vs. 40.2%, p = 0.02 Female related OPG % difference 10.2%, p = 0.05 |
Osteoprotegerin | [181] |
| Gupta et al. | Hungary | 2021 | 5D (HD) | 982 | Female higher | <3.2 vs. 3.2–4.39 vs. >4.39 pmol/L, male 63% vs. 57% vs. 52%, p = 0.015 | Osteoprotegerin | [165] |
| Nemeth et al. | Hungary | 2015 | 5T | 993 | Male lower | <3.2 vs. 3.2–4.39 vs. >4.39 pmol/L, male 63% vs. 58% vs. 52%, p = 0.02 | Osteoprotegerin | [176] |
| Chae et al. | Korea | 2018 | 1–5 | 1832 | Neutral | Quartile 1 (lowest) vs. 2 vs. 3 vs. 4, female 36.2% vs. 43.4% vs. 42.5% vs. 38.6%, p = 0.517 (mean, 6.76 pmol/L) | Osteoprotegerin | [115] |
| Jean et al. | France | 2011 | 5D (HD) | 1138 | Neutral | <50 vs. ≥50 pg/mL, female 43% vs. 40%, p > 0.05 | Parathyroid hormone | [169] |
| González-Parra E et al. | Spain | 2015 | 1–5 | 704 | Female higher in both | For PTH, male r = −0.084 (−0.155~−0.012), p = 0.0215 For FGF-23, male r = −0.191 (−0.301~−0.080), p = 0.0007 |
Parathyroid hormone and FGF-23 | [164] |
| Thambiah et al. | United Kingdom | 2012 | 3B-4 | 77 | Male higher | Male vs. female, mean 50.5 vs. 34 pmol/L, p = 0.015 Male β = 0.23, p = 0.024 |
Sclerostin | [186] |
| Kuo et al. | Taiwan | 2019 | 5D (PD) | 89 | Male higher | <80.2 vs. ≥80.2 pmol/L, male 36.4% vs. 62.2%, p = 0.015 Male OR 2.882 (1.219–6.815), p = 0.016 |
Sclerostin | [172] |
| Goncalves et al. | Brazil | 2014 | 5D (HD) | 91 | Neutral | High vs. low levels, male 68.9% vs. 52.2%, p = 0.103 Regression model, male OR 0.82 (0.39–1.75), p = 0.62 |
Sclerostin | [163] |
| Viaene et al. | Belgium | 2013 | 5D (HD) | 100 | Neutral | Low vs. high, female 47% vs. 35%, p = 0.2 | Sclerostin | [188] |
| Claes et al. | Belgium | 2013 | 1–5 | 154 | Male higher | Male gender significantly associated with higher levels (p = 0.006) | Sclerostin | [162] |
| Zou et al. | China | 2020 | 5D | 165 | Male higher | Among PD patients, male β = 0.259 (0.052–0.416), p = 0.012 | Sclerostin | [191] |
| Jean et al. | France | 2016 | 5D (HD) | 227 | Female lower | Female OR 0.16 (0.075−0.362) | Sclerostin | [66] |
| Evenpoel et al. | Belgium | 2015 | 5T | 268 | Male higher | Male gender significantly associated with higher levels (p = 0.002) | Sclerostin | [130] |
| Zhang et al. | China | 2019 | 5D (HD) | 105 | Neutral | Quartile 1 (lowest) vs. 2 vs. 3 vs. 4, male 57.7% vs.48.1% vs. 46.2% vs. 65.4%, p = 0.473 | Soluble Klotho | [189] |
| Buiten MS | Netherlands | 2014 | 5D | 127 | Female higher | <460 vs. >460 pg/mL, female 16% vs. 31%, p < 0.05 | Soluble Klotho | [159] |
| Cai et al. | China | 2015 | 5D (HD) | 129 | Neutral | ≤379 vs. 379–613 vs. 613–817 vs. >817 pg/mL, male 59.4% vs. 54.5% vs. 56.3% vs. 53.1%, p > 0.05 | Soluble Klotho | [129] |
| Jean et al. | France | 2008 | 5D (HD) | 253 | Female lower levels | Deficient vs. sufficient, female 53% vs. 28%, p < 0.05 | Vitamin D (25-OH-D) deficiency | [168] |
| Chang et al. | Republic of Korea | 2012 | 5D (HD) | 289 | Female lower levels | Female OR 3.892 (1.678–9.025), p = 0.002 | Vitamin D (25-OH-D) deficiency (<15 ng/mL) | [36] |
| Miscellaneous | ||||||||
| Kato et al. | Japan | 2009 | 5D (HD) | 68 | Neutral | No correlation with gender | Serum pre-B cell colony-enhancing factor/visfatin | [171] |
| He et al. | China | 2018 | 5D (HD) | 150 | Neutral | Male correlation coefficient 0.106, p = 0.114 | Serum irisin | [59] |
| Liabeuf et al. | France | 2013 | 2–5, 5D | 139 | Neutral | ≤0.041 vs. >0.041 mg/dL, male 63% vs. 57%, p = 0.5 | Free p-cresylglucuronide | [173] |
| Ulusoy et al. | Turkey | 2012 | 5D (HD) | 103 | Male higher | Pre-dialysis male vs. female levels, 314.7 ± 157.1 vs. 210.4 ± 115.4 ng/mL, p < 0.001 | Signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) | [187] |
| Wang et al. | Hong Kong | 2014 | 3–5 | 300 | Male lower | Male correlation coefficient −0.14, p = 0.02 | Tissue AGEs (surrogated by skin autofluorescence) | [107] |
ADPN, adiponectin; AGE, advanced glycation endproduct; CRP, C-reactive protein; Dp-ucMGP, dephosphorylated-uncarboxylated Matrix Gla protein; ECF, extracellular fluid; FGF-23, fibroblast growth factor-23; HD, hemodialysis; HDL, high density lipoprotein; HMW, high molecular weight; HR, hazard ratio; LDL, low density lipoprotein; LMW, low molecular weight; MGP, matrix Gla protein; OR, odds ratio; PD, peritoneal dialysis.
5. Overall Interpretations
In this literature review involving 167 studies of CKD-associated VC, we found that the majority identified males with a higher VC prevalence/severity compared to females, although stages of CKD, the approaches used to measure VC, and the number of cases per study may account for the heterogeneity in study findings. In patients with non-dialysis CKD, males might not necessarily have a higher VC prevalence/severity, especially when studies assessed VC surrogates, except for those focused on CAC. In patients with dialysis-dependent CKD, males still exhibited a higher VC risk, but heterogeneity persisted. Females from Asian countries exhibited a higher tendency of having carotid artery calcification relative to males. These findings appeared to be more uniform after multivariable adjustment, as studies in patients with non-dialysis dependent and dialysis-dependent CKD mostly supported a higher VC risk among males. Potential contributors to the observed gender-related differences can be multiple, including pericardial/total fat, lipid profile, inflammatory status, variations in MGP, soluble Klotho, vitamin D, sclerostin, PTH, FGF-23, and osteoprotegerin levels.
5.1. Intimal or Non-Intimal Calcification: A Potential Consideration for Gender-Related Differences in CKD-Associated VC Risk
Findings from our literature summary suggest that reports focusing on CAC more frequently identified a male preponderance in VC prevalence/risk (Supplementary Tables S1 and S2). Coronary atherosclerosis frequently begins with the retention of LDL and/or Lp(a) in subendothelial extracellular matrix, leading to local inflammation, cellular necrosis, and attraction of monocytes with phagocytosis of the degraded content. The necrotic core serves as the niche of calcification nucleation, assisted further by the enhanced release of pro-calcifying vesicles and the reduced expression of mineralization inhibitors by neighboring cells [194]. CAC is therefore deemed more akin to a symbol of atherosclerotic intimal calcification, although medial calcification exists simultaneously. On the other hand, calcifications involving smaller diameter vessels such as arteriovenous accesses, or digital arteries are more likely to be medially located and are pathophysiologically distinct from intimal type calcification [195]. Since male gender correlates with a higher risk of atherosclerosis and potentially atherosclerotic calcification [20], studies focusing on CAC may be inclined to derive the finding that males have an increased VC risk relative to females.
5.2. Reports Favoring a Higher Risk of CKD-Associated VC in Males: Plausible Mediators
We discovered that in CKD patients, especially those with non-dialysis status, males were at a higher risk of VC compared to females (Supplementary Table S2). It was further disclosed that males had a significantly higher proportion of pericardial fat and higher levels of serum ACE2, LDL, vitamin D, and sclerostin than females, but lower HDL, adiponectin, uncarboxylated MGP and soluble Klotho (Table 1). Prior studies showed that a higher amount of pericardial fat tissues was associated with a greater CAC severity at baseline and a higher speed of CAC progression, through their paracrine and endocrine effects of inducing inflammation, oxidative stress, and endothelial dysfunction [196]. A study disclosed that aortic ACE2 expression was significantly up-regulated in calcified aortas [197], and ACE2 presumably plays an important role in perpetuating local inflammation. Calcitriol supplementations have been reported to intensify the severity and accelerate the tempo of VC in models of CKD and DM [198,199], suggesting that differences in vitamin D levels may be partially responsible for gender-related differences in VC risk. Sclerostin, an inhibitor of the canonical Wnt/β-catenin pathway originally thought to be solely expressed in bones, is found to be up-regulated in VSMCs and serves as a predisposing factor for VC [200]. It is likely that higher expressions of sclerostin in male CKD patients contribute to their tendency of developing VC. Along the same line, anti-calcific factors in CKD such as MGP and Klotho [201,202] were also lower in male CKD patients (Table 1), further paving the ground for their pro-calcific tendency. Dyslipidemia, in the forms of high LDL or low HDL, is associated with an increased risk of VC progression through their pathogenic linkage to atherosclerosis and local inflammation [203,204]. From this perspective, male CKD patients may harbor several risk features that predispose themselves to VC development.
5.3. Reports Favoring Higher Risk of CKD-Associated VC in Females: Clinical Implications and Plausible Mediators
Few studies (5% to 7%) in our summary identified females as having an increased VC risk relative to males (Supplementary Tables S1 and S2), while they predominantly focused on aortic or carotid artery calcification and tended to originate from Asian countries. Experimental reports showed that through binding to estrogen receptor α, estrogen could inhibit receptor activator of nuclear factor κ ligand (RANKL) signaling, leading to VSMC trans-differentiation and calcification [205]. However, there are also studies suggesting that estrogen analogs could aggravate VSMC calcification [206]. These findings indicate that the influence of estrogen on VC can be complex and scenario-dependent, unlike that exerted by testosterone, which is consistently pro-calcific [207]. Several other features can further modify these gender-related differences in VC risk, including the higher serum phosphate, FGF-23, and PTH levels observed in female CKD patients (Table 1). Elevated levels of FGF-23 and PTH parallel the worsening of CKD-associated VC in clinical studies, and participate in VC pathogenesis according to experimental reports [208]. Consequently, alterations in these hormones may be responsible for the increased risk of CKD-associated VC in females, at least partially.
5.4. Gender-Related Differences in Osteoprotegerin Levels and CKD-Associated VC
We observed substantial heterogeneity in studies reporting gender-related differences in serum osteoprotegerin levels focusing on VC; there were reports yielding no gender-related difference, while others reported either higher levels in males or in females (Table 1). It is clear that studies enrolling more patient numbers (>300) tend to identify higher osteoprotegerin levels in females but not males with CKD. In vitro studies indicated that estrogen could stimulate the up-regulation of osteoprotegerin in osteoblasts, but the direction of effect may depend on the types of estrogen receptors [209]. Consequently, clinical findings that higher serum osteoprotegerin levels alternated between different genders are not unexpected, and a careful interpretation of results in the context of sample size and other influencing factors is needed.
6. Conclusions
Gender-related differences in various biological phenotypes do exist, and such differences greatly affect cardiovascular risk estimation and carry public health importance. The male gender is traditionally regarded as a risk trait for atherosclerosis, but whether this relationship applies to VC remains debatable. VC encompasses a complex molecular interplay between endothelial cells, VSMCs, adventitial fibroblasts, pericytes, and even infiltrating macrophages, further compounded by the influences of uremic milieu and pro-/anti-calcific factors. We systematically identified existing reports of CKD-associated VC or its potential regulators containing data about gender distributions, and synthesizing their messages. Though a higher risk of CKD-associated VC risk was observed in males among the majority of original investigations, heterogeneity in findings were found; multiple large-scale studies yielded neutral findings, and few reported a higher risk of CKD-associated VC among females. Clinically speaking, differences in findings of gender-related VC risk may result from variations in outcome assessment approaches, the anatomical segments of interest, sample size, and even ethnic origins of participants. Biologically speaking, plausible mediators of such gender-related difference may include body composition changes, lipid profiles, inflammatory status, and diversities in MGP, soluble Klotho, vitamin D, sclerostin, PTH, FGF-23, and even osteoprotegerin levels. Based on our findings, it may not be appropriate to assign a high-risk VC category to males with CKD, and more background in context should be contemplated before reaching conclusions.
Acknowledgments
We are grateful to the Second Core Laboratory, Department of Medical Research of the National Taiwan University Hospital and the National Taiwan University Center of Genomic and Precision Medicine for their technical input.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/healthcare9080979/s1, Table S1: Gender-related differences in the prevalence and/or severity of vascular calcification in existing studies, Table S2: Gender-related adjusted risk of vascular calcification in existing studies.
Author Contributions
Study design: P.Y.W. and C.-T.C.; data analysis: P.Y.W. and C.-T.C.; article drafting: P.Y.W., S.-Y.L., K.-V.C., C.-T.C. and J.-W.H. All authors have read and agreed to the published version of the manuscript.
Funding
The study is financially sponsored by the National Taiwan University Hospital BeiHu Branch (11001), the National Taiwan University Hospital Yunlin Branch, and the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-002-193-MY3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data for conducting this analysis are available upon reasonable request to the corresponding author and the first author.
Conflicts of Interest
None of the authors has relevant financial or non-financial competing interests to declare in relation to this article. The sponsors have no role in the study design, data collection, analysis, and result interpretation of this study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yano Y., O’Donnell C.J., Kuller L., Kavousi M., Erbel R., Ning H., D’Agostino R., Newman A.B., Nasir K., Hofman A., et al. Association of Coronary Artery Calcium Score vs. Age with Cardiovascular Risk in Older Adults: An Analysis of Pooled Population-Based Studies. JAMA Cardiol. 2017;2:986–994. doi: 10.1001/jamacardio.2017.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao C.-T., Han D.-S., Huang J.-W. Circulating microRNA-125b Levels Are Associated with the Risk of Vascular Calcification in Healthy Community-Dwelling Older Adults. Front. Cardiovasc. Med. 2021;8:624313. doi: 10.3389/fcvm.2021.624313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miedema M.D., Dardari Z.A., Nasir K., Blankstein R., Knickelbine T., Oberembt S., Shaw L., Rumberger J., Michos E.D., Rozanski A., et al. Association of Coronary Artery Calcium with Long-term, Cause-Specific Mortality Among Young Adults. JAMA Netw. Open. 2019;2:e197440. doi: 10.1001/jamanetworkopen.2019.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X.-R., Zhang J.-J., Xu X.-X., Wu Y.-G. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: A systematic review and meta-analysis. Ren. Fail. 2019;41:244–256. doi: 10.1080/0886022X.2019.1595646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mencke R., van der Vaart A., Pasch A., Harms G., Waanders F., Bilo H.J.G., van Goor H., Hillebrands J.-L., van Dijk P.R. Serum calcification propensity is associated with HbA1c in type 2 diabetes mellitus. BMJ Open Diabetes Res. Care. 2021;9:e002016. doi: 10.1136/bmjdrc-2020-002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhry M., Sidhu M.S., Bangalore S., Mathew R.O. Accelerated and intensified calcific atherosclerosis and microvascular dysfunction in patients with chronic kidney disease. Rev. Cardiovasc. Med. 2020;21:157–162. doi: 10.31083/j.rcm.2020.02.99. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.H., Rizvi A., Hartaigh B.Ó., Han D., Park M.W., Roudsari H.M., Stuijfzand W.J., Gransar H., Lu Y., Callister T.Q., et al. The Predictive Value of Coronary Artery Calcium Scoring for Major Adverse Cardiac Events According to Renal Function (from the Coronary Computed Tomography Angiography Evaluation for Clinical Outcomes: An International Multicenter [CONFIRM] Registry) Am. J. Cardiol. 2019;123:1435–1442. doi: 10.1016/j.amjcard.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Eelderink C., Te Velde-Keyzer C.A., Frenay A.S., Vermeulen E.A., Bachtler M., Aghagolzadeh P., van Dijk P.R., Gansevoort R.T., Vervloet M.G., Hillebrands J.-A., et al. Serum Calcification Propensity and the Risk of Cardiovascular and All-Cause Mortality in the General Population: The PREVEND Study. Arterioscler. Thromb. Vasc. Biol. 2020;40:1942–1951. doi: 10.1161/ATVBAHA.120.314187. [DOI] [PubMed] [Google Scholar]
- 9.Cano-Megías M., Guisado-Vasco P., Bouarich H., de Arriba-de la Fuente G., De Sequera-Ortiz P., Álvarez-Sanz C., Rodríguez-Puyol D. Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: A prospective long-term follow-up study. BMC Nephrol. 2019;20:188. doi: 10.1186/s12882-019-1367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z., Jiang A., Wei F., Chen H. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: A meta-analysis. BMC Cardiovasc. Disord. 2018;18:12. doi: 10.1186/s12872-018-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S.-C., Teh M., Huang J.-C., Wu P.-Y., Chen C.-Y., Tsai Y.-C., Chiu Y.-W., Chang J.-M., Chen H.-C. Increased Aortic Arch Calcification and Cardiomegaly is Associated with Rapid Renal Progression and Increased Cardiovascular Mortality in Chronic Kidney Disease. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-41841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handy C.E., Desai C.S., Dardari Z.A., Al-Mallah M.H., Miedema M.D., Ouyang P., Budoff M.J., Blumenthal R.S., Nasir K., Blaha M.J. The Association of Coronary Artery Calcium with Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc. Imaging. 2016;9:568–576. doi: 10.1016/j.jcmg.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S., Chao C., Huang J., Huang K. Vascular Calcification as an Underrecognized Risk Factor for Frailty in 1783 Community-Dwelling Elderly Individuals. J. Am. Hear. Assoc. 2020;9 doi: 10.1161/JAHA.120.017308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao C.-T., Yeh H.-Y., Tsai Y.-T., Chuang P.-H., Yuan T.-H., Huang J.-W., Chen H.-W. Natural and non-natural antioxidative compounds: Potential candidates for treatment of vascular calcification. Cell Death Discov. 2019;5:145. doi: 10.1038/s41420-019-0225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou Y.-C., Lu C.-L., Yuan T.-H., Liao M.-T., Chao C.-T., Lu K.-C. The Epigenetic Landscape of Vascular Calcification: An Integrative Perspective. Int. J. Mol. Sci. 2020;21:980. doi: 10.3390/ijms21030980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai Y.-T., Yeh H.-Y., Chao C.-T., Chiang C.-K. Superoxide Dismutase 2 (SOD2) in Vascular Calcification: A Focus on Vascular Smooth Muscle Cells, Calcification Pathogenesis, and Therapeutic Strategies. Oxidative Med. Cell. Longev. 2021;2021:1–9. doi: 10.1155/2021/6675548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disthabanchong S., Srisuwarn P. Mechanisms of Vascular Calcification in Kidney Disease. Adv. Chronic Kidney Dis. 2019;26:417–426. doi: 10.1053/j.ackd.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Chao C., Yeh H., Yuan T., Chiang C., Chen H. MicroRNA-125b in vascular diseases: An updated systematic review of pathogenetic implications and clinical applications. J. Cell. Mol. Med. 2019;23:5884–5894. doi: 10.1111/jcmm.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Zhao X., Wu H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler. Thromb. Vasc. Biol. 2020;40:1078–1093. doi: 10.1161/ATVBAHA.120.313131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calabrò P., Niccoli G., Gragnano F., Grove E.L., Vergallo R., Mikhailidis D.P., Patti G., Spaccarotella C., Katsiki N., Masiero G., et al. Are we ready for a gender-specific approach in interventional cardiology? Int. J. Cardiol. 2019;286:226–233. doi: 10.1016/j.ijcard.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Tani S., Matsuo R., Imatake K., Suzuki Y., Yagi T., Takahashi A., Matsumoto N., Okumura Y. Gender differences in the associations among fish intake, lifestyle, and non-HDL-C level in Japanese subjects over the age of 50 years: Anti-atherosclerotic effect of fish consumption. Nutr. Metab. Cardiovasc. Dis. 2021;31:1434–1444. doi: 10.1016/j.numecd.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Bairey Merz C.N., Shaw L.J., Reis S.E., Bittner V., Kelsey S.F., Olson M., Johnson B.D., Pepine C.J., Mankad S., Sharaf B.L., et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J. Am. Coll. Cardiol. 2006;47(Suppl. 3):S21–S29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 23.Tao X.-Y., Zuo A.-Z., Wang J.-Q., Tao F.-B. Effect of primary ovarian insufficiency and early natural menopause on mortality: A meta-analysis. Climacteric. 2016;19:27–36. doi: 10.3109/13697137.2015.1094784. [DOI] [PubMed] [Google Scholar]
- 24.Kim B.S., Chan N., Hsu G., Makaryus A.N., Chopra M., Cohen S.L., Makaryus J.N. Sex Differences in Coronary Arterial Calcification in Symptomatic Patients. Am. J. Cardiol. 2021;149:16–20. doi: 10.1016/j.amjcard.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Inker L.A., Astor B.C., Fox C.H., Isakova T., Lash J.P., Peralta C.A., Tamura M.K., Feldman H.I. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S., Neill K.D.O., Hood A.F., Evan A.P., Moe S.M. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am. J. Kidney Dis. 2001;37:1267–1276. doi: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 27.Alayoud A., El Amrani M., Belarbi M., El Kharras A., Chtioui M., Elfilali K. Facteurs de risque de progression des calcifications des artères coronaires après 5 ans d’évolution en dialyse. Ann. Cardiol. d’Angéiol. 2020;69:81–85. doi: 10.1016/j.ancard.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Al-Rifai R., Arabi A., Masrouji R., Daouk M. Prevalence of peripheral vascular calcifications in patients on chronic hemodialysis at a tertiary care center in Beirut: A pilot study. Leban. Med. J. 2011;59:117–121. [PubMed] [Google Scholar]
- 29.Asci G., Ok E., Savas R., Ozkahya M., Duman S., Töz H., Kayikcioglu M., Branscum A.J., Monier-Faugere M.-C., Herberth J., et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol. Dial. Transplant. 2011;26:1010–1015. doi: 10.1093/ndt/gfq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avramovski P., Avramovska M., Sotiroski K., Sikole A. Acute-phase proteins as promoters of abdominal aortic calcification in chronic dialysis patients. Saudi J. Kidney Dis. Transplant. 2019;30:376–386. doi: 10.4103/1319-2442.256845. [DOI] [PubMed] [Google Scholar]
- 31.Bae E., Seong E.Y., Han B.-G., Kim D.K., Lim C.S., Kang S.-W., Park C.W., Kim C.-D., Shin B.C., Kim S.G., et al. Coronary artery calcification in Korean patients with incident dialysis. Hemodial. Int. 2017;21:367–374. doi: 10.1111/hdi.12493. [DOI] [PubMed] [Google Scholar]
- 32.Ballotta E., Renon L., Toffano M., Piccoli A., Da Giau G. Patency and limb salvage rates after distal revascularization to unclampable calcified outflow arteries. J. Vasc. Surg. 2004;39:539–546. doi: 10.1016/j.jvs.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 33.Bellasi A., Block G.A., Ferramosca E., Ratti C., Raggi P. Integration of clinical and imaging data to predict death in hemodialysis patients. Hemodial. Int. 2013;17:12–18. doi: 10.1111/j.1542-4758.2012.00709.x. [DOI] [PubMed] [Google Scholar]
- 34.Bundy J.D., Cai X., Mehta R.C., Scialla J.J., De Boer I.H., Hsu C.-Y., Go A.S., Dobre M.A., Chen J., Rao P.S., et al. Serum Calcification Propensity and Clinical Events in CKD. Clin. J. Am. Soc. Nephrol. 2019;14:1562–1571. doi: 10.2215/CJN.04710419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao N., Chandra A., Raj G., Awasthi N.P., Srivastava D. Evaluation of the relationship between blood cell parameters and vascular calcification in dialysis-dependent end-stage renal disease patients. Saudi J. Kidney Dis. Transplant. 2020;31:136–143. doi: 10.4103/1319-2442.279933. [DOI] [PubMed] [Google Scholar]
- 36.Chang J.H., Ro H., Kim S., Lee H.H., Chung W., Jung J.Y. Study on the relationship between serum 25-hydroxyvitamin D levels and vascular calcification in hemodialysis patients with consideration of seasonal variation in vitamin D levels. Atherosclerosis. 2012;220:563–568. doi: 10.1016/j.atherosclerosis.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 37.Chao C.-T., Yeh H.-Y., Tsai Y.-T., Chiang C.-K., Chen H.-W. A combined microRNA and target protein-based panel for predicting the probability and severity of uraemic vascular calcification: A translational study. Cardiovasc. Res. 2021;117:1958–1973. doi: 10.1093/cvr/cvaa255. [DOI] [PubMed] [Google Scholar]
- 38.Chao C.-T., Liu Y.-P., Su S.-F., Yeh H.-Y., Chen H.-Y., Lee P.-J., Chen W.-J., Lee Y.-M., Huang J.-W., Chiang C.-K., et al. Circulating MicroRNA-125b Predicts the Presence and Progression of Uremic Vascular Calcification. Arter. Thromb. Vasc. Biol. 2017;37:1402–1414. doi: 10.1161/ATVBAHA.117.309566. [DOI] [PubMed] [Google Scholar]
- 39.Charitaki E., Davenport A. Aortic pulse wave velocity in haemodialysis patients is associated with the prescription of active vitamin D analogues. J. Nephrol. 2014;27:431–437. doi: 10.1007/s40620-014-0040-9. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Qureshi A.R., Parini P., Hurt-Camejo E., Ripsweden J., Brismar T., Barany P., Jaminon A.M., Schurgers L., Heimbürger O., et al. Does statins promote vascular calcification in chronic kidney disease? Eur. J. Clin. Investig. 2017;47:137–148. doi: 10.1111/eci.12718. [DOI] [PubMed] [Google Scholar]
- 41.Chen J., Budoff M.J., Reilly M., Yang W., Rosas S.E., Rahman M., Zhang X., Roy J.A., Lustigova E., Nessel L., et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients with Chronic Kidney Disease. JAMA Cardiol. 2017;2:635–643. doi: 10.1001/jamacardio.2017.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiu Y.-W., Adler S.G., Budoff M.J., Takasu J., Ashai J., Mehrotra R. Coronary artery calcification and mortality in diabetic patients with proteinuria. Kidney Int. 2010;77:1107–1114. doi: 10.1038/ki.2010.70. [DOI] [PubMed] [Google Scholar]
- 43.Choi S.R., Lee Y.-K., Cho A.J., Park H.C., Han C.H., Choi M.-J., Koo J.-R., Yoon J.-W., Noh J.W. Malnutrition, inflammation, progression of vascular calcification and survival: Inter-relationships in hemodialysis patients. PLoS ONE. 2019;14:e0216415. doi: 10.1371/journal.pone.0216415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chue C.D., Wall N.A., Crabtree N.J., Zehnder D., Moody W.E., Edwards N.C., Steeds R., Townend J.N., Ferro C.J. Aortic Calcification and Femoral Bone Density Are Independently Associated with Left Ventricular Mass in Patients with Chronic Kidney Disease. PLoS ONE. 2012;7:e39241. doi: 10.1371/journal.pone.0039241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Claes K.J., Heye S., Bammens B., Kuypers D.R., Meijers B., Naesens M., Vanrenterghem Y., Evenepoel P. Aortic calcifications and arterial stiffness as predictors of cardiovascular events in incident renal transplant recipients. Transpl. Int. 2013;26:973–981. doi: 10.1111/tri.12151. [DOI] [PubMed] [Google Scholar]
- 46.Coen G., Manni M., Agnoli A., Balducci A., Dessi M., De Angelis S., Jankovic L., Mantella D., Morosetti M., Naticchia A., et al. Cardiac Calcifications: Fetuin???A and Other Risk Factors in Hemodialysis Patients. ASAIO J. 2006;52:150–156. doi: 10.1097/01.mat.0000202606.44826.6b. [DOI] [PubMed] [Google Scholar]
- 47.Coll B., Betriu A., Martinez-Alonso M., Amoedo M.L., Arcidiacono M.V., Borras M., Valdivielso J.M., Fernández E. Large Artery Calcification on Dialysis Patients Is Located in the Intima and Related to Atherosclerosis. Clin. J. Am. Soc. Nephrol. 2011;6:303–310. doi: 10.2215/CJN.04290510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Craver L., Dusso A., Martinez-Alonso M., Sarro F., Valdivielso J.M., Fernández E. A low fractional excretion of Phosphate/Fgf23 ratio is associated with severe abdominal Aortic calcification in stage 3 and 4 kidney disease patients. BMC Nephrol. 2013;14:1–221. doi: 10.1186/1471-2369-14-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis B., Marin D., Hurwitz L.M., Ronald J., Ellis M.J., Ravindra K.V., Collins B.H., Kim C.Y. Application of a Novel CT-Based Iliac Artery Calcification Scoring System for Predicting Renal Transplant Outcomes. Am. J. Roentgenol. 2016;206:436–441. doi: 10.2214/AJR.15.14794. [DOI] [PubMed] [Google Scholar]
- 50.Deloach S.S., Joffe M.M., Mai X., Goral S., Rosas S.E. Aortic calcification predicts cardiovascular events and all-cause mortality in renal transplantation. Nephrol. Dial. Transplant. 2009;24:1314–1319. doi: 10.1093/ndt/gfn753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Iorio B.R., Bortone S., Piscopo C., Grimaldi P., Cucciniello E., D’Avanzo E., Ernesto A., Fiorentino M., Nicola C., Vincenzoet B. Cardiac Vascular Calcification and QT Interval in ESRD Patients: Is There a Link? Blood Purificat. 2006;24:451–459. doi: 10.1159/000095362. [DOI] [PubMed] [Google Scholar]
- 52.Disthabanchong S., Vipattawat K., Phakdeekitcharoen B., Kitiyakara C., Sumethkul V. Abdominal aorta and pelvic artery calcifications on plain radiographs may predict mortality in chronic kidney disease, hemodialysis and renal transplantation. Int. Urol. Nephrol. 2018;50:355–364. doi: 10.1007/s11255-017-1758-9. [DOI] [PubMed] [Google Scholar]
- 53.El Amrani M., Maoujoud O., Belarbi M., El Farouki M.R., Zajjari Y., Boukili Y., Bouzelmate H., Rbaibi A., El Kharras A., Salahedine T., et al. Dépistage et facteurs de risque des calcifications cardiaques chez l’hémodialysé: Apport du scanner multi-coupe ultra-rapide et de l’échocardiographie transthoracique. Ann. Cardiol. d’Angéiol. 2015;64:87–93. doi: 10.1016/j.ancard.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Etta P.K., Sharma R.K., Gupta A. Study of chronic kidney disease-mineral bone disorders in newly detected advanced renal failure patients: A Hospital-based cross-sectional study. Saudi J. Kidney Dis. Transplant. 2017;28:874–885. [PubMed] [Google Scholar]
- 55.Fabbian F., Catalano C., Orlandi V., Conte M.M., Lupo A., Catizone L. Evaluation of aortic arch calcification in hemodialysis patients. J. Nephrol. 2005;18:289–293. [PubMed] [Google Scholar]
- 56.Fayed A., Soliman A., El Mahdy H., Hamza W., Abdulazim D.O., Salem M.M., El Din U.A.S., on behalf of the Vascular Calcification Group (VCG) Intraoperative Arterial Biopsy in Incident Hemodialysis Patients: Differences Observed. Nephron. 2019;143:54–61. doi: 10.1159/000500486. [DOI] [PubMed] [Google Scholar]
- 57.Gunen Yilmaz S., Yilmaz F., Bayrakdar I., Harorli A. The Relationship between carotid artery calcification and pulp stone among hemodialysis patients: A retrospective study. Saudi J. Kidney Dis. Transplant. 2019;30:755–763. doi: 10.4103/1319-2442.265449. [DOI] [PubMed] [Google Scholar]
- 58.Harada P.H.N., Canziani M.E.F., Lima L.M., Kamimura M.A., Rochitte C.E., Lemos M.M., Cuppari L., Filho R.K., Draibe S.A., Santos R.D. Pericardial Fat Is Associated with Coronary Artery Calcification in Non-Dialysis Dependent Chronic Kidney Disease Patients. PLoS ONE. 2014;9:e114358. doi: 10.1371/journal.pone.0114358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He L., He W.-Y., La-Ta A., Yang W.-L., Zhang A.-H. Lower Serum Irisin Levels Are Associated with Increased Vascular Calcification in Hemodialysis Patients. Kidney Blood Press. Res. 2018;43:287–295. doi: 10.1159/000487689. [DOI] [PubMed] [Google Scholar]
- 60.He J., Reilly M., Yang W., Chen J., Go A.S., Lash J.P., Rahman M., DeFilippi C., Gadegbeku C., Kanthety R., et al. Risk Factors for Coronary Artery Calcium Among Patients with Chronic Kidney Disease (from the Chronic Renal Insufficiency Cohort Study) Am. J. Cardiol. 2012;110:1735–1741. doi: 10.1016/j.amjcard.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou J.-S., Lin Y.-L., Wang C.-H., Lai Y.-H., Kuo C.-H., Subeq Y.-M., Hsu B.-G. Serum osteoprotegerin is an independent marker of central arterial stiffness as assessed using carotid–femoral pulse wave velocity in hemodialysis patients: A cross sectional study. BMC Nephrol. 2019;20:184. doi: 10.1186/s12882-019-1374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al Humoud H., Al-Hilali N., Ahmad A.A.M.H., Ninan V.T., Nampoory M.R.N., Rizk A.M., Ali J.H., Johny K.V. Vascular Calcification in Dialysis Patients. Transplant. Proc. 2005;37:4183–4186. doi: 10.1016/j.transproceed.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 63.Jankovic A., Damjanovic T., Djuric Z., Marinkovic J., Schlieper G., Djuric P., Dragovic J.T., Bulatovic A., Mitrovic M., Popovic J., et al. Calcification in arteriovenous fistula blood vessels may predict arteriovenous fistula failure: A 5-year follow-up study. Int. Urol. Nephrol. 2017;49:881–887. doi: 10.1007/s11255-017-1515-0. [DOI] [PubMed] [Google Scholar]
- 64.Janković A., Damjanovic T., Djuric Z., Marinkovic J., Schlieper G., Tosic-Dragovic J., Djuric P., Popovic J., Floege J., Dimkovic N. Impact of Vascular Calcifications on Arteriovenous Fistula Survival in Hemodialysis Patients: A Five-Year Follow-Up. Nephron. 2015;129:247–252. doi: 10.1159/000380823. [DOI] [PubMed] [Google Scholar]
- 65.Jansson H., Saeed A., Svensson M.K., Finnved K., Hellström M., Guron G. Impact of Abdominal Aortic Calcification on Central Haemodynamics and Decline of Glomerular Filtration Rate in Patients with Chronic Kidney Disease Stages 3 and 4. Kidney Blood Press. Res. 2019;44:950–960. doi: 10.1159/000501687. [DOI] [PubMed] [Google Scholar]
- 66.Jean G., Chazot C., Bresson E., Zaoui E., Cavalier E. High Serum Sclerostin Levels Are Associated with a Better Outcome in Haemodialysis Patients. Nephron. 2016;132:181–190. doi: 10.1159/000443845. [DOI] [PubMed] [Google Scholar]
- 67.Jean G., Bresson E., Terrat J.-C., Vanel T., Hurot J.-M., Lorriaux C., Mayor B., Chazot C. Peripheral vascular calcification in long-haemodialysis patients: Associated factors and survival consequences. Nephrol. Dial. Transplant. 2009;24:948–955. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 68.Jean G., Bresson É., Lorriaux C., Mayor B., Hurot J.-M., Deleaval P., Chazot C. Increased Levels of Serum Parathyroid Hormone and Fibroblast Growth Factor-23 Are the Main Factors Associated with the Progression of Vascular Calcification in Long-Hour Hemodialysis Patients. Nephron. 2012;120:c132–c138. doi: 10.1159/000334424. [DOI] [PubMed] [Google Scholar]
- 69.Jiménez Villodres M., García Gutiérrez G., García Frías P., Rioja Villodres J., Martín Velázquez M., Sánchez Chaparro M.Á., López C.P., Valdivielso P. Fractional excretion of phosphorus and vascular calcification in stage 3 chronic kidney disease. J. Investig. Med. 2019;67:674. doi: 10.1136/jim-2018-000852. [DOI] [PubMed] [Google Scholar]
- 70.Kahn J., Ram L.M., Eberhard K., Müller H., Groselj-Strele A., Obermayer-Pietsch B. Calcification score evaluation in patients listed for renal transplantation. Clin. Transplant. 2017;31:e12888. doi: 10.1111/ctr.12888. [DOI] [PubMed] [Google Scholar]
- 71.Keyzer C.A., De Borst M., Berg E.V.D., Jahnen-Dechent W., Arampatzis S., Farese S., Bergmann I.P., Floege J., Navis G., Bakker S.J., et al. Calcification Propensity and Survival among Renal Transplant Recipients. J. Am. Soc. Nephrol. 2016;27:239–248. doi: 10.1681/ASN.2014070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim S.M., Jung I.M., Kim D., Lee J.P., So Y.H. Effect of Inflow Arterial Calcification on Arteriovenous Fistula Maturation. Ann. Vasc. Surg. 2019;58:331–337. doi: 10.1016/j.avsg.2018.10.057. [DOI] [PubMed] [Google Scholar]
- 73.Kim H.G., Song S.W., Kim T.Y., Kim Y.O. Risk factors for progression of aortic arch calcification in patients on maintenance hemodialysis and peritoneal dialysis. Hemodial. Int. 2011;15:460–467. doi: 10.1111/j.1542-4758.2011.00571.x. [DOI] [PubMed] [Google Scholar]
- 74.Kimura K., Saika Y., Otani H., Fujii R., Mune M., Yukawa S. Factors associated with calcification of the abdominal aorta in hemodialysis patients. Kidney Int. 1999;56:S238–S241. doi: 10.1046/j.1523-1755.1999.07163.x. [DOI] [PubMed] [Google Scholar]
- 75.Komatsu M., Okazaki M., Tsuchiya K., Kawaguchi H., Nitta K. Aortic Arch Calcification Predicts Cardiovascular and All-Cause Mortality in Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2014;39:658–667. doi: 10.1159/000368476. [DOI] [PubMed] [Google Scholar]
- 76.Lee C.-T., Huang C.-C., Hsu C.-Y., Chiou T.T.-Y., Ng H.-Y., Wu C.-H., Kuo W.-H., Lee Y.-T. Calcification of the Aortic Arch Predicts Cardiovascular and All-Cause Mortality in Chronic Hemodialysis Patients. Cardiorenal Med. 2014;4:34–42. doi: 10.1159/000360230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee C.-T., Lee Y.-T., Tain Y.-L., Ng H.-Y., Kuo W.-H. Circulating microRNAs and vascular calcification in hemodialysis patients. J. Int. Med. Res. 2019;47:2929–2939. doi: 10.1177/0300060519848949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee M.J., Park J.T., Park K.S., Kwon Y.E., Han S.H., Kang S.-W., Choi K.H., Oh K.-H., Park S.K., Chae D.W., et al. Normal body mass index with central obesity has increased risk of coronary artery calcification in Korean patients with chronic kidney disease. Kidney Int. 2016;90:1368–1376. doi: 10.1016/j.kint.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 79.Lioufas N.M., Pedagogos E., Hawley C.M., Pascoe E.M., Elder G.J., Badve S., Valks A., Toussaint N.D., on behalf of the IMPROVE-CKD Investigators Aortic Calcification and Arterial Stiffness Burden in a Chronic Kidney Disease Cohort with High Cardiovascular Risk: Baseline Characteristics of the Impact of Phosphate Reduction On Vascular End-Points in Chronic Kidney Disease Trial. Am. J. Nephrol. 2020;51:201–215. doi: 10.1159/000505717. [DOI] [PubMed] [Google Scholar]
- 80.Liu J., Zhang L., Zhou Y., Zhu D., Wang Q., Hao L. Aberrant activation of Wnt pathways in arteries associates with vascular calcification in chronic kidney disease. Int. Urol. Nephrol. 2016;48:1313–1319. doi: 10.1007/s11255-016-1291-2. [DOI] [PubMed] [Google Scholar]
- 81.Lockhart M.E., Robbin M.L., McNamara M.M., Allon M. Association of pelvic arterial calcification with arteriovenous thigh graft failure in haemodialysis patients. Nephrol. Dial. Transplant. 2004;19:2564–2569. doi: 10.1093/ndt/gfh414. [DOI] [PubMed] [Google Scholar]
- 82.London G.M., Pannier B., Marchais S.J. Vascular Calcifications, Arterial Aging and Arterial Remodeling in ESRD. Blood Purif. 2013;35:16–21. doi: 10.1159/000345172. [DOI] [PubMed] [Google Scholar]
- 83.Maharem D.A., Gomaa S.H., El Ghandor M.K., Mohamed E.I., Matrawy K.A., Zaytoun S.S., Nomeir H.M. Association of serum fetuin-A and fetuin-A gene polymorphism in relation to mineral and bone disorders in patients with chronic kidney disease. Egypt. J. Med. Hum. Genet. 2013;14:337–352. doi: 10.1016/j.ejmhg.2013.07.003. [DOI] [Google Scholar]
- 84.Mazzaferro S., Pasquali M., Pugliese F., Barresi G., Carbone I., Francone M., Sardella D., Taggi F. Serum Levels of Calcification Inhibition Proteins and Coronary Artery Calcium Score: Comparison between Transplantation and Dialysis. Am. J. Nephrol. 2007;27:75–83. doi: 10.1159/000099095. [DOI] [PubMed] [Google Scholar]
- 85.Merjanian R., Budoff M., Adler S., Berman N., Mehrotra R. Coronary artery, aortic wall, and valvular calcification in nondialyzed individuals with type 2 diabetes and renal disease. Kidney Int. 2003;64:263–271. doi: 10.1046/j.1523-1755.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 86.Miyatake N., Adachi H., Nomura-Nakayama K., Okada K., Okino K., Hayashi N., Fujimoto K., Furuichi K., Yokoyama H. Circulating CTRP9 correlates with the prevention of aortic calcification in renal allograft recipients. PLoS ONE. 2020;15:e0226526. doi: 10.1371/journal.pone.0226526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mizuiri S., Nishizawa Y., Yamashita K., Mizuno K., Ishine M., Doi S., Masaki T., Shigemoto K. Coronary artery calcification score and common iliac artery calcification score in non-dialysis CKD patients. Nephrology. 2018;23:837–845. doi: 10.1111/nep.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morena M., Dupuy A.-M., Jaussent I., Vernhet H., Gahide G., Klouche K., Bargnoux A.-S., Delcourt C., Canaud B., Cristol J.-P. A cut-off value of plasma osteoprotegerin level may predict the presence of coronary artery calcifications in chronic kidney disease patients. Nephrol. Dial. Transplant. 2009;24:3389–3397. doi: 10.1093/ndt/gfp301. [DOI] [PubMed] [Google Scholar]
- 89.Munguía P., Caramelo R., Rubio M., Sahdalá L., Arnaudas L., Paul J., Blasco A., Lou L., Aladren M., Sanjuan A., et al. Pre-Transplant Assessment of Vascular Calcification as a Risk Factor of Mortality, Graft Loss, and Cardiovascular Events in Renal Transplant Recipients. Transplant. Proc. 2015;47:2368–2370. doi: 10.1016/j.transproceed.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 90.Nitta K., Hanafusa N., Okazaki M., Komatsu M., Kawaguchi H., Tsuchiya K. Association Between Risk Factors Including Bone-Derived Biomarkers and Aortic Arch Calcification in Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2018;43:1554–1562. doi: 10.1159/000494441. [DOI] [PubMed] [Google Scholar]
- 91.Niu Q., Zhao H., Wu B., Tsai S., Wu J., Zhang M., Lu L., Qiao J., Men C., Zuo L., et al. Abdominal aortic calcification is superior to other arteries calcification in predicting the mortality in peritoneal dialysis patients—A 8 years cohort study. BMC Nephrol. 2019;20:1–13. doi: 10.1186/s12882-019-1593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niu Q., Yang S., Gan L., Zhao H., Zuo L. Different type and dosage of heparin were not associated with the progression of coronary artery calcification in haemodialysis patients. Nephrol. 2020;25:551–558. doi: 10.1111/nep.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okamoto T., Hatakeyama S., Kodama H., Horiguchi H., Kubota Y., Kido K., Momota M., Hosogoe S., Tanaka Y., Takashima T., et al. The relationship between poor nutritional status and progression of aortic calcification in patients on maintenance hemodialysis. BMC Nephrol. 2018;19:71. doi: 10.1186/s12882-018-0872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrovic M., Baralic M., Brkovic V., Arsenovic A., Stojanov V., Lalic N., Stanisavljevic D., Jankovic A., Radivojevic N., Pejanovic S., et al. Significance of acPWV for Survival of Hemodialysis Patients. Medicina. 2020;56:435. doi: 10.3390/medicina56090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qureshi A.R., Olauson H., Witasp A., Haarhaus M.L., Brandenburg V., Wernerson A., Lindholm B., Söderberg M., Wennberg L., Nordfors L., et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015;88:1356–1364. doi: 10.1038/ki.2015.194. [DOI] [PubMed] [Google Scholar]
- 96.Raggi P., Bellasi A., Gamboa C., Ferramosca E., Ratti C., Block G.A., Muntner P. All-cause Mortality in Hemodialysis Patients with Heart Valve Calcification. Clin. J. Am. Soc. Nephrol. 2011;6:1990–1995. doi: 10.2215/CJN.01140211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Renaud H., Atik A., Hervé M., Morinière P., Hocine C., Belbrik S., Fournier A. Evaluation of Vascular Calcinosis Risk Factors in Patients on Chronic Hemodialysis: Lack of Influence of Calcium Carbonate. Nephron. 1988;48:28–32. doi: 10.1159/000184864. [DOI] [PubMed] [Google Scholar]
- 98.Ribeiro S., Ramos A., Brandão A., Rebelo J.R., Guerra A., Resina C., Vila-Lobos A., Carvalho F., Remédio F., Ribeiro F. Cardiac valve calcification in haemodialysis patients: Role of calcium-phosphate metabolism. Nephrol. Dial. Transplant. 1998;13:2037–2040. doi: 10.1093/ndt/13.8.2037. [DOI] [PubMed] [Google Scholar]
- 99.Roca-Tey R., Páez R., Rivas A., Samon R., Ibrik O., Giménez I., Viladoms J. Prevalence and functional effect of arteriovenous fistula calcifications, evaluated by spiral CT in chronic haemodialysis patients. Nefrología. 2009;29:214–221. doi: 10.3265/NEFROLOGIA.2009.29.3.5103.EN.FULL. [DOI] [PubMed] [Google Scholar]
- 100.Schlieper G., Krüger T., Djuric Z., Damjanovic T., Markovic N., Schurgers L., Brandenburg V.M., Westenfeld R., Dimkovic S., Ketteler M., et al. Vascular access calcification predicts mortality in hemodialysis patients. Kidney Int. 2008;74:1582–1587. doi: 10.1038/ki.2008.458. [DOI] [PubMed] [Google Scholar]
- 101.Shu K.-H., Tsai I.-C., Ho H.-C., Wu M.-J., Chen C.-H., Yu T.-M., Chuang Y.-W., Huang S.-T. Coronary Artery Calcification in Kidney Transplant Recipients with Long-term Follow-up. Transplant. Proc. 2012;44:687–690. doi: 10.1016/j.transproceed.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 102.Sigrist M.K., Taal M.W., Bungay P., McIntyre C.W. Progressive Vascular Calcification over 2 Years Is Associated with Arterial Stiffening and Increased Mortality in Patients with Stages 4 and 5 Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 103.Stróżecki P., Odrowąż-Sypniewska G., Manitius J. Cardiac Valve Calcifications and Left Ventricular Hypertrophy in Hemodialysis Patients. Renal Fail. 2005;27:733–738. doi: 10.1080/08860220500243296. [DOI] [PubMed] [Google Scholar]
- 104.Tangvoraphonkchai K., Davenport A. Reduction in Aortic Pulse Wave Velocity Is Associated with a Short-Term Reduction in Dual-Energy X-Ray Absorptiometry Lumbar Spine Bone Mineral Density T Score. Blood Purif. 2019;48:346–350. doi: 10.1159/000501392. [DOI] [PubMed] [Google Scholar]
- 105.Tomiyama C., Carvalho A.B., Higa A., Jorgetti V., Draibe S.A., Canziani M.E.F. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J. Bone Miner. Res. 2010;25:499–504. doi: 10.1359/jbmr.090735. [DOI] [PubMed] [Google Scholar]
- 106.Turan M.N., Kircelli F., Yaprak M., Sisman A.R., Gungor O., Bayraktaroğlu S., Ozkahya M., Asci G., Floege J., Ok E. FGF-23 levels are associated with vascular calcification, but not with atherosclerosis, in hemodialysis patients. Int. Urol. Nephrol. 2016;48:609–617. doi: 10.1007/s11255-016-1231-1. [DOI] [PubMed] [Google Scholar]
- 107.Wang A.Y.-M., Wong C.-K., Yau Y.-Y., Wong S., Chan I.H.-S., Lam C.W.-K. Skin Autofluorescence Associates with Vascular Calcification in Chronic Kidney Disease. Arter. Thromb. Vasc. Biol. 2014;34:1784–1790. doi: 10.1161/ATVBAHA.114.303378. [DOI] [PubMed] [Google Scholar]
- 108.Wang A.Y.-M., Wang M., Woo J., Lam C.W.-K., Li P.K.-T., Lui S.-F., Sanderson J.E. Cardiac Valve Calcification as an Important Predictor for All-Cause Mortality and Cardiovascular Mortality in Long-Term Peritoneal Dialysis Patients: A Prospective Study. J. Am. Soc. Nephrol. 2003;14:159–168. doi: 10.1097/01.ASN.0000038685.95946.83. [DOI] [PubMed] [Google Scholar]
- 109.Wu C.-F., Lee Y.-F., Lee W.-J., Su C.-T., Lee L.J.-H., Wu K.-D., Chen P.-C., Kao T.-W. Severe aortic arch calcification predicts mortality in patients undergoing peritoneal dialysis. J. Formos. Med. Assoc. 2017;116:366–372. doi: 10.1016/j.jfma.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 110.Yoshikawa K., Abe H., Tominaga T., Nakamura M., Kishi S., Matsuura M., Nagai K., Tsuchida K., Minakuchi J., Doi T. Polymorphism in the human matrix Gla protein gene is associated with the progression of vascular calcification in maintenance hemodialysis patients. Clin. Exp. Nephrol. 2013;17:882–889. doi: 10.1007/s10157-013-0785-9. [DOI] [PubMed] [Google Scholar]
- 111.Zhou Y., Hellberg M., Kouidi E., Deligiannis A., Höglund P., Clyne N. Relationships between abdominal aortic calcification, glomerular filtration rate, and cardiovascular risk factors in patients with non-dialysis dependent chronic kidney disease. Clin. Nephrol. 2018;90:380–389. doi: 10.5414/CN109441. [DOI] [PubMed] [Google Scholar]
- 112.Abd alamir M., Radulescu V., Goyfman M., Mohler E.R., III, Gao Y.L., Budoff M.J. Prevalence and correlates of mitral annular calcification in adults with chronic kidney disease: Results from CRIC study. Atherosclerosis. 2015;242:117–122. doi: 10.1016/j.atherosclerosis.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adragao T., Pires A., Lucas C., Birne R., Magalhaes L., Gonçalves M., Negrao A.P. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol. Dial. Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 114.Bundy J.D., Cai X., Scialla J., Dobre M.A., Chen J., Hsu C.-Y., Leonard M.B., Go A.S., Rao P.S., Lash J.P., et al. Serum Calcification Propensity and Coronary Artery Calcification Among Patients with CKD: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2019;73:806–814. doi: 10.1053/j.ajkd.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chae S.Y., Chung W., Kim Y.H., Oh Y.K., Lee J., Choi K.H., Ahn C., Kim Y.-S. The Correlation of Serum Osteoprotegerin with Non-Traditional Cardiovascular Risk Factors and Arterial Stiffness in Patients with Pre-Dialysis Chronic Kidney Disease: Results from the KNOW-CKD Study. J. Korean Med. Sci. 2018;33:e322. doi: 10.3346/jkms.2018.33.e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dai L., Debowska M., Lukaszuk T., Bobrowski L., Barany P., Söderberg M., Thiagarajan D., Frostegård J., Wennberg L., Lindholm B., et al. Phenotypic features of vascular calcification in chronic kidney disease. J. Intern. Med. 2020;287:422–434. doi: 10.1111/joim.13012. [DOI] [PubMed] [Google Scholar]
- 117.Floege J., Raggi P., Block G.A., Torres P.U., Csiky B., Naso A., Nossuli K., Moustafa M., Goodman W.G., Lopez N., et al. Study design and subject baseline characteristics in the ADVANCE Study: Effects of cinacalcet on vascular calcification in haemodialysis patients. Nephrol. Dial. Transplant. 2010;25:1916–1923. doi: 10.1093/ndt/gfp762. [DOI] [PubMed] [Google Scholar]
- 118.Gelev S., Spasovski G., Trajkovski Z., Damjanovski G., Amitov V., Selim G., Dzekova P., Sikole A. Factors associated with various arterial calcifications in haemodialysis patients. Prilozi. 2008;29:185–199. [PubMed] [Google Scholar]
- 119.Kestenbaum B.R., Adeney K.L., de Boer I.H., Ix J.H., Shlipak M.G., Siscovick D.S. Incidence and progression of coronary calcification in chronic kidney disease: The Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009;76:991–998. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maia P.R.L., Medeiros A.M.C., Pereira H.S., Lima K.C., Oliveira P.T. Presence and associated factors of carotid artery calcification detected by digital panoramic radiography in patients with chronic kidney disease undergoing hemodialysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018;126:198–204. doi: 10.1016/j.oooo.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 121.Maréchal C., Coche E., Goffin E., Dragean A., Schlieper G., Nguyen P., Floege J., Kanaan N., Devuyst O., Jadoul M. Progression of Coronary Artery Calcification and Thoracic Aorta Calcification in Kidney Transplant Recipients. Am. J. Kidney Dis. 2012;59:258–269. doi: 10.1053/j.ajkd.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 122.Moldovan D., Moldovan I., Rusu C., Racasan S., Patiu I.M., Brumboiu A., Bondor C.I., Parvu L., Kacso I., Orasan R., et al. Vascular calcifications and renal osteodystrophy in chronic hemodialysis patients: What is the relationship between them? Int. Urol. Nephrol. 2011;43:1179–1186. doi: 10.1007/s11255-010-9841-5. [DOI] [PubMed] [Google Scholar]
- 123.Muntner P., Ferramosca E., Bellasi A., Block G.A., Raggi P. Development of a cardiovascular calcification index using simple imaging tools in haemodialysis patients. Nephrol. Dial. Transplant. 2007;22:508–514. doi: 10.1093/ndt/gfl609. [DOI] [PubMed] [Google Scholar]
- 124.Porter C.J., Stavroulopoulos A., Roe S.D., Pointon K., Cassidy M.J. Detection of coronary and peripheral artery calcification in patients with chronic kidney disease stages 3 and 4, with and without diabetes. Nephrol. Dial. Transplant. 2007;22:3208–3213. doi: 10.1093/ndt/gfm377. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y., Miao Y., Gong K., Cheng X., Chen Y., Zhao M.-H. Plasma Complement Protein C3a Level Was Associated with Abdominal Aortic Calcification in Patients on Hemodialysis. J. Cardiovasc. Transl. Res. 2019;12:496–505. doi: 10.1007/s12265-019-09885-2. [DOI] [PubMed] [Google Scholar]
- 126.Wang M., Li H., You L., Yu X., Zhang M., Zhu R., Hao C., Zhang Z., Chen J. Association of Serum Phosphorus Variability with Coronary Artery Calcification among Hemodialysis Patients. PLoS ONE. 2014;9:e93360. doi: 10.1371/journal.pone.0093360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bellasi A., Veledar E., Ferramosca E., Ratti C., Block G., Raggi P. Markers of vascular disease do not differ in black and white hemodialysis patients despite a different risk profile. Atheroscler. 2008;197:242–249. doi: 10.1016/j.atherosclerosis.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 128.Budoff M.J., Rader D.J., Reilly M., Mohler E.R., Lash J., Yang W., Rosen L., Glenn M., Teal V., Feldman H.I. Relationship of Estimated GFR and Coronary Artery Calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2011;58:519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai H., Lu R., Zhang M., Pang H., Zhu M., Zhang W., Ni Z., Qian J., Yan Y. Serum Soluble Klotho Level Is Associated with Abdominal Aortic Calcification in Patients on Maintenance Hemodialysis. Blood Purif. 2015;40:120–126. doi: 10.1159/000381937. [DOI] [PubMed] [Google Scholar]
- 130.Evenepoel P., Goffin E., Meijers B., Kanaan N., Bammens B., Coche E., Claes K., Jadoul M. Sclerostin Serum Levels and Vascular Calcification Progression in Prevalent Renal Transplant Recipients. J. Clin. Endocrinol. Metab. 2015;100:4669–4676. doi: 10.1210/jc.2015-3056. [DOI] [PubMed] [Google Scholar]
- 131.Fayed A., Elnokeety M.M., Attia K., Din U.A.S.E. Calcification of abdominal aorta in patients recently starting hemodialysis: A single-center experience from Egypt. Saudi J. Kidney Dis. Transplant. 2019;30:819–824. doi: 10.4103/1319-2442.265457. [DOI] [PubMed] [Google Scholar]
- 132.Filgueira A., Carvalho A.B., Tomiyama C., Higa A., Rochitte C.E., Santos R.D., Canziani M.E.F. Is Coronary Artery Calcification Associated with Vertebral Bone Density in Nondialyzed Chronic Kidney Disease Patients? Clin. J. Am. Soc. Nephrol. 2011;6:1456–1462. doi: 10.2215/CJN.10061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fusaro M., Gallieni M., Rebora P., Rizzo M.A., Luise M.C., Riva H., Bertoli S., Conte F., Stella A., Ondei P., et al. Atrial fibrillation and low vitamin D levels are associated with severe vascular calcifications in hemodialysis patients. J. Nephrol. 2016;29:419–426. doi: 10.1007/s40620-015-0236-7. [DOI] [PubMed] [Google Scholar]
- 134.Golembiewska E., Qureshi A.R., Dai L., Lindholm B., Heimbürger O., Söderberg M., Brismar T.B., Ripsweden J., Barany P., Johnson R.J., et al. Copeptin is independently associated with vascular calcification in chronic kidney disease stage 5. BMC Nephrol. 2020;21:1–8. doi: 10.1186/s12882-020-1710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gruppen M.P., Groothoff J.W., Prins M., van der Wouw P., Offringa M., Bos W.J.W., Davin J.C., Heymans H.S. Cardiac disease in young adult patients with end-stage renal disease since childhood: A Dutch cohort study. Kidney Int. 2003;63:1058–1065. doi: 10.1046/j.1523-1755.2003.00814.x. [DOI] [PubMed] [Google Scholar]
- 136.Ho T.-Y., Chen N.-C., Hsu C.-Y., Huang C.-W., Lee P.-T., Chou K.-J., Fang H.-C., Chen C.-L. Evaluation of the association of Wnt signaling with coronary artery calcification in patients on dialysis with severe secondary hyperparathyroidism. BMC Nephrol. 2019;20:1–9. doi: 10.1186/s12882-019-1543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ishimura E., Okuno S., Kitatani K., Maekawa K., Izumotani T., Yamakawa T., Jono S., Shoji T., Shioi A., Inaba M., et al. C-reactive protein is a significant predictor of vascular calcification of both aorta and hand arteries. Semin. Nephrol. 2004;24:408–412. doi: 10.1016/j.semnephrol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 138.Ishimura E., Okuno S., Kitatani K., Kim M., Shoji T., Nakatani T., Inaba M., Nishizawa Y. Different risk factors for peripheral vascular calcification between diabetic and non-diabetic haemodialysis patients—Importance of glycaemic control. Diabetologia. 2002;45:1446–1448. doi: 10.1007/s00125-002-0920-8. [DOI] [PubMed] [Google Scholar]
- 139.Manghat P., Souleimanova I., Cheung J., Wierzbicki A., Harrington D., Shearer M., Chowiencki P., Fogelman I., Nerlander M., Goldsmith D., et al. Association of bone turnover markers and arterial stiffness in pre-dialysis chronic kidney disease (CKD) Bone. 2011;48:1127–1132. doi: 10.1016/j.bone.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 140.Wada J., Nakayama K., Nakao K., Takatori Y., Inoue J., Kojo S., Akagi S., Fukushima M., Makino H. Long-term effect of cinacalcet hydrochloride on abdominal aortic calcification in patients on hemodialysis with secondary hyperparathyroidism. Int. J. Nephrol. Renov. Dis. 2013;7:25–33. doi: 10.2147/IJNRD.S54731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nishizawa Y., Mizuiri S., Yorioka N., Hamada C., Tomino Y. Determinants of coronary artery calcification in maintenance hemodialysis patients. J. Artif. Organs. 2015;18:251–256. doi: 10.1007/s10047-015-0823-3. [DOI] [PubMed] [Google Scholar]
- 142.Nishizawa Y., Jono S., Ishimura E., Shioi A. Hyperphosphatemia and vascular calcification in end-stage renal disease. J. Ren. Nutr. 2005;15:178–182. doi: 10.1053/j.jrn.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 143.Oprisiu R., Bunea D., Tarek S., Hedi B., Fournier A. Progression of vascular calcification and dyslipidemia in patients on chronic hemodialysis. Am. J. Kidney Dis. 2002;39:209. doi: 10.1053/ajkd.2002.30955. [DOI] [PubMed] [Google Scholar]
- 144.Pateinakis P., Papagianni A., Douma S., Efstratiadis G., Memmos D. Associations of fetuin-A and osteoprotegerin with arterial stiffness and early atherosclerosis in chronic hemodialysis patients. BMC Nephrol. 2013;14:122. doi: 10.1186/1471-2369-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raggi P., Boulay A., Chasan-Taber S., Amin N., Dillon M., Burke S.K., Chertow G.M. Cardiac calcification in adult hemodialysis patients: A link between end-stage renal disease and cardiovascular disease? J. Am. Coll. Cardiol. 2002;39:695–701. doi: 10.1016/S0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 146.Schlieper G., Brandenburg V., Djuric Z., Damjanovic T., Markovic N., Schurgers L., Krüger T., Westenfeld R., Ackermann D., Haselhuhn A., et al. Risk Factors for Cardiovascular Calcifications in Non-Diabetic Caucasian Haemodialysis Patients. Kidney Blood Press. Res. 2009;32:161–168. doi: 10.1159/000221064. [DOI] [PubMed] [Google Scholar]
- 147.Sharma R., Pellerin D., Gaze D.C., Mehta R.L., Gregson H., Streather C.P., Collinson P.O., Brecker S.J. Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis. 2007;191:348–354. doi: 10.1016/j.atherosclerosis.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 148.Sigrist M., Bungay P., Taal M., McIntyre C.W. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol. Dial. Transplant. 2006;21:707–714. doi: 10.1093/ndt/gfi236. [DOI] [PubMed] [Google Scholar]
- 149.Stavroulopoulos A., Porter C.J., Pointon K., Monaghan J.M., Roe S.D., Cassidy M.J. Evolution of coronary artery calcification in patients with chronic kidney disease Stages 3 and 4, with and without diabetes. Nephrol. Dial. Transplant. 2011;26:2582–2589. doi: 10.1093/ndt/gfq751. [DOI] [PubMed] [Google Scholar]
- 150.Sumida Y., Nakayama M., Nagata M., Nakashita S., Suehiro T., Kaizu Y., Ikeda H., Izumaru K. Carotid artery calcification and atherosclerosis at the initiation of hemodialysis in patients with end-stage renal disease. Clin. Nephrol. 2010;73:360–369. doi: 10.5414/CNP73360. [DOI] [PubMed] [Google Scholar]
- 151.Tamei N., Ogawa T., Ishida H., Ando Y., Nitta K. Serum fibroblast growth factor-23 levels and progression of aortic arch calcification in non-diabetic patients on chronic hemodialysis. J. Atheroscler. Thromb. 2011;18:217–223. doi: 10.5551/jat.5595. [DOI] [PubMed] [Google Scholar]
- 152.Turan M.N., Gungor O., Asci G., Kircelli F., Acar T., Yaprak M., Ceylan N., Demirci M.S., Bayraktaroglu S., Töz H., et al. Epicardial adipose tissue volume and cardiovascular disease in hemodialysis patients. Atherosclerosis. 2013;226:129–133. doi: 10.1016/j.atherosclerosis.2012.10.061. [DOI] [PubMed] [Google Scholar]
- 153.Vipattawat K., Kitiyakara C., Phakdeekitcharoen B., Kantachuvesiri S., Sumethkul V., Jirasiritham S., Stitchantrakul W., Disthabanchong S. Vascular calcification in long-term kidney transplantation. Nephrology. 2014;19:251–256. doi: 10.1111/nep.12210. [DOI] [PubMed] [Google Scholar]
- 154.Yamada S., Inaba M., Shidara K., Okada S., Emoto M., Ishimura E., Nishizawa Y. Association of glycated albumin, but not glycated hemoglobin, with peripheral vascular calcification in hemodialysis patients with type 2 diabetes. Life Sci. 2008;83:516–519. doi: 10.1016/j.lfs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 155.Jung H.H., Kim S.-W., Han H. Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol. Dial. Transplant. 2006;21:1915–1920. doi: 10.1093/ndt/gfl118. [DOI] [PubMed] [Google Scholar]
- 156.Aoun M., Makki M., Azar H., Matta H., Chelala D.N. High Dephosphorylated-Uncarboxylated MGP in Hemodialysis patients: Risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017;18:1–10. doi: 10.1186/s12882-017-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Axelsson J., Wang X., Ketteler M., Qureshi A.R., Heimbürger O., Bárány P., Lindholm B., Nordfors L., Stenvinkel P. Is Fetuin-A/α2-Heremans-Schmid Glycoprotein Associated with the Metabolic Syndrome in Patients with Chronic Kidney Disease? Am. J. Nephrol. 2008;28:669–676. doi: 10.1159/000121358. [DOI] [PubMed] [Google Scholar]
- 158.Block G.A., Hulbert-Shearon E.T., Levin N.W., Port F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am. J. Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 159.Buiten M.S., De Bie M.K., Krijger A.B.-D., Van Dam B., Dekker F., Jukema J.W., Rabelink T.J., Rotmans J.I. Soluble Klotho is not independently associated with cardiovascular disease in a population of dialysis patients. BMC Nephrol. 2014;15:197. doi: 10.1186/1471-2369-15-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chao C., Yuan T., Yeh H., Chen H., Huang J., Chen H. Risk Factors Associated with Altered Circulating MicroRNA-125b and Their Influences on Uremic Vascular Calcification Among Patients with End-Stage Renal Disease. J. Am. Hear. Assoc. 2019;8:e010805. doi: 10.1161/JAHA.118.010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen H.-Y., Chiu Y.-L., Hsu S.-P., Pai M.-F., Yang J.-Y., Peng Y.-S. Low serum fetuin A levels and incident stroke in patients with maintenance haemodialysis. Eur. J. Clin. Investig. 2013;43:387–396. doi: 10.1111/eci.12057. [DOI] [PubMed] [Google Scholar]
- 162.Claes K.J., Viaene L., Heye S., Meijers B., D’Haese P., Evenepoel P. Sclerostin: Another Vascular Calcification Inhibitor? J. Clin. Endocrinol. Metab. 2013;98:3221–3228. doi: 10.1210/jc.2013-1521. [DOI] [PubMed] [Google Scholar]
- 163.Gonçalves F.L.C., Elias R.M., Dos Reis L.M., Graciolli F.G., Zampieri F.G., Oliveira R.B., Jorgetti V., Moysés R.M.A. Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol. 2014;15:1–7. doi: 10.1186/1471-2369-15-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.González-Parra E., Álvaro A., Lorenzo O., Tarín N., González-Casaus M.L., Cristóbal C., Huelmos A., Fernandez J.L.T., Pello A.M., Carda R., et al. Important abnormalities of bone mineral metabolism are present in patients with coronary artery disease with a mild decrease of the estimated glomerular filtration rate. J. Bone Miner. Metab. 2016;34:587–598. doi: 10.1007/s00774-015-0706-y. [DOI] [PubMed] [Google Scholar]
- 165.Gupta V., Ekundayo O., Nemeth Z.K., Yang Y., Covic A., Mathe Z., Kovesdy C.P., Molnar M.Z., Mucsi I. Association between serum osteoprotegerin level and mortality in kidney transplant recipients—A prospective observational cohort study. Transpl. Int. 2021;34:844–854. doi: 10.1111/tri.13847. [DOI] [PubMed] [Google Scholar]
- 166.Holden R.M., Booth S.L., Tuttle A., James P.D., Morton A.R., Hopman W.M., Nolan R., Garland J.S. Sequence Variation in Vitamin K Epoxide Reductase Gene Is Associated with Survival and Progressive Coronary Calcification in Chronic Kidney Disease. Arter. Thromb. Vasc. Biol. 2014;34:1591–1596. doi: 10.1161/ATVBAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 167.Ikee R., Toyoyama T., Endo T., Tsunoda M., Hashimoto N. Impact of sevelamer hydrochloride on serum magnesium concentrations in hemodialysis patients. Magnes Res. 2016;29:184–190. doi: 10.1684/mrh.2016.0410. [DOI] [PubMed] [Google Scholar]
- 168.Jean G., Charra B., Chazot C. Vitamin D Deficiency and Associated Factors in Hemodialysis Patients. J. Ren. Nutr. 2008;18:395–399. doi: 10.1053/j.jrn.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 169.Jean G., Lataillade D., Genet L., Legrand E., Kuentz F., Moreau-Gaudry X., Fouque D. Association between Very Low PTH Levels and Poor Survival Rates in Haemodialysis Patients: Results from the French ARNOS Cohort. Nephron Clin. Pract. 2011;118:c211–c216. doi: 10.1159/000321642. [DOI] [PubMed] [Google Scholar]
- 170.Karsli Ceppioğlu S., Yurdun T., Canbakan M. Assessment of Matrix Gla Protein, Klotho Gene Polymorphisms, and Oxidative Stress in Chronic Kidney Disease. Renal Fail. 2011;33:866–874. doi: 10.3109/0886022X.2011.605534. [DOI] [PubMed] [Google Scholar]
- 171.Kato A., Odamaki M., Ishida J., Hishida A. Relationship between Serum Pre-B Cell Colony-Enhancing Factor/Visfatin and Atherosclerotic Parameters in Chronic Hemodialysis Patients. Am. J. Nephrol. 2009;29:31–35. doi: 10.1159/000148648. [DOI] [PubMed] [Google Scholar]
- 172.Kuo T.-H., Lin W.-H., Chao J.-Y., Wu A.-B., Tseng C.-C., Chang Y.-T., Liou H.-H., Wang M.-C. Serum sclerostin levels are positively related to bone mineral density in peritoneal dialysis patients: A cross-sectional study. BMC Nephrol. 2019;20:1–10. doi: 10.1186/s12882-019-1452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liabeuf S., Glorieux G., Lenglet A., Diouf M., Schepers E., Desjardins L., Choukroun G., Vanholder R., Massy Z.A., on behalf of the European Uremic Toxin (EUTox) Work Group Does P-Cresylglucuronide Have the Same Impact on Mortality as Other Protein-Bound Uremic Toxins? PLoS ONE. 2013;8:e67168. doi: 10.1371/journal.pone.0067168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Metry G., Stenvinkel P., Qureshi A.R.T., Carrero J.J., Yilmaz M.I., Bárány P., Snaedal S., Heimbürger O., Lindholm B., Suliman M.E. Low serum fetuin-A concentration predicts poor outcome only in the presence of inflammation in prevalent haemodialysis patients. Eur. J. Clin. Investig. 2008;38:804–811. doi: 10.1111/j.1365-2362.2008.02032.x. [DOI] [PubMed] [Google Scholar]
- 175.Nakashima A., Carrero J.J., Qureshi A.R.T., Hirai T., Takasugi N., Ueno T., Taniguchi Y., Lindholm B., Yorioka N. Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos. Int. 2011;22:1695–1701. doi: 10.1007/s00198-010-1377-0. [DOI] [PubMed] [Google Scholar]
- 176.Nemeth Z.K., Mardare N.G., Czira M.E., Deak G., Kiss I., Mathe Z., Remport A., Ujszaszi A., Covic A., Molnar M.Z., et al. Serum osteoprotegerin is associated with pulse pressure in kidney transplant recipients. Sci. Rep. 2015;5:14518. doi: 10.1038/srep14518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nishiura R., Fujimoto S., Sato Y., Yamada K., Hisanaga S., Hara S., Nakao H., Kitamura K. Elevated Osteoprotegerin Levels Predict Cardiovascular Events in New Hemodialysis Patients. Am. J. Nephrol. 2009;29:257–263. doi: 10.1159/000157629. [DOI] [PubMed] [Google Scholar]
- 178.Okamoto T., Tsutaya C., Hatakeyama S., Konishi S., Okita K., Tanaka Y., Imanishi K., Takashima T., Saitoh F., Suzuki T., et al. Low serum butyrylcholinesterase is independently related to low fetuin-A in patients on hemodialysis: A cross-sectional study. Int. Urol. Nephrol. 2018;50:1713–1720. doi: 10.1007/s11255-018-1957-z. [DOI] [PubMed] [Google Scholar]
- 179.Park S., Lee C.J., Jhee J.H., Yun H., Kim H., Jung S., Kee Y.K., Yoon C., Park J.T., Kim H.C., et al. Extracellular Fluid Excess Is Significantly Associated with Coronary Artery Calcification in Patients with Chronic Kidney Disease. J. Am. Hear. Assoc. 2018;7:e008935. doi: 10.1161/JAHA.118.008935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ramalho J., Petrillo E.M., Takeichi A.P.M., Moyses R.M.A., Titan S.M. Calcitriol and FGF-23, but neither PTH nor sclerostin, are associated with calciuria in CKD. Int. Urol. Nephrol. 2019;51:1823–1829. doi: 10.1007/s11255-019-02215-0. [DOI] [PubMed] [Google Scholar]
- 181.Scialla J.J., Leonard M.B., Townsend R.R., Appel L., Wolf M., Budoff M.J., Chen J., Lustigova E., Gadegbeku C.A., Glenn M., et al. Correlates of Osteoprotegerin and Association with Aortic Pulse Wave Velocity in Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011;6:2612–2619. doi: 10.2215/CJN.03910411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schlieper G.R., Westenfeld R., Krüger T., Cranenburg E.C.M., Magdeleyns E.J., Brandenburg V.M., Djuric Z., Damjanovic T., Ketteler M., Vermeer C., et al. Circulating Nonphosphorylated Carboxylated Matrix Gla Protein Predicts Survival in ESRD. J. Am. Soc. Nephrol. 2011;22:387–395. doi: 10.1681/ASN.2010040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sigrist M.K., Levin A., Er L., McIntyre C.W. Elevated osteoprotegerin is associated with all-cause mortality in CKD stage 4 and 5 patients in addition to vascular calcification. Nephrol. Dial. Transplant. 2009;24:3157–3162. doi: 10.1093/ndt/gfp253. [DOI] [PubMed] [Google Scholar]
- 184.Stenvinkel P., Wang K., Qureshi A.R., Axelsson J., Pecoits-Filho R., Gao P., Barany P., Lindholm B., Jogestrand T., Heimberger O., et al. Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int. 2005;67:2383–2392. doi: 10.1111/j.1523-1755.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 185.Stolic R.V., Jovanovic A., Trajkovic G.Z., Kostić M.M., Odalovic A.M., Sovtic S.R., Sipic M.V., Pajovic S.D., Sojevic-Timotijevic Z.N. Is low magnesium a clue to arteriovenous fistula complications in hemodialysis? Int. Urol. Nephrol. 2016;48:773–779. doi: 10.1007/s11255-015-1207-6. [DOI] [PubMed] [Google Scholar]
- 186.Thambiah S.C., Roplekar R., Manghat P., Fogelman I., Fraser W.D., Goldsmith D., Hampson G. Circulating Sclerostin and Dickkopf-1 (DKK1) in Predialysis Chronic Kidney Disease (CKD): Relationship with Bone Density and Arterial Stiffness. Calcif. Tissue Int. 2012;90:473–480. doi: 10.1007/s00223-012-9595-4. [DOI] [PubMed] [Google Scholar]
- 187.Ulusoy S., Özkan G., Mentese A., Yavuz A., Karahan S.C., Sümer A.U. Signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) level in hemodialysis patients and parameters affecting that level. Clin. Biochem. 2012;45:1444–1449. doi: 10.1016/j.clinbiochem.2012.07.103. [DOI] [PubMed] [Google Scholar]
- 188.Viaene L., Behets G., Claes K., Meijers B., Blocki F., Brandenburg V., Evenepoel P., D’Haese P.C. Sclerostin: Another bone-related protein related to all-cause mortality in haemodialysis? Nephrol. Dial. Transplant. 2013;28:3024–3030. doi: 10.1093/ndt/gft039. [DOI] [PubMed] [Google Scholar]
- 189.Zhang A.-H., Guo W.-K., Yu L., Liu W.-H. Relationship of Serum Soluble Klotho Levels and Echocardiographic Parameters in Patients on Maintenance Hemodialysis. Kidney Blood Press. Res. 2019;44:396–404. doi: 10.1159/000499200. [DOI] [PubMed] [Google Scholar]
- 190.Zhang Q., Sun L., Jin L. Association Between Angiotensin-Converting Enzyme 2 and Coronary Artery Calcification in Patients on Maintenance Hemodialysis Therapy. Ther. Apher. Dial. 2015;19:466–470. doi: 10.1111/1744-9987.12298. [DOI] [PubMed] [Google Scholar]
- 191.Zou Y., Yang M., Wang J., Cui L., Jiang Z., Ding J., Li M., Zhou H. Association of sclerostin with cardiovascular events and mortality in dialysis patients. Ren. Fail. 2020;42:282–288. doi: 10.1080/0886022X.2020.1741386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zou J., Yu Y., Wu P., Lin F.-J., Yao Y., Xie Y., Jiang G.-R. Serum phosphorus is related to left ventricular remodeling independent of renal function in hospitalized patients with chronic kidney disease. Int. J. Cardiol. 2016;221:134–140. doi: 10.1016/j.ijcard.2016.06.181. [DOI] [PubMed] [Google Scholar]
- 193.Chao C.-T., Lin S.-H. Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins. Toxins. 2020;12:812. doi: 10.3390/toxins12120812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nakahara T., Dweck M.R., Narula N., Pisapia D., Narula J., Strauss H.W. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging. 2017;10:582–593. doi: 10.1016/j.jcmg.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 195.Amann K. Media Calcification and Intima Calcification Are Distinct Entities in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 196.Lee J.J., Pedley A., Hoffmann U., Massaro J.M., O’Donnell C.J., Benjamin E.J., Long M.T. Longitudinal Associations of Pericardial and Intrathoracic Fat with Progression of Coronary Artery Calcium (from the Framingham Heart Study) Am. J. Cardiol. 2018;121:162–167. doi: 10.1016/j.amjcard.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sui Y.-B., Chang J.-R., Chen W.-J., Zhao L., Zhang B.-H., Yu Y.-R., Tang C.-S., Yin X.-H., Qi Y.-F. Angiotensin-(1-7) inhibits vascular calcification in rats. Peptides. 2013;42:25–34. doi: 10.1016/j.peptides.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 198.McCabe K.M., Zelt J.G., Kaufmann M., Laverty K., Ward E., Barron H., Jones G., Adams M.A., Holden R.M. Calcitriol Accelerates Vascular Calcification Irrespective of Vitamin K Status in a Rat Model of Chronic Kidney Disease with Hyperphosphatemia and Secondary Hyperparathyroidism. J. Pharmacol. Exp. Ther. 2018;366:433–445. doi: 10.1124/jpet.117.247270. [DOI] [PubMed] [Google Scholar]
- 199.Almeida Y.E., Fessel M.R., Carmo L.S.D., Jorgetti V., Farias-Silva E., Pescatore L.A., Gamarra L., Andrade M.C., Simplicio-Filho A., Mangueira C.L.P., et al. Excessive cholecalciferol supplementation increases kidney dysfunction associated with intrarenal artery calcification in obese insulin-resistant mice. Sci. Rep. 2020;10:87. doi: 10.1038/s41598-019-55501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.De Maré A., Maudsley S., Azmi A., Hendrickx J.O., Opdebeeck B., Neven E., D’Haese P.C., Verhulst A. Sclerostin as Regulatory Molecule in Vascular Media Calcification and the Bone-Vascular Axis. Toxins. 2019;11:428. doi: 10.3390/toxins11070428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roumeliotis S., Dounousi E., Salmas M., Eleftheriadis T., Liakopoulos V. Vascular Calcification in Chronic Kidney Disease: The Role of Vitamin K- Dependent Matrix Gla Protein. Front. Med. (Lausanne) 2020;7:154. doi: 10.3389/fmed.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hu M.C., Shi M., Zhang J., Quiñones H., Griffith C., Kuro-o M., Moe O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ceponiene I., Li D., El Khoudary S.R., Nakanishi R., Stein J.H., Wong N.D., Nezarat N., Kanisawa M., Rahmani S., Osawa K., et al. Association of Coronary Calcium, Carotid Wall Thickness, and Carotid Plaque Progression with Low-Density Lipoprotein and High-Density Lipoprotein Particle Concentration Measured by Ion Mobility (From Multiethnic Study of Atherosclerosis [MESA]) Am. J. Cardiol. 2021;142:52–58. doi: 10.1016/j.amjcard.2020.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Parhami F., Basseri B., Hwang J., Tintut Y., Demer L.L. High-density lipoprotein regulates calcification of vascular cells. Circ. Res. 2002;91:570–576. doi: 10.1161/01.RES.0000036607.05037.DA. [DOI] [PubMed] [Google Scholar]
- 205.Osako M.K., Nakagami H., Koibuchi N., Shimizu H., Nakagami F., Koriyama H., Shimamura M., Miyake T., Rakugi H., Morishita R. Estrogen inhibits vascular calcification via vascular RANKL system: Common mechanism of osteoporosis and vascular calcification. Circ. Res. 2010;107:466–475. doi: 10.1161/CIRCRESAHA.110.216846. [DOI] [PubMed] [Google Scholar]
- 206.Balica M., Boström K., Shin V., Tillisch K., Demer L.L. Calcifying subpopulation of bovine aortic smooth muscle cells is responsive to 17 beta-estradiol. Circulation. 1997;95:1954–1960. doi: 10.1161/01.CIR.95.7.1954. [DOI] [PubMed] [Google Scholar]
- 207.Woodward H.J., Zhu D., Hadoke P.W.F., MacRae V.E. Regulatory Role of Sex Hormones in Cardiovascular Calcification. Int. J. Mol. Sci. 2021;22:4620. doi: 10.3390/ijms22094620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ray M., Jovanovich A. Mineral Bone Abnormalities and Vascular Calcifications. Adv. Chronic Kidney Dis. 2019;26:409–416. doi: 10.1053/j.ackd.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 209.Jia J., Zhou H., Zeng X., Feng S. Estrogen stimulates osteoprotegerin expression via the suppression of miR-145 expression in MG-63 cells. Mol. Med. Rep. 2017;15:1539–1546. doi: 10.3892/mmr.2017.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data for conducting this analysis are available upon reasonable request to the corresponding author and the first author.