Abstract
Background:
PML, a rare disease of the central nervous system caused by JC polyomavirus (JCV) occurring in immunosuppressed people, is typically fatal unless adaptive immunity is restored. We hypothesized it is feasible and safe to use partially HLA-matched donor-derived BKV-specific T cells (PyVST) for immunotherapy in PML.
Methods:
Open-label, single-cohort, single-site pilot study of PyVST for the treatment of PML. (NCT02694783). Overlapping peptide libraries derived from Large T (LT) and Viral Protein 1 (VP1) of BK polyomavirus with high sequence homology to JCV counterparts were used to generate PyVST cross-recognizing JCV antigens. PyVST were manufactured from peripheral blood mononuclear cells of 1st degree relative donors. PyVST were administered at 1×106 PyVST/kg, followed by up to two additional infusions at 2×106 PyVST/kg. Safety monitoring period was 28 days after each infusion. Patients were followed with serial MRI and lumbar punctures for up to 12 months following last infusion.
Findings:
All administered PyVST (n=14) met release criteria and displayed in vitro recognition of cognate antigens. Twelve adults received at least one infusion; 10 received at least 2; and 7 received a total of 3 infusions. All infusions were tolerated well, and no serious treatment-related adverse events were observed. Seven patients survived PML for longer than one year following first infusion, whereas 5 died of PML within 3 months.
Interpretation:
PyVST were reliably generated from healthy related donors and safely used for adoptive immunotherapy of PML. Although not powered to assess efficacy, our data provide additional support for this strategy as a life-saving therapy for some patients.
Keywords: JC virus, polyomavirus, progressive multifocal leukoencephalopathy, immunodeficiency, adoptive cell transfer, virus-specific T cells, brain, infection
Background
Progressive multifocal leukoencephalopathy (PML) is a rare, debilitating disease of the central nervous system (CNS) caused by the JC virus (JCV). JCV is a member of the human polyomavirus (hPyV) family and is closely related to BK virus (BKV). Primary infection with JCV commonly establishes asymptomatic persistence in 50–70% of healthy adults.(1–3) In the setting of cellular immunodeficiency, JCV can reactivate from sites of latency and undergo sequential genomic rearrangements, allowing an otherwise benign virus to cause lytic infection of glial cells and the CNS manifestation of PML.(4) PML most often occurs in people with underlying HIV/AIDS, history of lymphoproliferative disease, or in the setting of immunosuppressive therapy for inflammatory diseases or transplant.(5)
PML presents with rapid progression of focal neurological deficits. There is no antiviral therapy, and survival is directly related to timely restoration of cellular immunity.(6,7)Correspondingly, the highest survival rates (>90%) are among patients with multiple sclerosis (MS) treated with natalizumab, a monoclonal antibody that blocks trafficking of lymphocytes into the CNS, in whom immune restoration is readily achieved upon discontinuation of treatment.(8) Similarly, combined antiretroviral therapies have increased survival of PML in AIDS from 10% to 75–80%.(9) In contrast, patients with hematological malignancy frequently develop profound chronic immune dysfunction due to the combined effects of the underlying malignancy and multiple lines of therapies that can be difficult to reverse; such patients face grave prognosis upon developing PML, with median survival of three months and mortality rates reaching 90%.(10)
Ex vivo expanded virus-specific T cells (VST) can mediate a potent therapeutic effect upon adoptive transfer into stem cell transplant (SCT) recipients and other immunocompromised individuals with viral reactivation.(11,12) Fully or partially HLA-matched allogeneic VST have been successfully used to control a variety of viruses, including cytomegalovirus (CMV), Epstein Barr virus, adenovirus, BKV, and human herpes virus-6, in many cases leading to complete recovery.(13–15) Recently, VST have been used to treat PML. The reported experience to date is encouraging but remains limited. The first report was a patient who received JCV-specific T cells manufactured from his allogeneic stem cell transplant donor.(16) The largest report to date is a retrospective of 9 patients treated with either allogeneic or autologous JCV-specific T cells.(17) A report of 3 patients treated with BKV-specific T cells provided proof-of-concept for use of T-cells derived from the related polyomavirus BKV.(18) On this basis, we hypothesized that partially matched PyVST generated from healthy 1st degree relatives of patients with PML can be safely used as adoptive immunotherapy in PML. Since JCV and BKV display significant sequence homology in their immunodominant Large T (LT) and VP1 antigens (83% and 78% respectively),(19) we used clinical grade BKV peptide libraries, readily available and previously used in a clinical trial in our institution, as immunogens to generate allogeneic PyVST from peripheral blood of donors. We report results of a pilot study establishing the feasibility and safety of this strategy in a cohort of 12 adults with PML.
Methods
Trial Design.
This open-label, single-cohort pilot study was conducted at the National Institutes of Health Clinical Center. PyVST were generated from partially matched 1st degree relatives of patients with PML (Supplementary Materials). A first dose of PyVST was administered at 1x106 T cells per kilogram; with administration up to 2 reinfusions, each at 2x106 T cells per kilogram. Reinfusions could be from same donor, or from different donors. Patients were monitored following each infusion with scheduled clinical, radiological, and laboratory testing. (Fig. 1)
Figure 1.
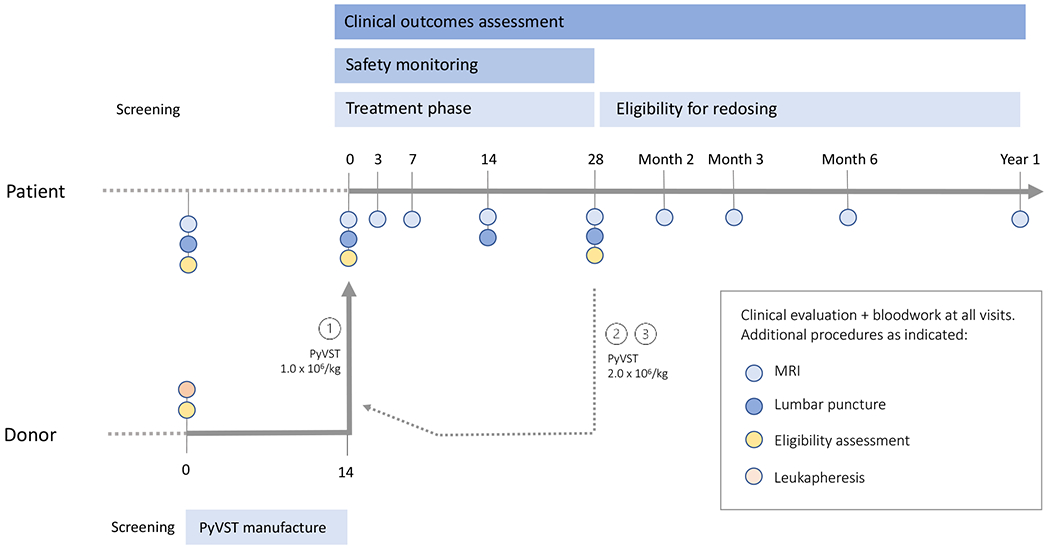
Trial schema.
Candidate patients and donors were screened for study eligibility contemporaneously. Selected donors underwent leukapheresis, and PyVST cultures were initiated either from cryopreserved peripheral blood mononuclear cells or freshly isolated cells. Final cell product was harvested at Day 14. Patients returned for baseline study visit and confirmation of eligibility criteria. First dose of PyVST was administered at 1x106 cells/kg followed by 28-day safety monitoring period with scheduled testing at Days 3, 7, 14 and 28. Patients were eligible for up to 2 additional doses of PyVST of 2x106 cells/kg, no less than 28 days from last infusion; each additional infusion was followed by 28-day safety monitoring as previously. Patients were followed for up to 1 year after last infusion with scheduled clinic visits and testing.
Study oversight.
This study was approved by the NIH Clinical Neurosciences Institutional Review Board and conducted under Food and Drug Administration (FDA) Investigational New Drug (IND) 16786. The study was registered on clinicaltrials.gov (NCT02694783). Safety of participants was overseen by an independent data safety and monitoring board, and an independent contract research organization provided on-site monitoring. There was no industry participation in this study.
Participants.
Eligible patients were 18 or older, with clinically definite PML per 2013 American Academy of Neurology consensus diagnostic criteria,(20) with clinical and radiological disease progression over the previous month, with mild to moderate disability defined by Modified Rankin Scale (mRS) score between 1 and 4. Patients previously treated for PML without benefit and demonstrating continued clinical and radiological disease progression were included; concurrent treatments were permitted if not immune modulatory or otherwise interfering with activity of PyVST. Patients with HIV infection, previous treatment with natalizumab, or otherwise readily reversible immunosuppressed state were excluded.
Up to three 1st degree relatives (i.e., parent, sibling, or child), age 18 or older, were contemporaneously screened as donors. While this minimized time to treatment, it also led to production of greater number of PyVST products than were ultimately used. Donors with medical contraindication to leukapheresis or those not meeting qualification as a transplant donor, including blood-borne infection or other serious medical condition, were excluded.
Donor candidates underwent high-resolution HLA typing. Best available HLA-matched donor was selected if other eligibility criteria were met, with at least haploidentical donors preferred and minimum of two HLA-matching alleles required. Further, when JCV serostatus was available, JCV Ab+ donors were preferred as it was predicted this might be associated with higher potency cell product.
All participants, patients, and donors gave written, informed consent. Patients were followed until end of study, death, or withdrawal from study, the last most commonly due to loss of ability to travel because of PML-related disability or progression of underlying disease. For patients no longer able to travel, best attempts were made to collect data regarding clinical course and survival.
Trial procedures and end points.
PyVST products were administered by intravenous syringe infusion, with inpatient monitoring for 7 days following each infusion. Patients returned to NIH for scheduled study visits (Fig. 1). Primary endpoints were feasibility and safety. Safety was monitored for 28 days following each infusion, with stopping rules based on rate of treatment-related serious adverse events. Secondary outcomes were collected for 12 months following last infusion and included: survival (overall and disease-specific); comprehensive neurological exam and functional performance scale scores (mRS; Karnofsky Performance Scale (KPS); Mini Mental Status Exam (MMSE); 25 foot timed walk (25FTW) and 9-hole PEG test (RPEG, LPEG); brain MR imaging, performed on 3-tesla scanners; JCV DNA detection in CSF using ultrasensitive multiplex quantitative polymerase-chain-reaction (PCR) assay with detection limit of 10 copies/ml; and immunophenotyping of PyVST products using multicolor flow cytometry and functional assays to determine magnitude of antiviral activity against surface viral antigens VP1 and LT. Details are provided in the Supplementary Materials.
Statistical analysis.
The planned analysis included descriptive statistics of PyVST products, incidence and severity of adverse events, and longitudinal course of secondary outcomes. All patients who received at least one PyVST infusion were included. Wilcoxon rank-sum and Student t tests were used to analyze differences between survivors and non-survivors; p values < 0·05 were considered statistically significant. No corrections for multiple testing were performed and no formal power calculation was conducted, therefore significant results under this framework are regarded as exploratory.
Results
Participants.
Between April 2016 and October 2018, 26 adult patients were screened for participation. Twelve patients received treatment with PyVST product derived from 14 matched donors. (Fig. 2) Baseline characteristics of treated patients and respective donors are summarized in Table 1. Median age of patients was 59 (range 35–72); 7 were female. Underlying condition predisposing to PML varied, with the most common being lymphoproliferative disease (n=6); systemic autoimmune disease treated with rituximab (n=2); and primary immunodeficiency (n=2). At first infusion, median time from PML symptom onset was 2 months (range 1–6 months). Median age of donors was 43 (range 19–64); donors were most often siblings (8/14).
Figure 2.
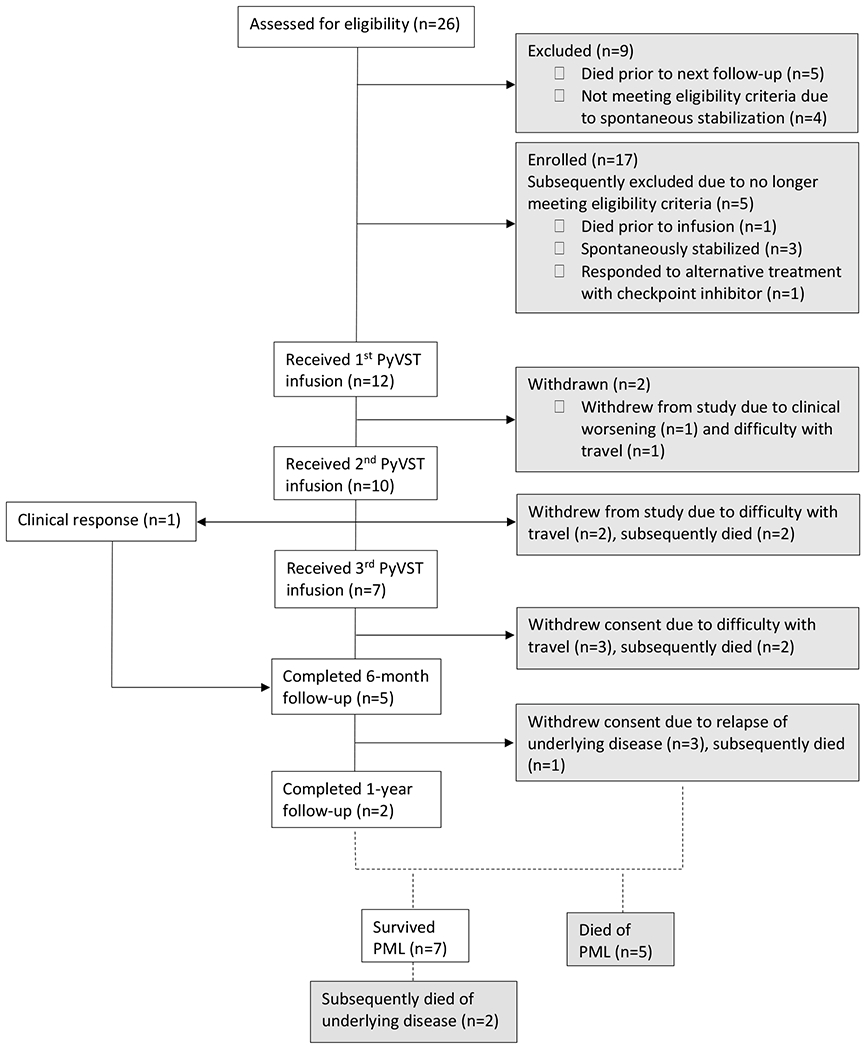
CONSORT diagram.
Table 1.
Patient and donor summary table.
| Patient Age/Sex |
Underlying disease Immune suppressive treatment history |
Absolute values CD4/CD8/CD19 at baseline |
Donor Age/Sex Degree of HLA-match DonorJCV Ab status |
Clinical Course (Time 0=first PyVST infusion) |
Standardized Clinical Scale Scores (baseline → final visit) • mRS • KPS • MMSE |
JCV CSF copies per ml (baseline → last value) Time from first PyVST infusion to last value |
|---|---|---|---|---|---|---|
|
1 62/F |
ICL/CVID/cyclic neutropenia Remote treatment with prednisone, methotrexate, TNF-inhibitor |
21/416/283 |
Donor 1: 39/F 6/10 match JCV Ab status unknown |
Neurological symptom onset: Month -6 Diagnosis of PML: Month -4 Died of complications of PML: Month 6 PML onset with rapid development of diplopia,severe truncal and appendicular ataxia, requiring bilateral assistive device for ambulation. Received 2 infusions of pembrolizumab, however symptoms continued to progress with worsening ataxia, cerebellar dysarthria, impaired eye movements, intractable hiccups; wheelchair-bound. Received two PyVST infusions from Donor 1 and one from Donor 2, with continued progression of symptoms. Withdrew from study and entered hospice care two months following last PyVST infusion. |
mRS 4→4 KPS 40→40 MMSE 26→28 |
27,429→77,774 Month 3 |
|
Donor 2: 64/F 2/10 match JC Ab negative | ||||||
|
2 61/F |
Microscopic polyangiitis Remote chronic prednisone Rituximab, last 2 months prior to PyVST |
706/101/0 | 40/F 5/10 match JCV Ab positive |
Neurological symptom onset: Month -1 Diagnosis of PML: Month -1 Survived PML: last communication > 36 months PML onset with progressive decline in memory and language over weeks, which developed into severe expressive aphasia, apraxia and mild right-sided weakness. Following first PyVST infusion, clinically stabilized. By Month 6 had improved use of right hand, further improved by Month 12. |
mRS 3→3 KPS 70→70 MMSE 22→28 |
609→Undetected Month 9 |
|
3 53/F |
CLL Remote rituximab, CFR, bendamustine, ibrutinib/obinutuzumab, CART, 2 months prior to PyVST |
45/19/0 | 58/F 10/10 match JCV Ab status unknown |
Neurological symptom onset: Month -2 Diagnosis of PML: Month -1 Died of complications of PML: Month 4 PML onset with progressive ataxia, abnormal eye movements with nystagmus, dysarthria with continued neurological deterioration following 2 PyVST infusions. By Day 14 post infusion 2, patient had developed severe bulbar dysfunction requiring NG tube and total care for ADL. Withdrew from study to enter hospice care within 1 month following second PyVST infusion. |
mRS 4→5 KPS 40→30 MMSE 28→4 |
62,963→109,880 Month 2 |
|
4 71/M |
Lymphoma (DLBC) Remote chemo (R-CHOP and intrathecal methotrexate; R-DICE x 3; R-BEAM) Autologous BMT |
217/426/53 | 44/M 5/10 match JCV Ab status unknown |
Neurological symptom onset: Month -2 Diagnosis of PML: Month -2 Presumed dead of complications of Stage IV lung cancer: > Month 12, date unknown PML onset with change in mental status, geographic disorientation with inability to find his way in his own home. Subsequently developed dense right sided weakness with inability to ambulate independently. Following first PyVST infusion, stabilized clinically. In the subsequent months, right hemiparesis improved such that by Month 6 follow-up was able to ambulate using a walker and had regained limited ability to use his right hand. At last communication, one year following completion of PyVST treatment, patient reported new diagnosis of Stage IV lung cancer and decision to enter hospice care. |
mRS 4→4 KPS 40→50 MMSE 14→18 |
333→18 Month 3 |
|
5 40/F |
SLE Remote azathioprine, prednisone, methotrexate, belimumab Rituximab, last 4 months prior to PyVST |
177/112/1 |
Donor 1: 39/M 5/10 match JCV Ab positive |
Neurological symptom onset: Month -2 Diagnosis of PML: Month -2 Died of complications of SLE: Month 14 PML onset with ataxia, slurred speech, right sided weakness and apraxia with rapid progression to being bed-bound, aphasic with cortical visual impairment. By three weeks after first PyVST infusion, clinical stabilization with subsequent improved cognition, vision and motor function following 2nd and 3rd infusions. By Month 6 following completion of PyVST treatment, able to ambulate using a walker, speech improved with recovery of limited reading and writing and use of tools. By Year 1, had experienced exacerbation of underlying SLE, leading to severe debilitation and inability to travel, and withdrew from study. |
mRS 4→4 KPS 50→50 MMSE 13→21 |
9564→1354 Month 7 |
|
Donor 2: 35/M 5/10 match JCV Ab positive | ||||||
|
6 40/F |
Follicular Lymphoma Bendamustine, Rituximab, last 6 months prior to PyVST |
230/134/0 | 64/F 6/10 match JCV Ab negative |
Neurological symptom onset: Month -6 Diagnosis of PML: Month -5 Died of complications of PML: Month 4 PML onset with progressive cortical visual impairment with quadrantopsia/alexia/simultag-nosia, developing into cortical blindness. Received a total of 3 PyVST infusions with no benefit and neurological symptoms progressed with left hemiparesis, bulbar symptoms and cognitive changes. One month following last PyVST infusion, withdrew from study and entered hospice care. |
mRS 3→4 KPS 60→40 MMSE 19→7 |
38,772→2,830,385 Month 2.5 |
|
7 60/M |
HBV/HDV Pegylated α interferon |
87/135/67 | 19/F 5/10 match JCV Ab positive |
Neurological symptom onset: Month -4 Diagnosis of PML: Month -3 Survived PML: last communication >24 months PML onset with right hand weakness and ataxia; symptoms progressed to dense right sided weakness, diplopia and dysarthria. Following first PyVST infusion, experienced initial worsening of symptoms with loss of ability to ambulate independently; following the 2nd and 3rd infusions, clinical stabilization and some improvement in speech and right sided weakness. By Month 6 after completion of PyVST treatment, experienced slight worsening of neurological symptoms, in context of progressive liver failure and ascites with no evidence of PML reactivation. Unable to return for Year 1 visit due to general medical debilitation. |
mRS 4→4 KPS 60→60 MMSE 25→28 |
782→Undetected Month 8 |
|
8 71/M |
CLL Remote ofatumumab, alemtuzumab, prednisone Rituximab, last 6 months prior to PyVST |
69/58/2 | 43/F 2/10 match JCV Ab positive |
Neurological symptom onset: Month -3 Diagnosis of PML: Month -2 Survived PML: last communication >16 months PML onset with progressive worsening dysarthric speech, left upper extremity weakness; dysphagia, requiring PEG. Received one PyVST infusion and withdrew from study 2 weeks later due to burden of trial participation. |
mRS 3→3 KPS 40→40 MMSE 28→29 |
362→533 Week 2 |
|
9 40/F |
SCID with spontaneous reversion mutation; hemolytic anemia Corticosteroids, rituximab, last 3 months prior to |
150/104/2 | 45/M 5/10 match JCV Ab positive |
Neurological symptom onset: Month -4 Diagnosis of PML: Month -3 Died of complications of PML: Month 2 PML onset with cognitive changes, apathy,slurred speech; progressive left hemiparesis and left hemianopsia; loss of ability to ambulate, decreased attentiveness, minimal to no verbalization. Received one PyVST infusion with no benefit; over subsequent weeks became increasingly less interactive with inability to take nutrition by mouth. By 2 months following PyVST infusion, withdrew from study and entered hospice care. |
mRS 4→4 KPS 40→40 MMSE 9→0 |
51,230→20,927 Month 1 |
|
10 35/F |
CVID untreated |
310/112/7 | 33/F 5/10 match JCV Ab negative |
Neurological symptom onset: Month -2 Diagnosis of PML: Month -1 Died of complications of PML: Month 2 PML onset with right UE weakness and expressive aphasia; progression to right hemiparesis and hemisensory loss. Received 2 infusions of PyVST with no benefit; neurological symptoms progressed to right hemiplegia, global aphasia and decreased attentiveness. By 2 weeks following 2nd PyVST infusion, withdrew from study and entered hospice care. |
mRS 4→4 KPS 50→40 MMSE 8→0 |
344,163→14,654 Month 2 |
|
11 57/M |
CLL untreated |
683/1,045/11,562 | 54/F 10/10 match JCV Ab negative |
Neurological symptom onset: Month -5 Diagnosis of PML: -3 Survived PML: last communication >24 months PML onset with left inferior quadrantopsia and subsequent development of cortical visual impairment; decreased dexterity left hand; partial complex seizures. Following first PyVST infusion, experienced progressive improvement in vision such that following second infusion regained limited ability to read. Progressive improvement in vision over following months. |
mRS 3→2 KPS 70→70 MMSE 28→29 |
74→32 Month 3 |
|
12 72/M |
CLL Remote obinutuzumab |
367/132/24 | 30/M 7/10 match JCV Ab positive |
Neurological symptom onset: Month -2 Diagnosis of PML: -1 Survived PML: last communication > 13 months PML onset with left sided ataxia, bulbar symptoms with dysarthria and dysphagia, ataxic gait. Continued slow worsening following first two PyVST infusions; by the third infusion, demonstrating improved strength and dysphagia. Withdrew from study following completion of PyVST treatment due difficulty with travel. At last communication, was neurologically stable13 months post PyVST treatment completion. |
mRS 2→3 KPS 70→50 MMSE 27→27 |
1,648→1,476 Month 2.5 |
Abbreviations: PyVST, polyomavirus specific T cells; M, male; F, female; Ab, antibody; MRS, Modified Rankin Scale (0 no signs or symptoms → 5 dead); KPS, Karnofsky Performance Scale (100 no signs or symptoms → 0 dead); MMSE, mini-mental status exam (30 best score → 0 worst score); CSF, cerebrospinal fluid; CLL, chronic lymphocytic leukemia; ICL, idiopathic lymphopenia; CVID, combined variable immunodeficiency; CAR, chimeric antigen receptor; NG, naso gastric; ADL, activities of daily living; DLBC, diffuse large B cell; RCHOP, rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine, prednisolone; R-DICE, rituximab, ifosfamide, carboplatin, etoposide phosphate; R-BEAM, rituximab, carmustine, etoposide, cytarabine, melphalan; BMT, bone marrow transplant; SLE, systemic lupus erythematosus; HBV, hepatitis B; HDV, hepatitis; PEG, percutaneous endoscopic gastrostomy.
PyVST Development, Manufacturing and Product Characteristics.
During preclinical development, we established and validated reliable methodology for ex vivo expansion of PyVST cells upon priming with BK LT and VP1 pepmixes that display high degree of sequence homology with counterpart JC LT and VP1 antigens (Fig. S1A). For the purpose of IND submission, validation runs were performed under current good manufacturing practice (cGMP) conditions using peripheral blood mononuclear cells (PBMC) obtained via apheresis from healthy deidentified volunteers. The resulting PyVST cell products displayed high degree of expansion and viability, and predominantly contained CD3+ T cells. While antigen-specific antiviral potency was not included in release criteria for this early-phase pilot study, all three cGMP-compliant validation products displayed robust activity against BKV antigens. Furthermore, TNF-α and IFN-γ production was observed upon stimulation of the PyVST cells with JCV LT and VP1 pepmixes (Fig. S1E), implying robust cross-reactivity and establishing the rationale for using this strategy to target PML.
For this study, 23 clinical PyVST products were successfully generated (Supplementary Materials); 14 were subsequently administered (Fig. 2). No manufacturing failures occurred; all final products met release criteria including sterility and negative mycoplasma testing. A variable degree of expansion (mean 7.6-fold, range 1·–17·1 fold) was observed during the manufacturing process (Fig. 3A), yielding between 3·4–34·2x108 total nucleated cells (TNC) (mean 15·x108 TNC), thus allowing cryopreservation of multiple aliquots of PyVST for repeat infusions. Phenotypic FACS analysis at harvest (Fig. 3B) revealed predominance of CD3+ T cells (mean 86·7%; range 64·1-99·0%), containing a mixture of CD4+ (mean 45·7%, range 7·1-88·5%) and CD8+ cells (mean 35·6%; range 8·7-74·3%), with negligible presence of CD14+ (myeloid cells) and CD19+(B) cells. CD3lo CD56hi NK cells were detected in the final PyVST at mean frequency of 11·73% (range 0·7-34·8%), indicating nonspecific expansion of NK cells, likely cytokine-driven. All final PyVST cell products displayed significant antigen specific TNF-α and IFN-γ cytokine production upon stimulation with cognate LT and VP1 pepmixes (Fig. 2C). Among CD3+ T cells, mean total (TNF-αhi) reactivity (LT plus VP1) was 20·3% (range 2·5-55·9%), with mean of 8·;1% of CD3+ T cells recognizing LT antigen (range 0·5-26·6%) and 12·3% recognizing VP1 (range 1·9-50·9%). The majority of infused PyVST reactivity was confined to the CD4+ T cell compartment (mean total reactivity 19·9%; range 2·4-54·2%) with 8.8% recognizing LT (range 1·2-27·6%) and 11·1% recognizing VP1 antigen (range 1·2-38·9%). In contrast, ex vivo generated PyVST cells contained relatively few CD8+ T cells displaying either anti-LT or VP1 activity (mean total reactivity 1·2%; range 0-8·0%). In a subset of 7 infused PyVST products for which clinical material was available for additional assays, cytolytic activity was identified among both CD4+ and CD8+ T cell compartments (Fig. S2), as measured by expression of CD107a alone and combined CD107a/IFNγ following stimulation with LT and VP1 pepmixes. Of note, in vitro potency of cell product, as measured by reactivity to LT and VP1 pepmixes, was not related to JCV Ab status of the donor.
Figure 3.
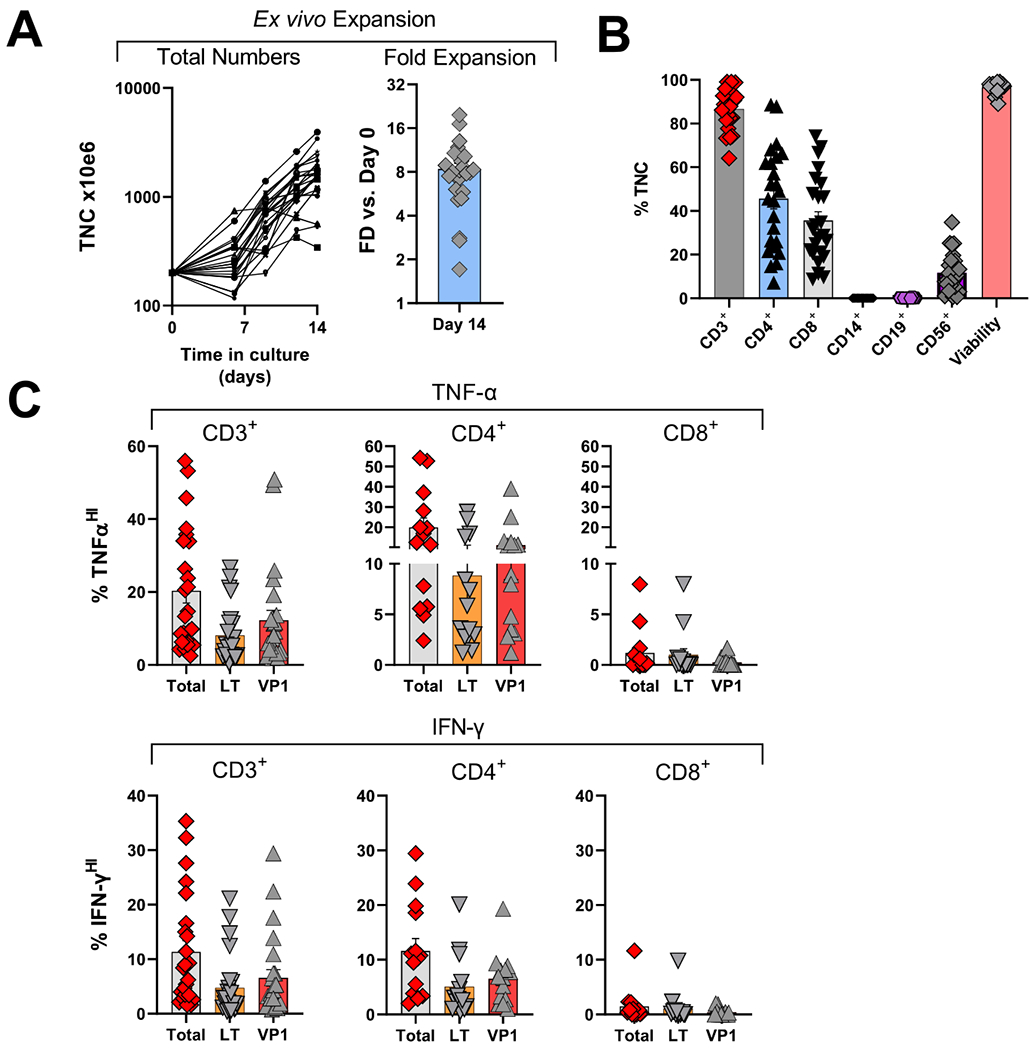
Characterization of clinical allogeneic PyVST products derived from matched healthy donors.
Peripheral blood mononuclear cells from healthy related donors were isolated from the apheresis product, stimulated with BK virus LT and VP1 pepmixes and expanded in G-rex tissue culture containers for 14 days. Upon harvest, as part of the release criteria, the resulting PyVST were enumerated, analyzed for identity, cellular content, and viability. Additionally, antiviral potency was assessed, measured by cytokine release. A. Expansion of PyVST cells as measured by absolute number of viable nucleated cells (left panel) and fold expansion relative to day 0 from the initiation of the culture (right panel). B. Composition and viability of the final PyVST products was evaluated by flow cytometry and trypan blue exclusion (respectively) on harvest (day 14 from the culture initiation). C. Antiviral activity of the final PyVST products in the viable CD3+, CD4+, and CD8+ T cell compartments was measured by intracellular staining for TNF-α and IFN-γ upon 6hr restimulation with BK virus LT and/or VP1 pepmixes in presence of brefeldin A and monesin using flow cytometry (infused products only shown). Unrelated pepmix was used as negative control.
PyVST administration.
Of 26 patients screened, 14 were ultimately excluded from participation and did not receive treatment with PyVST. Of these, one responded to experimental treatment with pembrolizumab(21); 6 died within weeks of first visit and prior to availability of PyVST; and 7 stabilized spontaneously and thereby did not meet inclusion criteria for treatment. Interestingly, 4 of the patients who stabilized spontaneously had underlying untreated sarcoidosis. Of the remaining 3, 2 had remote history of hematological malignancy and one had history of Sjögren Syndrome treated with rituximab. Twelve individuals met eligibility criteria and demonstrated clinical and radiological progression of PML up to the time of first PyVST administration. All 12 received at least one infusion; of these, 10 received a second infusion and 7 a third infusion (Fig. 2). Two individuals (Patients 1 and 5) received cell product from 2 distinct donors (Table 1; Fig. S3 and S7); this was pursued in the effort to optimize care with a different and possibly more potent product as both continued to worsen clinically and radiologically following first infusions. This strategy is based on practice developed in the setting of “off-the-shelf” VST infusions, where a switch to a different product is commonly made if first infusion induces suboptimal clinical response.(15)
Safety.
Treatment was tolerated well with no infusion reactions. Clinically relevant immune reconstitution inflammatory syndrome (IRIS) was not observed in any patient. Over the course of study, a total of 134 adverse events were documented; the majority were related to underlying predisposing disease, to progression of PML, or to intercurrent illness, and 15·7% were grade 3 or higher. No high-grade adverse event was deemed treatment-related by site investigators or the data safety monitoring board. Aside from neurological progression, transient fever was the single most common adverse event, experienced in 5 patients within one month from infusion (mean time to fever 24 days); most commonly this was attributed to intercurrent infection or aspiration (4/5). Nine hematological adverse events were observed in 6 patients, including increased white blood cell count (n=2), neutropenia (n=2), thrombocytosis (n=1), thrombocytopenia (n=2), and worsening of baseline anemia (n=2); none was deemed treatment-related (Table 2). There was one death during the 28-day safety monitoring period: Patient 10 withdrew from study to enter hospice care and died of PML at Day 18 following her second PyVST infusion.
Table 2.
Adverse event summary table
| Adverse Events | n (%) |
|---|---|
| Total events | 134 |
| Grade 3 or higher* | 21 (15·7%) |
| Fever | 5 (3·7%) |
| Headache | 3 (2·2%) |
| Hematological events | 6 (4·5%) |
| Increased WBC | 2 (1·5%) |
| Neutropenia | 2 (1·5%) |
| Thrombocytopenia | 2 (1·5%) |
| Thrombocytosis | 1 (0·7%) |
| Anemia | 1 (0·5%) |
| Pruritis | 1 (0·5%) |
| Neurological worsening due to PML | 25 (18·7%) |
| Worsening of underlying disease | 10 (7·5%) |
| Intercurrent illness | 21 (15·7%) |
no AE grade 3 or higher deemed treatment-related
Clinical Response.
Although this pilot study was not designed or adequately powered to determine efficacy, eligibility criteria were designed to enrich for patients with historically poor expectation of survival from PML. Most patients remained profoundly lymphopenic over the duration of study participation (Fig. S2–13), thus survival could not be directly correlated or attributed to reconstitution of endogenous immune competence. Seven patients survived from PML for at least one year from PyVST treatment initiation. Of these, 2 withdrew from the study prior to completing the on-site Day 28 post-infusion study visit, citing difficulty with travel (Patients 8 and 12, following 1 and 3 infusions respectively); at last contact, both were alive and neurologically stable at 16 and 13 months from last infusion, respectively. For all other survivors, post-treatment in-person evaluations demonstrated clinical stabilization or improvement on disability rating scales (mRS, KPS and MMSE) (Table 1). Survivors had progressive decline in CSF JCV load through last CSF analysis (86% median decline from baseline), typically at Day 28 following last PyVST infusion (Fig. 4B), with two patients achieving undetectable JCV PCR by this time. Among survivors, PML MRI lesion volume increased in the weeks following first infusion, typically peaking between 1–2 months, followed by a progressive decrease (67% median decline in lesion volume compared to baseline) (Fig. 4C; Supplementary Videos). Improvements in radiological and virological measures of PML disease activity were temporally correlated to infusions (Fig. S3–14 and Videos 1–12). It is important to note that decline in lesion volume by MRI likely does not signify lesion repair but rather reflects focal atrophy and parenchymal volume loss at the sites previously damaged by the infectious process. Stabilization and decline in MRI lesion volume does however indicate containment of the infection and indeed is only observed in patients who ultimately survive PML. Consistent with this is that the majority of survivors had minimal if any recovery of neurological deficits accrued despite decline in lesion burden.
Figure 4.
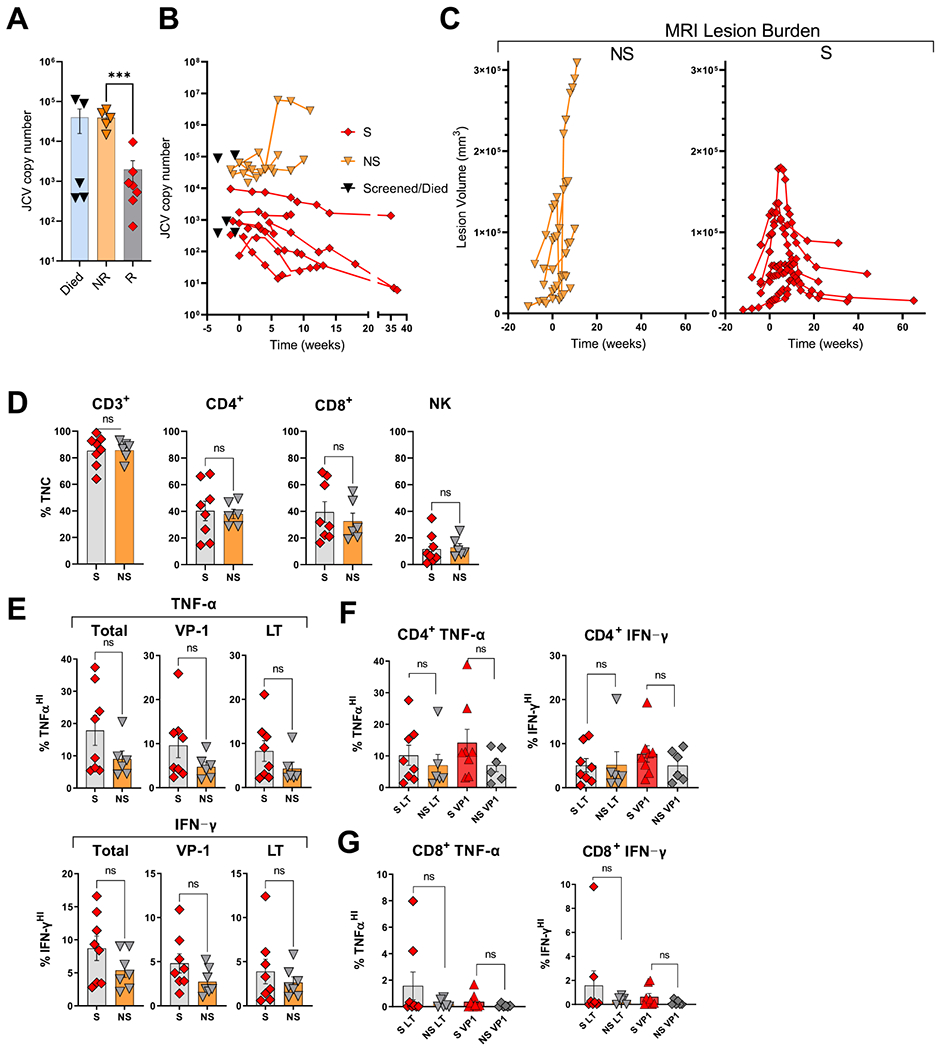
Comparison of survivors and non-survivors.
CSF JCV load at baseline (A) and longitudinally over course of the trial (B) in non-survivors (NS), survivors (S), and the 5 patients who underwent screening but died of PML within a few weeks of first visit, prior to receiving treatment with PyVST. C. Longitudinal MRI PML lesion burden over course of trial in non-survivors (NS) and survivors (S). D. Cell composition of the PyVST products received by survivors (S) and non-survivors (NS). E. Potency of PyVST received by survivors (S) and non-survivors (NS), measured as TNF-α and IFN-γ secretion upon stimulation with indicated pepmixes. F and G. Antiviral reactivity within CD4+ and CD8+ T cell compartments of PyVST products received by survivors (S) and non-survivors (NS).
Three survivors did not complete 1-year follow-up due to medical debilitation related to underlying disease. Patient 5 died of complications of lupus one year after completion of PyVST treatments; Patient 4 entered hospice due to newly diagnosed stage IV lung cancer 1 year after last infusion and was lost to follow-up; Patient 7 developed liver failure but was alive at last follow-up 2 years post last infusion.
Five patients died of PML 3 weeks–3 months after last PyVST infusion (median 2 months). These patients withdrew from study once physical disability precluded travel, leading to censoring of mRS and KPS scores; MMSE scores, however, almost uniformly documented substantial cognitive decline among non-responders leading up to last study visit. Correspondingly, PML MRI lesion burden steadily increased up until the last scan obtained (80% median increase in lesion volume compared to baseline; Table 1; Fig. 4C; Supplementary Figures 2–13 and Videos 1–12).
Compared to those that did not survive PML, median CSF JCV copy number at baseline was significantly lower among PML-survivors (609 vs 51,230; p=0·002), and subsequent viral load trajectories showed similar pattern (Fig. 4A–B). Note that of 5 patients who died of PML within several weeks of the screening visit and before treatment could be provided, 3 had viral loads comparable to patients who survived (Fig. 4A).
Older patients with lower baseline clinical disability, lower baseline PML lesion burden by MRI, and shorter PML disease duration appeared more likely to survive following treatment with PyVST, but none of these baseline factors predicted response on its own (Tables 1 and 3). No specific donor feature correlated with antiviral activity of derived PyVST. Specifically, in vitro potency of PyVST was not associated with donor age, and degree of HLA-match with donor was not related to patient survival (Table 3). Of interest, while donor JCV serostatus was not associated with greater in vitro potency of PyVST, patients who survived tended to have received products from seropositive donors.
Table 3.
Comparison of patient features between survivors and non-survivors.
| Survivors median (range) |
Non-Survivors median (range) |
p value* | |
|---|---|---|---|
| Patient age | 61 (40-72) | 40 (35-62) | p=0·08 |
| Baseline disability (mRS scale score) | 3 (2-4) | 4 (3-4) | p=0·23 |
| Baseline MRI lesion volume (cc) | 47,274 (9797-12,0932) | 53,660 (26,821-12,9678) | p=0·76 |
| Time from neurological symptom onset to treatment (months) | 2 (1-5) | 4 (2-6) | p=0·27 |
| Time from PML diagnosis to treatment (months) | 2 (1-3) | 3 (1-5) | p=0·5 |
| Baseline CSF JCV DNA (copies/ml) | 609(74-9,564) | 51230(27,429-344,163) | p=0·002 |
| Baseline CD4 count (cells/mm3) | 217(69-706) | 150 (21-310) | p=0·26 |
| Baseline CD8 count (cells/mm3) | 132 (58-1,045) | 112 (19-416) | p=0·6 |
| Degree of HLA match with donor (number of subjects) | |||
| Full match | 1 | 1 | .. |
| 50% or greater | 5 | 4 | .. |
| Less than 50% | 1 | 1 | .. |
Wilcoxon-rank sum
PyVST products received by survivors were not different in cell composition and relative frequency of CD4+ and CD8+ T cells than those received by non-survivors (Fig. 4D). In vitro potency, measured as mean frequency of TNF-α and IFN-γ secreting T cells in either CD3+, CD4+ or CD8+ T cell compartments following stimulation with cognate LT and VP1 pepmixes were not significantly different (Fig. 4E–G), albeit there was a trend towards higher activity of PyVST products given to survivors (total CD3+ reactivity: 17·9 vs 9·33%; p=0·06; 8·7 vs 5·37%; p=0·073 for TNF-α and IFN-γ respectively).
Discussion
PML is a devastating opportunistic brain infection that is uniformly fatal unless immune competence can be restored. Recently, general immune reconstitution strategies, including checkpoint inhibition, recombinant human interleukin-7 (rhIL7), and filgrastim, have shown some promise.(21–25) It is not clear, however, that such approaches can be successfully or safely applied across the range of patients susceptible to PML(7,26). Adoptive transfer of VST is particularly appealing as it may bypass intrinsic immune deficits by rapid reconstitution of the specific repertoire, while carrying little of exacerbation of underlying immune-mediated disease.
We report a pilot study of 12 patients with PML whose immune compromise could not otherwise be reversed, who were treated with adoptively transferred, donor-derived PyVST. While differences in relative frequency of antigen-specific T cells were observed between each individualized cell product, PyVST were successfully generated from all donors, supporting feasibility and reliability of this treatment approach. We used first degree relatives as donors, thus ensuring a relatively high degree of HLA matching, minimizing the likelihood of antigen escape, and enhancing likelihood of effective in vivo recognition of viral antigens upon transfer. On-demand ex vivo expansion requires extra manufacturing time for each patient as compared to ready-availability of minimally HLA-matched “off-the-shelf” cells that are pre-made and banked; however, third-party virus-specific banks are not yet widely available. In our study, 4-6 weeks were required to have final clinical product. It is conceivable that if banked VST become more available, a two-step treatment approach might be considered with rapid initial administration of minimally matched products followed by on-demand, highly matched products as needed.
In this study, the delay to treatment led to exclusion of 6 patients with rapid deterioration who died prior to receiving treatment. This was counterbalanced by the opportunity to identify and exclude 7 patients who spontaneously stabilized sufficiently so as to no longer require treatment. Fully appreciating the impact of any bias related to treatment delay will require a randomized, controlled study.
No specific donor features were associated with more robust antiviral products in vitro, including age and JCV serostatus. While early detection of JCV-specific CD8+ responses has been linked in literature to successful immune restoration and favorable clinical outcome in PML(27,28), PyVST products were predominantly characterized by LT and VP1-specific CD4+ cells. This is consistent with previously reported VST experience from healthy donors(17,18). While we were able to demonstrate cytolytic activity among CD4+ T cells in the subset of infused PyVST products tested, our results generally support the central role of T helper compartment in maintenance of immune competence and protection against many other latent viruses.(29)
Partially matched PyVST products were safe and well-tolerated and did not induce clinically manifest IRIS or GVHD. Seven of the 12 patients survived PML beyond one year from treatment initiation, exceeding survival expectations based on published literature. Excluding patients who died of underlying disease (2 in this cohort) or complication unrelated to VST (VZV encephalitis in (17)), disease-specific survival rates observed in this pilot study are comparable to those reported previously in PML treated with BK- (2 of 3 patients) and JC-VST (5 of 8 patients)(17,18); overall survival rates are similarly comparable. As previously reported(30,31), high baseline CSF viral load was associated with higher mortality in this cohort. However, low CSF viral load in several screened patients who died before treatment was possible, suggests that when immune suppression cannot be reversed, low viral load may identify a window of opportunity for treatment rather than a reliable prognostic biomarker of benign disease. Although not reaching statistical significance in this small cohort, survivors tended to have higher mean age, shorter time to treatment, higher baseline CD4 and CD8 counts, less baseline disability, lower baseline PML lesion burden by MRI, and more commonly received PyVST product derived from JCV Ab positive donors; these trends will need to be investigated in larger cohorts. Increasing potency of cell products and maximizing the potential in vivo activity are important areas for future development. In addition to optimization of manufacturing protocols, development of methods for identifying donors with most robust JCV-antiviral reactivity (including JCV Ab status or other biomarkers) or robust cross-reactivity with recipient antigen-presenting cells will be of great value. A limitation of this study is the lack of measurement of endogenous pre- and post-treatment JCV-antiviral activity in the patient recipient, which might have provided evidence of virus-specific immune reconstitution, either spontaneous or facilitated by PyVST treatment; longitudinal quantification of T cell subpopulations (Supplementary materials) provide some support for transient expansions temporally related to PyVST infusions.
Prospective clinical trials in PML have been scarce, limited by the rarity of this disease, its rapid course, lack of validated outcome measures, and design challenges related to heterogeneity of the patient populations affected. This pilot study highlighted the additional challenge of travel to the study site for patients with progressive disability, which led to early voluntary withdrawal even among patients who ultimately survived PML. Even when survival from PML was achieved, the underlying disease commonly continued to progress, leading to severe morbidity or death within the following year.
Important limitations of this study include the small sample size and the lack of a control arm. As a pilot study, the objective was to inform the design of a future adequately powered, controlled study, which will be essential to provide definitive evidence of efficacy. The excellent safety profile of VST may support less stringent or decentralized patient follow-up, which might improve access to treatment. Larger cohorts will be needed to address which patients, and which donors, are best suited for this treatment approach.
Supplementary Material
Evidence before this study
We searched PubMed for articles describing use of viral-specific T cells (VST) for treatment of progressive multifocal leukoencephalopathy (PML) published up until May 5, 2021, using the search terms “progressive multifocal leukoencephalopathy” AND “virus specific T cells” OR “T cell therapy” OR “adoptive transfer.” Only peer-reviewed, English-language reports of human cohort studies were considered. Only four publications reporting use of viral-specific T cells for treatment of PML were identified. Prior to the initiation of our study in 2016, there was a single case report, published in 2011, of a patient treated successfully using JCV-specific T cells generated from a 1st degree relative donor who had previously been the hematopoietic stem cell transplant donor.
Added value of this study
Although case series have since described the use of VST in 13 additional people, subsuming a variety of methodologies including third-party banked BKV-specific T cells, ex vivo expanded autologous and allogeneic JCV-specific T cells, and a single case of unexpanded JCV-reactive T cells isolated via cytokine capture strategy, our pilot study is the largest and only prospective study of VST conducted to date in PML. The study builds on prior reports demonstrating tolerability and safety of T cell products and supports feasibility of on-demand manufacture of VST for the treatment of this disease. The prospective design contributes additional rigor to previously published literature.
Implications of all the available evidence
Our data provide clear support for further development of VST for the treatment of PML and confirm an excellent safety profile. Our findings also highlight areas for future research, including optimization of donor selection and product manufacture, and provide valuable information for the design of upcoming studies. Though not reaching statistical significance in this small cohort, several features appear to have prognostic value that could be important for participant stratification in a future randomized study, including baseline disability and quantitative measurement of MRI lesion burden. Sample size calculations for future studies will also benefit from our detailed accounting of all screened study participants. Our study further emphasizes some of the challenges that will need to be considered in the design of future studies, in particular that PML-related disability often accumulates rapidly, leading to difficulty with travel to a central study site.
Funding:
This study was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke of the National institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing
Protocol and consent forms will be made available upon email request to the corresponding author (Irene Cortese; corteseir@ninds.nih.gov). All data requests should be submitted to IC for consideration. Access to available deidentified participant data may be granted 12 months after publication. Requesters will be asked to complete an application form detailing proposed use. A data-sharing agreement will need to be
Declaration of Interests
IC reports providing free consultative advice to Cellevolve, outside the submitted work. IC is a shareholder in Nouscom AG and Reithera AG, outside the submitted work. DSR reports non-financial support from Biogen, outside the submitted work. In addition, DSR has a patent “System and method of automatically detecting tissue abnormalities” (US Patent 9,607,392) issued, and a patent “Method of analyzing multisequence MRI data for analyzing brain abnormalities in a subject” (US Patent 9,888,876) issued. PM reports having received consultation fees from ATARA Biological and Astra Zeneca, outside the submitted work. JO, ESB, OAL, FA, JD, IO, BJB, NDZ, MKS, KB, LR, GN, YEA, BRS, MCM, EOM, SJ, DS, SH, SP, and AN report no competing interests.
References
- 1.Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DWG, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003September;71(1):115–23. [DOI] [PubMed] [Google Scholar]
- 2.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009March15;199(6):837–46. [DOI] [PubMed] [Google Scholar]
- 3.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. Atwood WJ, editor. PLoS Pathog. 2009March;5(3):e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson EM, Wortman MJ, Dagdanova AV, Lundberg PS, Daniel DC. Polyomavirus JC in the context of immunosuppression: a series of adaptive, DNA replication-driven recombination events in the development of progressive multifocal leukoencephalopathy. Clin Dev Immunol. 2013;2013(4):197807–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand P, Hotan GC, Vogel A, Venna N, Mateen FJ. Progressive multifocal leukoencephalopathy: A 25-year retrospective cohort study. Neurol Neuroimmunol Neuroinflamm. 2019November;6(6):e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. The Lancet Neurology. 2018May;17(5):467–80. [DOI] [PubMed] [Google Scholar]
- 7.Dunham SR, Schmidt R, Clifford DB. Treatment of Progressive Multifocal Leukoencephalopathy Using Immune Restoration. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2020July;17(3):955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifford DB, De Luca A, DeLuca A, Simpson DM, Arendt G, Giovannoni G, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. The Lancet Neurology. 2010April;9(4):438–46. [DOI] [PubMed] [Google Scholar]
- 9.Berger JR. Progressive multifocal leukoencephalopathy in acquired immunodeficiency syndrome: explaining the high incidence and disproportionate frequency of the illness relative to other immunosuppressive conditions. J Neurovirol. 2003;9Suppl 1(s1):38–41. [DOI] [PubMed] [Google Scholar]
- 10.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009May14;113(20):4834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migliori E, Chang M, Muranski P. Restoring antiviral immunity with adoptive transfer of ex-vivo generated T cells. Curr Opin Hematol. 2018November;25(6):486–93. [DOI] [PubMed] [Google Scholar]
- 12.Naik S, Nicholas SK, Martinez CA, Leen AM, Hanley PJ, Gottschalk SM, et al. Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes. J Allergy Clin Immunol. 2016May;137(5):1498–1505.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013June27;121(26):5113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Science translational medicine. 2014June25;6(242):242ra83–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017November1;35(31):3547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balduzzi A, Lucchini G, Hirsch HH, Basso S, Cioni M, Rovelli A, et al. Polyomavirus JC-targeted T-cell therapy for progressive multiple leukoencephalopathy in a hematopoietic cell transplantation recipient. Bone Marrow Transplant. 2011July;46(7):987–92. [DOI] [PubMed] [Google Scholar]
- 17.Berzero G, Basso S, Stoppini L, Palermo A, Pichiecchio A, Paoletti M, et al. Adoptive Transfer of JC Virus-Specific T Lymphocytes for the Treatment of Progressive Multifocal Leukoencephalopathy. Annals of Neurology. 2021April;89(4):769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muftuoglu M, Olson A, Marin D, Ahmed S, Mulanovich V, Tummala S, et al. Allogeneic BK Virus-Specific T Cells for Progressive Multifocal Leukoencephalopathy. The New England Journal of Medicine. 2018October11;379(15):1443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984August;51(2):458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013April9;80(15):1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortese I, Muranski P, Enose-Akahata Y, Ha S-K, Smith B, Monaco M, et al. Pembrolizumab Treatment for Progressive Multifocal Leukoencephalopathy. The New England Journal of Medicine. 2019April25;380(17):1597–605. [DOI] [PubMed] [Google Scholar]
- 22.Stefoski D, Balabanov R, Waheed R, Ko M, Koralnik IJ, Sierra Morales L. Treatment of natalizumab-associated PML with filgrastim. Ann Clin Transl Neurol. 2019May;6(5):923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alstadhaug KB, Croughs T, Henriksen S, Leboeuf C, Sereti I, Hirsch HH, et al. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. 2014August;71(8):1030–5. [DOI] [PubMed] [Google Scholar]
- 24.Miskin DP, Chalkias SG, Dang X, Bord E, Batson S, Koralnik IJ. Interleukin-7 treatment of PML in a patient with idiopathic lymphocytopenia. Neurol Neuroimmunol Neuroinflamm. 2016April;3(2):e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harel A, Horng S, Gustafson T, Ramineni A, Farber RS, Fabian M. Successful treatment of progressive multifocal leukoencephalopathy with recombinant interleukin-7 and maraviroc in a patient with idiopathic CD4 lymphocytopenia. J Neurovirol. 2018October;24(5):652–5. [DOI] [PubMed] [Google Scholar]
- 26.Beck ES, Cortese I. Checkpoint inhibitors for the treatment of JC virus-related progressive multifocal leukoencephalopathy. Curr Opin Virol. 2020February;40:19–27. [DOI] [PubMed] [Google Scholar]
- 27.Pasquier Du RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain : a journal of neurology. 2004September;127(Pt 9):1970–8. [DOI] [PubMed] [Google Scholar]
- 28.Gheuens S, Bord E, Kesari S, Simpson DM, Gandhi RT, Clifford DB, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011July;85(14):7256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jelcic I, Jelcic I, Kempf C, Largey F, Planas R, Schippling S, et al. Mechanisms of immune escape in central nervous system infection with neurotropic JC virus variant. Annals of neurology. 2016March;79(3):404–18. [DOI] [PubMed] [Google Scholar]
- 30.Dong-Si T, Gheuens S, Gangadharan A, Wenten M, Philip J, McIninch J, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol. 2015December;21(6):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bossolasco S, Calori G, Moretti F, Boschini A, Bertelli D, Mena M, et al. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin Infect Dis. 2005March1;40(5):738–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


