Abstract
Since identified in December 2019, COVID-19 has remained a pandemic across the globe. Although primarily a respiratory illness, the impact of COVID-19 on other end organs has been increasingly identified. The effect of COVID-19 on the liver has yet to be completely understood. We describe a case of COVID-19 leading to end-stage cholangiopathy and deceased donor liver transplantation (LT). A 64-year-old man with no underlying respiratory or liver disease presented with acute respiratory distress secondary to COVID-19 pneumonia requiring intubation. Several months after resolution of his respiratory symptoms, he developed transaminitis, worsening jaundice, abdominal pain and dark-coloured urine. Hepatic function remained severely impaired warranting LT 259 days following his initial COVID-19 diagnosis. Explant pathology demonstrated diffuse hepatic injury, onion skinning of the bile ducts and bile duct loss in scattered portal tracts. As more patients develop COVID-19-related complications, we suggest LT as an option for COVID-19-related end-stage liver disease.
Keywords: transplantation, COVID-19
Background
Post-COVID-19 cholangiopathy is a rare although increasingly described phenomenon. In a retrospective review of over 2000 patients admitted for COVID-19, less than 1% of patients demonstrated laboratory values consistent with cholestatic liver disease.1 Previously, COVID-19 has been associated with elevated liver function tests (LFTs), with the liver being the most commonly affected organ following the lungs. Epidemiological studies have found that elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are seen in 41.1% and 29.1% of patients, respectively.2–4 Still, post-COVID-19 cholangiopathy and liver failure are only beginning to be described in the literature, and only four cases of liver transplantation (LT) for post-COVID-19 cholangiopathy have been reported thus far. Of these, three have survived the immediate postoperative period, with the longest follow-up being 7 months.5–9 In this report, we describe a case of sclerosing cholangitis following COVID-19 leading to end-stage liver disease and deceased donor orthotopic LT 259 days after initial COVID-19 diagnosis, with normal allograft function on laboratory values 8 months postoperatively.
Case presentation
A 64-year-old man, body mass index of 29.8 kg/m2, with a history of hypertension on lisinopril, hyperlipidaemia on atorvastatin, insulin-dependent diabetes (HbA1c 7.1%), radical prostatectomy for prostate cancer, and no known liver disease was admitted to hospital with 6 days of progressive shortness of breath, whole body weakness and fevers. He had been diagnosed with COVID-19 2 days prior at a local emergency department, and when represented, was diagnosed with COVID-19-associated pneumonia warranting a hospital admission for inpatient care. He received hydroxychloroquine, azithromycin, tocilizumab and convalescent plasma. He continued to deteriorate and 13 days following his diagnosis, he was intubated and was transferred to intensive care unit (ICU) for acute hypoxic respiratory failure.
During his first week of intubation, he developed acute deep venous thrombi in both lower limbs which were initially treated with anticoagulation. An inferior vena cava (IVC) filter was placed after he developed a large iliopsoas haematoma. In addition, he developed line-related candidemia and received 2 weeks of fluconazole therapy during his ICU admission.
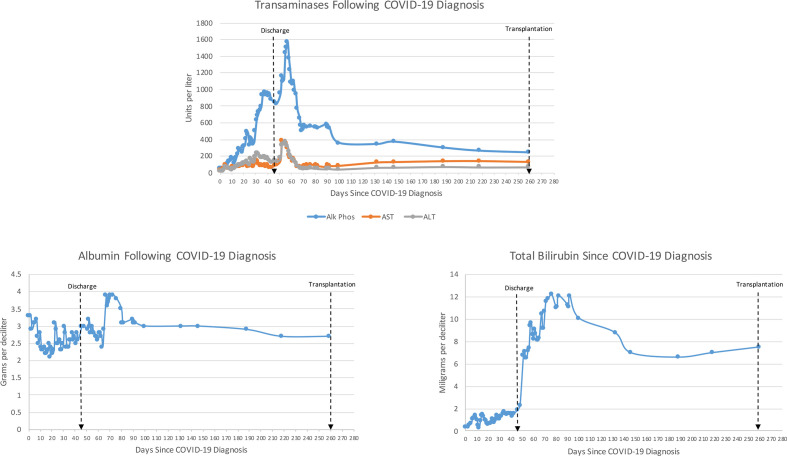
He was intubated for 14 days in total and following extubation, on day 24 following admission, elevated AST, ALT, alkaline phosphatase (ALP) and total bilirubin (TBIL) were noted on routine daily bloodwork. This was originally attributed to his medications, including his hydroxychloroquine, statin, acetaminophen and tocilizumab. All potential hepatotoxic medications were stopped, which resulted in a transient improvement in these labs. As he stabilised from his COVID-19 pneumonia and the line-related fungemia, his AST, ALT and ALP remained high (figure 1). An abdominal ultrasound scan was performed on day 34 following admission, which demonstrated mild gallbladder wall thickening but no evidence of cholelithiasis. Throughout his admission, no jaundice was reported on physical examination and his TBIL remained below 1.9 mg/dL, averaging 1.1 mg/dL. Without any further intervention, his AST, ALT, ALP and TBIL remained high but stable, and he was discharged on day 44 of his admission to an inpatient rehabilitation facility with hospitalist follow-up and plan for weekly blood tests.
Figure 1.
Trends of liver function tests following initial confirmation of COVID-19. Alk Phos, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
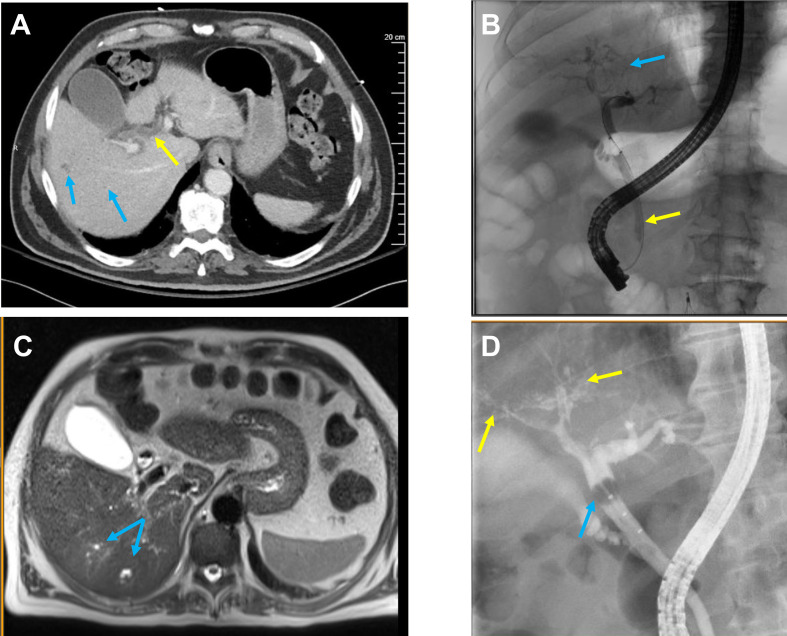
Throughout his inpatient rehabilitation course, his TBIL continued to trend upwards, and within a week of discharge, or day 51 following his diagnosis of COVID-19 pneumonia, his TBIL had risen from 1.6 mg/dL to 6.8 mg/dL. At this time, he saw a gastroenterologist who noted scleral icterus on examination. CT of the chest, abdomen and pelvis demonstrated dilation of the common bile duct and multiple subcentimetre low-density lesions in the right hepatic lobe (figure 2A), in contrast to his last abdominal CT from 2016 which had demonstrated no liver abnormalities. On day 52, he underwent endoscopic retrograde cholangiopancreatography (ERCP) which demonstrated irregular intrahepatic radicals consistent with cholangiopathy (figure 2B). Loose stone material was removed from the common bile duct and an 8.5 Fr biliary stent was inserted into the bile duct. He underwent magnetic resonance cholangiopancreatography (MRCP) on day 57 to further characterise the lesions in his right lobe, which demonstrated mild intrahepatic biliary ductal dilatation and mild patchy T2 hyperintensity within the right hemiliver, concerning for cholangitis (figure 2C). He underwent a repeat ERCP on day 150, which demonstrated relative ductopenia and subtle ductal beading consistent with secondary sclerosing cholangitis (figure 2D).
Figure 2.
Radiological imaging. (A) CT of the abdomen and pelvis with intravenous contrast taken day 51 from COVID-19 diagnosis demonstrating multiple subcentimetre low-density lesions in the right hepatic lobe (blue arrows) and dilatation of the common bile duct (yellow arrow). (B) Endoscopic retrograde cholangiopancreatography (ERCP) with intrahepatic radicals (blue arrow) and stent insertion (yellow arrow). (C) MRI of the abdomen day 164 from admission showing mild intrahepatic biliary ductal dilatation and mild patchy T2 hyperintensity in the right liver (blue arrow), possibly concerning for primary sclerosing cholangitis. Additionally, diffuse biliary hamartomas were visualised. (D) ERCP on day 150 with evidence of relative ductopenia of the right and left ducts with subtle irregularity and beading consistent with secondary sclerosing cholangitis (yellow arrow). A limited cholangiogram done during the procedure demonstrated a filling defect concerning for biliary casts (blue arrow).
Autoimmune and hepatitis panels were negative, including assessment of hepatitis B and C surface antigens and antibodies, antinuclear antibodies, IgG, mitochondrial antibodies and alpha-1 antitrypsin levels. Of note, no potential contributing social factors were identified. He had no history of alcohol use disorder. A diagnosis of secondary sclerosing cholangitis in critically ill patients (SSC-CIP) was made by hepatology and he was considered for LT.
Treatment
After undergoing standard pre-transplant multidisciplinary work-up, the patient was listed for transplant on day 224 following his initial COVID-19 pneumonia diagnosis (102 days following his SSC-CIP diagnosis). During his transplant work-up, pulmonology confirmed no significant long-term pulmonary sequelae from his COVID-19 illness. His immediate preoperative labs included a TBIL of 7.8 mg/dL, international normalised ratio of 1.4, creatinine of 1.1 mg/dL and sodium of 128 mmol/L, with a Model for End Stage Liver Disease score of 19. He underwent LT on day 259 after his initial diagnosis of COVID-19. Intraoperatively, his native liver was noted to be cholestatic and firm in appearance, and total ascites volume was 1 L.
Outcome and follow-up
His postoperative course was overall uncomplicated. He was extubated at the conclusion of the case and his nasogastric tube was removed on postoperative day 1. He was induced with solumedrol and maintained on tacrolimus, mycophenolic acid and prednisone. Liver ultrasound on postoperative day 1 showed patent vessels. His pain was well controlled with oral pain medications and he tolerated a regular diet by postoperative day 3. He was discharged home on postoperative day 4 with an AST of 107, ALT of 215 and TBIL of 3.7 mg/dL. At 8 months postoperatively, he reported doing well, with no evidence of rejection or infection, no interim hospitalisations, and improved appetite and energy, as well as having returned to work.
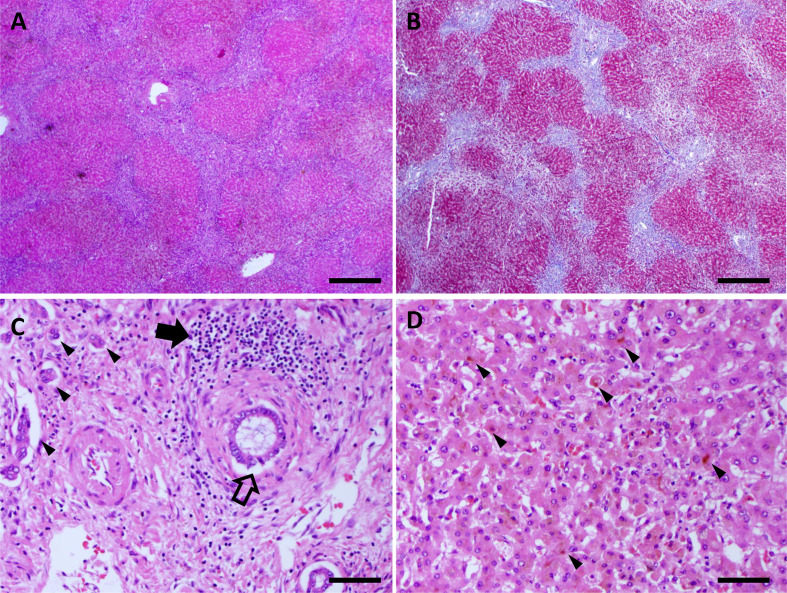
The liver explant was examined by a senior pathologist specialising in liver disease. There was diffuse hepatic injury and bridging fibrosis (figure 3A, B). Bile ducts showed onion skinning with nuclear disarray and cytoplasmic vacuolisation of the epithelium (figure 3C). A lymphoplasmacytic infiltrate was present in, and adjacent to, some bile ducts. Bile duct loss was noted in scattered portal tracts with associated ductular reaction. There was also evidence of intrahepatocellular cholestasis (figure 3D). Iron staining was negative for any abnormal iron deposition and there was no evidence of alpha-1 antitrypsin deficiency on Periodic acid Schiff with diastase staining (data not shown).
Figure 3.
Explant pathology. (A, B) H&E and trichrome stained slides demonstrating bridging fibrosis. Scale bar is 500 µm. (C) H&E stained slide showing portal tract with severe bile duct injury (open arrow), lymphoplasmacytic infiltrate (filled arrow) and ductular reaction (arrowheads). Scale bar is 50 µm. (D) H&E stained slide showing intracellular cholestasis (arrowheads). Scale bar is 50 µm.
Discussion
The current report presents a rare sequela of COVID-19 disease.8 Despite improvement from his COVID-19-associated pneumonia, this case highlights the ability of the virus to incur long-lasting hepatic injury and adds considerably to the literature given the emerging focus on long-term non-respiratory implications.
The question remains whether cholangiopathy following COVID-19 disease can be entirely described by SSC-CIP. The term SSC-CIP has been used to describe a heterogeneous group of liver disease seen in ICU patients without a history of hepatic impairment. Although the aetiologies of these patients’ ICU admissions may vary, their liver disease often presents with cholestasis associated with increases in TBIL, ALP and gamma-glutamyl transpeptidases on blood analysis.10 Biliary casts followed by diffuse intrahepatic duct strictures are often seen on MRCP and diffuse irregular strictures and dilations are noted on ERCP.11 Of these, our patient developed the described characteristic biochemical values and imaging findings on both MRI and ERCP. Symptomatically, patients often do not present until late in their disease course, that is, after their ICU admission, as did our patient. Mortality rates can reach up to 50% during ICU admission with hepatic failure being the most common cause of death. Of affected patients, 20% require LT.12
The literature surrounding post-COVID-19 cholangiopathy remains limited. Thus far, studies examining cholangiopathy pathology have found portal and periportal fibrosis, degenerative cholangiocyte injury, regenerative change, acute and/or chronic large duct obstruction, and epithelial necrosis of the cholangiocytes within the terminal bile ducts and marginal ductules.1 8 13 Although many of these features are shared with SSC-CIP, patients with a history of COVID-19 were noted to have a higher degree of cholangiocyte cytoplasmic vacuolisation and regenerative change.5 In the current case, the explant revealed features of bile duct loss and fibrosis which were compatible with a sclerosing cholangiopathy but cholangiocyte vacuolation was not determined. The challenge remains identifying histiological features unique to COVID-19-related hepatic injury. Postmortem histopathological liver changes from COVID-19-infected patients varied remarkably and involve the entire hepatic microstructure.14–17 Xu et al have reported severe microvesicular steatosis and portal inflammation on histological analysis which was again confirmed by Ji et al in their retrospective analysis of COVID-19 infection and fatty liver disease.16 18 Additional changes described are immune cell infiltration and patchy hepatic necrosis, both non-specific features of inflammatory liver injury. Thus, the elements underlying these phenomena are likely multifactorial.
Although several mechanisms of liver injury have been proposed, these remain speculative. It has been hypothesised that the binding of COVID-19 to ACE-2 receptors results in worsened localised injury, given that these receptors are found both in liver and bile duct cells.1 19 20 One case report has identified COVID-19 RNA in bile, suggesting cholangiocellular infection; however, other biliary studies have not been able to replicate this finding.13 21 The body’s systemic inflammatory response to the virus, as well as virus-induced hypoxia, may additionally contribute to liver injury via diffuse end-organ damage, akin to septic shock. In particular, the cytokine storm associated with COVID-19 may result in hepatocellular cholestasis secondary to tumor necrosis factor (TNF)-alpha, interleukin (IL)-1 and IL-6 downregulation of hepatobiliary uptake and excretion.21 22 IL-6 has also been associated with endotheliopathy which may result in further liver injury.13 Systemic viral infections, such as influenzas, have often resulted in transient transaminitis reflecting physiological immune activation without compromising hepatic function, a phenomenon known as ‘bystander hepatitis’.23 24 Another possibility is that medications administered to treat the virus, including acetaminophen and antivirals, may contribute to hepatotoxicity.25–27 Of note, in our patient, his disease progressed in spite of early termination of hepatotoxic medications. It is likely that specific molecular mechanisms of COVID-19-related injury will become more apparent as the body of knowledge surrounding the disease continues to grow.
The aetiology of our patient’s liver disease and favourable postoperative course presents post-COVID-19 cholangiopathy as a suitable indication for transplantation. This complication remains a rare complication and even fewer cases of transplantation after COVID-19 infection have been reported.28 As more patients recover from COVID-19, it is possible that they will have other viral sequelae, or sequelae from medications administered during treatment, requiring further work-up as described here. It has been widely reported that patients with underlying liver disease represent a higher risk of severe COVID-19 illness.29 Moreover, non-alcoholic fatty liver disease, obesity and increased age represent consistent risk factors for COVID-19 progression.18 23 With larger case series, factors predictive of long-term hepatic injury and failure may also come to light and clinical risk scoring systems using biophysical profiles and LFTs could potentially identify patients at risk of progressing to LT.
Learning points.
The true morbidity of COVID-19 remains uncertain. Long-term implications of the virus remain unknown in the setting of rapidly evolving disease presentations and possible emergence of new variants.
The most severe disease remains evident in patients with chronic underlying conditions and monitoring long-term effects of the virus merits focus not only to identify but also predict the occurrence of novel COVID-19-related entities.
Although liver complications have been reported, the overall consequences on liver health may have been underestimated, as this case demonstrates COVID-19-associated cholangiopathy warranting liver transplantation months after the initial respiratory insult.
As the pandemic continues, we welcome work towards categorising the longstanding and potentially irreversible outcomes of COVID-19 and research in understanding the hepatic microenvironment during infection.
Footnotes
Contributors: AL conducted data collection and wrote the manuscript. AW contributed pathology analysis and write-up. MBMD and WC led case report organisation, analysis and editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Faruqui S, Okoli FC, Olsen SK, et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol 2021;116:1414–25. 10.14309/ajg.0000000000001264 [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Jaiswal P, Kerakhan Y, et al. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol 2021;21:100273. 10.1016/j.aohep.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Fan J-G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol 2020;8:1–5. 10.14218/JCTH.2020.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portincasa P, Krawczyk M, Machill A. Hepatic consequences of COVID-19 infection. Lapping or biting? (1879-0828 (electronic)). Eur J Intern Med 2020:18–24. 10.1016/j.ejim.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth NC, Kim A, Vitkovski T, et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol 2021;116:1077–82. 10.14309/ajg.0000000000001154 [DOI] [PubMed] [Google Scholar]
- 6.Bangaru S. COVID-19 infection causing liver infarction, severe cholangiopathy, and biliary cast syndrome. Gastroenterology 2020;I:17–19. 10.33169/gastro.GOAOJ-I-104 [DOI] [Google Scholar]
- 7.Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep 2020;13:e237984. 10.1136/bcr-2020-237984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durazo FA, Nicholas AA, Mahaffey JJ, et al. Post-Covid-19 Cholangiopathy-A new indication for liver transplantation: a case report. Transplant Proc 2021;53:1132–7. 10.1016/j.transproceed.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meersseman P, Blondeel J, De Vlieger G, et al. Secondary sclerosing cholangitis: an emerging complication in critically ill COVID-19 patients. Intensive Care Med 2021. 10.1007/s00134-021-06445-8. [Epub ahead of print: 29 Jun 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruemmele P, Hofstaedter F, Gelbmann CM, Fau HF. Secondary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol 2009;6:287–95. 10.1038/nrgastro.2009.46 [DOI] [PubMed] [Google Scholar]
- 11.Gelbmann CM, Rümmele P, Wimmer M, Fau RP, Fau WM, et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol 2007;102:1221–9. 10.1111/j.1572-0241.2007.01118.x [DOI] [PubMed] [Google Scholar]
- 12.Martins P, Verdelho Machado M. Secondary sclerosing cholangitis in critically ill patients: an underdiagnosed entity. GE Port J Gastroenterol 2020;27:103–14. 10.1159/000501405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klindt C, Jensen B-E, Brandenburger T, et al. Secondary sclerosing cholangitis as a complication of severe COVID-19: a case report and review of the literature. Clin Case Rep 2021;9:e04068. 10.1002/ccr3.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portincasa P, Krawczyk M, Machill A, et al. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med 2020;77:18–24. 10.1016/j.ejim.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Wang RS, Qu GQ, et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi 2020;36:21–3. 10.12116/j.issn.1004-5619.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol 2020;33:1007–14. 10.1038/s41379-020-0536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji D, Qin E, Xu J, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol 2020;73:451–3. 10.1016/j.jhep.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020;159:768–71. 10.1053/j.gastro.2020.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Liu J, Lu M. Liver injury during highly pathogenic human coronavirus infections. (1478-3231 (electronic)). Liver Int.;40:998–1004. 10.1111/liv.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nardo AD, Schneeweiss-Gleixner M, Bakail M, et al. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int 2021;41:20–32. 10.1111/liv.14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreira JLdeS, Barbosa SMdeS, Gonçalves Júnior J, Júnior JG. Pathophysiology and molecular mechanisms of liver injury in severe forms of COVID-19: an integrative review. Clin Res Hepatol Gastroenterol 2021;45:101752. 10.1016/j.clinre.2021.101752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep 2020;2:100113. 10.1016/j.jhepr.2020.100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schütte A, Ciesek S, Wedemeyer H, et al. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol 2019;70:797–9. 10.1016/j.jhep.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 25.Feng G, Zheng KI, Yan Q-Q, et al. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol 2020;8:1–7. 10.14218/JCTH.2020.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keta-Cov research group . Intravenous ketamine and progressive cholangiopathy in COVID-19 patients. J Hepatol 2021;74:1243–4. 10.1016/j.jhep.2021.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deltenre P, Moreno C, Trépo E. Progressive cholangiopathy in Covid-19 patients: other possible diagnoses than ketamine-induced cholangiopathy should be considered. J Hepatol 2021. 10.1016/j.jhep.2021.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni AV, Parthasarathy K, Kumar P, et al. Early liver transplantation after COVID-19 infection: the first report. Am J Transplant 2021;21:2279–84. 10.1111/ajt.16509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]