Abstract
Simple Summary
Colorectal cancer (CRC) belongs to the most common cancer types. It is well known that half of all CRC possess missense mutations in the TP53 tumor suppressor gene. However, the entire signaling cascade upstream and downstream of the p53 protein may also contribute to CRC development, if relevant players in this signaling cascade lost their function. Besides p53 loss-of-function by mutations, epigenetic changes (DNA methylation, post translational modifications of histones, micro-RNAs) play a vital role in CRC development. In the present review, we concentrated on the epigenetic modifications related to the entire p53 signal transduction cascade upstream and downstream of p53. Indeed, numerous epigenetic aberrations influence the tumor suppressor function of p53 independent of missense mutations. Thus, the role of p53 for CRC development, therapy response and survival prognosis of patients may be much more complex than predicted earlier. Hence, we are in need to use novel diagnostic methods that are capable of evaluating the genetic and epigenetic changes in the “p53 signalome”, so that diagnosis and management of CRC will improve.
Abstract
Colorectal cancer (CRC) belongs to the most common tumor types, and half of all CRC harbor missense mutations in the TP53 tumor suppressor gene. In addition to genetically caused loss of function of p53, epigenetic alterations (DNA methylation, histone modifications, micro-RNAs) contribute to CRC development. In this review, we focused on epigenetic alterations related to the entire p53 signaling pathway upstream and downstream of p53. Methylation of genes which activate p53 function has been reported, and methylation of APC and MGMT was associated with increased mutation rates of TP53. The micro-RNA 34a activates TP53 and was methylated in CRC. Proteins that regulate TP53 DNA methylation, mutations, and acetylation of TP53-related histones were methylated in CRC. P53 regulates the activity of numerous downstream proteins. Even if TP53 is not mutated, the function of wildtype p53 may be compromised if corresponding downstream genes are epigenetically inactivated. Thus, the role of p53 for CRC development, therapy response, and survival prognosis of patients may be much more eminent than previously estimated. Therefore, we propose that novel diagnostic devices measuring the entirety of genetic and epigenetic changes in the “p53 signalome” have the potential to improve the predictive and prognostic power in CRC diagnostics and management.
Keywords: acetylation, carcinogenesis, methylation, micro-RNA, oncogene, signal transduction, tumor suppressor
1. Introduction
Since the early 19th century and the ground-breaking experiments of Gregor Mendel, genetics is fundamental in biology in general and also specifically in cancer biology [1]. Epigenetics emerged as new field, which complements many genetic mechanisms in evolution of life on earth, organismic homeostasis, and pathophysiology of diseases as well. Epigenetics focuses on changes in gene function, which are not caused by changes in DNA (mutation, recombination), but which are inherited from one cell to another. Epigenetic changes are related to the methylation of DNA or RNA, the modifications of chromatin and the expression of micro-RNAs.
DNA methylation is catalyzed by DNA-methyltransferases (DNMTs). DNMT1 maintains the methylation pattern of DNA, while DNMT3a and DNMT3b are responsible for de novo methylation. RNA methylation is catalyzed by DNMT2 [2,3]. There is a balance between methylation and demethylation of nucleic acids. Demethylation reactions are catalyzed by DNA methylase. DNMTs preferentially methylate cytosine within CpG islands. CpG islands are CG-rich DNA regions upstream of genes, which comprise gene promoters and regulate gene expression by different degrees of methylation. It has been estimated that about one half of all CpG islands is located in housekeeping genes and that about 40% of all gene promoters contain CpG islands [4,5]. CpG islands are domains for the recruitment of RNA polymerase II and transcription factors to initiate the transcription process [6]. CpG methylation favors the binding of methyl-binding proteins, which results in a nucleosome condensation and thereby inhibition of transcription.
DNA is bound to histones (histone 2A, histone 2B, histone 3, and histone 4), which together form the nucleosome. Histones can be methylated, acetylated, and/or phosphorylated at lysine, histidine and arginine residues. The acetylation of histones by histone acetyltransferases (HATs) opens the nucleosome conformation and thereby enables transcription by RNA polymerase II. The closure of open chromatin leading to transcriptional repression is conferred by methyl-binding proteins [7].
In contrast to HATs, histone deacetylases (HDACs) remove the acetyl group from the lysine residues and restore the interaction between DNA and histones. As a result, the histone-DNA architecture is stabilized leading to the inhibition of transcription process [8]. Similar to yeast deacetylases, HDACs are classified into four major types: class I HDACs (HDAC1-3, and HDAC8), class II HDACs (HDAC4-7, HDAC9, and HDAC10), class III HDACs (also named sirtuins) (SIRT1-7), and class IV which consists only of HDAC11 [9].
Micro-RNAs (miRNAs) are short, about 22 nucleotide long, non-coding RNA sequences, which are complementary to messenger RNA (mRNA) sequences [10]. Because of their complementarity, they can bind to corresponding mRNA species. Double-stranded miRNA-mRNA molecules are cleaved by RNases [11,12]. Thereby, miRNAs can silence gene expression.
Taken together, gene expression in mammals is epigenetically regulated by DNA methylation, histone acetylation, and micro-RNAs. As the basic scaffold of the DNA is neither changed by DNA-methylation, nor by histone acetylation and miRNAs, these modifications are not referred to as genetic mutations.
During the past years, it became evident that epigenetics plays an eminent role for carcinogenesis, because the silencing of tumor suppressor genes can happen both by classical genetic mechanisms (e.g., point mutations, chromosomal aberrations) as well as epigenetic modifications [13]. It is widely accepted that carcinogenesis evolves stepwise: (1) initiation with DNA mutation, (2) promotion with clonal expansion and proliferation of initiated cells, and (3) progression with chromosomal instability, neoangiogenesis, and metastasis [14,15]. Epigenetic changes can occur in all three stages of cancer development [13].
A central player in carcinogenesis is the tumor suppressor gene TP53. Its encoded protein p53 acts as transcription factor, which arrests the cell cycle after DNA damage and either contributes to DNA repair or triggers the induction of apoptosis [16,17]. Besides, p53 plays a prominent role in the regulation of cellular senescence and organismal aging [18,19,20]. Mutations cause a loss of function and are critical for the development of cancer, since damaged cells continue to divide and become malignant [21]. Remarkably, 50–60% of all colorectal cancers harbor mutations in the TP53 gene, most of which are missense mutations at certain hot spots of the gene [22].
In general, mutations can be categorized as driver mutations, which provide a survival advantage to tumor cells, and passenger mutations. In addition to mutations in TP53, other driver mutations in colorectal cancer (CRC) are found in genes of the WNT signaling pathway (e.g., APC and CTNNB1), TGF-β, DCC, SMAD, KRAS, RAF, BRAF, PI3K, PTEN as well as in the DNA mismatch repair genes hMSH3 and hMSH6, beyond other mutations [23].
Mutations in DNA mismatch repair genes do not only predispose to mutations in driver genes, but also to another characteristic feature of colorectal cancer, i.e., microsatellite instability (MSI) [24]. Because of impaired DNA mismatch repair, there is a predisposition for DNA mutations, and three phenotypes can be distinguished: MSI stable, MSI low, and MSI high, the latter one with a better prognosis than the others [25]. Epigenetic inactivation of other DNA repair genes contributes to mutations of driver genes in CRC (e.g., the mismatch repair genes hMLH1, hMSH2, and the gene coding for O6-methyguanine-DNA methyltransferase, MGMT) [26].
Interestingly, epigenetic alterations occur more frequently in colorectal cancer than gene mutations. A large number of hyper- and hypomethylations appear in genes and miRNAs of a majority of colorectal cancers [27]. It is estimated that 600–800 genes are transcriptionally silenced by CpG island methylation and that miRNAs also considerably affect transcriptional repression in this tumor entity [28,29]. The list of genes belonging to this CpG island hypermethylated phenotype (CIMP) is still growing and this phenotype is of clinical prognostic significance [30,31,32]. Several different epigenotypes have been defined in colorectal cancer with different prognostic outcome (high and low methylator phenotypes associated with specific high and low MSI and mutational profiles in driver genes [33,34]).
Even if CIMP epigenotypes can be identified consisting of different genetic and epigenetic profiles, there are numerous specific interactions between single genes (e.g., TP53) and aberrant methylation in p53-related genes.
A lot of attention has been paid on mutations in the TP53 gene and the related loss of function. Rather than genetic alterations, we focus in the present paper on epigenetic changes in the TP53 gene and also on epigenetics in the signaling cascade upstream and downstream of the p53 protein. If we assume that epigenetic changes in genes upstream and downstream of p53 also contribute to alterations in p53 signaling, the oncogenic potential of the entire epigenetically silenced p53 signaling pathways in addition to 50–60% mutations in TP53 itself is much larger than estimated yet. Therefore, we performed a systematic review on the interactions between p53 and methylation changes in genes upstream of the p53 signaling cascade or downstream genes, which are regulated by p53. Our overview is based on a PubMed search with the keywords “colorectal + epigenetic + p53” and “colorectal + methylator phenotype + p53” as of 19 May 2021.
2. Epigenetic Alterations Upstream of p53
2.1. Methylation Status of p53-Activating Genes
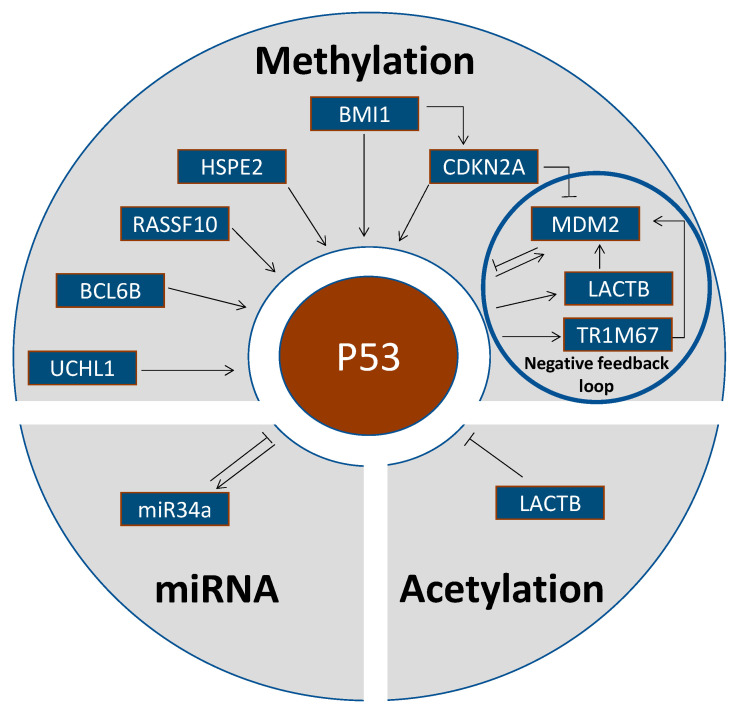
A number of genes mostly acting as tumor suppressors or oncogenes regulate the activity of p53 (Table 1, Figure 1).
Table 1.
Epigenetic alterations upstream of p53.
| Gene | Gene Name | Gene Function | Epigenetic Event | Reference |
|---|---|---|---|---|
| UCHL1 | ubiquitin carboxyl-terminal hydrolase L1 | Tumor suppressor. Carboxyl-terminal ubiquitin hydrolase regulating cellular ubiquitin levels | UCHL1 methylation in 22/31 CRC (=71%). UCHL1 binds to and stabilizes p53 by the ubiquitination pathway, UCHL1 demethylation caused growth inhibition, G2/M arrest and induction of apoptosis. | Yu et al., 2008 [35] |
| BCL6B | B-Cell CLL/Lymphoma 6 Member B | Tumor suppressor. BCL6B activates p53 signaling and induces apoptosis. | BCL6B was methylated in 81/102 CRC (=79%). | Hu et al., 2015 [36] |
| RASSF10 | Ras-association domain family member 10 | RASSF10 activates p53 signaling and sensitizes to docetaxel. RASSF10 demethylation induced apoptosis and inhibited proliferation. | RASSF10 was methylated in 54/89 CRC (=61%) and was positively associated with tumor stage and metastasis. | Jin et al., 2015 [37] |
| HPSE2 | Heparanase 2 | Tumor suppressor. HPSE2 regulates p53 signaling and G1 cell cycle arrest. | HPSE2 hypermethylation correlated with shorter survival times of CRC patients. | Zhang et al., 2021 [38] |
| BMI1 | B Lymphoma Mo-MLV insertion region 1 homologue | Proto-oncogene. BMI1 is an epigenetic repressor by chromatin remodeling. p14ARF is silenced by BMI1. | p14ARF and wild-type p53 were upregulated in BMI1-mutant CRC. p14ARF is required for the induction of p53 and apoptosis. | Maynard et al., 2014 [39] |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A | CDKN2A/p14ARF antagonizes MDM2-dependent p53 degradation. | p14ARF hypermethylation was increased in tumors with wildtype compared to mutated p53 without statistical significance. | Esteller et al., 2000 [40] |
| p14ARF promoter demethylation was associated with nuclear MDM2 expression (active state), p14ARF promoter hypermethylation with cytosolic MDM2 expression (inactive state). | Nuclear MDM2 expression was associated with p14ARF promoter demethylation in 33 CRC. | Esteller et al., 2001b [41] | ||
| Tumor suppressor. Regulates p53 protein stability by interaction with MDM2. p14ARF promoter hypermethylation abrogates wild-type p53 activity. | CDKN2A promoter hypermethylation was significantly correlated with restricted p53 overexpression and MDM2 overexpression. Epigenetic silencing of CDKN2A is a deregulating mechanism of the p53-MDM2-p14ARF pathway in CRC exhibiting restricted p53 overexpression. | Nyiraneza et al., 2012 [42] | ||
| APC | Adenomatous Polyposis Coli Protein | APC inhibits β-catenin, and β-catenin overexpression increases the TP53 mutation rate. | APC hypermethylation was associated with p53 mutation in 208 CRC. | Suehiro et al., 2008; Stamos and Weis, 2013 [43,44] |
| MGMT | O6-methylguanine-DNA methyltransferase | MGMT downregulation by promoter hypermethylation predisposes to p53 mutations. MGMT hypermethylation is associated with G > A mutations in TP53. | MGMT promoter hypermethylation was significantly correlated to G:C > A:T transition p53 mutations in 314 CRC. | Esteller et al., 2001a [45] |
| MGMT methylation was associated with G > A mutations in the TP53 gene. | Deng et al., 2008 [46] | |||
| No association between MGMT methylation and TP53 mutations in 261 CRC biopsies from Afro-American patients | Alonso et al., 2015 [47] | |||
| PTX3 | Pentraxin 3 | Tumor suppressor. PTX3 deficiency increases susceptibility to carcinogenesis by increasing DNA damage, p53 mutations, and inactivation of p53 downstream signaling (Mdm2, Bax, and Cdkn1a /p21). | Increasing PTX3 promoter methylation from normal colon epithelium to adenomas and CRC. | Bonavita et al., 2015 [48] |
| PCAF | P300/CBP-associated factor | Trimethylated histone H3K27 binding in the PCAF promoter attenuated its transcription. | Decreased PCAF impairs the acetylation of p53 and attenuates the p53-dependent transcription of p21, which results in the increased cyclin D1 expression and Retinobla-stoma 1 phosphorylation as well as increased resistance to 5-fluorouracil. | Liu et al., 2019 [49] |
| LACTB | Lactamase β | Tumor suppressor. LACTB binds to the p53 C terminus to inhibit p53 degradation by MDM2. | LACTB was significantly downregulated in CRC due to promoter methylation and histone deacetylation. | Zeng et al., 2018 [50] |
| TRIM67 | Tripartite motif containing 67 | Tumor suppressor. Upon stress, p53 binds to TRIM67 promoter and upregulates TRIM67 expression in a TRIM67/p53 self-amplifying loop. TRIMP67 interacts with the p53 C-terminus to inhibit p53 degradation by MDM2. | 108/138 CRC (=79%) downregulated TRIMP67 expression due to promoter methylation. Demethylation by treatment with 5-aza-2′-deoxycytidine restored TRIM67 expression in CRC cells. | Wang et al., 2019 [51] |
| COX2 | Cyclooxygenase 2 | Role in CRC progression. Converts arachidonic acid to prostaglandins | Methylation in 12/93 (=13%) CRC and 7/50 (=14%) colorectal ademonas. COX2 methylation was inversely related to p53 mutations. Functional relationship unclear | Toyota et al., 2000a [52] |
| CIMP | CpG island methylator phenotype | High levels of DNA methylation may predispose to carcinogenesis. | TP53 mutations in 10/41 (=24%) CIMP-positive CRC compared to 30/46 (=60%) CIMP-negative cases. Functional relationship unclear | Toyota et al., 2000b [53] |
| CIMP | CpG island methylator phenotype | CIMP correlated with wildtype p53. Functional relationship unclear | Konishi et al., 2007 [54] | |
| miR-34a | Micro-RNA 34a | miR-34a acts as translational repressor. | miR-34a activates p53 by inhibiting its acetylation by MTA2 and HDAC1. | Kaller et al., 2011 [55] |
| miR-34a methylation correlates with wild-type p53 in CRC and other tumor types. | Vogt et al., 2011 [56] |
Figure 1.
Epigenetic alterations upstream of p53.
Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) is a tumor suppressor protein, which stabilizes p53 expression. Its demethylation causes growth inhibition by G2/M cell cycle arrest and apoptosis induction. UCHL1 methylation has been reported in 22/31 CRC cases (=71%) [35].
B-Cell CLL/lymphoma 6 member B (BCL6B) is a tumor suppressor protein, which activates p53 signaling and induces apoptosis. The BCL6B gene was methylated in 81/102 CRC cases (=79%) [36].
Ras-association domain family member 10 (RASSF10) is a RAS-associated domain family member, which activates p53 signaling and sensitizes to anti-cancer drugs (e.g., docetaxel). Demethylation of RASSF10 induced apoptosis and inhibited proliferation in 54/89 CRC cases (=61%). The RASSF10 methylation status was positively associated with tumor stage and metastasis [37].
Heparanase 2 (HPSE2) acts as tumor suppressor, which regulates p53 signaling leading to G1 arrest of the cell cycle. Hypermethylation of HPSE2 significantly correlated with shorter survival times of CRC patients [38].
The proto-oncogene BMI1 (B lymphoma Mo-MLV insertion region 1 homologue) is an epigenetic repressor by remodeling chromatin. P14ARF and wildtype p53 were upregulated in BMI1-mutated CRC cases [39]. P14ARF is required for the induction of p53 expression and apoptosis.
The protein stability of p53 is regulated by p14ARF, which is also designated as cyclin-dependent kinase inhibitor 2A (CDKN2A). This protein interacts with MDM2 (mouse double minute 2 homologue) and thereby antagonizes the MDM2-dependent degradation of p53. Demethylation of the CDKN2A promoter was associated with MDM2 expression, while CDKN2A hypermethylation resulted in cytosolic translocation and inactivation of MDM2. Thereby, CDKN2A hypermethylation abrogated the activity of wildtype p53. CDKN2A hypermethylation was more frequently found in tumors with wildtype p53 than in those with mutated p53 [40]. CDKN2A demethylation was associated with nuclear (i.e., active) MDM2 expression in CRC [41]. In CRC, CDKN2A promoter hypermethylation was significantly correlated with p53 overexpression and MDM2 overexpression [42].
2.2. Methylation Status of p53-Inhibiting Genes
In addition to genes that activate p53, epigenetic regulation of genes, whose gene products inhibit p53, has also been reported (Table 1).
The tumor suppressor APC (adenomatous polyposis coli protein) inhibits β-catenin, and β-catenin overexpression increases the TP53 mutation rate. Hypermethylation of APC was associated with p53 mutation in 208 CRC cases [43,44].
O6-Methylguanine-DNA methyltransferase (MGMT) is a DNA repair protein whose downregulation by promoter hypermethylation predisposes genes to p53 mutations. MGMT promoter hypermethylation was significantly correlated to G > A transition mutations in the TP53 gene in 314 CRC patient samples [45]. This result was confirmed by other authors [46]. However, there was no association between MGMT methylation and TP53 mutations in 261 CRC biopsies from Afro-American patients, indicating that population-based differences may exist [47].
Deficiency of the tumor suppressor pentraxin 3 (PTX3) increases susceptibility to carcinogenesis by increasing DNA damage, p53 mutations and inactivation of p53 downstream signaling (MDM2, BAX, and CDKN1A/p21). Increasing rates of PTX3 promoter methylation were observed from normal colon epithelium to adenomas and CRC biopsies [48].
Trimethylated histone H3K27 binding in the PCAF (P300/CBP-associated factor) promoter attenuated transcription of this gene. Decreased PCAF impaired the acetylation of p53 and attenuated the p53-dependent transcription of p21, which resulted in increased cyclin D1 expression and Retinoblastoma 1 (RB1) phosphorylation as well as increased resistance to 5-fluorouracil [49].
Lactamase β (LACTB) is a tumor suppressor, which binds to the C-terminus of p53 to inhibit MDM2-mediated p53 degradation. LACTB was significantly downregulated in CRC due to promoter methylation [50].
TRIM67 (Tripartite motif containing 67) is also a tumor suppressor. Under stress conditions, p53 binds to the TRIM67 promoter and upregulates TRIM67 expression in a TRIM67/p53 self-amplifying loop. TRIM67 interacts with the C-terminus of p53 to inhibit MDM2-mediated p53 degradation. In 108/138 CRC (=79%), TRIM67 expression was downregulated due to promoter methylation. Demethylation by treatment with 5-aza-2′-deoxycytidine restored TRIM67 expression in CRC cells [51].
2.3. Methylation Status of Other Genes
Epigenetic alterations have been associated with p53 without explicitly describing the direct causative relationship to p53 function (Table 1 and Table 2).
Table 2.
In vitro and in vivo studies on epigenetics of p53 in CRC.
| Gene Name | Epigenetic Event | Cellular Function | Reference |
|---|---|---|---|
| Lactamase β (LACTB) | LACTB promoter methylation and histone deacetylation | Activation of CRC cell proliferation, migration, and invasion in HCT116 and HCT8 cells. Inhibited CRC xenograft growth and metastasis in vivo | Zeng et al., 2018 [50] |
| Deregulation of P53 | Abnormalities of miRNA expression | p53 effects (e.g., cell cycle arrest and apoptosis) were phenocopied in HCT-116 cells | Nugent et al., 2011 [76] |
| O6-methylguanine DNA methyltransferase | Aberrant methylation of APC, MGMT, hMLH1, P16, N33 | High levels of microsatellite instability in 208 CRC patients | Suehiro et al., 2008 [43] |
| Inhibitor of growth 2 (ING2) | ING2 is a cofactor of p300 for p53 acetylation | Positive regulation of p53-mediated replicative senescence in young fibroblasts | Pedeux, et al., 2020 [77] |
| NAD+-dependent protein deacetylase SIRT1 | Deacetylation of p53 | Decrease of cell proliferation and induction of apoptosis in vivo and in CRC patients | Stünkel et al., 2007 [78] |
Cyclooxygenase 2 (COX2) is a proinflammatory enzyme, which is involved in the progression of CRC. Methylation of the COX2 gene was found in 12/93 CRC biopsies (=13%) and 7/50 colorectal adenomas (=14%). COX2 methylation was inversely related to TP53 mutations, albeit the functional relevance of these mutations is not clear yet [52].
A CpG island methylator phenotype (CIMP) can be found in a fraction of CRC. CIMP was discussed as a predisposing factor for CRC carcinogenesis. TP53 mutations have been found in 10/41 CIMP-positive CRC (=24%) compared to 30/46 CIMP-negative cases (=60%) [53]. Another study also reported that CIMP correlated with wildtype p53 [54].
2.4. Acetylation Status of p53-Inhibiting Genes
LACTB was not only downregulated in CRC due to promoter methylation, but also due to histone deacetylation [50] (Table 1 and Table 2).
2.5. P53 Regulation by Micro-RNAs
Micro-RNAs are involved in the regulation of p53 and its network at multiple levels [57] (Table 1 and Table 2). This can occur by direct p53 targeting or indirectly by targeting p53 regulators (e.g., MDM2 and MDM4). Vice versa, p53 is a transcriptional regulator of numerous miRNAs, which contributes to its tumor suppressive function.
In tumor cells, including CRC, miRNAs can function in a tumor-suppressive (protective) or tumor-promoting (oncogenic) manner. MiR-339-5p was frequently downregulated and associated with poor patients’ prognosis; miR-339-5p and miR1827 directly repressed MDM2 expression through binding to MDM2 3′-UTR, which elevated p53 protein expression and p53-mediated apoptosis and senescence. In parallel, it also inhibited migration, invasion, and the growth of CRC xenografts [58]. Other tumor-suppressive miRNAs targeting the MDM2/p53 axis were miR193a-5p and miR-146a-5p [59]. MiR-1249 is a direct transcriptional target of p53, and p53-mediated induction of miR-1249 inhibited tumor growth, metastasis, and angiogenesis in vitro and in vivo [60]. Ectopic expression of miR-133a markedly increased p53 levels and induced p21 transcription and, thus, significantly suppressed CRC cell growth in vitro and in vivo, and sensitized cells to doxorubicin and oxaliplatin [61]. The p53-induced miR-34 microRNA family mediated repression of c-Kit by p53 via a conserved seed-matching sequence in the cKIT 3′-UTR; ectopic c-Kit expression conferred resistance of CRC cells to 5-FU, whereas ectopic miR-34a sensitized the cells to the drug [62]. MiR-34a directly inhibited the oncogenic receptor tyrosine kinase CSF1R, and p53 repressed CSF1R by inducing miR-34a. Accordingly, resistance of CRC cells to 5-FU was mediated by miRNA-34a silencing (via CpG-methylation) and the resulting elevated expression of CSF1R [63]. In CRC cells, the transcription factor AP4 was downregulated by p53, which was indirectly mediated by the tumor-suppressive miR-15a and miR-16-1, targeting the 3′-UTR of AP4 mRNA, inducing mesenchymal-epithelial transition (MET) and inhibiting CRC cell migration and invasion [64]. MiR-16 repressed CRC cell growth by decreasing Survivin (BIRC5) expression through a direct targeting of BIRC5, and p53 negatively modulated BCL-2 by controlling miR-1915 [65,66]. Also, miR-148b, whose transcription is directly activated by p53, bound specifically to the 3′-UTR of P55PIK mRNA and suppressed p55PIK expression, which abolished proliferation and cell cycle progression of CRC cells and decreased tumor growth in vivo [67]. Furthermore, miR-143 and miR-145 function in a tumor-suppressive way, and the major mediators of the oncosuppression were genes belonging to the growth factor receptor-mitogen-activated protein kinase network and to the p53 signaling pathway [68]. In rectal tumors, elevated expression of miR-150-5p and miR-196b-5p significantly increased patients’ survival [69]. In a genome-wide systematic approach, miR-30e, a direct transcriptional target of p53, was the most frequently deregulated miRNA in a p53-deficient background of CRC [70]. MiR-600 represents a direct negative regulator of p53 through binding the 3′ UTR of the TP53 mRNA. Its overexpression decreased endogenous levels of mutant p53 and inhibited cell proliferation, migration, and invasion in mutant p53-expressing CRC cells [71]. Furthermore, the WNT/cMYC axis signaling inhibited the expression of p53 by promoting a direct targeting of p53 by miR-552 leading to resistance to drug-induced apoptosis, suggesting that miR-552 may function as an oncogene [72]. Furthermore, miR-27a was also identified to be oncogenic in CRC cells. The overexpression of miR-27a, i.e., its binding to two putative binding sites on the 3′-UTR of the TP53 mRNA, resulted in the decreased p53 expression [73]. MiR-300 was a direct positive regulator of p53 through binding to the 3′UTR of TP53 in mutant p53 CRC cells. Both miR-300 and p53 induced EMT, thus being oncogenic [74]. One more CRC-promoting miRNA described so far is miR-150-5p repressing the p53 pathway [75].
Micro-RNA 34a acts as translational regulator, which activates p53 by inhibiting its acetylation by MTA2 (Metastasis Associated 1 Family Member 2) and HDAC1 [55]. The methylation of miR34a correlated with the p53 wildtype status in CRC and other tumor types [56].
3. Epigenetic Alterations at p53 as Target Site
3.1. Methylation of the TP53 Gene
The TP53 gene is a direct target site for epigenetic regulation (Table 3). The methylation inside and outside of exonic CG sequences of the TP53 gene was correlated with point mutations. Cigarette smoking increased the occurrence of methylation-associated mutations [79]. Methylation of the TP53 gene, histone modifications, chromatin remodeling, and non-coding RNAs were significantly associated with colitis-related carcinogenesis and tumor progression [80].
Table 3.
Epigenetic alterations at TP53 as target site.
| Gene | Gene Name | Gene Function | Epigenetic Event | Reference |
|---|---|---|---|---|
| TP53 | Tumor suppressor 53 | Tumor suppressor | Methylation inside and outside of exonic CG sequences was correlated with point mutations. Cigarette smoking increased the occurrence of methylation-associated mutations. | Kouidou et al., 2006 [79] |
| TP53 | DNA methylation, histone modifications, chromatin remodeling, and non-coding RNAs were significantly associated with colitis-related carcinogenesis and tumor progression. | Saraggi et al., 2017 [80] | ||
| POLE | Polymerase ε | Involved in DNA repair and chromosomal DNA replication. Methylated cytosines (5mCs) are frequently mutated. POLE exonuclease domain mutations increase 5mC mutagenesis and a mutator phenotype. | Highly methylated CpGs in TP53 contain mutation hotspots in POLE-mutated CRC | Poulos et al., 2017 [82] |
| PHF2 | PHD finger protein 2 | Tumor suppressor. Histone demethylase. PHF2 demethylates the repressive H3K9-Me2 mark in chromatin to induce p53 transcription. | PHF2 was downregulated in CRC and PHF2 correlated with p21 in cancers expressing functional p53. | Lee et al., 2015 [83] |
| LSD1 | LSD1demethylated K370me2 (dimethylated Lys370) of p53, which cannot bind to DNA and is inactivated. LSD1 inhibits p53-mediated apoptosis. | Huang et al., 2007 [84] | ||
| LSD1 | Histone lysine-specific demethylase 1 | Lysine-specific demethylases that removes histone methylations catalyzed by histone methyltransferases. | LSD1-knockout in HCT116 cells did not increase H3K4me2 (dimethyl-H3K4) or change stability or function of p53. | Jin et al., 2013 [85] |
| LSD2/KDM1B | Lysine-specific histone demethylase 1B | Oncogene. LSD2 directly binds to TP53 and represses p53 expression via H3K4me2 demethylation at the TP53 promoter. | LSD2 bound and inhibited p53 activity in CRC. | Cai et al., 2020 [86] |
| NRDC | Nardilysin, N-arginine dibasic convertase | NRDC is a dimethyl-H3K4 binding protein. | Nuclear NRDC bound to histone deacetylase 1 (HDAC1) and inhibited HDAC1 recruitment to the TP53 promoter and p53 acetylation. | Li et al., 2012; Kanda et al., 2018 [87,88] |
Polymerase epsilon (POLE) is a replicative polymerase important for efficient replisome assembly and strand synthesis, supporting tumor suppression [81]. POLE is involved in DNA repair and especially methylated cytosines (5mCs) are frequently mutated. Mutations in POLE exonuclease domain increase 5mC mutagenesis and a mutator phenotype. CRC with mutated POLE frequently contain highly methylated CpG islands in the TP53 gene [82].
PHD finger protein 2 (PHF2) is a tumor-suppressing histone demethylase. It demethylates the repressive H3K9-Me2 mark in chromatin to induce p53 transcription. PHF2 was downregulated in CRC and PHF2 correlated with p21 in cancers expressing functional p53 [83].
Histone lysine-specific demethylases 1 and 2 (LSD1, LSD2) are demethylases that bind to p53 and demethylate K370me2 at Lys370 (LSD1) or K3K4me2 in the TP53 promoter (LSD2). This leads to the inhibition of p53 function and p53-mediated apoptosis [83,84,85,86].
3.2. Acetylation of p53
In addition to methylation, epigenetic regulation of p53 can also take place by acetylation (Table 3). NRDC is a histone-binding protein that binds HDAC1 and inhibits HDAC1 recruitment to the TP53 promoter and p53 acetylation [87,88].
4. Epigenetic Alterations Downstream of p53
4.1. Methylation Status of p53 Downstream Genes
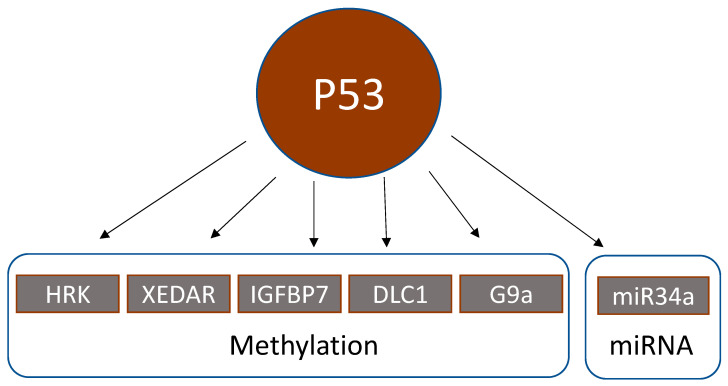
Genes that are activated by p53 still can lead to inactive downstream signaling, if they are methylated (Table 4, Figure 2).
Table 4.
Epigenetic alterations downstream of p53.
| Gene | Gene Name | Gene Function | Epigenetic Event | Reference |
|---|---|---|---|---|
| HRK | BCL-2-interacting protein | Proapoptotic p53 target protein, whose expression is reversible by methylation inhibitors (5-aza-deoxycytidine) and further enhanced by histone deacetylase inhibitors (trichostatin A, depsipeptide) | HRK promoter methylation significantly correlated with wildtype p53 in 58 CRC. | Obata et al., 2003 [89] |
| XEDAR | X-linked ectodermal dysplasia receptor | XEDAR is a member of the tumor necrosis factor receptor family. P53 upregulates XEDAR expression through two p53-binding sites within intron 1 of the XEDAR gene. Inactivation of XEDAR results in enhanced cell adhesion and spreading, and resistance to p53-induced apoptosis. | XEDAR downregulation by hypermethylation or TP53 mutations in CRC cell lines and biopsies | Tanikawa et al., 2009 [90] |
| IGFBP7 | Insulin-like growth factor-binding protein 7 | P53 induces expression of IGFBP7 upon binding to a p53 response element in intron 1 of IGFBP7. | IGFBP7 methylation was significantly higher in 83 CRC biopsies than in normal colonic tissue. 5-Aza-2′-deoxycytidine restored p53-induced IGFBP7 expression. IGFBP7 methylation significantly correlated with wildtype p53. | Suzuki et al., 2010 [91] |
| DLC1 | Deleted in liver cancer 1 | Tumor suppressor. The functional DLC1-i4 promoter is activated by wild-type p53. | DLC1 isoform 4 (DLC1-i4) was methylated in 2/4 CRC cell lines (=50%). Demethylation with 5-aza-2′-deoxycytidine restored DLC-i4 expression. | Low et al., 2011 [92] |
| G9a/KMT1C/EHMT2 | Lysine methyltransferase 1C, euchromatic histone methyltransferase 2 | Histone methyltransferase that catalyzes mono- and dimethylation of histone H3K9 | G9a dimethylated p53 at lysine 373. This transactivated PLK1 expression and increased proliferation | Zhang et al., 2018 [93] |
| miR-34b/c | Micro-RNA 34b/c | p53 activates the transcription of miR-34 family members. The miR-34b/c CpG island is a bidirectional promoter, regulating the expression of both miR-34b/c and B-cell translocation gene 4 (BTG4). Methylation of this promoter silenced BTG4 transcription. | miR-34b/c CpG island methylation in 100/111 (=90%) CRC | Toyota et al., 2008; Navarro and Lieberman, 2015 [98,99] |
Figure 2.
Epigenetic alterations downstream of p53.
The BCL-2-interacting protein HRK interacts with BCL-2 and is a pro-apoptotic p53 target protein. Its expression can be inhibited by the methylation inhibitor 5-aza-2′-deoxycytidine, an effect which is further enhanced by histone deacetylase inhibitors (trichostatin A, depsipeptide). The methylation of the HRK promoter significantly correlated with the p53 wildtype status in 58 CRC cases [89].
The X-linked ectodermal dysplasia receptor (XEDAR) is a member of the tumor necrosis factor receptor family. P53 upregulates XEDAR expression through two p53-binding sites within intron 1 of the XEDAR gene. Inactivation of XEDAR results in enhanced cell adhesion and spreading, and resistance to p53-induced apoptosis. The expression of XEDAR was down-regulated by promoter hypermethylation or TP53 mutations in CRC cell lines and clinical biopsies [90].
The expression of the insulin-like growth factor-binding protein 7 (IGFBP7) is induced by binding of p53 to a p53-responsive element in intron 7 of the IGFBP7 gene. IGFBP7 methylation was significantly higher in 83 CRC biopsies than in normal colonic tissue. 5-Aza-2′-deoxycytidine restored p53-induced IGFBP7 expression. IGFBP7 methylation significantly correlated with wildtype p53 [91].
DLC1 (deleted in liver cancer 1) is a tumor suppressor. The DLC1-i4 is an isoform whose promoter is activated by wildtype p53. DLC1-i4 was methylated in 2/4 CRC cell lines (=50%), and demethylation by 5-aza-2′-deoxycytidine restored its expression [92].
G9a (lysine methyltransferase 1C, euchromatic histone methyltransferase 2) is a histone methyltransferase, which demethylates the p53 protein at lysine 373. This reaction transactivated the expression of polo-like kinase 1 (PLK1) leading to increased proliferation [93].
4.2. Regulation of p53 by Acetylation
Several excellent reviews considered the various aspects of p53 acetylation. The acetylation of p53 at lysine 320 is achieved by histone acetyltransferase, PCAF (p300-CBP associated factor), which in most cases led to transactivation of the P21 (CIP1/WAF1/CDKN1A) promoter and increased histone acetylation after DNA damage [94,95]. Moreover, the addition of an acetyl group to p53 protein increased its stability, its binding to low affinity promoters, and other proteins [96]. On the other hand, inhibition of histone deacetylases (HDACs) resulted in increased p53 acetylation and eventually p53-dependent activation of apoptosis, cell cycle arrest, and senescence [97].
4.3. Regulation of Micro-RNAs by p53
Although not investigated to great detail, p53 can regulate micro-RNAs, which represents another level of gene regulation downstream of p53 (Table 4). P53 activated the transcription of miR-34 family members. The miR-34b/c CpG island is a bidirectional promoter, regulating the expression of both miR-34b/c and B-cell translocation gene 4 (BTG4). Methylation of this promoter silenced BTG4 transcription. The methylation of the miR34b/c CpG island was found in 100/111 CRC (=90%) [98,99].
5. Epigenetic Alterations and Clinical Outcome upon Standard Chemotherapy of CRC
In the past 10 years, a huge amount of data on epigenetic alterations in CRC has been published. Interest was placed in particular on putative clinical implications, emerging prognostic and diagnostic biomarkers, as well as on the implementation of the gained knowledge in precision (personalized) oncology [100,101,102,103,104,105].
Here, we focus on the impact of the CpG island methylator phenotype (CIMP), the most frequent epigenetically altered biomarker in CRC, on clinical outcome (disease progression, metastasis, and survival) of patients having received standard chemotherapy with 5-FU, oxaliplatin, and/or irinotecan. In a randomized controlled trial, significant differences in overall survival were not observed between patients with CIMP (+) vs. CIMP (−) tumors after adjuvant administration of oxaliplatin [106]. Thus, CIMP did not seem to be a prognostic biomarker in oxaliplatin-treated patients with resected CRC. On the other hand, in another clinical trial, CIMP was of prognostic value for stage III CRC patients treated with adjuvant oxaliplatin. A large cohort of well-defined patients with stage III CRC, CIMP (+) phenotype was associated with a shorter overall survival and a shorter survival after recurrence [107]. This was verified by another study, in which the CIMP (+) phenotype was an adverse prognostic marker in patients with metastatic disease treated with 5-FU/oxaliplatin and 5-FU/irinotecan [108]. Interestingly, in another clinical trial patients with stage III, CIMP-positive, MMR-intact CRC exhibited longer survival times, if irinotecan was added to combination therapy with 5-FU and leucovorin; i.e., those patients benefited most from the FOLFIRI protocol [109]. Furthermore, hypermethylation of p16 was predictive of clinical outcome in metastatic CRC patients treated with cetuximab and FOLFIRI, irrespective of KRAS mutation [110]. The progression-free survival of metastatic CRC patients with CIMP (+) tumors, who received sequential therapy with 5-FU/oxaliplatin (FOLFOX) as the first-line treatment, followed by irinotecan-based second-line treatment, was inferior to that of patients receiving the reverse sequence. It was concluded that the high mutation burden in EGFR-related genes in CIMP (+) tumors may cause a lower response to anti-EGFR antibody (cetuximab) therapy [111]. Recently, epigenetically regulated gene expression profiles revealed four molecular subtypes with prognostic and therapeutic implications in CRC [112]. A recent paper described a novel epigenetic signature of 8 hypermethylated genes that were able to identify metastatic CRC individuals with poor prognosis to oxaliplatin and irinotecan, characterized by CIMP (+) and MSI-like phenotype. The expression of the 8-genes signature and MSI-enriching genes was confirmed in oxaliplatin- and irinotecan-resistant CRC cell lines [113].
6. Conclusions and Perspective
While the significance of missense mutations in the TP53 gene has been recognized for a long time, the significance of epigenetic changes has only become clearer during the past few years. More and more data on epigenetic changes are being found not only in the TP53 gene itself, but also in genes upstream or downstream in the p53 signal transduction chain. Until now, it was assumed that about half of all CRC are affected by loss-of-function mutations in p53. If epigenetic changes upstream and downstream also have to be considered to contribute to CRC carcinogenesis, the significance of the entire p53 signaling pathway becomes much greater than previously assumed.
This is not only of prognostic relevance for the survival time of CRC patients, but also plays a prominent role for the response to therapy, since p53 is not only a factor that promotes carcinogenesis and tumor progression, but also determines the success of chemotherapy and radiotherapy of tumors [114].
Many studies showed the importance of epigenetics of TP53 in cancer in general. The methylation of TP53 and p53-related genes were well studied in comparison to acetylation and micro-RNAs. Therefore, more research is required to better understand how the epigenetics of p53 contributes to CRC carcinogenesis.
The entirety of all partners involved in signaling is referred to as the “signalome”. Therefore, we propose that the genetic and epigenetic characterization of the “p53 signalome” by the development of novel diagnostic devices based on biochips, DNA/RNA-sequencing, and other advanced technologies have the potential to significantly improve today’s diagnostic power in CRC management. For example, bisulfite treatment of genomic DNA in combination with high-throughput DNA sequencing enables the study of the genomic DNA methylation in general, and specifically, p53-dependent signaling pathway genes. This gold-standard (bisulfite-conversion) method has been adapted to fit with next-generation sequencing technologies and shotgun-sequencing approaches. Concerning the acetylation profile, the chromatin immunoprecipitation (ChIP) with high-throughput sequencing or DNA microarrays (ChIP-chip) is widely used.
Author Contributions
Conceptualization, T.E.; writing—original draft preparation, T.E.; figure drawings, M.D.; writing—review and editing, M.T.T., M.D. and T.E. All authors have read and agreed to the published version of the manuscript.
Funding
Support of the German Science Foundation grant [DFG TO 264/4-1] to M.T.T.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gayon J. From Mendel to epigenetics: History of genetics. C. R. Biol. 2016;339:225–230. doi: 10.1016/j.crvi.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Moore L.D., Le T., Fan G.P. DNA Methylation and Its Basic Function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards J.R., Yarychkivska O., Boulard M., Bestor T.H. DNA methylation and DNA methyltransferases. Epigenet. Chromatin. 2017;10:23. doi: 10.1186/s13072-017-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatemi M., Pao M.M., Jeong S., Gal-Yam E.N., Egger G., Weisenberger D.J., Jones P.A. Footprinting of mammalian promoters: Use of a CpG DNA methyltransferase revealing nucleosome positions at a single molecule level. Nucleic Acids Res. 2005;33:e176. doi: 10.1093/nar/gni180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee R., Vinson C. CpG methylation recruits sequence specific transcription factors essential for tissue specific gene expression. BBA-Gene Regul. Mech. 2012;1819:763–770. doi: 10.1016/j.bbagrm.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller F., Tora L. Chromatin and DNA sequences in defining promoters for transcription initiation. BBA-Gene Regul. Mech. 2014;1839:118–128. doi: 10.1016/j.bbagrm.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Sterner D.E., Berger S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000;64:435–459. doi: 10.1128/MMBR.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y., He C., Wang M.N., Ma X.L., Mo F., Yang S.Y., Han J.H., Wei X.W. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019;4:62. doi: 10.1038/s41392-019-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seto E., Yoshida M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. CSH Perspect. Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Lu Q., Chang C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020;1253:3–55. doi: 10.1007/978-981-15-3449-2_1. [DOI] [PubMed] [Google Scholar]
- 11.Bartel D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 13.Kanwal R., Gupta K., Gupta S. Cancer Epigenetics: An Introduction. Methods Mol. Biol. 2015;1238:3–25. doi: 10.1007/978-1-4939-1804-1_1. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Vousden K.H., Lane D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 17.Levine A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer. 2020;20:471–480. doi: 10.1038/s41568-020-0262-1. [DOI] [PubMed] [Google Scholar]
- 18.Rufini A., Tucci P., Celardo I., Melino G. Senescence and aging: The critical roles of p53. Oncogene. 2013;32:5129–5143. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]
- 19.Salama R., Sadaie M., Hoare M., Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mijit M., Caracciolo V., Melillo A., Amicarelli F., Giordano A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules. 2020;10:420. doi: 10.3390/biom10030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantovani F., Collavin L., Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama M., Oshima M. Mutant p53 in colon cancer. J. Mol. Cell Biol. 2019;11:267–276. doi: 10.1093/jmcb/mjy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raskov H., Soby J.H., Troelsen J., Bojesen R.D., Gogenur I. Driver Gene Mutations and Epigenetics in Colorectal Cancer. Ann. Surg. 2020;271:75–85. doi: 10.1097/SLA.0000000000003393. [DOI] [PubMed] [Google Scholar]
- 24.De’ Angelis G.L., Bottarelli L., Azzoni C., De’ Angelis N., Leandro G., Di Mario F., Gaiani F., Negri F. Microsatellite instability in colorectal cancer. Acta Bio-Med. Atenei Parm. 2018;89:97–101. doi: 10.23750/abm.v89i9-S.7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popat S., Hubner R., Houlston R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 26.Toyota M., Suzuki H. Epigenetic drivers of genetic alterations. Adv. Genet. 2010;70:309–323. doi: 10.1016/B978-0-12-380866-0.60011-3. [DOI] [PubMed] [Google Scholar]
- 27.Lao V.V., Grady W.M. Epigenetics and colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tessitore A., Cicciarelli G., Del Vecchio F., Gaggiano A., Verzella D., Fischietti M., Vecchiotti D., Capece D., Zazzeroni F., Alesse E. MicroRNAs in the DNA Damage/Repair Network and Cancer. Int. J. Genom. 2014;2014:820248. doi: 10.1155/2014/820248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y.P., Lei Q.Y. Metabolic recoding of epigenetics in cancer. Cancer Commun. 2018;38:25. doi: 10.1186/s40880-018-0302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang K.J., Min B.H., Ryu K.J., Kim K.M., Chang D.K., Kim J.J., Rhee J.C., Kim Y.H. The role of the CpG island methylator phenotype on survival outcome in colon cancer. Gut Liver. 2015;9:202–207. doi: 10.5009/gnl13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Advani S.M., Advani P., DeSantis S.M., Brown D., VonVille H.M., Lam M., Loree J.M., Mehrvarz Sarshekeh A., Bressler J., Lopez D.S., et al. Clinical, Pathological, and Molecular Characteristics of CpG Island Methylator Phenotype in Colorectal Cancer: A Systematic Review and Meta-analysis. Transl. Oncol. 2018;11:1188–1201. doi: 10.1016/j.tranon.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha Y., Kim S.Y., Yeo H.Y., Baek J.Y., Choi M.K., Jung K.H., Dong S.M., Chang H.J. Association of CHFR Promoter Methylation with Treatment Outcomes of Irinotecan-Based Chemotherapy in Metastatic Colorectal Cancer. Neoplasia. 2019;21:146–155. doi: 10.1016/j.neo.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagi K., Akagi K., Hayashi H., Nagae G., Tsuji S., Isagawa T., Midorikawa Y., Nishimura Y., Sakamoto H., Seto Y., et al. Three DNA methylation epigenotypes in human colorectal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010;16:21–33. doi: 10.1158/1078-0432.CCR-09-2006. [DOI] [PubMed] [Google Scholar]
- 34.Jass J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 35.Yu J., Tao Q., Cheung K.F., Jin H., Poon F.F., Wang X., Li H.Y., Cheng Y.Y., Rocken C., Ebert M.P.A., et al. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology. 2008;48:508–518. doi: 10.1002/hep.22343. [DOI] [PubMed] [Google Scholar]
- 36.Hu S., Cao B.P., Zhang M.Y., Linghu E.Q., Zhan Q.M., Brock M.V., Herman J.G., Mao G.P., Guo M.Z. Epigenetic silencing BCL6B induced colorectal cancer proliferation and metastasis by inhibiting P53 signaling. Am. J. Cancer Res. 2015;5:651–662. [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y., Cao B., Zhang M., Zhan Q., Herman J.G., Yu M., Guo M. RASSF10 suppresses hepatocellular carcinoma growth by activating P53 signaling and methylation of RASSF10 is a docetaxel resistant marker. Genes Cancer. 2015;6:231–240. doi: 10.18632/genesandcancer.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Xu C.X., Shi C., Zhang J.Y., Qian T., Wang Z., Ma R., Wu J.Z., Jiang F., Feng J.F. Hypermethylation of heparanase 2 promotes colorectal cancer proliferation and is associated with poor prognosis. J. Transl. Med. 2021;19:98. doi: 10.1186/s12967-021-02770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maynard M.A., Ferretti R., Hilgendorf K.I., Perret C., Whyte P., Lees J.A. Bmi1 is required for tumorigenesis in a mouse model of intestinal cancer. Oncogene. 2014;33:3742–3747. doi: 10.1038/onc.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteller M., Tortola S., Toyota M., Capella G., Peinado M.A., Baylin S.B., Herman J.G. Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res. 2000;60:129–133. [PubMed] [Google Scholar]
- 41.Esteller M., Cordon-Cardo C., Corn P.G., Meltzer S.J., Pohar K.S., Watkins D.N., Capella G., Peinado M.A., Matias-Guiu X., Prat J., et al. p14(ARF) silencing by promoter hypermethylation mediates abnormal intracellular localization of MDM2. Cancer Res. 2001;61:2816–2821. [PubMed] [Google Scholar]
- 42.Nyiraneza C., Sempoux C., Detry R., Kartheuser A., Dahan K. Hypermethylation of the 5′ CpG island of the p14ARF flanking exon 1beta in human colorectal cancer displaying a restricted pattern of p53 overexpression concomitant with increased MDM2 expression. Clin. Epigenet. 2012;4:9. doi: 10.1186/1868-7083-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Suehiro Y., Wong C.W., Chirieac L.R., Kondo Y., Shen L., Webb C.R., Chan Y.W., Chan A.S., Chan T.L., Wu T.T., et al. Epigenetic-genetic interactions in the APC/WNT, RAS/RAF, and P53 pathways in colorectal carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008;14:2560–2569. doi: 10.1158/1078-0432.CCR-07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stamos J.L., Weis W.I. The beta-Catenin Destruction Complex. CSH Perspect. Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteller M., Risques R.A., Toyota M., Capella G., Moreno V., Peinado M.A., Baylin S.B., Herman J.G. Promoter hypermethylation of the DNA repair gene O-6-methylguanine-DNA methyltransferase is associated with the presence of G:C to A: T transition mutations in p53 in human colorectal tumorigenesis. Cancer Res. 2001;61:4689–4692. [PubMed] [Google Scholar]
- 46.Deng G., Kakar S., Tanaka H., Matsuzaki K., Miura S., Sleisenger M.H., Kim Y.S. Proximal and distal colorectal cancers show distinct gene-specific methylation profiles and clinical and molecular characteristics. Eur. J. Cancer. 2008;44:1290–1301. doi: 10.1016/j.ejca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Alonso S., Dai Y.C., Yamashita K., Horiuchi S., Dai T., Matsunaga A., Sanchez-Munoz R., Bilbao-Sieyro C., Daz-Chico J.C., Chernov A.V., et al. Methylation of MGMT and ADAMTS14 in normal colon mucosa: Biomarkers of a field defect for cancerization preferentially targeting elder African-Americans. Oncotarget. 2015;6:3420–3431. doi: 10.18632/oncotarget.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonavita E., Gentile S., Rubino M., Maina V., Papait R., Kunderfranco P., Greco C., Feruglio F., Molgora M., Laface I., et al. PTX3 Is an Extrinsic Oncosuppressor Regulating Complement-Dependent Inflammation in Cancer. Cell. 2015;160:700–714. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Liu T., Wang X., Hu W.Q., Fang Z., Jin Y., Fang X.D., Miao Q.R. Epigenetically Down-Regulated Acetyltransferase PCAF Increases the Resistance of Colorectal Cancer to 5-Fluorouracil. Neoplasia. 2019;21:557–570. doi: 10.1016/j.neo.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng K.X., Chen X.X., Hu X.X., Liu X.X., Xu T., Sun H.L., Pan Y.Q., He B.S., Wang S.K. LACTB, a novel epigenetic silenced tumor suppressor, inhibits colorectal cancer progression by attenuating MDM2-mediated p53 ubiquitination and degradation. Oncogene. 2018;37:5534–5551. doi: 10.1038/s41388-018-0352-7. [DOI] [PubMed] [Google Scholar]
- 51.Wang S.Y., Zhang Y.Q., Huang J.Z., Wong C.C., Zhai J.N., Li C.G., Wei G.F., Zhao L.Y., Wang G.P., Wei H., et al. TRIM67 Activates p53 to Suppress Colorectal Cancer Initiation and Progression. Cancer Res. 2019;79:4086–4098. doi: 10.1158/0008-5472.CAN-18-3614. [DOI] [PubMed] [Google Scholar]
- 52.Toyota M., Shen L., Ohe-Toyota M., Hamilton S.R., Sinicrope F.A., Issa J.P.J. Aberrant methylation of the Cyclooxygenase 2 CpG island in colorectal tumors. Cancer Res. 2000;60:4044–4048. [PubMed] [Google Scholar]
- 53.Toyota M., Ohe-Toyota M., Ahuja N., Issa J.P.J. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc. Natl. Acad. Sci. USA. 2000;97:710–715. doi: 10.1073/pnas.97.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konishi K., Issa J.P.J. Targeting aberrant chromatin structure in colorectal carcinomas. Cancer J. 2007;13:49–55. doi: 10.1097/PPO.0b013e31803c72fe. [DOI] [PubMed] [Google Scholar]
- 55.Kaller M., Liffers S.T., Oeljeklaus S., Kuhlmann K., Roh S., Hoffmann R., Warscheid B., Hermeking H. Genome-wide Characterization of miR-34a Induced Changes in Protein and mRNA Expression by a Combined Pulsed SILAC and Microarray Analysis. Mol. Cell. Proteom. 2011;10:M111.010462. doi: 10.1074/mcp.M111.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogt M., Munding J., Gruner M., Liffers S.T., Verdoodt B., Hauk J., Steinstraesser L., Tannapfel A., Hermeking H. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 57.Liu J., Zhang C., Zhao Y., Feng Z. MicroRNA Control of p53. J. Cell. Biochem. 2017;118:7–14. doi: 10.1002/jcb.25609. [DOI] [PubMed] [Google Scholar]
- 58.Zhang C., Liu J., Wang X., Wu R., Lin M., Laddha S.V., Yang Q., Chan C.S., Feng Z. MicroRNA-339-5p inhibits colorectal tumorigenesis through regulation of the MDM2/p53 signaling. Oncotarget. 2014;5:9106, 9117. doi: 10.18632/oncotarget.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noorolyai S., Baghbani E., Maleki L.A., Kojabad A.B., Shanehbandi D., Shahgoli V.K., Mokhtarzadeh A., Baradaran B. Restoration of miR-193a-5p and miR-146 a-5p Expression Induces G1 Arrest in Colorectal Cancer through Targeting of MDM2/p53. Adv. Pharm. Bull. 2020;10:130–134. doi: 10.15171/apb.2020.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X.X., Zeng K.X., Xu M., Liu X.X., Hu X.X., Xu T., He B.S., Pan Y.Q., Sun H.L., Wang S.K. P53-induced miR-1249 inhibits tumor growth, metastasis, and angiogenesis by targeting VEGFA and HMGA2. Cell Death Dis. 2019;10:131. doi: 10.1038/s41419-018-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong Y.J., Zhao J.H., Wu C.W., Zhang L.J., Liu X.D., Kang W., Leung W.W., Zhang N., Chan F.K.L., Sung J.J.Y., et al. Tumor Suppressor Functions of miR-133a in Colorectal Cancer. Mol. Cancer Res. 2013;11:1051–1060. doi: 10.1158/1541-7786.MCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 62.Siemens H., Jackstadt R., Kaller M., Hermeking H. Repression of c-Kit by p53 is mediated by miR-34 and is associated with reduced chemoresistance, migration and stemness. Oncotarget. 2013;4:1399–1415. doi: 10.18632/oncotarget.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi X.L., Kaller M., Rokavec M., Kirchner T., Horst D., Hermeking H. Characterization of a p53/miR-34a/CSF1R/STAT3 Feedback Loop in Colorectal Cancer. Cell. Mol. Gastroenterol. Hepatol. 2020;10:391–418. doi: 10.1016/j.jcmgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi L., Jackstadt R., Siemens H., Li H.H., Kirchner T., Hermeking H. p53-induced miR-15a/16-1 and AP4 form a double-negative feedback loop to regulate epithelial-mesenchymal transition and metastasis in colorectal cancer. Cancer Res. 2014;74:532–542. doi: 10.1158/0008-5472.CAN-13-2203. [DOI] [PubMed] [Google Scholar]
- 65.Ma Q.Y., Wang X.Y., Li Z., Li B.S., Ma F.L., Peng L., Zhang Y., Xu A.G., Jiang B. microRNA-16 represses colorectal cancer cell growth in vitro by regulating the p53/survivin signaling pathway. Oncol. Rep. 2013;29:1652–1658. doi: 10.3892/or.2013.2262. [DOI] [PubMed] [Google Scholar]
- 66.Nakazawa K., Dashzeveg N., Yoshida K. Tumor suppressor p53 induces miR-1915 processing to inhibit Bcl-2 in the apoptotic response to DNA damage. FEBS J. 2014;281:2937–2944. doi: 10.1111/febs.12831. [DOI] [PubMed] [Google Scholar]
- 67.Wang G., Cao X., Lai S., Luo X., Feng Y., Wu J., Ning Q., Xia X., Wang J., Gong J., et al. Altered p53 regulation of miR-148b and p55PIK contributes to tumor progression in colorectal cancer. Oncogene. 2015;34:912–921. doi: 10.1038/onc.2014.30. [DOI] [PubMed] [Google Scholar]
- 68.Pagliuca A., Valvo C., Fabrizi E., di Martino S., Biffoni M., Runci D., Forte S., De Maria R., Ricci-Vitiani L. Analysis of the combined action of miR-143 and miR-145 on oncogenic pathways in colorectal cancer cells reveals a coordinate program of gene repression. Oncogene. 2013;32:4806–4813. doi: 10.1038/onc.2012.495. [DOI] [PubMed] [Google Scholar]
- 69.Slattery M.L., Mullany L.E., Wolff R.K., Sakoda L.C., Samowitz W.S., Herrick J.S. The p53-signaling pathway and colorectal cancer: Interactions between downstream p53 target genes and miRNAs. Genomics. 2019;111:762–771. doi: 10.1016/j.ygeno.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laudato S., Patil N., Abba M.L., Leupold J.H., Benner A., Gaiser T., Marx A., Allgayer H. P53-induced miR-30e-5p inhibits colorectal cancer invasion and metastasis by targeting ITGA6 and ITGB1. Int. J. Cancer. 2017;141:1879–1890. doi: 10.1002/ijc.30854. [DOI] [PubMed] [Google Scholar]
- 71.Zhang P.L., Zuo Z.G., Wu A.H., Shang W.J., Bi R.C., Jin Q.K., Wu J.B., Jiang L. miR-600 inhibits cell proliferation, migration and invasion by targeting p53 in mutant p53-expressing human colorectal cancer cell lines. Oncol. Lett. 2017;13:1789–1796. doi: 10.3892/ol.2017.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwak B., Kim D.U., Kim T.O., Kim H.S., Kim S.W. MicroRNA-552 links Wnt signaling to p53 tumor suppressor in colorectal cancer. Int. J. Oncol. 2018;53:1800–1808. doi: 10.3892/ijo.2018.4505. [DOI] [PubMed] [Google Scholar]
- 73.Maqbool R., Lone S.N., Ul Hussain M. Post-transcriptional regulation of the tumor suppressor p53 by a novel miR-27a, with implications during hypoxia and tumorigenesis. Biochem. J. 2016;473:3597–3610. doi: 10.1042/BCJ20160359. [DOI] [PubMed] [Google Scholar]
- 74.Wang L., Yu P.W. miR-300 promotes proliferation and EMT-mediated colorectal cancer migration and invasion by targeting p53. Oncol. Rep. 2016;36:3225–3232. doi: 10.3892/or.2016.5193. [DOI] [PubMed] [Google Scholar]
- 75.Liu F., Wang X.D. miR-150-5p represses TP53 tumor suppressor gene to promote proliferation of colon adenocarcinoma. Sci. Rep. 2019;9:6740. doi: 10.1038/s41598-019-43231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nugent M., Miller N., Kerin M.J. MicroRNAs in colorectal cancer: Function, dysregulation and potential as novel biomarkers. Ejso. 2011;37:649–654. doi: 10.1016/j.ejso.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Pedeux R., Sengupta S., Shen J.C., Demidov O.N., Saito S., Onogi H., Kumamoto K., Wincovitch S., Garfield S.H., McMenamin M., et al. ING2 regulates the onset of replicative senescence by induction of p300-dependent p53 acetylation. Mol. Cell. Biol. 2005;25:6639–6648. doi: 10.1128/MCB.25.15.6639-6648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stunkel W., Peh B.K., Tan Y.C., Nayagam V.M., Wang X., Salto-Tellez M., Ni B., Entzeroth M., Wood J. Function of the SIRT1 protein deacetylase in cancer. Biotechnol. J. 2007;2:1360–1368. doi: 10.1002/biot.200700087. [DOI] [PubMed] [Google Scholar]
- 79.Kouidou S., Malousi A., Maglaveras N. Methylation and repeats in silent and nonsense mutations of p53. Mutat. Res. Fundam. Mol. Mech. Mutagenes. 2006;599:167–177. doi: 10.1016/j.mrfmmm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 80.Saraggi D., Fassan M., Mescoli C., Scarpa M., Valeri N., Michielan A., D’Inca R., Rugge M. The molecular landscape of colitis-associated carcinogenesis. Dig. Liver Dis. 2017;49:326–330. doi: 10.1016/j.dld.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Meng X., Wei L., Devbhandari S., Zhang T., Xiang J., Remus D., Zhao X. DNA polymerase epsilon relies on a unique domain for efficient replisome assembly and strand synthesis. Nat. Commun. 2020;11:2437. doi: 10.1038/s41467-020-16095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poulos R.C., Olivier J., Wong J.W.H. The interaction between cytosine methylation and processes of DNA replication and repair shape the mutational landscape of cancer genomes. Nucleic Acids Res. 2017;45:7786–7795. doi: 10.1093/nar/gkx463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee K.H., Park J.W., Sung H.S., Choi Y.J., Kim W.H., Lee H.S., Chung H.J., Shin H.W., Cho C.H., Kim T.Y., et al. PHF2 histone demethylase acts as a tumor suppressor in association with p53 in cancer. Oncogene. 2015;34:2897–2909. doi: 10.1038/onc.2014.219. [DOI] [PubMed] [Google Scholar]
- 84.Huang J., Sengupta R., Espejo A.B., Lee M.G., Dorsey J.A., Richter M., Opravil S., Shiekhattar R., Bedford M.T., Jenuwein T., et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 85.Jin L.H., Hanigan C.L., Wu Y., Wang W., Park B.H., Wosterf P.M., Casero R.A. Loss of LSD1 (lysine-specific demethylase 1) suppresses growth and alters gene expression of human colon cancer cells in a p53- and DNMT1 (DNA methyltransferase 1)-independent manner. Biochem. J. 2013;449:459–468. doi: 10.1042/BJ20121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai S.X., Wang J.S., Zeng W., Cheng X.F., Liu L.H., Li W.H. Lysine-specific histone demethylase 1B (LSD2/KDM1B) represses p53 expression to promote proliferation and inhibit apoptosis in colorectal cancer through LSD2-mediated H3K4me2 demethylation. Aging. 2020;12:14990–15001. doi: 10.18632/aging.103558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li J., Chu M.Y., Wang S.S., Chan D., Qi S.K., Wu M., Zhou Z.L., Li J.W., Nishi E., Qin J., et al. Identification and Characterization of Nardilysin as a Novel Dimethyl H3K4-binding Protein Involved in Transcriptional Regulation. J. Biol. Chem. 2012;287:10089–10098. doi: 10.1074/jbc.M111.313965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanda K., Sakamoto J., Matsumoto Y., Ikuta K., Goto N., Morita Y., Ohno M., Nishi K., Eto K., Kimura Y., et al. Nardilysin controls intestinal tumorigenesis through HDAC1/p53-dependent transcriptional regulation. JCI Insight. 2018;3:e91316. doi: 10.1172/jci.insight.91316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Obata T., Toyota M., Satoh A., Sasaki Y., Ogi K., Akino K., Suzuki H., Murai M., Kikuchi T., Mita H., et al. Identification of HRK as a target of epigenetic inactivation in colorectal and gastric cancer. Clin. Cancer Res. 2003;9:6410–6418. [PubMed] [Google Scholar]
- 90.Tanikawa C., Furukawa Y., Yoshida N., Arakawa H., Nakamura Y., Matsuda K. XEDAR as a putative colorectal tumor suppressor that mediates p53-regulated anoikis pathway. Oncogene. 2009;28:3081–3092. doi: 10.1038/onc.2009.154. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki H., Igarashi S., Nojima M., Maruyama R., Yamamoto E., Kai M., Akashi H., Watanabe Y., Yamamoto H., Sasaki Y., et al. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis. 2010;31:342–349. doi: 10.1093/carcin/bgp179. [DOI] [PubMed] [Google Scholar]
- 92.Low J.S.W., Tao Q., Ng K.M., Goh H.K., Shu X.S., Woo W.L., Ambinder R.F., Srivastava G., Shamay M., Chan A.T.C., et al. A novel isoform of the 8p22 tumor suppressor gene DLC1 suppresses tumor growth and is frequently silenced in multiple common tumors. Oncogene. 2011;30:1923–1935. doi: 10.1038/onc.2010.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J., Wang Y.F., Shen Y.Y., He P.X., Ding J., Chen Y. G9a stimulates CRC growth by inducing p53 Lys373 dimethylation-dependent activation of Plk1. Theranostics. 2018;8:2884–2895. doi: 10.7150/thno.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barlev N.A., Liu L., Chehab N.H., Mansfield K., Harris K.G., Halazonetis T.D., Berger S.L. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell. 2001;8:1243–1254. doi: 10.1016/S1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 95.Sakaguchi K., Herrera J.E., Saito S., Miki T., Bustin M., Vassilev A., Anderson C.W., Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ito A., Lai C.H., Zhao X., Saito S., Hamilton M.H., Appella E., Yao T.P. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 2001;20:1331–1340. doi: 10.1093/emboj/20.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brooks C.L., Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell. 2011;2:456–462. doi: 10.1007/s13238-011-1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Navarro F., Lieberman J. miR-34 and p53: New Insights into a Complex Functional Relationship. PLoS ONE. 2015;10:e0132767. doi: 10.1371/journal.pone.0132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toyota M., Suzuki H., Sasaki Y., Maruyama R., Imai K., Shinomura Y., Tokino T. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008;68:4123–4132. doi: 10.1158/0008-5472.CAN-08-0325. [DOI] [PubMed] [Google Scholar]
- 100.Puccini A., Berger M.D., Naseem M., Tokunaga R., Battaglin F., Cao S., Hanna D.L., McSkane M., Soni S., Zhang W., et al. Colorectal cancer: Epigenetic alterations and their clinical implications. BBA-Rev. Cancer. 2017;1868:439–448. doi: 10.1016/j.bbcan.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grady W.M., Yu M., Markowitz S.D. Epigenetic Alterations in the Gastrointestinal Tract: Current and Emerging Use for Biomarkers of Cancer. Gastroenterology. 2021;160:690–709. doi: 10.1053/j.gastro.2020.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barchitta M., Maugeri A., Li Destri G., Basile G., Agodi A. Epigenetic Biomarkers in Colorectal Cancer Patients Receiving Adjuvant or Neoadjuvant Therapy: A Systematic Review of Epidemiological Studies. Int. J. Mol. Sci. 2019;20:3824. doi: 10.3390/ijms20153842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lam K., Pan K., Linnekamp J.F., Medema J.P., Kandimalla R. DNA methylation based biomarkers in colorectal cancer: A systematic review. Biochim. Biophys. Acta. 2016;1866:106–120. doi: 10.1016/j.bbcan.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Okugawa Y., Grady W.M., Goel A. Epigenetic Alterations in Colorectal Cancer: Emerging Biomarkers. Gastroenterology. 2015;149:1204–1225.e12. doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodriguez-Casanova A., Costa-Fraga N., Bao-Caamano A., Lopez-Lopez R., Muinelo-Romay L., Diaz-Lagares A. Epigenetic Landscape of Liquid Biopsy in Colorectal Cancer. Front. Cell Dev. Biol. 2021;9:622459. doi: 10.3389/fcell.2021.622459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohen S.A., Wu C., Yu M., Gourgioti G., Wirtz R., Raptou G., Gkakou C., Kotoula V., Pentheroudakis G., Papaxoinis G., et al. Evaluation of CpG Island Methylator Phenotype as a Biomarker in Colorectal Cancer Treated with Adjuvant Oxaliplatin. Clin. Colorectal Cancer. 2016;15:164–169. doi: 10.1016/j.clcc.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gallois C., Taieb J., Le Corre D., Le Malicot K., Tabernero J., Mulot C., Seitz J.F., Aparicio T., Folprecht G., Lepage C., et al. Prognostic Value of Methylator Phenotype in Stage III Colon Cancer Treated with Oxaliplatin-Based Adjuvant Chemotherapy. Clin. Cancer Res. 2018;24:4745–4753. doi: 10.1158/1078-0432.CCR-18-0866. [DOI] [PubMed] [Google Scholar]
- 108.Cha Y., Kim K.J., Han S.W., Rhee Y.Y., Bae J.M., Wen X., Cho N.Y., Lee D.W., Lee K.H., Kim T.Y., et al. Adverse prognostic impact of the CpG island methylator phenotype in metastatic colorectal cancer. Br. J. Cancer. 2016;115:164–171. doi: 10.1038/bjc.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shiovitz S., Bertagnolli M.M., Renfro L.A., Nam E., Foster N.R., Dzieciatkowski S., Luo Y.X., Lao V.V., Monnat R.J., Emond M.J., et al. CpG Island Methylator Phenotype Is Associated with Response to Adjuvant Irinotecan-Based Therapy for Stage III Colon Cancer. Gastroenterology. 2014;147:637–645. doi: 10.1053/j.gastro.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim S.H., Park K.H., Shin S.J., Lee K.Y., Kim T.I., Kim N.K., Rha S.Y., Roh J.K., Ahn J.B. p16 Hypermethylation and KRAS Mutation Are Independent Predictors of Cetuximab Plus FOLFIRI Chemotherapy in Patients with Metastatic Colorectal Cancer. Cancer Res. Treat. 2016;48:208–215. doi: 10.4143/crt.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X.F., Shimodaira H., Soeda H., Komine K., Takahashi H., Ouchi K., Inoue M., Takahashi M., Takahashi S., Ishioka C. CpG island methylator phenotype is associated with the efficacy of sequential oxaliplatin- and irinotecan-based chemotherapy and EGFR-related gene mutation in Japanese patients with metastatic colorectal cancer. Int. J. Clin. Oncol. 2016;21:1091–1101. doi: 10.1007/s10147-016-1017-6. [DOI] [PubMed] [Google Scholar]
- 112.Wang X., Liu J., Wang D., Feng M., Wu X. Epigenetically regulated gene expression profiles reveal four molecular subtypes with prognostic and therapeutic implications in colorectal cancer. Brief. Bioinform. 2021;22:bbaa309. doi: 10.1093/bib/bbaa309. [DOI] [PubMed] [Google Scholar]
- 113.Condelli V., Calice G., Cassano A., Basso M., Rodriquenz M.G., Zupa A., Maddalena F., Crispo F., Pietrafesa M., Aieta M., et al. Novel Epigenetic Eight-Gene Signature Predictive of Poor Prognosis and MSI-Like Phenotype in Human Metastatic Colorectal Carcinomas. Cancers. 2021;13:158. doi: 10.3390/cancers13010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hientz K., Mohr A., Bhakta-Guha D., Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8:8921–8946. doi: 10.18632/oncotarget.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]