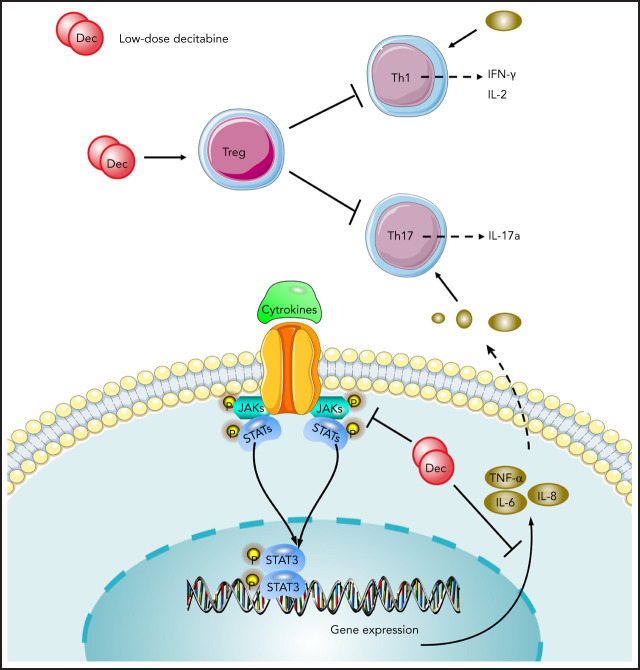
New treatment options are required for patients with refractory immune thrombocytopenia (ITP), a disease in which immune tolerance has been lost due to reduced regulatory T-cell (Treg) activity. Han et al demonstrate that the hypomethylating agent decitabine increases platelet counts in ITP by restoring T-cell homeostasis through modulation of Tregs, increasing their numbers and enhancing their immunosuppressive function.
Key Points
Low-dose decitabine augments the inhibitory effect of Treg cells and rebalances CD4+ T-cell subsets in ITP.
Decitabine restores immune tolerance in ITP by modulating Treg cells and inhibiting STAT3 activation.
Visual Abstract
Abstract
Our previous clinical study showed that low-dose decitabine exhibited sustained responses in nearly half of patients with refractory immune thrombocytopenia (ITP). The long-term efficacy of decitabine in ITP is not likely due to its simple role in increasing platelet production. Whether decitabine has the potential to restore immune tolerance in ITP is unknown. In this study, we analyzed the effect of decitabine on T-cell subpopulations in ITP in vitro and in vivo. We found that low-dose decitabine promoted the generation and differentiation of regulatory T (Treg) cells and augmented their immunosuppressive function. Splenocytes from CD61 knockout mice immunized with CD61+ platelets were transferred into severe combined immunodeficient mouse recipients to induce a murine model of ITP. Low-dose decitabine alleviated thrombocytopenia and restored the balance between Treg and helper T (Th) cells in active ITP mice. Treg deletion and depletion offset the effect of decitabine in restoring CD4+ T-cell subpopulations in ITP mice. For patients who received low-dose decitabine, the quantity and function of Treg cells were substantially improved, whereas Th1 and Th17 cells were suppressed compared with the pretreatment levels. Next-generation RNA-sequencing and cytokine analysis showed that low-dose decitabine rebalanced T-cell homeostasis, decreased proinflammatory cytokines, and downregulated phosphorylated STAT3 in patients with ITP. STAT3 inhibition analysis suggested that low-dose decitabine might restore Treg cells by inhibiting STAT3 activation. In conclusion, our data indicate that the immunomodulatory effect of decitabine provides one possible mechanistic explanation for the sustained response achieved by low-dose decitabine in ITP.
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by increased platelet destruction and impaired platelet production.1-5 Loss of immune tolerance is pivotal to the pathogenesis of ITP.6 CD4+CD25+Foxp3+ regulatory T (Treg) cells play an essential role in the maintenance of self-tolerance; their quantity and function are impaired in patients with ITP, which contributes to the excessive proliferation and activation of auto-reactive effector T (Teff) cells.7-9 The imbalance of CD4+ helper T (Th) cells is further evidenced in the development of ITP, along with the unrestricted activation of cytotoxic T lymphocytes (CTLs) and the production of antiplatelet autoantibodies.10,11 Restoring immune tolerance in patients with ITP by reversing the impairment of Treg cells and suppressing the expansion of Th1 and Th17 cells was shown to be an important mechanism for currently applied ITP-specific therapies.12-14
Decitabine is a hypomethylating agent that promotes cell differentiation at a low dose and is used for the treatment of myelodysplastic syndrome with a relatively high platelet response.15 Decitabine was shown to induce the murine megakaryoblastic cell line to differentiate into megakaryocyte-like polyploidy CD41-expressing cells in vitro and increase platelet release in BALB/C mice in vivo.16 In our previous studies, low-dose decitabine significantly increased the number of mature polyploidy megakaryocytes and also exhibited long-term clinical efficacy, which could not be explained simply by its role in promoting platelet production in patients with ITP.17,18 It has been reported that hypomethylating agents could decrease the production of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) while increasing the number of immunosuppressive Treg cells.19,20
Here, we investigated the effect of low-dose decitabine on the production and suppressive function of Treg cells derived from patients with ITP. In addition, active ITP murine models were established to evaluate the effect of low-dose decitabine on Treg and Teff cells in vivo. We collected sequential peripheral blood samples from patients with ITP receiving low-dose decitabine treatment and performed next-generation RNA-sequencing and cytokine assay to analyze the therapeutic effect and the underlying mechanism of low-dose decitabine.
Methods
Patients and control subjects
For in vitro studies, 40 patients with ITP (22 female subjects and 18 male subjects; age range, 19-75 years; median age, 45 years) (Table 1) were enrolled between March 2016 and December 2020 in the Department of Hematology, Qilu Hospital, Shandong University. Platelet counts ranged between 1 × 109/L and 28 × 109/L (median, 14 × 109/L). In parallel, 31 age- and sex-matched healthy volunteers (16 female subjects and 15 male subjects; age range, 22-73 years; median age, 43 years) were recruited, with platelet counts ranging from 149 × 109/L to 348 × 109/L (median, 243 × 109/L). For in vivo studies, 32 patients with ITP (19 female subjects and 13 male subjects; age range, 17-75 years; median age, 52 years; platelet counts from 1 × 109/L to 47 × 109/L [median, 8 × 109/L]) (Table 2) were enrolled and received decitabine as previously described.18 In brief, patients received decitabine at 3.5 mg/m2 intravenously for 3 days per cycle for 3 cycles with a 4-week interval (supplemental Figure 1, available on the Blood Website). Blood samples were taken before the first injection (day 0) and after 12 weeks of decitabine therapy (day 85), respectively. All patients fulfilled the diagnosis criteria and were free from previous therapies for at least 4 weeks.
Table 1.
Baseline characteristics of patients with ITP and healthy control subjects in the in vitro study
| Characteristic | Patients with ITP (n = 40) | Healthy controls subjects (n = 31) | P | |||
|---|---|---|---|---|---|---|
| Age, y | 45 (19-75) | 43 (22-73) | .630 | |||
| Sex | ||||||
| Female | 22 (55.00) | 16 (51.61) | .777 | |||
| Male | 18 (45.00) | 15 (48.39) | .777 | |||
| Baseline platelet count (109/L) | 14 (1-28) | 243 (149-348) | <.001 | |||
| ITP duration, mo | 14(1-224) | NA | NA | |||
| Previous therapies | ||||||
| ≤2 | 27 (67.50) | NA | NA | |||
| ≥3 | 13 (32.50) | NA | NA | |||
Data are expressed as median (range) or n (%). NA, not applicable.
Table 2.
Clinical characteristics of patients with ITP receiving decitabine treatment
| Antiplatelet antibodies | ||||||
|---|---|---|---|---|---|---|
| Patient no. | Sex/age (y) | Platelet count (109/L) | Course of disease (mo) | Anti-GPIIb/IIIa | Anti-GPIb/IX | Major previous drugs |
| 1 | M/75 | 6 | 8 | — | ND | Pred, IVIG, Dana |
| 2 | F/74 | 18 | 84 | — | — | IVIG, Pred, RTX |
| 3 | F/29 | 39 | 11 | — | — | Pred, TPO-RA |
| 4 | F/73 | 47 | 12 | — | — | Pred, rhTPO, RTX |
| 5 | M/71 | 1 | 12 | ND | ND | DXM, IVIG |
| 6 | M/38 | 5 | 324 | ND | ND | DXM, IVIG, Dana, RTX |
| 7 | M/19 | 17 | 12 | ND | ND | DXM, IVIG, Dana |
| 8 | M/53 | 16 | 6 | — | + | DXM, Dana |
| 9 | M/56 | 33 | 3 | + | + | DXM, IVIG, TPO-RA |
| 10 | F/50 | 16 | 4 | — | — | DXM, TPO-RA |
| 11 | F/67 | 2 | 12 | ND | ND | DXM, IVIG, rhTPO |
| 12 | M/52 | 11 | 20 | ND | ND | DXM, rhTPO, RTX |
| 13 | M/36 | 2 | 15 | ND | ND | DXM, IVIG, Dana |
| 14 | F/63 | 23 | 144 | — | — | rhTPO, DXM, IVIG, RTX |
| 15 | M/67 | 14 | 30 | — | — | DXM, IVIG, rhIL-11 |
| 16 | F/48 | 5 | 132 | — | — | DXM, rhTPO, SP |
| 17 | M/27 | 1 | 107 | + | — | Pred, IVIG, rhTPO, RTX |
| 18 | F/73 | 8 | 5 | — | — | MP, IVIG, Dana |
| 19 | F/52 | 28 | 240 | — | — | Pred, Dana, VCR, SP |
| 20 | F/57 | 2 | 24 | + | + | Pred, IVIG, Dana |
| 21 | F/52 | 8 | 284 | + | + | Pred, rhTPO, IVIG, CsA, TPO-RA |
| 22 | F/29 | 6 | 84 | — | — | DXM, IVIG, RTX |
| 23 | F/25 | 4 | 48 | ND | ND | DXM, IVIG, RTX |
| 24 | F/19 | 7 | 19 | ND | ND | DXM, rhTPO, IVIG, RTX, VCR |
| 25 | F/17 | 44 | 25 | — | — | DXM, rhTPO, IVIG |
| 26 | M/59 | 1 | 48 | — | — | DXM, rhTPO, IVIG, RTX |
| 27 | F/22 | 9 | 24 | ND | ND | DXM, rhTPO, IVIG |
| 28 | F/41 | 12 | 36 | — | — | DXM, CsA, RTX |
| 29 | M/54 | 6 | 348 | — | + | DXM, rhTPO, IVIG, RTX |
| 30 | M/28 | 1 | 11 | ND | ND | DXM, IVIG, rhTPO, RTX, VCR |
| 31 | F/21 | 6 | 16 | — | + | Pred, IVIG, CsA, rhIL-11 |
| 32 | F/55 | 13 | 12 | + | — | DXM, IVIG, rhTPO |
CsA, cyclosporine; Dana, danazol; DXM, dexamethasone; F, female; GP, glycoprotein; IVIG, intravenous immunoglobulin; M, male; ND, not determined; Pred, prednisone; rhIL-11, recombinant human interleukin-11; rhTPO, recombinant human thrombopoietin; RTX, rituximab; SP, splenectomy; TPO-RA, thrombopoietin receptor agonist; VCR, vincristine.
Each participant provided informed consent. This study was approved by the Medical Ethical Committee of Qilu Hospital of Shandong University.
Decitabine
For patients with ITP, decitabine (approved by China Food and Drug Administration) was dissolved with water for injection and then diluted with normal saline (0.9%). For in vitro and murine studies, decitabine (5-aza-2′-deoxycytidine; MilliporeSigma, St Louis, MO) was dissolved in water and then diluted with phosphate-buffered saline. Decitabine solution was prepared fresh for each use.
Active ITP murine model
Wild-type C57BL/6J mice (male, 8-12 weeks old) purchased from the Center for New Drug Evaluation of Shandong University were used as platelet donors. C57BL/6 CD61 knockout mice were provided by Junling Liu (Shanghai Jiaotong University School of Basic Medicine) and originally came from The Jackson Laboratory (Bar Harbor, ME; B6.129S2-Itgb3tm1Hyn/JSemJ, stock no. 008819). Severe combined immunodeficient (SCID) mice with C57BL/6 background (B6.Cg-Prkdcscid/SzJ, stock no. 001913, 6-8 weeks old) were purchased from The Jackson Laboratory and used as spleen cell transfer recipients.
CD61 knockout mice were transfused weekly with 108 platelets from wild-type C57BL/6J mice for 6 consecutive weeks. The spleens of CD61 knockout mice were removed and prepared into splenocyte suspensions. On the day of splenocyte transfer, SCID mice were subjected to 200 cGy total body irradiation to inhibit recipient innate immune responses and enhance engraftment. Within 3 hours of irradiation, SCID mice were injected intraperitoneally with 2 × 104 splenocytes of CD61 knockout mice.21-23 The successfully constructed murine model exhibited profound thrombocytopenia for 28 to 35 days after splenocyte transfusion. All mice were matched for sex and age, and randomized to groups. Different doses of decitabine were administrated intravenously 3 times a week for 3 weeks. The SCID mice were euthanized at day 28, and splenocytes were obtained for analysis (supplemental Figure 2).
All mouse experiments were approved by the Animal Care and Use Committee of Qilu Hospital of Shandong University and were conducted under their guidelines.
Isolation and culturing of peripheral blood mononuclear cells and CD4+ T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood of patients with ITP and healthy control subjects with Ficoll-Hypaque centrifugation (Amersham Biosciences, Piscataway, NJ). CD4+ T cells were sorted by using magnetic beads and MS Columns (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the isolated cells was >90% as measured by flow cytometry.
PBMCs or sorted CD4+ T cells were cultured in RPMI 1640 medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin and streptomycin (Solarbio, Beijing, China). Cells were stimulated with recombinant human interleukin-2 (IL-2; 5 ng/mL; R&D Systems, Minneapolis, MN), anti-human CD3 antibodies (1 ng/mL; eBioscience, San Diego, CA), and anti-human CD28 antibodies (1 ng/mL; eBioscience) and incubated for 72 hours (37°C, 5% carbon dioxide).24
Flow cytometry
A total of 1 × 106 cultured human PBMCs or CD4+ T cells were harvested from 24-well plates after coculture with different doses of decitabine (ie, 0, 1 nM, 10 nM, 100 nM, 1 μM, 10 μM). For ITP murine models, we prepared single-cell suspensions with a concentration of 1 × 106 cells/mL by pulverizing murine spleens. Cells were subsequently stained by anti-human or anti-mouse conjugated-antibodies (eBioscience) following the manufacturer’s instructions.
To analyze apoptosis, PBMCs were stained with fluorescein isothiocyanate–Annexin V and propidium iodide using a Cell Apoptosis Kit (BestBio, Shanghai, China). To enumerate Treg cells, which were defined as CD4+CD25+Foxp3+, single-cell suspensions were stained with a Regulatory T Cell Staining Kit (eBioscience). Furthermore, for CD4+ Teff cells, single-cell suspensions were processed by a T-cell Subset Staining Kit (eBioscience). Th1, Th2, Th17, and Th22 cells were defined as CD4+IFN-γ+, CD4+IL4+, CD4+IFN-γ–IL17+, and CD4+IFN-γ–IL22+, respectively. Cells were subsequently analyzed by using a Gallios Flow Cytometer equipped with Kaluza Analysis Software (Beckman Coulter, Brea, CA).
Immunosuppression assay of Treg cells from patients with ITP
CD4+CD25– T cells (Teff cells) and CD4+CD25+CD127– T cells (Treg cells) were isolated from PBMCs by using a CD4+CD25+CD127– Regulatory T Cell Isolation Kit (Stemcell Technologies, Vancouver, BC, Canada) according to the manufacturer’s instructions. CD4+CD25− T cells were labeled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE, 2.5 μM; MilliporeSigma) and seeded at 2 × 105 cells per well on a 96-well plate with or without Treg cells (Teff:Treg, 4:1). Cells were also stimulated with recombinant human IL-2 (5 ng/mL; R&D Systems), anti-human CD3 antibodies (1 ng/mL; eBioscience), and anti-human CD28 antibodies (1 ng/mL; eBioscience). In vitro, cells were harvested for proliferation analysis after incubation with 100 nM decitabine or phosphate-buffered saline for 6 days. We also sorted and cocultured Treg and Teff cells from patients before and after decitabine treatment to evaluate the suppressive function changes in vivo. Data were analyzed by using FlowJo software (FlowJo LLC, Ashland, OR).
Treg deletion and depletion in ITP murine models
For the deletion of Treg cells, CD4+CD25+ T cells were deleted from splenocytes of CD61 knockout mice using magnetic beads (Miltenyi Biotec) before the transfer to SCID mice. The deletion efficiency was >95% measured according to flow cytometry. For the depletion of CD25+ T cells in vivo, ITP mice were given an intraperitoneal injection of 400 μg monoclonal anti-mouse CD25 (IL-2Rα) antibody (clone: PC-61.5.3; BioXcell, Lebanon, NH) in 0.2 mL of phosphate-buffered saline 3 times a week for 3 weeks. Control mice received an equal amount of purified immunoglobulin G1 isotype antibody (BioXcell). The degree of CD25+ T-cell depletion was evaluated according to flow cytometry analysis of splenocytes on day 28. In general, we observed CD25+ T cells depletion efficiency of >95%.
Cytokine analysis
Serum samples of patients with ITP before and after decitabine treatment were tested for 11 cytokines by using V-PLEX Human Proinflammatory Panel 1 Kits and V-PLEX Human IL-17A Kit (Meso Scale Diagnostics, Rockville, MD). The level of TGF-β by using the R&D Elisa Assay Kit (R&D Systems) according to the manufacturer's instructions.
RNA-sequencing and western blot analysis
RNA of PBMCs from patients with ITP receiving decitabine treatment was extracted and sequenced on an Illumina HiSeq platform. PBMCs from patients with ITP were lysed in radio-immunoprecipitation assay buffer (BestBio) for western blot. The experimental protocols are described in detail in the supplemental Methods.
STAT3 and protein kinase B inhibition assay
In the in vitro studies, either cryptotanshinone (STAT3 inhibitor; TargetMol, Boston, MA) or ARQ 092 (protein kinase B [AKT] inhibitor; TargetMol) were dissolved in dimethyl sulfoxide and added to PBMC culture medium with a concentration of 10 μM or 500 nM, respectively. After 72 hours’ exposure, PBMCs were harvested for flow cytometry. For murine models, either cryptotanshinone or ARQ 092 was dissolved by using sterilized 0.5% carboxymethylcellulose sodium. SCID mice were treated with STAT3 inhibitor (20 mg/kg), AKT inhibitor (30 mg/kg), STAT3 inhibitor plus decitabine (0.03 mg/kg), AKT inhibitor plus decitabine, or decitabine. Inhibitor administration began from day 7 and was administered 3 times per week by gavage. After 3 weeks of administration, all mice were euthanized, and splenocytes were obtained for flow cytometry.
Statistical analysis
The results are expressed as median (range or interquartile range), mean ± standard deviation, or mean ± standard error of the mean. Statistical significance was determined by using Student t tests or analysis of variance, followed by Tukey’s multiple comparison test. In the case of heterogeneity of variances, groups were compared by using the Mann-Whitney U test and the Kruskal-Wallis nonparametric analysis of variance followed by Dunn’s multiple comparison test. For the comparison of classification measurements, the χ2 or Fisher’s exact test was used. All tests were performed by using GraphPad 8.0 system (GraphPad Software, La Jolla, CA). A value of P < .05 was considered statistically significant.
Results
Low-dose decitabine promoted the differentiation of Treg cells in vitro and enhanced their immunosuppressive capacity
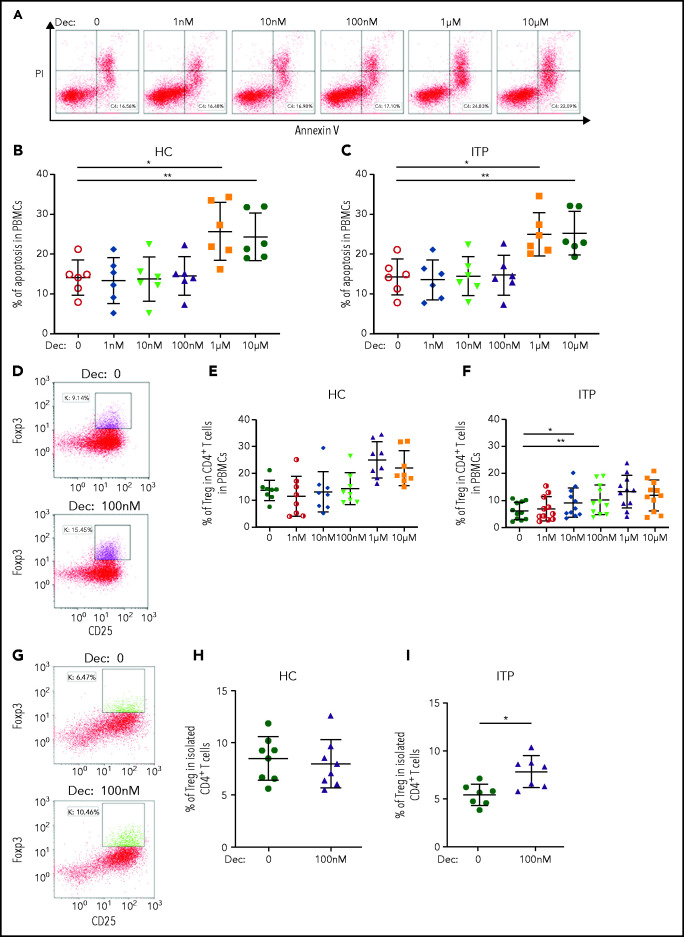
In the in vitro study, PBMCs from patients with ITP and healthy control subjects were isolated and cultured. The baseline Treg cells and CD4+ T-cell proliferation with or without stimulation in healthy control subjects and patients with IT are shown in supplemental Figure 3. After 72 hours’ culture with different concentrations of decitabine (0-10 μM) in the presence of T-cell receptor costimulation, PBMCs were stained with Annexin V and propidium iodide to detect the level of apoptosis. We found that high-dose decitabine (1 μM and 10 μM) predominately exhibited cytotoxicity (Figure 1A-C). Decitabine had no influence on the apoptosis of PBMCs from patients with ITP and healthy control subjects at 1 nM, 10 nM, or 100 nM. However, low-dose decitabine (10 nM and 100 nM) significantly elevated the percentage of Treg cells in patients with ITP without changing the percentage of CD4+ T cells in PBMCs (Figure 1D-F; supplemental Figure 4). Subsequently, to minimize the distraction of other cells, we sorted CD4+ T cells cocultured with decitabine and found that low-dose decitabine (100 nM) also significantly increased the number of Treg cells in ITP (Figure 1G-I).
Figure 1.
Low-dose decitabine had no influence on the apoptosis of PBMCs from patients with ITP or healthy control subjects, but significantly elevated the percentage of Treg cells in patients with ITP without changing the percentage of CD4+ T cells in PBMCs. (A) Representative dot plots of flow cytometry analysis of apoptosis in PBMCs from patients with ITP. The percentage of Annexin-positive and propidium iodide (PI)-negative cells in PBMCs showed the cell apoptosis rate. (B,C) Decitabine induced apoptosis of PBMCs from healthy control subjects and patients with ITP at doses of 1 μM and 10 μM (n = 6). (D-F) Percentages of Treg cells in CD4+ T cells were significantly increased by decitabine at the doses of 10 nM and 100 nM in patients with ITP (n = 11). (G-I) In cultured isolated CD4+ T cells, low-dose decitabine (100 nM) significantly increased the percentage of Treg cells in patients with ITP (I; n = 7), but not in healthy control subjects (H; n = 8). *P < .05, **P < .01. Dec, decitabine; HC, healthy controls; ns, not significant.
We sorted CD4+CD25+CD127– Treg cells from patients with ITP and healthy control subjects, and cultured with CFSE-labeled CD4+CD25– Teff cells in a 1:4 ratio to evaluate the suppressive activity of Treg cells in the presence of decitabine. The division index of Teff cells from patients with ITP was significantly decreased after coincubation with Treg cells and 100 nM decitabine compared with Treg cells in ITP (Figure 2; supplemental Figure 5), indicating an increased immunosuppressive function of decitabine-treated Treg cells. Decitabine itself had no apparent effect on the proliferation of Teff cells. These results suggest that the immunosuppressive function of Treg cells was significantly enhanced by low-dose decitabine in ITP.
Figure 2.
The inhibitory function of Treg cells was enhanced by low-dose decitabine in ITP in vitro. Teff cells (2 × 105 cells per well) were seeded in a 96-well plate with or without Treg cells (5 × 104 cells per well) in the presence of decitabine 100 nM. The cell division index was calculated based on the dilution of CFSE fluorescence measured by flow cytometry and represents the average number of cell divisions that Teff cells in the original population have undergone [division index = sum (i × N(i)/2i)/sum (N(i)/2i), “i” is division number (undivided = 0), and “N(i)” is the number of cells in division “i”]. (A,C) Representative histograms of CD4+CFSE+ Teff cells from one healthy control subject (A) and patient with ITP (C). (B,D) Treg cells suppressed the proliferation of Teff cells. Decitabine (100 nM) enhanced the inhibitory function of Treg cells in ITP (D; n = 24), but not in healthy control subjects (B; n = 13). *P < .05, **P < .01, ***P < .001. Dec, decitabine; HC, healthy control subjects; ns, not significant.
Low-dose decitabine ameliorated thrombocytopenia in active ITP mice with increased Treg cells in the spleen
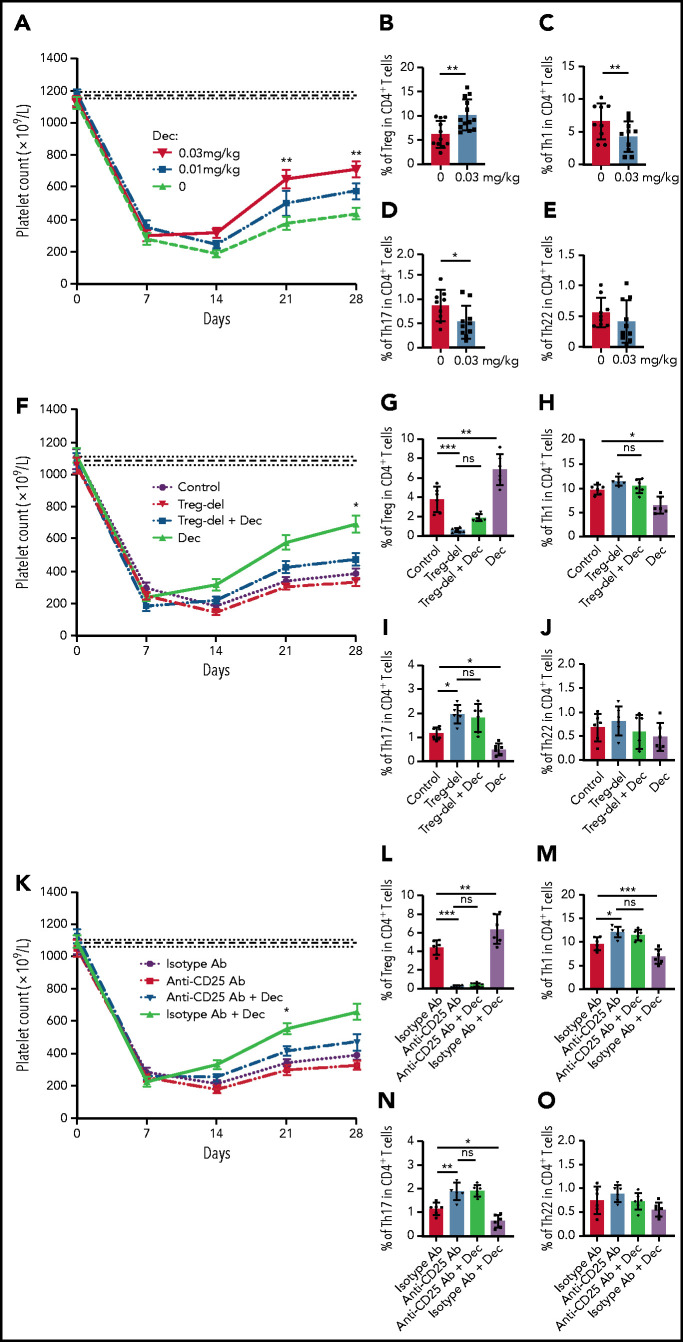
We further established an active ITP murine model to investigate whether our observations in vitro were reproducible in vivo. Seven days after the irradiation and splenocyte transfer, the platelet count decreased significantly in murine models. Splenocyte transfer itself did not affect platelet counts (supplemental Figure 6). Different doses of decitabine (0, 0.01 mg/kg, 0.03 mg/kg, 0.06 mg/kg, 0.1 mg/kg, 0.3 mg/kg, and 0.6 mg/kg) were administrated intravenously. High-dose decitabine (0.06 mg/kg, 0.1 mg/kg, 0.3 mg/kg, and 0.6 mg/kg) could not be tolerated in ITP mice (supplemental Figure 7). As a result, we chose 0.03 mg/kg as the dose of decitabine for ITP murine studies.
Our data showed a significant platelet increase at days 21 and 28 in the decitabine group at the dose of 0.03 mg/kg compared with control mice (Figure 3A). After 3 weeks of 0.03 mg/kg decitabine administration, spleens were harvested from ITP mice. Flow cytometry analysis revealed a significant increase in the percentage of Treg cells in CD4+ T cells in the spleen of decitabine-treated ITP mice compared with control mice (Figure 3B). We also identified a decrease of Th1 and Th17 cells in CD4+ T cells, but no significant difference was observed in Th22 cells (Figure 3C-E).
Figure 3.
Low-dose decitabine ameliorated thrombocytopenia in active ITP murine models via Treg cells. ITP models were established in irradiated SCID mice with engraftment of 2 × 104 splenocytes from CD61 knockout mice immunized against wild-type C57 mice platelets; platelet counts were then monitored every week for 4 weeks (mean ± standard error of the mean). (A) From day 7, decitabine (0, 0.01 mg/kg, 0.03 mg/kg) was administered. The horizontally dotted lines represent the baseline platelet counts of SCID mice (mean ± standard error of the mean). On days 21 and 28, significantly higher platelet counts were observed in the group administered the 0.03-mg/kg dose of decitabine compared with control group. (B) Decitabine-treated ITP mice had a significantly higher percentage of splenic Treg cells in CD4+ T cells compared with control mice (n = 11 in the control group and n = 13 in the decitabine group, respectively). The percentage of Th1 (C) and Th17 (D) in CD4+ T cells were decreased in decitabine-treated spleens compared with controls. No significance was found in Th22 cells (E) in splenic CD4+ T cells (n = 9 in the control group and n = 10 in the decitabine group). Treg deletion (F) by magnetic beads and depletion (K) by anti-CD25 antibody partly offset the effect of decitabine on increasing platelet counts of ITP mice (n = 6). Treg-deleted splenocyte-transferred mice (G) and anti-CD25 antibody-treated mice (L) were significantly depleted of Treg cells in the spleens on day 28 (n = 6). (H-J) After Treg deletion, decitabine had no significant effect on splenic Th1, Th17, or Th22 cells on day 28 in ITP mice (n = 6). (M-O) With Treg depletion by anti-CD25 antibody, decitabine had no significant effect on splenic Th1, Th17, or Th22 cells on day 28 in ITP mice (n = 6). *P < .05, **P < .01, ***P < .001. Dec, decitabine; ns, not significant.
Treg-deleted splenocyte-transferred mice and anti-CD25 antibody-treated mice were significantly depleted of Treg cells in the spleens (Figure 3G,L). Treg deletion and depletion therapy partly offset the effect of decitabine on increasing platelet counts (Figure 3F,K). After Treg deletion and depletion, decitabine had no significant effect on splenic Th1, Th17, or Th22 cells (Figure 3H-J, M-O).
Low-dose decitabine restored the balance of T-cell subsets in patients with ITP
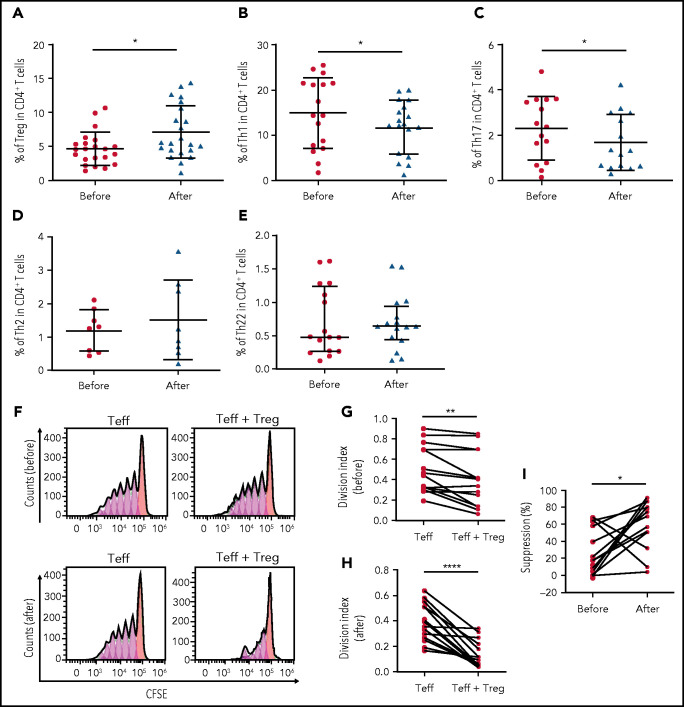
Peripheral blood samples were collected from 32 patients with ITP before and after decitabine treatment to analyze the effect of low-dose decitabine on T-cell homeostasis in vivo. After decitabine treatment, there was a significant increase in the number of CD25+Foxp3+ Treg cells in CD4+ T cells (Figure 4A). In contrast, decitabine significantly decreased the percentage of Th1 and Th17 cells in CD4+ T-cell populations, yet the amounts of Th2 and Th22 cells remained unchanged (Figure 4B-E). We also observed an increase in the suppressive function of Treg cells after decitabine treatment (Figure 4F-I).
Figure 4.
Immunologic responses to low-dose decitabine in patients with ITP. (A-E) Percentages of Treg, Th1, Th17, Th2, and Th22 cells in peripheral blood were analyzed by using flow cytometry. Treg cells (n = 22), Th1 cells (n = 17), Th17 cells (n = 15), Th2 cells (n = 8), and Th22 cells (n = 16) in CD4+ T cells before and after decitabine treatment are shown. (F) Ex vivo suppression assays were performed to examine the suppressive activity of Treg cells obtained from patients with ITP before and after decitabine treatment. CFSE-labeled CD4+CD25– Teff cells were stimulated and cocultured with Treg cells from each patient. Representative histograms show proliferation of CD4+ Teff cells with or without Treg cells before (above) or after (below) decitabine treatment. (G-H) Treg cell–mediated suppression of CD4+ Teff cell proliferation was measured using division index before and after decitabine treatment (n = 14). (I) Decitabine enhanced immunosuppressive function of Treg cells. *P < .05, **P < .01, ****P < .0001. NR, nonresponders; R, responders.
Low-dose decitabine regulates cytokines related to T-cell differentiation in patients with ITP
Serum was isolated from peripheral blood of 32 patients with ITP before and after decitabine treatment. Low-dose decitabine significantly reduced serum levels of TNF-α, IFN-γ, and IL-17a, as well as a panel of inflammatory cytokines and chemokines such as IL-6, IL-8, and IL-12, which were associated with T-cell differentiation (Table 3). We also found a significant increase in TGF-β and IL-2. However, there was no significant difference in serum levels of IL-1β, IL-4, IL-10, or IL-13.
Table 3.
Effect of low-dose decitabine on cytokines in patients with ITP (N = 32)
| Cytokine, pg/mL | Before decitabine | After decitabine | P |
|---|---|---|---|
| IL-1β | 0.34 (0.27-0.52) | 0.35 (0.24-0.44) | .111 |
| IL-2 | 0.63 (0.44-0.80) | 0.72 (0.53-0.90) | .027 |
| IL-4 | 0.05 (0.04-0.06) | 0.04 (0.04-0.06) | .536 |
| IL-6 | 1.38 (1.00-3.25) | 1.07 (0.76-1.48) | .005 |
| IL-8 | 4.88 (2.71-10.38) | 3.54 (2.34-7.83) | .029 |
| IL-10 | 0.78 (0.38-1.49) | 0.63 (0.41-0.79) | .410 |
| IL-12 | 0.37 (0.26-0.49) | 0.33 (0.24-0.41) | .026 |
| IL-13 | 0.63 (0.40-0.82) | 0.60 (0.43-0.85) | .733 |
| IL-17a | 2.01 (1.48-3.03) | 1.86 (1.09-2.50) | .046 |
| IFN-γ | 8.82 (6.11-13.12) | 6.06 (4.20-9.61) | .005 |
| TNF-α | 6.15 (4.72-9.25) | 5.75 (4.32-6.89) | .014 |
| TGF-β | 747.30 (616.00-1126.00) | 1109.00 (664.40-1976.00) | .029 |
Data are expressed as median (interquartile range).
RNA-sequencing analysis correlated with decitabine efficacy in patients with ITP
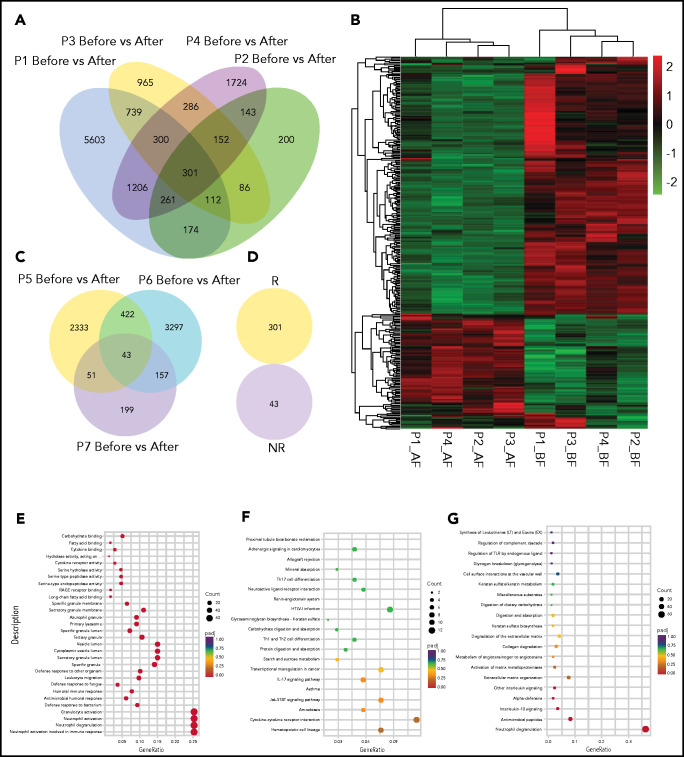
To assess the impact of low-dose decitabine on the gene expression of patients with ITP, we performed messenger RNA–sequencing analysis in 4 responders and 3 nonresponders. Compared with the pretreatment point, responders shared a significant differential expression of 301 genes after decitabine treatment (Figure 5A). These 2 time points were identified by unsupervised hierarchical clustering (Figure 5B). Nonresponders presented significant changes in 43 genes before and after decitabine treatment (Figure 5C), but no overlap of any gene was detected with responders (Figure 5D).
Figure 5.
Profiles and enriched pathway analysis of decitabine-induced genes. (A) Venn diagram of overlapping genes of decitabine-altered genes in 4 responders. (B) Heat map of decitabine-altered genes in 4 responders with unsupervised clustering. Red to green colors represent relatively high to low gene expression, respectively, and gray indicates below the detectable level. (C) Venn diagram of overlapping genes of decitabine-altered genes in 3 nonresponders. (D) No overlap of decitabine-altered genes was observed between responders and nonresponders. (E-G) Pathways were predicted by Gene Ontology (E), KEGG (F), and Reactome (G) databases based on the expression changes of decitabine altered genes in 4 responders. AF, after; BE, before; NR, nonresponders; R, responders.
These decitabine-altered genes in responders were linked with leukocyte migration and cytokine receptor activity according to Gene Ontology pathways analysis (Figure 5E). Based on Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, these genes were associated with cytokine–cytokine receptor interaction, hematopoietic cell lineage, IL-17 signaling pathway, Th1 and Th2 cell differentiation, Th17 cell differentiation, and the Jak-STAT signaling pathway (Figure 5F). Based on Reactome analysis (Figure 5G), these genes were linked with IL-10, which participated in Treg cell differentiation and Jak-STAT signaling pathway.
Low-dose decitabine inhibited STAT3 activation in ITP
Jak-STAT and PI3K-AKT pathways, which constitute a rapid signaling module that plays a vital role in the maintenance of CD4+ T-cell homeostasis, were shown to be overactivated in ITP. Notably higher levels of phosphorylated-STAT3 and phosphorylated-AKT have been reported.25,26 In our study, western blot analysis of cell lysates from PBMCs of patients with ITP after decitabine treatment revealed decreased STAT3 and AKT phosphorylation compared with pretreatment levels (Figure 6A-C).
Figure 6.
Low-dose decitabine inhibited the STAT3 signaling pathway. (A) Representative western blots of phosphorylated-STAT3 (p-STAT3), total STAT, phosphorylated-AKT (p-AKT), total AKT, and GAPDH in PBMCs from patients with ITP before and after decitabine treatment. (B-C) Graphs present densitometry data of relative phosphorylated protein to total protein (n = 3). Treg cells in CD4+ T cells from healthy control subjects (D; n = 10) and patients with ITP (E; n = 8) in the STAT3 inhibitor plus decitabine group were no different from those in the STAT3 inhibitor group in vitro. In the presence of AKT inhibitor, decitabine significantly increased the percentage of Treg cells from patients with ITP in vitro (G; n = 10), but not in the healthy control subjects (F; n = 8). (H) The platelet counts in the STAT3 inhibitor plus decitabine group were not significantly different from those in mice receiving STAT3 inhibitor (n = 6). (I) AKT inhibitor plus decitabine increased platelet counts in mice compared with AKT inhibitor on day 21 (n = 6). *P < .05. Dec, decitabine; ns, not significant.
STAT3 and AKT inhibitors were applied in vitro and in vivo. In the in vitro studies, the percentage of Treg cells in CD4+ T cells from patients with ITP and healthy control subjects in the STAT3 inhibitor plus decitabine group was no different from that in the STAT3 inhibitor group (Figure 6D-E). In murine studies, platelet counts in the STAT3 inhibitor plus decitabine group were not significantly different from those in mice receiving a STAT3 inhibitor (Figure 6H). However, in the presence of AKT inhibitor, decitabine significantly increased the percentage of Treg cells from patients with ITP in vitro (Figure 6G), but not in healthy control subjects (Figure 6F). In murine studies, AKT inhibitor plus decitabine increased platelet counts compared with AKT inhibitor on day 21 (Figure 6I). These results indicate that low-dose decitabine might restore Treg cells by inhibiting STAT3, but not AKT activation in ITP.
Discussion
ITP is an autoimmune disease with heterogeneous bleeding manifestation. The pathogenesis of ITP has not been fully elucidated, and management remains challenging.27,28 In this study, we identified the role and effects of low-dose decitabine on the production and suppressive function of Treg cells derived from patients with ITP. Using RNA-sequencing, Treg deletion/depletion, and pharmacologic inhibition of STAT3, we found that low-dose decitabine alleviated thrombocytopenia by increasing the number and improving the function of Treg cells and inhibiting STAT3 activation.
Abnormal T-cell homeostasis, including the deficiency of Treg cells and excessive activation of Teff cells, plays a critical role in the pathogenesis of ITP. Aberrant DNA methylation, a mechanism closely related to gene expression, was found to be involved in T-cell differentiation and development in ITP.29 The hypermethylation of CpG sites in the Foxp3 promoter region in pediatric patients with ITP mediated the dysfunction of Treg cells.30 Furthermore, detection of methylcytosine-binding domain (MBD) expression showed that messenger RNA expression of both MBD2 and MBD4 was decreased in PBMCs in patients with ITP compared with healthy control subjects.31
We recently reported that decitabine restored the methylation level and expression of programmed cell death protein 1 on CD8+ T cells and reduced CTL cytotoxicity to autologous platelets in ITP,32 providing another explanation regarding the effect of decitabine in addition to its role in increasing platelet production in ITP. However, the long-term efficacy of decitabine in ITP could not be elucidated by its roles in increasing platelet production or decreasing CTL-mediated platelet destruction. Of note, decitabine has been investigated as a sensitizer to immunotherapy and possesses the potent immunomodulatory properties through different pharmacologic mechanisms. Previously reported microarray analysis showed that the majority of decitabine upregulated genes belonged to immune processes and immune-related signaling pathways per Gene Ontology and KEGG analyses.33 Decitabine additionally augmented the expression of Foxp3 in CD4+CD25– T cells, which was necessary and sufficient for functional natural Treg cells, and have been exploited to prevent graft-versus-host disease in bone marrow transplantation models.34 The immunopharmacologic profile of decitabine by upregulation of Foxp3 has been repeatedly confirmed in rodent models of other autoimmune diseases such as type 1 diabetes and multiple sclerosis.35-37 Recent studies have also shown that decitabine restored the balance of Th1/Th2 cytokines with consequential promotion of an anti-inflammatory milieu and reduced the production of proinflammatory cytokines, including TNF-α and IFN-γ.38 In our study, we identified that decitabine increased the number and enhanced the immunosuppressive function of Treg cells from patients with ITP in vitro and in vivo. In addition to the effects on Treg cells, low-dose decitabine rebalanced CD4+ T-cell subpopulations by suppressing the differentiation of Th1 and Th17 cells in active ITP murine models, which might be one of the therapeutic mechanisms of increasing platelet counts in ITP models. Furthermore, in accordance with our in vitro and murine model study, low-dose decitabine significantly increased the number and enhanced the inhibitory function of Treg cells, and decreased Th1 and Th17 cells in patients with ITP. Treg deletion and depletion offset the effect of decitabine on restoring T-cell subpopulations, indicating that low-dose decitabine modulates T-cell homeostasis via Treg cells.
Previous studies have shown that other therapies, such as intravenous immunoglobulin and anti-CD20 antibody, could increase Foxp3 expression in CD4+ T cells and normalize Treg populations in ITP murine models.23,39 Similar results were reported in patients with ITP. Rituximab, thrombopoietin-receptor agonists, dexamethasone, and intravenous immunoglobulin showed profound effects on the improvement of Treg cells and more or less altered CD4+ T-cell subpopulations in patients with ITP.14,40-42 Guo et al43 examined whether allogeneic platelet transfusions had effects on immunopathology in ITP mice, and they found that T cell–mediated thrombocytopenia was alleviated by platelet transfusions. In our in vitro studies, in the absence of platelets, decitabine increased the number and enhanced the immunosuppressive function of Treg cells, indicating that the immunomodulatory effect of decitabine was independent on platelets.
Aberrant cytokine profiles are closely correlated with the loss of immune tolerance in ITP.44-46 Therapeutic options, such as high-dose dexamethasone, rituximab, and thrombopoietin-receptor agonists, are commonly associated with the correction of cytokine abnormalities.40,45,47 Consistent with the skewed Th1/Th2 and Treg/Th17 cells, the levels of IFN-γ, IL-2, and IL-17a were increased, whereas IL-4 and IL-10 levels were decreased in patients with ITP. Other cytokines, including IL-6, TGF-β, and TNF-α, have also been described as being involved in ITP. In the current study, TGF-β was significantly elevated by decitabine. On the contrary, TNF-α, IFN-γ, IL-17a, IL-12, IL-8, and IL-6 were reduced after decitabine administration. These data are consistent with the rebalance between Treg and Teff cells in ITP.
To further elucidate the potential mechanism of low-dose decitabine, next-generation RNA-sequencing was performed in ITP patients. Our data consistently showed that decitabine-altered genes in responders were significantly enriched in immunologic processes, including T-cell differentiation and cytokine signaling pathway. The Jak-STAT signaling pathway was identified based on KEGG analysis, which coincided with our observation that decitabine reduced the phosphorylated-STAT3 level and the inhibition of STAT3 dimmed the therapeutic effect of low-dose decitabine. The Jak-STAT signaling pathway is evolutionarily conserved in eukaryotes and involved in the initiation and maintenance of the balance between Treg and Teff cells.48 Laurence et al showed that STAT5 and STAT3 competed for promoter binding sites and had opposite functions in the regulation of T-cell differentiation.49-52 The activation of STAT3 by IL-6 and IL-21 not only plays a role in promoting Th17 cells, but also participates in the conversion of natural Treg cells to Teff cells.53 Our results are in line with these previously reported findings. Interestingly, decitabine exhibited a significant effect on Treg cells after AKT inhibition, indicating that the therapeutic effect of low-dose decitabine was not mainly dependent on AKT in ITP. The reduction of AKT phosphorylation by decitabine might be caused by the alteration of its upstream signaling molecules.
We are aware, however, that spontaneous remission may have occurred in patients with ITP receiving decitabine therapy in our study. Based on the recently updated American Society of Hematology 2019 guidelines and the 2019 international consensus report on ITP, the likelihood of a spontaneous remission is related to patient’s age and ITP course within the first year after the initial diagnosis.54-57 Among all patients who received decitabine therapy in our study, only a minority of them had ITP courses <12 months. Although the number of sequenced patients was relatively small, our study presents an important transcriptome basis for the understanding of decitabine in the management of patients with ITP.
In summary, low-dose decitabine increased the production of Treg cells and enhanced their immunosuppressive function in ITP in vitro and in vivo. Thrombocytopenia was ameliorated by low-dose decitabine with an increase of splenic Treg cells and reduction of Th1 and Th17 cells in ITP murine models. Our data suggest that the immunomodulatory effect of decitabine provides one possible mechanistic explanation for the sustained response achieved by low-dose decitabine in the management of adult patients with ITP.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The manuscript was edited by Alexandra H. Marshall (Marshall Medical Communications).
This work was supported by grants from the National Natural Science Foundation of China (82070122, 81900121, 81973994, and 81770133), the Major Research Plan of National Natural Science Foundation of China (91942306), the Major Research Plan of Shandong Province (2019GSF108240), and the State Key Clinical Specialty of China for Hematological Diseases and Shandong Provincial Clinical Medicine Research Center for Hematology.
Footnotes
Presented in abstract form at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017.
Requests for original data may be submitted to qlhouming@sina.com.
RNA-sequencing data are available in the Gene Expression Omnibus database (accession number GSE154703).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.H., Y.H., M.H., and H.Z. performed experiments, analyzed results, and wrote the paper; M.H. and H.Z. designed and funded the research; Y.Z., Y.L., T.Y., Y.S., H.W., and P.X. assisted the research; and G.L., T.S., X.H., X.L., L.L., and J.P. analyzed results and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ming Hou, Department of Hematology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, 107 Wenhuaxi Rd, 250012 Jinan, China; e-mail: qlhouming@sina.com.cn; or Hai Zhou, Shandong Key Laboratory of Immunohematology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, 107 Wenhuaxi Rd, Jinan, Shandong, 250012, China; e-mail: zhouhaisdu@163.com.
REFERENCES
- 1.Olsson B, Andersson PO, Jernås M, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9(9):1123-1124. [DOI] [PubMed] [Google Scholar]
- 2.McMillan R, Wang L, Tomer A, Nichol J, Pistillo J.. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103(4):1364-1369. [DOI] [PubMed] [Google Scholar]
- 3.Cines DB, Bussel JB, Liebman HA, Luning Prak ET.. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889-1899. [DOI] [PubMed] [Google Scholar]
- 5.Imbach P, Crowther M.. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med. 2011;365(8):734-741. [DOI] [PubMed] [Google Scholar]
- 6.Cines DB, Cuker A, Semple JW.. Pathogenesis of immune thrombocytopenia. Presse Med. 2014;43(4 pt 2):e49-e59. [DOI] [PubMed] [Google Scholar]
- 7.Nishimoto T, Kuwana M.. CD4+CD25+Foxp3+ regulatory T cells in the pathophysiology of immune thrombocytopenia. Semin Hematol. 2013; 50(suppl 1):S43-S49. [DOI] [PubMed] [Google Scholar]
- 8.Son BR, Kim JY.. Association of CD4(+)CD25(+)FoxP3(+) regulatory T cells with natural course of childhood chronic immune thrombocytopenic purpura. Korean J Pediatr. 2015;58(5):178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arandi N, Mirshafiey A, Jeddi-Tehrani M, et al. Alteration in frequency and function of CD4+CD25+FOXP3+ regulatory T cells in patients with immune thrombocytopenic purpura. Iran J Allergy Asthma Immunol. 2014;13(2):85-92. [PubMed] [Google Scholar]
- 10.Panitsas FP, Theodoropoulou M, Kouraklis A, et al. Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood. 2004;103(7):2645-2647. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie CGJ, Guo L, Freedman J, Semple JW.. Cellular immune dysfunction in immune thrombocytopenia (ITP). Br J Haematol. 2013;163(1):10-23. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Wang Z, Hu S, Zhao X, Cao L.. Correction of abnormal T cell subsets by high-dose dexamethasone in patients with chronic idiopathic thrombocytopenic purpura. Immunol Lett. 2013;154(1-2):42-48. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Mou W, Lu G, et al. Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 2011;93(1):91-98. [DOI] [PubMed] [Google Scholar]
- 14.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steensma DP.Myelodysplasia, megakaryocytes, and methylation. Leuk Res. 2004;28(8):775-776. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Yi Z, Wang S, Li Z.. The effect of decitabine on megakaryocyte maturation and platelet release. Thromb Haemost. 2011;106(2):337-343. [DOI] [PubMed] [Google Scholar]
- 17.Zhou H, Hou Y, Liu X, et al. Low-dose decitabine promotes megakaryocyte maturation and platelet production in healthy controls and immune thrombocytopenia. Thromb Haemost. 2015;113(5):1021-1034. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Qin P, Liu Q, et al. A prospective, multicenter study of low dose decitabine in adult patients with refractory immune thrombocytopenia. Am J Hematol. 2019;94(12):1374-1381. [DOI] [PubMed] [Google Scholar]
- 19.Kim HP, Leonard WJ.. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204(7):1543-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115(1):107-121. [DOI] [PubMed] [Google Scholar]
- 21.Chow L, Aslam R, Speck ER, et al. A murine model of severe immune thrombocytopenia is induced by antibody- and CD8+ T cell-mediated responses that are differentially sensitive to therapy. Blood. 2010;115(6):1247-1253. [DOI] [PubMed] [Google Scholar]
- 22.Hou Y, Feng Q, Xu M, et al. High-dose dexamethasone corrects impaired myeloid-derived suppressor cell function via Ets1 in immune thrombocytopenia. Blood. 2016;127(12):1587-1597. [DOI] [PubMed] [Google Scholar]
- 23.Aslam R, Hu Y, Gebremeskel S, et al. Thymic retention of CD4+CD25+FoxP3+ T regulatory cells is associated with their peripheral deficiency and thrombocytopenia in a murine model of immune thrombocytopenia. Blood. 2012;120(10):2127-2132. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Li H, Hao Y, et al. Decreased immunosuppressive actions of 1α, 25-dihydroxyvitamin D3 in patients with immune thrombocytopenia. Mol Immunol. 2016;78:89-97. [DOI] [PubMed] [Google Scholar]
- 25.Chen M, Yan R, Zhou K, et al. Akt-mediated platelet apoptosis and its therapeutic implications in immune thrombocytopenia. Proc Natl Acad Sci U S A. 2018;115(45):E10682-E10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Wang X, Yu S, Hou Y, Ma D, Hou M.. Neutralizations of IL-17A and IL-21 regulate regulatory T cell/T-helper 17 imbalance via T-helper 17-associated signaling pathway in immune thrombocytopenia. Expert Opin Ther Targets. 2015;19(6):723-732. [DOI] [PubMed] [Google Scholar]
- 27.Cuker A, Neunert CE.. How I treat refractory immune thrombocytopenia. Blood. 2016;128(12):1547-1554. [DOI] [PubMed] [Google Scholar]
- 28.Lambert MP, Gernsheimer TB.. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Xuan M, Yang R.. DNA methylation and primary immune thrombocytopenia. Semin Hematol. 2013;50(suppl 1):S116-S126. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Guo Z, Ma J, Ma J, Liu F, Wu R.. Foxp3 methylation status in children with primary immune thrombocytopenia. Hum Immunol. 2014;75(11):1115-1119. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZP, Gu DS, Zhou ZP, et al. Decreased expression of MBD2 and MBD4 gene and genomic-wide hypomethylation in patients with primary immune thrombocytopenia. Hum Immunol. 2011;72(6):486-491. [DOI] [PubMed] [Google Scholar]
- 32.Han P, Yu T, Hou Y, et al. Low-dose decitabine inhibits cytotoxic T lymphocytes-mediated platelet destruction via modulating PD-1 methylation in immune thrombocytopenia. Front Immunol. 2021;12:630693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Amoozgar Z, Huang J, et al. Decitabine enhances lymphocyte migration and function and synergizes with CTLA-4 blockade in a murine ovarian cancer model. Cancer Immunol Res. 2015;3(9):1030-1041. [DOI] [PubMed] [Google Scholar]
- 34.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116(1):129-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangano K, Fagone P, Bendtzen K, et al. Hypomethylating agent 5-aza-2'-deoxycytidine (DAC) ameliorates multiple sclerosis in mouse models. J Cell Physiol. 2014;229(12):1918-1925. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Wang J, Yu Y, et al. Decitabine inhibits T cell proliferation via a novel TET2-dependent mechanism and exerts potent protective effect in mouse auto- and allo-immunity models. Oncotarget. 2017;8(34):56802-56815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Q, Xu Y, Liu Y, et al. Induction of Foxp3 demethylation increases regulatory CD4+CD25+ T cells and prevents the occurrence of diabetes in mice. J Mol Med (Berl). 2009;87(12):1191-1205. [DOI] [PubMed] [Google Scholar]
- 38.Fagone P, Mazzon E, Chikovani T, et al. Decitabine induces regulatory T cells, inhibits the production of IFN-gamma and IL-17 and exerts preventive and therapeutic efficacy in rodent experimental autoimmune neuritis. J Neuroimmunol. 2018;321:41-48. [DOI] [PubMed] [Google Scholar]
- 39.Guo L, Kapur R, Aslam R, et al. CD20+ B-cell depletion therapy suppresses murine CD8+ T-cell-mediated immune thrombocytopenia. Blood. 2016;127(6):735-738. [DOI] [PubMed] [Google Scholar]
- 40.Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147-1150. [DOI] [PubMed] [Google Scholar]
- 41.Ling Y, Cao X, Yu Z, Ruan C.. Circulating dendritic cells subsets and CD4+Foxp3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur J Haematol. 2007;79(4):310-316. [DOI] [PubMed] [Google Scholar]
- 42.Wang SC, Yang KD, Lin CY, et al. Intravenous immunoglobulin therapy enhances suppressive regulatory T cells and decreases innate lymphoid cells in children with immune thrombocytopenia. Pediatr Blood Cancer. 2020;67(2):e28075. [DOI] [PubMed] [Google Scholar]
- 43.Guo L, Yang L, Speck ER, et al. Allogeneic platelet transfusions prevent murine T-cell-mediated immune thrombocytopenia. Blood. 2014;123(3):422-427. [DOI] [PubMed] [Google Scholar]
- 44.Semple JW, Milev Y, Cosgrave D, et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87(10):4245-4254. [PubMed] [Google Scholar]
- 45.Qu MM, Liu XN, Liu XG, et al. Cytokine changes in response to TPO receptor agonist treatment in primary immune thrombocytopenia. Cytokine. 2017;92:110-117. [DOI] [PubMed] [Google Scholar]
- 46.Andersson PO, Olsson A, Wadenvik H.. Reduced transforming growth factor-beta1 production by mononuclear cells from patients with active chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2002;116(4):862-867. [DOI] [PubMed] [Google Scholar]
- 47.Guo C, Chu X, Shi Y, et al. Correction of Th1-dominant cytokine profiles by high-dose dexamethasone in patients with chronic idiopathic thrombocytopenic purpura. J Clin Immunol. 2007;27(6):557-562. [DOI] [PubMed] [Google Scholar]
- 48.Villarino AV, Kanno Y, O’Shea JJ.. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18(4):374-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laurence A, Amarnath S, Mariotti J, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37(2):209-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA.. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178(1):280-290. [DOI] [PubMed] [Google Scholar]
- 51.Yao Z, Kanno Y, Kerenyi M, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109(10):4368-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujino M, Li XK.. Role of STAT3 in regulatory T lymphocyte plasticity during acute graft-vs.-host-disease. JAK-STAT. 2013;2(4):e24529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sailer T, Lechner K, Panzer S, Kyrle PA, Pabinger I.. The course of severe autoimmune thrombocytopenia in patients not undergoing splenectomy. Haematologica. 2006;91(8):1041-1045. [PubMed] [Google Scholar]
- 55.Schifferli A, Holbro A, Chitlur M, et al. ; Intercontinental Cooperative ITP Study Group (ICIS). A comparative prospective observational study of children and adults with immune thrombocytopenia: 2-year follow-up. Am J Hematol. 2018;93(6):751-759. [DOI] [PubMed] [Google Scholar]
- 56.Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia [published correction appears in Blood Adv. 2020;4(2):252]. Blood Adv. 2019;3(23):3829-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.