Abstract
Background
Long COVID is defined as the persistence of symptoms beyond 3 months after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. To better understand the long-term course and etiology of symptoms we analyzed a cohort of patients with COVID-19 prospectively.
Methods
Patients were included at 5 months after acute COVID-19 in this prospective, noninterventional, follow-up study. Patients followed until 12 months after COVID-19 symptom onset (n = 96; 32.3% hospitalized, 55.2% females) were included in this analysis of symptoms, quality of life (based on an SF-12 survey), laboratory parameters including antinuclear antibodies (ANAs), and SARS-CoV-2 antibody levels.
Results
At month 12, only 22.9% of patients were completely free of symptoms and the most frequent symptoms were reduced exercise capacity (56.3%), fatigue (53.1%), dyspnea (37.5%), and problems with concentration (39.6%), finding words (32.3%), and sleeping (26.0%). Females showed significantly more neurocognitive symptoms than males. ANA titers were ≥1:160 in 43.6% of patients at 12 months post–COVID-19 symptom onset, and neurocognitive symptom frequency was significantly higher in the group with an ANA titer ≥1:160 versus <1:160. Compared with patients without symptoms, patients with ≥1 long-COVID symptom at 12 months did not differ significantly with respect to their SARS-CoV-2 antibody levels but had a significantly reduced physical and mental life quality compared with patients without symptoms.
Conclusions
Neurocognitive long-COVID symptoms can persist ≥1 year after COVID-19 symptom onset and reduce life quality significantly. Several neurocognitive symptoms were associated with ANA titer elevations. This may indicate autoimmunity as a cofactor in etiology of long COVID.
Keywords: coronavirus disease 2019 (COVID-19), long COVID, life quality, ANA titers
Neurocognitive long-COVID symptoms can persist at least for 1 year after acute COVID-19 and reduce life quality significantly. Several neurocognitive symptoms were associated with ANA titer elevations. This may indicate autoimmunity as a cofactor in the etiology of long COVID.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the current coronavirus disease 2019 (COVID-19) pandemic that has affected more than 170 million people worldwide. Albeit the short-term outcomes in people hospitalized with COVID-19 are a major concern, another worrying aspect of this disease is the post-acute consequence of COVID-19, also termed long COVID. In the United Kingdom, the National Institute for Health and Care Excellence (NICE) established a definition for this condition, including signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis [1]. The etiology and pathophysiology of long COVID still needs to be clearly defined [2–4]. Patients with long COVID present with prolonged multisystem involvement and significant disability. Common symptoms in people with long COVID are sensory (loss of taste and anosmia), neurological (problems with concentration and “brain fog”), and cardiorespiratory (fatigue, dyspnea, reduced exercise capacity) problems. In patients who experience long COVID, 1 or more symptoms may be present.
A report from Italy found that 87% of people who recovered and were discharged from hospitals showed persistence of at least 1 symptom at 60 days post–COVID-19 onset [5]. In a prospective cohort study from China, a majority of formerly hospitalized patients (76%) reported at least 1 symptom 6 months after symptom onset, with fatigue being the most common (63%) [6]. In a large, international app-based cohort study, experiencing more than 5 symptoms during the first week of illness was associated with long COVID [7].
To date, little is known about the long-term course of symptoms, quality of life, laboratory parameters, and association of symptoms with SARS-CoV-2 antibody titers at 12 months after acute infection. Therefore, we performed a long-term follow-up of a cohort of patients with COVID-19, with the aim to analyze the development of symptoms over follow-up time and with a special focus on differences between subgroups defined by gender, age, severity of acute COVID-19 illness, and presence of antinuclear antibody (ANA) titers reflecting autoimmunity.
METHODS
Study Population
All patients with COVID-19 treated for acute COVID-19 as out- or inpatients at the Department of Internal Medicine IV of the University Hospital Heidelberg and with symptom onset between 22 February 2020 and 18 April 2020 were invited 10 to 18 weeks after COVID-19 symptom onset to participate in this prospective, noninterventional COVID-19 long-term follow-up study (Ethics Committee of University of Heidelberg: reference number: S-546/2020; DRKS00025089) (Supplementary Figure 1, Supplementary Table 1). In this study the long-term course of symptoms, laboratory and immunological parameters, and quality of life was analyzed at longitudinal follow-up time points starting 5 months after acute COVID-19 symptom onset.
Written informed consent according to the Declaration of Helsinki was obtained from all patients and the local ethics committees approved data collection and analysis. Inclusion criteria were status after polymerase chain reaction (PCR)–confirmed SARS-CoV-2-infection, prior out- or inpatient treatment (with live discharge) for acute COVID-19 at the Department of Internal Medicine IV, and age 18 years or older. The timeframes for the follow-up visits were as follows: 5 months (20–22 weeks post–symptom onset), 9 months (33–40 weeks post–symptom onset), and 12 months (50–54 weeks post–symptom onset). From the 96 patients seen at 5 and 12 months, only 80 had an intermediate follow-up examination at our outpatient clinic at 9 months post–COVID-19. Therefore, analysis of the development of symptoms focused on the comparison of the 5- and 12-month time points.
Disease severity of acute COVID-19 was divided into 4 categories: (1) mild, (2) moderate, (3) severe, and (4) critical disease, as previously described [8]. Symptom questionnaire and laboratory values from acute phase disease were available for all 146 patients initially included and analyzed retrospectively.
Assessment of Symptoms
The following symptoms were recorded in a structured, paper-based questionnaire with additional grading of the following present symptoms in 3 subjective grades (mild, moderate, severe): fever, sore throat, vomiting/nausea, diarrhea, decrease in taste, anosmia, cough, dyspnea, fatigue, headache, vertigo, cold, body aches, and shivering. In the follow-up examinations the following symptoms were additionally recorded: reduced exercise capacity, concentration problems, sleeping problems, anxiety, palpitations, hair loss, and (only at 9 and 12 months) difficulty finding words.
Assessment of Life Quality by the 12-Item Short Form Survey
For life quality assessment, study participants answered a standardized 12-item Short Form Survey (SF-12) questionnaire at the 5-, 9-, and 12-month time points. The SF-12 is a general health-related quality-of-life survey that measures general health status. Two scales are calculated according to a standardized evaluation with the help of an examination tool: the Physical Component Scale (PCS) and the Mental Component Scale (MCS). All scores were normalized against the 2009 US population reference scores. Although it has not been validated for patients post–COVID-19, the SF-12 has been used extensively and is demonstrated to be reliable and well validated [9, 10].
Serological Analyses
The laboratory parameters and ANA titers were determined in the accredited central laboratory and in the Department of Infectious Diseases of the Heidelberg University Hospital using standard operating procedures according to the manufacturers’ instructions. The ANA titers were determined during acute disease (until 16 days post–symptom onset; measurements were available for 71 of 96 patients) and at follow-up visits using an immunofluorescence testing kit (Euroimmun AG, Lübeck, Germany). Immunoglobulin (Ig) G (IgG) antibody levels against the S1 protein of SARS-CoV-2 were determined using an enzyme-linked immunosorbent assay (ELISA) kit (EI 2606–9601 G; Euroimmun AG) run on an Analyzer I instrument (Euroimmun AG). Multiplex SARS-CoV-2 serology was performed as recently described, and briefly, N and S1-RBD antibody levels measured in median fluorescence intensity (MFI) units [11]. Competition of sera with S1-ACE2 interaction was measured using the cPass surrogate SARS-CoV-2 neutralization test kit (L00847; Genscript, Piscataway, NJ, USA).
Statistical Analysis
Variables are described as median (interquartile range [IQR]) or absolute (relative, %) frequencies for metric or categorical variables, respectively. Comparisons between months 5 and 12 were performed by Wilcoxon signed-rank test for metric or McNemar test for categorical variables and restricted to patients who participated in both measurement times observed. Comparisons of symptom frequencies between subgroups were performed with a chi-square test (or Fisher’s exact test for symptoms with low absolute frequencies [<10]) for independent variables. Comparison of SARS-CoV-2 antibody levels between 5 and 12 months was performed with a Wilcoxon signed-rank test for dependent variables or for analysis between subgroups with a Mann-Whitney U test for independent variables. P values < .05 were considered statistically significant. Statistical analyses were performed with IBM SPSS Statistics for Windows, version 24.0 (IBM Corporation, Armonk, NY, USA).
RESULTS
Baseline Characteristics
Patient characteristics are outlined in Table 1. Of 146 patients initially consenting to study participation and seen at the 5-month time point, 50 were lost to follow-up at the 12-month follow-up visit and were therefore excluded from this 12-month long-term analysis (Supplementary Figure 1 and Supplementary Table 1).
Table 1.
Demographic Characteristics of the Study Population (n = 96) at the Time Point of Acute COVID-19
| Post–COVID-19 | % | |
|---|---|---|
| Age, median (IQR), y | 57 (50–63) | |
| Age group | ||
| <30 y | 3 | 3.1 |
| 31–40 y | 10 | 8.3 |
| 41–50 y | 12 | 10.4 |
| 51–60 y | 39 | 38.5 |
| 61–70 y | 20 | 25.0 |
| 71–80 y | 11 | 13.5 |
| >80 y | 1 | 1.0 |
| Gender (male) | 43 | 44.8 |
| Hospitalized | 31 | 32.3 |
| Coexisting conditions | ||
| Asthma | 12 | 12.5 |
| Hypertension | 35 | 35.1 |
| Cardiovascular disease | 4 | 4.2 |
| Diabetes mellitus type 2 | 7 | 7.3 |
| Active malignancy | 4 | 4.2 |
| Autoimmune disease | 5 | 5.2 |
| Depression | 7 | 7.3 |
| Neurocognitive disease | 0 | 0 |
| Adipositas (BMI >30 kg/m2) | 23 | 24.0 |
| Oxygen-support category | ||
| Supplemental oxygen via nasal tube | 21 | 21.9 |
| Noninvasive ventilation via high-flow nasal oxygen | 3 | 3.1 |
| Invasive ventilation | 4 | 4.2 |
| Disease severity | ||
| Mild | 15 | 15.6 |
| Moderate | 53 | 55.2 |
| Mild/moderate, ≥60 y | 29 | 30.2 |
| Mild/moderate, male | 25 | 26.0 |
| Severe | 24 | 25.0 |
| Critical | 4 | 4.2 |
| Severe/critical, ≥60 y | 15 | 15.6 |
| Severe/critical, male | 18 | 18.8 |
Data are presented as n (%) for categorical variables.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; IQR, interquartile range.
Ninety-six patients who had completed follow-up until 12 months post–COVID-19 were included in the analysis. Co-existing conditions at the time of acute COVID-19 are summarized in Table 1. Until 12 months post-COVID the following diagnoses had been newly established in the study cohort: hypertension (n = 7), diabetes mellitus type 2 (n = 3), asthma (n = 1), cardiovascular disease (n = 1), obstructive sleep apnea syndrome (n = 1), neuritis of nervus trigeminus (n = 1), perimyocarditis (n = 1), depression (n = 1), postural orthostatic tachycardia syndrome (n = 1), perianal abscess (n = 1), reactivation of varicella zoster virus (n = 1), autoimmune hepatitis (n = 1), and nonalcoholic steatohepatitis (n = 2).
Laboratory Findings
Laboratory findings of the study cohort are outlined in Table 2. At 5, 9, and 12 months after acute infection, inflammatory parameters (C-reactive protein [CRP], leukocytes, ferritin) were in the normal range for most of the patients.
Table 2.
Laboratory and Clinical Findings of Study Population 5, 9, and 12 Months Post–Symptom Onset of COVID-19
| Limits of Normal | Post–COVID-19 Month 5 (n = 96) | Post–COVID-19 Month 9 (n = 80) | Post–COVID-19 Month 12 (n = 96) | P | |
|---|---|---|---|---|---|
| Hemoglobin, g/dL | 13–17 | 14.2 (13.3–14.8) | 14.0 (13.3–14.9) | 14.3 (13.4–14.9) | .13 |
| Leukocytes, nL | 4–10 | 6.0 (5.2–7.6) | 6.1 (5.1–7.5) | 5.9 (5.1–7.5) | .24 |
| Lymphocytes, nL | 1.0–4.8 | 1.7 (1.4–2.2) | 1.7 (1.4–2.1) | 1.8 (1.4–2.2) | .04 |
| Creatinine, mg/dL | 0.6–1.2 | 0.8 (0.7–0.9) | 0.8 (0.6–0.9) | 0.8 (0.7–0.9) | .85 |
| CK, U/L | <170 | 111.0 (76.3–170.0) | 105.0 (80.5–153.5) | 105.5 (77.3–169.5) | .97 |
| GFR (CKD-EPI), mL/min/1.73 m2 | >60 | 92.0 (85.5–104.1) | 91.4 (79.7–103.3) | 92.0 (82.3–101.8) | .17 |
| LDH, U/L | <317 | 203.0 (176.0–231.0) | 200 (175.0–222.0) | 198.5 (175.3–213.8) | .003 |
| D-dimer, mg/L | <0.5 | 0.3 (0.2–0.6) | 0.3 (0.2–0.5) | 0.3 (0.2–0.5) | .24 |
| CRP, mg/L | <5 | 2.0 (2.0–2.4) | 2.0 (2.0–2.9) | 2.0 (2.0–2.5) | .50 |
| Ferritin, μg/L | <300 | 85.0 (36.8–168.8) | 95.0 (43.5–157.0) | 89.5 (44.8–154.8) | .06 |
| TnT (high sensitive), pg/mL | <14 | 6.0 (4.0–10.0) | 5.2 (3.0–8.9) | 5.9 (3.4–9.3) | .09 |
| BMI, kg/m2 | 25.7 (23.4–29.3) | 25.3 (23.2–28.7) | 25.8 (23.5–29.5) | Not calculated | |
| Heart rate, beats/minute | 75.0 (64.5–83.5) | 72.0 (65.0–79.3) | 71.0 (64.0–80.0) | .07 | |
| Blood pressure, mm Hg | |||||
| Systolic | 134.0 (121.0–143.5) | 135.5 (124.8–151.8) | 130.0 (118.0–142.0) | .36 | |
| Diastolic | 88.0 (81.0–95.5) | 89.5 (85.0–95.3) | 83.0 (80.0–90.0) | .008 |
Values are medians (IQRs) to summarize the respective variable to describe the classification with respect to the limits of normal. P values are shown for the comparison between month 5 and 12 based on Wilcoxon signed-rank test.
Abbreviations: BMI, body mass index; CK, creatine kinase; CKD, Chronic Kidney Disease; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; EPI, Epidemiology Collaboration equation; IQR, interquartile range; LDH, lactate dehydrogenase; TnT, high sensitive troponin T.
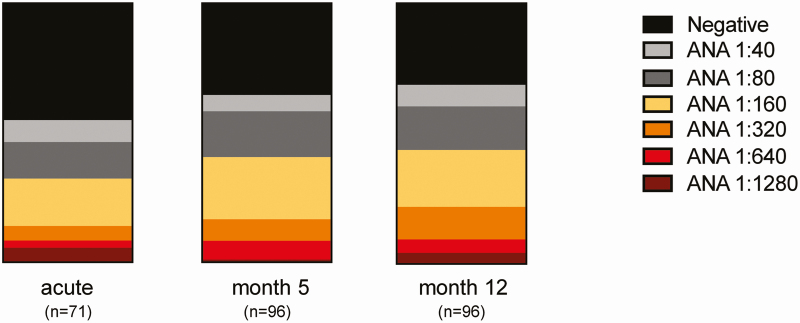
The distribution of ANA titers within the study group during acute COVID-19, as well as at 5 and 12 months post–symptom onset, is outlined in Figure 1 and Supplementary Table 2. The proportion of women who showed elevated ANA titers (≥1:160) was higher than that of men (Supplementary Figure 2A), whereas ANA titers did not differ significantly when grouping for acute disease severity (Supplementary Figure 2B).
Figure 1.
Distribution of ANA titers within study group at acute COVID-19 and 5 and 12 months post–symptom onset of COVID-19 presented in percentage of the total cohort (n = 96). Abbreviations: ANA, antinuclear antibody; COVID-19, coronavirus disease 2019.
Evaluation of Symptoms
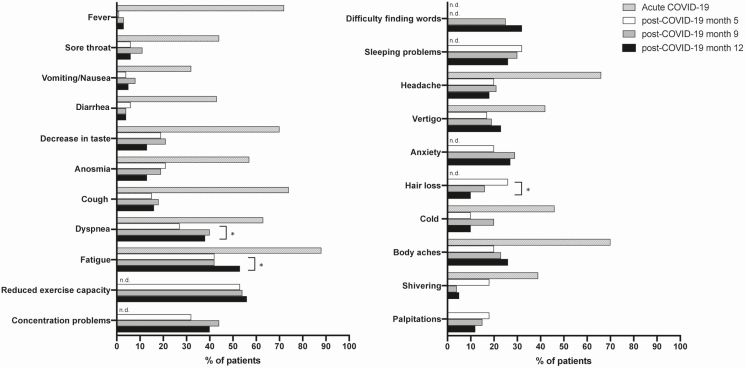
Patients answered a symptom questionnaire during acute COVID-19 and at follow-up visits at 5, 9, and 12 months (Figure 2). Symptoms already present before COVID-19 onset were not included in the analysis. The most frequent symptoms 5 months post–COVID-19 were reduced exercise capacity (53.1%), fatigue (41.7%), sleeping problems (32.3%), concentration problems (31.3%), and dyspnea (27.1%). At 5 and 12 months post–symptom onset, only 22.9% of patients were completely free of long-COVID symptoms. Between 5 months and 12 months after symptom onset, the reported symptom frequency decreased significantly only for hair loss (26.1% vs 10.4%, P = .022), but increased for fatigue (from 41.7% to 53.1%, P = .043) and dyspnea (from 27.1% to 37.5%, P = .041). For all other symptoms there were no significant changes in reported symptom frequencies between 5 and 12 months of follow-up. At 12 months, the most frequently reported symptoms were reduced exercise capacity, fatigue, dyspnea, concentration problems, problems finding words, and sleeping problems.
Figure 2.
Frequencies of symptoms (%) in the study cohort at acute COVID-19, as well as at 5, 9, and 12 months post–COVID-19 symptom onset. P values for the group differences between 5- and 12-month time points are based on McNemar test for dependent samples. Symptoms with significant differences are marked with an asterisk (*P < .05). Abbreviations: COVID-19, coronavirus disease 2019; n.d., not determined.
The number of positive acute symptoms was associated with long COVID at 12 months: patients with at least 1 persisting long-COVID symptom at 12 months post–COVID-19 had experienced significantly more positive symptoms during the acute phase (8; IQR, 6–11) than patients who were free of long-COVID symptoms (7; IQR, 4–9) (P = .009).
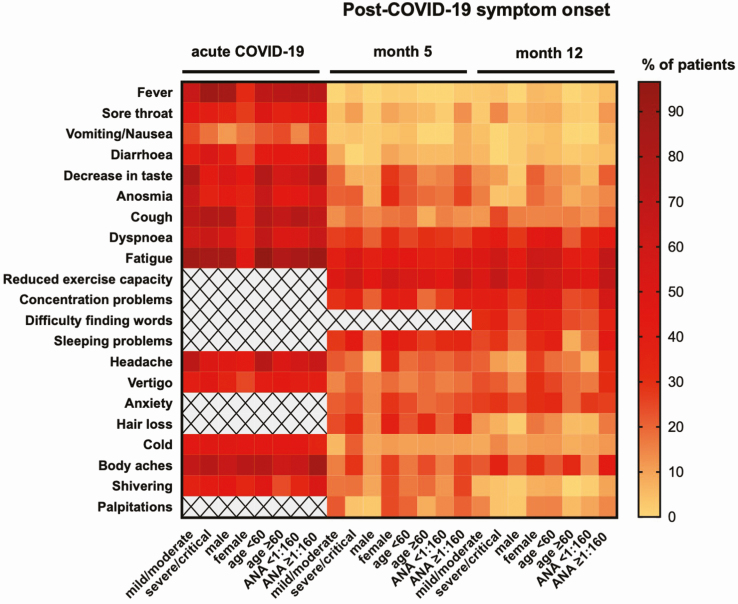
When stratifying by gender, a significantly higher proportion of females reported several symptoms compared with males at 12 months after COVID-19 (P for each, <.05) (Figure 3 and Supplementary Figure 3A). For most symptoms, the frequencies at 5 and 12 months post–acute infection did not differ between groups stratified for disease severity (Figure 3 and Supplementary Figure 3B). When grouping for age, younger patients (<60 years) reported dyspnea, sleeping problems, and concentration problems significantly more often than the group of patients aged 60 years and older (Figure 3 and Supplementary Figure 3C).
Figure 3.
Frequencies of symptoms (%) among the study population presented as a heatmap for the time of acute COVID-19 and at 5 and 12 months post–symptom onset. Symptom frequencies are stratified by disease severity (mild/moderate and severe/critical disease), gender (male and female), age (<60 and ≥60 years) and ANA titer (<1:160 vs ≥ 1:160). Gray fields with a cross indicate that this parameter was not analyzed at that specific time point. Abbreviations: ANA, antinuclear antibody; COVID-19, coronavirus disease 2019.
As autoimmunity might play a role in long COVID [13–15], we additionally determined ANA titers at each time point and grouped for ANA titer (<1:160 vs ≥1:160) [16]. At 12 months post–COVID-19, ANA titers were ≥1:160 in 43.6% of patients, and symptom frequency at that time was significantly higher in the group displaying an ANA titer ≥1:160 compared with the group with an ANA titer <1:160 for several symptoms (Figure 3 and Supplementary Figure 3D). As women tend to have higher levels of ANA positivity [12], we additionally analyzed male and female participants separately. At 12 months, females with ANA titers ≥1:160 had a significantly higher frequency of concentration problems than females with lower titers (66.7% vs 26.1%, respectively; P = .003). The same was true for body aches in women (46.7% vs 8.7%, respectively; P = .003). For males, there was no significant association of ANA titers and symptoms.
Assessment of Life Quality
To assess quality of life of the study cohort, the SF-12 questionnaire was used to evaluate the physical and mental health at 5, 9, and 12 months post–symptom onset.
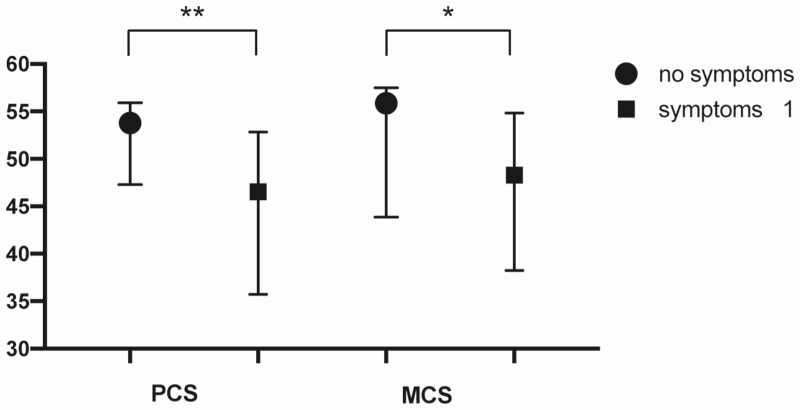
At 12 months, patients with at least 1 long-COVID symptom had a significantly reduced physical and mental life quality compared with patients without symptoms (PCS, P = .006; MCS, P = .031) (Figure 4).
Figure 4.
The PCS and MCS assessed by the SF-12 questionnaire for the study population subgrouped for patients with at least 1 symptom or no symptoms at 12 months post–COVID-19 symptom onset. Data are presented as medians and interquartile ranges, and P values for the group differences are based on the Mann-Whitney U test for independent samples. Significant differences are marked with an asterisk (*P < .05, **P < .01). Abbreviations: COVID-19, coronavirus disease 2019; MCS, Mental Component Scale; PCS, Physical Component Scale; SF-12, 12-item Short Form Survey.
The results of the PCS and MCS for the whole group and stratified by gender and disease severity are presented in Supplementary Figure 4A–F.
SARS-CoV-2–Specific Antibody Levels
Anti–SARS-CoV-2 anti-S1 IgG as determined by ELISA, IgG against SARS-CoV-2 nucleocapsid and S1-RBD (measured with multiplex SARS-CoV-2 serology), and the competition of sera with ACE2 binding to SARS-CoV-2 S1 (measured with a commercial ELISA–based surrogate neutralization assay) were analyzed 5, 9, and 12 months post–symptom onset. Eight patients had been vaccinated against SARS-CoV-2 shortly before the 12-month visit and were therefore excluded from the antibody titer analysis. Median antibody levels against nucleocapsid were reduced to approximately 70% compared with levels at 5 months, while S1-IgG measured with ELISA and S1-RBD measured with multiplex serology were reduced less (to 75% and 85%, respectively), and median relative ACE2 competition efficiency was only slightly reduced (Supplementary Figure 5). With the exception of 1 patient being seronegative against N and S1-RBD at all time points, all other patients were positive at the 12-month follow-up for S1-RBD IgG measured with multiplex serology, while 5.7% of patients showed sero-reversion for anti-nucleocapsid antibodies.
Comparison of patient groups stratified by disease severity revealed a significant difference between mild/moderate and severe/critical patient groups, with a higher level in the severe/critical group 5, 9, and 12 months post–COVID-19 for antibodies against S1 and S1-RBD, as well as for ACE2 competition efficiency, but not for nucleocapsid-specific antibodies at all 3 follow-up time points (Supplementary Figure 6). No relevant differences in antibody levels were observed when participants were stratified by ANA titers (</≥1:160; data not shown). When stratified by gender, females displayed significantly lower S1, S1-RBD, and nucleocapsid-specific antibody levels and ACE2 competition efficiency than males at 5 months post–COVID-19 (Supplementary Figure 6).
Next, we stratified for the presence of long-COVID symptoms at 12 months after symptom onset (≥ 1 symptom vs no symptoms at 12 months) when analyzing for differences in SARS-CoV-2 antibody titer levels and found no significant differences (Supplementary Figure 6).
DISCUSSION
We present a longitudinal follow-up cohort study of patients from Germany who were systematically assessed at 5, 9, and 12 months after COVID-19 symptom onset. To our knowledge, our study represents the longest post–COVID-19 follow-up study reported to date.
In our cohort, the most likely symptoms to persist until 12 months were reduced exercise capacity, fatigue, dyspnea, concentration problems, problems finding words, and sleeping problems. Patients reporting at least 1 long-COVID symptom had a significantly reduced physical and mental quality of life.
A similar pattern of persisting symptoms was reported from other studies evaluating an earlier time point after symptom onset. An Italian study with a mean follow-up of 60 days after symptom onset reported persistence of symptoms in 125 of 143 patients with COVID-19 discharged from the hospital. Fatigue (53.1%) and dyspnea (43.4%) were the most commonly reported symptoms, and a decline in quality of life, as measured by the EuroQol visual analog scale, was reported in 44.1% of patients in this study [5]. A French study in 150 patients with noncritical COVID-19 similarly reported persistence of symptoms in two-thirds of individuals at 60 days of follow-up [17]. Other studies reported similar findings [18–22].
The longest follow-up times published to date are studies with follow-up up to 7 months after symptom onset. In a prospective Chinese cohort study of 1733 patients at 6 months after symptom onset, fatigue, muscle weakness, sleep difficulties, and anxiety or depression were most frequently found [6]. In summary, our study cohort shows the typical pattern of symptoms reported from other studies and the predominance of women with long-COVID symptoms [6, 7].
Unexpectedly, in our patient cohort, symptom frequency remained unchanged for most neurocognitive symptoms analyzed. This high degree of symptom persistence may, in part, be due to a selection bias as 50 of the 146 patients initially included at 5 months were lost to the 12-month follow-up, likely due to being less symptomatic. In addition, we cannot exclude the contribution of an influence of the long duration of the pandemic and subsequent psychological impact. Nevertheless, in these 96 patients who were followed long term, most of the symptoms present at the 5-month time point persisted until the 12-month time point.
It is currently unclear why some patients experience long-term symptoms after COVID-19. Potential causes for different outcomes of infection are viral dose as well as host-dependent factors—for example, genetic susceptibility or induction of anti-inflammatory cells and proteins. For acute SARS-CoV-2 infection, the development of ANA IgA autoantibodies has been shown [14] and the presence of high-titer serum IgG antibodies targeting the GD1b ganglioside has been demonstrated in certain neurologically affected patients [13]. In a cohort of 31 patients with long COVID, functionally active autoantibodies targeting G-protein–coupled receptors were detected in a high proportion of patients experiencing a variety of post–COVID-19 symptoms [15]. Although the nature of the self-antigens recognized by autoimmune-like antibodies is diverse, a general characteristic shared by infections is the generation of ANAs, which appear during acute infection and may remain at lower levels during chronic infections [23]. Therefore, we analyzed our follow-up cohort for the presence of ANAs and the correlation of ANA positivity and long-COVID symptoms. We observed that patients with ANA titer elevations ≥1:160 at 12 months after acute infection had a significantly higher frequency of several long-COVID symptoms. Thus, we speculate that there is an underlying autoimmune component to the post–COVID-19 syndromes reported here, including neurocognitive symptoms and dyspnea. As expected [12], more women showed ANA positivity than men and the group of women with ANA titer elevations ≥1:160 displayed significantly higher symptom frequencies than the group of women with lower titers. As this observation was significant for female but not for male participants, autoimmune reactions might, in part, be responsible for the female predominance in long-COVID syndromes [6, 7].
Analysis of SARS-CoV-2 antibody titers showed a significant decline, especially for anti-nucleocapsid antibodies from 5 to 12 months after acute infection, while immunity-relevant anti–S1 antibodies declined only modestly and all patients remained positive in the ACE2 competition assay at 12 months. As an earlier report suggests that vaccination against SARS-CoV-2 alleviates long-COVID symptoms [24, 25], we analyzed for differences in antibody titer levels between patients stratified for the presence of symptoms at 12 months after symptom onset. We did not observe any differences between the groups in antibody levels for all analyzed antibodies.
The strength of our study is the long-term follow-up of patients with examination of all we report on the patients examined at 5 and 12 months, although more patients were initially included at 5 months. In addition, our study is, to date, the longest follow-up of patients post–COVID-19. Like for all observational studies, the increased willingness of symptomatic patients to take part in a follow-up study is a potential confounding factor. In line with this, reduced exercise capacity was reported, with a significantly lower frequency in the cohort of 50 patients lost to the 12-month follow-up than in the remaining group. We acknowledge this potential selection bias. The generalizability of the results may be limited by the single-center, unblinded, and nonrandomized design and the relatively small cohort size.
Conclusions
Neurocognitive long-COVID symptoms can persist at least until 1 year after COVID-19 symptom onset and reduce quality of life significantly. Several neurocognitive symptoms were associated with ANA titer elevations, rendering autoimmunity a potential cofactor in the etiology of long COVID. As the disease is poorly understood, it is too early to speculate about disease etiology and prognosis. Our results provide information useful to clinicians caring for patients post–COVID-19 disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. J. Seeßle, T. W., U. M., and B. M. were involved in the study concept and design, drafting of the manuscript, and study supervision. J. Seeßle, T. W., T. H., J. Simon, A. L., B. M., and U. M. were involved in acquisition of data. J Seeßle, T. W., M. K., J. Simon, B. M., and U. M. were involved in interpretation of data, statistical analysis, and revision of the manuscript for intellectual content. All authors read and approved the final version of the manuscript.
Acknowledgments. The authors acknowledge Jessica Langel, Petra Klöters-Plachky, Jutta Mohr, and Alexandra Hof for patient-related and technical support; Markus Zorn for laboratory analyses; and Sylvia Parthé, Paul Schnitzler, Maria Anders-Össwein, and Stefanie Wolf for support with serological analyses. The data will be made publicly available no later than the time of online publication.
Financial support. The work of T. W. was supported by a donation from the Dieter Morszeck Foundation.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.NICE COVID-19 rapid guidelines. PharmacoEcon Outcomes News 2021;877:33. doi: 10.1007/s40274-021-7682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27:601–15. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis A, Wamil M, Alberts J, et al. ; COVERSCAN Study Investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021; 11:e048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marx V. Scientists set out to connect the dots on long COVID. Nat Methods 2021; 18:449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020; 383:1757–66. [DOI] [PubMed] [Google Scholar]
- 9.Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ 2004; 13:873–84. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JA, Coons SJ. Comparison of the EQ-5D and SF-12 in an adult US sample. Qual Life Res 1998; 7:155–66. [DOI] [PubMed] [Google Scholar]
- 11.Butt J, Murugan R, Hippchen T, et al. From multiplex serology to serolomics—a novel approach to the antibody response ag ainst the SARS-CoV-2 proteome. Viruses 2021; 13: 749. doi: 10.3390/v13050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun 2012; 38:J109–19. [DOI] [PubMed] [Google Scholar]
- 13.Guilmot A, Maldonado Slootjes S, Sellimi A, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol 2021; 268:751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacchi MC, Tamiazzo S, Stobbione P, et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci 2021; 14:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent long-COVID-19 symptoms. J Transl Autoimmun 2021; 4:100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997; 40:1601–11. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2021; 27:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2020; 76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al. ; COVID19-ALC Research Group. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect 2021; 82:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021; 93:1013–22. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15:e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81:e4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera-Correa J, Rodriguez A. Divergent roles of antiself antibodies during infection. Trends Immunol 2018; 39:515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra PK, Bruiners N, Ukey R, et al. Vaccination boosts protective responses and counters SARS-CoV-2-induced pathogenic memory B cells. medRxiv [Preprint]. April 14, 2021. 2021. doi: 10.1101/2021.04.11.21255153. Accessed 4 May 2021. [DOI] [Google Scholar]
- 25.Arnold DT, Milne A, Samms E, et al. Symptoms after COVID-19 vaccination in patients with persistent symptoms after acute infection: a case series. Ann Intern Med 2021. doi: 10.7326/M21-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.