Abstract
Acrylamide is the product of the Maillard reaction, which occurs when starchy, asparagine-rich foods including potato or grain products and coffee are fried, baked, roasted, or heated. Studies in rodents provide evidence that acrylamide is carcinogenic and a male reproductive harmful agent when administered in exceedingly high levels. A 2002 study identified acrylamide in popular consumer food and beverage products, stimulating the European Union (EU) and California to legislate public notice of acrylamide presence in fried and baked foods, and coffee products. The regulatory legislation enacted in the EU and California has scientists working to develop foods and processes aimed at reducing acrylamide formation and advancing rapid and accurate analytical methods for the quantitative and qualitative determination of acrylamide in food and beverage products. The purpose of this review is to survey the studies performed on rodents and humans that identified the potential health impact of acrylamide in the human diet, and provide insight into established and emerging analytical methods used to detect acrylamide in blood, aqueous samples, and food.
1 |. INTRODUCTION
Acrylamide was first characterized by L. C. Maillard in 1912 as a product of the reaction between asparagine or amino acids that can produce acrylic acid and reducing sugars like glucose by a process referred to as the Maillard reaction (Maillard, 1912). The Maillard reaction occurs in nearly every cooking process of starchy foods that involve heat treatment over 140 °C for the purpose of browning. Cooking enhances the flavor and aroma of foods, by production of many desirable compounds via the Maillard reaction, but acrylamide is not one of these flavor or aroma-enhancing molecules (Tamanna & Mahmood, 2015; Tareke, Rydberg, Karlsson, Eriksson, & Törnqvist, 2002). In 1994, the International Agency for Research on Cancer (IARC) classified acrylamide as a neurotoxin and group 2A probable carcinogen, making its presence in myriad processed food products of notable concern to consumer advocacy groups (IARC, 1994). The US Food and Drug Administration (FDA) has since limited the use of polymeric food packaging containers made using acrylamide, where the material may come into contact with food or potable water sources (“Assessment of Health Risks from Exposure to Acryalmide,” 1990; “CFR – Code of Federal Regulations Title 21,” 2019; IARC, 1994). The state of California and the European Union (EU) have both passed initiatives that require food product labels to contain acrylamide disclosure, and the EU passed legislation in 2018 limiting the amount of acrylamide allowed in food to 750 μg/kg (“Acrylamide Levels in Food: Feedback to the European Commission,” 2019; Acrylamide, 2018). While the state of California does not currently set benchmark maximum levels of acrylamide in food products, Proposition 65 requires that businesses provide warning labels to the public if any of their food products contain chemicals that are known to potentially cause cancer or reproductive harm, to which acrylamide was first added in 1990 (“Acrylamide,” 2020a). It also sets a recommended maximum allowable dose level for reproductive toxicity of 140 μg/day, and sets guidance for industry to reduce acrylamide levels in food (“Acrylamide,” 2020b; Guidance for Industry Acrylamide in Foods, 2016). The purpose of this review is to provide a biomedical rationale for the in vivo impact of acrylamide in rodent and human studies, and offer readers an overview of commonly used analytical detection methods for acrylamide.
2 |. BIOMEDICAL RATIONALE
The Environmental Protection Agency (EPA) has determined a no observed adverse effect level (NOAEL) for acrylamide ingestion of 0.2 mg/kg per day (US EPA, 2010). In 2002, acrylamide was discovered in a variety of foods that were cooked at or above 120 °C, including potato chips, french fries, and bread; see Table 1 for acrylamide levels in these foods (Tareke et al., 2002). Since that time, acrylamide has been detected in many other food products, most of which are processed through heat treatment, as acrylamide forms as a byproduct of the Maillard reaction (Barutcu, Sahin, & Sumnu, 2009; NegoiţĂ, Iorga, Tamba, & CatanĂ, 2016; “Survey Data on Acrylamide in Food : Individual Food Products,” 2008). Many of these food items, namely, potato chips and french fries, exceed the recommended FDA NOAEL threshold at approximately 0.337 mg/kg per day and 0.739 mg/kg per day, respectively (see Table 1). The approximate values for acrylamide content in potato chips and french fries are within EU regulatory limits of 0.750 mg/kg. The ubiquitous presence of acrylamide in processed foods has led many scientists to search for a connection between acrylamide and human health ailments.
TABLE 1.
Known acrylamide-containing foods and their detected levels. Data adapted from 2015 and/or 2011 FDA data. https://www.fda.gov/food/chemicals/survey-data-acrylamide-food
| Product | Average levels of acrylamide (ppb) |
|---|---|
| French fry | 337 |
| Potato chips | 739 |
| Coffee beans, ground (not brewed) | 272 |
| Coffee (brewed) | <10 |
| Bread and bakery products | 31 |
| Cereals | 234 |
| Infant foods | 143 |
| Nut butters | 159 |
| Prunes and prune juice | 137 |
| Carrots | 36 |
| Onions, grilled | 70 |
| Plums | 59 |
| Hot cocoa mix | 17 |
| Chocolate products | 144 |
| Blood (endogenous levels) | 0.3–0.4 (Goempel et al., 2017) |
2.1 |. Acrylamide impact to rodent health
Studies in mice have shown that acrylamide, when ingested, is metabolized by cytochrome P450 into glycidamide (GA), which in turn forms adducts with deoxyribonucleic acid (DNA) and can lead to male infertility via inhibition of sperm protamines (Akmal et al., 2016; Li, Wang, Liu, Hu, & Chen, 2016). It was also found by Friedman et al., during a 1995 lifetime oncogenicity study in rats, that while no changes were observed in the rats by physical appearance, increased level of acrylamide ingestion was positively correlated with higher levels of male and female rat mortality over a period of 104 weeks, with higher incidence in male rats. Male rats that had been given high doses of acrylamide (2.0 mg/kg per day) in their drinking water experienced a 22 to 31% increase in mortality when compared with two different control groups. For each group, 102 rats comprised each control group and 204, 102, and 75 rats comprised the 0.1, 0.5, and 2.0 mg/kg per day rat groups, respectively. Female rats given high doses of acrylamide (3.0 mg/kg per day) were observed to have a mortality rate 35 to 49% higher than that of rats in two different control groups. For each female group, 50 each comprised the two control groups while 100 rats each comprised the 1.0 and 3.0 mg/kg per day experimental groups. In addition, this study provided evidence of an increase in benign tumor formation in female rat tissues, as compared with control rats with no added acrylamide (Friedman, Dulak, & Stedham, 1995). For example, fibroadenomas were found to have a 6.5-fold increase in presence when compared with control groups. While initially convincing, these rodent studies were performed at dosages two to three times higher than the maximum allowed levels of acrylamide-containing human food products according to EU guidelines (see Table 1). However, there is a growing concern for the potential carcinogenicity of acrylamide, due to increasing dependence of the human diet on fast food, often fried or grilled, and processed food and beverage products, including specialty coffees, which are sold in many quick-serve restaurants.
2.2 |. Acrylamide influence in human studies
In 2011, a proteomics study of blood plasma proteins was conducted to determine which human proteins bind to either acrylamide (AA) or glycidamide (GA) (C. H. Feng & Lu, 2011). Plasma was collected from healthy volunteers who had a standard diet and no history of smoking or occupational exposure to acrylamide. Total protein was isolated from plasma by addition of acetone prior to vortexing and centrifugation for 10 min at 10,000 rpm. The protein pellet was treated via alkylation conditions to identify which proteins coupled to acrylamide and glycidamide by incubating proteins with high concentrations of acrylamide or glycidamide for 60 min at 25°C. Proteins that were alkylated by acrylamide and/or glycidamide were separated and identified by liquid chromatography with tandem mass spectrometry (LC-MS/MS) and compared with non-alkylated, unmodified protein, control samples. The mass spectrometry results indicated that most acrylamide or glycidamide alkylations occurred at lysine residues, and to a lesser extent at the N-terminus of the protein, or in some cases at cysteine residues (Feng & Lu, 2011). The proteins found to be alkylated with acrylamide included immunoglobulin, serum albumin, lipid-free human apolipoprotein A-I, and Ig G1 H Nie. The authors assert that these proteins, especially immunoglobulin, were likely involved in the metabolism of acrylamide and glycidamide (Figure 1). However, further study is required to determine the exact metabolic processes.
FIGURE 1.
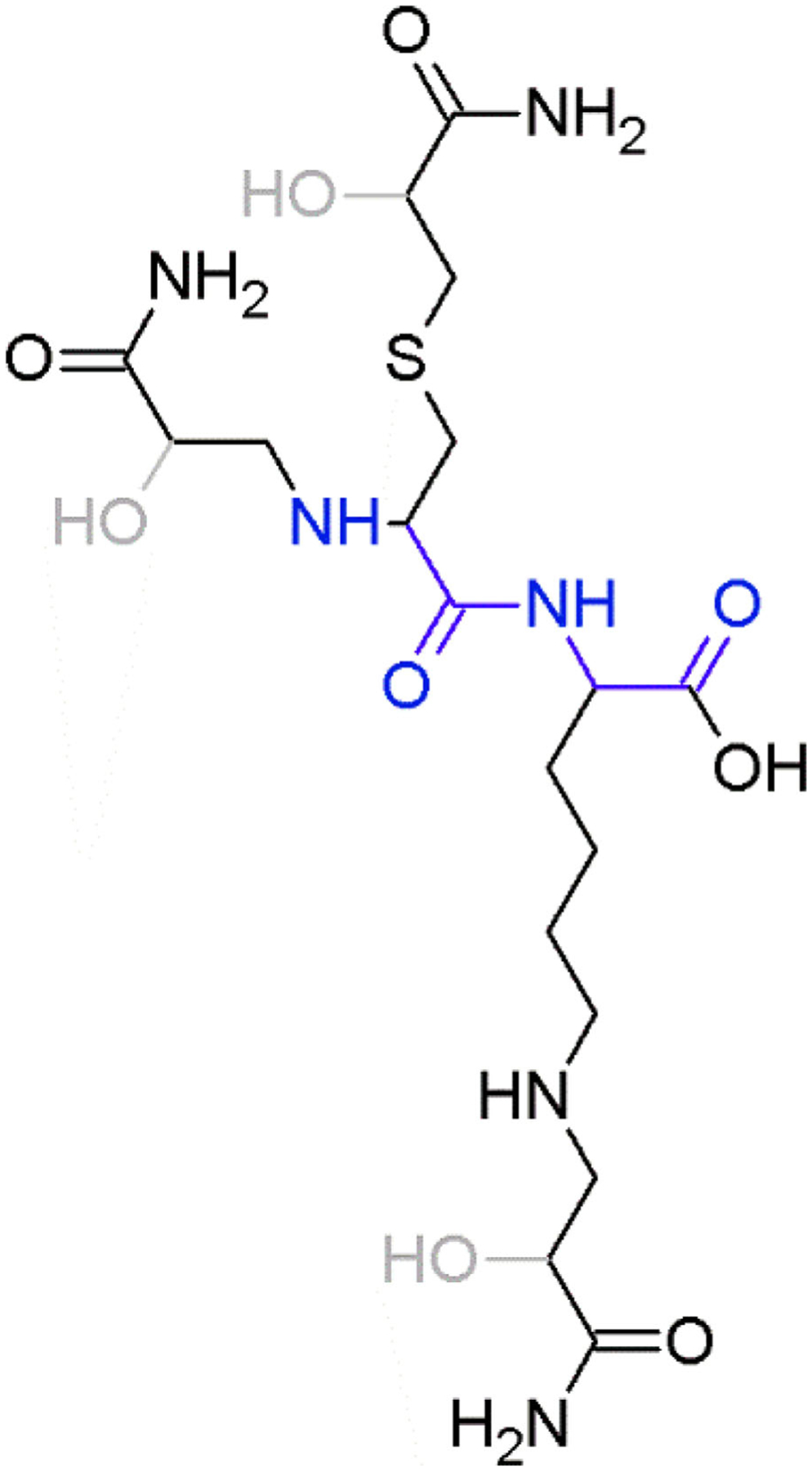
Representation of the alkylation product of acrylamide and glycidamide (hydroxyl groups in gray) on a peptide with (left to right) the N-terminus, cysteine and lysine. The peptide backbone is displayed in blue
2.2.1 |. Carcinogenicity of acrylamide in humans
Acrylamide studies in humans have been contradicting and inconclusive with regard to carcinogenicity (Graff et al., 2018; Levi et al., 2005; Mucci, Dickman, Steineck, Adami, & Augustsson, 2003; Mucci, Lindblad, Steineck, & Adami, 2004; Mucci, Sandin, & Magnusson, 2005; Pelucchi, Bosetti, Galeone, & La Vecchia, 2015). While experimentation using rodents has correlated high quantities of acrylamide ingestion to tumor formation and male infertility, human trials have been rare, and often limited to dietary studies, due to human health study regulations. Studies in mice provide data showing that acrylamide, when metabolized by cytochrome P450 (CP450), will produce the epoxide derivative, glycidamide (Figure 1). Glycidamide is a reactive compound that forms adducts with protamines, which are DNA binding proteins rich in the amino acid arginine, as well as DNA itself, specifically with guanine and adenine (Adler, Baumgartner, Gonda, Friedman, & Skerhut, 2000; Brewer, Corzett, & Balhorn, 2002; Li et al., 2016; Tyl & Friedman, 2003). The purpose of the protamine protein is to bind negatively charged DNA and render it inactive, thus halting transcription that may have been occurring. Introduction of the protamine protein ensures the male genome does not begin functioning once it fertilizes an embryonic egg (Balhorn, 2007; Brewer et al., 2002; Sega, Valdivia Alcota, Tancongco, & Brimer, 1989). Thus, when glycidamine binds to sperm, and blocks protamine function, male reproduction is adversely affected and reproduction is prevented. In 2017, it was reported that humans have an endogenous level of acrylamide in the bloodstream at all times of approximately 0.3–0.4 μg/kg body weight per day (Goempel et al., 2017). While high levels of acrylamide may cause health issues, humans potentially utilize an innate level of acrylamide. This realm of research is yet to be broadly reported in literature.
2.2.2 |. Toxicity of acrylamide in humans
Conversely, studies have also been conducted that compare dietary acrylamide with toxicity and its effects in humans (Fennell & Friedman, 2005; Virk-Baker, Nagy, Barnes, & Groopman, 2014). Table 1 contains a list of items (including food products, blood, and potable water) and their average levels of measured acrylamide. While the research and general findings involving the human toxicity, such as carcinogenicity and reproductive harm, of acrylamide have been controversial, legislation has been passed, and is expected to expand, to regulate warnings associated with acrylamide-containing products and acceptable levels to which acrylamide may be present in food. Mitigation strategies have been mandated, researched and are currently being developed, especially in potato research, to reduce or eliminate the amount of acrylamide formed in heat-treated products, and the topic continues to be extensively studied (Pan et al., 2020; Sharma, 2016).
2.3 |. Food processor acrylamide reduction efforts
In 2010, the U.S. potato industry named acrylamide its highest research priority, and urged the U.S. Department of Agriculture (USDA) National Institutes of Food and Agriculture to fund research aimed at identifying ways to minimize acrylamide formation in fried potato products (Bethke, 2018). By 2017, the EU and Great Britain mandated that food processors perform measurement and mitigation of acrylamide to ensure levels in food were below benchmark standards of 0.750 mg/kg (European Commission, 2017). Mitigation strategies developed since 2010 have ranged from the use of asparaginase enzymes to reduce the amount of acrylamide produced during cooking, to the addition of salt to alter pH and subsequent acrylamide formation, cultivar selection, fertilizer application, environmental effect on crop growth, and even storage conditions (Krishnapura, Belur, & Subramanya, 2016; Pedreschi, Mariotti, Granby, & Risum, 2011; Rannou, Laroque, Renault, Prost, & Sérot, 2016; Sanghvi et al., 2016; Sharma, 2016; Xu, Oruna-Concha, & Elmore, 2016; Zaheer & Akhtar, 2016). Many successes have occurred. For example, the INNATE potato, a genetically modified organism (GMO) developed by J. R. Simplot Co., works by reducing the production of asparaginase in potatoes, thereby reducing acrylamide formation by 58%–72% (“INNATE by Simplot,” 2017). Efforts to reduce acrylamide formation pre-cooking are ongoing and optimistic for the future.
Another very promising development in food processing to reduce acrylamide production in french fries and potato chips, involves the use of pulsed electric fields applied to potatoes prior to slicing and frying. The pulsed electric fields induce electroporation of plant cell membranes, causing more uniform composition of the potato for enhanced efficiency in cutting and reduced energy input for cooking. Potatoes that cook for shorter times at lower temperature absorb less oil and generate less acrylamide, thus producing a product that is healthier (less acrylamide) and more nutritious (less oil). Researchers in New Zealand demonstrated the benefits of pulsed electric fields on the processing of “kumara” sweet potato. Pre-treatment of potatoes by pulsed electric field, at an electric field strength of 1.2 kV/cm, resulted in nearly 20% less oil content and faster browning rate, compared with untreated potatoes (Liu et al., 2017). A second notable study of potato chips by Genovese et al. demonstrated a 30% reduction in acrylamide formation attributed to PEF pre-treatment of peeled potato slices that lowered reducing sugar and free asparagine content before frying (Genovese et al., 2019). Yet another report by Schouten et al. combined the use of PEF with Aureobasidium pullulans L1 yeast water suspension pretreatment to reduce acrylamide formation during french fry and potato chip processing (Schouten et al., 2020). Pulsed electric field emerging technology is being widely adopted throughout the food process industry to introduce economic and health benefits that were not available just a decade ago.
2.4 |. Conclusion of acrylamide biomedical findings
There has been no definitive study demonstrating the deleterious effect of acrylamide in humans at levels present in common foods, but there is mounting evidence to suggest that acrylamide deserves reporting on food labels and warrants strategies to minimize its presence in food products and packaging materials. The acrylamide studies introduced to this point in the review have focused on rodents, humans, food products, and environmental acrylamide exposure. From this point forward, the emphasis will be analytical methods of quantitative and qualitative analysis of acrylamide in blood, aqueous samples, and food products. Traditional and reliable acrylamide quantification protocols use gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) (Mastovska & Lehotay, 2006). These instruments are expensive, technician training to maintain, repair, and operate these systems is extensive, and sample preparation and analysis is tedious and time consuming. An overview of traditional and alternative acrylamide testing procedures is provided.
3 |. EXTRACTION METHODS
Extraction of acrylamide from food samples typically involves sample preparation in the form of homogenization and/or lyophilization, followed by the addition of an internal standard, commonly acrylamide-d3 or acrylamide-13C3. Commercially available, isotopically enriched, acrylamide standards generally have a purity of 98 to 99% with trace amounts of acrylamide derivatives constituting the impurity. In acrylamide studies involving rodent or primate blood, acrylamide is converted to glycidamide through metabolic processes, making glycidamide quantitation an integral part of the experiments (Besaratinia & Pfeifer, 2005; Li et al., 2016). Commercially available glycidamide, with CAS Number 5694-00-8, is often sold under the synonym oxirane carboxamide by companies including Sigma-Aldrich, BOC Sciences, Angene Chemical and others. A common theme for all analytical methods developed to obtain quantitative acrylamide and/or glycidamide levels from food, beverage, or biological fluids is the use of commercially available internal standards.
3.1 |. Food matrices
The quick, easy, cheap, effective, rugged, and safe (QuEChERs) method was first published in 2003 for the analysis of pesticide residues in varying food matrices, and has been adapted for the study of many different analytes including acrylamide (Anastassiades, Lehotay, Štajnbaher, & Schenck, 2003; AOAC, 2007; Bertuzzi, Rastelli, Mulazzi, & Pietri, 2017; De Paola et al., 2017). QuEChERs begins with homogenization of the sample, followed by addition of internal standard, and extraction with acetonitrile. Buffering salts, such as sodium chloride and magnesium sulfate, are then added for phase separation and pH adjustment, followed by agitation and centrifugation to make the raw extract. The upper phase is separated and subject to cleanup with a sorbent, commonly a primary secondary amine (PSA) or other solid-phase extraction (SPE) technique. PSA is an anion exchange sorbent that has a high affinity for fatty acids, organic acids, and sugars remaining in the extract (Stenerson, Wolford, & Shimelis, 2011). The eluent is centrifuged to provide an extract ready for analysis by gas chromatography (GC) or liquid chromatography (LC). Acrylamide is highly soluble in water and acetonitrile, making this method ideal for acrylamide extraction from food matrices when using GC or LC analytical techniques (Figure 2). The QuEChERs protocol or an adaptation of it has been widely implemented for acrylamide analysis and has been cited more than any other method (Bertuzzi et al., 2017; De Paola et al., 2017; Surma, Sadowska-Rociek, Cieślik, & Sznajder-Katarzyńska, 2017; Weijun, 2015). The modified QuEChERs protocol with additional hexanes or petroleum ether defatting step is shown in Figure 3 (Nguyen, van der Fels-Klerx, & van Boekel, 2017; Razia, Bertrand, Klaus, & Meinolf, 2016). Defatting with either nonpolar organic solvent efficiently solubilizes fats and oils, permitting easy removal of the solvent by reduced pressure evaporation to allow isolation of fat or oil content.
FIGURE 2.
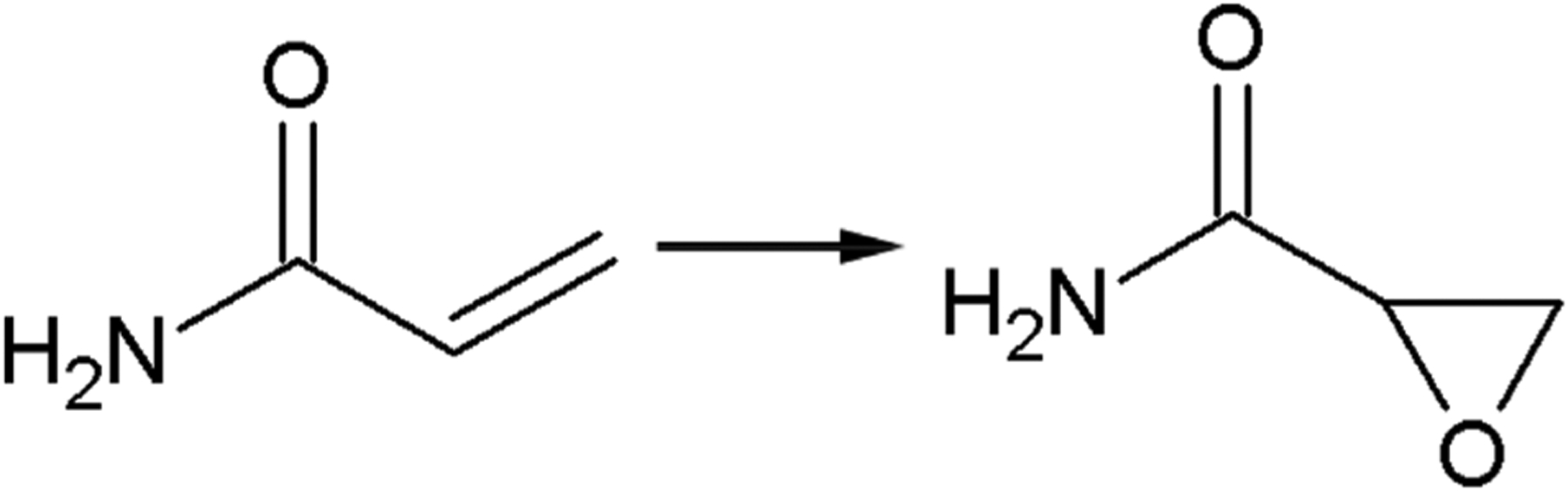
Metabolic conversion of acrylamide by CP450 to produce the epoxide glycidamide
FIGURE 3.
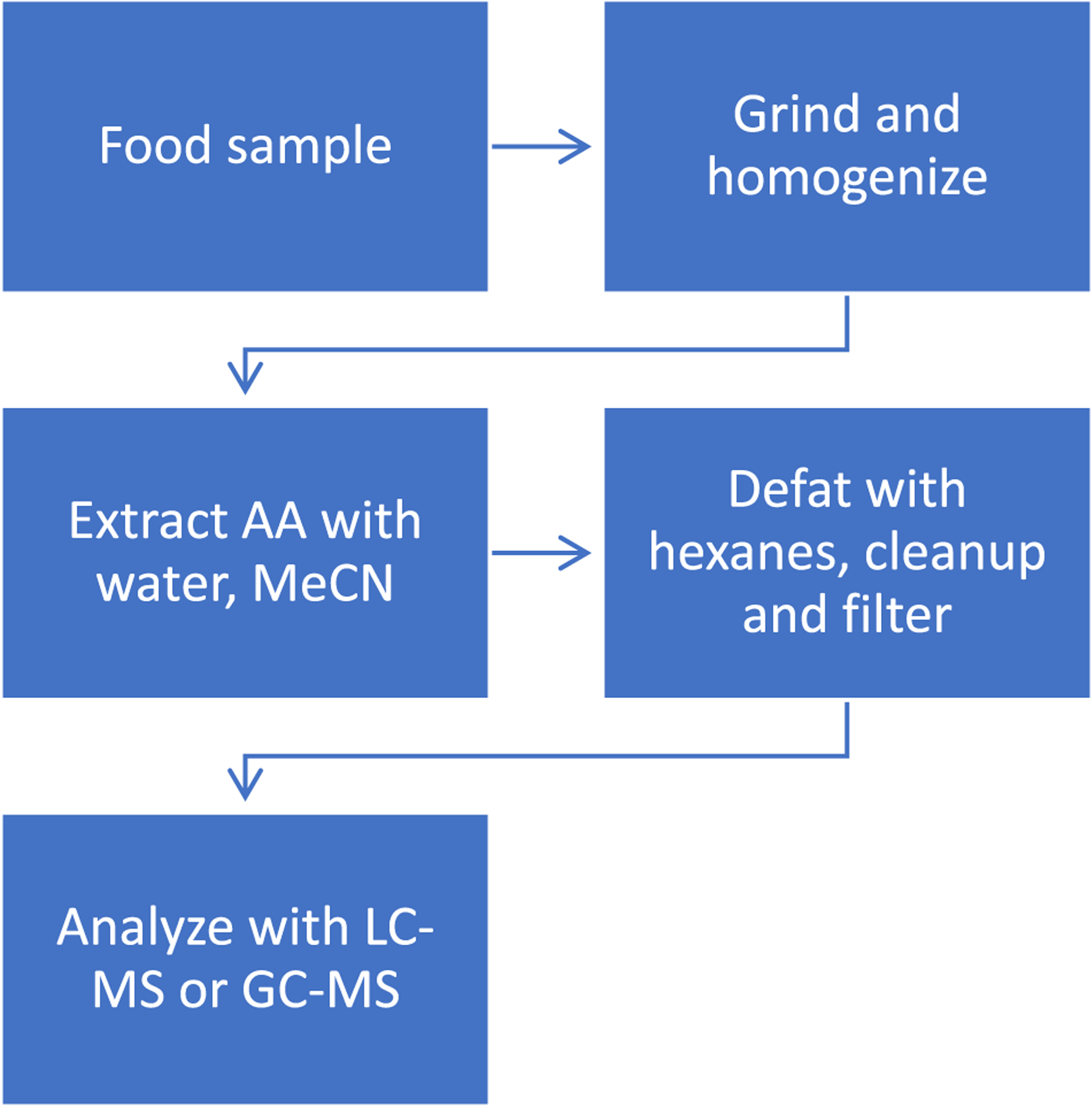
Sequence of steps for acrylamide extraction from food and analyte analysis by LC-MS or GC-MS
Alternative defatting processes to the use of nonpolar organic solvent and SPE include the use of Carrez I and II solutions (Asnaashari, Esmaeilzadeh Kenari, Farahmandfar, Taghdisi, & Abnous, 2018; Khan et al., 2017; NegoiţĂ & Culeţu, 2016; Oroian, Amariei, & Gutt, 2016; Raters & Matissek, 2018; Razia et al., 2016; Yadav, Chhillar, & Pundir, 2018). Carrez I contains a 15% (w/v) solution of potassium hexacyanoferrate (II) trihydrate (K4[Fe(CN)6•3H20), while Carrez II contains a 30% (w/v) solution of zinc sulfate heptahydrate (ZnSO4•7H20) (Emdmillipore, 2013). Carrez I and II solutions are commonly used in food analysis for the breakdown of emulsions (Emdmillipore, 2013). These solutions have been shown to be suitable on their own for cleanup of acrylamide-containing extracts from food, but extract purity assurance typically involves the addition of a defatting agent.
3.2 |. Blood matrices
Analysis of blood for acrylamide content is performed by physicians to determine the effects of diet, smoking, physical activity, workplace exposure, age, and sex on the amount of acrylamide or glycidamide present in a patient’s bloodstream. Acrylamide forms the epoxide glycidamide when metabolized by CP450 (Figure 1) (Li et al., 2016). Both acrylamide and glycidamide form adducts with the N-terminal valine (Val) in hemoglobin (Hb) and can easily be isolated by cleaving the N-terminal valine with Edman reagent, and purified by liquid–liquid extraction (Doerge et al., 2005; Ferrari et al., 2013; Goempel et al., 2017; Vesper et al., 2008). The modified Edman degradation, which normally involves phenylthiohydantoin (PTH), uses pentafluorophenyl isothiocyanate (PFPTH), because PFPTH is optimal for performing Edman degradation of adducts specifically at the N-terminal valine of hemoglobin (Rydberg, Lüning, Wachtmeister, Eriksson, & Törnqvist, 2002). In one study, 510 people from nine European countries, 30 smokers and 30 nonsmokers from each country (except France where only women were studied), half of each group being men and the other half women, between the ages of 41 and 60 and of undisclosed race, had their blood analyzed for evidence of acrylamide and/or glycidamide. The erythrocytes from their blood were hemolyzed, and the N-terminal valine residues were cleaved via modified Edman degradation to extract acrylamide and glycidamide hemoglobin adducts. After the reaction, AA-Val-PFPTH and GA-Val-PFPTH were quantified using high-pressure liquid chromatography (HPLC) (Vesper et al., 2008). This study concluded that in general smokers contained higher levels of blood acrylamide (121 pmol/g) and glycidamide (92.8 pmol/g) than nonsmokers (acrylamide 42.5 pmol/g and glycidamide 39.9 pmol/g). Interestingly, people from the United Kingdom and the Netherlands, both smokers and nonsmokers alike, were found to have higher acrylamide and glycidamide content than people of the other nine European countries included in this study. The authors accounted for this observation by stating that the British and Dutch participants compared with those from other countries may have high exposure to acrylamide in their diets.
In a separate study conducted at Stockholm University, acrylamide adducts were analyzed in both smokers and non-smokers; however, the population included in this study was limited to laboratory scientists who worked routinely with polyacrylamide gel electrophoresis (PAGE). They compared 22 laboratory technicians (15 of which were smokers) who regularly worked with PAGE to a control group consisting of 18 laboratory workers (10 of which were smokers) or office staff who did not work with PAGE (Bergmark, 1997). The derivatization of globin occurred in accordance with the protocol of Tornqvist et al., where PFPTH reagent was used in conjunction with the modified Edman degradation to measure AA-Val-PFPTH (Törnqvist, Kautiainen, Gatz, & Ehrenberg, 1988; Vesper et al., 2008). The results were such that non-smoking PAGE workers were found to have 1.74 times the acrylamide adducts to non-smoking controls, while smokers who did not work with PAGE had 3.74 times more acrylamide adducts when compared with non-smoking controls. Interestingly, PAGE workers who were also smokers were found to have a lower number of adducts to non-PAGE working smokers, but still had higher levels of acrylamide adducts than non-smoking PAGE workers. The authors did not comment about this anomaly.
Glycidamide has been found to covalently bind to DNA to form GA-DNA (Besaratinia & Pfeifer, 2005; Doerge et al., 2005). In order to isolate GA-DNA adducts, DNA is first isolated from whole tissue homogenates from laboratory mice using, for example, a Blood and Cell Culture DNA Maxi Kit from Qiagen (Middletown, MD, USA) with some changes to the standard protocol to maintain the viability of the labile GA-DNA adducts (Besaratinia & Pfeifer, 2005; Doerge et al., 2005). Specifically, the DNA sample is treated with different RNases and proteinases to maintain GA-DNA viability. The isolated GA-DNA samples are then analyzed by LC-MS.
3.3 |. Water matrices
Acrylamide is water-soluble, and therefore its presence in potable water sources is a concern because it is used as a flocculate for suspended matter in both irrigation and drinking water (Tepe & Çebi, 2017). Acrylamide is also suspected of being released into groundwater systems as a result of its industrial use in organic chemical, pesticide, and use as friction reducers in pipes, posing a potential threat to public health (Cavalli, Polesello, & Saccani, 2004; Tepe & Çebi, 2017). Detection of acrylamide often occurs by a process called “simple cleanup,” which is commonly used for aqueous samples from water treatment and groundwater sources. “Simple cleanup” involves the use of sorbents like PSA to remove organic acids and sugar impurities that may interfere with instrument measurement of acrylamide (Anastassiades et al., 2003). In 2014, a hydrophobic interaction liquid chromatography (HILIC) method was developed and employed to separate small polar molecules, such as acrylamide, from water sources and then analyze them in tandem with mass spectrometry (Backe, Yingling, & Johnson, 2014). Potable water samples can be solvent-exchanged into dichloromethane using acetonitrile as an intermediate solvent through a series of additions and evaporations of the aforementioned solvents, and then samples can be directly loaded onto a HILIC column without further sample preparation. The HILIC method provided a process by which acrylamide concentrations in the ng/L range could be measured at a local water treatment plant where the study was carried out.
4 |. DETECTION METHODS
4.1 |. LC-MS
Typical methods for quantification of acrylamide and glycidamide employ the use of HPLC systems that separate analytes by passing them through either C18 (see Table 2) or C8 capillary columns (Petrarca, Rosa, Queiroz, & Godoy, 2017). Differences in column size and packing material account for differing retention times for acrylamide and acrylamide internal standards (Table 2). However, acrylamide and acrylamide-d3 elute at the same retention time, making differentiation between the two isotypes dependent on the use of a mass selective detector. Mass spectrometry cannot easily provide accurate concentration information for acrylamide in the absence of a calibration curve, so acrylamide-d3 is routinely used as an internal standard or external standard to obtain a ratio of isotopically enriched to unenriched acrylamide for validation of quantitative measurement. Most commonly, acrylamide samples and acrylamide-d3 samples are run independently to obtain concentration levels that can be cross-validated. These samples can also be run as acrylamide-d3 spikes, so that a linear regression can be performed and the endogenous level of acrylamide determined from the line at x = 0 (Yang, Li, & Chambers, 2020). An alternative to acrylamide-d3 is the use of an internal standard that is similar in structure to acrylamide, such as methylacrylamide (MeAA), because it elutes at a different time than acrylamide and permits simultaneous quantification within a single sample run. However, this type of analysis is semi-quantitative because the retention times are different for the acrylamide and the internal standard. Internal standards for acrylamide and glycidamide hemoglobin adducts have included “heavy” versions of the AA/GA-Val-PFPTH, where deuterium is used in place of hydrogen atoms on AA/GA and 13C is used in place of carbon (Bergmark, 1997). Other groups have used octapeptides of AA/GA adducts with the eight N-terminal residues of hemoglobin as internal standards, permitting good separation on HPLC chromatograms (Lange, Anzenba, Muller, Maurin, & Balny, 1994). Experimental samples are able to be spiked with internal standard and quantified simultaneously, and in comparison with an internal standard of predetermined concentration. The applications lab at Agilent Technologies published a protocol for the analysis of acrylamide in peanut butter, using acrylamide-d3 as an internal standard following QuEChERS extraction (Mastovska & Lehotay, 2006; Yang et al., 2020). The approach used by the application scientists at Agilent Technologies for peanut butter analysis was the same as that used for the measurement of acrylamide in potato chips (Mastovska & Lehotay, 2006).
TABLE 2.
Survey of representative methods of analysis for acrylamide from different food matrices
| Detection method | Samples | Sample preparation | Internal standard | Column used | Method parameters (LC or GC) | Detector parameters (MS or MS/MS) | References |
|---|---|---|---|---|---|---|---|
| LC-MS | Roasted coffee, barley, potato crisps | QuEChers procedure followed by treatment with Al2O3 and purification on HLB column | AA-d3 | X-Select HSS T3 (2.5 μm particle size, 150 × 2.1 mm i.d., Waters Corp., Milford, MA, USA) | Water and MeCN (both acidified with 0.2% formic acid) as solvent A and B, respectively. Flow rate 0.2 ml min−1, 100% solvent A 3 min, linear gradient to 15% solvent B within 4 min, isocratic 2 min, conditioning column 7 min | ESI interface in positive mode, spray capillary voltage 4.2 kV, sheath and aux gas 35 and 12 psi, respectively, skimmer 12 V, temperature of heated capillary 270 °C, argon pressure 1.2 mTorr at CE of 12 V | Bertuzzi et al. (2017) |
| Dried fruits and edible deeds | Samples ground, QuEChers extraction, filtration through 0.22 μm PES membrane | None? | Gemini RP C18 column (Phenomenex, Torrance, CA, USA) (25 cm × 2 mm i.d. × 5 μm particle size × 110 A pore size) | Water and MeOH acidified with 0.1% formic acid as solvent A and B, respectively. Isocratic elution 7 min with flow rate of 0.25 ml min−1 | Positive ESI with curtain gas 20.0 pse, collision-activated dissociation 7.0, ionspray 5,500/0 V, 700.0 °C, nebulizer gas 70.0 pse, heater gas 30.0 psi, declustering potential 22.0 V, CE 14.1 V, CXP 4.1 V, CEP 6.0 V | De Paola et al. (2017) | |
| LC-MS/MS | Blood samples, serum (DNA-GA adducts) | GA-DNA isolation with Blood and Call Culture Maxi kits (Qiagen Co., Valencia, CA) or thawed blood serum sample purified with solid-phase extraction | (13C)3-Substituted AA, racemic (13C)3-GA | Aquasil column (2.1 mm × 150 mm, 5 μm particle size) (Thermo Hypersil-Keystone, Bellefonte, PA) | 300 μL min−1, step gradient 0.5% MeOH in 0.01% formic acid in water for 2.5 min, 30% MeOH in aqueous 0.1% formic acid for 2 min, re-equilibration for 5.5 min. | Ion source temperature 120 °C, desolvation gas temperature 400 °C, constant cone voltage 35 V, gas pressure (ar) 2–4 × 10−3 mbar | Doerge et al. (2005) |
| Hemolyzed erythrocytes | Cleave N-terminal valine with AA or GA attached with Edman reagent, followed by liquid–liquid extraction | n/a | n/a | n/a | n/a | (Ferrari et al., 2013) | |
| Food samples | Stable isotope dilution method (SIDA) | AA-d3 | Adapted from Ruenz. Et al with 1 μL injection volume | Ruenz et al. | DP 36 V, CEP 10 V, CE 15 V, CXP 7 V, ionspray voltage 4500 V, curtain gas 40 psi, temperature 550 °C | (Goempel et al., 2017) | |
| Blood samples | Hb isolation described by Schettgen et al. 2003; derivatization by modified Edman degradation generating pentafluorophenyl thiohydantoin derivatives | D7-AA-Val-PFPTH, D7-GA-Val-PFPTH | Kintex PFP column (100 × 3.1 mm, 100 Å, 2.6 μm, Phenomenex, Aschaffebburg, Germany) | 0.1% aqueous acetic acid and MeCN as solvents A and B, respectively, at 40 °C, MeCN concentration 10% for 0.5 min, increased to 40% for 0.6 min, and to 45% for 4.8 min, and reconditioning | DP −125/75 V, exit potential −10 V, CE −22 V/−22 V, CXP −17/−15 V, curtain gas 50 psi, ionspray voltage −4,500 V, temperature 500 °C | (Goempel et al., 2017) | |
| HPLC-UV | Vending machine snacks | Solid–liquid water extraction with water and derivatization with 2-mercaptobenzoic acid, followed by extraction with ethyl acetate, resolve residue in MeOH | AA (AA 99%, SIGMA-Fluka, St. Louis, MO, USA) | C18 RP column (250 × 4.6 mm, Phenomenex Inc, Torrance, CA, USA) | Detection wavelength of 238 nm, 20:80 MeCN and acetic acid as mobile phase | n/a | (Haouet, Pistolese, Branciari, Ranucci, & Altissimi, 2016) |
| UPLC-MS | Ground and homogenize samples. Spike with IS and perform solid–liquid extraction with Carrez I and II solutions, cation exchange with SPE column | AA-d3 (Sigma-Aldrich, Steinheim, Germany) | Waters Acquity BEH C18 column (50 mm × 2.1 mm i.d., particle size 1.7 μm) | Isocratic elution with 10% MeOH in 0.1% aqueous formic acid, flow rate 300 μL/min | Cone voltage 48 V, capillary voltage 3.5 kV, desolvation temperature 350 °C, source temperature 120 °C, desolvation gas flow rate 600 L h−1 | (Khan et al., 2017) | |
| LC-MS/MS | Infant and toddler foods | Homogenize samples, spike with IS, solid–liquid extraction with water, anion exchange with SPE column | AA-d5 (Cluzeau Info Labo) | Hypercarb chromatographic column (50 mm × 2.1 mm i.d., 5 μm particle size) (Thermo Fisher Scientific) | 1% MeOH in water with 0.2% formic acid and pure MeOH as solvents A and B, respectively, 200 μL min−1 flow rate with re-equilibration | ESI+, spray voltage 5 kV, curtain gas 25 psi, spray gas 15 psi, auxiliary gas 20 psi, source temperature 500 °C, CEP 10 V, CXP 15 V, CE 15 eV | (Lambert et al., 2018) |
| LC-MS/MS | Food samples *potato chip, sweet potato chip, crackers and snacks, peanut better, chocolate, chocolate syrup) | Spike sample with IS, solid–liquid extraction with MeCN and hexane (for defatting) | AA-d3 (Cambridge Isotope Laboratories Inc., Andover, MA, USA) | Aqua C18 Column (150 × 3 mm, 5 μm particle size, 125 A pore size), C18 4 × 3 mm guard column (Phenomenex; Torrance, CA) | 0.5% aqueous MeOH and 0.1% formic acid in 50% aqueous MeOH as solvents A and B, respectively, 200 μL min−1 flow rate for 8 min | ESI+, no other information available | (Mastovska & Lehotay, 2006) |
| HPLC | Food samples (potato, baked bread, potato chips, french fries, sweet bread, bun, rusk and biscuit) | Finely ground samples, solid–liquid extraction with MeOH, defatting with hexanes | L-asparaginase (Nocozymes, Acrylaway L), AA (Sigma-Aldrich, St. Louis, USA) | C18 column (250 mm × 4.6 mm × 5 μm i.d.) | Isocratic conditions with 97% MeCN, 3% acetic acid as mobile phase at 1 ml min−1 flow rate and detection at 210 nm at 28 °C | n/a | (Meghavarnam & Janakiraman, 2018) |
| LC-MS/MS | Food samples (wheat flour and biscuits) | Spike with IS, solid–liquid extraction with water, defatting with a spatula | AA-d3 (Cambridge Isotope Laboratories, Inc.) and asparagine-15N2 (Sigma-Aldrich, The Netherlands) | Hypercarb column (100 × 3 mm, 5 μm particle size) | 100% water and 100% MeOH as solvents A and B, respectively; 16 min per sample with a flow rate of 0.4 mL min−1 | ESI− for Asp, ESI+ for AA | (Nguyen et al., 2017) |
| LC-MS/MS | Food samples (biscuit powder) | Spike with IS, solid–liquid extraction with water | AA-d3 (Cambridge Isotope Laboratories, Inc.) and asparagine-15N2 (Sigma-Aldrich, The Netherlands) | Hypercarb column (100 × 3 mm, 5 μm particle size) | 100% water as mobile phase with 0.4 mL min−1 flow rate and 10 min run time per sample | ESI− for Asp, ESI+ for AA | (Nguyen, Van Der Fels-Klerx, Peters, & Van Boekel, 2016) |
| LC-MS | Baby food | Homogenized samples, extracted with MeCN, anion exchange with PSA sorbent | MethylAA (Sigma-Aldrich, St. Louis, MO, USA) | C8 column (150 mm × 2.1 mm i.d., 5 μm particle size; Zorbax Eclipse XDB, Agilent) at 30 °C | DI water and MeCN as solvent A and B, respectively, both acidified with 0.01% formic acid: 0 min, 5% B, 5 min, 50% B, 5.1 min, 100% B, flow rate 0.2 ml min−1 | ESI+ mode, source temperature 120 °C, desolvation temperature 400 °C, capillary voltage, 3 kV, cone voltage 25 V, gas flow 500 L h−1 (desolvation), 54 L h−1 (cone) and 0.2 mL min−1() | (Petrarca et al., 2017) |
| LC-MS/MS | Cocoa and cocoa products | Homogenize samples, spike with IS extract with MeCN followed by clean up with Carrez I and II solutions | AA-d3 (LGC Standards GmbH (Wesel, Germany) | Lichrospher 100 CH 5 μm (250 × 4 mm) (Merck, Darmstadt, Germany) | Isocratic elution with 0.5% MeCN in water with 0.1% formic acid; flow rate 0.25 ml min−1 and 20 μL injection volume | ESI+, capillary voltage 3 kV, cone voltage 40 V, source temperature 450 °C | (Raters & Matissek, 2018) |
| LC-MS/MS | Food samples (potato chips, mashed potatoes, rye flour) | Homogenize samples, spike with IS, solid–liquid extraction with MeCN, and cleanup with SPE sorbent | AA-d3 (Polymer Source Inc., Dorval, Quebec, Canada) | Hypercarb 5 μm (50 × 2.1 mm, guard column 5 μm, 10 × 2 mm) (Thermo Hypersil-Keystone, UK) | Water mobile phase with flow rate 400 μL min−1, 10 μL injection volume | ESI+, source temperature 125 °C, capillary voltage 2 kV, cone voltage 20 V, energies 9, 16, 20, 0, and 14 eV | (Rosén & Hellenäs, 2002) |
| LC-MS | Food samples | Stable isotope dilution method (SIDA) after homogenization | AA-d3 (Toronto Research Chemicals; Toronto, Canada) | Luna C8(2) (150 mm × 4.6 mm; 3 μm) and Luna C8(2) guard column (4 mm × 3 mm, Phenomenex, Aschaffenburg, Germany) | Injection volume 50 μL, flow rate 0.5 mL min−1; 0.05% aqueous formic acid and MeOH as solvents A and B, respectively; concentration was increased from 1% to 20% over 12 minutes | ESI+, DP 36 V, CEP 10 V, CE 15 V, CXP 7 V, curtain gas 40 psi, ionspray voltage 4,500 V, temperature 550 °C | (Ruenz, Bakuradze, Eisenbrand, & Richling, 2016) |
| HPLC | Fried potatoes | According to Wenzal et al. (2003), defatted with hexane, adding of MeCN and Carrex I and II solutions | AA | n/a | 50% MeCN in aqueous 1% formic acid | n/a | (Sanghvi et al., 2016) |
| LC-MS/MS | Foodstuffs; fish fillet (cod), lean beef, lean pork, chicken fillet, soy flour, grated potato, grated beetroot, spinach | Homogenized samples, add IS, solid–liquid extraction with water, anion exchange with SPE sorbent, add MeCN and add water | AA-13C3 | Hypercarb column (50 × 2 mm; ThermoHypersil) | Water as mobile phase, flow rate 0.2 mL min−1 for 6.1 min, wash with 80% aqueous MeCN 0.2 ml min−1, 10 min, reconditioning with water | ESI+, capillary voltage 3.2 kV, cone voltage 50 V, source temperature 125 °C, desolvation temperature 350 °C, cone gas flow 211 L h−1, desolvation gas flow 653 L h−1, 2.5 mbar gas psi | (Tareke et al., 2002) |
| HPLC-MS/MS | Blood samples (hemoglobin) | Cleave N-terminal Val of Hb with AA attached via Edman degradation, spike with IS, liquid-liquid extraction | AA-Val(13C515N)-HLTPEEK | n/a | n/a | n/a | (Vesper et al., 2008) |
| GC-MS | Potato flour, french fries | Homogenize samples, spike with IS, solid–liquid extraction with hexane, water and MeCN | AA (Sigma-Aldrich, Milquakee, WI, USA) | PEG column, no specifics given | n/a | Single ion monitoring mode, no other specifics given | (Adedipe et al., 2016) |
| GC-MS | Food samples (potato chip, sweet potato chip, crackers and snacks, peanut better, chocolate, chocolate syrup) | Spike sample with IS, solid–liquid extraction with MeCN and hexane (for defatting) | AA-d3 (Cambridge Isotope Laboratories Inc., Andover, MA, USA) | Stabilwax-DB capillary column (20 m, 0.32 mm i.d., 1 μm film thickness; Restek) | Analysis time 11 min (AA r.t. 9.3 min), inlet temperature 100 °C held for 3.1 min, 200 °C min−1 ramp to 150 °C, held for 2 min, return to 100 °C, split ratio 50:1 for 2.5 min, then 15:1 for rest of analysis, oven temperature 80 °C for 6.35 min, then 70 °C min−1 ramp to 200 °C for 2.94 min | MS transfer line temperature 170 °C, ion trap temperature 200 °C, manifold temperature 50 °C, MeOH chemical ionization reagent | (Mastovska & Lehotay, 2006) |
| GC-MS | Bread, biscuits and bakery products | Homogenize samples, solid–liquid extraction in water pH 4–5 with glacial acetic acid, clean up with Carrex I and II solutions, derivatization with KBr, HBr, sodium thiosulfate, extraction with ethyl acetate and hexane | AA-13C3 | PEG column (30 × 0.25 μm, 0.25 μm i.d., TraceGOLD TG-WaxMS, Thermo Fisher Scientific, USA) | He mobile phase with constant flow rate 1.6 ml min−1, 1 μL sample volume, split ratio 1:10, injector temperature 220 °C, AA r.t. 10.65 min | n/a | (NegoiţĂ & Culeţu, 2016) |
| GC-MS | Instant coffee | QuEChERS method with addition of hexane for defatting | AA-d3 (Sigma-Aldrich Chemi GmbH, Germany) | DB-5MS column (30 m × 0.25 μm; Agilent Technologies, USA) | Initial temperature 50 °C, 3 °C min−1 to 100 °C, and then 25 °C min−1 until 250 °C (takes 5 min), solvent delay 8 min, He carrier gas at flow rate 1.0 ml min−1 | Internal ionization mode, scan m/z from 45 to 500, emission current of ionization filament 15 μM, trap line temperature 180 °C, transfer line temperature 220 °C, SIM mode | (Surma et al., 2017) |
| GC-MS | Foodstuffs; fish fillet (cod), lean beef, lean pork, chicken fillet, soy flour, grated potato, grated beetroot, spinach | Homogenize samples, solid–liquid extraction with water, purification on carbon black column, spike with IS | N,N-dimethylAA (Sigma-Aldrich; Stockholm, Sweden) | BPX-10 fused silica capillary column (30 mx 0.25 mm i.d., 0.25 μm film thickness; SGE, Ringwood, Australia) | Isothermal for 1 min at 65 °C, 15 °C min−1 to 250 °C, isothermal 10 min | Splitless injection, injector temperature 250 °C | (Tareke et al., 2002) |
| UV spectrophotometry | Bread samples (white and brown) | Homogenize samples, spike with IS, add MgSO4 and NaCl, hexanes for defatting, and MeCN and water for extraction, anion exchange with PSA sorbent, followed by microextraction with MeCN and ultrasound, add HNO3 in EtOH | AA (Sigma) | n/a | Perform spectrophotometric determination at 530 nm against a blank | (Altunay et al., 2018) | |
| Fluorescence quenching biosensor | Potato fries | Homogenize and extract with water, defat with hexanes, purify with Carrex I and II solutions, spike with IS, incubate with ssDNA, add FAM-csDNA followed by AuNPs with incubation | AA (Sigma-Aldrich, USA) | n/a | Detect at 490 nm | n/a | (Asnaashari et al., 2018) |
| Glutathione S-transferase | Food samples: bread, potato chips, boiled potatoes | Homogenize samples, extract with EtOH and defat with hexane, spike with IS | AA (Sigma-Aldrich, Germany) | n/a | Detect spectrometrically at 340 nm using CDNB to produce a colored compound with GSH, get inhibition constant | n/a | (Bucur, Bucur, & Radu, 2018) |
| Fluormetric assay | Food samples: potato chips, fried bread sticks, bread and rice crust | Solid–liquid extraction with MeOH, defatting with hexanes, spike with AA | AA (supplier in supplementary) | ZnS quantum dots (QD) doped with Mn (II) ions are fluorescent source, are conjugated to AA to serve as fluorescent probe | Add AA-MIP, add AA extraction, let incubate. Record fluorescence at 470–750 nm upon excitation at 235 nm, slit width 10 nm, scan speed 200 nm min−1, excitation voltage 750 V | n/a | (Y. Liu et al., 2018) |
| HS-SPME coupled with IMS (Headspace solid-phase microextraction with ion mobility spectrometry) | Food samples: potato chips, fried potatoes | Homogenize samples, solid–liquid extraction with MeOH | AA (Aldrich, Steinheim, Germany) | Nanostructured polypyrrole fiber | n/a | [IMS] Corona voltage 3.0 kV, drift field 636 V cm−1, drift gas flow, N2 450 ml min−1, injection temperature 200 °C, shutter grid pulse 150 us, drift tube length 11 cm, calibrant ion NH4+(H2O)n, drift time (7.50 ms), reduced mobility 1.89 cm2V−1s−1 | (Pourmand, Ghaemi, & Alizadeh, 2017) |
| Hemoglobin nanoparticles for detection | Food samples: biscuits, cakes, chips, fried cereals, nuts, corn puffs | Homogenize, solid–liquid extraction in water, clean up with Carrez I and II solutions, measure in sodium acetate buffer | AA (SISCO Research Lab, Mumbai, India) | n/a | [three electrode system] Immobilize covalently HbNPS onto polycrystalline Au electron, which acts as a biosensor to detect AA concentration in processed foods | Current measured at 0.26 V with scan rate 20 mVs−1 vs Ag/AgCl | (Yadav et al., 2018) |
| AA-specific aptamers | n/a (methods paper with no validation with other methods yet) | n/a | AA (Sigma-Aldrich, St. Louis, MO, USA) | n/a | Use SELEX to identify AA-specific aptamers, incubate gold nanoparticles | Record on microplate reader from rage 400 to 750 nm | (Hu et al., 2018) |
| Hollow fiber liquid phase microextraction and chromatography-electron capture detection (HF-LPME) | Waste water samples | AA (Aldrich, Buchs, Switzerland) | (Sobhi et al., 2017) | ||||
| GC-MS | Waste water samples | Derivatization with bromine | AA (Aldrich, Buchs, Switzerland) | HP-5 MS capillary column (30 m × 0.25 mm, 0.25 μm, film thickness) (Agilent J&W Scientific, Folsom, CA, USA) | TIC mode, He carrier gas flow rate 1.0 ml min−1, 60 °C for 1 min, increased to 280 °C at 10 °C min−1 and held 5 min, increase to 300 °C at 50 °C min−1 held for 3 min. MS quadrupole source temperature 150 °C, MS source temperature 230 °C, m/z range 40–300 | (Sobhi et al., 2017) | |
| GC-MS | Food samples: biscuits, chips and cake | Homogenize, spike with IS, solid–liquid extraction with water, defat with petroleum ether, cleanup with Carrex I and II solutions, salt out with NaCl, extract with ethyl acetate | AA-d3 (Cambridge Isotope Laboratories, Andover, MA, USA) | DB-23 capillary column (30 m × 0.25 mm i.d.d., 0.25 μm film thickness; J&W Scientific Products GmbH, Koln, Germany) | He flow rate 1.0 ml min−1, column temperature 80 °C for 2 min, increase from 80 °C-220 °C at a rate of 10 °C min−1 | Splitless injection 240 °C, ion source temperature 200 °C | (Razia et al., 2016) |
| HS-SPME/GC-FID | Potato chips, french fries | Homogenize, extract with HS-SPME at 60 °C for 30 min, desorp at 230 °C for 2 min | AA (Merck; Darmstadt, Germany) | BP20 polar capillary column (PEG, 30 m × 0.25 mm × 0.25 μm; SGE Company) | N flow rate 2.3 ml min−1, start at 45 °C for 2 min, raise to 150 °C at a rate of 10 °C min−1 | Detector temperature 200 °C, injector temperature 230 °C | (Ghiasvand & Hajipour, 2016) |
| FAAS (flame atomic absorption spectrometry) | Food samples: chips, crackers, cereal-based baby foods | Homogenize, defat with hexane, extract with MeCN and water with NaCl and MgSO4, add IS as preconcentration with ammonia buffer at pH 9.0, separate aqueous phase, dilute in MeOH | AA (Sigma, St. Louis, MO, USA) | n/a | n/a | Wavelength 232.0 nm, 0.7 nm slit, lamp current 6 mA, burner height 7 mm, nebulizing flow rate 6 mL min−1 | (Altunay et al., 2016) |
| Microchip electrophoresis | Food samples; potato chip and french fry | Homogenize, spike with IS and extract with water, defat with hexane, dilute in borate buffer, and derivatize with Cy5 | AA (Sinopharm Chemical Reagent Co. Ltd; Shanghai, China) | Glass microchip with separation channel 60 mm × 45 mm, etched to depth of 25 μm and width 70 μm | n/a | n/a | (Wu, Chen, Wang, He, & Wang, 2016) |
| HS-GC-MS | Brewed coffee | AA (Sigma-Aldrich, St. Louis, MO, USA) | Mega-FFAP-EXT column (50 m × 200 μm × 20 μm; Legnane, MI, Italy) | HS at 205 °C, sample loop and transfer line temperatures 215 °C and 225 °C, respectively, injector temperature 250 °C with 5:1 split ratio, He gas flow 1.0 ml min−1, initial temperature 50 °C held 1 min, ramp of 2 °C min−1 to 165 °C, then increased to 250 °C at 7.5 °C min−1 held 2 min | EI mode at 70 eV, SIM (single ion monitoring) acquisition mode | (Zhang, Cagliero, Pierson, & Anderson, 2017) | |
| HPLC-UV | Food samples; potato chips, coffee beans, french fries | Homogenize, extract with water, defat with hexane, clean up with Carrez I and II solutions | AA (Sigma-Aldrich, Hamburg, Germany) | Zorbax C18 steel analytical column (250 × 4.6 mm, 4.5 μm, Hewlett Packard, Houston, USA) | Six mobile phases utilized: 1. Water/MeOH (90:10 v/v), 2. MeOH/MeCN/Water (10:10:80 v/v/v), 3. Water/MeCN (90:10 v/v), 4. Water/formic acid (99.9: 0.1 v/v), 5. Water/formic acid (90:10 v/v) 6. Water (100%) | DAD wavelength set to 190 and 240 nm | (Oroian et al., 2016) |
| LC-MS/MS | Food samples; rice porridge, apple juice and peanut butter | Homogenize sample, spike with IS, extract with water, clean up with sorbent | aa- 13 c 3 | Atlantis T3 column (2.1 × 100 mm i.d., 3 μm; Waters Corporation, Dublin, Ireland) | Mobile phase 30 mm ammonium formate in water and 100% MeCN as solvents A and B, respectively; 90% A for 2 min, 2–2.5 min held at 87% A, 2.5–4 min 87%–86% A, 7.5–8.5 min 66–50% A, 8.5–11 min held at 50% A, 11–13 min 50–0% A, flow rate 0.25 ml min−1, 5 μl injection volume | Ionspray voltage 55 kV at 550 °C, curtain gas 25 psi, nebulizing gas 50 psi, heating gas 50 psi, DP 31 V, CEP 4.5 V, CE 15 V, CEP 18 V, CXP 4 V | (Lee et al., 2015) |
| GC-NCD | Food samples; dry and unheated potato, french fries, potato chips | Homogenize samples, extract with MeCN and water, add NaCl and Mg SO4, defat with hexane, clean up with PSA sorbent | Acrylamide (Dr. Ehrenstorfer, Augsburg, Germany) | DBWaxEtr pre-column (5 m × 0.53 mm × 1 μm) and DBWaxEtr analytical column (15 m × 0.53 mm × 1 μm) (Agilent) | Injection volume 20 μl, splitless mode, 3 min purge time, MMI inlet temperature 70 °C (1 min), increased by 600 °C min−1 to 300 °C, oven temperature 60 °C (1 min), increased by 25 °C min−1 to 150 °C and held 2.5 min, flow rate 20 ml min−1 He, initial source pressure 9.1 psi, switching flow from pressure control started 45 ml min−1 (1 min), increased by 2 mL min−1 to 40 ml min−1 | Nitrogen chemiluminescence detector conditions 930 °C burner temperature, 5 ml min−1 hydrogen and 10 ml min−1 oxygen | (Weijun, 2015) |
4.2 |. GC-MS
GC-MS is less often reported in literature than LC-MS for acrylamide analysis; however, there are several notable acrylamide studies relevant to food products that have used GC-MS analysis. For acrylamide extraction, food samples such as peanut butter, potato chips, boiled meat, french fries, and even fried beef are subject to the same or nearly identical QuEChERs and solid–liquid or liquid–liquid extraction methods as were described for the LC-MS protocols (Adedipe, Johanningsmeier, Truong, & Yencho, 2016; Mastovska & Lehotay, 2006; NegoiţĂ & Culeţu, 2016; Surma et al., 2017; Tareke et al., 2002). The solubility characteristics of acrylamide complicate its extraction from food in that residual water is often present in the extract. For GC-MS sample preparation, any water remaining in the extract must be removed prior to injection into the instrument. This is due to the high expansion of water when heated, which can damage the column over time. The typical method of choice to remove water from the acrylamide extract is salting out with sodium chloride and magnesium sulfate, which allows acrylamide to partition into the acetonitrile layer from the water due to the formation of a density gradient (Mastovska & Lehotay, 2006).
GC-MS detection of acrylamide generally involves the use of internal standards like N,N-dimethylacrylamide, as well as derivatization by bromination or silylation (Sobhi, Ghambarian, Behbahani, & Esrafili, 2017; Surma et al., 2017; Tareke et al., 2002). Table 2 provides information about the various methodologies used on a GC-MS apparatus, as well as the relevant studies performed and internal standards used. Acrylamide polymerizes at temperatures at or above 138 °C, and decomposes between 175 °C and 300 °C (NIOSH, 2007), making derivatization a valuable way to accurately measure the acrylamide adduct that is resistant to polymerization or decomposition. Two recent publications reported successful use of a polyethylene glycol (PEG) GC column for the detection of acrylamide from food products (Adedipe et al., 2016; Ghiasvand & Hajipour, 2016). In the first of these studies, acrylamide detection in french fries by GC-MS was used as a validation step for a new method of acrylamide detection using near-infrared spectroscopy (NIR) (Adedipe et al., 2016). However, their validation results were not published in the study. In the second of these studies, GC-FID was used in conjunction with solid-phase microextraction to determine the acrylamide content of potato chips. In both studies, derivatization was deemed unnecessary because the PEG column was optimal for acrylamide analysis, purportedly because it is specifically designed for small polar analytes (Guide to GC Column Selection and Optimizing Separations, 2013). Adedipe et al. extracted acrylamide from french fries and compared analysis results from GC-MS to those of near-infrared spectroscopy. The focus of their study was to assess NIR for acrylamide detection, and GC-MS was used as a validation method (Adedipe et al., 2016; Rothweiler, Kuhn, & Prest, 2004). The GC protocol referenced by Adedipe et al. originated from an Agilent Technologies application note for the detection of native, nonderivatized acrylamide, using a pulsed splitless injection mode with an inlet temperature of 220 °C and a temperature ramp starting at 60 °C (held for 1 min) to 230 °C at a rate of 12 °C/min. The carrier gas was helium with a constant flow rate of 2.0 ml/min. Adedipe et al. utilized selective ion monitoring mode (SIM) for mass spectrometry in electrospray ionization (ESI) positive mode to select for ions going through the detector at 71, 55, and 44 m/z. These mass-to-charge ratios were selected because the most abundant acrylamide fragments consist of ions of these three masses (Rothweiler et al., 2004).
When analyzing the acrylamide content of potato chips and french fries, Ghiasvand et al. used a gas chromatogram equipped with a flame ionization detector (GC-FID), rather than a mass selective detector; in their protocol, the temperature ramp began from 45 °C (held for two minutes) and progressed to 150 °C at a rate of 10 °C/min (Ghiasvand & Hajipour, 2016). Nitrogen was used as the carrier gas with a flow rate of 2.3 ml/min, and detector and injector temperatures were held at 200 °C and 230 °C, respectively. However, if analyte derivatization did not reach 100%, accurate concentration determination was not achieved, which is a limitation of the GC method as compared with the LC protocol. The composition of column packing material changes the retention time for derivatized acrylamide and isotopically enriched acrylamide internal standards, making agreement between and within studies subject to component validation in each case (see Table 2).
GC-MS analysis of acrylamide, which does not readily volatilize, in combination with a non-PEG GC column, necessitates derivatization to avoid broad or tailing peaks in the gas chromatogram (Thermo Scientific Reagents, Solvents and Accessories, 2003). A sampling of acrylamide derivatization techniques has been published and is summarized in Table 3 (Kamankesh, Zokaei, Shojaei, & Mohammadi, 2017; Lan, 2016; Sobhi et al., 2017; Tareke et al., 2002). Silylation and bromination are the most commonly reported derivatization techniques used to analyze acrylamide. Silylation is performed by first adding acrylamide extract to a solution of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and heating for approximately one hour at 70 °C, followed by liquid–liquid extraction using a nonpolar solvent like hexane (Surma et al., 2017). BFTSA is typically used for derivatizing fatty acid amides, but since acrylamide is a short-chain amide, BFTSA has successfully been used to derivatize acrylamide (Her, 1985; Shanchun et al., 1994). Bromination can be accomplished with either hydrobromic acid (HBr) or bromine water. French fries from which acrylamide was extracted and derivatized by bromination led to quantitative determination of acrylamide by GC-MS (Tareke et al., 2002). French fries ground to a powder and frozen were lyophilized, followed by addition of deionized water, sonication, and stirring. The aqueous acrylamide extract was then filtered and the filtrate defatted with hexanes, followed by separation of the aqueous layer. Potassium bromide was added to derivatize the acrylamide, and excess bromine was decomposed by addition of 1 M sodium thiosulfate solution. Ethyl acetate was added to form a biphasic solution, where the brominated acrylamide went into the organic layer, and was isolated using a separatory funnel. The organic layer was dried with anhydrous sodium sulfate, filtered, and analyzed by GC-MS (Hashimoto, 1976; Zhu et al., 2008).
TABLE 3.
Derivatizing agents used for GC-MS
| Derivatizing agent for GC-MS | Resulting acrylamide molecular formula | Reference |
|---|---|---|
| Bromine | C3H4NOBr2 | (Sobhi et al., 2017; Tareke et al., 2002) |
| 9-hydroxyxanthene, aka xanthydrol | C16H12NO2 | (Kamankesh et al., 2017; Lan, 2016) |
| BSTFA (BTMSA-AA) | C9H22ONSi2 | (Surma et al., 2017) |
4.3 |. Computer-based visualization methods
Several methods using computer-based visualization have been developed to study the relationship between acrylamide formation and the browning of various food products such as potato chips, french fries, biscuits, and cookies (Dutta, Singh, & Ghosal, 2015; Gökmen, Şenyuva, Dülek, & Çetin, 2007; Gökmen & Mogol, 2010; Hu, Xu, Fu, & Li, 2015; Mogol & Gökmen, 2014). Computer-based visualization measures the increase in brown color upon frying or cooking to derive an approximate acrylamide concentration (Pedreschi, Mariotti, & Granby, 2014). These computational methods may offer valuable monitoring solutions for the baking, frying, and even fast-food industries, where legislative regulations may mandate acrylamide levels be minimized. A major limitation of computer-based visualization, however, is encountered for the coffee or fruit juice industry, as increased browning during coffee brewing or juicing is not necessarily a function of increased acrylamide formation.
4.4 |. Rapid detection methods
A review by Hu et al. provides an overview of rapid methods of acrylamide detection in potato chips, biscuits, and coffee, reported in literature up to 2015 and will therefore not be discussed in depth here (Hu et al., 2015). Hu et al. described reports of acrylamide detection including fluorescence, supramolecular, ELISA, and electrochemical methods. More recently, Pundir et al. reported on novel methods of acrylamide measurement with specific emphasis on biosensors (Pundir, Yadav, & Chhillar, 2019). The review by Pundir et al. covered electrochemical techniques, molecularly imprinted polymers, microbial cells, gravimetric sensors and piezoelectric sensors. For the purposes of the current review, we will limit our discussion to an updated overview of the rapid methods of acrylamide analysis developed since 2015, as well as those not covered by Pundir et al. in 2019, and summarize these approaches (see Table 2). In all cases of reported rapid acrylamide analysis protocols for food products, a consistent theme is result validation using either LC-MS or GC-MS.
4.4.1 |. SELEX method
Hu et al. developed a quartz crystal microbalance (QCM) method of acrylamide detection for the systematic evolution of ligands by exponential enrichment (SELEX) (Hu, Wang, Wang, Slavik, & Li, 2018). Single-stranded DNA (ssDNA) nucleotides of about 45 nt length were randomized and selected by binding with acrylamide-modified solid matrix. The sequences of the two best aptamers were determined to be (1) GCCGCCCCCAGTTGACCATGCCCACACCATGCCCTCACCGCGCAC, labeled in their paper as C14, and (2) TGGTCGTGGTGAGGTGCGTGTATGGGTGGTGGATGAGTGTGTGGC, labeled in their paper as A5. In this instance, Hu et al. performed a proof-of-concept study to show that an aptamer could be designed and utilized to detect acrylamide extracted from a potato matrix (Hu et al., 2016). Quartz crystals were modified with bovine serum albumin conjugated to acrylamide (BSA-AA), followed by incubation with DNA aptamers. Unbound ssDNA was washed away and the best two bound aptamers, A5 and C14, were analyzed further, and used to develop a colorimetric assay for acrylamide detection. The A5-AA and C14-AA were eluted and amplified via PCR, followed by cloning and sequencing. To ensure the specificity of their aptamers, Hu et al. tested the efficacy of their aptamers toward acrylamide derivatives methylacrylamide and methacrylic acid, and acrylic acid. Their designed aptamers had no affinity toward the evaluated derivatives, indicating high specificity for acrylamide, which was the desired result. For the colorimetric assay, gold nanoparticles (AuNPs) were synthesized and added to solutions of varying concentrations of acrylamide with steady concentration of aptamer. After incubation, sodium chloride solution was added and the absorbance measured using a microplate reader in the range of 400 to 750 nm. The results demonstrated that a DNA aptamer and AuNPs could provide a calibration curve for quantitative measurement of acrylamide in the linear range of 0.5–10 μg/L, with a detection limit of 0.390 μg/L.
4.4.2 |. Ionic liquid-based microextraction and spectrophotometry
UV-VIS spectrophotometry is a sensitive method for the detection of wavelengths of light that absorb in the visible to ultraviolet (UV) region (~100–700 nm) of the electro-magnetic spectrum. Molecules that are chromophores, or are covalently attached to chromophores, will be detected. Altunay et al. utilized UV-VIS spectrophotometry for the detection of acrylamide in white and brown bread, whole grain cereal, biscuits, chips, and coffee. For UV-VIS to detect acrylamide, the ion pairing reagent, phenosafranine (PSH+), was introduced to the sample, in phosphate buffer (pH = 7.5), to couple with acrylamide and serve as a spectrophotometric probe (Altunay, Elik, & Gürkan, 2018). The AA-PSH+ solution is incubated, acetonitrile and 1-butyl-3-methylimidazolium tetrafluoroborate (Bmim BF4) are added, and the solution is brought to a final volume of 15 mL with water. Centrifugation and removal of the bottom layer containing the AA-PSH+ complex is then brought to a final volume of 500 μL by addition of 0.1 M nitric acid in ethanol. Spectrophotometric determination of acrylamide concentration by UV-VIS can then measure the content of the sample at a maximum wavelength of 530 nm, with a detection limit of 0.7 μg/kg of sample.
4.4.3 |. Flame atomic absorption spectrometry
Altunay et al. also developed a method for the detection of acrylamide in potato chips, corn chips, cracker samples, and cereal-based baby foods using flame atomic absorption spectrometry (FAAS) (Altunay, Gürkan, & Orhan, 2016). FAAS is inexpensive and nontrivial for general use. For this method, the known acrylamide-containing samples were first concentrated by adding aqueous aliquots to an ammonia buffer solution at pH 9.0 along with fluorescein, Ni(II), ammonium chloride, and poly(ethyleneglycolmono-p-nonylphenylether) (PONPE). Ni(II) complexes with acrylamide permitting detection and quantitation of AA-Ni(II) by FAAS. The solution was placed in an ultrasonic bath at 40 °C for 5 min, followed by vortexing for 2.5 min at 3000 rpm to generate droplets. All of this was then followed by centrifugation at 4,000 rpm for 5 min, and the surfactant-rich phase diluted in methanol after cooling via refrigeration. The sample was analyzed using FAAS to detect AA-Ni(II) at 232 nm, providing acrylamide concentration determination upon comparison with a Ni(II) calibration curve. The detection limit of this method was found to be 0.053 μg/L in a linear range of 0.2 to 210 μg/L and a limit of quantification of 0.176 μg/L.
4.4.4 |. Fluorescence quenching biosensor, au nanoparticles
Asnaashari et al. developed a fluorescence quenching biosensor method for the detection of acrylamide in crushed potato fries using double-stranded DNA (dsDNA) and gold nanoparticles (AuNPs) (Asnaashari et al., 2018). This method is an adaptation of the fluorescence protocol proposed by Hu et al. that incorporated quantum dots (QDs) during acrylamide polymerization as a substitute for the use of a fluorescent tag (Hu et al., 2014). In the Asnaashari et al. protocol, ssDNA with the sequence 5′-ACG GCA ACA TGC CGA AGG AAC TAC CGG AAA CGG CAA ATC CTC G-3′ was used to bind free acrylamide. Acrylamide forms adducts with DNA by coupling 2-carboxyethyl on the nitrogen atoms of adenosine, cytosine, and guanosine residues (Besaratinia & Pfeifer, 2005, 2007; Doerge et al., 2005; Li et al., 2016; Shipp et al., 2006). The commercially available complementary sequence DNA (csDNA) to the ssDNA was oligomerized to fluorescein amidites (FAM) to make FAM-csDNA, with the sequence 5′-CGA GGA TTT GCC GTT TCC GGT AGT TCC TTC GGC ATG TTG CCG T-3′. FAM can be added to either the 5′ or 3′ end of DNA, though they did not specify which end of the DNA the FAM was added to. For this assay, acrylamide binds to ssDNA, which prevents fluorescent FAM-csDNA from binding to its complementary sequence. The AuNPs then bind the FAM-csDNA and quench fluorescence. The ssDNA that is bound to FAM-csDNA will remain fluorescent. The assay takes approximately one hour and fluorescence spectra are measured at λ = 490 nm, with a detection limit of 100 μM in the linear range of 0.5 to 100 μM. When comparing fluorescence between the biosensor itself and the biosensor plus acrylamide, fluorescence significantly decreased. After proof of concept, this method was used to study french fries through matrix spike calibration and back calculation to determine acrylamide concentration. French fries were ground and mixed with water and allowed to swell at 4 °C for 20 min, followed by centrifugation and defatting the supernatant with hexane. The defatted aqueous solution was then treated with Carrez I and II solutions followed by filtering and 100-fold dilution. While it was shown that this method is effective for acrylamide detection, and is efficient time-wise, the results were not validated using LC-MS or GC-MS protocols. Although the fluorescence quenching biosensor method is rapid, the acrylamide extraction from the food product remains time consuming, which may not be ideal to food processors. Further, materials and equipment are required that a food quality control laboratory may not have readily available or that may not have general application to other analyses.
4.4.5 |. NIR
NIR spectrometers are common in food analysis labs, because they are relatively inexpensive and easy to operate. NIR spectroscopy has been routinely used for determination of protein content in dairy products, fat, moisture, and salt content of cheese, and a wide variety of other food analysis applications (Laporte & Paquin, 1999; Margolies & Barbano, 2017; Osborne & Fearn, 1986; Siesler, Ozaki, Kawata, & Heise, 2002). NIR spectrometers can be used in reflectance mode for the analysis of solid samples, or transmittance mode to analyze liquid samples. Adedipe et al. demonstrated the proof of concept that NIR spectroscopy could be used to determine the acrylamide content in french fries (Adedipe et al., 2016). If a routine analysis method using NIR for acrylamide detection were to be optimized, the application could provide less expensive and more efficient testing capabilities for food quality control labs. Since NIR spectrometers are commonly available in food labs already, the instrument cost and employee training would be dramatically reduced. The NIR method reported by Adedipe et al. begins with establishment of a calibration curve for acrylamide in a lyophilized peeled and ground potato matrix. Acrylamide dissolved in acetonitrile was spiked into the freeze-dried potato flour, at different concentrations, to generate the calibration curve. Acrylamide quantitation was achieved in the linear range of 50–8,000 μg/kg at wavelengths of 400 to 2,500 nm, with a detection limit of 70 μg/kg. French fries made in the laboratory were lyophilized and ground into a potato powder. The potato powder was spiked with acrylamide that had been dissolved in acetonitrile in the same fashion as the potato flour model system used for acrylamide calibration. The difference between the model system and the french fries can be attributed to the acrylamide produced during the cooking process for the french fries. The french fry study by Adedipe et al. validated their NIR results using a GC-MS protocol from Agilent Technologies, but they did not report the acrylamide concentration values obtained for either the NIR or GC-MS method. The NIR method of acrylamide analysis is extremely promising for routine use in food processing facilities, and has efficiency advantages to LC-MS and GC-MS. For NIR to be broadly implemented, more studies must be conducted to validate acrylamide detection limits and applicability across different sample matrices, including coffee (beans or brew), cereal, crackers, and many others.
4.4.6 |. SPE combined with surface-enhanced Raman spectroscopy
Surfaced-enhanced Raman scattering (SERS) is a technique that employs the use of Raman scattering by molecules absorbed on metal surfaces (Le Ru, Blackie, Meyer, & Etchegoint, 2007). Cheng et al. in 2018 used SERS to detect acrylamide in fried foods such as croquette, potato chips, and fried instant noodles (Cheng et al., 2019). For the assay, AuNPs were synthesized according to their established protocol (Cheng et al., 2018). In this study, fried foods were pretreated by homogenization, and then acrylamide was extracted using QuECHeRS (Mastovska & Lehotay, 2006). The extract was mixed with the synthesized AuNPs and a Raman spectrum acquired in the wavenumber range of 500 to 2500 cm−1 at room temperature. The Raman peak at 1,478 cm−1 was used for qualitative and quantitative analysis of acrylamide. The limit of detection was determined to be 2 μg/kg and the limit of quantitation was 5 μg/kg in a range of linearity of 5 to 100 μg/kg. For validation, their own method was used with five different concentrations of acrylamide spiked into potato chips, croquettes, and fried instant noodles and analyzed, as well as LC-MS/MS. Their acrylamide concentrations were determined using unknowns and spiked samples versus LC-MS/MS protocols provided results that were highly similar, if not exact.
The most cost- and time-effective method for acrylamide detection will be researcher and lab resource dependent. While LC-MS and GC-MS methods are currently the most accurate, the instrumentation is expensive and equipment usage requires appreciable technician training. Ideally, NIR would be the fastest and cheapest method to determine acrylamide levels in food, as many industrial food laboratories already have NIR spectrometers, sample preparation is minimal, and data processing is automated. However, more research is required to validate NIR results with other detection methods and ensure that the method and results are robust and reliable.
5 |. CONCLUSION
The evidence for the carcinogenicity of acryalmide in rodents at levels that are two to three times higher than in human food products has provided sufficient evidence for the EU and the state of California to pass legislation requiring the labeling and potentially the quantification of acrylamide on food product nutritional labels. Food process industries are preparing to align their lab testing capabilities with anticipated regulatory mandates pertaining to acrylamide. Optimization of acrylamide measurement protocols that provide accurate and rapid results from food and beverage products are being developed to meet the need. Emerging technologies incorporating GMOs or pulsed electric fields are gaining popularity to reduce acrylamide production by lowering levels of reducing sugars and asparagine; however, many quick-service restaurants, including McDonald’s, do not serve any products made from GMO potatoes. The studies reported here are intended to offer researchers and industry scientists a plethora of testing options to choose from depending on their budget, instrumentation, location, and local or national legislation regarding acrylamide.
ACKNOWLEDGMENTS
Thank you to Mark Skinner, B.S. of the Department of Chemistry and Biochemistry at Boise State University, for providing references on bromination.
FUNDING
This research was funded by the Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grants #P20GM103408 (INBRE) and #P20GM109095 (COBRE in Matrix Biology) and the Idaho State Department of Agriculture grant of title “Fast, Accurate, and Economical Evaluation of Acrylamide Content in Fried Potato Products.”
Nomenclature:
- AA
acrylamide
- BSA
bovine serum albumin
- csDNA
complementary strand DNA
- DNA
deoxyribonucleic acid
- FAAS
flame atomic absorption spectrometry
- FAM
fluorescein amidites
- GA
glycidamide
- GC-MS
gas chromatography-mass spectrometry
- LC-MS
liquid chromatography-mass spectrometry
- NIR
near-infrared spectroscopy
- PAGE
polyacrylamide gel electrophoresis
- PFPTH
pentafluorophenyl isothiocyanate
- PTH
phenylthiohydantoin
- QCM
quartz crystal microbalance
- SELEX
selective evolution of ligands by exponential enrichment
- ssDNA
single-stranded DNA
REFERENCES
- Acrylamide. (2018). RetrievedMarch 9, 2019, from https://oehha.ca.gov/proposition-65/general-info/acrylamide
- Acrylamide. (2020a). RetrievedSeptember 8, 2020, from https://www.p65warnings.ca.gov/chemicals/acrylamide
- Acrylamide. (2020b). RetrievedSeptember 15, 2020, from https://oehha.ca.gov/proposition-65/chemicals/acrylamide
- Acrylamide Levels in Food: Feedback to the European Commission. (2019).
- Adedipe OE, Johanningsmeier SD, Truong V. Den, & Yencho GC (2016). Development and validation of a near-infrared spectroscopy method for the prediction of acrylamide content in french-fried potato. Journal of Agricultural and Food Chemistry, 64(8), 1850–1860. 10.1021/acs.jafc.5b04733 [DOI] [PubMed] [Google Scholar]
- Adler ID, Baumgartner A, Gonda H, Friedman MA, & Skerhut M (2000). 1-Aminobenzotriazole inhibits acrylamide-induced dominant lethal effects in spermatids of male mice. Mutagenesis, 15(2), 133–136.Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10719038 [DOI] [PubMed] [Google Scholar]
- Akmal M, Aulanni’am A, Widodo MA, Sumitro SB, Purnomo BB, & Widodo (2016). The important role of protamine in spermatogenesis and quality of sperm: A mini review. Asian Pacific Journal of Reproduction, 5(5), 357–360. 10.1016/j.apjr.2016.07.013 [DOI] [Google Scholar]
- Altunay N, Elik A, & Gürkan R (2018). Extraction and reliable determination of acrylamide from thermally processed foods using ionic liquid-based ultrasound-assisted selective microextraction combined with spectrophotometry. Food Additives and Contaminants – Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 35(2), 222–232. 10.1080/19440049.2017.1394585 [DOI] [PubMed] [Google Scholar]
- Altunay N, Gürkan R, & Orhan U (2016). A preconcentration method for indirect determination of acrylamide from chips, crackers and cereal-based baby foods using flame atomic absorption spectrometry. Talanta, 161, 143–150. 10.1016/j.talanta.2016.08.053 [DOI] [PubMed] [Google Scholar]
- Anastassiades M, Lehotay SJ, Štajnbaher D, & Schenck FJ (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of AOAC International, 86(2), 412–431. 10.1371/journal.pone.0029268 [DOI] [PubMed] [Google Scholar]
- AOAC. (2007). AOAC Official Method 2007. 01 Pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate [Applicable for the following pesticides in grapes, lettuces, and oranges: Atrazine, azoxystrobin, bifenthrin, carbaryl. Aoac in Ter Na Tional, 1–9.https://doi.org/10.1.04 [Google Scholar]
- Asnaashari M, Esmaeilzadeh Kenari R, Farahmandfar R, Taghdisi SM, & Abnous K (2018). Fluorescence quenching biosensor for acrylamide detection in food products based on double-stranded DNA and gold nanoparticles. Sensors and Actuators, B: Chemical, 265, 339–345. 10.1016/j.snb.2018.03.083 [DOI] [Google Scholar]
- Assessment of health risks from exposure to acryalmide. (1990). Office of Toxic Substances U.S. Environmental Protection Agency, 1–264. [Google Scholar]
- Backe WJ, Yingling V, & Johnson T (2014). The determination of acrylamide in environmental and drinking waters by large-volume injection-hydrophilic-interaction liquid chromatography and tandem mass spectrometry. Journal of Chromatography A, 1334, 72–78. 10.1016/j.chroma.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Balhorn R (2007). The protamine family of sperm nuclear proteins. Genome Biology, 8(9), article 227. 10.1186/gb-2007-8-9-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutcu I, Sahin S, & Sumnu G (2009). Acrylamide formation in different batter formulations during microwave frying. LWT— Food Science and Technology, 42(1), 17–22. 10.1016/j.lwt.2008.07.004 [DOI] [Google Scholar]
- Bergmark E (1997). Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chemical Research in Toxicology, 10(1), 78–84. 10.1021/tx960113p [DOI] [PubMed] [Google Scholar]
- Bertuzzi T, Rastelli S, Mulazzi A, & Pietri A (2017). Survey on acrylamide in roasted coffee and barley and in potato crisps sold in Italy by a LC–MS/MS method. Food Additives and Contaminants: Part B Surveillance, 10(4), 292–299. 10.1080/19393210.2017.1351498 [DOI] [PubMed] [Google Scholar]
- Besaratinia A, & Pfeifer GP (2005). DNA adduction and mutagenic properties of acrylamide. Mutation Research – Genetic Toxicology and Environmental Mutagenesis, 580(1–2), 31–40. 10.1016/j.mrgentox.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Besaratinia A, & Pfeifer GP (2007). A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis, 28(3), 519–528. 10.1093/carcin/bgm006 [DOI] [PubMed] [Google Scholar]
- Bethke PC (2018). Progress and successes of the specialty crop research initiative on acrylamide reduction in processed potato products. American Journal of Potato Research, 95(4), 328–337. 10.1007/s12230-018-9660-2 [DOI] [Google Scholar]
- Brewer L, Corzett M, & Balhorn R (2002). Condensation of DNA by spermatid basic nuclear proteins. Journal of Biological Chemistry, 277(41), 38895–38900. 10.1074/jbc.M204755200 [DOI] [PubMed] [Google Scholar]
- Bucur M-P, Bucur B, & Radu G-L (2018). Simple, selective and fast detection of acrylamide based on glutathione: S-transferase. RSC Advances, 8(42), 23931–23936. 10.1039/c8ra02252f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli S, Polesello S, & Saccani G (2004). Determination of acrylamide in drinking water by large-volume direct injection and ion-exclusion chromatography-mass spectrometry. Journal of Chromatography A, 1039(1–2), 155–159. 10.1016/j.chroma.2004.01.010 [DOI] [PubMed] [Google Scholar]
- CFR—Code of Federal Regulations Title 21. (2019). RetrievedNovember 9, 2020, from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=176%26showFR=1
- Cheng J, Su XO, Han C, Wang S, Wang P, Zhang S, & Xie J (2018). Ultrasensitive detection of salbutamol in animal urine by immunomagnetic bead treatment coupling with surface-enhanced Raman spectroscopy. Sensors and Actuators, B: Chemical, 255, 2329–2338. 10.1016/j.snb.2017.09.047 [DOI] [Google Scholar]
- Cheng J, Zhang S, Wang S, Wang P, Su XO, & Xie J (2019). Rapid and sensitive detection of acrylamide in fried food using dispersive solid-phase extraction combined with surface-enhanced Raman spectroscopy. Food Chemistry, 276, 157–163. 10.1016/j.foodchem.2018.10.004 [DOI] [PubMed] [Google Scholar]
- De Paola EL, Montevecchi G, Masino F, Garbini D, Barbanera M, & Antonelli A (2017). Determination of acrylamide in dried fruits and edible seeds using QuEChERS extraction and LC separation with MS detection. Food Chemistry, 217, 191–195. 10.1016/j.foodchem.2016.08.101 [DOI] [PubMed] [Google Scholar]
- Doerge DR, Gamboa Da Costa G, McDaniel LP, Churchwell MI, Twaddle NC, & Beland FA (2005). DNA adducts derived from administration of acrylamide and glycidamide to mice and rats. Mutation Research – Genetic Toxicology and Environmental Mutagenesis, 580(1–2), 131–141. 10.1016/j.mrgentox.2004.10.013 [DOI] [PubMed] [Google Scholar]
- Dutta MK, Singh A, & Ghosal S (2015). A computer vision based technique for identification of acrylamide in potato chips. Computers and Electronics in Agriculture, 119, 40–50. 10.1016/j.compag.2015.10.007 [DOI] [Google Scholar]
- Emdmillipore. (2013). Carrez clarification Carrez solution I. 49(1), 64271. [Google Scholar]
- European Commission. (2017). Commission Regulation (EU) 2017/2158: Establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Official Journal of the European Union, 304, 24–44.Retrieved from https://www.fsai.ie/uploadedFiles/Reg2017_2158.pdf [Google Scholar]
- Feng C-H, & Lu C-Y (2011). Modification of major plasma proteins by acrylamide and glycidamide: Preliminary screening by nano liquid chromatography with tandem mass spectrometry. Analytica Chimica Acta, 684(1–2), 89–95. 10.1016/j.aca.2010.10.042 [DOI] [PubMed] [Google Scholar]
- Fennell TR, & Friedman MA (2005). Comparison of acrylamide metabolism in humans and rodents. Advances in Experimental Medicine and Biology, 561, 109–116. [DOI] [PubMed] [Google Scholar]
- Ferrari P, Freisling H, Duell EJ, Kaaks R, Lujan-Barroso L, Clavel-Chapelon F, … Slimani N (2013). Challenges in estimating the validity of dietary acrylamide measurements. European Journal of Nutrition, 52(5), 1503–1512. 10.1007/s00394-012-0457-7 [DOI] [PubMed] [Google Scholar]
- Friedman MA, Dulak LH, & Stedham MA (1995). A lifetime oncogenicity study in rats with acrylamide. Fundamental and Applied Toxicology, 27(1), 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese J, Tappi S, Luo W, Tylewicz U, Marzocchi S, Marziali S, … Rocculi P (2019). Important factors to consider for acrylamide mitigation in potato crisps using pulsed electric fields. Innovative Food Science and Emerging Technologies, 55, 18–26. 10.1016/j.ifset.2019.05.008 [DOI] [Google Scholar]
- Ghiasvand AR, & Hajipour S (2016). Direct determination of acrylamide in potato chips by using headspace solid-phase microextraction coupled with gas chromatography-flame ionization detection. Talanta, 146, 417–422. 10.1016/j.talanta.2015.09.004 [DOI] [PubMed] [Google Scholar]
- Goempel K, Tedsen L, Ruenz M, Bakuradze T, Schipp D, Galan J, … Richling E (2017). Biomarker monitoring of controlled dietary acrylamide exposure indicates consistent human endogenous background. Archives of Toxicology, 91(11), 3551–3560. 10.1007/s00204-017-1990-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökmen V, Şenyuva HZ, Dülek B, & Çetin AE (2007). Computer vision-based image analysis for the estimation of acrylamide concentrations of potato chips and french fries. Food Chemistry, 101(2), 791–798. 10.1016/j.foodchem.2006.02.034 [DOI] [Google Scholar]
- Gökmen V, & Mogol BA (2010). Computer vision-based image analysis for rapid detection of acrylamide in heated foods. Quality Assurance and Safety of Crops and Foods, 2(4), 203–207. 10.1111/j.1757-837X.2010.00072.x [DOI] [Google Scholar]
- Graff RE, Cho E, Preston MA, Sanchez A, Mucci LA, & Wilson KM (2018). Dietary acrylamide intake and risk of renal cell carcinoma in two large prospective cohorts. Cancer Epidemiology Biomarkers & Prevention, 27(8), 979–982. 10.1158/1055-9965.EPI-18-0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidance for Industry Acrylamide in Foods. (2016). FDA food guidance.
- Guide to GC Column Selection and Optimizing Separations. (2013). Retrieved from https://www.restek.com/pdfs/GNBR1724-UNV.pdf
- Haouet N, Pistolese S, Branciari R, Ranucci D, & Altissimi MS (2016). Study of acrylamide level in food from vending machines. Italian Journal of Food Safety, 5(4), 191–193. 10.4081/ijfs.2016.6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A (1976). Improved method for the determination of acrylamide monomer in water by means of gas-liquid chromatography with an electron-capture detector. The Analyst, 101(1209), 932–938. 10.1039/an9760100932 [DOI] [PubMed] [Google Scholar]
- Her GR (1985). Quantitative methodology for corticosteroids based on chemical oxidation to electrophilic products for electron capture-negative chemical ionization using capillary gas chromatography-mass spectrometry. Analytical Biochemistry, 151(2), 292–298. [DOI] [PubMed] [Google Scholar]
- Hu Q, Fu Y, Xu X, Qiao Z, Wang R, Zhang Y, & Li Y (2016). A colorimetric detection of acrylamide in potato chips based on nucleophile-initiated thiol–ene Michael addition. The Analyst, 141(3), 1136–1143. 10.1039/C5AN01989C [DOI] [PubMed] [Google Scholar]
- Hu Q, Wang R, Wang H, Slavik MF, & Li Y (2018). Selection of acrylamide-specific aptamers by a quartz crystal microbalance combined SELEX method and their application in rapid and specific detection of acrylamide. Sensors and Actuators, B: Chemical, 273(January), 220–227. 10.1016/j.snb.2018.06.033 [DOI] [Google Scholar]
- Hu Q, Xu X, Fu Y, & Li Y (2015). Rapid methods for detecting acrylamide in thermally processed foods: A review. Food Control, 56, 135–146. 10.1016/j.foodcont.2015.03.021 [DOI] [Google Scholar]
- Hu Q, Xu X, Li Z, Zhang Y, Wang J, Fu Y, & Li Y (2014). Detection of acrylamide in potato chips using a fluorescent sensing method based on acrylamide polymerization-induced distance increase between quantum dots. Biosensors and Bioelectronics, 54, 64–71. 10.1016/j.bios.2013.10.046 [DOI] [PubMed] [Google Scholar]
- IARC. (1994). Acrylamide. IARC Monographs, 60, 389–433. [PMC free article] [PubMed] [Google Scholar]
- INNATE by Simplot. (2017). RetrievedSeptember 9, 2020, from http://www.innatepotatoes.com/
- Kamankesh M, Zokaei M, Shojaei S, & Mohammadi A (2017). Determining the amount of acrylamide in potato chips using xanthydrol as a derivative representative with gas chromatography-mass spectrometry. Nutrition and Food Sciences Research, 3(1), 51–56. 10.18869/acadpub.nfsr.3.1.51 [DOI] [Google Scholar]
- Khan MR, Alothman ZA, Naushad M, Alomary AK, Alfadul SM, Alsohaimi IH, & Algamdi MS (2017). Occurrence of acrylamide carcinogen in Arabic coffee Qahwa, coffee and tea from Saudi Arabian market. Scientific Reports, 7, 1–8. 10.1038/srep41995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapura PR, Belur PD, & Subramanya S (2016). A critical review on properties and applications of microbial l-asparaginases. Critical Reviews in Microbiology, 42(5), 720–737. 10.3109/1040841X.2015.1022505 [DOI] [PubMed] [Google Scholar]
- Lambert M, Inthavong C, Hommet F, Leblanc JC, Hulin M, & Guérin T (2018). Levels of acrylamide in foods included in ‘the first French total diet study on infants and toddlers.’ Food Chemistry, 240), 997–1004. 10.1016/j.foodchem.2017.08.035 [DOI] [PubMed] [Google Scholar]
- Lan L (2016). Determination of acrylamide in foods by GC-MS using 9-hydroxyxanthene as derivating agent. Xiandai Yufang Yixue, 43(7), 1003–8507. [Google Scholar]
- Lange R, Anzenba P, Muller S, Maurin L, & Balny C (1994). Interaction of tryptophan residues of cytochrome P450scc with a highly specific fluorescence quencher, a substrate analogue, compared to acrylamide and iodide. European Journal of Biochemistry, 226(3), 963–970. [DOI] [PubMed] [Google Scholar]
- Laporte MF, & Paquin P (1999). Near-infrared analysis of fat, protein, and casein in cow’s milk. Journal of Agricultural and Food Chemistry, 47(7), 2600–2605. 10.1021/jf980929r [DOI] [PubMed] [Google Scholar]
- Le Ru EC, Blackie E, Meyer M, & Etchegoint PG (2007). Surface enhanced Raman scattering enhancement factors: A comprehensive study. Journal of Physical Chemistry C, 111(37), 13794–13803. 10.1021/jp0687908 [DOI] [Google Scholar]
- Lee K-J, Lee G-H, Kim H, Oh M-S, Chu S, Hwang IJ, … Park H-M (2015). Determination of heterocyclic amines and acrylamide in agricultural products with liquid chromatography-tandem mass spectrometry. Toxicological Research, 31(3), 255–264. 10.5487/TR.2015.31.3.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Galeone C, Pelucchi C, La Vecchia C, Giacosa A, Negri E, … Talamini R (2005). Dietary acrylamide and human cancer. International Journal of Cancer, 118(2), 467–471. 10.1002/ijc.21336 [DOI] [PubMed] [Google Scholar]
- Li D, Wang P, Liu Y, Hu X, & Chen F (2016). Metabolism of acrylamide: Interindividual and interspecies differences as well as the application as biomarkers. Current Drug Metabolism, 17(4), 317–326. 10.2174/1389200216666151015115007 [DOI] [PubMed] [Google Scholar]
- Liu T, Dodds E, Leong SY, Eyres GT, Burritt DJ, & Oey I (2017). Effect of pulsed electric fields on the structure and frying quality of “kumara” sweet potato tubers. Innovative Food Science and Emerging Technologies, 39, 197–208. 10.1016/j.ifset.2016.12.010 [DOI] [Google Scholar]
- Liu Y, Hu X, Bai L, Jiang Y, Qiu J, Meng M, … Ni L (2018). A molecularly imprinted polymer placed on the surface of graphene oxide and doped with Mn (II) -doped ZnS quantum dots for selective fluorometric determination of acrylamide. Microchimica Acta, 185, 48. 10.1007/s00604-017-2543-2 [DOI] [PubMed] [Google Scholar]
- Maillard L (1912). Action of amino acids on sugars. Formation of melanoidins in a methodical way. Comptes Rendus de l’Academnie Des Sci, 154, 66–68. [Google Scholar]
- Margolies BJ, & Barbano DM (2017). Determination of fat, protein, moisture, and salt content of Cheddar cheese using mid-infrared transmittance spectroscopy. Journal of Dairy Science, 101(2), 924–933. 10.3168/jds.2017-13431 [DOI] [PubMed] [Google Scholar]
- Mastovska K, & Lehotay SJ (2006). Rapid sample preparation method for LC-MS/MS or GC-MS analysis of acrylamide in various food matrices. Journal of Agricultural and Food Chemistry, 54(19), 7001–7008. 10.1021/jf061330r [DOI] [PubMed] [Google Scholar]
- Meghavarnam AK, & Janakiraman S (2018). Evaluation of acrylamide reduction potential of L-asparaginase from Fusarium culmorum (ASP-87) in starchy products. LWT – Food Science and Technology, 89, 32–37. 10.1016/j.lwt.2017.09.048 [DOI] [Google Scholar]
- Mogol BA, & Gökmen V (2014). Computer vision-based analysis of foods: A non-destructive colour measurement tool to monitor quality and safety. Journal of the Science of Food and Agriculture, 94(7), 1259–1263. 10.1002/jsfa.6500 [DOI] [PubMed] [Google Scholar]
- Mucci LA, Dickman PW, Steineck G, Adami HO, & Augustsson K (2003). Dietary acrylamide and cancer of the large bowel, kidney, and bladder: Absence of an association in a population-based study in Sweden. British Journal of Cancer, 88(1), 84–89. 10.1038/sj.bjc.6600726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci Lorelei A., Lindblad P, Steineck G, & Adami HO (2004). Dietary acrylamide and risk of renal cell cancer. International Journal of Cancer, 109(5), 774–776. 10.1002/ijc.20011 [DOI] [PubMed] [Google Scholar]
- Mucci Lorelei A., Sandin S, & Magnusson C (2005). Acrylamide intake and breast cancer risk in Swedish women. Journal of the American Medical Association, 293(11), 1326–1327. [DOI] [PubMed] [Google Scholar]
- NegoiţĂ M, & Culeţu A (2016). Application of an accurate and validated method for identification and quantification of acrylamide in bread, biscuits and other bakery products using GC-MS/MS System. Journal of the Brazilian Chemical Society, 27(10), 1782–1791. 10.5935/0103-5053.20160059 [DOI] [Google Scholar]
- NegoiţĂ M, Iorga E, Tamba R, & CatanĂ L (2016). Investigation regarding influence of different food ingredients addition on the acrylamide level in bread. Romanian Biotechnological Letters, 21(5), 11974–11981. [Google Scholar]
- Nguyen HT, van der Fels-Klerx H. J. Ine., & van Boekel MAJS (2017). Acrylamide and 5-hydroxymethylfurfural formation during biscuit baking. Part II: Effect of the ratio of reducing sugars and asparagine. Food Chemistry, 230, 14–23. 10.1016/j.foodchem.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Van Der Fels-Klerx HJ, Peters RJB, & Van Boekel MAJS (2016). Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: Part I: Effects of sugar type. Food Chemistry, 192, 575–585. 10.1016/j.foodchem.2015.07.016 [DOI] [PubMed] [Google Scholar]
- NIOSH. (2007). Niosh pocket guide to chemical hazards. Safety and health, (2005). 10.1109/ICNN.1993.298588 [DOI] [PubMed] [Google Scholar]
- Oroian M, Amariei S, & Gutt G (2016). Food additives and contaminants : Part B. Acrylamide in Romanian food using HPLC-UV and a health risk assessment. Food Additives & Contaminants: Part B, 8(2), 136–141. 10.1080/19393210.2015.1010240 [DOI] [PubMed] [Google Scholar]
- Osborne BG, & Fearn T (1986). Near infrared spectroscopy in food analysis. Essex: Longman Scientific and Technical; John Wiley & Sons, Inc. [Google Scholar]
- Pan M, Liu K, Yang J, Hong L, Xie X, & Wang S (2020). Review of research into the determination of acrylamide in foods. Foods, 9(4), 524. 10.3390/foods9040524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreschi F, Mariotti MS, & Granby K (2014). Current issues in dietary acrylamide: Formation, mitigation and risk assessment. Journal of the Science of Food and Agriculture, 94(1), 9–20. 10.1002/jsfa.6349 [DOI] [PubMed] [Google Scholar]
- Pedreschi F, Mariotti S, Granby K, & Risum J (2011). Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. LWT – Food Science and Technology, 44(6), 1473–1476. 10.1016/j.lwt.2011.02.004 [DOI] [Google Scholar]
- Pelucchi C, Bosetti C, Galeone C, & La Vecchia C (2015). Dietary acrylamide and cancer risk: An updated meta-analysis. International Journal of Cancer, 136(12), 2912–2922. 10.1002/ijc.29339 [DOI] [PubMed] [Google Scholar]
- Petrarca MH, Rosa MA, Queiroz SCN, & Godoy HT (2017). Simultaneous determination of acrylamide and 4-hydroxy-2,5-dimethyl-3(2H)-furanone in baby food by liquid chromatography–tandem mass spectrometry. Journal of Chromatography A, 1522, 62–69. 10.1016/j.chroma.2017.09.052 [DOI] [PubMed] [Google Scholar]
- Pourmand E, Ghaemi E, & Alizadeh N (2017). Determination of acrylamide in potato-based foods using headspace solid-phase microextraction based on nanostructured polypyrrole fiber coupled with ion mobility spectrometry: A heat treatment study. Analytical Methods, 9(35), 5127–5134. 10.1039/c7ay01506b [DOI] [Google Scholar]
- Pundir CS, Yadav N, & Chhillar AK (2019). Occurrence, synthesis, toxicity and detection methods for acrylamide determination in processed foods with special reference to biosensors: A review. Trends in Food Science and Technology, 85, 211–225. 10.1016/j.tifs.2019.01.003 [DOI] [Google Scholar]
- Rannou C, Laroque D, Renault E, Prost C, & Sérot T (2016). Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Research International, 90, 154–176. 10.1016/j.foodres.2016.10.037 [DOI] [PubMed] [Google Scholar]
- Raters M, & Matissek R (2018). Acrylamide in cocoa: A survey of acrylamide levels in cocoa and cocoa products sourced from the German market. European Food Research and Technology, 244(8), 1–8. 10.1007/s00217-018-3051-2 [DOI] [Google Scholar]
- Razia S, Bertrand M, Klaus V, & Meinolf GL (2016). Investigation of acrylamide levels in branded biscuits, cakes and potato chips commonly consumed in Pakistan. International Food Research Journal, 23(5), 2187–2192. [Google Scholar]
- Rosén J, & Hellenäs K-E (2002). Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry. The Analyst, 127(7), 880–882. 10.1039/b204938d [DOI] [PubMed] [Google Scholar]
- Rothweiler B, Kuhn E, & Prest H (2004). Gas chromatography/mass spectrometry approaches to the analysis of acrylamide in foods, (79), 14. https://gcms.cz/labrulez-bucket-strapi-h3hsga3/paper/5989-0602EN.pdf. Last accessed on January 16, 2021. [Google Scholar]
- Ruenz M, Bakuradze T, Eisenbrand G, & Richling E (2016). Monitoring urinary mercapturic acids as biomarkers of human dietary exposure to acrylamide in combination with acrylamide uptake assessment based on duplicate diets. Archives of Toxicology, 90(4), 873–881. 10.1007/s00204-015-1494-9 [DOI] [PubMed] [Google Scholar]
- Rydberg P, Lüning B, Wachtmeister CA, Eriksson L, & Törnqvist M (2002). Applicability of a modified Edman procedure for measurement of protein adducts: Mechanisms of formation and degradation of phenylthiohydantoins. Chemical Research in Toxicology, 15(4), 570–581. 10.1021/tx000247+ [DOI] [PubMed] [Google Scholar]
- Sanghvi G, Bhimani K, Vaishnav D, Oza T, Dave G, Kunjadia P, & Sheth N (2016). Mitigation of acrylamide by l-asparaginase from Bacillus subtilis KDPS1 and analysis of degradation products by HPLC and HPTLC. SpringerPlus, 5(1), 533. 10.1186/s40064-016-2159-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten MA, Genovese J, Tappi S, Di Francesco A, Baraldi E, Cortese M, … Romani S (2020). Effect of innovative pre-treatments on the mitigation of acrylamide formation in potato chips. Innovative Food Science and Emerging Technologies, 64(April), 102397. 10.1016/j.ifset.2020.102397 [DOI] [Google Scholar]
- Sega GA, Valdivia Alcota RP, Tancongco CP, & Brimer PA (1989). Acrylamide binding to the DNA and protamine of spermiogenic stages in the mouse and its relationship to genetic damage. Mutation Research, 216, 221–230. [DOI] [PubMed] [Google Scholar]
- Shanchun J, O’Leary T, Volkman JK, Huizhi Z, Rongfen J, Suhua Y, … Ronghua J (1994). Origins and simulated thermal alteration of sterols and keto-alcohols in deep-sea marine sediments of the Okinawa Trough. Organic Geochemistry, 21(3–4), 415–422. 10.1016/0146-6380(94)90203-8 [DOI] [Google Scholar]
- Sharma GK (2016). A review on acrylamide mitigation strategies in various processed foods. International Journal of Advanced Research, 4(7), 1025–1040. 10.21474/IJAR01 [DOI] [Google Scholar]
- Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, … Van Landingham C (2006). Acrylamide: Review of toxicity data and dose-response analyses for cancer and non-cancer effects. Critical Reviews in Toxicology, 36(6), 481–608. 10.1080/10408440600851377 [DOI] [PubMed] [Google Scholar]
- Siesler HW, Ozaki Y, Kawata S, & Heise HM (Eds.). (2002). Near-infrared spectroscopy (1st ed.). Weinheim: Wiley-VCH. [Google Scholar]
- Sobhi HR, Ghambarian M, Behbahani M, & Esrafili A (2017). Application of modified hollow fiber liquid phase microextraction in conjunction with chromatography-electron capture detection for quantification of acrylamide in waste water samples at ultra-trace levels. Journal of Chromatography A, 1487, 30–35. 10.1016/j.chroma.2017.01.051 [DOI] [PubMed] [Google Scholar]
- Stenerson BK, Wolford R, & Shimelis O (2011). Extraction and analysis of agricultural pesticides from oranges using the “QuEChERS” method. Reporter EU, 23(7), 1–4. [Google Scholar]
- Surma M, Sadowska-Rociek A, Cieślik E, & Sznajder-Katarzyńska K (2017). Optimization of QuEChERS sample preparation method for acrylamide level determination in coffee and coffee substitutes. Microchemical Journal, 131, 98–102. 10.1016/j.microc.2016.11.021 [DOI] [Google Scholar]
- Survey Data on Acrylamide in Food: Individual Food Products. (2008). FDA. Retrieved from https://www.fda.gov/food/foodborneillnesscontaminants/chemicalcontaminants/ucm053549.htm
- Tamanna N, & Mahmood N (2015). Food processing and maillard reaction products: Effect on human health and nutrition. International Journal of Food Science, 2015, 526762. 10.1155/2015/526762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareke E, Rydberg P, Karlsson P, Eriksson S, & Törnqvist M (2002). Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of Agricultural and Food Chemistry, 50(17), 4998–5006. 10.1021/jf020302f [DOI] [PubMed] [Google Scholar]
- Tepe Y, & Çebi A (2017). Acrylamide in environmental water: A review on sources, exposure, and public health risks. Exposure and Health, 11, 3–12. 10.1007/s12403-017-0261-y [DOI] [Google Scholar]
- Thermo Scientific Reagents, Solvents and Accessories. (2003). 10.1002/ejoc.201200111 [DOI]
- Törnqvist M, Kautiainen A, Gatz RN, & Ehrenberg L (1988). Hemoglobin adducts in animals exposed to gasoline and diesel exhausts 1. Alkenes. Journal of Applied Toxicology, 8(3), 159–170. 10.1002/jat.2550080303 [DOI] [PubMed] [Google Scholar]
- Tyl RW, & Friedman MA (2003). Effects of acrylamide on rodent reproductive performance. Reproductive Toxicology, 17(1), 1–13. 10.1016/S0890-6238(02)00078-3 [DOI] [PubMed] [Google Scholar]
- US EPA. (2010). Acrylamide CASRN 79-06-1 | IRIS | US EPA, ORD. Us Epa, Iris, 1–33. Retrieved from https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=286 [Google Scholar]
- Vesper HW, Slimani N, Hallmans G, Tjønneland A, Agudo A, Benetou V, … Strömberg U (2008). Cross-sectional study on acrylamide hemoglobin adducts in subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Journal of Agricultural and Food Chemistry, 56(15), 6046–6053. 10.1021/jf703750t [DOI] [PubMed] [Google Scholar]
- Virk-Baker MK, Nagy TR, Barnes S, & Groopman J (2014). Dietary acrylamide and human cancer: A systematic review of literature. Nutrition and Cancer, 66(5), 774–790. 10.1080/01635581.2014.916323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijun Y (2015). Direct determination of acrylamide in food by gas chromatography with nitrogen chemiluminescence detection. Journal of Separation Science, 38(13), 2272–2277. 10.1002/jssc.201500060 [DOI] [PubMed] [Google Scholar]
- Wu M, Chen W, Wang G, He P, & Wang Q (2016). Analysis of acrylamide in food products by microchip electrophoresis with online multiple-preconcentration techniques. Food Chemistry, 209, 154–161. 10.1016/j.foodchem.2016.04.065 [DOI] [PubMed] [Google Scholar]
- Xu F, Oruna-Concha MJ, & Elmore JS (2016). The use of asparaginase to reduce acrylamide levels in cooked food. Food Chemistry, 210, 163–171. 10.1016/j.foodchem.2016.04.105 [DOI] [PubMed] [Google Scholar]
- Yadav N, Chhillar AK, & Pundir CS (2018). Preparation, characterization and application of haemoglobin nanoparticles for detection of acrylamide in processed foods. International Journal of Biological Macromolecules, 107(PartA), 1000–1013. 10.1016/j.ijbiomac.2017.09.070 [DOI] [PubMed] [Google Scholar]
- Yang Y, Li G, & Chambers T (2020). Quantitative analysis of acrylamide in peanut butter using LC triple quadrupole mass spectrometry. https://www.chromatographyonline.com/view/quantitative-analysis-acrylamide-peanut-butter-using-lc-triple-quadrupole-mass-spectrometry. Last accessed on January 16, 2021.
- Zaheer K, & Akhtar MH (2016). Potato production, usage, and nutrition—A review. Critical Reviews in Food Science and Nutrition, 56(5), 711–721. 10.1080/10408398.2012.724479 [DOI] [PubMed] [Google Scholar]
- Zhang C, Cagliero C, Pierson SA, & Anderson JL (2017). Rapid and sensitive analysis of polychlorinated biphenyls and acrylamide in food samples using ionic liquid-based in situ dispersive liquid-liquid microextraction coupled to headspace gas chromatography. Journal of Chromatography A, 1481, 1–11. 10.1016/j.chroma.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li G, Duan Y, Chen S, Zhang C, & Li Y (2008). Application of the standard addition method for the determination of acrylamide in heat-processed starchy foods by gas chromatography with electron capture detector. Food Chemistry, 109(4), 899–908. 10.1016/j.foodchem.2008.01.020 [DOI] [PubMed] [Google Scholar]


