Abstract
Erythropoietin-producing human hepatocellular receptors (EPHs) compose the largest known subfamily of receptor tyrosine kinases (RTKs). They bind and interact with the EPH family receptor interacting proteins (ephrins). EPHs/ephrins are implicated in a variety of physiological processes, as well as in cancer pathogenesis. With neoplastic disease remaining a leading cause of death world-wide, the development of novel biomarkers aiding in the field of diagnosis, prognosis, and disease monitoring is of utmost importance. A multitude of studies have proven the association between the expression of members of the EPH/ephrin system and various clinicopathological parameters, including disease stage, tumor histologic grade, and patients’ overall survival. Besides their utilization in timely disease detection and assessment of outcome, EPHs/ephrins could also represent possible novel therapeutic targets. The aim of the current review of the literature was to present the existing data regarding the association between EPH/ephrin system expression and the clinical characteristics of malignant tumors.
Keywords: EPHs, ephrins, biomarkers, cancer, diagnosis, prognosis, treatment
1. Introduction
Neoplastic disease remains a leading cause of death, with approximately 19.3 million new cancer cases diagnosed in 2020 worldwide, accompanied by an estimated 10 million cancer-related deaths [1]. Furthermore, according to the National Cancer Institute, an estimated 39.5% of men and women will be diagnosed with cancer at some point during their lifetime [2]. Such statistics indicate the heavy impact of neoplasia on patients’ welfare, healthcare systems, and socio-economic stability. Despite ongoing advances in therapeutic interventions, further research is called for to combat the immense challenge imposed by neoplasia. Modern studies continue to shed light on the molecular mechanisms implicated in key steps of cancer pathogenesis, such as tumor formation and growth, invasion, lymph- and angiogenesis, as well as distant dissemination. The development of novel, specialized treatment strategies renders the understanding of these molecular pathways and their clinical impact of paramount importance. Furthermore, the well-established positive effect of early therapeutic intervention on the outcome of cancer patients underlines the need for the discovery of diagnostic, prognostic and predictive biomarkers contributing to timely detection of disease progression and re-currence, as well as assessment of therapeutic outcome. Scientists have shifted their focus on molecules participating in physiologic processes that, when deregulated, can enhance carcinogenesis. Among them, EPHs, with their well-established roles in proliferation, angiogenesis, and cell motility, are speculated to represent key factors in cancer pathogenesis. Therefore, a great number of studies attempted to assess their utilization as diagnostic and prognostic biomarkers, as well as therapeutic targets.
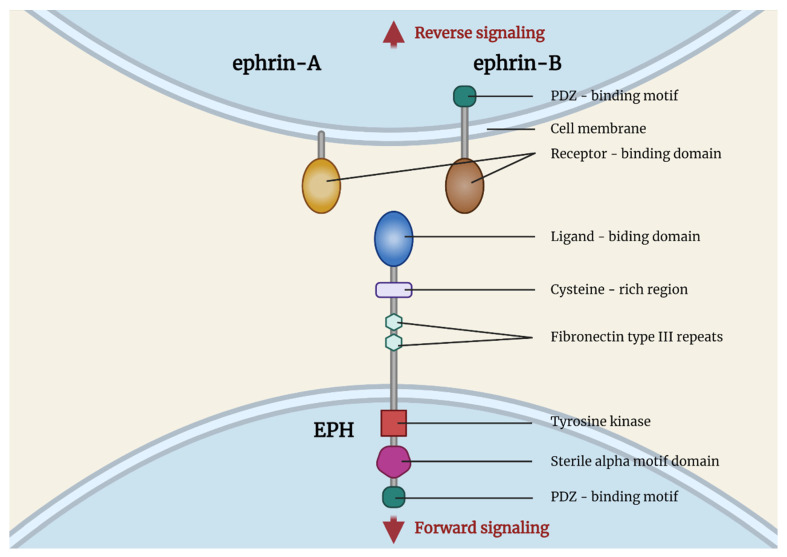
EPHs compose the largest known subfamily of receptor tyrosine kinases. They bind and interact with the EPH family receptor interacting proteins (ephrins). EPHs are membrane-bound proteins, consisting of an extracellular ephrin-binding domain, a transmembrane region, and a cytoplasmic component that, upon activation, following interaction with a ligand, initiates its RTK activity, activating downstream signaling molecular pathways. Ligand binding activation requires cell-to-cell interaction. Subsequently, as a response, signaling cascades are triggered in the cytoplasm of both the EPH-expressing cell and the ephrin-bearing one, a process termed as backward signaling (Figure 1). Nine EPHA receptors (EPHA1 to EPHA8 and EPHA10), that bind 5 ephrin-A ligands (ephrin-A1 to ephrin-A5), along with 5 EPHB receptors (EPHB1 to EPHB4 and EPHB6), that interact with 3 ephrin-B ligands (ephrin-B1 to ephrin-B3), are expressed in humans. EPHs of a specific subgroup (A or B) show a higher affinity for binding with ephrins belonging to the same subgroup, although inter-subgroup interaction has been frequently observed.
Figure 1.
Structure of EPHs/ephrins. Created with BioRender.com (accessed on 2 August 2021).
EPHs/ephrins participate in wide variety of processes in human physiology. They play a pivotal role in cell migration, axon guidance, and synapse formation during embryonic development. They are also implicated in mechanisms like cell adhesion, motility, and cell-matrix interactions, as well as lymphangiogenesis and angiogenesis in response to hypoxia [3].
All the aforementioned processes are vital for tumorigenesis, as cell motility is required for cancer cells’ invasion, vessel infiltration, and spreading to distant sites. Moreover, tumors cannot grow beyond a few millimeters without the sprouting of new vessels to provide them with oxygen and nutrients [4]. As accumulating data clearly underlined the clinical implications of EPH/ephrin tumor profiles, many in vivo and in vitro studies focused on revealing the molecular mechanisms through which members of the EPH/ephrin family exert their tumor promoting and tumor suppressive properties. In glioblastoma cell lines, EPHB4, along with its ligand, ephrin-B2, were observed to enhance angiogenesis through interacting with VGFR2 and Notch signaling pathways [5]. EPHA3, on the contrary, seems to enhance apoptosis of lung carcinoma cells via phosphorylation of members of the PI3K/BMX/STAT3 signaling pathway [5]. In breast cancer cells, phosphorylation of EPHA3 via Src kinase, due to trastuzumab treatment, induces the activation of PI3K/Akt and MAPK pathways, leading to trastuzumab resistance [5].
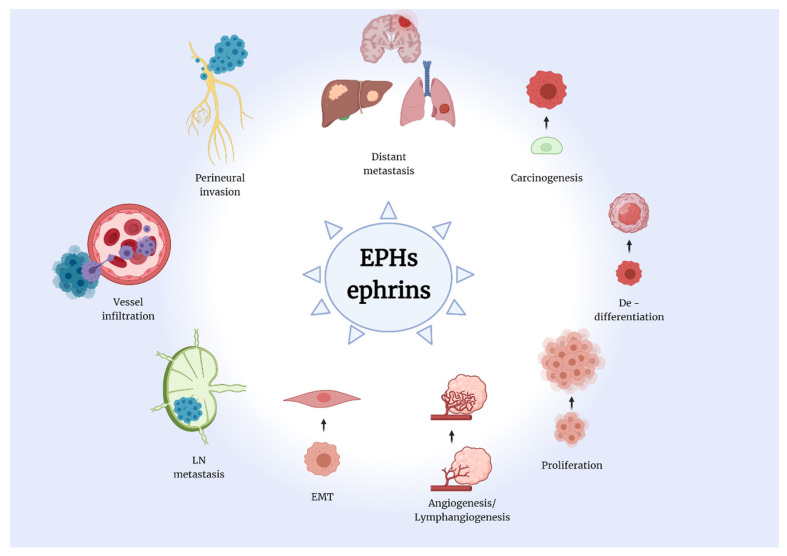
Therefore, the implication of the EPH/ephrin system in cell motility, cell-to-cell and cell-matrix interaction, as well as lymph- and angiogenesis renders them a possible key factor in carcinogenesis and an excellent biomarker candidate of tumor promoting and suppressive properties (Figure 2). Indeed, a plethora of studies have attempted to reveal the link between EPH/ephrins and tumorigenesis, as well as tumor characteristics and behavior. Specifically, in solid tumors, researchers have proven the close relationship between the various members of the EPH/ephrin system expression and disease onset and progression. Moreover, therapeutic agents that suppress those mechanisms could represent excellent possible targets for the development of novel, specialized anti-cancer intervention strategies.
Figure 2.
The Eph/ephrin system is implied in a multitude of tumorigenic processes. EPH: erythropoietin-producing human hepatocellular receptors, ephrins: EPH family receptor interacting proteins, EMT: epithelial-mesenchymal transition, LN: lymph nodes. Created with BioRender.com (accessed on 2 August 2021).
In the current review, data regarding the association between EPH/ephrin expression and the clinical characteristics of neoplasia were presented.
2. Methodology
We searched, via the pubmed engine, information regarding the role of the members of the EPH/ephrin family in carcinogenesis of solid tumors, focusing on the clinical impact of their expression. The majority of researchers investigated the protein expression of EPHs/ephrins through immunohistochemistry (IHC), IHC in tissue microarrays (TMA IHC), and Western Blot, as well as their mRNA expression, utilizing methods such as the Polymerase Chain Reaction (PCR), reverse-transcription PCR (RT-PCR), and quantitative PCR (q PCR), in a series of tumor specimens from patients with malignant disease. The presence or absence of expression was assessed, as well as the location (nuclear, cytoplasmic or membranous), the staining intensity, and the percentage of positive cells to overall tumor cell population. In addition, observations were made regarding different patterns of expression, such as diffuse staining, positive clusters of cells, and increased staining intensity on the tumor invasive front in deeper normal tissue layers. Moreover, most studies investigated the differences in EPHs/ephrins expression by including normal and non-neoplastic tissue samples, either from tissues adjacent to the malignant ones or from healthy individuals. The researchers then proceeded to associate their observations on EPHs/ephrins gene and protein expression with a wide range of clinicopathological parameters, including disease stage, histological grade, presence of lymph node (LN) or distant metastasis, overall survival (OS), and disease-free survival (DFS). Furthermore, the patterns of EPH/ephrin system members expression were correlated to a variety of histological characteristics, such as invasion of vessels, perineural infiltration, Microvessel Density (MVD), level of tumor neo-angiogenesis, and lymphangiogenesis and tumor cell proliferative capacity.
3. Head and Neck Tumors
3.1. Brain
The impact of the EPH/ephrin system expression in brain tumors is among the most extensively studied, with results suggesting that the aforementioned biomolecules play an overwhelmingly tumor-promoting role on brain cancer pathogenesis [6,7,8,9,10,11,12].
3.1.1. EPHAs
EPHA2 expression was assessed in normal brain samples and glioma tissues through either IHC or RT-PCR in three studies [6,7,8]. EPHA2 expression was then correlated with tumor histological features, various clinicopathological parameters, and patients’ outcomes. EPHA2 overexpression was positively linked to tumor size [8], higher histological grade of tumors [6,8], peritumoral edema levels [8], and poor patients prognosis [7,8]. EPHA4 expression, on the other hand, exhibited no significant difference between glioblastoma multiforme (GBM) and non-tumoral white matter tissue [9]. EPHA7 immunohistochemical expression was notably absent in the 10 normal brain tissues included in the study and strong in 14 out of 32 cases of primary or recurrent GBM tissues investigated. EPHA7 expression was positively associated with tumor MVD, increased age, and decreased OS [10].
3.1.2. EPHBs
In a large study that included 171 tumor samples (29 astrocytomas, 82 GBM, 49 oligodendrogliomas, 11 oligoastrocytomas) and 24 non-tumorous brain specimens from epileptogenic patients, EPHB1, B2, B3, B4, and B6 expression was assessed by quantitative real-time RT-PCR [11]. EPHB1 seemed to have a beneficial influence, since its expression correlated with longer patient OS and was upregulated in oligodendrogliomas, which notably have a more favorable outcome compared to other infiltrating glioma tumors. EPHB2 and EPHB3 expression was reported as higher in neoplastic tissues compared to normal ones, and EPHB4 expression was correlated with shorter patient OS. EPHB4’s negative impact was also underlined in another series of 96 primary glioma patients [12], as its expression was positively associated with higher grade and lower Karnofsky Performance Score. Interestingly, EPHB6, along with EPHB1, seems to suppress tumorigenesis. In the aforementioned study by Teng et al [11], EPHB6 expression was reported as lower in neoplastic tissues compared with non-tumorous ones.
3.1.3. Ephrins
Ephrin-A1 expression was associated with lower grade gliomas, while patients with tumors positive for EPHA2 and negative for ephrin-A1 exhibited shorter OS and PFS. On the other hand, Tu et al correlated the elevated ephrin-B2 expression to higher-grade tumors and low Karnofsky Performance Score and designated it as an independent negative prognostic factor for progression-free survival of glioma patients [12]. Likewise, ephrin-B3 seems to negatively impact Central Nervous System neoplasia, with its expression in gliomas reported 2.5-fold higher in tumor samples compared with controls and linked to higher grade tumors [9]. Information concerning the clinical impact of EPH/ephrin expression in brain tumors is summarized in Table 1.
Table 1.
EPHs/ephrins (bold) studied in solid tumors of head and neck and correlations with clinicopathological parameters.
| EPHs/Ephrins | Malignant Tissues | Benign Control Tissues | Methods | Results | Refs |
|---|---|---|---|---|---|
| BRAIN | |||||
| EPHA2 | 78 primary glioma samples | IHC |
|
[4] | |
| 21 glioblastoma samples | 5 non-tumorous human brain samples | RT-PCR real-time RT-PCR |
|
[5] | |
| 43 glioma samples | IHC MRI |
|
[6] | ||
| EPHA4 | 31 GBM samples | 28 non-tumoral white matter samples | q RT-PCR |
|
[7] |
| EPHA7 | 32 stage IV GBM samples (26 primary, 6 recurrent) | 10 normal brain samples | IHC |
|
[8] |
| EPHB1 | 171 tumor samples (29 astrocytomas, 82 GBM, 49 oligodendrogliomas, 11 oligoastrocytomas) | 24 non-neoplastic brain samples from epileptogenic patients | q real-time RT-PCR |
|
[9] |
| EPHB2 | 171 tumor samples (29 astrocytomas, 82 GBM, 49 oligodendrogliomas, 11 oligoastrocytomas) | 24 non-neoplastic brain samples from epileptogenic patients | q real-time RT-PCR |
|
[9] |
| EPHB3 | 171 tumor samples (29 astrocytomas, 82 GBM, 49 oligodendrogliomas, 11 oligoastrocytomas) | 24 non-neoplastic brain samples from epileptogenic patients | q real-time RT-PCR |
|
[9] |
| EPHB4 | 171 tumor samples (29 astrocytomas, 82 GBM, 49 oligodendrogliomas, 11 oligoastrocytomas) | 24 non-neoplastic brain samples from epileptogenic patients | q real-time RT-PCR |
|
[9] |
| 96 primary glioma samples | IHC |
|
[10] | ||
| EPHB6 | 171 tumor samples (29 astrocytomas, 82 GBM, 49 oligodendrogliomas, 11 oligoastrocytomas) | 24 non-neoplastic brain samples from epileptogenic patients | q real-time RT-PCR |
|
[9] |
| ephrin-A1 | 78 primary gliomas samples | IHC |
|
[4] | |
| ephrin-B2 | 96 primary glioma samples | IHC |
|
[10] | |
| ephrin-B3 | 31 GBM samples | 28 non-tumoral white matter samples | q RT-PCR |
|
[7] |
| 7 grade IV glioblastoma samples, 3 glioma samples (1 oligodendroglioma grade II, 1 astrocytoma grade II and 1 astrocytoma grade III) | IHC |
|
|||
| SALIVARY GLANDS | |||||
| EPHA2 | 49 ACC samples | 10 normal salivary gland samples | IHC Western blot real-time RT-PCR |
|
[11] |
| 1 case of ACC | IHC RT-PCR |
|
[12] | ||
| ephrin-A1 | 49 primary ACC samples | 10 normal salivary gland samples | IHC Western blot real-time RT-PCR |
|
[11] |
| 1 case of ACC | IHC RT-PCR |
|
[12] | ||
| THYROID | |||||
| EPHA2 | 59 thyroid carcinoma samples (47 papillary, 5 follicular, 5 medullary, 2 anaplastic) | 72 benign tissue samples (61 hyperplastic nodules, 11 Hashimoto thyroiditis samples) | IHC |
|
[13] |
| 74 thyroid carcinoma samples (68 papillary, 6 follicular) | 14 follicular adenoma samples | IHC |
|
[14] | |
| EPHA4 | 59 thyroid carcinoma samples (47 papillary, 5 follicular, 5 medullary, 2 anaplastic) | 72 benign tissue samples (61 hyperplastic nodules, 11 hashimoto thyroiditis samples) | IHC |
|
[13] |
| EPHB4 | 56 thyroid carcinoma samples (46 papillary, 10 follicular) | 71 benign thyroid tissues (56 hyperplastic nodules, 12 hashimoto thyroiditis tissues) | IHC |
|
[15] |
| 21 papillary carcinoma samples | surrounding normal thyroid samples | cDNA microarray Western blot IHC |
|
[16] | |
| EPHB6 | 56 thyroid carcinoma samples (46 papillary, 10 follicular) | 71 benign thyroid samples (56 hyperplastic nodules, 12 hashimoto thyroiditis samples) | IHC |
|
[15] |
| ephrin-B2 | 21 papillary carcinoma samples | surrounding normal thyroid samples | cDNA microarray Western blot IHC |
|
[16] |
| 66 malignant thyroid samples (23 follicular carcinomas, 18 follicular variant of papillary carcinomas, 25 papillary carcinomas) | 4 normal thyroid samples, 26 hyperplastic nodules, 27 follicular adenomas | q real-time PCR | ephrin-B2 mRNA expression elevated in higher TNM stage neoplasms | [17] | |
IHC: immunohistochemistry, RT-PCR: reverse transcription-polymerase chain reaction, q RT-PCR: quantitative reverse transcription-polymerase chain reaction, OS: overall survival, GBM: glioblastoma multiforme, MVD: microvessel density, ACC: adenoid cystic carcinoma, EMT: epithelial-mesenchymal transition, cDNA: complementary DNA, LN: lymph nodes.
3.2. Salivary Glands
In the single study of EPH/ephrin expression in salivary gland tumors, EPHA2 and ephrin-A1 expression were assessed by IHC, Western blot, and real-time RT PCR in 49 primary Adenoid Cystic Carcinomas (ACCs) and 10 normal salivary gland tissues [13]. EPHA2 and ephrin-A1 expression was reported as higher in ACC tissues compared with non-neoplastic ones, and positively correlated with increased MVD. EPHA2 and ephrin-A1 overexpression, along with elevated MVD, was associated with the tumor TNM stage and the presence of perineural and perivascular invasion. Of note, solid type ACCs, which are accompanied by worse prognoses, exhibited higher EPHA2 and ephrin-A1 expression and increased MVD compared with cribriform and tubular subtypes [13].
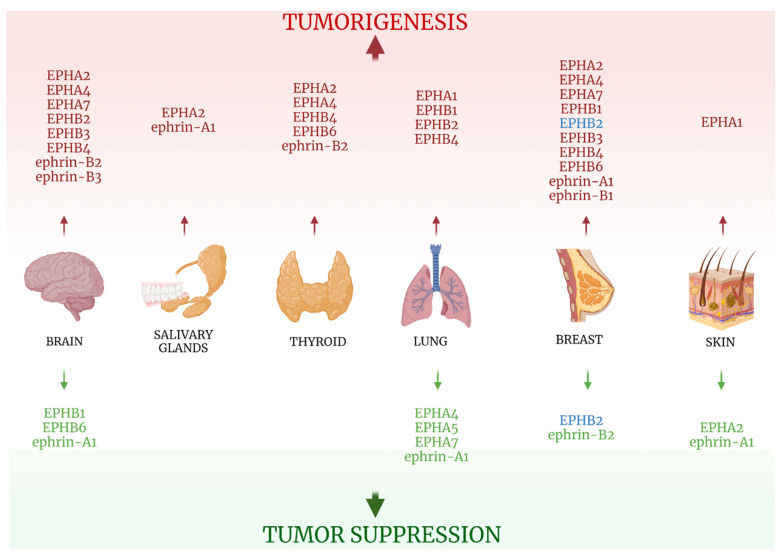
Fukai et al reported a case of a 29-year-old male ACC patient that presented with perineural spread of the tumor along the mandibular nerve [14]. Tumor cells showed characteristics of Epithelial-Mesenchymal Transition (EMT) and a high expression of EPHA2 (but not ephrin-A1), using IHC, suggesting a possible link between the receptor and aggressive tumor phenotypes. Table 1 and Figure 3 summarize the data concerning EPH/ephrin expression and salivary gland tumors clinicopathological characteristics.
Figure 3.
EPH/ephrin family role in solid tumors of the brain, salivary glands, thyroid, lung, breast, and skin. Red-colored EPHs/ephrins enhance tumorigenesis, while green-colored ones suppress it. EPHB2 in breast cancer can play both roles, depending on its location: cytoplasmic EPHB2 expression promotes carcinogenesis, while membranous EPHB2 expression favors tumor-suppression. Created with BioRender.com (accessed on 2 August 2021).
3.3. Thyroid
3.3.1. EPHAs
EPHA2 expression was reported as higher in thyroid carcinoma compared with non-cancerous thyroid tissue in two studies [15,16], although no further association with clinicopathological parameters was noted, while EPHA4 showed no difference in expression levels at all [15].
3.3.2. EPHBs
In a study that enrolled 46 papillary and 10 follicular thyroid carcinoma tissues, as well as 71 benign thyroid samples, EPHB4 expression was found to be higher in malignant specimens compared with their benign counterparts, while its overexpression was associated with larger tumor sizes [17]. In consistency with those findings, Sharma et al also correlated EPHB4 increased expression with malignancy, as well as with the presence of LN metastasis [18]. It is noteworthy that increased expression of an EPH may hinder tumorigenesis of a certain type of tumor but promote carcinogenesis of another one. The study conducted by Giaginis et al [17] underlined the association between elevated EPHB6 expression and malignancy, tumor size, presence of LN metastasis, capsular, vascular, and lymphatic invasion, as well as increased recurrence rate. Those results are in contrast with the beneficial role of EPHB6 in glioma pathogenesis [11].
3.3.3. Ephrins
Ephirn-B2 protein overexpression was correlated with malignant status and LN metastasis [18], while its elevated mRNA expression was linked to higher TNM stage cases [19].
All information on EPH/ephrin expression on thyroid neoplastic tissues is summarized in Table 1 and Figure 3.
4. Thoracic and Skin Tumors
4.1. Lung
The role of EPH/ephrin system in lung carcinogenesis has been extensively studied in a number of works that incorporated a large numbers of specimens.
4.1.1. EPHAs
EPHA1 overexpression was associated with high tumor proliferative capacity, assessed by the Ki67 index, probably revealing a link between its upregulation with more aggressive tumor phenotypes [20]. Intriguingly, EPHA2, largely reported for its negative impact on many solid tumors’ pathogenesis, seems to play a beneficial role in lung carcinogenesis. Two studies, which jointly included 420 patients with stage I Non-Small-Cell Lung Carcinoma (NSCLC), suggested that high EPHA2 expression was correlated with well-differentiated tumors, reduced smoking status, adenocarcinoma histological type, stage IA tumors, as well as the presence of Epidermal Growth Factor Receptor (EGFR) gene mutations. Both groups of researchers indicated EPHA1 as a positive prognostic factor for NSCLC patients [21,22]. EPHA4, EPHA5, and EPHA7 also exert a positive influence on lung cancer tumorigenesis. In the aforementioned study by Giaginis et al, EPHA4 was associated with low tumor stage, decreased presence of inflammation, and favorable OS. Additionally, EPHA5 overexpression was correlated with tumor proliferative capacity and longer OS and EPHA7 overexpression correlated with tumor proliferative capacity and older age, presence of fibrosis, as well as with smaller tumor size [20].
4.1.2. EPHBs
EPHs of the B subgroup appear to overwhelmingly contribute to lung cancer tumorigenesis. EPHB1 expression, measured through IHC and Western blot in 60 NSCLC specimens, was higher in cancer tissues compared with non-neoplastic ones. Additionally, EPHB1 expression was higher in patients with metastatic disease and shorter OS [23]. EPHB2 exhibited elevated expression in cancer NSCLC tissues, being correlated with increased depth of invasion, higher TNM stage, and decreased patient OS [24]. EPHB4 overexpression was linked to low tumor differentiation, high Ki67 index, and presence of LN metastasis in a series of 93 lung adenocarcinoma tissues [25].
As far as ephrins are concerned, few data currently exist linking their action to lung carcinogenesis. Ephrin-A1 was reported to have no impact on clinical outcome in two studies [21,26], while a third study associated its overexpression with reduced smoking status, adenocarcinoma histological type, high level of tumor differentiation, and the presence of EGFR gene mutations, attributing to the ligand a positive influence in tumorigenesis [22]. Ephrin-B2 expression was absent in all 93 lung adenocarcinoma tissues in the aforementioned study [25], and ephrin-B3 expression, while observed as higher in non-squamous tumors, exhibited no effect on patient OS [21]. Table 2 and Figure 3 include information regarding the clinical impact of EPH/ephrin expression in lung cancer.
Table 2.
EPHs/ephrins (bold) studied in solid tumors of lung, breast, and skin and correlations with clinicopathological parameters.
| EPHs/Ephrins | Malignant Tissues | Benign Control Tissues | Methods | Results | Refs |
|---|---|---|---|---|---|
| LUNG | |||||
| EPHA1 | 88 NSCLC (56 lung adenocarcinoma, 32 squamous cell carcinoma) | TMA IHC |
|
[20] | |
| EPHA2 | 225 NSCLC node-negative stage I samples | TMA IHC |
|
[21] | |
| 195 stage I NSCLC samples | Real-time RT-PCR |
|
[22] | ||
| EPHA4 | 88 NSCLC (56 lung adenocarcinoma, 32 squamous cell carcinoma) | TMA IHC |
|
[20] | |
| EPHA5 | 88 NSCLC (56 lung adenocarcinoma, 32 squamous cell carcinoma) | TMA IHC |
|
[20] | |
| EPHA7 | 88 NSCLC (56 lung adenocarcinoma, 32 squamous cell carcinoma) | TMA IHC |
|
[20] | |
| EPHB1 | 60 NSCLC samples | Non-cancerous tissues | Western blot, IHC |
|
[23] |
| EPHB2 | 126 lung adenocarcinoma samples | corresponding non-cancerous lung tissues | Quantum dots IHC |
|
[24] |
| EPHB4 | 93 lung adenocarcinoma samples | IHC |
|
[25] | |
| ephrin-A1 | 225 NSCLC node-negative stage I samples | TMA IHC |
|
[21] | |
| 195 stage I NSCLC samples | Real-time RT-PCR |
|
[22] | ||
| 244 NSCLC samples | IHC |
|
[26] | ||
| ephrin-B2 | 93 lung adenocarcinoma samples | IHC |
|
[25] | |
| ephrin-B3 | 225 NSCLC node-negative stage I samples | TMA IHC |
|
[21] | |
| BREAST | |||||
| EPHA2 | mRNA expression and patients’ survival data from 412 patients from databases | mRNA and survival data analysis |
|
[28] | |
| TMA IHC in 126 invasive ductal adenocarcinoma samples | 8 normal/benign epithelium samples | TMA IHC | |||
| EPHA4 | mRNA expression and patients’ survival data from 412 patients from databases | mRNA and survival data analysis |
|
[28] | |
| TMA IHC in 126 invasive ductal adenocarcinoma samples | 8 normal/benign epithelium samples | TMA IHC | |||
| No number mentioned | GOBO and ONCOMINE databases used to investigate EPHA4 mRNA expression and clinical correlations |
|
[29] | ||
| EPHA7 | mRNA expression and patients’ survival data from 412 patients from databases | mRNA and survival data analysis |
|
[28] | |
| TMA IHC in 126 invasive ductal adenocarcinoma samples | 8 normal/benign epithelium samples | TMA IHC | |||
| EPHA8 | mRNA expression and patients’ survival data from 412 patients from databases | mRNA and survival data analysis |
|
[28] | |
| TMA IHC in 126 invasive ductal adenocarcinoma samples | 8 normal/benign epithelium samples | TMA IHC | |||
| EPHB1 | 3554 breast cancer samples | mRNA expression data and survival information from the KM plotter database |
|
[30] | |
| EPHB2 | Cohort 1: 65 LN positive breast cancer samples |
RT-PCR |
|
[27] | |
| Cohort 2: 216 breast cancer samples |
IHC | ||||
| 3554 breast cancer samples | mRNA expression data and survival information from the KM plotter database |
|
[30] | ||
| 94 breast carcinoma samples | 9 normal breast tissue samples | IHC Semi-quantitative RT-PCR |
|
[31] | |
| EPHB3 | 3554 breast cancer samples | mRNA expression data and survival information from the KM plotter database |
|
[30] | |
| EPHB4 | mRNA expression and patients’ survival data from 412 patients from databases | mRNA and survival data analysis |
|
[28] | |
| TMA IHC in 126 invasive ductal adenocarcinoma samples | 8 normal/benign epithelium samples | TMA IHC | |||
| 3554 breast cancer samples | mRNA expression data and survival information from the KM plotter database |
|
[30] | ||
| 94 breast carcinoma samples | 9 normal breast tissue samples | IHC Semi-quantitative RT-PCR |
|
[31] | |
| 216 breast cancer samples | IHC |
|
[32] | ||
| EPHB6 | mRNA expression and patients’ survival data from 412 patients from databases | mRNA and survival data analysis |
|
[28] | |
| TMA IHC in 126 invasive ductal adenocarcinoma samples | 8 normal/benign epithelium samples | TMA IHC | |||
| ephrin-A1 | mRNA expression and patients’ survival data from 412 patients from databases | mRNA and survival data analysis |
|
[28] | |
| TMA IHC in 126 invasive ductal adenocarcinoma samples | 8 normal/benign epithelium samples | TMA IHC | |||
| ephrin-B1 | 96 samples | Gene expression data from SAGA and MicMa databases |
|
[33] | |
| 75 breast cancer samples (71 ductal adenocarcinoma, 4 lobular adenocarcinoma) | IHC |
|
[34] | ||
| ephrin-B2 | 216 breast cancer samples | IHC |
|
[32] | |
| SKIN | |||||
| EPHA1 | 56 basal cell carcinomas, 32 squamous cell carcinomas | 10 normal skin samples | IHC qRT-PCR |
|
[35] |
| EPHA2 | 202 vertical growth phase melanomas 68 separate samples of local (skin), regional (LNs) and distant metastasis from 58 patients with recurrent disease |
TMA IHC |
|
[36] | |
| ephrin-A1 | 202 vertical growth phase melanomas 68 separate samples of local (skin), regional (LNs) and distant metastasis from 58 patients with recurrent disease |
TMA IHC |
|
[36] | |
NSCLC: non-small cell lung carcinoma, TMA: tissue microarrays, IHC: immunohistochemistry, LN: lymph nodes, RT-PCR: reverse transcription-polymerase chain reaction, EGFR: epidermal growth factor receptor, DFS: disease-free survival, ALK: anaplastic lymphoma kinase, OS: overall survival.
4.2. Breast
The established, immense impact of breast tumors’ molecular signature in disease prognosis and treatment regimens has shifted the focus of the scientific community on those molecular mechanisms implicated in the disease pathogenesis and the search for novel biomarkers that influence the clinical outcome. Among them, the expression and effect of the EPH/ephrin system in breast cancer tumorigenesis has been extensively studied, with overexpression of all members contributing to worse disease prognosis [27,28,29,30,31,32,33,34].
4.2.1. EPHAs
EPHA2 expression has been reported as higher in cancer tissues compared with non-cancerous epithelium, elevated EPHA2 expression being associated with reduced OS, while EPHA2 and ephrin-A1 co-expression are correlated with recurrence rates [28]. EPHA4 overexpression was also associated with malignancy and cases with reduced OS [28]. In addition, data mining from the GOBO and ONCOMINE databases indicated that elevated EPHA4 mRNA expression correlated with stage 3 and 4 tumors, poor tumor differentiation, shortened relapse-free survival, positive LN status, and the highly aggressive basal-like subtype [29]. EPHA7 exhibited higher expression in cancer tissues compared with normal ones and in samples from patients with reduced OS as well [28], while EPHA8 expression exhibited no significant increase in cancerous tissues compared with normal ones and did not affect clinical outcomes [28].
4.2.2. EPHBs
EPHB1 expression showed no correlation with clinical parameters [30]. On the other hand, the varying impact of EPHB2 expression in breast cancer patients’ clinical outcomes reflects the complex role of EPH/ephrin molecular mechanisms in tumorigenesis. EPHB2 exhibited both membranous and cytoplasmic staining in IHC, showing an inverse correlation between the two patterns. Cytoplasmic expression of EPHB2 negatively affected patients’ outcomes, as it was positively associated with shorter OS, histological grade, and HER2 expression. On the contrary, membranous EPHB2 staining was correlated with longer patient OS [27]. Other studies yielded contradictory results regarding the influence of EPHB2 expression in breast cancer pathogenesis, with Wu et al linking its overexpression to decreased OS and DFS [31], and another group of researchers associating it with improved patient prognosis [30]. Mu et al associated elevated mRNA EPHB3 expression with worse OS and EPHB4 mRNA overexpression with improved RFS [30]. In contrast, other studies underlined that EPHB4 overexpression is correlated with malignancy and reduced OS [28], higher histological grade and stage [31], as well as with shorter metastasis-free survival [32]. Despite its beneficial influence in many neoplastic diseases, EPHB6 seems to promote tumorigenesis in the breast, as its expression was reported as higher in malignant tissues and was correlated with reduced OS [28].
4.2.3. Ephrins
Ephrin-A1 and EPHA2 co-expression is associated with recurrence [27]. Similarly, ephrin-B2 expression is associated with the clinically aggressive basal-like breast cancer subtype and HER2(+) tumors [33], while its overexpression is associated with the presence of LN metastasis, HER2 positivity, and the triple-negative breast cancer subtype [34]. On the other hand, the protective role of ephrin-B2 was underlined through the association of its expression with high estrogen receptor expression, low HER2 expression, low histological grade, and good patient’ prognosis [32].
All information retrieved on the clinical impact EPH/ephrin system expression is presented in Table 2 and Figure 3.
4.3. Skin
EPHA1 expression in 56 basal cell and 32 squamous cell carcinoma was examined through IHC and q RT-PCR and compared to 10 normal skin tissues. Cancer tissues exhibited downregulation of EPHA1 in relation to normal tissues, and EPHA1 decreased expression associated with increased tumor thickness in squamous cell carcinoma cases [35].
On the other hand, EPHA2 overexpression in melanoma tissues was correlated with increased melanoma thickness and increased tumor cell proliferative capacity, assessed as the Ki67 index. In addition, high expression of its ligand, ephrin-A1, was also correlated with increased melanoma thickness, as well as decreased patient survival [36].
Data from the two studies on EPH/ephrins and skin cancer are presented in Table 2 and Figure 3.
5. Gastrointestinal Tract Neoplasia
5.1. Esophagus
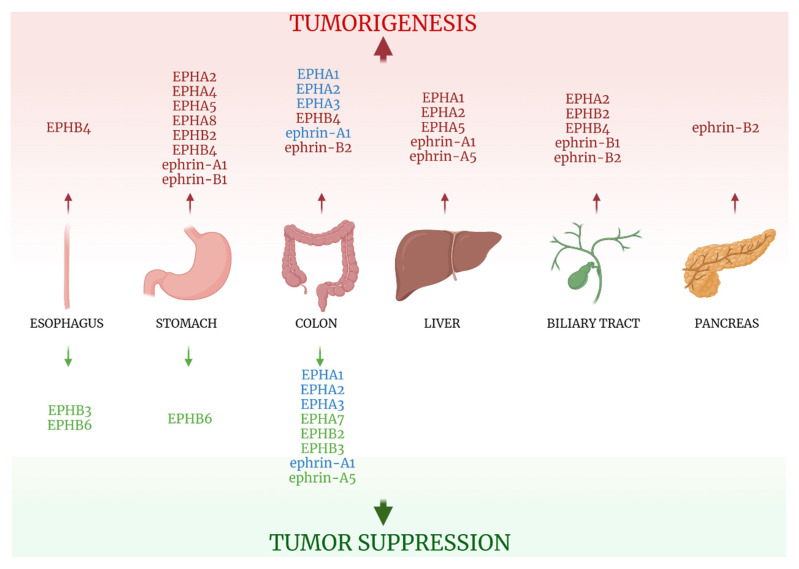
A limited amount of information exists concerning the clinical impact of the EPH/ephrin system expression in esophageal carcinomas. Schauer et al investigated the expression of EPHB3 and E-cadherin in healthy esophagi and Barrett’s carcinoma patients, reporting that both were reduced in adenocarcinoma tissues compared with normal or dysplastic ones [37]. Simultaneous expression of the two proteins showed an inverse correlation with tumor stage. Interestingly, when EPHB3 was expressed, redistribution of E-cadherin from the cytoplasm to the membrane was observed, revealing a possible mechanism through which EPHB3 might exert its speculated protective role, as E-cadherin membranous expression stabilizes cell to cell adhesion, decreasing tumor cells’ invasion capabilities, and disrupts EMT transition and cell proliferation. Other studies reported that EPHB4 was gradually overexpressed, from preneoplastic lesions to gastroesophageal cancers, and was correlated positively with advanced tumor stage. On the other hand, EPHB6 expression was down-regulated in neoplastic tissues, in accordance with its tumor-suppressive properties observed in other cancers [38]. Information concerning the clinical impact of the EPH/ephrin system expression in esophageal cancers is summarized in Table 3 and Figure 4.
Table 3.
EPHs/ephrins (bold) studied in solid tumors of the gastrointestinal tract, liver, biliary tract, and pancreas and correlations with clinicopathological parameters.
| EPHs/Ephrins | Malignant Tissues | Benign Control Tissues | Methods | Results | Refs |
|---|---|---|---|---|---|
| ESOPHAGUS | |||||
| EPHB3 | 141 Barrett’s carcinoma samples |
20 healthy esophagi samples |
IHC q PCR |
|
[37] |
| EPHB4 | 31 samples (GEJ and gastric carcinomas) from 30 patients (1 patient had 1 GEJ carcinoma and 1 gastric cancer in stomach corpus) | Paired normal samples | IHC q RT-PCR |
|
[38] |
| EPHB6 | 31 samples (GEJ and gastric carcinomas) from 30 patients | Paired normal samples | IHC q RT-PCR |
|
[38] |
| STOMACH | |||||
| EPHA2 | 176 gastric adenocarcinoma samples | Paired adjacent normal tissues | Real-time RT-PCR IHC Western blot |
|
[39] |
| 46 adenocarcinomas, 2 adenosquamous carcinomas, 1 neuroendocrine carcinoma | Corresponding non-tumor samples | Semiquantitative RT-PCR IHC Western blot |
|
[40] | |
| 91 gastric adenocarcinomas | 7 gastrointestinal stromal tumor samples | IHC Western blot |
|
[41] | |
| 107 gastric adenocarcinoma samples (54 of them received adjuvant chemotherapy) | Normal paired gastric samples | Proteome analysis (LC-MS/MS) mRNA (real-time RT-PCR) IHC |
|
[44] | |
| EPHA4 | 24 paired fresh gastric adenocarcinoma samples, 74 fresh frozen paraffin embedded gastric adenocarcinoma samples, 55 gastric adenocarcinoma samples in tissue microarrays | Adjacent non-tumor samples from 24 gastric adenocarcinoma specimens | Real-time RT-PCR RT-PCR IHC |
|
[42] |
| EPHA5 | 107 gastric adenocarcinoma samples (54 of them received adjuvant chemotherapy) | Normal paired gastric samples | Proteome analysis (LC-MS/MS) mRNA (real-time RT-PCR) IHC |
|
[44] |
| EPHA8 | 206 gastric cancer samples | 32 normal gastric mucosa, 60 paracancerous samples | IHC Western blot |
|
[43] |
| EPHB2 | 107 gastric adenocarcinoma samples (54 of them received adjuvant chemotherapy) | Normal paired gastric samples | Proteome analysis (LC-MS/MS) mRNA (real-time RT-PCR) IHC |
|
[44] |
| EPHB4 | 107 gastric adenocarcinoma samples (54 of them received adjuvant chemotherapy) | Normal paired gastric samples | Proteome analysis (LC-MS/MS) mRNA (real-time RT-PCR) IHC |
|
[44] |
| 31 gastric and GEJ carcinomas | Paired normal samples | Quantitative real-time RT-PCR IHC |
|
[38] | |
| EPHB6 | 31 gastric and GEJ carcinomas | Paired normal samples | Quantitative real-time RT-PCR IHC |
|
[38] |
| 152 gastric carcinoma samples | IHC |
EPHB6 expression:
|
[46] | ||
| ephrin-A1 | 176 gastric adenocarcinoma samples | Paired adjacent normal samples | Real-time RT-PCR IHC Western blot |
|
[39] |
| 46 adenocarcinomas, 2 adenosquamous carcinomas, 1 neuroendocrine carcinoma | Paired non-tumor samples | Semiquantitative RT-PCR IHC Western blot |
|
[40] | |
| ephrin-B1 | 29 gastric carcinoma samples | Matched normal samples | Semiquantitative RT-PCR |
|
[45] |
| COLON | |||||
| EPHA1 | 53 CRC samples | adjacent normal samples | IHC q real-time PCR |
|
[48] |
| 125 CRC specimens, 53 paired normal colon-CRC samples |
18 normal colon samples, paired normal samples from the 53 CRC patients | q real-time PCR Flow cytometry Western blot IHC |
|
[49] | |
| EPHA2 | 53 CRC samples | adjacent normal samples | IHC q real-time PCR |
|
[48] |
| 37 CRC samples | 37 paired normal samples | IHC Semi-quantitative RT-PCR |
|
[50] | |
| EPHA3 | 53 CRC samples | adjacent normal tissues | IHC q real-time PCR |
|
[48] |
| 68 CRC samples | paired adjacent normal mucosa | IHC |
|
[51] | |
| EPHA7 | 53 CRC samples | adjacent normal samples | IHC q real-time PCR |
|
[48] |
| EPHB2 | 345 primary CRCs 98 LN metastasis 82 liver metastasis |
100 adenomas 111 normal mucosa |
IHC |
|
[54] |
| 159 samples of stage 3 CRC | IHC |
|
[55] | ||
| 28 primary CRCs 39 metastasis 342 paired primary CRCs and normal samples in tissue microarrays |
28 normal colon samples 148 colorectal adenomas |
IHC |
|
[56] | |
| 4000 samples from 138 different tumor types, 1476 samples of colon cancer |
76 different normal samples | IHC |
|
[57] | |
| 36 CRCs | 30 Dysplastic aberrant foci, (dACF) 31 small (<5 mm) adenomas, 12 large (>5 mm) adenomas |
IHC |
|
[58] | |
| EPHB3 | 610 CRC samples formalin-fixed 32 fresh colorectal cancer samples |
IHC q real-time PCR |
|
[59] | |
| EPHB4 | 20 colon carcinoma samples | adjacent uninvolved mucosa | IHC RT-PCR |
|
[53] |
| 200 CRC samples | 50 adjacent non-tumor samples | IHC |
|
[60] | |
| ephrin-A1 | 53 CRC samples | adjacent normal samples | IHC q real-time PCR |
|
[48] |
| 37 CRC samples | 37 paired normal samples | IHC Semi-quantitative RT-PCR |
|
[50] | |
| ephrin-A5 | 53 CRC samples | adjacent normal samples | IHC Quantitative real-time PCR |
|
[48] |
| 72 colon malignancies (68 adenocarcinomas, 4 non-Hodgkin lymphomas) | 10 normal colon samples 14 benign colon lesions (adenomas and polyps) |
IHC Western blot q real-time PCR RT-PCR |
|
[52] | |
| ephrin-B2 | 20 colon carcinoma samples | adjacent uninvolved mucosa | IHC RT-PCR |
|
[53] |
| 200 CRC samples | 50 adjacent non-tumor samples | IHC |
|
[60] | |
| LIVER | |||||
| EPHA1 | 20 HCC samples | Non-cancerous adjacent tissues | IHC Northern blot |
|
[61] |
| EPHA2 | 139 HCC samples | Real-time q RT-PCR |
|
[63] | |
| 40 HCC samples | Cirrhotic non-tumorous samples | IHC Northern blot RT-PCR |
|
[62] | |
| EPHA5 | 250 HCC samples | Paired normal samples | IHC |
|
[64] |
| ephrin-A1 | 20 HCC samples | Non-cancerous adjacent samples | IHC Northern blot |
|
[61] |
| 139 HCC samples | Real-time q RT-PCR |
|
[63] | ||
| 40 HCC samples | Cirrhotic non-tumorous samples | IHC Northern blot RT-PCR |
|
[62] | |
| ephrin-A5 | 142 paired HCC and peritumoral liver samples | Paired peritumoral samples | Real-time q RT-PCR |
|
[65] |
| BILIARY TRACT | |||||
| EPHA2 | 30 ICC samples, 5 LN metastasis from 5 of the above patients |
Normal adjacent samples | PCR-based Sanger sequencing |
|
[66] |
| EPHB2 | 50 cholangiocarcinoma samples | IHC | High
|
[67] | |
| EPHB4 | 50 cholangiocarcinoma patients | IHC |
|
[67] | |
| ephrin-B1 | 50 cholangiocarcinoma samples | IHC | High
|
[67] | |
| ephrin-B2 | 50 cholangiocarcinoma samples | IHC |
|
[67] | |
| PANCREAS | |||||
| ephrin-B2 | 179 PDAC samples (mRNA data) | 171 normal pancreatic samples (mRNA data) | Statistical analysis | Data analysis of mRNA expression of the 179 PDAC and 171 normal pancreatic tissues showed that:
|
[68] |
| 54 PDAC samples | 54 adjacent normal samples | IHC Quantitative real-time PCR Western blot |
IHC, qRT-PCR and western blot analysis of the 54 PDAC tissues showed that:
|
||
q PCR: quantitative polymerase chain reaction, IHC: immunohistochemistry, q RT-PCR: quantitative reverse transcription-polymerase chain reaction, q real time PCR: quantitative real time transcription-polymerase chain reaction, GEJ: gastroesophageal junction, LN: lymph nodes, OS: overall survival, DFS: disease-free survival, MVD: microvessel density, GCSC: gastric cancer tissues stromal cells, GSC: normal gastric tissue stromal cells, ICC: intrahepatic cholangiocarcinoma, PDAC: pancreatic ductal adenocarcinoma.
Figure 4.
EPH/ephrin family role in solid tumors of organs of the gastrointestinal tract, liver, biliary tract, and pancreas. Red-colored EPHs/ephrins enhance tumorigenesis, while green-colored ones suppress it. It is unclear if EPHA1, EPHA2, EPHA3, and ephrin-A5 enhance tumorigenesis or tumor suppression (blue font). See text and Table 3. Created with BioRender.com (accessed on 2 August 2021).
5.2. Stomach
5.2.1. EPHAs/Ephrin-As
The clinical impact of EPH/ephrin member proteins’ expression in gastric cancer is among the most extensively studied. Most researchers utilized a variety of methods, such as IHC, western blot, or RT-PCR, to assess EPH/ephrin protein or mRNA expression and proceeded to correlate it with clinicopathological parameters.
Yuan et al [39] studied the expression of EPHA2 and ephrin-A1 in 176 gastric adenocarcinoma specimens, underlining that they were significantly overexpressed in cancer tissues when compared with normal ones. Expression of both proteins was correlated with increased TNM stage and LN metastasis rate, with EPHA2 overexpression also linked to deeper levels of tumor invasion and found to be an independent poor prognostic factor, as its increased expression correlated with decreased OS. Nakama et al, however, observed that while EPHA2/ephrin-A1 genes expression was associated with tumors that invaded in deeper layers of the stomach and had undefined borders or infiltrated diffusely, no correlation existed regarding the tumor size, patients’ age, vessel infiltration, and lymph node status [40]. On the contrary, in a large study that included 91 gastric adenocarcinoma samples, high EPHA2 protein expression was associated with advanced tumor stage, size, LN metastasis, TNM stage, and lymphovascular invasion [41].
EPHA4 and EPHA8 also seem to hold tumorigenic properties. Both proteins’ expression was linked to depth of malignant infiltration of the stomach and lower OS [42,43], with high EPHA8 expression additionally accompanied by lower tumor differentiation, as well as higher TNM stages and distant metastasis rate.
Interestingly, overexpression of a number of EPHs, belonging in A and B subgroups, was also observed in gastric stromal cells associated with gastric adenocarcinoma [44]. Such results possibly prove that EPHs exert their carcinogenic properties through their involvement in cell adhesion and matrix-cell interactions, creating a direct clinical impact. Ephrin ligands expressed on tumor cells could possibly help them interact with stromal cells expressing EPHs, promoting carcinogenic properties such as increased cell motility that would enhance tissue infiltration or triggering molecular cascades that promote cell proliferation. The EPH/ephrin system may represent a key factor in the creation of a tumor-promoting microenvironment, and further study regarding their expression in non-tumorous tissues surrounding cancer cells is called for.
5.2.2. EPHBs/Ephrin-Bs
EPHs of the B subgroup and their ligands also seem to play a role in gastric cancer pathogenesis. Ephrin-B1 expression was positively correlated with the tumor differentiation level [45]. EPHB4 expression, that was reported higher in Barrett esophagus, was also recorded elevated in gastroesophageal carcinomas, tumors of higher stage and in malignant cells at the tumor invasion front [38]. On the other hand, two studies underline the protective role of EPHB6, as its expression was found to be downregulated in tumor cells [38], being negatively associated with tumor stage, LN metastasis, and a poor level of differentiation [46].
Lastly, ephrin-B1 expression was reported more frequent in poorly differentiated adenocarcinomas than well-differentiated ones [45].
Data from research on EPH/ephrin expression and clinicopathological features in gastric cancer are presented in Table 3 and Figure 4.
5.3. Colon
Colorectal cancer (CRC) is the third most common malignancy in the world and the third leading cause of cancer-related deaths [47]. The heavy clinical impact of the disease has led to extensive research on its pathogenesis, including the tumorigenic role of the EPH/ephrin system. Strikingly, many EPHs and their ligands seem to have a protective function [48,49,50,51,52,53,54,55,56,57,58,59,60].
5.3.1. EPHAs
EPHA1, although increased in CRC tissues compared with normal ones, exhibited decreased expression in advanced TNM disease stages [48,49], with one study correlating its low expression to poor OS [49]. Similarly, EPHA2 was found to be upregulated in CRC tissues compared with their non-cancerous counterparts, but its downregulation was prevalent in more advanced disease stages [48,50]. Moreover, EPHA2 overexpression was observed in tumors less than 5 centimeters in diameter compared with larger ones [50]. EPHA3 and EPHA7 exhibited loss of expression in 96% of the cases in one of the three previous studies [48]. On the contrary, Li et al [51] reported that EPHA3 not only was overexpressed in CRC tissues compared with adjacent normal mucosa, but was also positively linked to patients’ age, poor differentiation level of the tumor, and LN metastasis.
5.3.2. EPHBs
The inhibitory role of EPHB2 in colon carcinogenesis is among the ones most thoroughly studied. Multiple works have investigated its clinical impact, associating its high expression levels with lower tumor stage [54,57], lower histological grade [58], higher grade of histological differentiation [54], low LN metastasis rate [57], longer OS and DFS [54,56,57], and lower recurrence and death rates [55]. In a large series of tissue specimens from CRC patients, scientists have demonstrated that from normal colon mucosa to preneoplastic lesions, to malignant tissues, to the tumor invasion front, to metastasis, EPHB4 expression is progressively reduced [54,57,58].
Jan et al examined the expression of EPHB3 in a total of 642 specimens, reporting its attenuation during transition from adenoma to carcinoma and its further decline as the tumor invaded into deeper tissues. Statistical analysis linked EPHB3 expression to tumor differentiation, lymphovascular invasion, TNM stage, microsatellite instability and better OS [59].
In contrast, EPHB4 was presented in one study as being among the few members of the EPH/ephrin family that favor tumorigenesis. While its expression was minimal to absent in normal colon tissues, it was strongly expressed in CRC and associated with tumor depth of invasion, presence of LN and distant metastasis and TNM stage [60].
5.3.3. Ephrins
Ephrin-A1 expression, although higher in CRC tissues compared with normal ones [48,50], was correlated to early-stage cancers and smaller tumor size [50]. Ephrin-A5 expression, albeit found higher than its EPHA receptors [48], was significantly reduced in colon cancer tissues and was negatively associated with tumor differentiation and clinical stage [52]. Ephrin-B2 expression, contrary to the seemingly tumor suppressive properties of ephrin-A5, was found elevated in CRC samples, with frequent enhancement on the luminal surface of carcinoma epithelium [53]. All information on EPH/ephrin expression on CRC tissues is summarized in Table 3 and Figure 4.
5.4. Liver
All of the EPH/ephrins investigated in Hepatocellular Carcinoma (HCC), with the only exception of ephrin-A5, seem to have a negative impact on carcinogenesis and patients’ clinical outcomes. Ephrin-A1 expression was reported as lowest in normal liver tissues, elevated in cirrhotic liver specimens, and further enhanced in HCC [61,62]. Its expression was correlated with Alpha Fetoprotein expression, which was associated with poor OS [61]. Wada et al also linked ephrin-A1 expression to poorer patients’ prognosis [63]. EPHA1 exhibited strong expression in HCC specimens [61], and EPHA2 expression was associated with decreased differentiation and shorter OS [62], as well as with microscopic portal invasion. A study that examined the expression of three kinases (Anaplastic Lymphoma Kinase, Fibroblast Growth Factor Receptor 2, and EPHA5) in a series that included 250 HCC cases, underlined that coactivation of all three of them led to a worse prognosis [64]. On the contrary, Wang et al [65] examined the mRNA expression of the two alternative isoforms of ephrin-A5, large ephrin-A5 (ephrin-A5L), and small ephrin-A5 (ephrin-A5S), through means of quantitative real-time PCR, reporting them lower in HCC compared with peritumoral tissues. Ephrin-A5S expression positively correlated with old age and histological grade, and its expression in peritumoral tissues was associated with better DFS and OS. Data are presented in Table 3 and Figure 4.
5.5. Biliary Tract
Limited data is currently available regarding the role of EPHs and their ligands in biliary tract neoplasia and its clinical significance. Sheng et al observed that the EPHA2 gene was frequently mutated in primary intrahepatic cholangiocarcinoma (ICC) tumors, with mutations even more likely to occur in cases with lymph node metastasis [66]. Another study included 50 ICC patients to examine EPHB2, EPHB4, ephrin-B1, and ephrin-B2 expression, as well as tumors’ MVD through CD34 IHC staining [67]. An association between metastasis status and EPHB2 expression, EPHB2/ephrin-B1 co-expression, EPHB2/ephrin-B2 co-expression, EPHB4 expression along with high MVD and lastly ephrin-B2 expression and high MVD, was reported.
Information regarding the EPH/ ephrin system’s role in biliary tract neoplasia is summarized on Table 3 and Figure 4.
5.6. Pancreas
The only study examining the clinical significance of EPHs/ephrins members expression in pancreatic cancer is the one conducted by Zhu et al [68] and concerned the ephrin-B2 gene expression in pancreatic ductal adenocarcinoma (PDAC). The researchers gathered information on ephrin-B2 mRNA expression from 179 PDAC patients and 171 normal pancreatic tissues. Statistical analysis showed that ephrin-B2 expression was higher in PDAC tissues when compared with normal ones and correlated with shorter OS and DFS. Furthermore, samples from 54 PDAC tissues and adjacent uninvolved tissues were tested for ephrin-B2 expression through IHC, western blot, and q real-time PCR analysis. Ephrin-B2 expression was again reported higher in cancerous tissues, and its overexpression was linked to tumor TNM stage and high Ki67 expression. Table 3 and Figure 4 summarize the study.
6. Urinary Tract Neoplasia
6.1. Kidney
6.1.1. EPHAs
EPHA1 and EPHA2 proteins expression, although lower in cancer tissues compared with matched non-malignant ones, was observed as higher in metastatic lesions than in primary tumor specimens. Moreover, positive staining of both EPHs was associated with aggressive tumor features, while positive EPHA1 staining was also linked to poor patient OS [69]. Wang et al studied the IHC expression of EPHA3 in 68 samples of Clear Cell Renal Cell Carcinoma (CCRCC) and adjacent normal kidney tissues, reporting its tumor suppressive properties. EPHA3 exhibited strong immunostaining in normal renal tubes, negative expression in 72% of the CCRCC cases and decreased intensity of immunostaining in positive malignant cases. Loss of EPHA3 expression was positively correlated with tumor size and TNM stage [70].
6.1.2. EPHBs/Ephrins
Interestingly, immunohistochemical expression of EPHB4 in the venous endothelium and ephrin-B2 in the arterial endothelium was reported greater in tumoral areas compared with non-tumorous ones [71], possibly underlining the role of the EPH/ephrin system in tumor angiogenesis. Lastly, while ephrin-A1 expression was found to be increased in cancer tissues, its low staining was linked to more aggressive tumor features [69]. All information is presented in Table 4 and Figure 5.
Table 4.
EPHs/ephrins (bold) studied in solid tumors of the urinary tract and prostate and correlations with clinicopathological parameters.
| KIDNEY | |||||
| EPHs/Ephrins | Malignant Tissues | Benign Control Tissues | Methods | Results | Refs |
| EPHA1 | mRNA from 75 malignant samples | matched non-malignant samples | q PCR |
|
[69] |
| protein expression from 241 malignant samples (primary and metastatic) | non-malignant samples | TMA IHC | |||
| EPHA2 | mRNA from 75 malignant samples | matched non-malignant samples | q PCR |
|
[69] |
| protein expression from 241 malignant samples (primary and metastatic) | non-malignant samples | TMA IHC | |||
| EPHA3 | 68 CCRCC samples | adjacent normal kidney samples | IHC |
|
[70] |
| EPHB4 | 12 kidney cancer samples | IHC staining of arterial and venous vessels in tumoral and non-tumoral tissues |
|
[71] | |
| ephrin-A1 | mRNA from 75 malignant samples | matched non-malignant samples | q PCR |
|
[69] |
| protein expression from 241 malignant samples | non-malignant samples | TMA IHC | |||
| ephrin-B2 | 12 kidney cancer samples | IHC staining of arterial and venous vessels in tumoral and non-tumoral tissues |
|
[71] | |
| BLADDER | |||||
| EPHs/Ephrins | Malignant Tissues | Benign Control Tissues | Methods | Results | Refs |
| EPHA2 | 64 TCC samples | 13 normal urothelium samples | IHC |
|
[72] |
| EPHB2 | 40 bladder TCC samples | adjacent non-tumorous samples | IHC Western blot |
|
[73] |
| EPHB4 | 40 bladder TCC samples | adjacent non-tumorous samples | IHC Western blot |
|
[73] |
| 33 bladder cancer samples | IHC staining of arterial and venous vessels in tumoral and non-tumoral tissues |
|
[71] | ||
| ephrin-A1 | 64 TCC samples | 13 normal urothelium samples | IHC |
|
[72] |
| ephrin-B2 | 410 bladder TCC samples | Ephrin-B2 expression data from the cancer genome atlas (TCGA) |
|
[74] | |
| 33 bladder cancer samples | IHC staining of arterial and venous vessels in tumoral and non-tumoral tissues |
|
[71] | ||
| PROSTATE | |||||
| EPHs/Ephrins | Malignant Tissues | Benign Control Tissues | Methods | Results | Refs |
| EPHA5 | 22 PCa samples, 23 paired cancerous and non-cancerous samples | 39 BPH samples, 23 paired non-cancerous samples | IHC q RT-PCR Methylation-specific PCR |
|
[75] |
| EPHA6 | 112 PCa tumor samples | 58 BPH samples | q real-time PCR |
|
[76] |
| EPHB4 | 20 PCa samples | IHC staining of arterial and venous vessels in tumoral and non-tumoral tissues |
|
[71] | |
| ephrin-B2 | 20 PCa samples | IHC staining of arterial and venous vessels in tumoral and non-tumoral tissues |
|
[71] | |
q PCR: quantitative polymerase chain reaction, TMA: tissue microarrays, IHC: immunohistochemistry, q RT-PCR: quantitative reverse transcription-polymerase chain reaction, CCRCC: clear cell renal cell carcinoma, TCC: transitional cell carcinoma, OS: overall survival, DFS: disease-free survival, BPH: benign prostate hyperplasia, PCa: prostate carcinoma.
Figure 5.
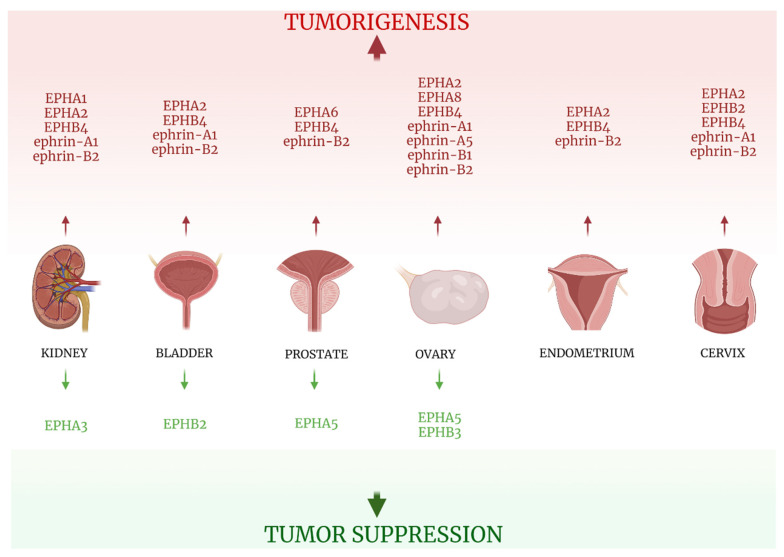
EPH/ephrin family role in solid tumors of organs of the urinary tract, prostate, and gynecological tract. Red-colored EPHs/ephrins enhance tumorigenesis, while green-colored ones suppress it. Created with BioRender.com (accessed on 2 August 2021).
6.2. Bladder
6.2.1. EPHAs
The EPH/ephrin system is aberrantly expressed in normal urothelium, with its deregulation implicated in the pathogenesis of transitional cell carcinoma (TCC). EPHA2, similarly to its role in kidney cancer, seems to promote TCC development, as it exhibited low expression in normal urothelial cells and was highly expressed in advancing stages of bladder cancer [72].
6.2.2. EPHBs
TCC tissues showed loss of EPHB2, which is expressed in normal urothelium. On the other hand, TCC specimens exhibited gain of EPHB4 expression, which is absent in normal urothelium, with the signal strength correlating with the highest tumor stage [73]. In addition, EPHB4 expression in venous endothelium was increased in tumor sections compared with benign ones [71].
6.2.3. Ephrins
Ephrin-B2 expression was associated with poor OS and reduced DFS [74] and noted as higher in arterial endothelium of cancer specimens compared with normal ones [71]. Data on EPH/ephrin expression in TCC specimens are presented in Table 4 and Figure 5.
6.3. Prostate
6.3.1. EPHAs
Few research data have focused on the role of the EPH/ephrin system in Prostate Cancer (PCa) pathogenesis. EPHA5 is speculated to play a protective role, as loss or downregulation of its expression was reported in 62.2% of PCa specimens but only in 5.1% of Benign Prostate Hyperplasia (BPH) samples. Furthermore, patients with an elevated Gleason score or T3-T4 disease stage exhibited higher rates of EPHA5 methylation [75]. EPHA6 mRNA expression, on the other hand, was elevated in PCa tissues compared with benign ones, and was correlated with vascular and neural invasion, as well as with increased serum levels of prostate-specific antigen and high TNM stage [76].
6.3.2. EPHBs/Ephrin-Bs
In the study conducted by Özgür et al, that enrolled 20 PCa specimens, EPHB4 expression in venous endothelium and ephrin-B2 expression in arterial endothelium was elevated in tumor areas, compared with benign ones [71].
Information concerning EPH/ephrin expression and its clinical impact in prostate cancer is summarized in Table 4 and Figure 5.
7. Gynecological Tumors
7.1. Ovary
Gynecological tract neoplasia, along with breast cancer, is greatly impacted by deregulation of the EPH/ephrin system, with its role in ovarian cancer pathogenesis being amongst the most extensively studied.
7.1.1. EPHAs
EPHA1 and EPHA2 expression were reported by Herath et al as having no impact on patients’ survival [77]. However, in a large study that included 118 epithelial ovarian carcinoma (EOC) samples, EPHA2 overexpression was correlated with tumors of higher histological grade and shorter patient OS [78]. EPHA5, in consistency with its tumor suppressive properties on other solid tumors [20,75], exhibits a protective role in ovarian cancer, with its negative or weak expression linked to poor clinical outcomes. Downregulation of EPHA5 seems to contribute to carcinogenesis, since from normal fallopian tissues and benign ovarian neoplasms to borderline tumors and serous carcinomas, a progressive loss of EPHA5 expression was noted [79]. EPHA8 overexpression was reported in EOC tissues and was additionally associated with older age, higher disease stage, positive LNs, presence of distant metastasis, positive ascetic fluid, and high CA-125 levels [80].
7.1.2. EPHBs
EPHB3 also seems to play a beneficial role in ovarian carcinogenesis, with its expression reduced in serous carcinoma tissues compared with benign ones and its downregulation linked to the histological grade and FIGO stage of serous tumors [81]. High EPHB4 expression, on the other hand, was correlated with clinical stage and low patients’ OS [82].
7.1.3. Ephrins
Overexpression in cancer tissues of all of the ligands studied (ephrin-A1, -A5, -B1, and -B2) was linked to adverse clinical outcomes and dismal prognoses.
Ephrin-A1 and ephrin-A5 elevated expression in tumorous specimens was linked to decreased survival [77], while ephrin-B1 overexpression, more frequently observed in high-grade carcinomas, was correlated with an increased recurrence rate and decreased OS [83]. Lastly, ephrin-B2 overexpression in cancer tissues was associated with the clinical stage and low patient OS [82].
Data from studies on the role of the EPH/ephrin system on ovarian cancer clinical outcomes are presented in Table 5 and Figure 5.
Table 5.
EPHs/ephrins (bold) studied in solid tumors of the gynecological tract and correlations with clinicopathological parameters.
| EPHs/Ephrins | Malignant Tissues | Benign Control Tissues | Methods | Results | Refs |
|---|---|---|---|---|---|
| OVARY | |||||
| EPHA1 | 24 ovarian carcinoma samples | 4 benign ovarian samples | q real-time RT-PCR |
|
[77] |
| EPHA2 | 24 ovarian carcinoma samples | 4 benign ovarian samples | q real-time RT-PCR |
|
[77] |
| 118 EOC samples | IHC RT-PCR |
|
[78] | ||
| EPHA5 | 61 ovarian serous carcinoma samples | 24 benign ovarian serous tumors, 42 serous borderline ovarian tumors, 20 normal fallopian tubes | IHC |
|
[79] |
| EPHA8 | 20 fresh-frozen specimens: 20 EOC samples | 40 fresh frozen samples: 20 normal ovarian samples, 20 normal fallopian tube samples |
q RT-PCR |
|
[80] |
| 125 paraffin-embedded EOCs | 98 samples paraffin-embedded: 30 borderline samples, 30 benign ovarian tumors, 20 normal fallopian tubes, 18 normal ovarian samples | TMA IHC | |||
| EPHB3 | 50 ovarian serous carcinomas | 17 serous borderline tumors, 19 normal fallopian tubes | IHC |
|
[81] |
| EPHB4 | 72 ovarian cancer samples | IHC Real-time RT-PCR |
|
[82] | |
| ephrin-A1 | 24 ovarian carcinoma samples | 4 benign ovarian samples | q real-time RT-PCR |
|
[77] |
| ephrin-A5 | 24 ovarian carcinoma samples | 4 benign ovarian samples | q real-time RT-PCR |
|
[77] |
| ephrin-B1 | 27 serous adenoCa, 14 mucinous adenoCa, 15 clear cell adenoCa, 15 endometrioid adenoCa | 11 serous cystadenomas, 5 mucinous cystadenomas, 10 serous borderline tumors, 15 muscinous borderline tumors | IHC Western blot |
|
[83] |
| ephrin-B2 | 72 ovarian cancer samples | IHC Real-time RT-PCR |
|
[82] | |
| ENDOMETRIUM | |||||
| EPHA2 | 139 endometrial endometrioid carcinoma samples | 10 benign endometrial | IHC |
|
[84] |
| EPHB4 | 68 endometrial cancer samples | 16 normal endometrium samples | IHC Real-time RT-PCR |
|
[85] |
| 11 ER (+)/PR (+) endometrial carcinoma samples, 33 ER (-)/PR (-) endometrial carcinoma samples | 10 adenomyosis samples, 2 simple endometrial hyperplasia samples, 20 atypical endometrial hyperplasia samples | IHC Western blot |
|
[86] | |
| ephrin-B2 | 68 endometrial cancer samples | 16 normal endometrium samples | IHC Real-time RT-PCR |
|
[85] |
| 11 ER (+)/PR (+) endometrial carcinoma samples, 33 ER (-)/PR (-) endometrial carcinoma samples | 10 adenomyosis samples, 2 simple endometrial hyperplasia samples, 20 atypical endometrial hyperplasia samples | IHC Western blot |
|
[86] | |
| CERVIX | |||||
| EPHA2 | 206 squamous cell cervical cancer samples | IHC |
|
[87] | |
| 217 early squamous cell cervical carcinoma samples | IHC |
|
[88] | ||
| EPHB2 | 20 HGSIL, 53 cervical squamous cell carcinomas | 25 normal cervical samples | IHC |
|
[89] |
| EPHB4 | 90 cervical carcinoma samples, 15 CIN samples | 15 normal cervix samples | IHC |
|
[90] |
| 62 uterine cervical cancer samples | IHC Real-time RT-PCR |
|
[82] | ||
| ephrin-A1 | 206 squamous cell cervical cancer samples | IHC |
|
[87] | |
| 217 early squamous cell cervical carcinoma samples | IHC |
|
[88] | ||
| ephrin-B2 | 90 cervical carcinoma samples, 15 CIN samples | 15 normal cervix samples | IHC |
|
[90] |
| 62 uterine cervical cancer samples | IHC Real-time RT-PCR |
|
[82] | ||
q real time PCR: quantitative real time transcription-polymerase chain reaction, IHC: immunohistochemistry, TMA: tissue microarrays, OS: overall survival, FIGO: International Federation of Gynaecology and Obstetrics, er: estrogen receptor, pr: progesterone receptor, LN: lymph nodes, Ca: carcinoma, MVD: microvessel density, EOC: epithelial ovarian carcinomas, CIN: cervical intraepithelial neoplasm, HGSIL: high-grade squamous intraepithelial lesion.
7.2. Endometrium
Kamat et al reported EPHA2 overexpression by IHC in 48% of 139 endometrial endometrioid carcinoma tissues, but only in 10% of the 10 benign endometrial specimens. In addition, EPHA2 was positively associated with a plethora of adverse clinical characteristics, such as high disease stage, high tumor grade, increased depth of myometrial invasion, low estrogen and progesterone receptors expression, high Ki67 index, and shorter OS [84]. EPHB4 upregulation were correlated with increased clinical stage, tumor dedifferentiation, deeper myometrial invasion, and low OS [85]. Additionally, according to Dong et al, EPHB4 and ephrin-B2 was associated with estrogen receptor expression [86].
Table 5 and Figure 5 summarize the three studies regarding EPHs/ephrins and endometrial carcinoma.
7.3. Cervix
Two large studies, that each incorporated more than 200 Squamous Cell Carcinoma (SCC) specimens, investigated the protein expression of EPHA2 and its ligand, ephrin-A1, through IHC. The first group reported that EPHA2 overexpression was correlated with shorter patient OS [87], while no such association was reached in the second study [88]. However, both studies supported that high ephrin-A1 expression was correlated with poor patient OS. EPHB2 expression correlated with cancer progression, as the percentage of ratio of EPHB2 positive cells to the number of all cells was 28% in normal tissues, 40% in High Grade Squamous Intraepithelial Lesion tissues and 69.8% in SCCs. Furthermore, EPHB2 was positively correlated with tumor stage [89]. Likewise, EPHB4 and ephrin-B2 expression were reported higher in cervical cancer and Cervical Intraepithelial Neoplasia specimens and associated with tumor diameter, with EPHB4 additionally associated with disease stage. EPHB4 and ephrin-B2 may promote tumorigenesis by driving neoangiogenesis, as their strong protein expression is correlated with MVD [90]. Another study linked EPHB4 and ephrin-B2 overexpression to higher tumor stage, LN metastasis, higher tumor size and poorer prognoses [82]. Table 5 and Figure 5 summarize information on EPH/ephrins and cervical cancer.
8. Pediatric Neoplasia
8.1. Sarcomas
The limited number of studies on the EPH/ephrin system’s clinical impact on soft tissue tumors concerns Embryonal Rhabdomyosarcoma (ERM) tumors. EPHA2 and ephrin-A1 were reported to be significantly upregulated in ERM tissues compared with normal skeletal muscle specimens [91]. In contrast, EPHB4 overexpression was correlated with positive prognoses (longer OS) for ERM patients [92]. Data are presented on Table 6.
Table 6.
EPHs/ephrins (bold) studied in solid pediatric tumors and correlations with clinicopathological parameters.
| EPHs/Ephrins | Malignant Tissues | Benign Control Tissues | Methods | Results | Refs |
|---|---|---|---|---|---|
| RHABDOMYOSARCOMA | |||||
| EPHA2 | 14 ERM samples | normal skeletal muscle samples | real-time RT-PCR |
|
[91] |
| EPHB4 | Not mentioned | Data from the Intergroup Rhabdomyosarcoma Study Group (IRSG)-IV Affymetrix U95 GeneChip database regarding EphB4 expression in human ERMs |
|
[92] | |
| ephrin-A1 | 14 ERM samples | normal skeletal muscle samples | real-time RT-CR |
|
[91] |
| NEUROBLASTOMA | |||||
|
EPHB6
ephrin-B2 ephrin-B3 |
50 neuroblastoma samples | q RT-PCR |
|
[93] | |
| WILMS TUMOR | |||||
| EPHB2 | 25 primary Wilms tumor samples | q PCR |
|
[94] | |
ERM: embryonal rhabdomyosarcoma, real time RT-PCR: real time reverse transcription-polymerase chain reaction, q RT-PCR: quantitative reverse transcription-polymerase chain reaction.
8.2. Neuroblastoma
Tang et al [93] investigated, through q PCR, the gene expression of EPHB6, ephrin-B2 and ephrin-B3 in 50 neuroblastoma tissues, associating it with favorable outcomes (longer OS), in contrast to the negative role of the aforementioned EPH/ephrin members in central nervous system neoplasia [9,12]. The study is summarized in Table 6.
8.3. Wilms Tumor
The investigation of EPHB2 gene expression by q PCR in 25 primary Wilms tumors revealed elevated EPHB2 expression in stages 2–4 compared to stage 1 Wilms tumors, probably showing a negative impact on carcinogenesis [94]. The study’s results are presented on Table 6.
9. Conclusions
EPHs/ephrins seem to heavily impact key molecular steps of carcinogenesis. Their role designates them as possible biomarkers, useful in clinical practice for their accuracy, but also for timely detection of the presence of a neoplasm.
Different members of the EPH/ephrin family carry out distinct actions in different stages of neoplasia. Some members exert their role at early steps of tumorigenesis, aiding tumor cells’ proliferation or enhancing the appropriate cell-matrix interactions that allow cancer cells to locally invade surrounding normal tissues. Others are implicated in the creation of the microenvironment that will allow spreading of the tumor to distant sites, carrying out their action in later disease stages. The distinct roles of the EPH/ephrin system members indicate the diversity of their usefulness in the clinical setting. The expression level evaluation of an individual EPH/ephrin could be utilized to investigate not only the presence of neoplasia, but also the assessment of parameters such as the stage of the disease, the size of the tumor, and the overall disease burden. The EPH/ephrin molecular profile of a tumor should also indicate, in some cases, tumor origin. Moreover, evaluation of the EPH/ephrin molecular signature of a tumor can accurately estimate patients’ prognoses. Determination of fluctuation in their levels could contribute to monitoring treatment efficacy and outcome, as well as to early detection of disease recurrence.
The well-established tumor-promoting or tumor-suppressive properties of the various EPH/ephrin members renders them potential targets of therapeutic interventions. The development of therapeutic agents blocking EPHs/ephrins that enhance carcinogenesis or enhance the expression of those that suppress it represents a promising future treatment strategy. In addition, researchers have proven that different tumors exhibit distinct profiles of EPH/ephrin protein expression, with some cases showing significant upregulation of a certain member and others extensive loss. The colon is a characteristic example, where the majority of EPHs/ephrins seem to have a protective function regarding neoplastic transformation. Most studies underlined that colon cancer tissues exhibit loss of EPHs/ephrins expression of both subgroups A and B [48,49,50,51,52,53,54,55,56,57,58,59,60]. The molecular profile of a tumor could be utilized in order to create a specialized treatment regimen that will benefit each individual patient’s prognosis. Antibodies targeting members of the EPH/ephrin system have already been tested in clinical trials. Anti-EPHA2 monoclonal antibody DS-8895a has been tested on esophageal and gastric cancer patients and EPHA3 antibody IIIA4 (Ifabotuzumab/KB004) on GBM cases and patients with hematologic malignancies. As far as antibody-drug conjugates are concerned, an anti-EPHA2 1C1 antibody was evaluated as an auristatin conjugate, MEDI-547, in patients with solid tumors. Furthermore, anti-ephrin-A4-Calicheamicin (PF-06647263) has been tested on 60 patients, the majority of whom were diagnosed with ovarian or breast cancer [95]. Research has also been conducted towards the creation of a protein compound with the ability to block EPHB4-ephrin-B2 interaction. The final product, sEphB4-HAS, underwent clinical trial that included 70 patients with various malignancies. Four patients exhibited a response to some extent (1 of 3 Kaposi Sarcoma patients, 2 out of 17 head and neck cancer patients and 1 out of 8 hepatocellular cancer patients), half of the patients had stable disease, and the rest demonstrated disease progression [96,97]. The therapeutic agents targeting members of the EPH/ephrin system that have currently undergone clinical testing are summarized on Table 7.
Table 7.
Agents (bold) targeting EPHs/ephrins tested in clinical trials.
| Therapeutic Agent | Target | Category | Malignancy | Reference |
|---|---|---|---|---|
| DS-8895a | EPHA2 | Antibody, antagonist | Esophageal and gastric cancer | [95] |
| Ifabotuzumab (KB004) | EPHA3 | Antibody, antagonist | Hematologic malignancies | [95] |
| MEDI-547 | EPHA2 | Antibody-drug conjugate, agonist | Solid tumors (various) | [95] |
| PF-06647263 | ephrin-A4 | Antibody-drug conjugate, antagonist | Ovarian and breast cancer (majority) | [95] |
| sEphB4-HSA | ephrin-B2 | Protein complex, antagonist | Solid and hematologiccancers | [96,97] |
The role of the microenvironment in tumorigenesis on the organ of origin, as well as the spreading of tumor to distant sites, is often overlooked. EPHs/ephrins are among the molecules implicated in interactions between malignant cells and stromal cells that enhance cancer pathogenesis. It is speculated that EPH/ephrin expression on stromal cells promotes invasion of tumor cells in normal surrounding tissues and also provides a positive feedback for malignant cells’ proliferation. EPHA1, EPHA2, EPHA3, EPHA5, EPHB2 and EPHB4 expression has been reported as 2-fold higher in stromal cells isolated from gastric cancer tissues compared with normal gastric tissue stromal cells [44]. Further research on the field of EPH/ephrin stromal cells’ molecular profile is called for, as interrupting those mechanisms of communication between tumor cells and cellular components of surrounding connective tissues could represent possible treatment strategies.
In practice, researchers have established that the varying expression patterns of the EPH/ephrin system heavily impact a multitude of clinicopathological parameters. Large studies that enrolled hundreds of patients revealed the correlation between EPHs/ephrins gene or protein expression and disease stage, histological grade and, most importantly, OS and DFS. In vitro studies reported change in cells’ biological behavior when certain EPH/ephrin genes where either upregulated or silenced. However, many questions remain unanswered regarding the role of the EPH/ephrin family in the molecular pathways of neoplasia. The upregulation of a certain EPH can enhance carcinogenesis in one organ, while suppressing it in a different one [56,57,58,67]. Furthermore, some studies yielded contradictory results regarding the role of EPHs/ephrins in solid tumors. While EPHA1 and EPHA2 showed significantly increased expression in CRC specimens [46,47,48], they were also associated with lower stage tumors [46,47,48], and, in the case of EPHA1, with longer patient OS [48]. Moreover, different groups of researchers reported contradicting results on the effect of a distinct EPH/ephrin in tumorigenesis of an organ, as is the case of EPHA1 in ovarian tumors [75,76]. Such discrepancies underline the need of further research on the subject.
In conclusion, the EPH/ephrin system represents a large family of biomolecules with great possible applications in the fields of diagnosis, prognosis, disease monitoring, and treatment of neoplasia, with an established important clinical impact.
Abbreviations
| EPH | erythropoietin-producing human hepatocellular receptors |
| Ephrin | EPH family receptor interacting proteins |
| RTK | receptor tyrosine kinases |
| IHC | immunohistochemistry |
| TMA | tissue microarrays |
| PCR | polymerase chain reaction |
| RT-PCR | reverse transcription-polymerase chain reaction |
| q PCR | quantitative reverse transcription- polymerase chain reaction |
| LN | lymph nodes |
| OS | overall survival |
| DFS | disease-free survival |
| MVD | microvessel density |
| GBM | glioblastoma |
| ACC | adenoid cystic carcinoma |
| EMT | epithelial-mesenchymal transition |
| NSCLC | non-small-cell lung carcinoma |
| EGFR | epidermal growth factor receptor |
| CRC | colorectal carcinoma |
| HCC | hepatocellular carcinoma |
| PDAC | pancreatic ductal adenocarcinoma |
| CCRCC | clear cell renal cell carcinoma |
| TCC | transitional cell carcinoma |
| PCa | prostate carcinoma |
| BPH | benign prostate hyperplasia |
| EOC | epithelial ovarian carcinoma |
| SCC | squamous cell carcinoma |
| ERM | embryonal rhabdomyosarcoma |
Author Contributions
Conceptualization: A.P., E.D. and S.T.; Writing–original draft preparation: A.P.; writing, review and editing: A.P., E.D., D.G., A.G.S. and A.S.; visualization: A.P.; supervision: S.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Statistics—National Cancer Institute. [(accessed on 13 June 2021)]; Available online: https://www.cancer.gov/about-cancer/understanding/statistics.
- 3.Rudno-Rudzińska J., Kielan W., Frejlich E., Kotulski K., Hap W., Kurnol K., Dzierżek P., Zawadzki M., Hałoń A. A review on Eph/ephrin, angiogenesis and lymphangiogenesis in gastric, colorectal and pancreatic cancers. Chin. J. Cancer Res. 2017;29:303–312. doi: 10.21147/j.issn.1000-9604.2017.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones D. Parallels of Resistance between Angiogenesis and Lymphangiogenesis Inhibition in Cancer Therapy. Cells. 2020;9:762. doi: 10.3390/cells9030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kou C.-T.J., Kandpal R.P. Differential Expression Patterns of Eph Receptors and Ephrin Ligands in Human Cancers. BioMed Res. Int. 2018;2018:7390104. doi: 10.1155/2018/7390104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Wang L., Gu J.-W., Li B., Liu W.-P., Wang Y.-G., Zhang X., Zhen H.-N., Fei Z. Up-regulation of EphA2 and down-regulation of EphrinA1 are associated with the aggressive phenotype and poor prognosis of malignant glioma. Tumor Biol. 2010;31:477–488. doi: 10.1007/s13277-010-0060-6. [DOI] [PubMed] [Google Scholar]
- 7.Liu F., Park P.J., Lai W., Maher E., Chakravarti A., Durso L., Jiang X., Yu Y., Brosius A., Thomas M., et al. A Genome-Wide Screen Reveals Functional Gene Clusters in the Cancer Genome and Identifies EphA2 as a Mitogen in Glioblastoma. Cancer Res. 2006;66:10815–10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 8.Suo F., Zhong B., Lu F., Dong Z. The combined use of EphA2/MMP-2 expression and MRI findings contributes to the determination of cerebral glioma grade. Oncol. Lett. 2019;18:5607–5613. doi: 10.3892/ol.2019.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royet A., Broutier L., Coissieux M.-M., Malleval C., Gadot N., Maillet D., Gratadou-Hupon L., Bernet A., Nony P., Treilleux I., et al. Ephrin-B3 supports glioblastoma growth by inhibiting apoptosis induced by the dependence receptor EphA4. Oncotarget. 2017;8:23750–23759. doi: 10.18632/oncotarget.16077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L.-F., Fokas E., Juricko J., You A., Rose F., Pagenstecher A., Engenhart-Cabillic R., Janko J. Increased expression of EphA7 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. BMC Cancer. 2008;8:79. doi: 10.1186/1471-2407-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng L., Nakada M., Furuyama N., Sabit H., Furuta T., Hayashi Y., Takino T., Dong Y., Sato H., Sai Y., et al. Ligand-dependent EphB1 signaling suppresses glioma invasion and correlates with patient survival. Neuro Oncol. 2013;15:1710–1720. doi: 10.1093/neuonc/not128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu Y., He S., Fu J., Li G., Xu R., Lu H., Deng J. Expression of EphrinB2 and EphB4 in glioma tissues correlated to the progression of glioma and the prognosis of glioblastoma patients. Clin. Transl. Oncol. 2012;14:214–220. doi: 10.1007/s12094-012-0786-2. [DOI] [PubMed] [Google Scholar]
- 13.Shao Z., Zhu F., Song K., Zhang H., Liu K., Shang Z. EphA2/EphrinA1 mRNA Expression and Protein Production in Adenoid Cystic Carcinoma of Salivary Gland. J. Oral Maxillofac. Surg. 2013;71:869–878. doi: 10.1016/j.joms.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Fukai J., Fujita K., Yamoto T., Sasaki T., Uematsu Y., Nakao N. Intracranial extension of adenoid cystic carcinoma: Potential involvement of EphA2 expression and epithelial-mesenchymal transition in tumor metastasis: A case report. BMC Res. Notes. 2014;7:131. doi: 10.1186/1756-0500-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karidis N.P., Giaginis C., Tsourouflis G., Alexandrou P., Delladetsima I., Theocharis S. Eph-A2 and Eph-A4 expression in human benign and malignant thyroid lesions: An immunohistochemical study. Med. Sci. Monit. 2011;17:BR257–BR265. doi: 10.12659/MSM.881929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin C., Cau W., Zhang Y., Mghanga F.P., Lan X., Gao Z., An R. Correlation of Clinicopathological Features and Expression of Molecular Markers With Prognosis After 131I Treatment of Differentiated Thyroid Carcinoma. Clin. Nucl. Med. 2012;37:e40–e46. doi: 10.1097/RLU.0b013e31823905e4. [DOI] [PubMed] [Google Scholar]
- 17.Giaginis C., Alexandrou P., Poulaki E., Delladetsima I., Troungos C., Patsouris E., Theocharis S. Clinical Significance of EphB4 and EphB6 Expression in Human Malignant and Benign Thyroid Lesions. Pathol. Oncol. Res. 2015;22:269–275. doi: 10.1007/s12253-014-9879-2. [DOI] [PubMed] [Google Scholar]
- 18.Sharma G.K., Dhillon V.K., Masood R., Maceri D.R. Overexpression of EphB4, EphrinB2, and epidermal growth factor receptor in papillary thyroid carcinoma: A pilot study. Head Neck. 2014;37:964–969. doi: 10.1002/hed.23694. [DOI] [PubMed] [Google Scholar]
- 19.Kebebew E., Peng M., Reiff E., Duh Q.-Y., Clark O.H., McMillan A. Diagnostic and prognostic value of angiogenesis-modulating genes in malignant thyroid neoplasms. Surgery. 2005;138:1102–1110. doi: 10.1016/j.surg.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Giaginis C., Tsoukalas N., Bournakis E., Alexandrou P., Kavantzas N., Patsouris E., Theocharis S. Ephrin (Eph) receptor A1, A4, A5 and A7 expression in human non-small cell lung carcinoma: Associations with clinicopathological parameters, tumor proliferative capacity and patients’ survival. BMC Clin. Pathol. 2014;14:8. doi: 10.1186/1472-6890-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efazat G., Novak M., Kaminskyy V.O., De Petris L., Kanter L., Juntti T., Bergman P., Zhivotovsky B., Lewensohn R., Hååg P., et al. Ephrin B3 interacts with multiple EphA receptors and drives migration and invasion in non-small cell lung cancer. Oncotarget. 2016;7:60332–60347. doi: 10.18632/oncotarget.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa M., Miyahara R., Sonobe M., Horiuchi M., Mennju T., Nakayama E., Kobayashi M., Kikuchi R., Kitamura J., Imamura N., et al. Higher expression of EphA2 and ephrin-A1 is related to favorable clinicopathological features in pathological stage I non-small cell lung carcinoma. Lung Cancer. 2012;76:431–438. doi: 10.1016/j.lungcan.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Wang L., Peng Q., Sai B., Zheng L., Xu J., Yin N., Feng X., Xiang J. Ligand-independent EphB1 signaling mediates TGF-β-activated CDH2 and promotes lung cancer cell invasion and migration. J. Cancer. 2020;11:4123–4131. doi: 10.7150/jca.44576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao C., Wang A., Lu F., Chen H., Fu P., Zhao X., Chen H. Overexpression of junctional adhesion molecule-A and EphB2 predicts poor survival in lung adenocarcinoma patients. Tumor Biol. 2017;39:1010428317691000. doi: 10.1177/1010428317691000. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Sun Y., Wang X., Wang J., Zhu Y. The expressions of EphB4 and ephrinB2 in lung adenocarcinomas: A high level of the EphB4 protein is associated with lymph node metastasis. Int. J. Clin. Exp. Pathol. 2019;12:3447–3452. [PMC free article] [PubMed] [Google Scholar]
- 26.Rud A.K., Boye K., Øijordsbakken M., Lund-Iversen M., Halvorsen A.R., Solberg S.K., Berge G., Helland A., Brustugun O.T., Mælandsmo G.M. Osteopontin is a prognostic biomarker in non-small cell lung cancer. BMC Cancer. 2013;13:540. doi: 10.1186/1471-2407-13-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husa A.-M., Magić Z., Larsson M., Fornander T., Pérez-Tenorio G. EPH/ephrin profile and EPHB2 expression predicts patient survival in breast cancer. Oncotarget. 2016;7:21362–21380. doi: 10.18632/oncotarget.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brantley-Sieders D.M., Jiang A., Sarma K., Badu-Nkansah A., Walter D.L., Shyr Y., Chen J. Eph/Ephrin Profiling in Human Breast Cancer Reveals Significant Associations between Expression Level and Clinical Outcome. PLoS ONE. 2011;6:e24426. doi: 10.1371/journal.pone.0024426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hachim I.Y., Villatoro M., Canaff L., Hachim M., Boudreault J., Haiub H., Ali S., Lebrun J.-J. Transforming Growth Factor-beta Regulation of Ephrin Type-A Receptor 4 Signaling in Breast Cancer Cellular Migration. Sci. Rep. 2017;7:14976. doi: 10.1038/s41598-017-14549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mu X., Huang O., Jiang M., Xie Z., Chen D., Zhang X. Prognostic value of ephrin B receptors in breast cancer: An online survival analysis using the microarray data of 3554 patients. Oncol. Lett. 2019;18:742–750. doi: 10.3892/ol.2019.10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Q., Suo Z., Risberg B., Karlsson M.G., Villman K., Nesland J.M. Expression of Ephb2 And Ephb4 in Breast Carci-noma. Pathol. Oncol. Res. 2004;10:26–33. doi: 10.1007/BF02893405. [DOI] [PubMed] [Google Scholar]
- 32.Magic Z., Perez-Tenorio G. Ephrin-B2 inhibits cell proliferation and motility in vitro and predicts longer metastasis-free survival in breast cancer. Int. J. Oncol. 2019;55:1275–1286. doi: 10.3892/ijo.2019.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández-Nogueira P., Bragado P., Almendro V., Ametller E., Rios J., Choudhury S., Mancino M., Gascón P. Differential expression of neurogenes among breast cancer subtypes identifies high risk pa-tients. [(accessed on 2 August 2021)];Oncotarget. 2015 7:5313–5326. doi: 10.18632/oncotarget.6543. Available online: www.impactjournals.com/oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin H., Lu C., Tang Y., Wang H., Wang H., Wang J. Enhanced expression of EphrinB1 is associated with lymph node metastasis and poor prognosis in breast cancer. Cancer Biomark. 2013;13:261–267. doi: 10.3233/CBM-130356. [DOI] [PubMed] [Google Scholar]
- 35.Hafner C., Becker B., Landthaler M., Vogt T. Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod. Pathol. 2006;19:1369–1377. doi: 10.1038/modpathol.3800660. [DOI] [PubMed] [Google Scholar]
- 36.Straume O., Akslen L.A. Importance of Vascular Phenotype by Basic Fibroblast Growth Factor, and Influence of the Angiogenic Factors Basic Fibroblast Growth Factor/Fibroblast Growth Factor Receptor-1 and Ephrin-A1/EphA2 on Mela-noma Progression. Am. J. Pathol. 2002;160:1009–1019. doi: 10.1016/S0002-9440(10)64922-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schauer M.C., Stoecklein N.H., Theisen J., Kröpil F., Baldus S., Hoelscher A., Feith M., Bölke E., Matuschek C., Budach W., et al. The simultaneous expression of both ephrin B3 receptor and E-cadherin in Barrett‘s adenocarcinoma is associated with favorable clinical staging. Eur. J. Med. Res. 2012;17:10. doi: 10.1186/2047-783X-17-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liersch-Löhn B., Slavova N., Buhr H.J., Bennani-Baiti I.M. Differential protein expression and oncogenic gene network link tyrosine kinase ephrin B4 receptor to aggressive gastric and gastroesophageal junction cancers. Int. J. Cancer. 2015;138:1220–1231. doi: 10.1002/ijc.29865. [DOI] [PubMed] [Google Scholar]
- 39.Yuan W.-J., Ge J., Chen Z.-K., Wu S.-B., Shen H., Yang P., Hu B., Zhang G.-W., Chen Z.-H. Over-Expression of EphA2 and EphrinA-1 in Human Gastric Adenocarcinoma and Its Prognostic Value for Postoperative Patients. Dig. Dis. Sci. 2008;54:2410–2417. doi: 10.1007/s10620-008-0649-4. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura R., Kataoka H., Sato N., Kanamori M., Ihara M., Igarashi H., Ravshanov S., Wang Y.-J., Li Z.-Y., Shimamura T., et al. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42–47. doi: 10.1111/j.1349-7006.2005.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong H.N., Won Y.J., Shim J.H., Kim H.J., Han S.H., Kim B.S., Kim H.S. Cancer-associated fibroblasts promote gastric tumorigenesis through EphA2 activation in a ligand-independent manner. J. Cancer Res. Clin. Oncol. 2018;144:1649–1663. doi: 10.1007/s00432-018-2683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oki M., Yamamoto H., Taniguchi H., Adachi Y., Imai K., Shinomura Y. Overexpression of the receptor tyrosine kinase EphA4 in human gastric cancers. World J. Gastroenterol. 2008;14:5650–5656. doi: 10.3748/wjg.14.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Zhou N., Li P., Wu H., Wang Q., Gao X., Wang X., Huang J. EphA8 acts as an oncogene and contributes to poor prognosis in gastric cancer via regulation of ADAM10. J. Cell. Physiol. 2019;234:20408–20419. doi: 10.1002/jcp.28642. [DOI] [PubMed] [Google Scholar]
- 44.Kikuchi S., Kaibe N., Morimoto K., Fukui H., Niwa H., Maeyama Y., Takemura M., Matsumoto M., Nakamori S., Miwa H., et al. Overexpression of Ephrin A2 receptors in cancer stromal cells is a prognostic factor for the relapse of gastric cancer. Gastric Cancer. 2014;18:485–494. doi: 10.1007/s10120-014-0390-y. [DOI] [PubMed] [Google Scholar]
- 45.Kataoka H., Tanaka M., Kanamori M., Yoshii S., Ihara M., Wang Y.-J., Song J.-P., Li Z.-Y., Arai H., Otsuki Y., et al. Expression profile of EFNB1, EFNB2, two ligands of EPHB2 in human gastric cancer. J. Cancer Res. Clin. Oncol. 2002;128:343–348. doi: 10.1007/s00432-002-0355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J., Bin Xu B., Xu G., Zhang X., Yang X., Wang J. Reduced EphB6 protein in gastric carcinoma and associated lymph nodes suggests EphB6 as a gastric tumor and metastasis inhibitor. Cancer Biomark. 2017;19:241–248. doi: 10.3233/CBM-160256. [DOI] [PubMed] [Google Scholar]
- 47.Rawla P., Sunkara T., Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herath N.I., Spanevello M.D., Doecke J.D., Smith F.M., Pouponnot C., Boyd A.W. Complex expression patterns of Eph receptor tyrosine kinases and their ephrin ligands in colorectal carcinogenesis. Eur. J. Cancer. 2012;48:753–762. doi: 10.1016/j.ejca.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Herath N.I., Doecke J., Spanevello M.D., Leggett B., Boyd A.W. Epigenetic silencing of EphA1 expression in colorectal cancer is correlated with poor survival. Br. J. Cancer. 2009;100:1095–1102. doi: 10.1038/sj.bjc.6604970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kataoka H., Igarashi H., Kanamori M., Ihara M., Wang J.-D., Wang Y.-J., Li Z.-Y., Shimamura T., Kobayashi T., Maruyama K., et al. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136–141. doi: 10.1111/j.1349-7006.2004.tb03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M., Yang C., Liu X., Yuan L., Zhang F., Wang M., Miao D., Gu X., Jiang S., Cui B., et al. EphA3 promotes malignant transformation of colorectal epithelial cells by upregulating oncogenic pathways. Cancer Lett. 2016;383:195–203. doi: 10.1016/j.canlet.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Wang T.-H., Chang J.-L., Ho J.-Y., Wu H.-C., Chen T.-C. EphrinA5 suppresses colon cancer development by negatively regulating epidermal growth factor receptor stability. FEBS J. 2011;279:251–263. doi: 10.1111/j.1742-4658.2011.08419.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu W., Ahmad S.A., Jung Y.D., Reinmuth N., Fan B.S.F., Bucana C.D., Ellis L.M. Coexpression of ephrin-Bs and their receptors in colon carcinoma. Cancer. 2002;94:934–939. doi: 10.1002/cncr.10122. [DOI] [PubMed] [Google Scholar]
- 54.Guo D.L., Tsui W.Y., Chan A.S., Ho C., Leung S.Y., Zhang J., Yuen S.T., Ji J., Chen X. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours. Carcinogenesis. 2005;27:454–464. doi: 10.1093/carcin/bgi259. [DOI] [PubMed] [Google Scholar]
- 55.Drucker A., Arnason T., Sen R.Y., Aljawad M., Thompson K., Huang W.-Y. Ephrin B2 Receptor and Microsatellite Status in Lymph Node-Positive Colon Cancer Survival. Transl. Oncol. 2013;6:520–527. doi: 10.1593/tlo.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jubb A.M., Zhong F., Bheddah S., Grabsch H.I., Frantz G.D., Mueller W., Kavi V., Quirke P., Polakis P., Koeppen H. EphB2 is a Prognostic Factor in Colorectal Cancer. Clin. Cancer Res. 2005;11:5181–5187. doi: 10.1158/1078-0432.CCR-05-0143. [DOI] [PubMed] [Google Scholar]
- 57.Lugli A., Spichtin H., Maurer R., Mirlacher M., Kiefer J., Huusko P., Azorsa D., Terracciano L., Sauter G., Kallioniemi O., et al. EphB2 Expression across 138 Human Tumor Types in a Tissue Microarray: High Levels of Expression in Gastrointestinal Cancers. Clin. Cancer Res. 2005;11:6450–6458. doi: 10.1158/1078-0432.CCR-04-2458. [DOI] [PubMed] [Google Scholar]
- 58.Batlle E., Bacani J., Begthel H., Jonkheer S., Gregorieff A., Van De Born M., Malats N., Sancho E., Boon E., Pawson T., et al. EphB receptor activity suppresses colorectal cancer progression. Nat. Cell Biol. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 59.Jang B.G., Kim H.S., Bae J.M., Kim W.H., Hyun C.L., Kang G.H. Expression Profile and Prognostic Significance of EPHB3 in Colorectal Cancer. Biomolecules. 2020;10:602. doi: 10.3390/biom10040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lv J., Xia Q., Wang J., Shen Q., Zhang J., Zhou X. EphB4 promotes the proliferation, invasion, and angiogenesis of human colorectal cancer. Exp. Mol. Pathol. 2016;100:402–408. doi: 10.1016/j.yexmp.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Iida H., Honda M., Kawai H.F., Yamashita T., Shirota Y., Wang B.-C., Miao H., Kaneko S. Ephrin-A1 expression contributes to the malignant characteristics of -fetoprotein producing hepatocellular carcinoma. Gut. 2005;54:843–851. doi: 10.1136/gut.2004.049486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui X.-D., Lee M.-J., Yu G.-R., Kim I.-H., Yu H.-C., Song E.-Y., Kim D.-G. EFNA1 ligand and its receptor EphA2: Potential biomarkers for hepatocellular carcinoma. Int. J. Cancer. 2009;126:940–949. doi: 10.1002/ijc.24798. [DOI] [PubMed] [Google Scholar]
- 63.Wada H., Yamamoto H., Kim C., Uemura M., Akita H., Tomimaru Y., Hama N., Kawamoto K., Kobayashi S., Eguchi H., et al. Association between ephrin-A1 mRNA expression and poor prognosis after hepatectomy to treat hepatocellular carcinoma. Int. J. Oncol. 2014;45:1051–1058. doi: 10.3892/ijo.2014.2519. [DOI] [PubMed] [Google Scholar]
- 64.Wang X., Zhang M., Ping F., Liu H., Sun J., Wang Y., Shen A., Ding J., Geng M. Identification and Therapeutic Intervention of Coactivated Anaplastic Lymphoma Kinase, Fibroblast Growth Factor Receptor 2, and Ephrin Type-A Receptor 5 Kinases in Hepatocellular Carcinoma. Hepatology. 2019;69:573–586. doi: 10.1002/hep.29792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang T.-H., Ng K.-F., Yeh T.-S., Wang Y.-L., Liang K.-H., Yeh C.-T., Chen T.-C. Peritumoral Small EphrinA5 Isoform Level Predicts the Postoperative Survival in Hepatocellular Carcinoma. PLoS ONE. 2012;7:e41749. doi: 10.1371/journal.pone.0041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheng Y., Wei J., Zhang Y., Gao X., Wang Z., Yang J., Yan S., Zhu Y., Zhang Z., Xu D., et al. Mutated EPHA2 is a target for combating lymphatic metastasis in intrahepatic cholangiocarcinoma. Int. J. Cancer. 2018;144:2440–2452. doi: 10.1002/ijc.31979. [DOI] [PubMed] [Google Scholar]
- 67.Khansaard W., Techasen A., Namwat N., Yongvanit P., Khuntikeo N., Puapairoj A., Loilome W. Increased EphB2 expression predicts cholangiocarcinoma metastasis. Tumor Biol. 2014;35:10031–10041. doi: 10.1007/s13277-014-2295-0. [DOI] [PubMed] [Google Scholar]
- 68.Zhu F., Dai S.-N., Xu D.-L., Hou C.-Q., Liu T.-T., Chen Q.-Y., Wu J.-L., Miao Y. EFNB2 facilitates cell proliferation, migration, and invasion in pancreatic ductal adenocarcinoma via the p53/p21 pathway and EMT. Biomed. Pharmacother. 2020;125:109972. doi: 10.1016/j.biopha.2020.109972. [DOI] [PubMed] [Google Scholar]
- 69.Toma M.I., Erdmann K., Diezel M., Meinhardt M., Zastrow S., Fuessel S., Wirth M.P., Baretton G.B. Lack of Ephrin Receptor A1 Is a Favorable Independent Prognostic Factor in Clear Cell Renal Cell Carcinoma. PLoS ONE. 2014;9:e102262. doi: 10.1371/journal.pone.0102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X., Xu H., Cao G., Wu Z., Wang J. Loss of EphA3 Protein Expression Is Associated With Advanced TNM Stage in Clear-Cell Renal Cell Carcinoma. Clin. Genitourin. Cancer. 2017;15:e169–e173. doi: 10.1016/j.clgc.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 71.Özgür E., Heidenreich A., Dagtekin O., Engelmann U., Bloch W. Distribution of EphB4 and EphrinB2 in normal and malignant urogenital tissue. Urol. Oncol. Semin. Orig. Investig. 2011;29:78–84. doi: 10.1016/j.urolonc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 72.Abraham S., Knapp D.W., Cheng L., Snyder P.W., Mittal S.K., Bangari D.S., Kinch M., Wu L., Dhariwal J., Mohammed S.I. Expression of EphA2 and Ephrin A-1 in Carcinoma of the Urinary Bladder. Clin. Cancer Res. 2006;12:353–360. doi: 10.1158/1078-0432.CCR-05-1505. [DOI] [PubMed] [Google Scholar]
- 73.Li X., Choi W.W., Yan R., Yu H., Krasnoperov V., Kumar S.R., Schuckman A., Klumpp D.J., Pan C.-X., Quinn D., et al. The Differential Expression of EphB2 and EphB4 Receptor Kinases in Normal Bladder and in Transitional Cell Carcinoma of the Bladder. PLoS ONE. 2014;9:e105326. doi: 10.1371/journal.pone.0105326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oweida A., Bhatia S., Hirsch K., Calame D., Griego A., Keysar S., Pitts T., Sharma J., Eckhardt G., Jimeno A., et al. Ephrin-B2 overexpression predicts for poor prognosis and response to therapy in solid tumors. Mol. Carcinog. 2016;56:1189–1196. doi: 10.1002/mc.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li S., Zhu Y., Ma C., Qiu Z., Zhang X., Kang Z., Wu Z., Wang H., Xu X., Zhang H., et al. Downregulation of EphA5 by promoter methylation in human prostate cancer. BMC Cancer. 2015;15:18. doi: 10.1186/s12885-015-1025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li S., Ma Y., Xie C., Wu Z., Kang Z., Fang Z., Su B., Guan M. EphA6 promotes angiogenesis and prostate cancer metastasis and is associated with human prostate cancer pro-gression. [(accessed on 2 August 2021)];Oncotarget. 2015 6:22587–22597. doi: 10.18632/oncotarget.4088. Available online: www.impactjournals.com/oncotarget/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herath N.I., Spanevello M.D., Sabesan S., Newton T., Cummings M., Duffy S., Lincoln D., Boyle G., Parsons P.G., Boyd A.W. Over-expression of Eph and ephrin genes in advanced ovarian cancer: Ephrin gene expression correlates with shortened survival. BMC Cancer. 2006;6:144. doi: 10.1186/1471-2407-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han L., Dong Z., Qiao Y., Kristensen G.B., Holm R., Nesland J.M., Suo Z. The clinical significance of EphA2 and Ephrin A-1 in epithelial ovarian carcinomas. Gynecol. Oncol. 2005;99:278–286. doi: 10.1016/j.ygyno.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 79.Chen X., Wang X., Wei X., Wang J. EphA5 protein, a potential marker for distinguishing histological grade and prognosis in ovarian serous carcinoma. J. Ovarian Res. 2016;9:83. doi: 10.1186/s13048-016-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu X., Xu Y., Jin Q., Wang W., Zhang S., Wang X., Zhang Y., Xu X., Huang J. EphA8 is a prognostic marker for epithelial ovarian cancer. Oncotarget. 2016;7:20801–20809. doi: 10.18632/oncotarget.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao W., Zhang Q., Wang Y. EphB3 protein is associated with histological grade and FIGO stage in ovarian serous carcinomas. APMIS. 2017;125:122–127. doi: 10.1111/apm.12646. [DOI] [PubMed] [Google Scholar]
- 82.Alam S.M., Fujimoto J., Jahan I., Sato E., Tamaya T. Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian cancers. Br. J. Cancer. 2008;98:845–851. doi: 10.1038/sj.bjc.6604216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castellvi J., García-Jiménez A., de la Torre J., Hernandez J., Gil-Moreno A., Xercavins J., Cajal S.R.Y. Ephrin B expression in epithelial ovarian neoplasms correlates with tumor differentiation and angiogenesis. Hum. Pathol. 2006;37:883–889. doi: 10.1016/j.humpath.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 84.Kamat A.A., Coffey D., Merritt W.M., Nugent E., Urbauer D., Lin Y.G., Edwards C., Broaddus R., Coleman R.L., Sood A.K. EphA2 overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer. 2009;115:2684–2692. doi: 10.1002/cncr.24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alam S., Fujimoto J., Jahan I., Sato E., Tamaya T. Overexpression of ephrinB2 and EphB4 in tumor advancement of uterine endometrial cancers. Ann. Oncol. 2006;18:485–490. doi: 10.1093/annonc/mdl414. [DOI] [PubMed] [Google Scholar]
- 86.Dong L.-D., Cheng X.-L., Zhou L., Huang Q., Li J.-C., Yi C.-J. Overexpression of erythropoietin-producing hepatocyte receptor B4 and ephrin-B2 is associated with estrogen receptor expression in endometrial adenocarcinoma. Oncol. Lett. 2017;13:2109–2114. doi: 10.3892/ol.2017.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu D., Suo Z., Kristensen G.B., Li S., Troen G., Holm R., Nesland J.M. Prognostic value of EphA2 and EphrinA-1 in squamous cell cervical carcinoma. Gynecol. Oncol. 2004;94:312–319. doi: 10.1016/j.ygyno.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 88.Holm R., Van de Putte G., Suo Z., Lie A.K., Kristensen G.B. Expressions of EphA2 and EphrinA-1 in early squamous cell cervical carcinomas and their relation to prognosis. Int. J. Med. Sci. 2008;5:121–126. doi: 10.7150/ijms.5.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao Q., Liu W., Cai J., Li M., Gao Y., Lin W., Li Z. EphB2 promotes cervical cancer progression by inducing epithelial-mesenchymal transition. Hum. Pathol. 2014;45:372–381. doi: 10.1016/j.humpath.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Zhang S., Jiang T., Liang M. Expression of Eph B4 and Ephrin B2 in cervical cancer tissues and angiogenesis. Int. J. Gynecol. Obstet. 2007;96:46–47. doi: 10.1016/j.ijgo.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 91.Megiorni F., Gravina G.L., Camero S., Ceccarelli S., Del Fattore A., Desiderio V., Papaccio F., McDowell H.P., Shukla R., Pizzuti A., et al. Pharmacological targeting of the ephrin receptor kinase signalling by GLPG1790 in vitro and in vivo reverts oncophenotype, induces myogenic differentiation and radiosensitizes embryonal rhabdomyosarcoma cells. J. Hematol. Oncol. 2017;10:161. doi: 10.1186/s13045-017-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Randolph M.E., Cleary M.M., Bajwa Z., Svalina M.N., Young M.C., Mansoor A., Kaur P., Bult C.J., Goros M.W., Michalek J.E., et al. EphB4/EphrinB2 therapeutics in Rhabdomyosarcoma. PLoS ONE. 2017;12:e0183161. doi: 10.1371/journal.pone.0183161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang X.X., Zhao H., Robinson M.E., Cohen B., Cnaan A., London W., Cohn S., Cheung N.-K.V., Brodeur G.M., Evans A.E., et al. Implications of EPHB6, EFNB2, and EFNB3 expressions in human neuroblastoma. Proc. Natl. Acad. Sci. USA. 2000;97:10936–10941. doi: 10.1073/pnas.190123297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chetcuti A., Aktas S., Mackie N., Ulger C., Toruner G., Alkan M., Catchpoole D. Expression profiling reveals MSX1 and EphB2 expression correlates with the invasion capacity of Wilms tumors. Pediatr. Blood Cancer. 2011;57:950–957. doi: 10.1002/pbc.23003. [DOI] [PubMed] [Google Scholar]
- 95.Janes P.W., Vail M.E., Gan H.K., Scott A.M. Antibody Targeting of Eph Receptors in Cancer. Pharm. 2020;13:88. doi: 10.3390/ph13050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lodola A., Giorgio C., Incerti M., Zanotti I., Tognolini M. Targeting Eph/ephrin system in cancer therapy. Eur. J. Med. Chem. 2017;142:152–162. doi: 10.1016/j.ejmech.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 97.El-Khoueiry A., Gitlitz B., Cole S., Tsao-Wei D., Goldkorn A., Quinn D., Nieva J., Dorff T., Oswald M., Berg J., et al. A first-in-human phase I study of sEphB4-HSA in patients with advanced solid tumors with expansion at the maximum tolerated dose (MTD) or recommended phase II dose (RP2D) Eur. J. Cancer. 2016;69:S11. doi: 10.1016/S0959-8049(16)32623-5. [DOI] [Google Scholar]